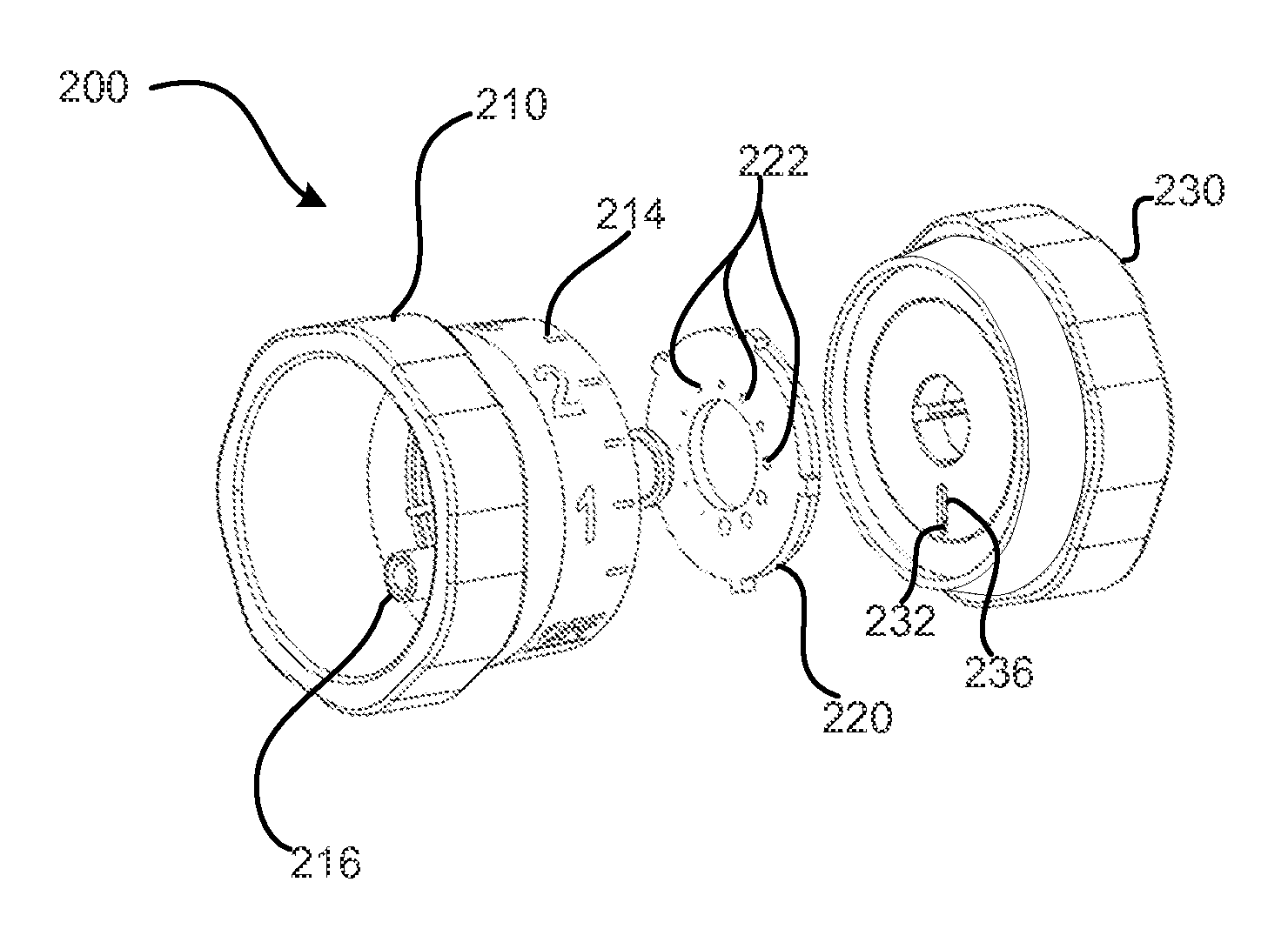
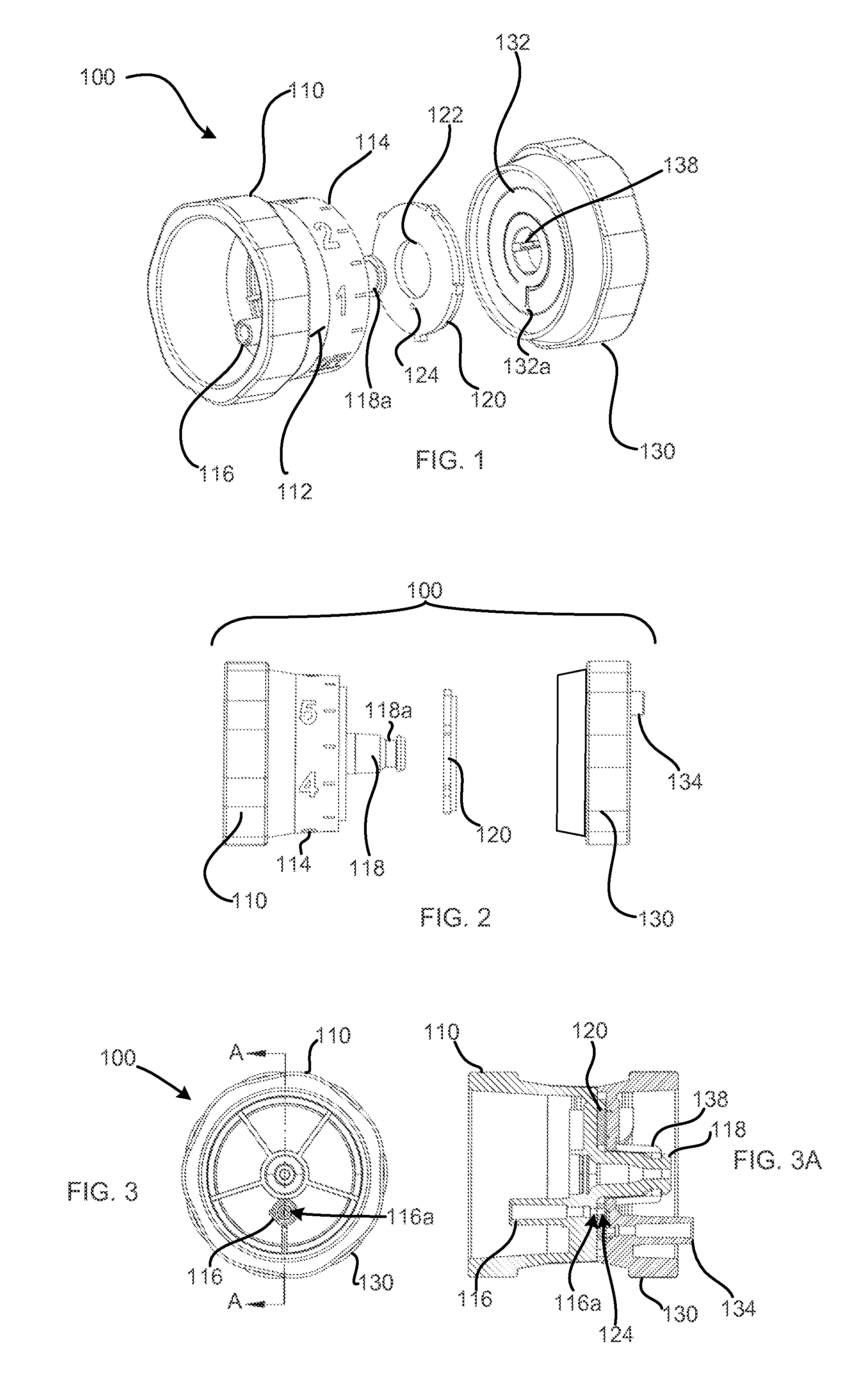
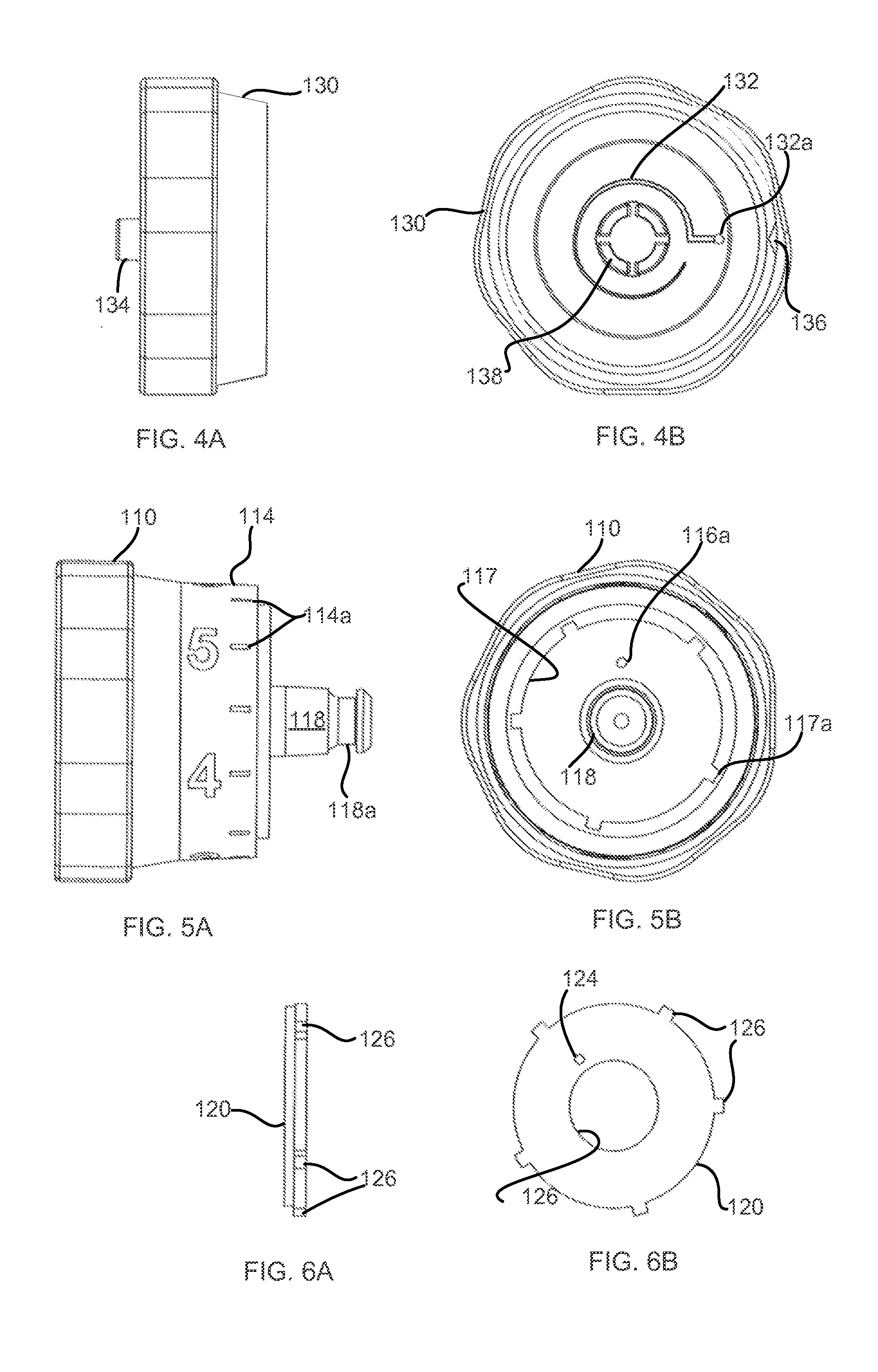
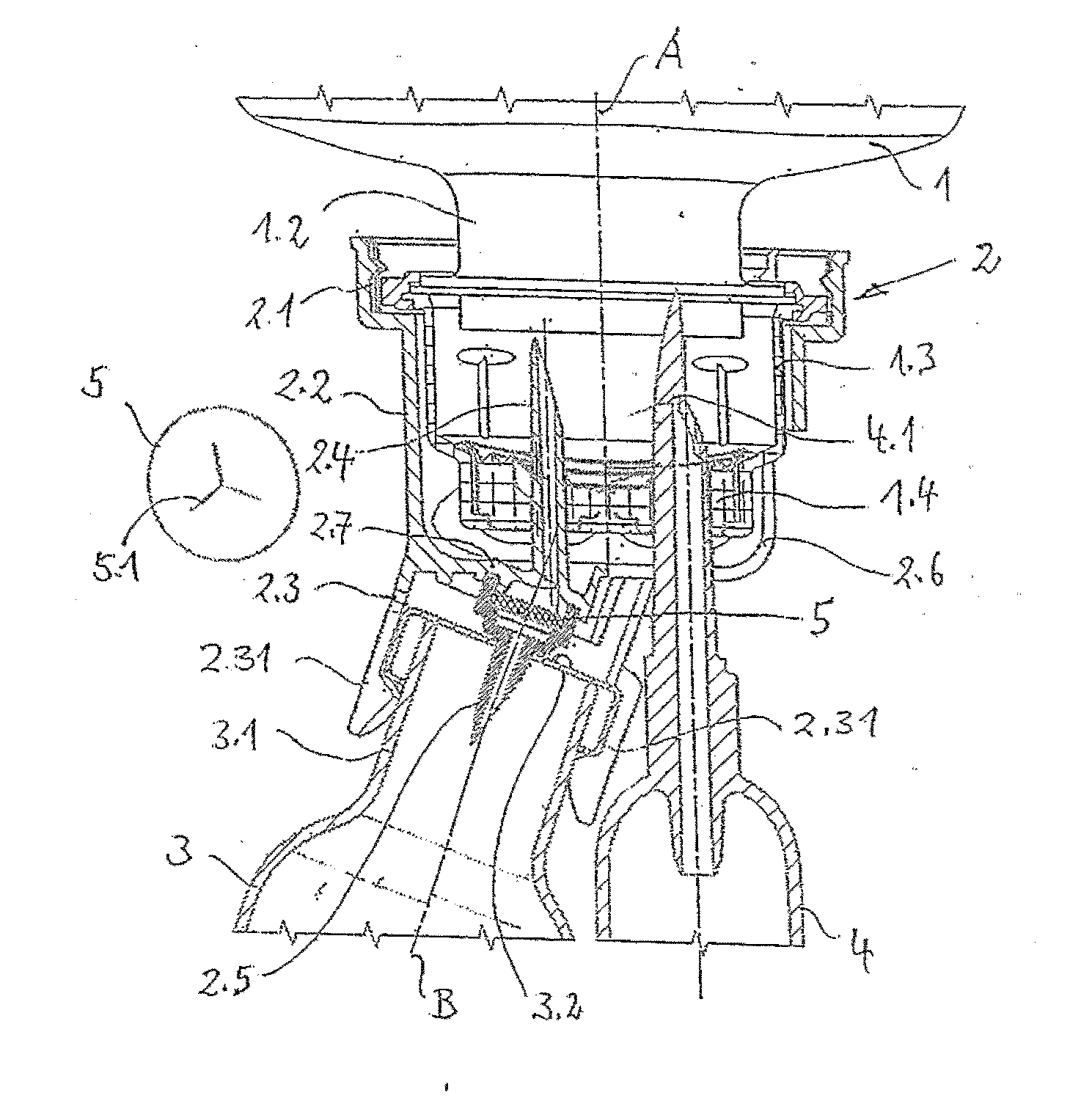
Patents
Literature
2206 results about "Infusion solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
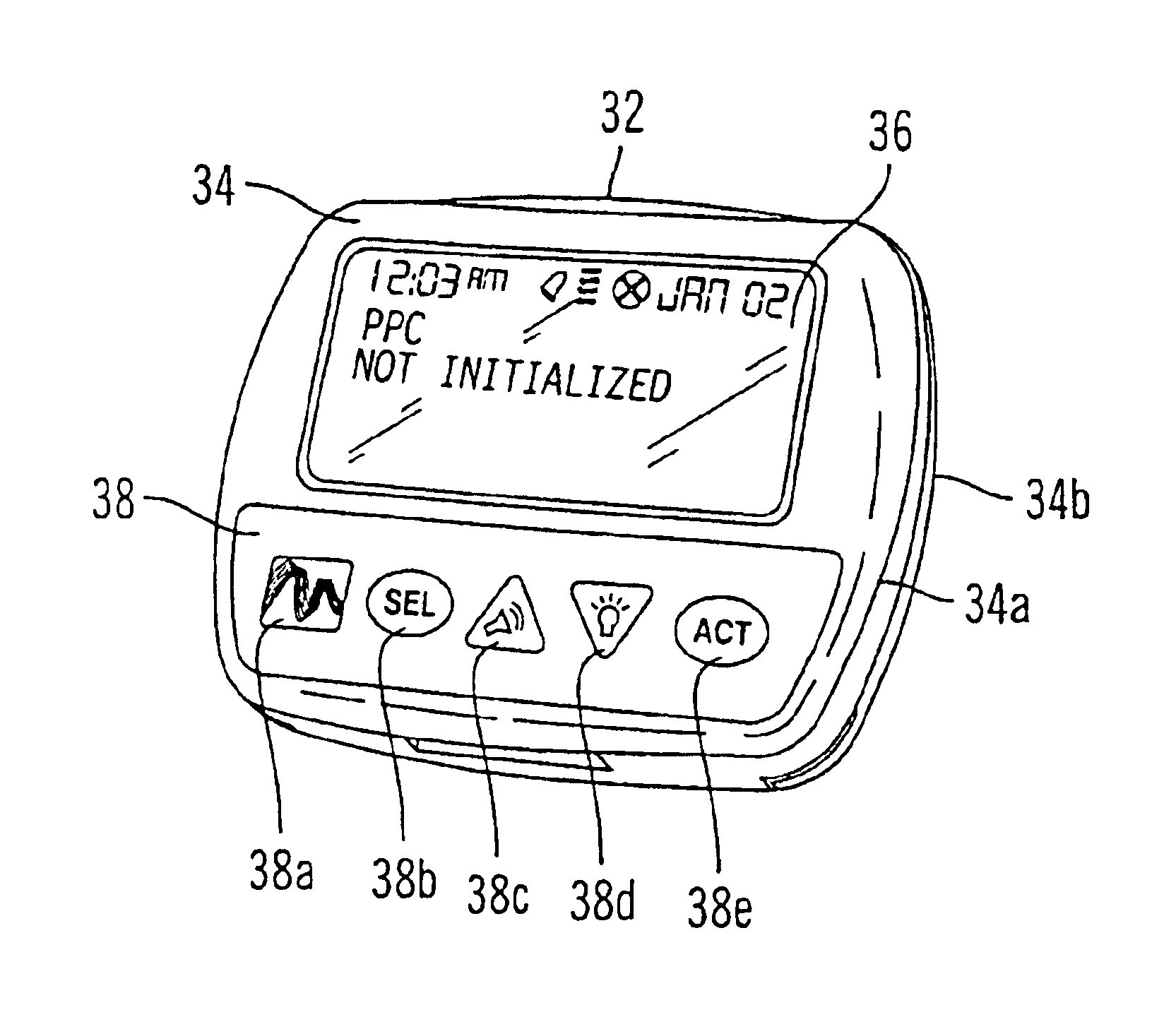
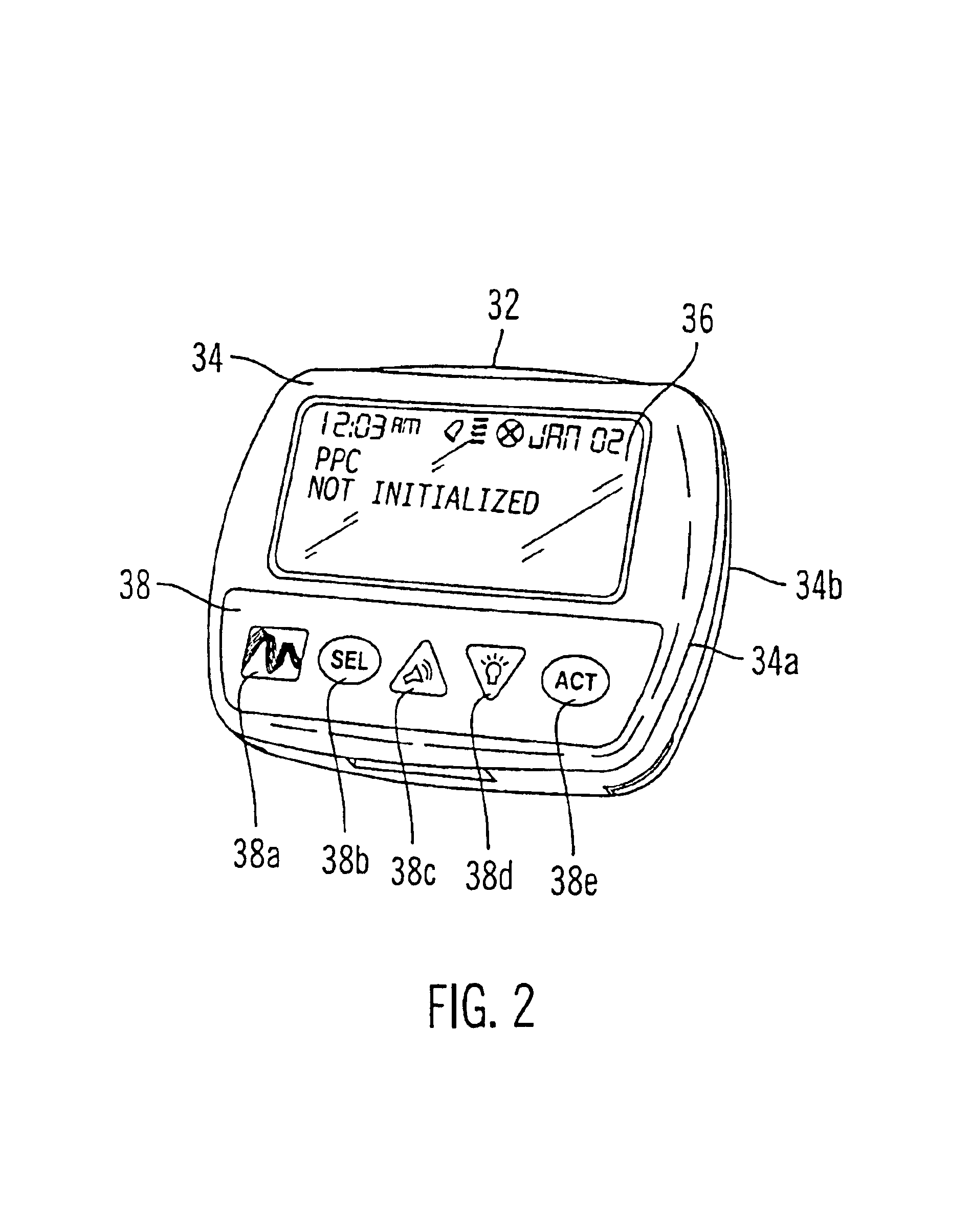
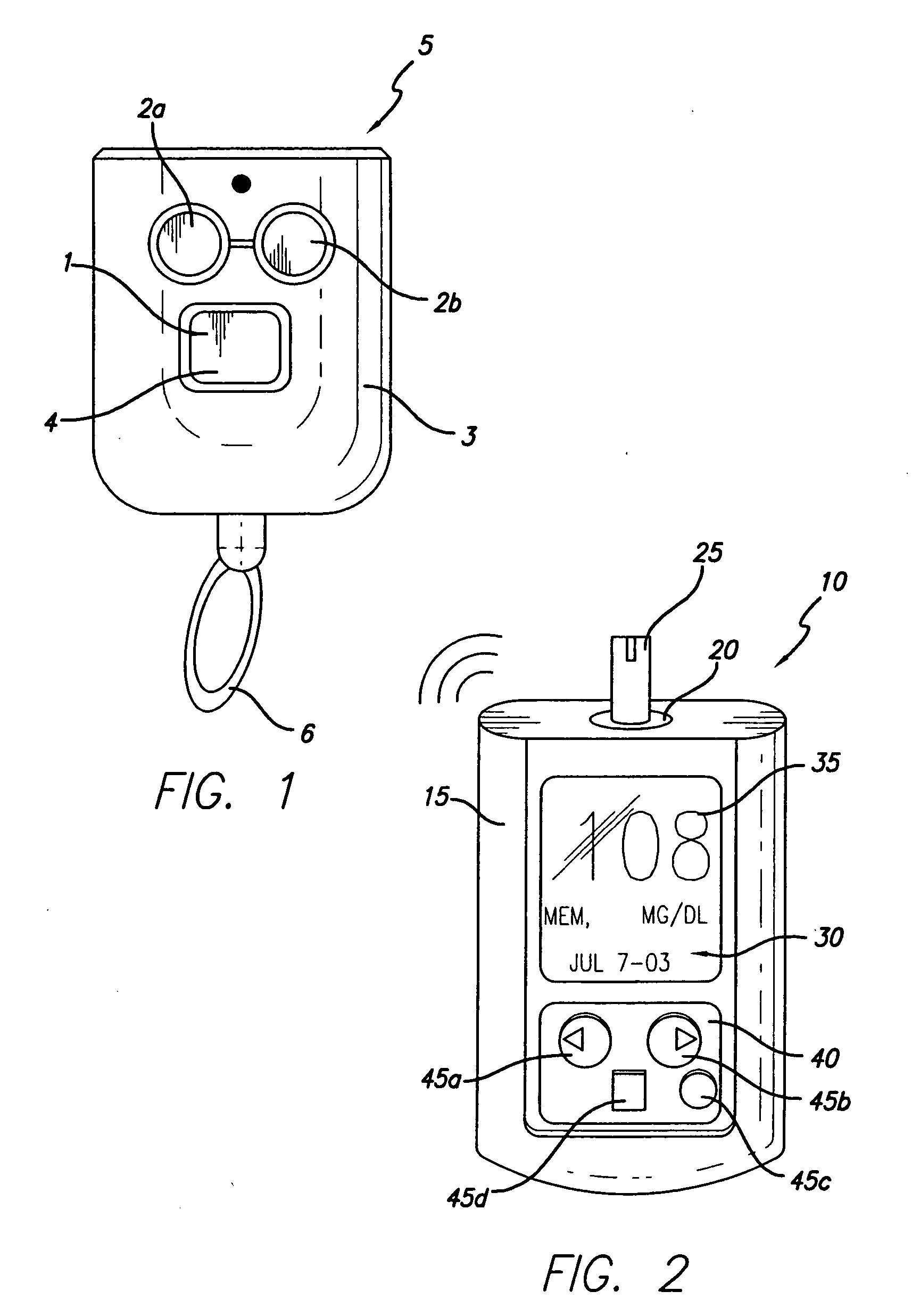
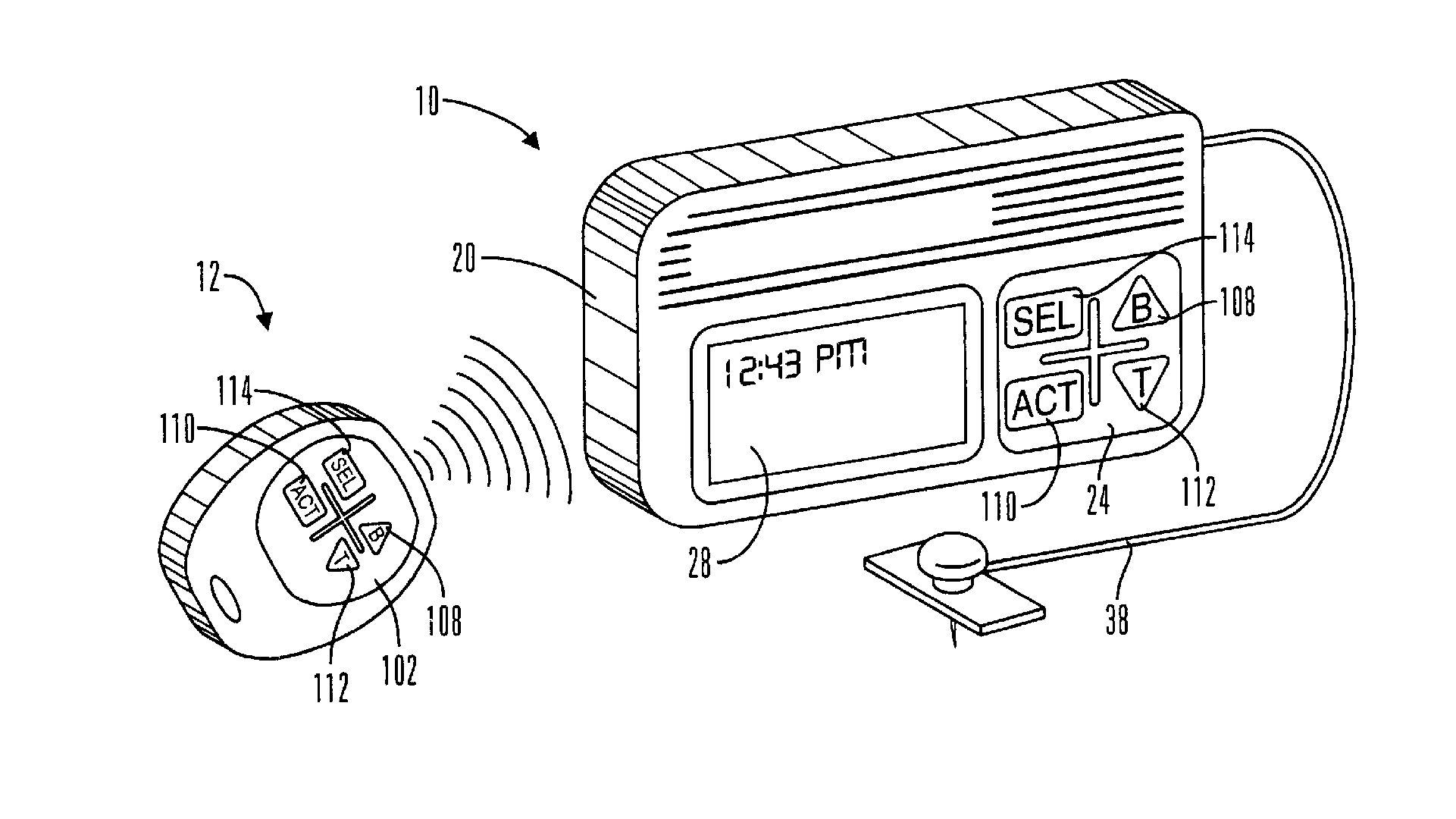
