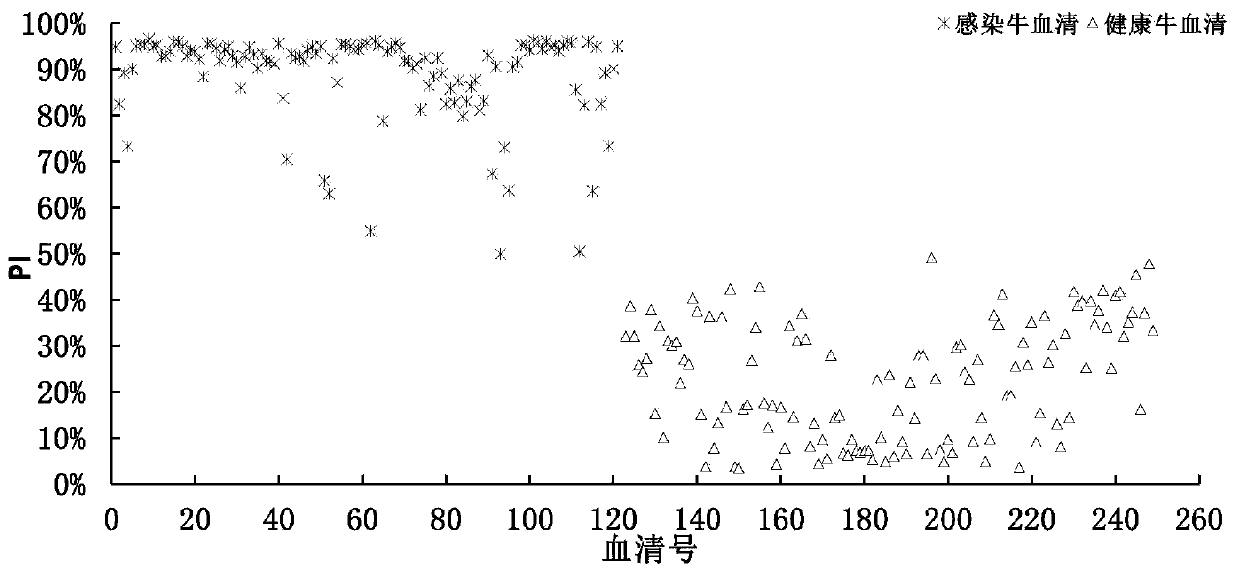
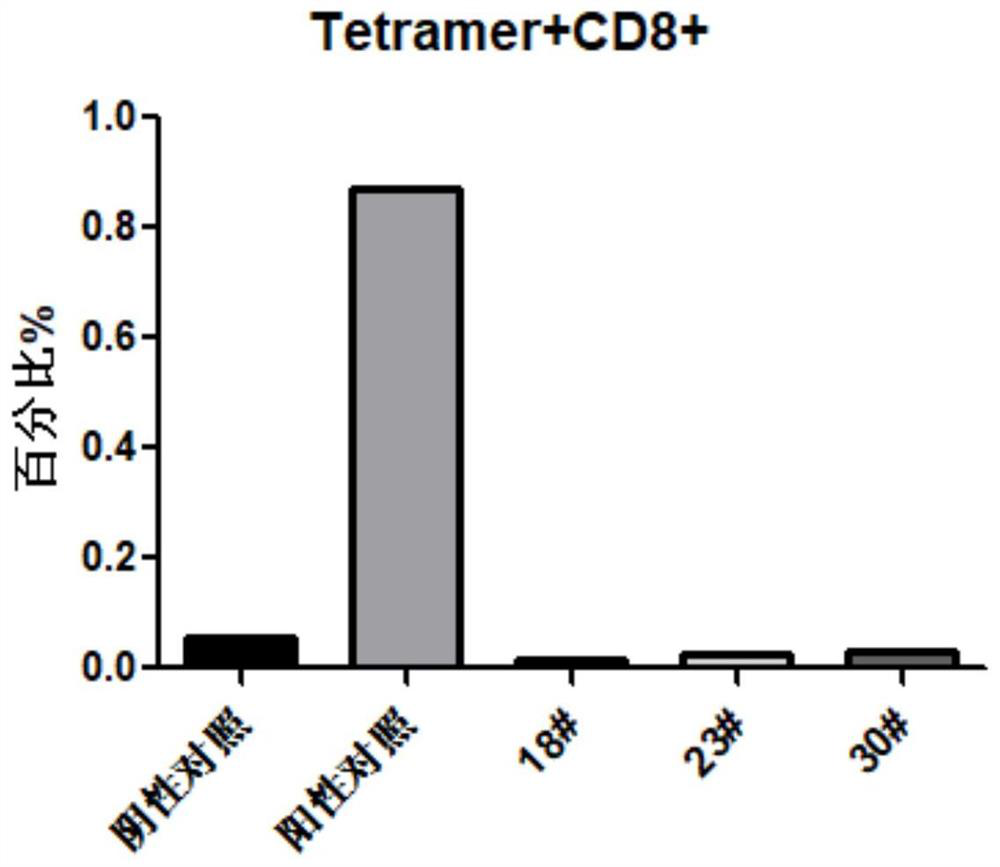
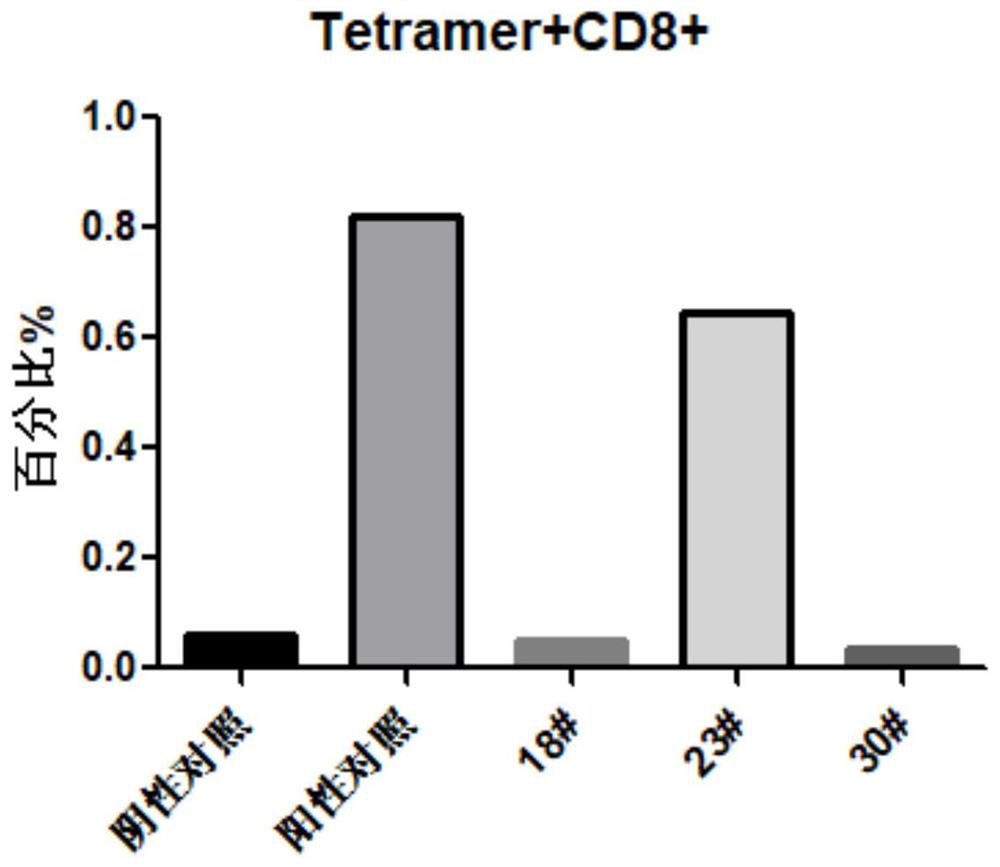
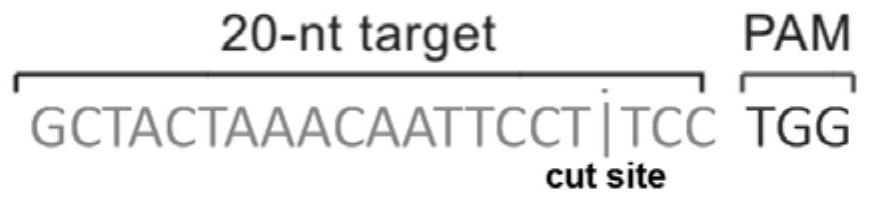
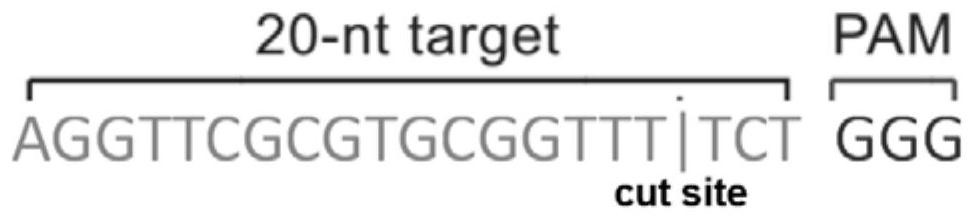
Patents
Literature
41 results about "Virus Structural Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Packaging cell
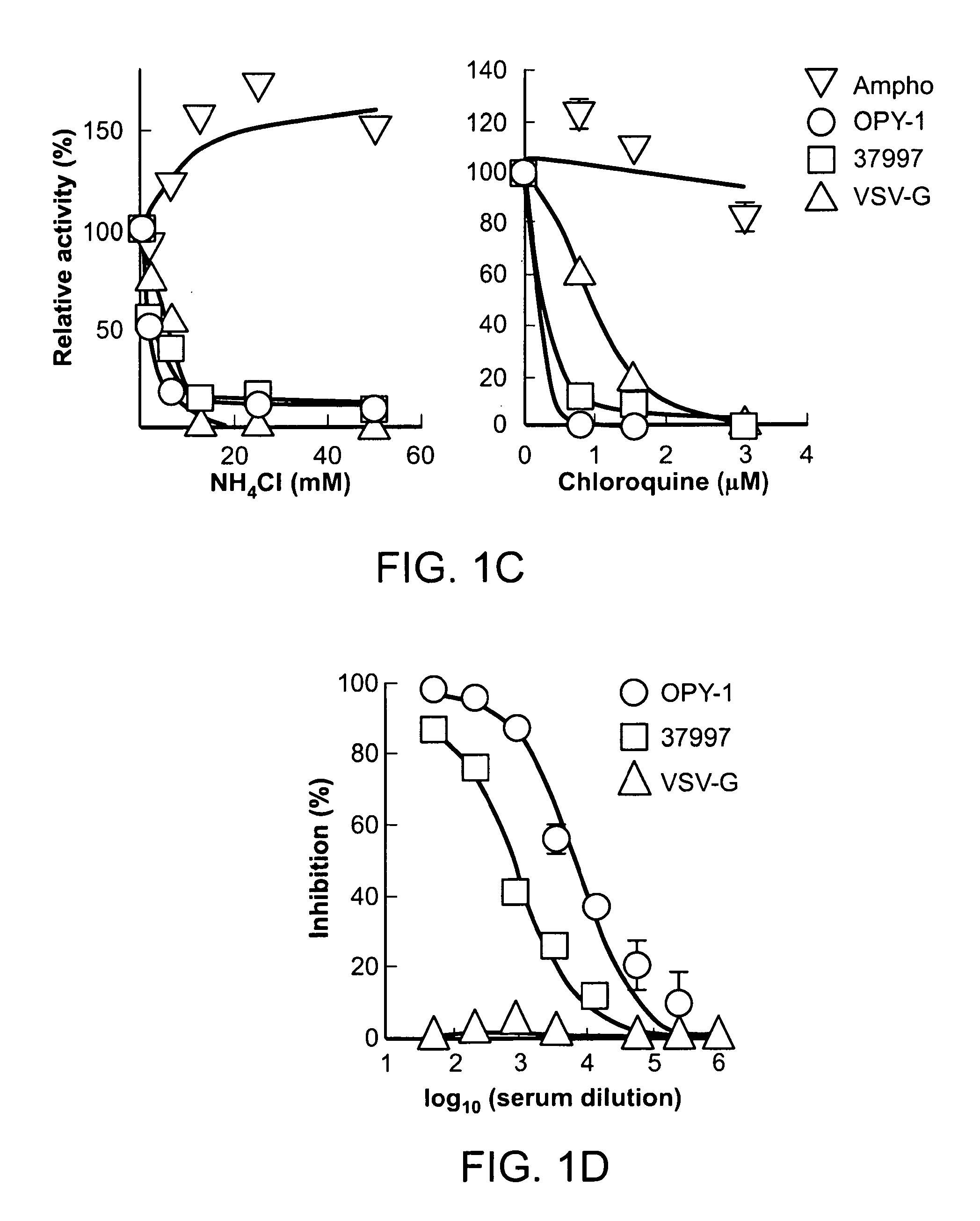
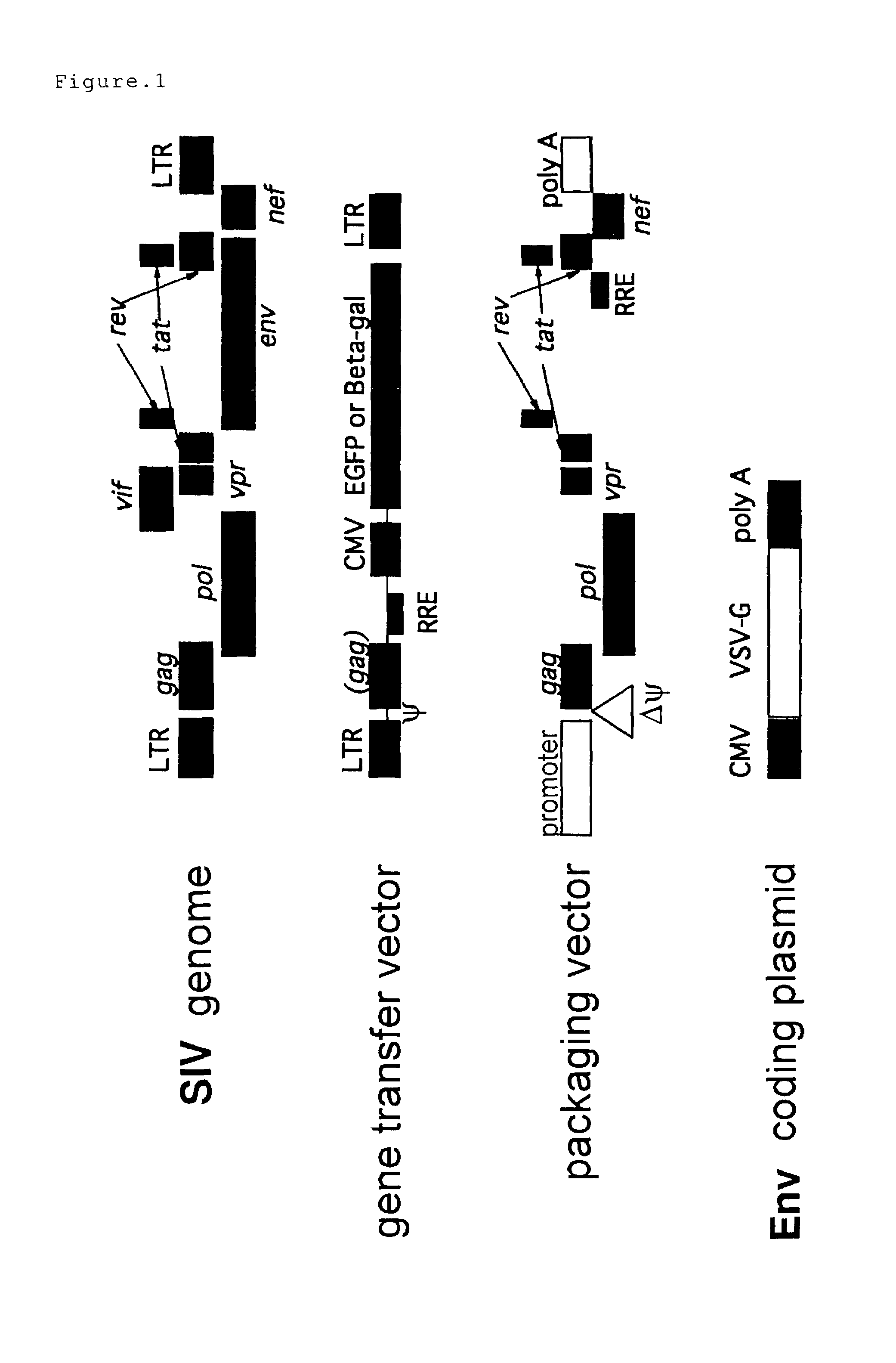
A virus-producing cell sustaining the ability to produce viruses at high titer is successfully constructed by expressing the virus structural gene under the regulation of EF1α promoter. In this virus-producing cell, the virus structural gene is ligated to a selection marker gene via IRES and domains other than the protein coding domain are eliminated from the DNA encoding virus structural proteins. Thus, reduction of the titer due to cell passages can be prevented and emergence of wild type viruses caused by unfavorable recombination of the virus genome can be inhibited.
Owner:CHUGAI PHARMA CO LTD
SARS-CoV virus structural protein infusion protein and its high yield expression and purification and uses
InactiveCN1884303ASsRNA viruses positive-senseAntibody mimetics/scaffoldsProtein tagStructural protein
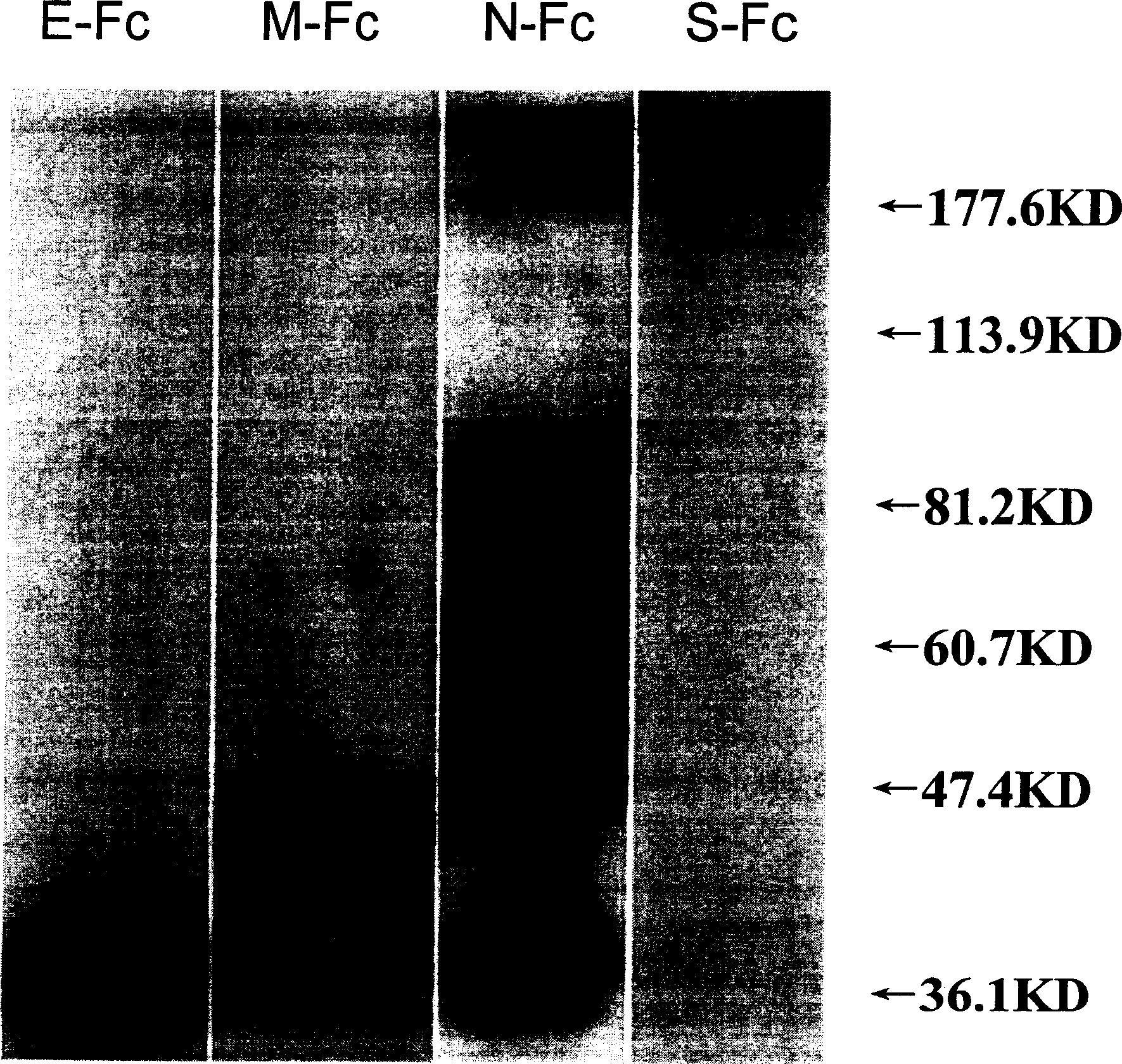
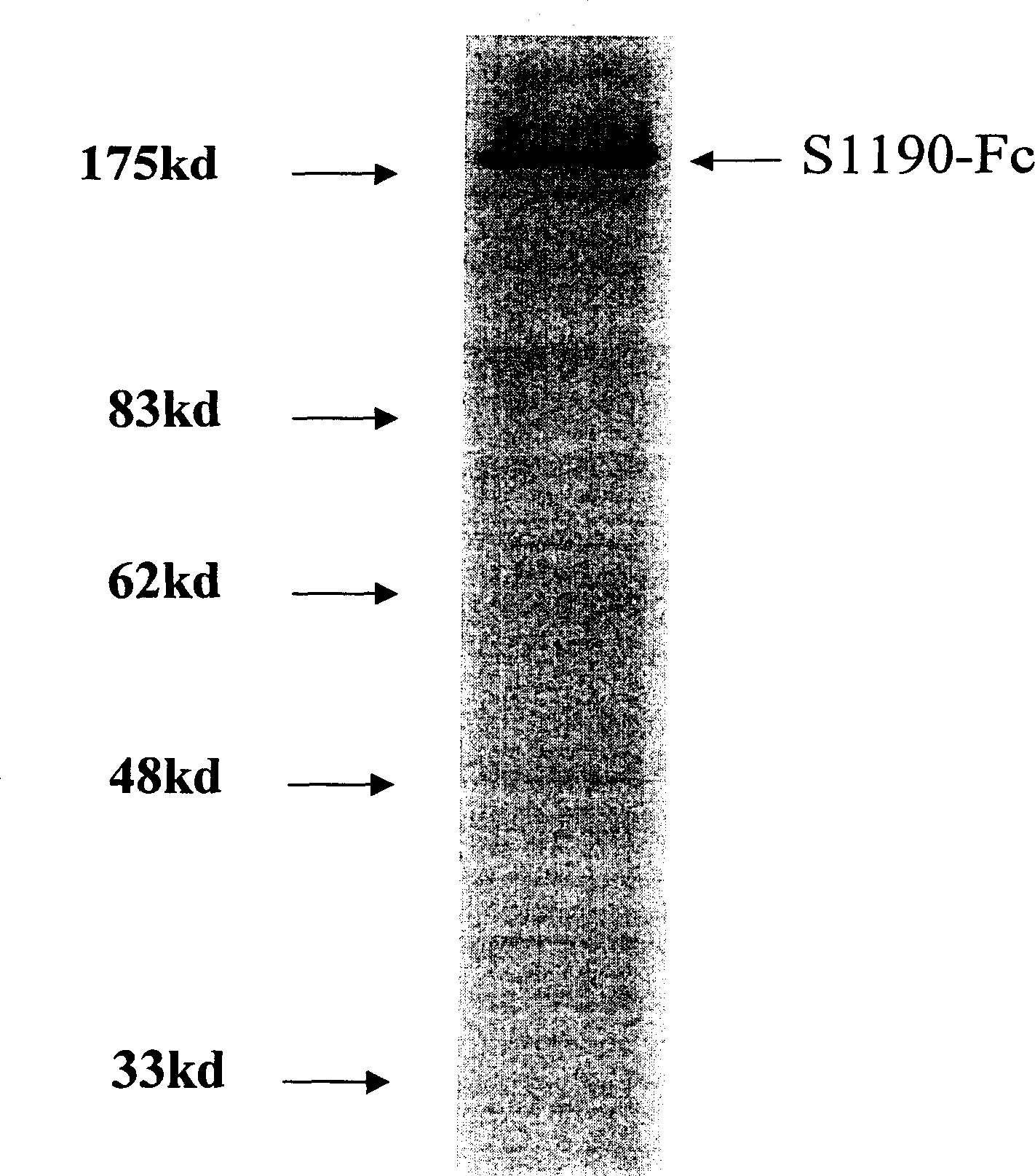
