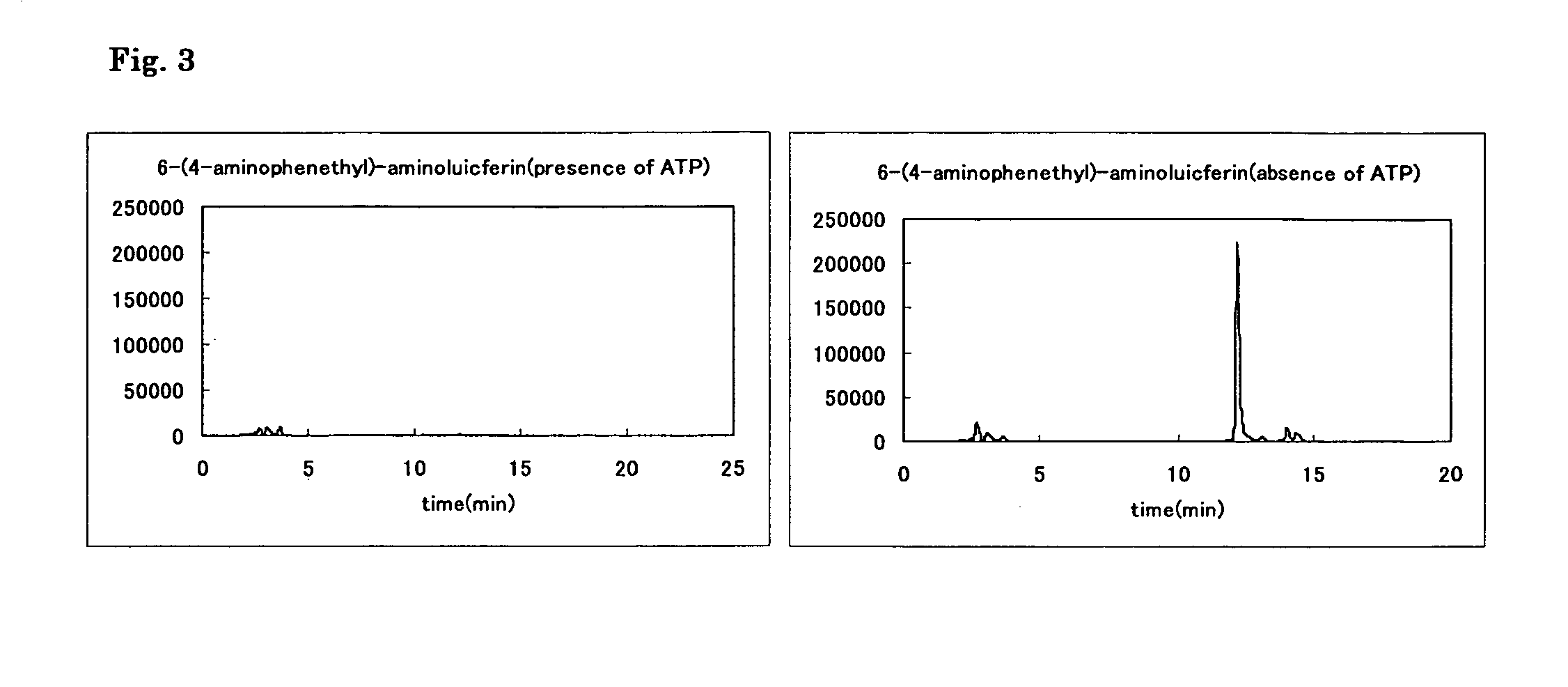
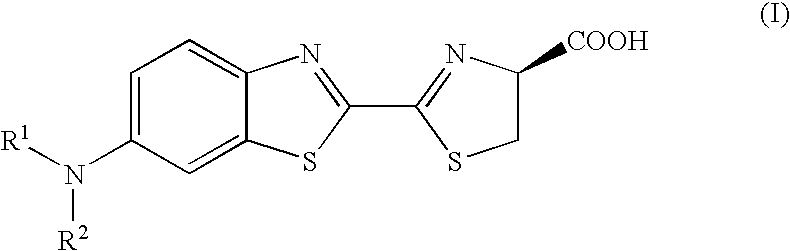
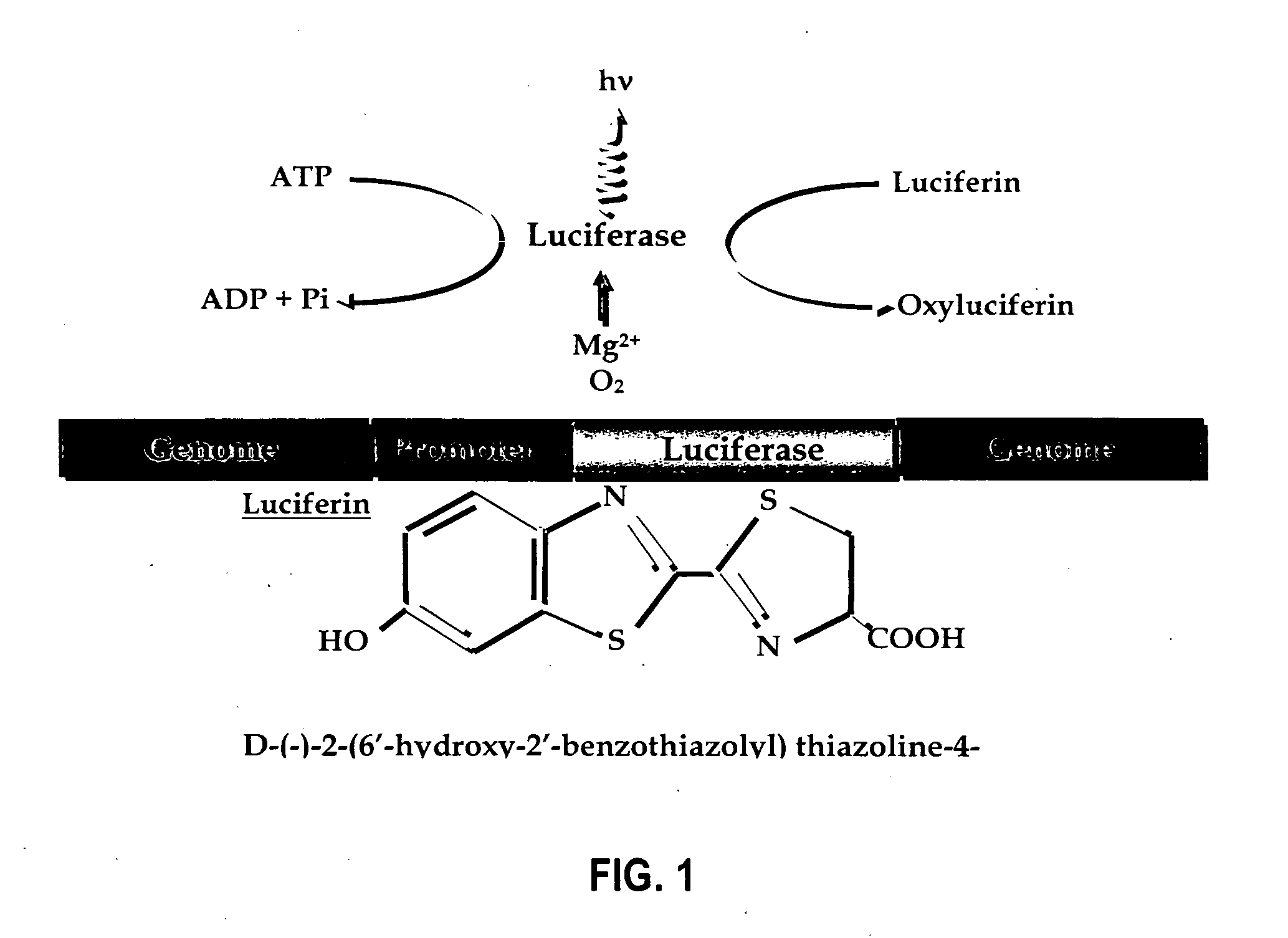
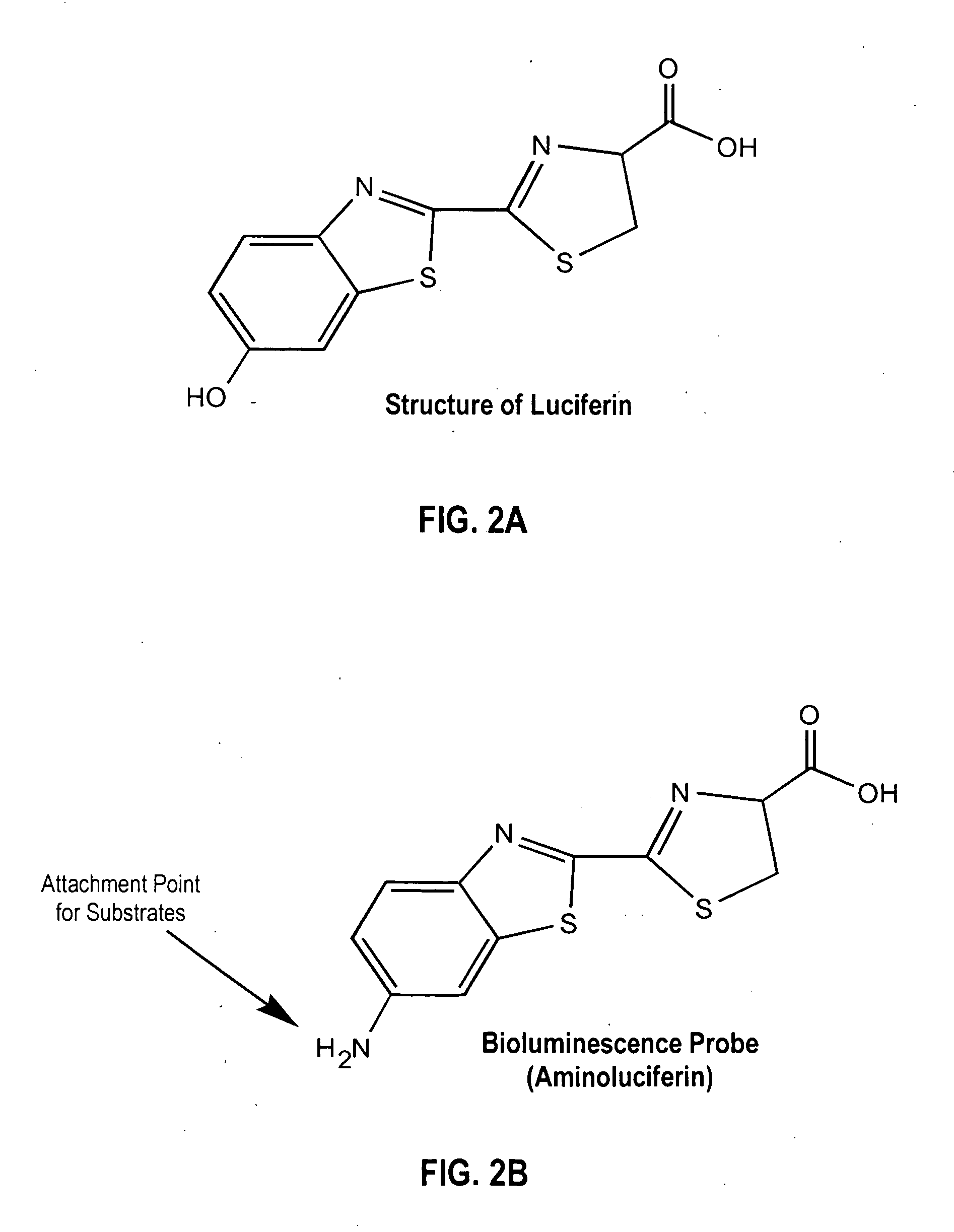
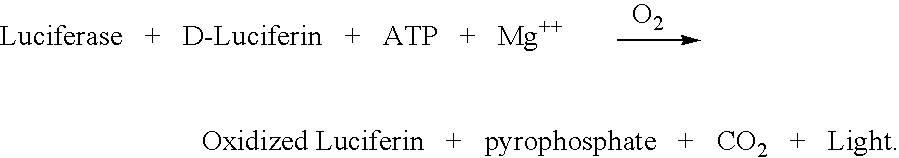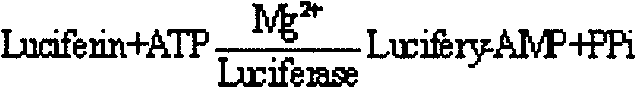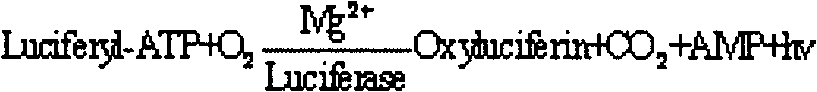Patents
Literature
139 results about "Luciferase Gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
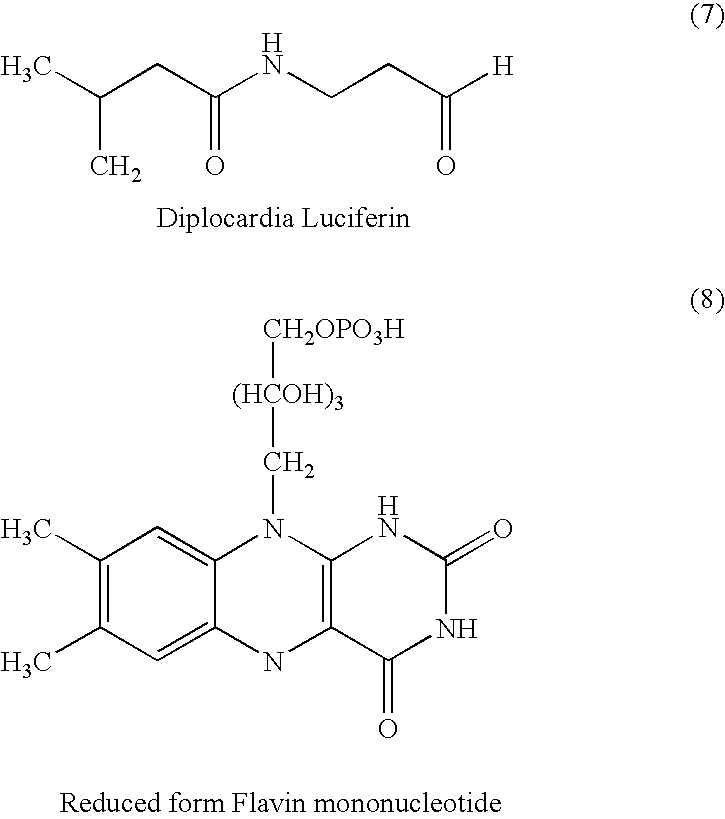
Luciferases can be produced in the lab through genetic engineering for a number of purposes. Luciferase genes can be synthesized and inserted into organisms or transfected into cells. Mice, silkworms, and potatoes are just a few of the organisms that have already been engineered to produce the protein.
