Patents
Literature
46 results about "Vaccine manufacturing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process
InactiveCN105031634AKeep healthyAvoid pollutionAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineVaccine manufacturing
The invention discloses an A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process. The problem that highly-toxic chemical reagents, such as cyanogen bromide, are used in the vaccine manufacturing process in the prior art and accordingly are harmful to protection of human health and environment is solved. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process comprises the following steps of 1 cultivating Neisseria meningitidis, 2 utilizing the cultivated Neisseria meningitidis to extract crude polysaccharide, 3 purifying the crude polysaccharide to prepare refined Neisseria meningitidis polysaccharide and 4 activating and deriving the refined polysaccharide. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process has the advantages that the extraction rate of the polysaccharide from a capsule is higher, the precipitation rate of the polysaccharide is higher, the recovery rate and purity of the refined polysaccharide are high, the derivation effect is better and the like.
Owner:CHENGDU OLYMVAX BIOPHARM
Recombinant baculovirus expressing porcine circovirus type 3 truncated Cap protein and construction method and primers thereof
ActiveCN109207522AAchieve and improve expressionProven good expressionVirus peptidesFermentationVaccine manufacturingShuttle plasmid
The invention belongs to the technical field of vaccine manufacturing. The invention discloses a recombinant baculovirus rBac-[delta]Cap expressing porcine circovirus type 3 truncated Cap protein, with the accession number of the recombinant baculovirus being CGMCC No. 15691. The invention also discloses primers used for constructing the recombinant baculovirus, and a constructing method of the recombinant baculovirus. The method comprises the following steps: 1, constructing pSK-SPCV3 containing PCV3 whole genome; 2. Constructing a transfer vector; 3. constructing a shuttle plasmid; 4. obtaining recombinant baculovirus, wherein the positive shuttle plasmid Bacmid-[delta]Cap transfects Sf9 cells to obtain the recombinant baculovirus rBac-[delta]Cap expressing the porcine circovirus type 3truncated Cap protein. The method can conveniently and quickly construct the recombinant baculovirus expressing the PCV3 truncated Cap protein, and can confirm that the expressed truncated Cap proteinhas good biological activity, and immunizes mice to find out that the recombinant baculovirus has good immunogenicity.
Owner:YANGZHOU UNIV
Recombinant protein comprising starch binding domain and use thereof
A recombinant protein is prepared comprising a polypeptide of interest and a starch binding domain (SBD). The said SBD is obtainable from glucoamylase of fungi genus Rhizopus. The said recombinant protein comprising the said SBD can be purified by contacting with an affinity matrix such as starch, the SBD binds to the affinity matrix to isolate the recombinant protein. The recombinant protein can be purified by separating the association between the SBD and the affinity matrix by acid, alkaline, salt, or sugar. The polypeptide of interest may be an antibody, an antigen, a therapeutic compound, an enzyme, or a protein and may apply in pathogen destruction, vaccine producing, and oral care product manufacturing. The SBD further provides as a tool to screen or identify polysacchrides.
Owner:SIMPSON BIOTECH CO LTD
VII type Newcastle disease virus L gene mutation attenuated vaccine strain and preparation method thereof
ActiveCN104974989AHigh titerGenetically stableMicroorganism based processesViruses/bacteriophagesVaccine manufacturingGenes mutation
The present invention relates to a VII type Newcastle disease virus L gene mutation attenuated vaccine strain produced by using a reverse genetic operation technology, and a preparation method thereof, and belongs to the fields of biological products and biotechnology. According to the present invention, the VII type Newcastle disease virus L gene mutation attenuated vaccine strain has characteristics of significantly reduced virulence, high titer on chicken embryos, genetic stability and high antigen matching with epidemic virus, is suitable for mass production of the vaccine, can be used for vaccine manufacturing, and provides broad application prospects in the Newcastle disease incidence and prevalence control field compared with the conventional vaccine (gene I type and gene II type).
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
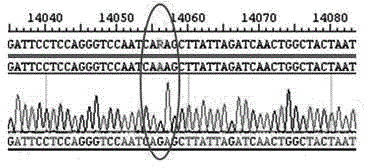
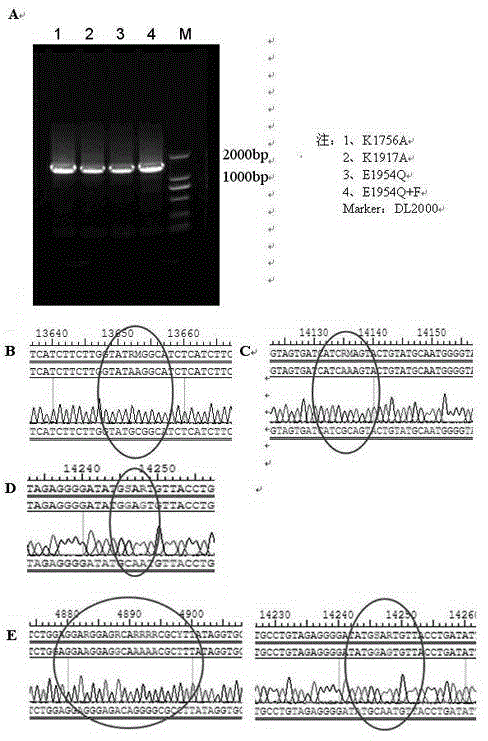
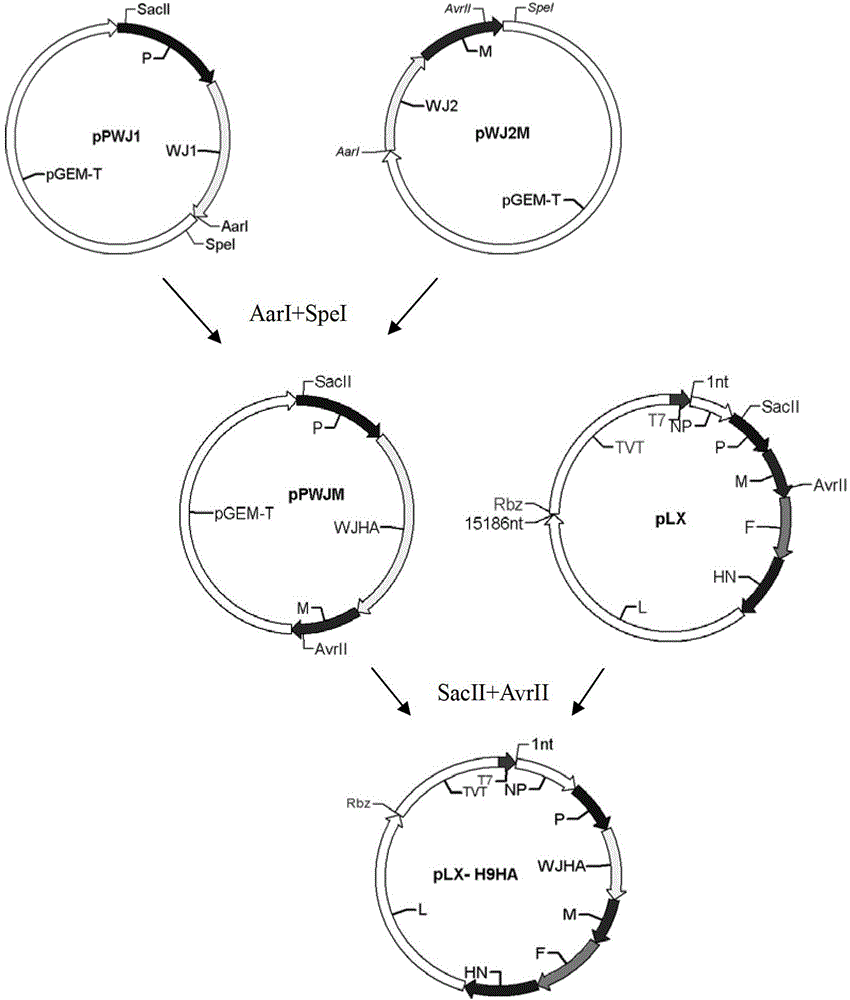
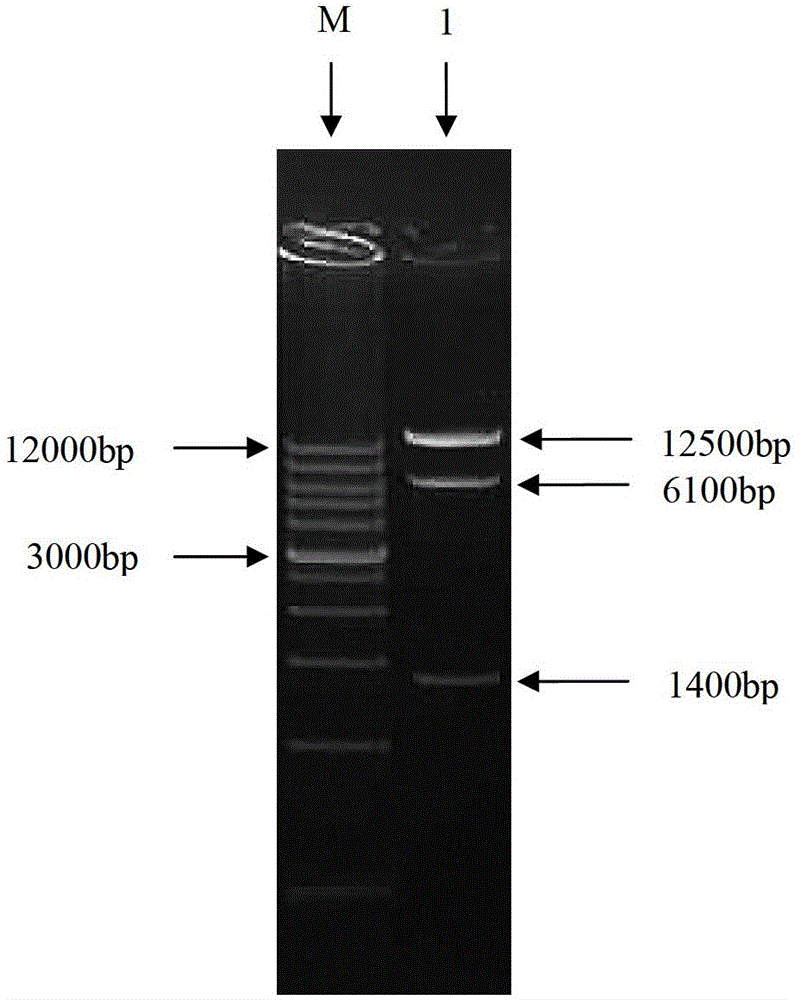
Newcastle disease virus rLX/H9HA and construction method and application thereof
InactiveCN103146751ASuitable for mass productionImprove reproductive performanceMicroorganism based processesVector-based foreign material introductionVaccine manufacturingHemagglutinin
The invention discloses a recombination newcastle disease virus rLX / H9HA of H9 subtype avian influenza virus hemagglutinin albumen. A preservation number is CGMCCNo: 6652. According to the method, a reverse genetic operation platform of an established newcastle disease virus (DVA) weak poison LX strain is used, an HA gene of an H9N2 subtype avian influenza virus (AIV) epidemic strain A / Chicken / Jiangsu / WJ57 / 2012 is inserted in a genome overall length transcription carrier pLX of the LX strain, and thus a recombination newcastle disease virus genome overall length cDNA clone Plx-H9HA containing a H9N2 subtype AIVHA gene. A recombinant virus rLX / H9HA obtained through transfection on a chick embryo has high breeding geometric mean titer, can still express HA albumen stably through continuous passing of multiple generations, is suitable for mass production of vaccines, and can be used for vaccine manufacturing.
Owner:YANGZHOU UNIV
Recombinant baculovirus expressing porcine circovirus type 3 Cap protein and construction method and primers thereof
ActiveCN109207441AStimulate immune responseHigh titerVirus peptidesMicroorganism based processesVaccine manufacturingShuttle plasmid
The invention belongs to the technical field of vaccine manufacturing. The invention discloses a recombinant baculovirus expressing a porcine circovirus type 3 Cap protein. The invention also discloses primers for constructing a recombinant baculovirus expressing a porcine circovirus type 3 Cap protein. A method for constructing the recombinant baculovirus-expressing porcine circovirus type 3 Capprotein is also disclosed and comprises that follow steps of: 1, constructing a pSK-SPCV3 inluding a PCV3 whole genome; 2. constructing a transfer vector; 3. Constructing a shuttle plasmid; 4, obtaining the recombinant baculovirus. Sf9 cells are transfected with positive shuttle plasmid Bacmid-Cap to obtain the recombinant baculovirus rBac-Cap expressing porcine circovirus type 3 Cap gene. The Capprotein expressed by the invention has good biological activity and is found to have good immunogenicity by immunizing mice.
