Patents
Literature
44 results about "Serial passage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serial passage refers to the process of growing bacteria or a virus in iterations. For instance, a virus may be grown in one environment, and then part of that virus can be removed and put into a new environment. This process is repeated with as many stages as desired, and then the final product is studied, often in comparison with the original virus.
Genetically stable recombinant modified vaccinia ankara (RMVA) vaccines and methods of preparation thereof
ActiveUS20100316667A1Elicit immune responseVectorsSugar derivativesHeterologousModified vaccinia Ankara
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Genetically stable recombinant modified vaccinia ankara (rMVA) vaccines and methods of preparation thereof
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Larimichthys crocea liver cell line and establishment method thereof
InactiveCN103992981AImprove stabilityGood repeatabilityMicroorganism based processesVertebrate cellsSerial passageDigestion
The invention discloses a larimichthys crocea liver cell line and an establishment method thereof. The larimichthys crocea liver cell line is preserved in the China center for type culture collection (CCTCC) On October 31, 2013 and has a preservation number of CCTCC NO: C2013157. The larimichthys crocea liver cell line can realize serial passage, can provide a large amount of larimichthys crocea liver cell lines, can be used for pathogenic characteristic research, vaccine development, functional gene research and breeding research, has excellent properties, comprises fibroblast-like cells and mainly comprises cells having parapodiums stretching out and irregular shapes. The larimichthys crocea liver cell line has the advantages of exuberant division, short passage time, easy digestion, good adherence rate and hunger resistance. The establishment method has good repeatability. The larimichthys crocea liver cell line has good stability.
Owner:JIMEI UNIV
Feruloyl-esterase-producing Bacillus licheniformis strain and application thereof
ActiveCN104894017ALow carbon sourceLower requirementBacteriaHydrolasesBacillus licheniformisSerial passage
The invention discloses a feruloyl-esterase-producing Bacillus licheniformis strain and application thereof. The collection number of the Bacillus licheniformis DBM12 strain is CGMCC No.8672. The Bacillus licheniformis strain has the advantages of lower requirements for the carbon source and nitrogen source, short fermentation time, high safety and no toxicity, and can be used in food / feed industry. The Bacillus licheniformis strain has favorable hereditary stability, and is simple to operate. The feruloyl esterase synthesis capacity is basically unchanged after serial passage by more than 10 generations.
Owner:QINGDAO BEIBAO OCEAN TECH CO LTD
Multivalent dengue virus vaccine
InactiveUS20070087015A1Enhance immune responseSufficient amountSsRNA viruses positive-senseViral antigen ingredientsSerial passageSerotype
The present invention provides vaccine compositions of attenuated dengue virus. More specifically, the attenuated virus is produced by serial passage in PDK cells. The invention also provides methods for stimulating the immune system of an individual to induce protection against all four dengue virus serotypes by administration of attenuated dengue-1, dengue-2, dengue-3, and dengue-4 virus.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Efficient cell culture system for hepatitis C virus genotype 5A
Owner:HVIDOVRE HOSPITAL
Lentivirus vector expression system of luciferase and application thereof
InactiveCN102477445AVector-based foreign material introductionForeign genetic material cellsLymphatic SpreadSerial passage
The invention relates to a lentivirus vector expression system of luciferase and an application thereof. Particularly speaking, in the invention a luciferase lentivirus expression system with FG12-Luc2 as core plasmid is successfully constructed, and a PVTT-1 cell line from tumor thrombus tissue of liver cancer patients is established by the system. Through the lentivirus expression system, liver cancer tissue is successfully marked; and by experimental methods such as subcutaneous transplantation and orthotopic liver cancer transplantation, a mouse orthotopic transplantation liver cancer metastasis model is established which is suitable for serial passage, is applicable to in-vivo imaging, and can realize real-time monitoring of the growth and metastasis conditions of tumors in the body.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Efficient cell culture system for hepatitis c virus genotype 1a and 1b
The present inventors developed hepatitis C virus 1a / 2a and 1b / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and NS2 were replaced by the corresponding genes of the genotype Ia reference strain H77C or TN or the corresponding genes of the genotype Ib reference strain J4. Sequence analysis of recovered 1a / 2a and 1b / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in e.g. p7, NS2 and / or NS3. In addition, the inventors demonstrate the possibility of using adaptive mutations identified for one HCV isolate in generating efficient cell culture systems for other isolates by transfer of mutations across isolates, subtypes or major genotypes. Furthermore neutralization studies showed that viruses of e.g. genotype 1 were efficiently neutralized by genotype Ia, 4a and 5a serum, an effect that could be utilized e.g. in vaccine development and immunological prophylaxis. The inventors in addition demonstrate the use of the developed systems for screening of antiviral substances in vitro and functional studies of the virus, e.g. identification of receptors required for HCV entry
Owner:HVIDOVRE HOSPITAL
Avian nephropathogenic infectious bronchitis virus strain as well as vaccine composition, preparation method and application thereof
ActiveCN105802918AEffective controlProtection against virusesInactivation/attenuationMicroorganism based processesDiseaseSerial passage
The invention belongs to the field of veterinary biological products, and relates to a strain of avian nephropathogenic infectious bronchitis virus containing a gene coding sequence SEQ ID No.2 which codes a protein and an attenuated virus strain. Serial passage of the virus strain is carried out to 110-140th generation in chicken embryos, and the attenuated virus strain is obtained. The invention also relates to a vaccine composition which is prepared by using the virus strain, the attenuated virus strain and antigen proteins thereof, as well as a preparation method and an application thereof. A vaccine composition containing inactivated totivirus antigen, live attenuated totivirus antigen and subunit antigen can be used for effectively preventing diseases related to avian nephropathogenic infectious bronchitis.
