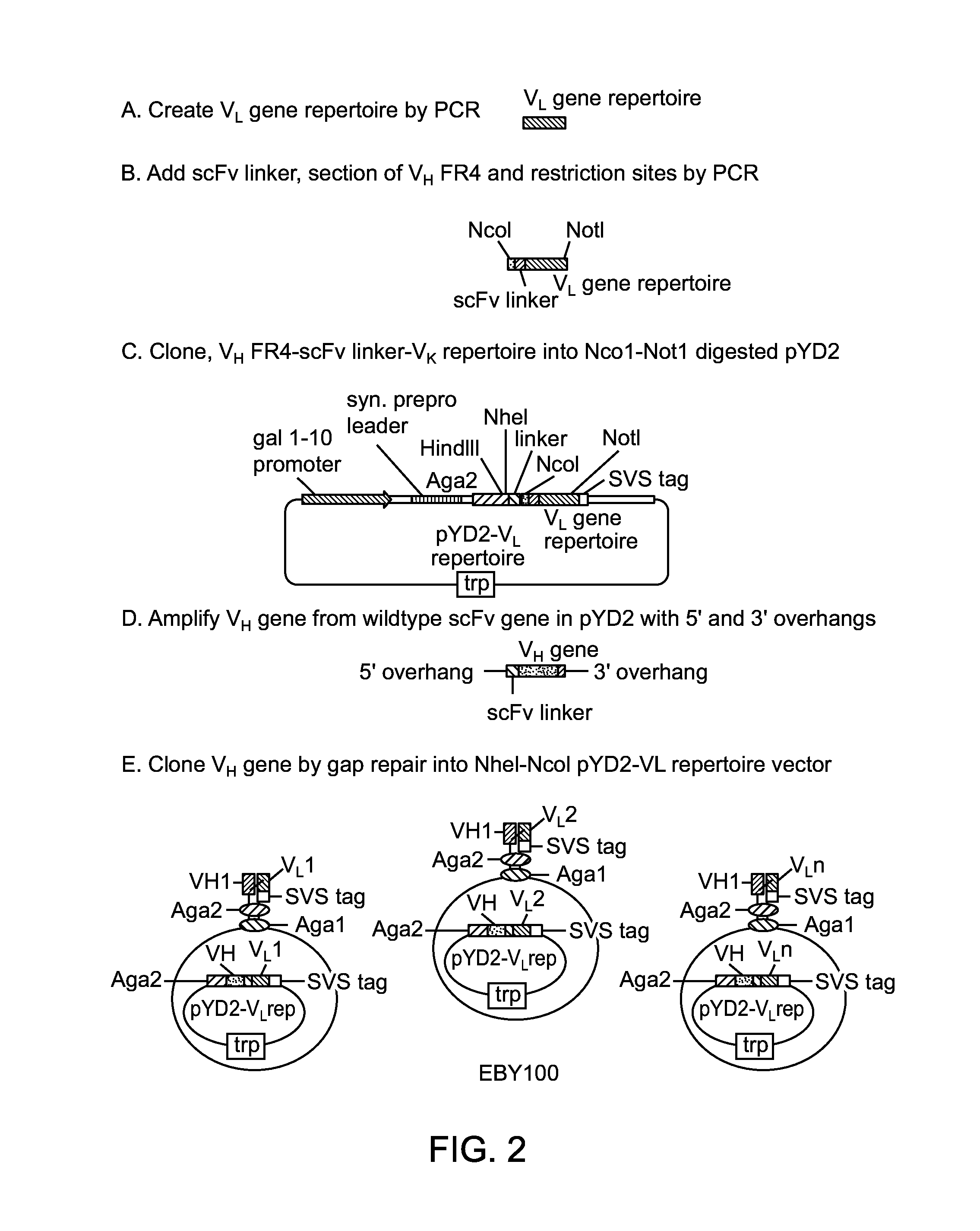
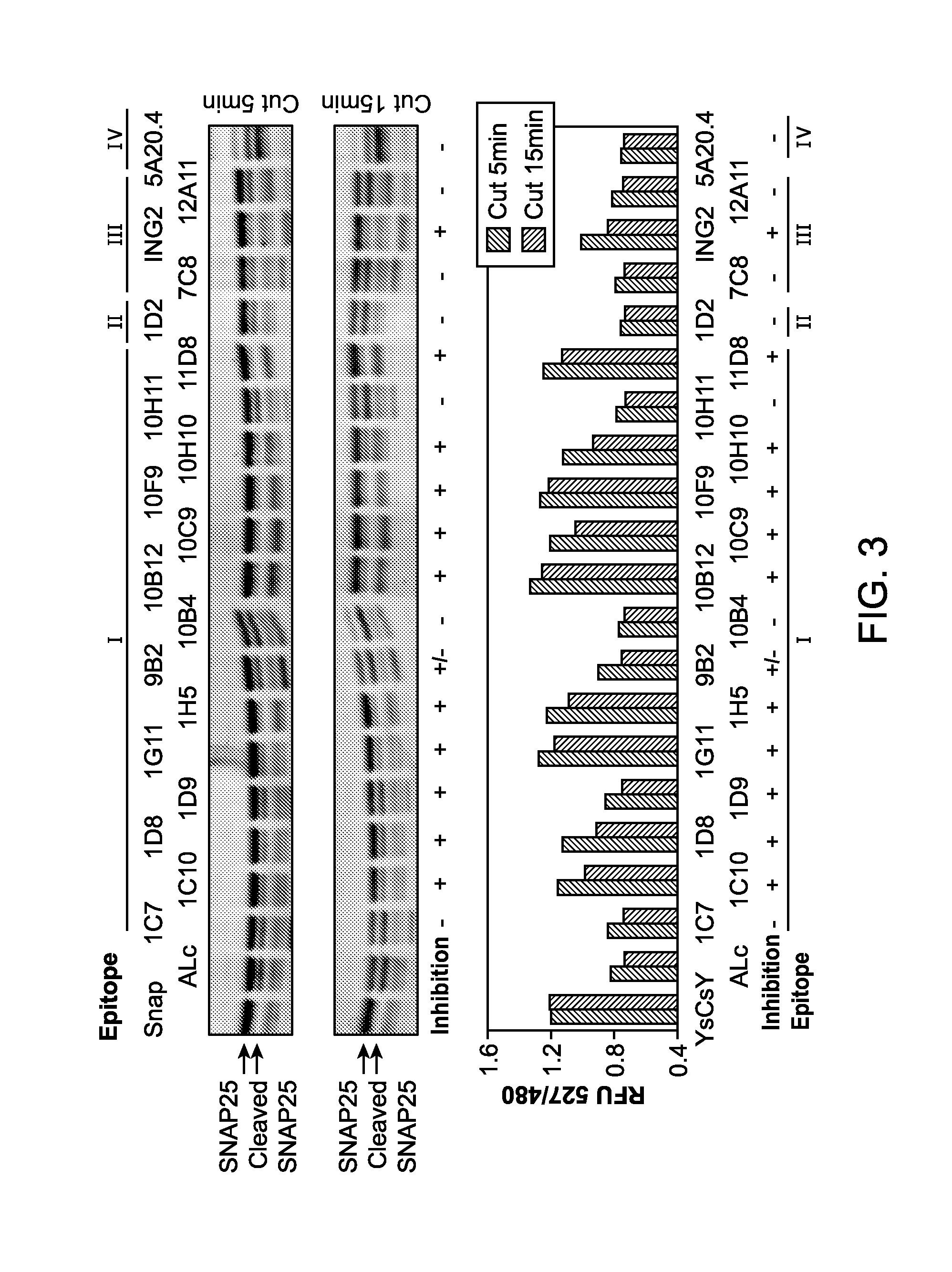
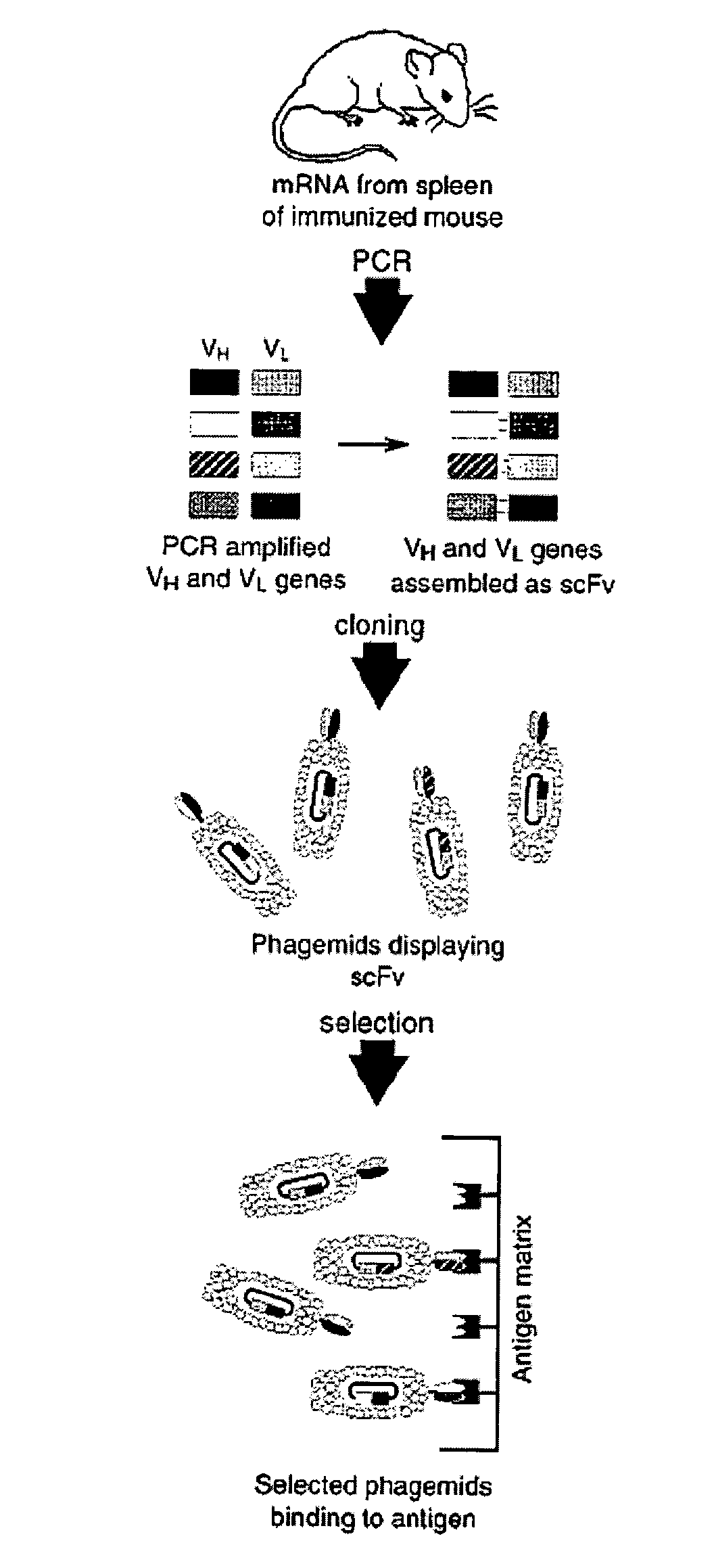
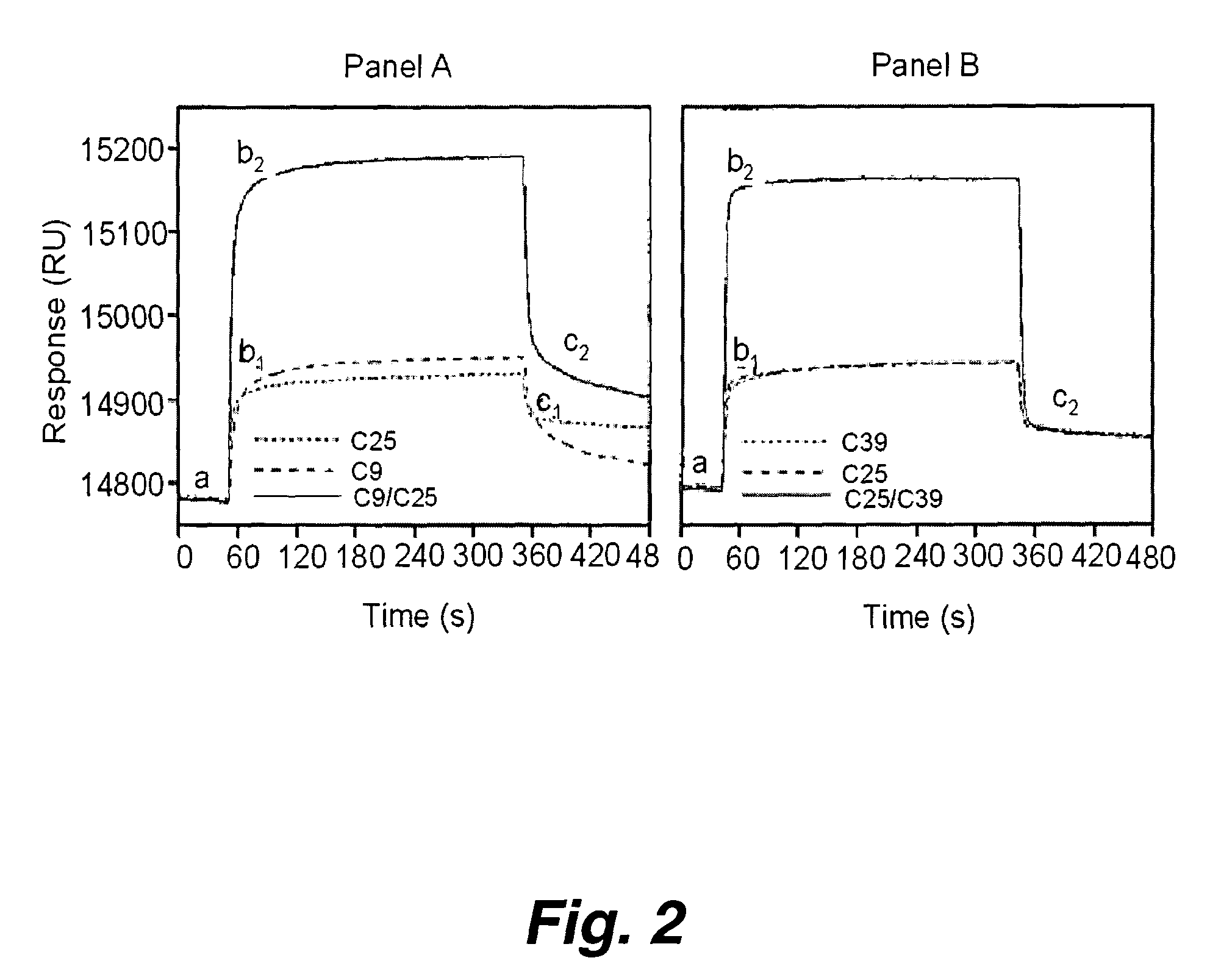
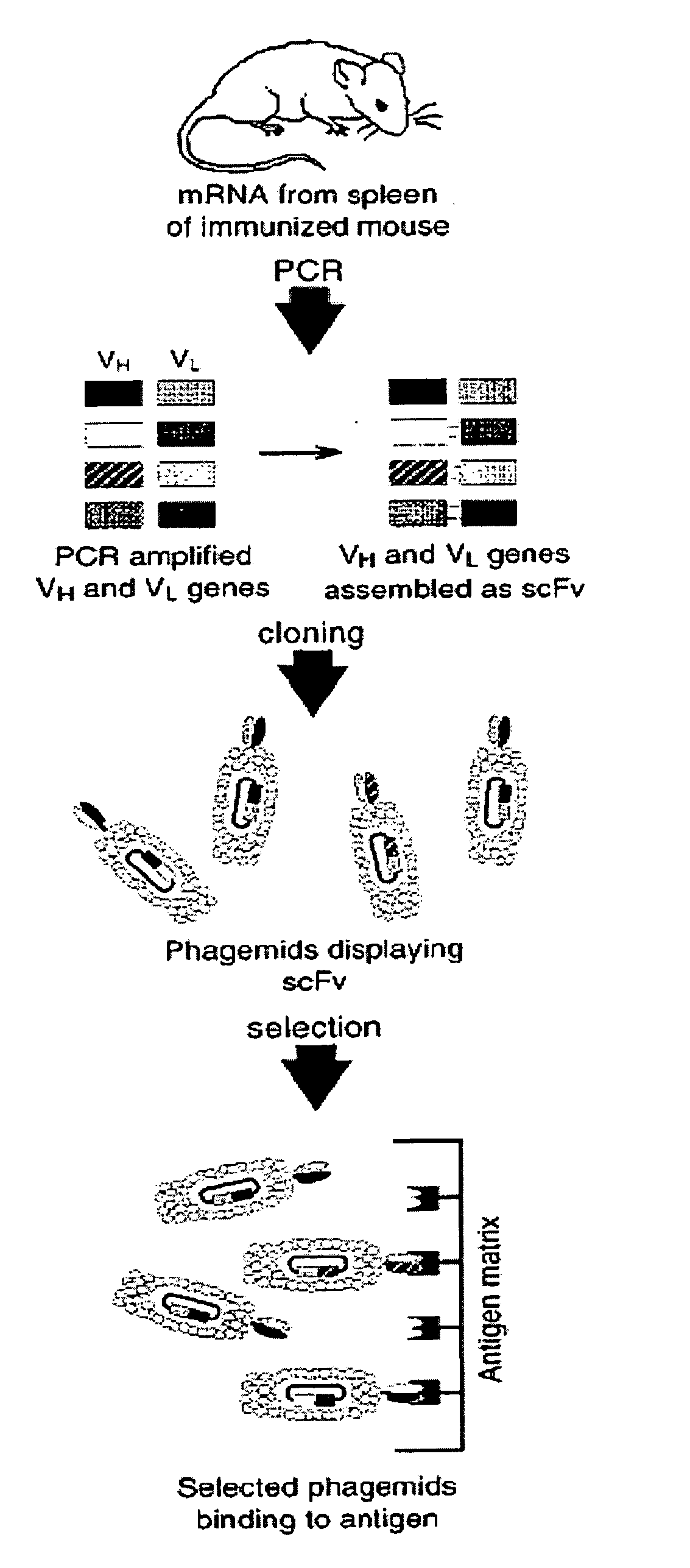
