Patents
Literature
331 results about "Recombinant vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccine generated using recombinant DNA technology is called recombinant vaccine. While there are various types of vaccines made possible by recombinant DNA technology, recombinant vaccines can be classified into two major categories.
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Vaccines and immunotherapeutics using codon optimized IL-15 and methods for using the same
InactiveUS8178660B2Sugar derivativesGenetic material ingredientsImmunotherapeutic agentRecombinant vaccines
Nucleic acid molecules that encode IL-15 or fragments thereof, which express protein at a higher level than nucleic acid molecules with native coding sequences for IL-15 are disclosed. Nucleic acid molecules with additional modifications such as the absence of coding sequences for IL-15 signal sequences and / or the absence of IL-15 untranslated sequences and / or inclusion of non-IL-15 signal sequences are also disclosed. Vectors, including plasmids and viral vectors, comprising such nucleic acid molecules; and to host cells comprising such nucleic acid molecules are disclosed as well as methods of using such nucleic acid molecules alone or in combination with nucleic acid sequences encoding immunogens which are part of the nucleic acid molecules and / or part of a different nucleic acid molecule. Recombinant vaccines and live attenuated pathogens encoding fusion proteins, and methods of using the same, are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant vaccine against botulinum neurotoxin
InactiveUS7081529B2Fast and efficient purificationBacterial antigen ingredientsBacteriaVaccinationRecombinant vaccines
This invention is directed to preparation and expression of synthetic genes encoding polypeptides containing protective epitopes of botulinum neurotoxin (BoNT). The invention is also directed to production of immunogenic peptides encoded by the synthetic genes, as weel as recovery and purification of the immunogenic peptides from recombinant organisms. The invention is also directed to methods of vaccination against botulism using the expressed peptides.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Live listeria-based vaccines for central nervous system therapy
InactiveUS20110223187A1Bacterial antigen ingredientsAntibody mimetics/scaffoldsCns tumorRecombinant vaccines
