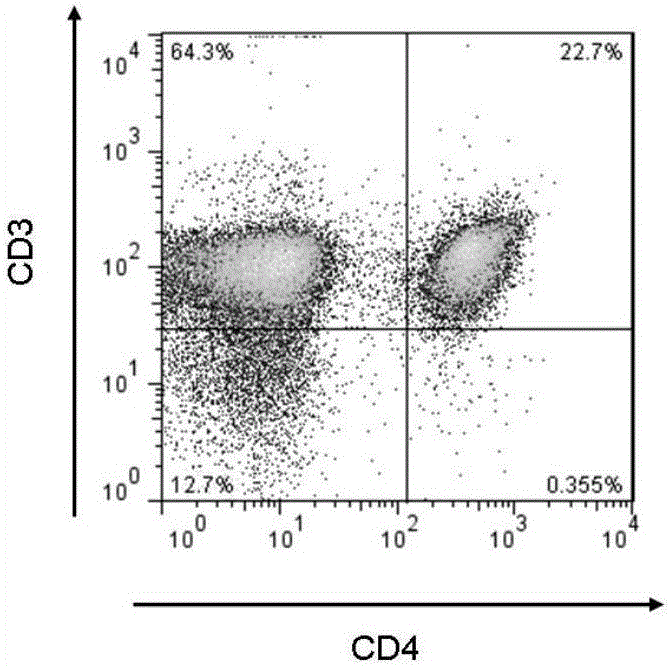
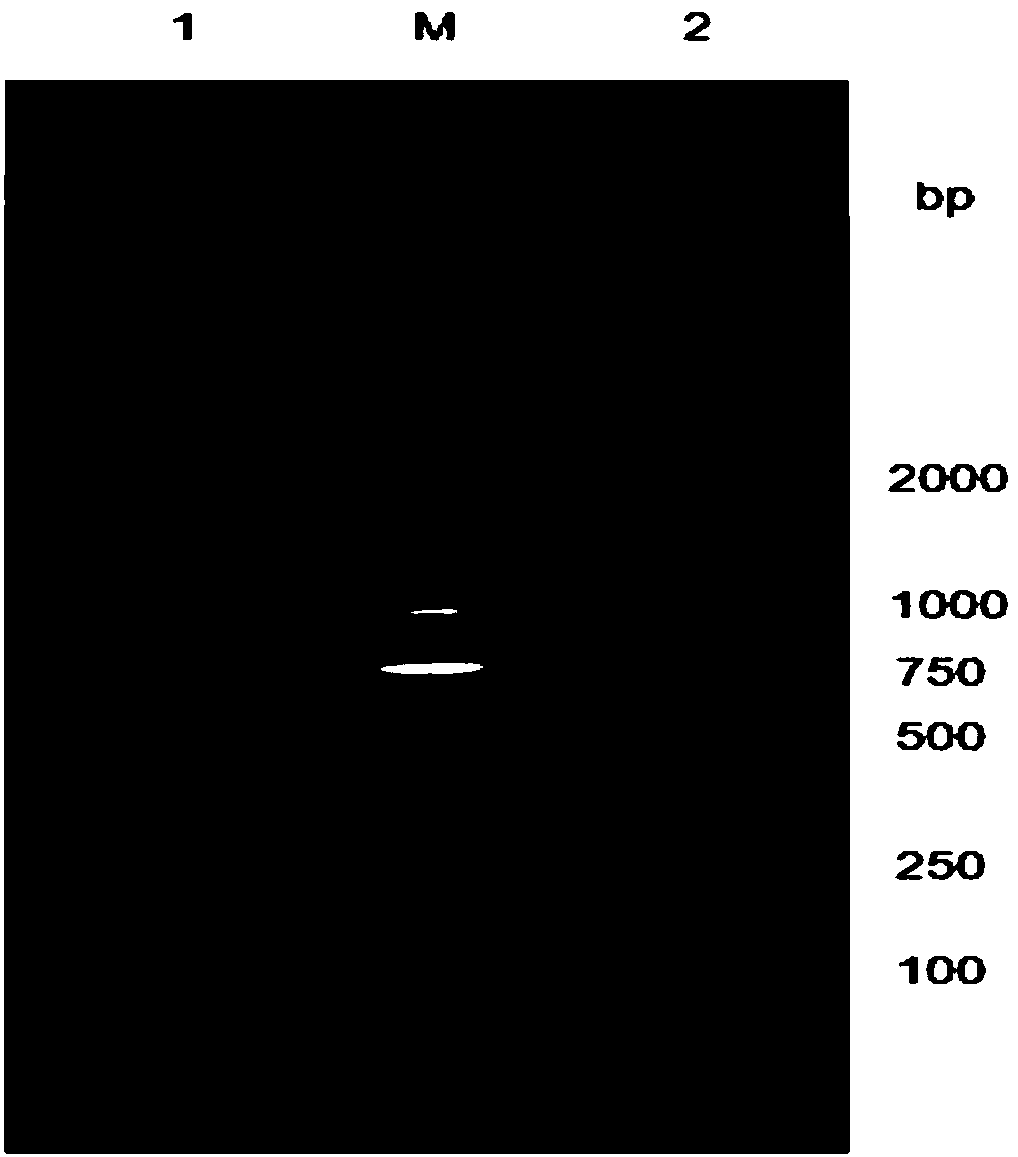
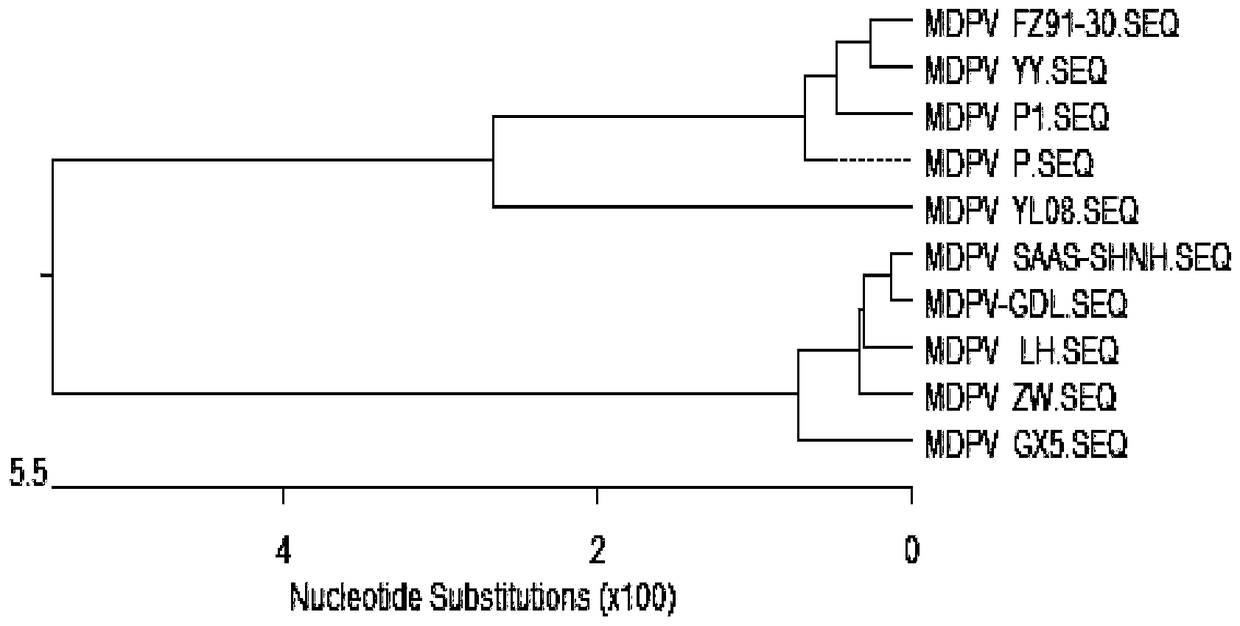
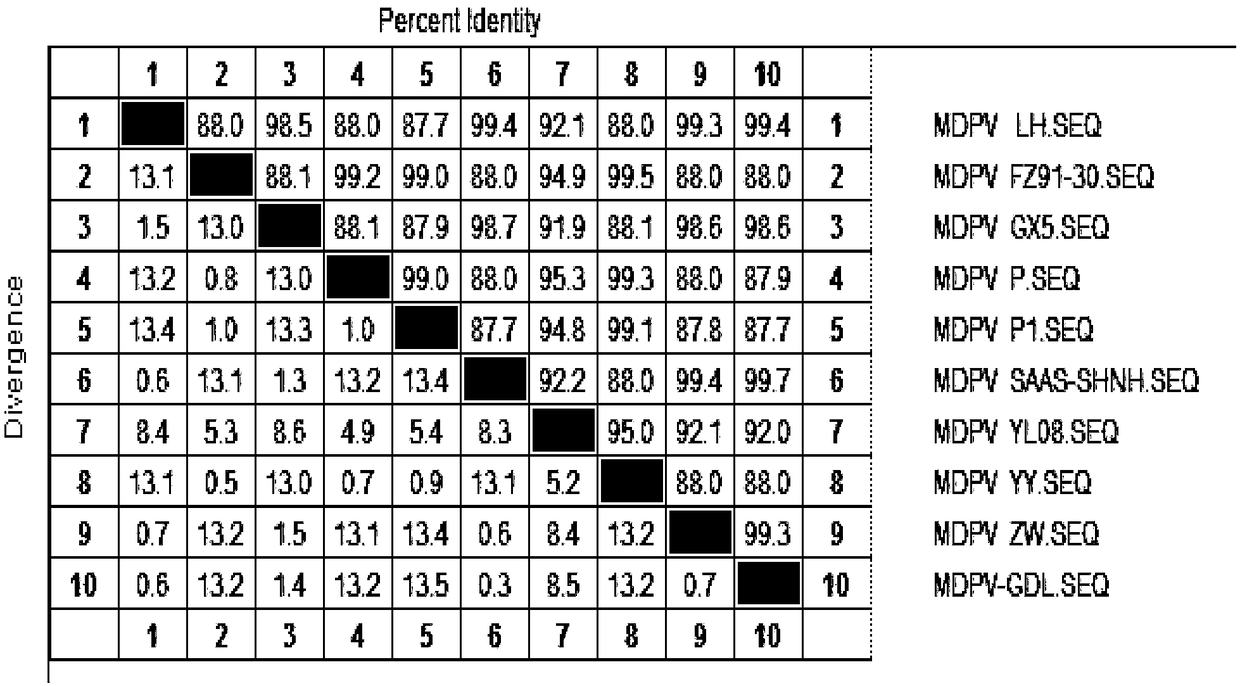
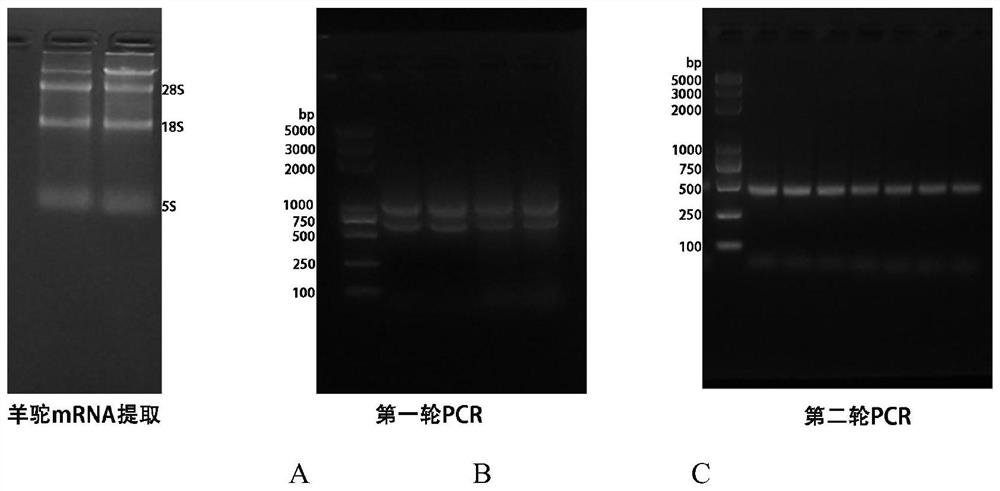
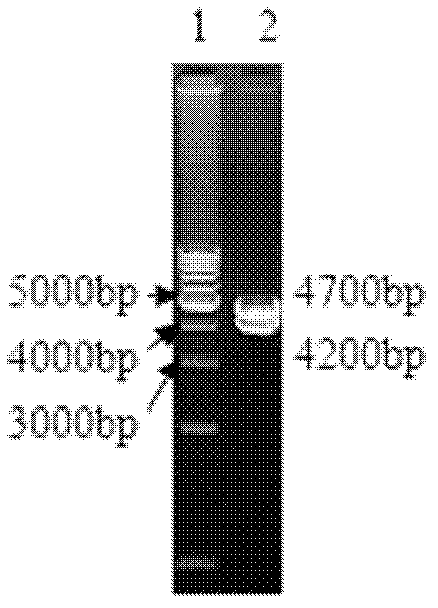
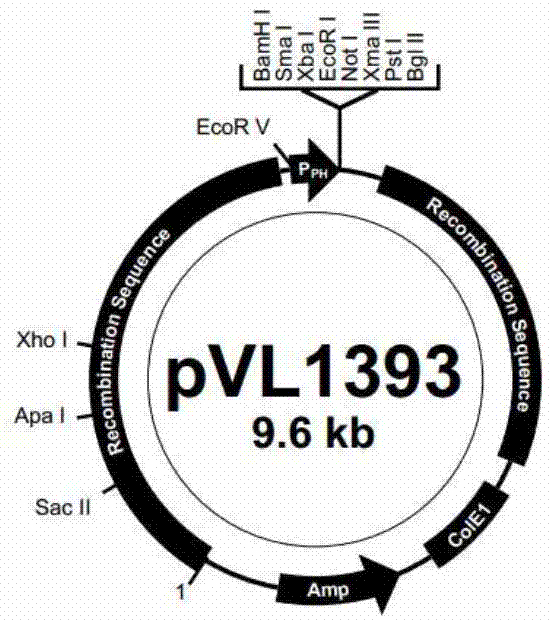
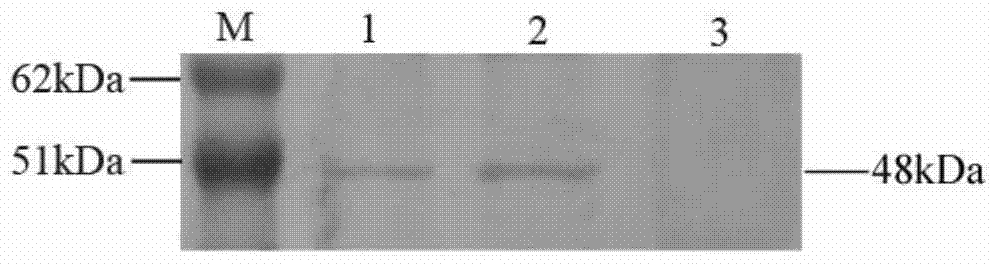
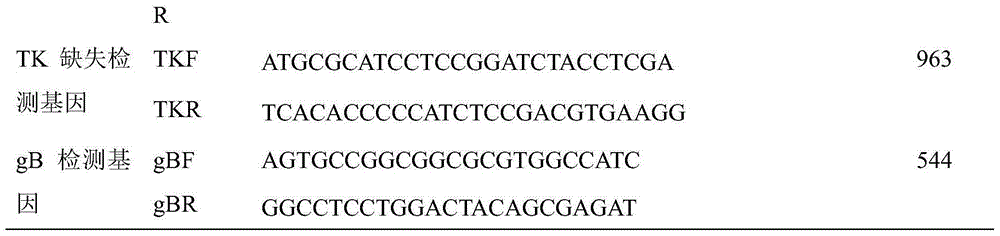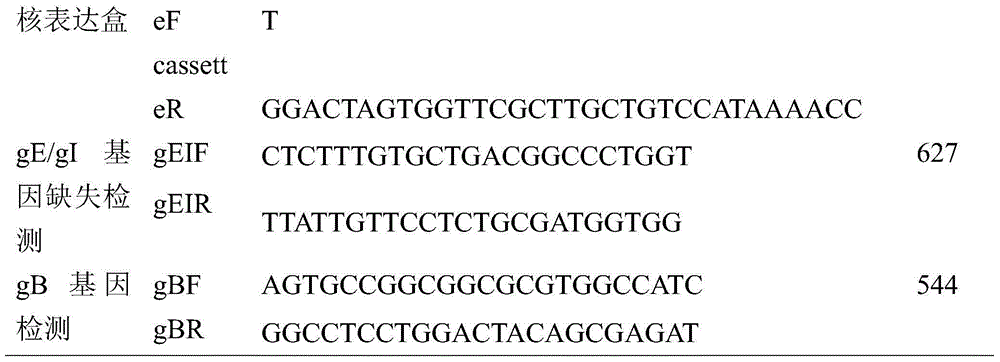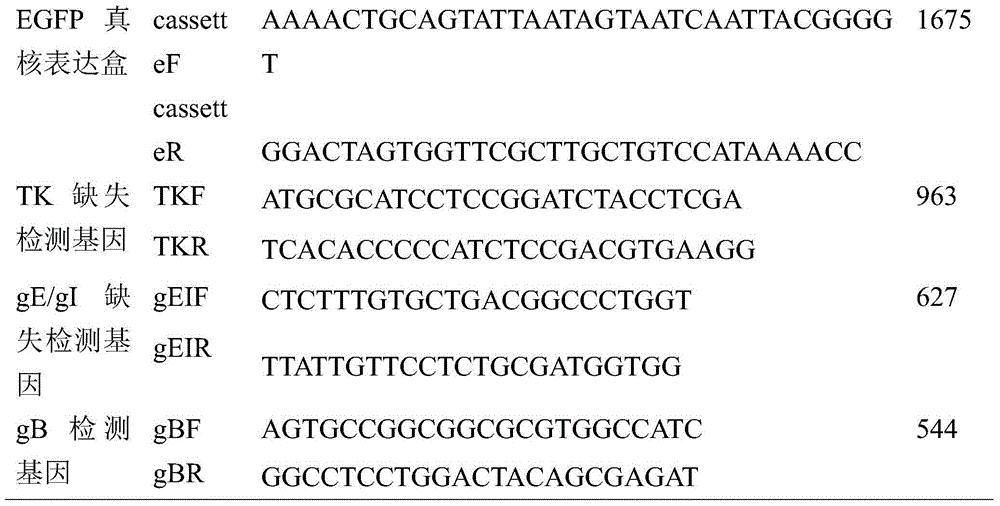Patents
Literature
81results about How to "Effective immune protection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
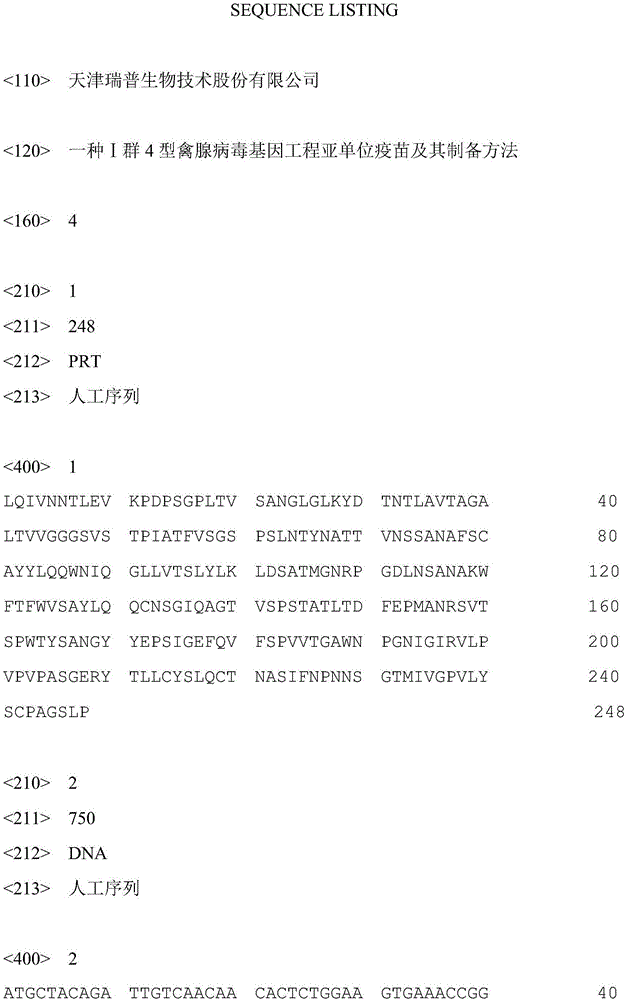
I-group 4-type aviadenovirus genetic engineering subunit vaccine and preparation method thereof
InactiveCN106344919AGood prospects for commercial developmentLow costViral antigen ingredientsVirus peptidesSequence analysisInclusion bodies
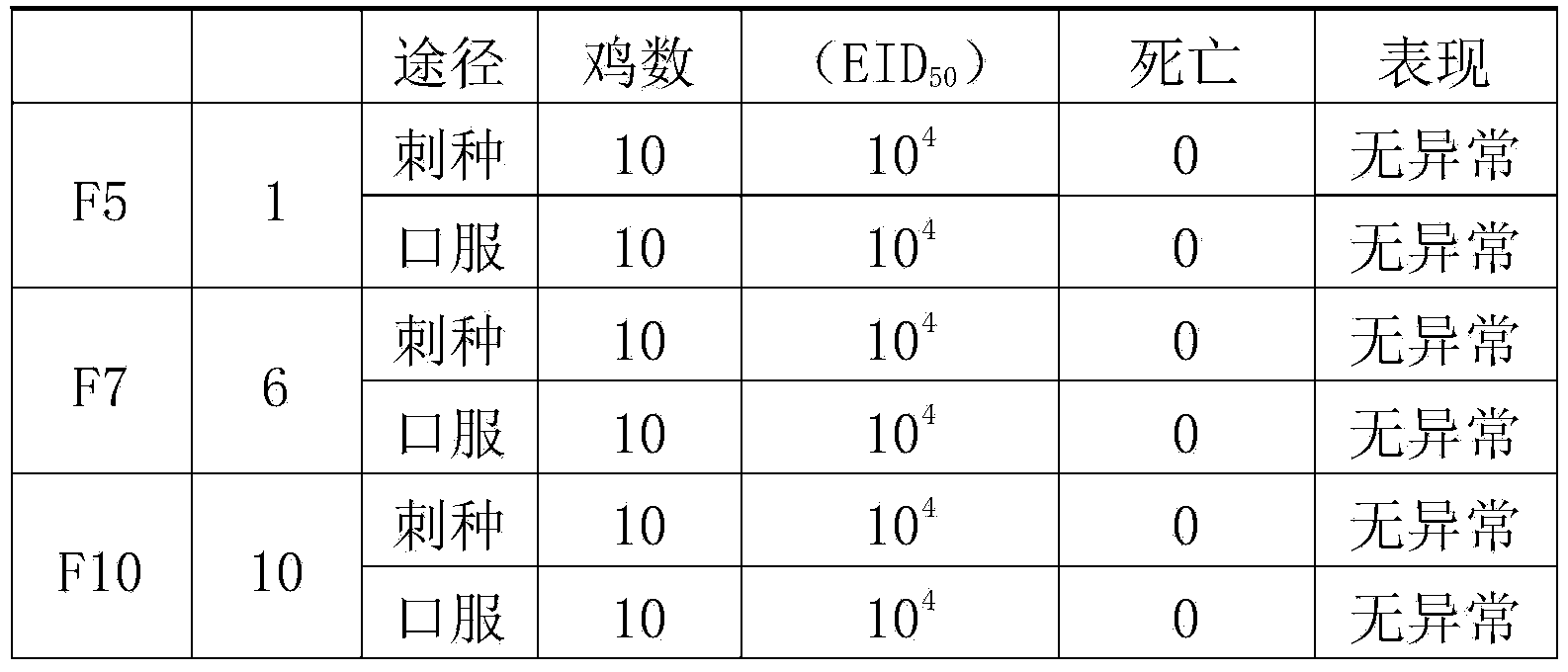
The invention provides an I-group 4-type aviadenovirus genetic engineering subunit vaccine and a preparation method thereof. According to the technical scheme, the preparation method comprises the following steps: cloning an encoding gene of fibrous protein C-terminal from an I-group 4-type aviadenovirus genome according to a PCR technology and performing sequence analysis; cloning the gene to an expression vector pET-32a, transforming escherichia coli, constructing engineering bacteria, and inducing the engineering bacteria by isopropyl-beta-D-thiogalactopyranoside to express the fibrous protein C-terminal; performing lysis on an engineering bacterial cell, performing centrifugal separation on an inclusion body of the engineering bacterial cell, dissolving urea and diluting for renaturation; preparing the vaccine according to the conventional preparation method of a mineral oil adjuvant inactivated vaccine. According to the I-group 4-type aviadenovirus genetic engineering subunit vaccine prepared by the method, the immune effect of the vaccine is evaluated by a serological method and an immunity challenge method, and the result indicates that the aviadenovirus inactivated vaccine prepared by the method can provide effective immunoprotection and has a good commercialized development prospect.
Owner:TIANJIN RINGPU BIO TECH
Porcine pseudorabies virus vaccine
ActiveCN104826103AEffective immune protectionMeet the standard of non-virulence reversionAntiviralsAntibody medical ingredientsAntigenVirulent characteristics
The invention aims at providing a porcine pseudorabies virus vaccine. The porcine pseudorabies virus vaccine comprises an antigen and a protective agent, wherein the antigen contains an attenuated virus strain which is prepared after deleting virulence genes by a porcine pseudorabies virus strain with the collection number of CGMCC No. 10266. The prepared vaccine can effectively prevent porcine pseudorabies; furthermore, because the porcine pseudorabies virus as the antigen is a gene-deleted strain, by continuous passage of horizontally transmitted infections in mouse bodies, no virulence reversion occurs, and the genetic stability is realized, thereby being in line with the standard of having no virulence reversion in the porcine pseudorabies virus deleted vaccine strain; and the prepared vaccine can provide effective immune protection, and has great commercialization development prospects.
