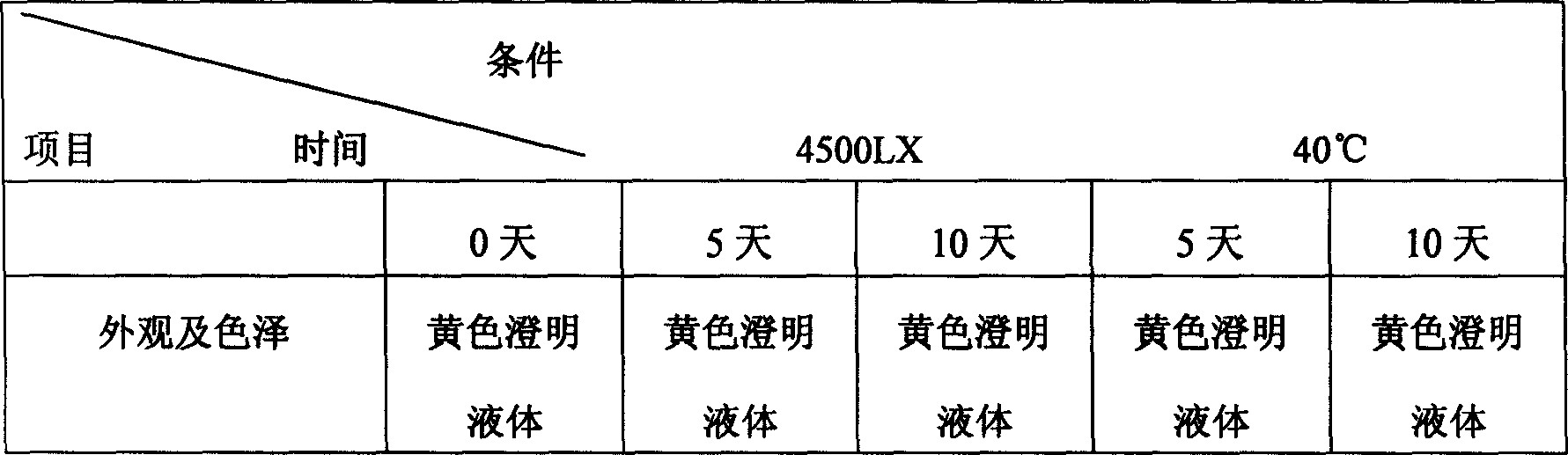
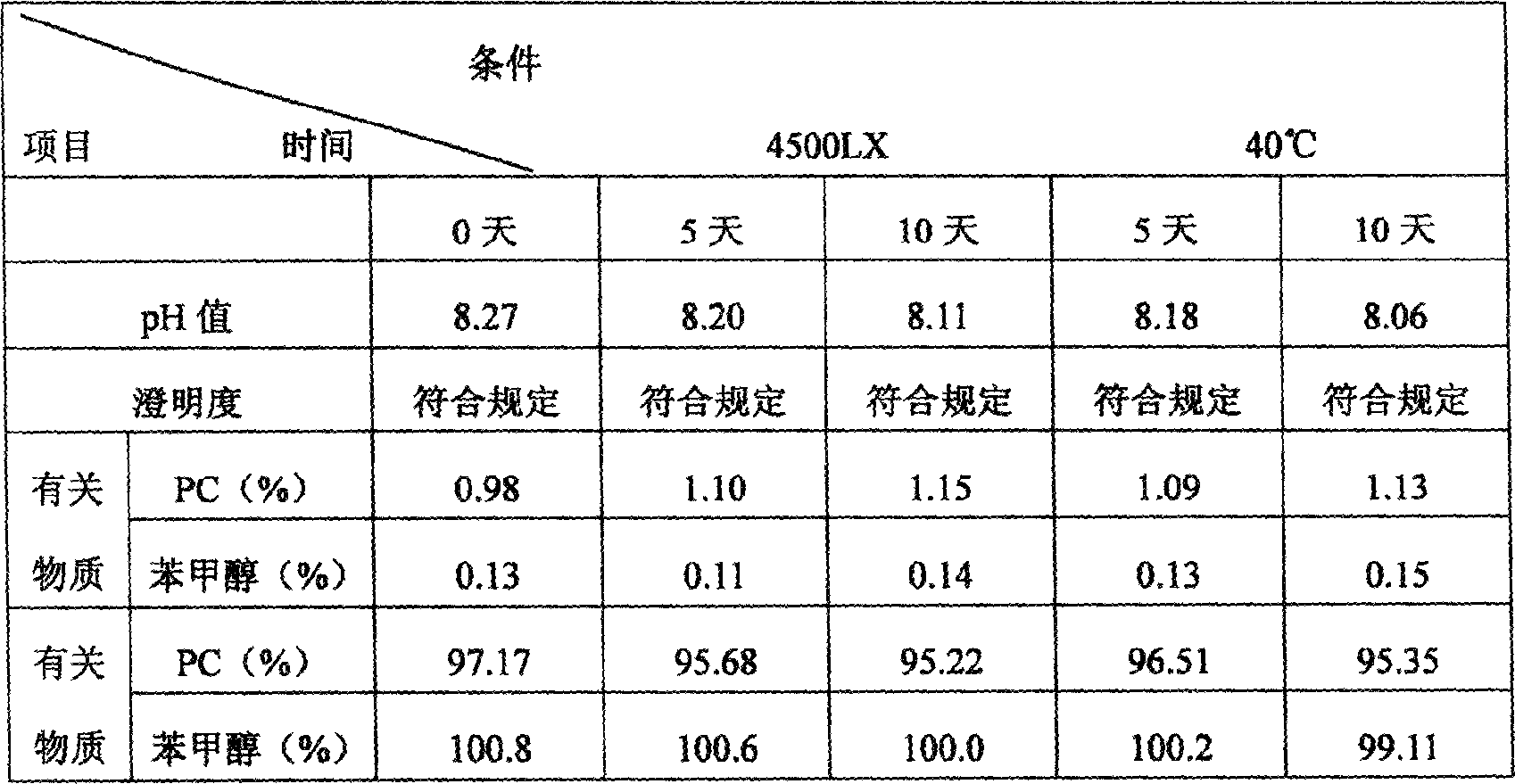
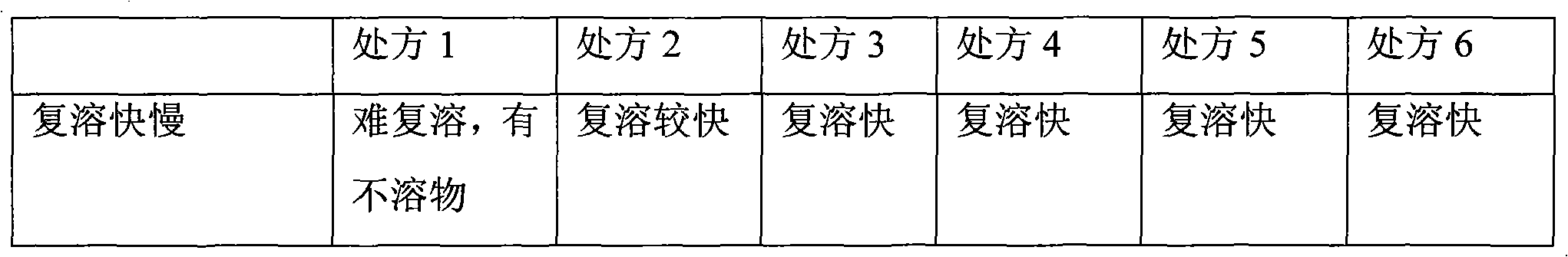
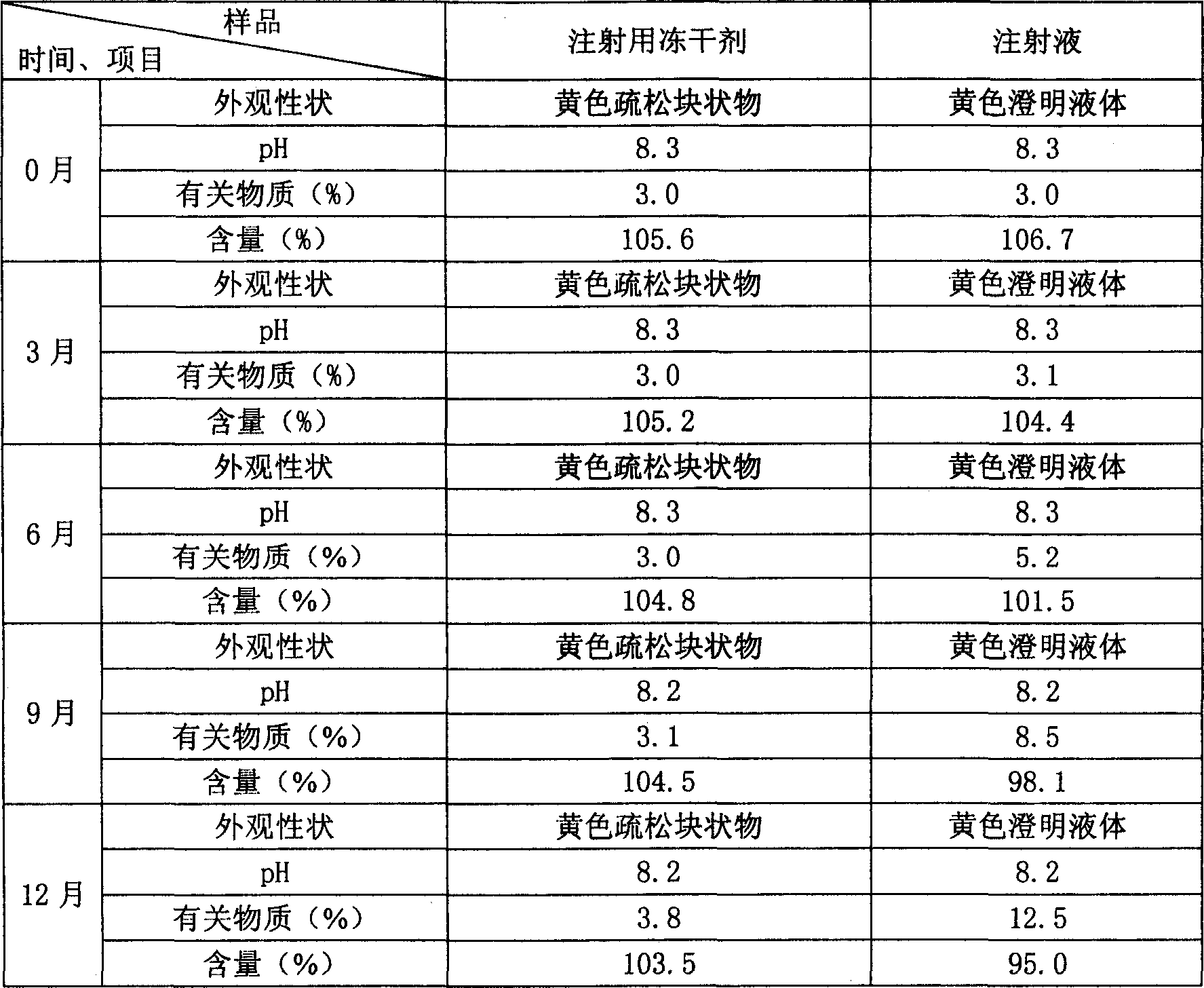
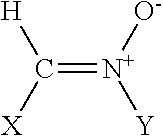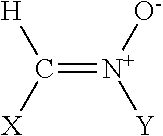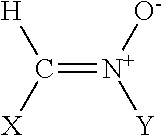Patents
Literature
69 results about "Polyene phosphatidylcholine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatment of skin damage using polyenylphosphatidycholine
InactiveUS6979459B1Avoid skin damageCosmetic preparationsBiocideNatural sourcePolyene phosphatidylcholine
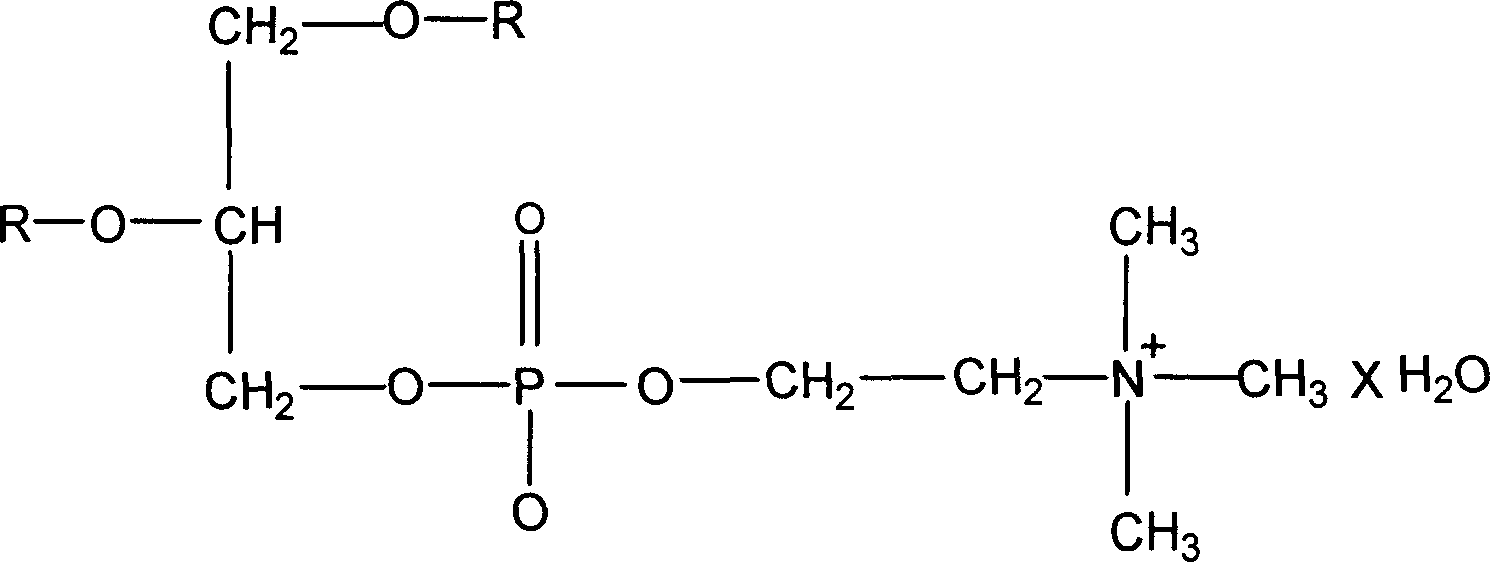
Polyenylphosphatidylcholine is topically applied to treat skin damage, such as contact dermatitis (particularly diaper area dermatitis), atopic dermatitis, xerosis, eczema (including severe hand and foot eczema), rosacea, seborrhea, psoriasis, thermal and radiation burns, other types of skin inflammation, and aging. Typical compositions contain from about 0.25% to about 10% of a polyenylphosphatidylcholine preparation obtained form natural sources such as soybean oil which contains at least about 25% by weight, preferably about 40% or more, dilinoeoylphosphatidylcholine.