This invention is directed to methods for treating a central nervous system (CNS) tumor or cancer using live Listeria-based recombinant vaccines.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
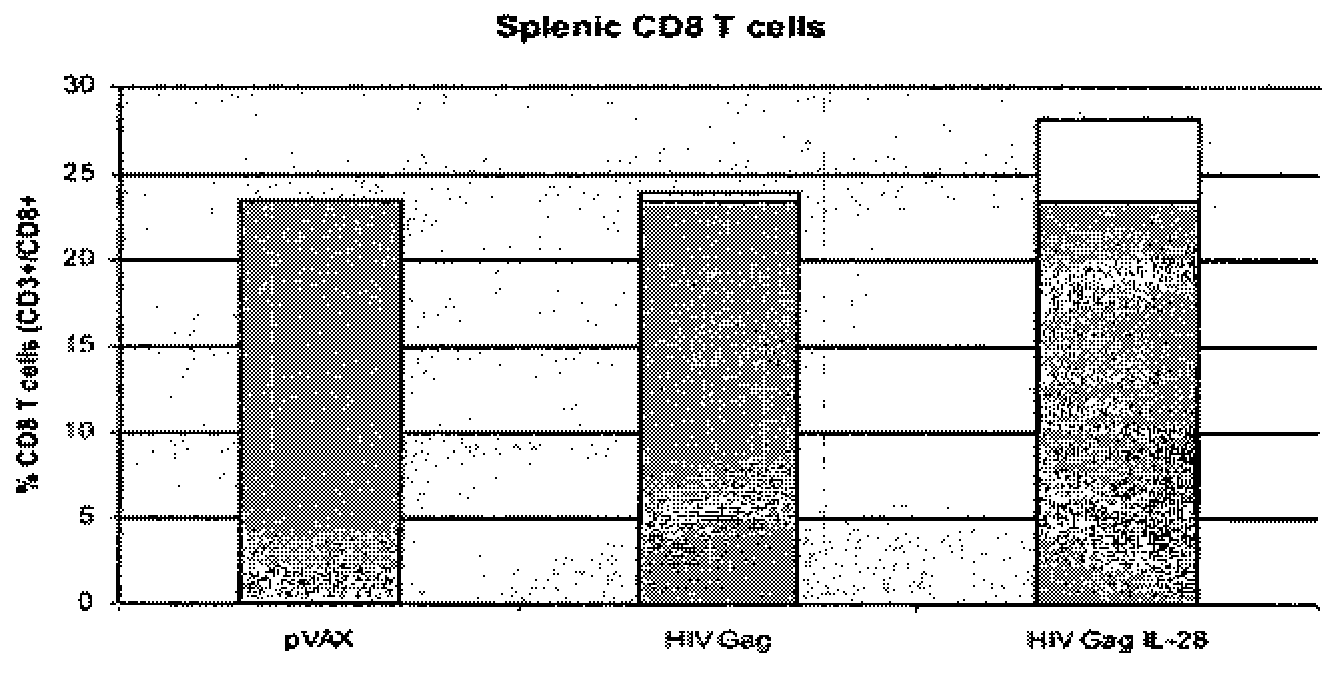
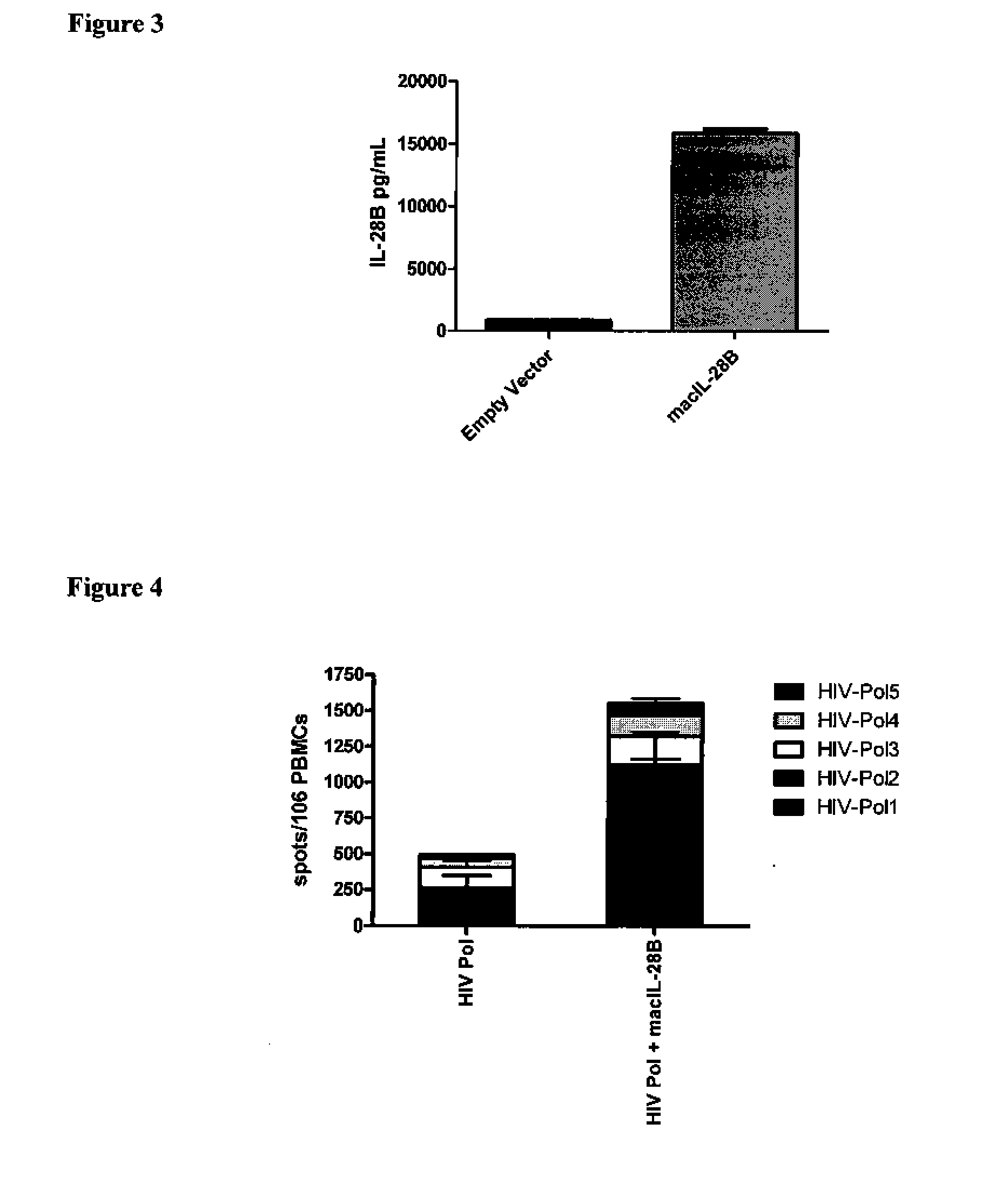
Vaccines and immunotherapeutics using il-28 and compositions and methods of using the same
ActiveUS20110104105A1SsRNA viruses negative-senseAntibacterial agentsImmunotherapeutic agentRecombinant vaccines
Compositions, recombinant vaccines and live attenuated pathogens comprising one or more isolated nucleic acid molecules that encode an immunogen in combination with an isolated nucleic acid molecule that encodes IL-28 or a functional fragment thereof are disclosed. Methods of inducing an immune response in an individual against an immunogen, using such compositions are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant vaccines comprising immunogenic attenuated bacteria having RpoS positive phenotype
InactiveUS7083794B2Improve balanceImproving immunogenicityAntibacterial agentsBiocideSalmonella entericaSalmonella serotype typhi
Attenuated immunogenic bacteria having an RpoS+ phenotype, in particular, Salmonella enterica serotype Typhi having an RpoS+ phenotype and methods therefor are disclosed. The Salmonella have in addition to an RpoS+ phenotype, an inactivating mutation in one or more genes which render the microbe attenuated, and a recombinant gene capable of expressing a desired protein. The Salmonella are attenuated and have high immunogenicity so that they can be used in vaccines and as delivery vehicles for genes and gene products. Also disclosed are methods for preparing the vaccine delivery vehicles.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Vaccines, immunotherapeutics and methods for using the same
InactiveUS8119395B1Enhance and modulate immune responseUseful for immunotherapyAntibacterial agentsVirusesDiseaseAntigen
Improved vaccines which include a nucleotide sequence that encodes immunomodulating protein operably linked to regulatory elements are disclosed. The improved vaccines include DNA vaccines, recombinant vaccines for delivering foreign antigen and live attenuated vaccines. Methods of immunizing individuals are disclosed. Compositions for and methods of treating individuals with autoimmune diseases are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Vaccines and methods for using the same
