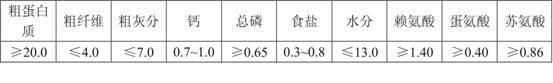
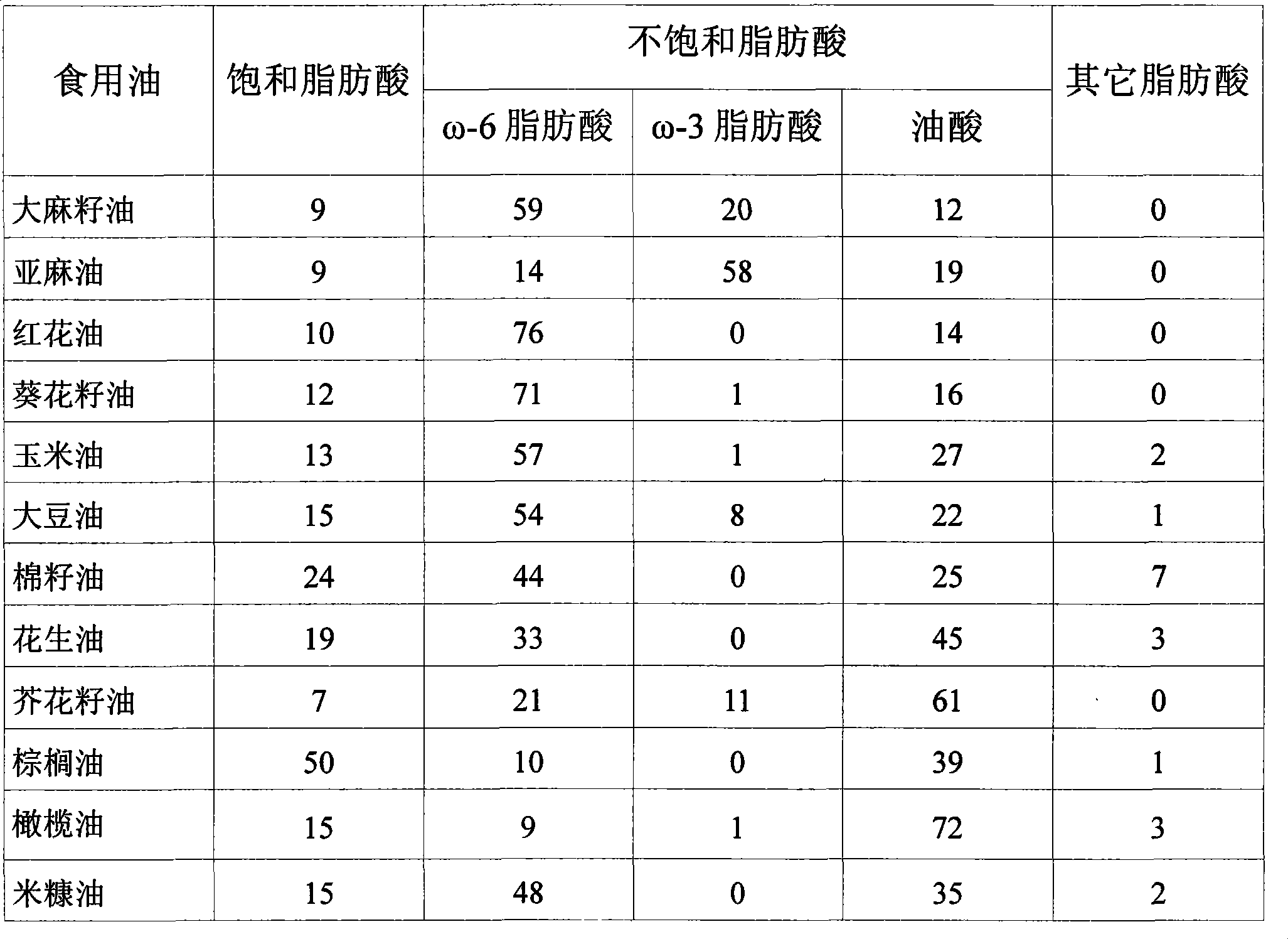
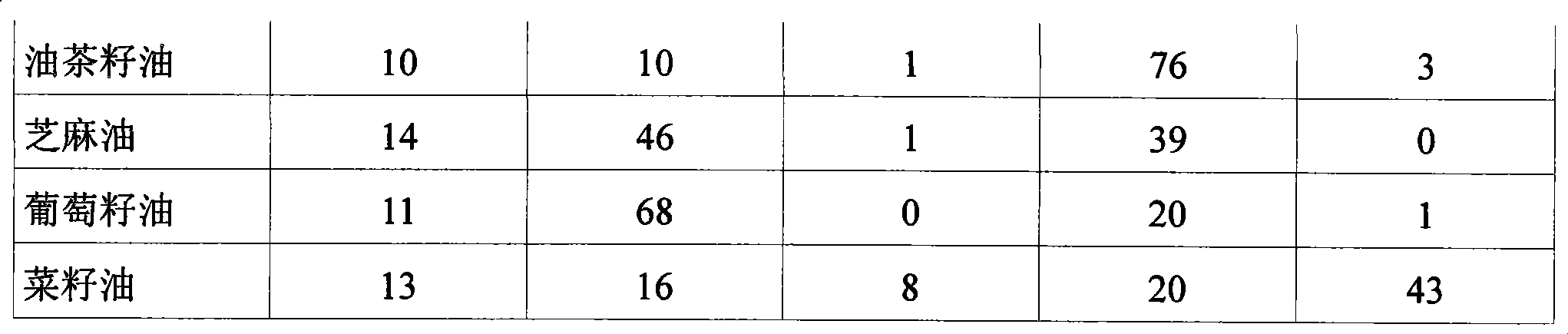
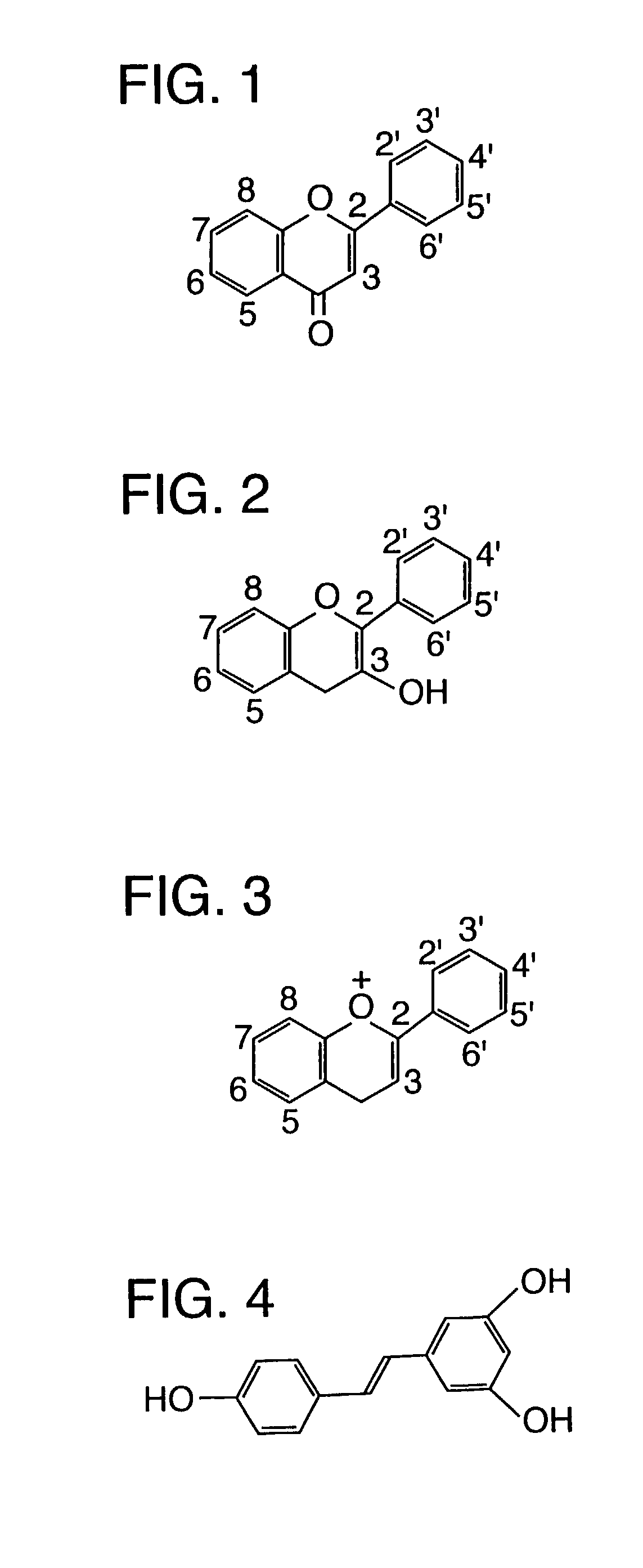
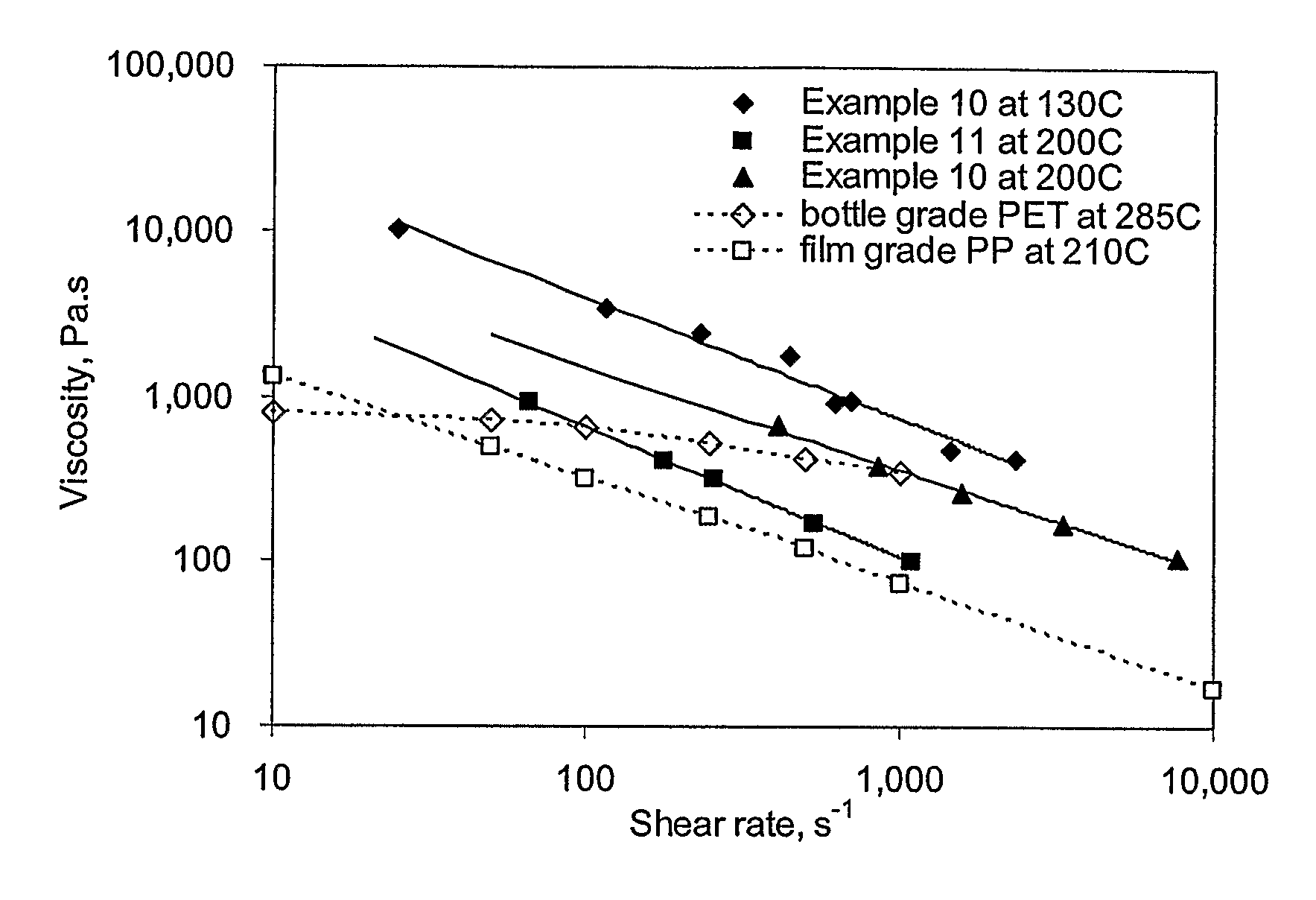
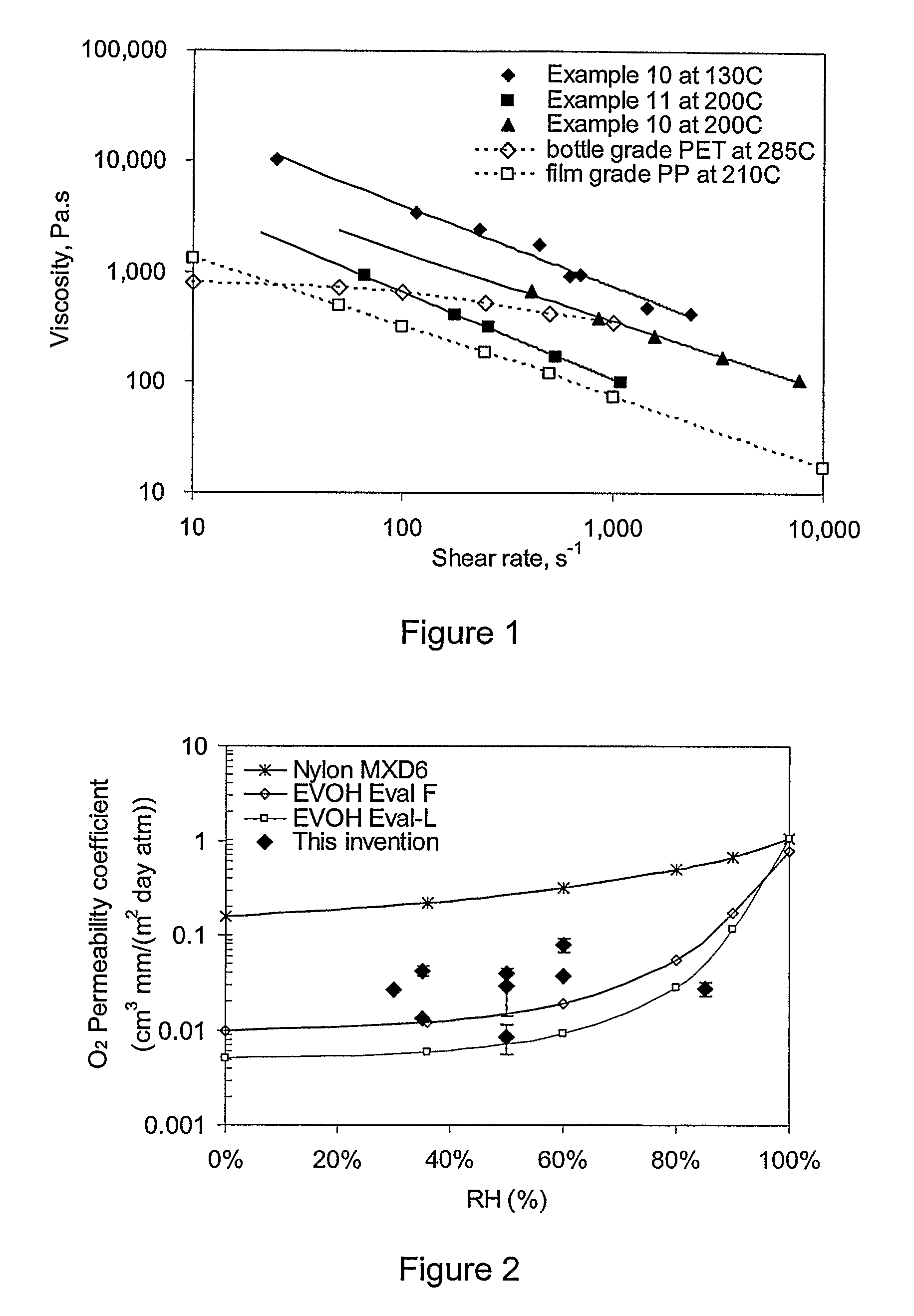
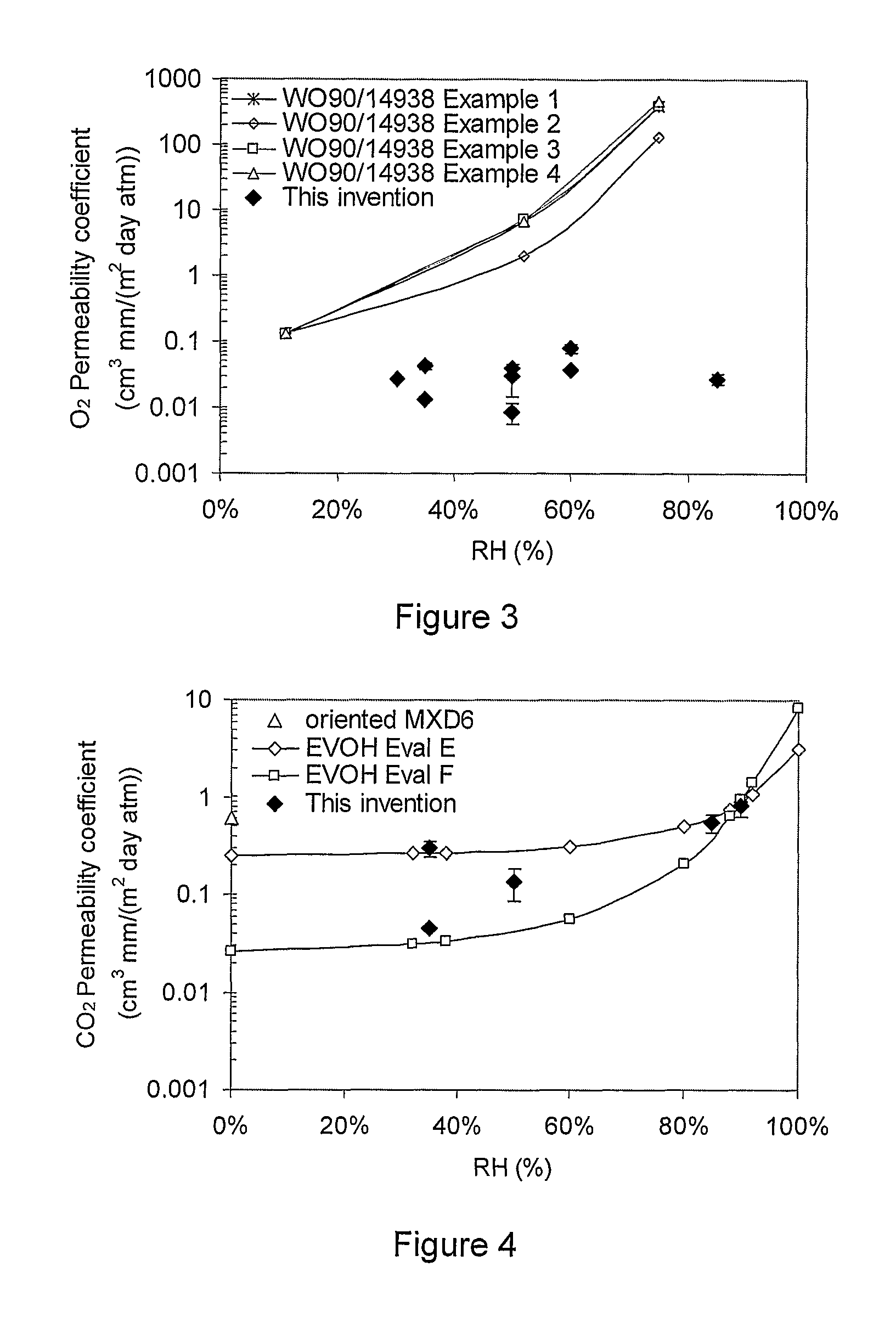
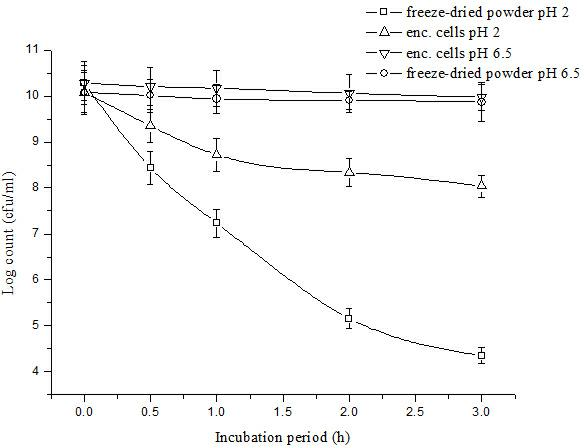
Patents
Literature
7197 results about "SOYBEAN SEED OIL" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Soybean oil is a vegetable oil extracted from the seeds of the soybean (Glycine max). It is one of the most widely consumed cooking oils.