Owner:N V PERRICONE
Polyene phosphatidyl choline injection and method for preparing the same
The invention discloses a polyene phosphatidyl choline injection and the preparation method. The components and the ratio (portion) of the polyene phosphatidyl choline injection of the invention is: 465 portion of polyene phosphatidyl choline injection, 88 portion of benzyl zlcohol, 50 to 800 portion of glycocholic acid, cholic acid or tween-80, 50 to 80 portion of alcohol, propanediol or glycerin, 15 to 200 portion of sodium hydroxide or sodium carbonate, 0.5 to 5 portion of 2,6-D-itert-butyl-p-cresol, 0.8 to 8 portion of Tertiary butyl-4-hydroxyl anisole, 3 to 25 portion of Vitamin E by weight. The polyene phosphatidyl choline injection of the invention has good clarity, high stability, simple preparation process and easy operation.
Owner:SICHUAN HAISCO PHARMA CO LTD +1
Freeze dried polyene lecithin powder for injection and its prepn
ActiveCN1771914AImprove solubilityImprove drug activityPowder deliveryOrganic active ingredientsPolyene phosphatidylcholineSodium dehydrocholate
The present invention discloses one kind of freeze dried polyene phosphatidylcholine powder for injection and its preparation process. It features that the preparation contains polyene phosphatidylcholine and solubilizer sodium deoxycholate, sodium dehydrocholate or sodium cholate in the weight ratio of 0.5-1.5.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Soft polyene phosphatidylcholinecapsule and its prepn process
ActiveCN1887279AThe preparation process is stable and feasibleNot easy to stabilizeDigestive systemCapsule deliveryPolyene phosphatidylcholineDrug product
The present invention relates to soft polyene phosphatidylcholine capsule and its preparation process, and belongs to the field of medicine preparing technology. The preparation process include mixing polyene phosphatidylcholine in the recipe amount, vitamins and co-solvent, antioxidant under medicine GMP condition; adding supplementary material and mixing; sieving, subpacking, sealing, scrubbing capsule and air drying to obtain soft capsule. Encapsulating polyene phosphatidylcholine, which is easy to oxidize, sensitive to light and heat and unstable in water solution, can raise the stability of the medicine and reduce the stimulation to gastrointestinal tract.
Owner:国仁健康制药(北京)有限公司
Polyene phosphatidyl choline lyophilized powdered injection
The invention discloses a polyene phosphatidyl choline freeze dried injection for the treatment of hepatic diseases, which comprises polyene phosphatidyl choline as the main ingredients and pharmaceutically acceptable carrying agents, wherein the carrying agent can be auxiliary solvent selected from sodium cholate, sodium deoxycholate, sodium deoxycholate, hydroxypropyl-beta-cyclodextrin, hydroxyethyl-beta-cyclodextrin and / or methyl cyclodextrin. The injection has higher stability.
Owner:GUANGDONG XIANQIANG PHARMA
Preparation process of polyene phosphatidylcholine injection
InactiveCN1602878ANo obvious degradationDigestive systemPhosphorous compound active ingredientsPolyene phosphatidylcholineActivated carbon
The invention discloses a method for preparing polyene phosphatidyl choline injection, including mixing polyene phosphatidyl choline, solubilizer, and injection water in proportion, stirring them into a colloidal dispersive system, adding in activated carbon to absorb, and adding proper antiseptic. It is a low-cost, simple-technique preparing method.
Owner:北京瑞伊人科技发展有限公司 +1
Polyvinyl-phosphorylcholine elaioplast preparation and its making method
InactiveCN1895224ANo vascular irritationEasy to useDigestive systemPhosphorous compound active ingredientsVitamin CCholesterol
A polyene phosphatidylcholine liposome with high curative effect is prepared through dissolving polyene phosphatifylcholine, phosphatide and cholesterol in organic solvent, constant-temp drying for removing solvent, filming, adding vitamin, stabilizer and mannitol or glucose solution, dissolving, ultrasonic vibration or homogenizing, filtering by membrane for removing bacteria, loading in containers, filling N2 or H2 and sealing.
Owner:裴泽军
A lyophilized powder injection of polyene phosphatidylcholine and preparation method thereof
The invention relates to a phosphatidyl choline freeze powder injection and relative preparation, wherein said formula is formed by phosphatidyl choline, solubilizer, support agent and other additive components; the phosphatidyl choline content is 10-60%; the solubilizer can colalin, glycocholic acid, cholaic acid, deoxycholic acid, anthrodsoxycholic acid, or taurodeoxycholic acid and relative salt, loxapine and tween; the support agent can be freeze protector. The invention has high stability, which can be stored for 1 year at normal temperature, without allergic and excitation.
Owner:四川思达康药业有限公司
High safety polyene phosphatidyl choline for injection and preparation method thereof
InactiveCN101940586ASolve the Stability ConundrumReduce dosageOrganic active ingredientsPowder deliveryPolyene phosphatidylcholineMedicine
The invention provides high safety polyene phosphatidyl choline for injection and a preparation method thereof. Each injection contains the polyene phosphatidyl choline, vitamin B6, vitamin B12, nicotinamide, sodium pantothenate, a solubilizer and cryoprotectant. The injection is characterized in that the weight ratio of the solubilizer to the polyene phosphatidyl choline is 0.4-0.6:1, and is 0.5:1 preferably. The dosage of the solubilizer is reduced, the potential safety problem is reduced, the problem of re-dissolution is solved by optimizing the formula and the process, and the excellent re-dissolution is maintained on the premise of reducing the dosage of the solubilizer.
Owner:北京中海康医药科技发展有限公司
Polyenic phosphatide acylcholine freeze-dried powder injecta medicine
The present invention relates to a polyene phosphatidylcholine freeze-dried powder injection. It is formed from polyene phosphatidylcholine as effective medicinal component and auxiliary component including necessary solubiliser and freeze-drying protective agent. Its weight composition includes 0.1-1.5 portions of polyene phosphatidylcholine, the weight ratio of solubilizer and polyene phosphatidylcholine is 0.1-2.5:1 and the weight ratio of freeze-drying protective agent and polyene phosphatidylcholine is 0.1-10:1. According to the requirement the pharmaceutically-acceptable vitamin as other auxiliary component also can be added.