Owner:SHANDONG SINDER TECH
Recombinant porcine pseudorabies virus gE/gI double-gene-deleted strain and application thereof
ActiveCN104877972AGenetically stableEffective immune protectionInactivation/attenuationAntiviralsAntigenPseudorabies
The invention aims at providing a porcine pseudorabies virus vaccine. The porcine pseudorabies virus vaccine consists of an antigen and a protective agent, wherein the antigen comprises an attenuated virus strain which is prepared by deleting virulence genes gE and gI of a porcine pseudorabies virus strain with a collection number of CGMCC No.10266. The prepared vaccine provided by the invention can be used for effectively preventing porcine pseudorabies; moreover, porcine pseudorabies virus which is taken as the antigen is a gene-deleted strain, and after the porcine pseudorabies virus is subjected to continuous passage in a mouse body by virtue of horizontal transmission infection, no virulence enhancement phenomenon can be caused, and thus the gene-deleted strain is stable in heredity, and can meet the standard that a porcine pseudorabies virus deleted vaccine strain does not have virulence enhancement; and the prepared vaccine can be used for providing effective immune protection, and has very good commercialized development prospects.
Owner:SHANDONG SINDER TECH
Immunoloregulation type DNA vaccine capable of preventing Eimeria necatrix
InactiveCN101422605AGood immune protectionEffective immune protectionGenetic material ingredientsAntiparasitic agentsAntigenEmoia loyaltiensis
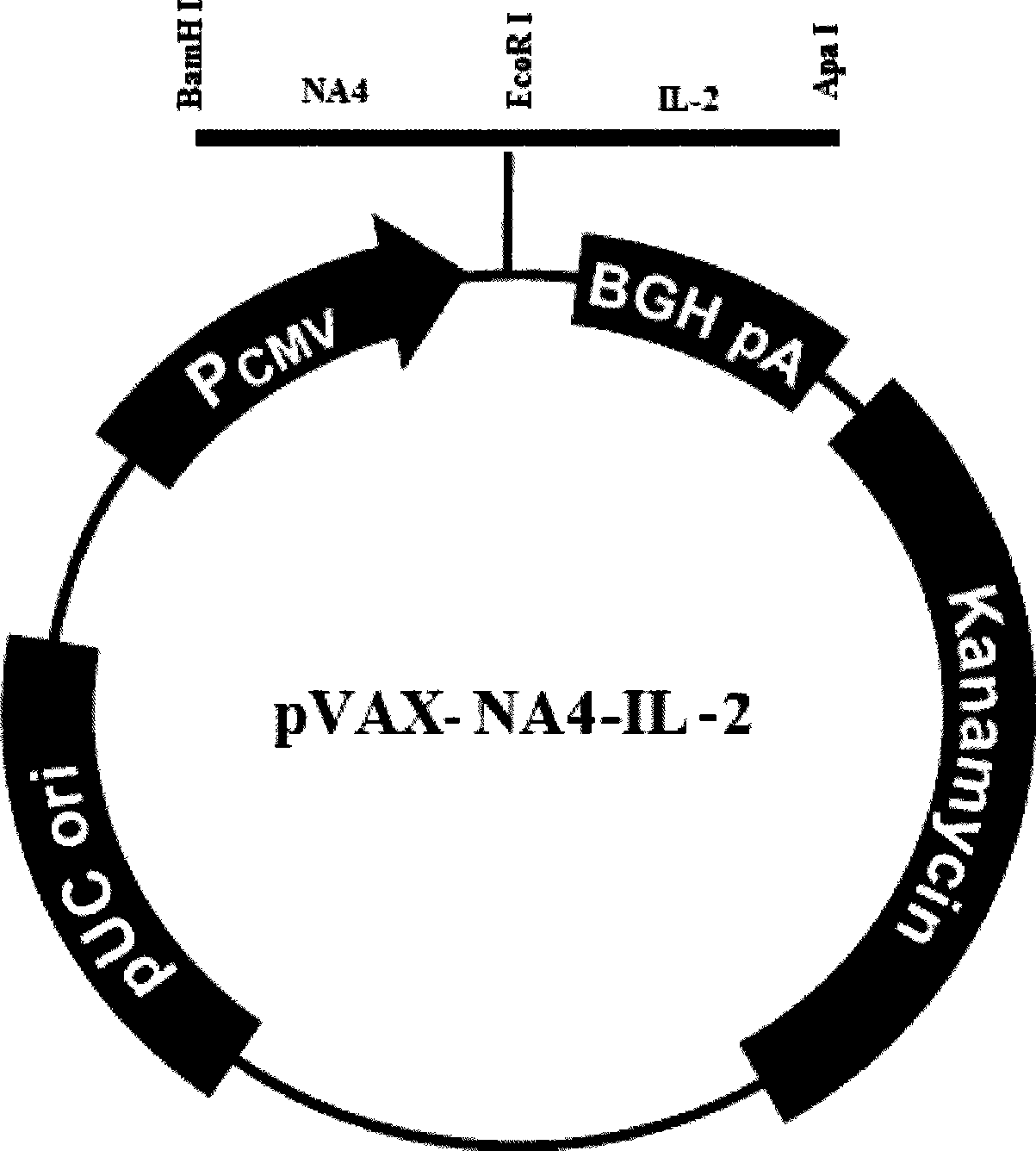
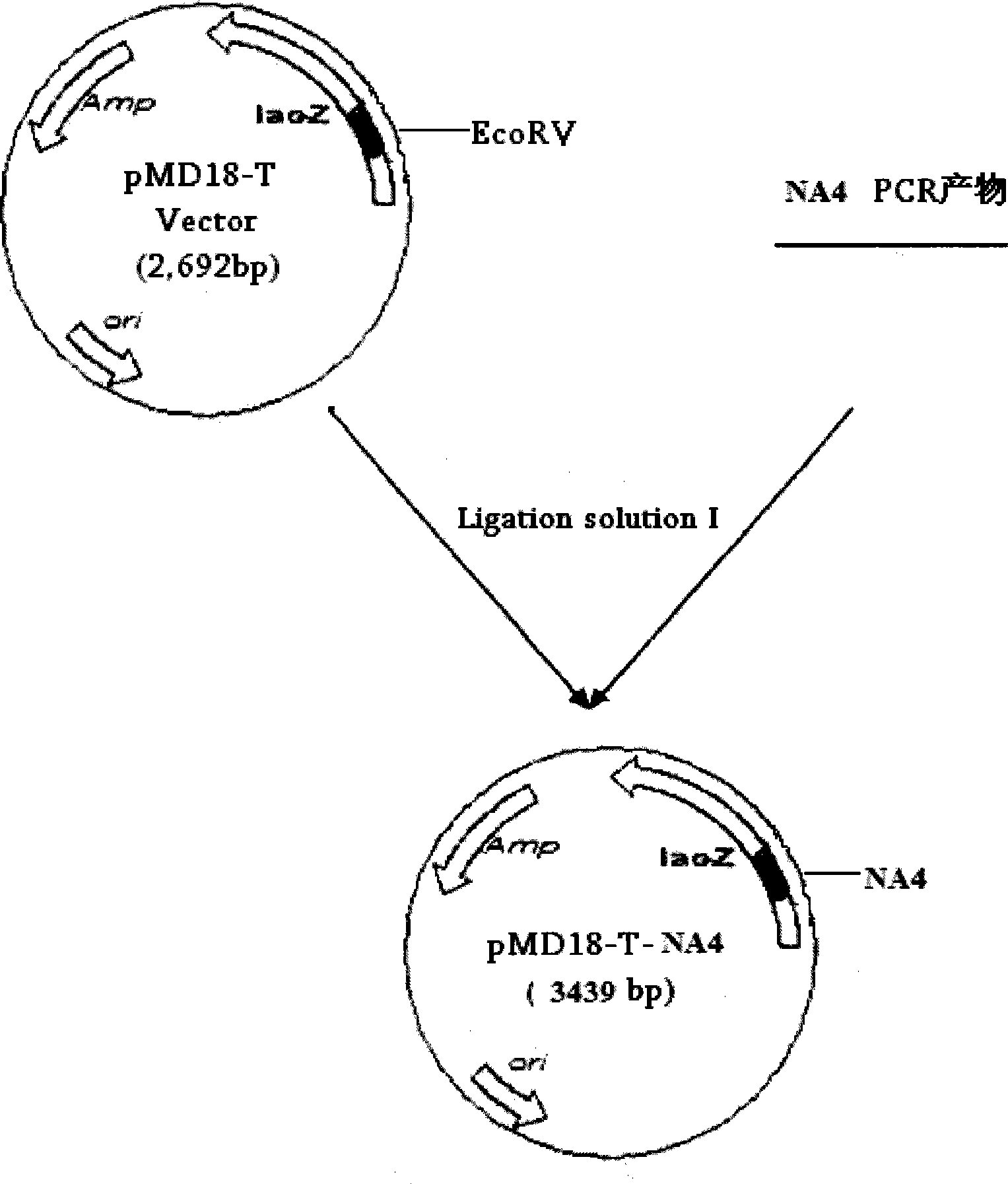
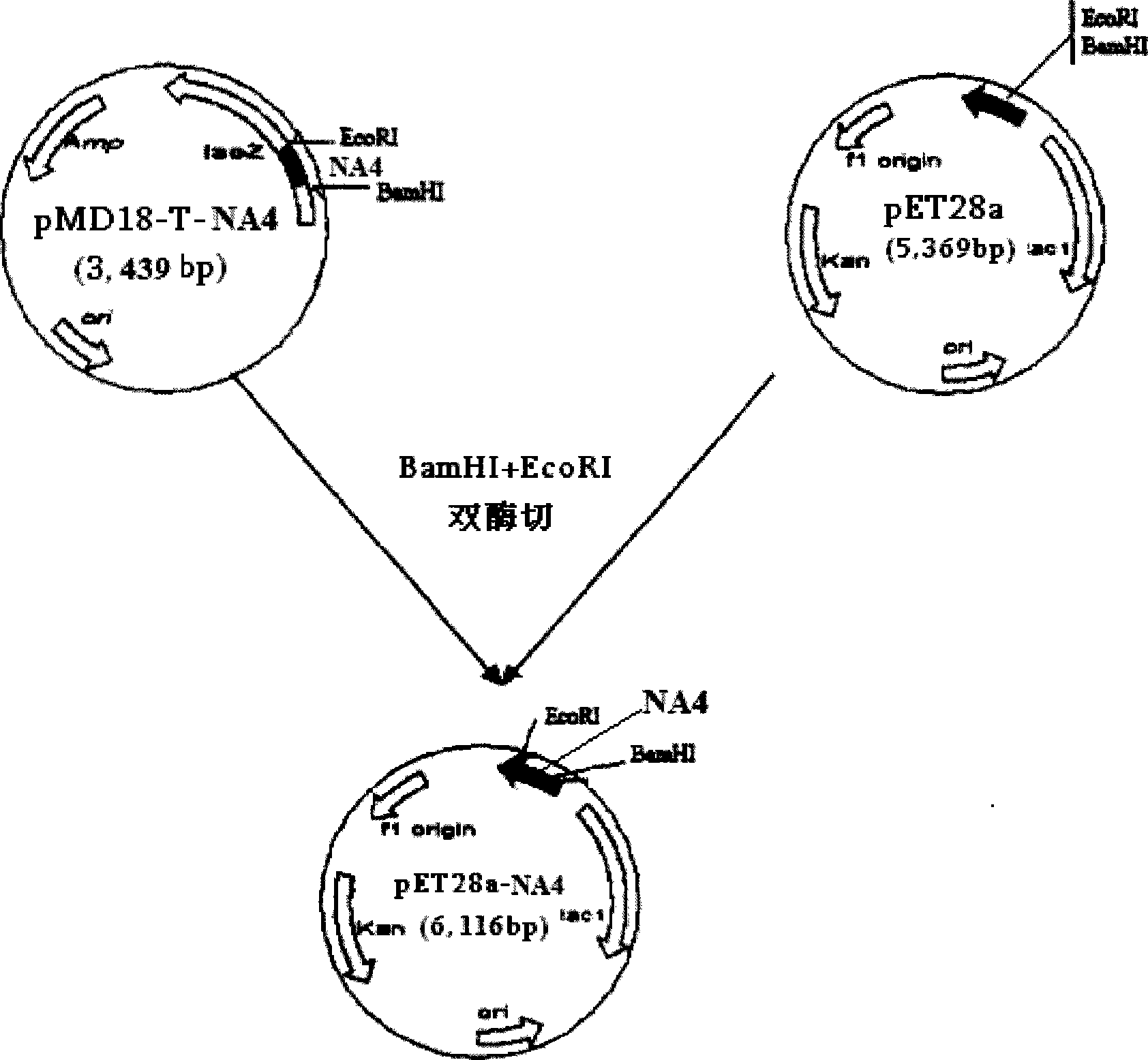
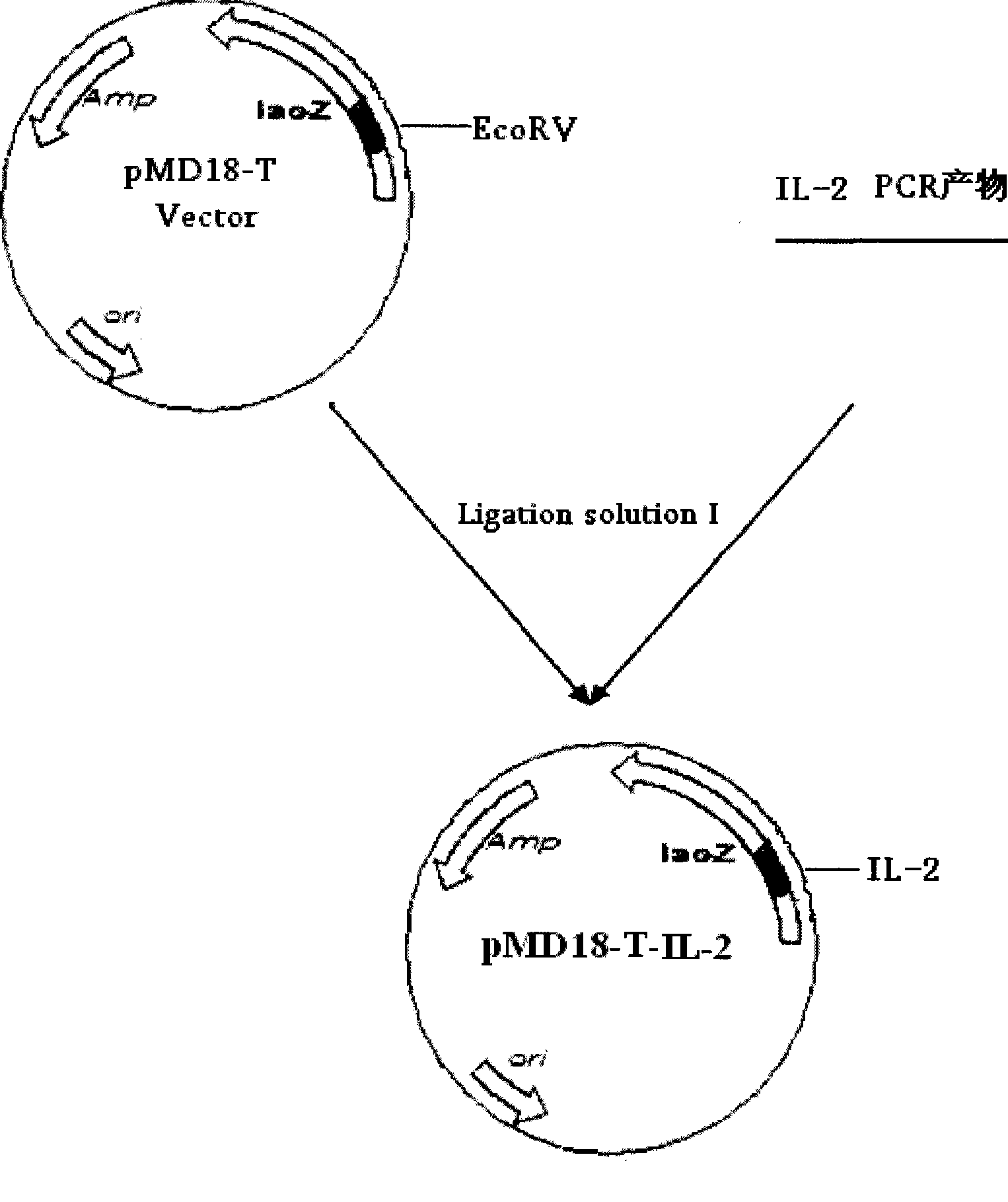
The invention relates to a chicken toxic Eimeria acervulina immunoregulation type DNA vaccine, which belongs to the technical field of biological veterinary medicines. The molecular biology technology is used for connecting toxic Eimeria sporozoite surface antigen NA4 genes and chIL-2 genes in series to construct a fusion expressing carrier pVAX-LDH-IL-2. The vaccine comprises the inserted toxic Eimeria sporozoite surface antigen NA4 genes and the chIL-2 genes. The vaccine comprises a plurality of T cell immune response elements, and has the advantages of high safety, long keeping time in bodies, simple preparation, good heat stability, convenient storage and transportation, time saving and labor saving when in use and the like, thus preventing and curing chicken coccidiosis effectively. Tests prove that the immunoregulation type DNA vaccine pVAX-NA4-IL-2 has good immune protection effect in coccidian resistant infections.