ActiveUS20100166787A1SsRNA viruses negative-senseOrganic active ingredientsRecombinant vaccinesBioinformatics
Improved anti-HIV immunogens and nucleic acid molecules that encode them are disclosed, Immunogens disclosed include those having consensus sequences for HIV Subtype A Envelope protein, those having consensus sequences for HIV Subtype B Envelope protein, those having consensus sequences for HIV Subtype C Envelope protein, those having consensus sequences for HIV Subtype D Envelope protein, those having consensus sequences for HIV Subtype B consensus Nef-Rev protein, and those having consensus sequences form HIV Gag protein subtypes A, B, C and D. Improved anti-HPV immunogens and nucleic acid molecules that encode them; improved anti-HCV immunogens and nucleic acid molecules that encode them; improved hTERT immunogens and nucleic acid molecules that encode them; and improved anti-Influenza immunogens and nucleic acid molecules that encode them are disclosed. Pharmaceutical composition, recombinant vaccines comprising and live attenuated pathogens are disclosed as well methods of inducing an immune response in an individual against HIV, HPV, HCV, hTERT and Influenza are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant avian herpesvirus useful in vaccine production
InactiveUS6913751B2SsRNA viruses negative-senseSsRNA viruses positive-senseInfectious laryngotracheitisInfectious laryngotracheitis virus
The present invention provides a novel avian herpesvirus (NAHV) vector and recombinant vaccines made therefrom that are useful to immunize avian species against Marek's disease, infectious laryngotracheitis and Newcastle disease. Methods of immunizing an avian species against Marek's disease, infectious laryngotracheitis and Newcastle disease are also provided.
Owner:SCHERING PLOUGH ANIMAL HEALTH
Recombinant vaccine viruses expressing il-15 and methods of using the same
InactiveUS20060147419A1Stimulates proliferationStimulates differentiationBiocideGenetic material ingredientsAdjuvantMammal
The invention is directed to compositions capable of augmenting the immunogenicity of a vaccine. The composition, or adjuvant, is administered to a mammal in need thereof in sequential or concurrent combination with a vaccine antigen. In one preferred aspect, the adjuvant is provided in the form of a recombinant poxvirus vector, such as a vaccinia virus vector, which comprises a nucleic acid sequence encoding IL-15.