Owner:PU LIKE BIO ENG
Vaccines for diseases of fish
Fish are immunized by a mass vaccination method, such as by immersion in water containing an attenuated strain of a pathogenic bacterium that does not effectively cause disease in fish when the non-attenuated pathogenic bacterium is exposed to the fish by immersion. An illustrative example of the method is for immunizing against coldwater disease caused by Flavobacterium psychrophilum, which may be attenuated by serial passage in media containing increasing amounts of an antibiotic, such as rifampicin.
Owner:IDAHO UNIV OF
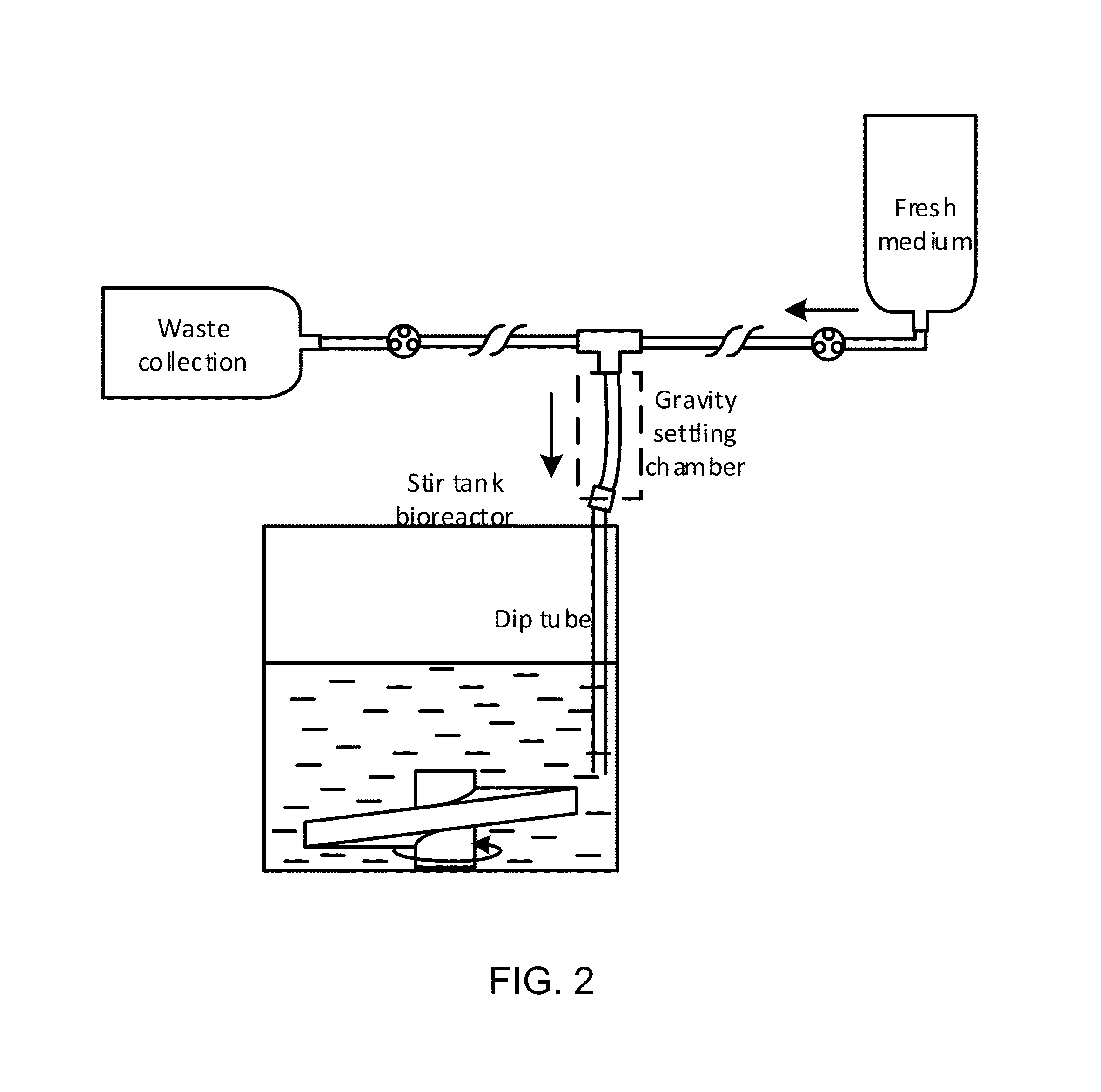
Pluripotent stem cell expansion and passage using a stirred tank bioreactor
ActiveUS20160215257A1Reduce human interventionReduce cross-contaminationBiological substance pretreatmentsBiomass after-treatmentSerial passageCell aggregation
Provided herein are novel methods for expansion and passaging of cell aggregates comprising stem cells and / or differentiated cells and comprising the use of closed systems in stirred tank bioreactors. The methods of the invention permit closed system serial passage expansion of pluripotent stem cells and / or progeny thereof with associated pluripotency markers and differentiation potential.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
O-type foot and mouth disease virus acid-resistant mutant strain, capsid protein carried by O-type foot and mouth disease virus acid-resistant mutant strain, coding gene of capsid protein and use of O-type foot and mouth disease virus acid-resistant mutant strain and capsid protein
ActiveCN103849637ADecreased immune efficiencyImproving immunogenicityGenetic material ingredientsVirus peptidesSerial passageVaccine Production