Owner:YANGZHOU UNIV
Nucleic acid vaccine for preventing AIDS
InactiveCN1631441ASimple production processEffective immune protectionViral antigen ingredientsGenetic material ingredientsVaccine manufacturingNucleotide
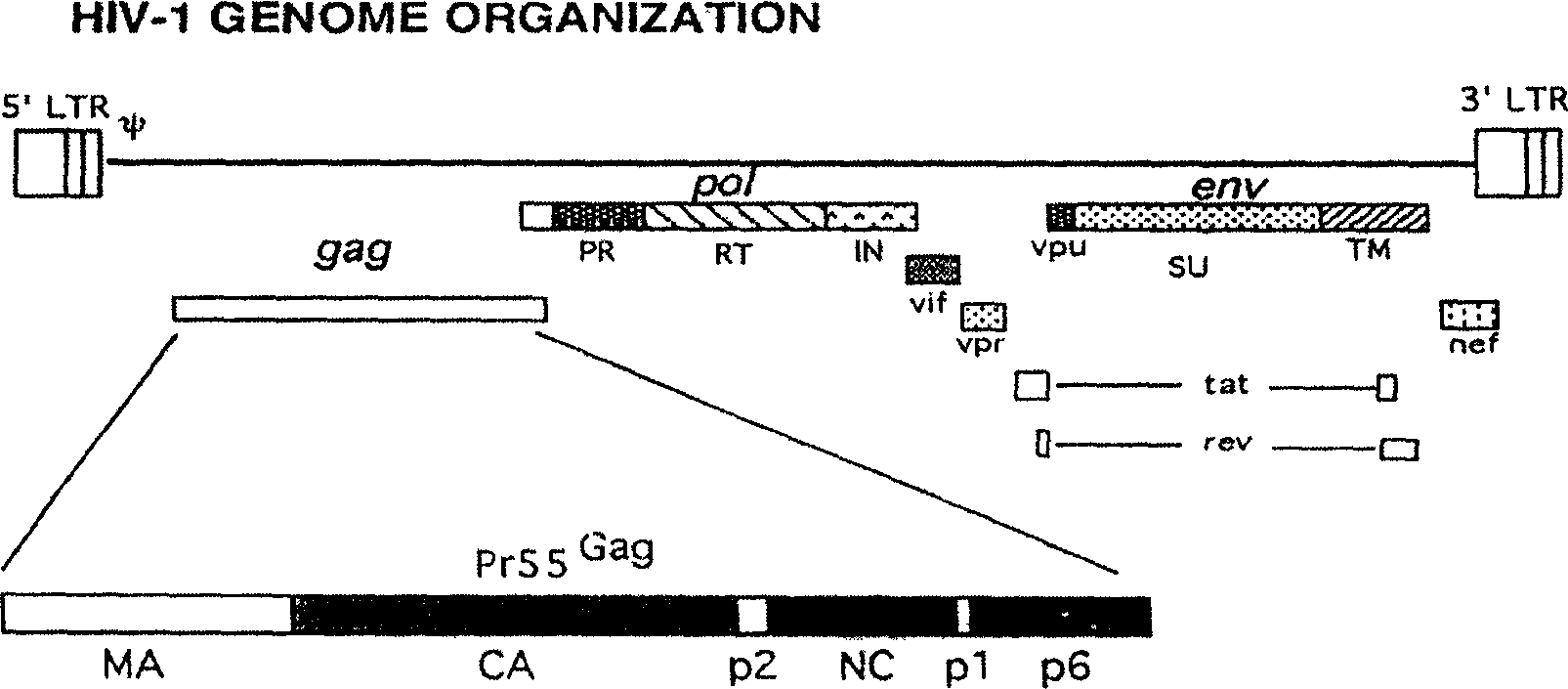
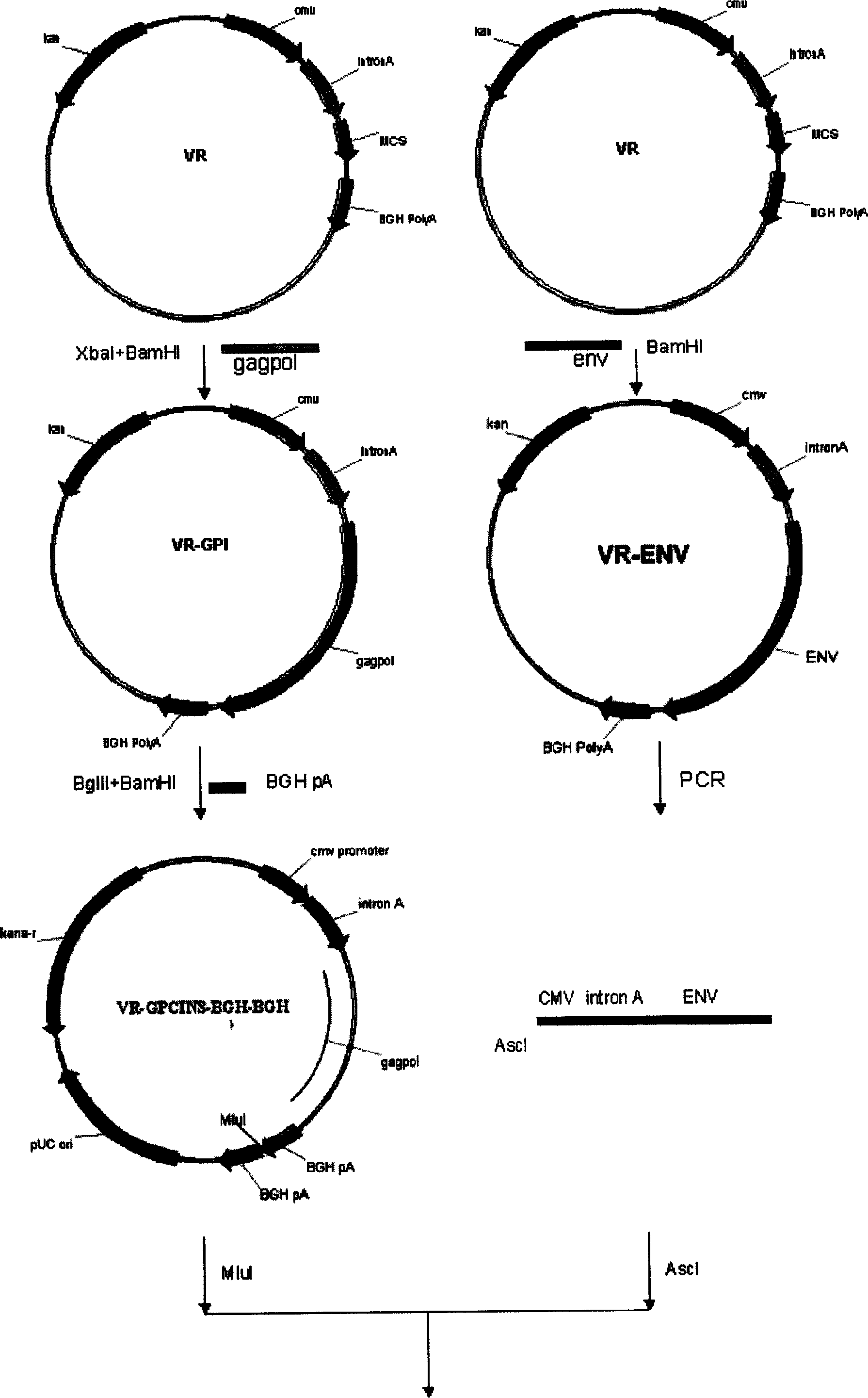
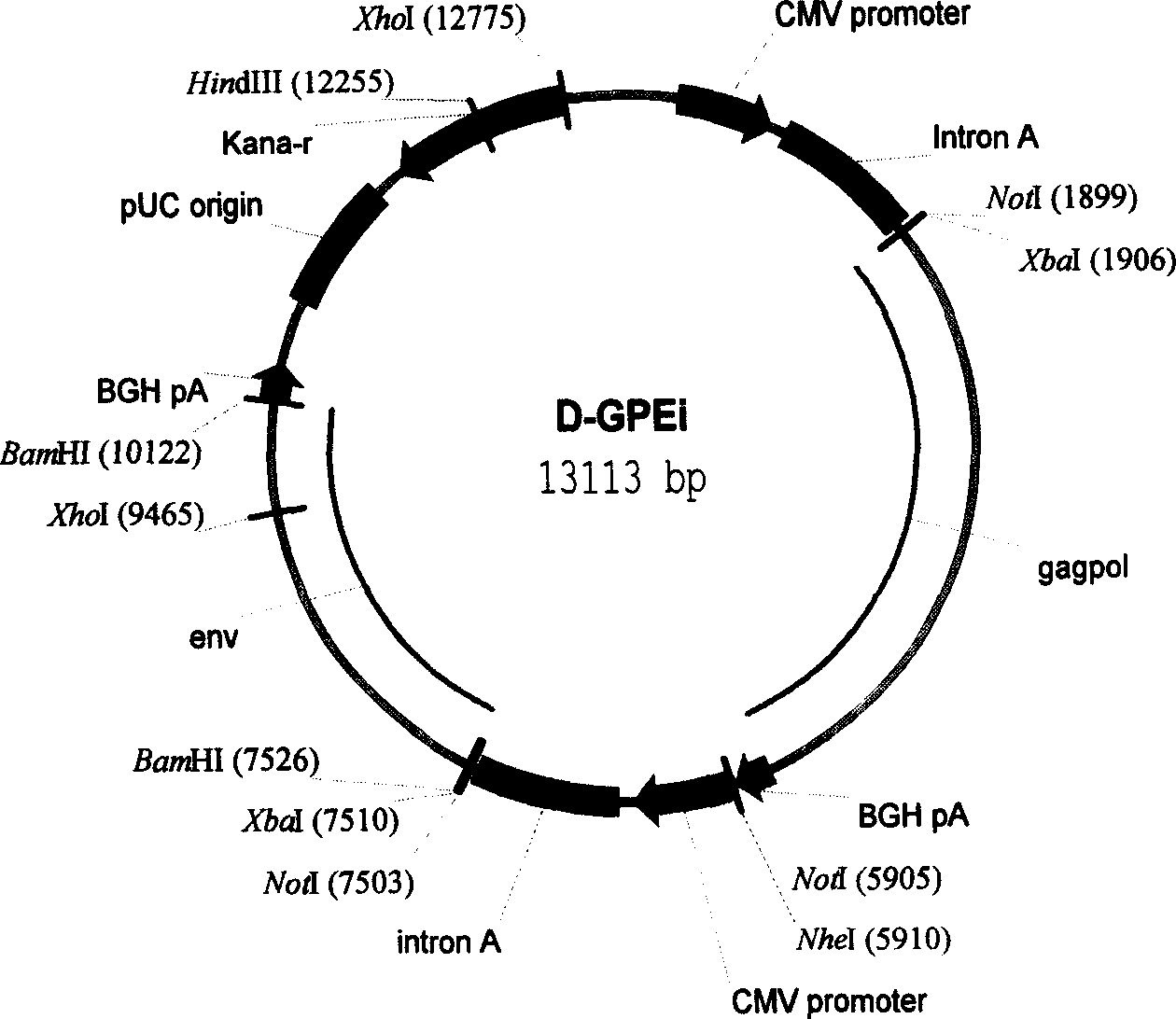
The invention relates to a DNA vaccine for preventing AIDS, the vaccine is the recombinant nucleotide of Gag, Pol and Env nucleotide sequence through the artificial modification of structural protein containing encoded Human Immunodificidncy Virus-1 (HIV-1), these vaccines are used as the transcript units for the biological synthesis of the virus protein antigen when applied in vivo. The beneficial effects of the invention include, (1) improved DNA vaccine manufacturing technique, (2) no integration indication detected, (3) the vaccine target antigen includes almost all the HIV-1 structural protein, such as GagPol and Env.
Owner:长春百克药业有限责任公司
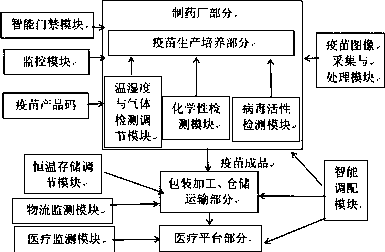
Preventive vaccine anti-counterfeiting traceability system based on Internet of Things and block chain
InactiveCN110717767ADatabase distribution/replicationCommerceVaccine manufacturingAcquisition apparatus
The invention relates to the technical field of vaccine anti-counterfeiting traceability, and discloses a preventive vaccine anti-counterfeiting traceability system based on the Internet of Things anda block chain, and the system comprises a local block database server A which is provided with vaccine traceability software in an installation and operation manner and is installed at a vaccine manufacturing place; and comprises first video acquisition equipment used for monitoring the whole process from raw materials to product manufacturing of vaccines; first vaccine detection and analysis equipment used for synchronously detecting basic preventive immune indexes of packaged vaccines in a product packaging process; date printing equipment used for printing a production date at the opening part of the sealing bag before the product packaging is about to be finished; wherein the block database of the vaccine traceability software stores video information of the whole process of manufacturing and packaging the sealed packaged vaccine printed with the specific production date and basic preventive immune index information. The vaccine anti-counterfeiting traceability system solves the technical problem that an existing vaccine anti-counterfeiting traceability system cannot fundamentally guarantee that vaccines inoculated by an inoculator belong to qualified products.
Owner:谢坚
Vaccine tracing and monitoring system and method
PendingCN110910152AEnsure safetyImprove favorabilityClosed circuit television systemsSatellite radio beaconingVaccine manufacturingDisease
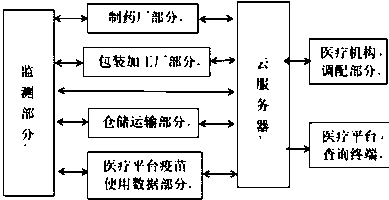
The invention relates to the technical field of vaccine traceability and monitoring, in particular to a traceability and monitoring system and method for vaccine production and manufacturing, packaging and processing, storage and transportation, use of a medical platform, whole-course tracking and recording of import and export information and displaying of the information to related personnel ofa medical platform. Information of each link of a tracing and monitoring system is uploaded to a cloud public platform, so that vaccine information transparency is realized. A cloud platform collectsinformation of the whole process from vaccine production and manufacturing to vaccine use of a consumer platform. Once adverse conditions such as diseases occur due to the use of the vaccine in vaccination, all public information of the vaccine production process can be checked through a website or a mobile phone app, so that a vaccinator can accurately find out problem links, accurately correctresponsibility and reduce disputes of all parties. When a certain vaccine is used sharply due to outbreak of epidemic situations in a certain area, the cloud platform can send out a deploying alarm signal to surrounding hospitals or areas and manufacturers without epidemic situations in time according to data, so that the vaccine is supplemented in time, and the social stability is maintained.