Owner:UNITED STATES OF AMERICA
Recombinant vaccine against West Nile Virus
ActiveUS20050255127A1Provide securityPractical and convenientSsRNA viruses positive-senseViral antigen ingredientsAdjuvantRecombinant vaccines
An immunogenic or vaccine composition to induce an immune response or protective immune response against West Nile virus (WNV) in an animal susceptible to WNV. The composition includes a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector contains heterologous nucleic acid molecule(s), expresses in vivo in the animal WNV antigen, immunogen or epitope thereof, e.g., WNV E; WNV prM and E; WNV M and E; WNV prM, WNV M and E, WNV polyprotein prM-E, WNV polyprotein M-E, or WNV polyprotein prM-M-E. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL LTD
Newcastle disease virus vectored avian vaccines
The present invention encompasses engineered Newcastle Disease Virus (NDV) vaccines or compositions. The vaccine or composition may be a recombinant vaccine. The invention also encompasses recombinant vectors encoding and expressing avian pathogen antigens, more specifically avian influenza proteins, epitopes or immunogens. Such vaccines or compositions can be used to protect animals, in particular avian, against disease.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
A type foot-and-mouth disease recombinant vaccine strains and preparation method and application thereof
ActiveCN103266091AMultiple phenotype improvements and enhancedPhenotype Improvement and EnhancementMicroorganism based processesAntiviralsAntigenDisease
The invention relates to A type foot-and-mouth disease recombinant vaccine strains prepared by using a reverse genetic manipulation technology and a preparation method and application of the strains. One strain is an A type foot-and-mouth disease recombinant vaccine strain with high titer, antigen matching property and immune protection rate, and the other strain is an A type foot-and-mouth disease recombinant non-pathogenic vaccine strain with high titer, antigen matching property and immune protection rate and without pathogenicity for a host; an antigen nucleotide sequence of each of the vaccine strains is shown as SEQ ID NO: 1; eukaryotic plasmids of viruses can be saved by using a reverse genetic manipulation system; after pigs and cattle are immunized by using the inactivated vaccines prepared from the prepared recombinant vaccine strains, the bodies can be effectively stimulated to produce immune response, and an immune protection effect is provided for the bodies of the pigs and the cattle; through a 10,000-times cattle median infectious dose (BID50) challenge assay of A type AISA topological strains, the immune protection rate reaches 100 percent, and the median protective dose (PD50) is 10.81 to 13.59; and the recombinant vaccine strains can be applied to prevention and control of A type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Vaccines and immunotherapeutics using codon optimized il-15 and methods for using the same
InactiveUS20100003277A1Sugar derivativesViral antigen ingredientsImmunotherapeutic agentRecombinant vaccines
Nucleic acid molecules that encode IL-15 or fragments thereof, which express protein at a higher level than nucleic acid molecules with native coding sequences for IL-15 are disclosed. Nucleic acid molecules with additional modifications such as the absence of coding sequences for IL-15 signal sequences and / or the absence of IL-15 untranslated sequences and / or inclusion of non-IL-15 signal sequences are also disclosed. Vectors, including plasmids and viral vectors, comprising such nucleic acid molecules; and to host cells comprising such nucleic acid molecules are disclosed as well as methods of using such nucleic acid molecules alone or in combination with nucleic acid sequences encoding immunogens which are part of the nucleic acid molecules and / or part of a different nucleic acid molecule. Recombinant vaccines and live attenuated pathogens encoding fusion proteins, and methods of using the same, are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant novel coronavirus as well as preparation method and application thereof
ActiveCN111560354ASolve the shortage problemSsRNA viruses negative-senseSsRNA viruses positive-senseAntigen epitopeAntigen