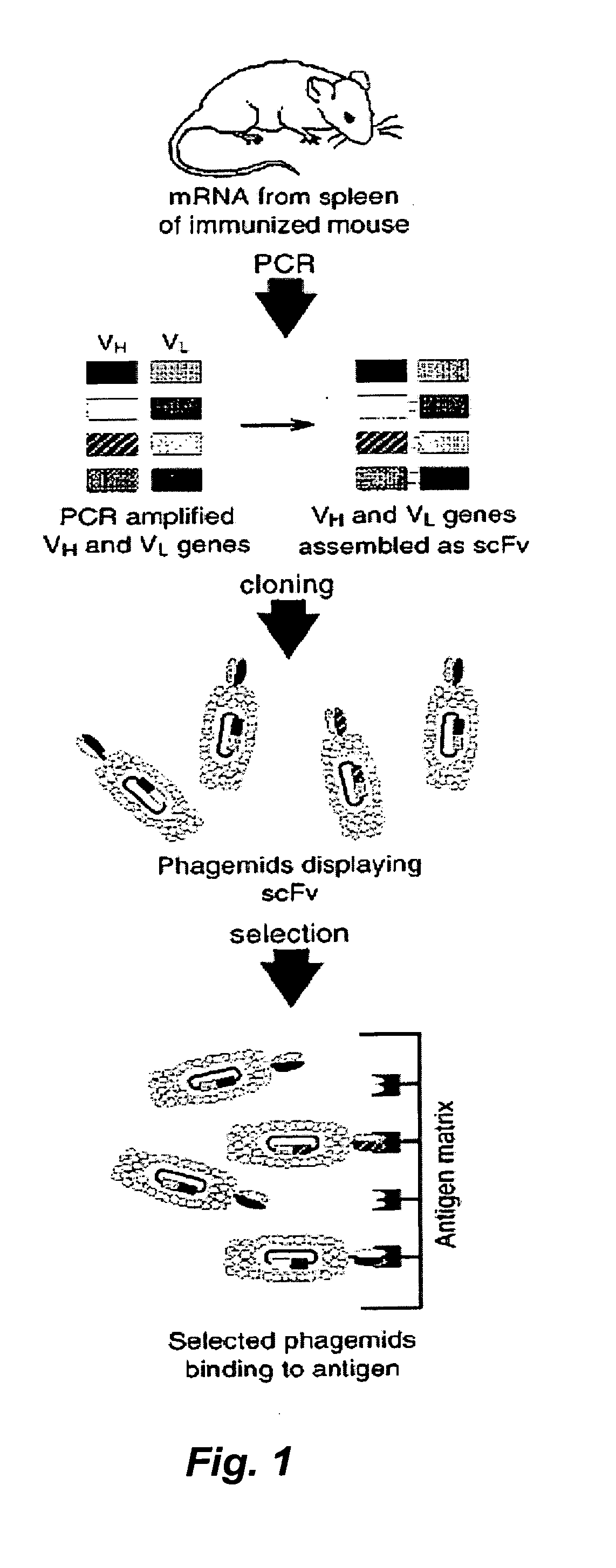
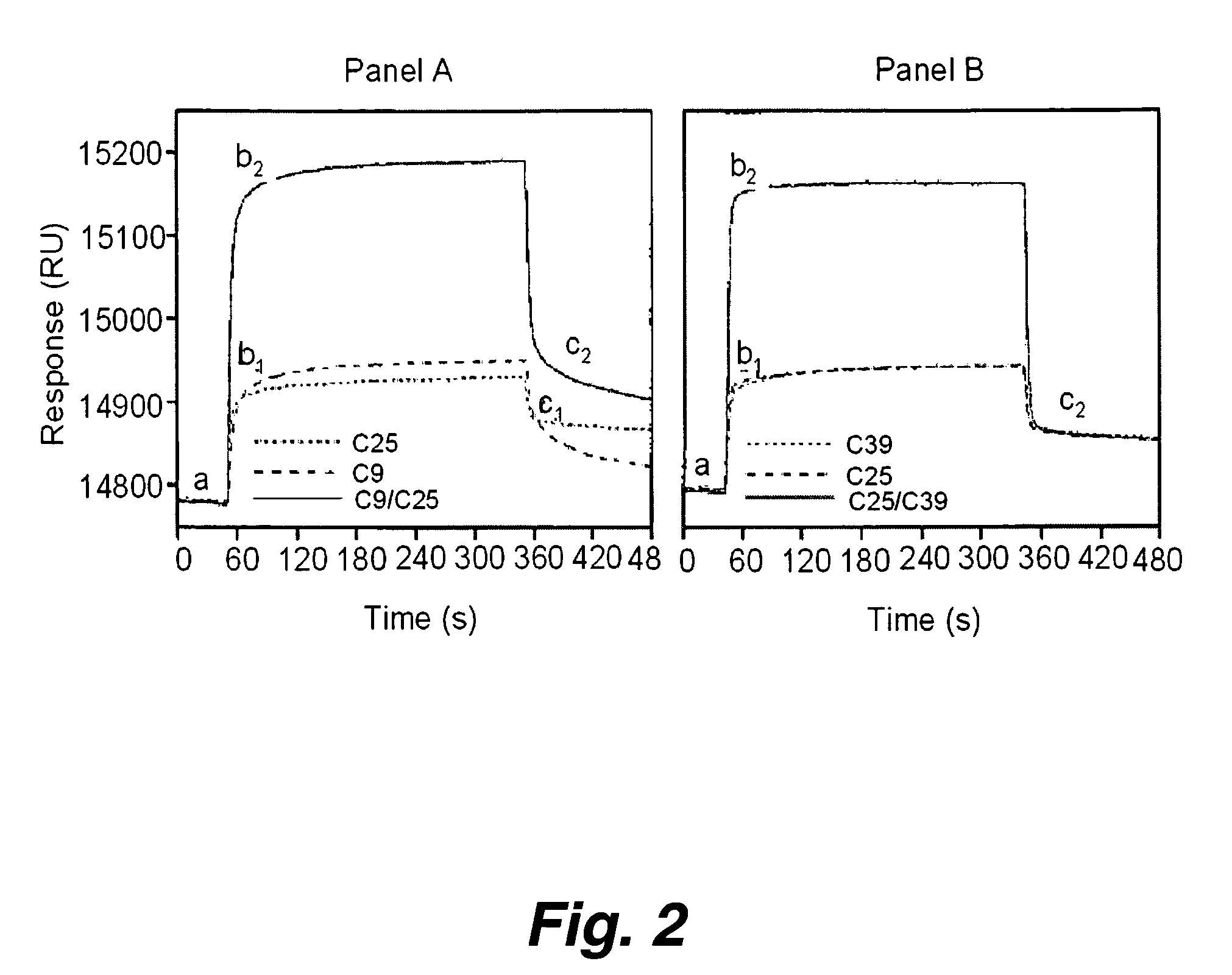
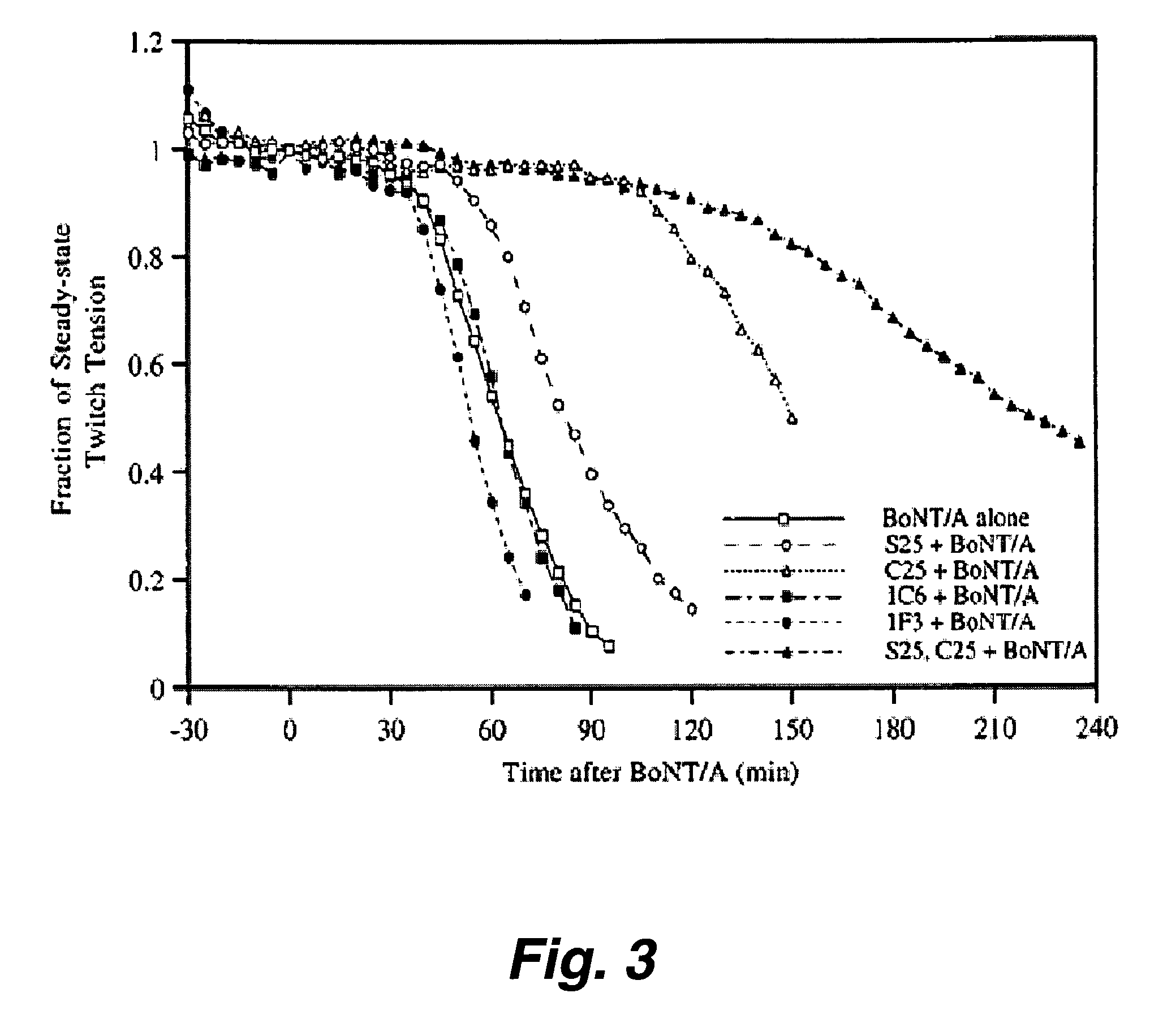
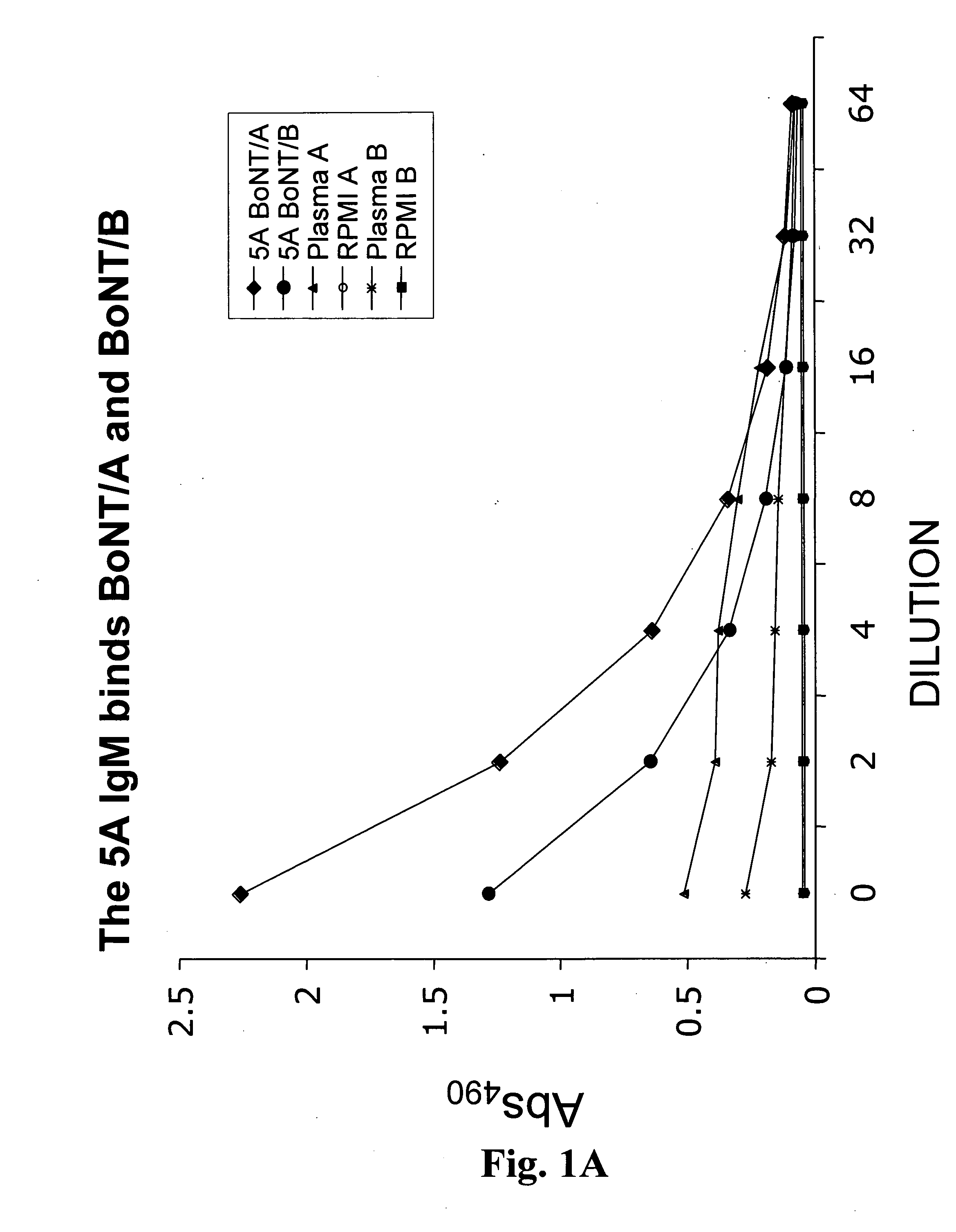
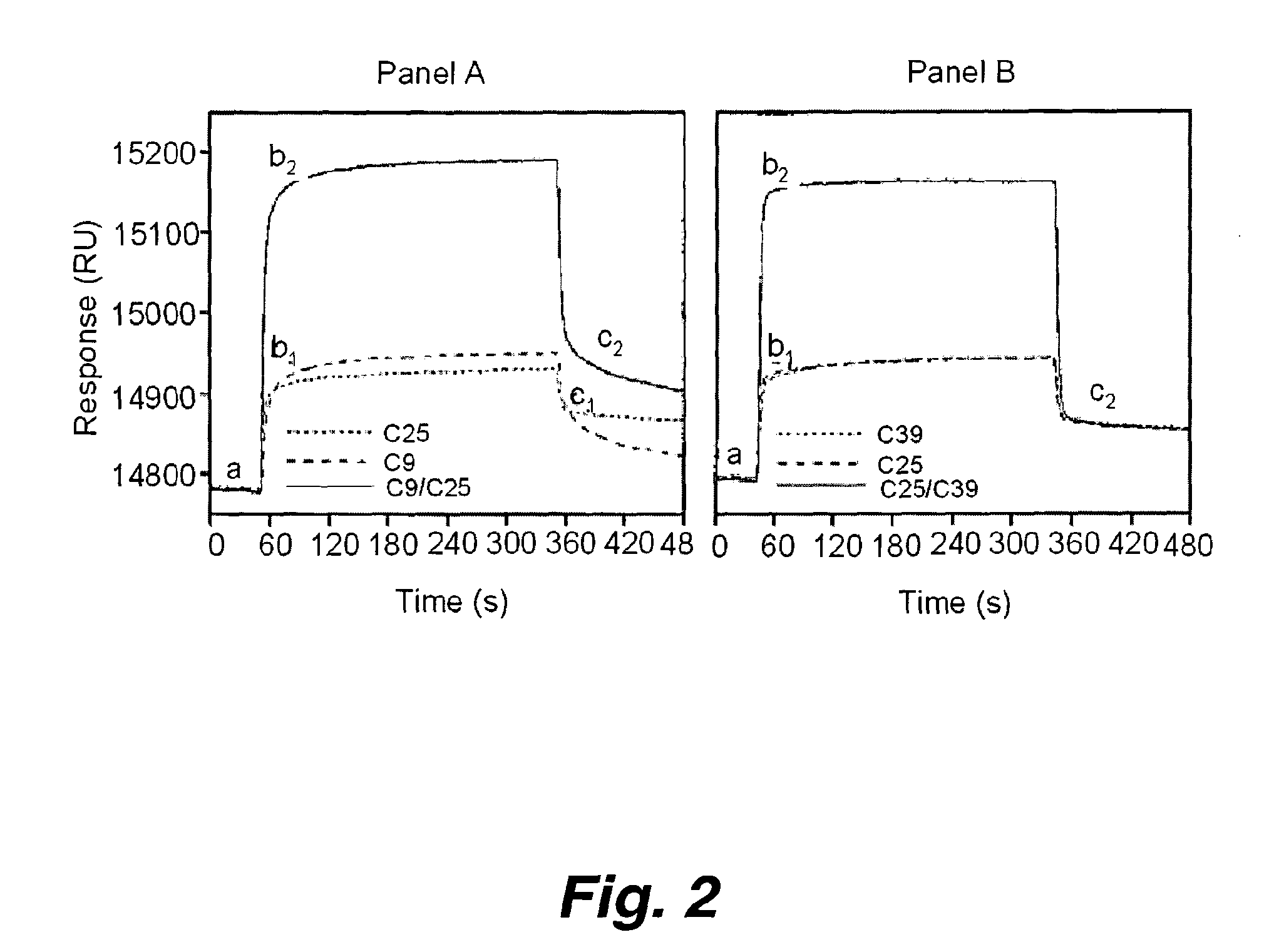