The invention relates to fusion protein of SARS-Cov virus structural protein and its high expression and purification in mammalian cell and its usage. The construction of said fusion protein is X-Y-z, and X is S or M or E or N selected from SARS-CoV virus construction protein, or their arbitrary short form; Y is the connection part with 0-20 amino acid; Z is Fc and its modification or other protein tag. The invention also provides the method of expressing and purifying said fusion protein in mammalian cell for the batch preparation or industrial production. The fusion protein can be used to prepare genetic engineering vaccine preventing SARS-CoV virus infection, solvent box for checking SARS-CoV virus, and to sift drug anti SARS-CoV virus infection with S protein combined with its acceptor ACE2. The invention can detect combination of S protein of SARS-CoV virus with ACE2, which reduces ACE2 expression, and result in or exacerbate acute lung damage, then it modifies combined segment, which can improve safety of preventing SARS-CoV virus vaccine.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
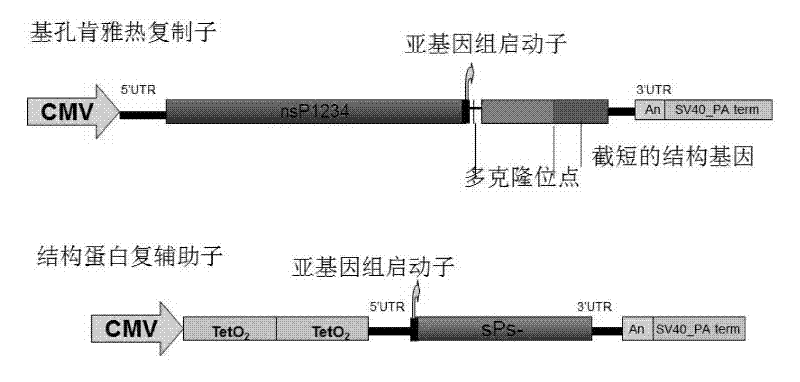
Virus-like particles (VLPs) prepared from chikungunya virus structural proteins