Owner:NANJING AGRICULTURAL UNIVERSITY
Recombinant porcine pseudorabies virus TK/gE/gI three-gene-deleted vaccine
ActiveCN104830810AEffective immune protectionMeet the standard of non-virulence reversionInactivation/attenuationAntiviralsAntigenVirulent characteristics
The invention aims at providing a porcine pseudorabies virus vaccine. The porcine pseudorabies virus vaccine comprises an antigen and a protective agent, wherein the antigen contains an attenuated virus strain which is prepared after deleting virulence genes, namely TK, gE and gI, by a porcine pseudorabies virus strain with the collection number of CGMCC No. 10266. The prepared vaccine can effectively prevent porcine pseudorabies; furthermore, because the porcine pseudorabies virus as the antigen is a gene-deleted strain, by continuous passage of horizontally transmitted infections in mouse bodies, no virulence reversion occurs, and the genetic stability is realized, thereby being in line with the standard of having no virulence reversion in the porcine pseudorabies virus deleted vaccine strain; and the prepared vaccine can provide effective immune protection, and has great commercialization development prospects.
Owner:SHANDONG SINDER TECH
Preparation and application of new goose astrovirus egg yolk antibody
ActiveCN109265540AImprove securityEffective immune protectionEgg immunoglobulinsImmunoglobulins against virusesAntigenAntibody titer
The invention provides an egg yolk antibody prepared by using new goose astrovirus. The egg yolk antibody is prepared by taking inactivated vaccine prepared by using a new goose astrovirus GD 0122 strain with the preservation number of CCTCC No:V201820 as an antigen. The prepared antibody is good in safety, after a gosling is inoculated with the antibody, no local or systemic adverse reactions caused by the injection of the antibody occur; the prepared antibody is evaluated by adopting an antibody titer determination and challenge protection test, the result shows that the agar diffusion antibody titer of the prepared antibody is not lower than 1:16, after the antibody is injected, the gosling can resist the attack of virulent virus, and the prepared antibody can provide effective immune protection for geese and has a good commercial development prospect.
Owner:SHANDONG SINDER TECH +1
Veterinary clostridium septicum toxin, preparation method thereof and special culture medium
ActiveCN108342434AEffective immune protectionEasy to prepareAntibacterial agentsBacterial antigen ingredientsTiterToxin