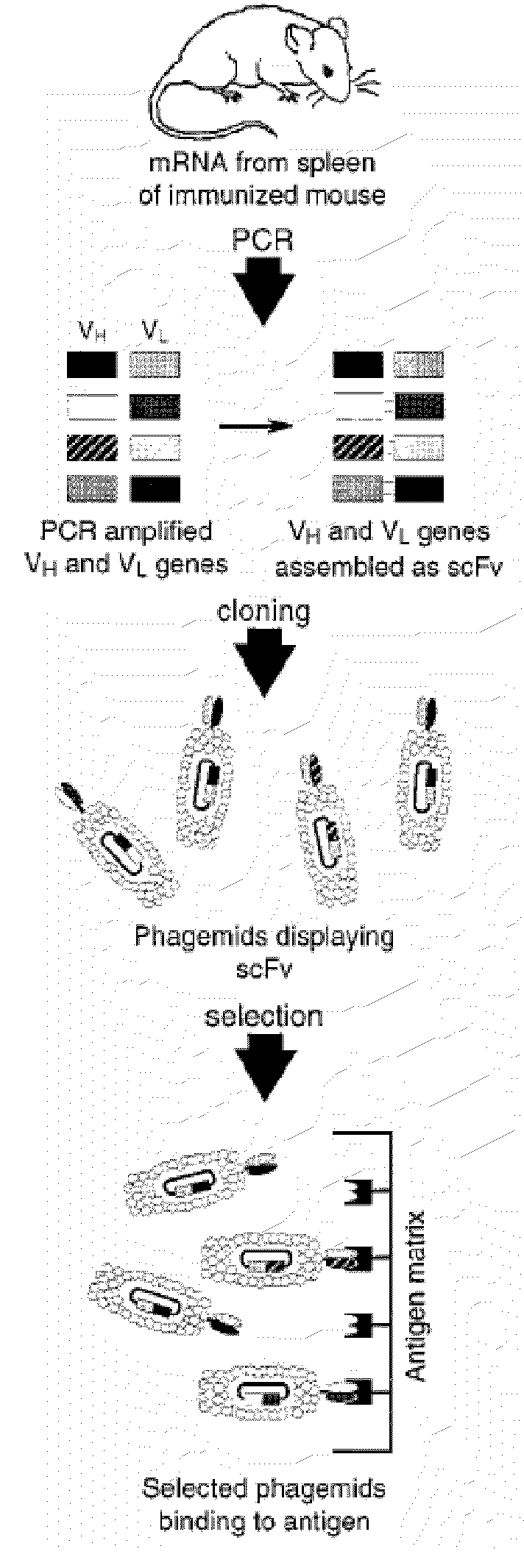
Patents
Literature
35 results about "Botulism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A rare but serious bacterial infection caused by Clostridium botulinum.