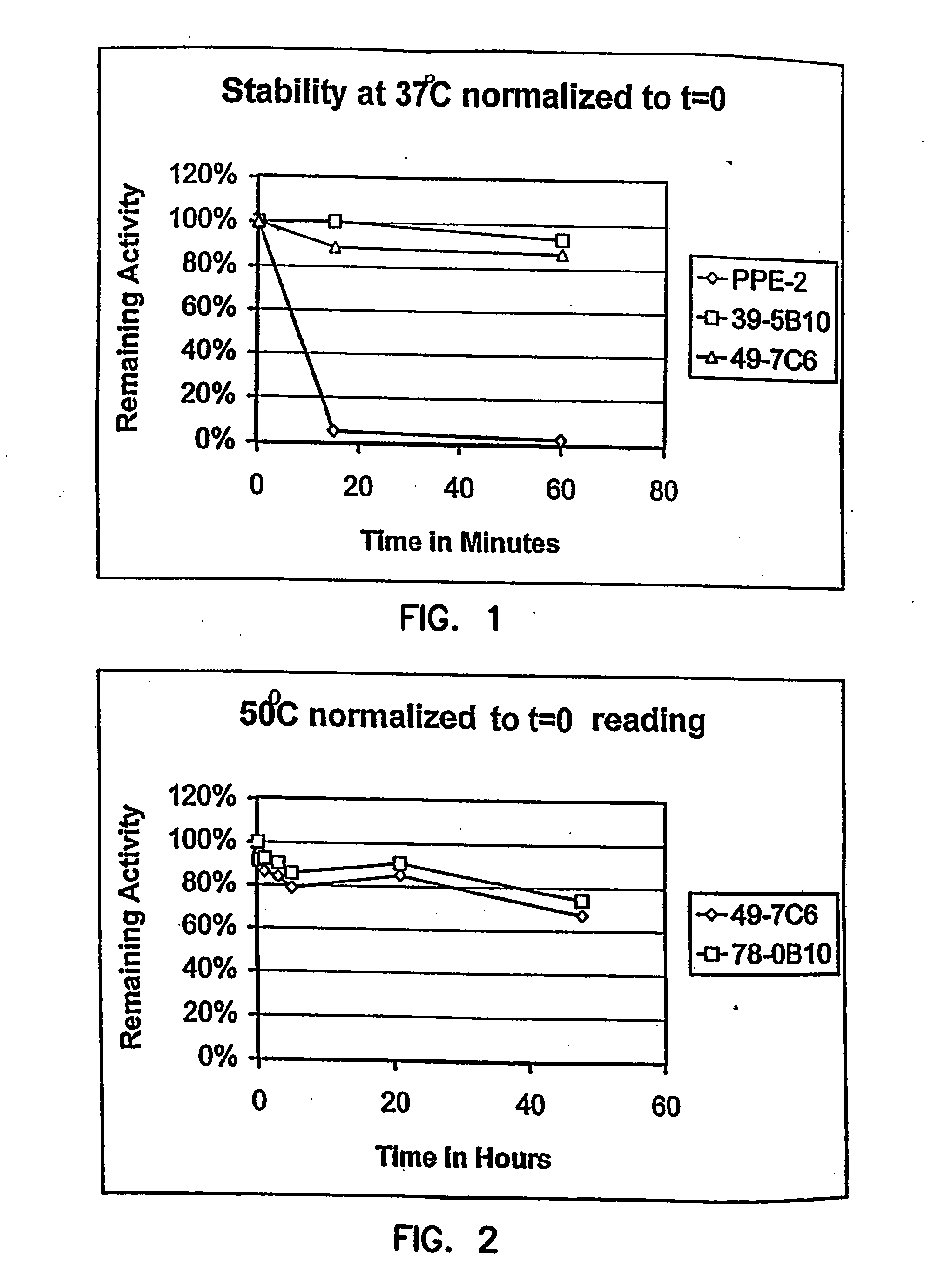
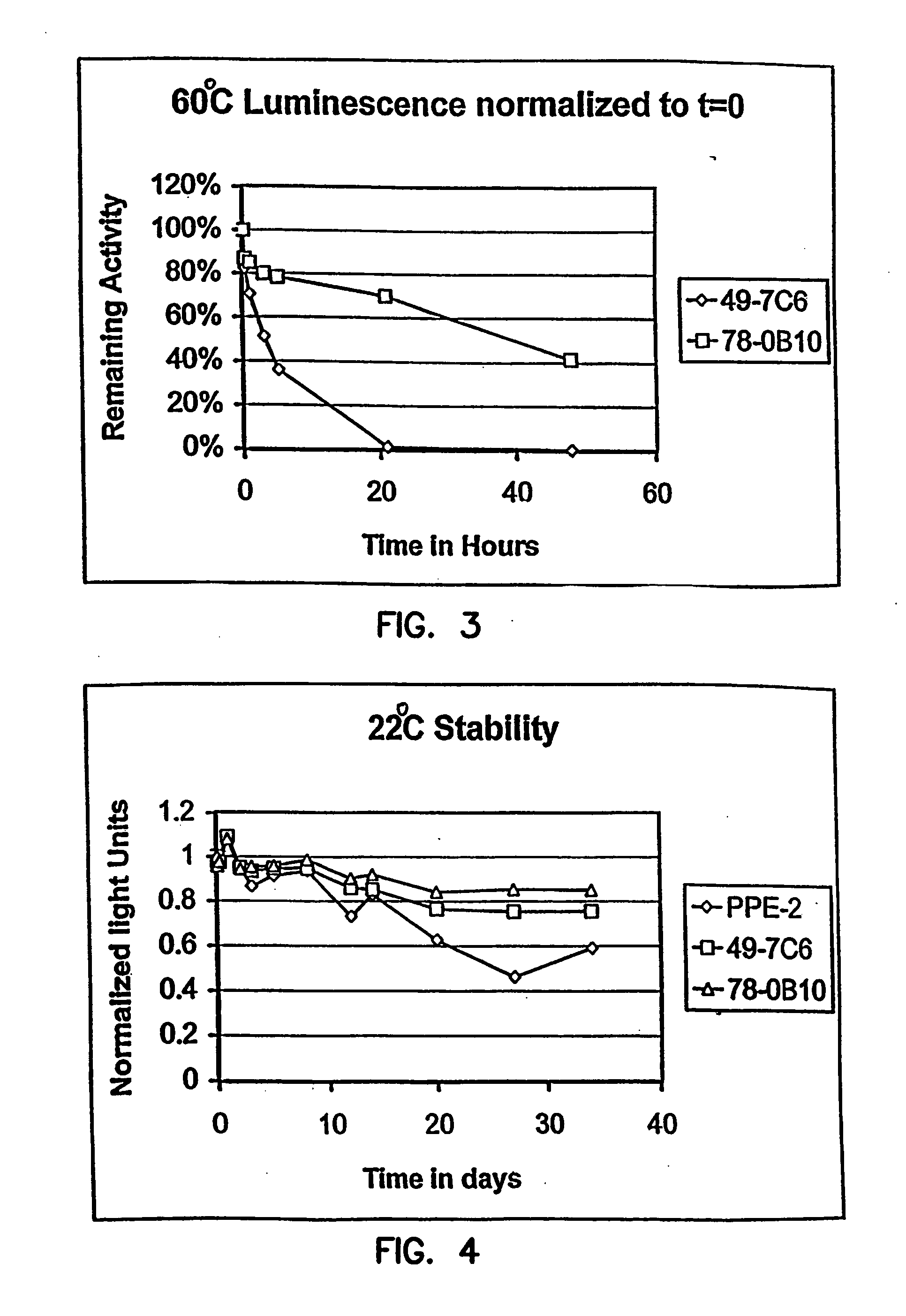
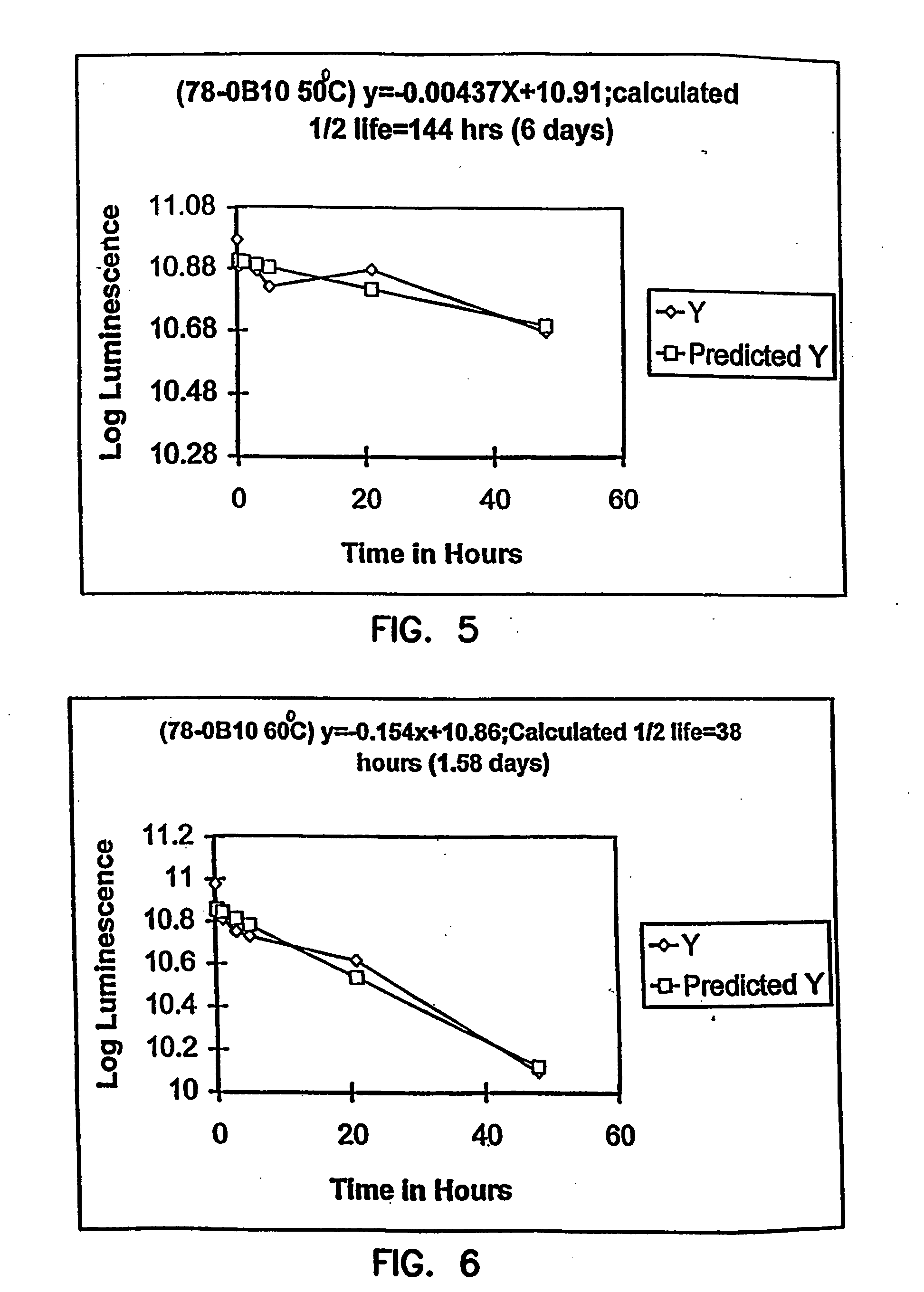
Thermostable luciferases and methods of production
InactiveUS20060183212A1High luminous intensityLost less than 5% luminescence activityFungiNanotechBiotechnologyLuciferase Gene
Luciferase enzymes with greatly increased thermostability, e.g., at least half lives of 2 hours at 50° C., cDNAs encoding the novel luciferases, and hosts transformed to express the luciferases, are disclosed. Methods of producing the luciferases include recursive mutagenesis. The luciferases are used in conventional methods, some employing kits.
Owner:PROMEGA CORP
In-vitro evaluation cell model of skin sensitization of compound and construction method of cell model
ActiveCN108103098AEffective resolutionSensitization Study SpecificHydrolasesDrug screeningSkin sensitizationLuciferase Gene
The invention discloses an in-vitro evaluation cell model of skin sensitization of a compound and a construction method of the cell model. The construction method of the cell model comprises the following steps of: designing and constructing an sgRNA (small guide ribonucleic acid) expression vector with CRISPR / Cas9 (clustered regularly interspaced short palindromic repeats / CRISPR associated protein 9), designing and constructing a homologous recombinant vector capable of knocking a reporter gene connected with a spontaneous lysis peptide sequence into an expression cassette of an HMOX1 (heme oxygenase (decycling) 1) gene, and cotransfecting a cell with the homologous recombinant vector, hCas9 (humanized Cas9) plasmid and the sgRNA expression vector for monoclone enlarging culture to form the cell model. The HaCaT cell model with a luciferase gene knocked in before a termination codon of the HMOX1 gene by a combined CRISPR / CAS9 cell monoclone technology. The cell model achieves synchronous expression of the luciferase gene and the HMOX1 gene, so that a sensitization compound and a non-sensitization compound are effectively differentiated, and the more specific and sensitive cell model is provided for compound sensitization studies.
Owner:SOUTH CHINA UNIV OF TECH
Novel luciferin derivatives
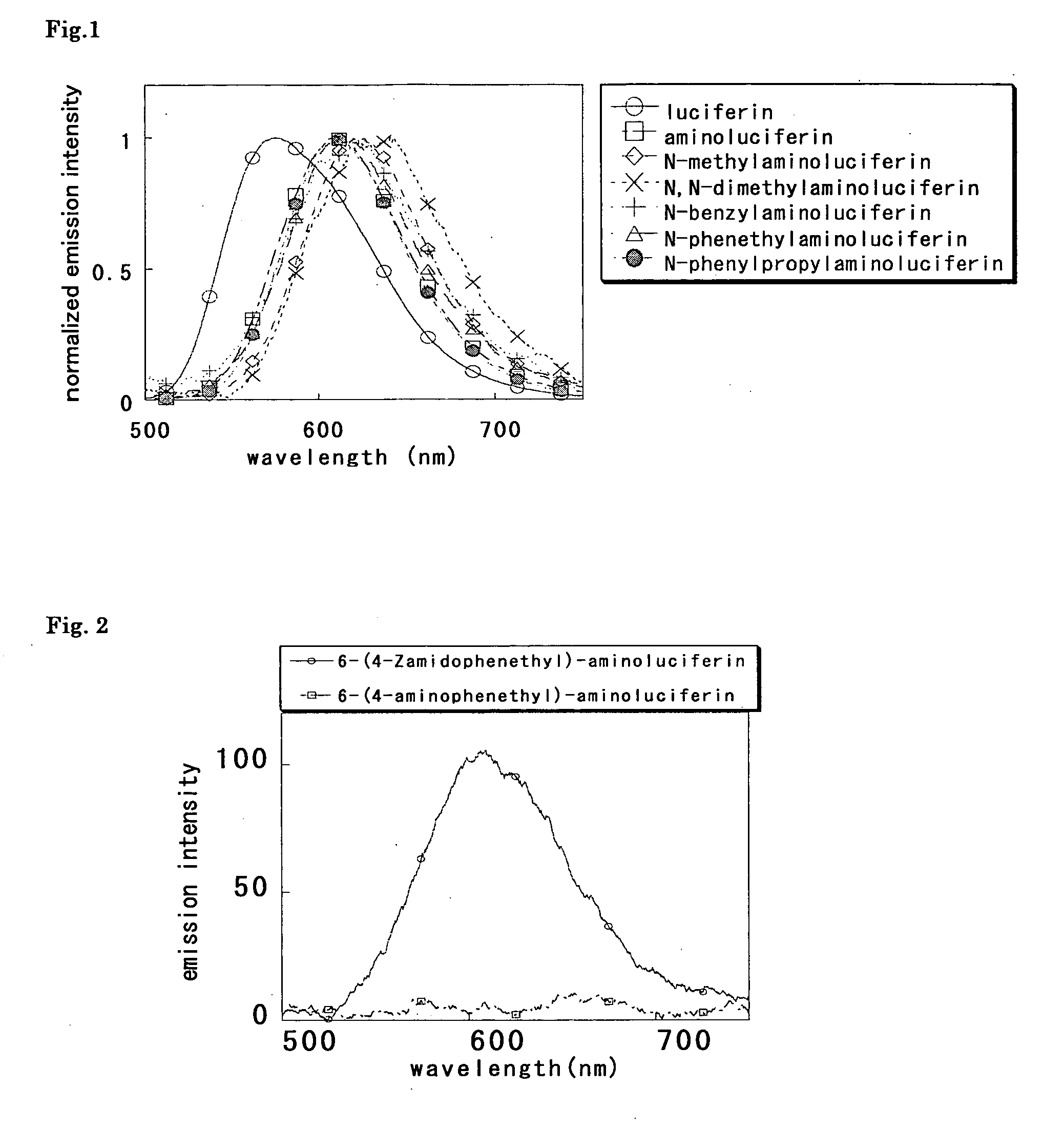
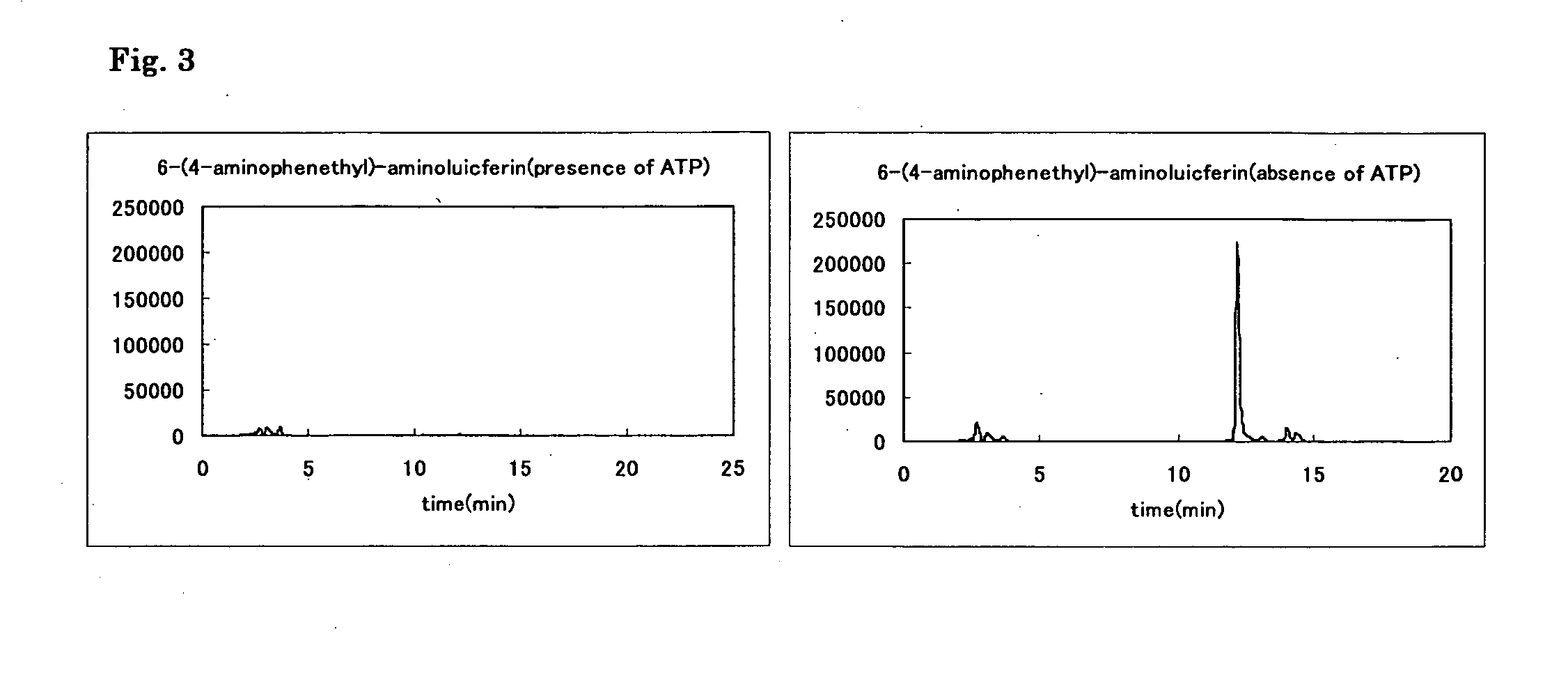
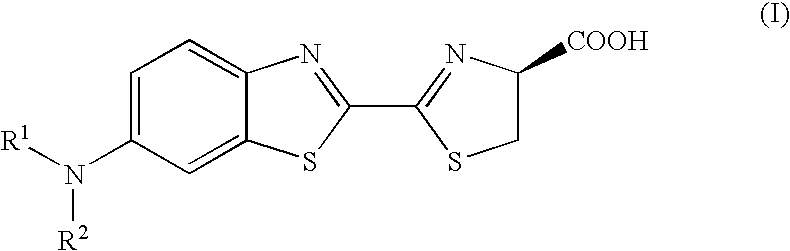
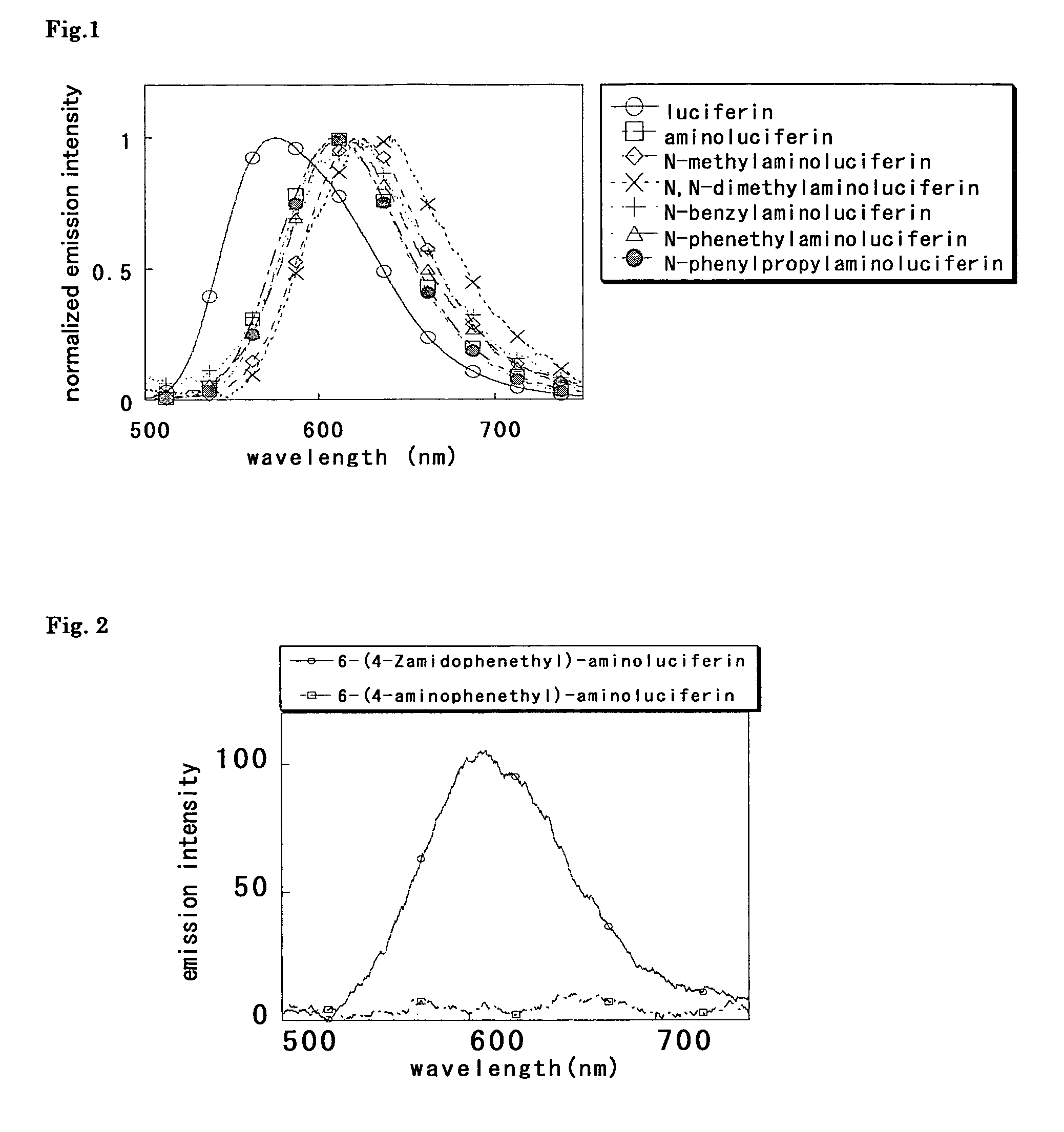
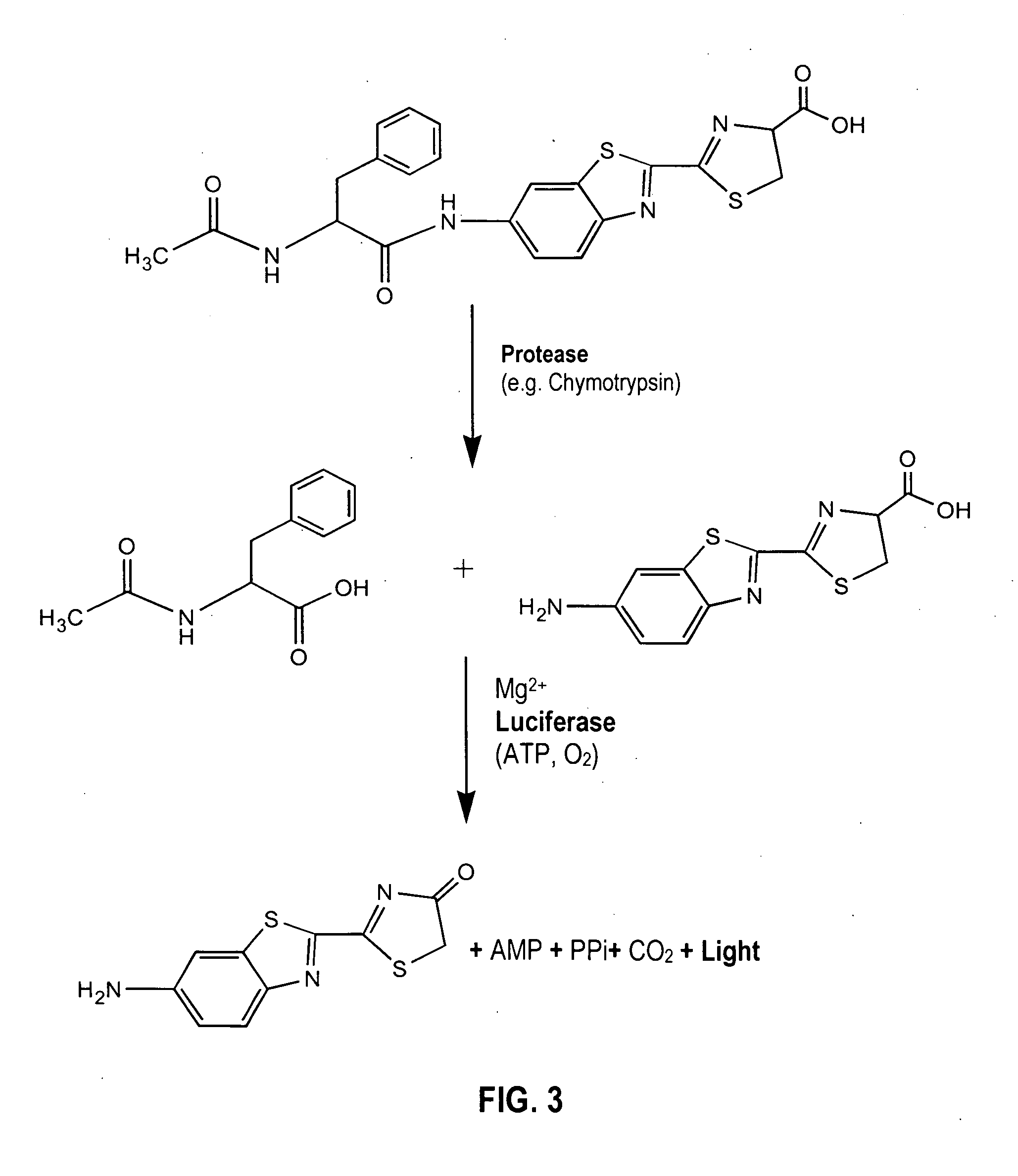
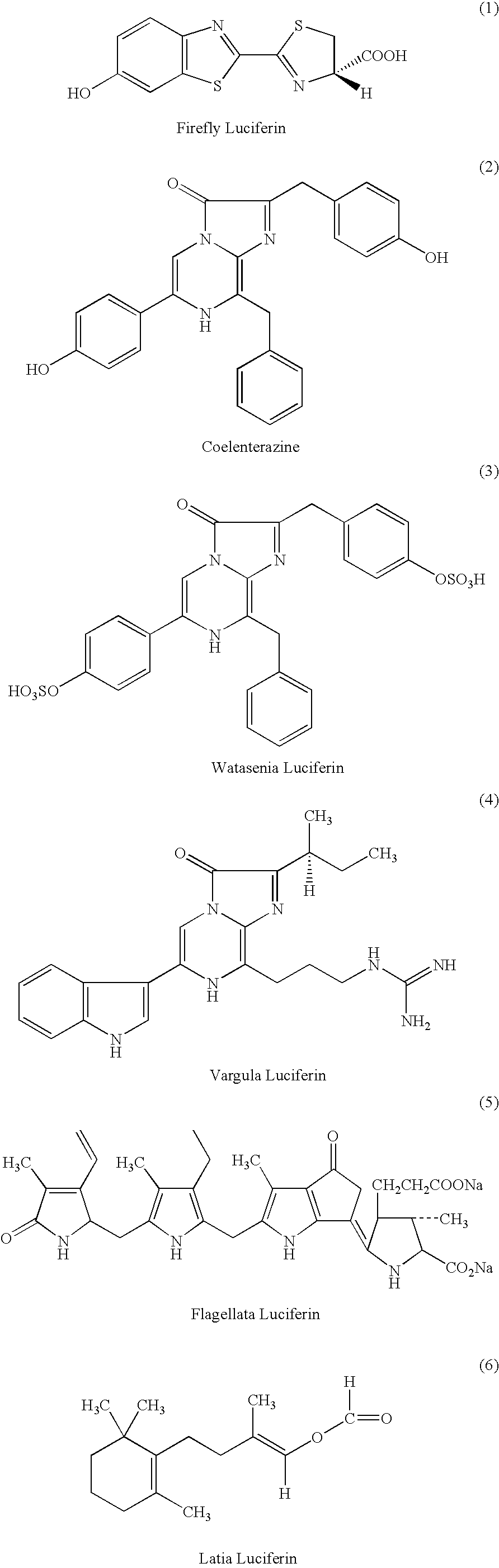
A compound represented by the following general formula (I) or a salt thereof: [wherein R1 and R2 represent hydrogen atom, a C1-6 alkyl group, or a group represented by the following formula (A): [wherein X1 and X2 represent hydrogen atom, or a group represented as —N(R3)(R4) (R3 and R4 represent hydrogen atom, a C1-6 alkyl group, a C1-6 alkylcarbonyl group, or a C1-6 alkyloxycarbonyl group); and n represents an integer of 1 to 6], provided that R1 and R2 do not simultaneously represent hydrogen atom), which is a novel luciferin derivative that serves as a luciferase substrate.