Owner:UNIV OF JINAN
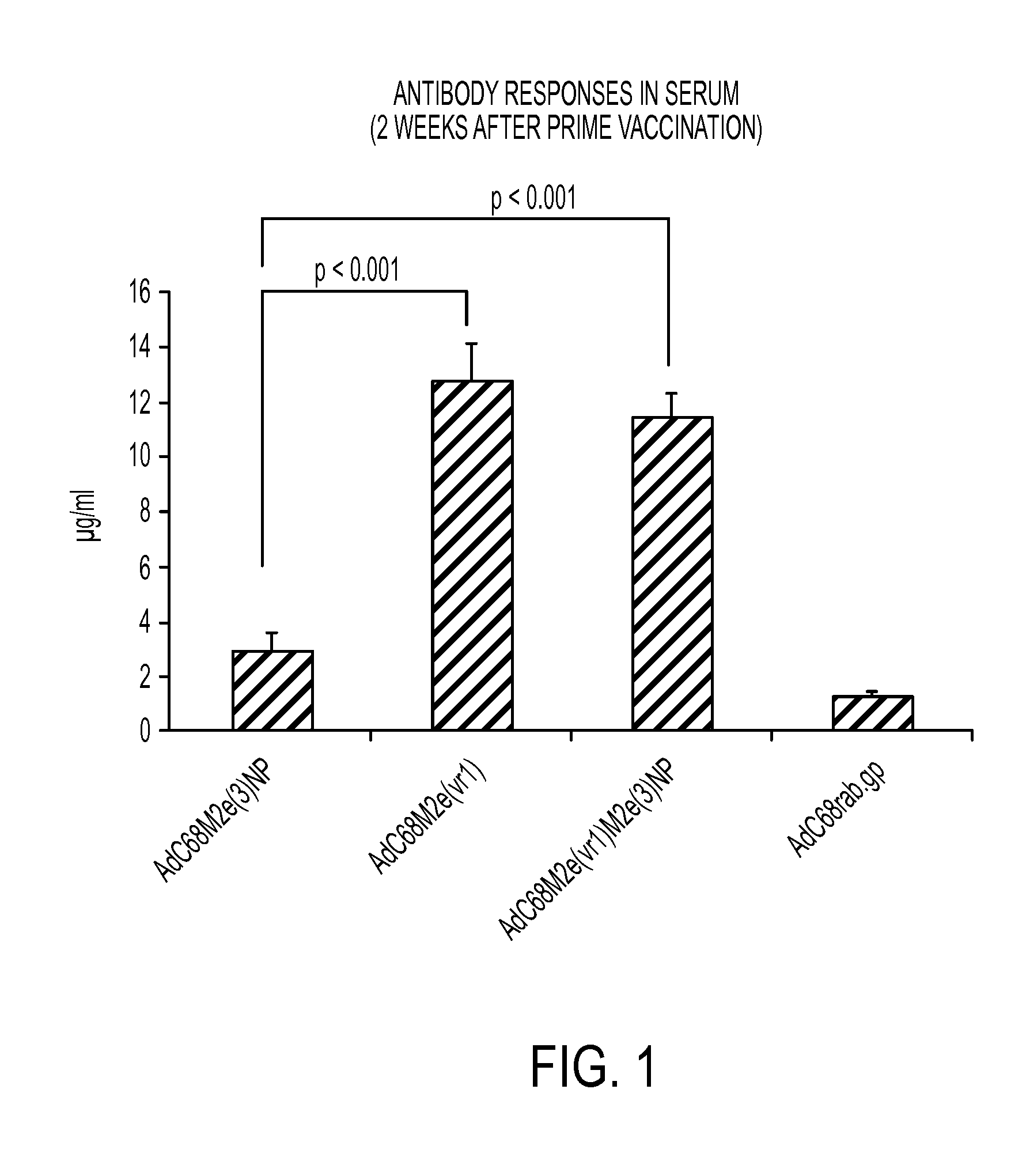
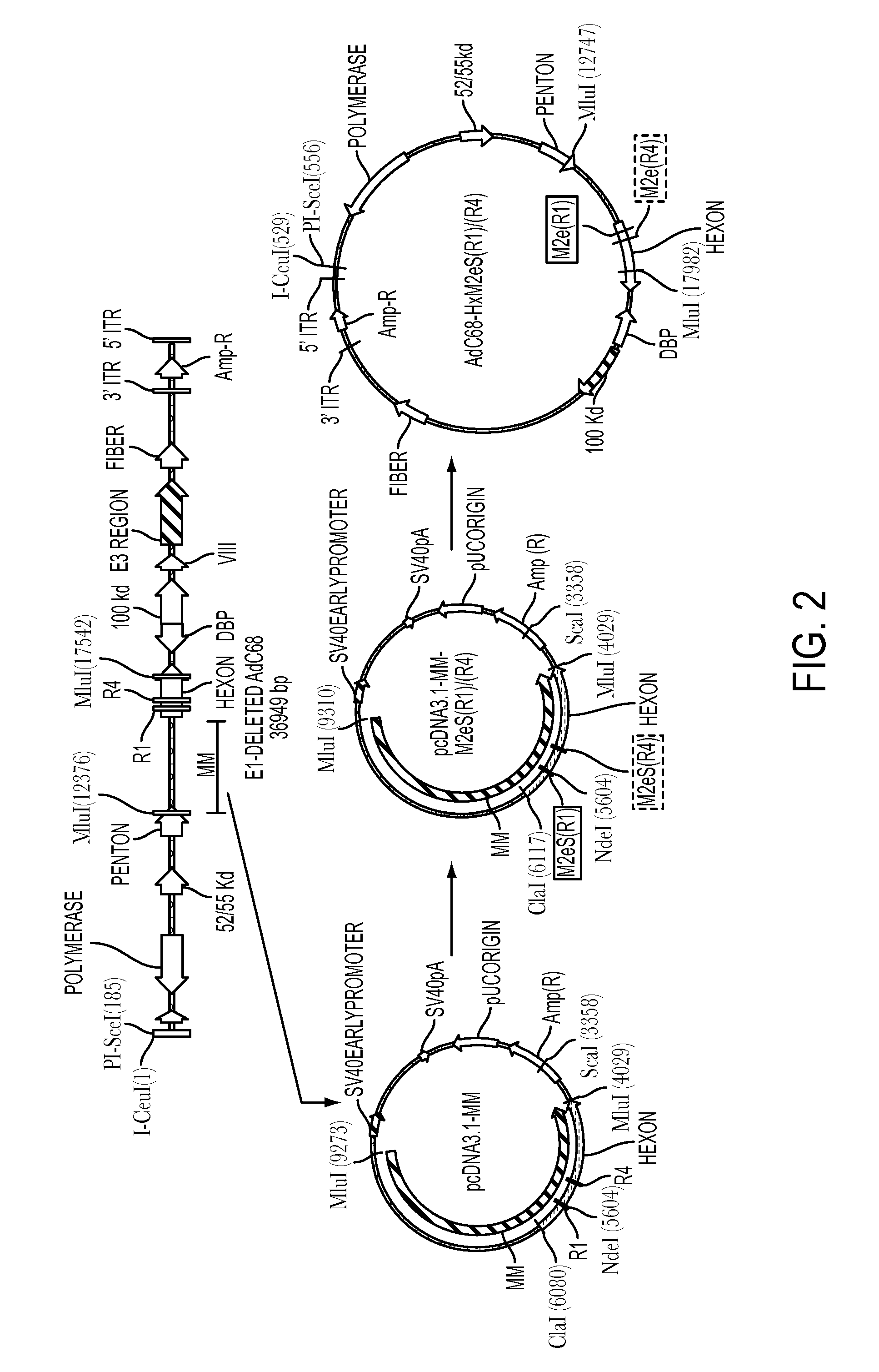
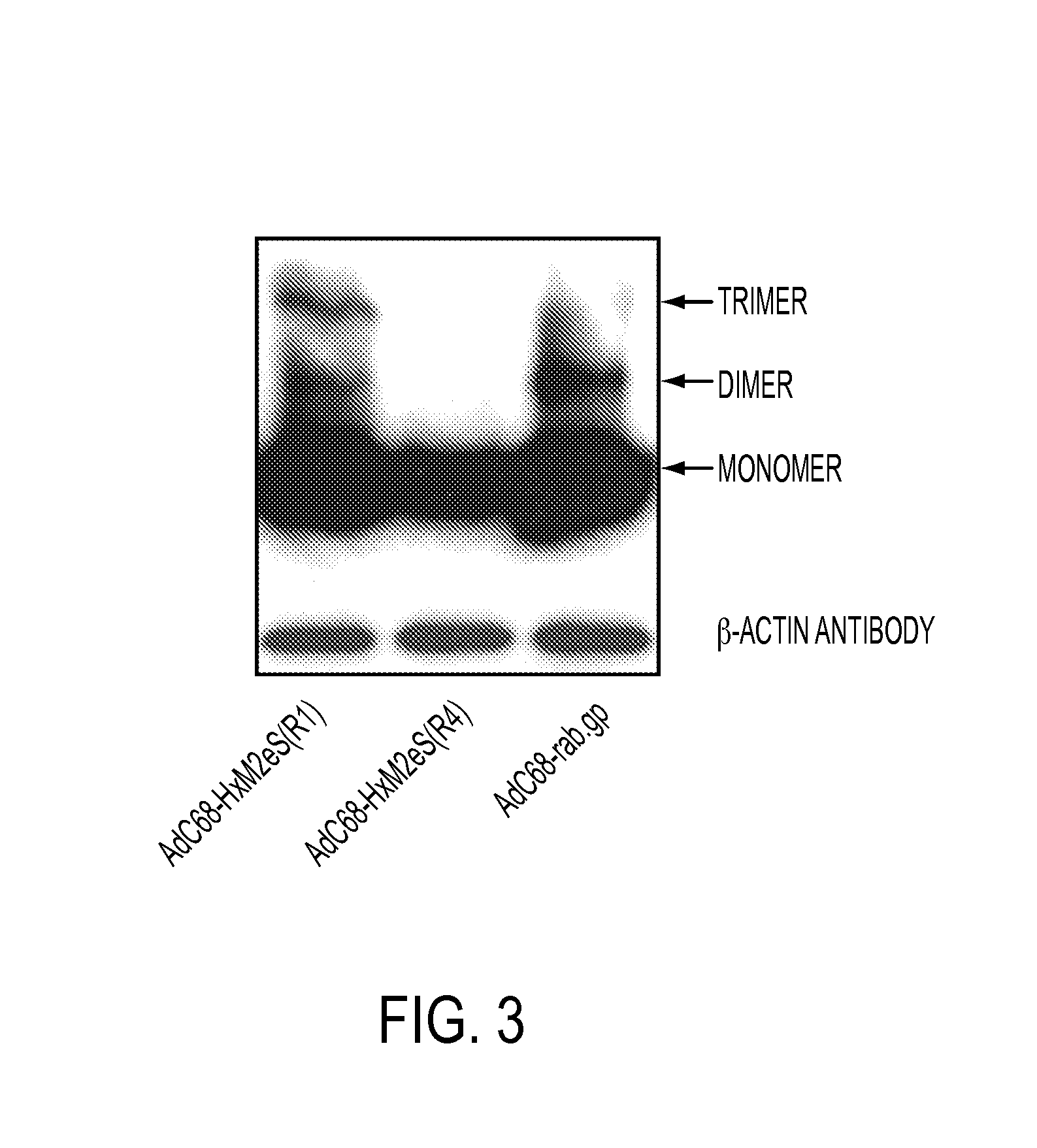
Influenza vaccines containing modified adenovirus vectors
InactiveUS20140377295A1Improve protectionProvide protectionSsRNA viruses negative-sensePeptide/protein ingredientsVaccine manufacturingPandemic
This disclosure provides universal influenza vaccines which can provide extended protection for several years, provide improved protection to circulating influenza strains that were not predicted accurately for annual vaccine manufacturing, and provide protection against newly emerging strains of influenza virus which carry the potential for establishing global pandemics.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY
Mink hemorrhagic pneumonia and botulism combined inactivate vaccine and preparing method thereof
ActiveCN105749266AStrong targetingImprove protectionAntibacterial agentsBacterial antigen ingredientsVaccine manufacturingMink
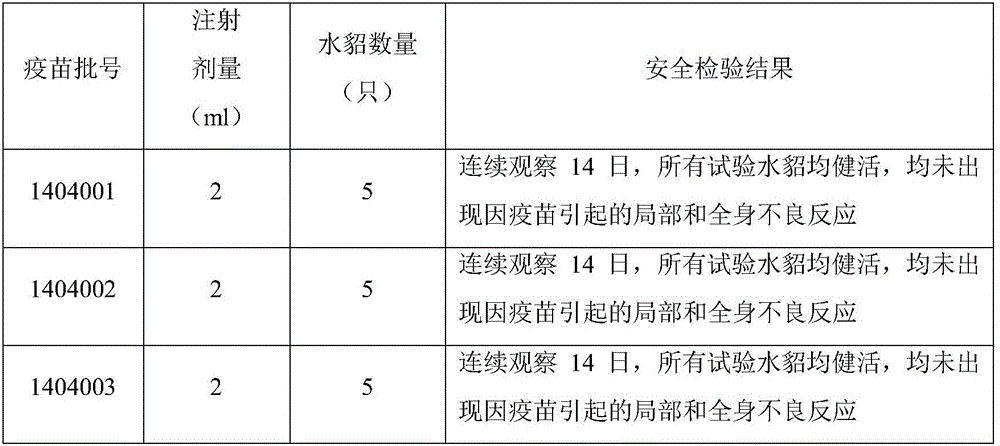
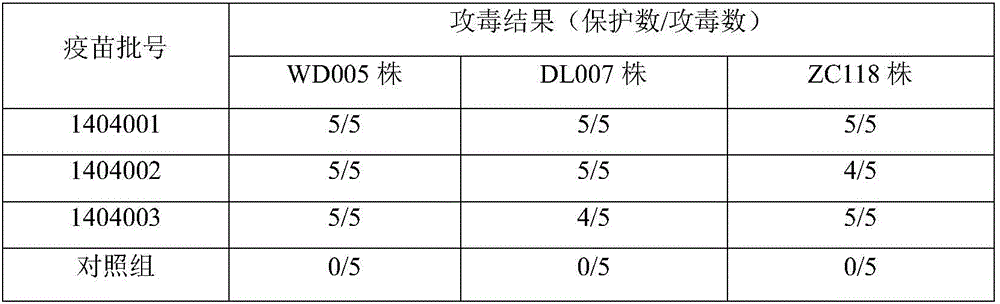
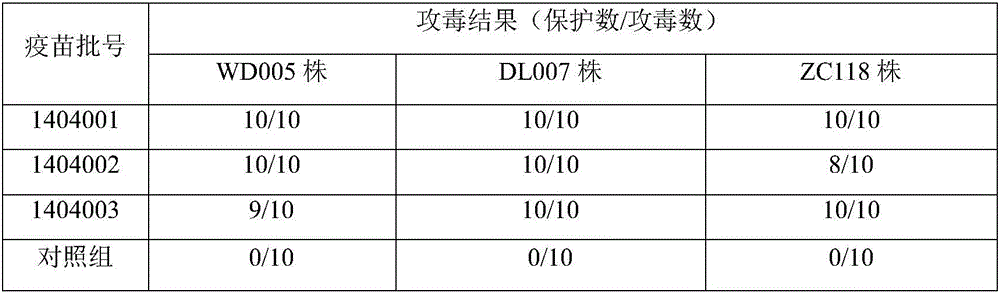
The invention relates to a mink hemorrhagic pneumonia and botulism combined inactivate vaccine and a preparing method thereof.Strain WD005, strain DL007 and strain ZC118 of pseudomonas aeruginosa for vaccine manufacturing and detecting are obtained through clinical isolation, and the strains are higher in pertinence and more comprehensive in protection for current mink hemorrhagic pneumonia epidemic serotype; by means of virulence tests and immunogenicity tests, the immunogenicity is good; a strain C-type clostridium botulinum C62-4 has the advantage of being superior in immunogenicity.The combined inactivate vaccine can prevent attack of G-type pseudomonas aeruginosa, B-type pseudomonas aeruginosa, C-type pseudomonas aeruginosa and C-type clostridium botulinum to minks at the same time, and has the advantages that the number of inoculation times is reduced, and using is convenient.The labor intensity of immunization is relieved, the immune cost is reduced, the stress reaction of animals is reduced, and the vaccine is more economical and reliable.
