Preparation method of oral solid preparation containing polyene phosphatidyl choline
A technology of polyene phosphatidylcholine and solid preparation, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, and pill delivery, etc., to facilitate the detection of finished product loading, improve the yield of finished products and the accuracy of quality. , the effect of facilitating the intermediate quantitative control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dosage form: drop pills, specification 76mg / grain (calculated as polyene phosphatidylcholine)
[0021] Put the polyene phosphatidylcholine in a low-temperature pulverizer, pulverize it to 100 mesh at a temperature of -60°C to -70°C, and sieve it; take 326g of polyene phosphatidylcholine and 33g of folic acid, and then granulate it under nitrogen. granules, dried under reduced pressure at 15°C and 0.01MPa; take the dry matter and add it to molten 977g stearic acid, stir quickly until the mixture is uniform, drip, cool, and wash the pills to obtain 3000 drop pills, each drop pill Contents weigh approximately 0.45g.
Embodiment 2
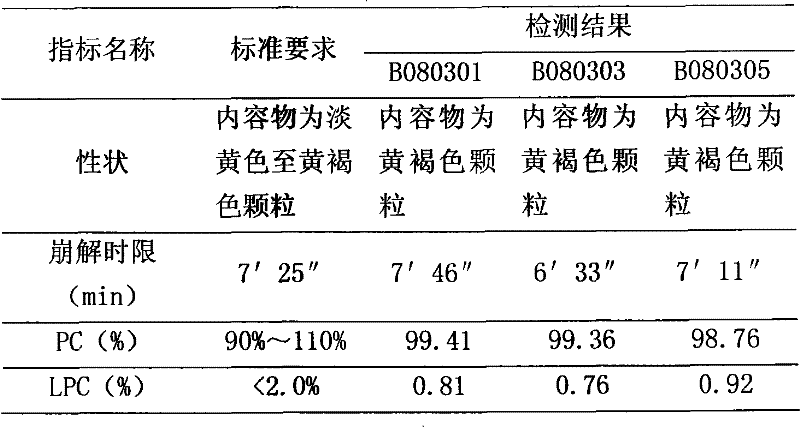
[0023] Dosage form: capsule, specification: 57mg / capsule (calculated as polyene phosphatidylcholine)
[0024] Put the polyene phosphatidylcholine in a low-temperature pulverizer, grind it to 100 mesh at a temperature of -40°C to -50°C, and sieve it; take 326g of polyene phosphatidylcholine and mix it with 33g of vitamin C, and then mix it with 1629g of starch, Microcrystalline cellulose 1303g, mix in equal increments, detect the content of intermediates, measure the bulk density, pack in capsules or enteric-coated capsules, make 4000 capsules in total, and the content of each capsule weighs about 0.8g.
Embodiment 3
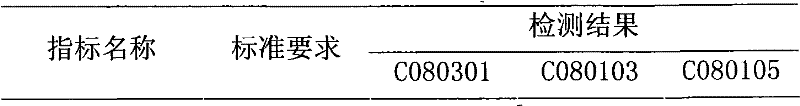
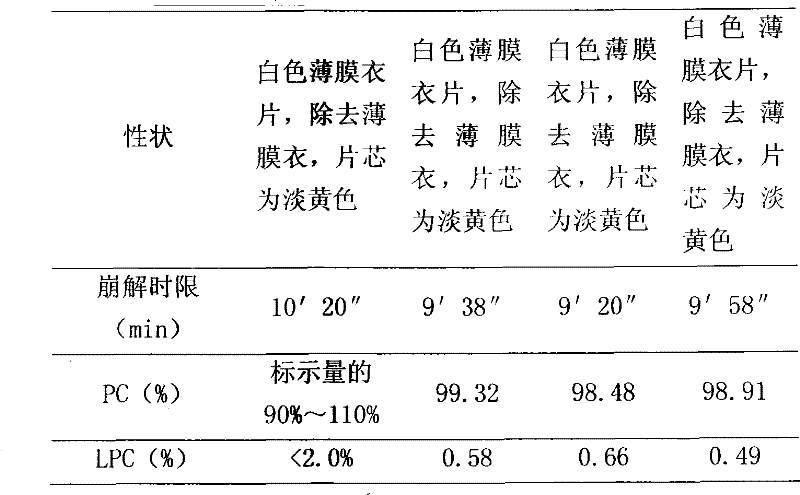
[0026] Dosage form: tablet, specification: 76mg / tablet (calculated as polyene phosphatidylcholine)
[0027] Put the polyene phosphatidylcholine in a low-temperature pulverizer, pulverize it to 100 mesh at a temperature of -70~-80°C, and sieve it; take 326g of polyene phosphatidylcholine and 33g of folic acid, and mix them with 977g of pregelatinized starch And carboxymethyl starch sodium 326g, mix by equal increment method, granulate, granulate, 20 ℃, 0.08MPa decompression drying 3 hours, tabletting, film-coating, make 3000 tablets altogether. Each piece weighs about 0.56g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com