Owner:SICHUAN KELUN PHARMA CO LTD +1
Polyene phosphatidyl choline powder injection and its preparation method
InactiveCN1839851ANon-reactiveNo hemolysisPowder deliveryOrganic active ingredientsFreeze-dryingSolvent
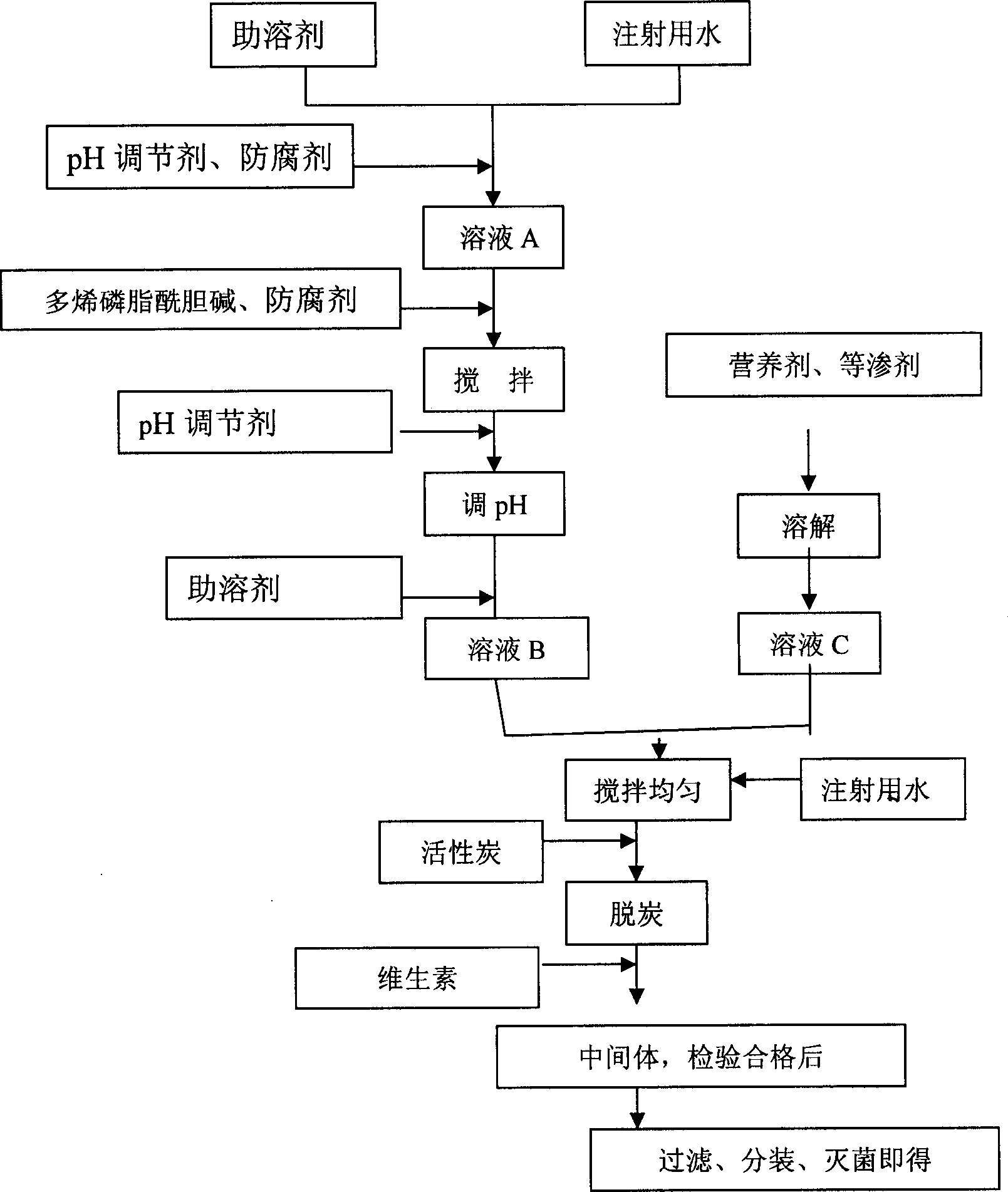
The invention relates to a freeze-dried powder injection of polyene phosphatidyl choline and its preparation, which comprises charging into a finite amount of water for injection by auxiliary solvent, pH regulator, polyene phosphatidyl choline, anti-oxidant agent, homogenizing through stirring, adjusting pH to 7.0-10.0, charging right amount of excipient, auxiliary solvent, and water for injection, charging activated charcoal and vitamins to obtain intermediate products, filtering with filter membrane and split charging.
Owner:AOLING BODA MEDICINE SCI & TECH DEV BEIJING
Method for preparing Polyene Phosphatidylcholine injection liquid
InactiveCN1961886AOrganic active ingredientsDigestive systemPolyene phosphatidylcholinePolymer science
The invention relates to a process for preparing polyene phosphatidyl choline injection, which consists of mixing polyene phosphatidyl choline, solubilizing agent and water for injection proportionally, charging right amount of preservative agent and anti-oxidizing agent, and mixing to colloid form dispersible system.
Owner:ZHEJIANG HAILISHENG PHARM CO LTD
Polyene phosphatidylcholine freeze-dried preparation injection and its preparing method
InactiveCN1820757AImprove stabilityRaise storage temperaturePowder deliveryOrganic active ingredientsPolyene phosphatidylcholineFreeze-drying
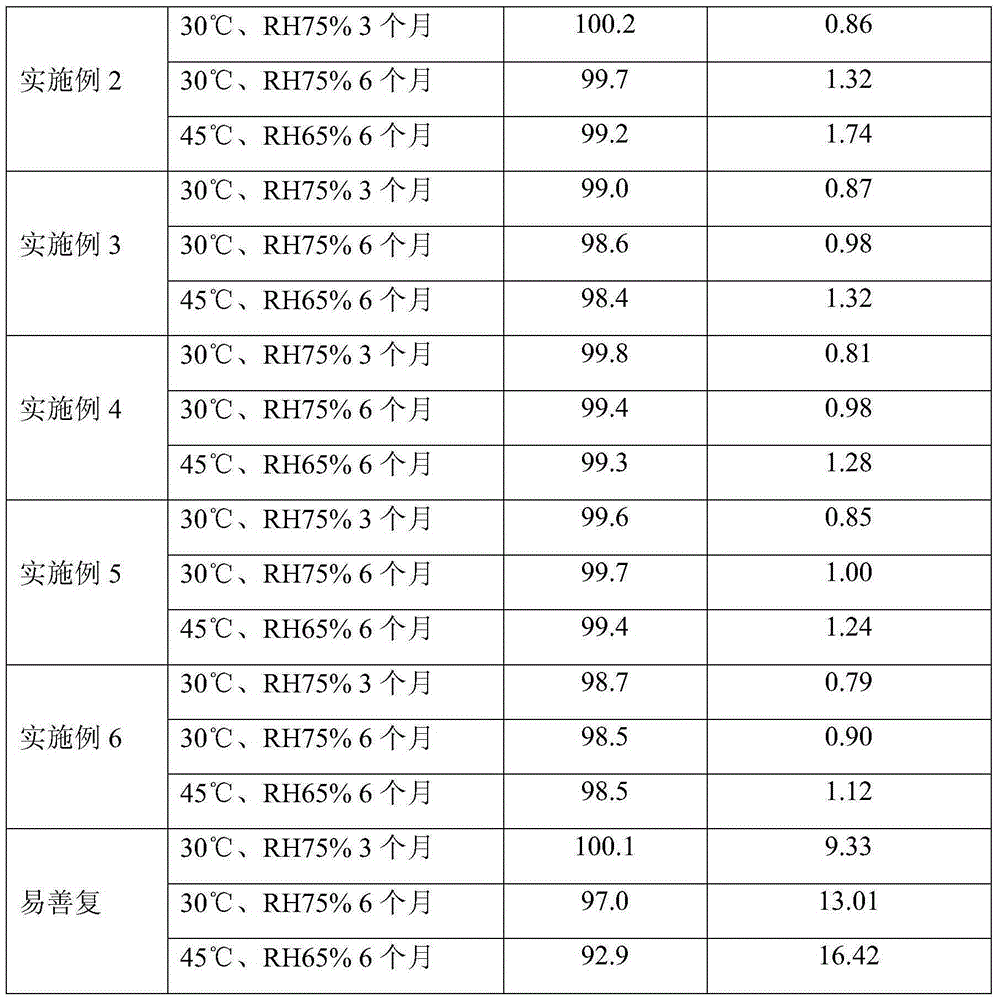
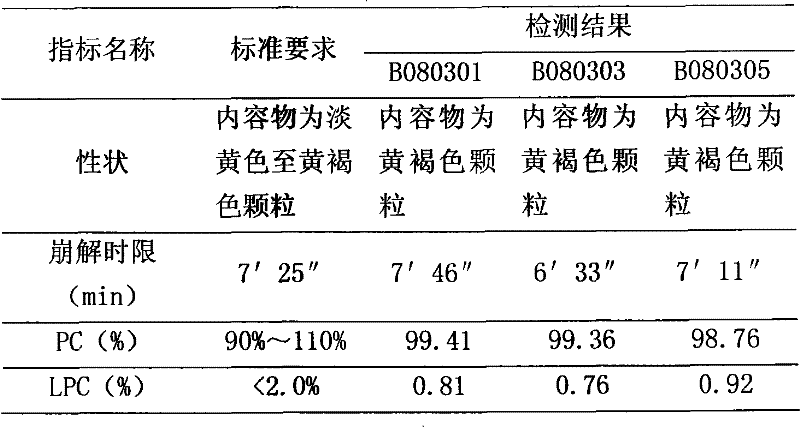
The present invention discloses freeze dried polyene phosphatidylchline preparation for injection and its preparation process. The freeze dried polyene phosphatidylchline preparation contains polyene phosphatidylchline in 30-60 wt% and medicinal supplementary material 40-70 wt%. The test on stability shows that the preparation of the present invention has excellent stability and high storage temperature up to 25deg.c. The present invention has simple and practical preparation process and stable and reliable product.