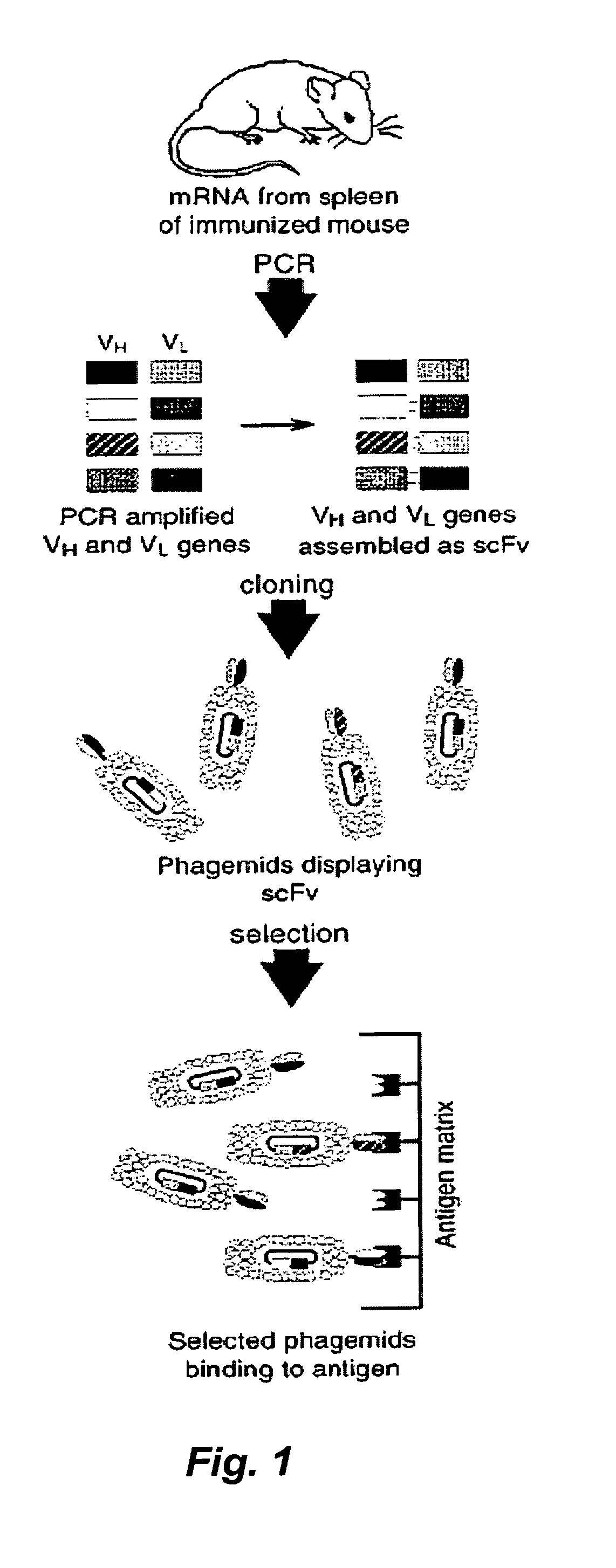
Recombinant vaccine against botulinum neurotoxin
InactiveUS7081529B2Fast and efficient purificationBacterial antigen ingredientsBacteriaVaccinationRecombinant vaccines
This invention is directed to preparation and expression of synthetic genes encoding polypeptides containing protective epitopes of botulinum neurotoxin (BoNT). The invention is also directed to production of immunogenic peptides encoded by the synthetic genes, as weel as recovery and purification of the immunogenic peptides from recombinant organisms. The invention is also directed to methods of vaccination against botulism using the expressed peptides.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
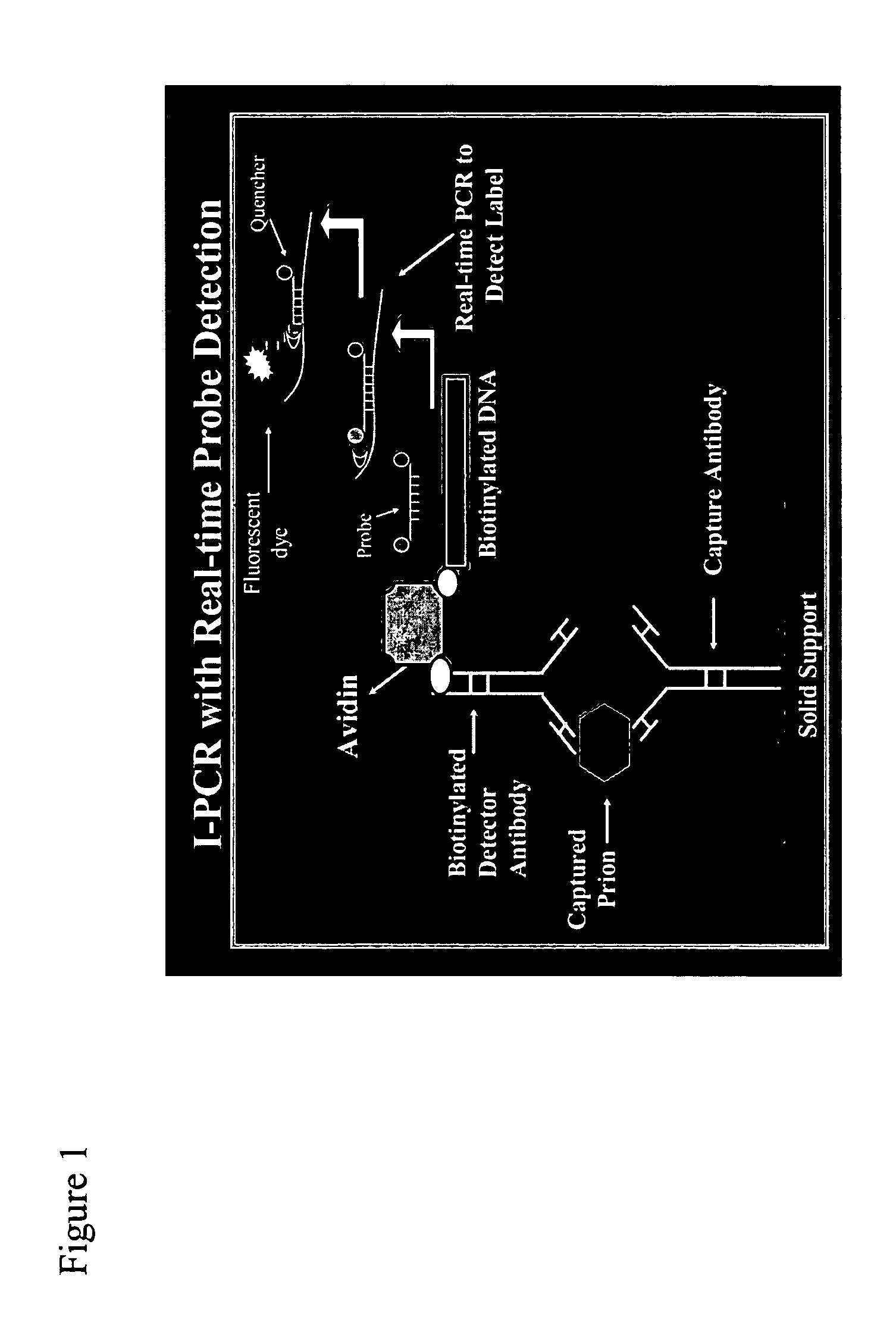
Immuno-PCR method for the detection of a biomolecule in a test sample
InactiveUS20050239108A1Simple and highly sensitiveSimple and highly methodMicrobiological testing/measurementAssay labelsHuman bodyNucleic acid amplification technique
The invention relates to methods and kits for detecting and / or monitoring biological molecules in a test sample. For example, the invention relates to methods and kits for detecting and / or monitoring HIV p24 antigen in human body fluid, biological toxins such as ricin or botulism in an environmental or biological sample, and prion protein from human, deer or bovine, such as PrPSC, in a biological sample. The antigen detection signal is boosted by amplification of a polynucleotide linked to a detector molecule using methods for nucleic acid amplification technology.
Owner:UNIV OF MARLAND BALTIMORE +1
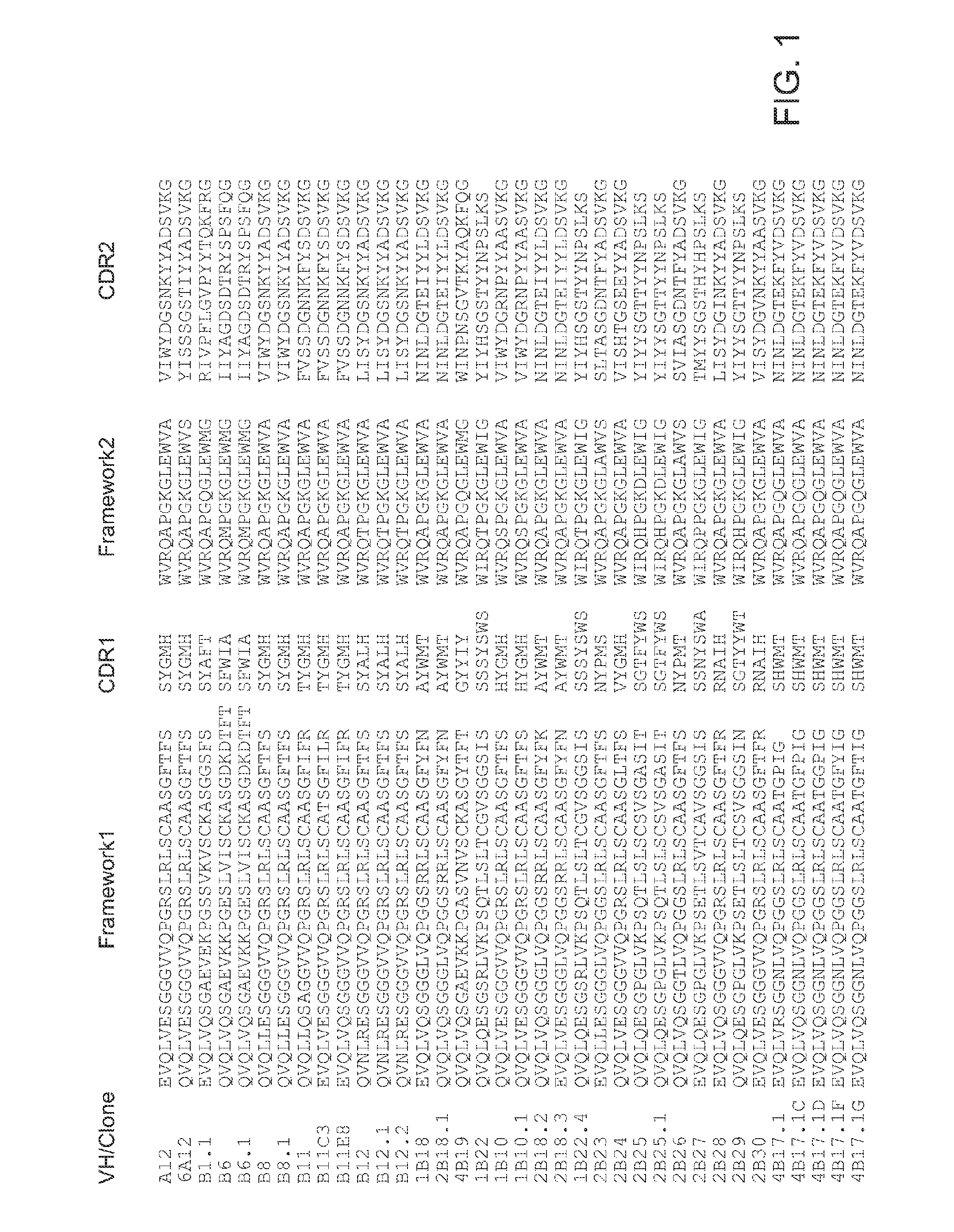
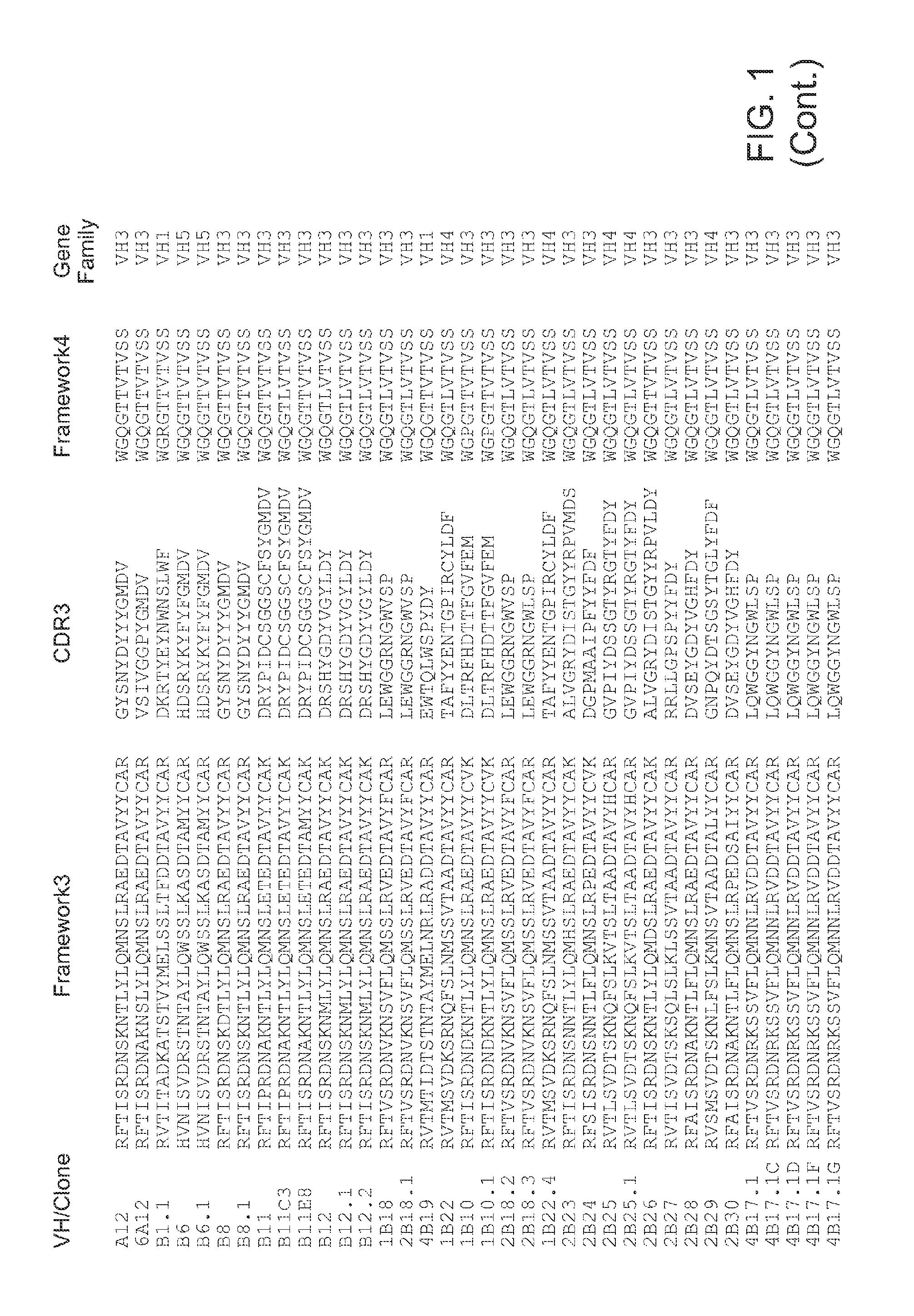