Soft capsule preparation
A soft capsule preparation which comprises a dispersion of (2E,4E,6E,10E)-3,7,11,15-tetramethyl-2,4,6,10,14-hexadecapentaenoic acid in a vegetable oil filled in a soft capsule comprising a shell having a light blocking effect. The soft capsule preparation preferably comprises polyoxyethylene sorbitan monooleate and glycerol monostearate and the like as surfactants. As the vegetable oil, soybean oil, sesame oil, a mixture thereof, or the like may be used.
Owner:LEBER +1
Treatment of skin wounds using polyenylphosphatidylcholine and alkanolamines
InactiveUS6932963B2Minimize scarringReduce inhibitionBiocideCosmetic preparationsSOYBEAN SEED OILTriethanolamine
Polyenylphosphatidylcholine in combination with an alkanolamine are topically applied to treat skin wounds, promote healing, and minmize scar formation. Typical compositions contain from about 0.25% to about 12% of a polyenylphosphatidylcholine preparation obtained from natural sources such as soybean oil which contains at least about 25% by weight, preferably about 40% or more, dilinoeoylphosphatidylcholine, and from about 0.1% to about 10% by weight of an alkanolamine such as ethylaminoethanol, methylaminoethanol, dimethylaminoethanol, isopropanolamine, triethanolamine, isopropanoldimethylamine, ethylethanolamine, 2-butanolamine, choline, serine, or mixtures thereof. Dimethylaminoethanol is a particularly preferred alkanolamine. Tyrosine is an adjunct ingredient in many embodiments.
Owner:N V PERRICONE
Food spreads
The present invention is of a food spread containing a mixture of at least one edible oil of natural or synthetic origin and a monoglyceride. The oil is preferably one or more of the oils from the group of olive oil, avocado oil, canola oil, soybean oil, sunflower oil, nut oils, walnut oil, peanut oil, safflower oil, cottonseed oil, coconut oil, rice bran oil, mustardseed oil, camelina oil, chia oil, flaxseed oil, perilla oil, fish oil, palm oil, sesame oil, wheatgerm oil, jojoba oil or corn oil. More preferably an oil such as avocado oil, fish oil, palm oil or olive oil is used and most preferably the oil is olive oil and fish oil. The monoglycerides used are preferably derivatives of oleic, or palmitic acid. The oil is present preferably in an amount of from about 85 to about 98 percent and most preferably in an amount of from about 93 to about 96 percent. The more monoglyceride used, the greater the degree of solidity of the food spread at room temperature. It is therefore possible to produce a desired degree of solidity, by changing the proportion of monoglyceride.
Owner:DR EGER OLIVE OIL PROD IND
Nutrition bar
InactiveUS20050181019A1Stable and goodExtended shelf lifeBiocideHeavy metal active ingredientsRice proteinIngested food
A nutrition bar comprising about 10% wt or more of soy and / or rice protein, at least one transition metal or transition metal compound, and about 2% wt or more of a humectant, and wherein the at least one transition metal or transition metal compound is in a substantially water insoluble form at 20° C. or the nutrition bar has an Aw of 0.45 or less or about 1% wt or more of the soy and / or rice protein is in the form of nuggets and the humectant is selected from polyols. The bars have elevated levels of soy and / or rice protein, yet do not suffer unacceptable from a deterioration in taste or other organoleptic properties over time. In other aspects, a nutrition bar or other food which incorporates pro-oxidants and / or polyunsaturated fatty acids or their sources in encapsulated form, especially as microcapsules. The pro-oxidants may be metal salts such as copper, manganese, iron and / or zinc salts. Sources of omega-3 fatty acids include fish oil. Processes for preparing the polyunsaturated fatty acid capsules are also disclosed. The polyunsaturated fatty acid capsules / microcapsules are prepared by forming an emulsion of the unsaturated fatty acid with a carrier, spray drying the emulsion to form a powder and encapsulating powder, especially with a fluid bed. The invention is especially useful for encapsulating polyunsaturated fatty acids, or oil sources thereof, most preferably omega-3 and omega-6 fatty acids, such as arachidonic acid, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), lineoleic acid, linolenic acid (alpha linolenic acid), and gamma-linolenic acids, fish oil, and oil sources of C18:2 and C18:3 fatty acids such as canola oil, soybean oil or blends thereof.
Owner:SLIM FAST FOODS COMPANY A DIV OF CONOPCO
PVC foam wood/plastic composite material and manufacturing method thereof
The invention discloses a PVC foam wood / plastic composite material and relates to the technical field of composite materials. The PVC foam wood / plastic composite material is made from the following raw materials in parts by weight: 20-70 parts of PVC resin powder, 0-55 parts of wood flour, 5-40 parts of calcium carbonate powder, 0.1-0.5 part of sodium bicarbonate, 0.3-0.6 part of an azo-compound or hydrazine derivative, semicarbazide compound or nitroso-compound, 2-10 parts of a foaming regulator, 1.5-6 parts of a composite stabilizer, 0.7-2.1 parts of a lubricant, 0.5-1.2 part of soybean oil, 2.5-5.5 parts of an impact modifier, and 0.5-3 parts of a processing agent. The PVC foam wood / plastic composite material provided by the invention has the advantages of strong chemical stability, high strength, resistance to acid / alkaline corrosion, resistance to water seepage, flame retardancy and low cost.
Owner:山东宜群木塑科技有限公司
Fatty Acid Desaturases From Primula
ActiveUS20080063691A1High nutritional valueIncrease SDA contentBacteriaOxidoreductasesDouble bondOmega-6 fatty acid
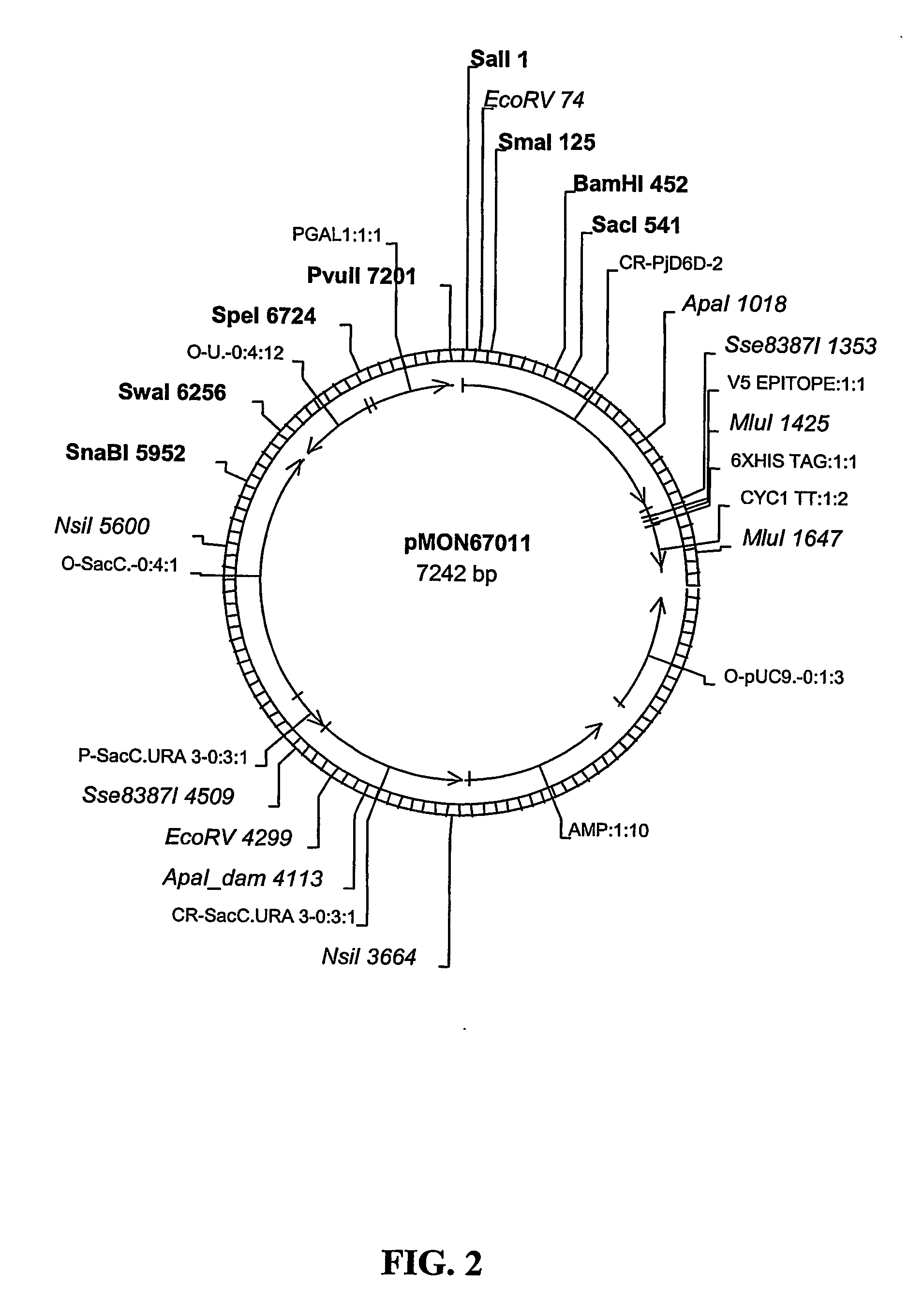
The invention relates generally to methods and compositions concerning desaturase enzymes that modulate the number and location of double bonds in long chain poly-unsaturated fatty acids (LC-PUFA's). In particular, the invention relates to methods and compositions for improving omega-3 fatty acid profiles in plant products and parts using desaturase enzymes and nucleic acids encoding for such enzymes. In particular embodiments, the desaturase enzymes are Primula Δ6-desaturases. Also provided are improved soybean oil compositions having SDA and a beneficial overall content of omega-3 fatty acids relative to omega-6 fatty acids.