Owner:YAOPHARMA CO LTD
Polyene phosphatidyl choline infusion solution and its preparing method
InactiveCN1781488ANon-irritatingThe preparation process is stable and feasibleOrganic active ingredientsDigestive systemDiseaseHemolysis
The present invention belongs to the field of medicine preparation technology, relates to medicine preparation for treating several kinds of liver and gallbladder diseases and its preparation, and is especially one kind of polyene phosphatidyl chloine infusion solution and its preparation process. The preparation process of the polyene phosphatidyl chloine infusion solution includes: adding polyene phosphatidyl chloine, co-solvent, pH regulator, antioxidant and other supplementary material into injection water; regulating pH value to 6.5-10.0; adding active carbon and filtering, adding vitamins to obtain intermediate; content meat, filtering with 0.1-0.45 micron membrane and packing. The stability test shows that the preparation process is stable and feasible; and the safety test shows that the polyene phosphatidyl chloine infusion solution has no allergic reaction, no hemolysis effect, and no irritation on blood vessel.
Owner:AOLING BODA MEDICINE SCI & TECH DEV BEIJING
Composition of Essentiale Phospholipids injection and preparation method thereof
ActiveCN1951389AAvoid adverse reactions of accumulation poisoningAvoid hydrolysisOrganic active ingredientsPowder deliveryPolyene phosphatidylcholinePhospholipid
The invention relates to a compound of phosphatide bilineurine powder injection and relative preparation. Wherein, the inventive product can avoid benzenemethanol, to be stably used. And its components contain phosphatide bilineurine, cholate and support agent. The invention uses special method and treatment. The inventive product via frozen and dried is made into white or yellow powder injection, which can be diluted before vein injection.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Glycyrrhizic acid double salt and preparation thereof
ActiveCN101081861ASimplify Preparing PrescriptionsSimple processOrganic active ingredientsDigestive systemPolyene phosphatidylcholineEssential phospholipids
The present invention relates to medicine technology, and is especially one kind of complex glycyrrhizinate and its preparation process. The complex glycyrrhizinate has the liver protecting effects of glycyrrhizic acid or glycyrrhizinate and essential phospholipids combined together, and thus hepatosis treating effect higher than that of each of glycyrrhizic acid or glycyrrhizinate and essential phospholipids.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Polyene phosphatidylcholine capsule and preparation method thereof
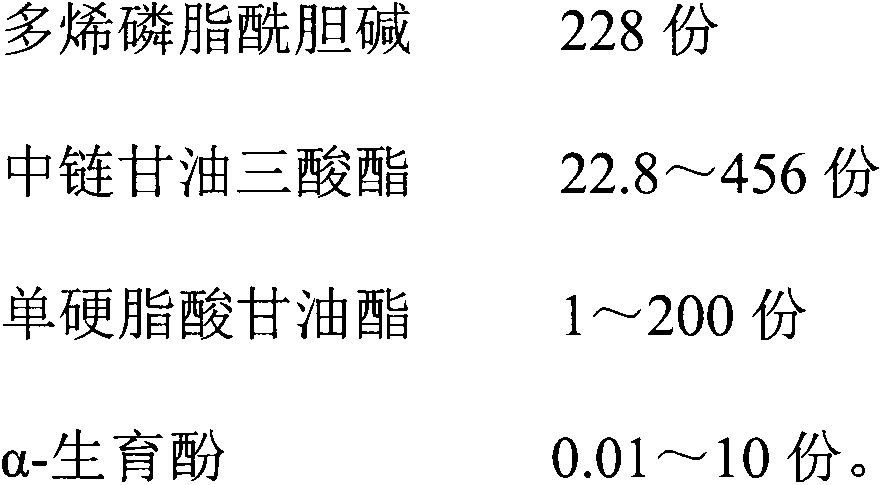
ActiveCN103908440ASmall rate of changeSimple processOrganic active ingredientsDigestive systemPolyene phosphatidylcholineMedicine
The invention relates to a polyene phosphatidylcholine capsule and a preparation method thereof, and belongs to the field of medicine preparations. Polyene phosphatidylcholine has unstable characteristic, different-degree phosphatide hydrolysis is generated during long-term storage of polyene phosphatidylcholine, and leads to content reduction of polyene phosphatidylcholine and generation increase of a toxic substance lysophosphatidylcholine. The invention provides the polyene phosphatidylcholine capsule which comprises polyene phosphatidylcholine and medium-chain triglyceride, and is substantially improved in stability compared with a current marked product Essentiale N. The polyene phosphatidylcholine capsule also comprises one or more pharmaceutically accepted excipient. Additionally, the invention also aims at providing a method for preparing the polyene phosphatidylcholine capsule, and the method is simple in preparation technology and is convenient for industrialized production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Polyene phosphatidylcholine high-capacity injection
InactiveCN101244070ARegulate energy balancePromote regenerationOrganic active ingredientsDigestive systemPolyene phosphatidylcholineActive component
The invention discloses a polyene phosphatidylcholine large volume injection which is prepared by the mixture of polyene phosphatidylcholine or soybean phospholipid as the active component and the pharmaceutical carrier; the content proportion of the active component in the injection is 0.0465% to 0.93%. The invention is characterized in that the polyene phosphatidylcholine injection can be used to intravenous infusion directly. The polyene phosphatidylcholine large volume injection has the advantage that the diluting process in the present medication condition is omitted; cross infection is avoided; the clinical application level and safety is improved; and the convenient use is guaranteed.
