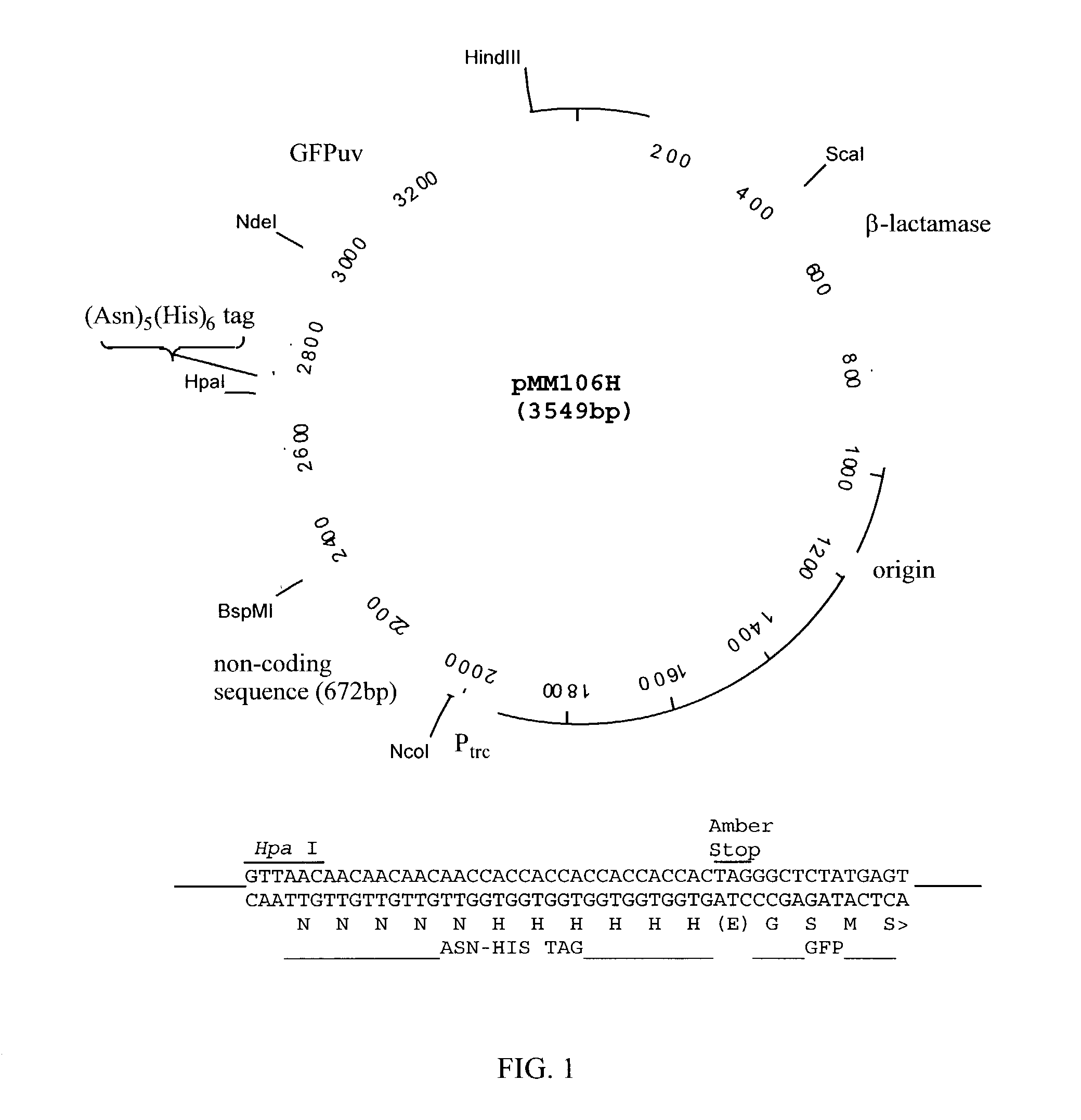
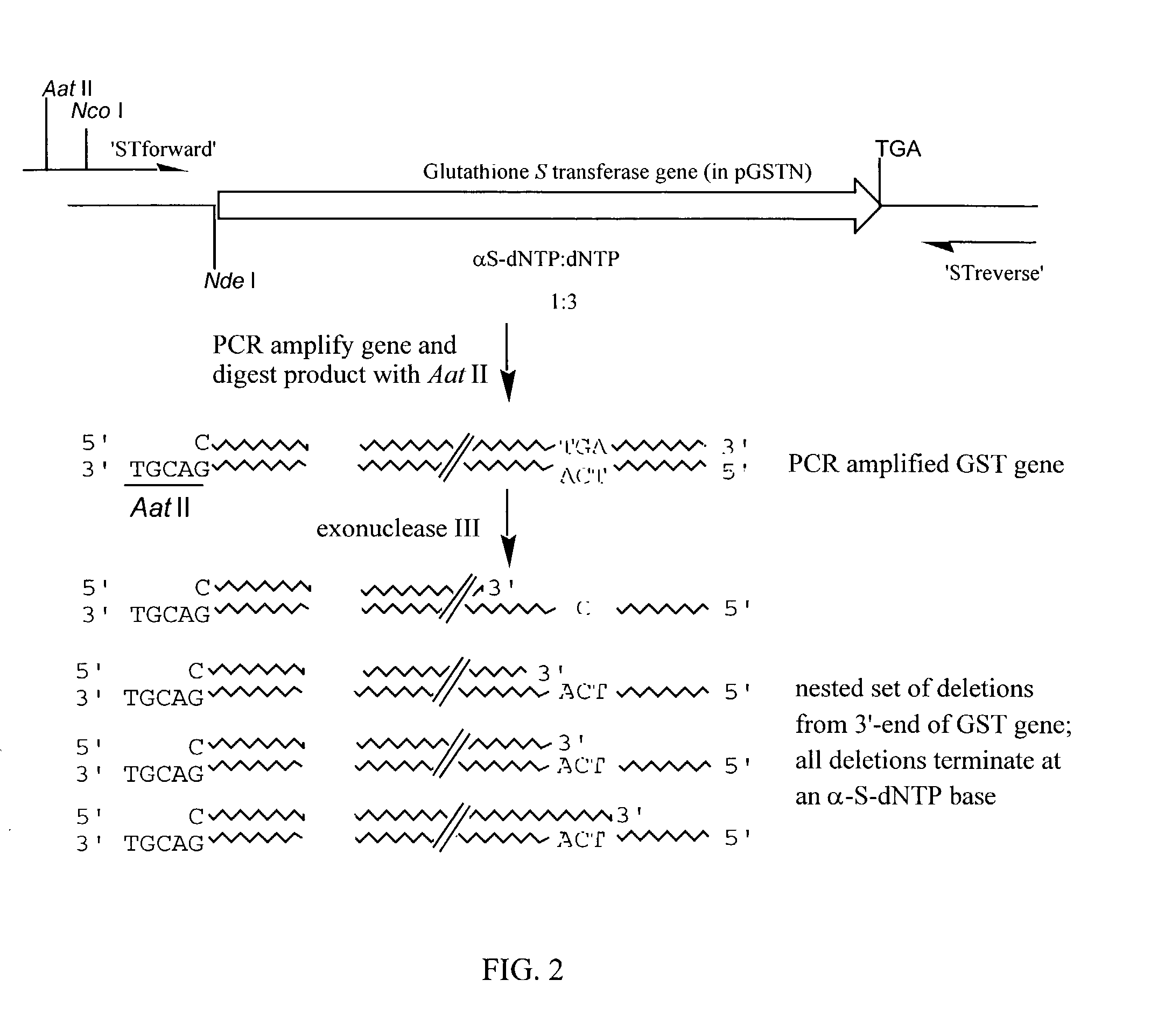
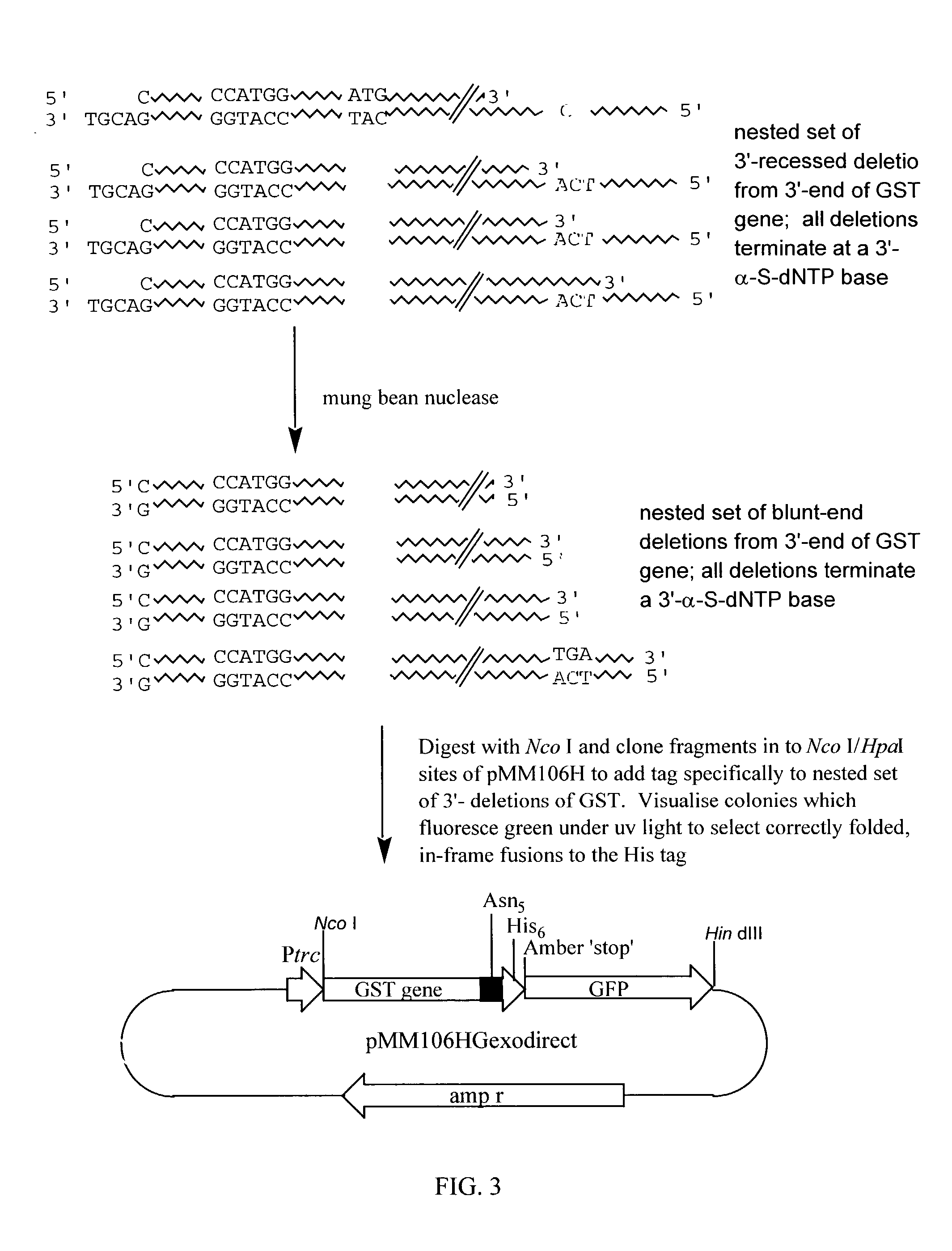
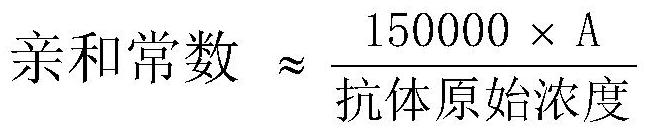Patents
Literature
143 results about "Protein tag" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein tags are peptide sequences genetically grafted onto a recombinant protein. Often these tags are removable by chemical agents or by enzymatic means, such as proteolysis or intein splicing. Tags are attached to proteins for various purposes.