Owner:TOKYO UNIV OF THE
Luciferase expression cassettes and methods of use
InactiveUS6737245B1Improve translationBroadening the range of wavelength of light emittedFungiSugar derivativesLuciferase GeneBacterial luciferase
The present invention relates to bacterial luciferase expression cassettes suitable for conferring bioluminescence properties on Gram-positive bacteria, cells transformed with such cassettes, and methods of making and using such cassettes.
Owner:XENOGEN CORP
Apparatus and methods for chemiluminescent assays
InactiveUS6881554B2Reduce pressureBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismLuciferin
Disclosed is a device and methods for the rapid chemiluminescence assay of surfaces to detect the presence of microbial contamination. The device and methods are suitable for use by untrained personnel under the relatively harsh and variable conditions found in the field, for example in fast food restaurants and other food preparation areas. The chemiluminescence reaction that is the source of the analytical signal in the disclosed assay device and method is preferably based on a luciferase / luciferin system.
Owner:NEOGEN CORP
Luciferin derivatives
Owner:TOKYO UNIV OF THE
Luciferyl peptide substrate
InactiveUS20060073529A1Promote localizationPeptide/protein ingredientsMicrobiological testing/measurementLuciferase GeneCell membrane
Provided are luciferyl peptide substrates that are produced by attaching specifically prepared peptide conjugates to luciferin, and / or its analogs and derivatives. The luciferyl peptide substrates are incapable of penetrating cell membranes and tissue barriers. Cleavage of the peptide conjugates from the luciferyl peptide substrates releases the luciferin, which upon contact with luciferase emits photons for easy detection. The luciferyl peptide substrates may be used in assays to detect pathogens, test protease inhibitors, probe cell physiology, assess protease activity in oncogenesis, and improve specific and regulated drug delivery.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Renilla reniformis fluorescent proteins, nucleic acids encoding the fluorescent proteins and the use thereof in diagnostics, high throughput screening and novelty items
Isolated and purified nucleic acids encoding green fluorescent proteins from Renilla reniformis and the green fluorescent protein encoded thereby are also provided. Mutants of the nucleic acid molecules and the modified encoded proteins are also provided. Compositions and combinations comprising the green fluorescent proteins and / or the luciferase are further provided.
