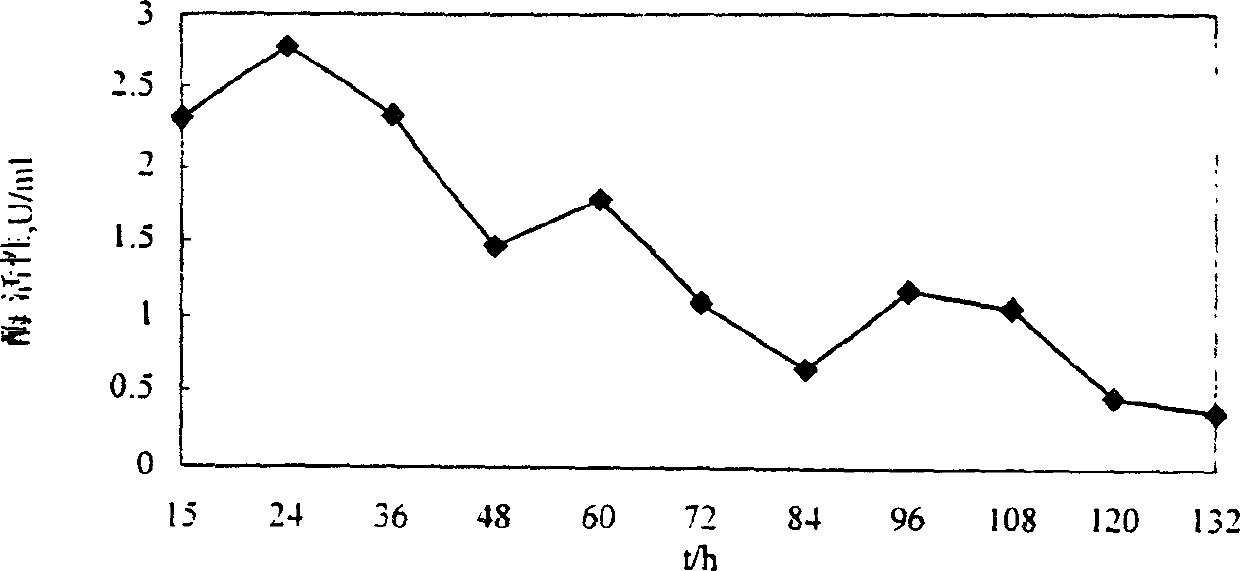
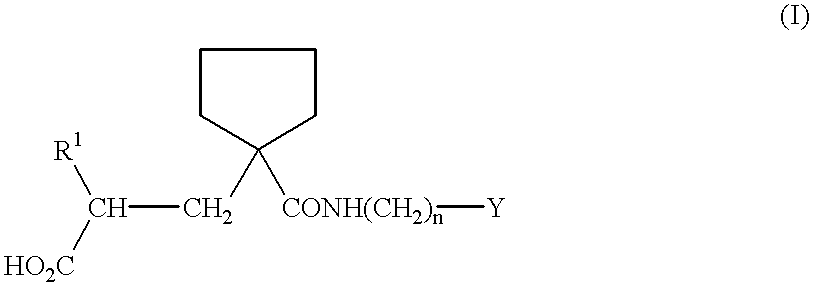Patents
Literature
225 results about "Endopeptidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
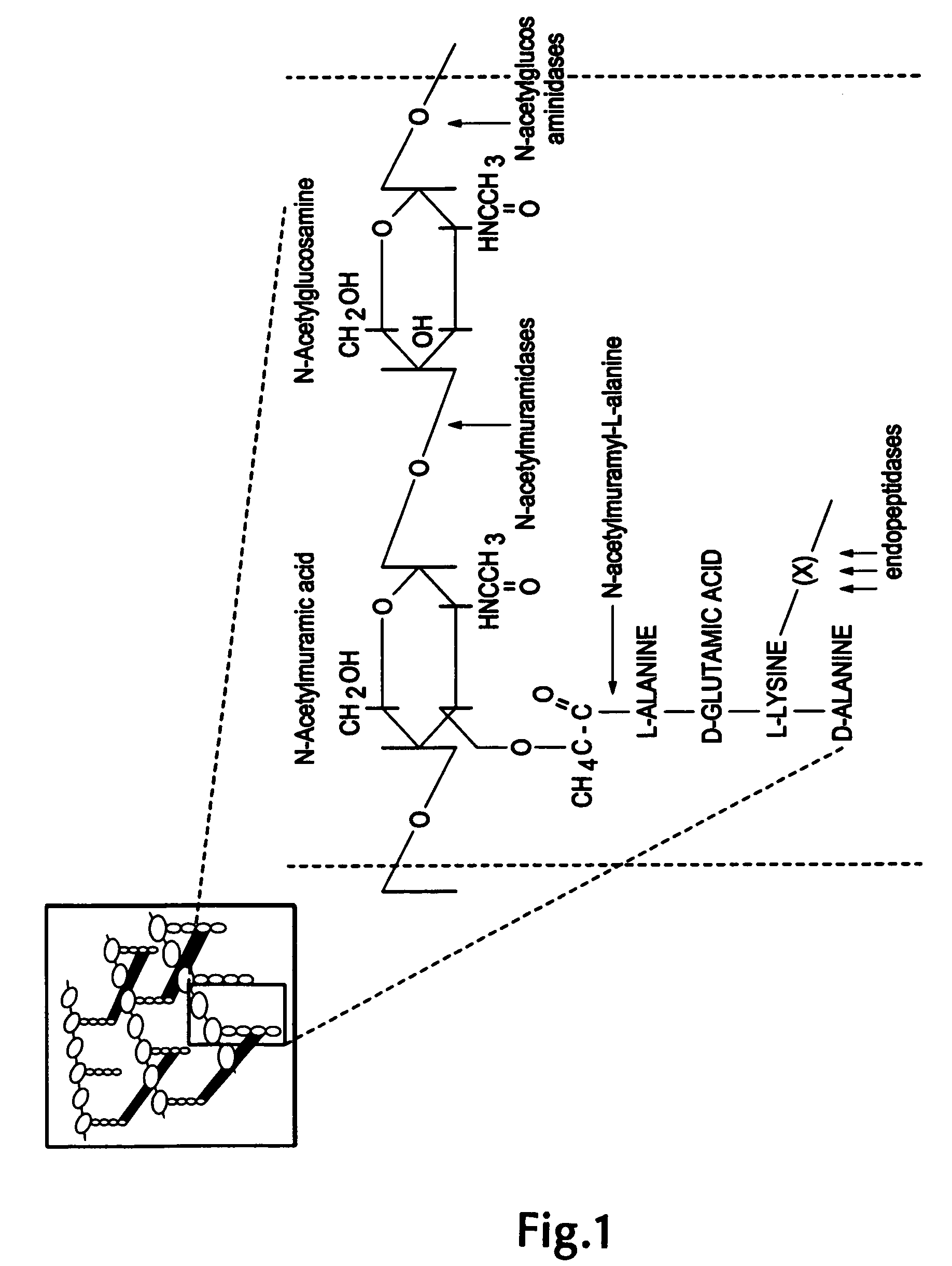
Endopeptidase or endoproteinase are proteolytic peptidases that break peptide bonds of nonterminal amino acids (i.e. within the molecule), in contrast to exopeptidases, which break peptide bonds from end-pieces of terminal amino acids. For this reason, endopeptidases cannot break down peptides into monomers, while exopeptidases can break down proteins into monomers. A particular case of endopeptidase is the oligopeptidase, whose substrates are oligopeptides instead of proteins.
Novel compounds that inhibit dipeptidyl peptidase (DPP-IV) and neprilysin (NEP) and/or angiotensin converting enzyme (ACE)
This invention relates to novel compounds, compositions containing the compounds, that inhibit dipeptidyl peptidase (especially DPP-IV) and neprilysin (NEP, neutral endopeptidase) as well as dipeptidyl peptidase (especially DPP-IV) and angiotensin converting enzyme (ACE) and / or dipeptidyl Peptidase (especially DPP-IV) and vasopeptidases (especially ACE and NEP). These compounds and pharmaceutical compositions thereof are useful for the treatment as well as the prevention of type 2 diabetes mellitus.
Owner:MORPHOCHEM AG
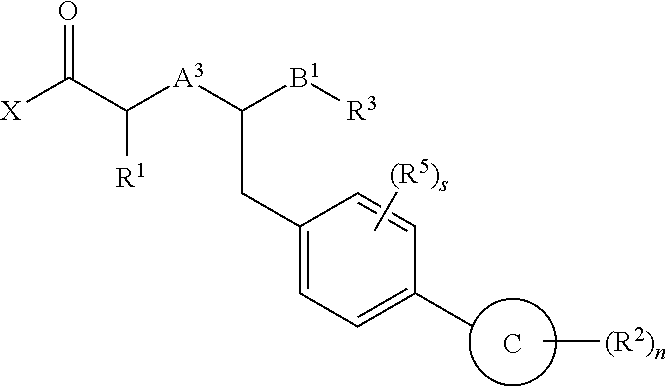
Method of treating contrast-induced nephropathy
InactiveUS20120122844A1Treating and preventing and ameliorating contrast-induced nephropathyImprove the level ofBiocideOrganic chemistryNephrosisActive agent
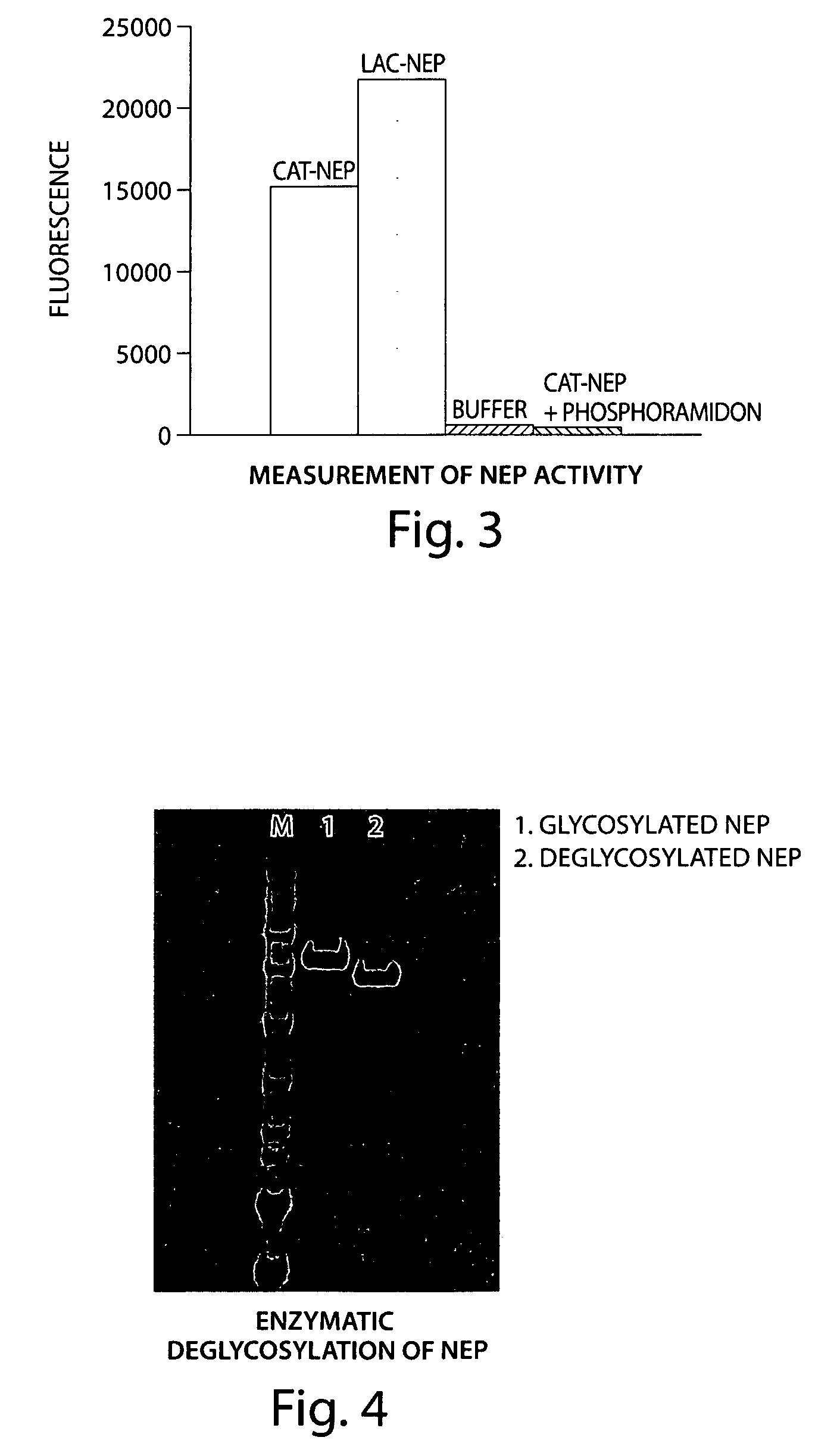
The present invention provides the use of a neutral endopeptidase inhibitor, in the manufacture of a medicament for the treatment, amelioration and / or prevention of contrast-induced nephropathy. The invention also relates to the use of a compound of Formula I:wherein R1, R2, R3, R5, X, A3, B1, s and n are defined herein, for the treatment, amelioration and / or prevention of contrast-induced nephropathy. The present invention further provides a combination of pharmacologically active agents for use in the treatment, amelioration and / or prevention of contrast-induced nephropathy.