Owner:MONSANTO TECH LLC
Plant mixed oil with rational proportion of fatty acid
InactiveCN1448061AReasonable intakeImprove nutritional structureEdible oils/fatsEmbryoPolyunsaturated fatty acid
The blended vegetable oil with reasonable fatty acid proportion consists of rape seed oil 20-65 wt%; any combination of corn embryo oil, peanut oil, sunflower seed oil and safflower seed oil 15-71 wt%; any combination of soybean oil and linseed oil 2-42 wt% and sesame oil 0-5 wt%. In the blended vegetable oil product, the fatty acids to constitute fat includes saturated fatty acid 7-17 wt%, unsaturated monofatty acid 41-47 wt%, unsaturated n-6 polyfatty acid 35-39 wt% and unsaturated n-3 polyfatty acid 5-9 wt% with the total unsaturated polyfatty acid accounting for 41-47 wt%. It is designed based on the fat and fatty acid taking amount recommended by the Chinese nutriology institute and has reasonable fatty acid proportion and important significance in reducing cardiac vascular diseases and cancer.
Owner:SOUTHSEAS OILS & FATS INDAL CHIWAN
High-antiwear cutting fluid
InactiveCN104277900AImprove anti-wear performanceLow costLubricant compositionLanthanum fluoridePolyethylene glycol
The invention discloses a high-antiwear cutting fluid, which comprises the following raw materials in parts by weight: 10-25 parts of soybean oil, 5-15 parts of rapeseed oil, 8-25 parts of 2-ethylhexyl oleate, 15-30 parts of fatty alcohol-polyoxyethylene ether, 3-8 parts of disodium sulphonatoacetate, 1-5 parts of copper / silica composite nano materials, 5-12 parts of citric acid-modified lanthanum fluoride nanoparticles, 20-40 parts of an imidazoline-ammonium salt corrosion inhibitor, 3-15 parts of glycerin, 2-15 parts of triethanolamine, 2-6 parts of borax, 0.5-1.5 parts of benzotriazole, 8-20 parts of polyethylene glycol and 30-50 parts of water. The high-antiwear cutting fluid is excellent in antiwear, cooling, lubrication, cleaning and antirust functions, and is low in cost and strong in stability.
Owner:CHAOHU GUANGFENG METAL PROD
Healthy food spreads
InactiveUS6117476AGreat degree of solidityUnlimited shelf lifeEdible oils/fats with aqeous phaseFood borneMonoglyceride
The present invention is of a food spread containing a mixture of an edible oil of natural origin and a monoglyceride. The oil is preferably one or more of the oils from the group of olive oil, avocado oil, canola oil, soybean oil, sunflower oil, peanut oil, safflower oil, cottonseed oil, coconut oil, rice bran oil, mustardseed oil, camelina oil, chia oil, flaxseed oil, perilla oil, fish oil or corn oil. More preferably an oil such as avocado oil or olive oil is used and most preferably the oil is olive oil. The monoglycerides used are preferably derivatives of oleic, or palmitic acid. The ratio of oil to monoglyceride is preferably from about 9 to about 1 to from about 49 to about 1 and most preferably from about 15 to about 1 to from about 24 to about 1. The more monoglyceride used, the greater the degree of solidity of the food spread at room temperature. It is therefore possible to produce a desired degree of solidity, by changing the proportion of monoglyceride.
Owner:DR EGER OLIVE OIL PROD IND
Method for preparing hard polyurethane foam plastics with soybean oil
ActiveCN101314632AImprove water resistanceImprove heat resistancePolyesterFatty acid glycerol esters
The invention provides a method for preparing hard polyurethane foam plastics from soybean oil. The method comprises the steps as follows: (1) carrying out epoxidation including the sub-steps of subjecting soybean oil and epoxidizing agent to reaction to obtain epoxidized soybean oil; (2) carrying out ring-opening reaction including the sub-steps of subjecting epoxidized soybean oil and nucleophilic reagent of reactive hydrogen to the ring-opening reaction of epoxy chemical bond in the presence of a catalyst to obtain mixed hydroxyl fatty acid glyceride; (3) carrying out alcoholysis and esterification including the sub-steps of adding alcohol, heating for alcoholysis to obtain mixed hydroxyl fatty acid monoester, i.e. soybean oil based polyol, and esterifying with organic acid and acid anhydride to generate polyester polyol; and (4) sequentially adding 80-150 weight parts of isocyanate, 0.3-4 weight parts of triethanolamine and 0.5-4 weight parts of foam stabilizer to 100 weight parts of soybean oil based polyol, intensively stirring, adding 0.5-3 weight parts of distilled water, and uniformly foaming while stirring at the high speed.
Owner:NANTONG HAIERMA TECH CO LTD
Anti-stress health compound feed for jewfish
ActiveCN104664174APromote digestionPromotes fast digestionClimate change adaptationAnimal feeding stuffAnti stressAnimal science
The invention discloses an anti-stress health compound feed for jewfish. The feed consists of an animal protein source, a plant protein source, a fat source raw material, a sugar source raw material, composite vitamin, composite mineral salt, hepatinica, a Chinese herbal medicine compound preparation, probiotic powder, a composite immunopotentiator, antimicrobial peptide, an acidifying agent and a premixed compound additive, wherein the animal protein source comprises one or more of fish meal, euphausiid meal and chicken meat meal; the plant protein source comprises one or more of seaweed meal, fermented soybean meal, soy protein concentrate and corn protein powder; the fat source comprises one or more of soybean oil, fish oil and phosphatide oil. The feed disclosed by the invention has the characteristics of being rapid in growth, low in food coefficient, good in stability in water, rapid to eat, convenient to use, low in feeding cost, resistant to stress, healthy and the like.
Owner:珠海海龙生物科技有限公司
High molecular weight, lipophilic, orally ingestible bioactive agents in formulations having improved bioavailability
InactiveUS20050169988A1Absorption of large and highLarge and high weightAntibacterial agentsOrganic active ingredientsActive agentTriglyceride
Orally ingestible bioactive agents are disclosed which contain a triglyceride matrix and one or more polyphenols that improve the bioavailability of the bioactive agent. In particular non-limiting examples, the bioactive agent is a ubiquinone (such as Coenzyme Q), the triglyceride matrix is a soybean oil matrix, and the composition further includes additional anti-oxidants.
Owner:SHAKLEE CORP
Artificial compound feed with function of preventing tilapia mossambica streptococcicosis
ActiveCN101971928AImprove digestibilityImprove antibacterial propertiesFood processingClimate change adaptationSodium BentoniteAntioxidant
The invention relates to a tilapia mossambica feed, in particular to an artificial compound feed for tilapia mossambica. An artificial compound feed with function of preventing tilapia mossambica streptococcicosis comprises the following components in percentage by mass: 5 to 10 percent of fish meal, 20 to 30 percent of bean pulp, 20 to 30 percent of rape pulp, 10 to 15 percent of cotton pulp, 10 to 15 percent of DDGS (maize alcohol lees and residual liquid dry substance), 5 to 10 percent of rice bran, 15 to 20 percent of flour, 5 to 10 percent of maize, 1 to 2 percent of soybean oil, 0.5 to 1.5 percent of phospholipid oil, 1 to 2 percent of calcium hydrogen phosphate, 1 to 10 percent of bentonite, 0.5 to 1 percent of multi-dimensional multi-mineral premix, 0.1 to 0.5 percent of choline chloride, 0.01 to 0.05 percent of antioxidant, 0.1 to 0.5 percent of mildew-proof agent and 1 to 5 percent of composite Chinese medicinal additive. Multiple antibacterial Chinese medicinal herb additives are added into the feed, so the feed improves the antibacterial capability of the tilapia mossambica and can prevent the tilapia mossambica streptococcicosis.
Owner:GUANGZHOU PANYU DACHUAN FEED
Compound feed for 0-21-days-old broiler chicken and preparation method of compound feed
ActiveCN102657292AEnhance immune functionImprove digestibilityAnimal feeding stuffDiseaseAntimicrobial peptides
The invention discloses a compound feed for 0-21-days-old broiler chicken and a preparation method of the compound feed. The compound feed is prepared from various raw materials of corn, flour, soybean meal, 60% of corn protein powder, soybean oil, limestone, calcium hydrophosphate, salt, antimicrobial peptide for the poultry, choline chloride, methionine, threonine, lysine, compound enzyme of corn and soybean meal, premixed vitamin compound, premix compound of trace elements and traditional Chinese medicine addictives. The preparation method for the compound feed comprises the following steps of: weighing all raw materials; putting the raw materials into a pulverizer to be pulverized; putting the pulverized raw materials into a mixer to be uniformly mixed; and granulating the mixed raw materials in a granulator after mixing. The compound feed has the beneficial effects that the compound feed disclosed by the invention is added with the traditional Chinese medicine addictives, so thatthe compound feed can meet the all nature requirements of the green food, diseases can be prevented, the immunity of the organism is enhanced, and the production performance of the broiler chicken can be improved so as to create favorable social value and economic benefits.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Compound feed for teaching piglets to eat foods other than breast milk
InactiveCN102178115AHealthy micro-ecological balance environmentAvoid damageFood processingAnimal feeding stuffDiseaseSucrose
The invention provides compound feed for teaching piglets to eat foods other than breast milk, which is used for weaning piglets and teaching the piglets to eat foods other than breast milk, and comprises the following components in part by weight: 212.4 parts of corn starch, 154.0 parts of bulked corn, 100.0 parts of 46 percent of bulked bean pulp, 40 parts of fish meal, 100 parts of wheat flour, 5 parts of calcium powder, 21 parts of calcium hydrophosphate, 2 parts of table salt, 20 parts of soybean oil, 5 parts of lysine, 2 parts of threonine, 1.5 parts of choline chloride, 10 parts of premix, 0.2 part of complex enzyme, 0.3 part of pig Duowei, 0.2 part of sweetener, 0.3 part of flavouring agent, 3 parts of baking soda, 20 parts of cane sugar, 30 parts of glucose, 50 parts of egg powder, 70 parts of soy protein concentrate, 50 parts of fermented bean pulp, 6 parts of acidizer, 100 parts of whey powder, 0.5 part of mould removing agent, 0.3 part of antioxidant and 0.3 part of mildewpreventive. In the invention, the problems of incomplete immune functions, low disease resistance, inadequate digestive ferment, incomplete upper gastrointestinal development, low digestion and absorption ability, susceptibility to diarrhea and the like of piglets.