Owner:GAUS
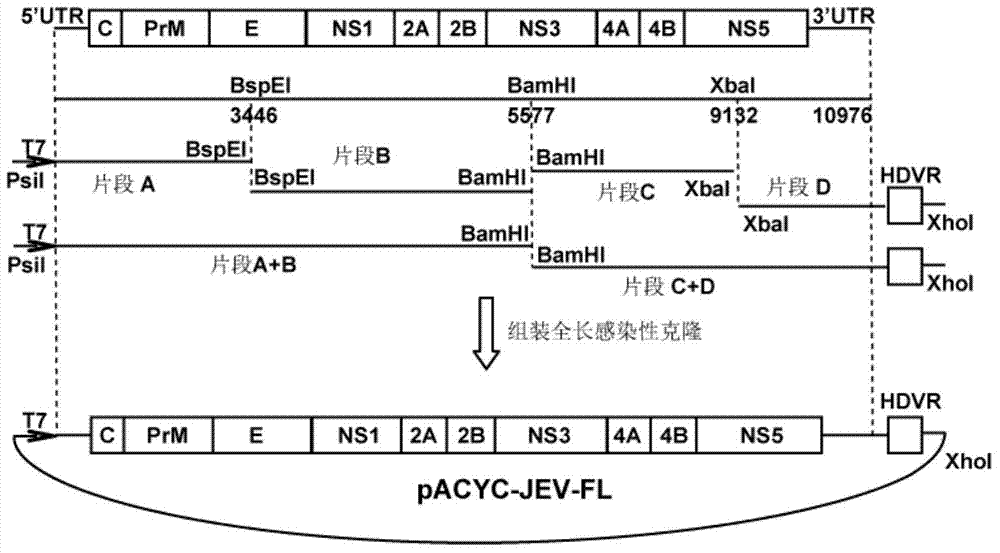
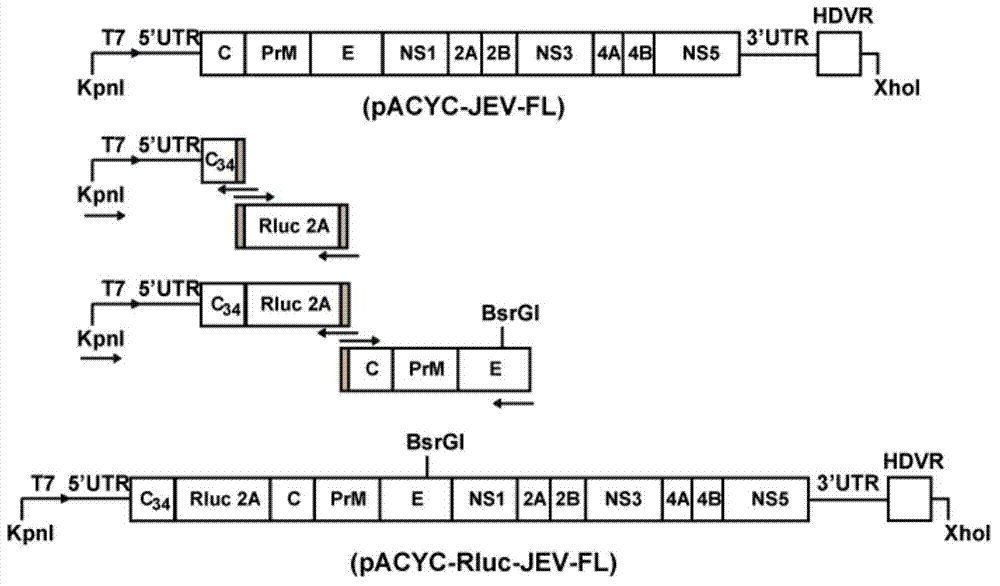
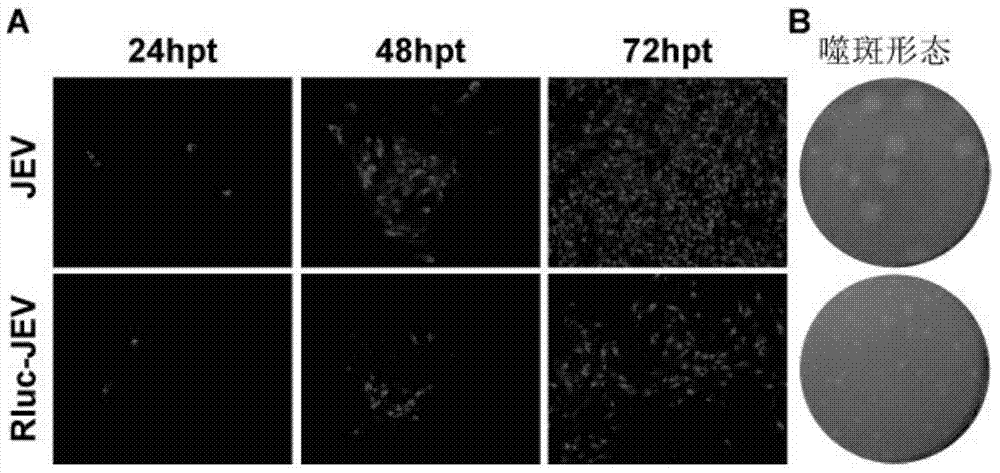
Japanese encephalitis virus (JEV) infectious clone with luciferase gene and building method and application thereof
InactiveCN103497972ASolve the phenomenon of not copyingFast growing trendMicrobiological testing/measurementFluorescence/phosphorescenceLuciferase GeneVaccine evaluation
The invention discloses a Japanese encephalitis virus (JEV) infectious clone with a luciferase gene, and a building method and application thereof. The JEV infectious clone with the luciferase gene is prepared by the following steps: (1) sectional synthesis of a JEV SA14 strain gene sequence; (2) assembling and building of the JEV infectious clone; (3) building of the JEV infectious clone with the luciferase gene. It is proved that a Rluc-JEV report virus with the same growth tendency as the JEV virus can be saved by the JEV infectious clone with the luciferase gene built by the method, and the JEV infectious clone has wide application value in the aspects of an animal model, virus replication and pathogenesis, drug screening and drug action mechanism, live animal imaging and vaccine evaluation and the like through the experiments of immunofluorescence, Rluc activity detection, virus bacteriophage plaque, drug inhibition, live animal imaging, vaccine evaluation and the like.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Luciferase and photoprotein
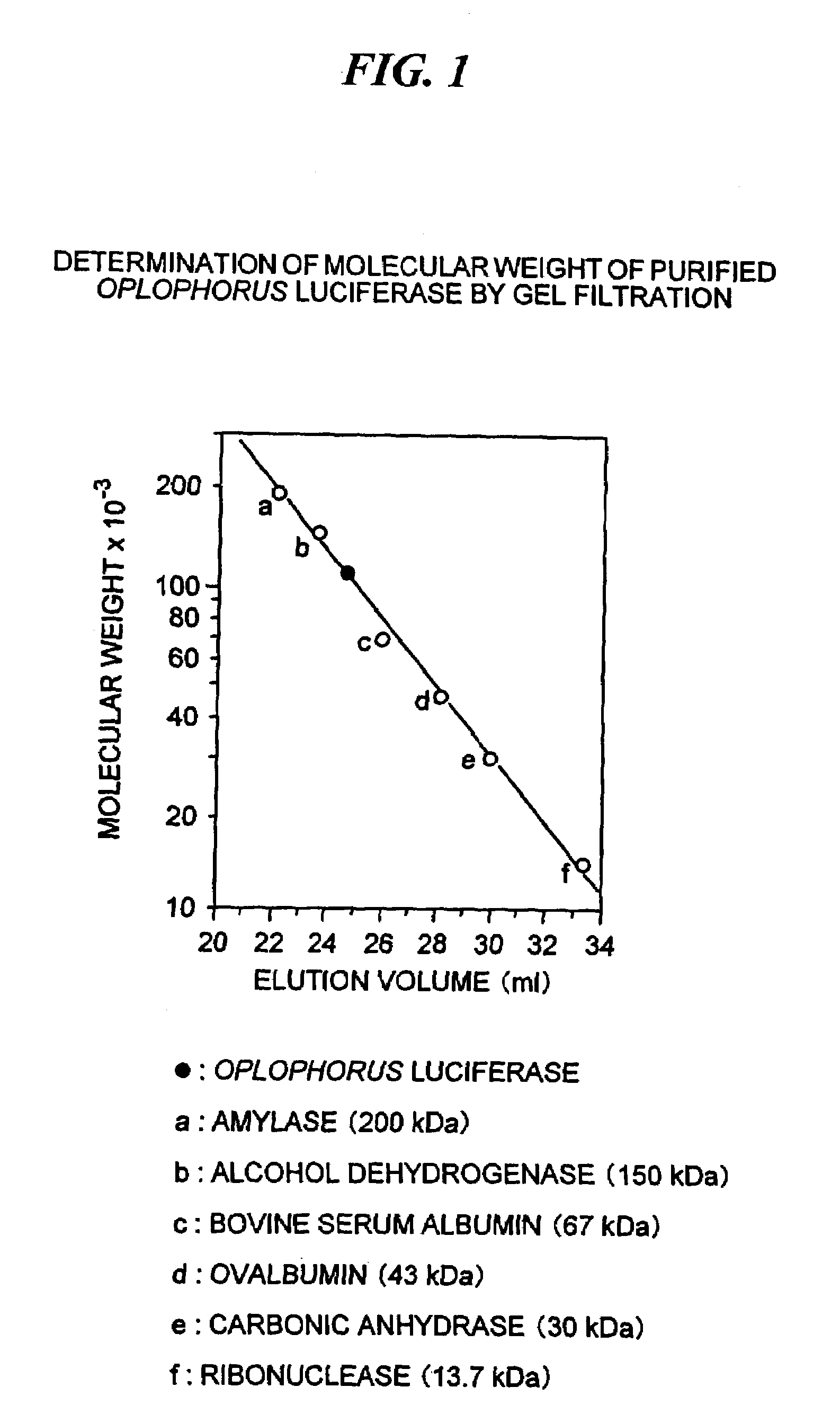
The present invention provides a polynucleotide or polynucleotides encoding Oplophorus luciferase which is composed of 19 kDa and 35 kDa proteins, or the 19 kDa photoprotein, the recombinant secretional Oplophorus luciferase or the 19 kDa photoprotein encoded by the polynucleotide(s), an expression vector containing the polynucleotide(s) and a host transformed with the vector.Further, the invention provides a method for producing the recombinant Oplophorus luciferase or the photoprotein.These proteins could be recombinantly produced by culturing the host cell or by in vitro translation system using the recombinant expression vector.
Owner:JNC CORP
Method and reagent for sequencing
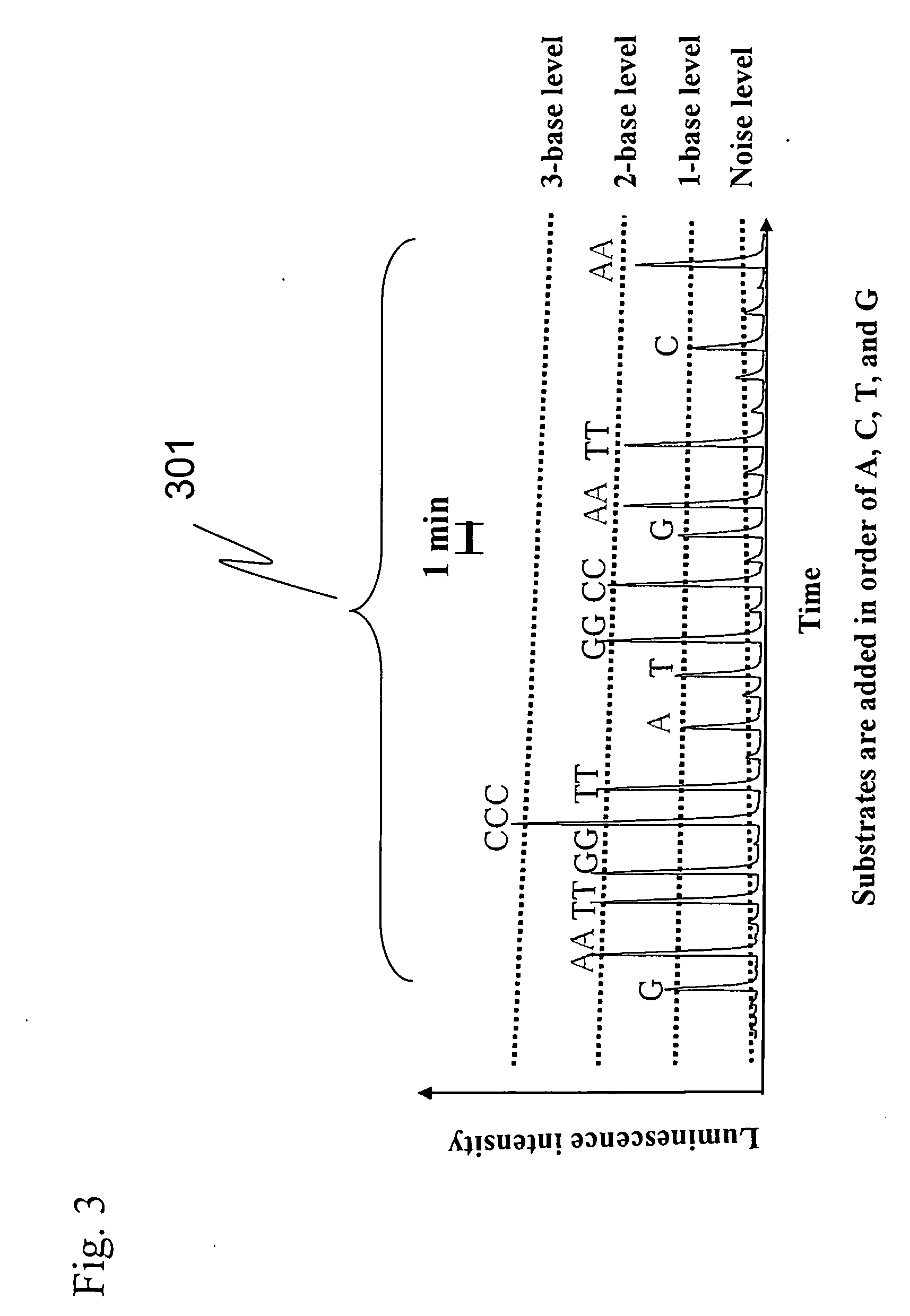
InactiveUS20070166729A1Reduce the amount requiredLow costMicrobiological testing/measurementLuciferase GenePyrophosphate
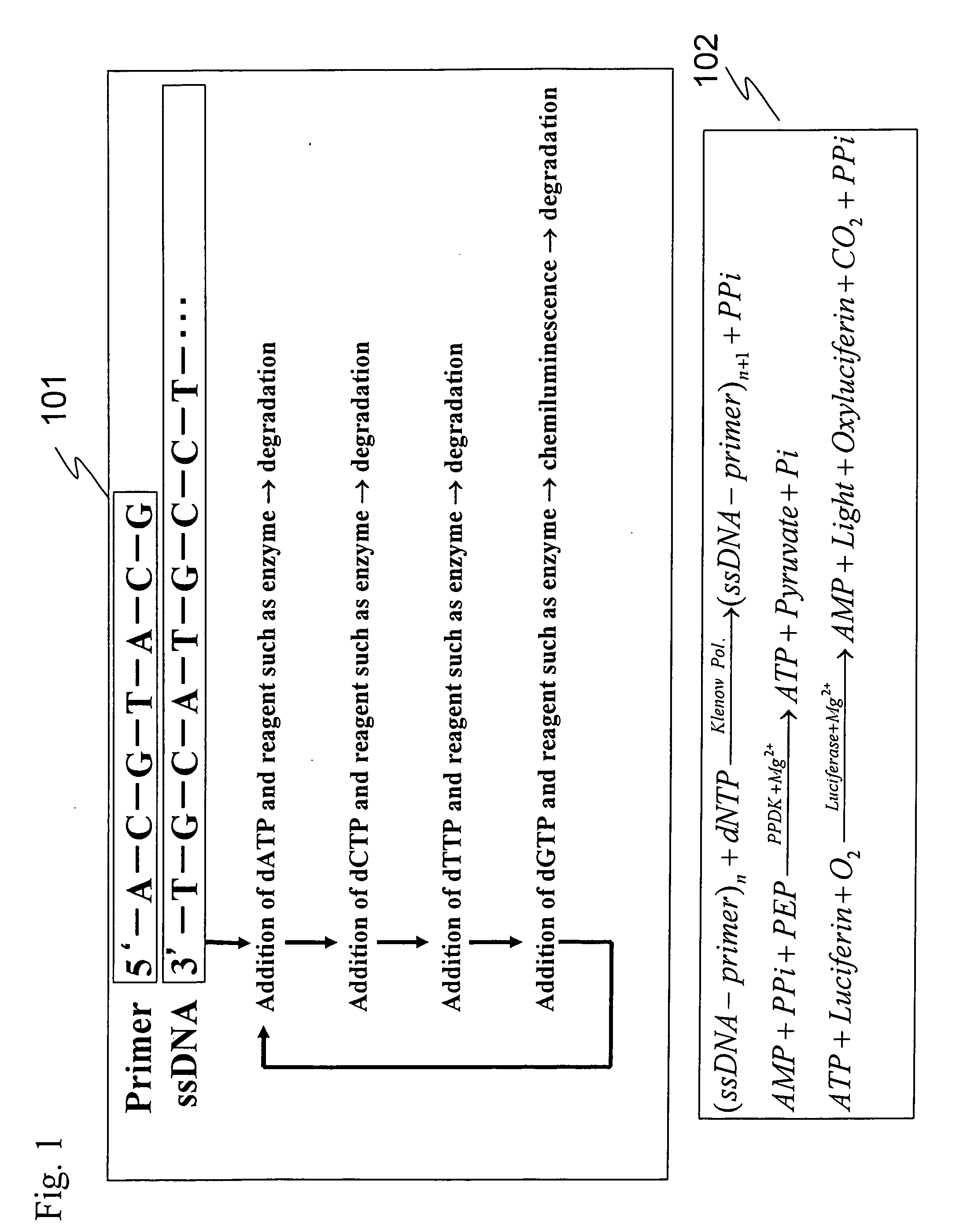
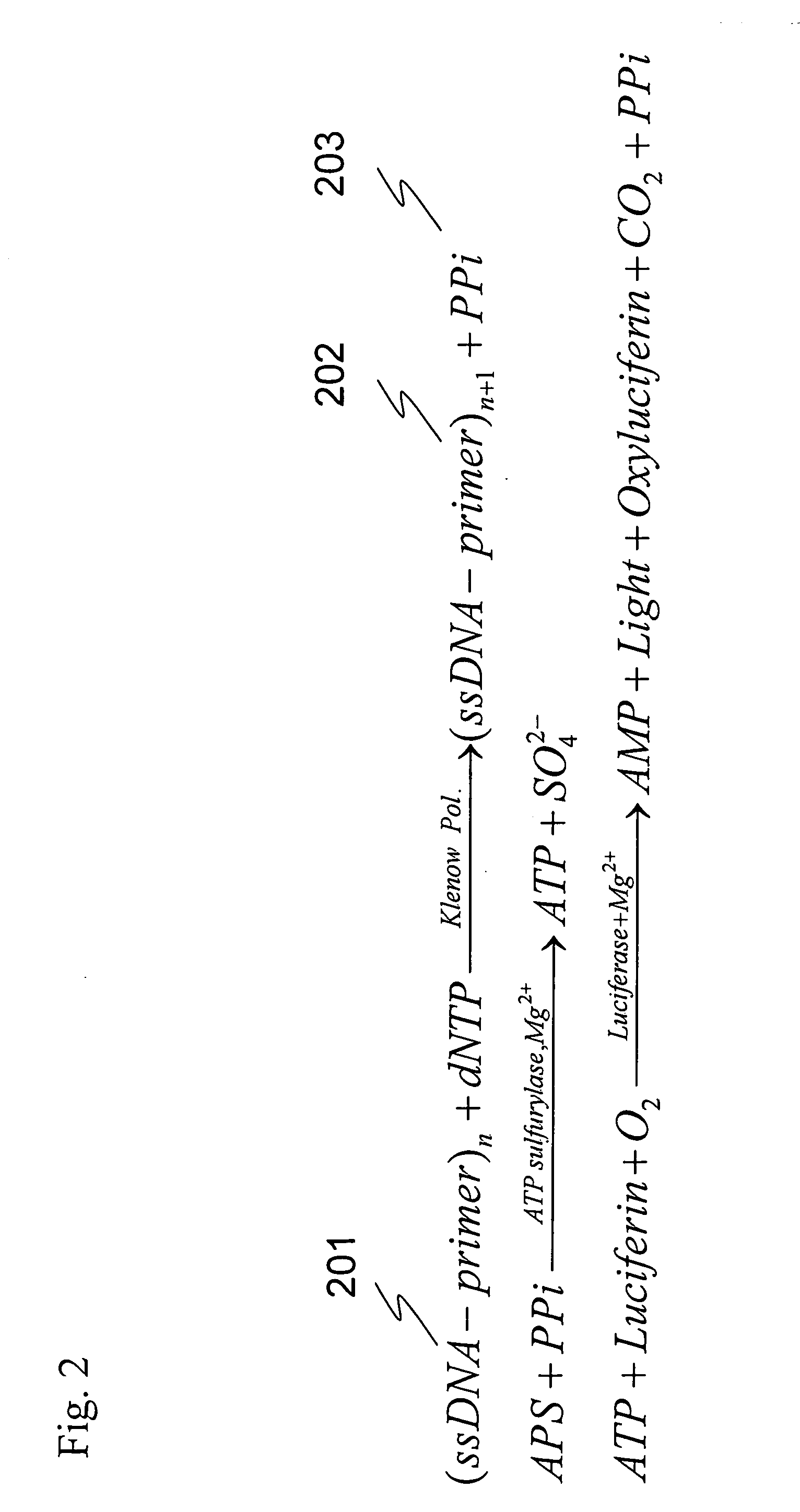
The present invention provides: a method for nucleic acid analysis including the steps of subjecting a reaction solution containing a sample nucleic acid to complementary strand synthesis with the sample nucleic acid as a template, reacting pyrophosphate produced in the complementary strand synthesis with 30 to 800 μM AMP in the coexistence of pyruvate phosphate dikinase to produce ATP, performing luciferase reaction with the ATP as a reaction substrate, and detecting chemiluminescence generated in the luciferase reaction to determine the presence or absence of the complementary strand synthesis; and a kit therefor.