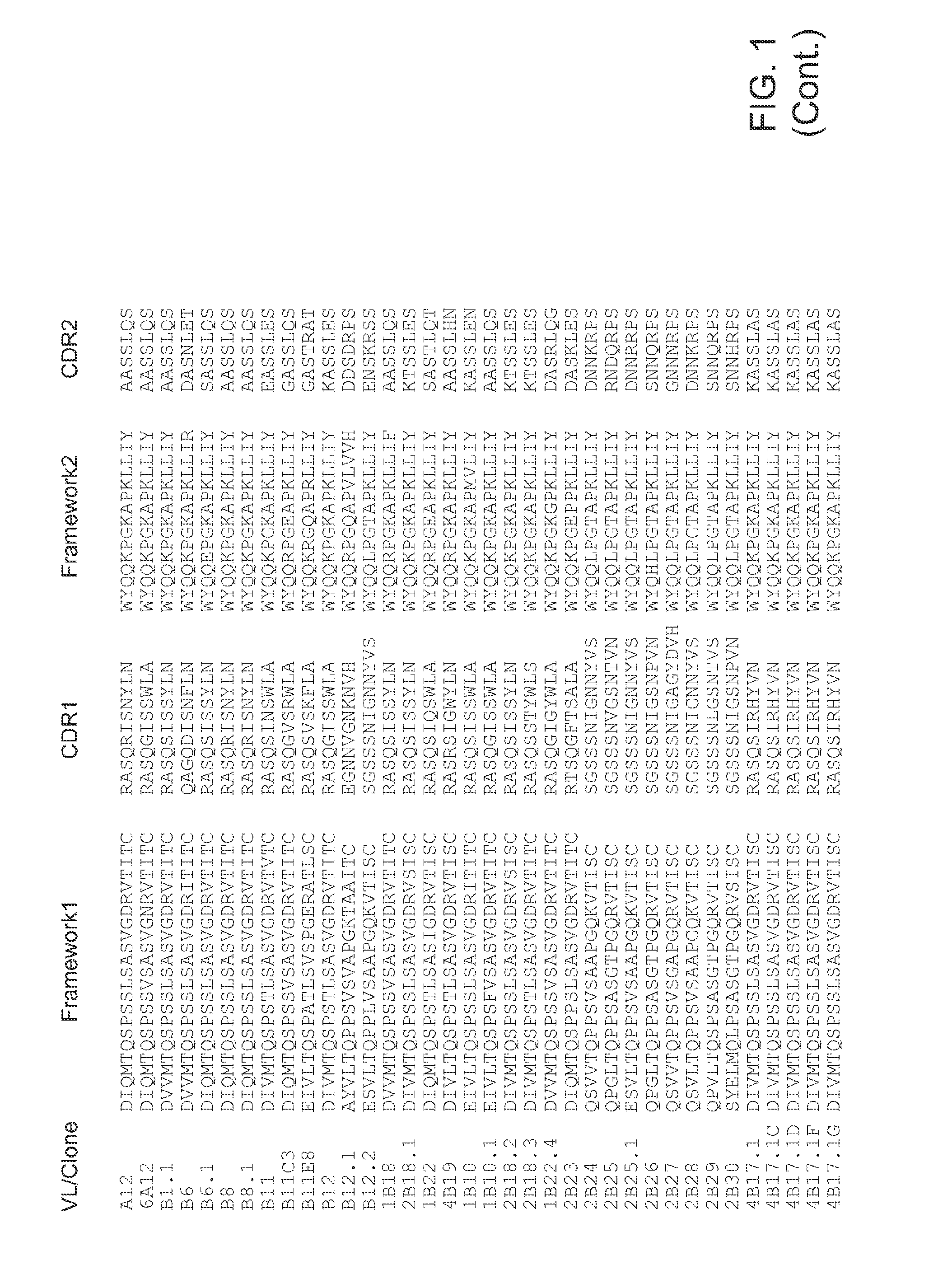
Antibodies for Botulinum Neurotoxins
The present disclosure provides antibodies that specifically bind to botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / C, BoNT / D, BoNT / E, BoNT / F, BoNT / G, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
InactiveUS7563874B2Mitigate and eliminate symptomBiocidePeptide/protein ingredientsMedicineBotulinum Neurotoxin Type A
Owner:RGT UNIV OF CALIFORNIA
Antibodies that Neutralize Botulinum Neurotoxins
This disclosure provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
ActiveUS20100166773A1Low toxicityExtension of timeAntibacterial agentsImmunoglobulins against bacteriaMedicineNeutralizing epitope
This invention provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
ActiveUS7700738B2Low toxicityExtension of timeAntibacterial agentsAntinoxious agentsAntiendomysial antibodiesBotulinum Neurotoxin Type A
This invention provides antibodies that specifically bind to and neutralize botulinum neurotoxin type A (BoNT / A) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Monoclonal antibodies that neutralize botulinum neurotoxin
InactiveUS20100222555A1Reduce development riskSugar derivativesImmunoglobulins against bacteriaMedicineBotulinum Neurotoxin Type B
This invention provides antibodies that specifically bind to botulinum neurotoxin type A (BoNT / A) and / or botulinum neurotoxin type B (BoNT / B) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism. Also included in the invention are diagnostic and therapeutic assays directed to botulinum neurotoxins.
Owner:THOMAS JEFFERSON UNIV
Methods and systems for multi-antibody therapies