Owner:AGRI SCI & TECH INST CO LTD OF CHENGDU WEST HOPE GRP
Edible mixed oil
ActiveCN101422200AReduce chronic diseaseEdible oils/fatsFood preparationVegetable oilOMEGA-3 POLYUNSATURATED FATTY ACIDS
The invention relates to an edible blend oil, which aims at solving the problem of the improper ratio between Omega-6 polyunsaturated fatty acid and Omega-3 polyunsaturated fatty acid in the current single plant edible oil and provides an edible plant blend oil with the ratio between Omega-6 polyunsaturated fatty acid and Omega-3 polyunsaturated fatty acid being the best, meeting the health need of Chinese residents and simultaneously being capable of controlling the amount and stability of saturated fatty acids. The technical keys are as follows: the edible blend oil is prepared with a hemp seed oil and another edible plant oil or other edible plant oils according to weight percentage; wherein, the hemp seed oil is 8 to 85 and the edible plant oil is 15 to 92; and the other edible plant oil can be as follows: grape seed oil, olive oil, camellia oil, rice bran oil, safflower oil, canola oil, sunflower oil, corn oil, linseed oil, sesame oil, rapeseed oil, soybean oil, peanut oil and other edible plant oils. The content of the saturated fatty acids does not exceed 15 percent and the ratio between Omega-6 and Omega-3 is 4-6:1.
Owner:淮安市淮安区综合检验检测中心
High molecular weight, lipophilic, orally ingestible bioactive agents in formulations having improved bioavailability
InactiveUS20030044474A1Promote absorptionExtended shelf lifeOrganic active ingredientsBiocideActive agentTG - Triglyceride
Orally ingestible bioactive agents are disclosed which contain a triglyceride matrix and one or more polyphenols that improve the bioavailability of the bioactive agent. In particular non-limiting examples, the bioactive agent is a ubiquinone (such as Coenzyme Q), the triglyceride matrix is a soybean oil matrix, and the composition further includes additional anti-oxidants.
Owner:SHAKLEE CORP
Compound feed for piglets
The invention discloses a compound feed for piglets, which consists of the following components in part by weight: 20 to 30 parts of corn, 20 to 30 parts of soybean meal, 5 to 10 parts of sesame meal, 5 to 10 parts of fermented soybean meal, 10 to 20 parts of wheat bran, 10 to 20 parts of rice bran, 10 to 15 parts of DDGS, 5 to 10 parts of sepiolite powder, 1 to 2 parts of soybean oil and 1 to 2 parts of traditional Chinese medicine preparation, wherein the traditional Chinese medicine preparation consists of the following components: 3 to 7 parts of oyster, 3 to 7 parts of radish seed, 3 to 7 parts of the stem of noble dendrobium, 3 to 7 parts of fragrant solomonseal rhizome, 3 to 7 parts of codonopsis pilosula, 3 to 7 parts of Indian buead, 3 to 7 parts of lily, 3 to 7 parts of Chinese angelica, 3 to 7 parts of white paeony root, 3 to 7 parts of officinal magnolia bark, 3 to 7 parts of bitter orange, 3 to 7 parts of cuttlebone and 3 to 7 parts of cablin potchouli herb. Since the compound feed is added with the traditional Chinese medicines and scientifically prepared, the compound feed has the function of spleen activation and appetite stimulation, and can reduce the diarrhoea of piglets, enhance disease-resisting capability and increase the growing speed of piglets.
Owner:许雪姣
Blend oil, preparation method and application thereof
InactiveCN101766235AImprove stabilityFix stability issuesEdible oils/fatsFood preparationBeef TallowRice bran oil
The invention provides a blend oil, a preparation method and application thereof. The blend oil, counted in 100 parts by weight, comprises 0.1-5 parts of phytosterol ester, 0.01-5 parts of microalgae DHA and 90-99.8 parts of other oil components; the other oil components are sorted from three or more than three of soybean oil, colza oil, maize oil, sunflower oil, peanut oil, sesame oil, tea seed soil, palm oil, cottonseed oil, rice bran oil, olive oil, safflower oil, linseed oil, cannabis oil, pig tallow, beef tallow, or microalgae oil.
Owner:嘉里特种油脂(上海)有限公司
Nursing feedings for roaster
InactiveCN101077135AMeet nutritional needsImprove immunityFood processingAnimal feeding stuffAnimal scienceSoybean meal
The present invention discloses one kind of feed for sucking pig. The feed for sucking pig consists of corn, puffed corn, soybean oil, soybean dregs, puffed soybean and other 20 kinds of material in certain weight proportion. The feed for sucking pig can provide sucking pig with sufficient nutrients, raise the immunological and digestive capacity of sucking pig, increase its feed intake, reduce its ablactation reactions and promote its growth.
Owner:侯彦卫
Feed for improving sow oestrum and increasing farrowing amount
ActiveCN101120731AImprove fertilityImprove conception rateFood processingAnimal feeding stuffAnimal sciencePhosphate
The present invention discloses a feed for promoting sows aphrodisiac and more farrowing, which belongs to the technical field of pig feed. The feed comprises the components as the weight of 600 to 700 shares of corn, 220 to 280 shares of soybean meal, 20 to 50 shares of corn gluten meal, 20 to 50 shares of fish meal, 10 to 30 shares of soybean oil, 5 to 15 shares of dicalcium phosphate, 10 to 20 shares of rock powder, 3 to 5 shares of salt, 2 to 4 shares of lysine, 0.2 to 0.5 shares of methionine, 0.2 to 0.5 shares of threonine, 2 to 4 shares of minerals vitamin and 5 to 15shares of additives. The sows can definitely have more than four kilograms daily meal after weaning with the feed and feeding about six days, the main function of the present invention is to early estrus, and more litter. Using the feed can make sow early estrus and more litter, and strengthen the needs of nutrition for sows breeding, supplement the iron and zinc trace elements, and enhance the ability of the breeding sows.
Owner:沂水和美华福泰华饲料有限公司
Barrier film
InactiveUS7854994B2Improve the level ofImprove homogeneityFibre treatmentBottlesPolyethylene terephthalate glycolPolyethylene oxide
A barrier composition which is injection mouldable and able to be made into a transparent film or incorporated (by co-extrusion and / or lamination) into multi-layer film products, the composition on dry basis: a) from 45 to 90% by weight of a starch and / or a modified starch selected from starches modified by reaction with a hydroxyl alkyl group, an acetate or a dicarboxylic acid anhydride or a grafting polymer; b) from 4 to 12% by weight of a water soluble polymer selected from polyvinyl alcohol, polyvinylacetate, and copolymers of ethylene and vinylalcohol which have a melting point compatible with the molten state of the starch components c) from 5 to 45% by weight of a non-crystallising mixture of sorbitol and at least one other plasticizer selected from glycerol, maltitol, xylitol, mannitol, glycerol trioleate, epoxidised linseed or soybean oil, tributyl citrate, acetyl tri-ethyl citrate, glyceryl triacetate, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate; polyethylene oxide or polyethylene glycol; d) from 0.3 to 2.5% by weight of a C12-22 fatty acid or salt; e) from 0.25% to 3% of an emulsifier system having a hydrophilic lipophilic balance value between 2 and 10. The barrier film may be co-injection moulded with polyethylene terephthalate (PET) or polylactic acid (PLA) for blow moulding into beverage bottles, with polyethylene (PE) or polypropylene (PP) or biodegradable polymers for high gas-barrier containers or closures, or may be co-extruded with polyethylene, polypropylene or polylactic acid for thin film packaging applications or for blow-moulded containers.
Owner:PLANTIC TECH
Bifidobacterium microcapsule and preparing method thereof
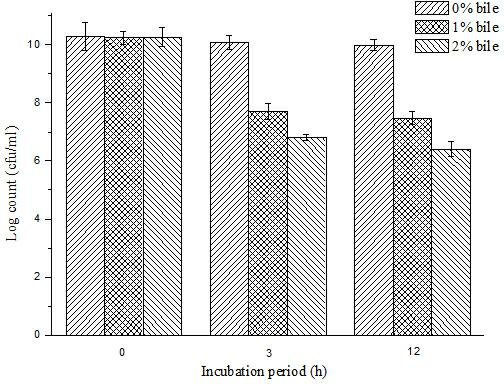
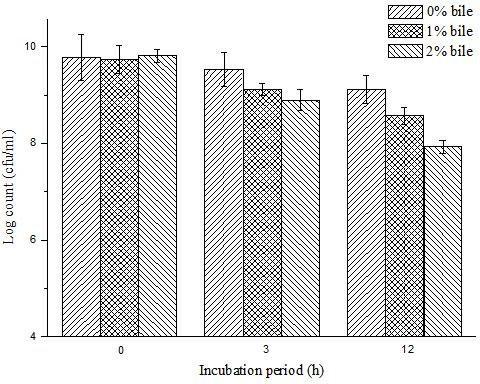
ActiveCN102210659AResistant to gastric acidBile salt resistantMetabolism disorderBacteria material medical ingredientsFreeze-dryingHigh survival rate
The invention specifically relates to a bifidobacterium microcapsule and a preparing method thereof. The bifidobacterium is obligatorily anaerobic and very sensitive to oxygen, PH, temperature, humidity, and other adverse external environment, thereby being very hard to remain activity during production, storage and transport; besides, if taken orally in a form of dry bifidobacterium powder, the bifidobacterium cannot tolerate low-pH value gastric acid, bile salt, and other environments, so a purpose that massive survived bifidobacteria arrive at an intestinal tract and colonise on the intestinal mucosa is hard to be guaranteed. The preparing method disclosed by the invention comprises the following steps of: adding freeze-dried bifidobacterium powder into a sodium alginate solution and then mixing in soybean oil, emulsifying the mixed solution and standing; centrifugally collecting micro-capsules; and drying the micro-capsules by using a vacuum freeze-drying technology. The bifidobacterium microcapsule disclosed by the invention has the advantages of gastric acid resistance, bile salt resistance and entericsolubility, high survival rate of bifidobacterium, simple preparation method, strong practicality, convenience for industrial production, and excellent storage stability and solves the problem of short storage period of probiotics preparations.
