MAPWA fusion antibacterial peptide, preparation method and application thereof
A technology of antibacterial activity and recombinant bacteria, applied in the direction of biochemical equipment and methods, applications, hybrid peptides, etc., can solve problems of immeasurable practical significance and economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1. Construction of novel fusion antimicrobial peptide gene MAPWA and construction of prokaryotic expression plasmid
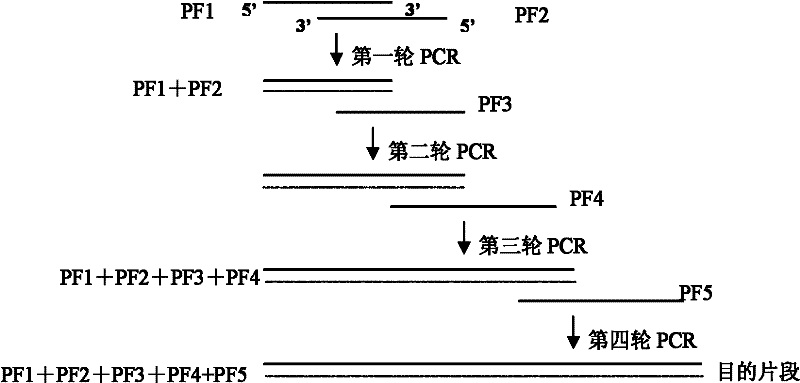
[0096] The cDNA of Magainin (MA) and the cDNA of Phytoacetin (PWA) were transformed by Gene Splicing by Overlap Extension (GSOE) to construct the MAPWA fusion gene, and the pG-MAPWA prokaryotic expression vector was constructed. PCR and BamHI / EcoRI double enzyme digestion identification, sequencing results confirmed that the MA / PWA fusion gene has been successfully cloned into the prokaryotic expression vector pGEX-4T-1. Specific steps are as follows:
[0097] 1. Design and synthesis of overlapping primers
[0098]According to the cDNA base sequences of the mature peptides encoding Magainin and PWA respectively, five primers were designed and artificially synthesized. PF1 contains BamHI and XhoI restriction sites; the 3' end of PF2 contains a sequence complementary to the 3' end of PF1; The 3' end of PF3 contains a sequence complementary to the...
Embodiment 2
[0131] Example 2, Prokaryotic expression and expression activity research of MAPWA fusion gene
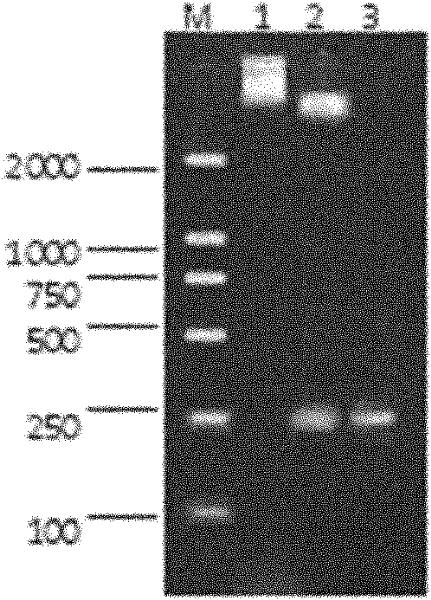
[0132] The MAPWA fusion gene expression plasmid was transformed into Escherichia coli BL21(DE3), and the expression of fusion protein was induced by IPTG. The expressed protein with GST tag was analyzed by SDS-PAGE, and its molecular weight was in line with the expected 33.9KDa. The expression product of the fusion protein exists in a soluble form in the supernatant of broken cells, and a large amount of expressed protein is purified by affinity chromatography; the fusion protein significantly inhibits Escherichia coli, Staphylococcus aureus, and pneumococcus in in vitro antibacterial experiments and growth of Pseudomonas aeruginosa. Specific steps are as follows:
[0133] 1. Induced expression of fusion protein and SDS-PAGE electrophoresis analysis
[0134] 1. Experimental steps: Pick a single colony containing pG-MAPWA and pGEX-4T-1 transformants and inoculate it in 2ml of LB l...
Embodiment 3
[0158] Example 3. Construction of Yeast Expression Vector Fused with Antimicrobial Peptide MAPWA and Study on its Expression Activity
[0159] The fusion antimicrobial peptide MAPWA gene was cloned into the baker's yeast secretion expression vector pYCa (pYES2 / CT was inserted into the a-factor secretion signal peptide), transformed into yCY3 baker's yeast, and high-efficiency expression strains were screened out; after the recombinant strain was induced to express the fusion antimicrobial peptide, The expression supernatant was analyzed for antibacterial activity and purified protein electrophoresis, and it was found that the fusion antimicrobial peptide existed in the expression supernatant, with a molecular weight of approximately 7.9kDa; the secretion and expression of the antimicrobial peptide was realized; the produced fusion antimicrobial peptide was effective against E. Pseudomonas, Staphylococcus aureus, and Streptococcus pneumoniae all had significant antibacterial act...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com