ActiveUS9353353B2Avoid infectionRelieve symptomsSsRNA viruses positive-senseAntipyreticDiseaseVirus-like particle
The invention features compositions and methods for the prevention or treatment of one or more strains of Chikungunya virus, as well as other alphavirus-mediated diseases.
Owner:UNITED STATES OF AMERICA
Vector for the expression of two foreign genes
InactiveUS6979568B1Increase transcriptionInhibit expressionBiocideVectorsLentivirusStructural protein
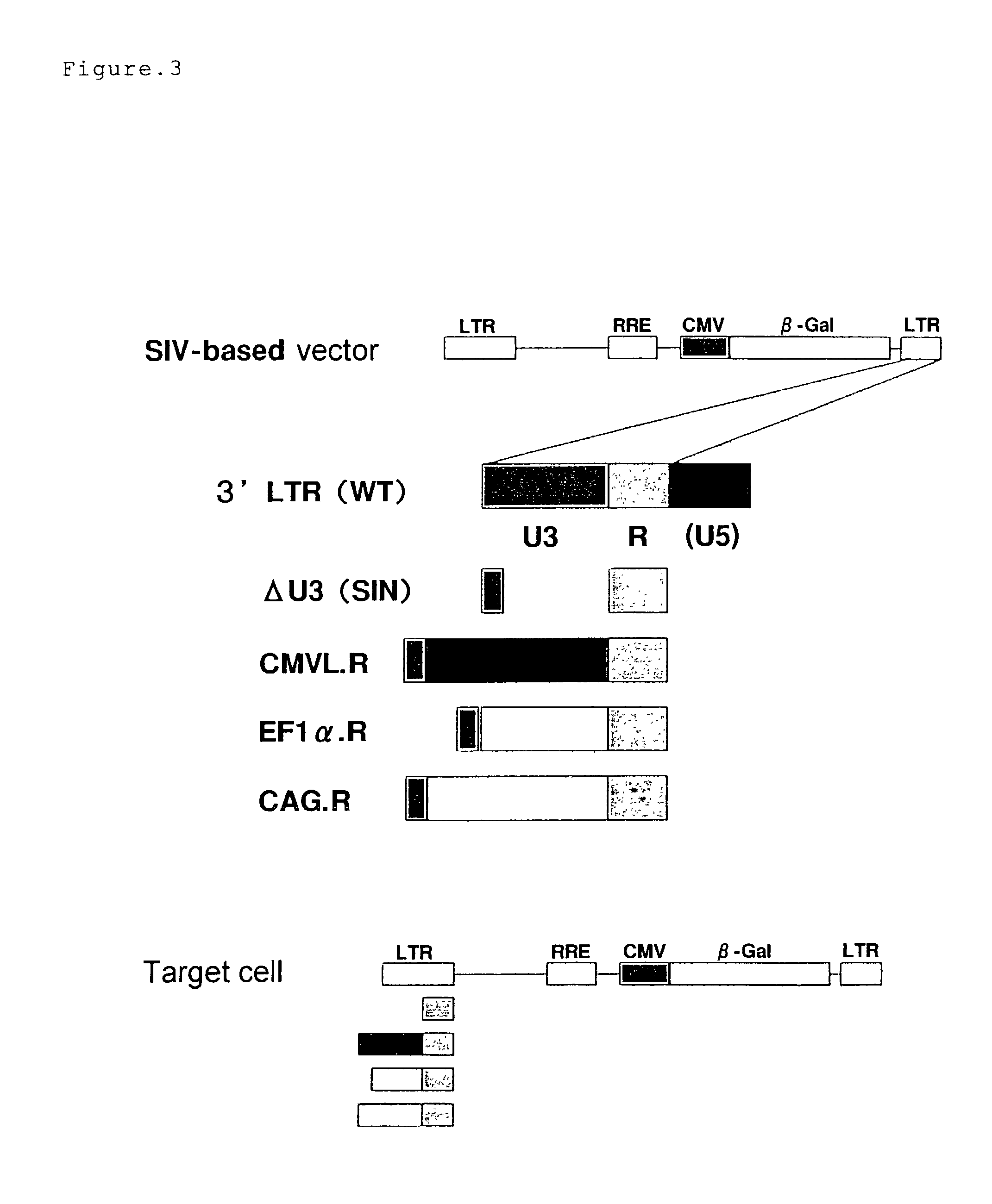
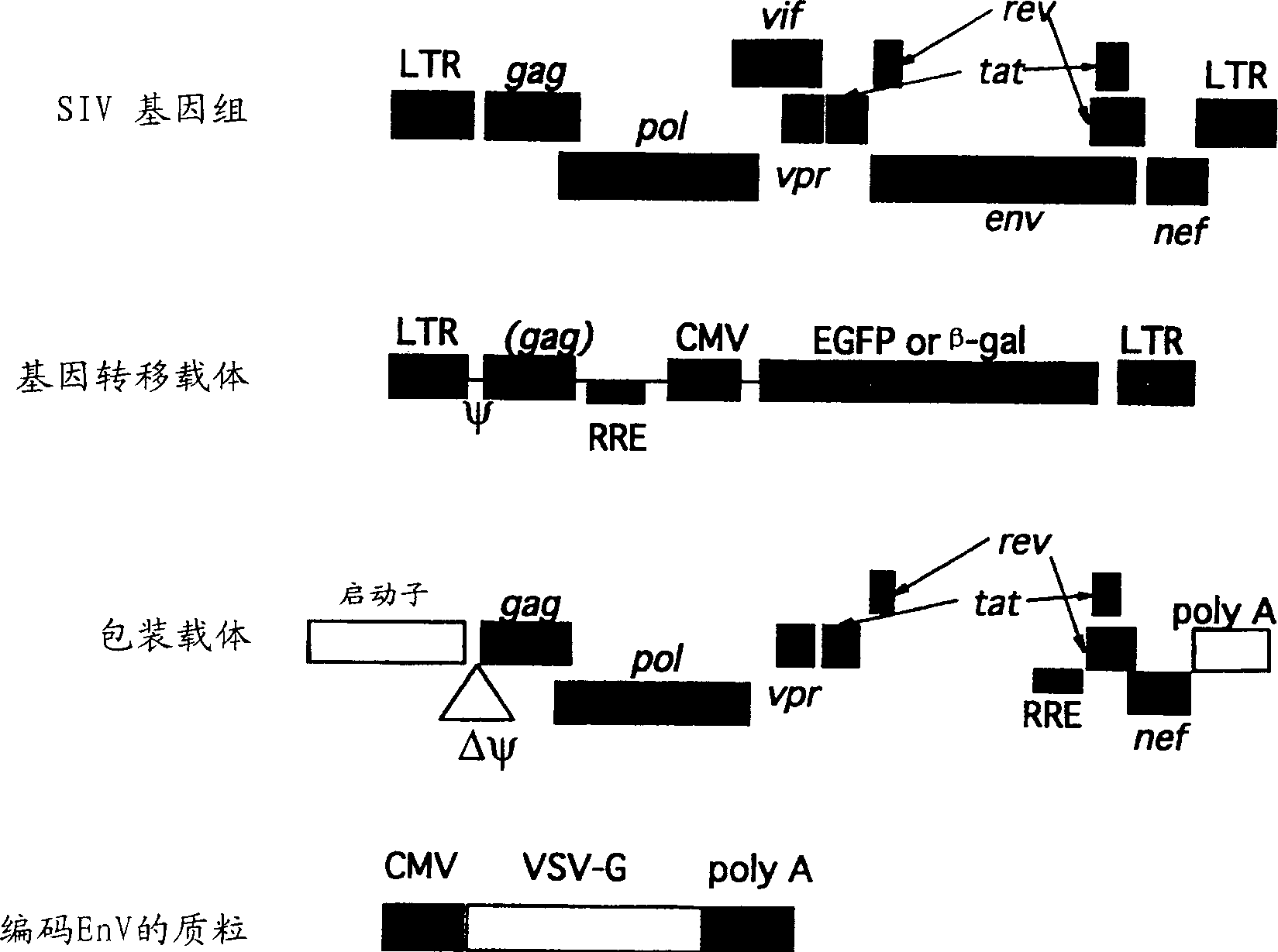
A vector expressing two foreign genes by using RRE sequence and controlling the ratio of the expression doses of these genes owing to the modification is provided. This vector, which can be provided as a lentivirus vector based on SIVagm, is constructed by modifying a virus-origin expression regulatory sequence into another expression regulatory sequence so as to eliminate the dependency on the virus-origin protein. Although this vector has a packaging signal, it has been modified so that the risk of the occurrence of wild strains due to gene recombination is lowered and no virus structural protein is expressed. This vector is highly useful as a gene therapeutic vector with a need for transferring two genes while controlling the expression doses or expression dose ration thereof.