Owner:SHAANXI GIANT BIOTECHNOLOGY CO LTD
Spicy beef paste and manufacturing method thereof
InactiveCN103652824AHigh nutritional valueRich in nutritional valueFood ingredient functionsFood preparationNutritive valuesMonosodium glutamate
The invention relates to a spicy beef paste and a manufacturing method thereof, especially to a spicy beef paste with beef, capsicum and thick broad-bean sauce as main raw materials and a manufacturing method thereof. The spicy beef paste is prepared from the following raw materials by weight: 65 parts of beef, 65 parts of thick broad-bean sauce, 50 parts of capsicum, 120 parts of soybean oil, 25 parts of peanut, 20 parts of Laoganma hot and spicy sauce, 10 parts of sweet fermented flour sauce, 6 parts of sesame, 5 parts of garlic, 5 parts of ginger, 3 parts of cumin, 3 parts of white sugar, 3 parts of Chinese prickly ash, 3 parts of Wangshouyi thirteen-spices, 2 parts of essence of chicken and 1 part of monosodium glutamate. The manufacturing method comprises the following procedures: dicing, cooking and stir-frying of beef; stir-frying and grinding of capsicum; stir-frying and grinding of peanut; decocting of the raw materials. The invention has the following advantages: the manufacturing method is simple; the prepared spicy beef paste has a high nutritive value and can guarantee excellent taste, a long shelf-life and uneasy deterioration without addition of other essence and additives.
Owner:BALIKUN XINDI NATIVE PROD DEV CO LTD
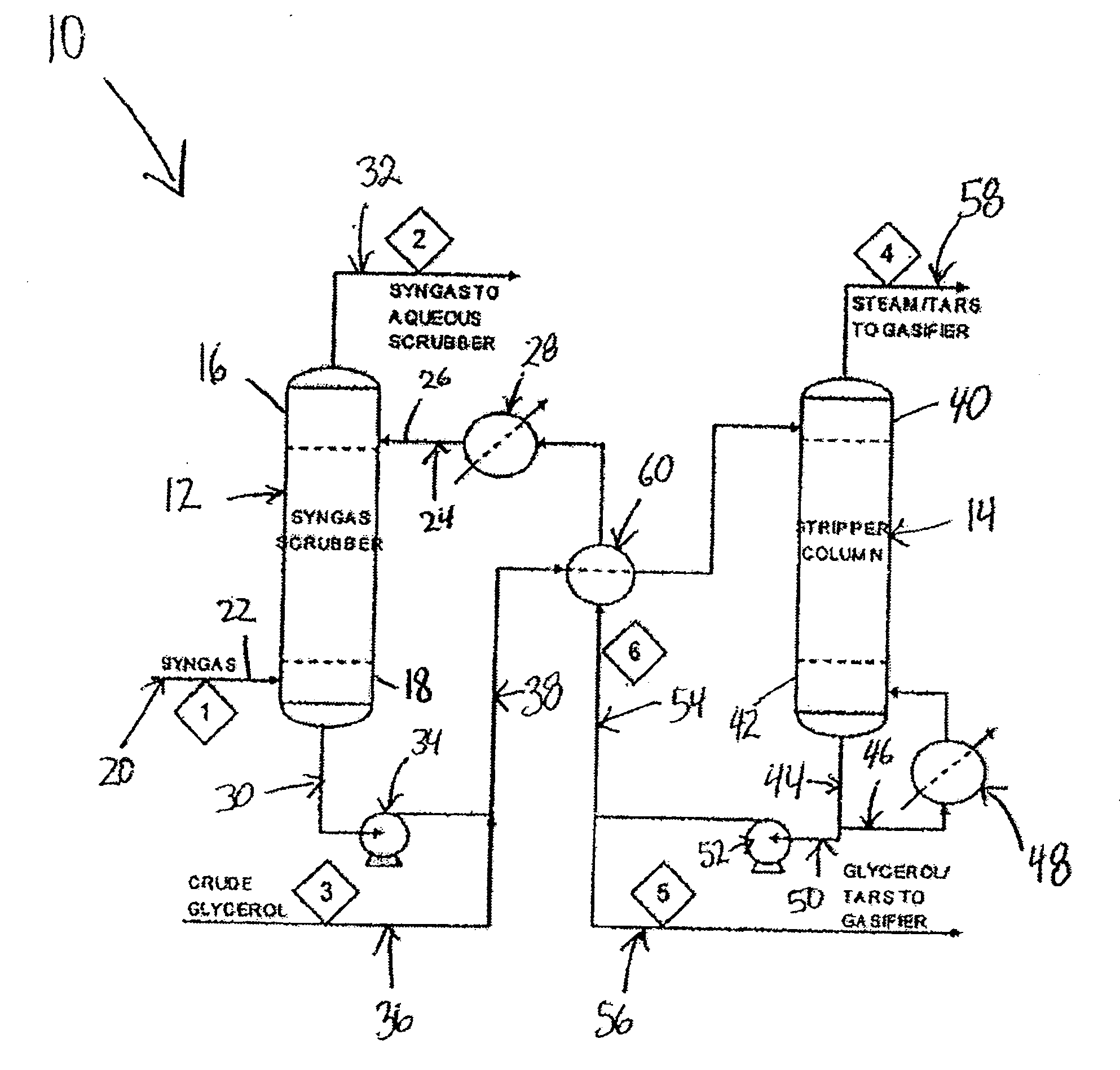
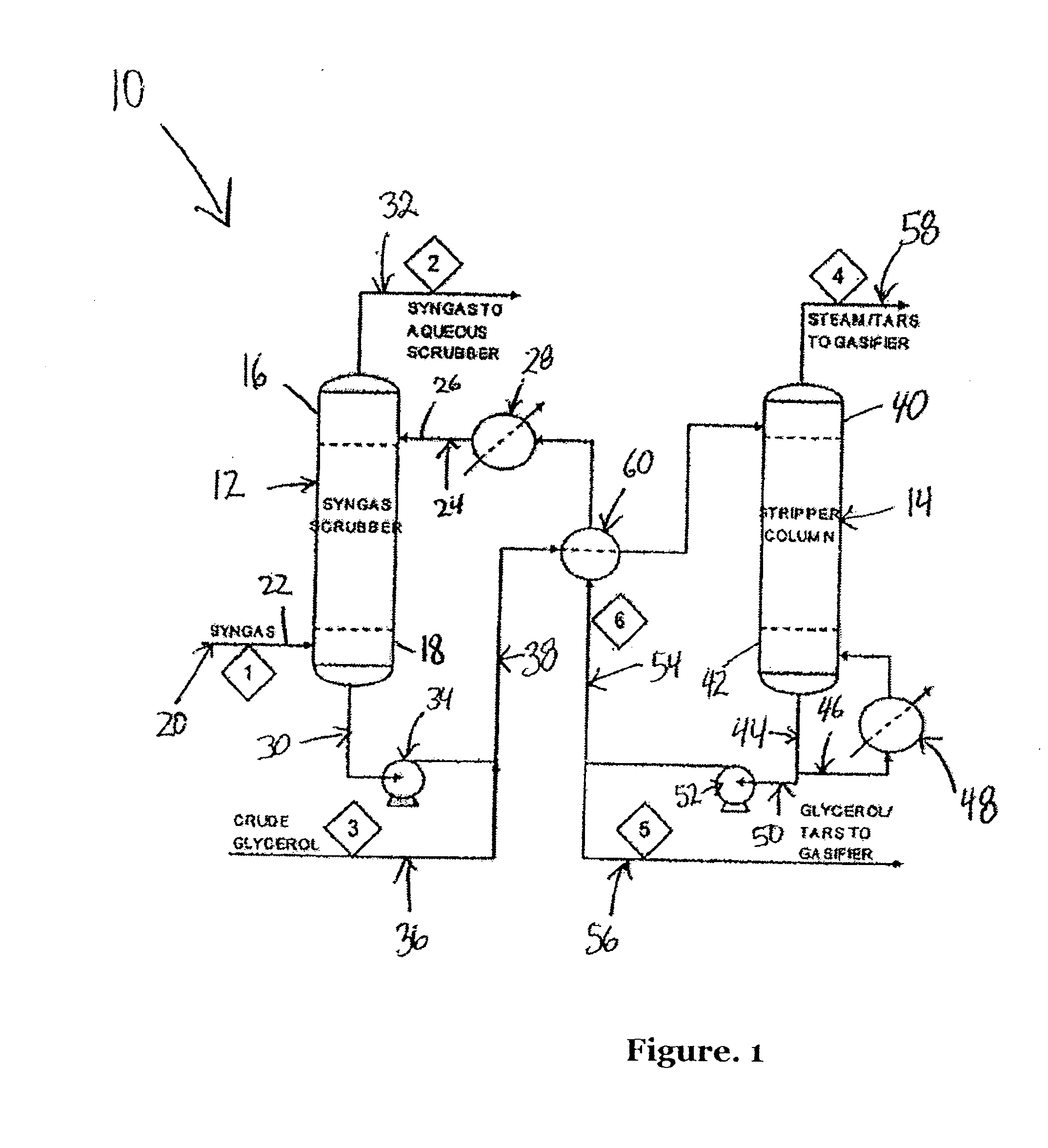
Process for removing tar from synthesis gas
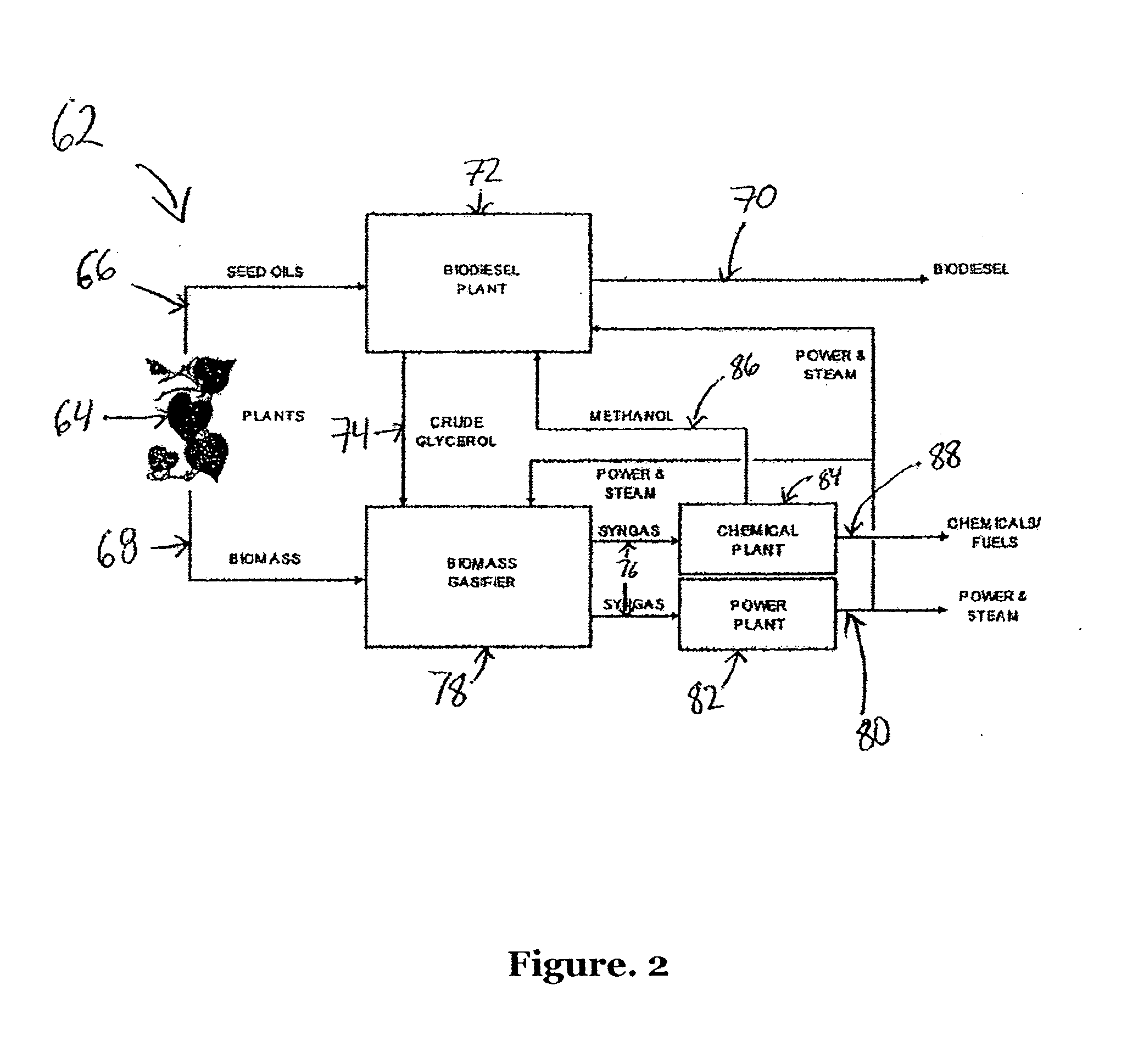
A process and system for removing tars from synthesis gas uses glycerol produced as a byproduct of biodiesel manufacture. The biodiesel may be made from various oil feedstocks such as canola, rapeseed, or soybean oils. Associated with the harvesting of these crops may be the ready availability of byproduct biomass