The invention discloses an O-type foot and mouth disease virus acid-resistant mutant strain, a capsid protein carried by the O-type foot and mouth disease virus acid-resistant mutant strain and a coding gene of the capsid protein and also discloses a key amino acid site for determining O-type foot and mouth disease virus acid-resistant characteristics and a use of the key amino acid site. An O-type foot and mouth disease virus parent strain is subjected to serial passage screening under acid stress so that a mutant strain having strong acid resistance is obtained, and an analysis result shows that a VP1 N17D mutational site is a key amino acid site for determining O-type foot and mouth disease virus acid-resistant characteristics, the O-type foot and mouth disease virus only with the VP1 N17D mutational site has the acid resistance the same as that of the mutant strain and also has a good replication capability and immunogenicity. The key amino acid site for determining O-type foot and mouth disease virus acid-resistant characteristics can be used for reconstruction of acid resistance of a foot and mouth disease virus vaccine and for exploitation of a novel foot and mouth disease gene engineering vaccine. The acid-resistant mutant strain rN17D can be used as a high-quality inactivated vaccine-production parent strain, can improve content of a 146S effective antigen in the inactivated vaccine and can improve vaccine immune efficacy.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Multivalent dengue virus vaccine
The present invention provides vaccine compositions of attenuated dengue virus. More specifically, the attenuated virus is produced by serial passage in PDK cells. The invention also provides methods for stimulating the immune system of an individual to induce protection against all four dengue virus serotypes by administration of attenuated dengue-1, dengue-2, dengue-3, and dengue-4 virus.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