Polychromic laser scanning system and method of use
InactiveUS20050280817A1Avoid distractionRadiation pyrometrySpectrum investigationFluorescenceProtein tag
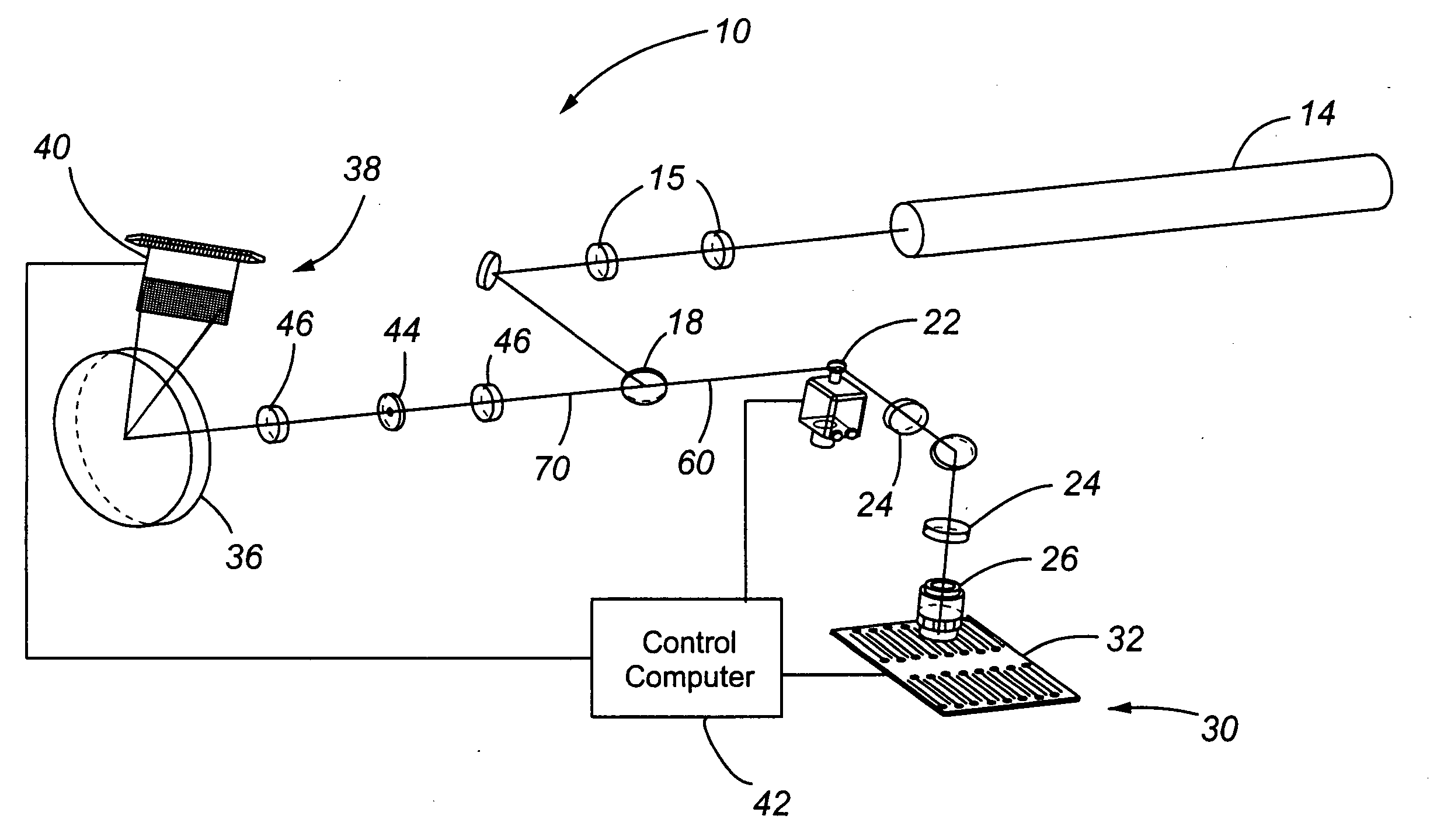
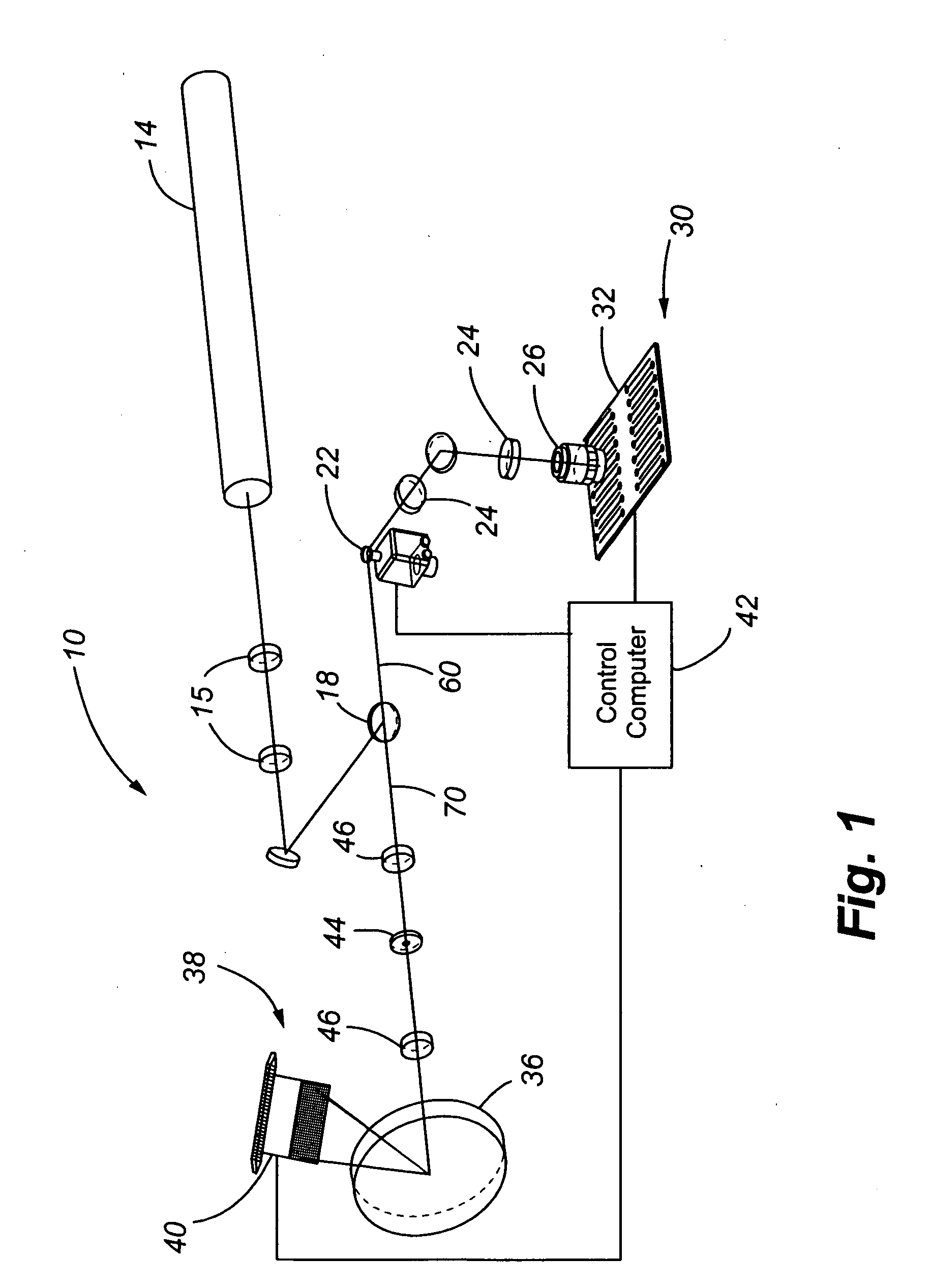
A system for laser scanning provides spectral flexibility needed for the spectroscopic monitoring of highly multiplexed samples, such as cellular and particle assays in whole blood or other suspensions. In accordance with embodiments of the present invention, the system comprises a scanner to direct an excitation laser through a sample, an objective to collect light emitted by the sample in response to the excitation laser, a spectrograph to disperse the emitted light over a plurality of wavelengths as a spectrum, and a charge coupled device for detecting the spectrum. The system can be used with samples having a variety of reporter tags, including one or more SERS tags, fluorescent organic and protein tags, and quantum dot tags. A laser scanning apparatus and method of using the same is also provided.
Owner:PPD BIOMARKER DISCOVERY SCI
Method for producing tagged genes, transcripts and proteins
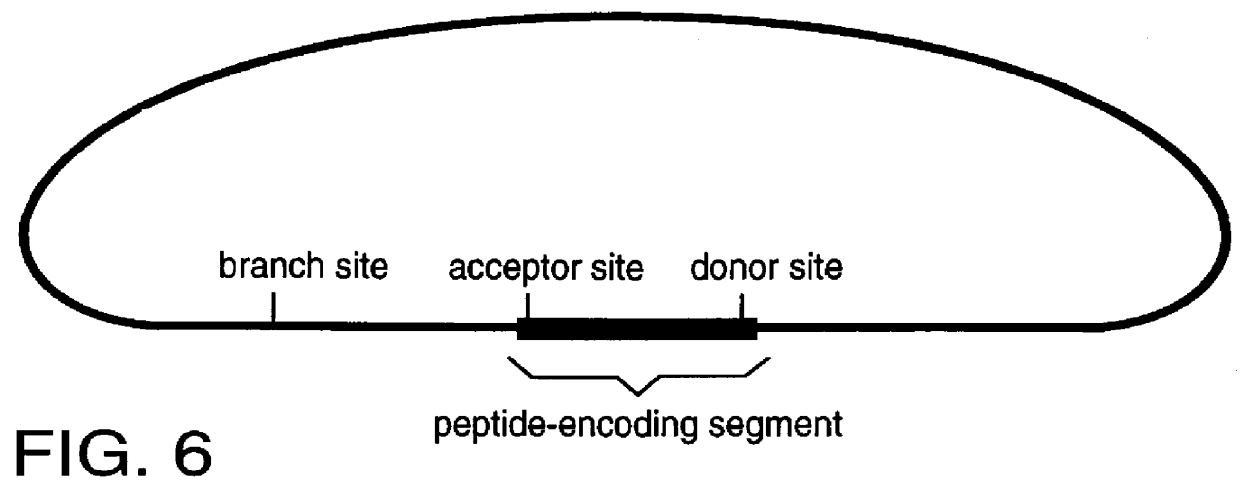
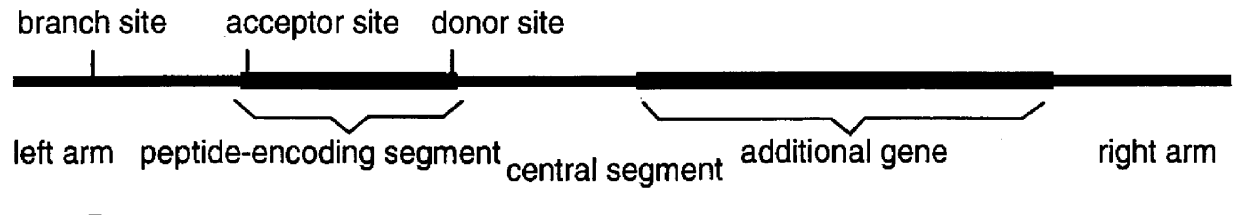
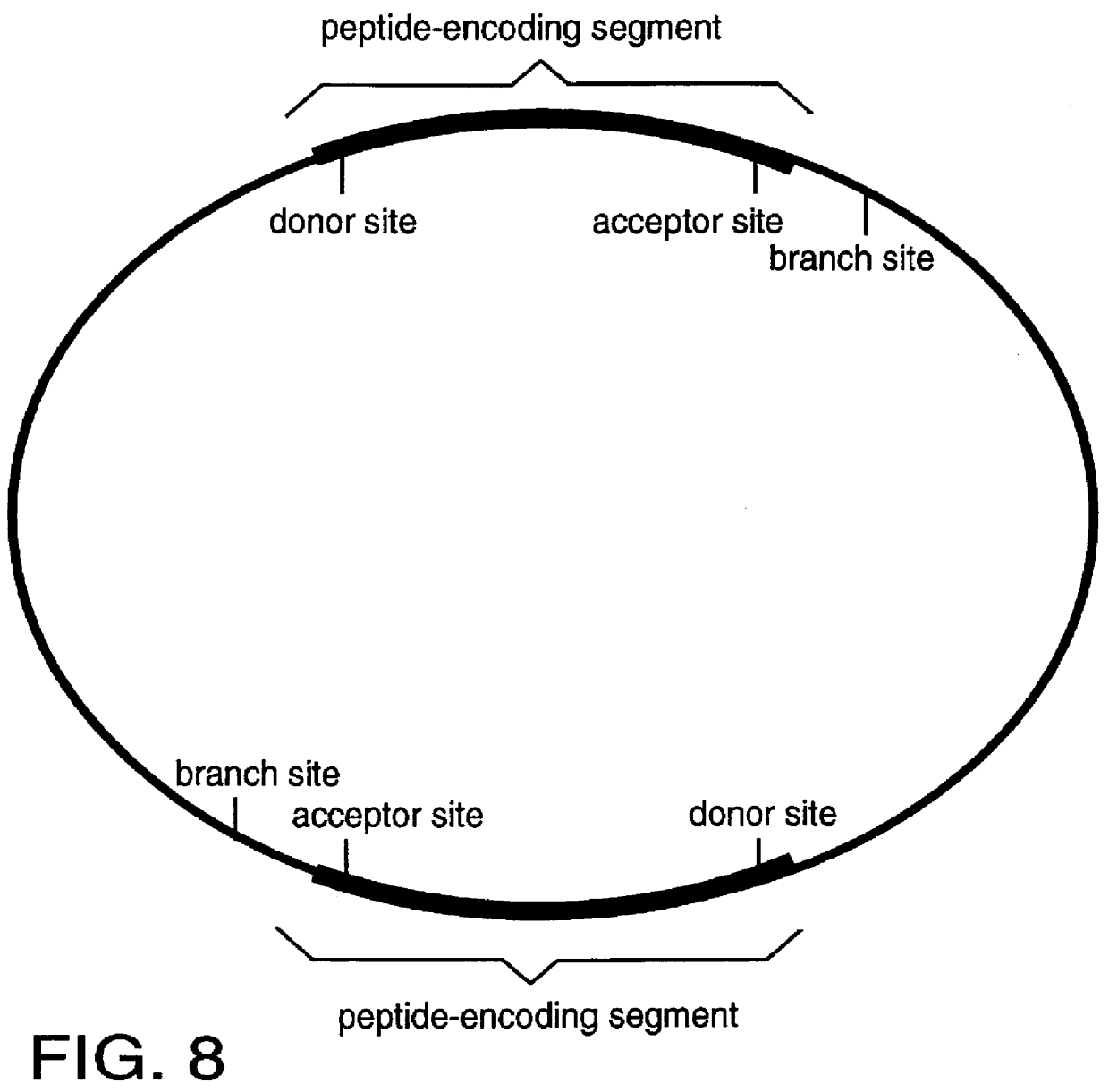
The invention described here is a method whereby a molecular tag is put on a gene, transcript and protein in a single recombinational event. The protein tag takes the form of a unique peptide that can be recognized by an antibody or other specific reagent, the transcript tag takes the form of the sequence of nucleotides encoding the peptide that can be recognized by a specific polynucleotide probe, and the gene tag takes the form of a larger sequence of nucleotides that includes the peptide-encoding sequence and other associated nucleotide sequences. The central feature of the invention in its essential form is that the tag-creating DNA has a structure such that when it is inserted into an intron within a gene it creates two hybrid introns separated by a new exon encoding the protein tag. A major virtue of the method is that it allows one to identify new proteins or protein-containing structures, and, having done so, to readily identify and analyze the genes encoding those proteins.