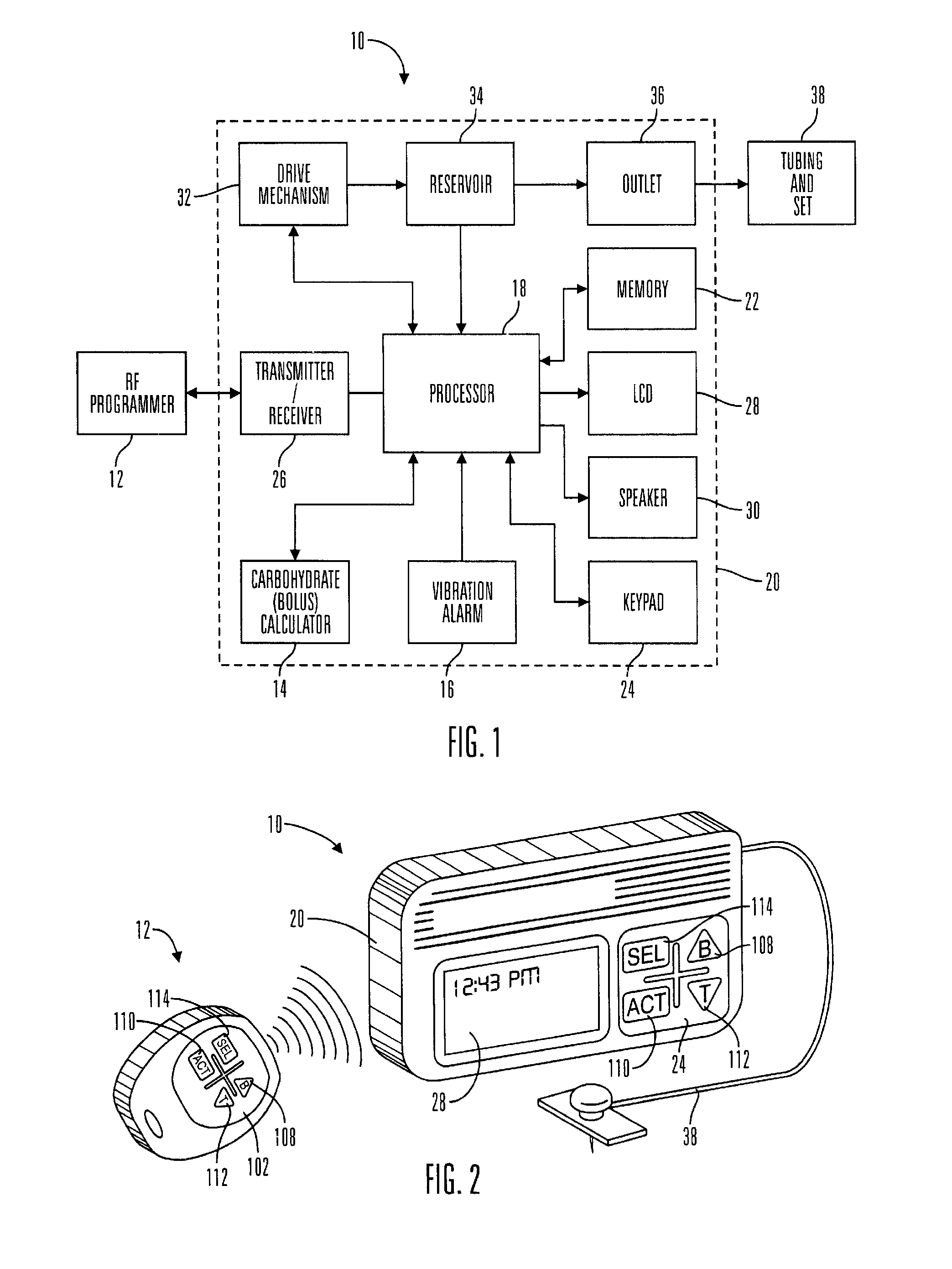
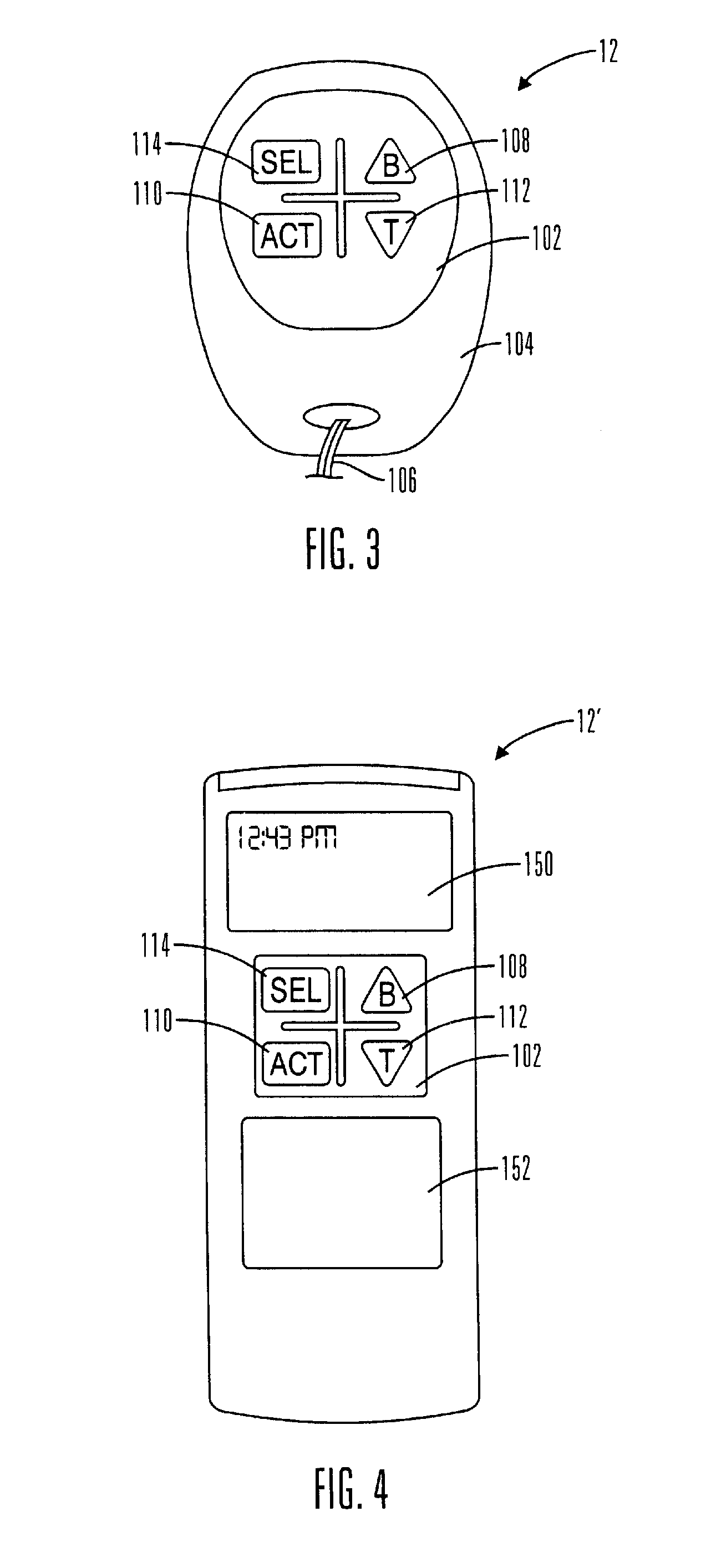
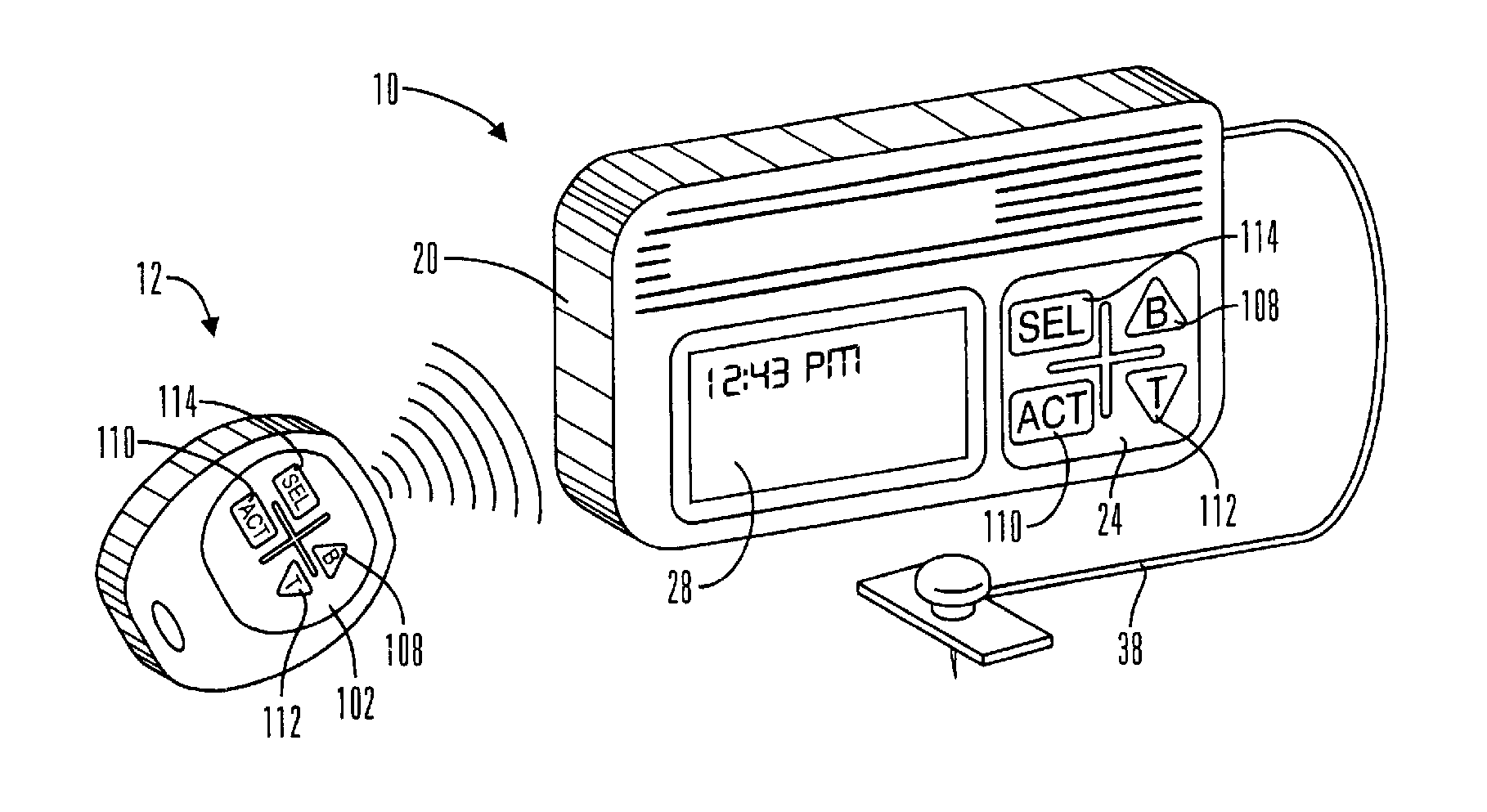
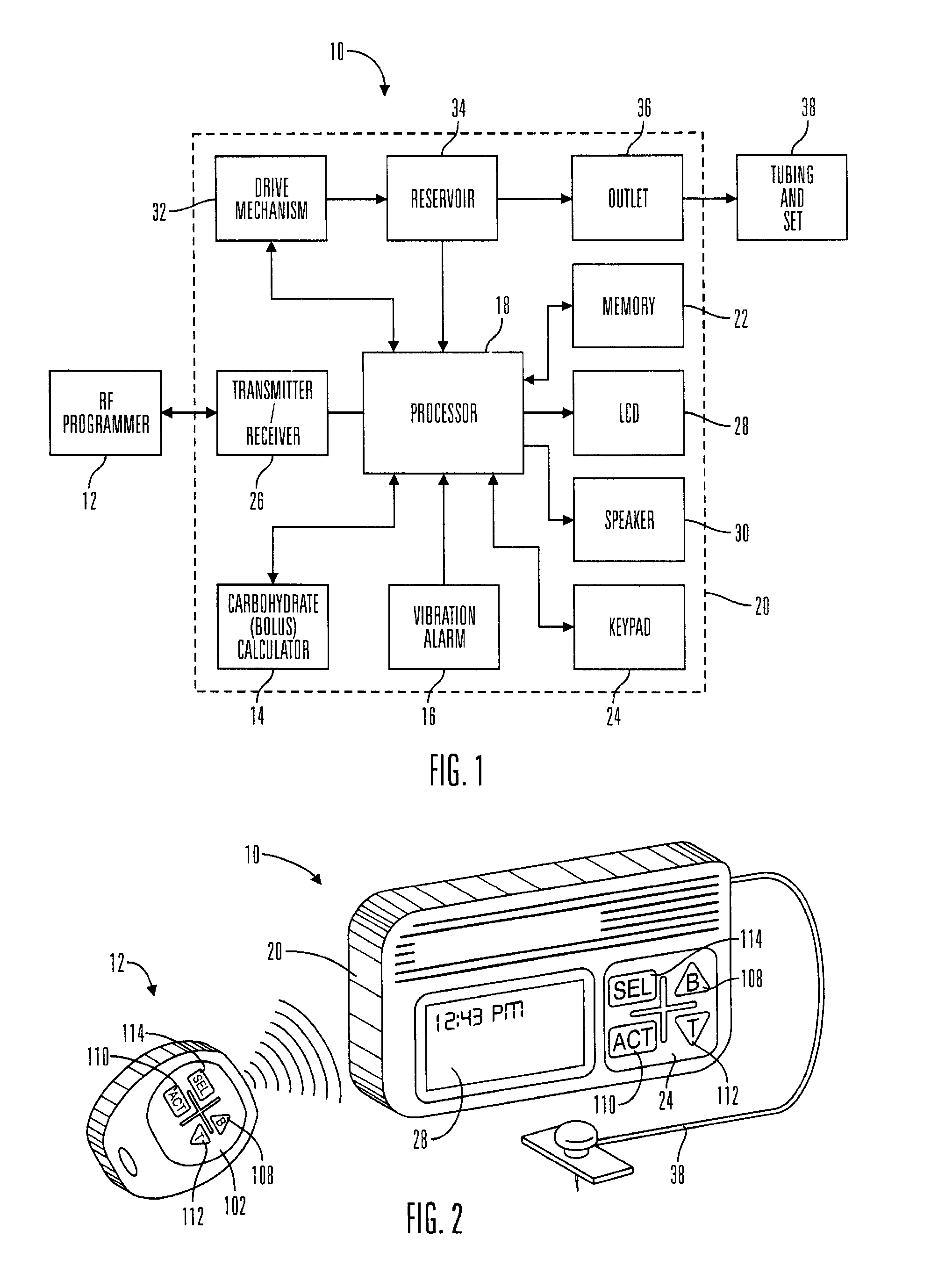
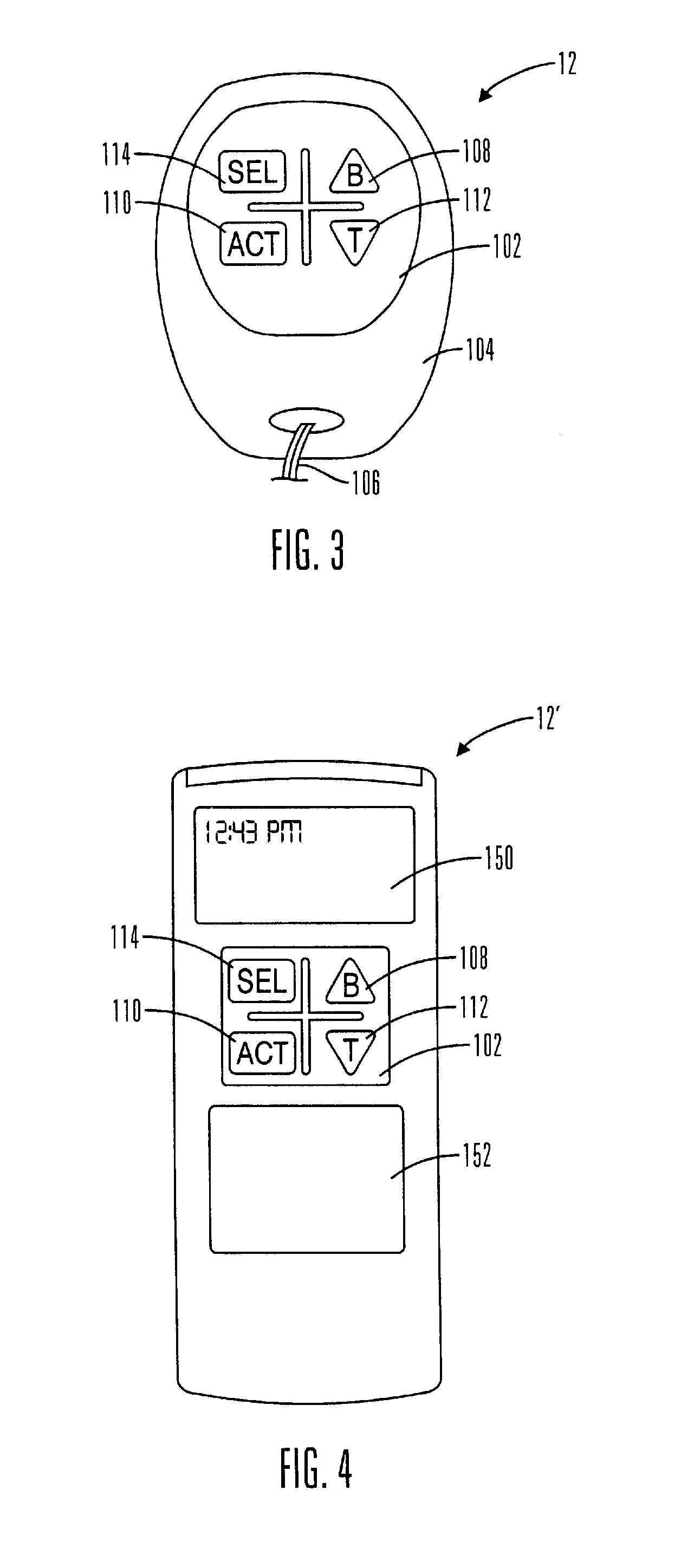
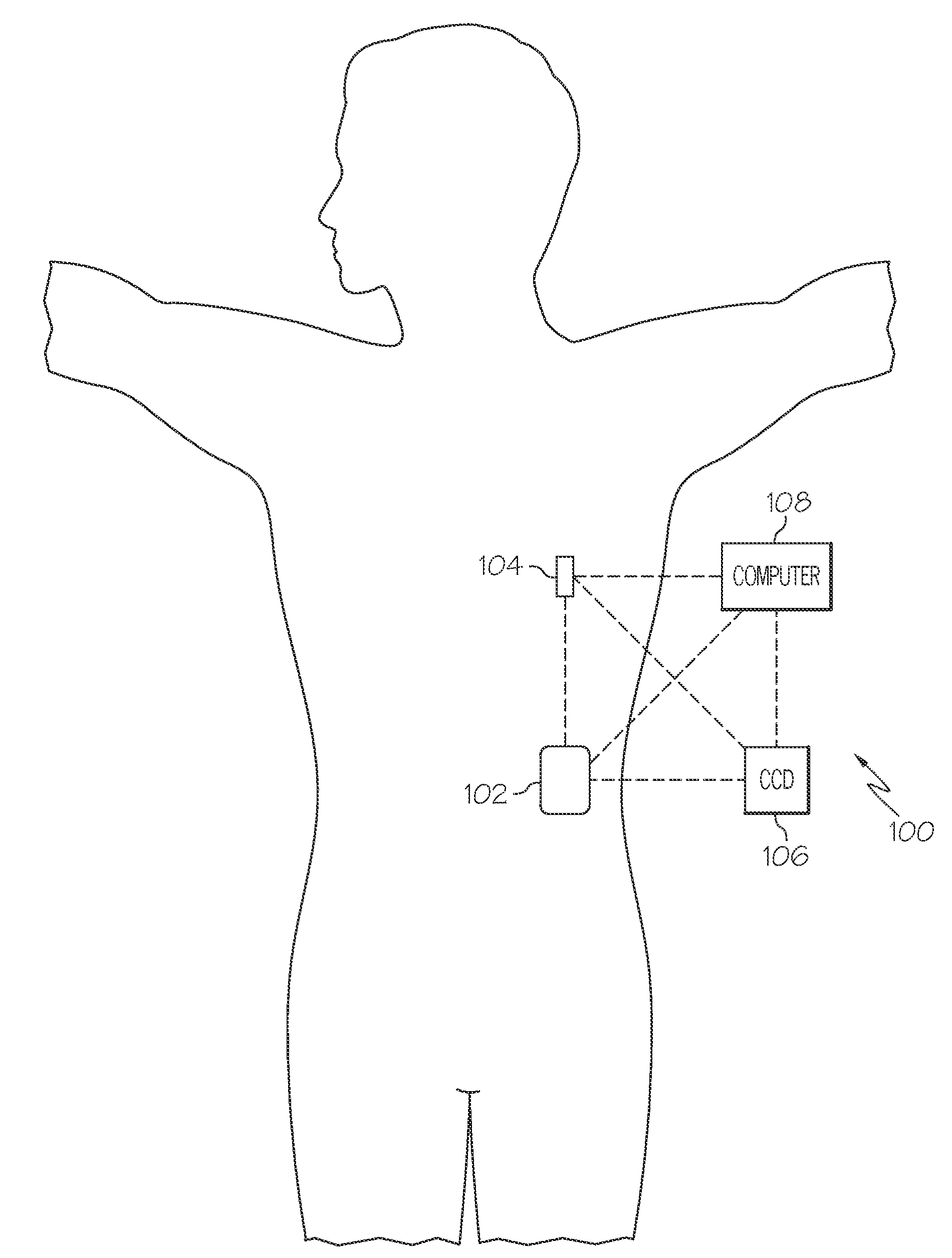
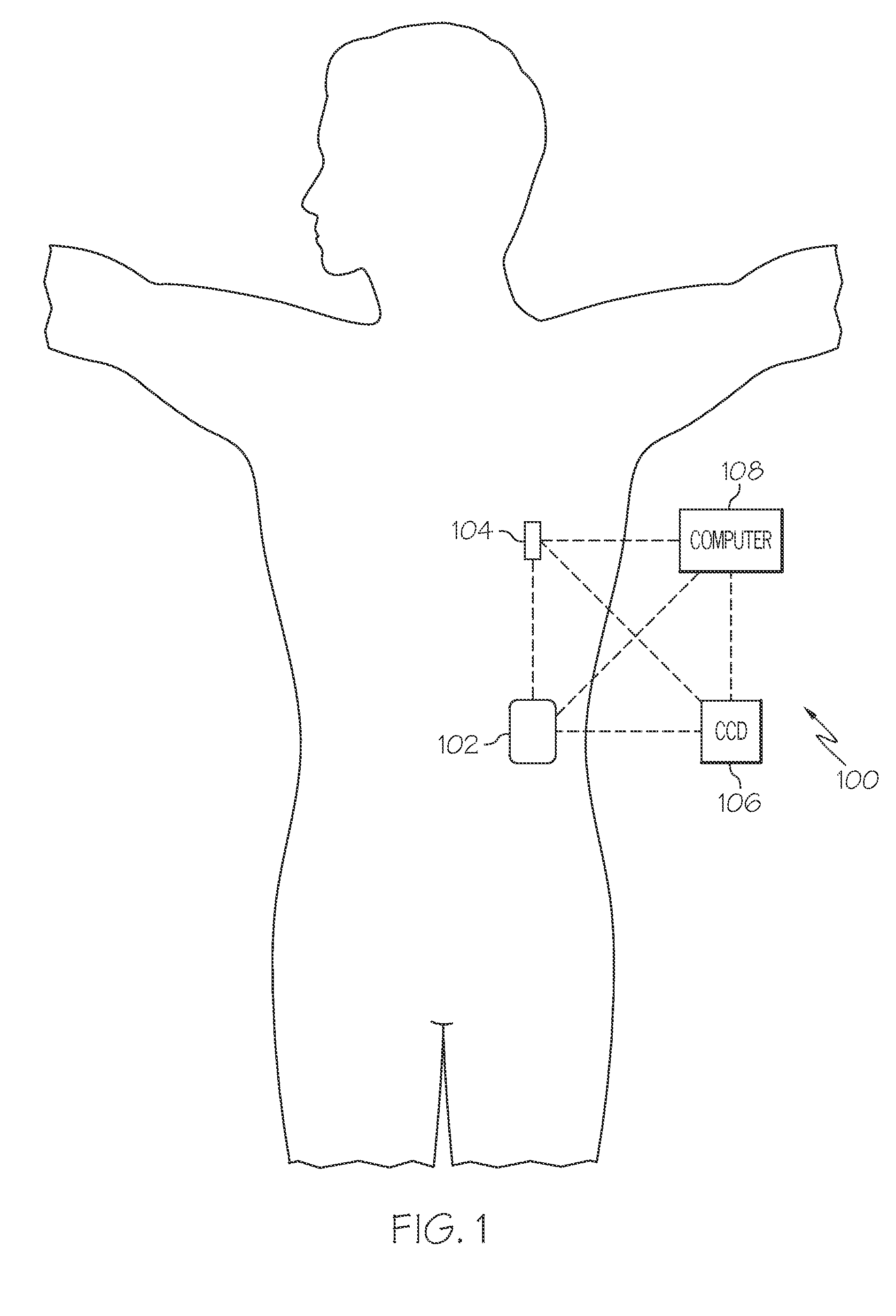
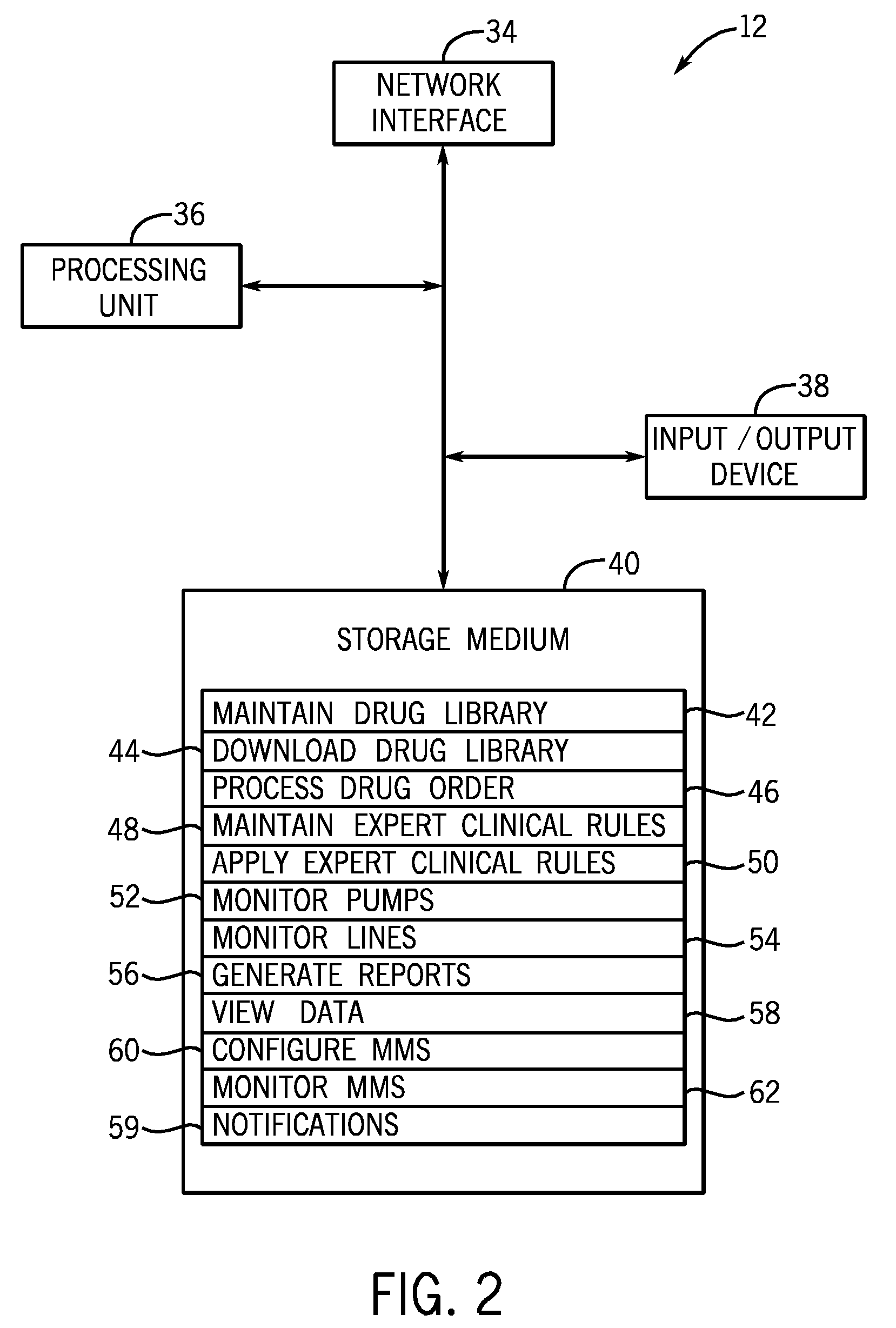
Microprocessor controlled ambulatory medical apparatus with hand held communication device