Owner:QILU ANIMAL HEALTH PROD
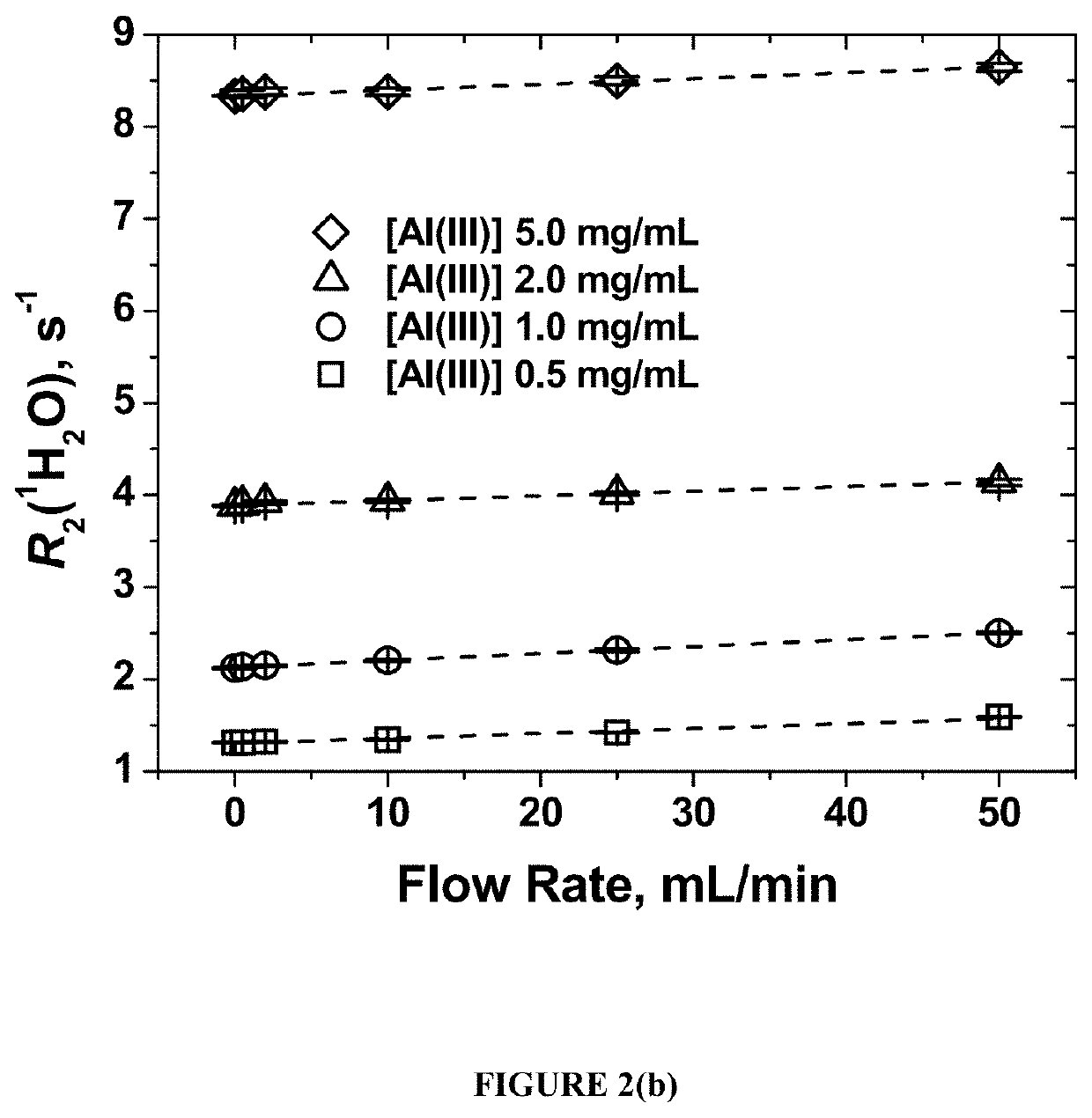
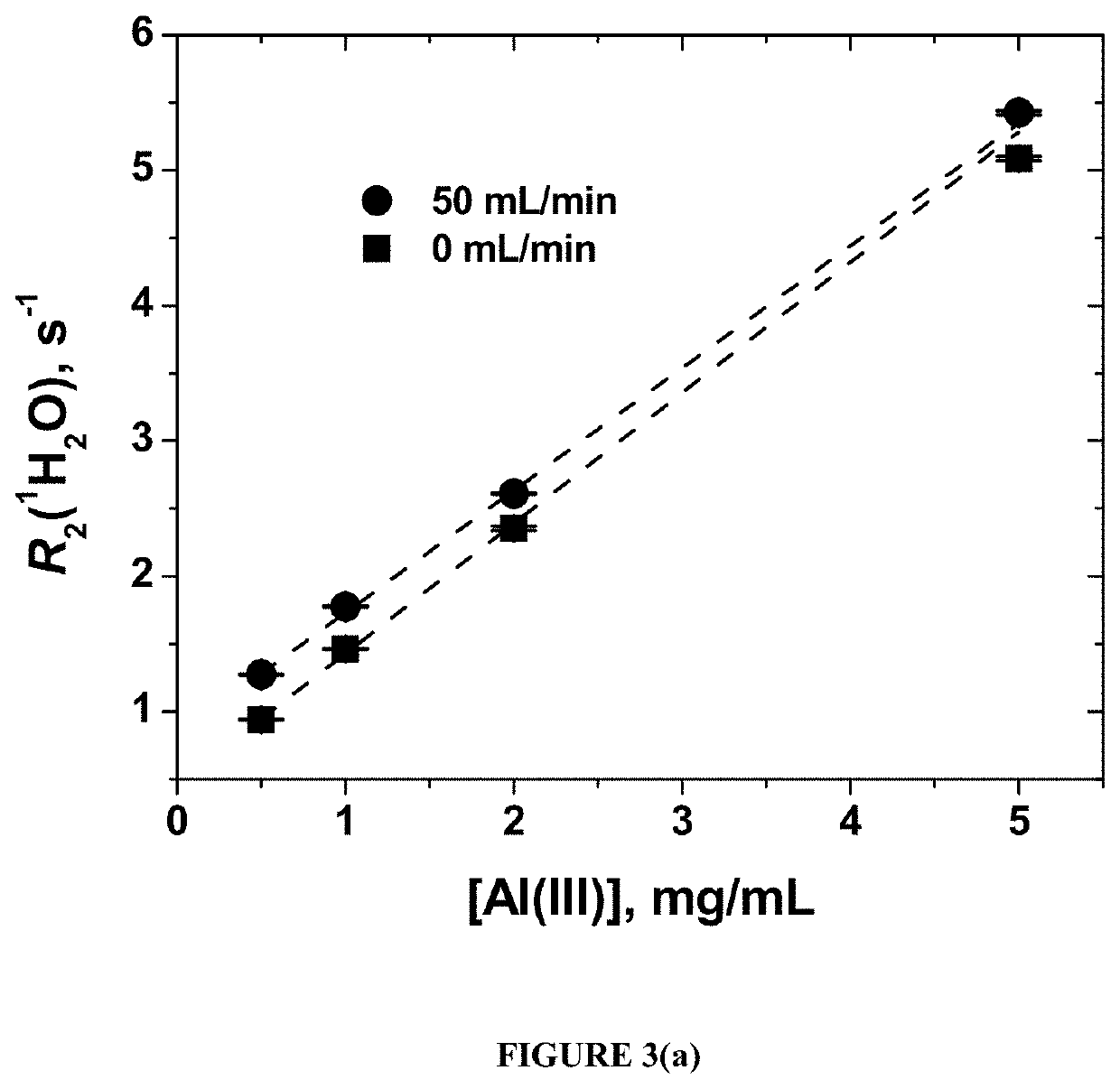
In situ, real-time in-line detection of filling errors in pharmaceutical product manufacturing using water proton nmr
ActiveUS20210010962A1Measurements using NMR spectroscopyAnalysis using nuclear magnetic resonanceVaccine manufacturingDrug product
A method of using the transverse relaxation rate (R2) of solvent NMR signal to detect filling errors of an alum-containing product in real-time in-line during manufacturing, for example during a fill-finish unit operation. This technique can be used for quality control in vaccine manufacturing to ensure the delivery of the correct concentration of alum-containing product to the product container such as a vial or pre-filled syringe.
Owner:UNIV OF MARYLAND BALTIMORE
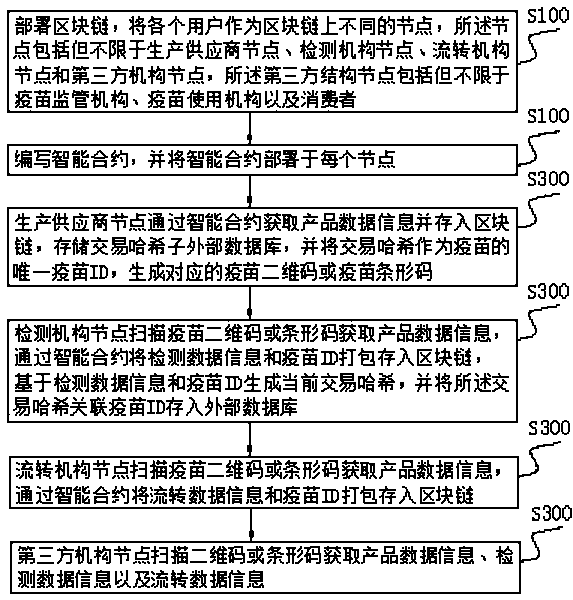
Vaccine manufacturing process supervision method based on block chain
InactiveCN111242651AMaster moreOpen and transparentDrug and medicationsDigital data protectionVaccine manufacturingThird party
The invention discloses a vaccine manufacturing process supervision method based on a block chain, belongs to the field of vaccine monitoring, and aims to solve the technical problem of how to supervise the vaccine manufacturing process. The method comprises the following steps: a block chain is deployed, and different users are taken as different nodes in the block chain; an intelligent contractis compiled, and the intelligent contract is deployed at each node; a production supplier node acquires product data information through the intelligent contract and stores the product data information into the block chain to generate a corresponding vaccine two-dimensional code or vaccine bar code; a detection department node scans the vaccine two-dimensional code or bar code to obtain product data information, and stores a transaction hash associated vaccine ID into an external database; a circulation structure node scans the vaccine two-dimensional code or bar code to obtain product data information, and the circulation data information and the vaccine ID are packaged and stored in the block chain through the intelligent contract; and a third-party department node scans the two-dimensional code or the bar code to obtain the product data information, the detection data information and the circulation data information.
Owner:山东爱城市网信息技术有限公司
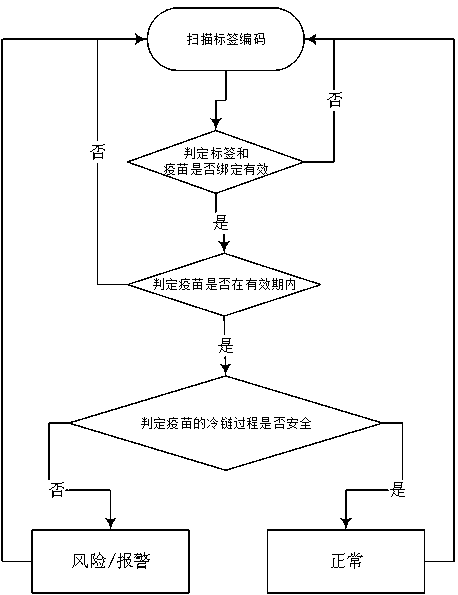
A method for judging vaccine safety by using microelectronic label
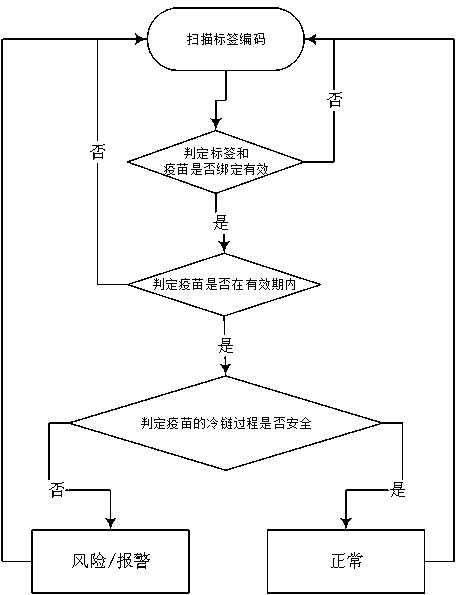
PendingCN111178470AFast trackStrong targetingDigital data information retrievalCo-operative working arrangementsVaccine manufacturingCold chain
The invention relates to a method for judging vaccine safety by using a microelectronic label. Codes on the microelectronic label comprise a label classification identification code and a GTIN code. The microelectronic label is edited and written into before the cold chain process; the cold chain process information is in a cold chain storage and transportation process; a microelectronic label automatically records and stores the vaccine information and actively or passively uploads the vaccine information to a database, label codes are bound with vaccine manufacturer information, vaccine information, cold chain rules and cold chain process information set in the database to form a corresponding relationship, and the method for judging the vaccine safety comprises the following steps: reading the codes; judging whether the label and the vaccine are bound effectively or not; judging whether the vaccine is valid or not; judging whether the cold chain process of the vaccine is safe or not according to the cold chain rule, wherein judgment meeting the cold chain rule is safe, and judgment not meeting the cold chain rule is unsafe. Considering various aspects of the vaccine cold chainprocess, the method has the advantages of high reliability and reasonable flow setting, has strong pertinence to the strictly controlled mode of vaccines, can also be used in similar fields, and hasgood popularization value.