Owner:JARVIK JONATHAN W
SARS-CoV virus structural protein infusion protein and its high yield expression and purification and uses
InactiveCN1884303ASsRNA viruses positive-senseAntibody mimetics/scaffoldsProtein tagStructural protein
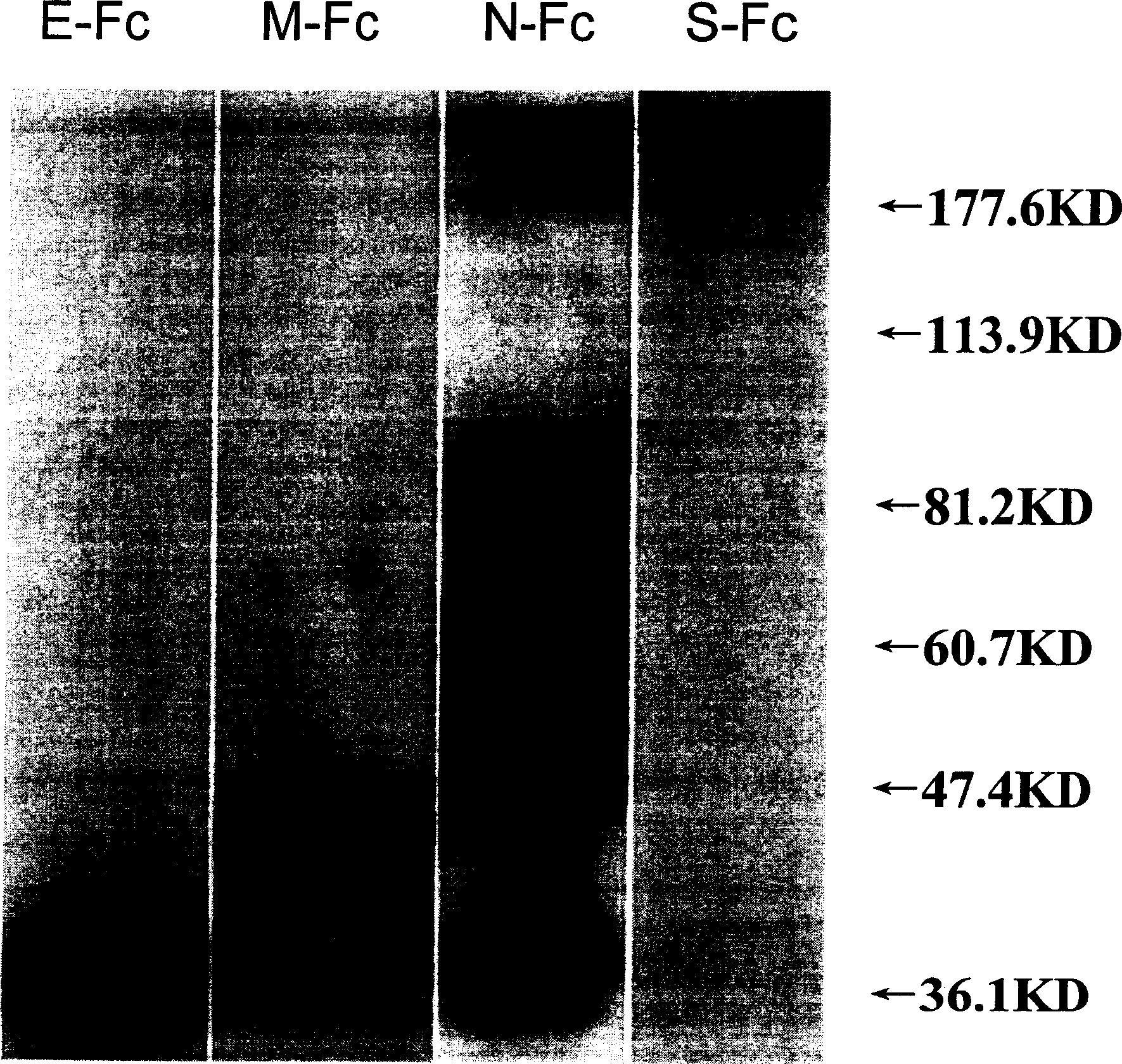
The invention relates to fusion protein of SARS-Cov virus structural protein and its high expression and purification in mammalian cell and its usage. The construction of said fusion protein is X-Y-z, and X is S or M or E or N selected from SARS-CoV virus construction protein, or their arbitrary short form; Y is the connection part with 0-20 amino acid; Z is Fc and its modification or other protein tag. The invention also provides the method of expressing and purifying said fusion protein in mammalian cell for the batch preparation or industrial production. The fusion protein can be used to prepare genetic engineering vaccine preventing SARS-CoV virus infection, solvent box for checking SARS-CoV virus, and to sift drug anti SARS-CoV virus infection with S protein combined with its acceptor ACE2. The invention can detect combination of S protein of SARS-CoV virus with ACE2, which reduces ACE2 expression, and result in or exacerbate acute lung damage, then it modifies combined segment, which can improve safety of preventing SARS-CoV virus vaccine.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Method for producing proteins tagged at the N- or C-terminus
Owner:SENSE PROTEOMIC LTD
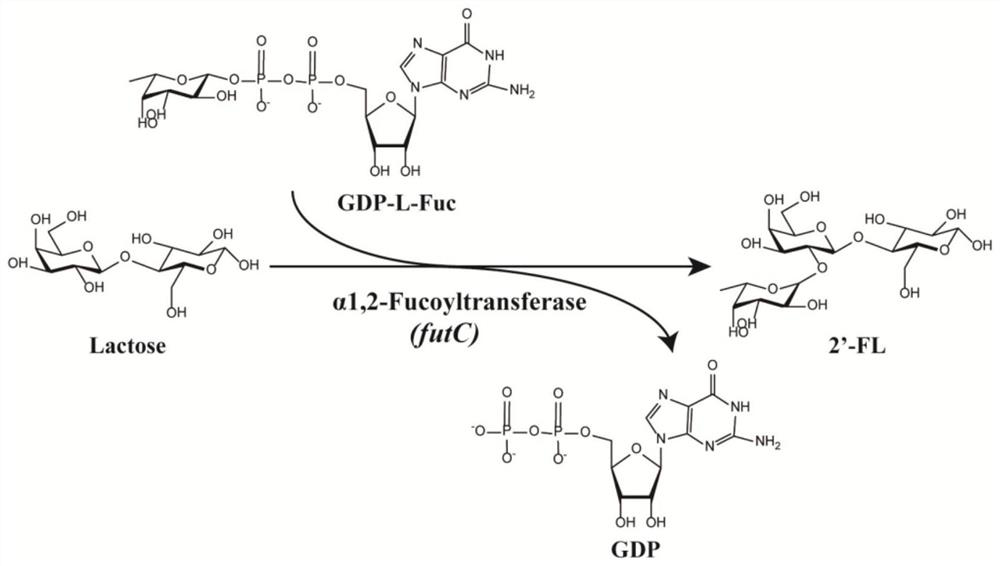
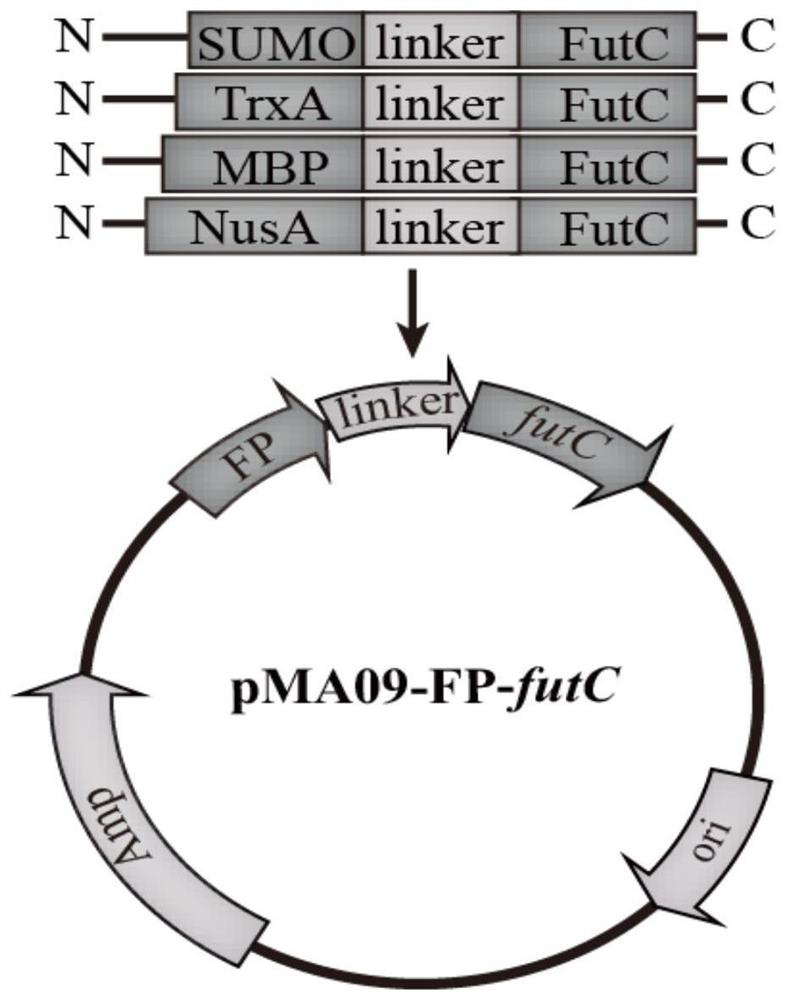
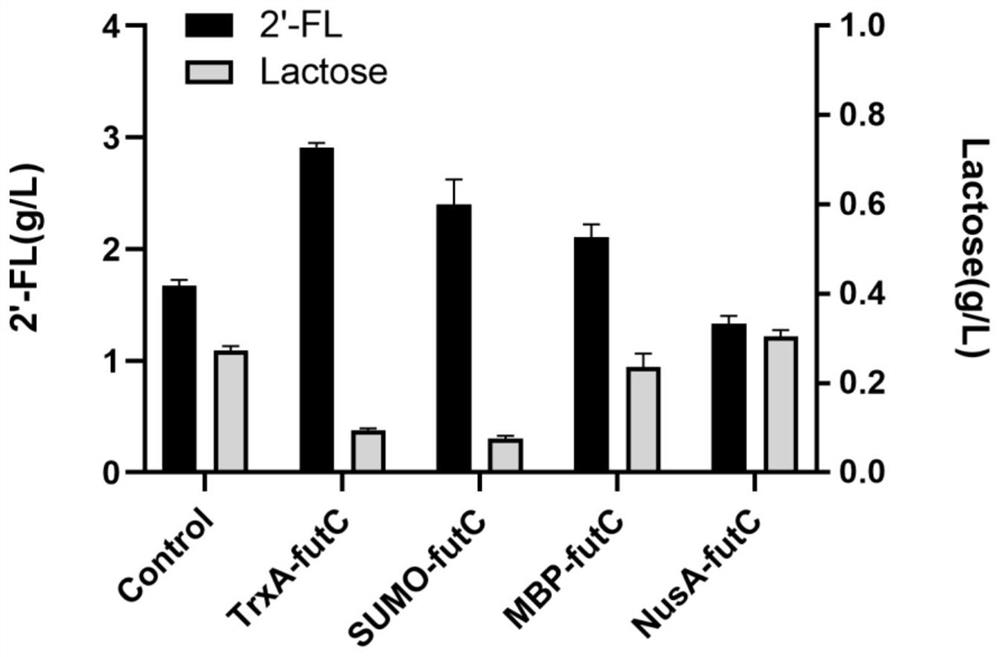
Method for increasing yield of 2'-fucosyllactose in recombinant escherichia coli
PendingCN112322565AImprove conversion efficiencyEasy to synthesizeBacteriaAntibody mimetics/scaffoldsEscherichia coliProtein tag
The invention discloses a method for increasing the yield of 2'-fucosyllactose in recombinant escherichia coli, and belongs to the technical field of gene engineering. According to the method, four different protein labels are fused at the N ends of alpha-1, 2-fucosyltransferase FutC respectively by adopting flexible Linker, and the constructed fusion protein FP-futC can improve the yield of 2'-FLcatalyzed and synthesized by alpha-1,2-fucosyltransferase to different extents. The yield of the 2'-FL synthesized by adopting the TrxA-futC fusion protein is the highest and reaches 2.94 g / L, the consumption of substrate lactose reaches 3.55 g / L, and the lactose conversion rate is 0.58 mol / mol. In the control group, the yield of the 2'-FL synthesized by the recombinant escherichia coli obtainedby plasmid pMA09-futC constructed without protein tags is only 1.71 g / L, the lactose consumption is 2.88 g / L, and the lactose conversion rate is only 0.42 mol / mol. Furthermore, the TrxA-futC fusion protein gene is integrated to a yjiP site on an escherichia coli MG1655 genome to obtain a plasmid-free 2'-FL genetically engineered strain MG-26 delta yjiP:: trxA-futC, the yield of the 2'-FL after shake-flask fermentation reaches 3.85 g / L, and the lactose conversion rate is 0.68 mol / mol.
Owner:JIANGNAN UNIV +1
Establishment and application of wheat germ cell-free protein synthesis system for high level expression of snake venom kininogenase
InactiveCN102732548AReduce incubation timeEfficient expressionHydrolasesVector-based foreign material introductionCell freeHigh level expression
The present invention provides a wheat germ cell-free protein synthesis system for high level expression of recombination snake venom kininogenase. According to the system, snake venom kininogenase gene of agkistrodon halys pallas is cloned through reverse transcription, wheat germ cell-free expression plasmid pCS<2+> / VK is constructed, a wheat germ extract is prepared, an in vitro transcription and translation system is established, the snake venom kininogenase is expressed and the system is optimized. The N terminal of the exogenous protein expressed by the expression system has the His protein tag, and a Ni column elution method is adopted to carry out purification, such that the subsequent purification of the recombinant protein is substantially simplified, the purification efficiency is high, and the cost is low. The exogenous protein further has biological activity, and provides the foundation for the next clinical study.
Owner:CHINA AGRI UNIV +1
Method for separating natural sequence nerve growth factor from mixture
The invention discloses a method for separating a natural sequence nerve growth factor from a mixture. A solution containing a human or mouse nerve growth factor is separated and purified through metal ion chelating chromatography to obtain the natural sequence nerve growth factor; the metal ion chelating chromatography resin comprises a supporting medium, a chelating ligand and metal ions; the metal ions are chelated on the chelating ligand; the elution condition is to change the concentration gradient of protein and metal ion coordinate bound competitive materials. Strong cation exchange chromatography can be conducted before metal ion chelating chromatography; after metal ion chelating chromatography, efficient strong cation exchange chromatography can be conducted. According to the method, the nerve growth factor with natural sequence and without any protein tags can be purified and separated rapidly.
Owner:山东衍渡生物科技有限公司
SNAP-tag protein tag fluorescence probe with quick specific marking ability
ActiveCN109400609ASynthetic raw materials are cheapSimple methodOrganic chemistryFluorescence/phosphorescenceProtein targetBinding site
The invention relates to an SNAP-tag protein tag fluorescence probe with quick specific marking ability. The fluorescence probe takes 4-amino-1,8-naphthalimide as a fluorophore and benzyloxy as a binding site. A structure of the fluorescence probe is shown as formula (1) as shown in the specification. The probe is derived from environment sensitive fluorophore naphthalimide, and can specifically react with an SNAP-tag protein; and the fluorescence intensity is increased by 12 times after a reaction. A reaction rate of the fluorescence enhanced SNAP-tag fluorescence probe reaches 13247-15625M<-1>s<-1>; the speed of the fluorescence probe is equivalent to that of a commercial fluorescence substrate requiring a cell elution step; and the fluorescence probe has the highest marking speed in fluorescence enhanced SNAP-tag fluorescence probes reported at present. The probe can achieve specific marking of a target protein fused with an SNAP-tag in a living cell in short time to achieve elution-free fluorescence imaging. The probe can be widely applied in the fields of protein marking, protein identification, interaction between the protein and micromolecules / macromolecules, cell fluorescence imaging and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Gene modification based method for expressing exogenous drug through probiotic and application of method
InactiveCN108660148ATo achieve the purpose of treating tumor diseasesImprove securityTumor rejection antigen precursorsBacteriaEscherichia coliNutritional deficiency
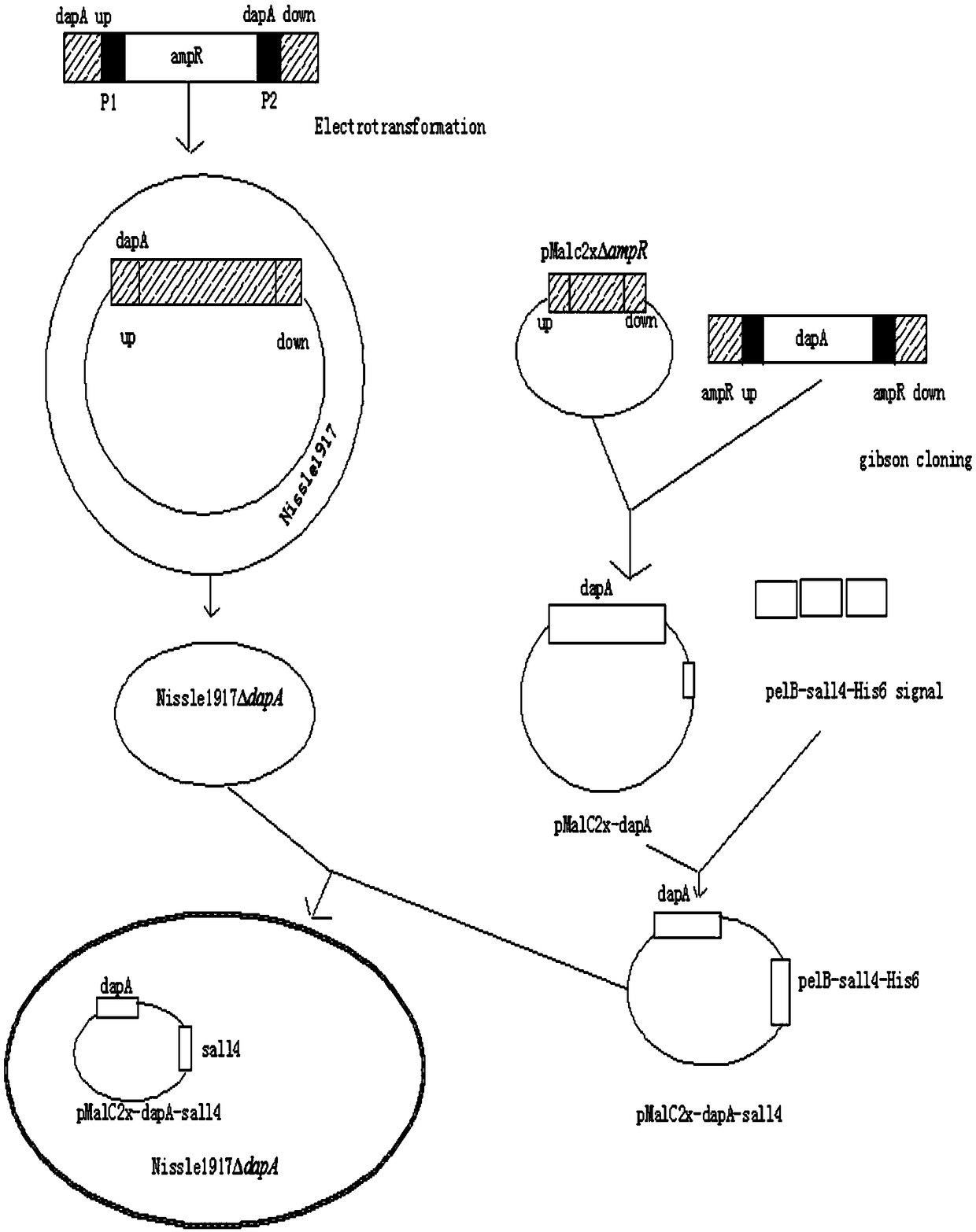
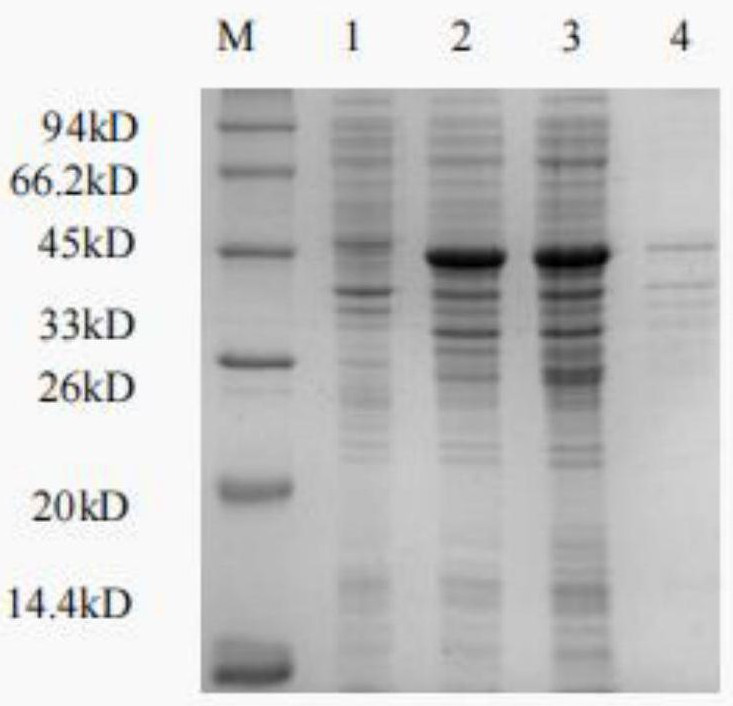
The invention discloses a gene modification based method for expressing an exogenous drug through a probiotic and an application of the method, the homologous recombination and the one-step seamless cloning are used to knock out an indispensable gene dapA of escherichia coli Nissle1917 to obtain an escherichia coli nutritional deficiency strain Nissle1917deltadapA, the dapA gene is cloned to construct a complementary plasmid pMalc2x-dapA of the nutritional deficiency strain, the complementary plasmid pMalc2x-dapA is electrically shocked to convert into the strain Nissle1917deltadapA, an amicillin resistance gene ampR of a pMalc2x carrier is substituted through the homologous recombination to obtain a plasmid balance system without an antibiotics resistance marker, 12 amino polypeptide sequence genes of an amino terminal sall4 are synthesized in vitro at a sall4 amino terminal, a pelB signal peptide gene sequence of an erwinia carotovora pectinase is fused at the N end, a gene sequenceof a His protein tag is added at the C end, the gene sequences are closed into the complementary plasmid pMalc2x-dapA together to obtain an exogenous drug expression plasmid pMalc2x-dapA-sall4, the plasmid is imported into the escherichia coli nutritional deficiency strain Nissle1917deltadapA, the obtained strain effectively expresses the sall4 polypeptide, and the method has an obvious effect ontreating the liver cancer.