Owner:NOVARTIS AG
Pharmaceutical compositions including ACE/NEP inhibitors and bioavailability enhancers
InactiveUS6890918B2Enhance the oral bioabsorption of saidBiocideAngiotensinsAngiotensin-converting enzymeOrganic acid
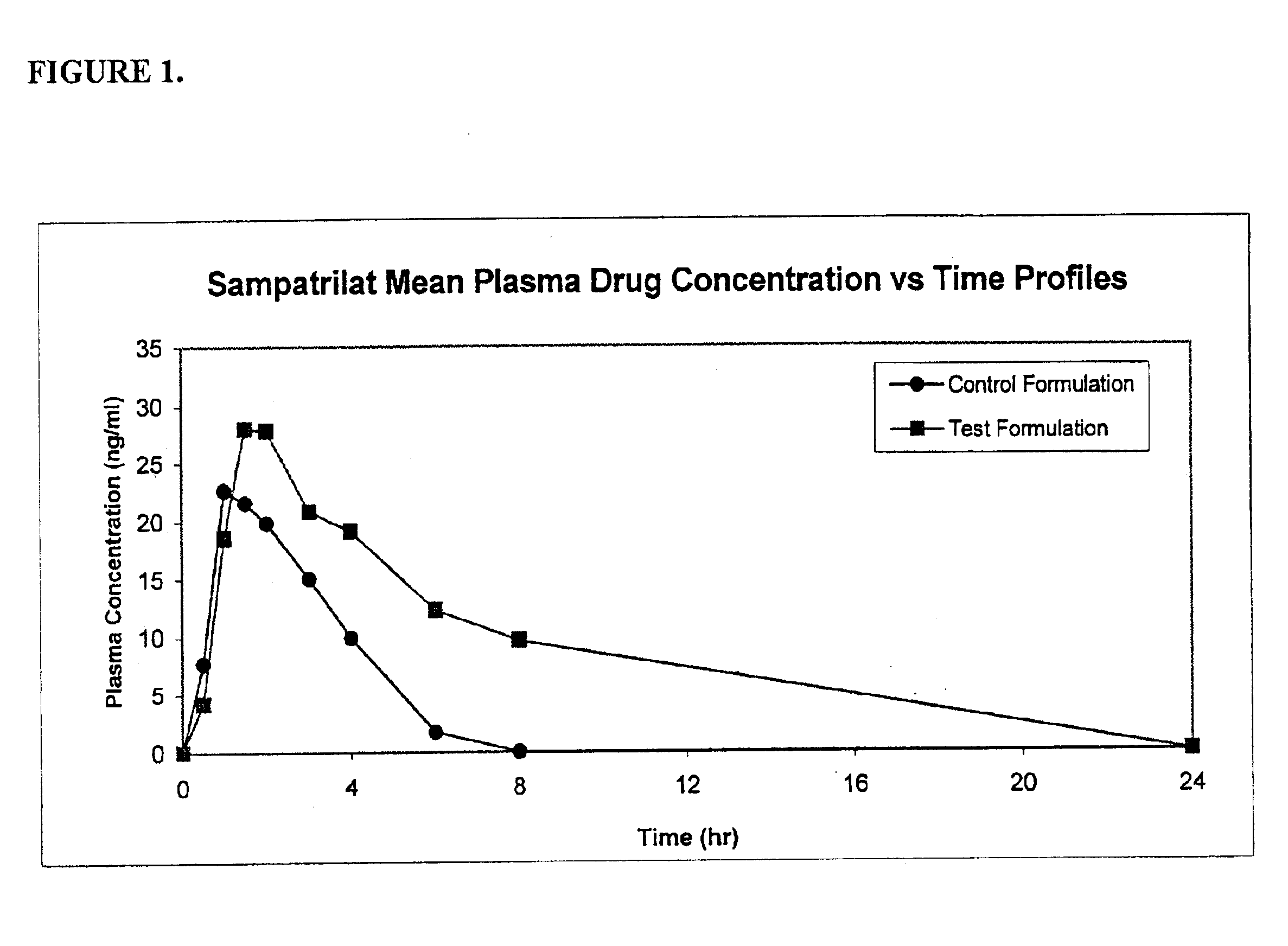
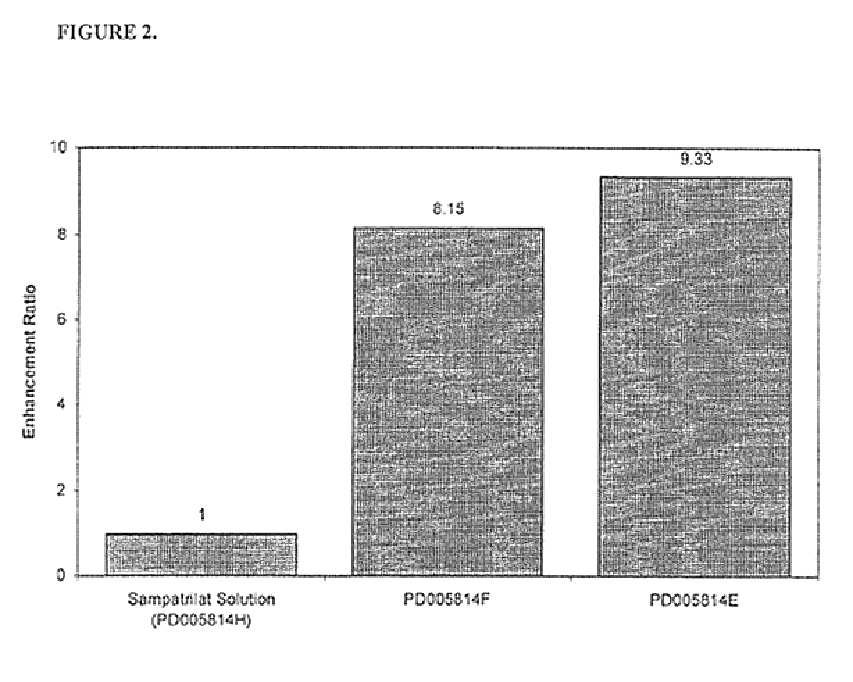
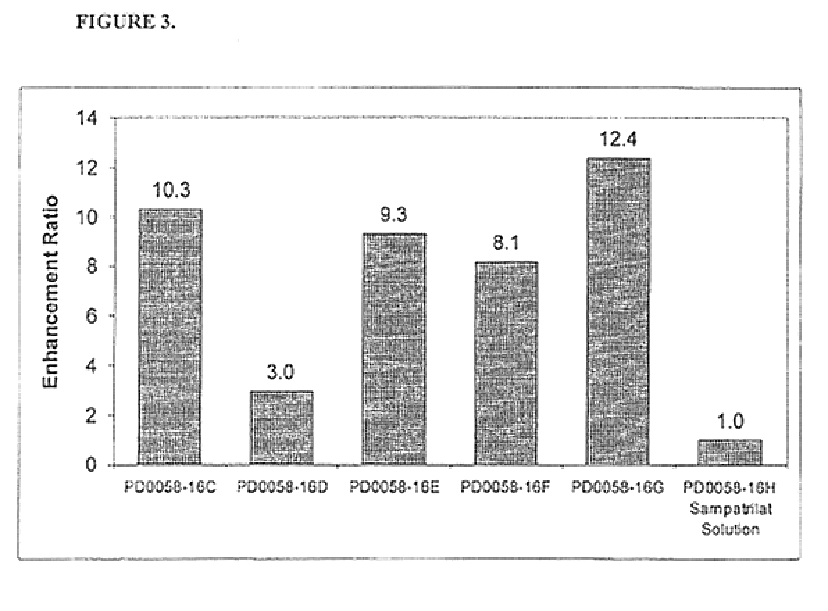
A pharmaceutical composition comprising an inhibitor of angiotensin converting enzyme and neutral endopeptidase, such as sampatrilat, and at least one bioavailability enhancer such as an organic acid, e.g., ascorbic acid. Such a composition has improved systemic bioavailability.
Owner:SUPERNUS PHARM INC
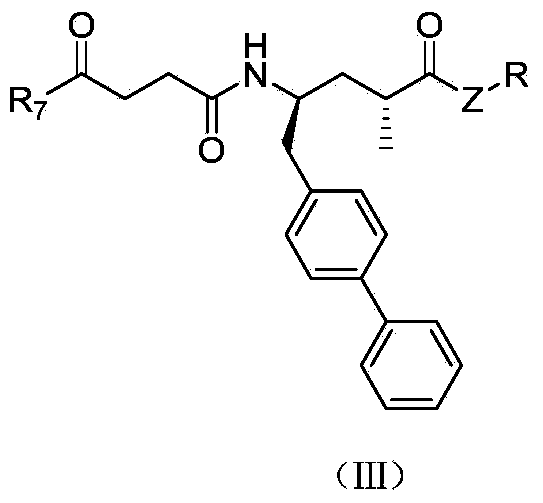
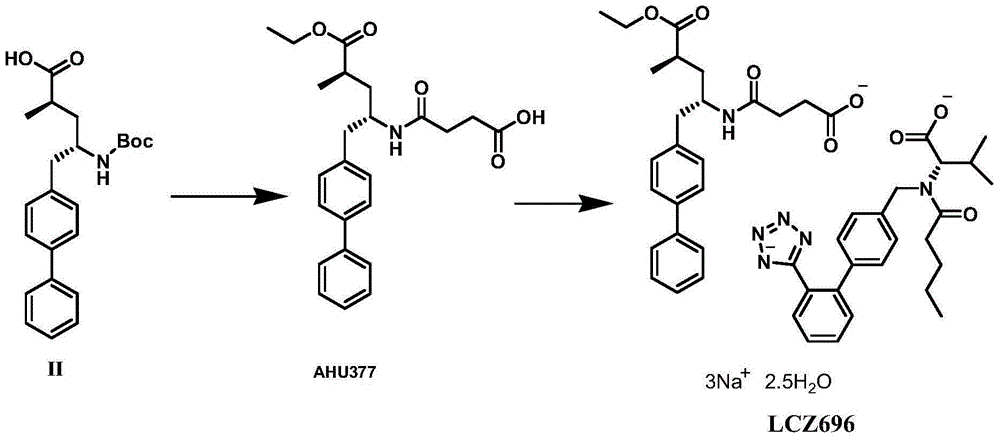
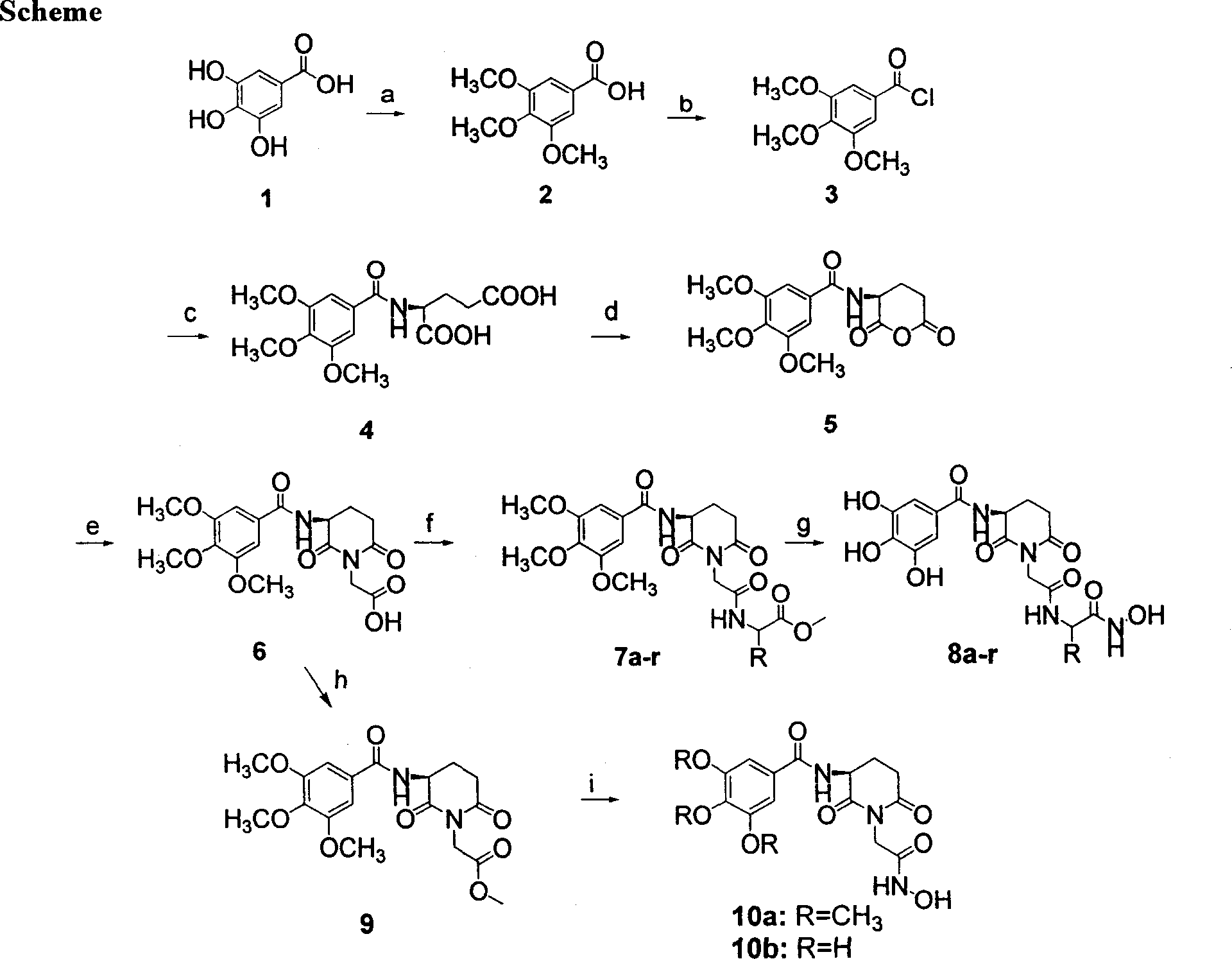
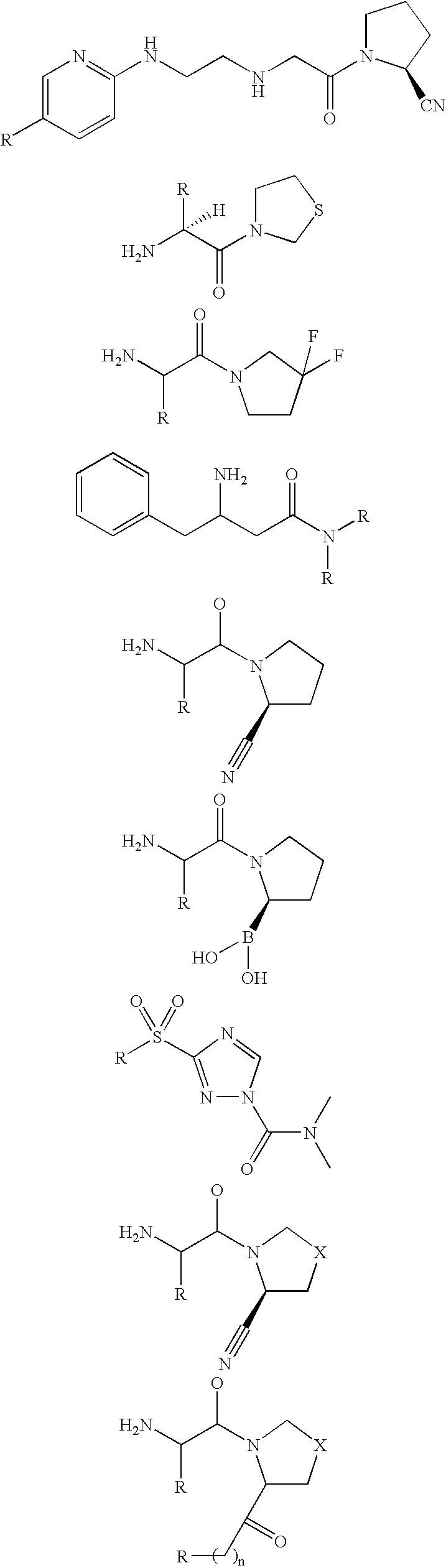
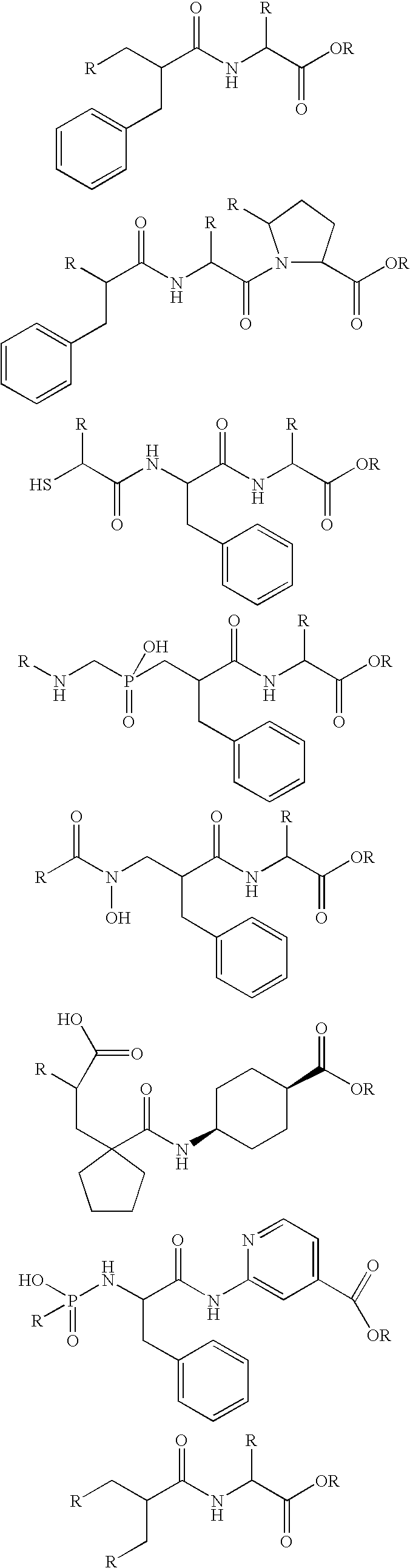
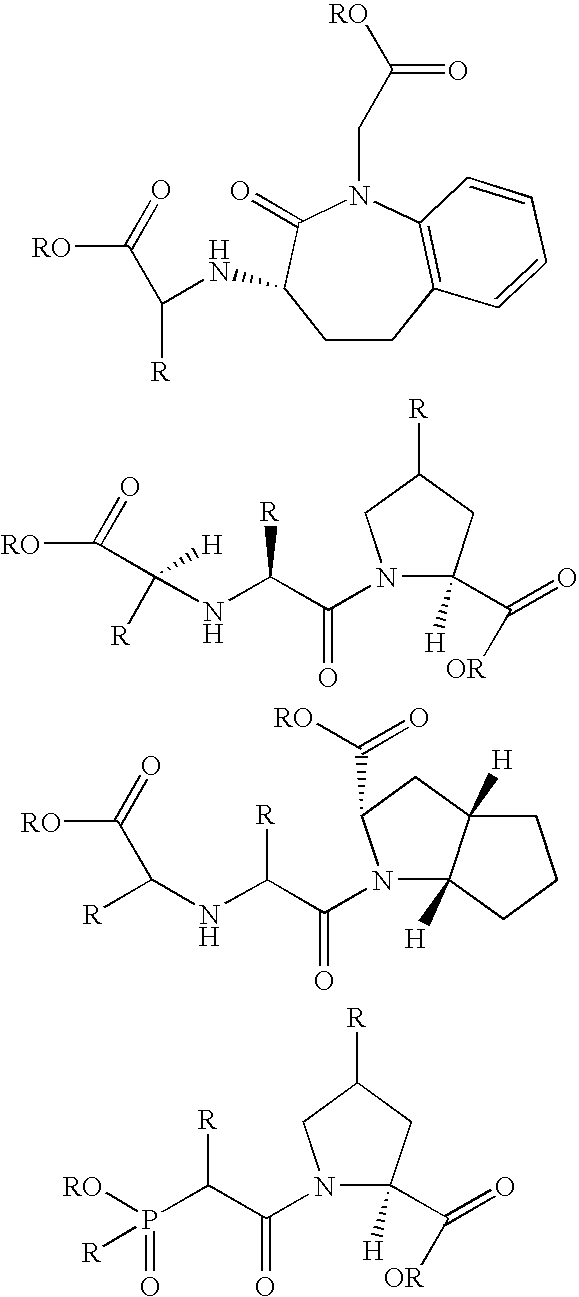
Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations
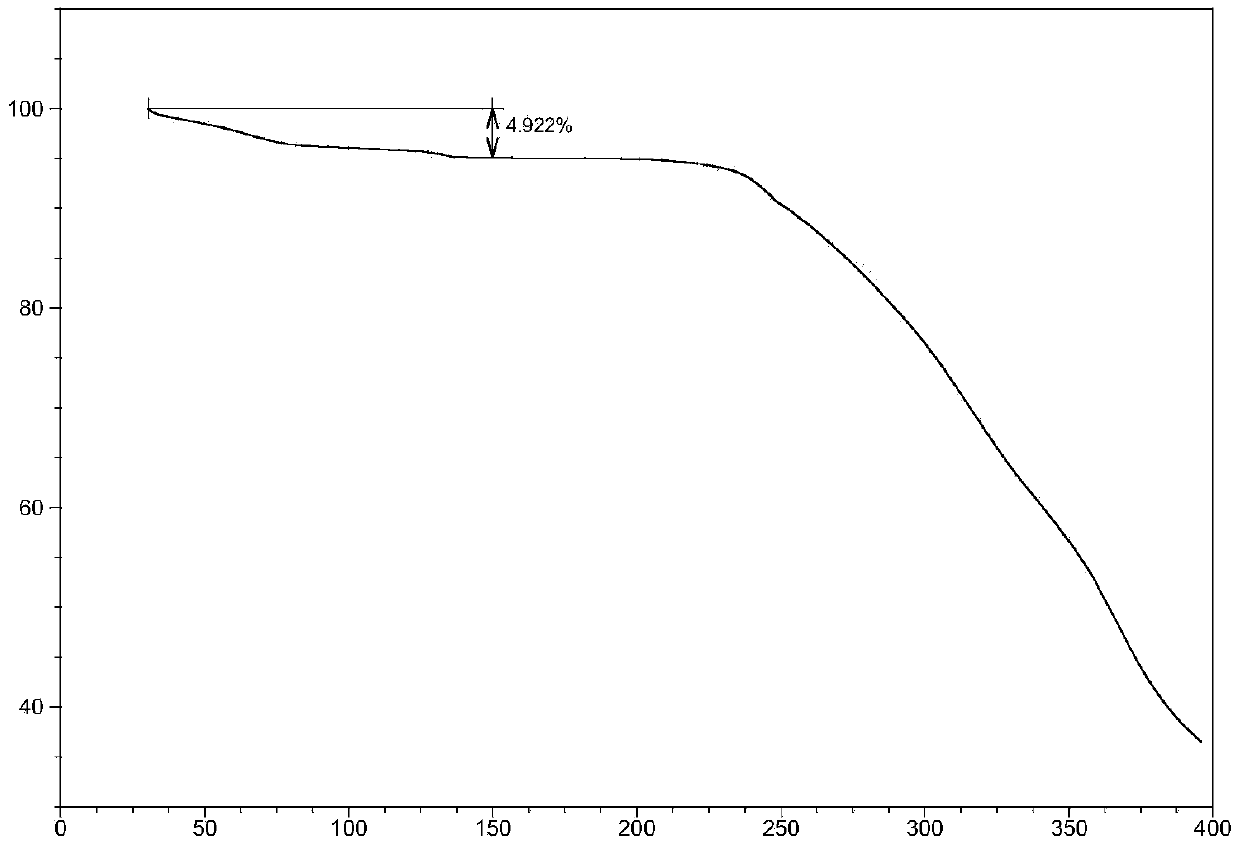
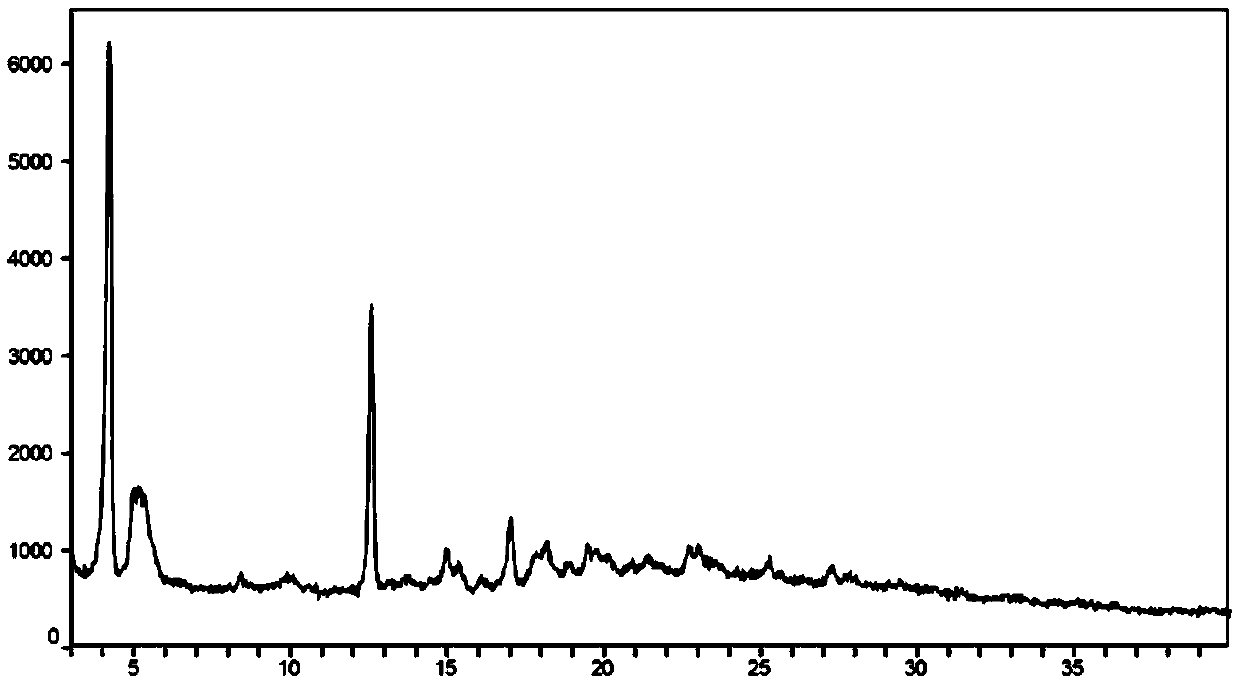
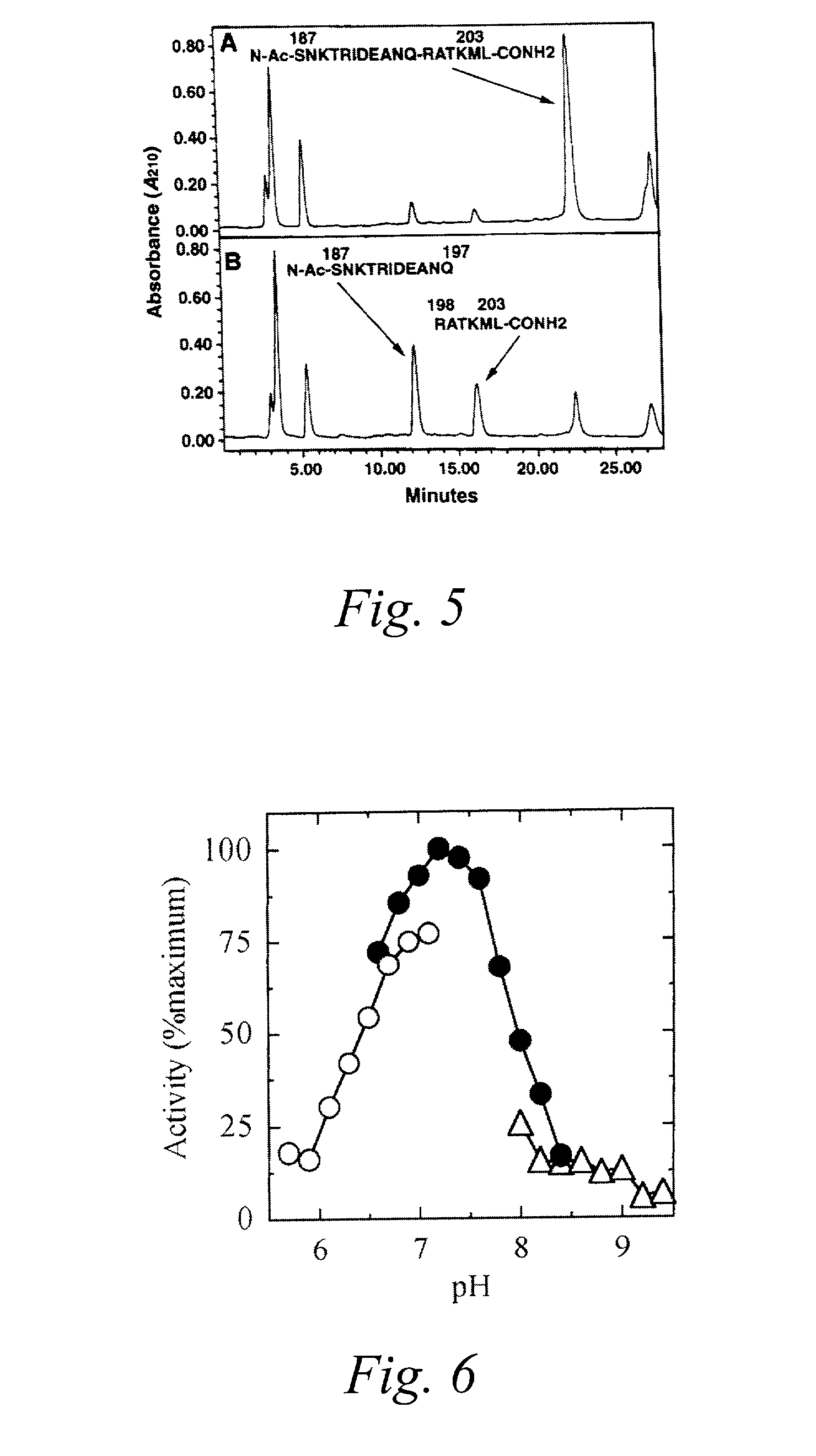
A compound of an angiotensin receptor antagonist (ARB), a neutral endopeptidase inhibitor (NEPi) and one or more monovalent cations are useful for the treatment of hypertension and / or heart failure. ARB includes S—N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine in the anion form, NEPi includes (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester in the anion form and cation includes monovalent cations such as Na+. The compound includes trisodium [3-((1S,3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3′-methyl-2′-(pentanoyl{2″-(tetrazol-5-ylate)biphenyl-4′-ylmethyl}amino)butyrate] hemipentahydrate.