The invention discloses a recombinant novel coronavirus as well as a preparation method and application thereof. The invention firstly discloses a recombinant virus. Any one of an NS gene of an influenza virus, an HA gene of the influenza virus and an NA gene of the influenza virus of the recombinant virus is replaced. The invention further discloses application of the recombinant virus in preparation of a product for preventing and / or treating diseases caused by influenza virus and / or SARS-CoV-2. According to the invention, the SARS-CoV-2 antigen epitope and the influenza virus genome are operated from the gene level; the recombinant SARS-CoV-2 vaccine strain taking the influenza virus as the carrier is prepared on the basis of an RG technology, so that the problem of SARS-CoV-2 vaccine shortage is solved. The recombinant novel coronavirus will be a new milestone in the field of coronavirus vaccines, and the SARS-CoV-2 chimeric vaccine can protect more people from being harmed by theinfluenza virus and SARS-CoV-2.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL +1
Recombinant vaccine against bluetongue virus
ActiveUS20070280960A1Provide securityPractical and convenientAntibacterial agentsViral antigen ingredientsAntigenAdjuvant
The present invention relates to an immunogenic or vaccine composition to induce an immune response or protective immune response against Orbiviruses, more specifically bluetongue virus (BTV) in an animal susceptible to BTV infection. The composition may include a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector may contain heterologous nucleic acid molecule(s), expresses in vivo in the animal BTV antigen, immunogen or epitope thereof, e.g., BTV VP2; BTV VP2 and VP5; BTV VP2 and VP5 and VP3 and / or VP7. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.AGACAGTGGTCAATTCCAATGGTACTGTTTGACGATAC
Owner:MERIAL LTD +3
Establishing method of bacterial artificial chromosome recombinant duck plague virus rescue system platform and application
InactiveCN105802922ALower titerDoes not affect the replication cycleVirus peptidesNucleic acid vectorBacteroidesRecombinant vaccines
The invention discloses an establishing method of a bacterial artificial chromosome recombinant duck plague virus rescue system platform and application of the platform. A bacterial artificial chromosome recombinant duck plague virus is obtained by inserting a recombinant duck plague virus transfer vector pUC18 / EGFP-TKAB-BAC11 in a TK domain, wherein the recombinant duck plague virus transfer vector contains a TK gene left-right homologous arm, a reporter gene EGFP and a bacterial artificial chromosome core function component. By means of the platform, the in-vitro biologics characteristics of a UL55 gene-deleted strain established through an inside-bacterium two-step RED recombination method and a back mutation strain and parent strain of the UL55 gene-deleted strain are quite close; the functions are not related to positioning of a UL26.5 gene in a cell. The method is beneficial to development of pathogenic mechanism and gene function research of DPV CHv and is beneficial to the duck plague virus prevention and the research and application of recombinant duck plague virus vaccines of other poultry infectious diseases based on the platform; in addition, due to the fact that the recombinant virus carries a TK deletion mark and an EGFP gene, a mark vaccine can be developed to clinically distinguish a wild virus and a recombinant vaccine virus.
Owner:SICHUAN AGRI UNIV
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
Ehrlichia canis genes and vaccines
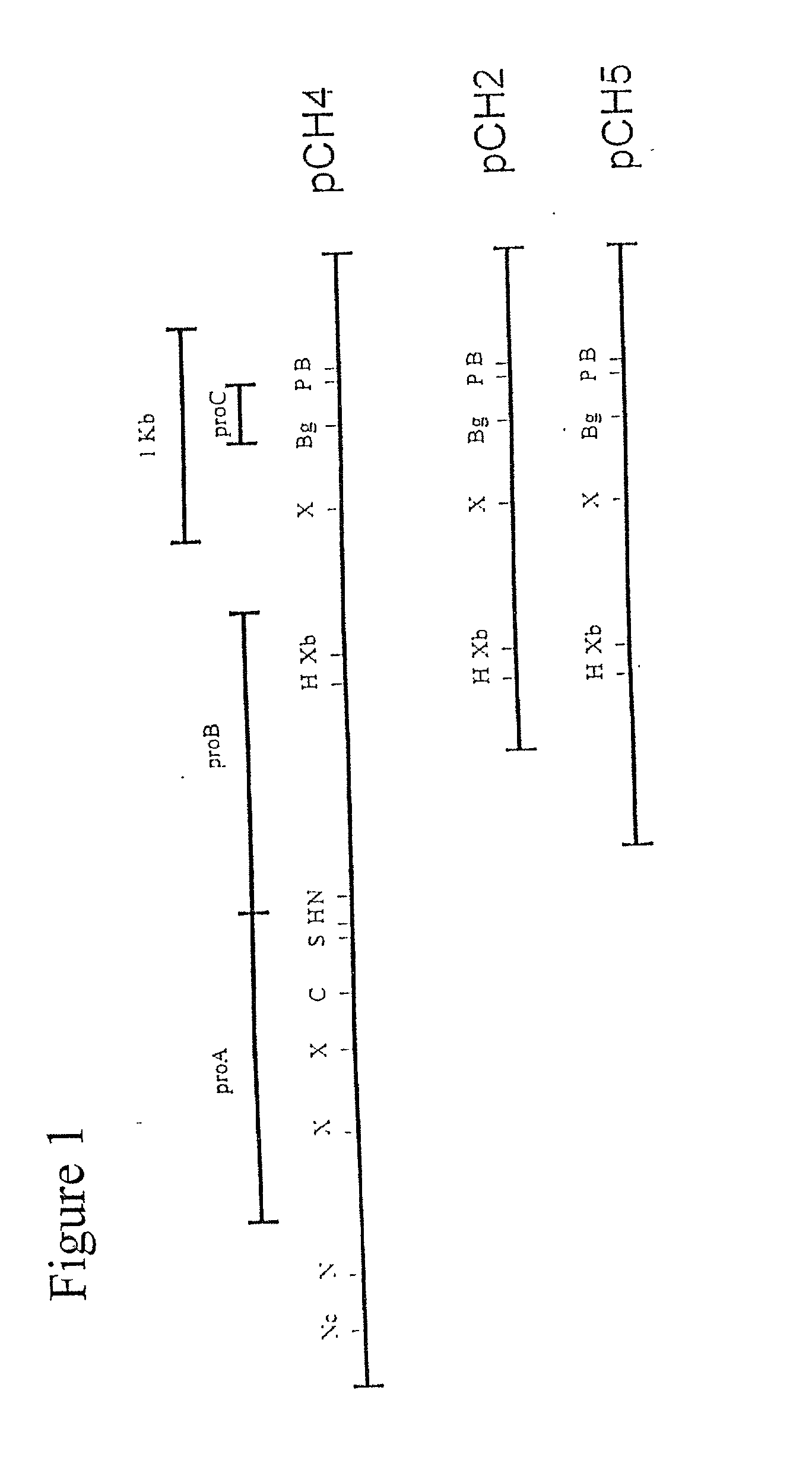
This invention provides the sequence of 5,299 nucleotides from the E. canis genome. There are four proteins, ProA, ProB, MmpA, and a cytochrome oxidase homolog, as well as a partial lipoprotein signal peptidase homolog at the carboxy terminus, coded for in this cloned fragment. The antigenic properties of these proteins allow them to be used to create a vaccine. An embodiment of this invention includes the creation of a DNA vaccine, a recombinant vaccine, and a T cell epitope vaccine. Another embodiment of this invention includes the use of serological diagnosis techniques.