Owner:中义(泰州)医药科技有限公司
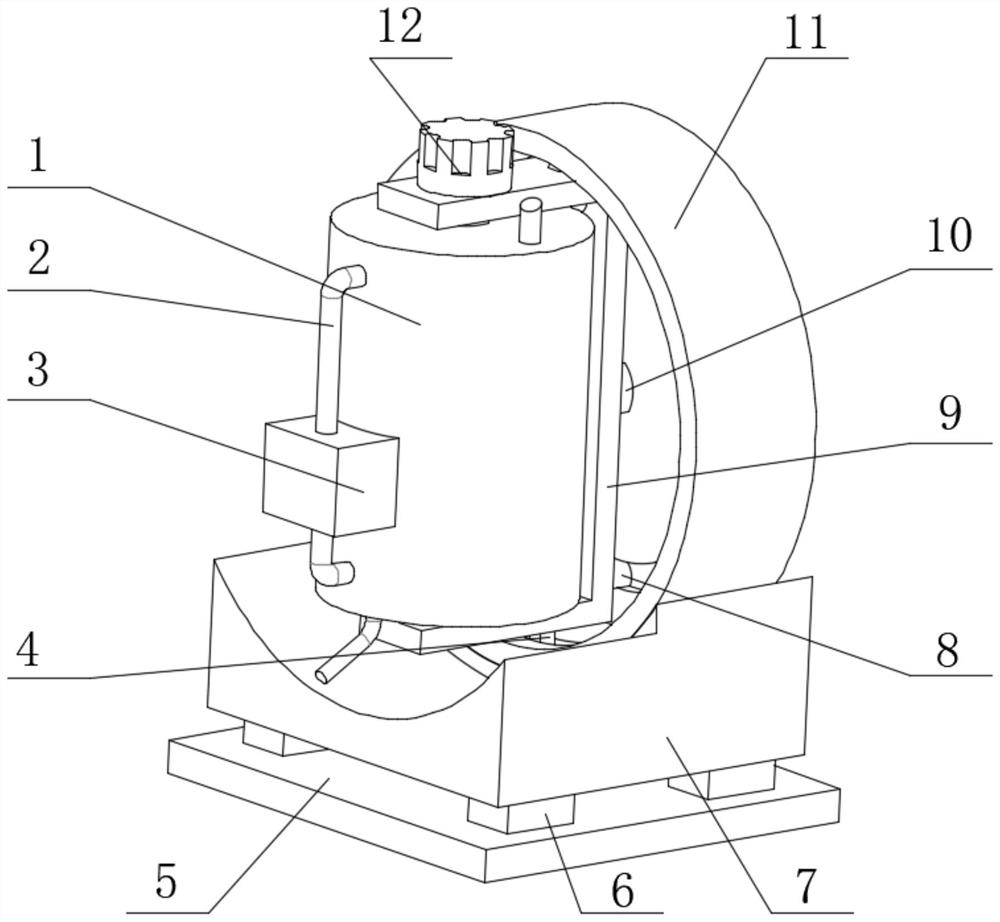
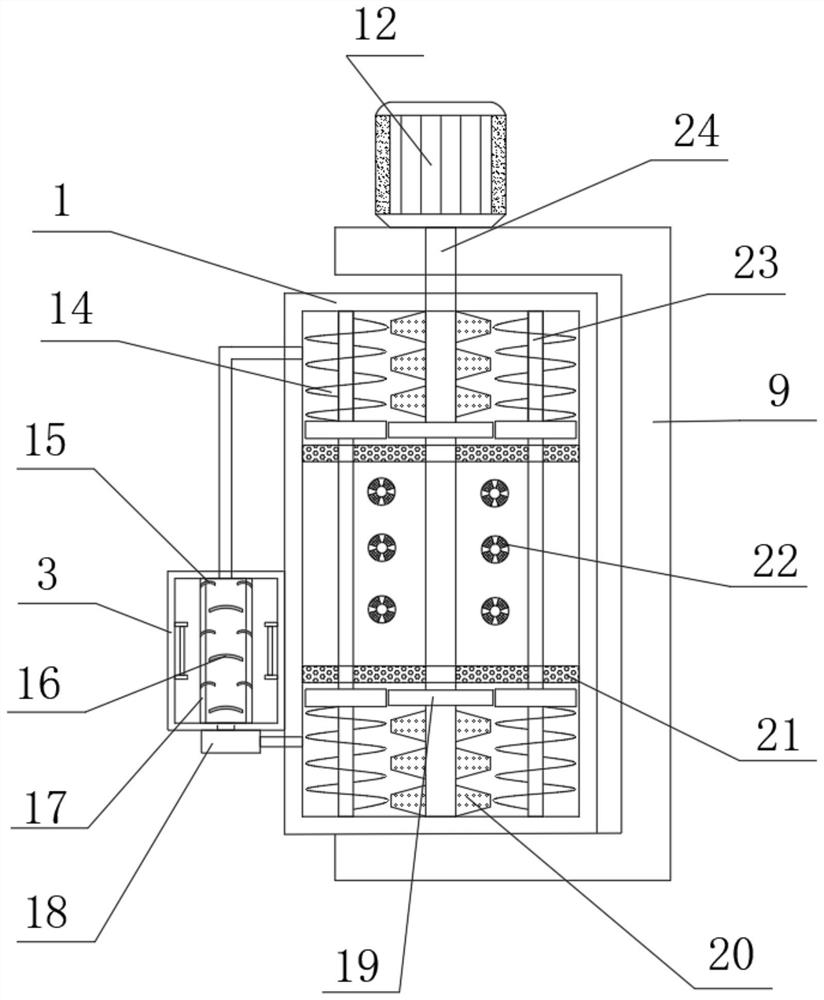
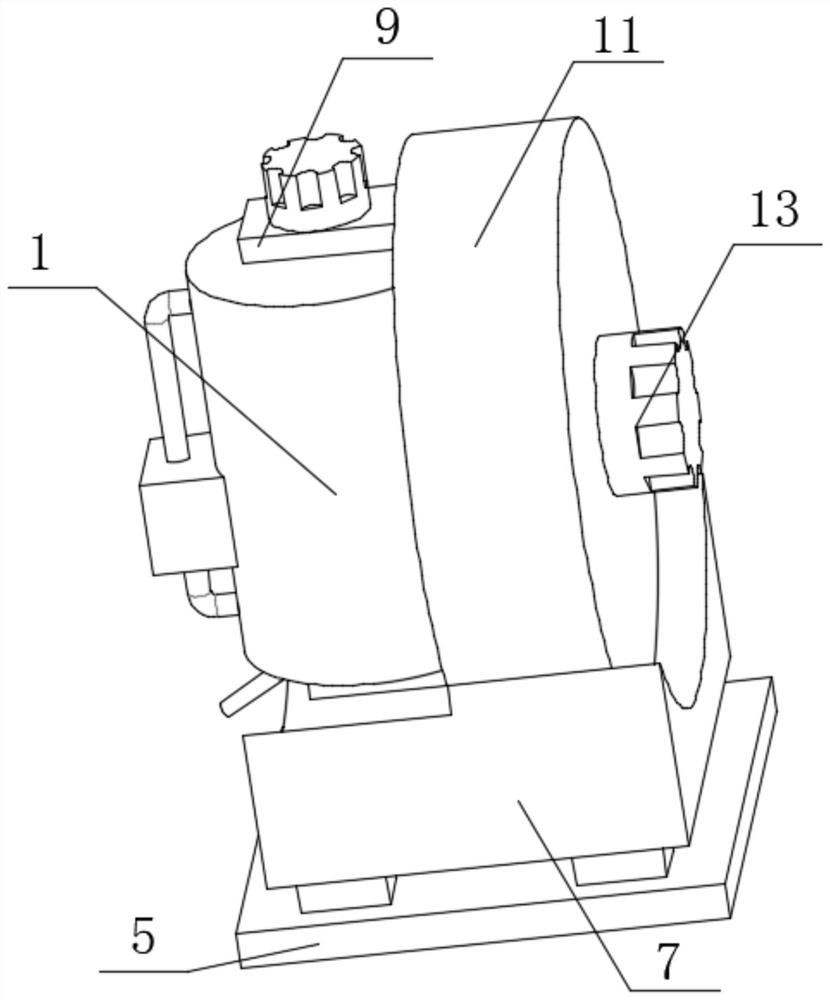
Oscillating device for animal vaccine production
PendingCN114432936AAchieve the effect of placementTo achieve a vibrating effectShaking/oscillating/vibrating mixersTransportation and packagingVaccine manufacturingAnimal science
The invention discloses a vibration device for animal vaccine production, which comprises a support table, a vibration mechanism is mounted on the side wall of the support table, the vibration mechanism comprises a motor I, the vibration mechanism further comprises a vibration table, a plurality of support blocks are fixedly mounted on the surface of the vibration table, and a rotating shaft is rotatably mounted at the top of each support block. And an adjusting mechanism is rotationally mounted at the top of the rotating shaft. During placement, animal vaccines are placed in the test tubes, the clamping buckles are opened, the groove covers are opened through the handles after the clamping buckles are opened, the groove covers rotate along the rotating shafts, then the placement grooves are opened, then the test tubes are placed in the placement holes in the placement grooves, then the groove covers are closed, and when the groove covers are closed, the pressing plates abut against the test tubes; and the test tube is buffered, extruded and positioned through the spring II of the pressing plate, so that the placement effect is achieved.
Owner:云南生物制药有限公司
Egg sorting equipment used before vaccine seeding
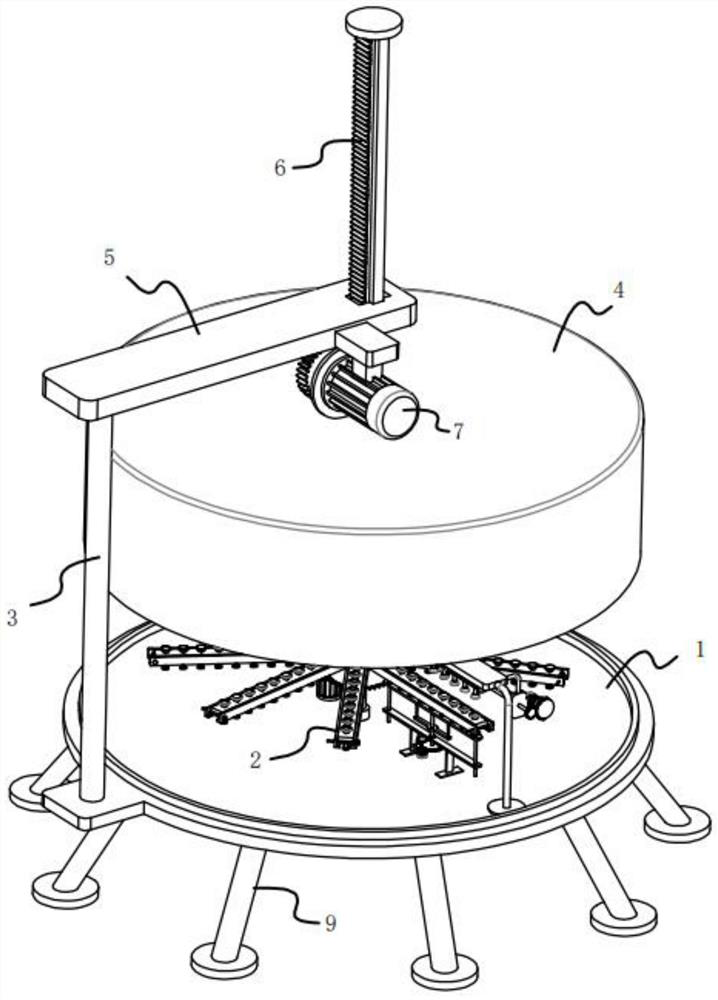
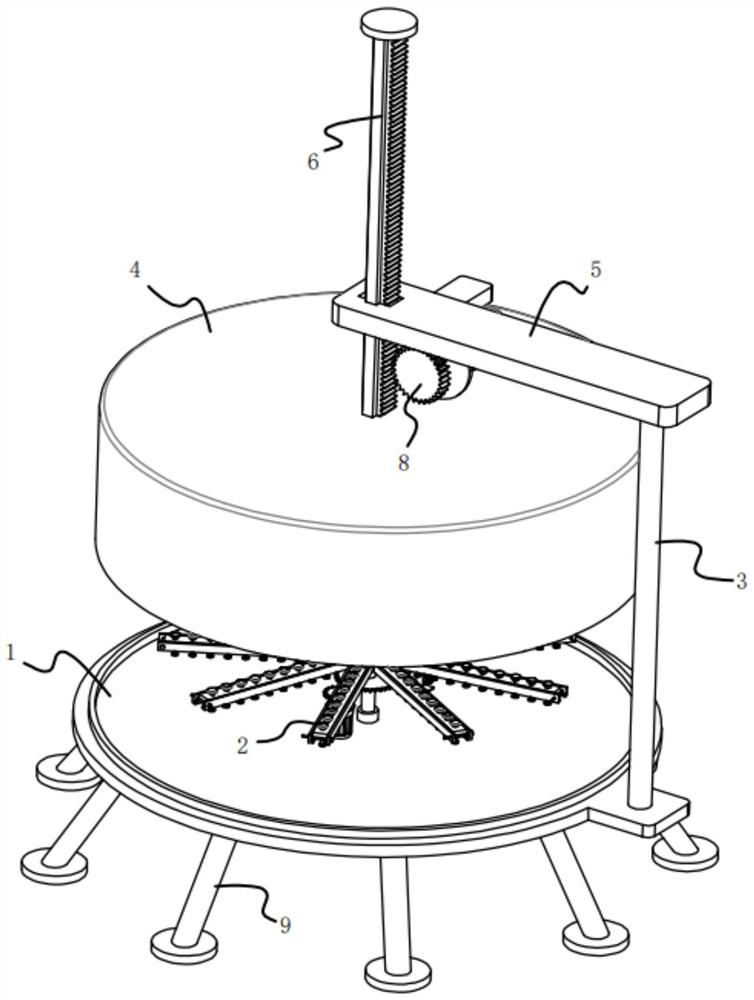
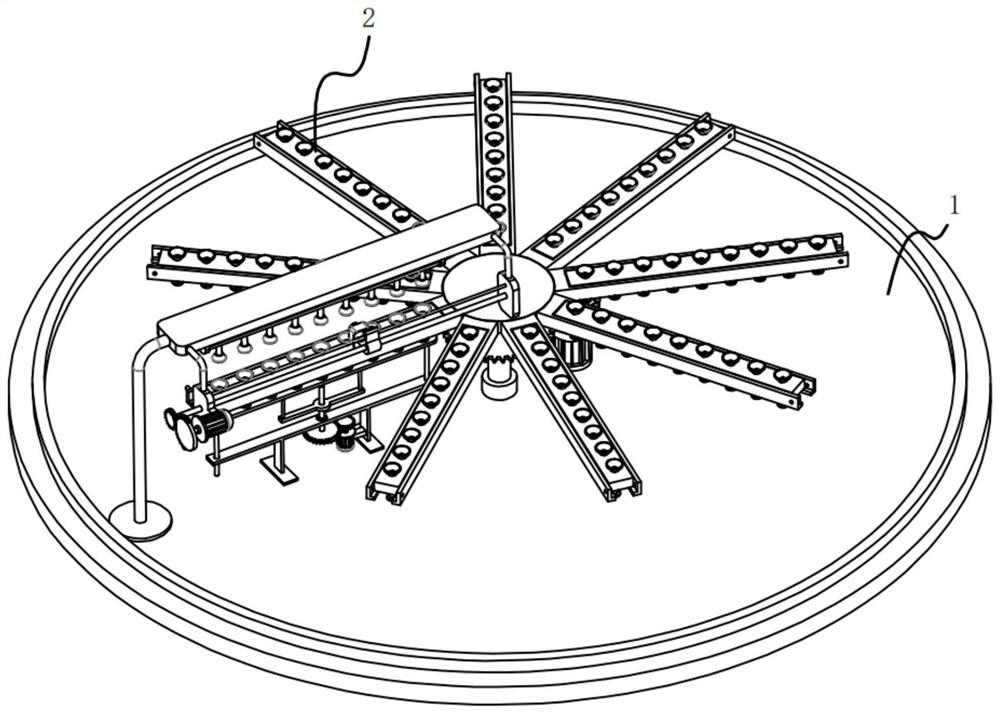
ActiveCN113396839AAvoid missed inspectionsReduce investmentClimate change adaptationAvicultureVaccine manufacturingAnimal science
The invention relates to the technical field of vaccine production, in particular to egg sorting equipment before vaccine seeding. The egg sorting equipment comprises an irradiation lamp, a connecting frame, a stepping motor, a sorting box and a feeding structure, wherein the horizontal section of the sorting box is circular, the sorting box is fixed through the connecting frame mounted outside the sorting box, the stepping motor is fixedly mounted on a motor frame, the feeding structure is located outside the sorting box, and the irradiation lamp is fixedly mounted on the extension line of the axis of the sorting box through a lamp holder. According to the egg sorting equipment, a plurality of eggs are placed on the inner side of a support, the obliquely-arranged support enables the eggs to slide to the bottom of the inner side of the support under the action of gravity and enter the upper end of a conveying plate on the inner side of a square frame through the through holes, and when the eggs at the upper end of the conveying plate are linearly arranged and evenly distributed at the upper end of the inner side of the conveying plate, in the process, the investment of personnel is reduced, and the condition of egg missing detection is avoided.