Owner:NOVARTIS PHARM CORP
Antifouling paint composition comprising rosin and enzyme
An antifouling paint composition comprising an enzyme, such as endopeptidase, Subtilisin (EC 3.4.21.62) and Alcalase(R), and a rosin compound, wherein the enzyme is effective to reduce or prevent fouling by aquatic organisms of a surface coated with the composition. Also disclosed is a method for preventing fouling of a surface by aquatic organisms.
Owner:BIOLOCUS
Treatment of male sexual dysfunction
InactiveUS20020028799A1Increase heightSpeed up the processBiocideOrganic chemistryPhosphodiesterasePeptidase Inhibitors
The present invention relates to the use of neutral endopeptidase inhibitors (NEPi) and a combination of NEPi and phosphodiesterase type 5 (PDE5) inhibitor for the treatment of male sexual dysfunction, in particular MED.
Owner:PFIZER INC
Novel crystalline form of angiotensin receptor antagonist and neutral endopeptidase inhibitor supramolecular complex
ActiveCN105037289AGood water solubilityImprove stabilityOrganic chemistry methodsCarboxylic acid amide separation/purificationSolubilityWater soluble
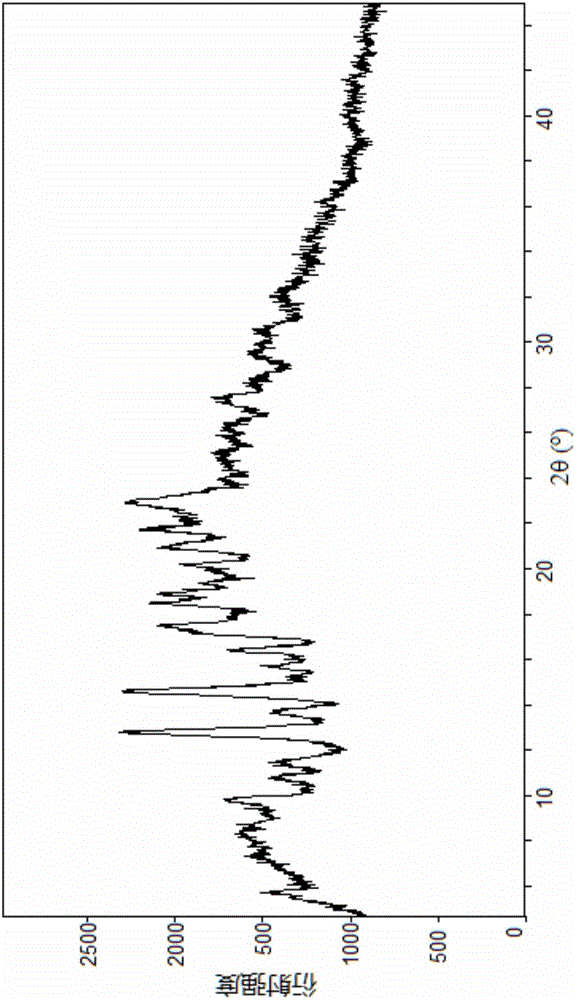
The present invention discloses a novel crystalline form of an angiotensin receptor antagonist and neutral endopeptidase inhibitor supramolecular complex. The novel crystalline form of the supramolecular complex has high water solubility and good stability. Experiments show that the moisture absorption and intrinsic dissolution extent and speed are superior to those of a conventional crystalline supramolecular complex.