InactiveUS6873268B2Enhance user interfaceReduce system sizeEnergy efficient ICTElectrotherapyDrugs infusionHand held
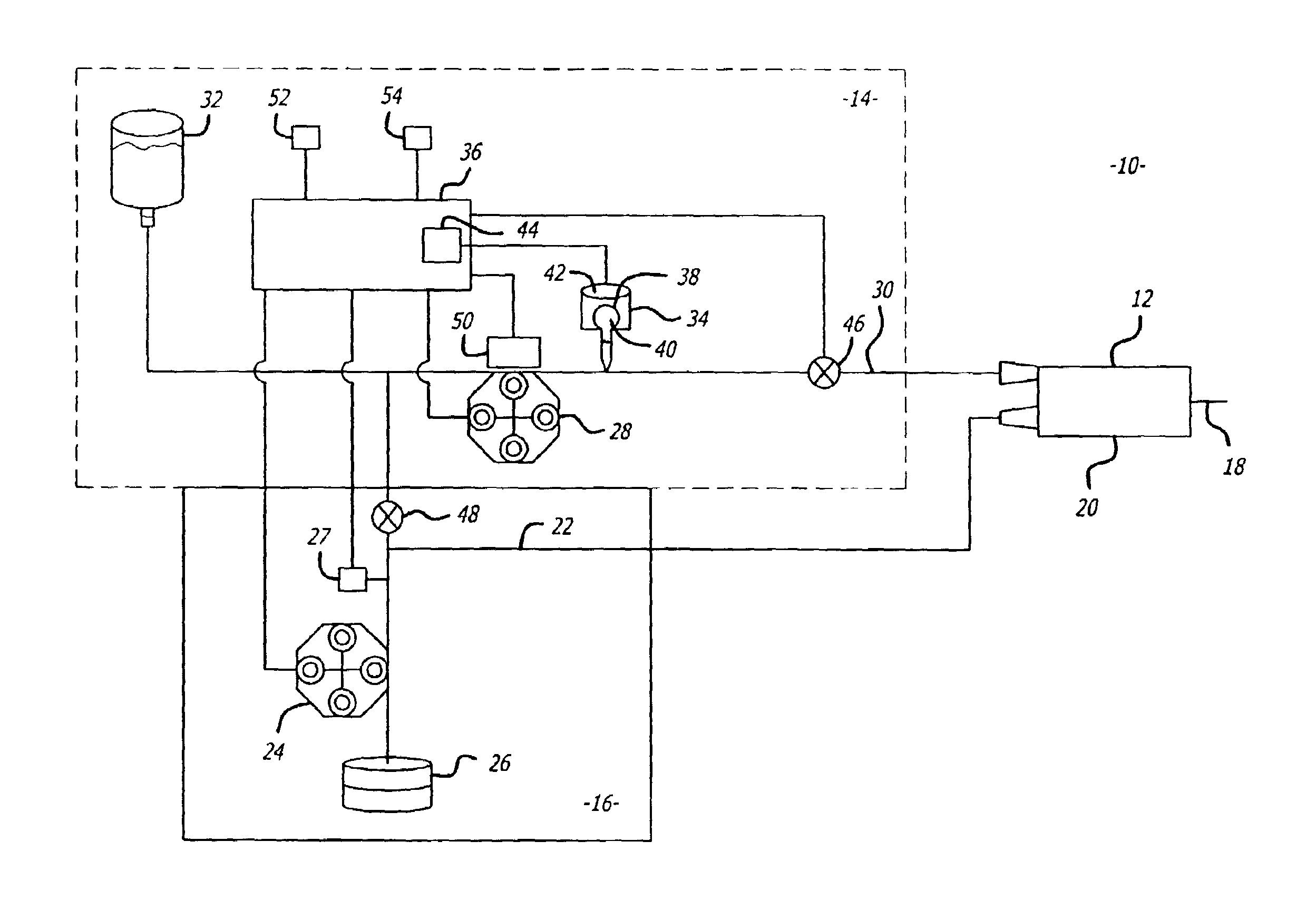
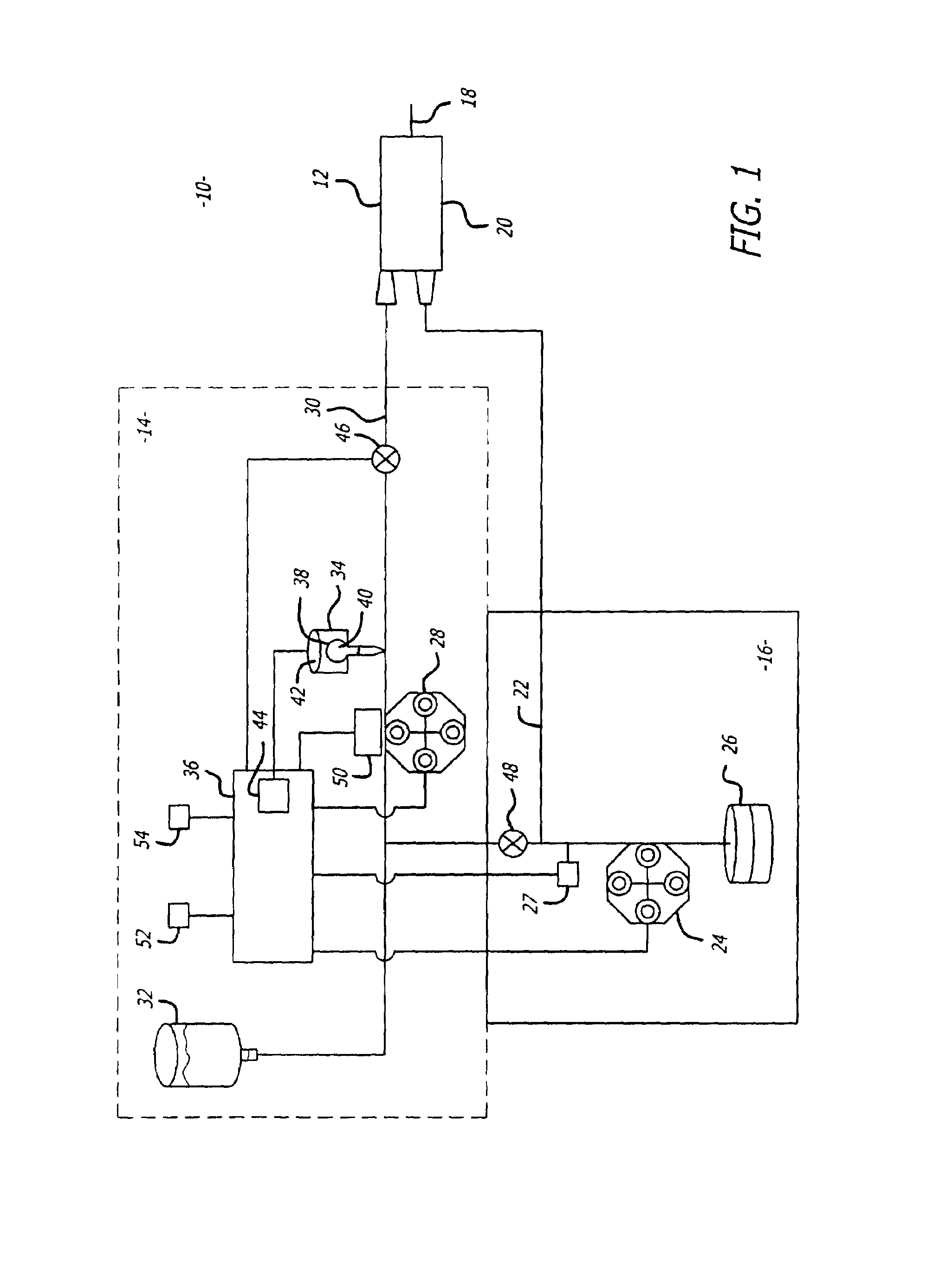
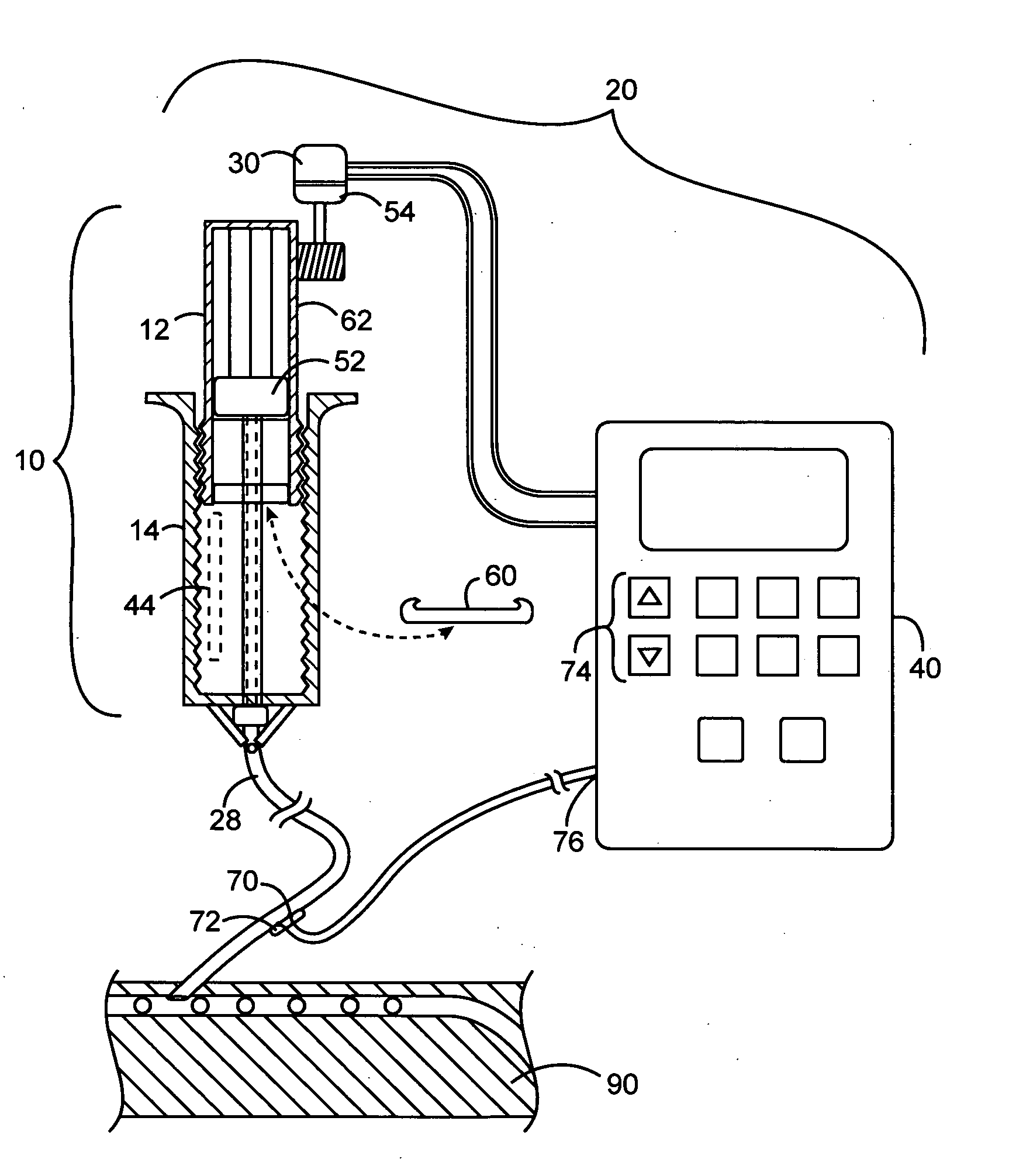
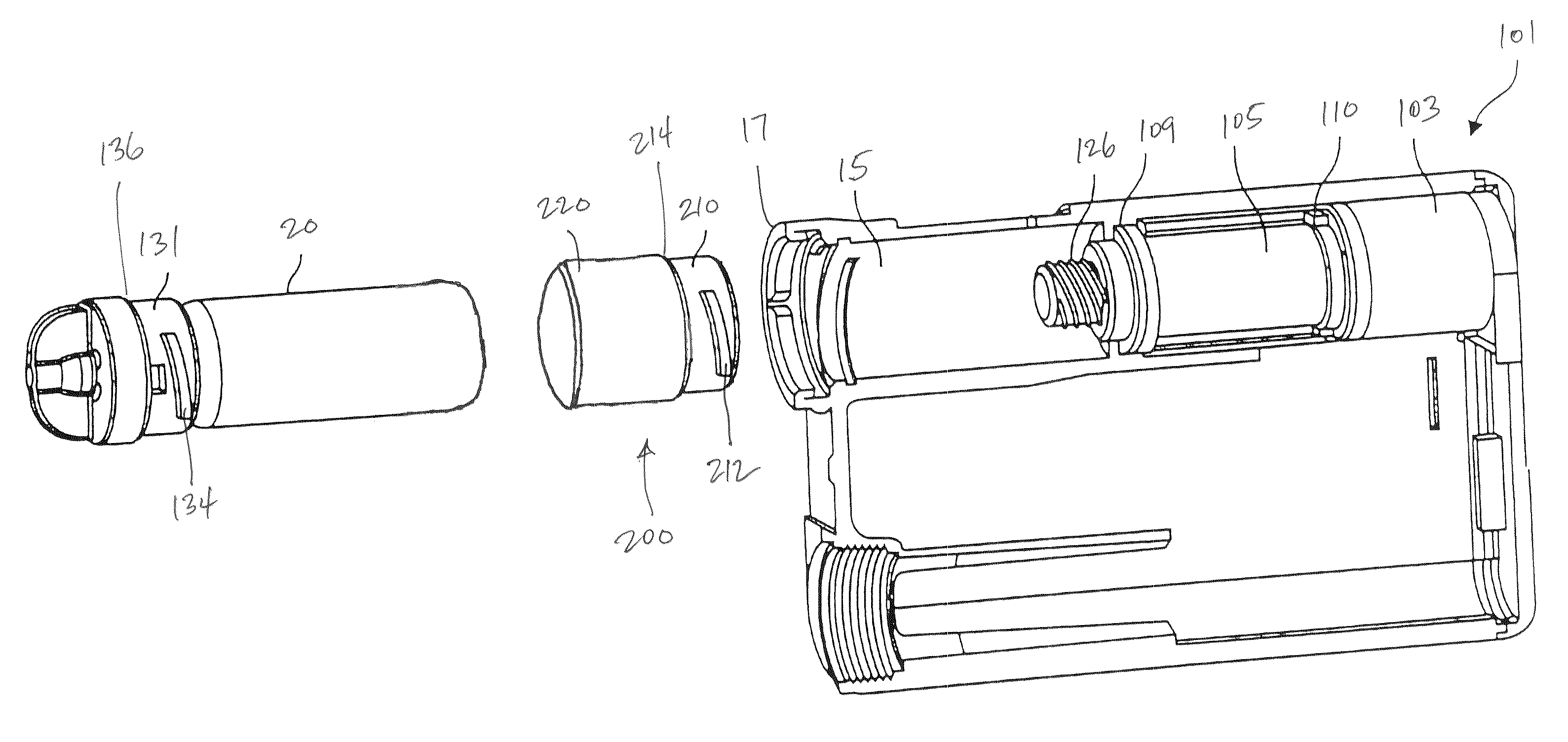
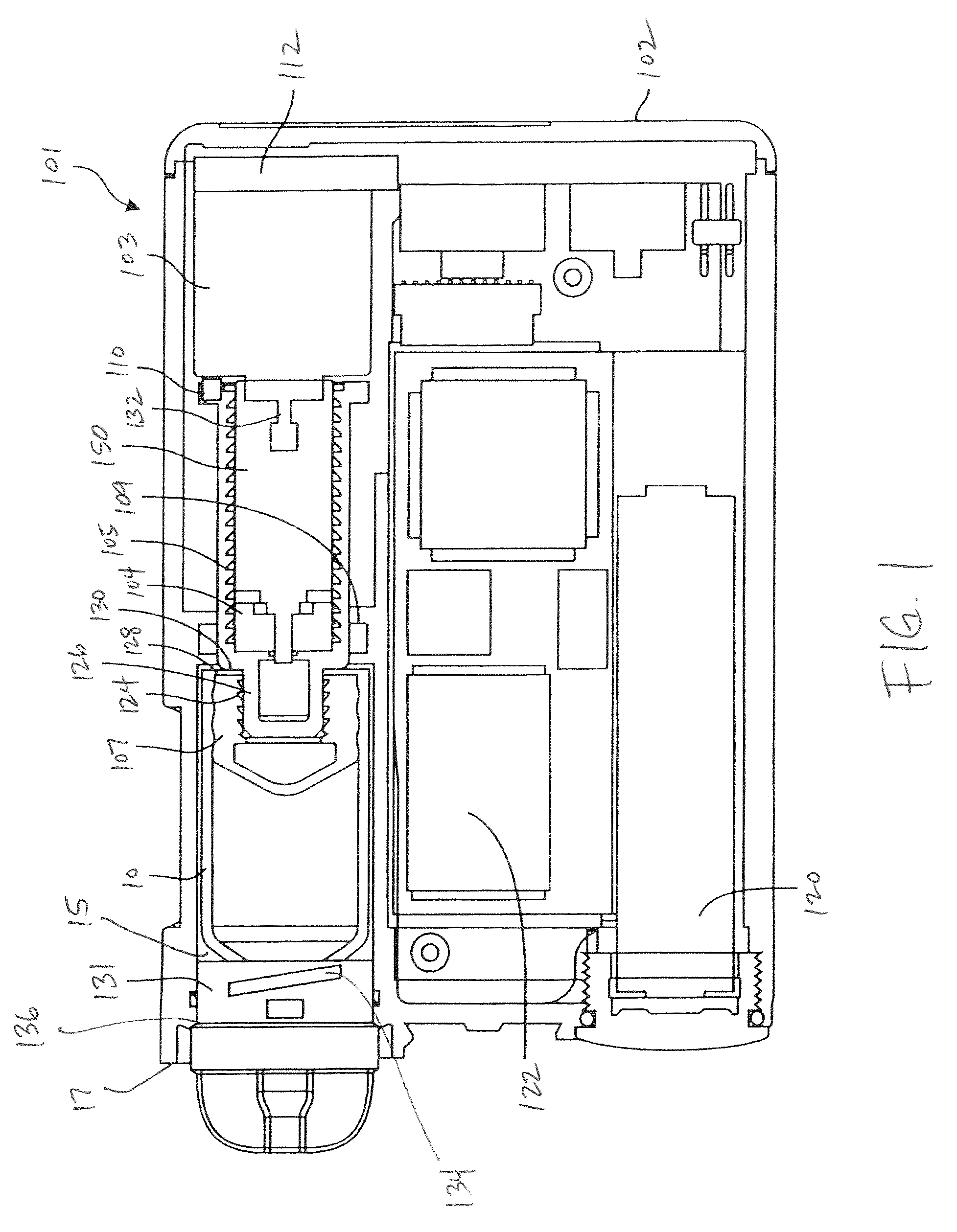
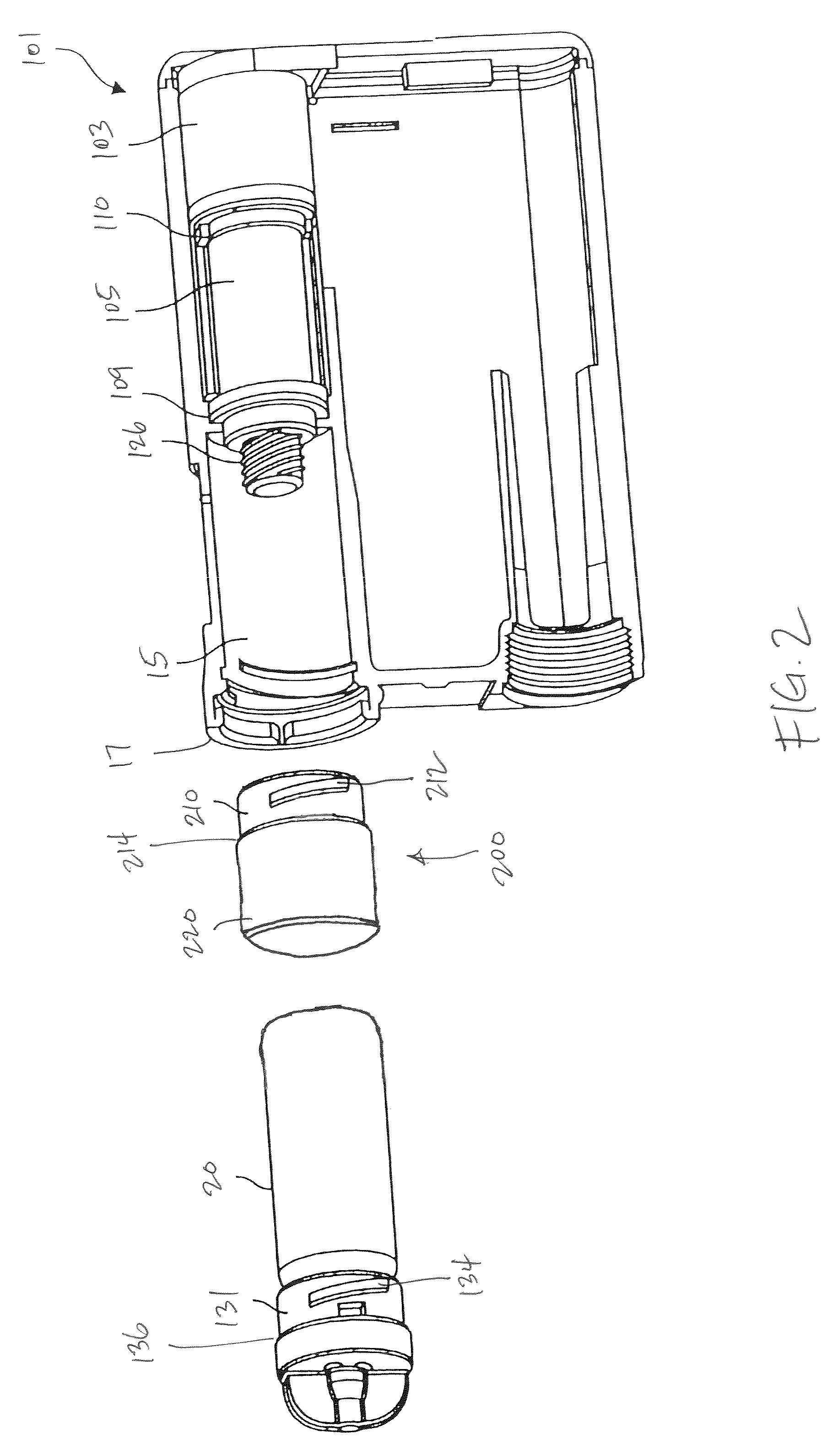
An implantable infusion pump possesses operational functionality that is, at least in part, controlled by software operating in two processor ICs which are configured to perform some different and some duplicate functions. The pump exchanges messages with an external device via telemetry. Each processor controls a different part of the drug infusion mechanism such that both processors must agree on the appropriateness of drug delivery for infusion to occur. Delivery accumulators are incremented and decremented with delivery requests and with deliveries made. When accumulated amounts reach or exceed, quantized deliverable amounts, infusion is made to occur. The accumulators are capable of being incremented by two or more independent types of delivery requests. Operational modes of the infusion device are changed automatically in view of various system errors that are trapped, various system alarm conditions that are detected, and when excess periods of time lapse between pump and external device interactions.