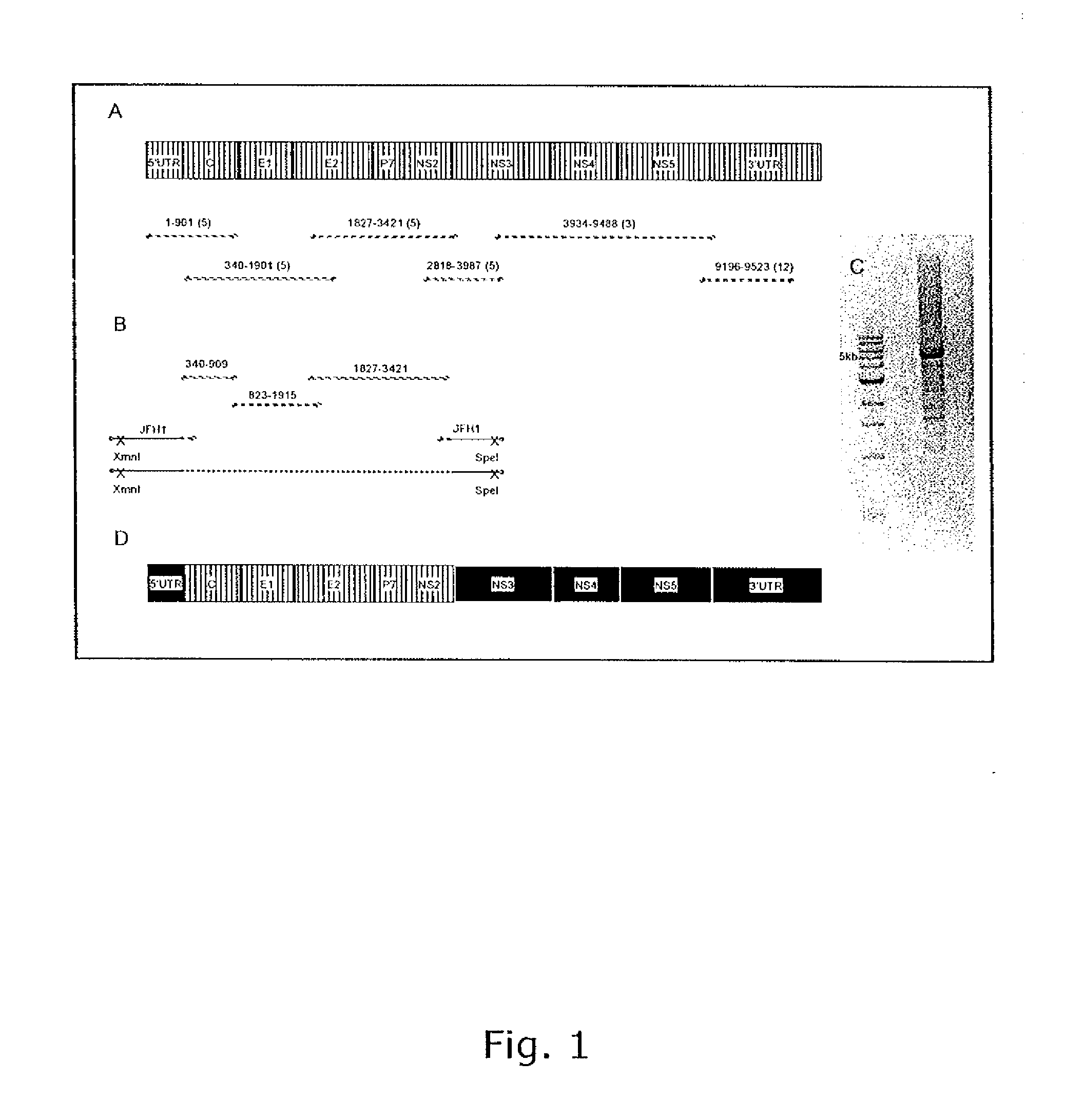
Efficient cell culture system for hepatitis c virus genotype 5a
InactiveUS20110021611A1Organic active ingredientsSsRNA viruses positive-senseSequence analysisSerial passage
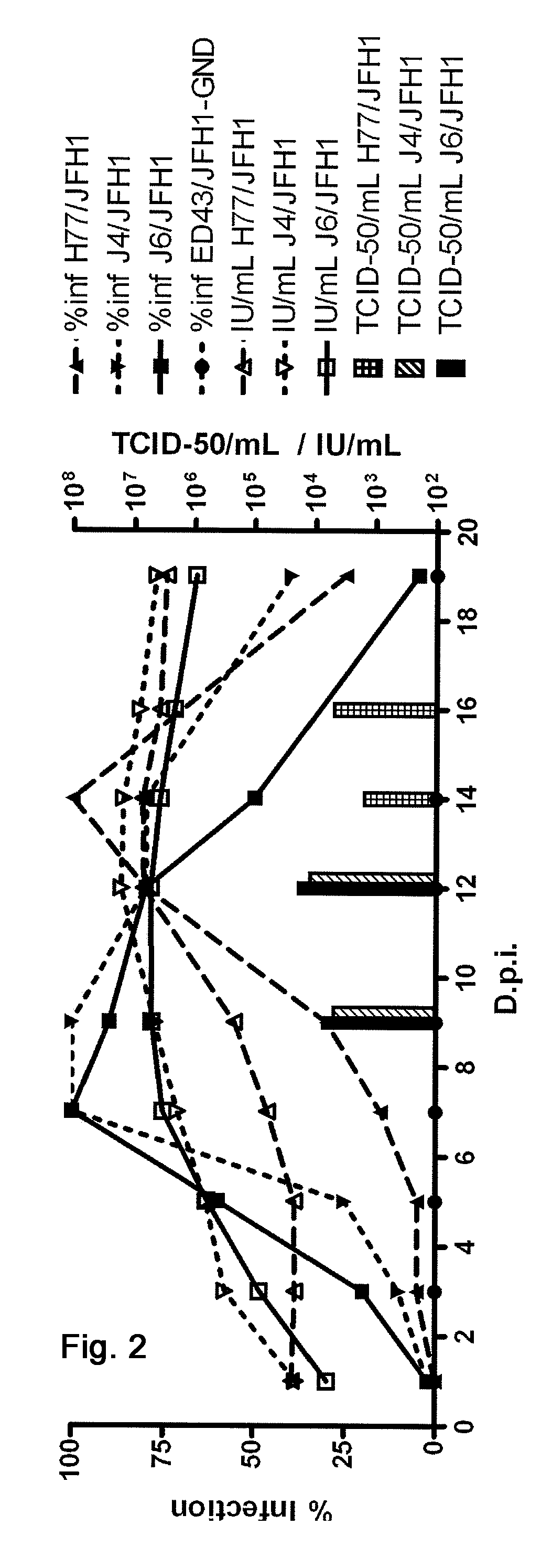
The present inventors developed 5a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and all of or part of NS2 were replaced by the corresponding genes of the genotype 5a reference strain SA13. Compared to the J6 / JFH control virus, after transfection of in vitro transcripts in Huh7.5 cells, production of infectious viruses was delayed. However, in subsequent viral passages efficient spread of infection and HCV RNA titers as high as for J6 / JFH were obtained. Infectivity titers were at all time points analyzed comparable to J6 / JFH control virus. Sequence analysis of recovered 5a / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in p7, NS2 and / or NS3. Infectivity of the 5a / 2a viruses was CD81 and SR-BI dependant, and the recombinant viruses could be neutralized by chronic phase sera from patients infected with genotype 5a. Conclusion: The developed 5a / 2a viruses provide a robust in vitro tool for research in HCV genotype 5, including vaccine studies and functional analyses of an increasingly important genotype in South Africa and Europe.
Owner:HVIDOVRE HOSPITAL
Varicella attenuation live vaccine
ActiveCN101161286AImprove securityHigh viral titerAntiviralsAntibody medical ingredientsInfected cellKanamycin