Owner:EAST CHINA UNIV OF SCI & TECH
Biaryl-substituted 4-aminobutyric acid derivatives, and preparation method and application thereof
ActiveCN104230865AGood pharmacokinetic activityOrganic active ingredientsSugar derivativesMedicinal chemistryEndopeptidase
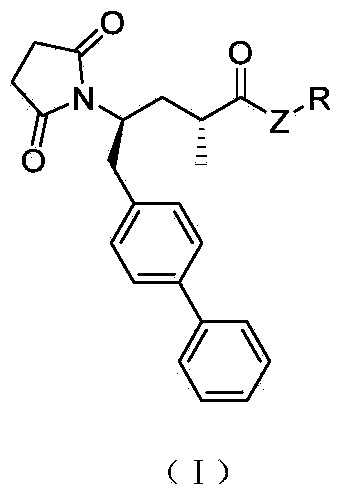
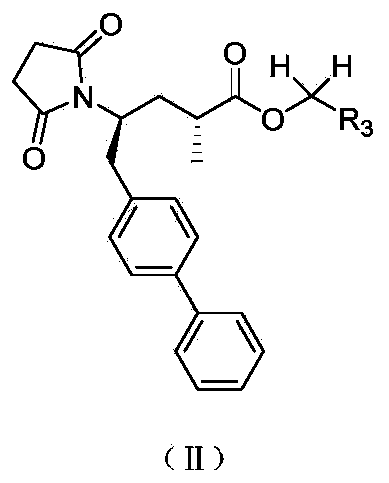
The invention relates to biaryl-substituted 4-aminobutyric acid derivatives, and a preparation method and application thereof, particularly biaryl-substituted 4-aminobutyric acid derivatives disclosed as Formula (I), and a preparation method and application thereof, an intermediate of biaryl-substituted 4-aminobutyric acid derivatives prepared by cyclization reaction and a pharmaceutical composition containing the biaryl-substituted 4-aminobutyric acid derivatives. The biaryl-substituted 4-aminobutyric acid derivatives have NEP (neutral endopeptidase) inhibition activity, and can be used as an NEP inhibitor.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Nucleic acid encoding endolysin fusion protein
The invention concerns a recombinant nucleic acid molecule encoding an antimicrobial fusion peptidoglycan endopeptidase. The recombinant nucleic acid molecule according to the invention is formed from a nucleic acid encoding a bacterial endopeptidase (lysostaphin) from Staphylococcus simulans and a nucleic acid encoding a second endopeptidase (endolysin) module from Group B streptococcal bacteriophage B30. The encoded fusion endopeptidase has antimicrobial activity and kills both Staphylococcus bacteria and Streptococcus bacteria.