Owner:HITACHI LTD
Recombinant cell strain for detecting dioxin compounds according to behaviour of leuciferinase gene
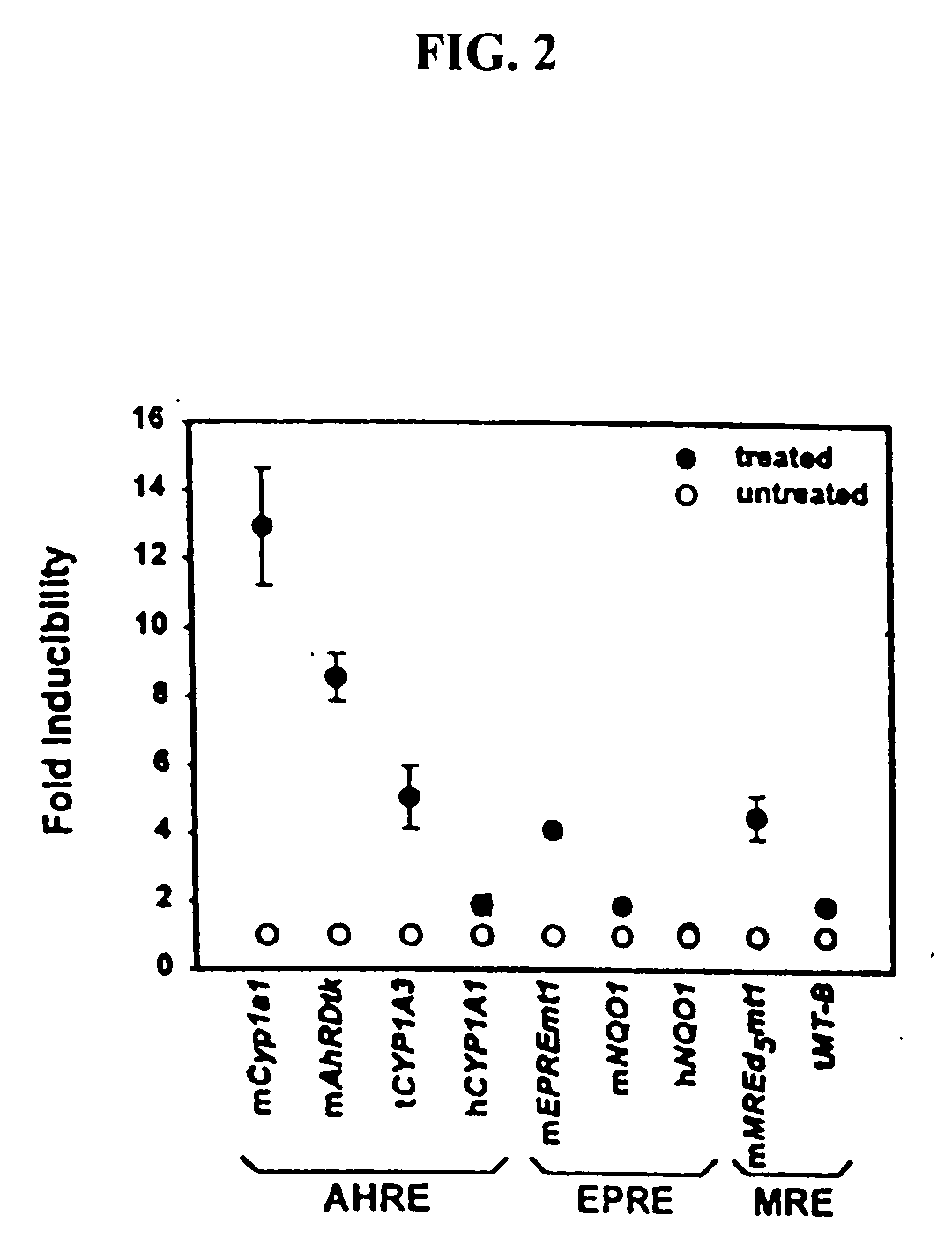
The present invention relates to one recombinant cell strain with foreign leuciferinase gene, and is especially one DNA segment of transfected mouse liver cancer cell strain Hepalcle7-M4P2 and Hepale7-MP and with dioxin reaction segment specific nucleotide sequence í‹cacgcíŒ and í‹gcgtgíŒ. The DNA segment is designed based on Mus musculus CYP1 A1 gene promoter area and partial fore end sequence of the structural gene. Therefore, the recombinant cell strain and the cultured descendant may be used in detecting dioxin compounds from the sample collected from natural environment by means of biological analysis process.
Owner:CHENG SHIU UNIVERSITY
Cell systems and methods for detecting proliferation acitvity
The invention provides proliferative response indicator cell having a vertebrate cell having a luciferase encoding nucleic acid and a heterologous proliferation factor receptor encoding nucleic acid, wherein each of the encoding nucleic acids are operationally linked to expression elements for co-expression of a luciferase polypeptide and a heterologous proliferation factor receptor. The invention also provides a method of determining a cell proliferative response to a proliferation factor. The method includes: (a) contacting a vertebrate cell expressing luciferase and a proliferation factor receptor with a proliferation factor for sufficient time for the proliferation factor to bind to the proliferation factor receptor; (b) culturing the contacted cell expressing luciferase for at least one generation, and (c) measuring the amount of light emission, wherein the luciferase expression is driven from a promoter non-responsive to the proliferation factor and the light emission directly correlates with proliferation factor-mediated cell proliferation. The proliferation factor can be a growth factor, a cytokine or a hormone or an agonist or antagonist thereof. The methods of the invention additionally include determining the effect of an inhibitor of the proliferation factor. Cells used in the method are contacted with a proliferation factor in the presence of a sample suspected of containing an inhibitor of the proliferation factor. The inhibitor can be neutralizing antibody, or binding fragment thereof, to the proliferation factor. The methods of the invention also are applicable as an indicator of cell health or viability. The invention further provides a diagnostic system. The diagnostic system includes a plurality of different vertebrate cell lines each encoding a luciferase gene and a different proliferation factor receptor, the luciferase gene being operationally linked to a promoter non-responsive to a proliferation factor bound by the proliferation factor receptor, wherein light emission from each of the different cell lines being characterized as directly correlating with proliferation factor-mediated cell proliferation.
Owner:AMGEN INC
Single molecule-format bioluminescent probe
InactiveUS8124424B2Easy to introduceConvenient and accurateSugar derivativesMicrobiological testing/measurementLuciferase GeneLigand binding domain
The claimed invention comprises a single molecule-format bioluminescent probe for detecting a target-specific ligand in a living cell, which comprises, a ligand-binding molecule of which conformation is changed upon binding to the ligand, wherein the ligand-binding molecule comprises a ligand-binding domain (LBD) of a nuclear receptor and an LBD-interacting domain that is a co-activator peptide of said nuclear receptor, and an N-terminal polypeptide and a C-terminal polypeptide of a click beetle luciferase (N-CBLuc and C-CBLuc), which flank each end of the ligand-binding molecule, respectively, wherein the N-CBLuc and the C-CBLuc self-complement to generate a luminescent signal only upon binding of the ligand to the ligand-binding molecule.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Cell line screened by anti-oxidant or chemical preventive agent, construction and application
InactiveCN102899351AIncrease vitalityIncreased sensitivityMicrobiological testing/measurementVector-based foreign material introductionAntioxidative responseNeomycin
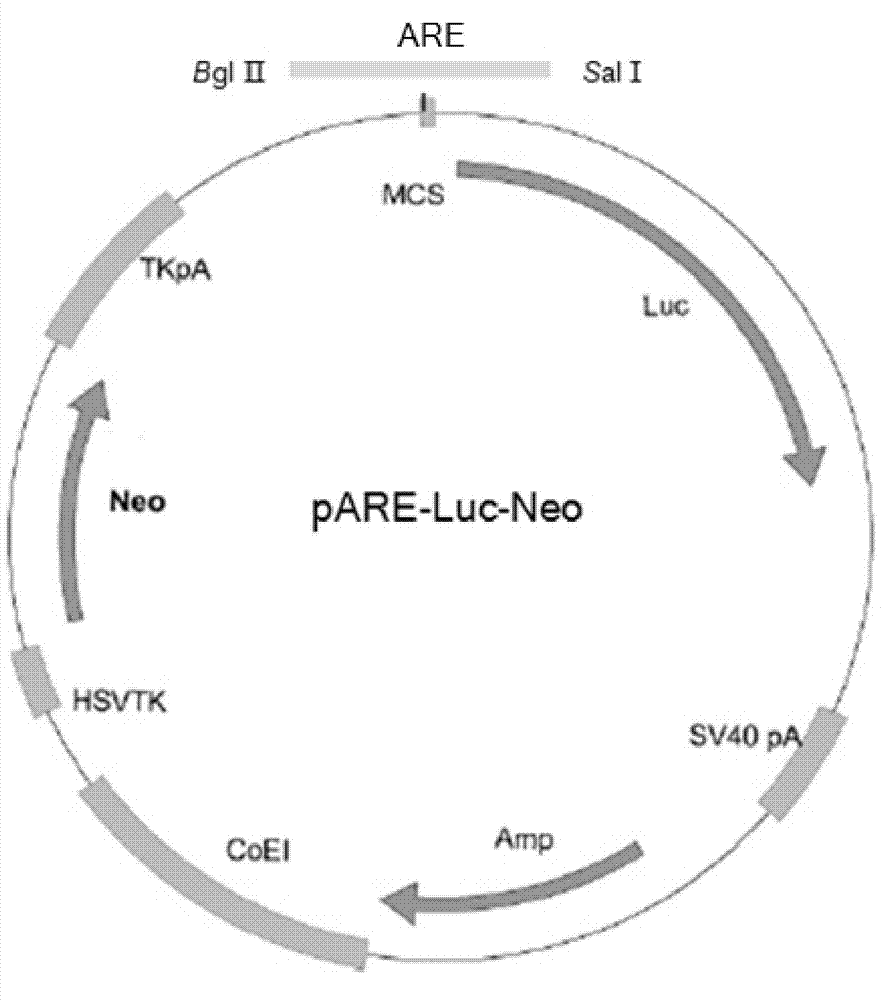
The invention discloses a recombinant plasmid and a construction method, the recombinant plasmid contains an antioxidation reaction element, luciferase gene and neomycin resistant gene. The invention also discloses a cell line screened by an anti-oxidant or a chemical preventive agent, a construction and an application, and the cell line is the cell integrated with the above recombinant plasmid. The provided cell line integrates the ARE gene in a recombinant plasmid carrier pARE-Luc-Neo, luciferase gene and neomycin resistant gene to a genome of a host cell Hek293, by following with subcultring of cell (more than 10 generation), the detection of a luciferase expression is stable, compared with a transient transfection method, and the construction provided by the invention is more convenient, sensitive and reliable.