Owner:MEDTRONIC MIMIMED INC
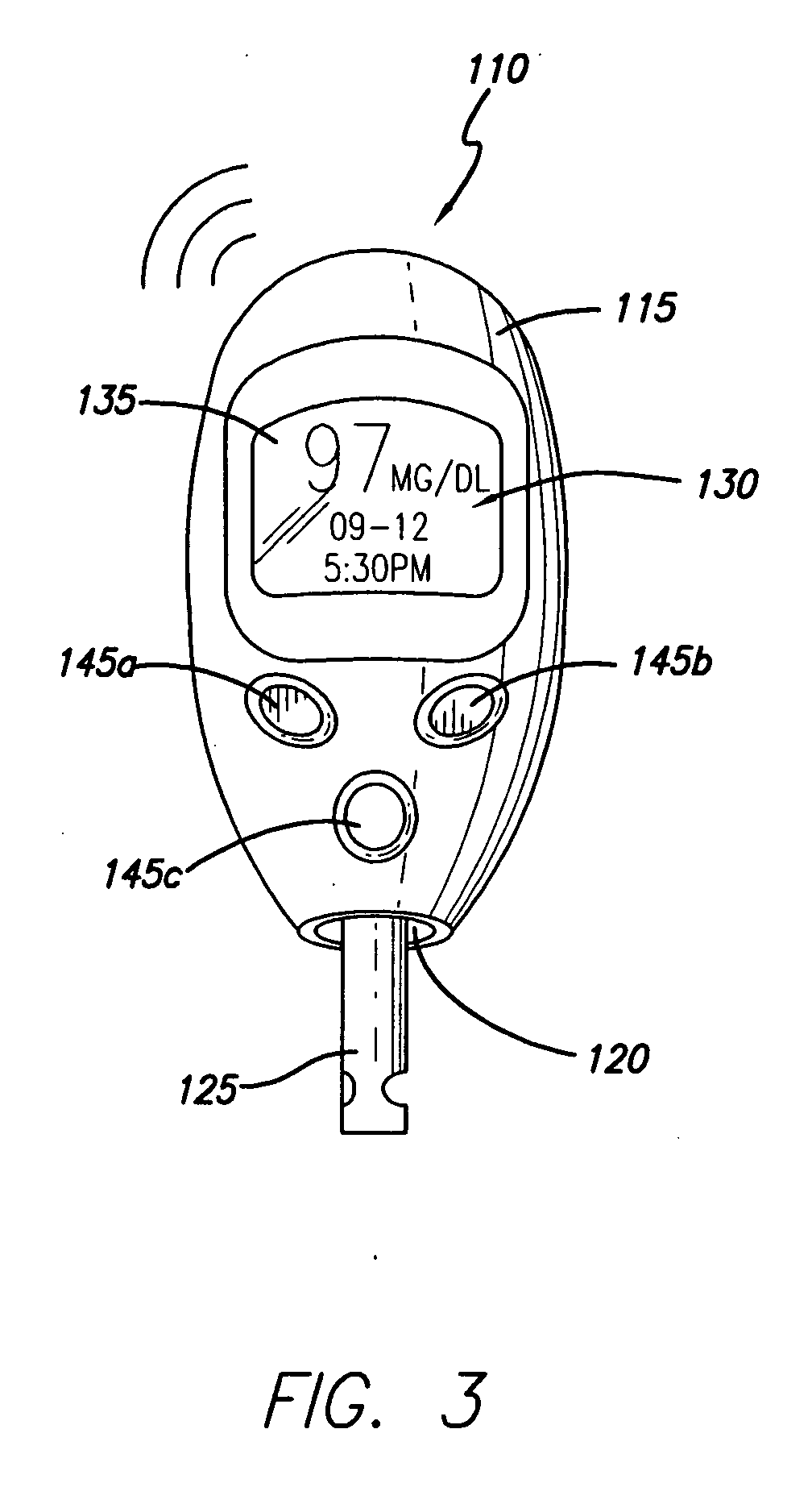
Watch controller for a medical device
InactiveUS20070093786A1Minimizes potential for errorEasy to watchMedical devicesPharmaceutical delivery mechanismCommunications systemMonitoring and control
An infusion system that includes a watch controller device and a communication system to transmit the communications from the watch controller device to an infusion device pump that controls delivery of fluids to the user's body. More particularly, these apparatuses and methods are for providing convenient monitoring and control of the infusion pump device in determining the appropriate amount of insulin to deliver.
Owner:MEDTRONIC MIMIMED INC
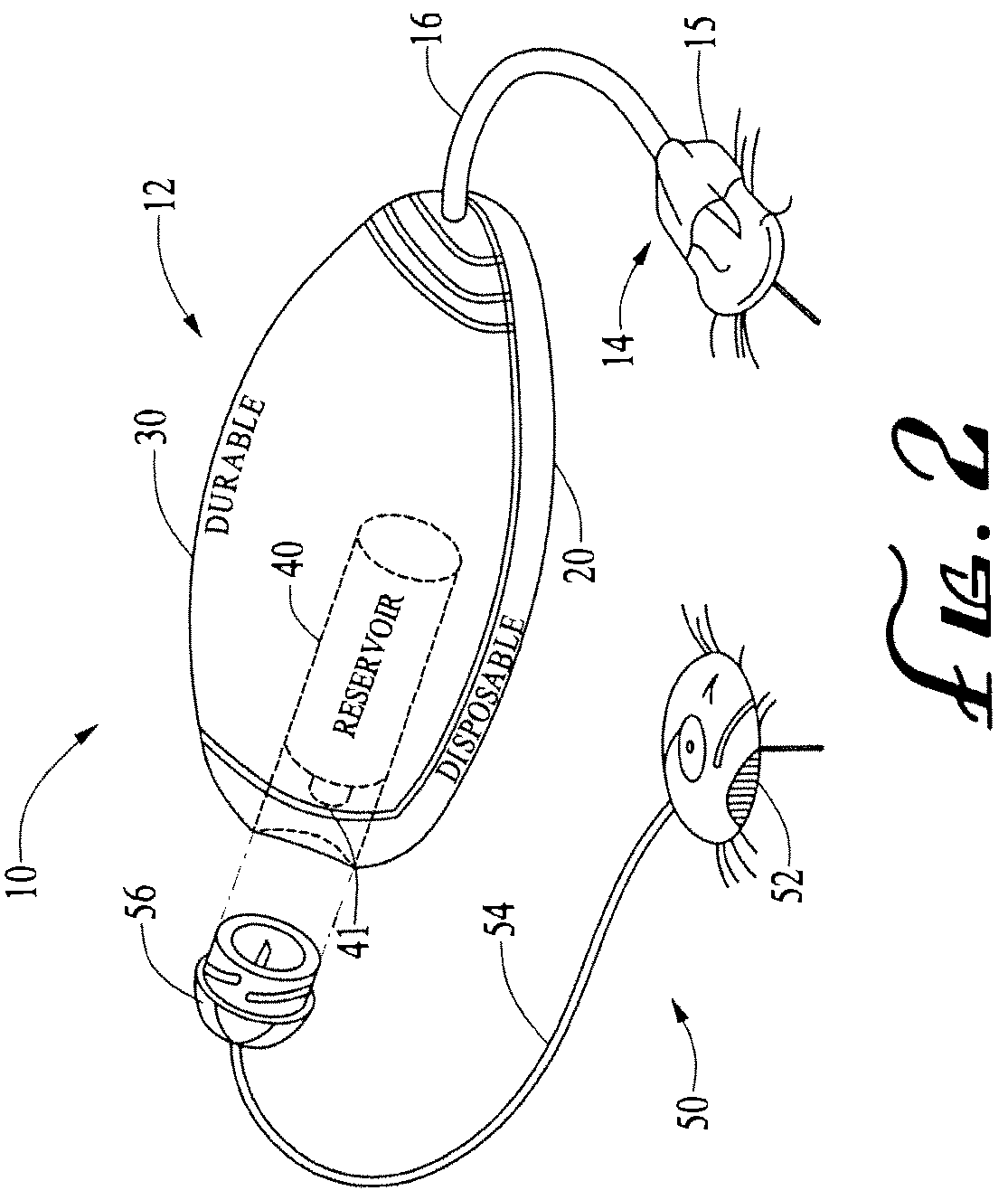
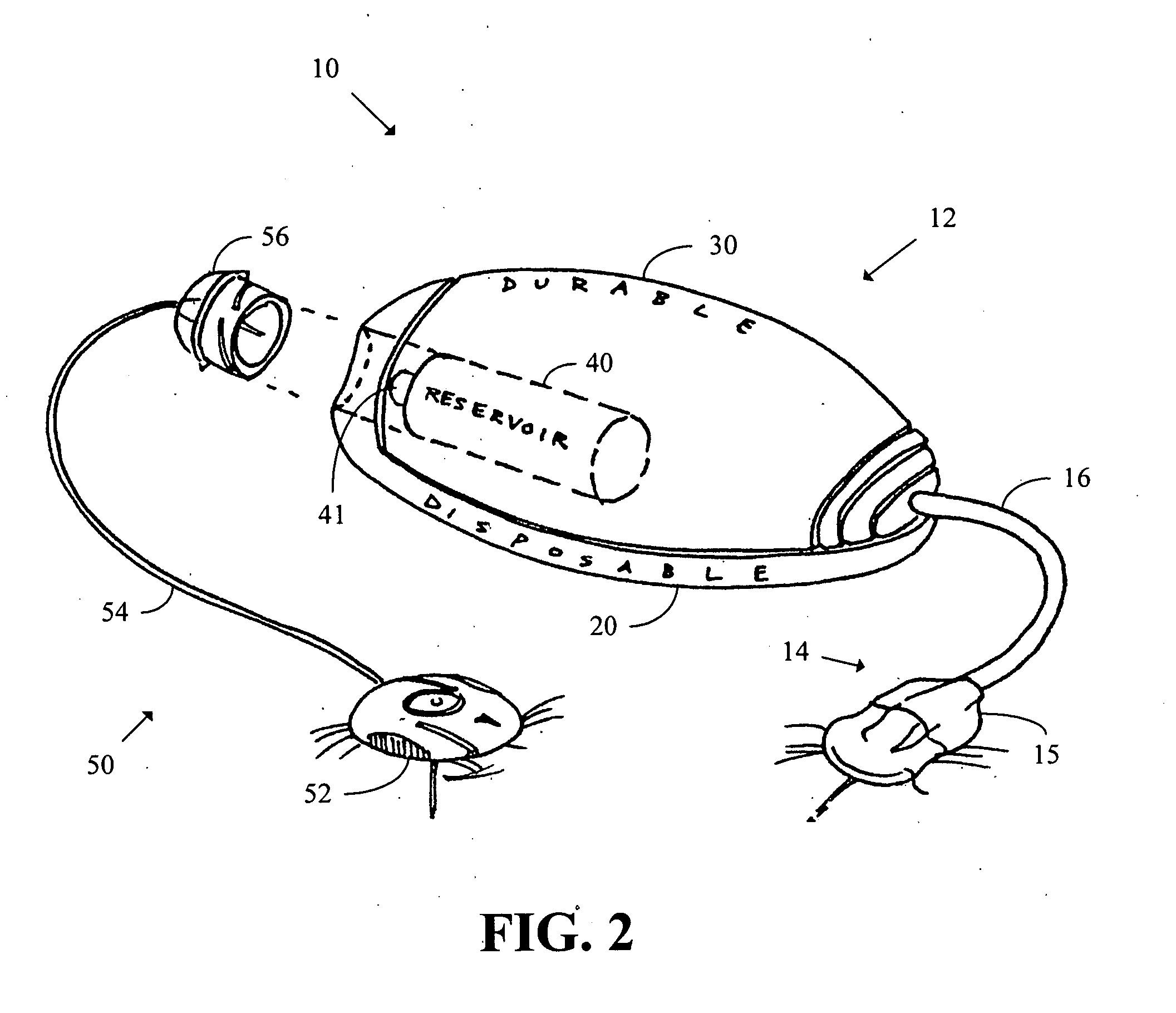
Systems and methods allowing for reservoir filling and infusion medium delivery
ActiveUS7828764B2Reduce internal volumeIncrease the internal volumePharmaceutical containersMedical devicesSurgeryMechanical engineering
A system includes a durable portion with a durable housing and a separable disposable portion with a disposable housing that selectively engage with and disengage from each other. The disposable housing secures to a patient and may be disposed of after it has been in use for a prescribed period. Components that normally come into contact with a patient or with an infusion medium may be part of the disposable portion to allow for disposal after a prescribed use. A reservoir for holding the infusion medium may be part of the disposable portion, and may be supported by the disposable housing. The durable portion may include other components such as electronics for controlling delivery of the infusion medium from the reservoir, and a drive device including a motor and drive linkage.
Owner:MEDTRONIC MIMIMED INC
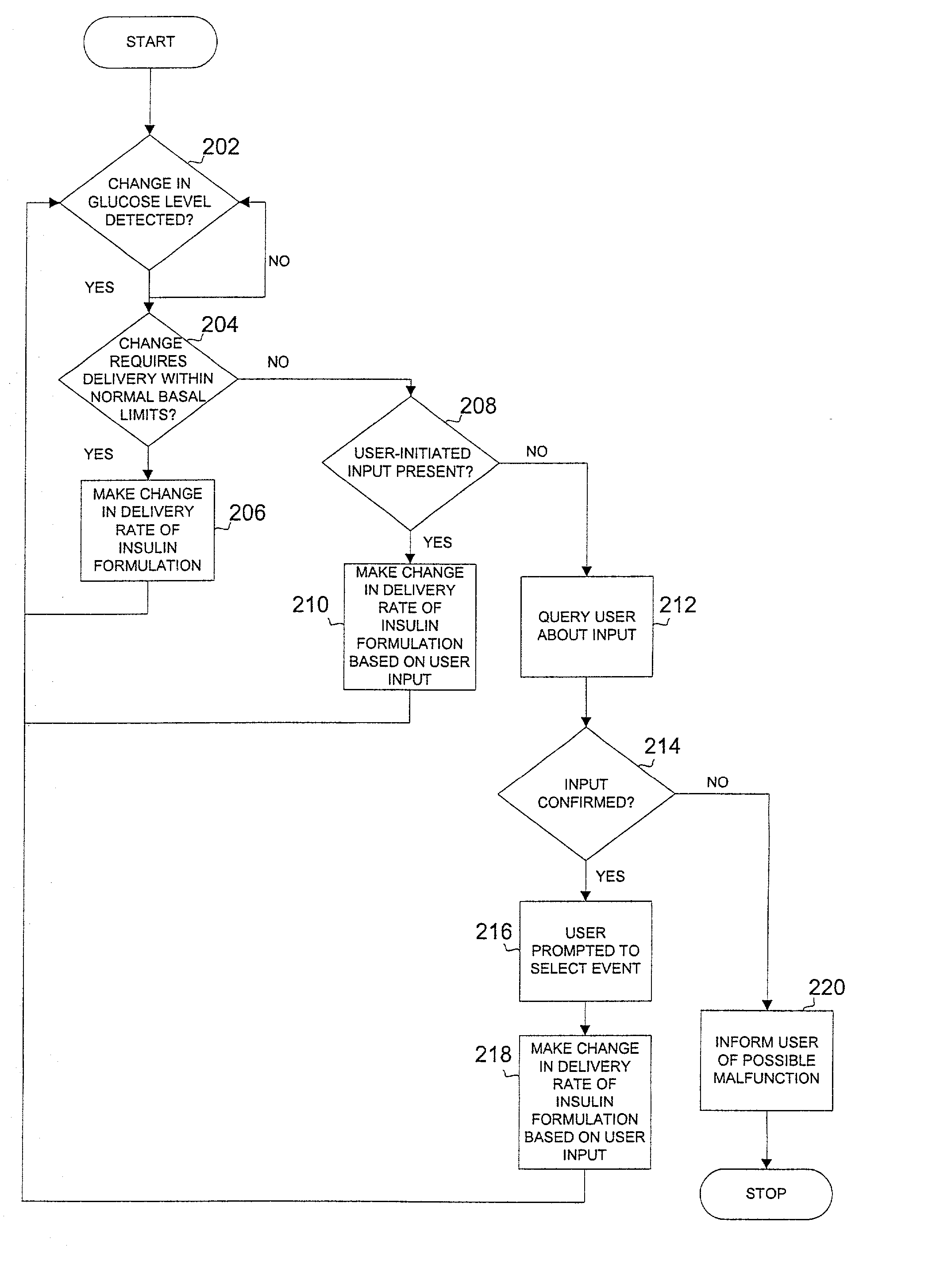
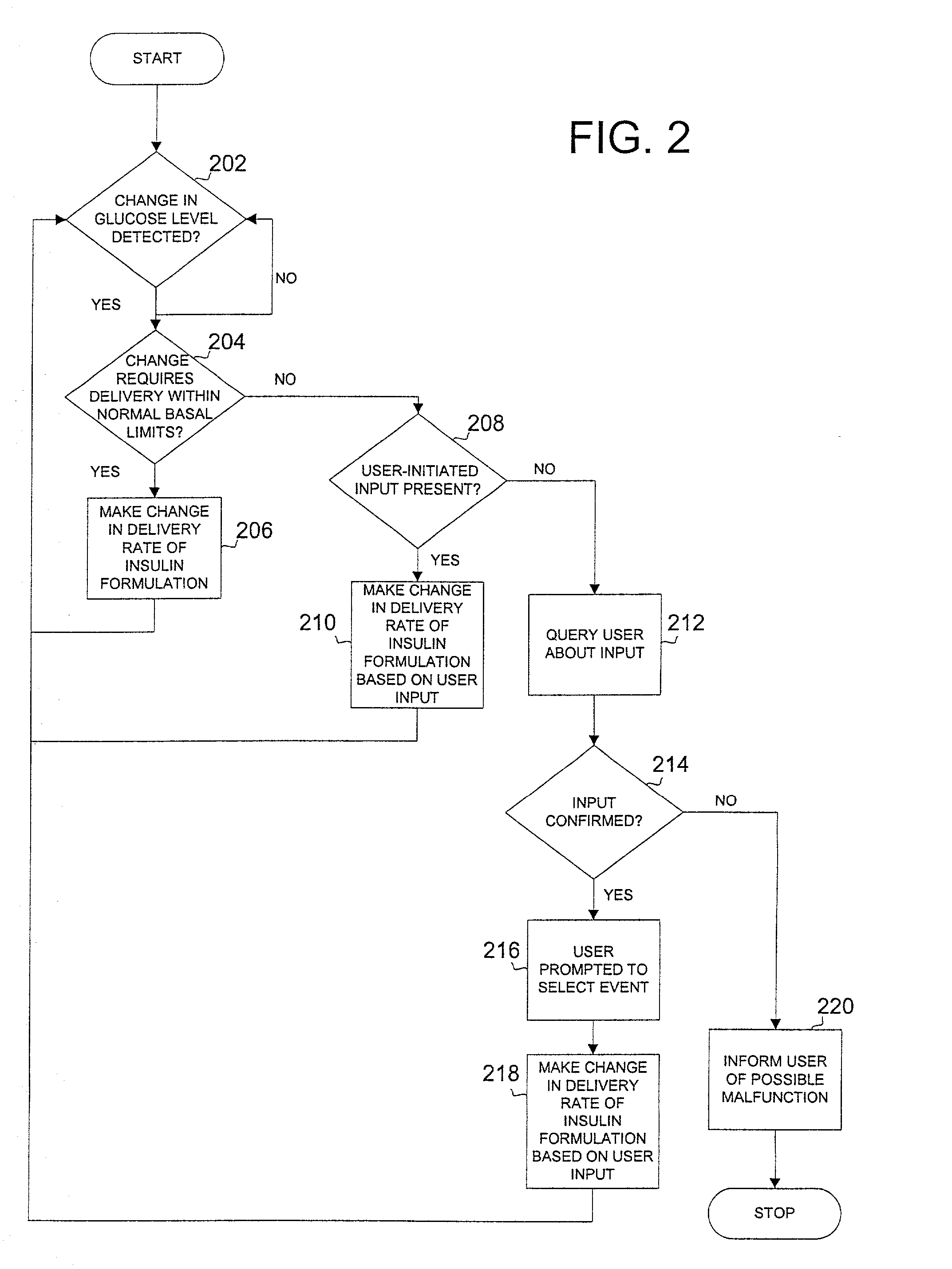
Safety limits for closed-loop infusion pump control
A system and process for providing safety limits on the delivery of an infusion formulation by an infusion pump system in response to a sensed biological state. The safety limits may comprise user-initiated event signals corresponding to events that may significantly affect the biological state. The safety limits may further comprise user-initiated event ranking signals for respective events which specify a degree, quantity, or measure for the respective event. The user-initiated event and event ranking signals may be communicated to a computing element associated with the infusion pump by an associated communication device having a user interface which comprises a plurality of user-selectable operators for entering information about the events and event rankings.