Owner:US SEC AGRI
Pegylated glutenase polypeptides
InactiveUS20090304754A1Lower Level RequirementsHigh activityHydrolasesPeptide/protein ingredientsCrystallographyProlyl endopeptidase
Owner:IMMUNOGENICS LLC
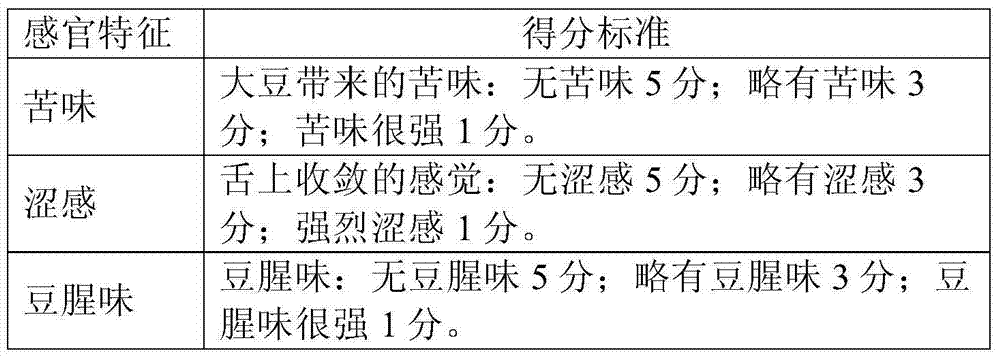
Method for preparing soybean peptides through enzymolysis of soy protein
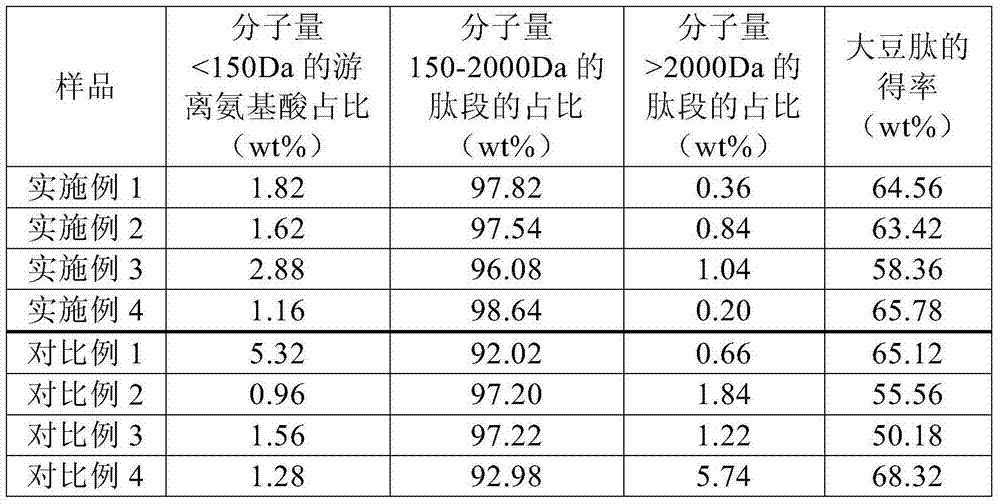
ActiveCN104719611AReduce bitternessHigh yieldVegetable proteins working-upAlkaline proteaseNeutral proteinase
The present invention relates to a method for preparing soybean peptides through enzymolysis of soy protein, wherein the obtained soybean peptides have characteristics of low bitter taste and high yield, and the contents of free amino acids and the peptides having a molecular weight of less than 150 Da or greater than 2000 Da are low. The method comprises: adding water to soybean protein to prepare a 4-12 wt% soybean separation protein liquid, adjusting the temperature and the pH value, sequentially adding a proper amount of alkaline proteinase adopted as the endopeptidase, neutral proteinase and flavourzyme adopted as the exopeptidase, carrying out an enzymolysis reaction, adjusting the pH value of the enzymolysis liquid, carrying out enzyme inactivation, centrifugating, taking the supernatant, sterilizing, and carrying out spray drying to obtain the soybean peptide.
Owner:COFCO NUTRITION & HEALTH RES INST +1
Methods and compositions involving endopeptidases PepO2 and PepO3
The present invention concerns the methods and compositions involving endopeptidase enzymes, especially PepO2 and PepO3 from L. helveticus, and their use in reducing bitterness by cleaving bitter peptides. In particular embodiments of the invention, these methods and compositions apply to the cheesemaking process. The invention also concerns the use of PepO2 and / or PepO3 polypeptides in the treatment or prevention of celiac sprue or as a food additive.
Owner:WISCONSIN ALUMNI RES FOUND +1
Recombinant light chains of botulinum neurotoxins and light chain fusion proteins for use in research and clinical therapy
Botulinum neurotoxins, the most potent of all toxins, induce lethal neuromuscular paralysis by inhibiting exocytosis at the neuromuscular junction. The light chains (LC) of these dichain neurotoxins are a new class of zinc-endopeptidases that specifically cleave the synaptosomal proteins, SNAP-25, VAMP, or syntaxin at discrete sites. The present invention relates to the construction, expression, purification, and use of synthetic or recombinant botulinum neutoroxin genes. For example, a synthetic gene for the LC of the botulinum neurotoxin serotype A (BoNT / A) was constructed and overexpressed in Escherichia coli. The gene product was purified from inclusion bodies. The methods of the invention can provide 1.1 g of the LC per liter of culture. The LC product was stable in solution at 4° C. for at least 6 months. This rBoNT / A LC was proteolytically active, specifically cleaving the Glu-Arg bond in a 17-residue synthetic peptide of SNAP-25, the reported cleavage site of BoNT / A. Its calculated catalytic efficiency kcat / Km was higher than that reported for the native BoNT / A dichain. Treating the rBoNT / A LC with mercuric compounds completely abolished its activity, most probably by modifying the cysteine-164 residue located in the vicinity of the active site. About 70% activity of the LC was restored by adding Zn2+ to a Zn2+-free, apo-LC preparation. The LC was nontoxic to mice and failed to elicit neutralizing epitope(s) when the animals were vaccinated with this protein. In addition, injecting rBoNT / A LC into sea urchin eggs inhibited exocytosis-dependent plasma membrane resealing.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
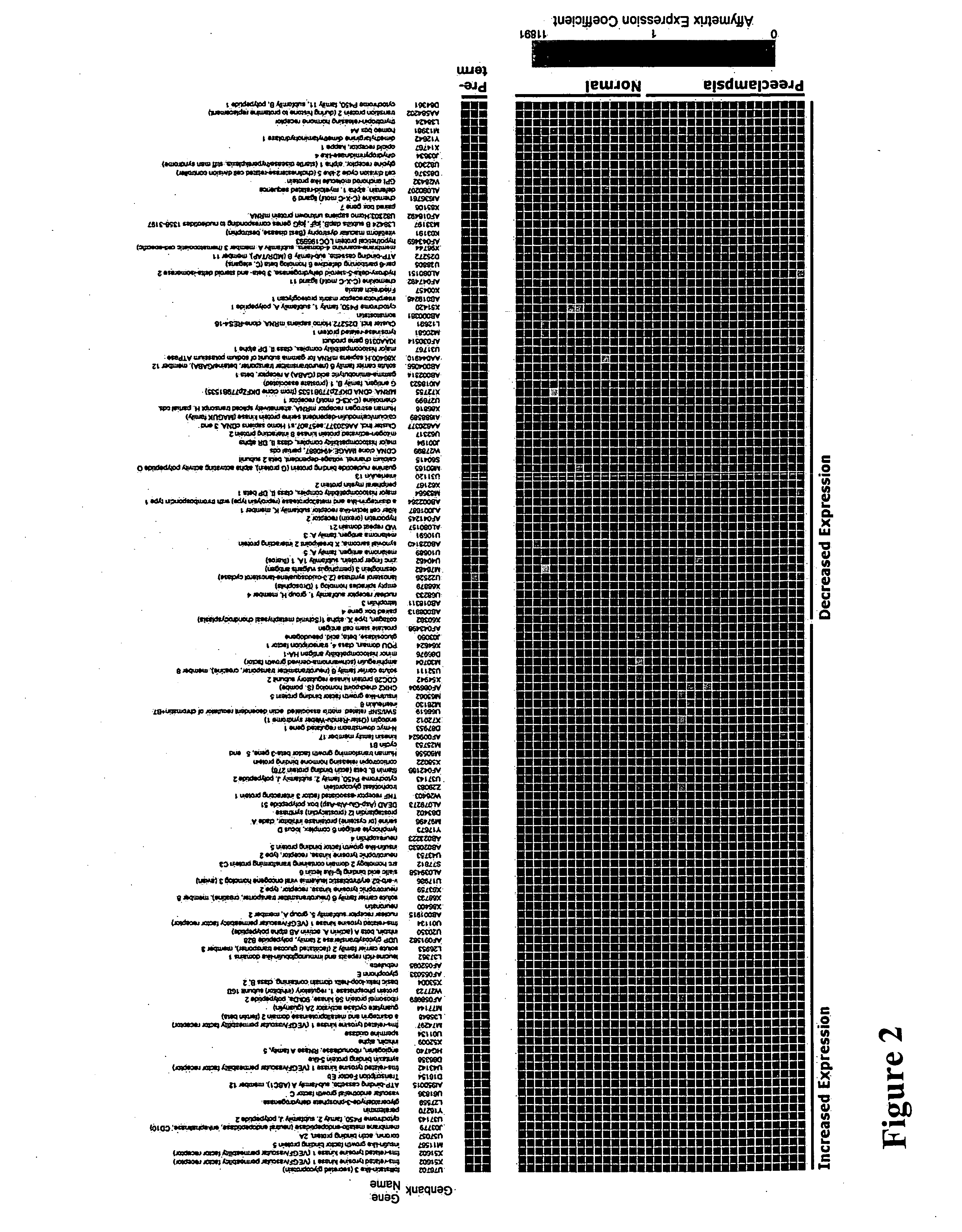
Nucleic acids and polypeptides useful for diagnosing and treating complications of pregnancy
ActiveUS20060166277A1Diagnosing and effectively treatingSave maternalMicrobiological testing/measurementDisease diagnosisPregnancyUdp glycosyltransferase
Disclosed herein are methods for diagnosing or treating pregnancy related hypertensive disorders that include the use of a polypeptide or a nucleic acid encoding a polypeptide selected from the following: follistatin related protein, interleukin 8, inhibin A, VEGF-C, angiogenin, beta fertilin, hypothetical protein, leukocyte associated Ig-like receptor secreted protein, erythroid differentiation protein, adipogenesis inhibitory factor, corticotropin releasing factor binding protein, alpha-1 anti-chymotrypsin, insulin-like growth factor binding protein-5, CD33L, cytokine receptor like factor 1, platelet derived endothelial growth factor, lysyl hydroxylase isoform 2, stanniocalcin precursor, secreted frizzled related protein, galectin-3, alpha defensin, ADAM-TS3, cholecystokinin precursor, interferon stimulated T-cell alpha chemoattractant precursor, azurocidin, sperminine oxidase, UDP glycosyltransferase 2 family polypeptide B28, neurotrophic tyrosine kinase receptor 2, neutral endopeptidase, CDC28 protein kinase regulatory subunit 2, beta glucosidase, lanosterol synthase, calcium / calmodulin-dependent serine protein kinase, estrogen receptor-alternatively spliced transcript H, chemokine (CX3C motif) receptor 1, tyrosinase-related protein 1, hydoxy-delta-5-steroid dehyrogenase, dihydropyramidinase-like-4, and cytochrome P450-family 11.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Whey protein hydrolysate
ActiveUS20080254505A1Pleasant tasteGreat tasteDepsipeptidesPeptide preparation methodsSports drinkClinical nutrition
The present invention relates to a method of producing a whey protein hydrolysate using a microbial endopeptidase which specifically cleaves on the carboxy terminal side of arginine or lysine. The invention also relates to use of such whey protein hydrolysate in sports drinks or in clinical nutrition.