The invention discloses a veterinary clostridium septicum toxin, a preparation method thereof and a special culture medium. Every 100 ml of culture medium is prepared from 1.5-2.0 g of proteose peptone, 1.5-2.0 g of casein peptone, 0.5-0.75 g of yeast extract powder, 0.14-0.28 mg of ZnSO4.7H2O, 0.5-0.75 g of Na2HPO4.12H2O, 0.03-0.045 g of KH2PO4, 1-1.5 g of glucose and the balance water; the pH value of the culture medium is 7.5-8.0. The veterinary clostridium septicum toxin is obtained by inoculating a production strain of clostridium septicum into the culture medium, collecting a culture object, conducting centrifugation and filtering a supernatant. By means of the method, the highest toxicity can be raised up to 10 times that of the vaccine-making standard of regulations for veterinarybiological products in China, and the output-input ratio can be increased to 20 times that of an original traditional process. Moreover, the neutralizing titers of toxoid vaccines prepared by using the veterinary clostridium septicum toxin to corresponding serums of rabbits and sheep are also increased to 8 times that of the regulation standard respectively.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
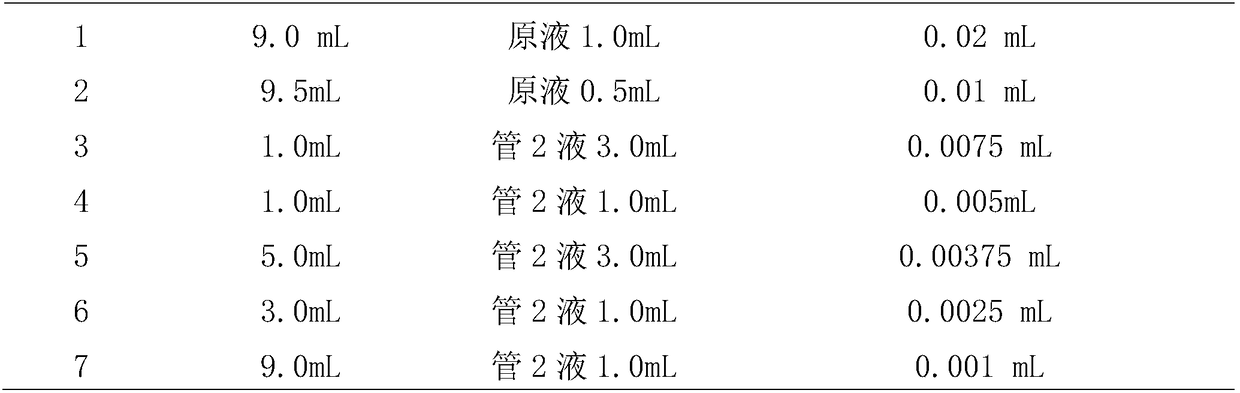
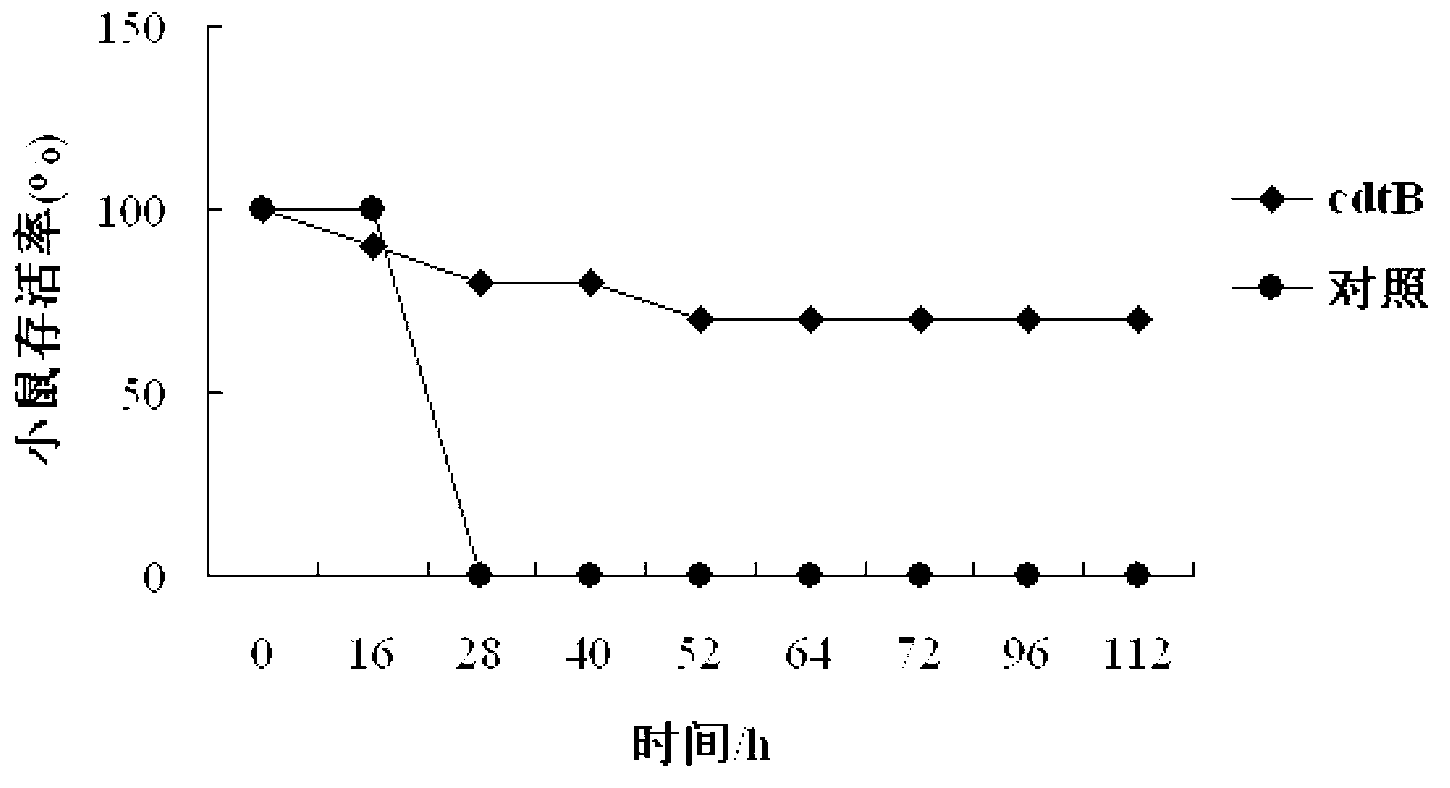
Haemophilus parasuis (Hps) immunoprotecive antigen CdtB
The invention relates to identification of a Haemophilus parasuis (Hps) immunoprotecive antigen, separation and cloning of a protein gene and application of a protein coded by the Hps immunoprotecive antigen in a vaccine. According to the invention, a new protein CdtB having immunogenicity is separated from Hps (the culture collection number is CVCC3361), and the nucleotide sequence is shown as SEQ ID NO:2 in a sequence table and is formed by coding 277 amino acids. The CdtB is a new protein having immunogenicity and can provide effective immunoprotection for Hps infection in mice. The invention also comprises preparation of an Escherichia coli recombinant bacterium BL21 / Hps-CdtB expressing the immunogenicity protein gene CdtB. The recombinant Hps CdtB protein expressed by the invention has favorable safety and protection efficacy, and the immunoprotection effect is up to 70%.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Nucleic acid vaccine for preventing AIDS
InactiveCN1631441ASimple production processEffective immune protectionViral antigen ingredientsGenetic material ingredientsVaccine manufacturingNucleotide
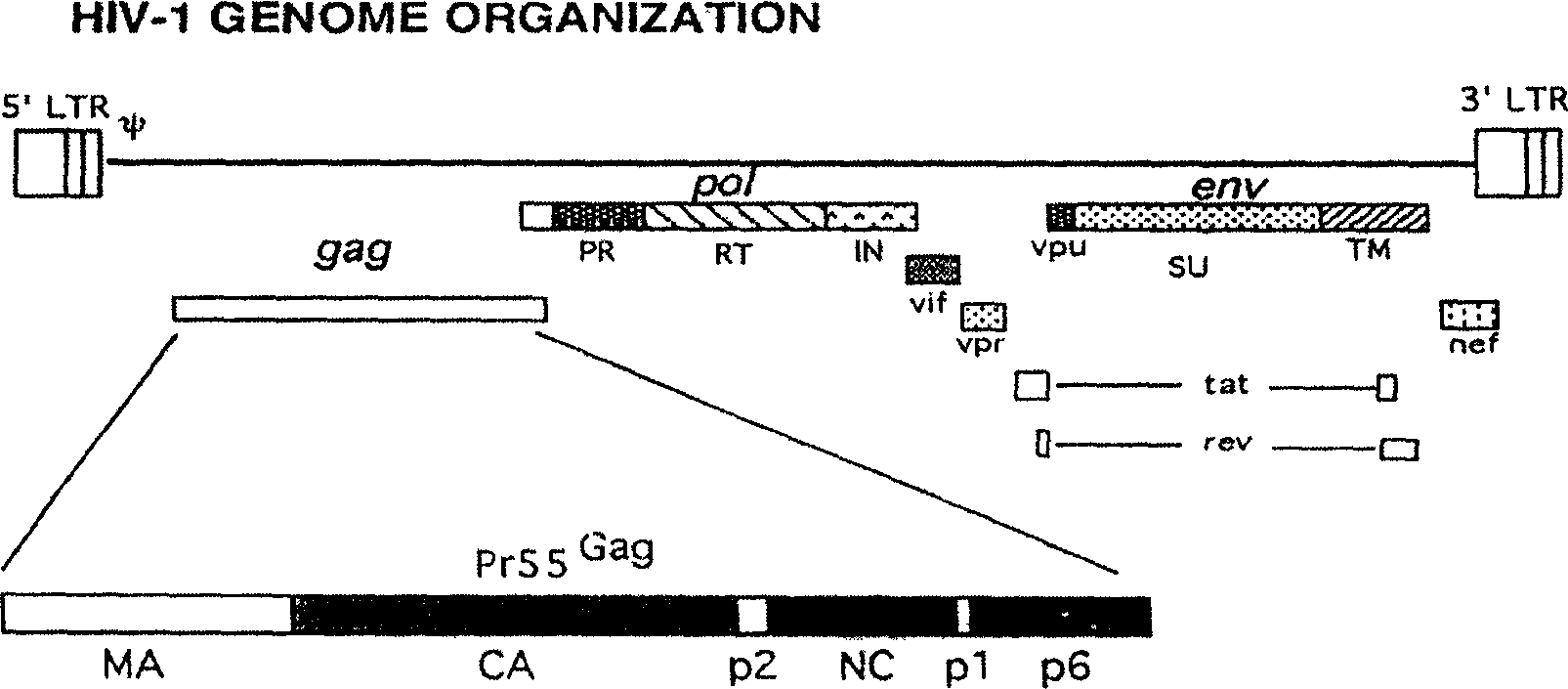
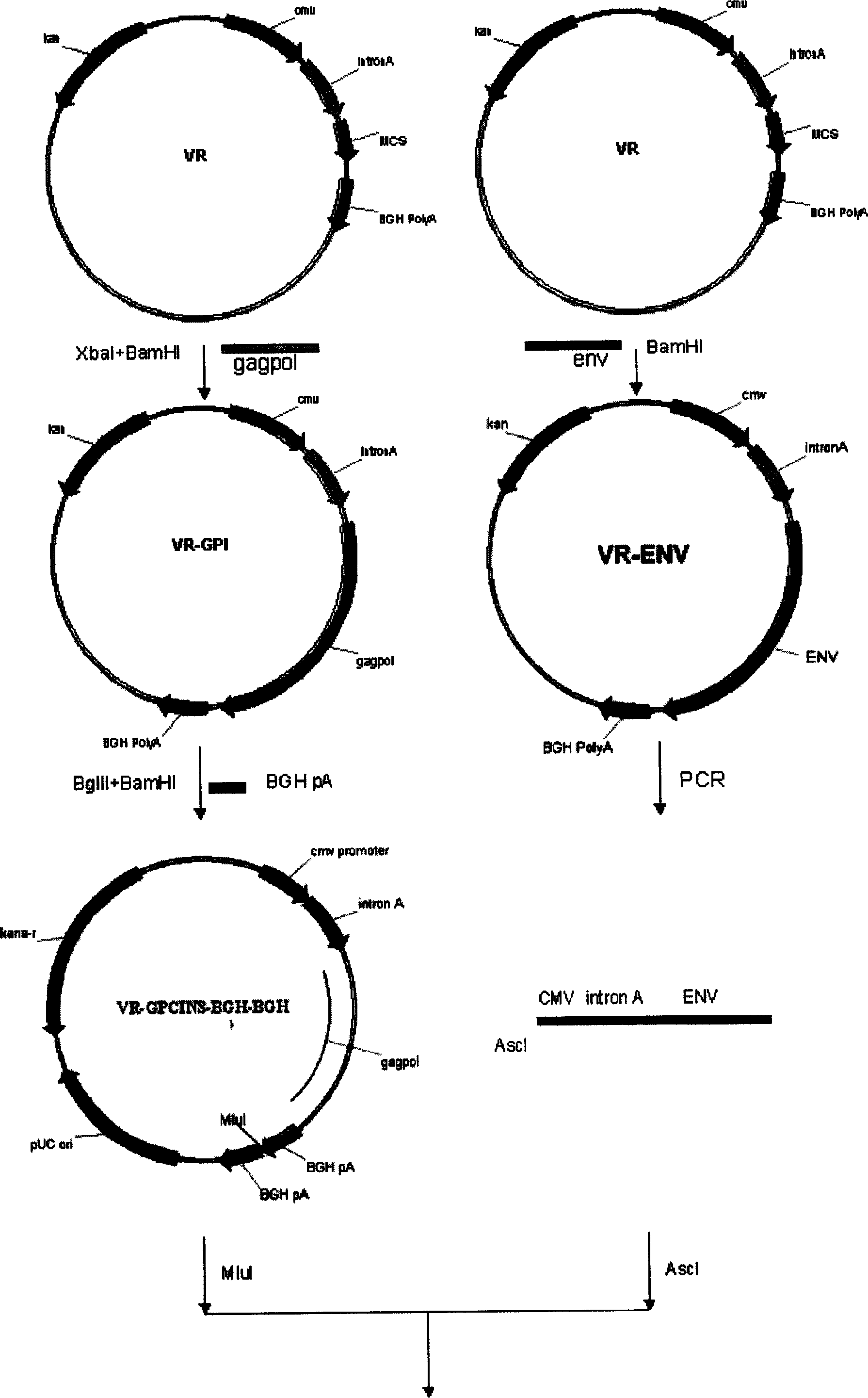
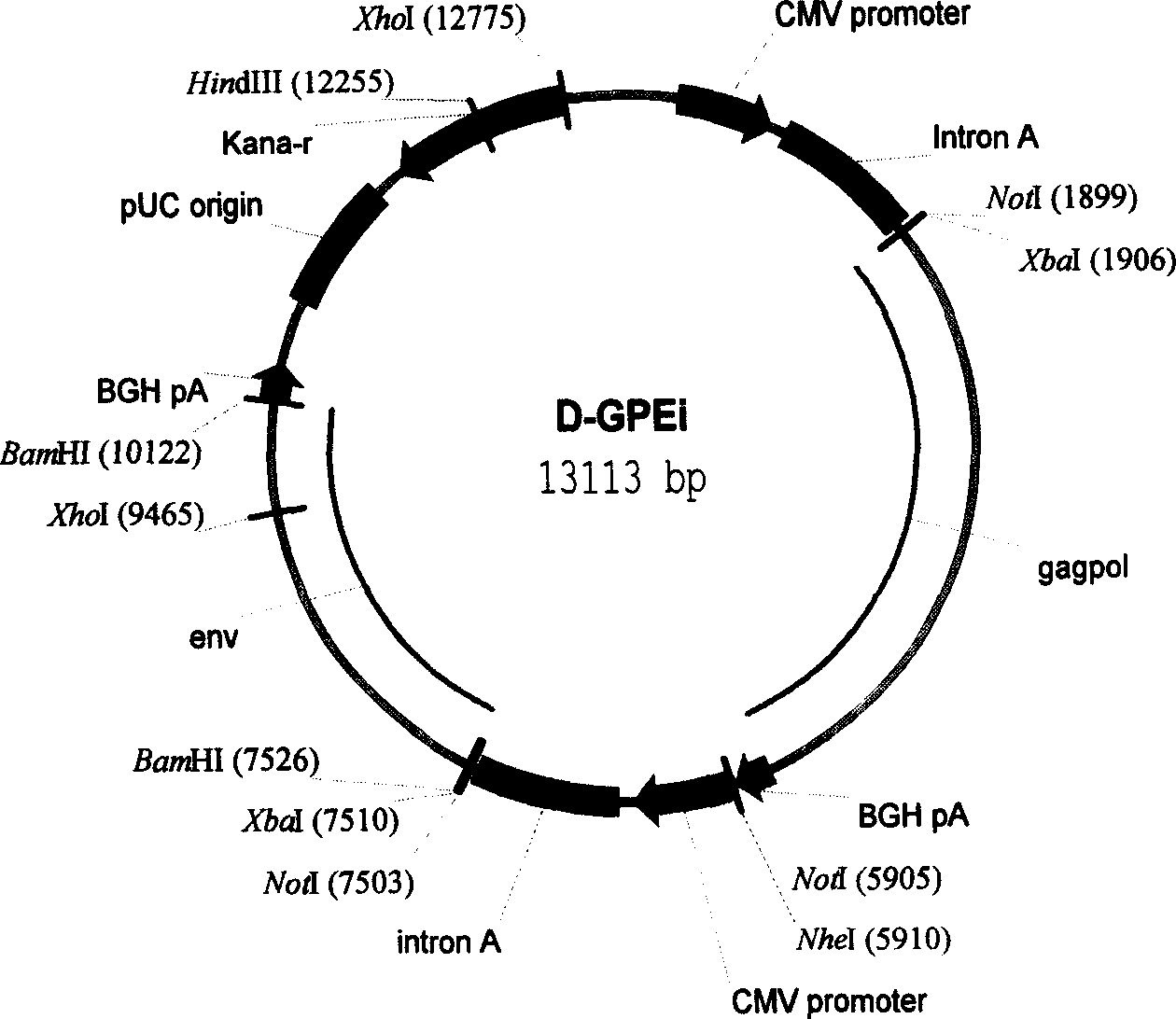
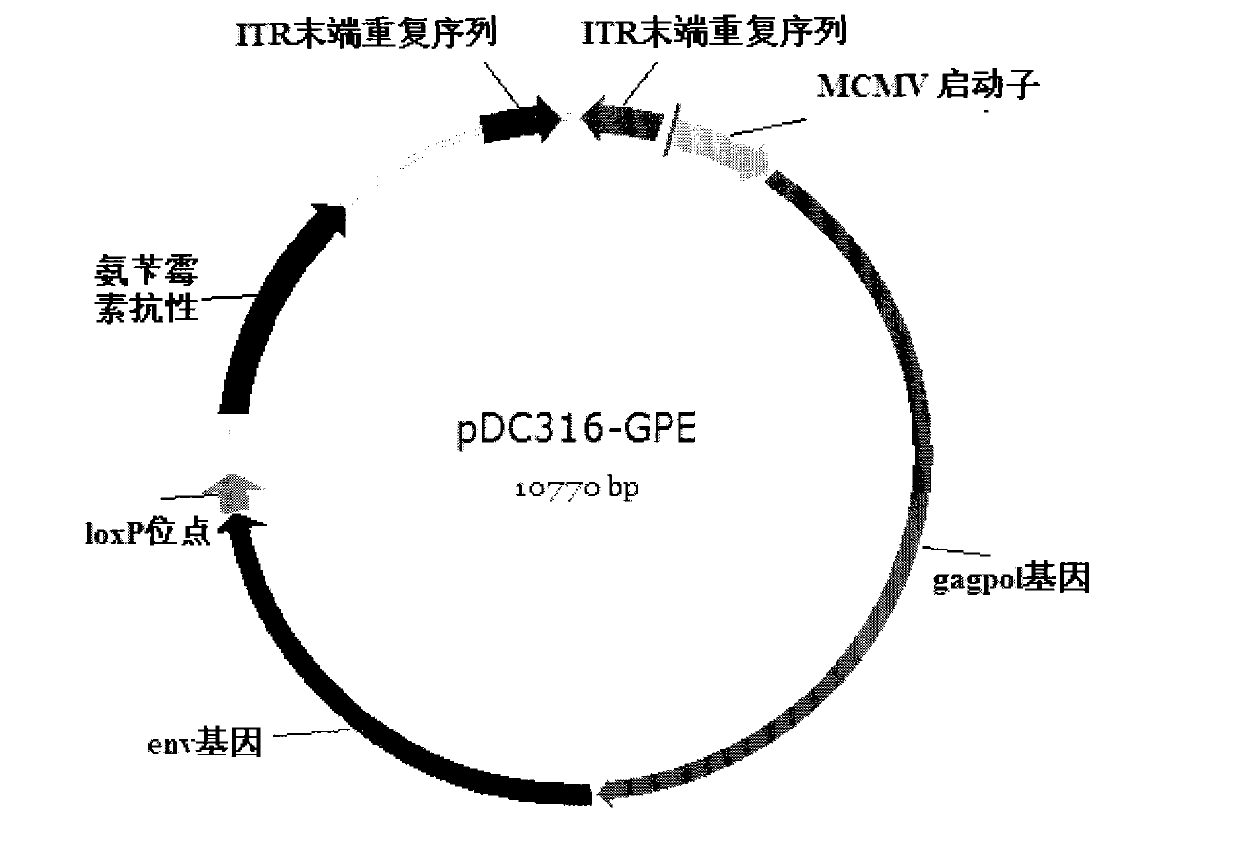
The invention relates to a DNA vaccine for preventing AIDS, the vaccine is the recombinant nucleotide of Gag, Pol and Env nucleotide sequence through the artificial modification of structural protein containing encoded Human Immunodificidncy Virus-1 (HIV-1), these vaccines are used as the transcript units for the biological synthesis of the virus protein antigen when applied in vivo. The beneficial effects of the invention include, (1) improved DNA vaccine manufacturing technique, (2) no integration indication detected, (3) the vaccine target antigen includes almost all the HIV-1 structural protein, such as GagPol and Env.