Owner:TIANJIN YAOYU BIOLOGICAL TECH
Microbial cell sensor for detecting arsenic bioavailability degree
InactiveCN102796693AHigh sensitivityLow costBacteriaMicrobiological testing/measurementEscherichia coliMicroorganism
The present invention relates to a microbial cell sensor for detecting arsenic bioavailability degree, which is suitable for detecting bioavailability degree of arsenic in water and soil. The microbial cell sensor comprises: Escherichia coli as a host cell carrying recombinant plasmid. The recombinant plasmid is pUC18 plasmid containing arsenic resistant system ars promoter, arsenic resistant system controlling gene arsR, luciferase gene luc and rrnb terminator tandem sequence. The response time of the sensor on the arsenic is 30 minutes, the lowest detection concentration of the arsenic is 0.01 micromole / L, the highest detection concentration is 100 micromole / L. The microbial cell sensor has features of high sensitivity, fast response speed, low cost, and simple operation, and can be widely used in detecting the arsenic polluting the environment and evaluating the risk.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Lentivirus vector expression system of luciferase and application thereof
InactiveCN102477445AVector-based foreign material introductionForeign genetic material cellsLymphatic SpreadSerial passage
The invention relates to a lentivirus vector expression system of luciferase and an application thereof. Particularly speaking, in the invention a luciferase lentivirus expression system with FG12-Luc2 as core plasmid is successfully constructed, and a PVTT-1 cell line from tumor thrombus tissue of liver cancer patients is established by the system. Through the lentivirus expression system, liver cancer tissue is successfully marked; and by experimental methods such as subcutaneous transplantation and orthotopic liver cancer transplantation, a mouse orthotopic transplantation liver cancer metastasis model is established which is suitable for serial passage, is applicable to in-vivo imaging, and can realize real-time monitoring of the growth and metastasis conditions of tumors in the body.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
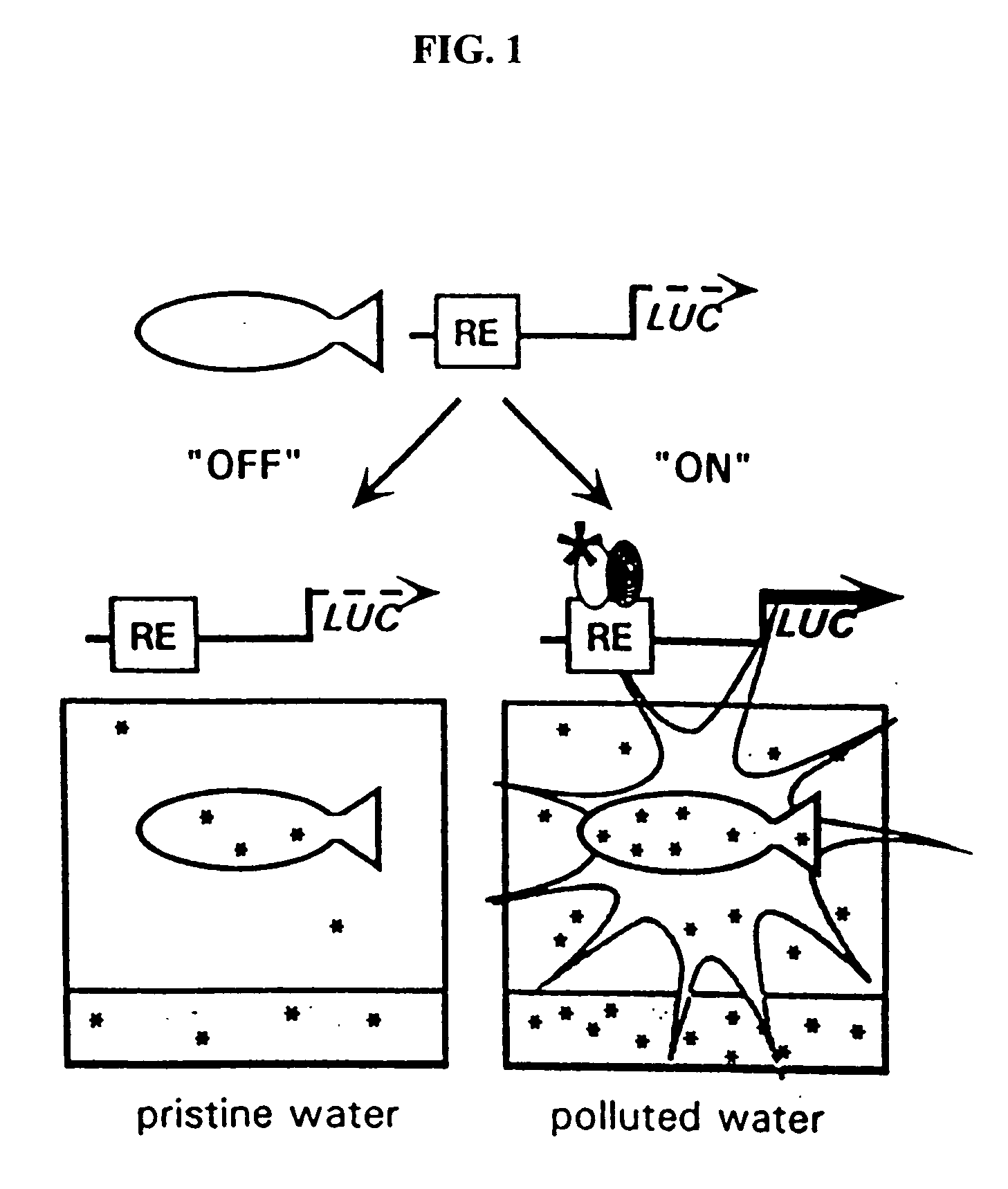
Transgenic animals for monitoring water quality
InactiveUS20060143718A1Reduce cost per sampleIncreases time required data acquisitionPollution detectorsChemiluminescene/bioluminescenceLuciferase GeneWater quality
The present invention provides methods and systems that uses transgenic zebrafish with an easily assessable reporter gene under the control of pollutant-inducible DNA response elements. Transgenic zebrafish, carrying pollution-inducible response elements, are placed in the water to be tested, and the contaminants become bioconcentrated (generally 1,000- to 40,000-fold, relative to the water) in the tissues of the fish thereby activating specific response elements, which up-regulate the LUC reporter gene. Fish are then removed from the test water and placed immediately in a luminometer cuvette and incubated with luciferin. Luciferin is rapidly taken up into the tissues of the fish, oxidized by luciferase, and light is produced. The luminescence is proportional to the environmental concentration of the pollutant (to which the fish had been exposed), which drives the expression of the LUC gene by means of the various DNA motifs. The luminescence is quantitated in the luminometer. In each response element-containing construct, a specific class of polluting chemicals, allowing for differential identification of pollutants in a complex mixture activates the expression of the LUC gene. This assay does not require killing the fish and allows for repeated analysis of the same site with the same fish. The sensitivity of the system can be manipulated by varying the sequence of the response element.
Owner:NEBERT DANIEL
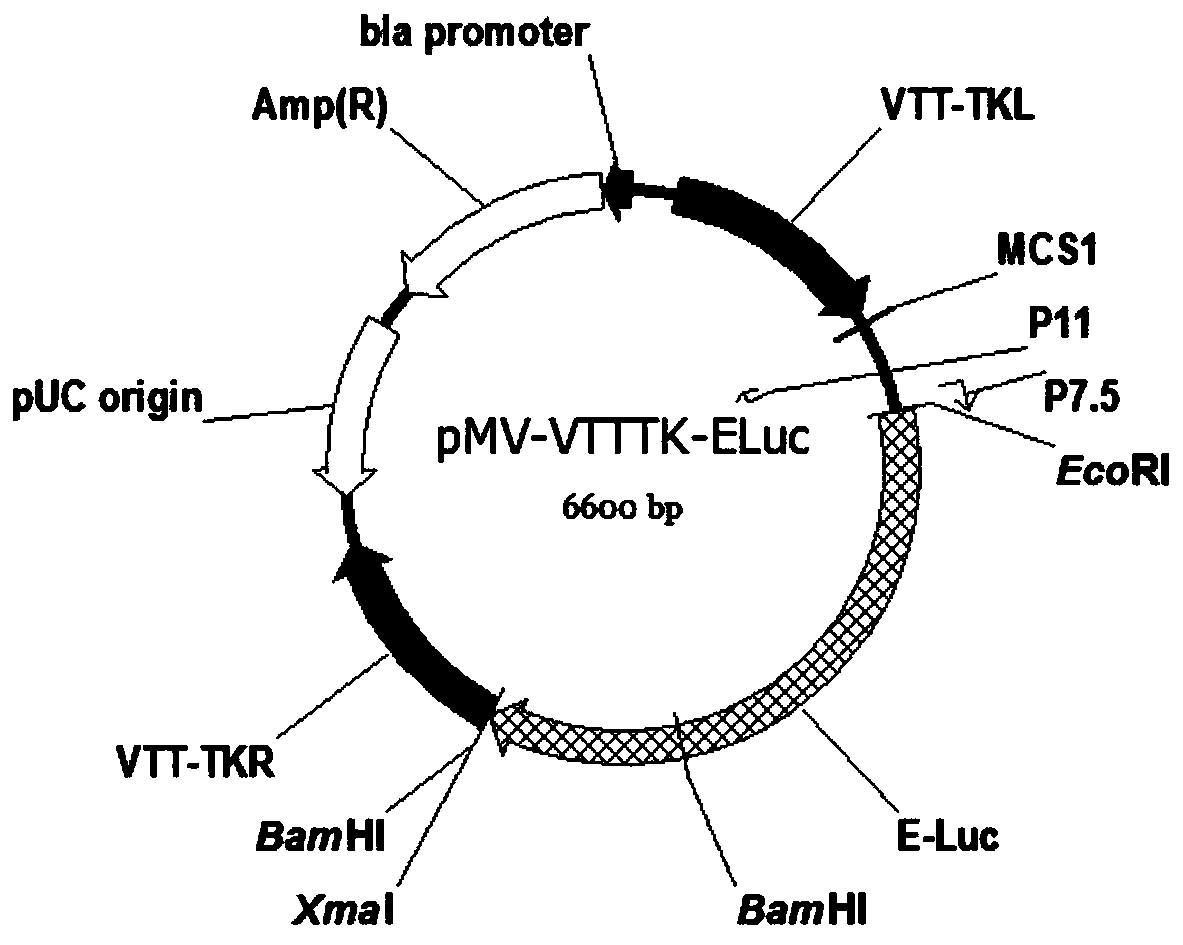
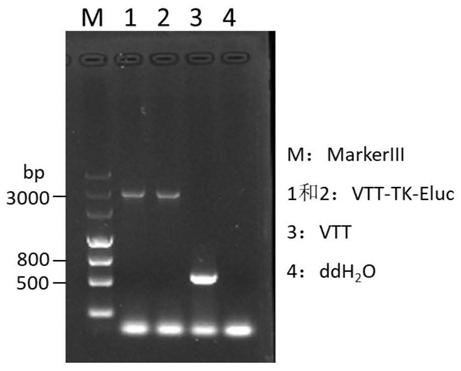
TK (Thymidine Kinase) gene removed recombinant VTT (Tian Tan strain) oncolytic VACA (Vaccinia Virus) and preparation and application thereof
PendingCN109810953ALow toxicityImprove tumor selectivityStable introduction of DNAViruses/bacteriophagesMelanomaWild type
The invention discloses a TK (Thymidine Kinase) gene removed recombinant VTT (Tian Tan strain) oncolytic VACA (Vaccinia Virus). A VTT oncolytic VACA is subjected to recombination transformation, a TKgene is removed, and an EGFP (Enhanced Green Fluorescent Protein) gene and a Luciferase gene are expressed. The invention further discloses a preparation method of the TK gene removed recombinant VTToncolytic VACA, which comprises the steps of: recombining a wild type VTT virus and a targeting plasmid aiming at the TK gene of the VTT virus in cells to obtain virus suspension; screening and purifying the virus suspension to obtain the attenuated VTT oncolytic VACA. According to the TK gene removed recombinant VTT oncolytic VACA, which is disclosed by the invention, the TK gene is used as an attenuation target, the EGFP gene and the Luciferase gene are expressed, virus virulence is reduced, and tumor selectivity of the virus is improved; the TK gene removed recombinant VTT oncolytic VACA has a function of monitoring distribution of virus particles in a body, provides a novel antitumor drug for the clinic, can be used for researching and developing a live vaccine and is applicable to various tumors such as the lung cancer, the liver cancer, the melanoma and the like.
Owner:西安彤盛生物科技有限公司
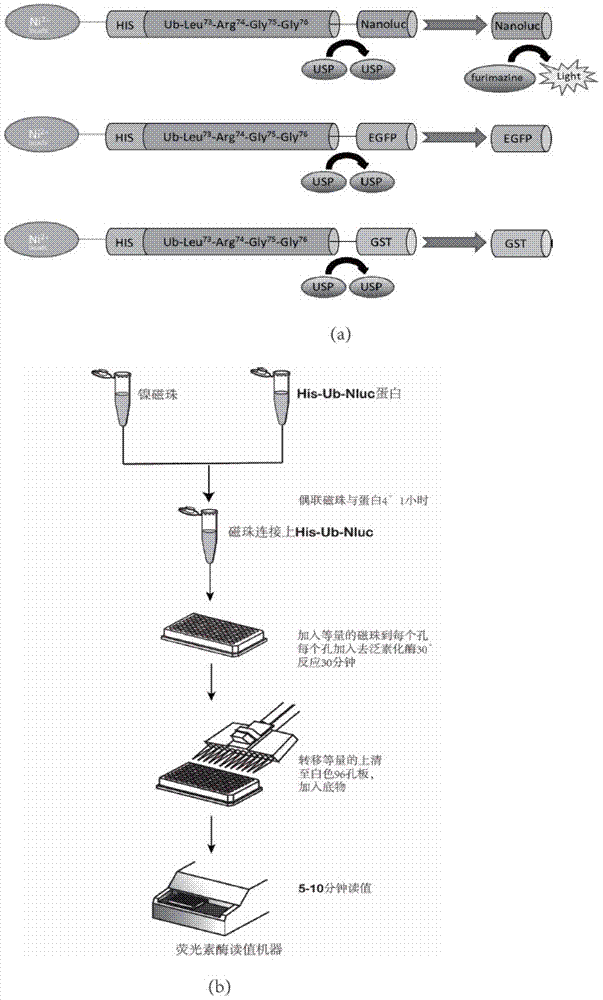
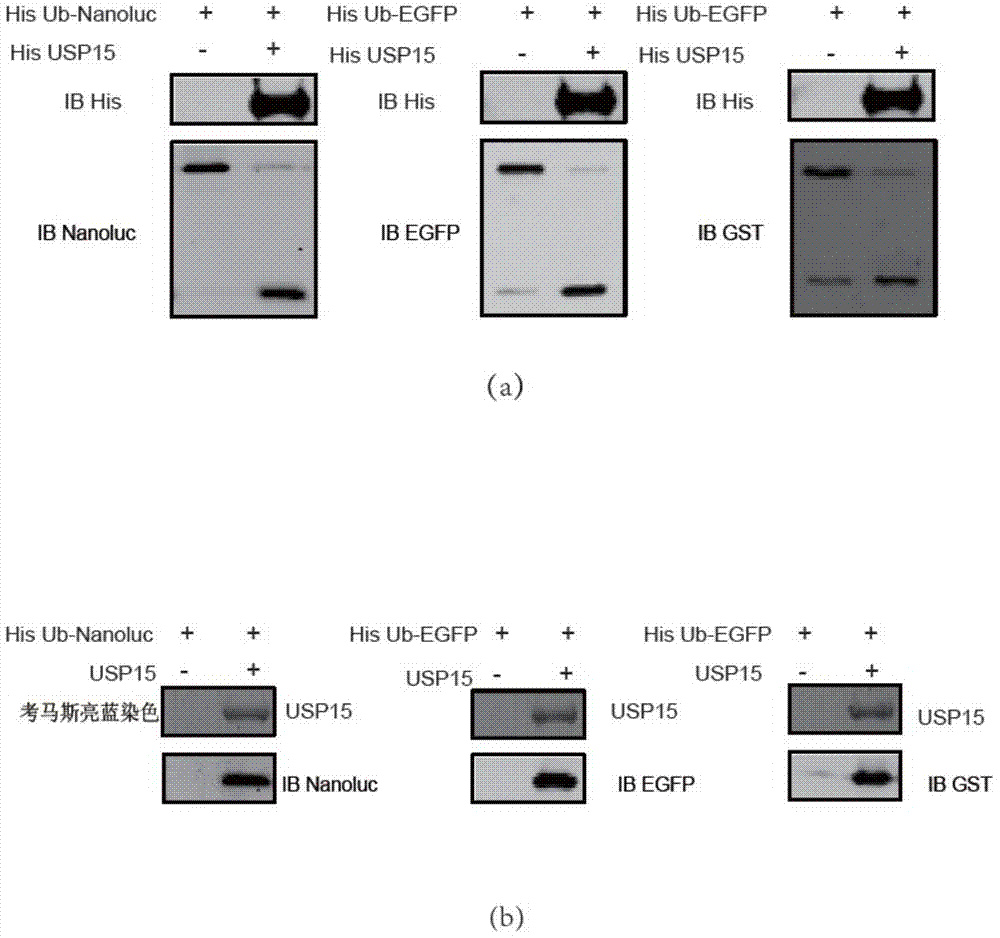
Ub-Nanoluc reporter gene system and Ub-Ub-GS-Nanoluc reporter gene system, constructions and applications thereof
InactiveCN104844704AMicrobiological testing/measurementOxidoreductasesLuciferase GeneEnzyme inhibitor
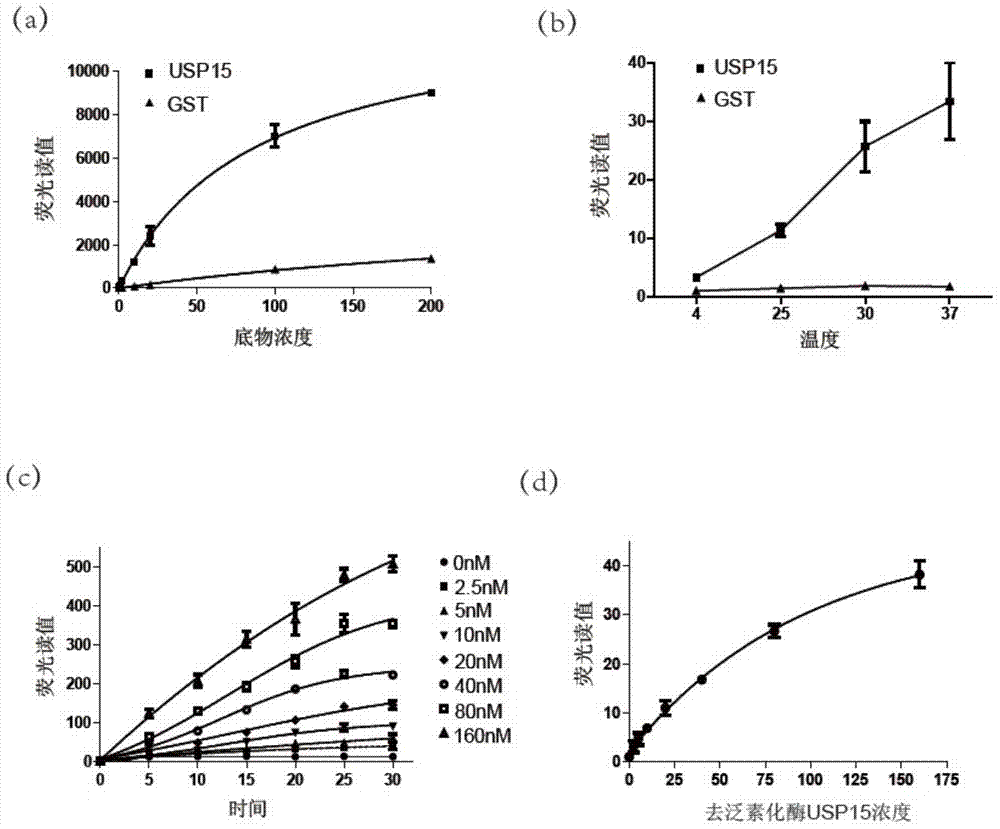
The present invention discloses an Ub-Nanoluc reporter gene system and an Ub-Ub-GS-Nanoluc reporter gene system, and applications of the Ub-Nanoluc reporter gene system and the Ub-Ub-GS-Nanoluc reporter gene system in deubiquitination enzyme activity detection and deubiquitination enzyme inhibitor throughput-screening, wherein the Ub-Nanoluc reporter gene DNA sequence is represented by SEQ ID NO.1, and the Ub-Ub-GS-Nanoluc reporter gene DNA sequence is represented by SEQ ID NO.4. According to the present invention, the high sensitivity characteristic of luciferase Nanoluc is utilized to provide the method for deubiquitination enzyme activity detection and deubiquitination enzyme inhibitor throughput-screening by using the chemical luminescent reporter gene so as to provide the new action target point and the new ideal for screening and research of new drugs including anticancer drugs, and provide broad application prospects, great enormous benefits, and great social benefits.