Owner:NOVOZYMES AS
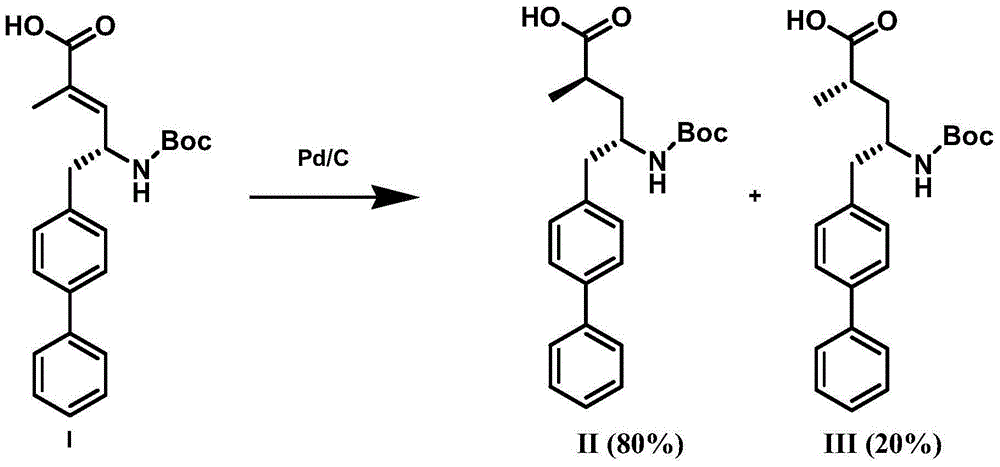
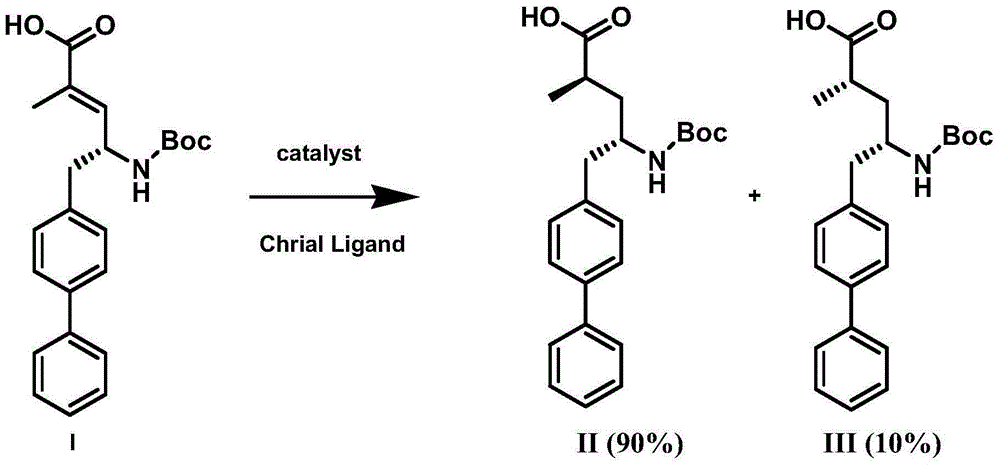
Preparation method of NEP (neutral endopeptidase) inhibitor intermediate
ActiveCN105061263ACarbamic acid derivatives preparationOrganic compound preparationHydrogenTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of an NEP (neutral endopeptidase) inhibitor intermediate, further discloses a selective reduction preparation method of the NEP inhibitor intermediate (2R, 4S)-5-([1,1'-biphenyl]-4-yl)-4-((t-butyloxycarboryl)amino)-2-methylpentanoic acid in presence of a chiral ligand, and particularly discloses a diastereoselective hydrogenation synthetic method with hydrogen under the condition of presence of a transition metal catalyst and the chiral ligand. The metal catalyst used in the method is cheap, the chiral ligand is obtained easily, high yield is realized, a high-purity product is produced preferably, and the product with the proportion of diastereoisomers being 90:10 is produced preferably.
Owner:苏州楚凯药业有限公司
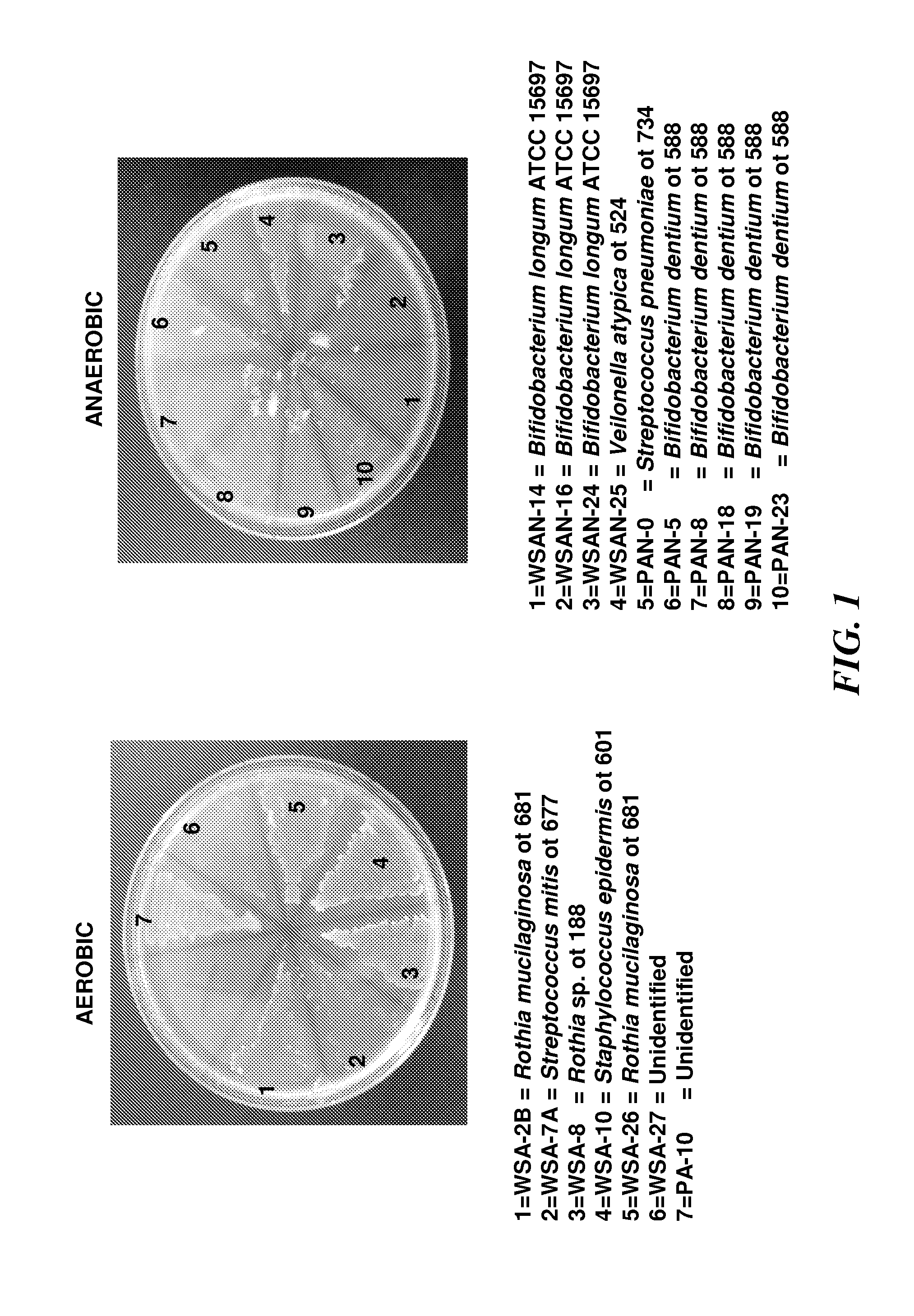
Rothia species glutamine endopeptidases and use thereof
InactiveUS20120230976A1Improve enzyme stabilityIncrease enzyme activityMilk preparationDough treatmentMedicineDermatitis herpetiformis
Disclosed are glutamine endopeptidase enzymes from Rothia sp. bacteria that are naturally associated with the oral cavity, formulations comprising the glutamine endopeptidase enzymes and the use thereof for the treatment, prevention of allergic reaction and diagnosis of gluten allergy related diseases such as Celiac Sprue, gluten allergy and / or dermatitis herpetiformis.
Owner:TRUSTEES OF BOSTON UNIV
Crystalline ARB-NEPi dicationic compound and preparation method and application thereof
The present invention discloses a crystalline ARB-NEPi dicationic compound and a preparation method and application thereof. In particular, the present invention relates to the crystalline ARB-NEPi dicationic compound having the formula of NEPi.Nax.Ky.ARB.ZH2O. The present invention also relates to a pharmaceutical composition containing an effective amount of the crystalline dicationic compound and application of the pharmaceutical composition in the preparation of drugs for treatment or prevention of neutral-endopeptidase-related diseases, cardiovascular diseases, hypertension, acute and chronic heart failures, congestive heart failure, left ventricular dysfunction, hypertrophic cardiomyopathy, diabetic cardiomyopathy, supraventricular arrhythmia and ventricular arrhythmia, atrial fibrillation, atrial flutter or detrimental vascular remodeling, and the like, and the pharmaceutical composition has broad application prospects.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Administration of neutral endopeptidase to treat inflammatory bowel disease
InactiveUS20060029590A1Reduce N-linked glycosylationReducing and preventing symptomPeptide/protein ingredientsTissue cultureInflammatory bowel diseaseBacteria
Administration of recombinant, truncated mammalian NEP or certain bacterial homologues of this protein is therapeutically effective in the treatment of inflammatory bowel disease.
Owner:CATALYST BIOSCIENCES INC
Composition, methods and reagents for the synthesis of a soluble form of human PHEX
InactiveUS7427498B2Easy to prepareEasy to purifyImmobilised enzymesBioreactor/fermenter combinationsPhosphoric acidMutant
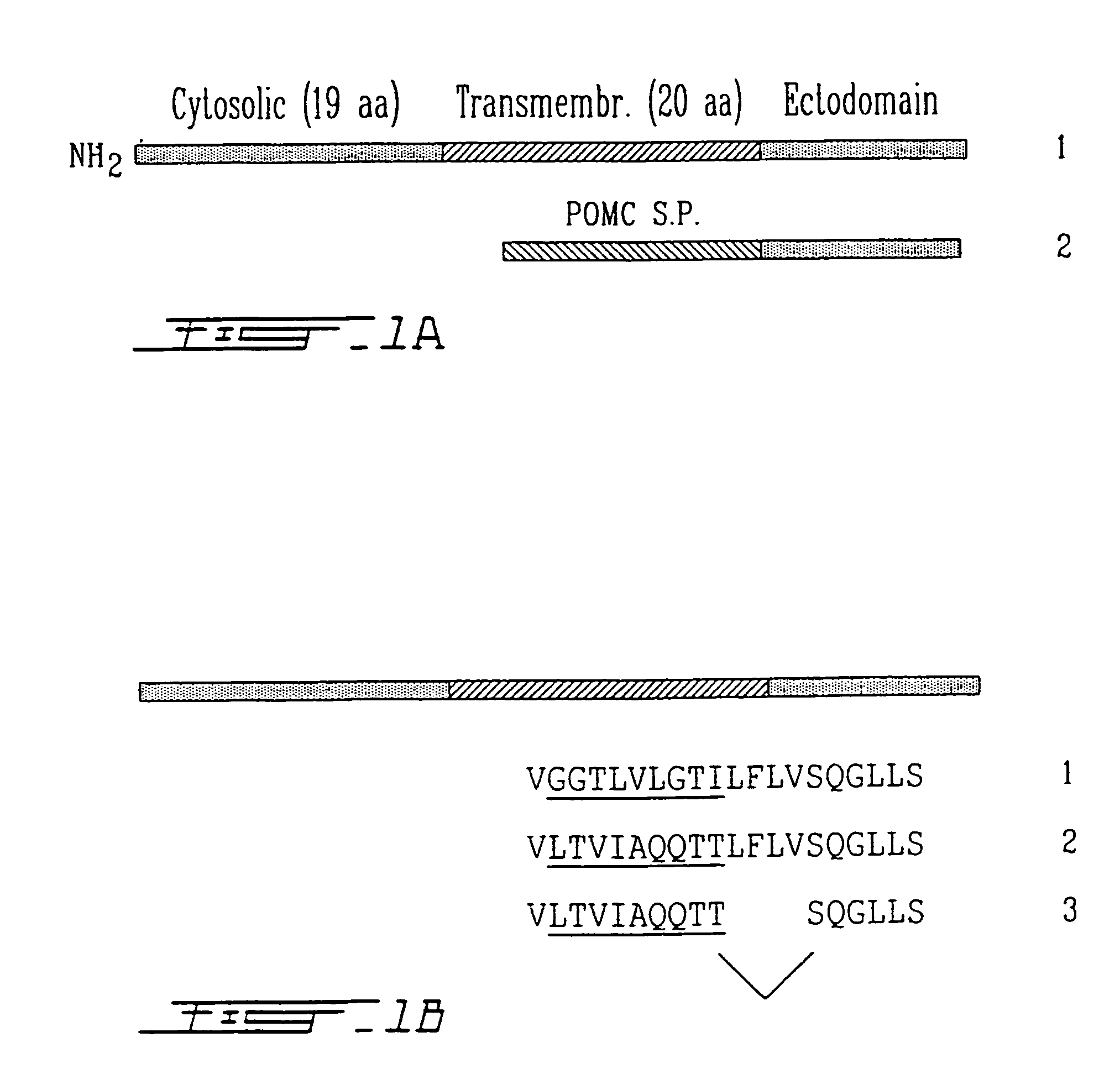
This invention relates to a soluble form of PHEX, PHEX being a type II integral membrane glycoprotein. This enzyme is the gene product of a phosphate-regulating gene with homologies to endopeptidases on the X chromosome. To produce a soluble form of PHEX, the transmembrane anchor domain has been modified to encode a signal peptidase coding sequence. The soluble PHEX therefore comprises the active ectodomain. An inactive mutant of PHEX is also an object of this invention. Both soluble and inactive mutant forms of PHEX can be used to screen ligands to PHEX. These ligands can also be used as substrates or inhibitors of PHEX. PHEX being phosphaturic, an inhibitor thereof will be used to treat phosphaturia and / or hypophosphatemia. On the opposite, a substrate for PHEX or PHEX itself can be used to treat hyperphosphatemia.
