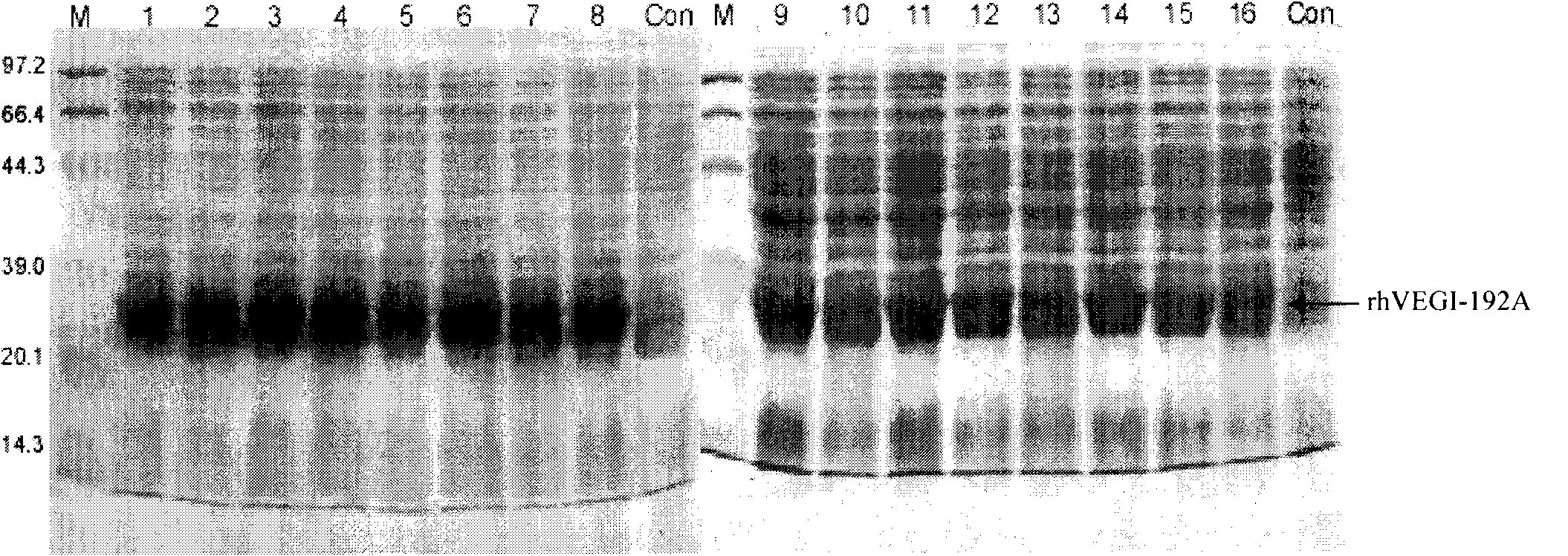Preparation of recombinant protein of human vascular endothelial cell growth inhibition factor rhVEGI-192A
A technology of VEGI-192A, a growth inhibitor, applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 2: IPTG-induced expression of rhVEGI-192A in Escherichia coli and screening of highly expressed clones.
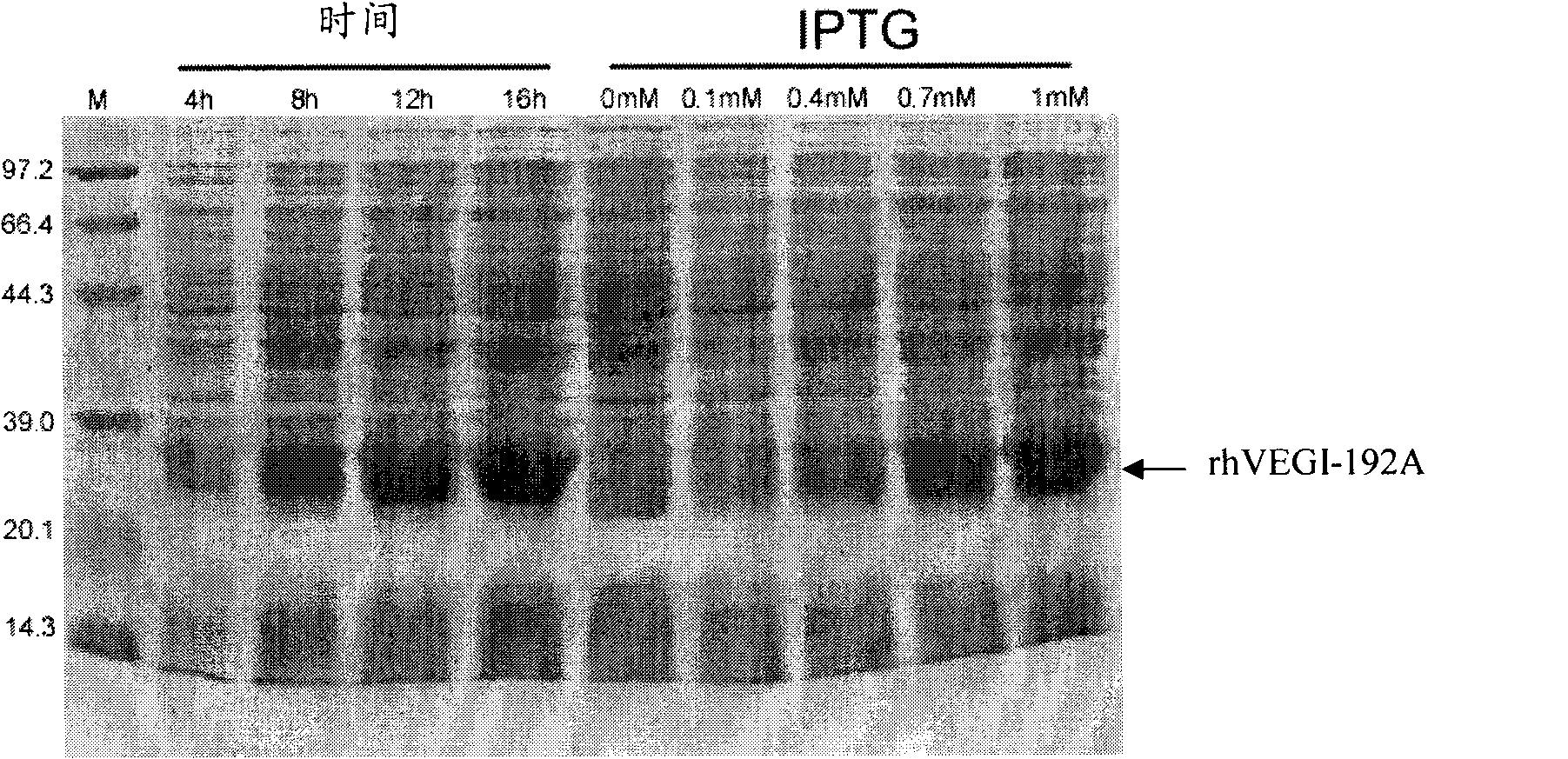
[0040] 1. IPTG induction: Use the expression vector pET-30a-rhVEGI-192A to transform the competent cell BL21(DE3)pLysS, randomly pick 16 clones and colonize them in 3 mL LB medium, add kanamycin to a final concentration of 50 μg / ml , 37 ° C recovery culture overnight. The next day, measure the density of each tube of bacterial liquid. If the OD600 is between 0.6 and 1, add IPTG to a certain final concentration, and induce culture for 4 hours. The negative control is induced without adding IPTG. The recombinant expression was analyzed by SDS-PAGE. 183 clonal colonies were obtained.
Embodiment 2
[0041] 2. LB medium: 1% peptone, 0.5% yeast extract, 1% sodium chloride, pH7.0; carbenicillin: 50mg / ml; IPTG solution: 100mmol / L;
[0042] 3. Screening high-expression clones of rhVEGI-192A protein: Using high-throughput SDS-PAGE method, 16 single colonies with the highest expression efficiency were screened from 183 clonal colonies. figure 1 It is the SDS-PAGE results of the lysates of 16 colony colonies obtained from the screening of high-expression clones in the IPTG-induced expression system, in which 1 to 16 are high-expression clone colony groups, C is the clone colony group without IPTG induction, and M is protein Molecular weight standard, the host bacterium is BL21(DE3)pLysS.
[0043] While carrying out subsequent expression and purification procedures, the screened clones were preserved at low temperature - 80°C. After SDS-PAGE, gel scanning software was used to analyze that the expression of the target protein accounted for 44% of the total bacterial protein.
[...
Embodiment 3
[0046] According to the above results, we concluded that the optimal conditions for the expression of rhVEGI-192A protein in BL21(DE3)pLysS host bacteria were the addition of IPTG: 1mM, and the selected culture time: 16h.