useful as feedstock for gasification. In addition, methanol may be sourced from the gasification of biomass to exploit a potential synergy between biodiesel manufacture and biomass gasification. The present invention develops those synergies further by making use of a byproduct stream from the manufacture of biodiesel to remove tars from the gasifier synthesis gas and to provide a useful end use for the byproduct.
Owner:IHI E&C INT
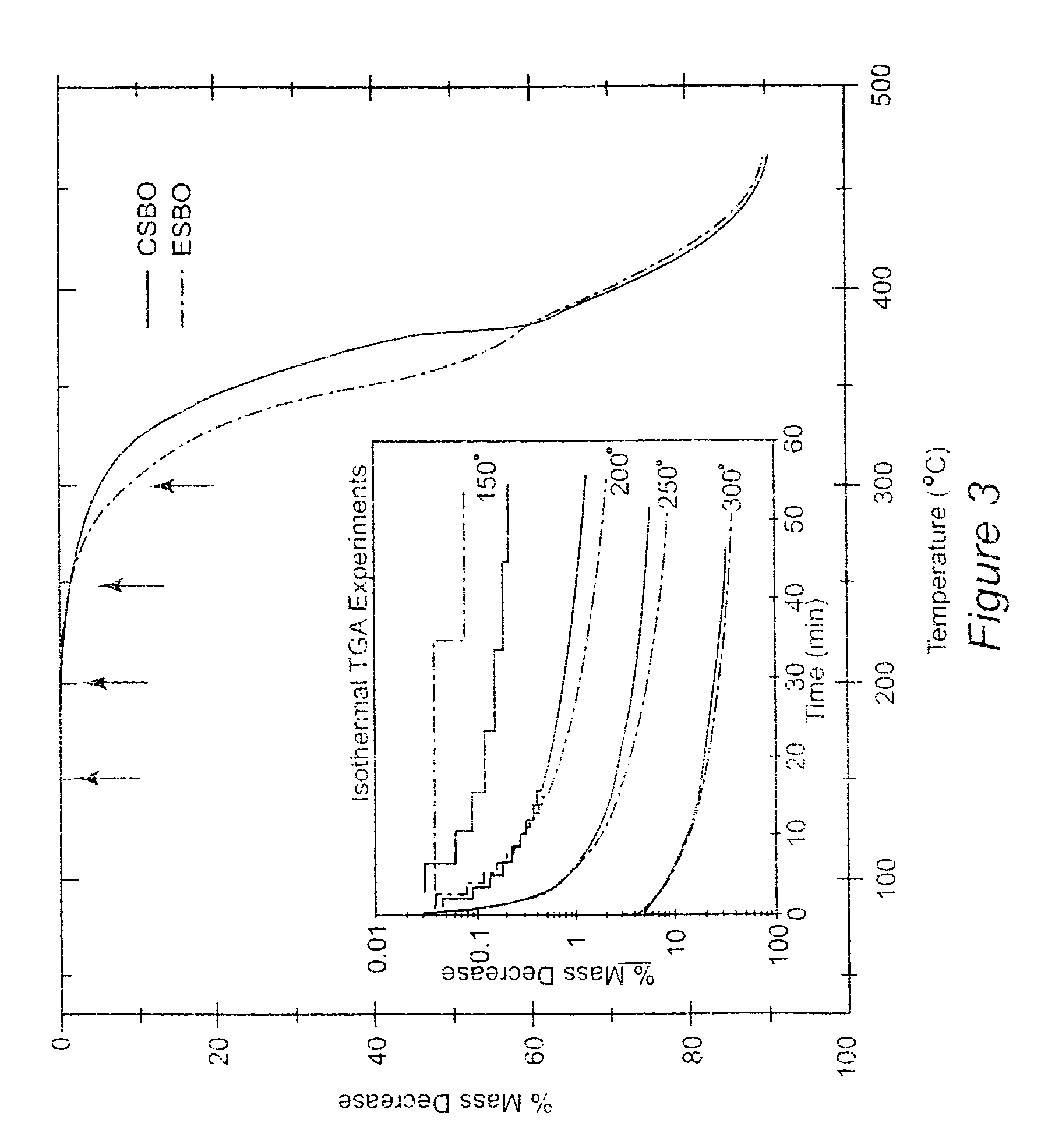
Nonisocyanate polyurethane materials, and their preparation from epoxidized soybean oils and related epoxidized vegetable oils, incorporation of carbon dioxide into soybean oil, and carbonation of vegetable oils
Novel carbonated vegetable oils (such as carbonated soybean oil) are made by reacting carbon dioxide with an epoxidized vegetable oil. The carbonated vegetable oils advantageously may be used for producing nonisocyanate polyurethane materials.
Owner:VIRGINIA TECH INTPROP INC
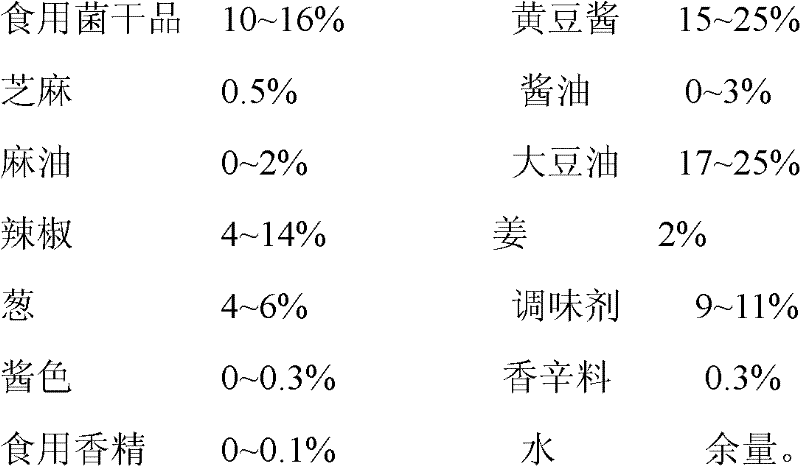
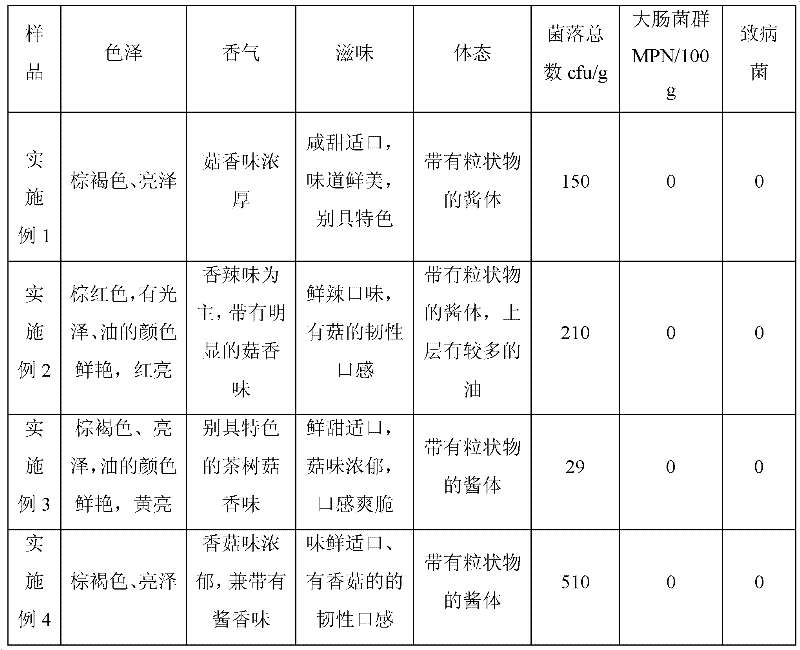
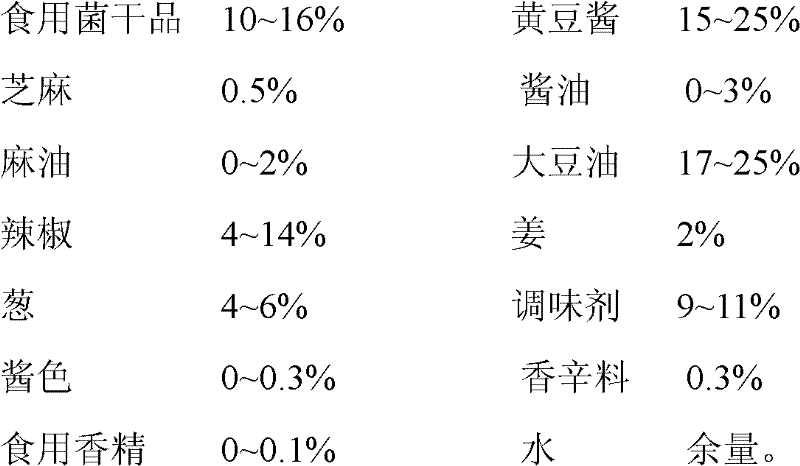
Assorted mushroom sauce and preparation method thereof
The invention provides assorted mushroom sauce which is prepared by the following components by weight: 10-16% of edible mushroom dry materials, 15-25% of soybean sauce, 0.5% of sesame, 0-3% of soy sauce, 0-2% of sesame oil, 17-25% of soybean oil, 4-14% of capsicum, 2% of ginger, 4-6% of scallion, 9-11% of flavoring agents, 0-0.3% of caramel, 0.3% of spice, 0-0.1% of edible essence, and the balance of water. The assorted mushroom sauce provided by the invention maintains original flavor and nutrition of mushrooms; various raw materials and ingredients are added; the prepared seasoning sauce not only can be eaten together with rice or bread, is nutritive and convenient, but also can be used for cooking and seasoning, can enhance the fragrance and delicious taste of dishes, and improve the attraction of dishes.