The present invention relates to herpes virus family medical microorganism. The present invention is prepared by the following method: (1) serial passage MRC-5 cell; (2) inoculating to cell and continuous infecting; (3) adding culture solution; (4) washing cell surface; (5) eluting infected cell, centrifugal separating, adding vaccine fluid containing stabilizing agent to refrigerate; (6) ultrasonic crashing and centrifugal separating to acquire virus stock solution; (7) combining and diluting, and at once processing to pack and freeze-dry, preparing attenuated pox vaccine. The stabilizing agent is prepared by dissolving NaCl, KCl, KH2PO4, Na2HPO4.12H2O, saccharose, sodium glutamate, kalamycin, erythromycin and deionized water into the water with weight ratio 3:0.1:0.2:3.3:50:0.4:0.1:0.03:1000. The present invention has good advantages of safety and immunogenicity.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Bacterial strain capable of realizing high yield of L-alanine
InactiveCN106047740AIncrease productionImprove stabilityBacteriaMicroorganism based processesEscherichia coliSerial passage
The invention provides a bacterial strain capable of realizing high yield of L-alanine, belonging to the field of bioengineering technology. The bacterial strain capable of realizing high yield of L-alanine is named as Escherichia coli (EcQLS1) with an accession number of CGMCC No. 1764. The Escherichia coli (EcQLS1) strain is obtained through a combination of normal-temperature normal-pressure plasma mutation and high-flux screening, can greatly improve the yield of L-alanine and has good stability, wherein the yield of L-alanine has no obvious change after serial passage of the strain for five generations. Results of shake-flask experiments show that the yield of L-alanine reaches 120 g / L. The bacterial strain provided by the invention totally meets industrial mutation and screening demands on an L-alanine strain, is applicable to industrial fermentation production and has significant economic value.