Owner:DNAVEC RES
Direct Attachment of Polypeptides to Virus Like Particles
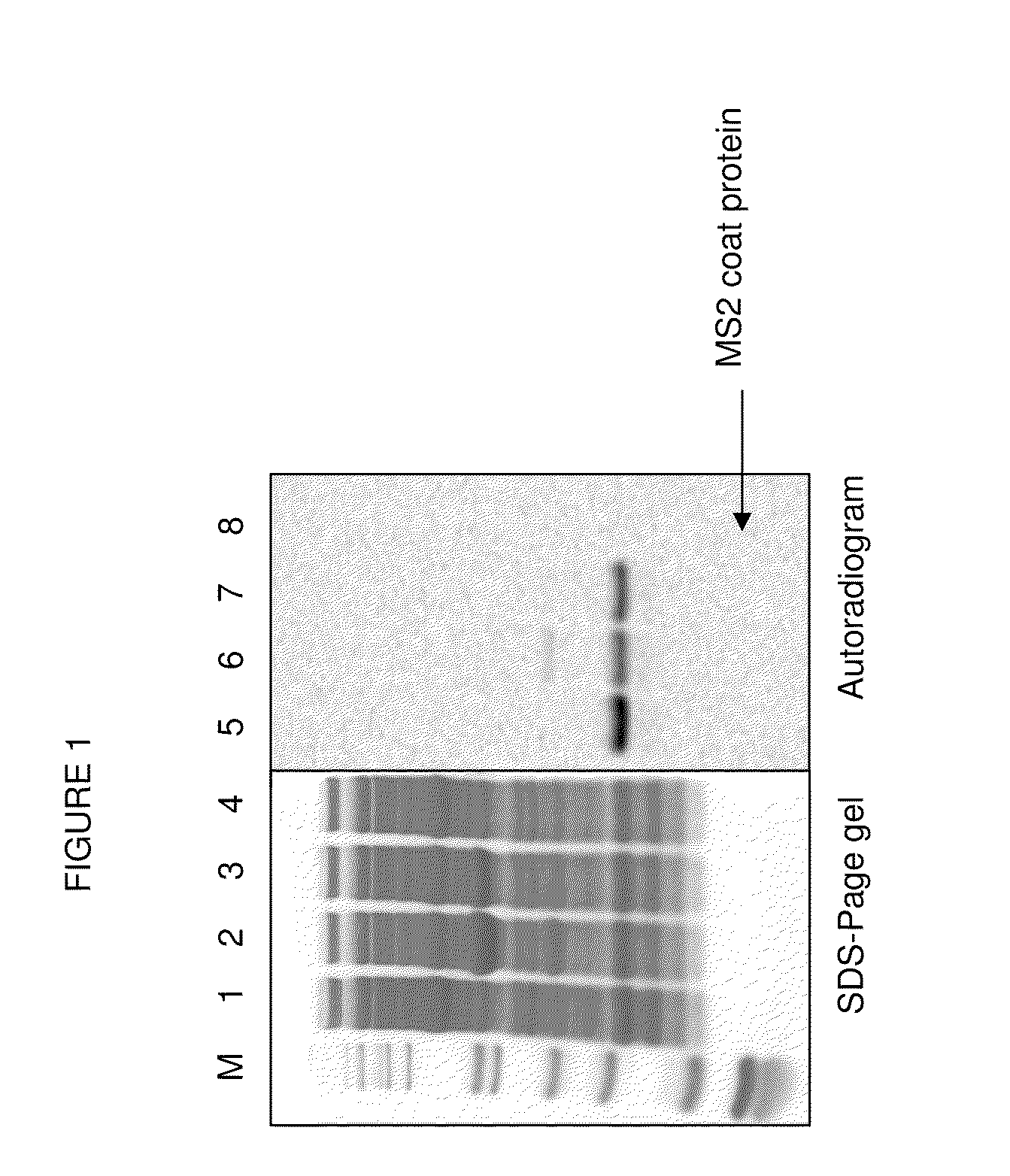
Compositions and methods are provided for the control of direct protein attachment to virus like particles where virus structural proteins that have been modified to comprise an unnatural amino acid at a pre-determined site are reacted with one or more “display” polypeptides that also comprise an unnatural amino acid at a pre-determined site in a one step reaction. The compositions of the invention are useful for various purposes where it is desirable to efficiently and directly attach multiple polypeptides to a single carrier entity, particularly where two or more different polypeptides are attached to a single carrier.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Rapid quantitative detection card for canine distemper virus antibody and using method
The invention discloses a rapid quantitative detection card for a canine distemper virus antibody and a using method. The rapid quantitative detection card comprises a detection card shell and a teststrip assembled in the detection card shell. The test strip comprising a plastic base plate with pressure-sensitive adhesive. A sample pad, a marker pad, a nitrocellulose membrane and absorbent paperare sequentially pasted on the base plate. The marker pad is composed of a carrier base layer and a marker, wherein the marker is a membrane formed by spraying the carrier base layer with lanthanide fluorescent detection microspheres and lanthanide fluorescent quality control microspheres. The part, coated with a canine distemper virus H protein antigen, of the nitrocellulose membrane is a detection line. The part, coated with an anti-Chicken IgY antibody, of the nitrocellulose membrane is a quality control line. The marker is fluorescent detection microspheres marked with a canine distemper virus structural protein H protein recombinant antigen and fluorescent quality control microspheres marked with the anti-Chicken IgY antibody. By the adoption of the rapid quantitative detection card,on-site rapid quantitative determination of the canine distemper virus antibody can be achieved, and the practical value and the promotional value are higher.
Owner:杭州微瑞科技有限公司
Novel genetic engineering vaccine of porcine Seneca virus as well as preparation method and application of novel genetic engineering vaccine
ActiveCN110279855AEasy to assembleGood antigenicitySsRNA viruses positive-senseVirus peptidesVaccine ProductionImmunogenicity
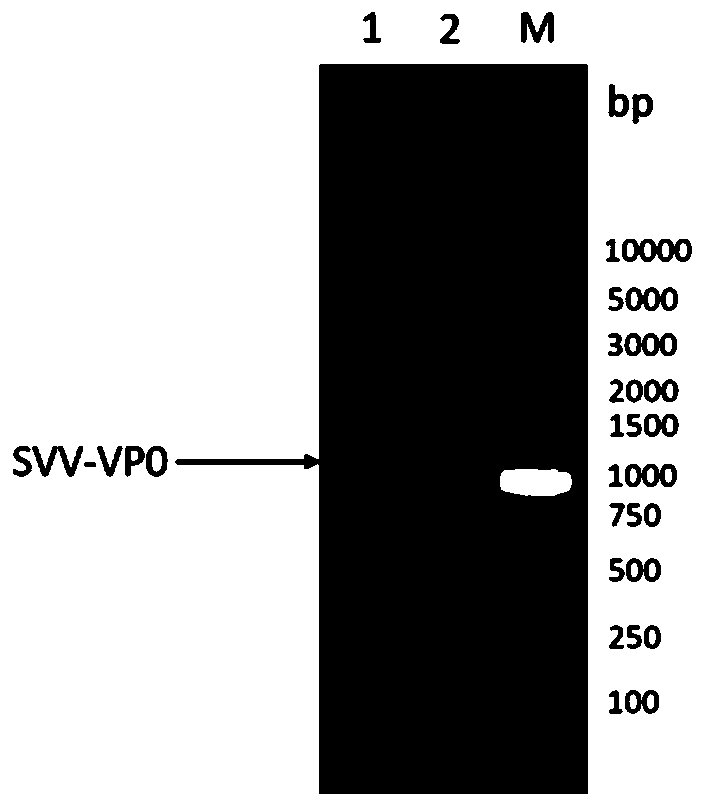
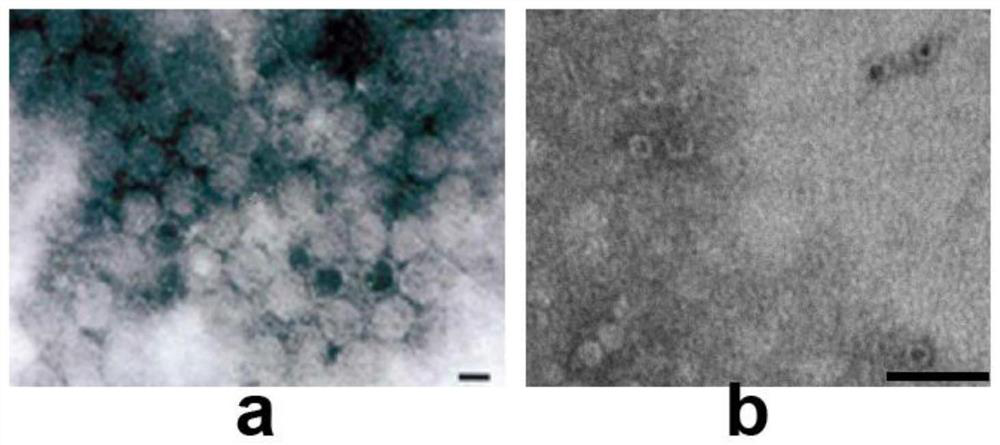
The invention discloses an immunological composition which comprises porcine Seneca virus structural protein VP3 and VP1 proteins, as well as porcine Seneca virus structural protein VP2 and / or VP4 protein. Further, the immunological composition can further comprise a porcine Seneca virus structural protein VP0. The immunological composition can be used for preparing a novel genetic engineering subunit vaccine of porcine Seneca virus, the antigenicity, immunogenicity and function of the vaccine are similar to those of natural proteins, the expression level is relatively high, the immunogenicity is strong, and no pathogenicity is caused to animals; the vaccine can be prepared by large-scale serum-free suspension culture in a bioreactor, thereby greatly reducing the cost of vaccine production.
Owner:苏州世诺生物技术有限公司
Hepatitis E virus-like particle vaccine preparation method and application
ActiveCN104857510AEasy to prepareEasy to zoom inDigestive systemImmunoglobulins against virusesEscherichia coliDisease
The invention discloses a hepatitis E virus-like particle vaccine preparation method, and belongs to the biological medicine technical field. According to the method, hepatitis E virus truncated capsid protein gene codon optimization is performed, prokaryotic expression system escherichia coli strain B834-pRARE2 is used, by fusion of purification tag MBP expression, different cracking agent screening and combination, bacterial membrane extraction, maltose amylose affinity chromatography, molecular sieve chromatography for removal of MBP and non-virus structural protein, and promotion of virus-like particle assembly, the hepatitis E virus-like particle purity is more than 99.0%, and is free of protein label and bioactive (in the form of non-inclusion body, and soluble). The hepatitis E virus-like particle vaccine produces complete protection to pig, dog and chicken HEV infection, cross protective antigens are between different genotype HEV virus, and the hepatitis E virus-like particle vaccine can be used as an animal vaccine to prevent the diseases and infection caused by pig, dog and poultry HEV.