Owner:奇元科技(武汉)有限公司
Anti-Calponin protein monoclonal antibody and cell strain, preparation method and application thereof
ActiveCN113234155AStrong specificityIncreased sensitivityImmunoglobulins against animals/humansMicroorganism based processesEscherichia coliAntigen
The invention relates to a monoclonal antibody capable of recognizing a human Calponin antigen, a secretory cell strain, a preparation method of the monoclonal antibody and application of the secretory cell strain in immunodetection. According to the technical scheme, amino acids from the first site to the 297th site at the C tail end of the Calponin protein are selected as antigen peptides, codon optimization is carried out, a gene segment suitable for being expressed in escherichia coli BL21 is formed, and finally the obtained recombinant protein comprises a Calponin protein segment and a histidine protein tag. A mouse is immunized by the recombinant protein, and the mouse hybridoma cell strain 24H5 capable of efficiently secreting the anti-Calponin protein monoclonal antibody and the anti-Calponin protein monoclonal antibody secreted by the cell strain are obtained through cell fusion, screening and subcloning. The antibody obtained by the scheme has high specificity and sensitivity, can specifically recognize cells expressing Calponin protein, and is suitable for immunological detection, especially immunohistochemical detection.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Link application of protein tag
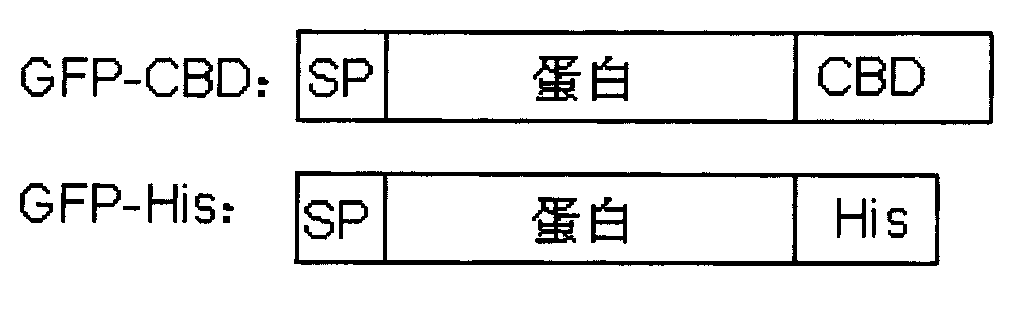
InactiveCN102757501AEasy to fixEfficient purificationChemical industryPeptide preparation methodsProtein targetProtein tag
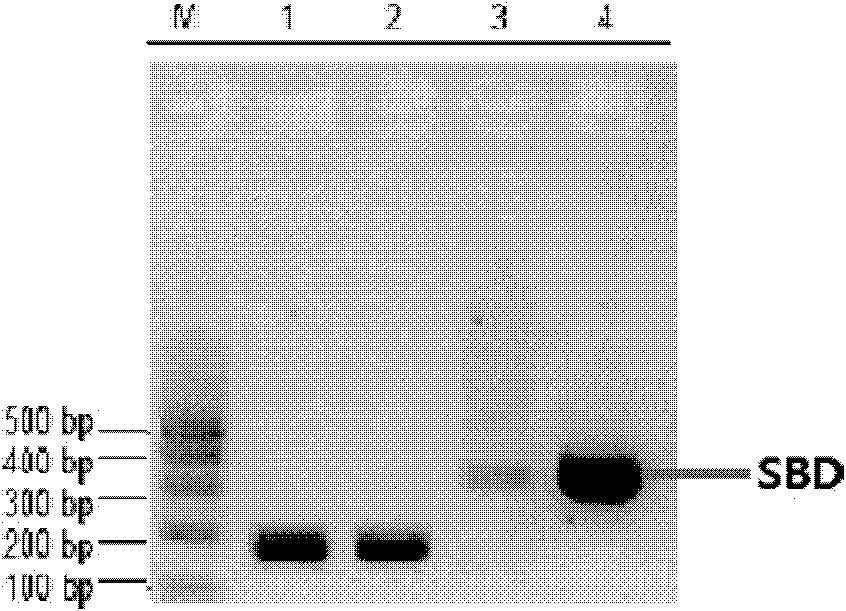
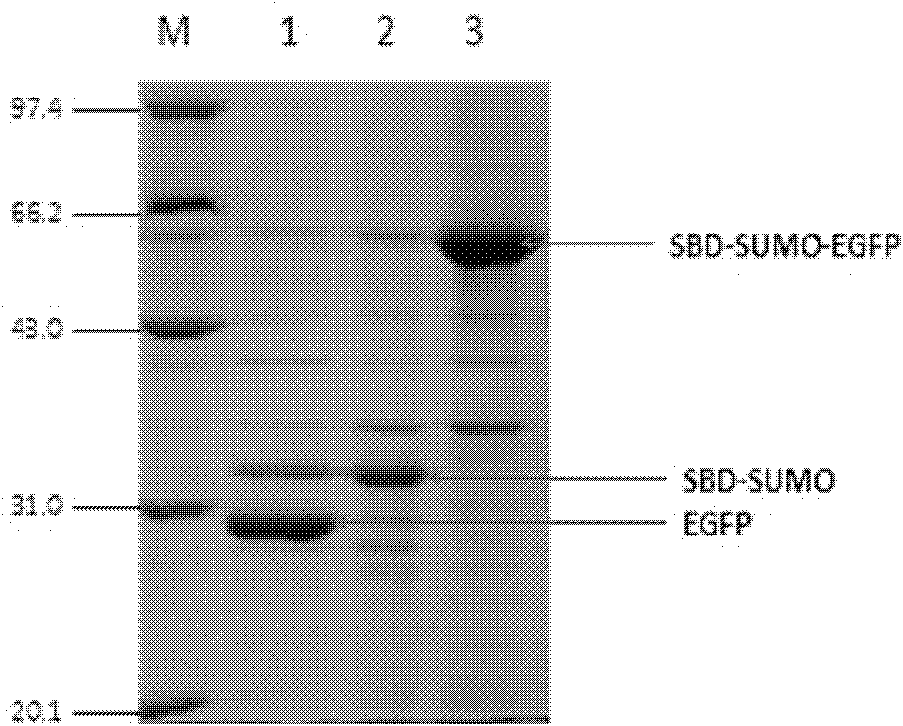
The invention relates to a link application of a tag. The invention develops a brand new protein tag, i.e., a starch binding domain (SBD). After a target protein is fused with the SBD which can be well combined with a starch substrate, the SBD can be applied to fixation or purification of the target protein as well as observation of mutual actions between the target protein and other proteins. The SBD is a cheap and reliable tag protein.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Monoclonal antibody for resisting CA125 protein and cell strain, preparation method and application thereof
ActiveCN113061186AStrong specificityIncreased sensitivityTumor rejection antigen precursorsMicroorganism based processesEscherichia coliAntigen
The invention relates to a monoclonal antibody capable of recognizing a human CA125 antigen, a secretory cell strain, a preparation method of the monoclonal antibody and application of the secretory cell strain in immunodetection. According to the technical scheme, 13837-14184 amino acids at the C tail end of the CA125 protein are selected as the antigen peptide, codon optimization is carried out, a gene segment suitable for being expressed in escherichia coli BL21 (DE3) is formed, and the finally obtained recombinant protein contains a CA125 protein segment and a histidine protein tag. A mouse is immunized by the recombinant protein, and the mouse hybridoma cell strain 20E3 capable of efficiently secreting the anti-CA125 protein monoclonal antibody and the anti-CA125 protein monoclonal antibody secreted by the cell strain are obtained through cell fusion, screening and subcloning. The antibody obtained by the scheme has high specificity and sensitivity, can specifically recognize cells expressing CA125 protein, and is suitable for immunological detection, especially immunohistochemical detection.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Method for improving solubility of foot-and-mouth disease protein for immunization
The present invention belongs to the technical field of preparation of a vaccine, and particularly relates to a method for improving the solubility of a foot-and-mouth disease protein for immunization. The method is by adding an His6-MBP protein tag in the C terminal of the foot-and-mouth disease protein by the mean genetic engineering in FMD protein by genetic engineering, so as to increase the solubility of the foot-and-mouth disease protein; the His6 represents the 6 histidine tags, and MBP represents a maltase binding protein. The present invention also provides an immune FMD protein CDG276 aiming at type A FMD prepared by the method. Compared to the FMDV VPI protein prepared by the existing genetic engineering, the FMD protein prepared by the invention has obvious increased the expression level; and due to the addition of soluble label maltose binding protein (MBP), the expressed FMDVA1 soluble protein has significantly increased solubility; therefore, the method has good application value.
Owner:HENAN AGRICULTURAL UNIVERSITY
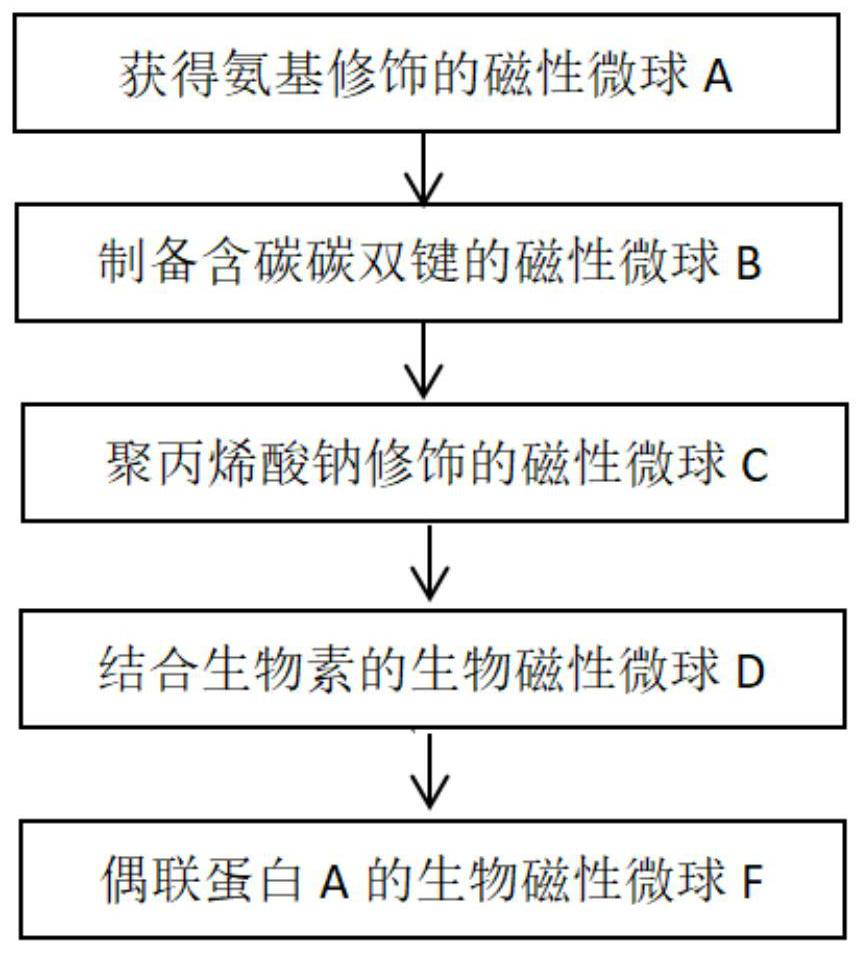
Biomagnetic microspheres as well as preparation method and application thereof
InactiveCN112877387AIncrease activity spaceImprove throughputPeptide preparation methodsFermentationProtein tagMicrosphere
The invention relates to biomagnetic microspheres, which comprises a magnetic microsphere body, at least one polymer with a linear main chain and a branched chain is arranged on the outer surface of the magnetic microsphere body, one end of the linear main chain is fixed to the outer surface of the magnetic microsphere body, the other end of the polymer is dissociated on the outer surface of the magnetic microsphere body, and biotin is connected to the tail end of a branch chain of the polymer of the biological magnetic microspheres. The invention also provides an improvement based on the biomagnetic microsphere provided in the first aspect, and a preparation method and an application thereof. The biomagnetic microspheres are convenient to operate and use and can be rapidly dispersed and rapidly settled in a solution, and large-scale experimental equipment such as a high-speed centrifugal machine does not need to be used; the product has the advantages of simple operation and wide application, can be connected with selective purification media (such as avidin, affinity protein, polypeptide / protein tag and the like) through biotin, and can be universally applied to separation and purification of proteins including but not limited to target objects such as antibody substances and the like on a large scale.