Owner:LEE KUM KEE XIN HUI FOOD
Preparation of silica reinforced polyisoprene-rich rubber composition and tire with component thereof
This invention relates to the preparation of a cis 1,4-polyisoprene rubber-rich rubber composition containing precipitated silica which has been pre-treated with a fatty alcohol and / or epoxidized soybean oil together with an organosilane containing polysulfide coupling agent and to tires having at least one component comprised of such rubber composition. The invention particularly relates to a process of (a) preparing a natural rubber-rich rubber composition comprised of pre-treating precipitated silica aggregates prior to blending with, or in the presence of, dry natural rubber with a fatty alcohol and / or epoxidized soybean oil to the exclusion of sulfur curative for the natural rubber, mixing an organalkoxysiloxane based polysulfide coupling agent with said dry natural rubber coincidentally with or subsequent to said precipitated silica aggregate fatty alcohol and / or epoxidized soybean oil pre-treatment to form a composite thereof, to the exclusion of sulfur curative, followed by (b) mixing the resulting rubber mixture with sulfur curative and (c) curing the resulting rubber composition.
Owner:THE GOODYEAR TIRE & RUBBER CO
Hydrosilation in high boiling natural vegetable oils
An improved process is provided for the preparation of siloxane-oxyalkylene and siloxane-alkyl copolymer compositions via a hydrosilation reaction in the presence of high boiling point natural vegetable oils as the reaction solvent. The reaction solvent need not be removed from the block copolymer product, and indeed is beneficial to remain with the copolymer particularly when the copolymer is used as a surfactant for polyurethane foam formulations. Soybean oil and linseed oil are the preferred high boiling natural oil solvents when the copolymer product is to be used in the preparation of the surfactants for polyurethane foams. High resiliency polyurethane foam prepared with these natural oils present in the surfactant preparation afforded improved compression sets, wet compression sets and humid aged compression sets. Additionally, the use of the surfactants made with natural oils as a reaction solvent or consequently surfactants post diluted with natural oils when employed in the preparation of polyurethane foam afforded foams with greatly reduced amounts of "glass fogging".
Owner:GENERAL ELECTRIC CO
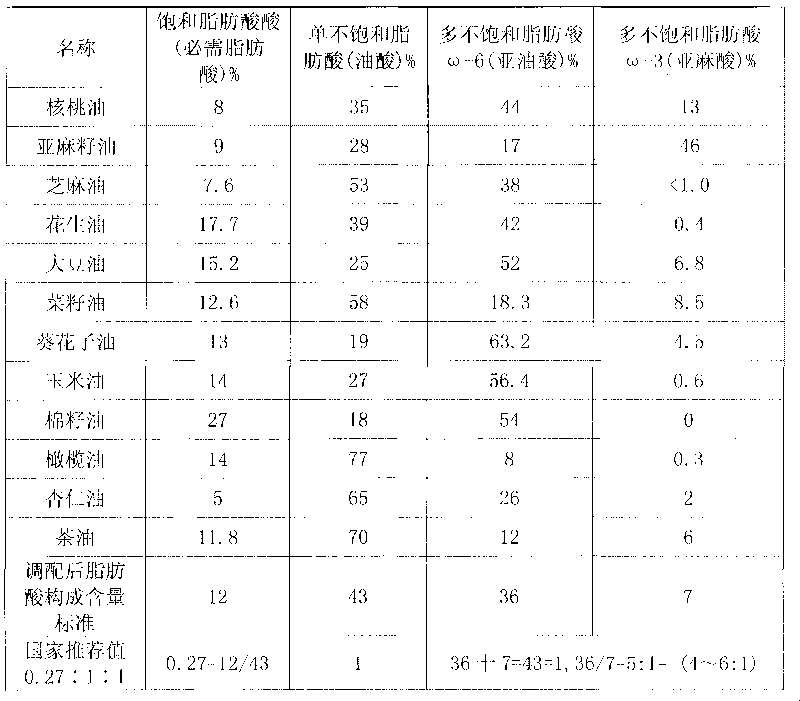
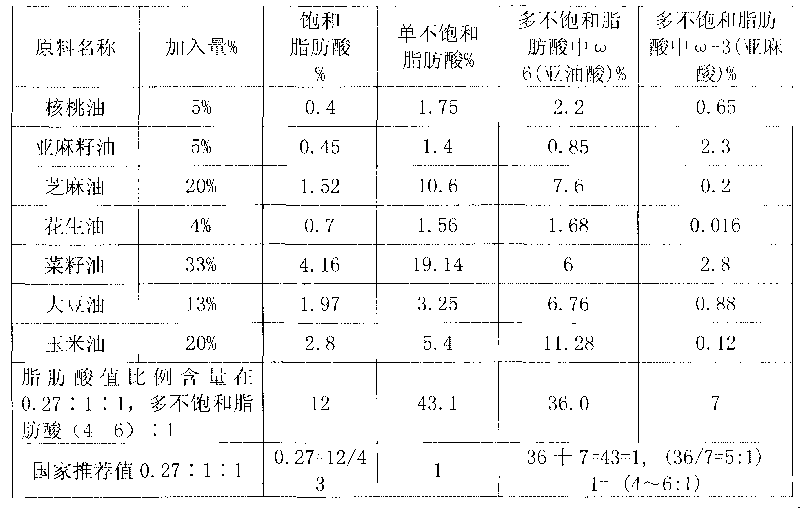
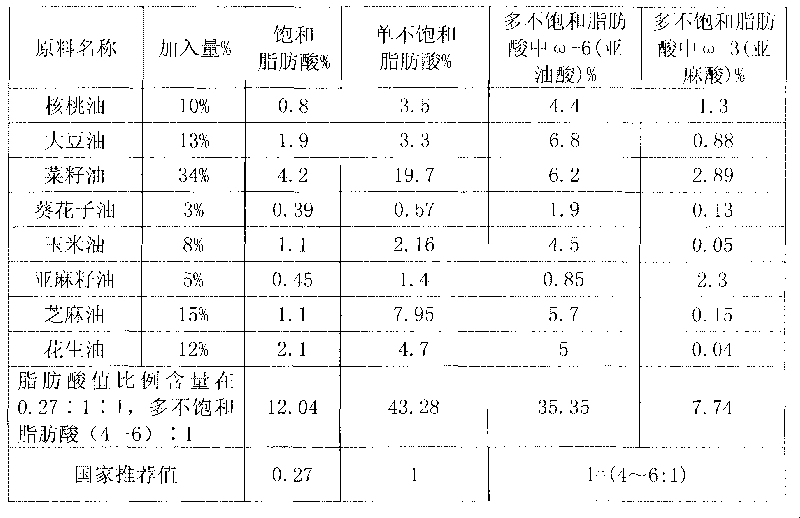
Blend oil with proportional fatty acid prepared by walnut oil and other plant oils
InactiveCN101690526AReduce contentReduce concentrationEdible oils/fatsFood preparationVegetable oilCholesterol
The invention relates to blend oil with proportional fatty acid prepared by walnut oil and other plant oils, which is composed of 5-20% of walnut oil by weight and 80-95% of common plant oils by weight, wherein the common plant oils are selected from any 6 to 11 from peanut oil, soybean oil, sunflower seed oil, teal oil, cotton seed oil, corn oil, almond oil, olive oil, tea oil, linseed oil and colza oil. The ratio of three fatty acids in the blend oil provided by the invention can reach 0.27:1:1, wherein the ratio of omega-6 (linoleic acid) to omega-3 (linolenic acid) in polyunsaturated fatty acids is (4-6):1, and the blend oil in the invention also can overcome the disadvantages that walnut oil is expensive in price and little in market acceptance space while common plant oil has single nutrition and is difficult to supplement fatty acids essential to human body, therefore, by long term administration of the walnut blend oil, cholesterol can be effectively reduced, hyperlipaemia can be reduced, heart cerebrovascular disease can be prevented, and the holistic health level of people can be improved.
Owner:祁景泉
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com