Owner:张澍 +1
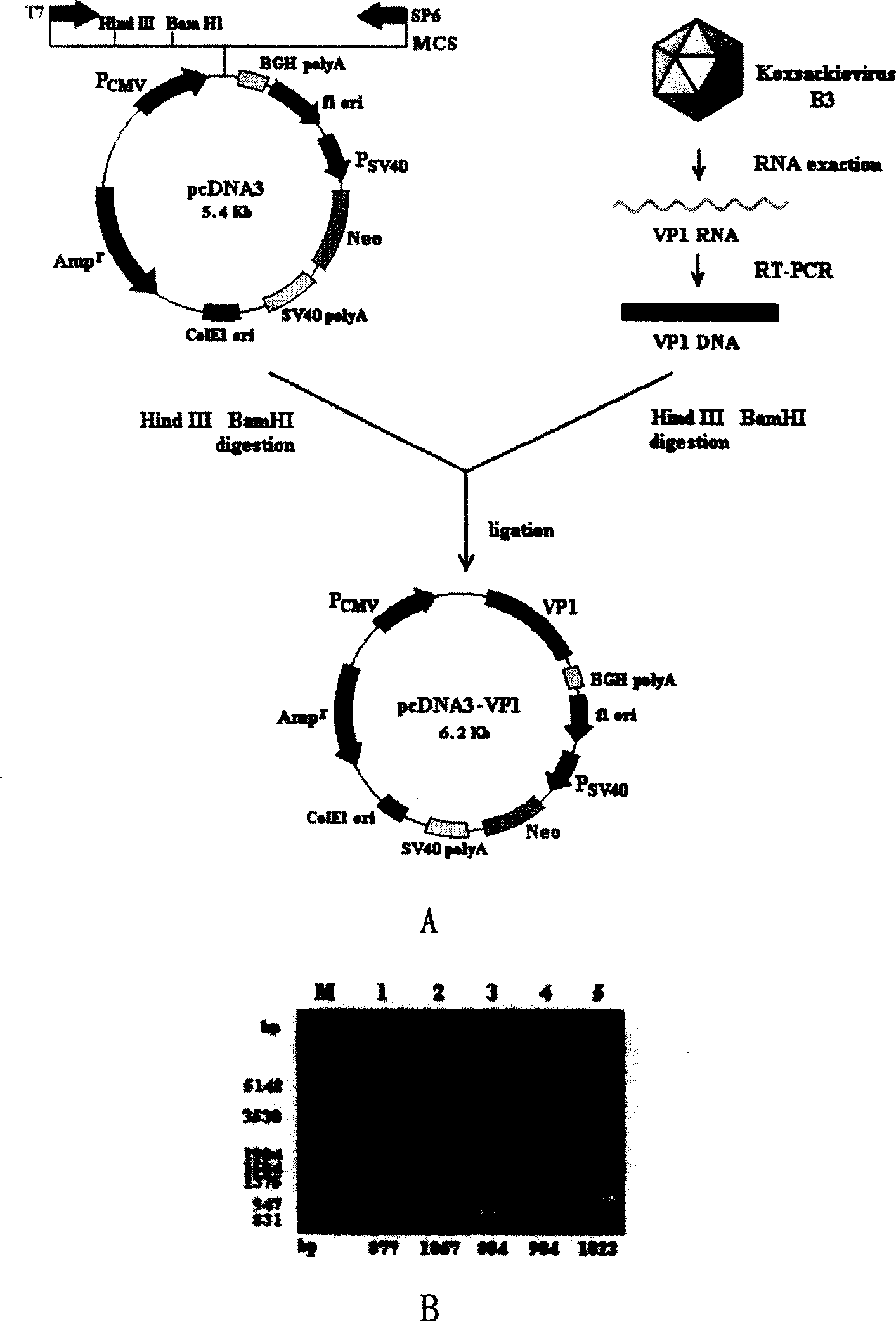
Viral myocarditis gene vaccine, its preparation method and application
The present invention discloses a viral myocarditis gene vaccine, Said vaccine is constructed by using B3 type Coxsackie virus structural protein VPI gene and pcDNA carrier together, and its exterior is covered with a biological polysaccharide. It also discloses a method for preparing said gene vaccine and the application of said gene vaccine in preparation of medicine for preventing viral myocarditis.
Owner:上海欣安基因免疫与疫苗研究开发有限公司
General foot-and-mouth disease virus structural protein antibody and blocking ELISA detection reagent kit thereof
ActiveCN110642945ASimple compositionConducive to specific bindingBiological material analysisImmunoglobulins against virusesDiseaseFoot mouth disease virus
The invention provides a general foot-and-mouth disease virus structural protein antibody and a blocking ELISA detection reagent kit thereof, and belongs to the technical field of virus detection. Thegeneral foot-and-mouth disease virus structural protein antibody and the blocking ELISA detection reagent kit thereof comprise the following composition of an antigen coating elisa plate and a biotinmarker concentrating monoclonal antibody, wherein a monoclonal antibody in the biotin marker concentrating monoclonal antibody is a monoclonal antibody E32, and the antigen coating elisa plate is anFMDV antigen indirectly coated through a monoclonal antibody F104. The E32 antibody and the F104 antibody are specifically combined to between-genotype conservative epitope in FMDV structural proteinVP2 protein, the sensitivity and the specificity are high, the general foot-and-mouth disease virus structural protein antibody and the blocking ELISA detection reagent kit thereof are suitable for detection of structural protein antibodies after O, A and Asia1 type FMDV infected or inactivated vaccine immunity, and are a new method for monitoring FMD non-immune animal community infection conditions, and a complete set of identifying and diagnosing method is provided for a marker vaccine having FMDV structural protein VP2 epitope deletion.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Virus like particle comprising PD-1 antigen or PD-1 ligand antigen
ActiveUS9637532B2Immunoglobulin superfamilySsRNA viruses positive-senseVirus-like particleStructural protein
The present invention provides a virus like particle comprising a virus structural protein and an antigen derived from PD-1 or a ligand of PD-1, and a composition or kit comprising thereof, its use in immune response etc.
Owner:VLP THERAPEUTICS LLC
Sensitization polystyrene nano microsphere for detection of canine parvo virus structural protein VP2 antibody, and preparation method and application thereof
ActiveCN109212230AGood antigenicityImprove hydrophilicityBiological material analysisBiological testingMicrospherePolystyrene
The invention discloses a sensitization polystyrene nano microsphere for detection of a canine parvo virus structural protein VP2 antibody, and a preparation method and application thereof. A surfaceof the sensitization polystyrene nano microsphere is coupled with a canine parvo virus recombinant VP2 protein, the recombinant VP2 protein is a truncated protein of a canine parvo virus VP2 protein and located at the 365-486 digits of the canine parvo virus VP2 protein, and an amino acid sequence of the recombinant VP2 protein is shown as the SEQ ID NO.3. The protein contains a main epitope domain of the VP2 protein and has high antigenicity, excellent hydrophilicity and relatively strong specificity and immunogenicity. With the recombinant protein used as an antigen, a sensitization color polystyrene nano microsphere is prepared, and the sensitization color polystyrene nano microsphere is used for detecting a canine parvo virus serum antibody. Experiments show that the prepared sensitization microsphere cannot generate an autoagglutination phenomenon and has excellent repeatability and stable property. A detection method provided by the invention is suitable for clinical fast detection of single or multiple dog serum samples for pets, and compared with a commercial detection kit, the detection method is simple and convenient to operate, and a detection result has relatively highaccuracy and coincidence rate.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Vector for expressing two foreign genes
A vector expressing two foreign genes by using RRE sequence and controlling the ratio of the expression doses of these genes owing to the modification is provided. This vector, which can be provided as a lentivirus vector based on SIVagm, is constructed by modifying a virus-origin expression regulatory sequence into another expression regulatory sequence so as to eliminate the dependency on the virus-origin protein. Although this vector has a packaging signal, it has been modified so that the risk of the occurrence of wild strains due to gene recombination is lowered and no virus structural protein is expressed. This vector is highly useful as a gene therapeutic vector with a need for transferring two genes while controlling the expression doses or expression dose ration thereof.
Owner:DNAVEC RES
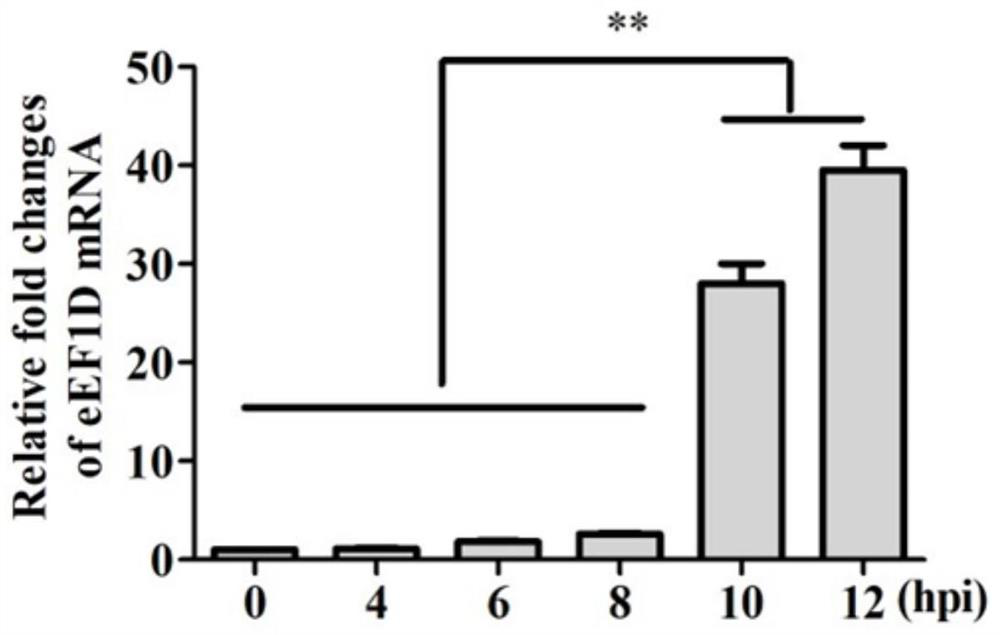
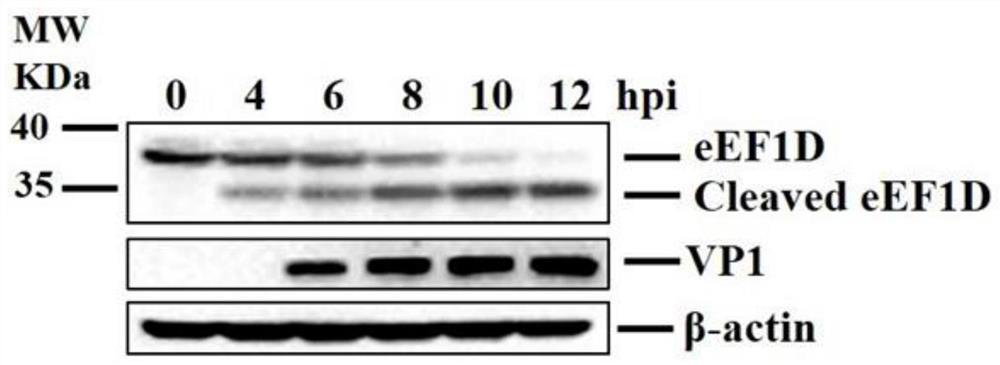
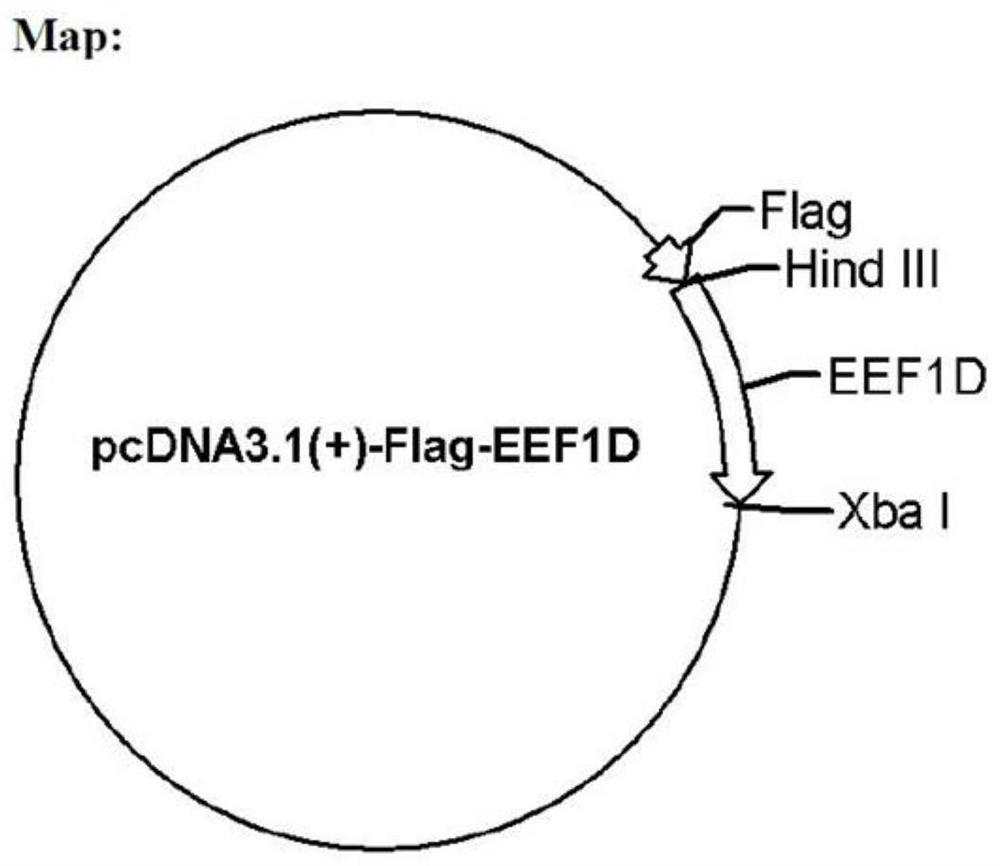
Application of eEF1D protein in preparation of medicine for preventing or treating foot-and-mouth disease virus infection
ActiveCN111793721AHigh expressionReduce contentMicrobiological testing/measurementAntiviralsDiseaseFoot mouth disease virus
The invention belongs to the technical field of biology, and particularly relates to application of an eEF1D protein in preparation of a medicine for preventing or treating foot-and-mouth disease virus infection. The invention finds that the expression of the eEF1D mRNA is increased and the content of the eEF1D protein is reduced after the foot-and-mouth disease virus infection, namely, the eEF1DmRNA and the eEF1D protein can be used as detection markers for the foot-and-mouth disease virus infection and are used for evaluating whether the foot-and-mouth disease virus infection occurs or not;secondly, the eEF1D protein can remarkably reduce the structural protein VP1 of the foot-and-mouth disease virus, the expression of mRNA of the foot-and-mouth disease virus and the titer of the foot-and-mouth disease virus, remarkably inhibits the replication of the foot-and-mouth disease virus, and can be used for preventing or treating the infection of the foot-and-mouth disease virus; and finally, after knocking down the eEF1D protein, the expression of the foot-and-mouth disease virus structural protein VP1 is promoted, and the expression of the foot-and-mouth disease virus mRNA and the virus titer are improved. Therefore, the eEF1D protein can be knocked down in a production cell strain through a genetic engineering means, and a cell line with better performance than that of the foot-and-mouth disease virus vaccine produced by the existing production cell line is obtained and is used for producing foot-and-mouth disease virus vaccines.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Mud crab bicistronic mRNA virus structural protein 1, monoclonal antibody thereof and application