Owner:桂勇 +1
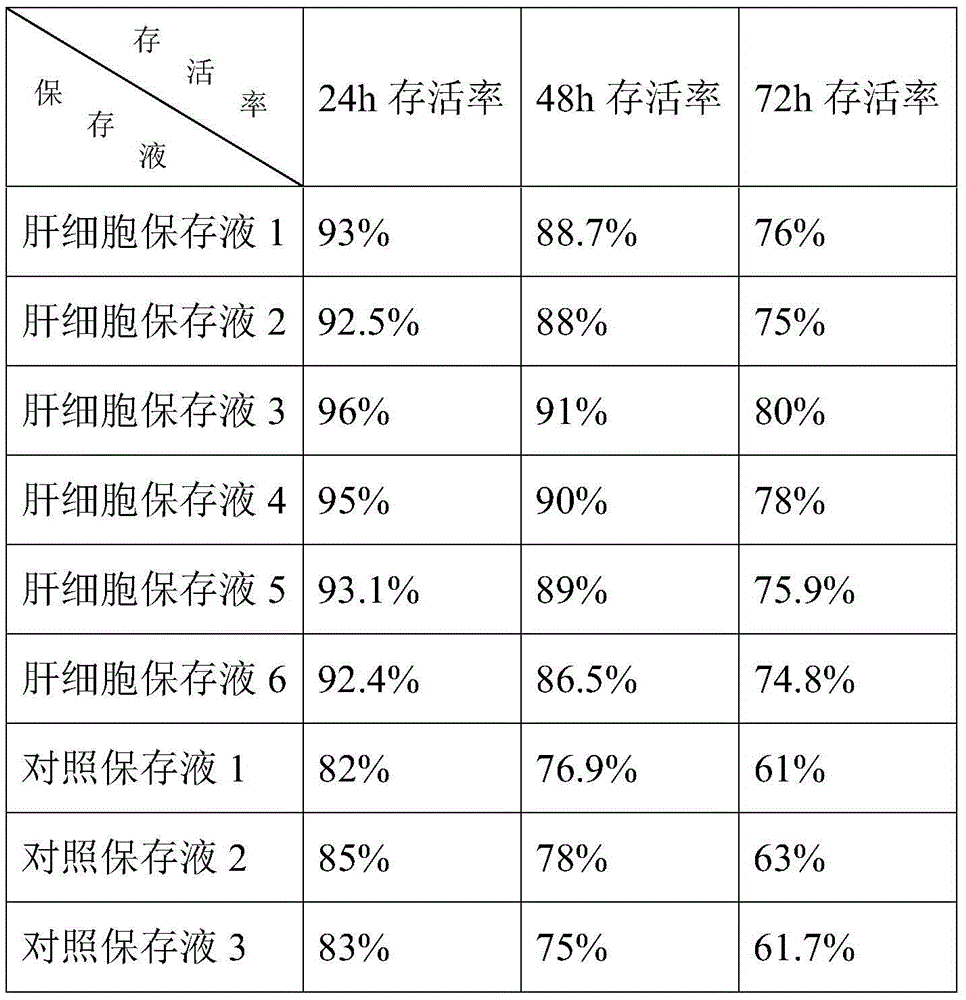
Liver cell preserving fluid and preparation method and application
ActiveCN105532646AComposition is stableIncrease osmotic pressureDead animal preservationHydroxyethyl starchVitamin C
The invention discloses a liver cell preserving fluid and a preparation method and application thereof. The liver cell preserving fluid which is stable in ingredient, diverse in nutrient substance and strong in solution permeating system buffering power is formed by mixing hydroxyethyl starch 130 / 0.4 sodium chloride injection, glucose, sodium glutamate, vitamin C, phenol red, glucurolactone, polyene phosphatidyl choline, a cell buffering solution and an amino acid mixed solution. The liver cell preserving fluid can not only provide nutrition for liver cells, can also well maintain cell activity and accordingly plays a better effect in subsequent clinical use.
Owner:WUHAN TOGO MEDITECH CO LTD
Polyene phosphatidyl choline composition for injection and preparation method thereof
InactiveCN103816118ADefinite curative effectImprove securityOrganic active ingredientsDigestive systemPolyene phosphatidylcholineCholesterol
The invention provides a polyene phosphatidyl choline composition for injection and a preparation method thereof. The composition is composed of methoxy polyethylene glycol-phosphatidyl ethanolamine, cholesterol, water for injection, soybean oil, a glycine-HCl buffer solution, and is prepared through a compound compatibility method. The polyene phosphatidyl choline composition has the advantages of reduced safety hazard and improved drug effect.
Owner:HAISCO PHARMA GRP INC
Polyene phosphatidyl choline intravenous preparation and preparation method thereof
InactiveCN101756895ASolve cumbersome operationsAvoid pollutionPowder deliveryOrganic active ingredientsFreeze-dryingFiltration
The invention relates to a polyene phosphatidyl choline intravenous preparation (comprising infusion and freeze-drying) and a preparation method thereof. The raw materials of the polyene phosphatidyl choline intravenous preparation comprise polyene phosphatidyl choline, sodium cholate and xylitol which can accept the adjustment osmotic pressure. The process comprises the following steps: preparing a colloidal dispersion system from the polyene phosphatidyl choline and the sodium cholate according to the prescription under the conditions required by the process; carrying out active carbon decoloration on the colloidal dispersion system, then carrying out rough filtration on the obtained product under the condition of adding nitrogen, adding the xylitol according to the prescription into the obtained solution to prepare concentrated solution for later use; and adding water for injection to make up to volume, and preparing the infusion by the processing procedures of filtering, filling, adding the stopper, pressing the cover, sterilizing, detecting leakage and the like, or preparing the freeze-drying preparation by the processing procedures of filtering, filling, freeze-drying, adding the stopper, pressing the cover and sterilizing. The prepared infusion solution finished product has convenient clinical use, better stability on low temperature storage or clinical use, no turbidity or emulsification phenomenon, and better safety performance.
Owner:北京瑞伊人科技发展有限公司 +1
Polyene phosphatidyl choline injection, and preparation method thereof
The invention belongs to the field of medicinal preparation, and specifically relates to a polyene phosphatidyl choline injection, and a preparation method thereof. The polyene phosphatidyl choline injection comprises polyene phosphatidyl choline, a solubilizer, an antiseptic, and a stabilizing agent; the solubilizer is selected from deoxycholic acid, deoxysodium cholate, dehydrocholic acid, sodium dehydrocholate, cholic acid, or sodium cholate; and the stabilizing agent is selected from amino acid, or a sodium salt thereof, or trometamol. Compared with a control group without the stabilizing agent, stability of the polyene phosphatidyl choline injection after high temperature sterilization and long-term storage is increased significantly, formula and preparation method are both simple, and the polyene phosphatidyl choline injection is convenient for industrialized production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Oral solid preparation containing polyene phosphatidyl choline and preparation method thereof
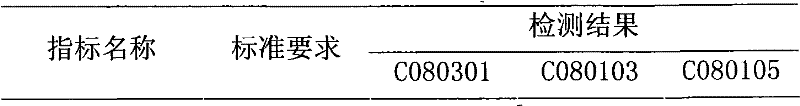
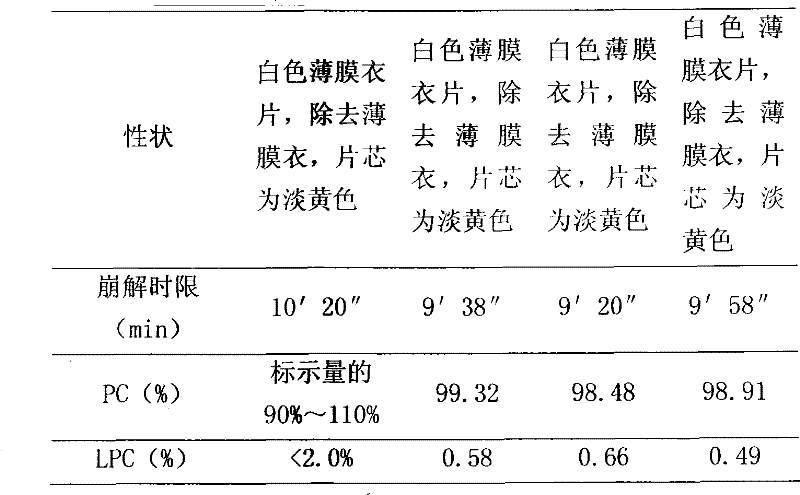
ActiveCN103251568AImprove stabilityGood dissolution effectOrganic active ingredientsDigestive systemPolyene phosphatidylcholineHard Capsule
The invention relates to an oral solid preparation containing polyene phosphatidyl choline and a preparation method thereof. The preparation method comprises the following steps: dissolving polyene phosphatidyl choline in ethanol; adequately mixing the polyene phosphatidyl choline with a matrix, an antioxygen and a sorbefacient in a protective state; carrying out vacuum drying and volatilizing to remove ethanol, and preparing pellets by a pill dropping machine; and packaging the pellets coated or packing in hard capsules or tabletting and coating to obtain the oral solid preparation containing polyene phosphatidyl choline. The oral solid preparation containing polyene phosphatidyl choline provided by the invention can enhance the stability of polyene phosphatidyl choline, reduce the gastrointestinal irritation by the medicine and improve the dissolving-out performance of polyene phosphatidyl choline, so that the equipment cost is saved to the benefit of industrialized production.
