Preparation method and application of engineering bacteria for efficiently expressing growth hormone
A growth hormone and high-efficiency expression technology, applied in the field of genetic engineering, can solve the problems of poor formation of disulfide bonds, easy degradation of recombinant proteins, low secretion efficiency, etc., to facilitate protein purification, avoid inclusion bodies or precipitation, and have Beneficial for purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) HG16122-CF (http: / / www.sinobiologicalcdn.com / reagent / HG16122-CF.pdf) was used as the template plasmid (shown in SEQ ID NO: 1), and the upstream primer 1 containing the HRV3C protease cleavage site and Containing PST1 restriction endonuclease restriction site downstream primer 2, the hGH gene is amplified by PCR, after purification, the amplified hGH gene is obtained, wherein:
[0023] The upstream primer 1 is: CTGGAAGTCCTGTTTCAGGGACCCTTCCCAACCATTCCCTTATCCAG (shown in SEQ ID NO: 2);
[0024] Downstream primer 2 is: CGGCCAGTGCCAAGCTTGCCTGCAGCTAGAAGCCACAGCTGCCCTCC (shown in SEQ ID NO: 3);
[0025] The KOD FX PCR reaction system is: 94°C for 2min, 98°C for 10sec, 62°C for 30Sec, 68°C for 50sec, repeated for 35 cycles; 68°C for 5min; 4°C hold.
[0026] 2) Using the upstream primer 1 of the reverse complementary sequence of the homologous arm of the PST1 restriction site and the downstream primer 2 of the reverse complementary sequence containing the HRV3C protease cleava...
Embodiment 3
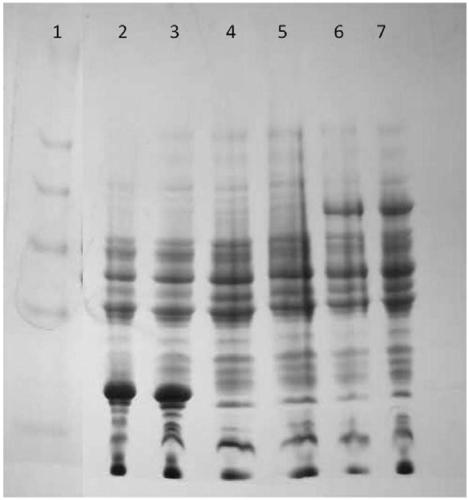
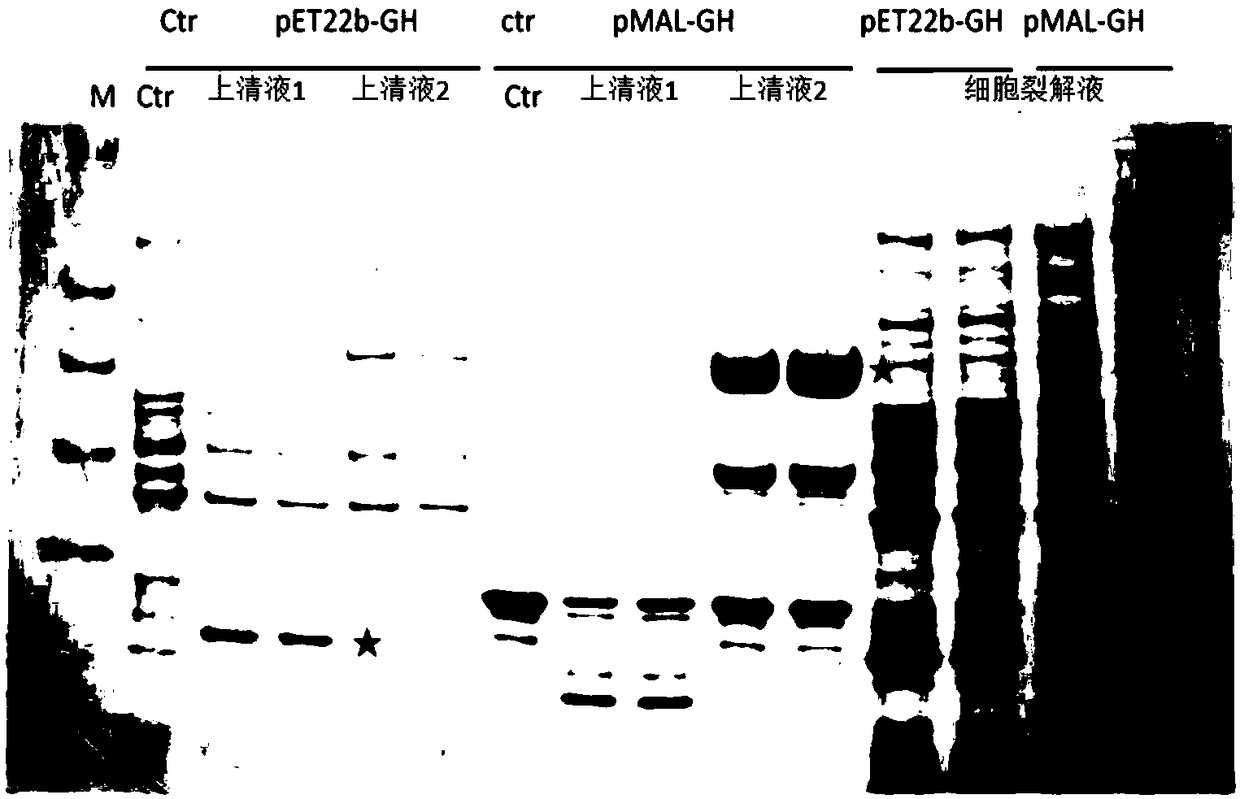
[0042] Small amount of expression of the recombinant genetically engineered bacteria in Example 1 and Comparative Example 1:
[0043] The recombinant genetically engineered bacteria 1 and 2 were subjected to the following treatments respectively:
[0044] 1) The selected monoclonal strains were inoculated into 5 ml ampicillin-resistant TB liquid medium, and cultured at 37° C. for 24 hours.
[0045] 2) The next day, take 1ml of the bacterial solution and transfer it to 100ml of TB liquid medium, and culture the bacteria to OD at 37°C 600 When =0.7-0.9, add IPTG to a final concentration of 0.2mM, and induce expression at 18°C for 16h.
[0046] 3) Take 100 μl of bacterial liquid and directly add 30 μl of SDS loading buffer, and heat at 95° C. for 20 minutes. Then take out 10 μl for SDS-page electrophoresis to detect the expression of hGH;
[0047] 4) The remaining bacterial liquid was centrifuged at 6000 rpm for 20 minutes to collect bacterial precipitates.
[0048] 5) Coll...
Embodiment 4
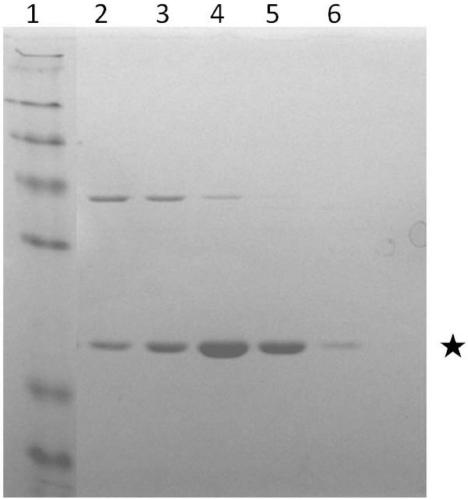
[0052] Example 4: Massive expression of hGH protein
[0053] 1) Inoculate 10 ml of TB liquid medium with the engineering bacteria 1 in Example 1, and incubate at 37° C. for 24 hours.
[0054] 2) The next day, transfer 10ml of the bacterial solution to 1L of TB liquid medium, and continue to grow the bacteria to OD at 37°C 600 =0.7-0.9, add IPTG to its final concentration of 0.2mM, induce expression at 18°C for 16h, and collect the bacteria.
[0055] 3) Resuspend the collected bacteria with 100ml of hypertonic solution (buffer I: 30mM Tris, 20% W / V sucrose, 1mM EDTA), and after stirring at 4°C for 30min, centrifuge at 8000g for 20min, discard the supernatant, and then Use 100ml hypotonic solution (buffer II: 5mM MgSO 4 ) to resuspend the cells, stir at 4°C for 10-30min, centrifuge again at 8000g for 20min, and collect the hypotonic supernatant.
[0056] 4) Take 2ml of dextrin ligand affinity chromatography medium and put it into a gravity column, wash with binding buffer (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com