Owner:QILU UNIV OF TECH +1
Construction and ultralow temperature freezing preservation method of fin cell line of schizothorax grahami
ActiveCN103436490AEasy to operateActiveDead animal preservationVertebrate cellsSerial passageGermplasm
The invention relates to construction and ultralow temperature freezing preservation method of a fin cell line of schizothorax grahami, and belongs to the technical field of culture and ultralow temperature ultralow temperature freezing preservation of cells of fresh water aquatic organisms. The method comprises the following steps of: culturing in a DMEM(Dulbecco Modified Eagle Medium) / F12 culture solution which contains fetal calf serum and cell growth factors and has the pH value of 7.0-7.2 by taking the ventral fin tissue of the schizothorax grahami as a material and adopting a tissue explant method; carrying out subculture by adopting a trypsin digestion method. The method particularly comprises the steps of cell culture solution preparation, primary culture and subculture. The construction method disclosed by the invention is short in primary culture time consumption and large in cell quantity and can be used for chromosome analysis; the constructed fin cell line of the schizothorax grahami is in a fiber-like form, can be subjected to serial passage and directly applied to biological characteristic research, meets the requirements for germplasm resource conservation and theoretical research and application of the schizothorax grahami and is suitable for constructing the fin cell lines of other fishes.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Construction method for Hexagrammos otakii cell line
InactiveCN102492650AThe process steps are simpleEasy to operateVertebrate cellsArtificial cell constructsInsulin-like growth factorSerial passage
The invention discloses a construction method for a Hexagrammos otakii cell line. The construction method comprises the following steps: 1, selecting a DMEM / F12 medium, adding a fetal calf serum, a human basic fibroblast growth factor, an I type insulin-like growth factor and chondroitin sulfate to prepare a cell culture solution, and storing the cell culture solution in a 4DEG C environment; 2, carrying out double resisting and rinsing processing on tissues of the Hexagrammos otakii on a super-clean workbench, and carrying out primary culture in a culture bottle; and 3, preparing a cell suspension after cells grow into a single layer, and adding the culture solution to subculture. The method of the invention has the advantages of simple step and easy operation; the morphology of the constructed tissue cell line of Hexagrammos otakii fins (HOFs), Hexagrammos otakii lips (HOLs) and Hexagrammos otakii kidneys (HOKs) likes a fiber, the serial passage of the cell line can be realized, and the Hexagrammos otakii cell line can be directly applied to pathogenic characteristic researches, vaccine developments and function gene researches, so needs for application researches of theoretical researches, virus vaccine developments and the like of Hexagrammos otakii are satisfied; and the construction method is also suitable for constructing cell lines of fins, lips and kidneys of other fishes.
Owner:DALIAN OCEAN UNIV
System, method and application for saving swine enteric alphacoronavirus
ActiveCN110468155ASsRNA viruses positive-senseViral antigen ingredientsSerial passageBiological property
The invention discloses a system, a method and application for saving swine enteric alphacoronavirus. The system comprises a recombinant transcription vector containing whole genome DNA of a swine enteric alphacoronavirus and helper plasmids containing SeACoV-N genes. For the system, the method and the application disclosed by the invention, an SADS-CoV / SeACoV reverse genetic system is successfully constructed, produced viruses can be subjected to serial passage, and reviving viruses and parental viruses have consistent biological characteristics. Establishment of the reverse genetic system ishindered by the instability and the toxicity of a virus genome sequence; and the saving method has a potential application value in reverse genetics research and vaccine creation of the swine coronavirus and has a wide application prospect in the aspects of virus gene function research and the like.
Owner:ZHEJIANG UNIV