Owner:长春百克药业有限责任公司
Immunoregulation DNA vaccine capable of preventing chicken Eimeria maxima
InactiveCN101396556AImprove securityLong lastingGenetic material ingredientsAntiparasitic agentsAntigenWhite blood cell
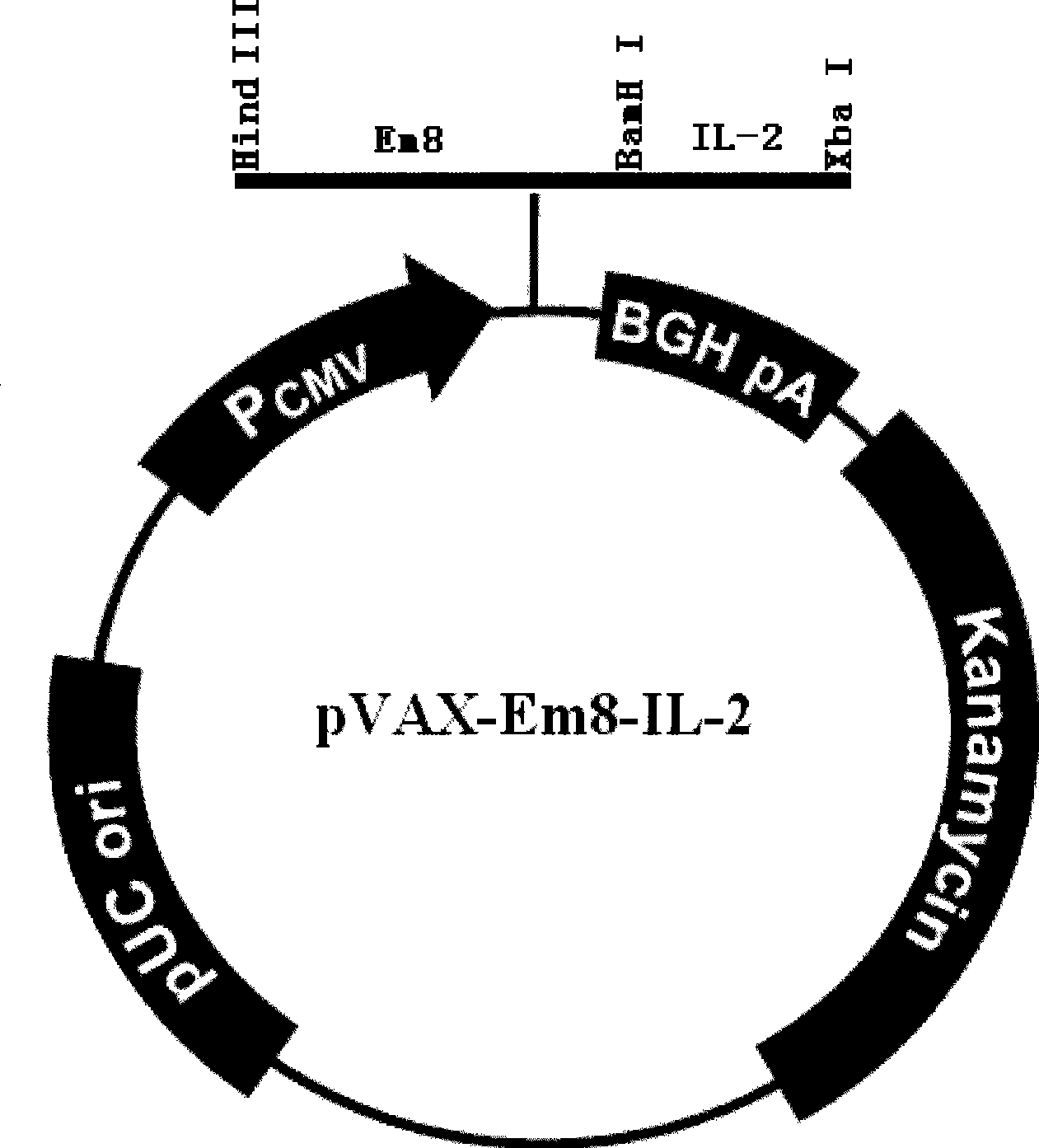
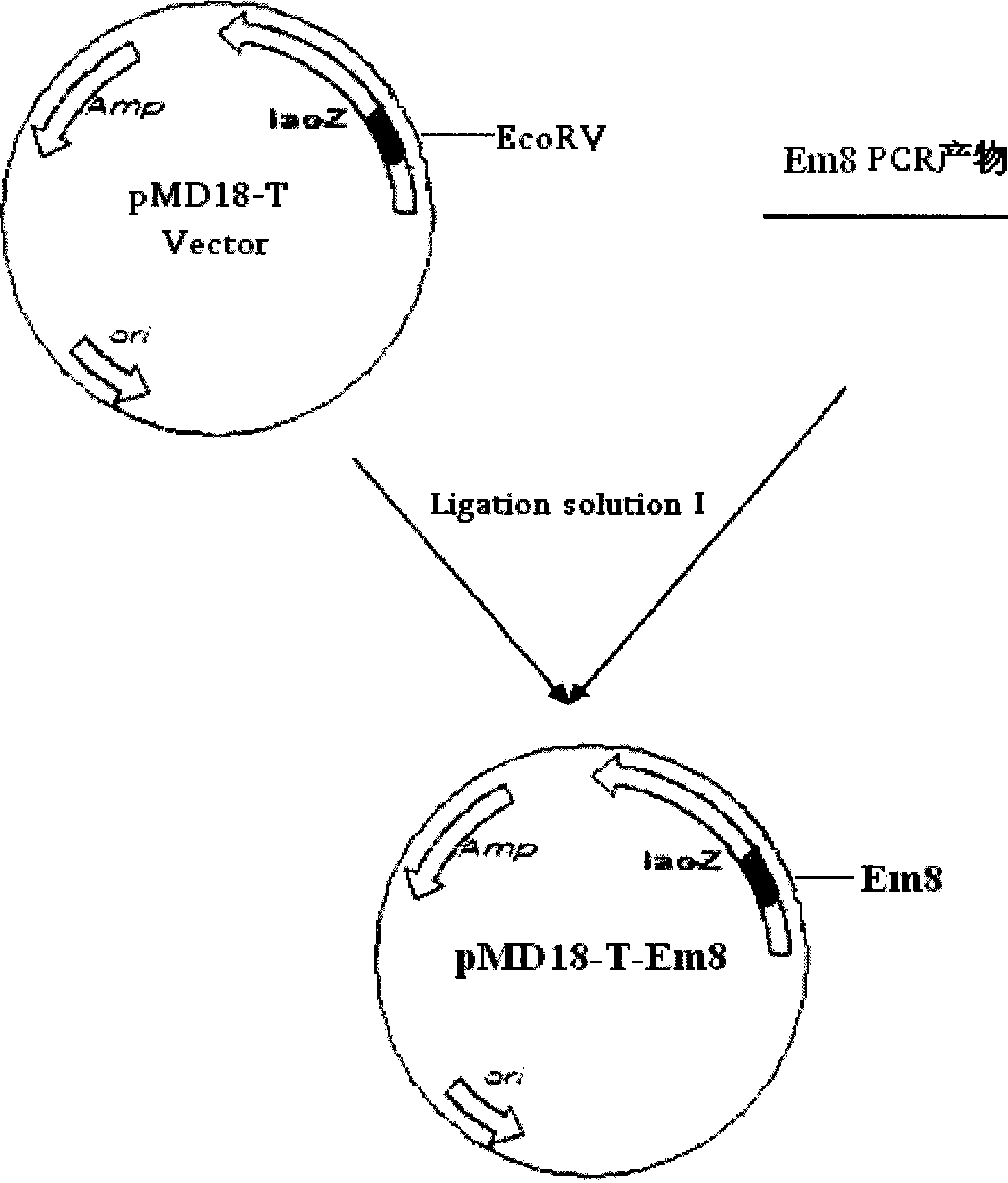
The invention relates to an Eimeria maxima DNA vaccine of chicken, belonging to the field of biological veterinary drugs. A gene fragment Em8 of an antigen EmTFP250 at the spore stage of the Eimeria maxima of the chicken is cloned by utilizing the molecular biology technology, and the fragment has higher antigen index and the centralized T cell epitope. The gene fragment is connected with a chicken interleukin-2 (chIL-2) gene in series, thereby constructing a vaccine expression vector pVAX-Em8-IL-2. The vaccine contains a plurality of T cell immune response elements and has the advantages of high safety, long maintenance time in vivo, simple preparation, good thermal stability, convenient storage and transport, time-saving and effort-saving use, and the like. Experiments prove that the immune regulatory DNA vaccine pVAX-Em8-IL-2 has good immune protection effect on the anti-Eimeria maxima infection.
Owner:NANJING AGRICULTURAL UNIVERSITY
Recombined adenovirus vaccine for preventing AIDS
InactiveCN102559609AImprove expression levelGood securityViral antigen ingredientsGenetic material ingredientsAdenovirus vaccineGene expression
The invention provides a replication defective recombinant adenovirus vector vaccine for preventing AIDS. The recombinant adenovirus vaccine contains a nucleotide sequence which can encode human immunoddficiency virus-1 (HIV-1) structural proteins. A transcription unit contained in the recombinant adenovirus vaccine encodes HIV-1B / C recombinant complete core protein Gag, enzyme proteins coding Pol and outer membrane protein Env. In addition, in order to improve the efficiency of gene expression, the recombinant adenovirus ultimately selects modified Gag and Pol genes and wild type Env gene.
Owner:JILIN UNIV
Methods and compositions for inducing protective immunity against RSV infection
ActiveUS11229692B2Effective immune protectionSsRNA viruses negative-senseViral antigen ingredientsEngineeringRSV Infections
Compositions, vaccines and methods using adenovirus vectors for inducing protective immunity against a respiratory syncytial virus (RSV) infection are described.
Owner:JANSSEN VACCINES & PREVENTION BV
Pseudorabies virus gene engineering gB recombinant attenuated vaccine strain and application thereof
ActiveCN106929485AImproving immunogenicityEasy to solveViral antigen ingredientsVirus peptidesAttenuated vaccineGene engineering
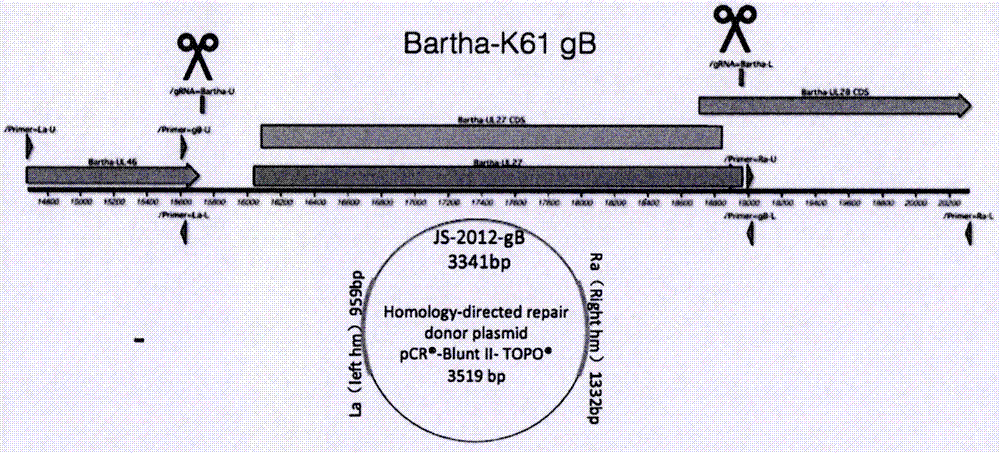
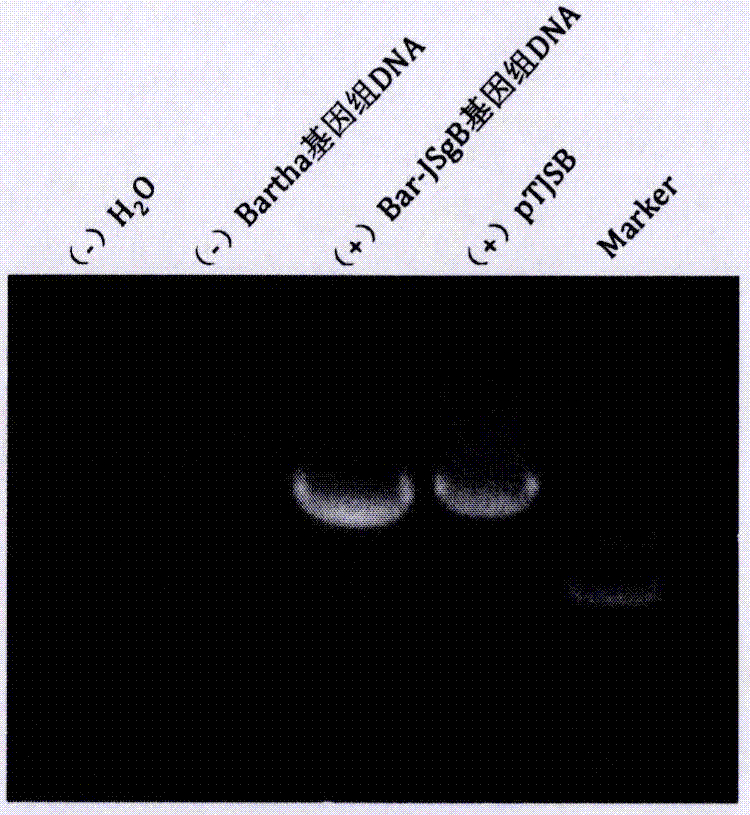
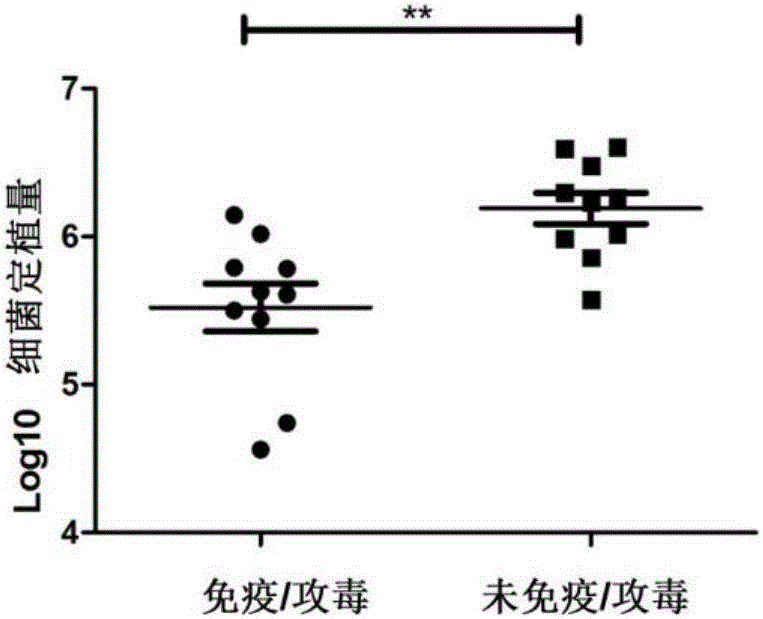
The invention discloses a pseudorabies virus gene engineering gB recombinant attenuated vaccine strain and application thereof. CRISPR / Cas9 is combined with techniques such as homologous recombination, and a genome of a pseudorabies virus vaccine strain Bartha-K61 is taken as a framework, and a recombinant pseudorabies virus strain Bar-JS-gB (BJB) is successfully acquired by replacing a gB gene of the Bartha-K61 with a gB gene of an epidemic pseudorabies virus variant JS-2012 in China. The strain BJB has stable heredity, and an immune mouse processed by an inactivated vaccine prepared by taking the strain BJB as a vaccine strain has relatively good capacity of effectively resisting attacking of a virulent strain JS-2012 than an immune mouse processed by an inactivated vaccine prepared from the Bartha-K61. Therefore, the strain BJB has relatively good immunogenicity, can be used for effectively controlling the prevalence of a PRV variant in China and can play an important role on control of pseudorabies.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Helicobacter pylori dominant antigen assembly based on CD4+T cell immunity and screening method
InactiveCN106480003AImprove cleanlinessMild immunopathological damageAntibacterial agentsBacterial antigen ingredientsProtective antigenScreening method
The invention relates to a helicobacter pylori dominant antigen assembly based on CD4+T cell immunity and a preparation method. The dominant antigen assembly comprises the following three components and homologous protein of the three components: inosine monophosphate dehydrogenase, type II citrate synthase and urease B subunit, wherein the amino acid sequences are shown as SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3. The screened dominant antigen assembly based on CD4+T cell immunity has the obvious immune protection effect, has the protection effect superior to that of H.pylori holoprotein antigen, has strong capacity of scavenging helicobacter pylori, and causes extremely slight pathological injuries. The three immunity dominant antigens provided by the invention can induce the body to generate strong immune response reaction aiming at antigens, therefore, through the means of inducing the body to generate the response aiming at the immunoprotecive dominant antigens, or directly immunizing the body by adopting the protective antigens, the effective immune protection effect can be achieved on the helicobacter pylori infection, and the scheme can be used for the further study on the preventive and therapeutic polyvaccines of helicobacter pylori.