Owner:CORNELL RES FOUNDATION INC
Needle-free administration of FeLV vaccines
ActiveUS20060034867A1Improve securityEasy and less-expensive to useViral antigen ingredientsMicrobiological testing/measurementNeedle freeLiquid jet
The invention provides a novel method of vaccination of an animal of the felidae family against feline leukemia. The FeLV recombinant vaccine based on viral vector with the aid of a liquid jet needle-free injector can result in distribution of the vaccine essentially in the dermis and the hypodermis of the animal.
Owner:MERIAL INC
Vaccines for human papilloma virus and methods for using the same
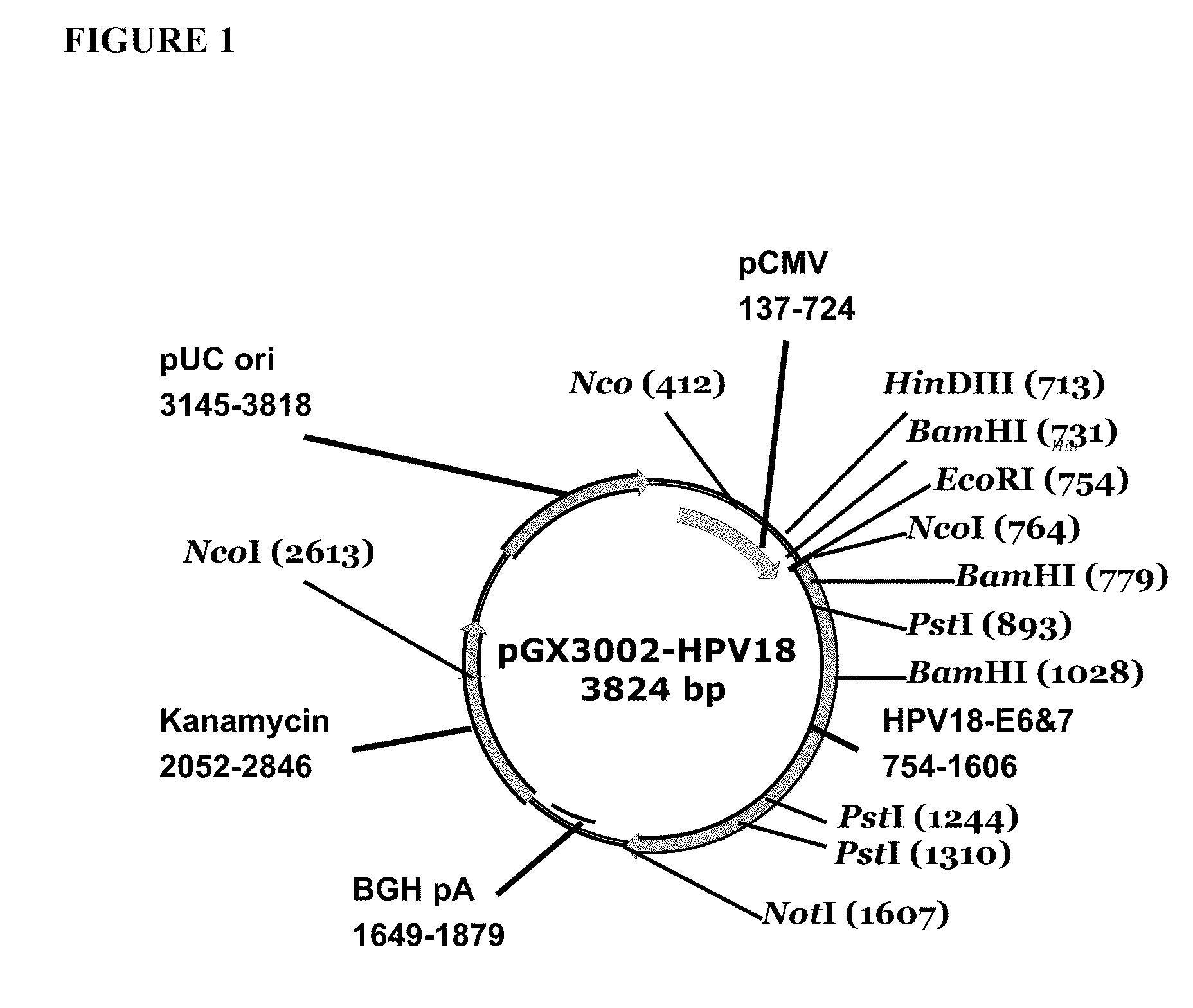
ActiveUS20100189730A1Improved immunogenic targetImproved immunogenic targetsSsRNA viruses negative-senseOrganic active ingredientsRecombinant vaccinesAttenuated vaccine
Improved anti-HPV immunogens and nucleic acid molecules that encode them are disclosed. Immunogens disclosed include those having consensus HPV 18 E6 and E7. Pharmaceutical composition, recombinant vaccines comprising and live attenuated vaccines are disclosed as well methods of inducing an immune response in an individual against HPV are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Hetero type pentamer recombinant vaccine
A heteropentamer composed of a fusion monomer (32) comprising a fusion protein of an antigenic amino acid sequence and an amino acid sequence of a monomer of a mucous membrane-binding protein and a nonfusion monomer (20) of an amino acid sequence of a monomer of a mucous membrane-binding protein. A homopentamer composed of a fusion monomer including a fusion protein of an antigen derived from an envelope protein of Japanese encephalitis virus and an amino acid sequence of a monomer of a mucous membrane-binding protein. These heteropentamer and the homopentamer can be used as a vaccine.
Owner:ADVANCED MEDICAL BIOLOGICAL SCI INST +1
Hcv vaccines and methods for using the same
ActiveUS20120034256A1Improved immunogenic targetOrganic active ingredientsSsRNA viruses positive-senseRecombinant vaccinesAttenuated vaccine
Owner:INOVIO PHARMA +1
Genetically engineered rabies recombinant vaccine for immunization of stray dogs and wildlife
InactiveUS7074413B2Reduce absorptionEnhance immune responseSsRNA viruses negative-senseVectorsWildlifeRecombinant vaccines