Owner:MEDTRONIC MIMIMED INC
Multi-position infusion set device and process
An infusion set for subcutaneous delivery of an infusant. The infusion set may include a base removably attachable to an infusion site and a connector temporarily lockable to the base. The connector can engage the base in a plurality of orientations. The connector locks into the base after at least partial rotation of the connector about the base. The connector may include flexible arms which unlock the connector from the base. The base includes a cannula for insertion through the infusion site. The connector includes a tubing for passing the infusant. The infusant is subcutaneously passable from the tubing through the cannula when the connector is attached to the base. The infusion set may also include a hub removably attachable to the base that includes a needle that extends through the base and the cannula and a guard removably attachable to the base opposite the hub for surrounding the needle.
Owner:MEDTRONIC MIMIMED INC
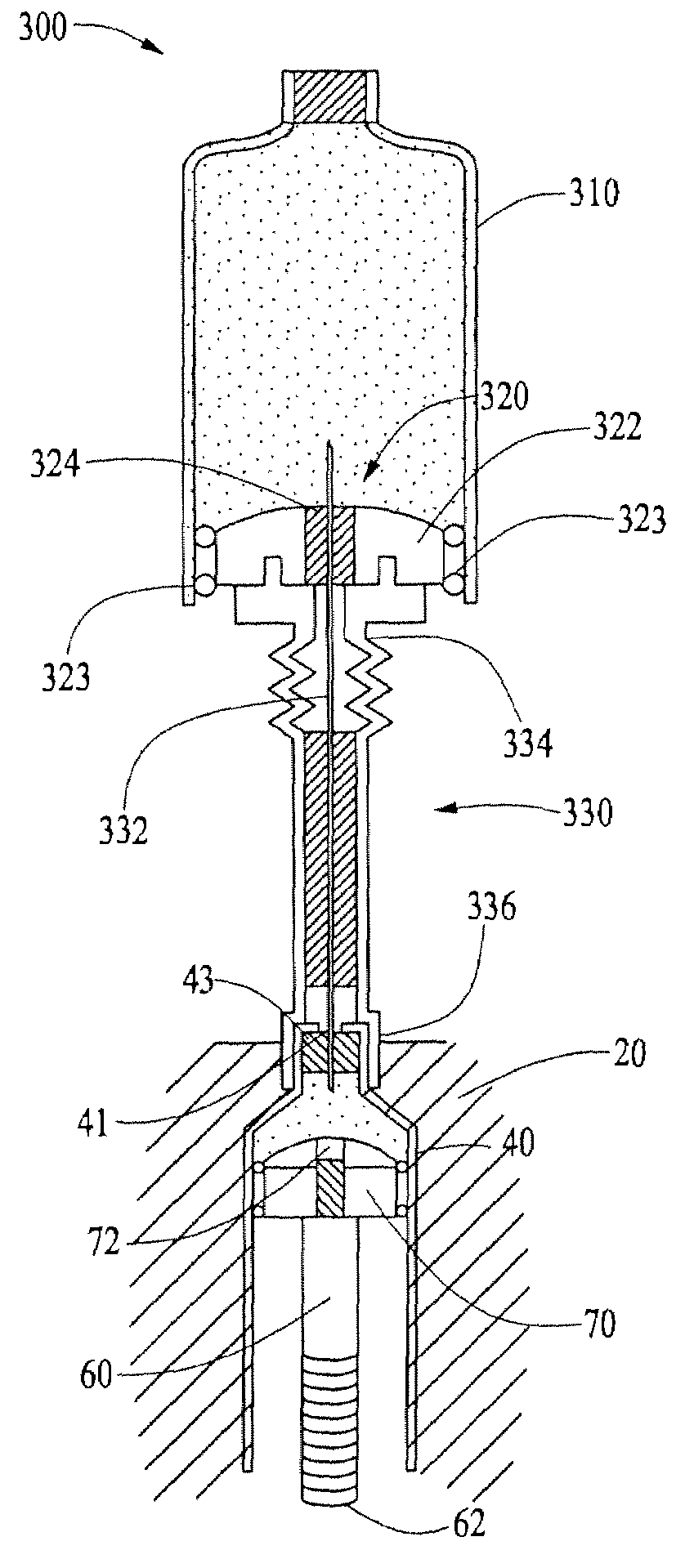
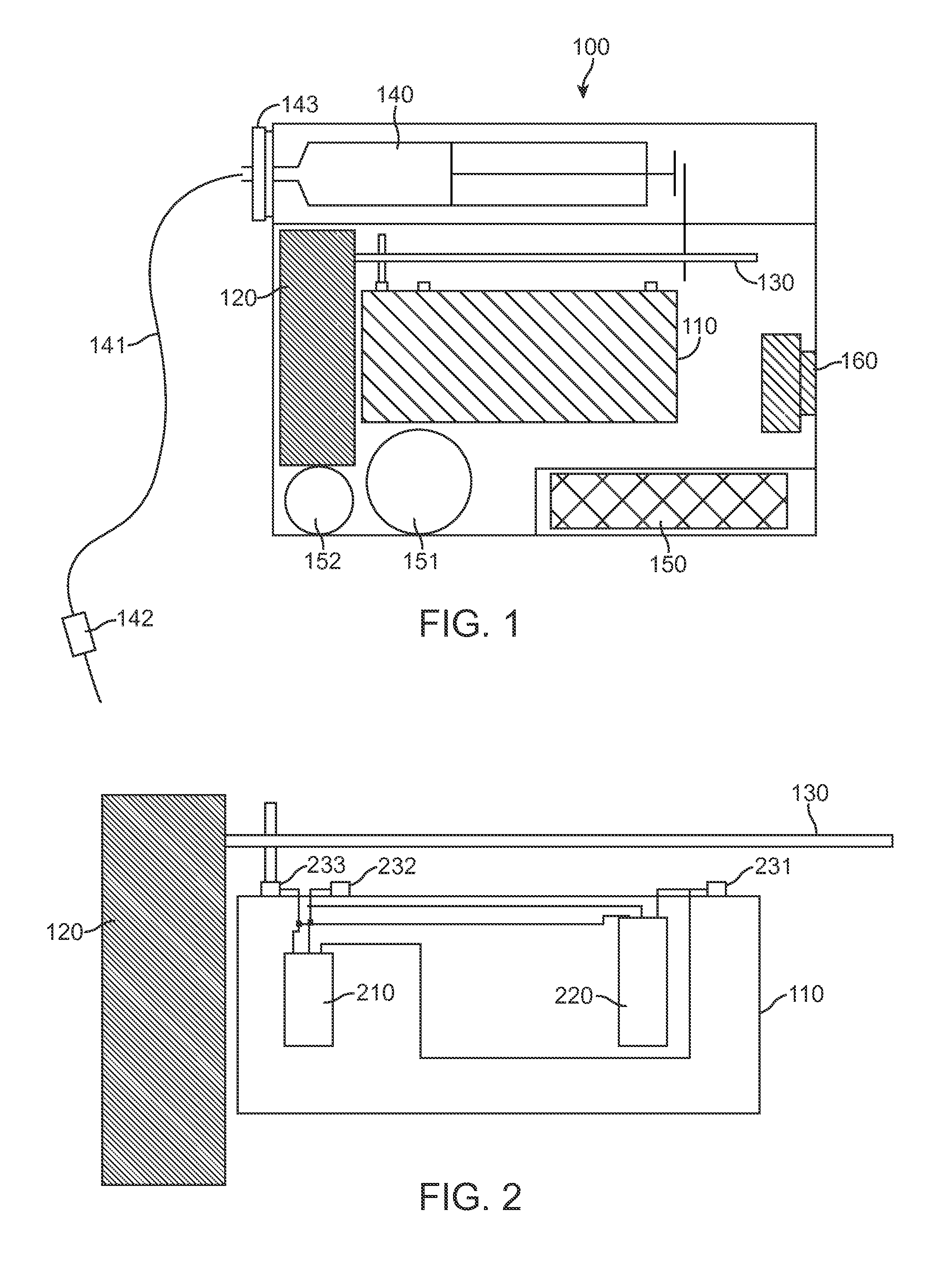
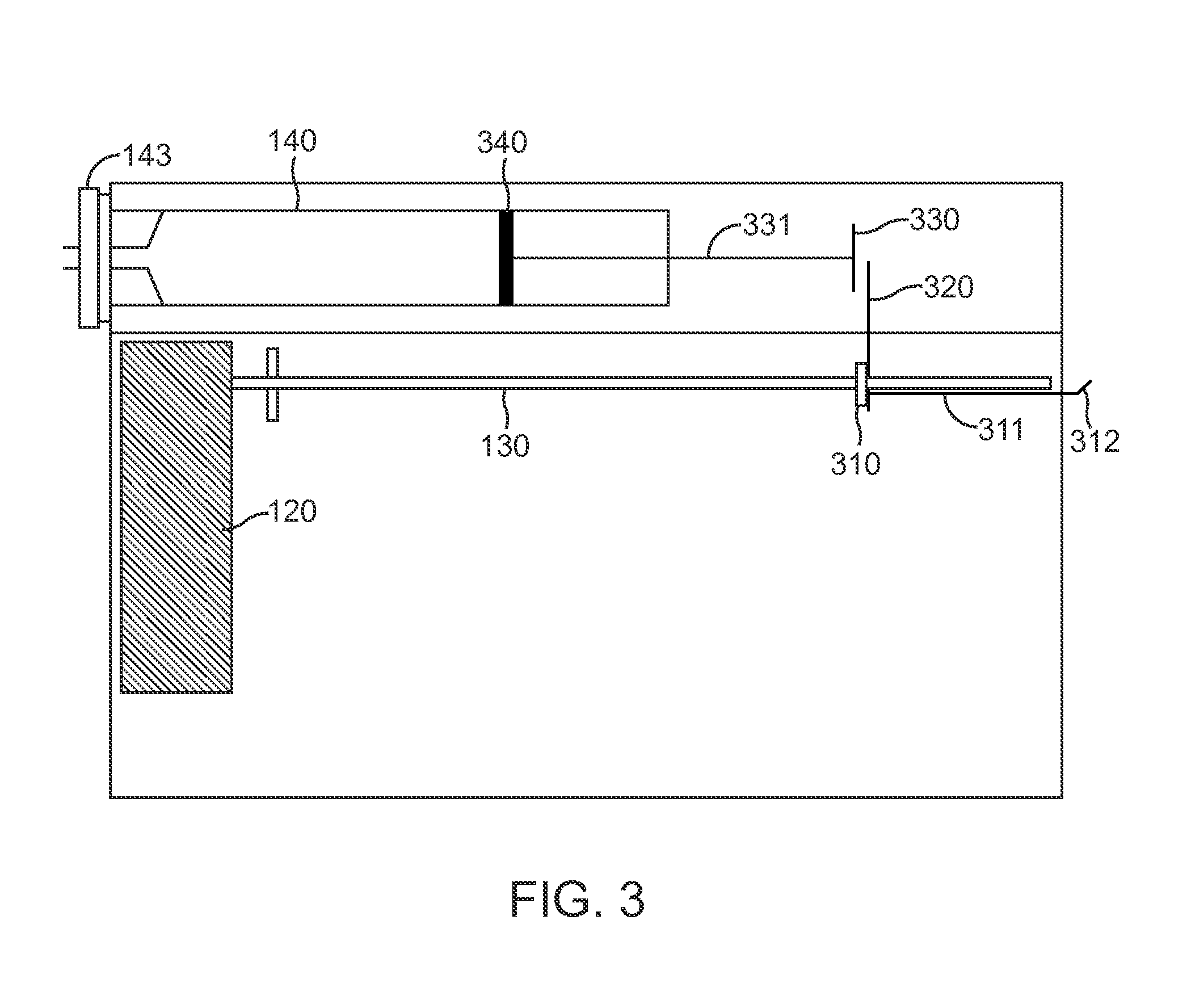
Optical displacement sensor for infusion devices
InactiveUS7498563B2Other blood circulation devicesMaterial analysis by optical meansDetector arrayEngineering
An optical sensor for a delivery device having a piston that displaces a substance, such as a fluid, from a reservoir. The optical sensor has a light source and a detector array for imaging encoding features disposed along a plunger rod coupled to the piston. By virtue of the pattern of encoding features, an absolute position of the plunger rod relative to a fiducial position may be determined uniquely. Thus, the volume of fluid remaining in the reservoir, the rate of fluid delivery, and proper loading of the reservoir may be accurately ascertained. Additionally, the encoding may serve to uniquely identify a version of the reservoir which may be supplied in various versions corresponding, for example, to differing concentrations of a therapeutic agent to be dispensed.
Owner:EUGLY DIABETES CARE LLC
Systems and methods allowing for reservoir filling and infusion medium delivery
ActiveUS20080097321A1Reduce internal volumeIncrease the internal volumeInfusion syringesPharmaceutical containersSurgeryMechanical engineering
A system includes a durable portion with a durable housing and a separable disposable portion with a disposable housing that selectively engage with and disengage from each other. The disposable housing secures to a patient and may be disposed of after it has been in use for a prescribed period. Components that normally come into contact with a patient or with an infusion medium may be part of the disposable portion to allow for disposal after a prescribed use. A reservoir for holding the infusion medium may be part of the disposable portion, and may be supported by the disposable housing. The durable portion may include other components such as electronics for controlling delivery of the infusion medium from the reservoir, and a drive device including a motor and drive linkage.
Owner:MEDTRONIC MIMIMED INC
Air trap for a medical infusion device
InactiveUS20090107335A1Easy to useOptimize locationLiquid degasificationDialysis systemsEngineeringVertical axis
An air trap for a blood circuit and method for removing air from blood in a dialysis unit. The air trap may include a blood inlet supply line, a blood outlet supply line, and a container having an approximately spherical internal wall, an inlet at a top end of the container connected to the blood inlet supply line, and an outlet at a bottom end of the container connected to the blood outlet supply line. The inlet may be offset from a vertical axis of the approximately spherical internal wall such that blood entering the container is directed to flow in a spiral-like path. The inlet port may be arranged to introduce blood into the container in a direction that is approximately tangential to the approximately spherical inner wall of the container and / or in a direction that is approximately perpendicular to the vertical axis of the container.