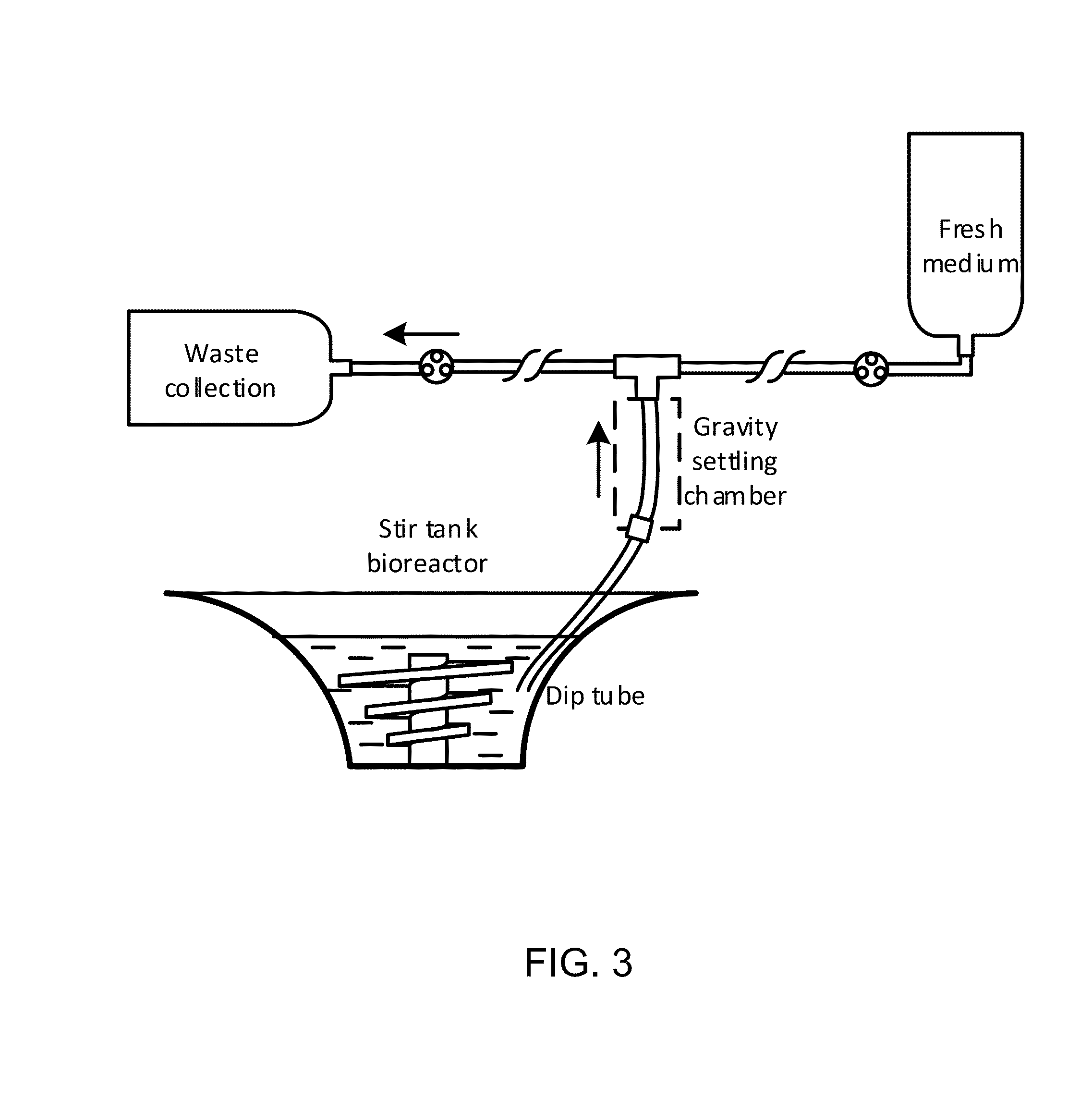
Pluripotent stem cell expansion and passage using a rocking platform bioreactor
ActiveUS20160340633A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSerial passageCell aggregation
Provided herein are novel methods for expansion and passaging of cell aggregates comprising stem cells and / or differentiated cells and comprising the use of closed systems on rocking platform bioreactors. The methods of the invention permit closed system serial passage expansion of pluripotent stem cells and / or progeny thereof with associated pluripotency markers and differentiation potential.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Adaptive mutations allow establishment of JFH1-based cell culture systems for hepatitis C virus genotype 4A
The present inventors developed three 4a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and all of or part of NS2 were replaced by the corresponding genes of the genotype 4a reference strain ED43. The 4a / 2a junction in NS2 was placed after the first transmembrane domain (α), in the cytoplasmic part (β) or at the NS2 / NS3 cleavage site (y). Following transfection of Huh7.5 cells with RNA transcripts, infectious viruses were produced in the ED43 / JFH1-β and -y cultures only. Compared to the 2a control virus, production of infectious viruses was significantly delayed. However, in subsequent passages efficient spread of infection and high HCV RNA titers were obtained. Infectivity titers were approximately 10-fold lower than for the 2a control virus. Sequence analysis of recovered 4a / 2a recombinants from 3 serial passages and subsequent reverse genetic studies revealed a vital dependence on a mutation in the NS2 4a part. ED43 / JFH1-γ further depended on a second NS2 mutation. Infectivity of the 4a / 2a viruses was CD81 dependent. Conclusion: The developed 4a / 2a viruses provide a robust in vitro tool for research in HCV genotype 4, including vaccine studies and functional analyses of an increasingly important genotype in the Middle East and Europe.
Owner:HVIDOVRE HOSPITAL
Pluripotent stem cell expansion and passage using a rocking platform bioreactor
ActiveUS9944894B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSerial passageCell aggregation
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Human adipose derived insulin making mesenchymal stem cells for treating diabetes mellitus
InactiveUS20110008301A1Simple and efficientCost-effectiveBiocidePancreatic cellsSerial passageMesenchymal stem cell
The invention provides a novel therapeutic composition comprising of insulin producing mesenchymal stem cells obtained from human adipose tissue along with Hematopoietic stem cells for the treatment of diabetic patients especially insulinopenic patients. The invention also describes a simple and efficient process for the isolation, proliferation and differentiation of insulin producing mesenchymall stem cells from human adipose tissue. Unfiltered extract of adipose tissue is used in the process with a medium totally free from xenogenic material; the serial passages of the cells are avoided in the process.
Owner:TRIVEDI H L +1
Efficient cell culture system for hepatitis C virus genotype 1A and 1B
The present inventors developed hepatitis C virus 1a / 2a and 1b / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and NS2 were replaced by the corresponding genes of the genotype Ia reference strain H77C or TN or the corresponding genes of the genotype Ib reference strain J4. Sequence analysis of recovered 1a / 2a and 1b / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in e.g. p7, NS2 and / or NS3. In addition, the inventors demonstrate the possibility of using adaptive mutations identified for one HCV isolate in generating efficient cell culture systems for other isolates by transfer of mutations across isolates, subtypes or major genotypes. Furthermore neutralization studies showed that viruses of e.g. genotype 1 were efficiently neutralized by genotype Ia, 4a and 5a serum, an effect that could be utilized e.g. in vaccine development and immunological prophylaxis. The inventors in addition demonstrate the use of the developed systems for screening of antiviral substances in vitro and functional studies of the virus, e.g. identification of receptors required for HCV entry.