Owner:KANGMA SHANGHAI BIOTECH LTD
Anti-CD5 protein monoclonal antibody as well as cell strain, preparation method and application thereof
ActiveCN113105547AStrong specificityIncreased sensitivityBiological material analysisMicroorganism based processesEscherichia coliCD5
The invention relates to a monoclonal antibody capable of recognizing a human CD5 antigen, a secretory cell strain, a preparation method of the monoclonal antibody and application in immunodetection. According to the technical scheme, amino acids from the 398th site to the 495th site of the C terminal of the CD5 protein are selected as antigen peptides, codon optimization is carried out, a gene segment suitable for being expressed in escherichia coli BL21 is formed, and the finally obtained recombinant protein comprises a CD5 protein segment and a histidine protein tag. A mouse is immunized by the recombinant protein, and the mouse hybridoma cell strain 11H3 capable of efficiently secreting the anti-CD5 protein monoclonal antibody and the anti-CD5 protein monoclonal antibody secreted by the cell strain are obtained through cell fusion, screening and subcloning. The antibody obtained by the scheme has high specificity and sensitivity, can specifically recognize cells expressing CD5 protein, and is suitable for immunological detection, especially immunohistochemical detection.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Fusion chitinase capable of efficiently degrading alpha-chitin and related biological material and application thereof
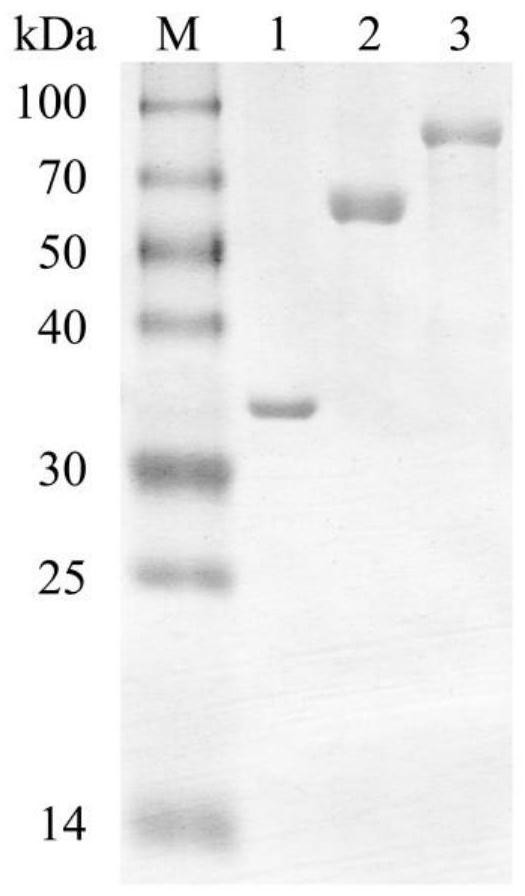
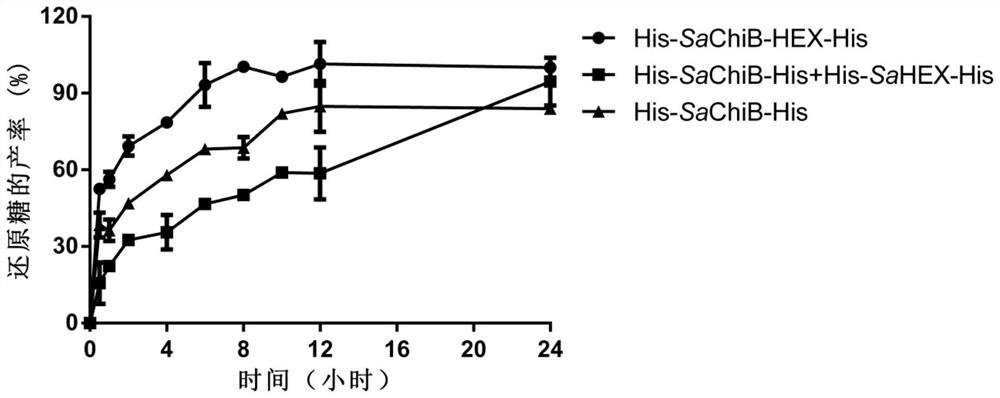
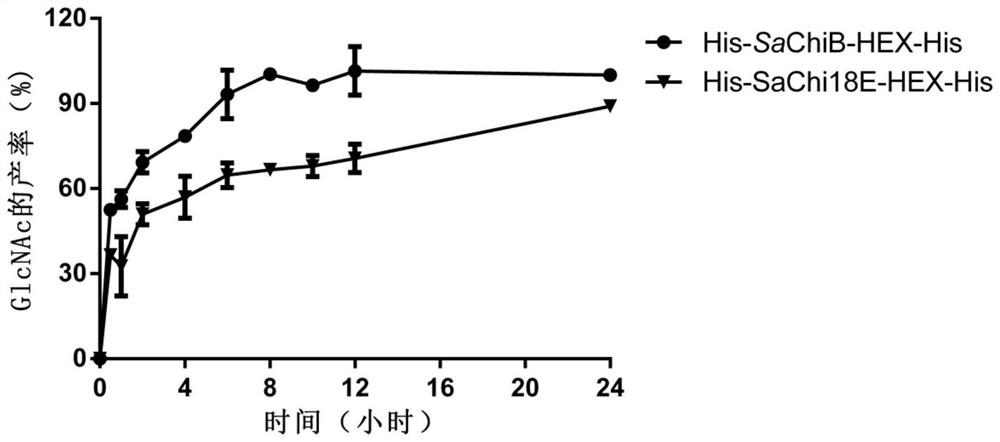
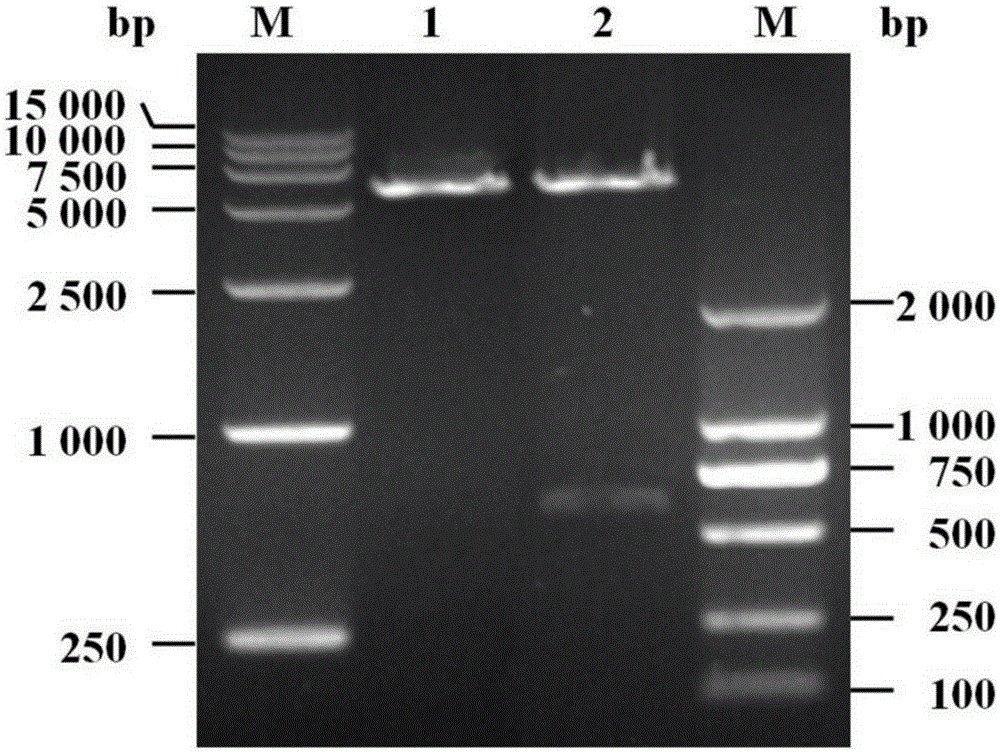
The invention discloses fusion chitinase capable of efficiently degrading alpha-chitin and a related biological material and application thereof. The fusion chitinase is a protein obtained by fusing beta-N-acetyl hexosaminase and chitinase, wherein the chitinase activity of the fusion chitinase is higher than that of the beta-N-acetyl hexosaminase and that of the chitinase; the chitinase is D1) or D2), wherein the D1) is a protein with 53rd-322nd amino acid residues of which the amino acid sequence is shown in SEQ ID No.2; and the D2) is a fusion protein obtained by fusing a protein tag at the carboxyl terminal or / and the amino terminal of the protein shown in D1). The fusion chitinase has a degradation effect on various types of chitin, and has relatively high activity on colloid chitin, alpha-chitin and fungal cell wall chitin; and the fusion chitinase has the capability of completely hydrolyzing chitin and generating N-acetyl glucosamine. The fusion chitinase can be widely applied to biological medicine, biological agriculture, cosmetics, energy industry, etc.
Owner:INST OF AGRI RESOURCES & REGIONAL PLANNING CHINESE ACADEMY OF AGRI SCI
Target protein expression with laetiporus sulphureus mushroom lectin N-acetyllactosamine binding domain as fusion tag and purification method thereof
ActiveCN105296506AAchieve one-step purificationReduce purification costsBacteriaPeptide preparation methodsN-AcetyllactosaminePurification methods
The invention discloses an artificially-synthesized LSL gene for encoding a laetiporus sulphureus mushroom lectin N-acetyllactosamine binding domain and an expression vector and host cell containing the LSL gene. The nucleotide sequence of the LSL gene is SEQ ID NO. 1. The invention further discloses a target protein expression with LSL used as the fusion tag and a purification method thereof. The method specifically relates to LSL gene synthesis, construction of the expression vector containing the LSL gene and the target protein gene, construction of the expression vector containing the LSL gene and a protease gene, expression and purification of fusion protein, LSL protein tag removal, target protein purification and the like. The method is simple and easy to implement, one-step purification of target protein is achieved, high-purity target protein can be obtained, the protein purification cost is lowered, the method can be widely applied to activity protein in the fields of biological medicine, veterinary medicine and the like and large-scale preparation of vaccine antigen in the industry of biological products, and high practical value is achieved.
Owner:HENAN AGRICULTURAL UNIVERSITY
Protein related to flowering time of plants and applications of protein
ActiveCN111620936AIncrease valueEarly floweringPlant peptidesFermentationBiotechnologyNicotiana tabacum
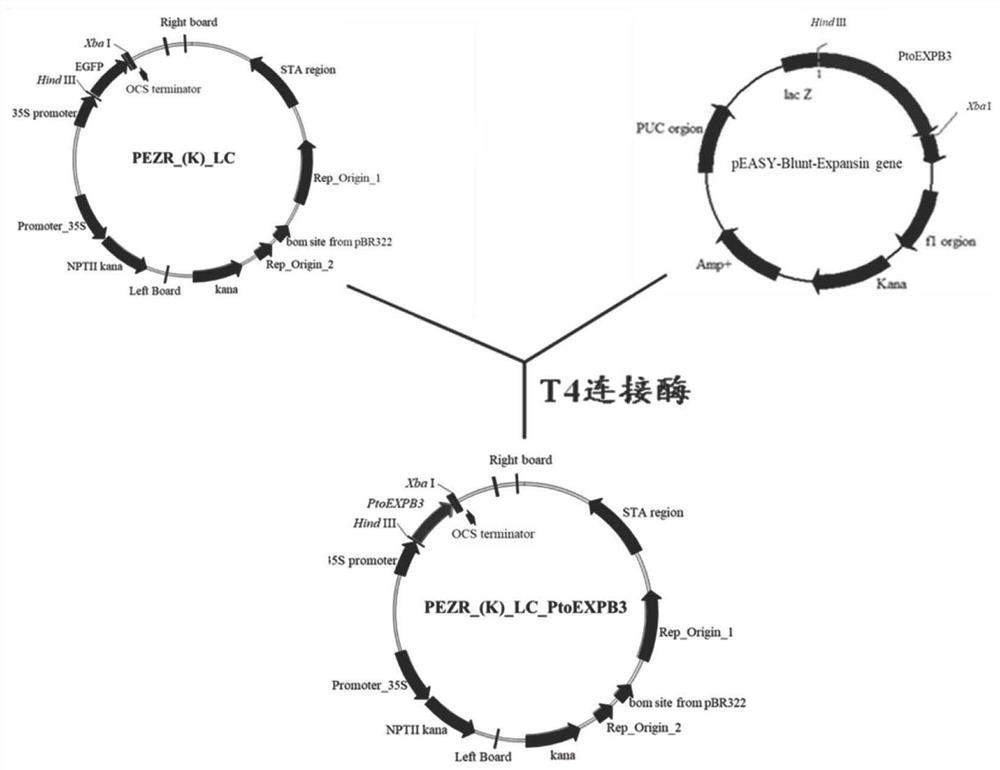
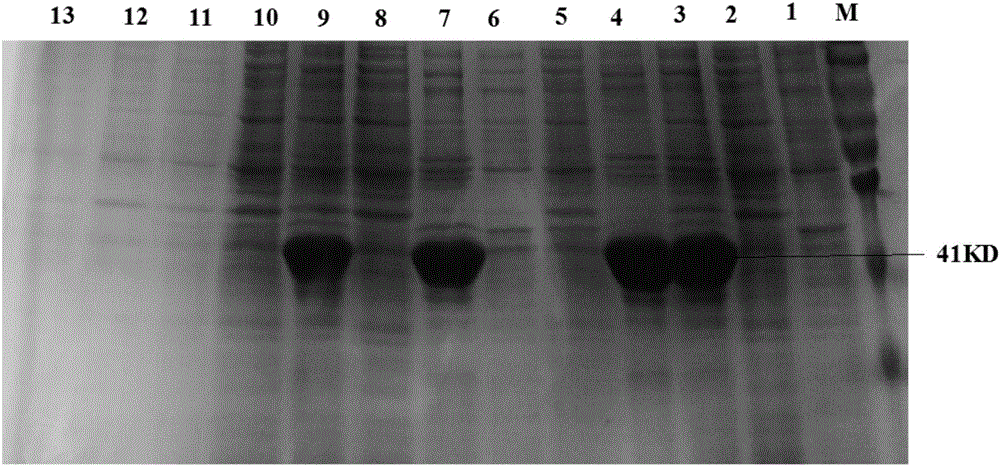
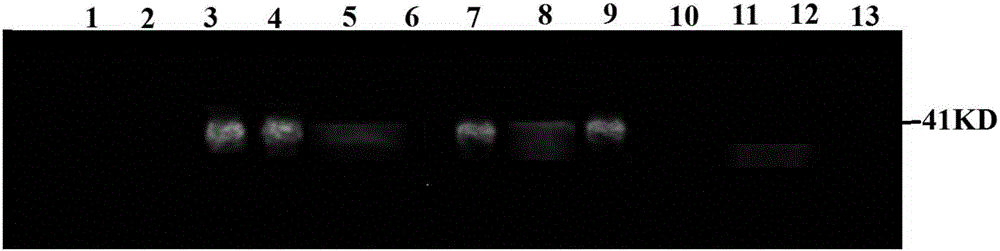
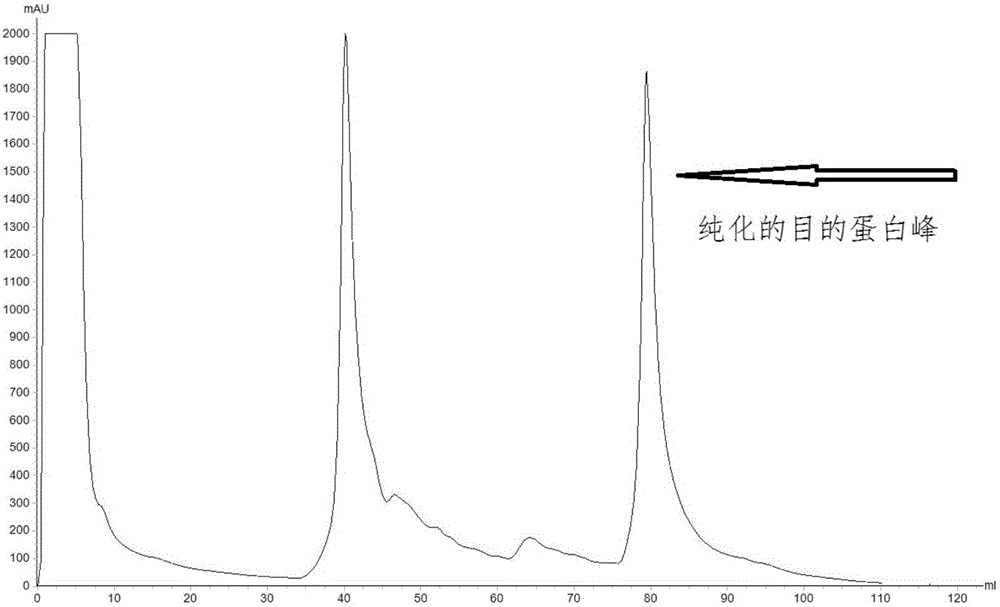
The invention relates to protein related to the flowering time of plants and applications of the protein. The protein is shown as follows: A1) the amino acid sequence of the protein is shown as a sequence 2 in a sequence table; A2) the protein obtained by substituting and / or deleting and / or adding one or more amino acid residues to the amino acid sequence shown as the sequence 2 in the sequence table has more than 90% identity with the protein shown in the A1) and is related to the flowering time of plants; and A3) the fusion protein is obtained by linking the N-terminal or / and C-terminal of the A1) or the A2) and protein tags. An extended protein gene PtoEXPB3 is obtained from populus tomentosa. The gene is introduced in tobacco; and compared with wild type tobacco, the flowering time oftransgenic tobacco flowers can be advanced by 21 days, so that the PtoEXPB3 can have the function of early flowering and can be taken as an important gene resource of the molecular breeding of ornamental plants.