InactiveCN101717434AVirus peptidesImmunoglobulins against virusesVirus Structural ProteinsAmino acid
The invention discloses a mud crab bicistronic mRNA virus structural protein 1, a monoclonal antibody thereof and the application. The amino acid sequence of the mud crab bicistronic mRNA virus structural protein 1 is SEQ ID NO: 3 and the nucleotide sequence is SEQ ID NO: 1. By researching and analyzing MCDV, the invention firstly obtains the amino acid sequence, the nucleotide sequence and the monoclonal antibody of the structural protein 1, locates the structural protein 1 and provides a new research direction for researching virus of mud crab bicistronic mRNA. The monoclonal antibody of the structural protein 1 can be used in monitoring MCDV, preparing a virus kit for detecting MCDV and preparing a virus colloidal gold test paper for detecting MCDV.
Owner:SUN YAT SEN UNIV
Replication-defective A virus expression vector system and vaccine preparation method
InactiveCN102443603AReduce the risk of useAvoid not being able to expand cultureViral antigen ingredientsAntiviralsNegative strandViral Vaccine
The invention discloses a replication-defective A virus expression vector system and a vaccine preparation method. The vector system consists of a replication-defective A virus vector of a deleted structural gene and minus strand complementary plasmids or cells containing a controllable virus A structural gene. In the preparation method, an exogenous gene target is introduced by constructing the replication-defective A virus vector of a deleted virus structural protein; and the replication-recombinant A virus can be continuously produced in a large scale by combining the minus strand complementary plasmids or cells containing the controllable A virus structural gene, so that the problem of low virus yield of a conventional A virus system can be solved, a target of preparing recombinant virus in a large scale is realized, and exogenous protein and virus sample particles can be expressed in scale. The replication-defective A virus expression vector system disclosed by the invention is suitable for large-scale production of DNA (deoxyribonucleic acid) vaccine, vector virus vaccine and exogenous protein expression.
Owner:SOUTH CHINA UNITED VACCINE INST
O-type foot-and-mouth disease virus structural protein VP1 broad-spectrum neutralizing antibody as well as preparation method and application thereof
ActiveCN114316036APrevent intrusionAvoid infectionClimate change adaptationImmunoglobulins against virusesDiseaseBALB/c
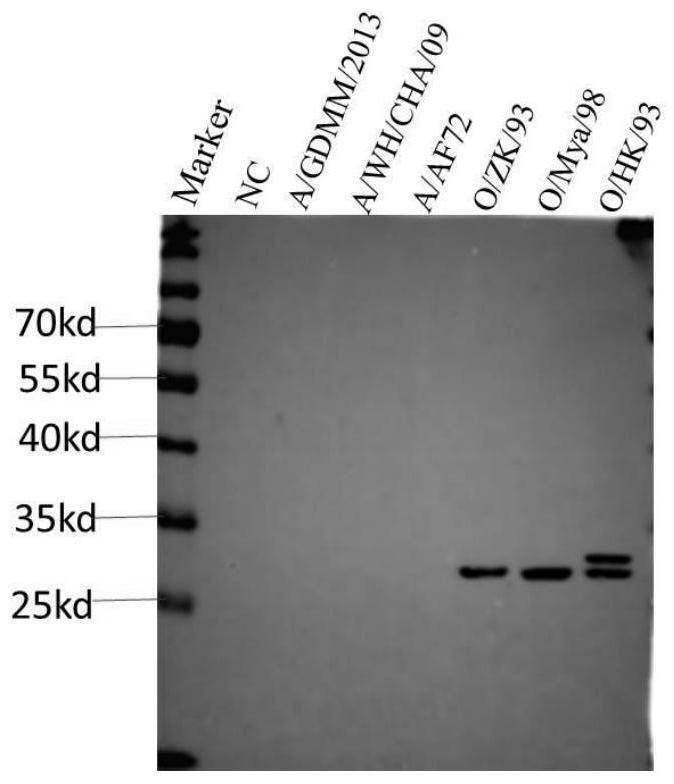
The invention relates to the technical field of biology, in particular to an O-type foot-and-mouth disease virus broad-spectrum neutralizing antibody, a preparation method and application. O-type foot and mouth disease virus structural protein VP1 is used as an immunogen to immunize Balb / c mice, a positive hybridoma cell line capable of efficiently secreting a monoclonal antibody is obtained through cell fusion and screening, a mouse monoclonal antibody 9H6-VP1 is obtained, and the monoclonal antibody 9H6-VP1 is an IgG1 type monoclonal antibody. An immunoblotting test shows that the monoclonal antibody 9H6-VP1 has broad-spectrum reactivity to O-type viruses of different pedigree (O / ZK / 93, O / Mya / 98 and O / HK / 93), and does not react with a type A strain; a virus neutralization experiment shows that the monoclonal antibody has neutralization activity on O / ZK / 93, O / Mya / 98 and O / HK / 93, and can prevent virus invasion and protect an organism from infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Virus-like particle as well as preparation method and application thereof
PendingCN114317571AImproving immunogenicityEnsure structural stabilityAntiviralsAntibody medical ingredientsSwine vesicular diseaseDisease
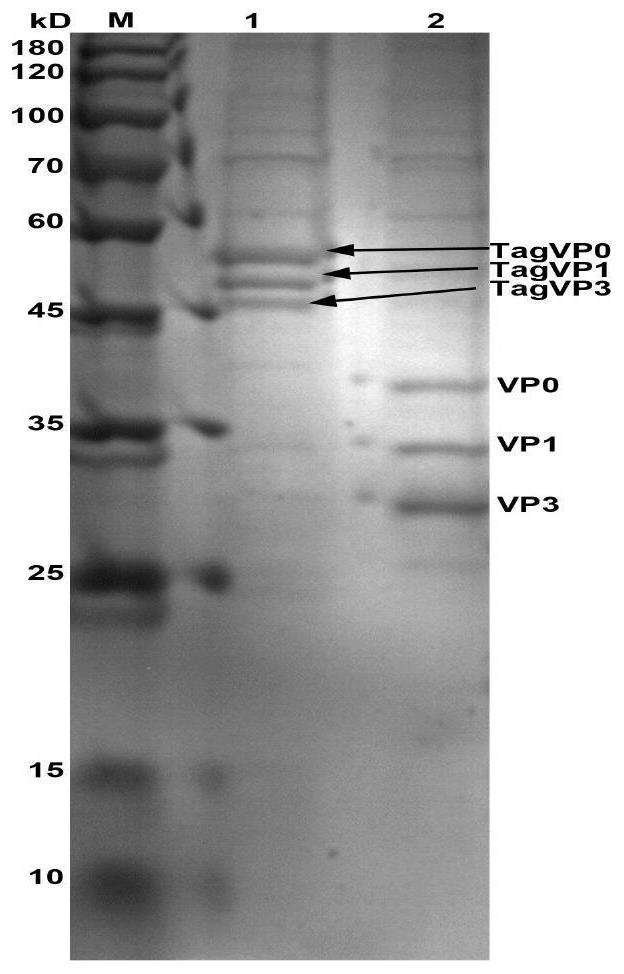
The invention provides a virus-like particle as well as a preparation method and application thereof, and belongs to the technical field of agricultural science, animal husbandry and veterinary science. Small ubiquitin-like modified proteins are used as tag proteins to promote correct folding of virus structural proteins VP0, VP1 and VP3, meanwhile, the structural stability of the proteins is guaranteed, mass expression of the structural proteins is achieved, and after in-vitro self-assembly of the three structural proteins, a large number of virus-like particles similar to natural viruses in performance are obtained. Immunogenicity detection shows that the prepared virus-like particles have high immunogenicity and can be used as reserve vaccines to be applied to prevention and control of swine vesicular disease or foot-and-mouth disease virus transmission.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Novel genetically engineered vaccine for swine seneca virus, preparation method and application thereof
ActiveCN110279855BEasy to assembleGood antigenicitySsRNA viruses positive-senseVirus peptidesVaccine ProductionEngineered genetic
The invention discloses an immunological composition which comprises porcine Seneca virus structural protein VP3 and VP1 proteins, as well as porcine Seneca virus structural protein VP2 and / or VP4 protein. Further, the immunological composition can further comprise a porcine Seneca virus structural protein VP0. The immunological composition can be used for preparing a novel genetic engineering subunit vaccine of porcine Seneca virus, the antigenicity, immunogenicity and function of the vaccine are similar to those of natural proteins, the expression level is relatively high, the immunogenicity is strong, and no pathogenicity is caused to animals; the vaccine can be prepared by large-scale serum-free suspension culture in a bioreactor, thereby greatly reducing the cost of vaccine production.