ActiveUS20100278830A1Simple processImprove effectivenessAntibacterial agentsAntimycoticsCancer cellToxin
The present invention relates to methods and systems for administering antibody therapeutic agents. The methods include administering one or more (e.g., two or three) binding agents, wherein each of the binding agents has a binding region that is specific to a portion of a disease agent and one or more copies of a tag. The binding agents can be specific to one or more portions of the same or different disease agents. The tag is the same for each of the binding agents. The methods include administering an anti-tag antibody, wherein the anti-tag antibody has an anti-tag region that is specific to the tag, and can have an immunoglobulin (e.g., IgA, IgD, IgE, IgG, and IgM.). Disease agents include bacterial proteins, viral proteins, cancer cells, and proteins or toxins produced therefrom. In particular, the present invention includes methods and systems for binding agents that are specific to neurotoxins that cause botulism.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Anti-Botulism Antibody Coformulations
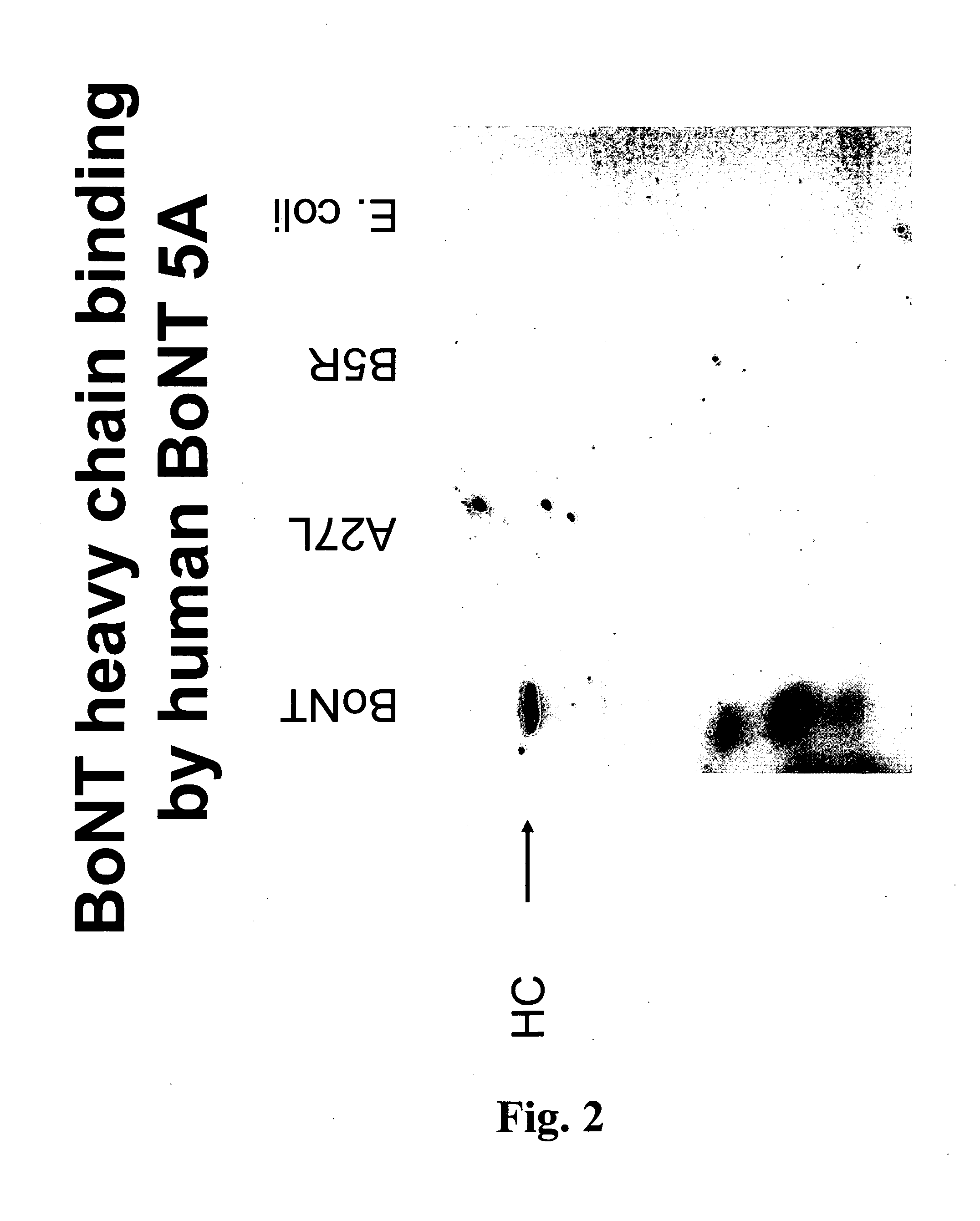
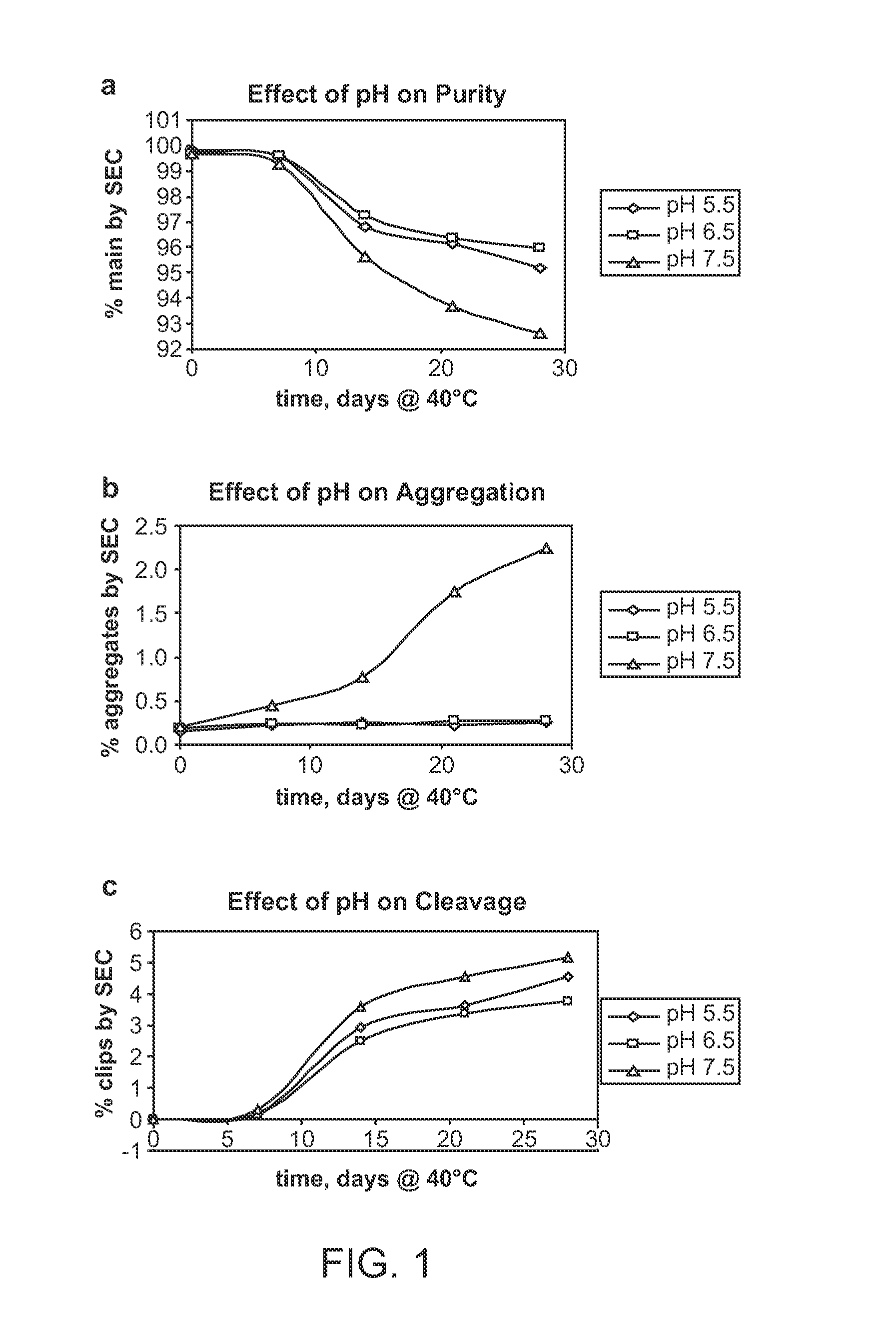
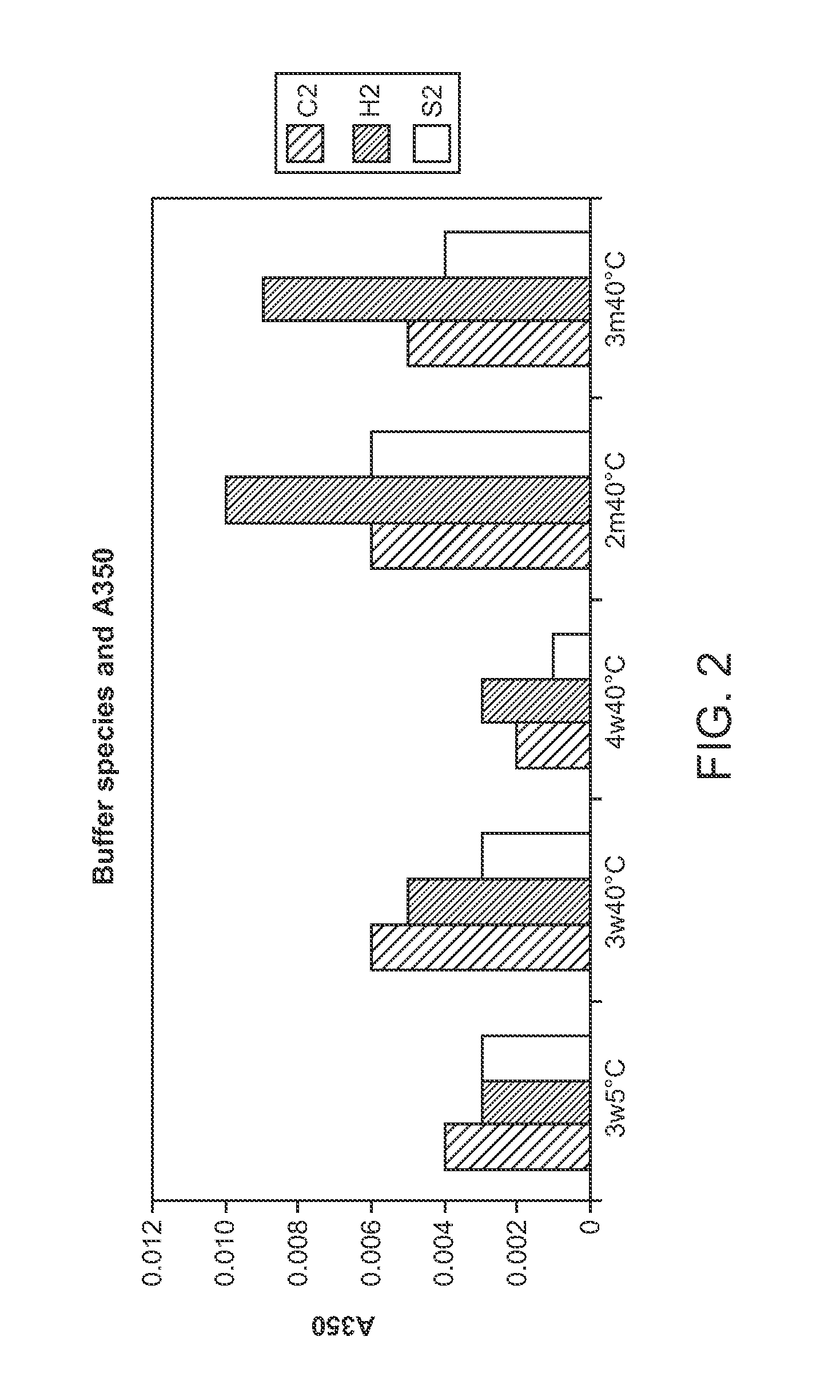
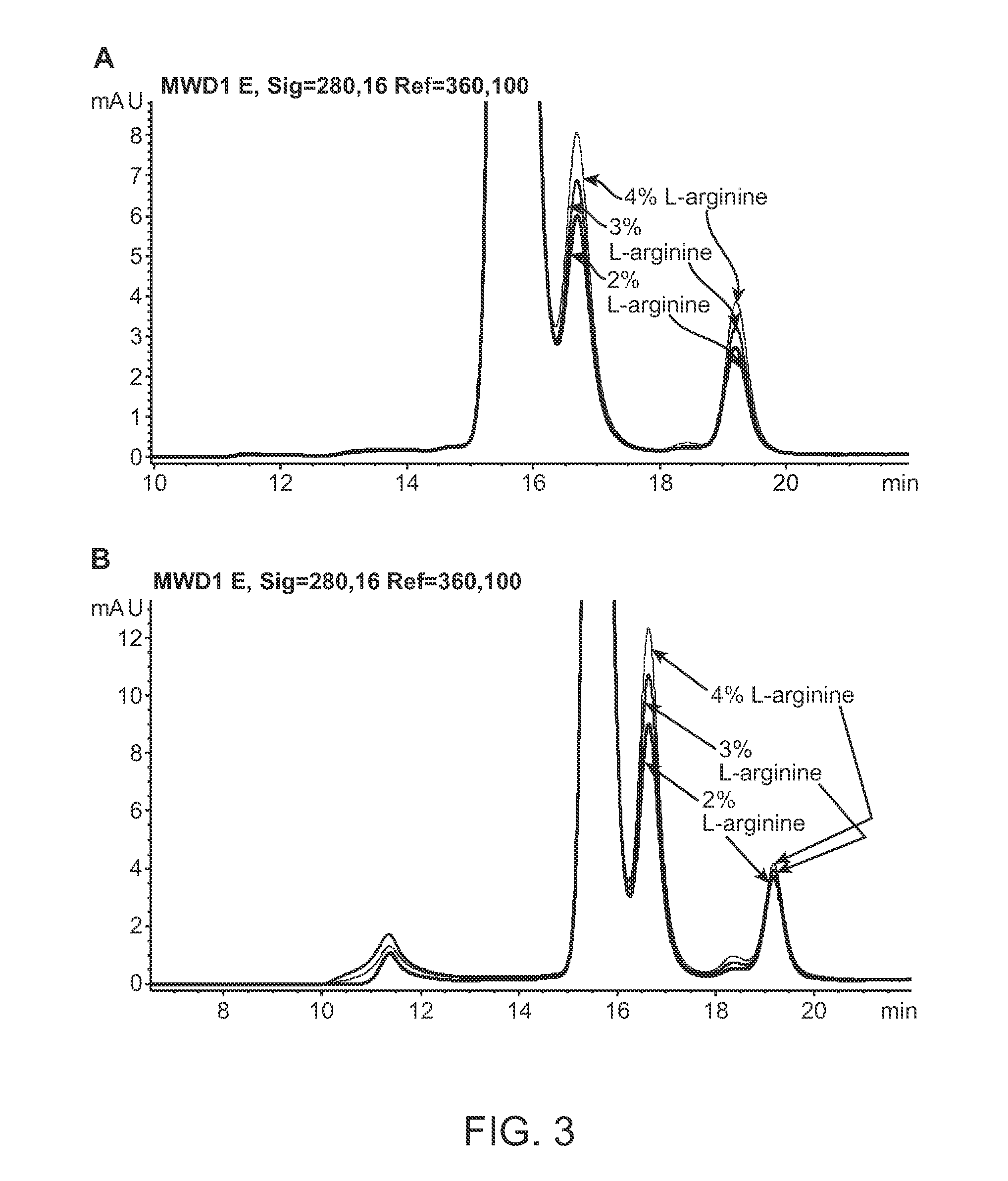
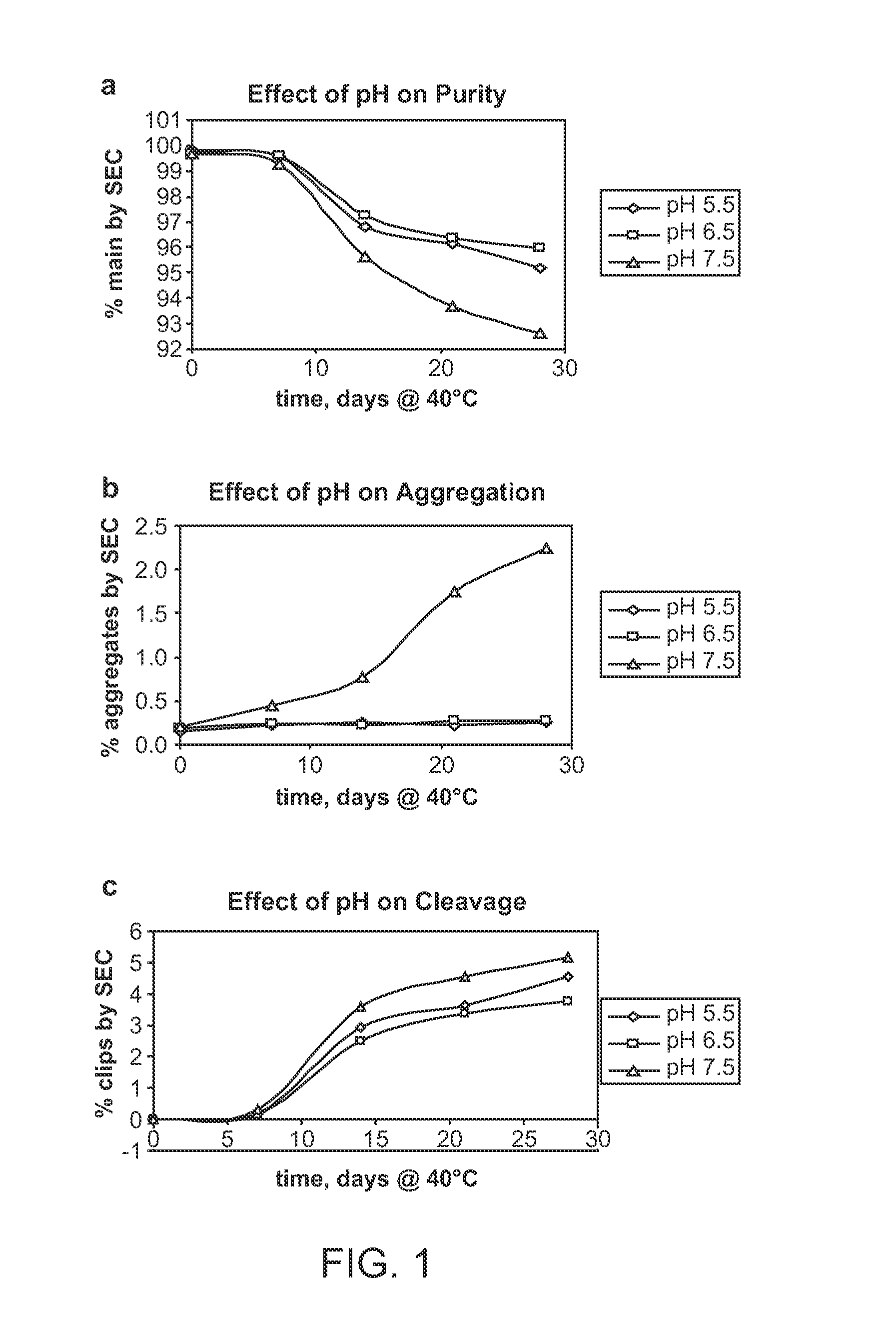
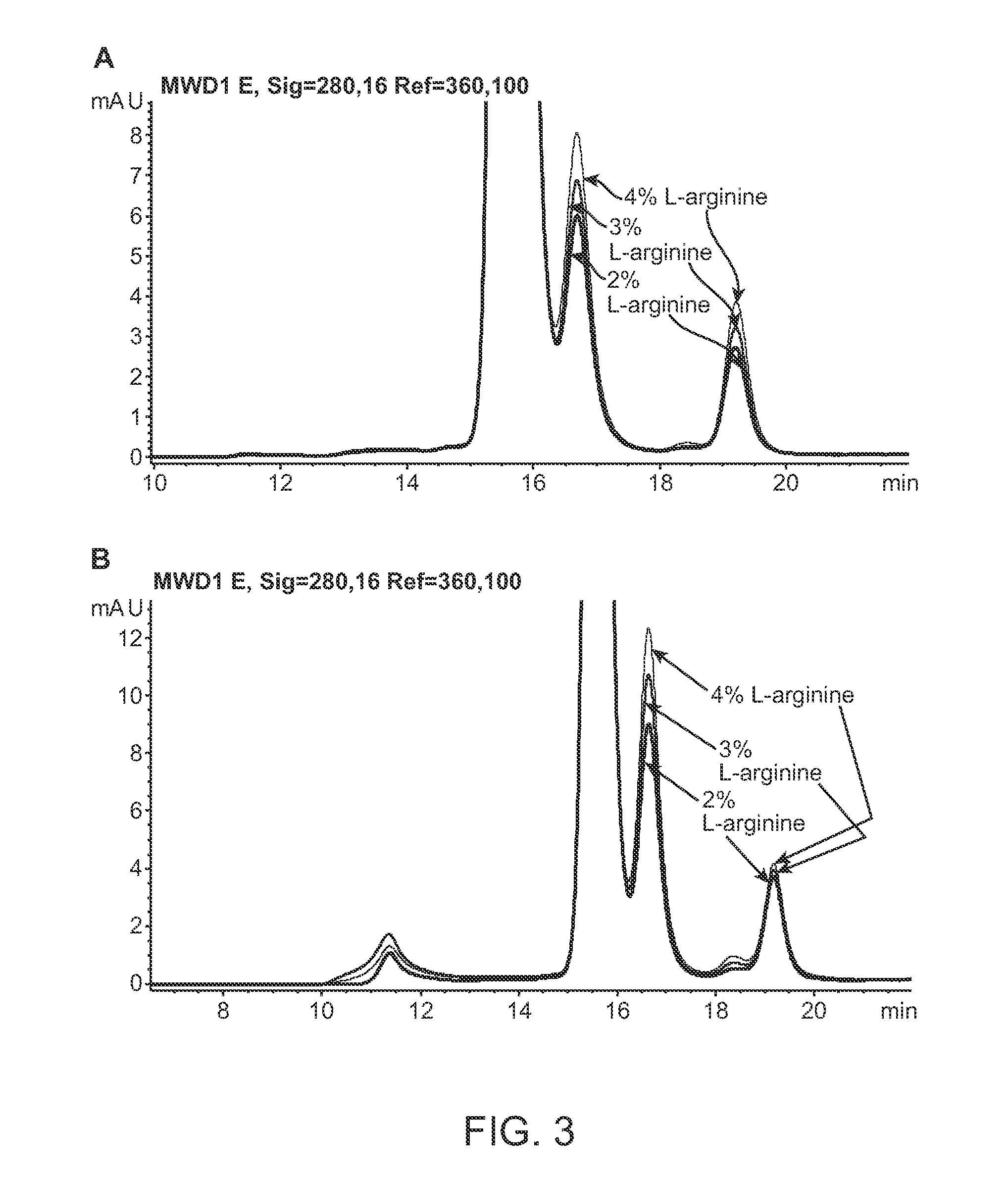
This invention relates to stable formulations of multiple antibodies comprising a plurality of anti-botulism antibodies and an effective amount of a succinate buffer, an effective amount of arginine, wherein the antibodies are present in substantially equal concentrations and the pH of the formulation is between about 5 and about 6.5.