Owner:BEIJING FORESTRY UNIVERSITY
Recombinant alpha protein for inhibiting clostridium perfringens infection and preparation method and application thereof
InactiveCN106008684AHigh expressionOptimizing expression conditionsAntibacterial agentsBacterial antigen ingredientsSolubilityProtein tag
The invention discloses recombinant alpha protein for inhibiting clostridium perfringens infection and a preparation method and application thereof. The recombinant alpha protein is shown in (a) or (b) or (c), wherein the protein in (a) is composed of amino acid sequences shown as SEQ ID No.2; the protein in (b) is composed of amino acid sequences shown at the sites No.51-No.353 of SEQ ID No.2; the fusion protein in (c) is obtained by fusing protein tags at carboxyl terminals or / and amino terminals of the protein shown in (a) or (b). The recombinant alpha protein enables animals to have a higher serum antibody level and resist attack of clostridium perfringens after the animals are immunized with the recombinant alpha protein. The recombinant alpha protein is good in solubility and easy to purify and can serve as a diagnostic antigen to be prepared into a monoclonal antibody or be used for further research on protein functions and conformation relations.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
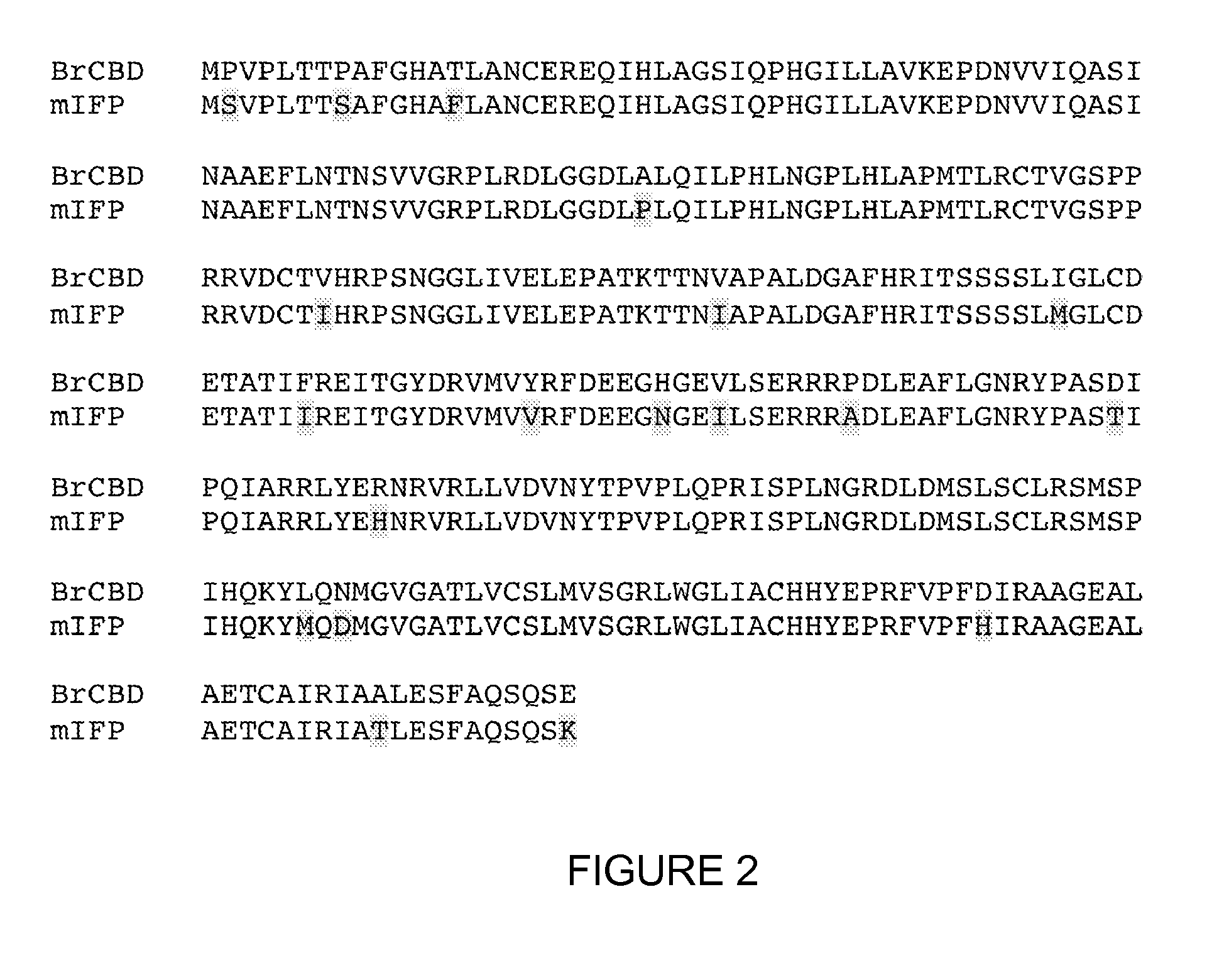
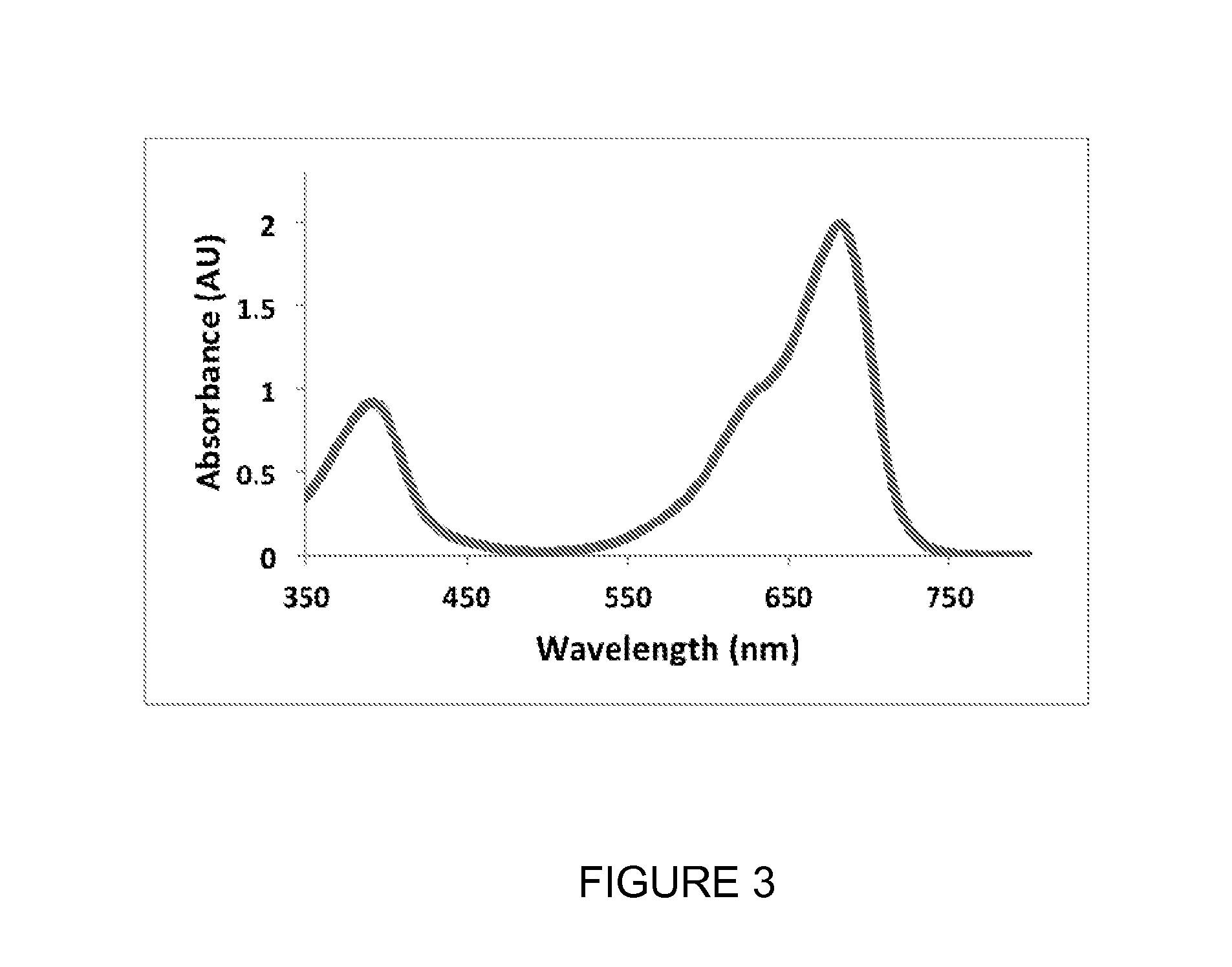
Monomeric and bright infrared fluorescent proteins
The invention described herein features variants related to infrared fluorescent proteins, in particular to mutants of a phytochrome from the bacterium Bradyrhizobium sp. ORS278. The variants show approximately a ten-fold increase in brightness compared to other known infrared fluorescent proteins. The variants are monomeric, allowing them to be used as a protein tag without disrupting the function of the tagged protein of interest.
Owner:RGT UNIV OF CALIFORNIA
Preparation method and application of engineering bacteria for efficiently expressing growth hormone
InactiveCN109402134AImprove solubilityReduce translation rateBacteriaMicroorganism based processesEscherichia coliProtein tag
The invention discloses a preparation method of engineering bacteria for efficiently expressing growth hormone. The preparation method comprises the following steps: 1) amplifying human growth hormonehGH gene by a PCR method to obtain an amplified hGH gene; 2) connecting the amplified hGH gene in step 1) to a pMal-p2x vector to obtain recombinant plasmids pMal-hGH; 4) guiding the recombinant plasmids in step 3) into escherichia coli BL21 (DE3), inducing protein expression by IPTG, and after it determines that expression is correct, obtaining the engineering bacteria for efficiently expressingthe growth hormone. The pMal-p2x is taken as vectors separately to obtain recombinant plasmids pMal-p2x-GH, an amino terminal of the recombinant plasmids of pMal-p2x-GH is provided with an MBP protein tag, therefore, the recombinant plasmids are guided into a receptor of the escherichia coli, hGH and MBP tags can be subjected to fusion expression, therefore, hGH is brought to a peripheral mass space, purification of hGH protein is facilitated, moreover, due to the oxidizing environment of peripheral mass, disulfide bonds can be formed correctly by the hGH protein, and thus, the protein has good activity.