Owner:苏州沃美生物有限公司
Process for designing inhibitors of influenza virus structural protein-1
InactiveUS20100081126A1SsRNA viruses negative-sensePeptide/protein ingredientsInfluenza aInfluenza prevention
Owner:RUTGERS THE STATE UNIV
Cloning, construction and application of new coronavirus SARS-CoV-2 subgenome replicon based on bacterial artificial chromosome
PendingCN114231544ASolve hard requirementsImprove screening efficiencySsRNA viruses positive-senseMicrobiological testing/measurementStructural proteinPharmaceutical Substances
The invention belongs to the technical field of biological medicines, and relates to cloning, construction and application of a new coronavirus SARS-CoV-2 subgenome replicon based on a bacterial artificial chromosome BAC. The invention provides an expression product cloned by a new coronavirus subgenome replicon, the expression product does not contain structural protein S, M or E of the new coronavirus, and the invention also comprises a coding sequence, a preparation method and application of the expression product. The invention relates to DNA (Deoxyribose Nucleic Acid) cloning of a new coronavirus (SARS-CoV-2) with a Gaussian luciferase reporter gene based on a bacterial artificial chromosome BAC (Bacterial Artificial Chromosome); four DNA clones respectively containing four new coronavirus gene segments and a reporter gene Gluc; the invention also discloses a preparation method of a new coronavirus (SARS-CoV-2) subgenome replicon by removing virus structural proteins S, M and E and adding a reporter gene. The system provided by the invention can be used for screening new coronavirus antiviral drugs.
Owner:FUDAN UNIV
A-type foot-and-mouth disease subunit vaccine as well as preparation method and application thereof
ActiveCN112076314AImprove stabilityImprove protection efficiencySsRNA viruses positive-senseViral antigen ingredientsEscherichia coliDisease
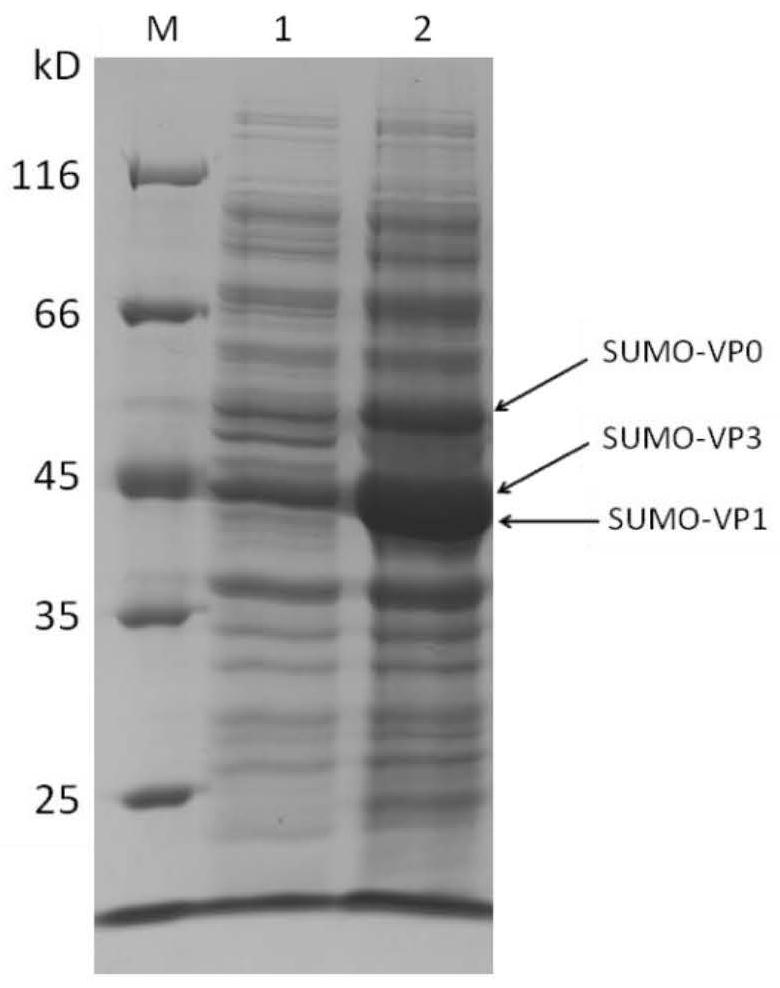
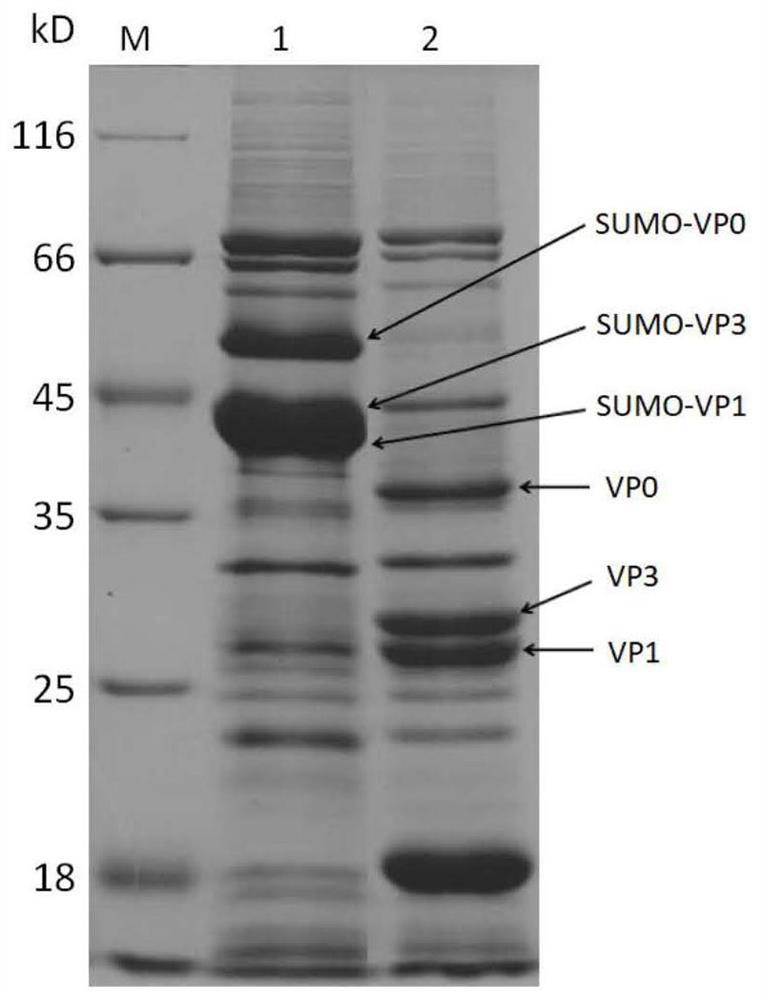
The invention relates to the technical field of molecular biology, in particular to an A-type foot-and-mouth disease subunit vaccine as well as a preparation method and application thereof. Accordingto the invention, optimization design is performed based on amino acid sequences of three kinds of structural proteins VP0, VP3 and VP1 of an A-type foot-and-mouth disease virus epidemic strain (A / GDMM / 2013) in 2013 in China, a single plasmid is screened out by means of small ubiquitin-related modifier (SUMO) fusion protein to simultaneously express the three kinds of structural proteins efficiently, uniformly and solubly in escherichia coli, and the three kinds of virus structural proteins are successfully self-assembled in vitro; and finally obtained target protein accounts for about 30% orabove of total bacterial protein, and the maximum yield of the purified target protein can reach 150 mg / L. The foot-and-mouth disease vaccine prepared with the method disclosed by the invention has agood protection effect on A-type foot-and-mouth disease epidemic viruses in China, and the minimum full-protection immune dose of the vaccine can be as low as 20 micrograms per vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Chicken Infectious Bursal Disease Virus Structural Protein vp2 Suitable for Expression in the Middle Silk Gland of Bombyx mori and Its Expression Vector and Application
ActiveCN104017814BConsistent structureIncrease contentVirus peptidesMicroorganism based processesAnimal virusInfectious bursal disease virus IBDV
The invention discloses a chicken infectious bursal disease virus structural protein VP2 applicable to silkworm middle silk gland expression as well as an expression vector and application thereof. A gene sequence of the chicken infectious bursal disease virus structural protein VP2 is as shown in SEQ ID NO.1; a coded amino acid sequence of the chicken infectious bursal disease virus structural protein VP2 is as shown in SEQ ID NO.2; the sequence for coding the chicken infectious bursal disease virus structural protein VP2, and a promoter and a terminator of an excretion sericin 1 gene form an expression frame, and expression is enhanced by an enhancer hr3; meanwhile, a piggyback transposition arm and a fluorescence-screening marker gene constitute the expression vector; the expression vector can express the chicken infectious bursal disease virus structural protein VP2 in the silkworm middle silk gland, has immunogenicity, and can stimulate a mouse to generate antibodies, so that a foundation is laid up for expressing an animal virus subunit vaccine by utilizing a silkworm middle silk gland biological reactor.
Owner:SOUTHWEST UNIV
A type 1 bovine viral diarrhea virus-like particle and its preparation and application
ActiveCN108456663BOptimize spatial structureStimulate immune responseSsRNA viruses positive-senseViral antigen ingredientsEscherichia coliBovine Viral Diarrhea Viruses
The invention discloses a type 1 bovine viral diarrhea virus (BVDV‑1) virus-like particle, a preparation method and application thereof. By combining the structural proteins C and E of BVDV‑1 rns , E1, and E2 coding genes were cloned into pFastBacDual to construct pFBD‑BVDV‑1 recombinant baculovirus transfer vector, transposed in Escherichia coli DH10Bac to obtain recombinant baculovirus vector Bac‑BVDV‑1, and transfected insect cells to obtain recombinant baculovirus Baculo‑BVDV‑1, infected insect cells expressing BVDV‑1 structural proteins C, E rns , E1, and E2, which are automatically assembled into BVDV‑1 virus-like particles (VLPs) in cells. The virus-like particle obtained by the invention is closer to the morphological structure of the natural BVDV virus particle, and can stimulate the body to produce a better immune response after being inoculated with animals, so as to achieve better immune effect. The obtained virus-like particles can be directly purified by sucrose density gradient centrifugation. Compared with other expression systems, it avoids the cumbersome process of separately purifying the virus structural proteins and then assembling them outside the cells, which is conducive to improving efficiency and saving costs. Infectious BVDV live virus is not used in the medium, which improves safety.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Polypeptide preparations
PendingCN113817028ASsRNA viruses positive-senseViral antigen ingredientsTechnology researchViral structural protein
On the basis of AI-Deep-Learning technical research, activation of an MHC-I immune system is taken as a target, research is carried out aiming at CoViD-19 virus structural protein, polypeptide sequences with the length of 9-14 amino acids are predicted, the polypeptides can obviously activate the immune system of a body, and a foundation is laid for designing a safe and effective polypeptide vaccine.
