Recombinant epsilon protein for inhibiting clostridium perfringens infection and preparation method and application thereof
A technology of Clostridium perfringens and protein, which is applied in biochemical equipment and methods, recombinant DNA technology, chemical instruments and methods, etc., and can solve problems such as difficulty in increasing the renaturation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1. Soluble expression of ε-hisY
[0089] 1. Synthetic genes
[0090] The present application designed three kinds of recombinant ε genes, respectively ε-hisY gene shown in SEQ ID No.1, ε-hisW gene shown in SEQ ID No.3, and pmε-hisW gene shown in SEQ ID No.4.
[0091] Both the ε-hisY gene and the ε-hisW gene encode the protein ε-his shown in SEQ ID No.2. The pmε-hisW gene encodes the protein pmε-hisW shown in SEQ ID No.5. ε-his is a protein obtained by deleting amino acid residues 52-59 of pmε-hisW.
[0092] Synthesize the ε-Y gene shown in the 151-1113 of SEQ ID No.1 (the protein shown in the 51-370 amino acid residues of coding SEQ ID No.2) by chemical synthesis method, SEQ ID No .3 the epsilon-W gene shown in No. 151-1113 (coding the protein shown in No. 51-370 amino acid residues of SEQ ID No.2), shown in No. 151-1137 of SEQ ID No.4 pmε-W gene (encodes protein pmε-W represented by amino acid residues 51-378 of SEQ ID No.5).
[0093] 2. Construction of re...
Embodiment 2
[0112] Embodiment 2, animal immune protective test of ε-his
[0113] 1. Preparation of anti-Clostridium perfringens vaccine
[0114] The ε-his protein purified by molecular sieves in Example 1 was dissolved in sterile PBS to obtain an ε-his solution with an ε-his concentration of 1000 μg / mL for immunization. The ε-his solution and Freund's adjuvant were mixed in an equal volume of 1:1, and emulsified to prepare an oil emulsion vaccine, which was named the first vaccine. The ε-his solution and incomplete Freund's adjuvant were mixed in an equal volume of 1:1, and emulsified to prepare an oil emulsion vaccine, which was named the second-immunity vaccine.
[0115] Take out the B-type Clostridium perfringens virulent strain C58-5 and the D-type Clostridium perfringens virulent strain C60-11 purchased from the China Veterinary Drug Administration. In the ultra-clean workbench, use 75% alcohol cotton ball to carefully wipe the outer wall of the ampoule that preserves the bacteria,...
Embodiment 3
[0134] Example 3, Optimization of ε-his Induced Expression Conditions
[0135] 1. Optimization of induction temperature and time
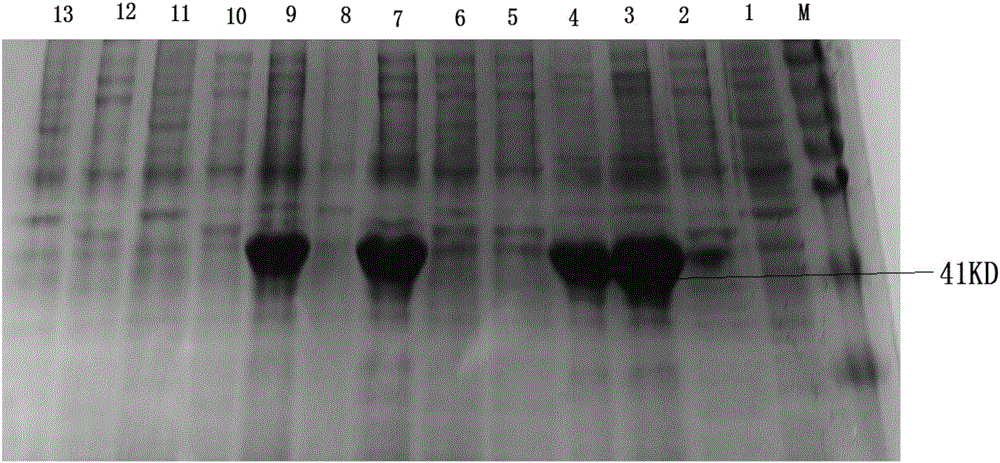
[0136] Inoculate BL21(DE3) / pET30a-ε-Y in LB liquid medium containing 50 μg / ml kanamycin (add kanamycin to LB liquid medium until the concentration of kanamycin is 50 μg / ml to obtain culture medium) at 37°C, using a Thermo MaxQ6000 full-temperature shaker at 200rpm to shake and cultivate to OD 600 When the value (the LB liquid medium containing 50 μg / ml kanamycin was used as the blank control) reached 0.6, isopropylthio-β-D-galactoside (IPTG) was added to induce the following six kinds of expression respectively. The first induced expression was induced with 0.75 mM IPTG for 1 hour at 37°C. The second induced expression was induced with 0.75 mM IPTG for 2 hours at 37°C. The third induced expression was induced with 0.75 mM IPTG for 4 hours at 37°C. The fourth induced expression was induced with 0.75 mM IPTG for 5 hours at 37°C. The fifth induce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com