Owner:湖南百尔泰克生物科技有限公司
Monoclonal antibody against DOG1 protein and cell strain, preparation method and application thereof
ActiveCN113045652AStrong specificityIncreased sensitivityImmunoglobulins against animals/humansTissue cultureEscherichia coliAntigen
The invention relates to a monoclonal antibody capable of recognizing a human DOG1 antigen, a secretory cell strain, a preparation method and application thereof in immunological detection. According to the technical scheme, amino acids at the positions 53-291 at C-terminal of the DOG1 protein are selected as antigen peptides, codon optimization is carried out, a gene fragment suitable for being expressed in escherichia coli BL21 (DE3) is formed, and finally an obtained recombinant protein contains a DOG1 protein fragment and a histidine protein tag. Mice are immunized by the recombinant protein, and a mouse hybridoma cell strain 19F2 capable of efficiently secreting the anti-DOG1 protein monoclonal antibody and the anti-DOG1 protein monoclonal antibody secreted by the cell strain are obtained through cell fusion, screening and subcloning. The antibody obtained by the scheme has high specificity and sensitivity, can specifically recognize cells expressing the DOG1 protein, and is suitable for immunological detection, especially immunohistochemical detection.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
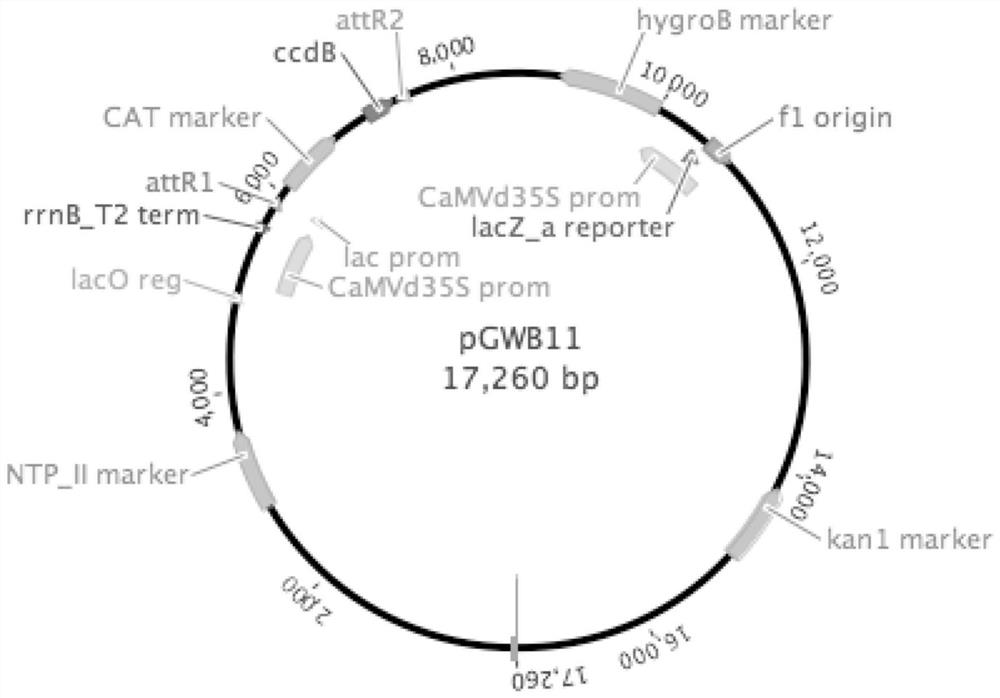
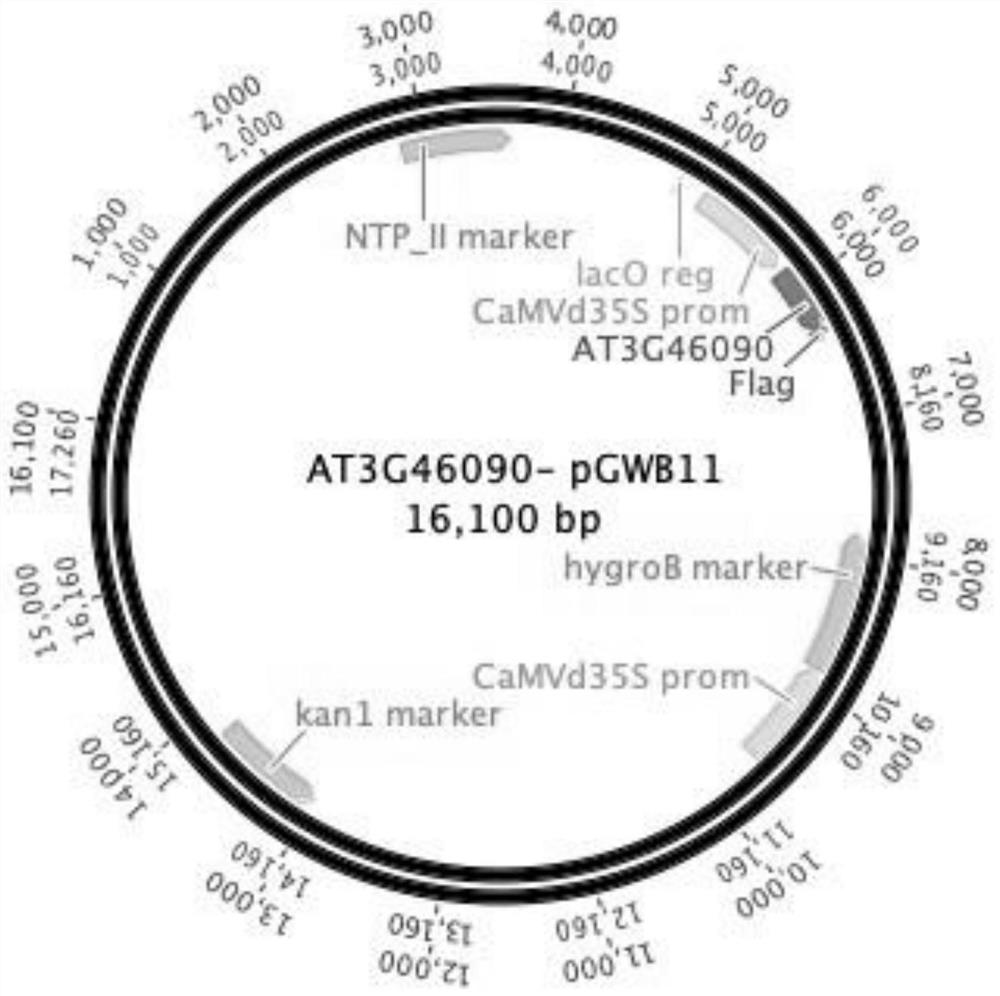
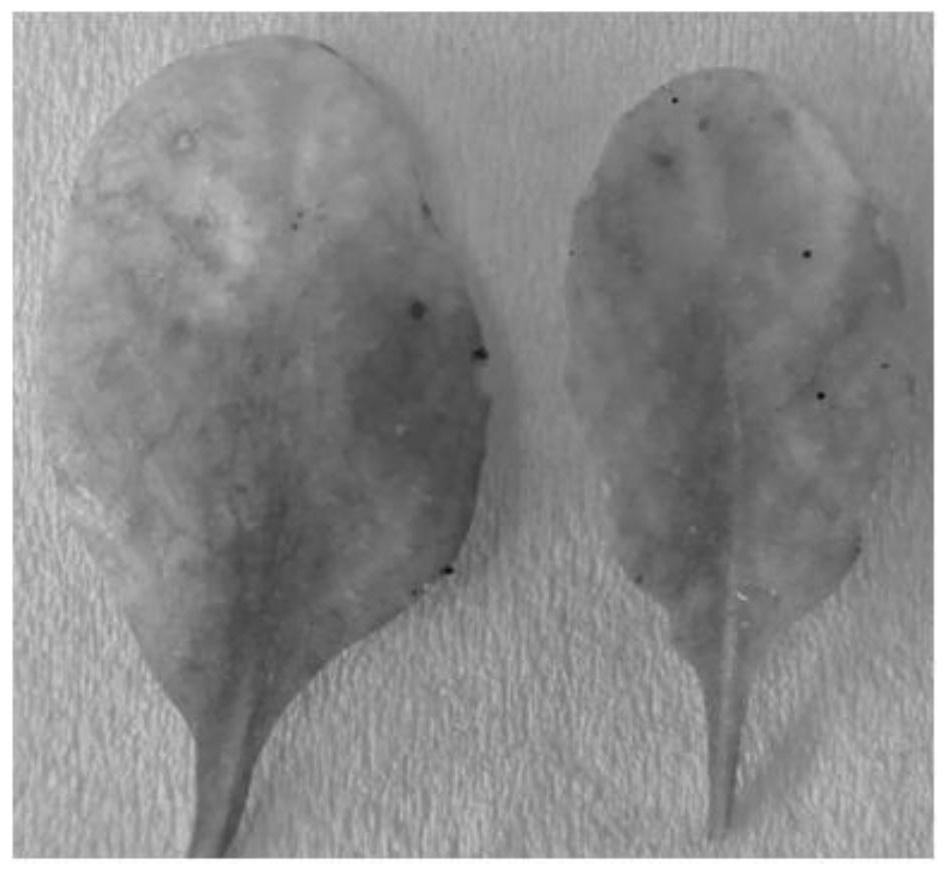
Application of arabidopsis transcription factor AT3G46090 gene in cultivation of disease-resistant transgenic plants
The invention provides the application of an Arabidopsis thaliana transcription factor, namely the AT3G46090 gene in cultivation of disease-resistant transgenic plants. The CDS sequence of the Arabidopsis thaliana AT3G46090 gene is constructed to upper stream with an FLAG protein tag through the Gateway technology, Arabidopsis thaliana plants are transformed, screened and identified, and finally, the transgenic plants are obtained. The screened plants are analyzed, and it is found that the AT3G46090 gene has the effect of enhancing the resistance of the plants to the pseudomonas syringae tomato subspecies, the expression of the gene affects the disease resistance phenotype of the plants, and homologous genes in crops possibly have the same function, so the gene has important significance in production.
Owner:XUZHOU NORMAL UNIVERSITY
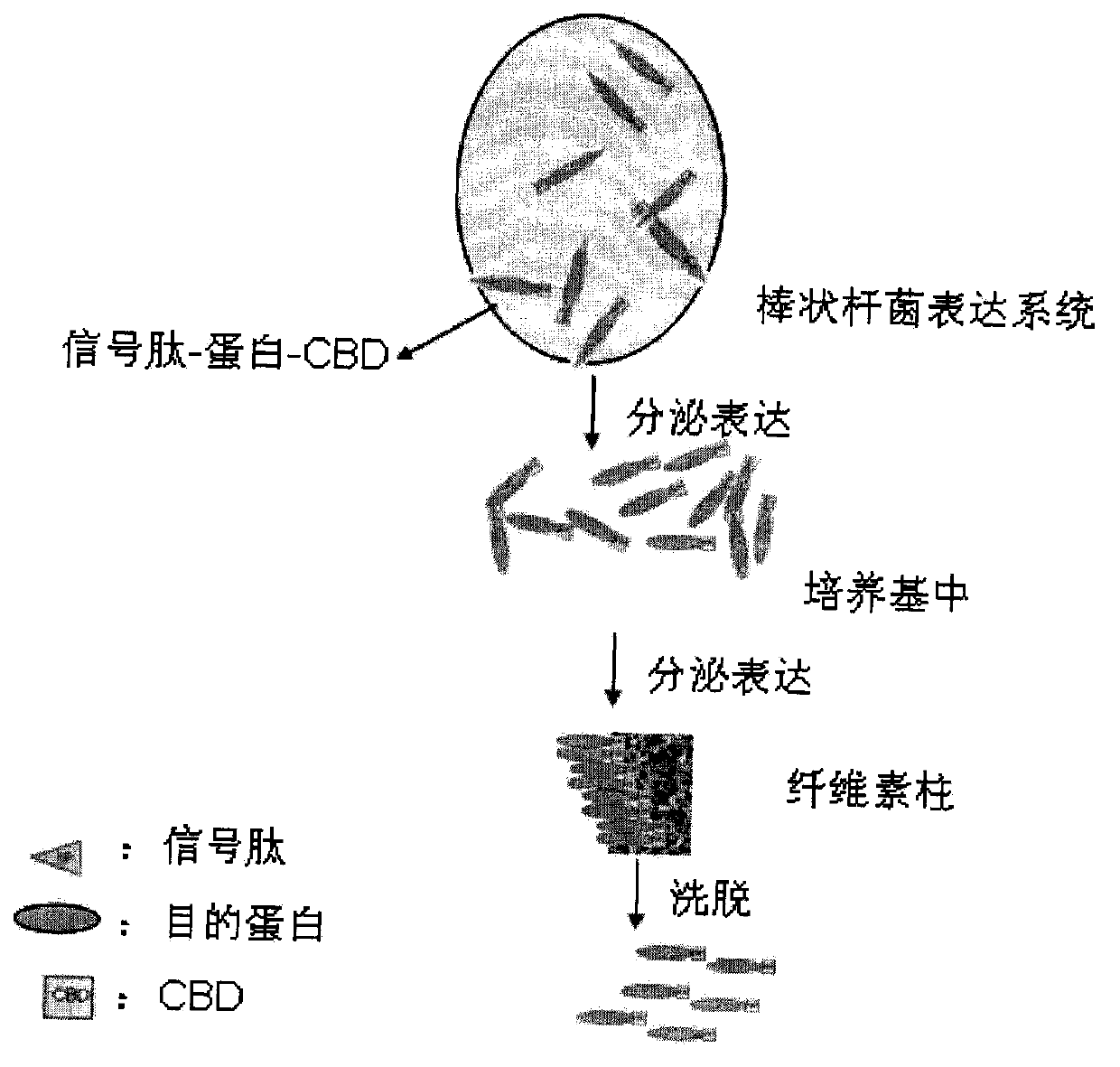
Application of cellulose binding domain in secretory expression system of corynebacteria
InactiveCN103966252ASimple purification processLow costPeptide preparation methodsVector-based foreign material introductionCelluloseProtein target
The invention specifically relates to a method of using the cellulose binding domain of Trichoderma reesei as an affinity tag for secretory expression and purification of recombinant protein in a corynebacteria expression system. The method comprises the following steps: 1) optimizing and synthesizing a codon sequence coding the cellulose binding domain of Trichoderma reesei glucoside hydrolase I; 2) cloning the sequence synthesized in the step 1 to a corynebacteria secretory expression vector as a protein tag sequence; 3) inserting a target protein sequence into the tag vector obtained in the step 2; 4) transforming the recombinant vector obtained in the step 3 to corynebacteria for secretory expression of protein; and 5) with CBD as the affinity tag, purifying recombinant protein secreted by the recombinant bacterium by using a cellulose column. According to the method, fragmentation of thalli is not needed, a low-cost cellulose column filling material is directly used for protein purification of an induced medium, so production cost for protein is reduced.
Owner:SHAOXING INST OF TECH COLLEGE OF ENG PEKING UNIV BIOENG CENT
Genetic engineering bacterium expressing snake venom kininogenase as well as constructing method and application thereof
The invention provides a genetic engineering bacterium expressing recombinant snake venom kininogenase efficiently. A snake venom kininogenase gene in Agkistrodon halys pallas is cloned through reverse transcription, a pronucleus recombinant expression plasmid pET30a / VK (venom kininogenase) is constructed and then is transferred into escherichia coli, and the strains which can secrete and express efficiently are screened out. A terminal N of foreign protein expressed by the expression system is provided with an His protein tag, purification is carried out by adopting a Ni column eluting method, follow-up purification operation of recombinant protein is greatly simplified, purity of the obtained recombinant protein reaches up to more than 90%, and purification efficiency is high and cost is low, thus the genetic engineering bacterium disclosed by the invention is applicable to large-scale preparation of protein.
Owner:CHINA AGRI UNIV +1
MAPWA fusion antibacterial peptide, preparation method and application thereof
InactiveCN102199215AHigh nutritional valueImprove disease resistanceAntibacterial agentsFungiGenetic engineeringTechnical support
The invention discloses a MAPWA fusion antibacterial peptide, its preparation method and application. The fusion antibacterial peptide is a protein as in 1) or 2) or 3) as follows: 1) a protein composed of amino acid sequences shown in sequence 2 of the sequence table; 2) a protein from the fusion of a GST tag and the protein in 1); 3) a protein derived from 1) or 2) with antibacterial activity, specifically obtained by substituting and / or deleting and / or adding to the amino acid residue sequences comprising the protein in 1) or 2) by one or several amino acid residues. In the invention, an antibacterial peptide with strong antibacterial effect is obtained through genetic engineering technology, thus finishing a high expression study in yeast cells. The study shows that the antibacterial peptide can endure the high temperature of feed pelleting. With a creative production process for recombinant yeast fermentation provided in the invention, a further in-depth study is expected to develop the antibacterial peptide into an efficient, safe and multifunctional biological feed so as to replace or reduce antibiotic additives and improve the nutritional value of the feed. Therefore, a new technical support is provided for the sustainable development of animal husbandry of our country.