Owner:HEFEI BOSIKC PHARMTECH CO LTD
Polyene phosphatidyl choline capsule and preparation process thereof
ActiveCN106619560AImprove stabilityEnsure safetyOrganic active ingredientsDigestive systemPolyene phosphatidylcholineHard Capsule
The present invention belongs to the field of pharmaceutical preparations, and mainly relates to a polyene phosphatidyl choline capsule and a preparation process thereof, wherein the polyene phosphatidyl choline capsule contains polyene phosphatidyl choline and soybean oil, and can further contain other pharmaceutically acceptable excipients. Compared to the polyene phosphatidyl choline capsule in the prior art, the polyene phosphatidyl choline capsule of the present invention has good stability so as to well ensure the medication safety and the medication efficacy, and polyene phosphatidyl choline capsule is produced by using the liquid filling hard capsule technology so as to provide advantages of simple production process, small equipment occupation area, low cost, high automation degree, less impurity, and the like.
Owner:SICHUAN HAISCO PHARMA CO LTD
Preparation method of oral solid preparation containing polyene phosphatidyl choline
InactiveCN101756907BImprove yieldImprove accuracyOrganic active ingredientsDigestive systemPolyene phosphatidylcholineMedicine
Owner:北京瑞伊人科技发展有限公司 +1
Topical nitrone spin trap compositions for psoriasis
Psoriasis is treated by application of a composition containing a nitrone spin trap such as α-phenyl t-butyl nitrone (PBN) and derivatives thereof. Preferred compositions and method of treatments further comprise at least one adjunctive ingredient including fatty acid esters of ascorbic acid such as ascorbyl palmitate and ascorbyl stearate, and polyenylphosphatidylcholine.
Owner:PERRICONE NICHOLAS V
Oral solid preparation containing polyene phosphatidylcholine and its preparation process
ActiveCN103251568BImprove stabilityGood dissolution effectOrganic active ingredientsDigestive systemPolyene phosphatidylcholineHard Capsule
The invention relates to an oral solid preparation containing polyene phosphatidyl choline and a preparation method thereof. The preparation method comprises the following steps: dissolving polyene phosphatidyl choline in ethanol; adequately mixing the polyene phosphatidyl choline with a matrix, an antioxygen and a sorbefacient in a protective state; carrying out vacuum drying and volatilizing to remove ethanol, and preparing pellets by a pill dropping machine; and packaging the pellets coated or packing in hard capsules or tabletting and coating to obtain the oral solid preparation containing polyene phosphatidyl choline. The oral solid preparation containing polyene phosphatidyl choline provided by the invention can enhance the stability of polyene phosphatidyl choline, reduce the gastrointestinal irritation by the medicine and improve the dissolving-out performance of polyene phosphatidyl choline, so that the equipment cost is saved to the benefit of industrialized production.
Owner:HEFEI BOSIKC PHARMTECH CO LTD
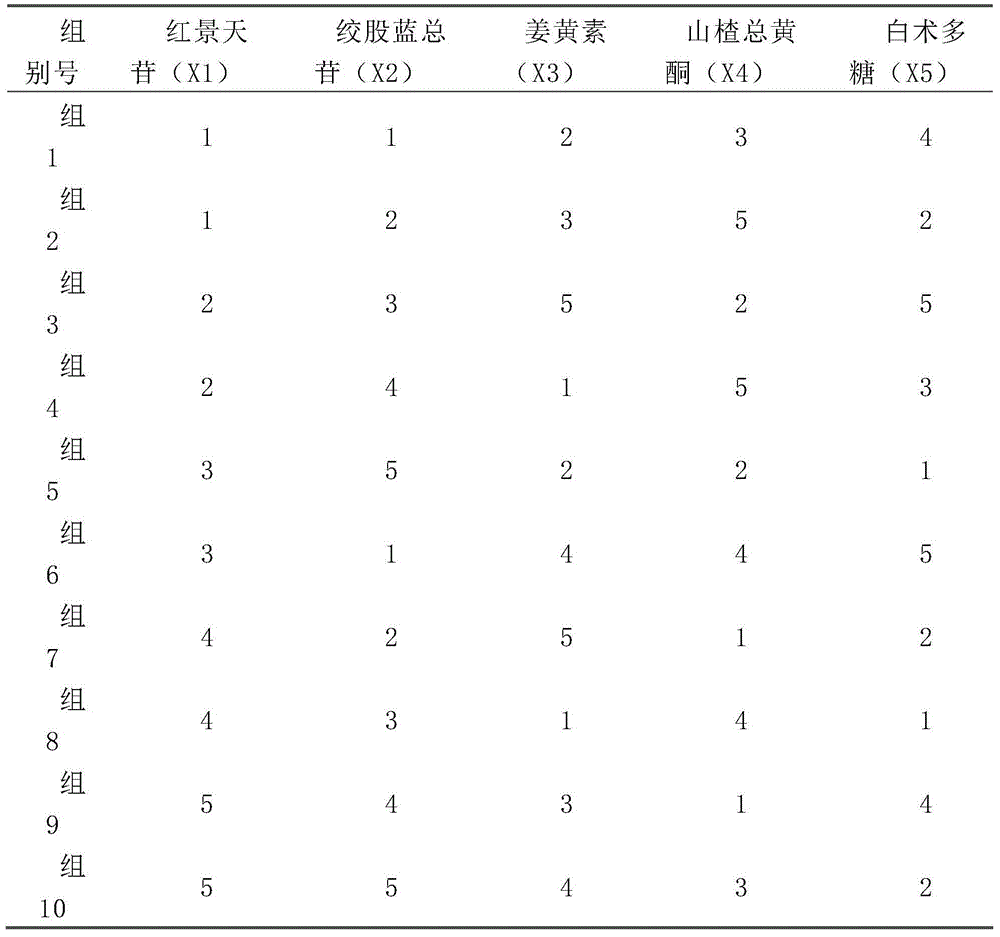
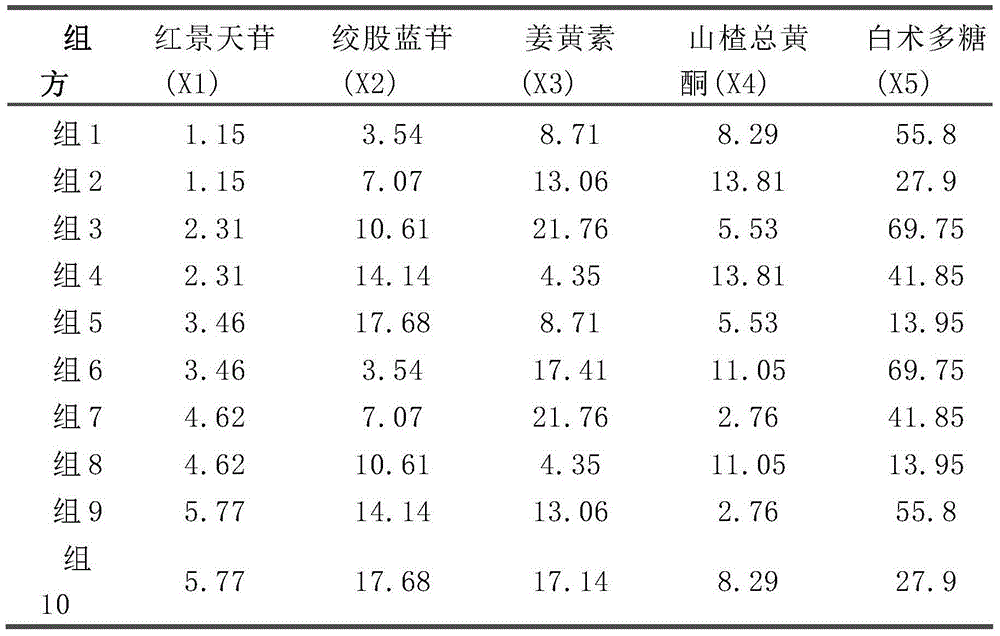
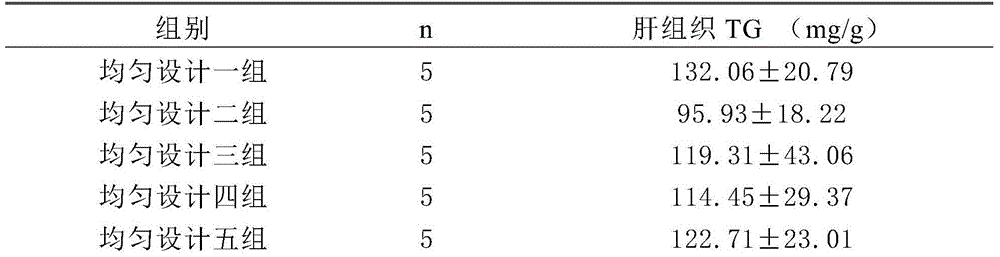
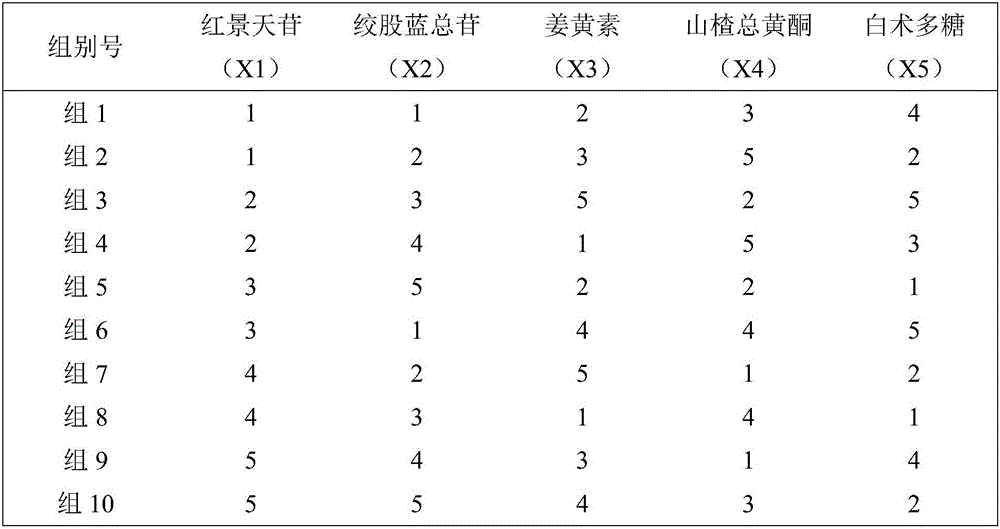
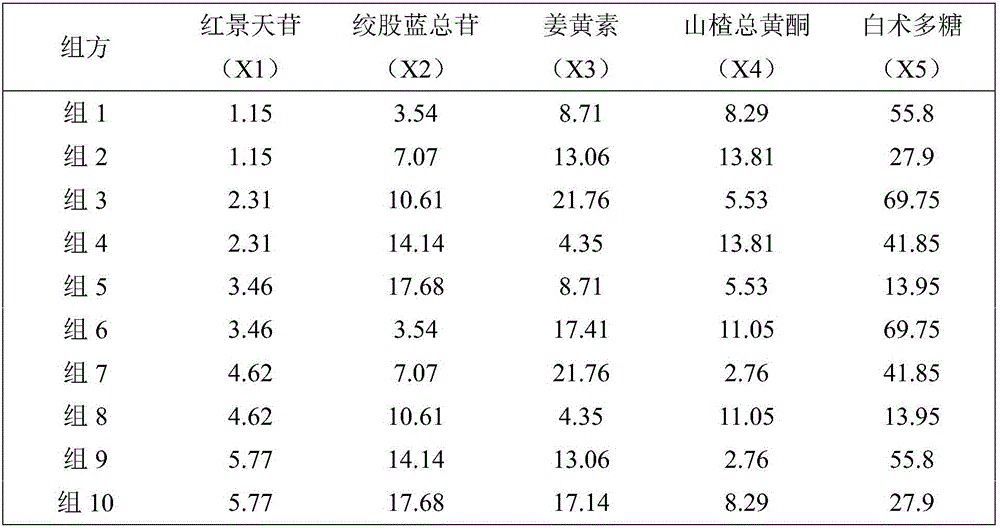
Traditional Chinese medicine effective component compound for preventing and treating fatty liver