Owner:ALEXION PHARMA INC
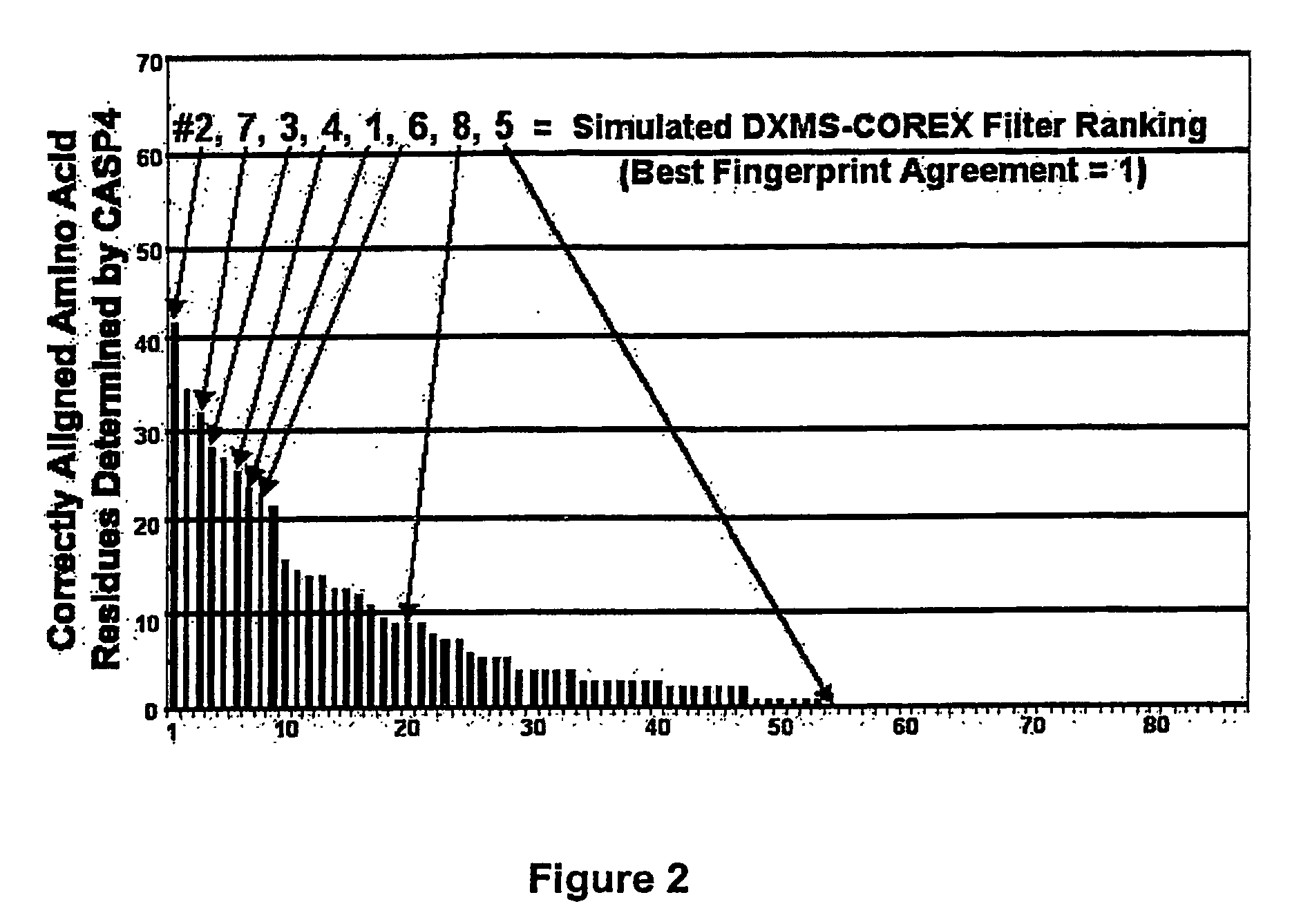
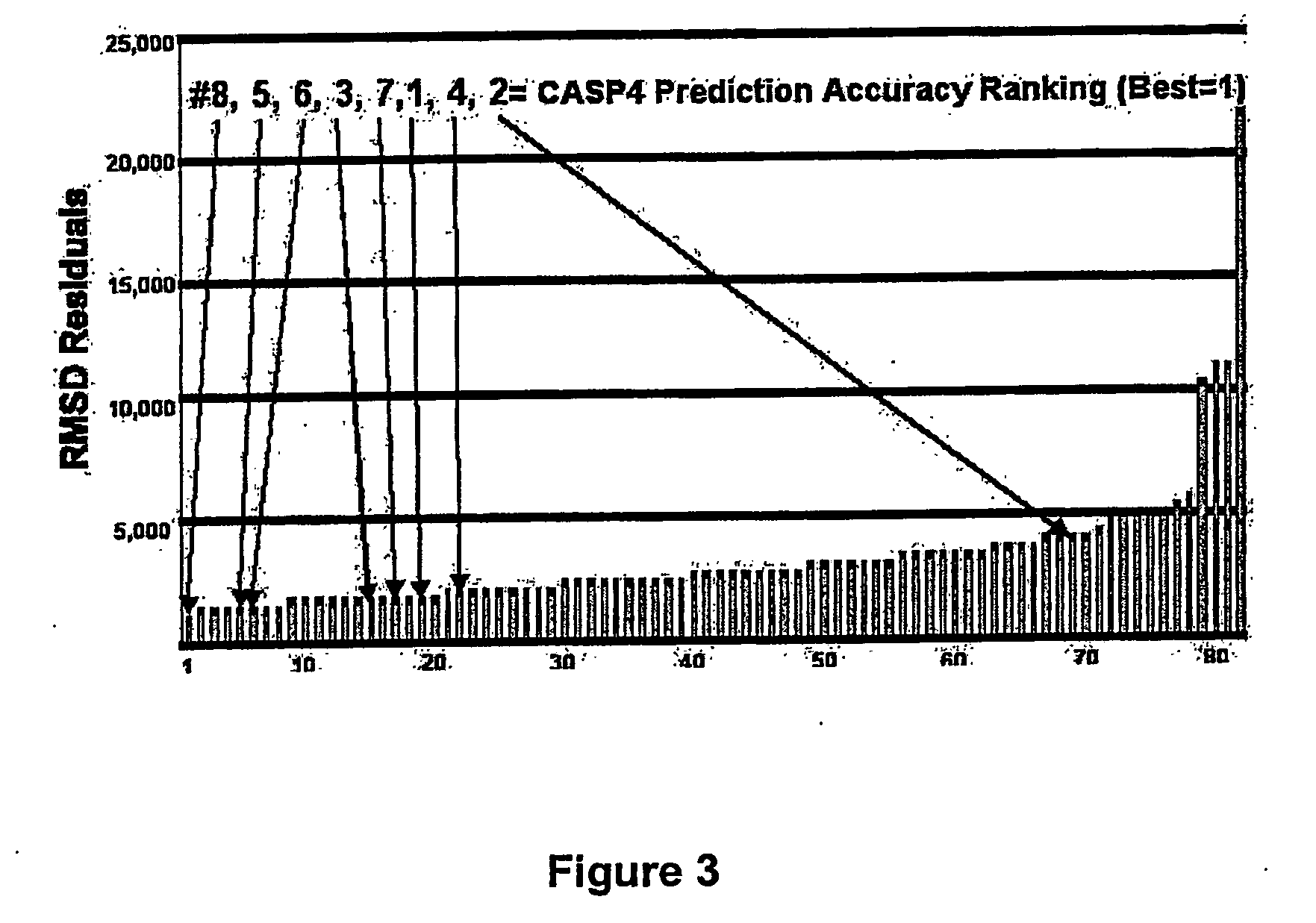
Methods for the determination of protein three-dimensional structure employing hydrogen exchange analysis to refine computational structure prediction
InactiveUS20070122864A1Improve accuracySpeeding calculationMicrobiological testing/measurementBiological material analysisCrystallographyHydrogen exchange
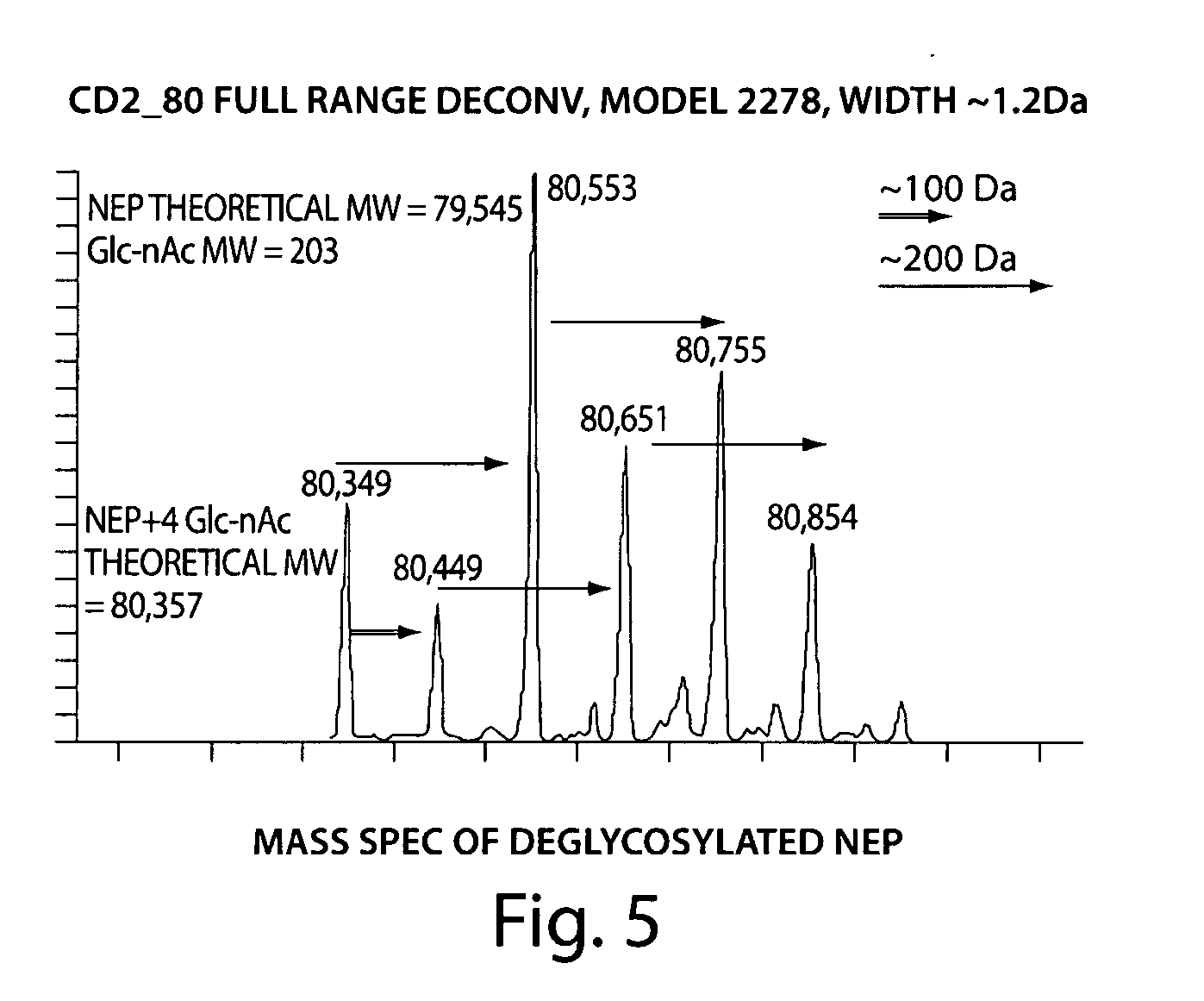
The present invention provides methods of structure prediction and / or determination of a protein of interest of unknown structure. Invention methods compare calculated rates of amide hydrogen exchange for a set of predicted possible structures for said protein with experimental hydrogen exchange analysis of said protein to identify valid protein structures based on similarities between hydrogen exchange profiles. In preferred methods, hydrogen exchange analysis is performed by determining the quantity of isotope and / or rate of exchange of peptide amide hydrogen(s) with isotope on a labeled protein, by generating a population of sequence-overlapping endopeptidase fragments of a protein labeled with a hydrogen isotope other than 1H under conditions of slowed hydrogen exchange, and then deconvoluting fragmentation data acquired from said population of sequence-overlapping endopeptidase-generated fragments.