Owner:ARMY MEDICAL UNIV
Duck adenovirus 2 and DPV (Muscovy duck parvovirus) disease combined inactivated vaccine
ActiveCN108465107AImprove securityEffective immune protectionViral antigen ingredientsAntiviralsDiseaseAntigen
The invention provides a duck adenovirus 2 and DPV (Muscovy duck parvovirus) disease combined inactivated vaccine. Antigens are inactivated duck adenovirus 2 and DPV, wherein the preservation number of the DPV is CCTCC NO:V201812, and the preservation number of the duck adenovirus 2 is CCTCC No:V201633. The prepared duck adenovirus 2 and DPV disease combined inactivated vaccine is good in safety and any local and general side effects caused by the vaccine are prevented. The analysis for character, safety test and efficacy test data in retention period tests indicates that all indexes are stable and effective; the immune effect of the vaccine is evaluated with a serological method and an immune challenge method, a result shows that the combined inactivated vaccine can provide effective immune protection for ducks and has good commercialized development prospect.
Owner:SHANDONG SINDER TECH +1
Live avian encephalomylitis and henpox combined vaccine
ActiveCN103721253AMeet the standard of non-virulence reversionEffective immune protectionAntiviralsAntibody medical ingredientsAntigenSerial passage
The invention provides a live avian encephalomylitis and henpox combined vaccine. The combined vaccine consists of an antigen and a protective agent, wherein the antigen comprises henpox virus and avian encephalomylitis virus with the preservation number of CGMCC (China General Microbiological Culture Collection Center) No.8505. The combined vaccine prepared by the invention can be used for preventing avian encephalomylitis and henpox at the same time to achieve the aim of preventing two viruses with one injection. Furthermore, The avian encephalomylitis virus screened by the invention is an attenuated virus, performs serial passage in an avian body through horizontal transmitted infection, does not have the phenomenon of virulence return, is stable in heredity, and accords with the standard of no virulence return of an avian encephalomylitis attenuated vaccine strain; the prepared vaccine can provide effective immune protection, and has good commercial development prospect.
Owner:YEBIO BIOENG OF QINGDAO
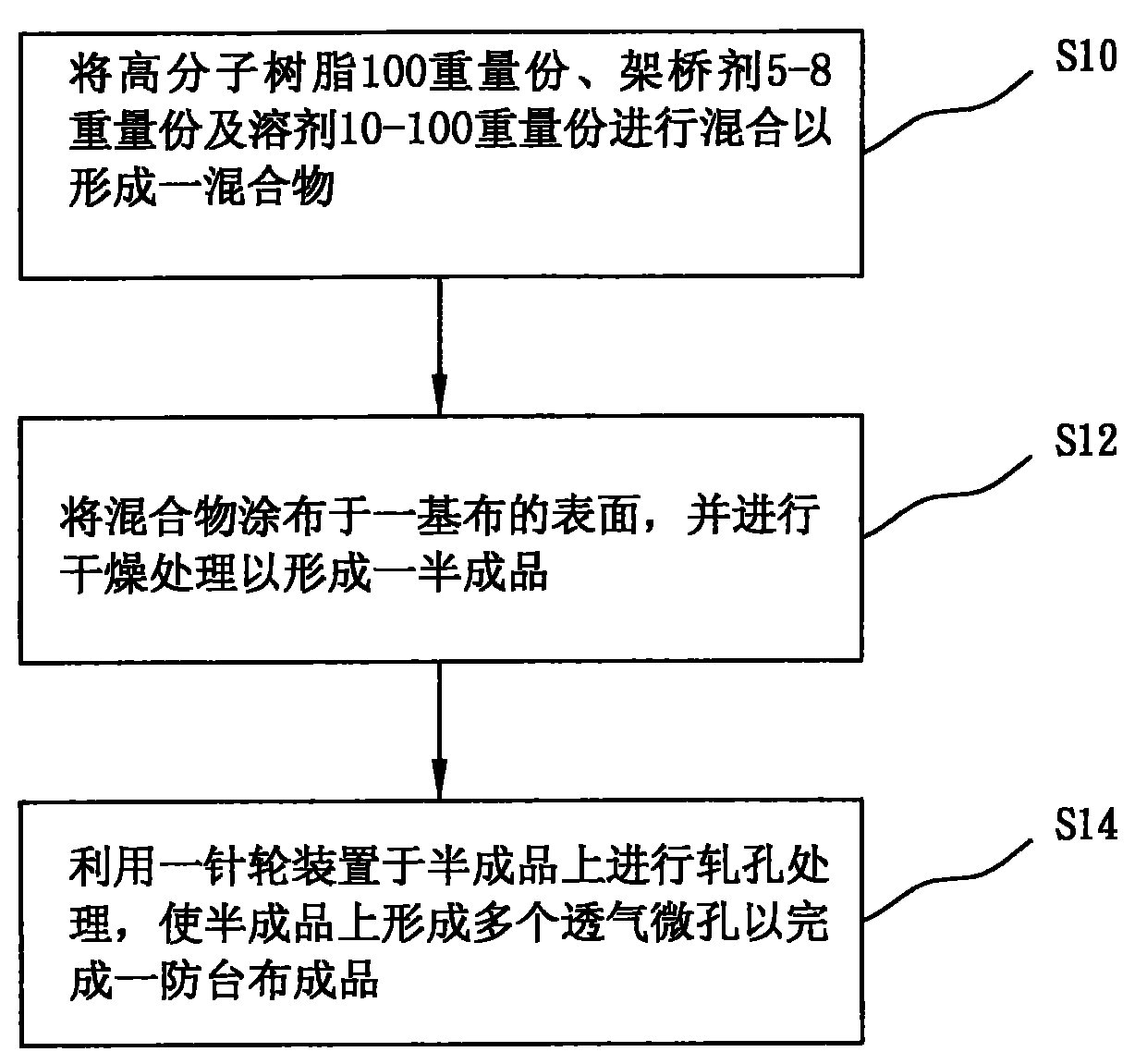
Method for manufacturing high-strength typhoon prevention cloth
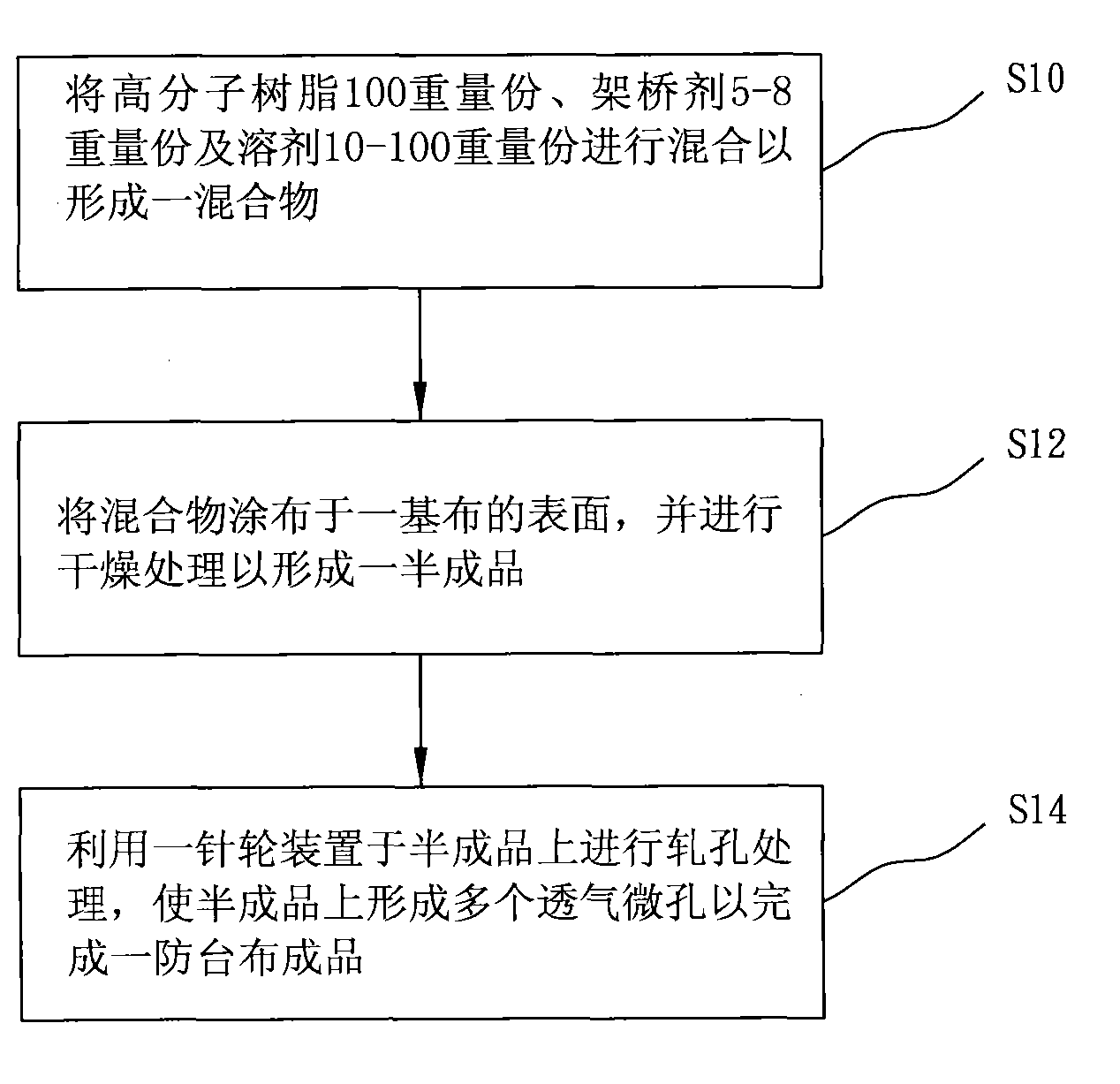
The invention provides a method for manufacturing high-strength typhoon prevention cloth, which comprises the following steps of: sufficiently mixing 100 parts by weight of polymer resin, 5 to 8 parts by weight of bridging agent and 10 to 100 parts by weight of solvent; uniformly coating the mixture on the surface of a foundation; carrying out drying treatment on the foundation to form a semifinished product, so that the semifinished product has functions of fire resistance, solarization resistance, waterproofness, antibacterial and antifungal performance and the like; and carrying out hole rolling treatment on the semifinished product by selecting a pin wheel device with suitable pin diameter and pitch to form a plurality of breathable micropores on the semifinished product, so that the manufacturing of the typhoon prevention cloth with air permeability can be completed.
Owner:FORMOSAN RUBBER GRP
Immunity-protective Acinetobacter baumannii surface antigen SurAl
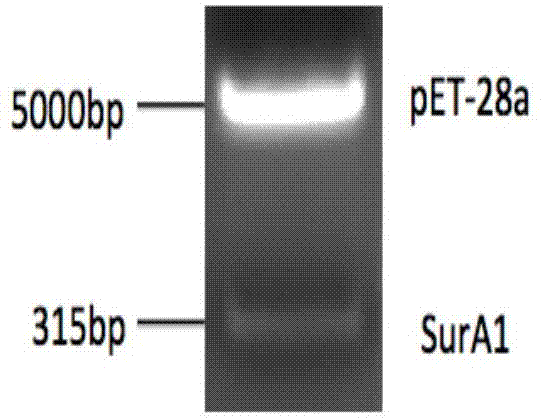
ActiveCN104725492AImmunoprotectiveEffective immune protectionAntibacterial agentsBacterial antigen ingredientsAntigenNucleotide
The invention relates to immunity-protective Acinetobacter baumannii surface antigen SurAl and belongs to bacterial surface antigen recombinant proteins. The amino acid sequence of the immunity-protective Acinetobacter baumannii surface antigen SurAl is shown in SEQ ID No. 1; the nucleotide of the immunity-protective Acinetobacter baumannii surface antigen SurAl is encoded, under a sequence shown in SEQ ID No.2. The immunity-protective Acinetobacter baumannii surface antigen SurAl has low toxicity to human pulmonary epithelial cells A549, provides effective immunity protection for mice from being infected by Acinetobacter baumannii, and can be used as a candidate antigen for the development of subunit vaccines of Acinetobacter baumannii.
Owner:JILIN UNIV
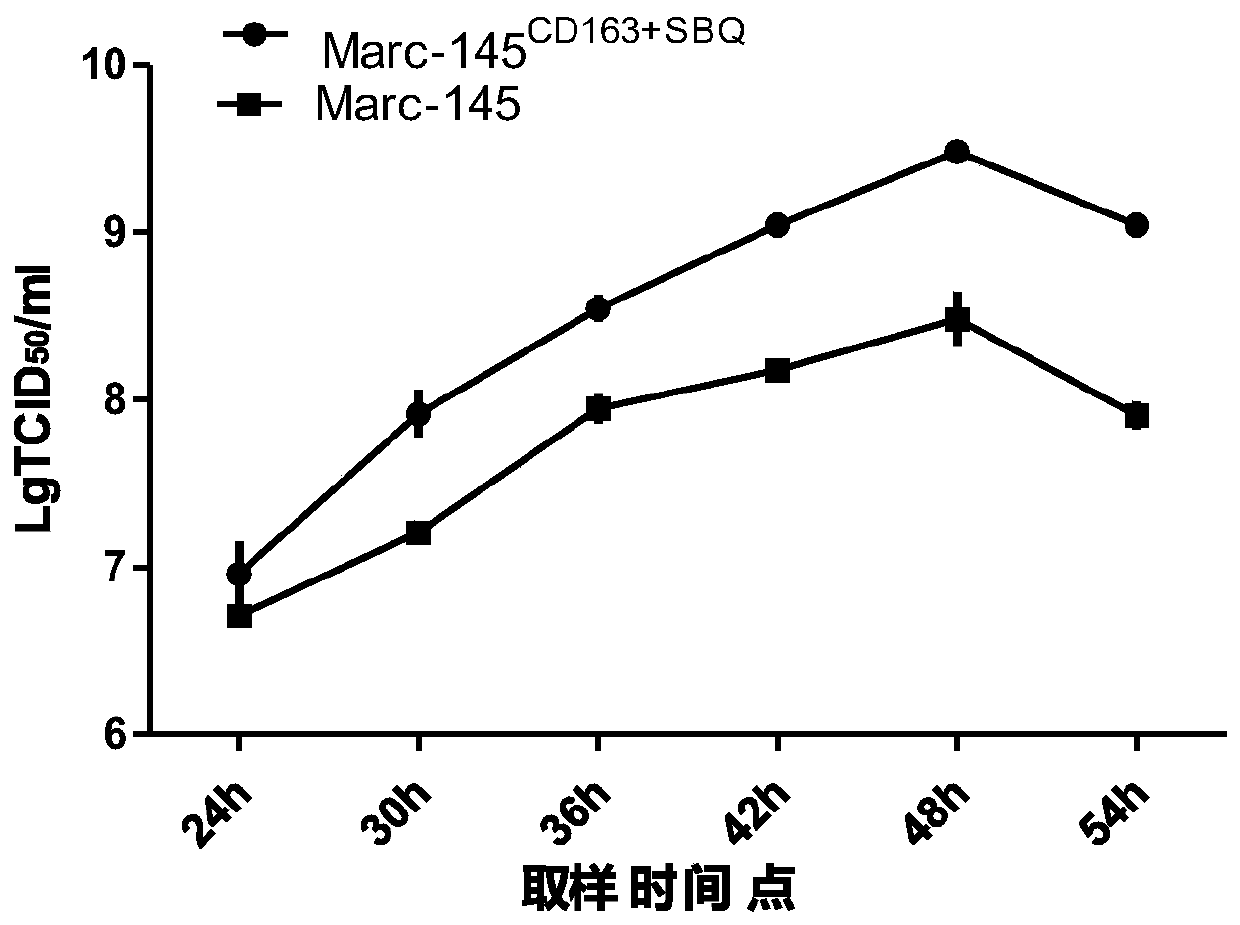
Construction and application of Marc-145 stable cell strain
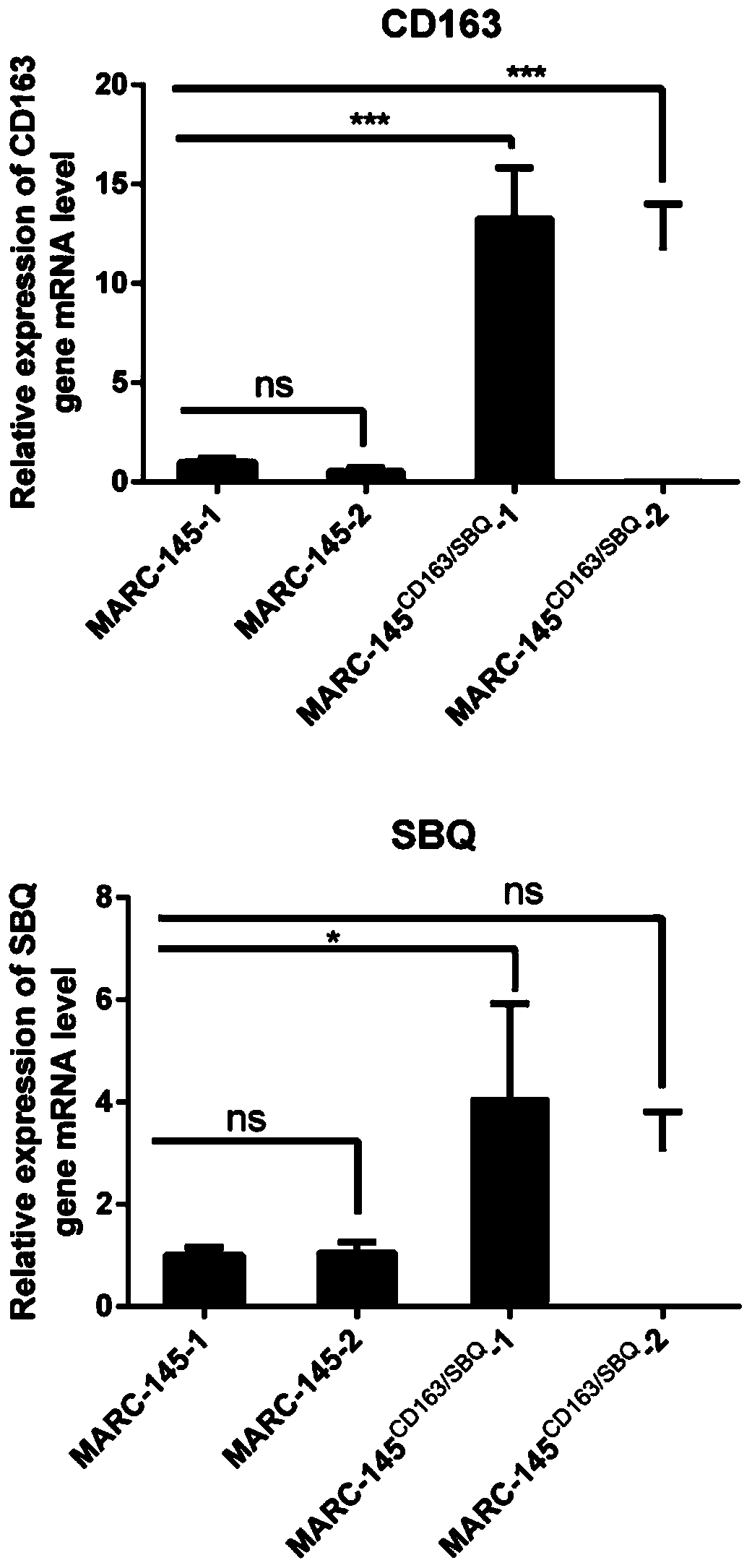
PendingCN110423782AGood genetic stabilityTransgenic genes are not lostSsRNA viruses positive-senseGenetically modified cellsGenetic engineeringViral vector
The invention relates to the technical fields of cytobiology, genetic engineering and animal biological products, in particular to a construction method and application of a Marc-145 stable cell strain. The invention provides the construction method of the cell strain for porcine reproductive and respiratory syndrome virus (PRRSV) reproduction, the construction method comprises the following stepsthat 1), a CD163 gene and a SBQ gene are correspondingly constructed to an lentiviral vector, and a recombination lentiviral vector is obtained; 2), the recombination lentiviral vector and a virus rescue auxiliary plasmid are together transmitted into a mammalian cell, the cell is cultured to obtain recombination viruses; and 3) the recombination viruses in the steps 2) infect a Marc-145 cell, and the Marc-145 stable cell strain is obtained. The invention further provides use of the cell. An inheritable character of the cell is stable, and the cell is sensitive to PRRSV, and has very good application prospects.