[0047] Example 4: Auto-induced expression of rhVEGI-192A in Escherichia coli and screening of high-expression clones.
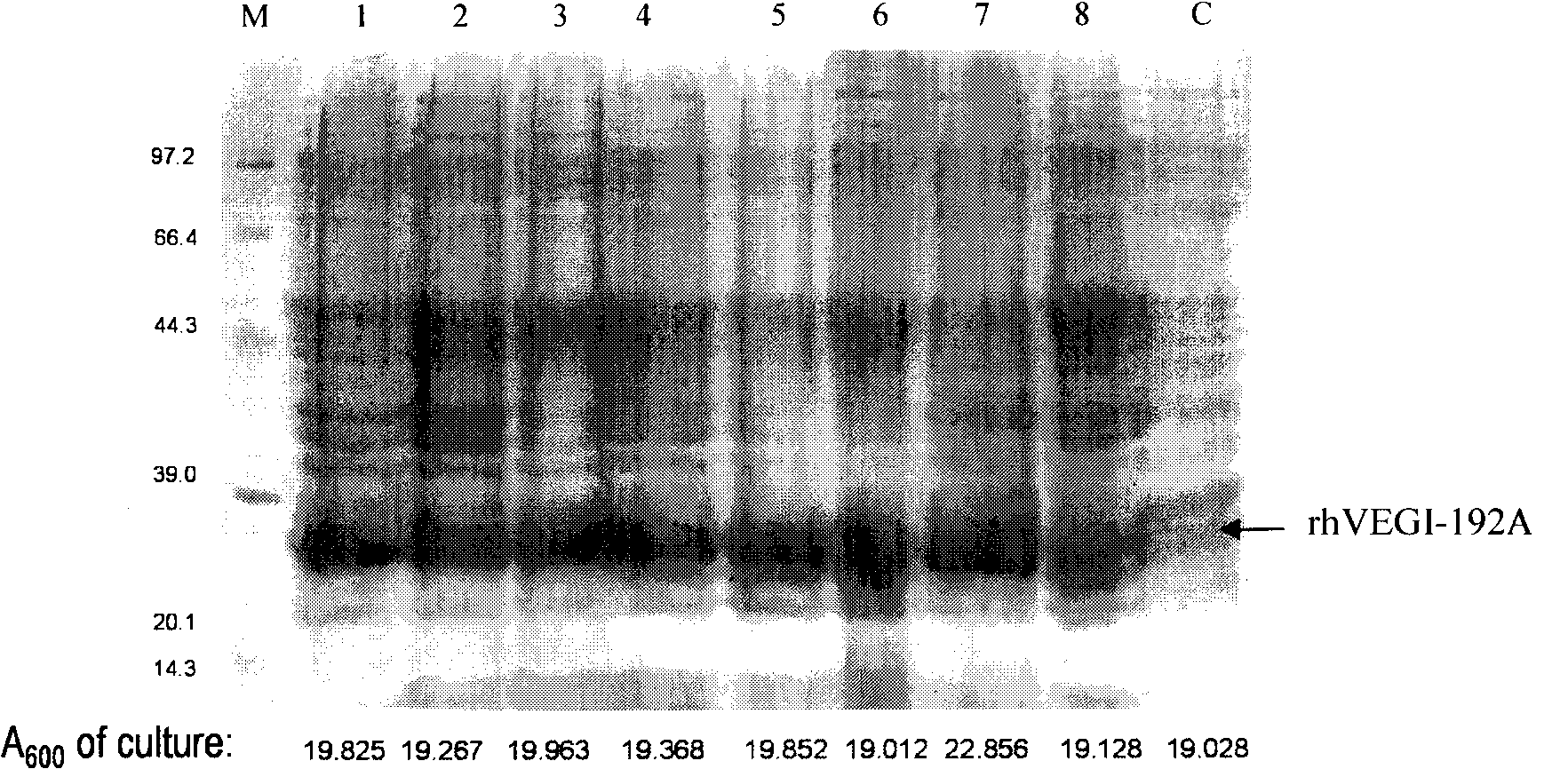
[0048] 1. Automatic induction: Use the expression vector pET-30a-rhVEGI-192A to transform the competent cell BL21(DE3)pLysS, randomly pick a certain number of clone colonies into 3mL LB medium, add kanamycin to a final concentration of 50μg / ml , 37 ° C recovery culture overnight. The bacterial cell pellet was collected by centrifugation the next day, and the recombinant expression was analyzed by SDS-PAGE.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com