Owner:杭州华杰禽业有限公司
Packaging system for vaccine production and use method thereof
PendingCN114590438APrevents the risk of re-leaking openingsBio-packagingBelt grinding machinesVaccine manufacturingEngineering
The invention relates to the field of vaccine production, in particular to a packaging system for vaccine production and a using method of the packaging system. According to the packaging system for vaccine production and the using method of the packaging system, the risk that in the packaging process of vaccine production, a vaccine bottle opening with an aluminum package leaks again can be prevented. According to the technical scheme, the packaging system for vaccine production comprises two transverse moving frames, clamping plates, connecting rods and an arc frame, the bottom of each transverse moving frame is rotationally connected with one connecting rod, the middle of each connecting rod is fixedly connected with one clamping plate, and the clamping plates are fixedly connected with the arc frame; a plurality of arc frames are evenly arranged on the upper side and the lower side of the rear side of each clamping plate.
Owner:宋四喜
Method for preparing infectious bronchitis viral antigen by adopting non-immune eggs
ActiveCN110404063AHigh poison priceQuality improvementSsRNA viruses positive-senseViral antigen ingredientsVaccine manufacturingVaccine antigen
The invention belongs to the technical field of vaccine antigens, and discloses a method for preparing infectious bronchitis viral antigen by adopting non-immune eggs. The method comprises the following steps that infectious bronchitis non-immune eggs are adopted, pre-incubation conditions are controlled for pre-incubation; toxic strains of infectious bronchitis virus are inoculated on a chick embryo, and the inoculation dose is 105.0EID50 / piece; after inoculation, post-incubation conditions are controlled for post-incubation, inspecting is still conducted after inoculation for 24 h, a dead embryo is discarded, after inoculation for 36 h, the chick embryo is placed into a cold storage chamber at 2-8 DEG C, and a virus allantoic fluid is obtained after 12 hours of cold storage; the virus titer of the obtained virus allantoic fluid is detected, and the virus allantoic fluid is concentrated and purified to obtain the infectious bronchitis viral antigen. According to the method, SPF eggs are replaced by the non-immune eggs, meanwhile the process conditions of the infectious bronchitis virus on the propagation of the non-immune eggs are determined, the quality of vaccine is ensured, thecost of vaccine research and production is also lowered, the huge reference value can be provided for vaccine manufacturing enterprises, and application prospects are broad.
Owner:商丘美兰生物工程有限公司
Method of extracting immunologic adjuvant component from cochinchina momordica seed
InactiveCN101224231ASimple extraction processHigh yieldOrganic active ingredientsImmunological disordersVaccine manufacturingHemolysis
The invention discloses a method for exacting immune adjuvant from cochinchina momordica seed. After grinding traditional Chinese medicine cochinchina momordica seed, the obtained material is degreased by petroleum ether and extracted in a reflux way by using ethanol; the extraction liquid is filtered and concentrated by decompression; then adsorption macroporous resin column purification is carried out, so as to obtain extract powder of cochinchina momordica seed. The obtained extract powder is light yellow with faint scent. The preparation method of the invention improves the yielding rate four times as much as the prior method. Physical and chemical analysis and hemolysis test all show that the extract contains triterpene saponin. The obtained triterpene saponin from cochinchina momordica seed of the invention has low toxicity and test mice have no adverse reaction after oral taking or intramuscular injecting; therefore, the invention has wide application prospect in the vaccine manufacturing field.
Owner:ZHEJIANG UNIV
Stirring equipment for pneumonia vaccine production
InactiveCN112076659AAvoid accumulationWell mixedRotating receptacle mixersTransportation and packagingVaccine manufacturingRotational axis
The invention discloses stirring equipment for pneumonia vaccine production. The equipment comprises a base, a mounting shell is mounted on one side of the outer wall of the top of the base, a motor is mounted on the outer wall of one side of the mounting shell, an output shaft is mounted on the outer wall of one side of the output end of the motor, and a mounting frame is mounted on the outer wall of one side of the output shaft; and a first limiting circular groove is formed in the circumferential inner wall of the mounting shell, and first limiting rods are arranged on the inner walls of the top and the bottom of the first limiting circular groove correspondingly. According to the stirring equipment, through a rotating shaft and a driving gear arranged on a servo motor, a circular shafton a driven gear can be driven to rotate, spiral blades on the circular shaft mix raw materials in the stirring shell, the rotating directions of the spiral blades at the top and the bottom of the circular shaft are opposite, the raw materials can be moved to the middle, and the sufficient mixing is achieved; the accumulation of the raw materials is avoided, trapezoidal stirring plates on the outer wall of the circumference of the rotating shaft are used for mixing the raw materials again, so that the mixing efficiency is improved.
Owner:AB&B BIO TECH CO LTD JS
Peptide-based vaccine generation
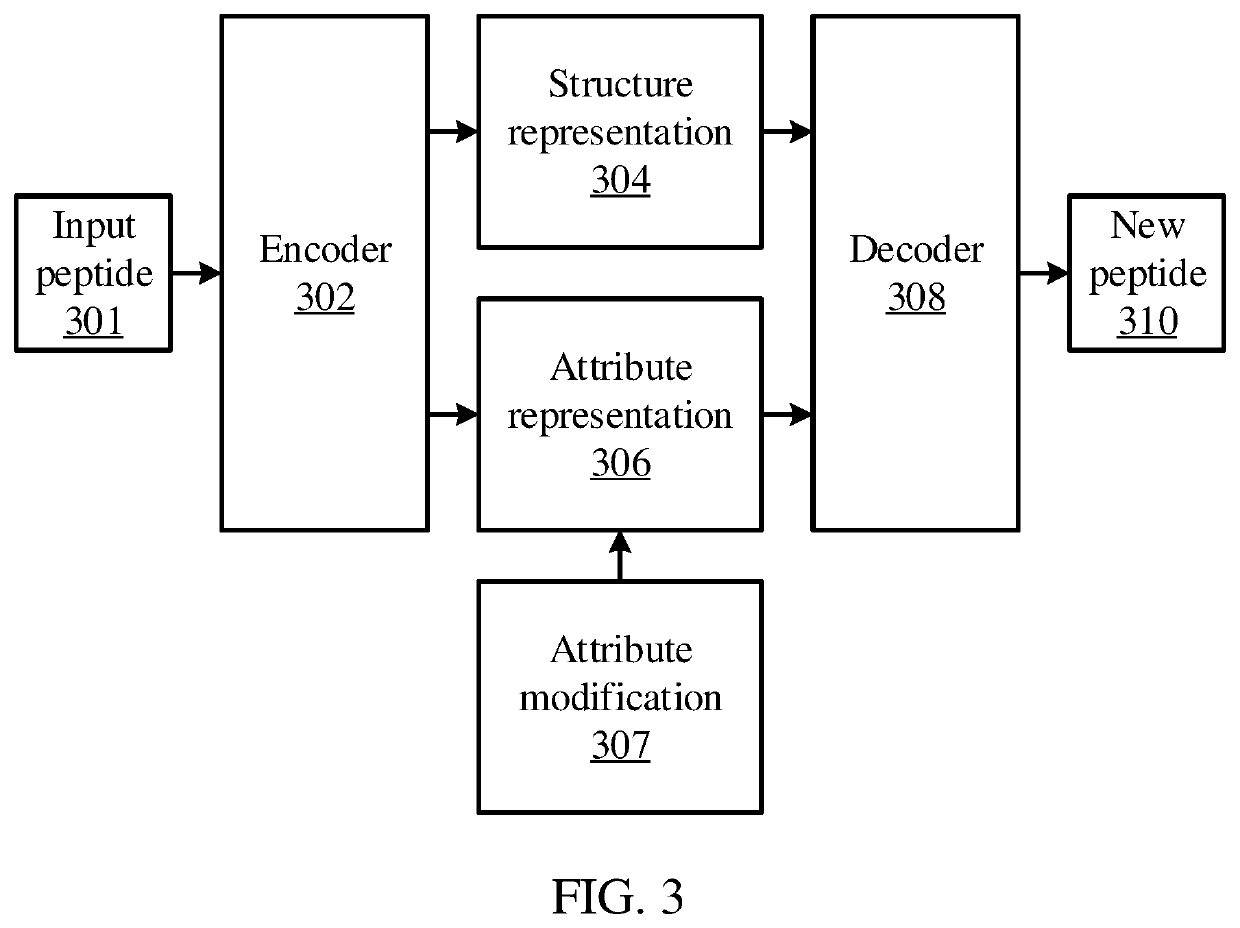
PendingUS20220130490A1Improve vaccine efficacyGood curative effectBiostatisticsNeural architecturesVaccine manufacturingPeptide sequence
Methods and systems for generating a peptide sequence include transforming an input peptide sequence into disentangled representations, including a structural representation and an attribute representation, using an autoencoder model. One of the disentangled representations is modified. The disentangled representations, including the modified disentangled representation, are transformed to generate a new peptide sequence using the autoencoder model.
Owner:NEC LAB AMERICA
Method for preparing influenza vaccine
InactiveCN104436185ASolve yourselfSolve pollutionMicroorganism based processesAntiviralsVaccine manufacturingBioreactor
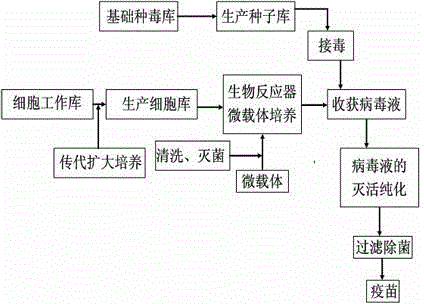
The invention discloses a method for preparing an influenza vaccine. The method comprises the following steps: preparing virus seeds; conducting micro-carrier suspension cultivation on Vero cells in a bioreactor; breeding a vaccine-made virus solution; inactivating and purifying the virus solution. The method adopts a cell line to replace a chicken embryo tissue for cultivation to prepare avian influenza viruses, and the problem that a chicken embryo itself has high possibility of being polluted by exogenous viruses can be solved; by the strict control on raw materials and the cultivation condition, the purity of prepared vaccine is guaranteed, and safety of the vaccine is ensured; the method adopts the bioreactor for vaccine preparation, is high in automation degree and simple and stable in preparation technology, and requires few operators required; the method can greatly reduce the preparation cost, is prevented from being restricted by raw material supply, and is short in preparation cycle; by adopting the method to prepare the vaccine, environmental pollution is less and is easy to handle; the conventional chick embryo preparation method can produce a great number of waste embryos and other waste, is great in handling difficulty, and relates to the problems of biological safety and public hygiene.