Owner:成都史纪生物制药有限公司
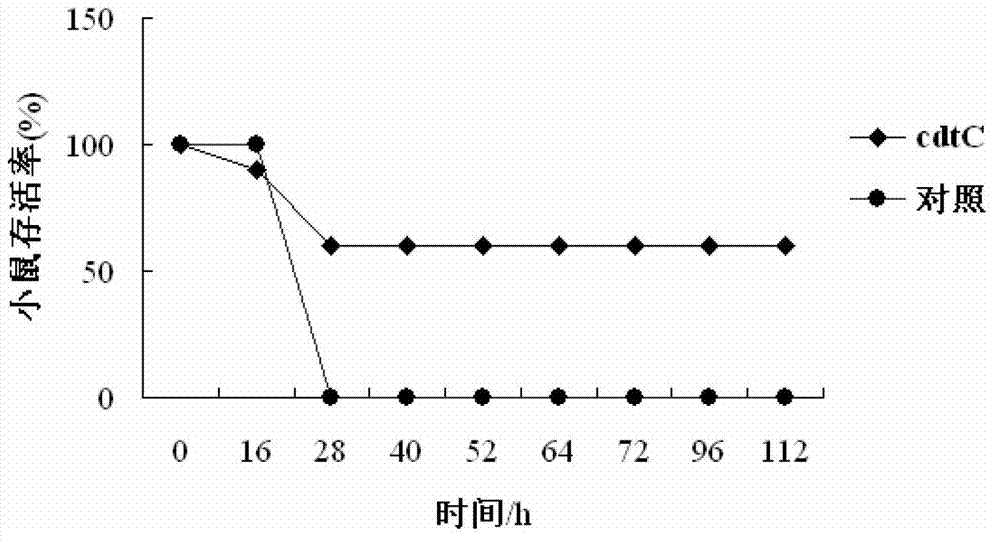
Haemophilus parasuis immunoprotective antigen CdtC
InactiveCN103288933AEffective immune protectionAntibacterial agentsBacteriaProtective antigenEscherichia coli
The invention relates to identification of a Haemophilus parasuis immunoprotective antigen, separation and cloning of protein gene, and application of coding protein thereof in vaccines. According to the invention, a new immunogenic protein CdtC is separated from Haemophilus parasuis (Hps) (the culture collection number is CVCC3361), the nucleotide sequence is disclosed as SEQ ID NO:2 in the sequence table, and the protein CdtC codes 176 amino acids. The CdtC is a new immunogenic protein, and can provide effective immunoprotection for mice infected by Haemophilus parasuis. The invention also relates to preparation of Escherichia coli recombinant bacterium BL21 / Hps-CdtC for expressing the immunogenic protein gene CdtC. The recombinant Haemophilus parasuis CdtC protein expressed in the invention has favorable safety and protection efficacy, and the immunoprotection effect reaches 60%.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant modified vaccinia virus ankara (MVA) filovirus vaccine
ActiveUS20170304427A1Effective immune protectionSsRNA viruses negative-senseViral antigen ingredientsAntigenRegimen
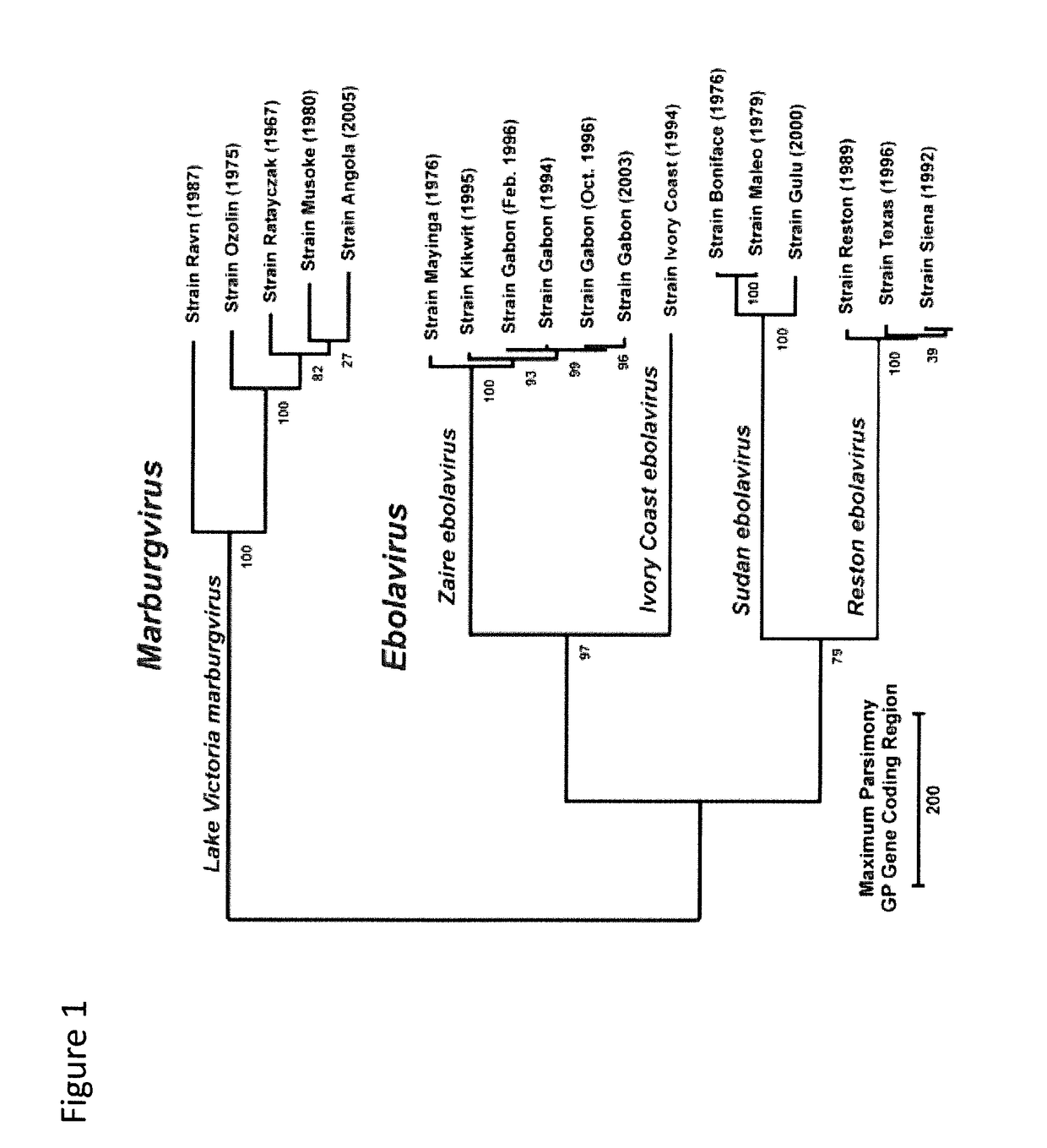
The present invention relates to an improved filovirus vaccine comprising a recombinant modified vaccinia virus Ankara-based (MVA-based) vaccine against filovirus infection and to related products, methods and uses. Specifically, the present invention relates to genetically engineered (recombinant) MVA and FPV vectors comprising at least one heterologous nucleotide sequence encoding an antigenic determinant of a Marburg virus (MARV) or Ebola virus glycoprotein. Specifically, the invention relates to recombinant MVA comprising Ebola virus glycoprotein and virion protein 40. The invention also relates to products, methods and uses thereof as well as prime / boost regimens of MVA and genetically engineered (recombinant) FPV, e.g., suitable to induce a protective immune response in a subject.
Owner:BAVARIAN NORDIC AS
Antiangiogenic Peptides
InactiveUS20080039384A1Short half-lifeReduced activityOrganic active ingredientsSenses disorderPeptideAmino acid residue
The present invention refers to a pharmaceutical composition comprising an isolated antiangiogenic peptide or a recombinant protein comprising the antiangiogenic peptide, wherein the peptide is between 11 and 40 amino acids in length and having antiangiogenic activity, the peptide comprising the amino acid sequence: X1-X2-X3-X4-X5-X6-X7-X8-X9-X10-X11-X12-X13-X14, wherein X1 is any amino acid residue compatible with forming a helix; X2 is an amino acid residue of: Leu, Ile, Val; X3 is an amino acid residue of: Arg, Lys, His, Ser, Thr; X4 is an amino acid residue of: Ile, Leu, Val; X5 is any amino acid residue compatible with forming a helix; X6 is an amino acid residue of: Leu, Ile, Val; X7 is an amino acid residue of: Leu, Ile, Val, Ser, Thr; X8 is any amino acid residue compatible with forming a helix; X9 is any amino acid residue compatible with forming a helix; X10 is an amino acid residue of: Gln, Glu, Asp, Arg, His, Lys, Asn; X11 is an amino acid residue of: Ser, Thr; X12 is an amino acid residue of: Trp, Tyr, Phe; X13 is an amino acid residue of: Leu, Ile, Val, Asn, Gln; X14 is an amino acid residue of: Glu, Gln, Asp, Asn.
Owner:UNIV LIEGE +1
Attenuated strain of duck reovirus and application of attenuated strain
ActiveCN111172122AImprove securityIncreased proliferationInactivation/attenuationAntibody medical ingredientsVero cellBiomedical engineering
The invention relates to an attenuated strain of a duck reovirus and application of the attenuated strain. The attenuated strain Q of the duck reovirus is obtained by attenuating a virulent strain 17117 of the duck reovirus through continuous passage on a Vero cell; a proliferation level on the Vero cell is high; and after being vaccinated, one-day old ducklings have no obvious clinical symptoms and pathological changes, and thus, the attenuated strain is high in safety. By vaccinating the one-day old ducklings with live vaccines prepared from the attenuated strain Q, effective immune protection can be generated.
Owner:QILU ANIMAL HEALTH PROD
Nano antibody for resisting methicillin-resistant staphylococcus as well as preparation method and application of nano antibody
InactiveCN113980127AImprove specific recognition abilityImprove specific binding abilityAntibacterial agentsBiological material analysisComplementarity determining regionBiomedicine
The invention provides a nano antibody targeting a methicillin-resistant staphylococcus LspA protein as well as a preparation method and application of the nano antibody. The provided nano antibody mainly recognizes a binding motif region combined with the methicillin-resistant staphylococcus LspA, and comprises a framework region FR and complementary determining regions CDR1, CDR2 and CDR3. One specific amino acid sequence of the nano antibody is SEQ ID NO: 1. The nano antibody disclosed by the invention can be efficiently and specifically combined with methicillin-resistant staphylococcus LspA protein, and the affinity can reach a nanomole level; and meanwhile, the nano antibody provided by the invention can obtain an excellent effect in methicillin-resistant staphylococcus detection or diseases caused by methicillin-resistant staphylococcus, and can be applied to the fields of biology and medicine.