Owner:XOMA TECH LTD
Antibodies that Neutralize Botulinum Neurotoxins
This disclosure provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Antibodies for botulinum neurotoxins
The present disclosure provides antibodies that specifically bind to botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / C, BoNT / D, BoNT / E, BoNT / F, BoNT / G, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
InactiveUS20090324606A1Mitigate and eliminate symptomImmunoglobulins against bacteriaAntibody ingredientsEpitopeMedicine
This invention provides antibodies that specifically bind to and neutralize botulinum neurotoxin type A (BoNT / A) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
InactiveUS20090123481A1Mitigate and eliminate symptomImmunoglobulins against animals/humansImmunoglobulins against bacteriaEpitopeMedicine
This invention provides antibodies that specifically bind to and neutralize botulinum neurotoxin type A (BoNT / A) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
This invention provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Anti-botulism antibody coformulations
This invention relates to stable formulations of multiple antibodies comprising a plurality of anti-botulism antibodies and an effective amount of a succinate buffer, an effective amount of arginine, wherein the antibodies are present in substantially equal concentrations and the pH of the formulation is between about 5 and about 6.5.
Owner:XOMA TECH LTD
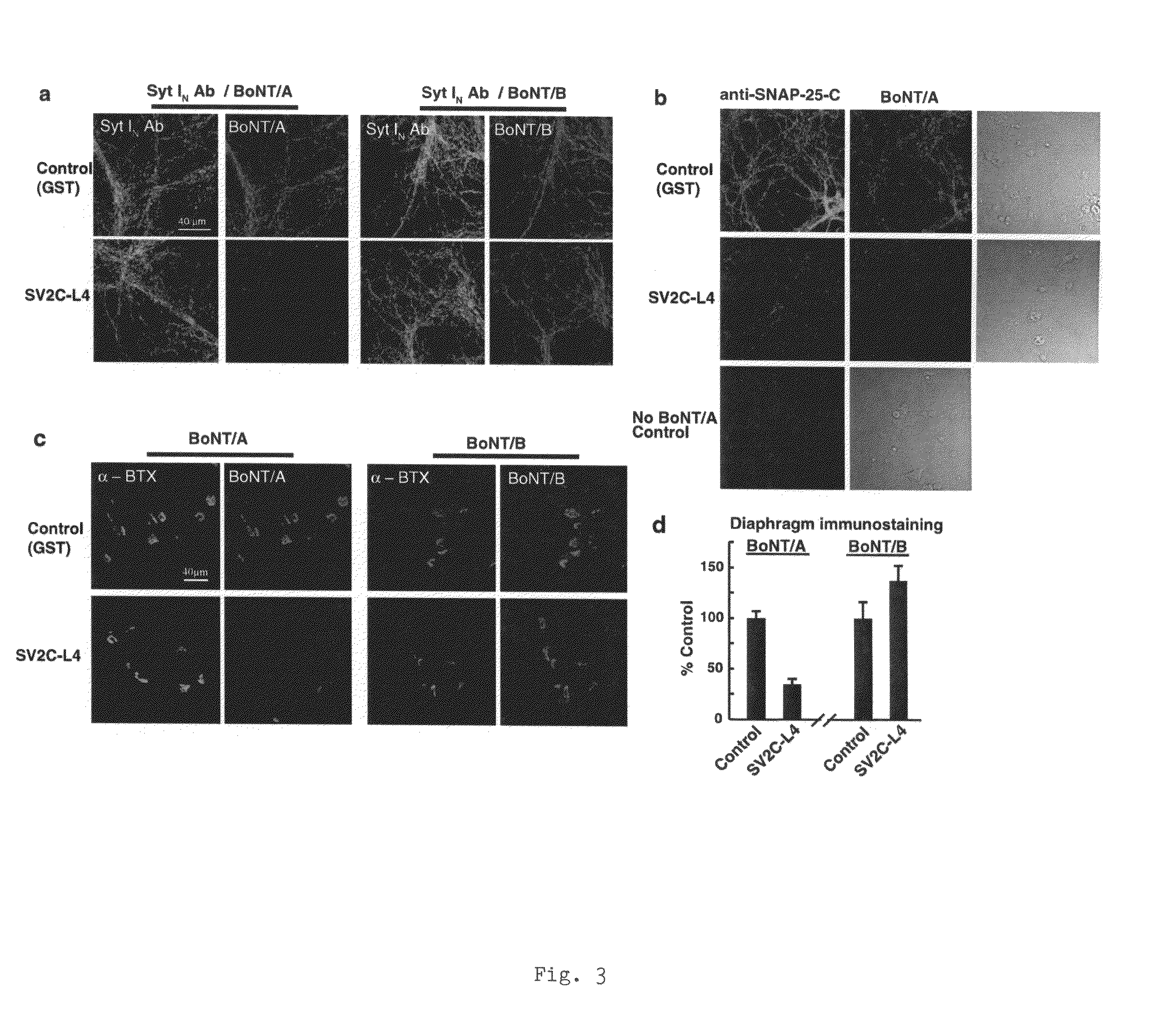
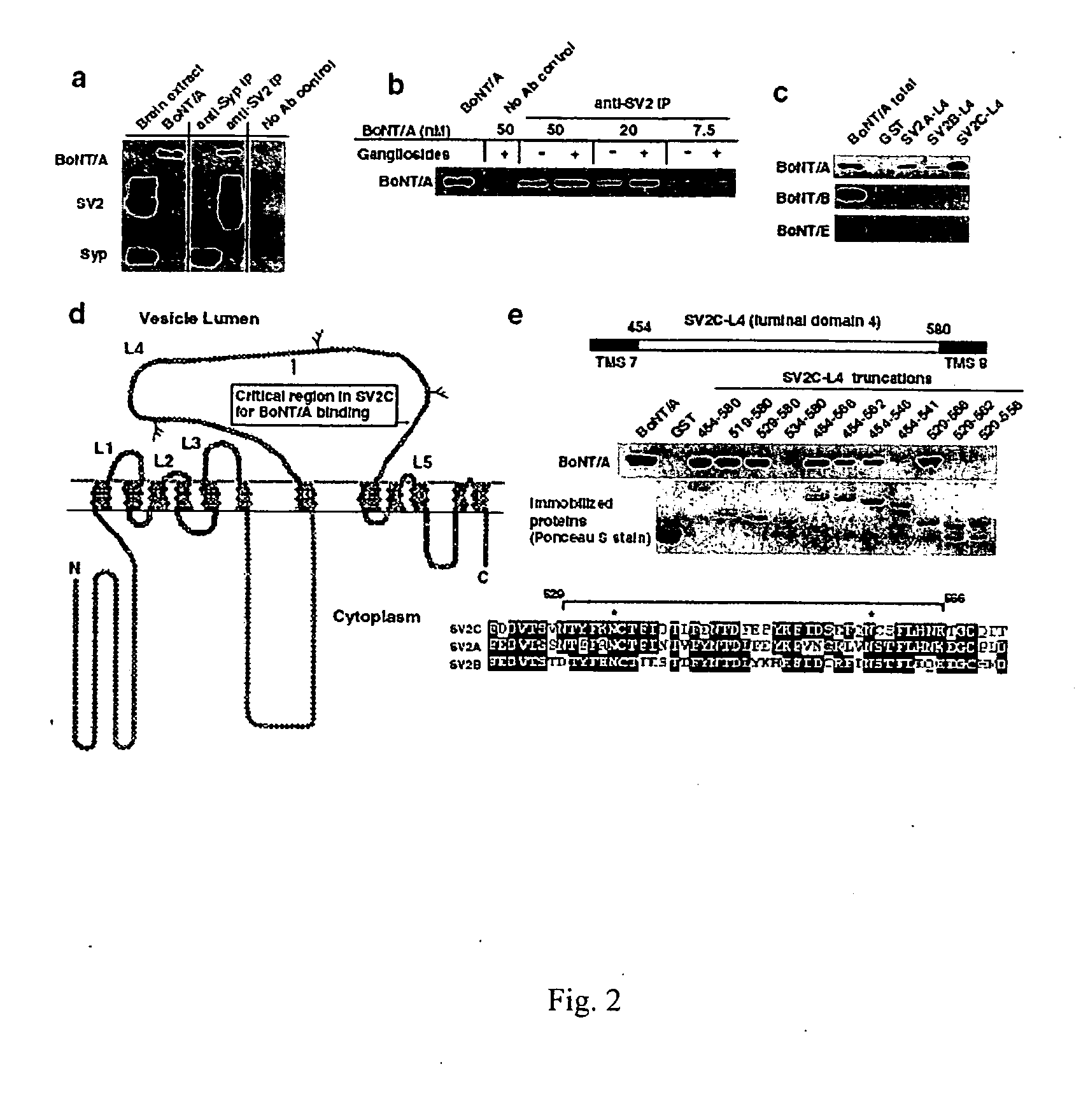
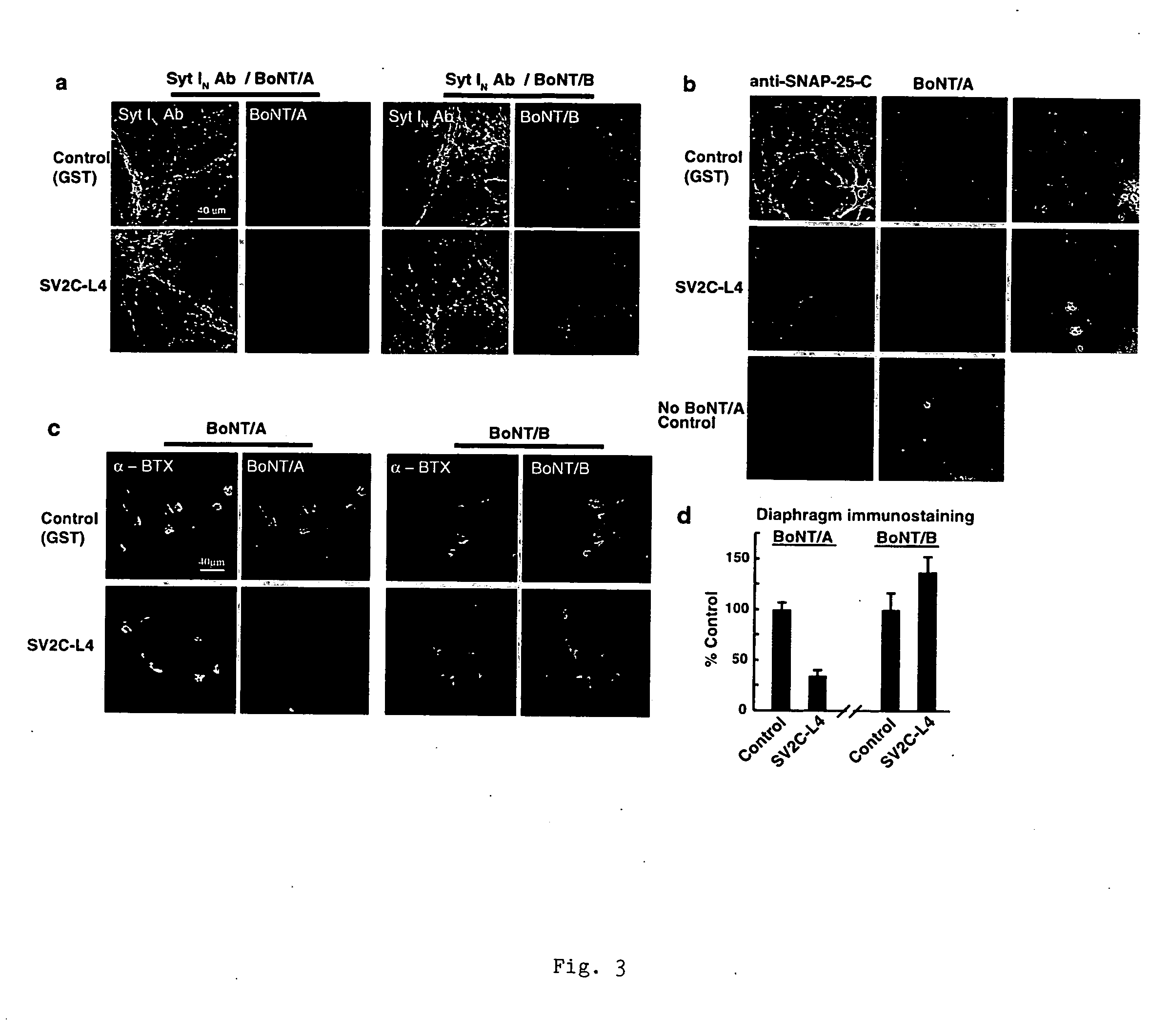
Botulinum neurotoxin A receptor and the use thereof
The present invention is based on the identification of synaptic vessel glycoprotein SV2 as the BoNT / A receptor and the further identification of various BoNT / A-binding fragments of SV2. The disclosure here provides new tools for diagnosing and treating botulism.