Owner:QINGDAO MARINE BIOPHARMACEUTICAL RES INST
Trivalent recombinant adenovirus vaccine for foot-and-mouth disease and construction method of trivalent recombinant adenovirus vaccine
PendingCN113897392AKnockout avoidanceLow immunogenicitySsRNA viruses positive-senseHydrolasesDiseaseSpecific immunity
The invention discloses a trivalent recombinant adenovirus vaccine for foot-and-mouth disease and a construction method of the trivalent recombinant adenovirus vaccine. The trivalent recombinant adenovirus vaccine is obtained by constructing a recombinant adenovirus vector co-expressed by three antigen genes of a foot-and-mouth disease virus and packaging the recombinant adenovirus vector by AY293-6015 cells, and the trivalent recombinant adenovirus vaccine can be used for simultaneously preventing three serotype foot-and-mouth diseases. E1, E3 and E4 genes of an adenovirus vector are knocked out through CRISPR, and shuttle plasmids in E1 and E4 regions are constructed and are separately used for expressing A-P12A, OBY-P12A and OXJ-P12A-3C-mutant genes; and three foot-and-mouth disease virus structural proteins share one 3C protease to form a VLP, compared with a first-generation adenovirus vector, the vector capacity is increased by about 3kb, a recombinant adenovirus with high titer can be obtained, and the vaccine capacity can be greatly improved when the recombinant adenovirus vector is used for preparing a recombinant adenovirus vaccine for the foot-and-mouth disease. Due to a mode of simultaneously expressing independent-complete-structure protein antigens of three foot-and-mouth disease epidemic strains on one adenovirus vector, foot-and-mouth disease virus A type, O type BY strains and O type XJ strains can be simultaneously prevented, and the specific immune response to the foot-and-mouth disease virus can be enhanced.
Owner:JIAXING ANYU BIOTECH CO LTD
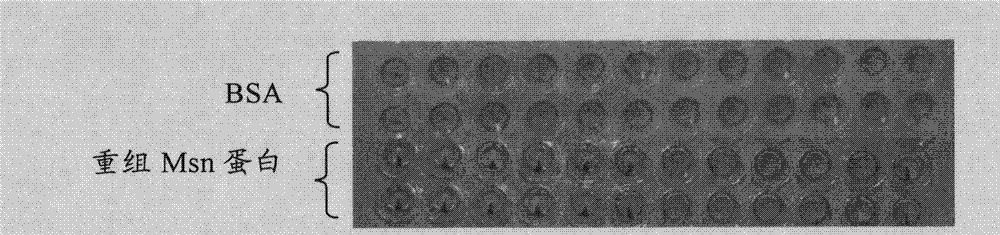
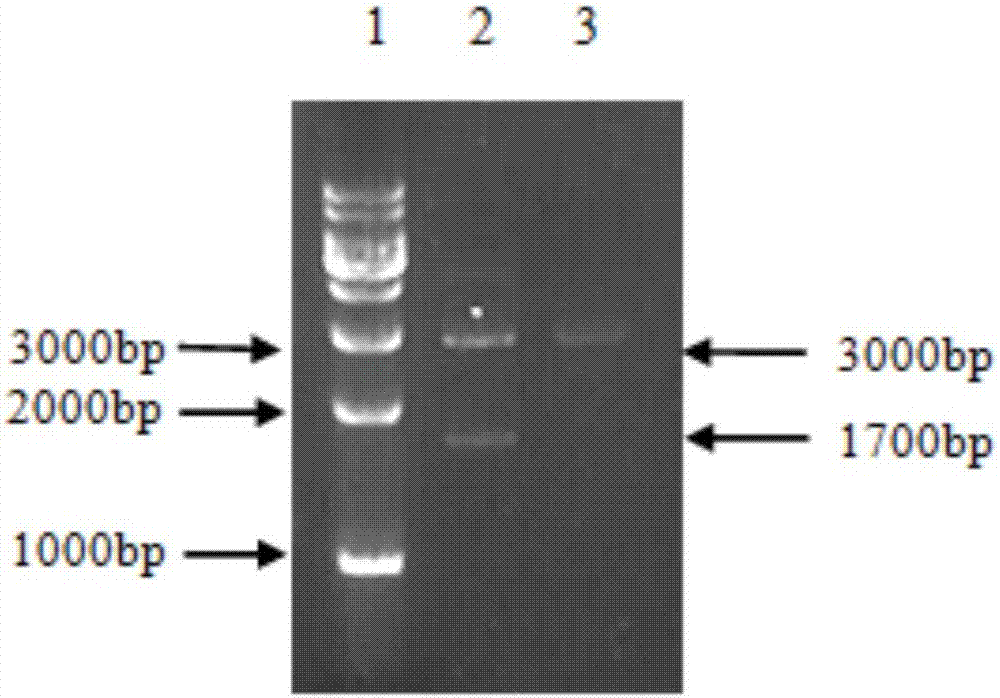
Protein bonded with rabbit hemorrhagic disease virus VP60 protein and application thereof
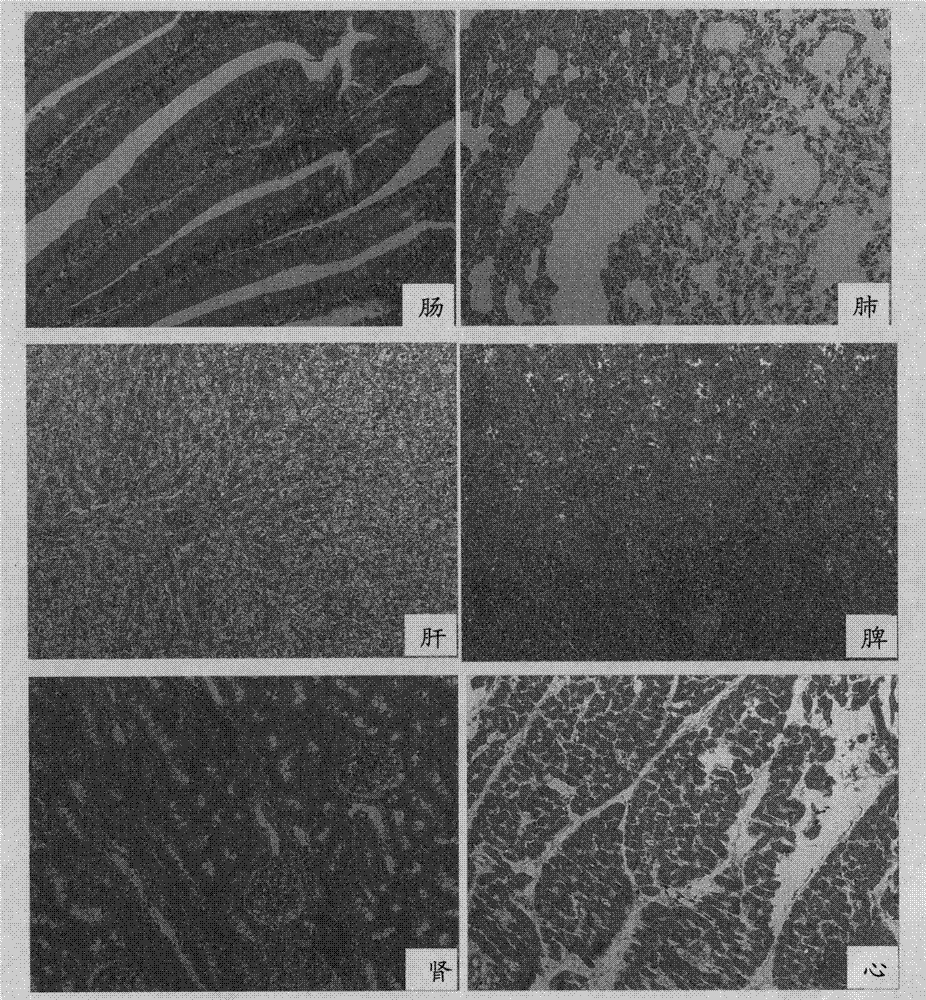
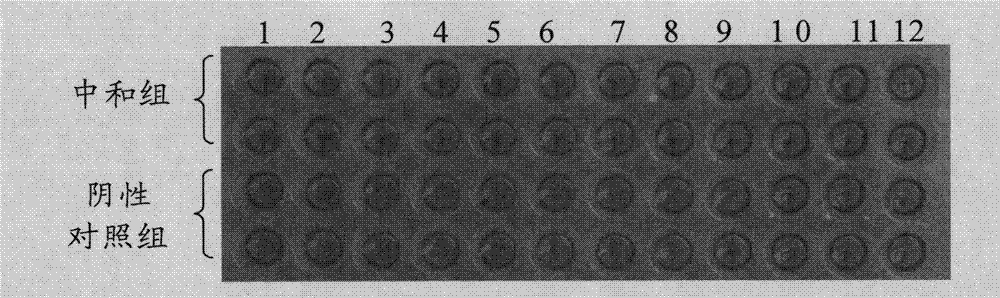
The invention discloses a protein bonded with rabbit hemorrhagic disease virus (RHDV) VP60 protein and application thereof. The protein is characterized by comprising at least one sequence which is shown as SEQ ID NO: 1. In the invention, moesin (MSN) which interacts with rabbit hemorrhagic disease virus structural protein VP60 is tagged from a rabbit liver tissue complementary DNA (cDNA) librarythrough a yeast two-hybrid technology. Immunoprecipitation tests verify that the protein has the function of being bonded with virus particles in vitro; and the purified MSN protein can inhibit the agglutination effect of 8 hemagglutination units of virus antigen on human O-shaped erythrocytes. The protein and the rabbit hemorrhagic disease virus are incubated at room temperature; an anti-RHDV negative rabbit is inoculated through oral administration and nasal dripping, still survives after 8 days and is healthy-looking; and pathological anatomy and excisional biopsy show that each visceral organ is normal. The rabbit which is infected by the virus dies in 3 days. Thus, the protein can be used for preparation of medicaments for treating rabbit hemorrhagic disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Infectious bursal disease virus recombinant ubiquitinated DNA vaccine and preparation method thereof
InactiveCN107190020ALong development timeLow costViral antigen ingredientsPharmaceutical delivery mechanismVp2 geneInfectious bursitis
The invention discloses a recombinant plasmid for expressing infectious bursal disease virus structural protein VP2; the recombinant plasmid mainly comprises infectious bursal disease virus structural protein VP2 gene, avian Ub gene and eukaryotic expression vector pVAX1. Therefore, the inventor designs and establishes a corresponding infectious bursal disease virus recombinant ubiquitinated DNA vaccine and a preparation method thereof. Compared with the prior art, the vaccine has the advantages of good manufacture simplicity, low cost, high safety, and good immunization effect. Tests indicate that the vaccine is effective in avoiding and decreasing damage and chicken mortality of immune organs, such as bursa of Fabricius, due to infectious bursal disease.
Owner:GUANGXI UNIV
Antibody m19 of O-type foot-and-mouth disease virus structural protein as well as preparation method and application of antibody m19
ActiveCN114316037AHigh sensitivityImprove featuresImmunoglobulins against virusesTissue cultureDiseaseBALB/c
The invention relates to the technical field of biology, in particular to an O-type foot and mouth disease virus structural protein antibody, a preparation method and application. The O-type foot-and-mouth disease virus 146s is used as an immunogen to immunize a Balb / c mouse, a hybridoma cell line capable of efficiently secreting the monoclonal antibody is obtained through cell fusion, and the mouse monoclonal antibody m19 is obtained. Indirect immunofluorescence tests show that the monoclonal antibody m19 has good reactivity to O-type strains of different lineages and does not react with A-type strains of different lineages. The O-type foot-and-mouth disease virus structural protein solid-phase competitive ELISA detection kit is established by utilizing the monoclonal antibody m19 and O-type structural protein rabbit antiserum, compared with an existing antibody, the sensitivity and specificity of a detection result are remarkably improved, the kit is suitable for detection of structural protein antibodies after O-type foot-and-mouth disease infection or inactivated vaccine immunization, and meanwhile, the kit can be used for detecting the structural protein antibodies after O-type foot-and-mouth disease infection or inactivated vaccine immunization. And the method is a novel method for monitoring the infection condition of the non-immune area of the foot-and-mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com