Owner:CHENGDU VERO BIOTECH
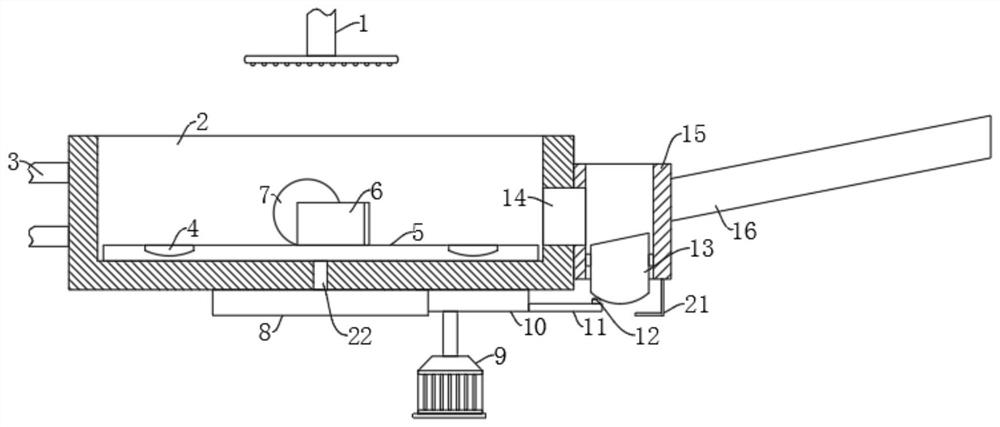
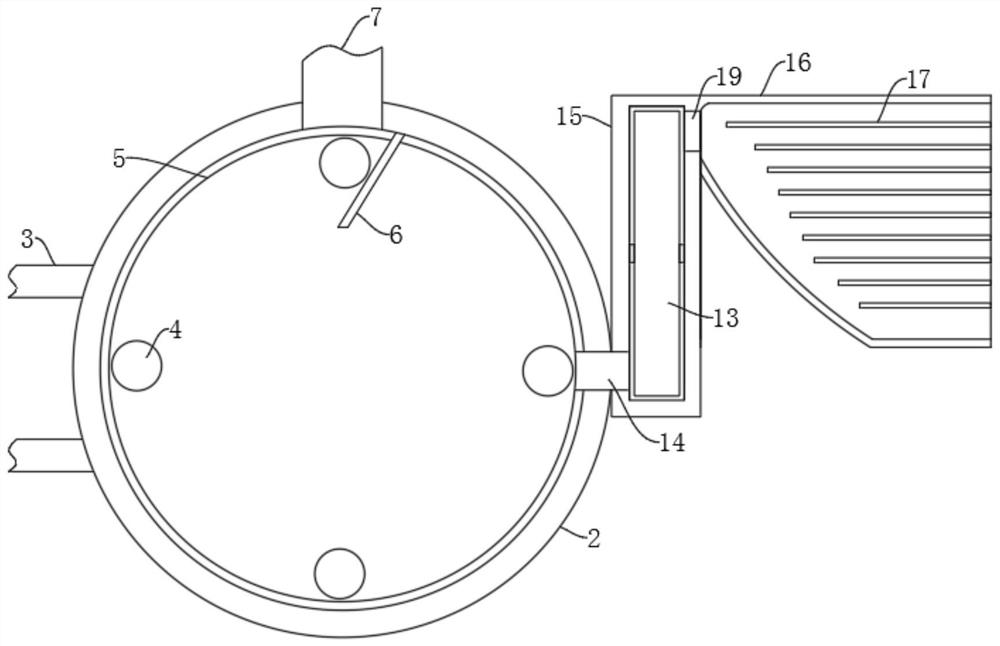
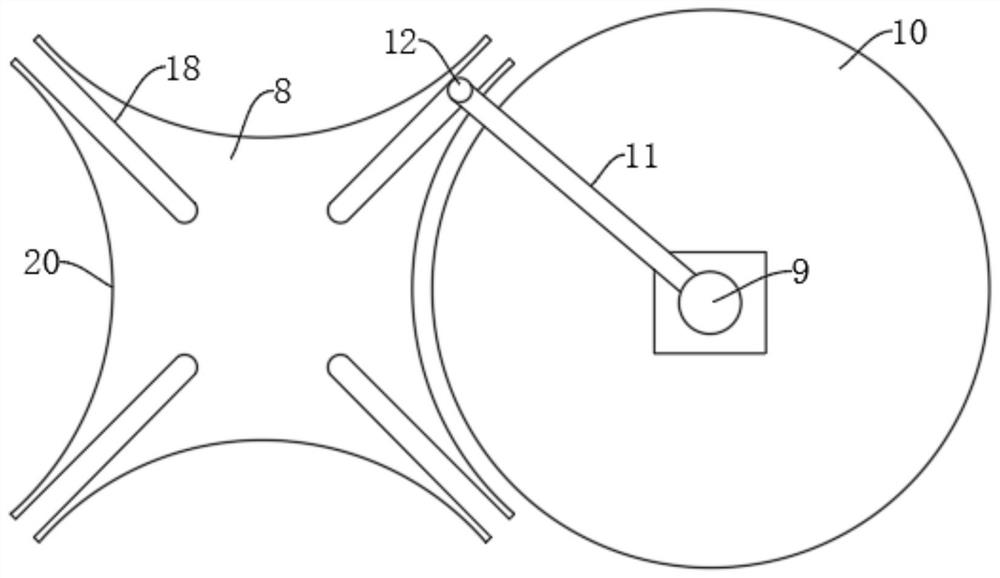
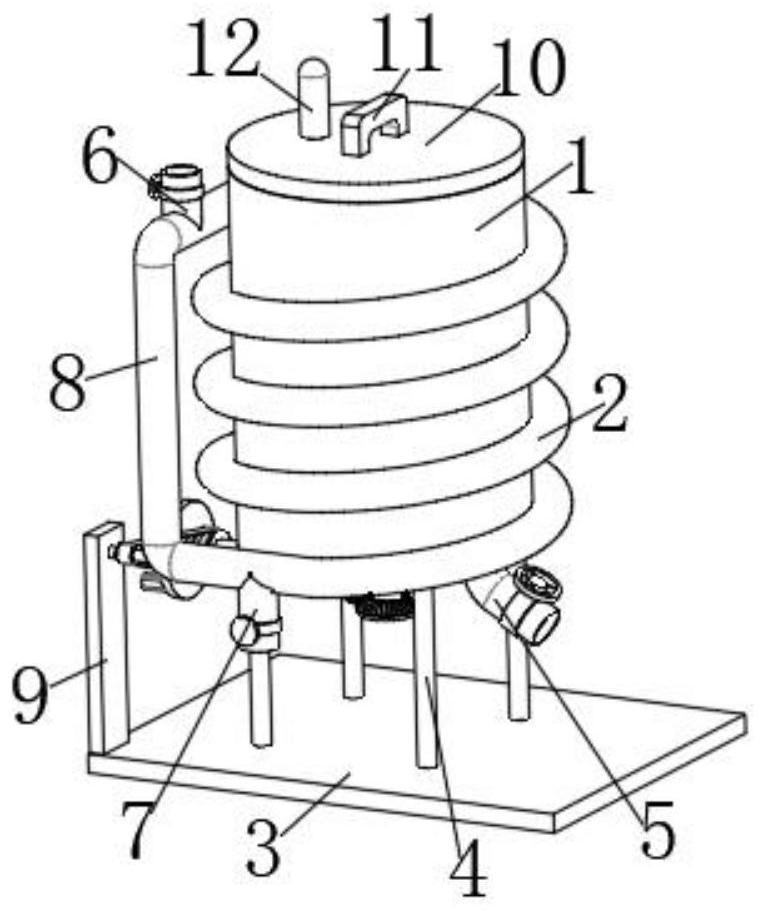
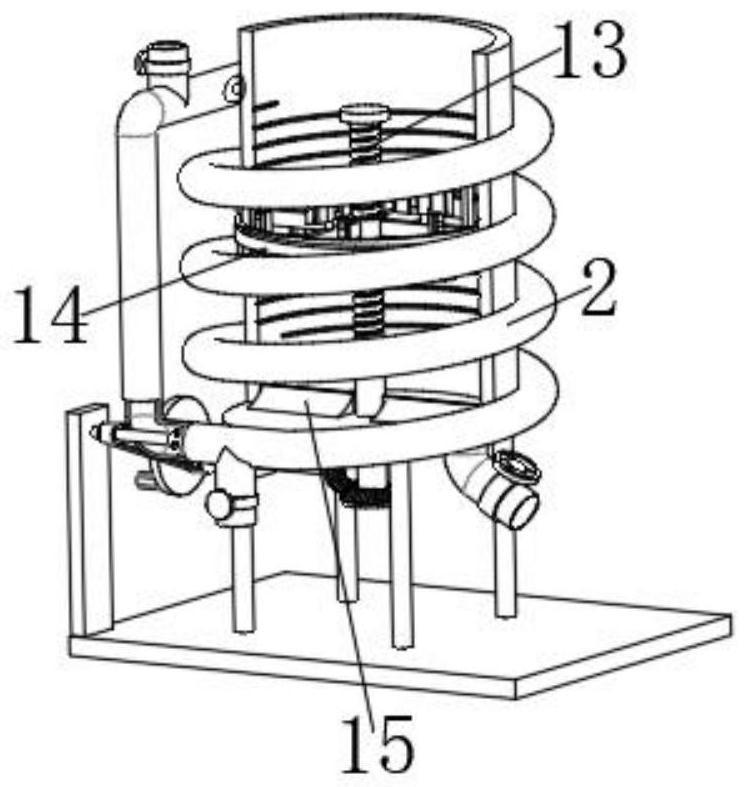
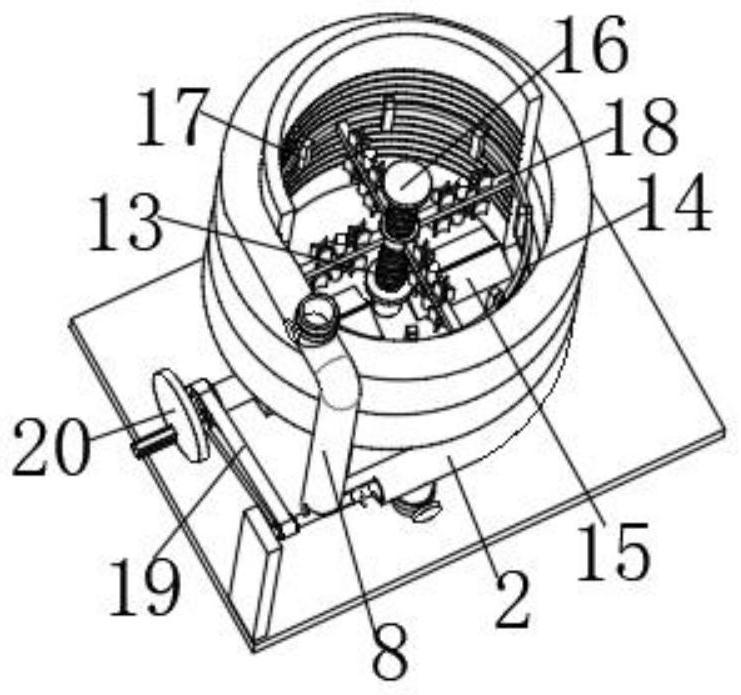
Vaccine manufacturing equipment and vaccine manufacturing process
PendingCN114749055AImprove qualitySpeed up the flowRotary stirring mixersTransportation and packagingVaccine manufacturingProcess engineering
The invention discloses vaccine manufacturing equipment and a vaccine manufacturing process, and relates to the technical field of vaccine manufacturing devices.The vaccine manufacturing equipment comprises a stirring box, a stud is rotationally installed at the bottom of the stirring box, a vaccine stirring and mixing part is installed on the stud, and four supporting columns are fixedly installed at the bottom of the stirring box. The device is reasonable in structure, vaccine agents can be fully mixed and stirred and cannot be adhered to the stirring device, the agents deposited or adhered to the bottom of the stirring box can be scraped by a scraper, the agents can be fully mixed with the other agents, the quality of manufactured vaccines is better, water in the condensation pipe and the guide pipe circularly flows at the same time, and the efficiency is improved. Heat generated by stirring can be taken away, vaccines cannot be inactivated due to temperature rise, the stirring effect of the vaccine stirring device is better, medicaments cannot be polluted by the external environment, the vaccines are more stable in the manufacturing process, and the stirring device is easy to operate, convenient to clean, wide in application range and high in practicability.
Owner:张 家斌
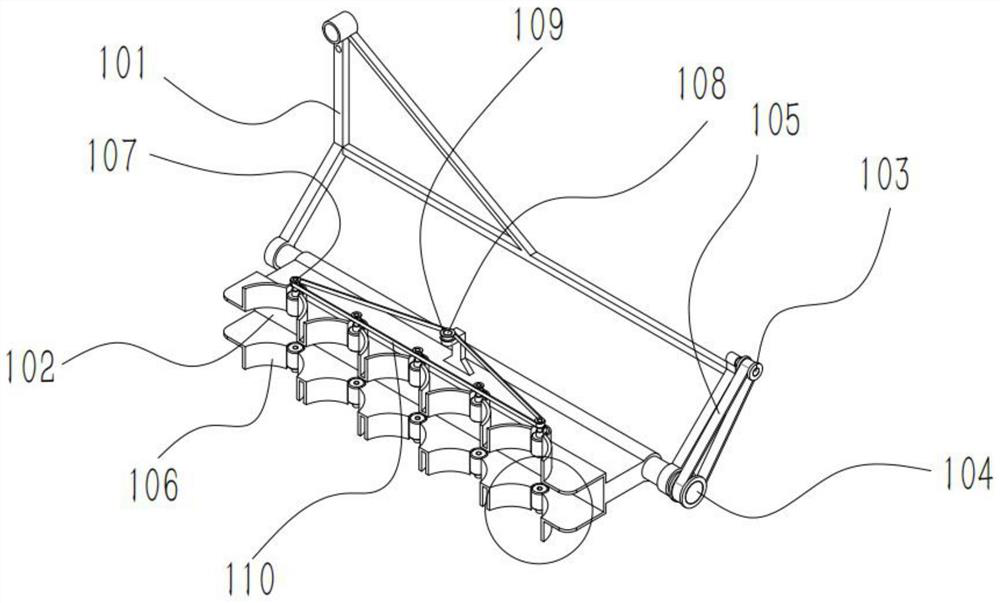
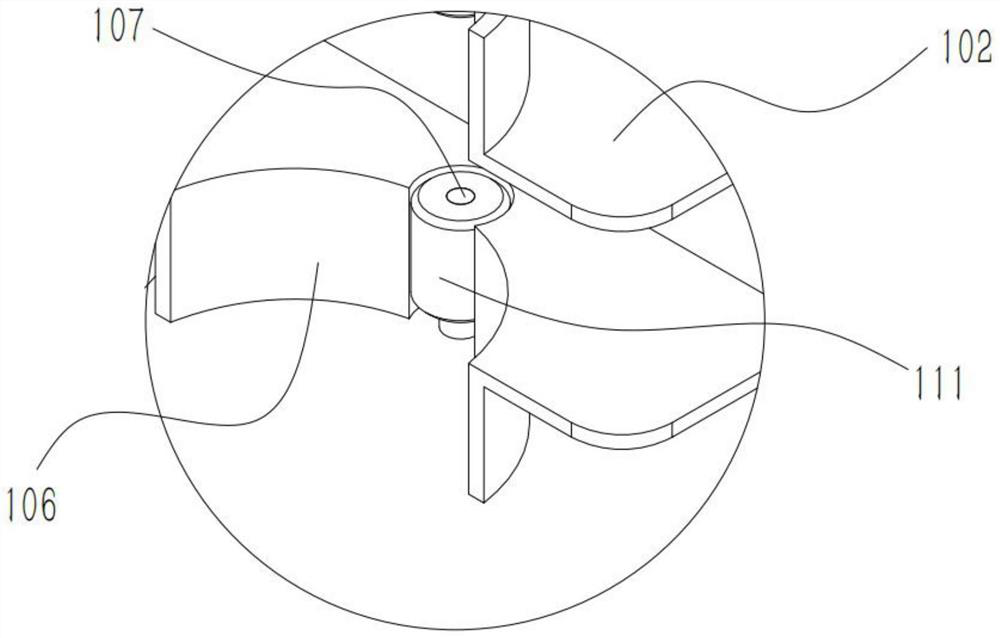
Automatic egg trolley positioning device in vaccine production process
PendingCN114194874APrecise positioningNormal pick and placeLoading/unloadingVaccine manufacturingTGE VACCINE
The invention relates to an automatic positioning device for an egg trolley in a vaccine production process, which comprises an L-shaped positioning bracket, and the long side and the short side of the L-shaped positioning bracket are matched with the long side and the wide side of the egg trolley; the inner side and the outer side of the long edge of the L-shaped positioning support are each provided with a front face pressing mechanism used for tightly pressing the egg trolley on the long edge of the L-shaped positioning support. A side face pressing mechanism is arranged on the outer side of the long edge of the L-shaped positioning support and used for pressing the egg trolley on the short edge of the L-shaped positioning support. According to the device, the egg vehicle can be accurately positioned, so that a robot can normally take and place egg trays, and the production efficiency is improved.