Owner:WISCONSIN ALUMNI RES FOUND
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
ActiveUS20110171235A1Low toxicityExtension of timeAntibacterial agentsImmunoglobulins against bacteriaAntiendomysial antibodiesBotulinum Neurotoxin Type A
This invention provides antibodies that specifically bind to and neutralize botulinum neurotoxin type A (BoNT / A) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Detoxified recombinant botulinum neurotoxin
ActiveUS8586081B2Development is reduced and preventedSymptom is reduced and preventedBacterial antigen ingredientsPeptide/protein ingredientsEscherichia coliAntidote
The present invention relates to the isolation of polypeptides derived from the Clostridium botulinum neurotoxin and the use thereof as immunogens for the production of vaccines and antitoxins, as well as research and drug development applications. Clostridium botulinum is responsible for food bone botulism, a severe and often deadly disease. Botulinum neurotoxin binds to neural cells and are translocated into the cytosol. The toxin then prevents neurotransmitter release by cleaving a SNARE protein. A double mutant E224A / E262 full length botulinum neurotoxin Type A holo form was successfully cloned and purified, which lacks the endopeptidase activity involved in the toxic action of the BoNT, and thus leading to its detoxification (DR BoNT / A). This new molecule can be used as an antidote against botulism, and also as a vaccine candidate for botulism. Due to the poor availability and extreme toxicity of native holo-toxin, a nontoxic form of the holo-toxin is highly desired for research and vaccine development. The full length DR BoNT / A protein has been expressed in E. coli as a soluble form.
Owner:SINGH BAL RAM
Recombinant vaccine against botulinum neurotoxin
InactiveUS20070037257A1Fast and efficient purificationBacterial antigen ingredientsSugar derivativesVaccinationRecombinant vaccines
This invention is directed to preparation and expression of synthetic genes encoding polypeptides containing protective epitopes of botulinum neurotoxin (BoNT). The invention is also directed to production of immunogenic peptides encoded by the synthetic genes, as well as recovery and purification of the immunogenic peptides from recombinant organisms. The invention is also directed to methods of vaccination against botulism using the expressed peptides.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Composition for neutralizing botulinus toxin type-a, and human Anti-botulinus toxin type-a antibody
Provided herein is a means which is effective for botulism diseases and the prevention of the botulism diseases. Specifically provided is a plurality of human anti-botulinum toxin type-A antibodies having different epitopes from one another. Also specifically provided is a composition for neutralizing botulinum toxin type-A, which comprises a combination of two or more of the antibodies and which has a high neutralizing activity.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES
Methods and systems for multi-antibody therapies
ActiveUS8349326B2Many symptomSimple processAntibacterial agentsPeptide/protein ingredientsCancer cellMedicine
Owner:TRUSTEES OF TUFTS COLLEGE
IgY antibody for resisting botulinus toxin substrate peptide SubA as well as preparation method and application thereof
InactiveCN101648997AImprove stabilityEasy to storeEgg immunoglobulinsImmunoglobulins against bacteriaChemical synthesisEndopeptidase activity
The invention discloses an IgY antibody for resisting botulinus toxin substrate peptide, which can be prepared by the following steps: preparing SNAP25 short peptide subA by a chemical synthesis method or a gene recombination expression method; collecting eggs by subA immune laying chicken and extracting and purifying yelk immunoglobulin by a biochemical method. The antibody has the characteristics of high specificity of combining substrate linear short peptide subA, but not combining integral substrate SNAP25, has the capability of especially identifying zymolytic SNAP25 of A botulinus toxin,can be used as a detection reagent to be applied to the detection of the A botulinus toxin and the activity analysis of neurovirulence or endopeptidase as well as the poisoning diagnosis of clinicalkreotoxism, and has good market prospect and social significance.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Methods And Systems For Multi-Antibody Therapies
ActiveUS20110129474A1Simple processImprove effectivenessAntibacterial agentsPeptide/protein ingredientsCancer cellToxin
The present invention relates to methods and systems for administering antibody therapeutic agents. The methods include administering one or more (e.g., two or three) binding agents, wherein each of the binding agents has one or more monomers that have a binding region that is specific to a portion of a disease agent and one or more copies of a tag. The binding agents can be specific to one or more portions of the same or different disease agents. The tag is the same for each of the binding agents. The methods include administering an anti-tag antibody, wherein the anti-tag antibody has an anti-tag region that is specific to the tag, and can have an immunoglobulin (e.g., IgA, IgD, IgE, IgG, and IgM.). Disease agents include bacteria, bacterial proteins, viruses, viral proteins, cancer cells, and proteins or toxins produced therefrom or from other sources such as snakes, insects, plants, etc. In particular, the present invention includes methods and systems for binding agents having one or more monomers that are specific to neurotoxins that cause botulism.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Mink hemorrhagic pneumonia and botulism combined inactivate vaccine and preparing method thereof
ActiveCN105749266AStrong targetingImprove protectionAntibacterial agentsBacterial antigen ingredientsVaccine manufacturingMink
The invention relates to a mink hemorrhagic pneumonia and botulism combined inactivate vaccine and a preparing method thereof.Strain WD005, strain DL007 and strain ZC118 of pseudomonas aeruginosa for vaccine manufacturing and detecting are obtained through clinical isolation, and the strains are higher in pertinence and more comprehensive in protection for current mink hemorrhagic pneumonia epidemic serotype; by means of virulence tests and immunogenicity tests, the immunogenicity is good; a strain C-type clostridium botulinum C62-4 has the advantage of being superior in immunogenicity.The combined inactivate vaccine can prevent attack of G-type pseudomonas aeruginosa, B-type pseudomonas aeruginosa, C-type pseudomonas aeruginosa and C-type clostridium botulinum to minks at the same time, and has the advantages that the number of inoculation times is reduced, and using is convenient.The labor intensity of immunization is relieved, the immune cost is reduced, the stress reaction of animals is reduced, and the vaccine is more economical and reliable.
Owner:QILU ANIMAL HEALTH PROD
Methods and systems for multi-antibody therapies
ActiveUS8865169B2Simple processImprove effectivenessAntibacterial agentsAntimycoticsCancer cellToxin
The present invention relates to methods and systems for administering antibody therapeutic agents. The methods include administering one or more (e.g., two or three) binding agents, wherein each of the binding agents has a binding region that is specific to a portion of a disease agent and one or more copies of a tag. The binding agents can be specific to one or more portions of the same or different disease agents. The tag is the same for each of the binding agents. The methods include administering an anti-tag antibody, wherein the anti-tag antibody has an anti-tag region that is specific to the tag, and can have an immunoglobulin (e.g., IgA, IgD, IgE, IgG, and IgM.). Disease agents include bacterial proteins, viral proteins, cancer cells, and proteins or toxins produced therefrom. In particular, the present invention includes methods and systems for binding agents that are specific to neurotoxins that cause botulism.
Owner:TRUSTEES OF TUFTS COLLEGE
Botulinum neurotoxin a receptor and the use thereof
InactiveUS20100249372A1Reduced BoNT/A bindingReduce the binding forceAntibacterial agentsPeptide/protein ingredientsReceptorGlycoprotein
The present invention is based on the identification of synaptic vessel glycoprotein SV2 as the BoNT / A receptor and the further identification of various BoNT / A-binding fragments of SV2. The disclosure here provides new tools for diagnosing and treating botulism.
Owner:WISCONSIN ALUMNI RES FOUND
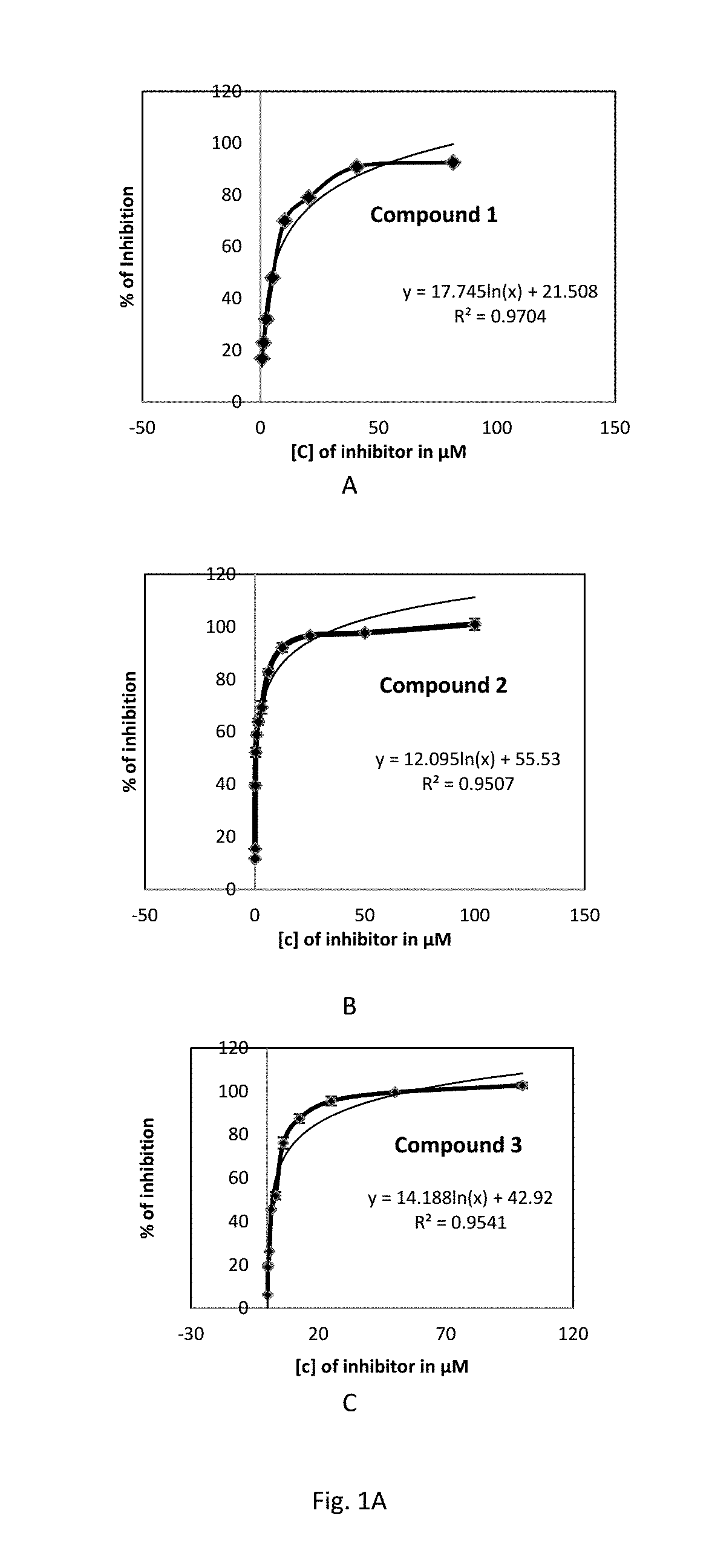
Botulinum neurotoxin inhibitors
Present invention discloses a method of treating an individual suffering from botulism by inhibiting botulinum neurotoxins.
Owner:PRIME BIO INC
Detoxified Recombinant Botulinum Neurotoxin
ActiveUS20090155348A1Development is reduced and preventedSymptom is reduced and preventedBacterial antigen ingredientsPeptide/protein ingredientsEscherichia coliAntidote
The present invention relates to the isolation of polypeptides derived from the Clostridium botulinum neurotoxin and the use thereof as immunogens for the production of vaccines and antitoxins, as well as research and drug development applications. Clostridium botulinum is responsible for food bone botulism, a severe and often deadly disease. Botulinum neurotoxin binds to neural cells and are translocated into the cytosol. The toxin then prevents neurotransmitter release by cleaving a SNARE protein. A double mutant E224A / E262 full length botulinum neurotoxin Type A holo form was successfully cloned and purified, which lacks the endopeptidase activity involved in the toxic action of the BoNT, and thus leading to its detoxification (DR BoNT / A). This new molecule can be used as an antidote against botulism, and also as a vaccine candidate for botulism. Due to the poor availability and extreme toxicity of native holo-toxin, a nontoxic form of the holo-toxin is highly desired for research and vaccine development. The full length DR BoNT / A protein has been expressed in E. coli as a soluble form.
Owner:SINGH BAL RAM
Human monoclonal antibody that binds/neutralizes botulinum neurotoxin type b
ActiveCN104837866BGood effectImprove securityAntibacterial agentsNervous disorderAntigen Binding FragmentAntigen binding
Isolated anti-botulinum neurotoxin type B monoclonal antibody and antigen-binding fragments thereof having a neutralization activity against a botulinum neurotoxin type B are provided, including a human monoclonal antibody. Hybridomas that produce such antibodies or fragments thereof are also provided by the present invention, as well as methods of producing such hybridomas and method of producing antibodies or fragments thereof from such hybridomas. Pharmaceutical compositions and kits including antibodies or fragments thereof for at least one of the prevention, the treatment, and the detection of botulinum neurotoxin type B poisoning are further provided. Methods of inhibiting or treating botulism in a human subject are provided, as are methods of detecting botulinum neurotoxin type B.
Owner:OSAKA UNIV +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com