InactiveCN104606275AGood control effectActive substance is clearDigestive systemKetone active ingredientsSalidrosidePolyene phosphatidylcholine
Disclosed is a traditional Chinese medicine effective component compound for preventing and treating fatty liver. The traditional Chinese medicine effective component compound is characterized in comprising salidroside, gypenosides, curcumin and bighead atractylodes rhizome polysaccharide, wherein the ratio of each component is 4-10 parts of salidroside, 15-20 parts of gypenosides, 3-8 parts of curcumin and 65-75 parts of bighead atractylodes rhizome polysaccharide. Dosage forms of the traditional Chinese medicine effective component compound for preventing and treating fatty liver comprise tablets, capsules, pills, oral liquid or suspensions. The traditional Chinese medicine effective component compound for preventing and treating fatty liver in the invention is safe and effective, has definite active substances, and has more significant effect on preventing and treating fatty liver compared with a component compound containing a single component and Western medicine of polyene phosphatidylcholine capsule.
Owner:NINGBO NO 2 HOSPITAL
Preparation method for high-purity polyene phosphatidylcholine
ActiveCN108101938AReduce dosageGrow fastGroup 5/15 element organic compoundsPhosphatide foodstuff compositionsPolyene phosphatidylcholineSilica gel
The invention discloses a preparation method for high-purity polyene phosphatidylcholine. According to the method, high-purity polyene phosphatidylcholine is prepared by subjecting soyabean lecithin powder to adsorption by neutral alumina and silica gel respectively. Polyene phosphatidylcholine prepared by using the method is low in peroxide value and high in purity; the preparation method is simple in process operation and beneficial for industrial production; and the purity and the content of polyene phosphatidylcholine can reach 98% or more and 95% or more, respectively, and all the relatedsubstances are qualified.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A traditional Chinese medicine effective component composition for preventing and treating fatty liver
InactiveCN104606275BGood control effectActive substance is clearDigestive systemKetone active ingredientsWestern medicinePolyene phosphatidylcholine
Disclosed is a traditional Chinese medicine effective component compound for preventing and treating fatty liver. The traditional Chinese medicine effective component compound is characterized in comprising salidroside, gypenosides, curcumin and bighead atractylodes rhizome polysaccharide, wherein the ratio of each component is 4-10 parts of salidroside, 15-20 parts of gypenosides, 3-8 parts of curcumin and 65-75 parts of bighead atractylodes rhizome polysaccharide. Dosage forms of the traditional Chinese medicine effective component compound for preventing and treating fatty liver comprise tablets, capsules, pills, oral liquid or suspensions. The traditional Chinese medicine effective component compound for preventing and treating fatty liver in the invention is safe and effective, has definite active substances, and has more significant effect on preventing and treating fatty liver compared with a component compound containing a single component and Western medicine of polyene phosphatidylcholine capsule.
Owner:NINGBO NO 2 HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com