Owner:EAST CHINA NORMAL UNIV
Modified luciola cruciata luciferase gene and protein
InactiveUS20100093029A1Improve thermal stabilityHigh expressionSugar derivativesDepsipeptidesLuciola cruciataLuciferases
A codon optimized and stabilized luciferase gene based upon the sequence of the natural luciferase gene isolated from Luciola cruciata (Japanese firefly) and a novel recombinant DNA characterized by incorporating this new gene coding for a novel luciferase into a vector DNA for improved activities in mammalian cells, are disclosed. This new luciferase exhibits long-wavelength light emission, as well as improved thermostability and higher expression levels in mammalian cell systems, compared to native luciferase.
Owner:MARKER GENE TECH
ATP-metry based on intracellular adenyl nucleotides for detecting and counting cells, use and implementing method for determining bacteria in particular devoid of atp
InactiveUS20080014607A1Less expensiveMeet needsMicrobiological testing/measurementLuciferinIntracellular
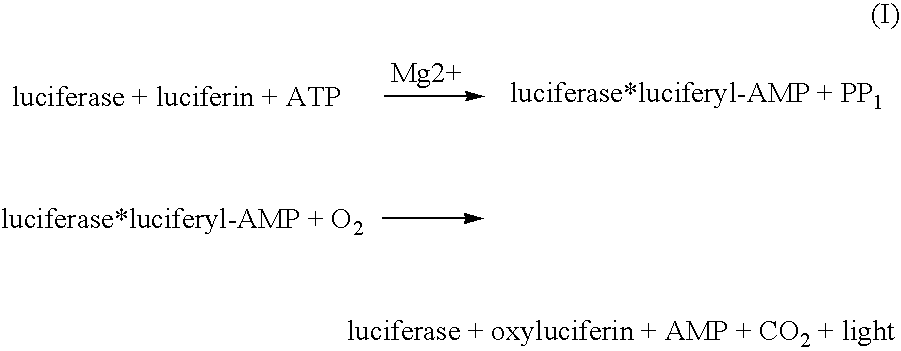
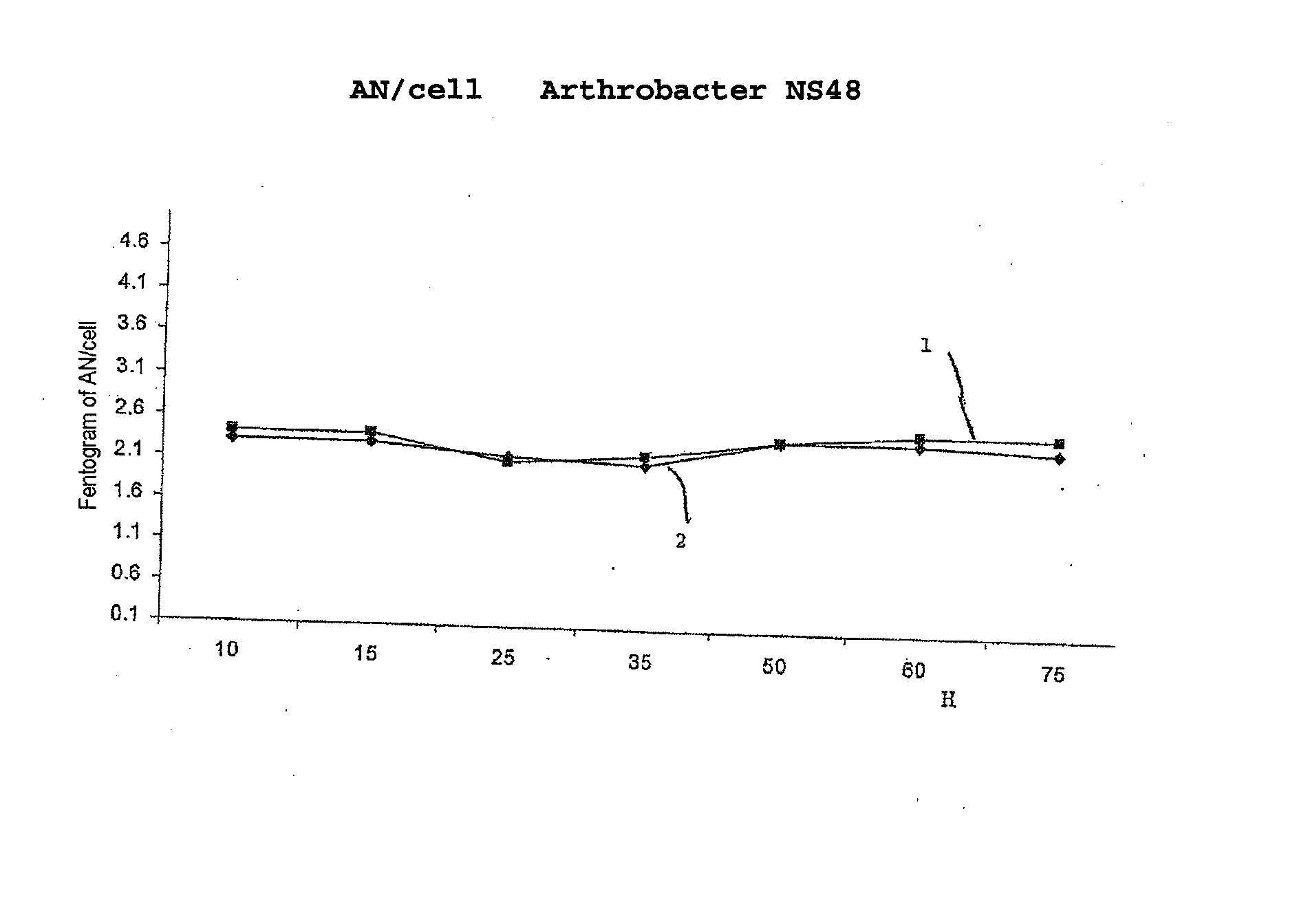
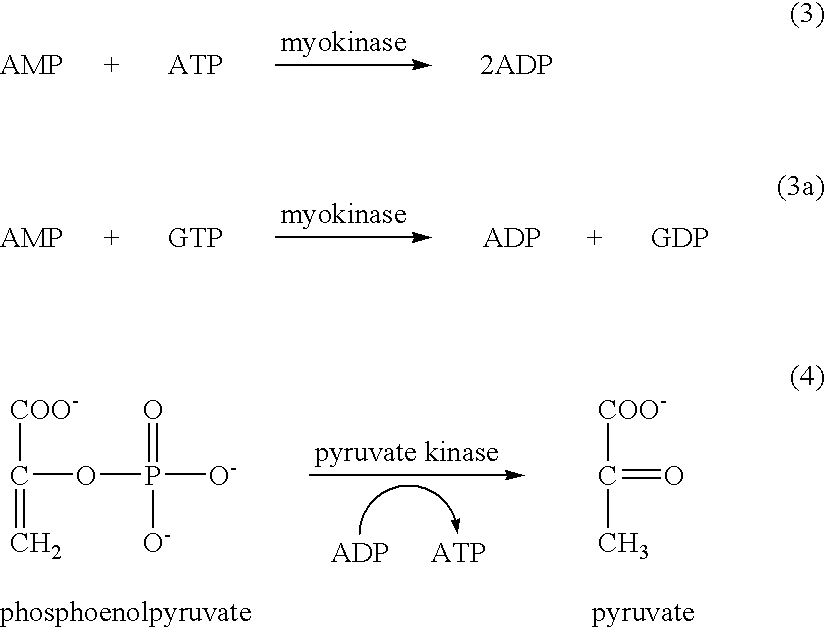
The invention concerns the use of bioluminescence dependent on the reaction (1): luciferin+ATP+O2+Mg2++luciferase→oxyluciferin+photons for detecting and counting living cells of a given species potentially present in a liquid sample, said use being characterized in that it consists in measuring the total free intracellular adenyl nucleotides (AN) content, expressed in ATP form, of living cells of a given non-viral species, taking into account the fact that the sum of free intracellular ATP, ADP and AMP of said family is constant according to the relationship (2): [AN]=[ATP]+[ADP]+[AMP]=Cte after transforming the free intracellular ATP, ADP and AMP by using myokinase and pyruvate kinase, said measurement being performed (i) without adding ATP and (ii) after adding a known amount of ATP. The invention also concerns a method for detecting and counting cells by ATP-metry.
Owner:TESTLIFE TECH
Light-activated in vitro assay process for luciferase bioluminescence
InactiveUS20040063165A1Easy to detectConvenient amountMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementLuciferase GenePhotonics
There is provided a process of inducing luminescent emission from a luciferase bioluminescent reaction particularly useful in binding assays. A luciferase bioluminescent combination, together with an inactive, caged trigger compound such as a cofactor, is subjected to photonic radiation, so as to release the trigger compound in active form, and thereby cause substantially instantaneous reaction of the active trigger compound so released with the luciferase combination, to induce photonic emission which can be detected and measured.
Owner:CARDIOGENICS
Covalent modification and conjugation of luciferase
A process for reversible chemical modification of the luciferase, a process for the covalent conjugation of a reversibly modified luciferase to a chemical moiety (a protein or a binding partner such as biotin or an antibody), a process for reactivation of the reversibly-modified and inactivated luciferase, a process for making the said luciferase conjugates and a bioluminescent assay method that uses covalently conjugated firefly luciferase are taught. The present invention also relates to a composition comprising a reversibly modified luciferase, as well as a composition comprising a reversibly modified luciferase covalently conjugated to a chemical moiety.
Owner:CARDIOGENICS
Reference internal type dual-luciferase reporter vector and application thereof
InactiveCN102094037AWill not interfere with each otherSimplify the transfection stepMicrobiological testing/measurementVector-based foreign material introductionMirna targetPolymerase chain reaction
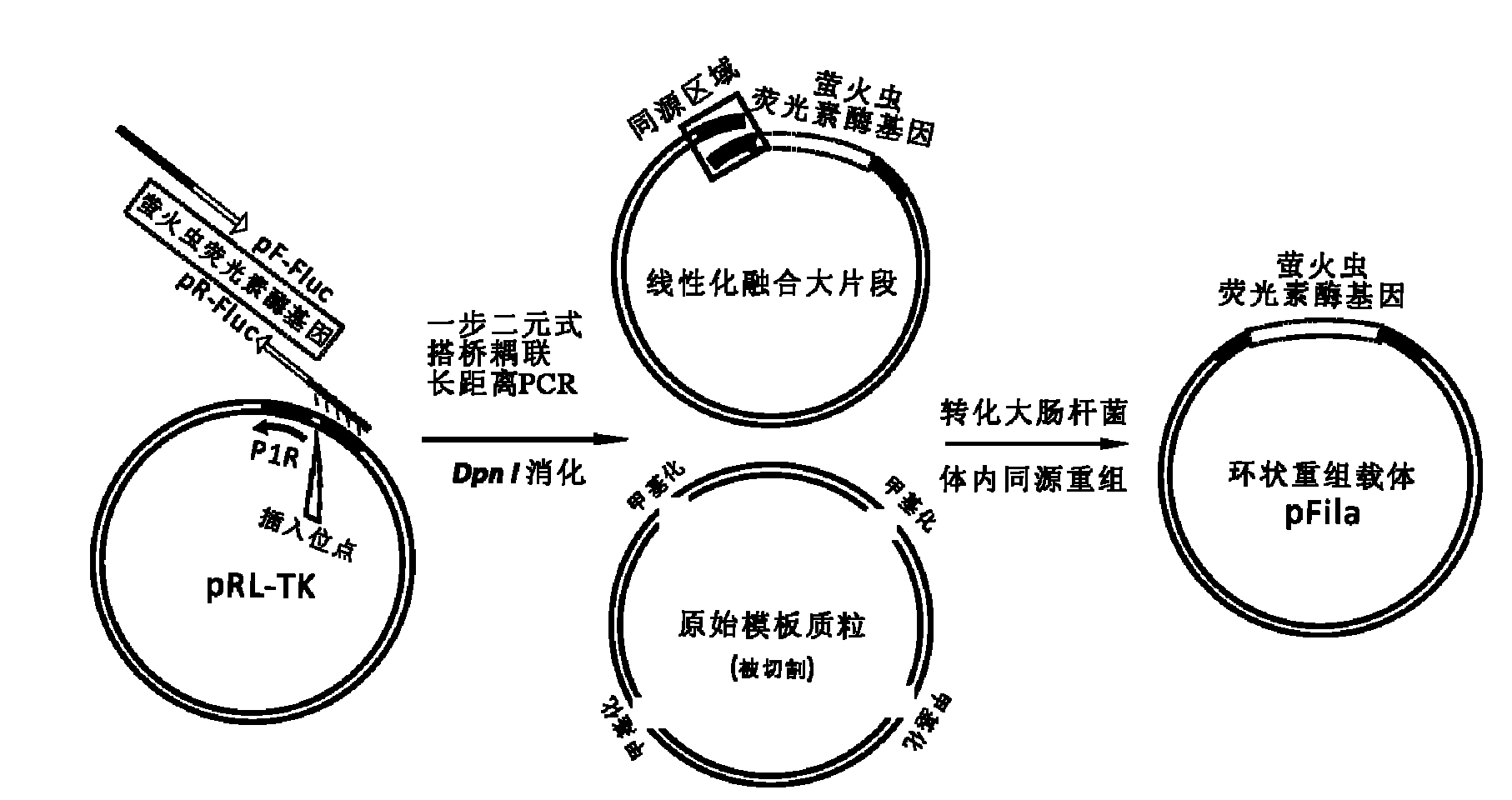
The invention discloses a reference internal type dual-luciferase reporter vector and an application thereof. Firefly luciferase gene is fused between the renilla luciferase gene of the pRL-TK vector and the ampicillin resistance gene, the two luciferases are on the same vector, and the firefly luciferase gene is used as reference. The one-step binary bridging coupling long-distance polymerase chain reaction (PCR) and the Escherichia coli vivo homologous recombination method are utilized to place the firefly luciferase gene and the renilla luciferase gene on the same vector; and monoclonal sites are introduced in the 3' untranslated region of the renilla luciferase gene for conveniently cloning the target segment, thus the reference internal type dual-luciferase reporter vector can be constructed. The vector of the invention has the advantages of high repeatability, little multiple-pore variation, convenient operation and precise quantification. The vector of the invention is suitablefor the screening, identification and confirmation of the miRNA target molecule and also suitable for the quantitative analysis of the activity change of miRNA in cellular level.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