Owner:成都市金之源生物技术有限公司 +1
Recombinant epsilon protein for inhibiting clostridium perfringens infection and preparation method and application thereof
InactiveCN106008682AOptimizing expression conditionsHigh expressionAntibacterial agentsBacterial antigen ingredientsProtein tagADAMTS Proteins
The invention discloses recombinant epsilon protein for inhibiting clostridium perfringens infection and a preparation method and application thereof. The recombinant epsilon protein is (a) protein formed by amino acid sequences shown in SEQ ID No.2 or (b) protein formed by amino acid sequences from the 51 site to the 370 site in SEQ ID No.2 or fusion protein obtained by fusing protein tags on carboxyl terminals or / and amino terminals of the (a) protein or the (b) protein. After animals are immune to the recombinant epsilon protein, the animals can generate the high serum antibody level and can resist attack of clostridium perfringens. The recombinant epsilon protein is good in dissolvability and easy to purify and can be taken as a diagnostic antigen or prepared into a monoclonal antibody or used for further studying the protein function and the conformation relationship.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
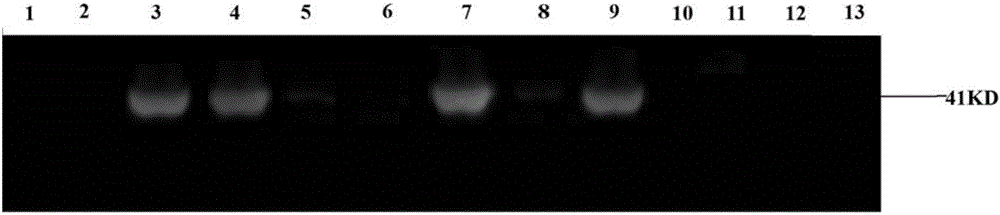
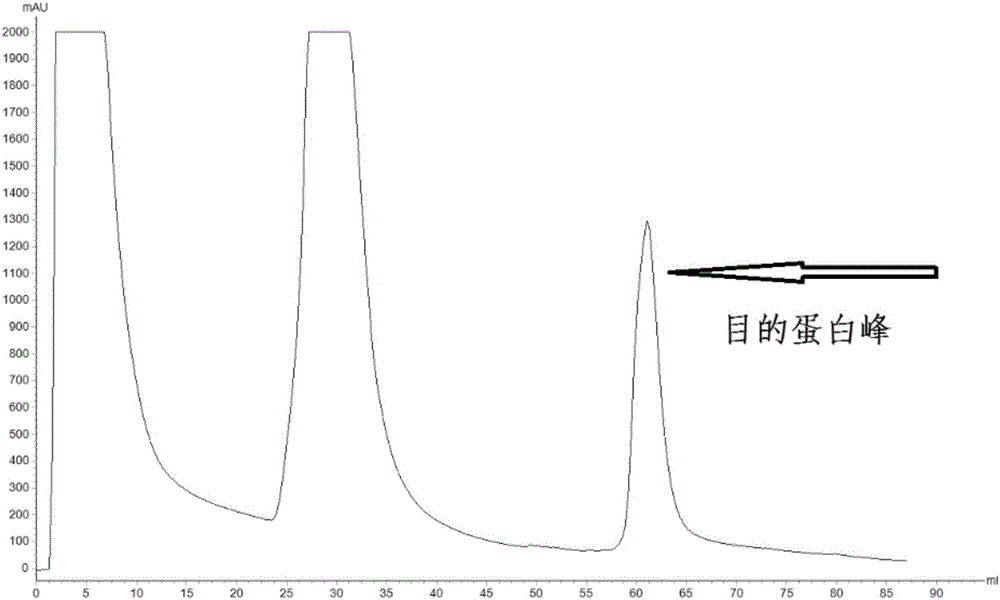
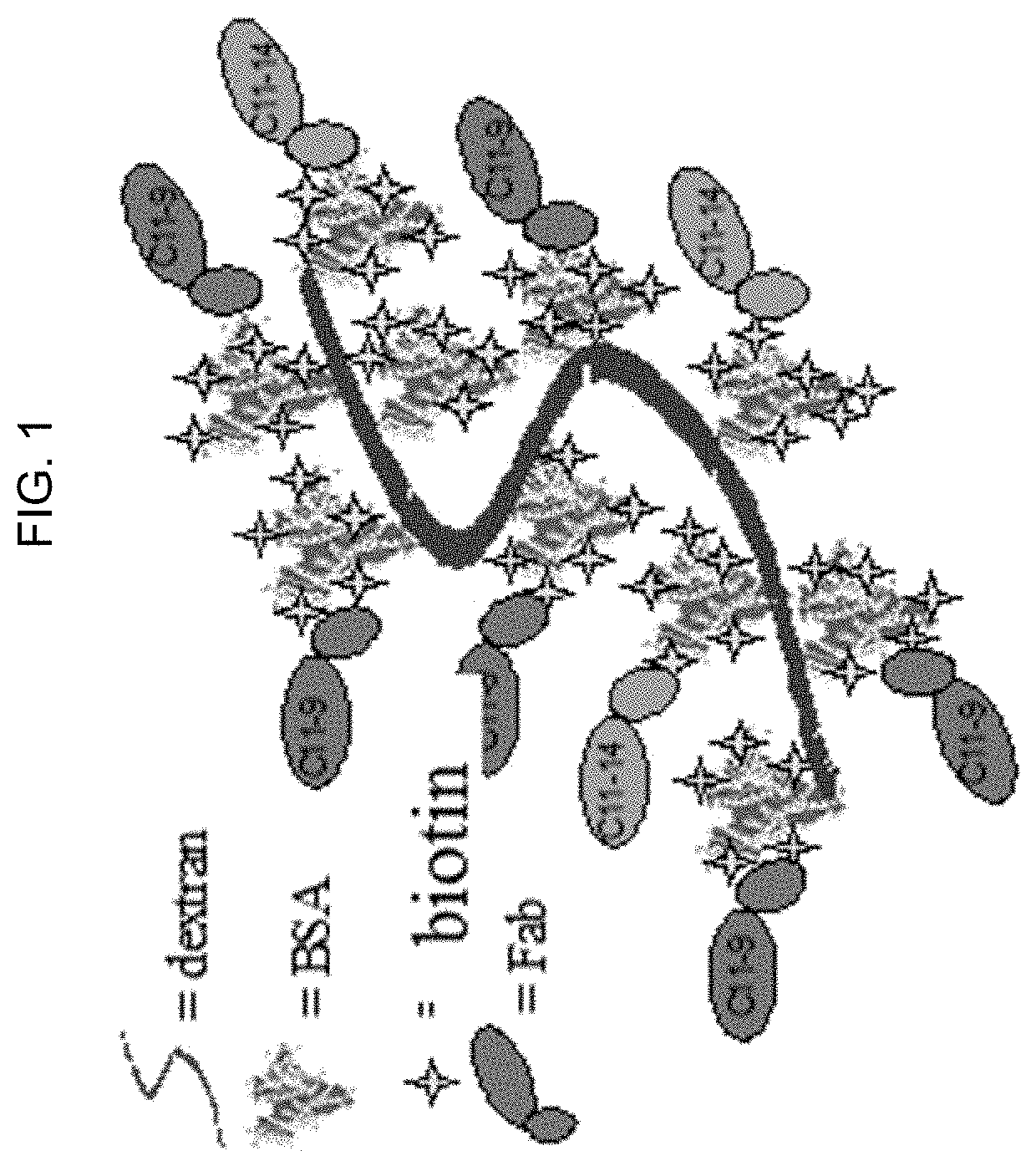
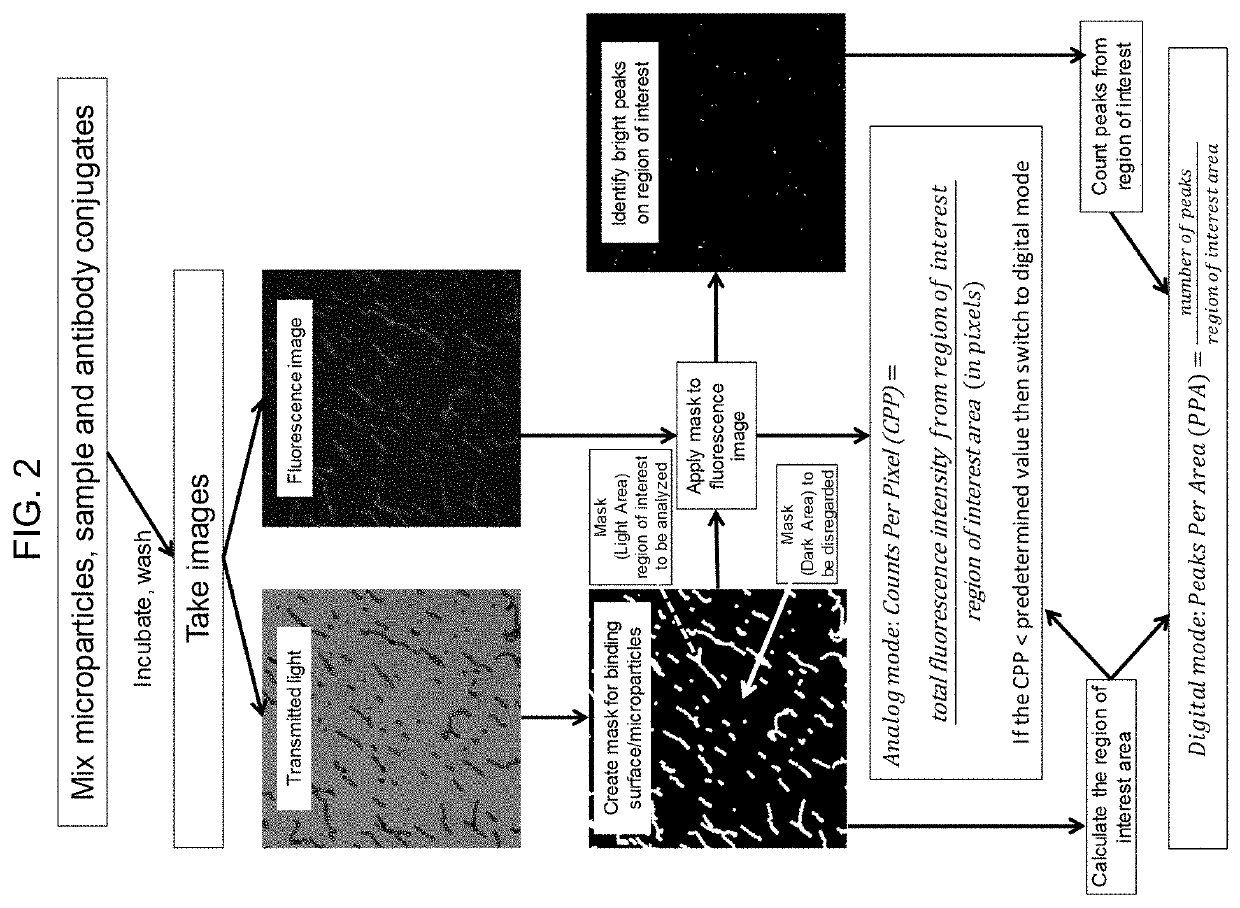
Direct detection of single molecules on microparticles
The disclosure provides methods of analyzing an analyte of interest in a biological sample using fluorescent agents and macroconjugates which comprise a core containing a cross-linked polymer or protein, tags, specific binding members or fragments thereof, and optionally carrier proteins. Also provided are methods of analyzing two or more analytes of interest in a biological sample in a single assay using microparticles and detection conjugates comprising different fluorophore labels, acquiring transmitted light and fluorescent images of the microparticles, and using a customized image analysis process to analyze the acquired images.
Owner:ABBOTT LAB INC
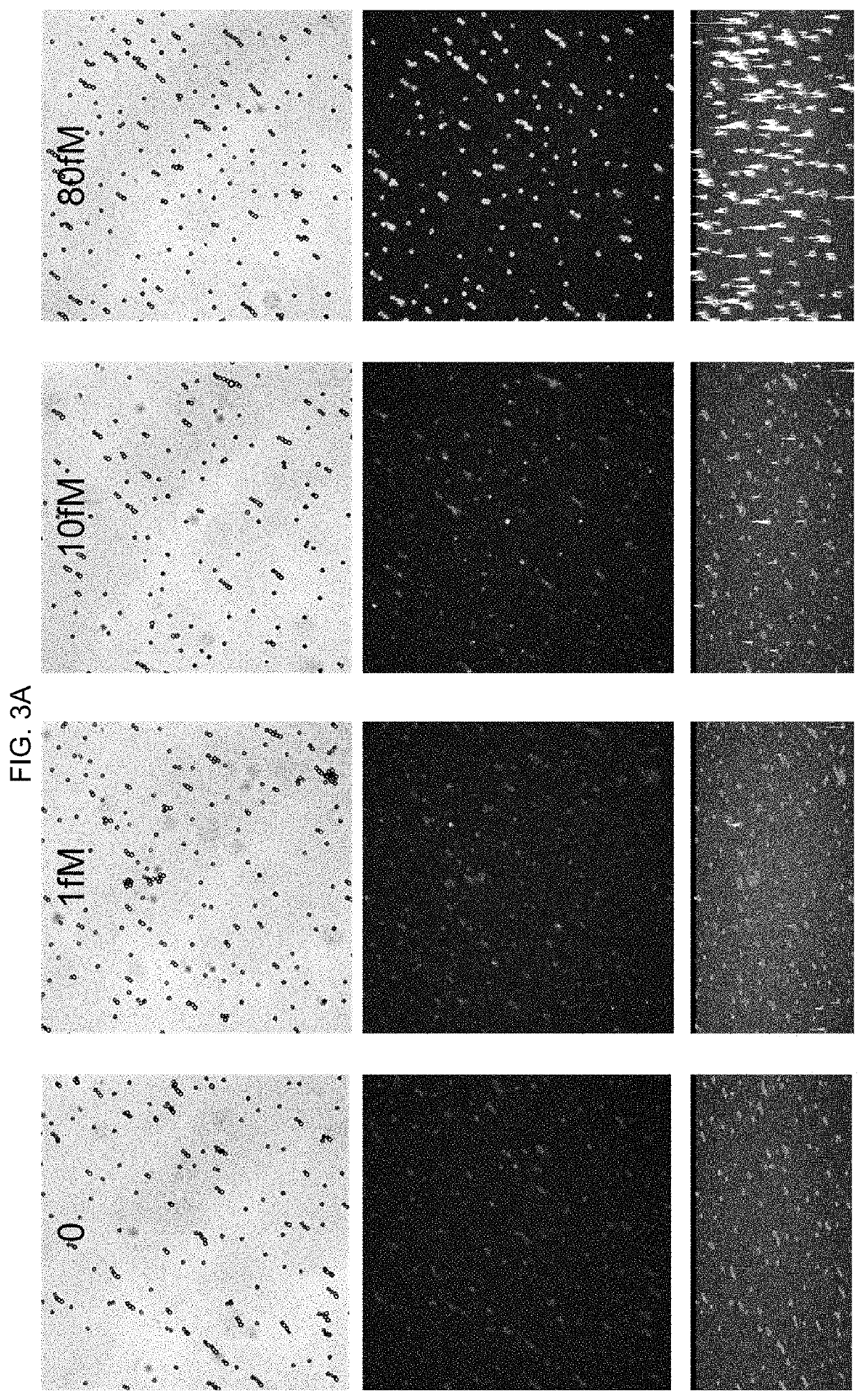
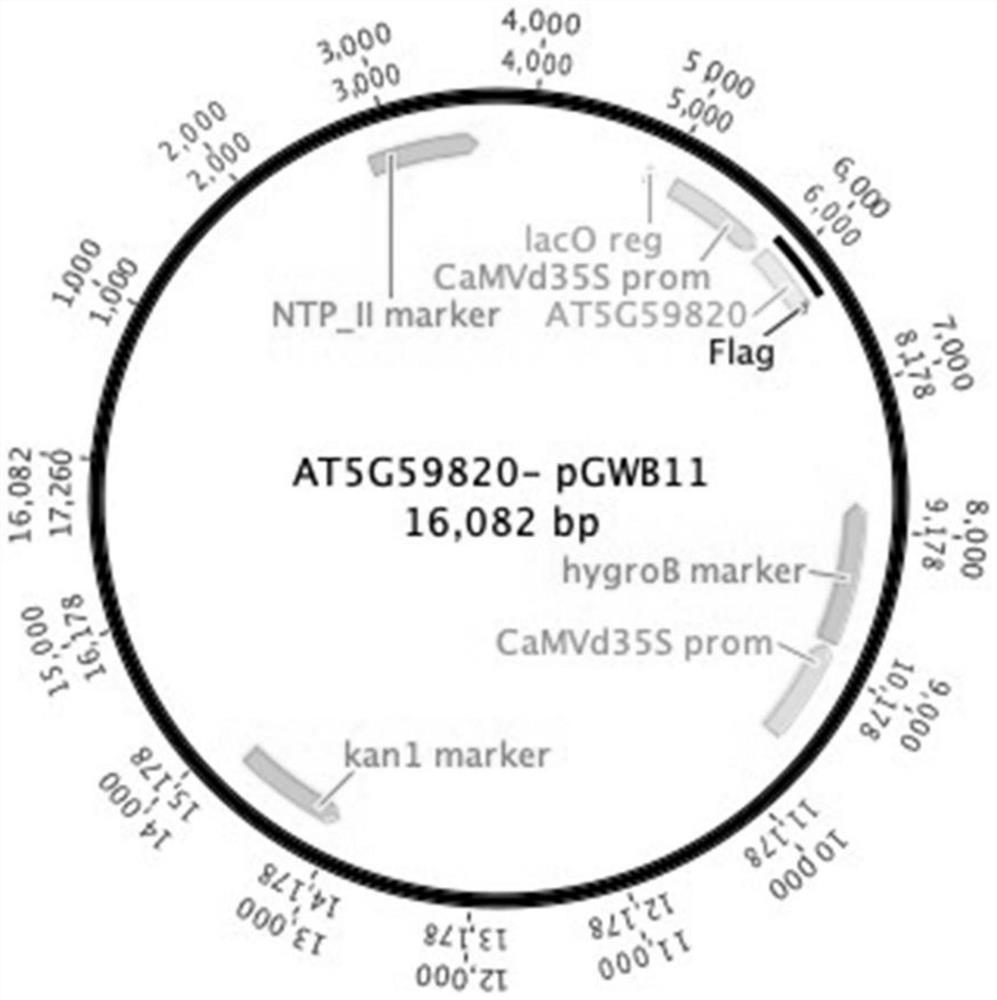
Application of arabidopsis transcription factor AT5G59820 gene in cultivation of disease-resistant transgenic plants
ActiveCN112795570AImprove disease resistanceBacteriaAntibody mimetics/scaffoldsBiotechnologyProtein tag
The invention relates to an application of arabidopsis thaliana transcription factor AT5G59820 gene in cultivation of disease-resistant transgenic plants, the CDS sequence of the arabidopsis thaliana transcription factor AT5G59820 gene is constructed to the upstream with an FLAG protein tag through the Gateway technology, the arabidopsis thaliana plant is transformed, screened and identified, and finally the transgenic plants are obtained. The screened plants are analyzed and found to have the effect of enhancing the resistance of arabidopsis thaliana to tomato pseudomonas syringae subspecies, that is, the gene affects the disease resistance phenotype of the plants, and homologous genes in crops possibly have the same or similar functions, so that the gene has important significance in production.
Owner:XUZHOU NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com