Owner:WUHAN XINHAO INTELLIGENT TECH CO LTD
Automatic egg shelling method and device
ActiveCN111037102BEasy tap to removeHigh production efficiency in shellingLaser beam welding apparatusVaccine manufacturingEggshell
Owner:上海赛摩电气有限公司
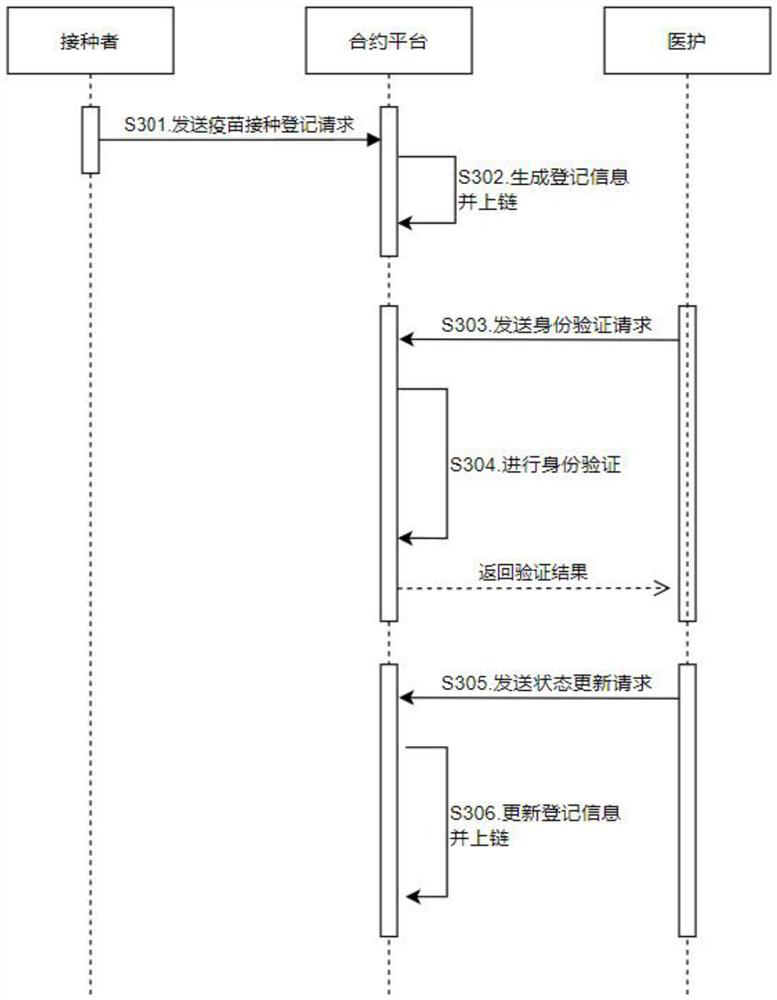
Vaccination verification system and method based on intelligent contract, and contract platform
InactiveCN114817903AReduce the involvement of human factorsDatabase distribution/replicationDigital data protectionVaccine manufacturingVaccination
The invention discloses a vaccination verification system, a vaccination verification method and a vaccination verification contract platform based on an intelligent contract, and the vaccination verification system, the vaccination verification method and the vaccination verification contract platform based on the intelligent contract can realize decentration of an application program. According to the technical scheme, high mutual trust among the first client serving as an inoculator, the second client serving as a medical care and the contract platform is achieved, participation of human factors is reduced, the phenomena of information leakage and masquerading are avoided, rapid popularization of vaccines is facilitated, and in addition, vaccine manufacturers can also carry out vaccine production planning according to registration information.
Owner:方伟杭
Influenza virus vaccine and method of making
PendingUS20200188507A1Improve scalabilityIncrease HA thermostabilitySsRNA viruses negative-senseHydrolasesVaccine manufacturingHemagglutinin
A composition for increasing vaccine yields due to increased virus growth in mutation comprising a vaccine strain bearing the Y161F mutation in hemagglutinin (HA). Y161F in HA increases HA thermostability without changing its original antigenic properties and enhances its binding affinity in the vaccine production platforms used in influenza vaccine manufacturing. A method for optimizing preparation of influenza vaccine seed strains which can further lower the cost of vaccines and increase profits for the vaccine companies, and also maintain antigenic stability during vaccine deliveries.
Owner:MISSISSIPPI STATE UNIVERSITY
Embryonated egg post hatching device for poultry vaccine production
InactiveCN111657182AConvenient and accurate real-time detectionAvoid damagePoultry incubationVaccine manufacturingAnimal science
The invention relates to the technical field of vaccine production, in particular to an embryonated egg post hatching device for poultry vaccine production. The embryonated egg post hatching device for poultry vaccine production comprises a hatching tray, wherein a hatching assembly is fixedly arranged on the upper surface of the hatching tray; a support post is fixedly arranged on the upper surface of the hatching tray; a top plate is fixedly arranged on the top end of the support post; a rack is arranged on the upper surface of the top plate in a penetrating way; a hatching cover covering the hatching assembly is fixedly arranged at the bottom end of the rack; a first electric motor arranged in a way of corresponding to the rack is fixedly arranged on the lower surface of the top plate;a gear engaged with the rack is fixedly arranged on the tail end of an output shaft of the first motor; and a plurality of uniformly distributed support legs are fixedly arranged on the lower surfaceof the hatching tray. Through an embryonated egg detection module arranged in the hatching device, the detection can be performed without taking embryonated eggs out of the device; and meanwhile, allof the embryonated eggs can be detected by one industrial camera, so that the cost is lower.
Owner:沈涛
Automatic egg shelling method and device
ActiveCN111037102AEasy tap to removeHigh production efficiency in shellingLaser beam welding apparatusVaccine manufacturingEggshell
The invention discloses an automatic egg shelling method and device. The method comprises the following steps of identifying a cavity range of an egg to be shelled; etching an opening track accordingto a range of the cavity; knocking an eggshell part of the cavity to generate a crack consistent with the opening track; and separating the cavity eggshell part. A stress structure of the egg is destroyed through the etched opening track. When the eggshell of the cavity part is knocked, the eggshell of the cavity part can be easily knocked and removed, and the eggshell with a chick embryo part isnot influenced. Compared with a traditional wall breaking and shelling process, by using the automatic egg shelling method and device of the invention, automatic egg shelling production efficiency ishigher, quality stability is better, and quality and labor cost in a vaccine manufacturing process is reduced.
Owner:上海赛摩电气有限公司
Method for preparing human Japanese encephalitis inactivated vaccine and vaccine
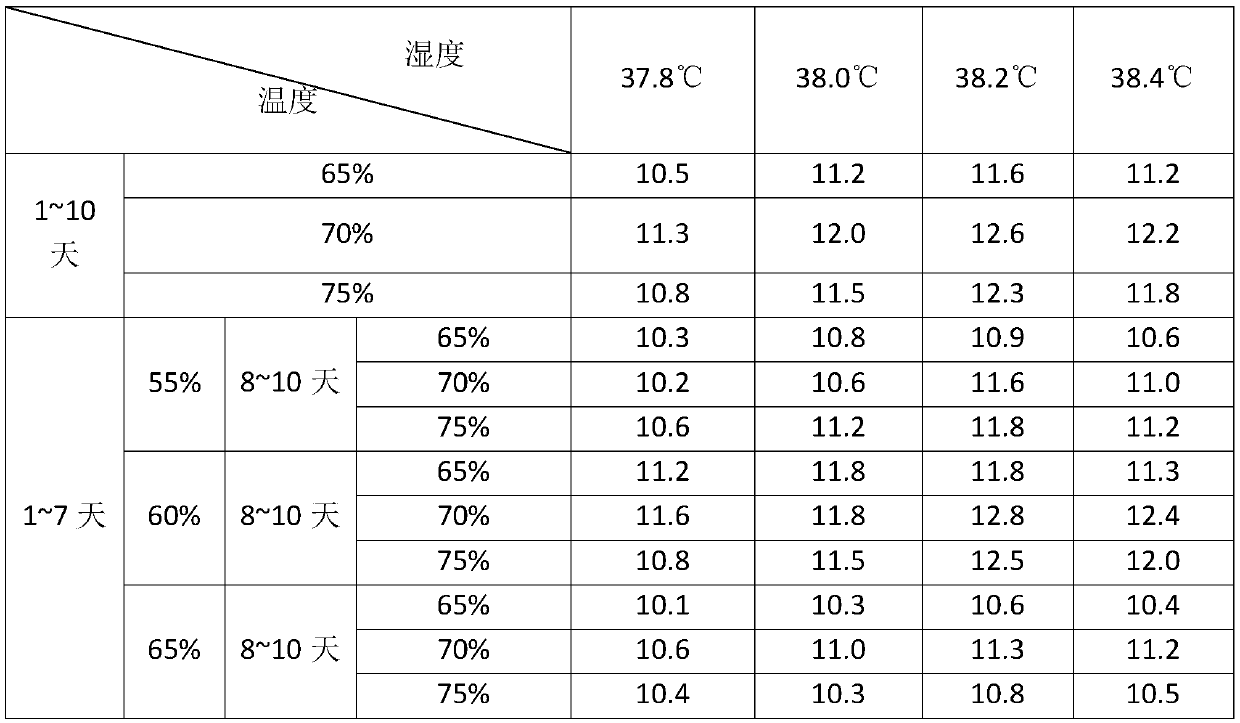
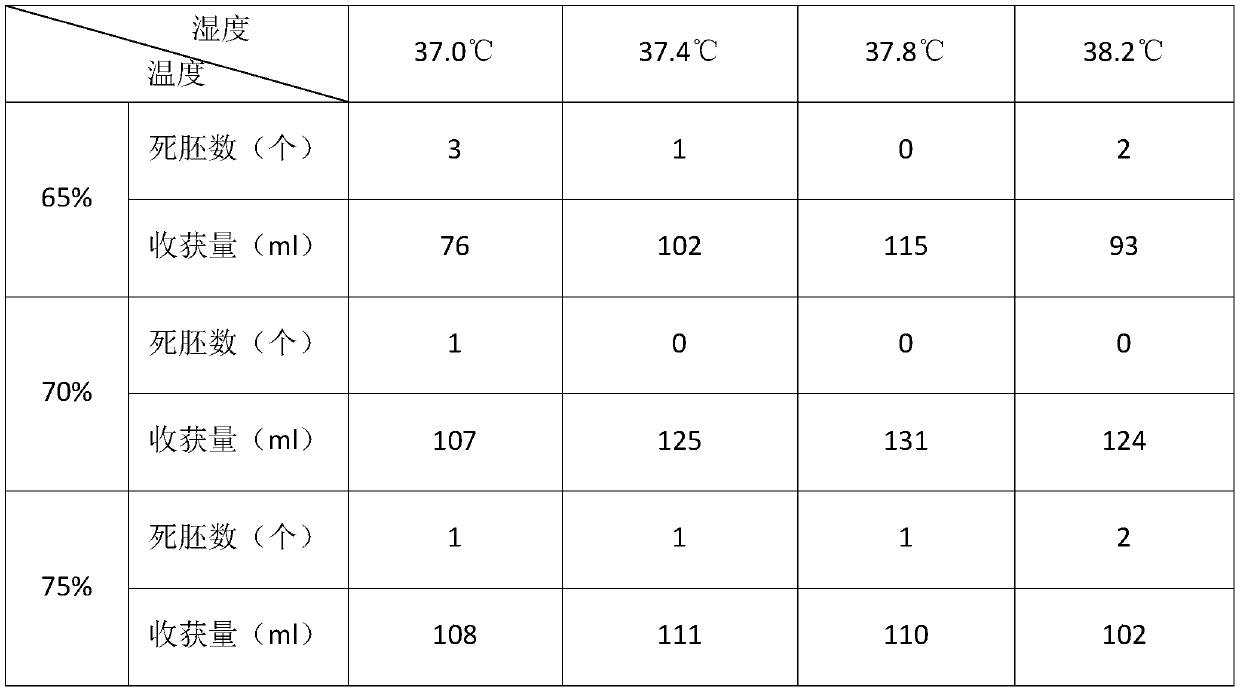
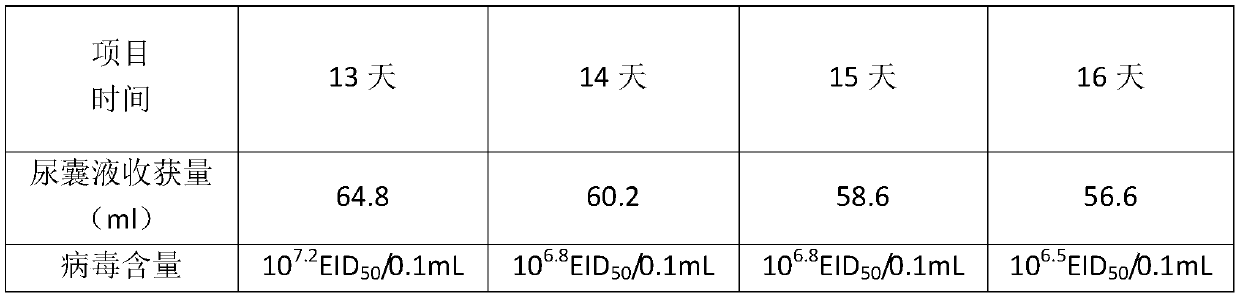
PendingCN114272366ALittle side effectsImprove securityViral antigen ingredientsMicroorganism based processesVaccine manufacturingJapanese encephalitis
The invention discloses a method for preparing a human encephalitis B inactivated vaccine and the vaccine, and belongs to the field of vaccine manufacturing, the method for preparing the human encephalitis B inactivated vaccine sequentially comprises the following steps: S1, serum-free adaptive domestication of Vero cells on a culture flask; s2, performing serum-free multiplication culture on the Vero cells on the bioreactor; s3, inoculating JEV on the Vero cells in the bioreactor; s4, harvesting, inactivating, concentrating and purifying viruses; s5, carrying out freeze-drying treatment on the vaccine stock solution; the Japanese encephalitis inactivated vaccine has the effects of improving the safety of the Japanese encephalitis inactivated vaccine for people and reducing the side effects of the Japanese encephalitis inactivated vaccine for people.
Owner:LIAONING CHENGDA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com