Owner:THE UNIV OF TEXAS MEDICAL BRANCH +1
Cyclin imide peptidyl metalloprotease inhibitor and its application
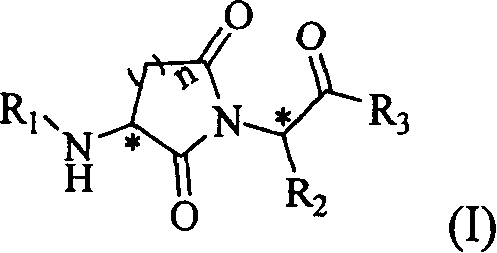
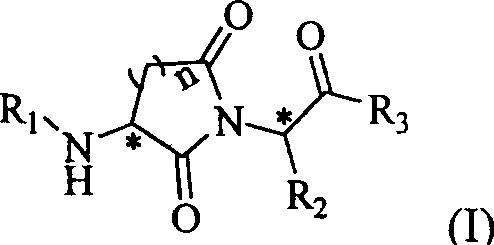
InactiveCN1974554ANo cytotoxic activityHigh cytotoxic activityOrganic active ingredientsDipeptidesEndopeptidaseDrug
The present invention provides one kind of powerful peptide-like metalloprotease inhibitor, which exhibits obvious selectivity between endopeptidase and exopeptidase and may be used in treating diseases with abnormal active expression of metalloprotease effectively. Specifically, the present invention relates to the peptide-like compound in the general expression as shown, and its optical isomers, pharmaceutically acceptable salts, solvates and medicine precursor. The present invention also relates to the medicine composition containing the peptide-like compound and its medicinal use.
Owner:SHANDONG UNIV
Method of manufacturing meat extract, and meat extract
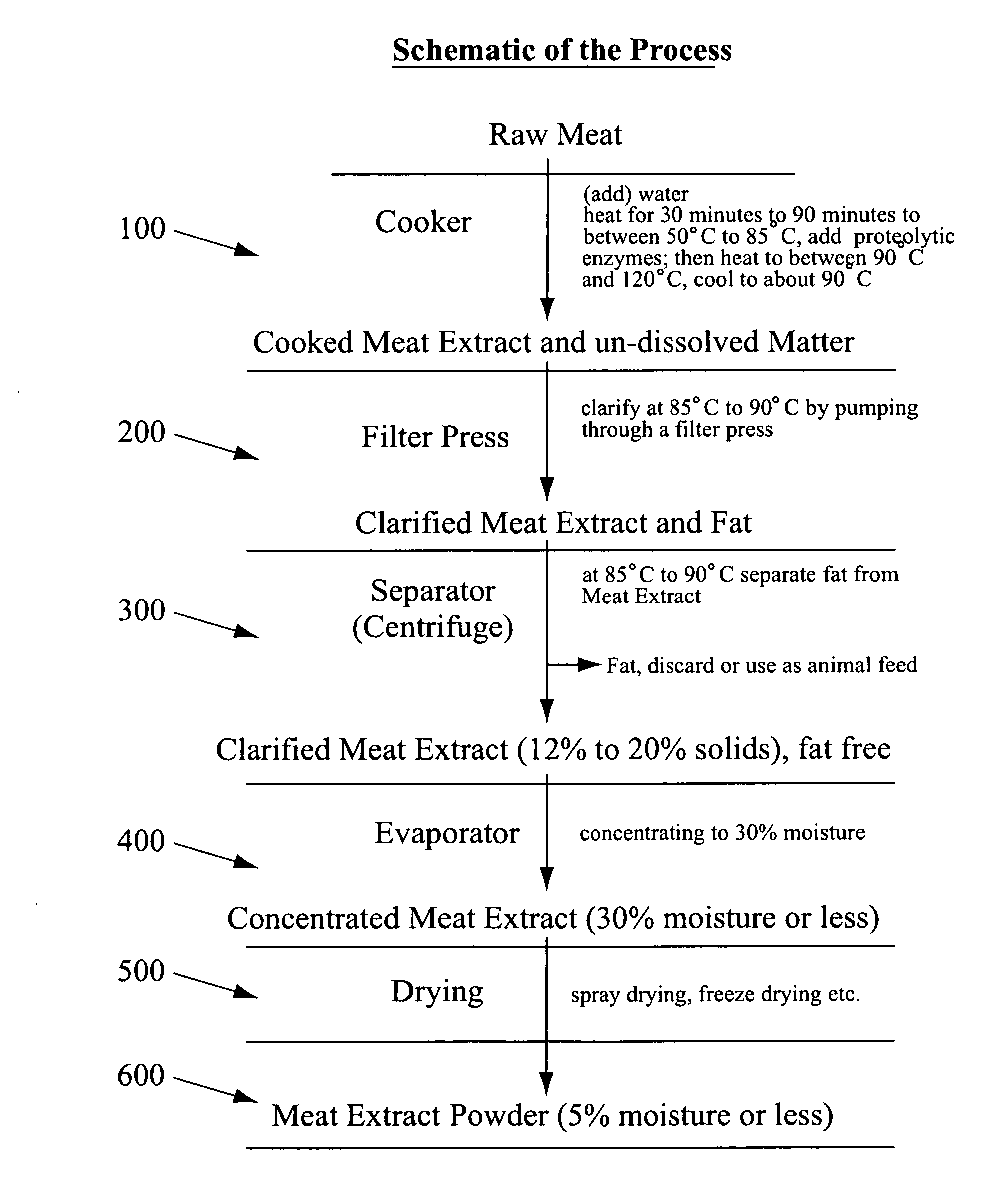
InactiveUS20110250316A1Overcome deficienciesSolid waste disposalFood preparationSalt contentEssential amino acid
Meat extract (warm and / or cold blooded animal) having a protein content of at least about 50% or greater and is substantially fluid or pourable at ambient temperature of about 15-25 degrees C. or more. The meat extract may have a viscosity ranging between about 1000 cP to no more than about 40000 cP for temperatures of about 20-80 degrees C. The meat extract may have a protein content of at least about 50%-about 70% with an extract moisture content of about 25%-40%, and a salt content of less than about 5%, e.g., less than 1%. The protein may have a minimum of about 22% of total essential amino acids, e.g., about 3.0% of L-Leucine and / or about 0.2% of L-Tryptophan. Also disclosed is a process of manufacturing a meat extract by extracting meat with water and at least one enzyme, e.g., a proteolytic enzyme, e.g., at least one endopeptidase (amino-endopeptidase and / or at least one carboxy-endopeptidase) and / or at least one exopeptidase, e.g., at least one amino-exopeptidase and / or at least one carboxy-exopeptidase. The extracting may takes place at an extraction temperature of between about 45° C. and about 80° C., e.g., for a period of between about 30 minutes and about 5 hours, and may be followed by subsequent heating to a temperature of between about 90° C. and about 120° C., e.g., between about 15 minutes and about 1 hour.
Owner:NUTRITION RES & MFG
Protein conjugate having an endopeptidase- cleavable bioprotective moiety
InactiveUS20120121613A1Extended half-lifeFactor VIIPeptide/protein ingredientsOrganic chemistryEndopeptidase
The invention is directed to a procoagulant conjugate having an endopeptidase-activatable procoagulant protein moiety and one or more bioprotective moieties, which are conjugated to one another by a linker that is cleaved by an endopeptidase in situ to release the bioprotective moiety. The invention is also directed to therapeutic uses of the procoagulant conjugate and methods of making the conjugate.
Owner:BAYER HEALTHCARE LLC
Rothia species glutamine endopeptidases and use thereof
The invention relates to glutamine endopeptidase enzymes from Rothia spp. bacteria that are naturally associated with the oral cavity, formulations comprising the glutamine endopeptidase enzymes and the use thereof for the treatment, prevention of allergic reaction and diagnosis of gluten allergy related diseases such as Celiac Sprue, gluten allergy and / or dermatitis herpetiformis.
Owner:TRUSTEES OF BOSTON UNIV
Improved preparation method of sacubitril intermediate
InactiveCN106397273ACarbamic acid derivatives preparationOrganic compound preparationPalladium catalystSacubitril
The invention relates to an improved preparation method of an important intermediate (2R, 4S)-5-(biphenyl-4-yl)-4-tert-butoxycarbonylamino-2-methyl pentanoic acid (that is a compound shown in the formula III) for preparing sacubitril which is a neutral endopeptidase (NEP) inhibitor and of an analog or salt of the intermediate. The method mainly comprises that in the presence of a palladium catalyst, hydrogenation is carried out to make the compound shown in the formula III and having high optical purity and the analog or salt of the compound. The improved method is available in raw materials, mild in reaction condition, and simple and convenient to operate. The product quality is excellent and the cost is low, so that the method is suitable for industrial mass production.
Owner:SICHUAN HAISCO PHARMA CO LTD
Preparation method for insulin aspart through recombinant expression by using yeast
ActiveCN105087724AImprove digestion efficiencyLow miscut rateMicroorganism based processesInsulinsEnzyme digestionThreonine
The invention discloses a preparation method for insulin aspart through recombinant expression by using yeast, and concretely relates to a preparation method for insulin aspart through recombinant expression by using pichia yeast. Concretely, the method comprises the following technological process: effectively secreting and expressing human aspart proinsulin, performing lysyl endopeptidase single enzyme digestion to obtain insulin aspart deleting B30, coupling with a threoninate, and performing deprotection, anti-phase purification and crystallization. The method is relatively suitable for industrialized preparation of recombinant insulin aspart.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
Glucagon analog for treatment of metabolic diseases
ActiveCN109836488AGood enzyme resistance and stabilityLong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDipeptidyl peptidase
The present invention relates to the field of biopharmaceuticals, and in particular to a glucagon analog for treating metabolic diseases. The structural formula is: H-X2-X3-GTFTSD-X10-SKYLD-X16-X17-AAQ-DFVQWLMN-X29-X<z> or H-S-Q-GTFTSD-Y-SKYLD-X16-X17-AAQ-DFVQWLMN-X29-Xz-NH2. The described glucagon analogue has a GLP-1 / GCG / GIP triple receptor agonist activity and better enzyme resistant stabilityfor neutral endopeptidase (NEP) and dipeptidyl peptidase-4(DPP-4), and has a longer half-life in vivo and duration of action compared with natural glucagon, GLP-1 and GIP.
Owner:ZHEJIANG DOER BIOLOGICS CO LTD
Aspergillus niger inulin endopeptidase gene and recombinant Pichia strain for expressing same
The invention concerns aspergillus niger 9891 CGMCC NOú‘0991, which produces alantin endonuclease, and clones alantin endonuclease gene. The result of target gene fragment sequence analysis indicates that open reading frame of gene not containing signal peptide is 1485bp, and codes 509 amino acid, the said protein molecular weight is 55.9KD. The homolog of said gene with counterpart of aspergillus niger (Ohtak.et alú¼ 1998)and Aspergillus ficuum(Uhmú¼t.ú¼et alú¼1998) are respectively 92úÑ and 95úÑ. The alantin endonuclease gene is inserted into Pichia pastoris expression vector, thus recombinant of transformed Pichia pastoris is obtained. The said gene expresses in Pichia pastoris, and expressed product has its function. The expression amount of good recombinant I3-50 is 84 times higher than alantin endonuclease of initial strain. Recombinant yeast is induced and fermented. The analysis about recombinant enzyme shows that its optimum PH is 5.5ú¼ and optimum reaction temperature is 55íµ..
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES +1
Recombinant light chains of botulinum neurotoxins and light chain fusion proteins for use in research and clinical therapy
InactiveUS20070104737A1High A+T rich base compositionFungiBacteriaEndopeptidaseNeuromuscular junction
Botulinum neurotoxins, the most potent of all toxins, induce lethal neuromuscular paralysis by inhibiting exocytosis at the neuromuscular junction. The light chains (LC) of these dichain neurotoxins are a new class of zinc-endopeptidases that specifically cleave the synaptosomal proteins, SNAP-25, VAMP, or syntaxin at discrete sites. The present invention relates to the construction, expression, purification, and use of synthetic or recombinant botulinum neutoroxin genes. For example, a synthetic gene for the LC of the botulinum neurotoxin serotype A (BoNT / A) was constructed and overexpressed in Escherichia coli. The gene product was purified from inclusion bodies. The methods of the invention can provide 1.1 g of the LC per liter of culture. The LC product was stable in solution at 4° C. for at least 6 months. This rBoNT / A LC was proteolytically active, specifically cleaving the Glu-Arg bond in a 17-residue synthetic peptide of SNAP-25, the reported cleavage site of BoNT / A. Its calculated catalytic efficiency kcat / Km was higher than that reported for the native BoNT / A dichain. Treating the rBoNT / A LC with mercuric compounds completely abolished its activity, most probably by modifying the cysteine-164 residue located in the vicinity of the active site. About 70% activity of the LC was restored by adding Zn2+ to a Zn2+-free, apo-LC preparation. The LC was nontoxic to mice and failed to elicit neutralizing epitope(s) when the animals were vaccinated with this protein. In addition, injecting rBoNT / A LC into sea urchin eggs inhibited exocytosis-dependent plasma membrane resealing.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00000.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00001.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka.patsnap.com/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-C00001.png)