Live, attenuated recombinant rabies virus vaccines are generated using reverse genetics to combine the antigenic determinants that render the rabies virus non-pathogenic with the determinants that are responsible for the elicitation of an effective anti-rabies immune response. These vaccines do not affect the antigenic, and therefore the immunogenic, properties of the virus. The present invention further relates to recombinant rabies virus vaccines that express a pro-apoptotic protein, such as cytochrome c, to increase the capacity to induce apoptosis, thereby enhancing the protective immunity against rabies. This new generation of live rabies virus vaccines represents a safe and effective approach to the eradication of rabies in wildlife, and subsequently humans and livestock.
Owner:UNITED STATES OF AMERICA +1
Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and preparation method thereof
ActiveCN101775399AImproving immunogenicityFacilitate presentationGenetic material ingredientsVirus peptidesAdjuvantRecombinant vaccines
The invention discloses an Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and a preparation method thereof. The recombinant vaccine comprises the following components: proteins coded by foot-and-mouth disease virus multi-epitope genes and the fusion genes of carrier proteins, and foot-and-mouth disease virus 3D proteins. The preparation method comprises the following steps: diluting the proteins expressed by the foot-and-mouth disease virus multi-epitope genes and the fusion genes of the carrier proteins and the foot-and-mouth disease virus 3D proteins, mixing the diluted proteins uniformly, adding an adjuvant into the mixture to emulsify the mixture. Animal models and animal immune effect experiments show that the bovine Asia1 epitope recombinant vaccine can make comprehensive immune protective response, can induce injected and immunized bovine and guinea pigs to generate high level neutralizing antibodies, and can also induce cell immune response, so the recombinant vaccine can effectively protect animals against the virulent attack of the foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant Swine pox virus (SPV) vector vaccine for the expression of Streptococcus equi subsp zooepidemicus (SEZ) M-like protein (SzP)
InactiveCN102198268ANot pathogenicEasy Security EvaluationAntibacterial agentsBacterial antigen ingredientsProtein targetAdjuvant
The invention belongs to the field of biological pharmacy. The invention provides a recombinant vaccine, comprising a SPV and one pharmaceutically acceptable vector and / or adjuvant or a plurality of such vectors and / or adjuvants. The SPV comprises an SPV vector and the encoding genes of SzP. The recombinant SPV vaccine provided in the invention can proliferate in large quantities in immune animalbodies, express target protein SzP and induce the generation of high titer antibodies in animal bodies, and exerts a good protective effect on immune animals.