Owner:HVIDOVRE HOSPITAL
Live avian encephalomylitis and henpox combined vaccine
ActiveCN103721253AMeet the standard of non-virulence reversionEffective immune protectionAntiviralsAntibody medical ingredientsAntigenSerial passage
The invention provides a live avian encephalomylitis and henpox combined vaccine. The combined vaccine consists of an antigen and a protective agent, wherein the antigen comprises henpox virus and avian encephalomylitis virus with the preservation number of CGMCC (China General Microbiological Culture Collection Center) No.8505. The combined vaccine prepared by the invention can be used for preventing avian encephalomylitis and henpox at the same time to achieve the aim of preventing two viruses with one injection. Furthermore, The avian encephalomylitis virus screened by the invention is an attenuated virus, performs serial passage in an avian body through horizontal transmitted infection, does not have the phenomenon of virulence return, is stable in heredity, and accords with the standard of no virulence return of an avian encephalomylitis attenuated vaccine strain; the prepared vaccine can provide effective immune protection, and has good commercial development prospect.
Owner:YEBIO BIOENG OF QINGDAO
Spontaneously immortalized piglet oral mucosa epithelial cell line and construction method thereof
InactiveCN105838662AHigh purityPromote growthCell dissociation methodsEpidermal cells/skin cellsGenes mutationSerial passage
The invention discloses a spontaneously immortalized piglet oral mucosa epithelial cell line and a construction method thereof. Oral mucosa epithelial tissue of a newly-born unfed piglet is subjected to primary culture and continuous passage culture so that a high-purity and good-stability spontaneously immortalized piglet oral mucosa epithelial cell line is obtained. The spontaneously immortalized piglet oral mucosa epithelial cell line can fast grow and has stable performances. 110th generation of cells still keep form characteristics of primary cells. A karyotype analysis result shows that the cells do not produce mutation. The spontaneously immortalized piglet oral mucosa epithelial cell line is self-formed after serial passage, does not produce exogenous gene insertion and gene mutation, can be used as a basic cell in vaccine research and development and does not produce a biological safety risk. The cell line provides a good cell model for molecular biology and virology research and can be used for porcine virus vaccine research and development.
Owner:张彦明
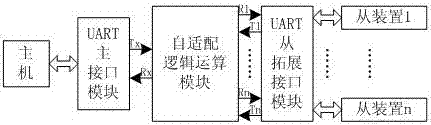
A channel self-adapting device and method for uart serial port expansion
ActiveCN104216854BEasy to expandImprove switching efficiencyTransmissionElectric digital data processingSerial passageLogical operations
The invention discloses a path self-adapting device and method for UART serial port expansion. Between the UART main interface and the UART slave expansion interface module, an adaptation logic operation module is added, and the adaptation logic operation module can intelligently measure, distinguish and Execute the occupation and release operation of the UART uplink channel, realize the self-adaptation operation of the independent channel with high switching efficiency, fast speed, short idle time and complete intelligent automation, without affecting the communication speed, convenient for UART serial port expansion, and has a good application prospect.
Owner:NANJING PANENG TECHNOLOGY DEVELOPMENT CO LTD
Vaccines for diseases of fish
Owner:IDAHO UNIV OF
Method for producing an adapted virus population from an African green monkey kidney cell line
InactiveUS6117667ARestricted and narrow tissue culture host rangeAvoid developmentSsRNA viruses negative-senseSsRNA viruses positive-senseEnterovirusSerial passage
The invention described herein provides a method for selecting a novel African Green monkey kidney (AGMK) cell substrate, its cultivation and serial passage and its subsequent characterization. The invention provides a method for the use of the cell substrate in the isolation, growth and serial passage of a large number of viruses, particularly rotaviruses, enteroviruses, respiratory viruses and hepatitis A virus. The invention provides a method for the utilization of this AGMK cell substrate for the production of live and killed virus vaccines.
Owner:NOVAVAX
Vaccines for porcine epidemic diarrhea virus infections
ActiveUS20180008699A1Reduce incidenceReduce severitySsRNA viruses positive-senseViral antigen ingredientsSerial passageImmunogenicity
Isolated porcine epidemic diarrhea virus (PEDV) deposited under ATCC Accession No. PTA-121847, and attenuated strains generated by serial passage in culture of the deposited strain. Immunogenic compositions for reducing the incidence or severity of clinical symptoms from PEDV infection, and methods of making and using the same.
Owner:KANSAS STATE UNIV RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com