Owner:南京中爱人工智能与生命科学研究院有限公司
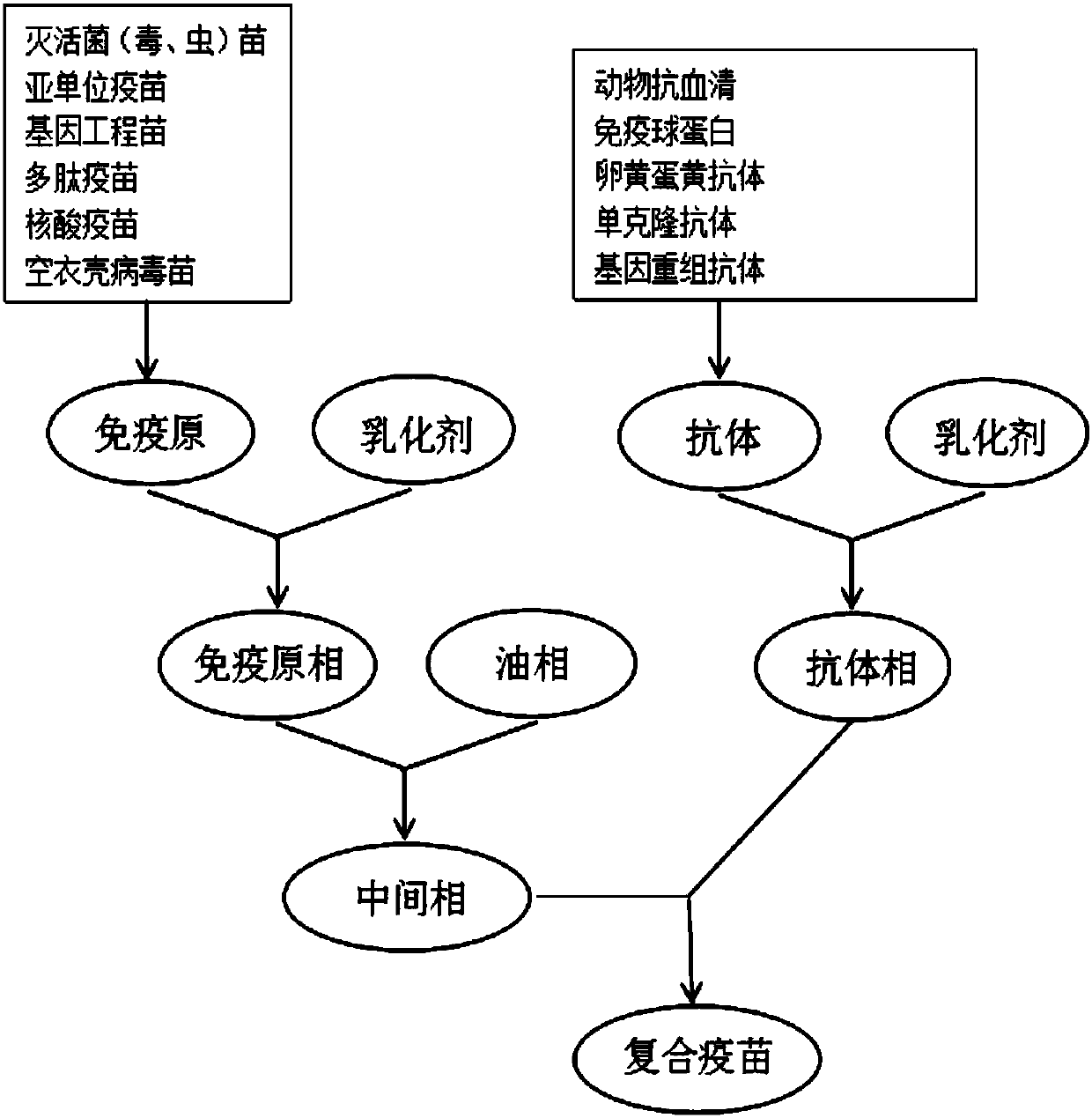
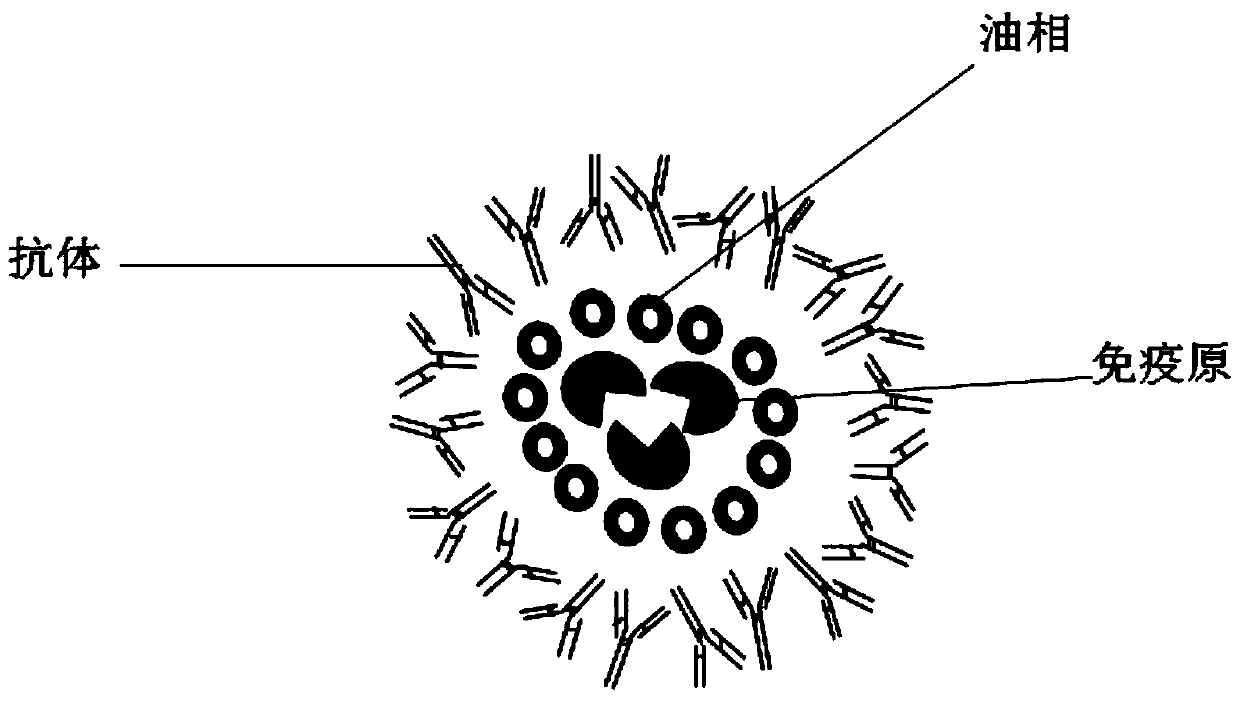
Immunogen and antibody composite vaccine and preparation method thereof
InactiveCN107714648AEfficient releaseImprove stabilityAntibody ingredientsAntiinfectivesOil phaseActive immunity
The invention discloses an immunogen and antibody composite vaccine and a preparation method thereof. The composite vaccine consists of an immunogen at the center, an oil phase for coating the immunogen at the middle, and an antibody for coating the oil phase at the outer part, therefore, a water-in-oil-in-water (W / O / W) type emulsion system is formed. Within several hours after the composite vaccine is injected, the antibody is firstly and quickly released in the early stage, and then is absorbed by an organ and enters blood for immunization; the immunogen is then released, and is induced to generate effective active immunity within 4 to 6 days before the antibody loses the immunity. The composite vaccine has the advantage that seamless connection between specific passive immunity and specific active immunity is realized, adding complementation is achieved, and an effect can be taken quickly.
Owner:重庆更尚科技有限公司
Chicken Marek's disease Meq gene deleted vaccine strain, construction method thereof, and application thereof
ActiveCN102363769BNon-pathogenicGenetic stabilityMicroorganism based processesViruses/bacteriophagesTGE VACCINEOncogene
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method and product of chicken infectious bursal disease virus antigen
ActiveCN104761624AEffective immune protectionHigh expressionViral antigen ingredientsVirus peptidesVp2 geneInfectious bursitis
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Construction method of multi-pathogen mycoplasma ovine pneumonia nucleic acid vaccine
InactiveCN113430214ASolve many problems faced by R&DEffective immune protectionAntibacterial agentsBacterial antigen ingredientsInfection inducedGenetic engineering
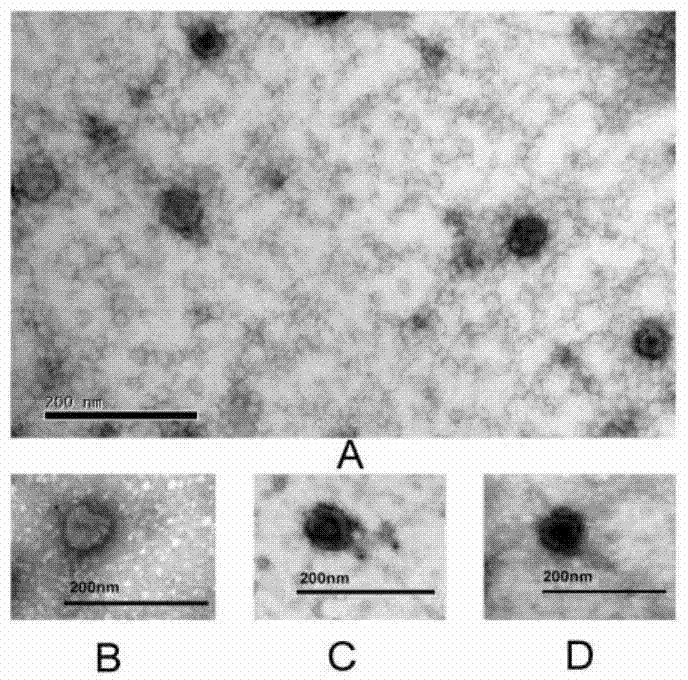
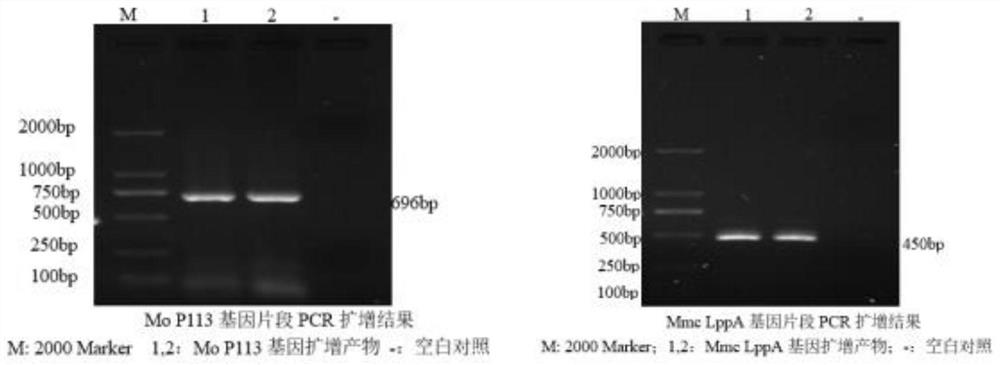
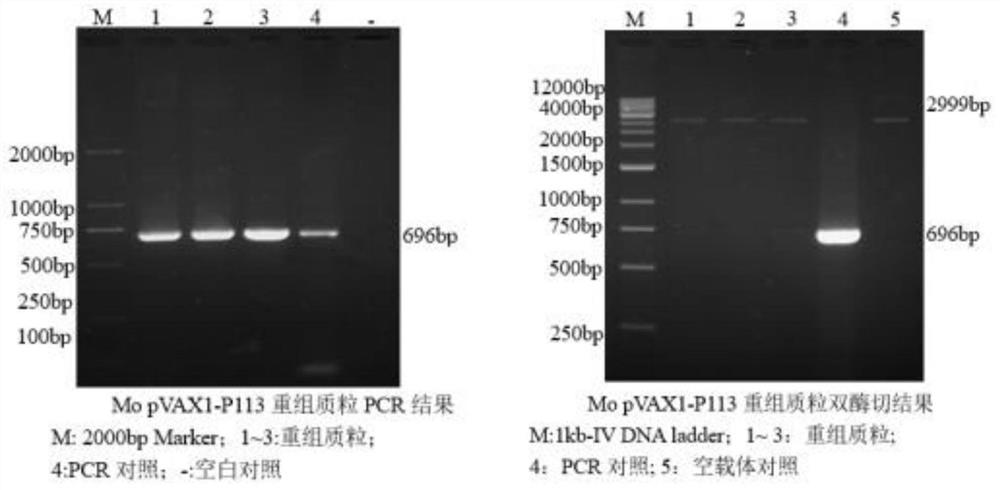
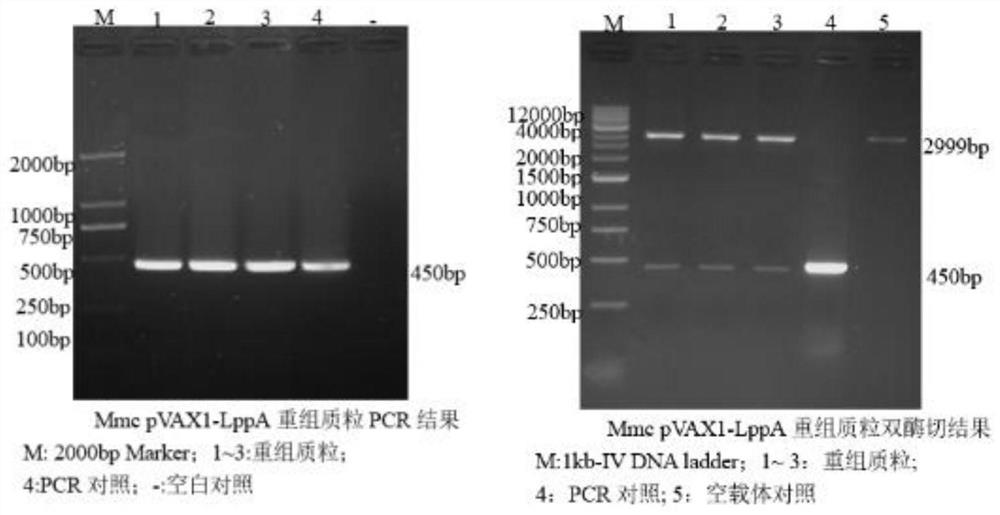
The invention discloses a construction method of a multi-pathogen mycoplasma ovine pneumonia nucleic acid vaccine. The construction method comprises the following steps of: step 1, extracting a Mo / Mmc genome; step 2, obtaining a MoP113 / MmcLppA gene; and step 3, constructing a pVAX1-P113-LppA co-expression vector and expressing in mammalian cells. According to the construction method disclosed by the invention, the pVAX1-P113-LppA co-expression vector is constructed through a gene engineering method on the basis of mycoplasma ovine pneumonia multi-epitope antigen genes MoP113 and MmcLppA which are screened out at an early stage; an indirect immunofluorescence technology is used for verifying an expression condition of recombinant plasmids in MDBK (MDBK); the nucleic acid vaccine is developed by selecting two different antigen genes and an immune protection effect can be effectively provided aiming at mycoplasma ovine pneumonia caused by Mo and Mmc infection; and a plurality of problems of current vaccine research and development are solved.
Owner:GUIZHOU UNIV
Preparation method of viral vaccine expressing plasmodium ovale AMA1 protein
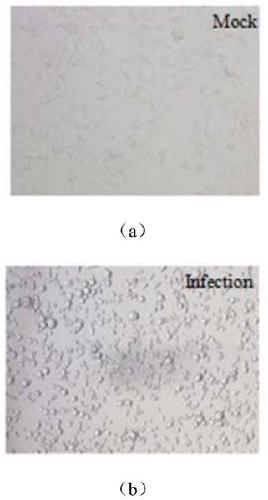
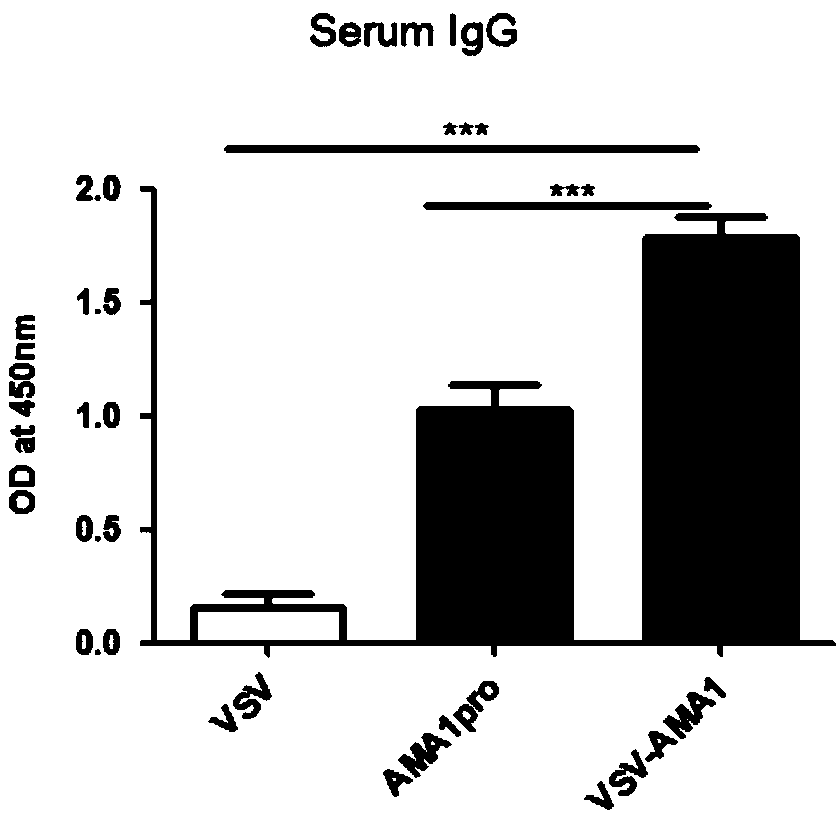
ActiveCN109022456AStrong immune responseEffective immune protectionSsRNA viruses negative-sensePeptidesImmune cycleStructural protein
Belonging to the technical field of virology and genetic engineering, the invention discloses a preparation method of a viral vaccine expressing plasmodium ovale AMA1 protein. According to the invention, a gene expressing plasmodium ovale apical membrane protein-1 is inserted into a vesicular stomatitis virus VSV genome vector to construct the recombinant virus vector skeleton plasmid XN2-AMA1, apoxvirus containing T7RNA polymerase is employed to infect the baby hamster kidney cell (BHK-21), then the plasmid XN2-AMA1 and the structural protein plasmids pN, pP, pL are utilized for cotransfection of BHK-21 cells to realize rescue of recombinant virus in cells, virus-like particles expressing AMA1 are packaged, and the recombinant virus rVSV-AMA1, i.e. the vaccine can be formed. The vaccineprovided by the invention has the advantages of simple operation, high production titer and short immune cycle, can induce strong humoral and cellular immune response, and has the great potential of clinical application.
Owner:JIANGNAN UNIV
Vaccine of encephalomyelitis of poultry
ActiveCN103705916AEffective immune protectionMeet the standard of non-virulence reversionAntiviralsAntibody medical ingredientsAntigenHorizontal transmission
The invention provides vaccine of encephalomyelitis of poultry. The vaccine comprises antigen and protective agent, wherein the antigen comprises low virulent strain of encephalomyelitis of poultry with preservation number CGMCC No.8505. The vaccine prepared by the invention can effectively prevent encephalomyelitis of poultry; furthermore, the virus of the encephalomyelitis of poultry is taken as the antigen, is low virulent strain, is serially subcultured in a chicken body through horizontal transmission and infection, does not become strong again in toxicity, has stable heredity, and meets the standard that the attenuated vaccine of the encephalomyelitis of poultry does not become strong again in toxicity, so that the prepared vaccine can provide effective immune protection and has good commercialized development prospect.
Owner:YEBIO BIOENG OF QINGDAO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com