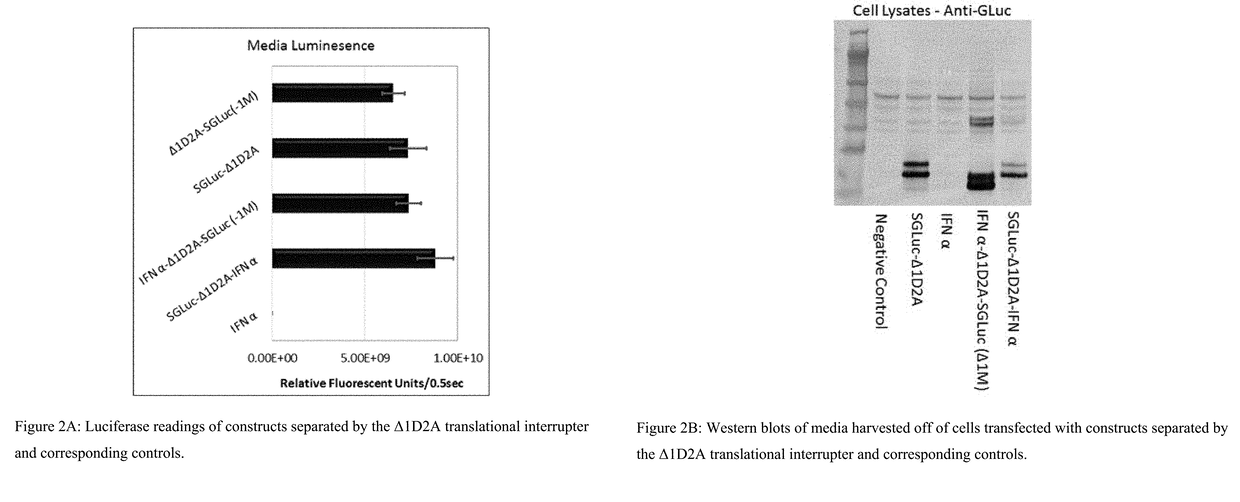
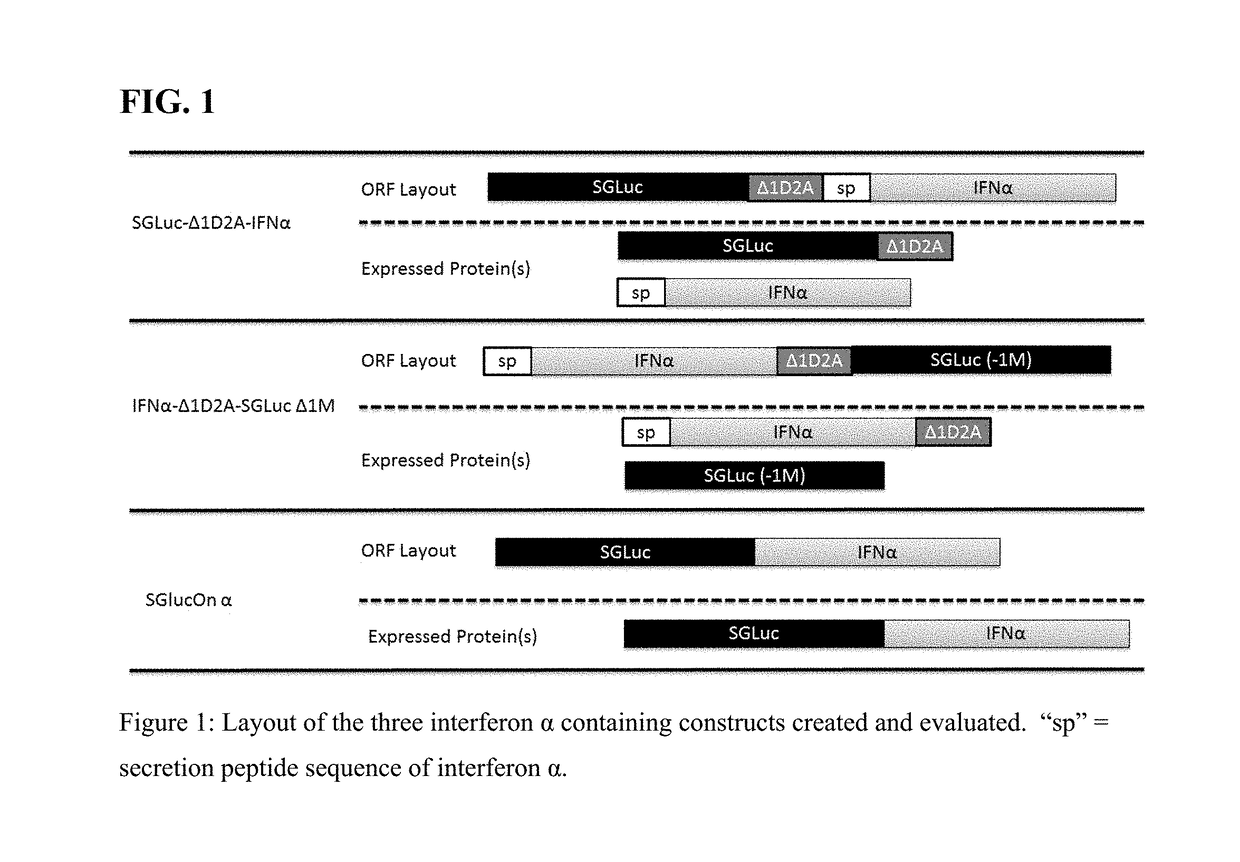
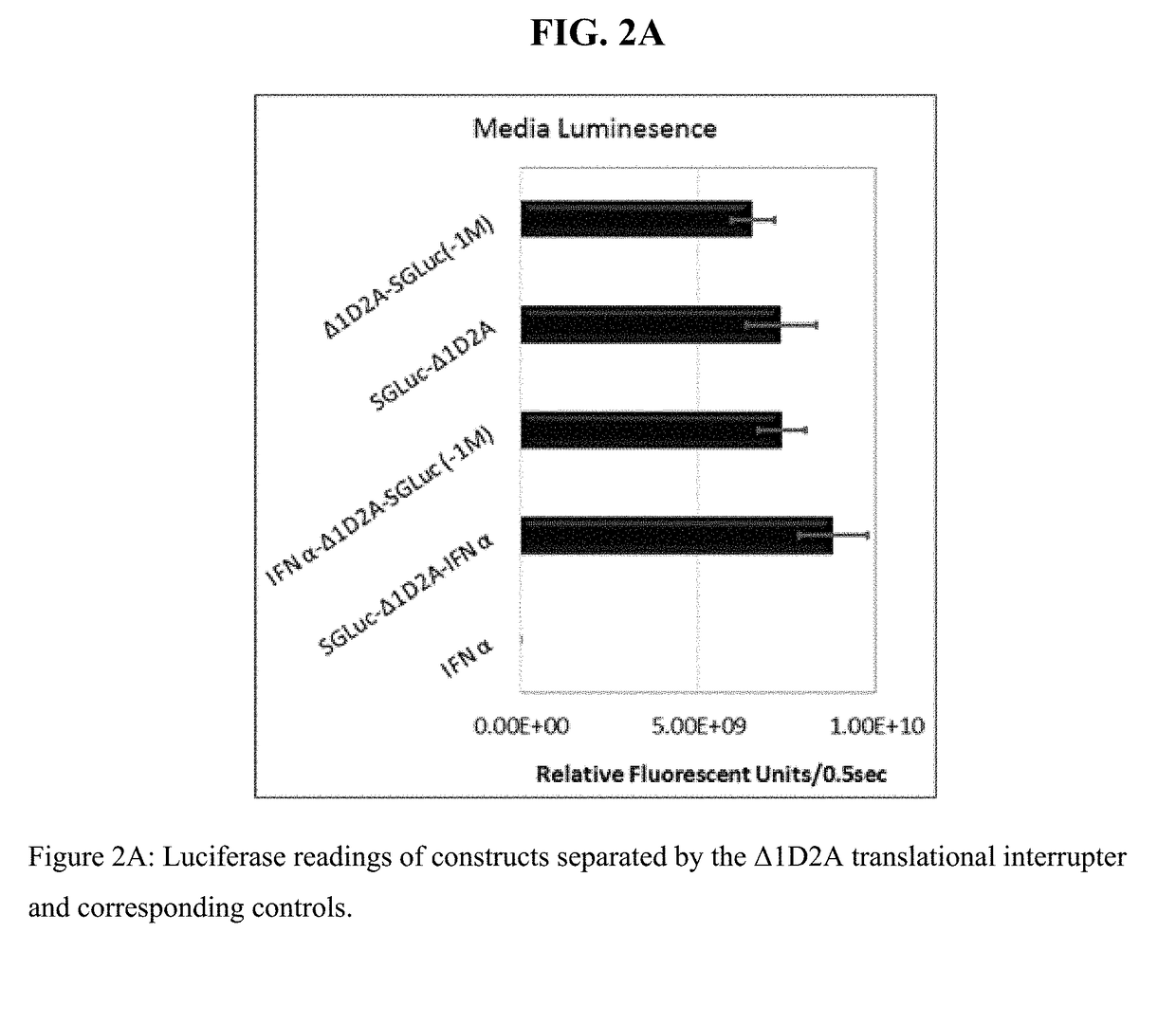
Fusion protein comprising gaussia luciferase, translation interrupter sequence, and interferon amino acid sequences
ActiveUS20180066267A1The process is simple and fastIncrease productionCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsBiotechnologyNucleotide
Polynucleotides encoding fusion proteins comprising interferons and luciferases which have biotherapeutic, diagnostic, and quality control applications in biotechnological, medical, and veterinary fields.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF HOMELAND SECURITY
Dual assay for evaluating activity and cytotoxicity of compounds in the same population of cells
Methods are provided for evaluating the activity and cytoxicity of a compound in the same population of cells. These dual activity / cytoxicity methods are amenable for use in a high-throughput format. Also provided are humanized Renilla luciferase genes useful for the dual activity / cytoxicity assays and for use in a variety of reporter constructs.
Owner:AGOURON PHARMA INC
Firefly luciferase and encoding gene and obtaining method thereof
ActiveCN103409380AHigh catalytic activityHigh activityBacteriaMicroorganism based processesHeat stabilityWild type
The invention relates to firefly luciferase and an encoding gene and an obtaining method thereof. The defect that the original firefly luciferase in North America is poor in heat stability and cannot be used at a high temperature is overcome; and the original firefly luciferase in North America is mutated, so that mutative enzyme of which the heat stability is obviously improved is obtained; and the application value is improved. An amino acid sequence is SEQ ID NO.1; and a protein deoxyribonucleic acid (DNA) sequence in encoding is SEQ ID NO.2. An encoding gene and a preparation method of firefly luciferase mutant in North America are provided. By the method, a recombinant expression vector containing a wild firefly luciferase gene is built; and the protein is purified by using an affinity chromatography method. The method is simple and feasible, simple to operate, and low in cost.
Owner:TIANJIN UNIV
Method for determining ATP
InactiveCN102818801AHigh sensitivityImprove accuracyChemiluminescene/bioluminescencePreparing sample for investigationCuvetteAtp content
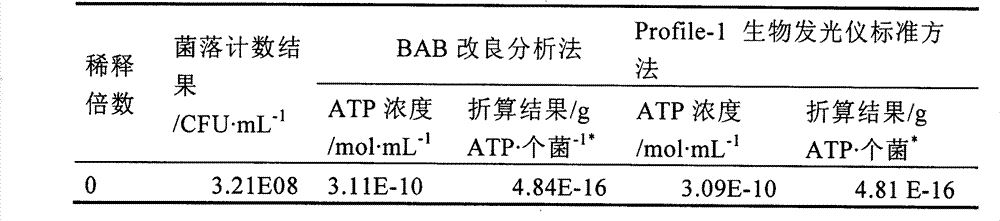
The invention relates to a method for determining ATP. The method is used for determining ATP content in bacteria-containing water body, soil, sludge, etc. The method comprises the following steps of a) preparing a to-be-measured solution of a sample; b) adding a BAB solution in the to-be-measured solution; c) sucking up a filtered filtrate and putting into a filtering cuvette with luciferase added therein already; and d) performing an ATP determination by using a Profile-1 biological luminescent instrument. Compared with a conventional method, the method provided by the invention is superior to a standard analysis method installed in the Profile-1 biological luminescent instrument, is particularly suitable for determining ATP in the soil, and makes up disadvantages of the standard analysis method installed in the Profile-1 biological luminescent instrument.
Owner:TONGJI UNIV
Gene encoding novel luciferase
InactiveUS20090233320A1Type is limitedWiden the optionsSugar derivativesMicrobiological testing/measurementNucleotideLuciferase Gene
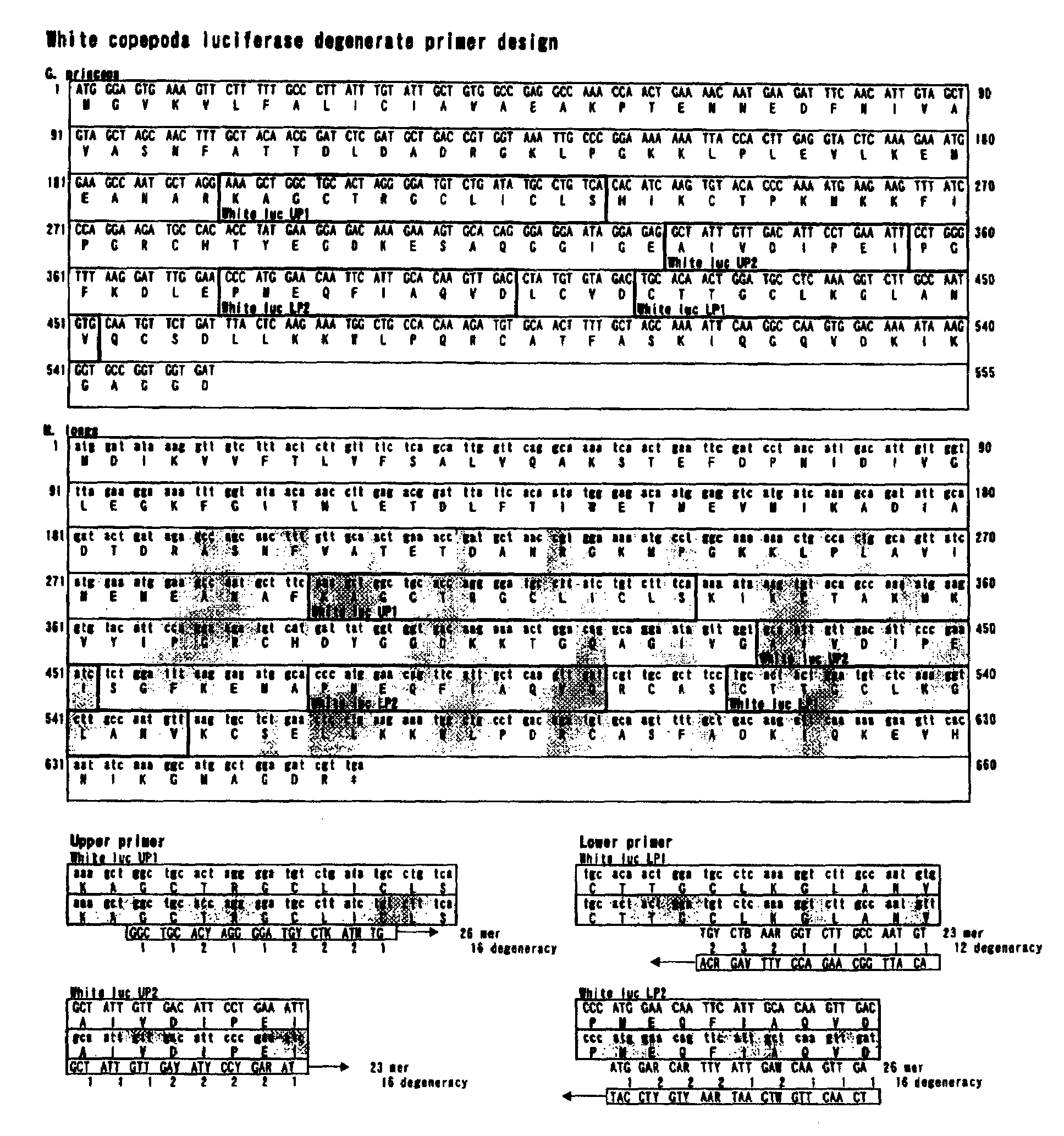
The present invention provides genes encoding novel luciferases having at least the properties of: being capable of using coelenterazine as their luminescent substrates; and being capable of being recombinantly expressed in a mammal cell as a host and produced to be secreted to the outside of the host cell. Specifically, the gene encoding novel luciferases according to the present invention is a DNA molecule comprising a nucleotide sequence encoding any of the full-length amino acid sequences of two types of luciferase proteins, luciferase 1 and luciferase 2, from M. pacifica, and is, for example, a gene encoding the following full-length amino acid sequence of the luciferase 1.MMEIQVLFAL ICFALVQANP TENKDDIDIV GVEGKFGTTD60LETDLFTIVE DMNVISRDTNLANSDADRGK MPGKKLPLEV LIEMEANARK AGCTRGCLIC120LSKIKCTAKM KVYIPGRCHDYGGDKKTGQA GIVGAIVDIP EISGFKELGP MEQFIAQVDL180CADCTTGCLK GLANVKCSALLKKWLPDRCA SFADKIQSEV DNIKGLAGDR210
Owner:NEC SOLUTION INNOVATORS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com