Owner:NANJING AGRICULTURAL UNIVERSITY
HPV vaccines and methods for using the same
ActiveUS8168769B2SsRNA viruses negative-senseSsRNA viruses positive-senseHPV vaccinesRecombinant vaccines
Improved anti-HIV immunogens and nucleic acid molecules that encode them are disclosed, Immunogens disclosed include those having consensus sequences for HIV Subtype A Envelope protein, those having consensus sequences for HIV Subtype B Envelope protein, those having consensus sequences for HIV Subtype C Envelope protein, those having consensus sequences for HIV Subtype D Envelope protein, those having consensus sequences for HIV Subtype B consensus Nef-Rev protein, and those having consensus sequences form HIV Gag protein subtypes A, B, C and D. Improved anti-HPV immunogens and nucleic acid molecules that encode them; improved anti-HCV immunogens and nucleic acid molecules that encode them; improved hTERT immunogens and nucleic acid molecules that encode them; and improved anti-Influenza immunogens and nucleic acid molecules that encode them are disclosed. Pharmaceutical composition, recombinant vaccines comprising and live attenuated pathogens are disclosed as well methods of inducing an immune response in an individual against HIV, HPV, HCV, hTERT and Influenza are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Sheep aphthovirus Asial type multi-epitope recombinant vaccine and preparation method thereof
ActiveCN101864434AComprehensive immune efficiencyComprehensive evaluation of immune efficiencyGenetic material ingredientsVirus peptidesAdjuvantAdditive ingredient
The invention discloses a sheep aphthovirus Asial type multi-epitope recombinant vaccine and a preparation method thereof. The recombinant vaccine comprises the following ingredients: a protein encoded by aphthovirus multi-epitope genes and carrier protein fusion genes, and an aphthovirus 3D protein. The preparation method comprises the following steps: respectively diluting the protein encoded by the aphthovirus multi-epitope genes and the carrier protein fusion genes and the aphthovirus 3D protein; then, uniformly mixing the diluted proteins; adding auxiliary agents into the mixture; carrying out emulsification; and obtaining the sheep aphthovirus Asia1 type multi-epitope recombinant vaccine. Animal model and animal immune efficiency experiments show that the sheep Asial epitope recombinant vaccine of the invention can generate overall immunoprotection reaction, injected immune sheep can be induced to generate high-level neutralizing antibodies, and can also induce the cellullar immunologic response. The recombinant vaccine of the invention can effectively protect animals from the virulent strain attack of the aphthovirus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
I-group 4-type fowl adenovirus DNA vaccine and application thereof
InactiveCN106729694AEffective immune protectionGood prospects for commercial developmentDigestive systemAntiviralsFiberRecombinant vaccines
The invention provides an I-group 4-type fowl adenovirus DNA vaccine and application thereof. According to the technical scheme, based on experimental means, research finds and shows that fiber protrusions have good immunity prototypes, in this way, with 4-type fowl adenovirus fibrous protein C-terminal genes being antigen substances, codon optimization is carried out on the basis of a natural sequence, an eukaryotic expression vector pCAGGS is cloned, and the DNA vaccine pCAGoptiFAV4C is constructed. According to the researched fowl adenovirus NDA vaccine, a method of gene engineering fermentation is adopted for preparing antigens, and therefore the I-group 4-type fowl adenovirus DNA vaccine is low in cost, pure in antigen and safe to use. By the utilization of a serology method and an immunity virus attack method, the immunity effect of the vaccine is evaluated. The result shows that the vaccine can provide effective immune protection for fowl, and has good commercial development prospects. Compared with a traditional vaccine, the DNA vaccine achieves the safety of subunit vaccines and inactivated vaccines, and also has the features of simultaneous induction of humoral immunity and cellular immune response, wherein the features only belong to attenuated vaccines or recombinant vaccines.
Owner:TIANJIN RINGPU BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com