Owner:DEKA PROD LLP
Patch-Like Infusion Device
InactiveUS20070203454A1Conveniently worn against skinAutomatic syringesMedical devicesInfusion setInfusion solution
A system and method for a patch-like, self-contained substance infusion device which provides one or more substantially hidden patient needles which can be placed in fluid communication with a fluid reservoir subassembly that includes a rigid bladder portion used in conjunction with a non-distensible bladder film, such as a metallized film. Simple removal of an interlock allows a disk, or Belleville spring assembly to apply an essentially even and constant pressure to the contents of the fluid reservoir assembly, and allows the device to then be attached to a skin surface via an adhesive contact surface. A push button activation assembly is provided which can then be used to release and seat one or more spring-loaded patient needles into the skin surface, and establish a fluid communication path between the patient needles and the pressurized fluid reservoir contents thereby delivering an infusion into the skin.
Owner:BECTON DICKINSON & CO
Method of hydration; infusion packet system(s), support member(s), delivery system(s), and method(s); with business model(s) and Method(s)
InactiveUS20020012689A1Constant deliveryUniform deliveryBiocideOrganic active ingredientsDiagnostic Radiology ModalityDietary supplement
Liquid activated infusion packet(s) / system, promoting hydration, containing active and / or inactive ingredients and / or a support member(s). Infusion Packet(s) / System is one or more individual compartments, and / or group(s), whereby the enveloping material(s) may be totally or partially dissolvable, edible, transparent, opaque, decorated, etc. Further, including of one or more: color(s), flavor(s), aroma(s), pharmaceutical(s), nutraceutical(s), dietary supplement(s), enzyme(s), pre / pro-biotic(s), amino-acid(s), soluble-fiber(s), diagnostic agent(s) etc. regardless of form, + / - effervescence, + / - uniform / controlled-release encapsulations into liquid for humans and / or animals. Enveloping material may be in whole and / or in combination; non-synthetic / porous, and / or synthetic porous / non-porous with deliberate perforations. Infusion Packet(s) / System + / - tag, support member for assistance, consumer compliance: promotion, advertising, education, entertainment, (toy / game), etc. Manual and / or power operated parts, lights, noise, etc. Additionally incorporated; unique business modalities with test market opportunities and / or the ability to provide income and / or esteem for the health challenged.
Owner:STILLMAN SUZANNE JAFFE
Disposable infusion device filling apparatus and method
An infusion system includes a disposable wearable infusion device and a filler device. The disposable infusion device has a body arranged to be adhered to a patient's skin and a reservoir for holding a liquid medicant to be infused into the patient. The filler device is arranged to detachably receive the infusion device body and to transfer a volume of the liquid medicant to the infusion device reservoir. The filler device may be part of a service device arranged to detachably receive the infusion device and which also includes a cannula driver and a cannula for providing the infusion device with a cannula and deploying the cannula to beneath a patient's skin.
Owner:CALIBRA MEDICAL
Infusion array ablation apparatus
An infusion array ablation apparatus includes an elongated delivery device having a lumen and an infusion array positionable in the lumen. The infusion array includes an RF electrode and at least a first and a second infusion member. Each infusion member has a tissue piercing distal portion and an infusion lumen. At least one of the first or second infusion members is positionable in the elongated delivery device in a compacted state and deployable from the elongated delivery device with curvature in a deployed state. Also, at least one of the first or second infusion members exhibits a changing direction of travel when advanced from the elongated delivery device to a selected tissue site. At least one infusion port is coupled to one of the elongated delivery device, the infusion array, the first infusion member or the second infusion member.
Owner:ANGIODYNAMICS INC
Constant ocular pressure active infusion system
An irrigation system for a medical device. The irrigation system may include a pump that can pump irrigation fluid from a reservoir through an irrigation line. The system may further have a controller coupled to the pump and an accumulator pressure sensor that senses the pressure of the irrigation line. The controller can vary the speed of the pump in response to a change in the line pressure to control the irrigation line pressure. Additionally, the controller can monitor the fluidic resistance of the system by determining the pump speed and corresponding flowrate of the pump. The controller can provide one or more safety output signals if the fluidic resistance exceeds a threshold value(s).
Owner:BUIVISION
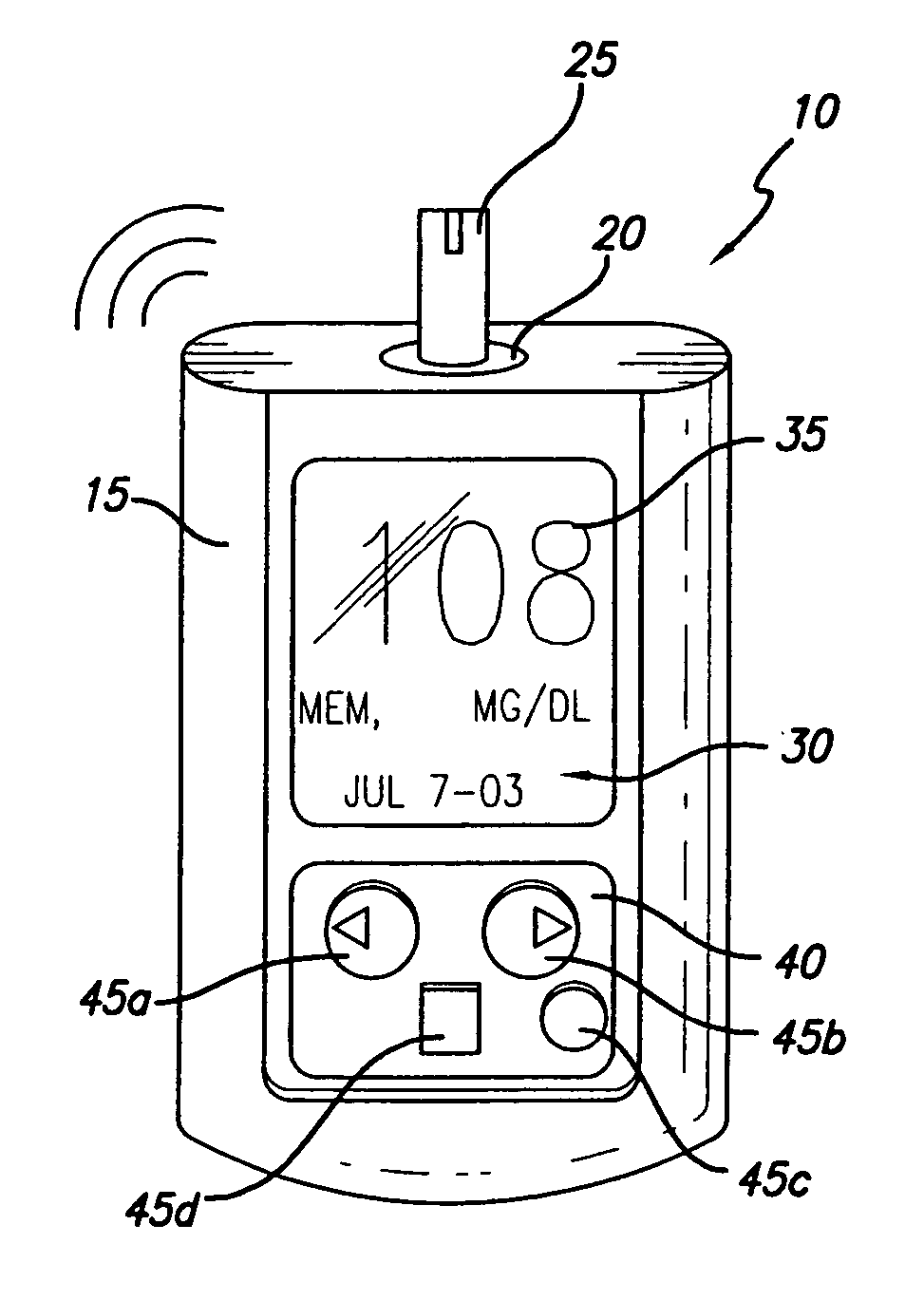
Quantitative chronological medical infusion device
InactiveUS20060079831A1Reduce riskReduced glucose levelMedical devicesIntravenous devicesLine sensorHigh rate
The present invention is a medical infusion and aspiration system delivering precisely timed and accurately calculated, adjusted pulsated delivery in high rates of flow delivering an effective profile of pulses tailored to provide momentary spikes of levels of freely available medicines based upon the uptake of the medicine and optimally on real time measurements of the medicine or response of the patient, termed Quantitative Chronological Delivery. The system comprises any pumping mechanism, and optimally a pumping mechanism, and a cassette or cartridge having a reservoir area where the plunger rotates as it advances in reference to the cartridge to provide additional accuracy and overcome the forces of inertia and slip-stick as well as eliminate backlash. Optimally, the systems incorporates an encoded area and an opening for connection to an infusion tube with an in-line sensor area where sampling probes are located. The infusion is adjusted in both amount and duration between pulses to provide quantitatively controlled, chronologically optimized infusion. A motor causes bi-directional pumping to allow for samples to be presented to the sensor area. The system accuracy allows for more concentrated medicines, as a sealed container can eliminate the need for diluting or withdrawing medicine to load a reservoir, and achieves extraordinary accuracy without error correcting software or expensive volumetric measurement and control systems.
Owner:BIONICA INT
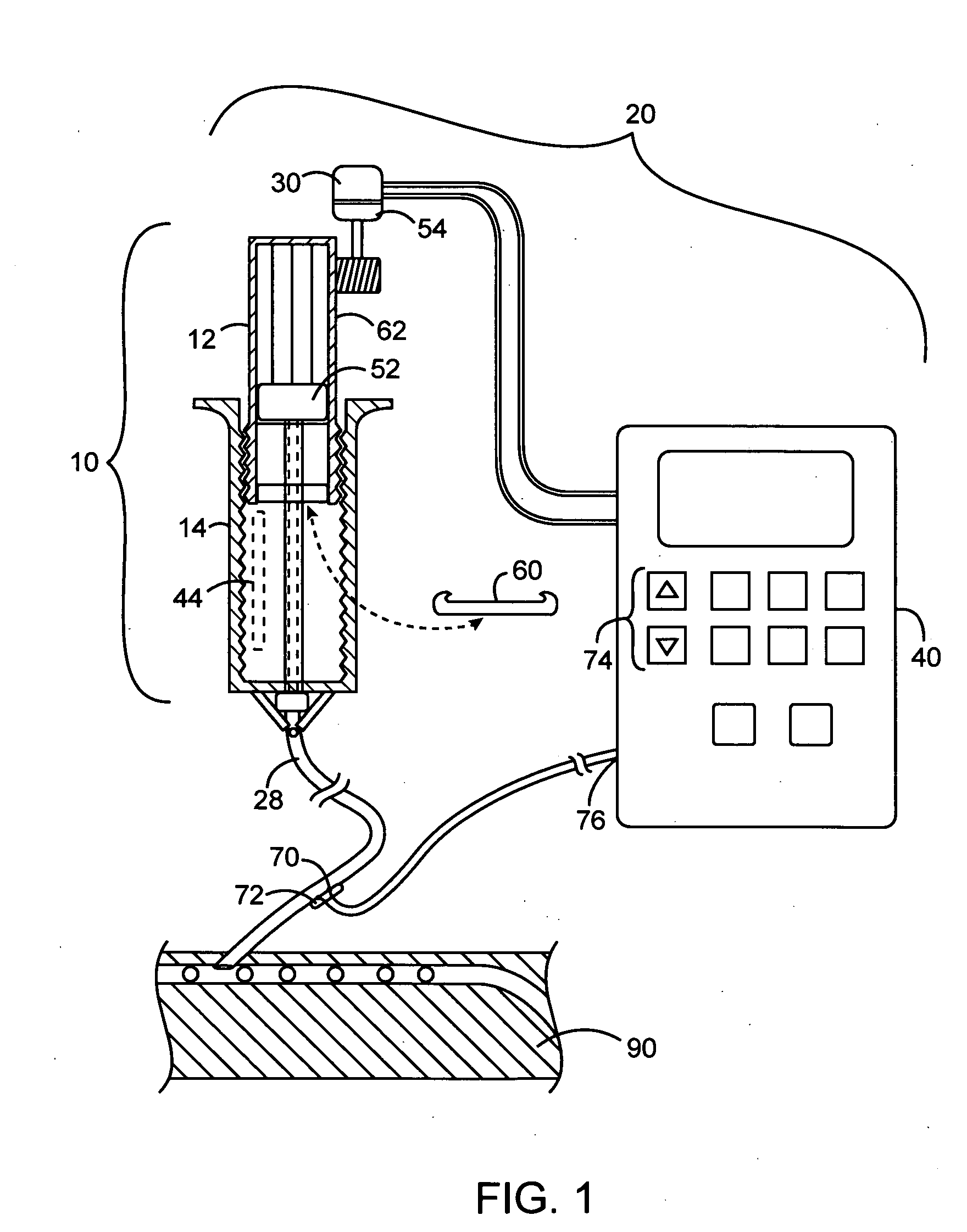
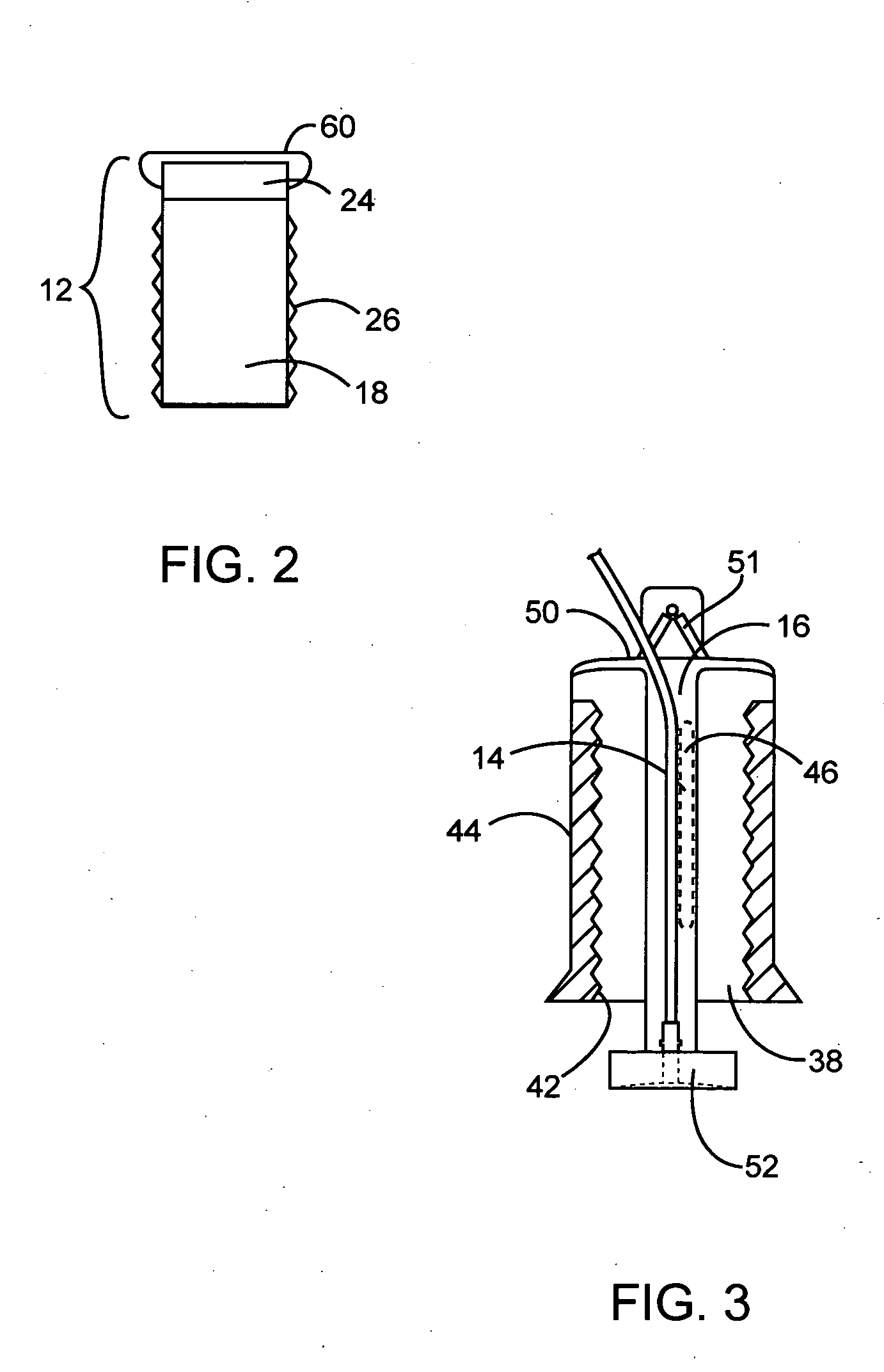
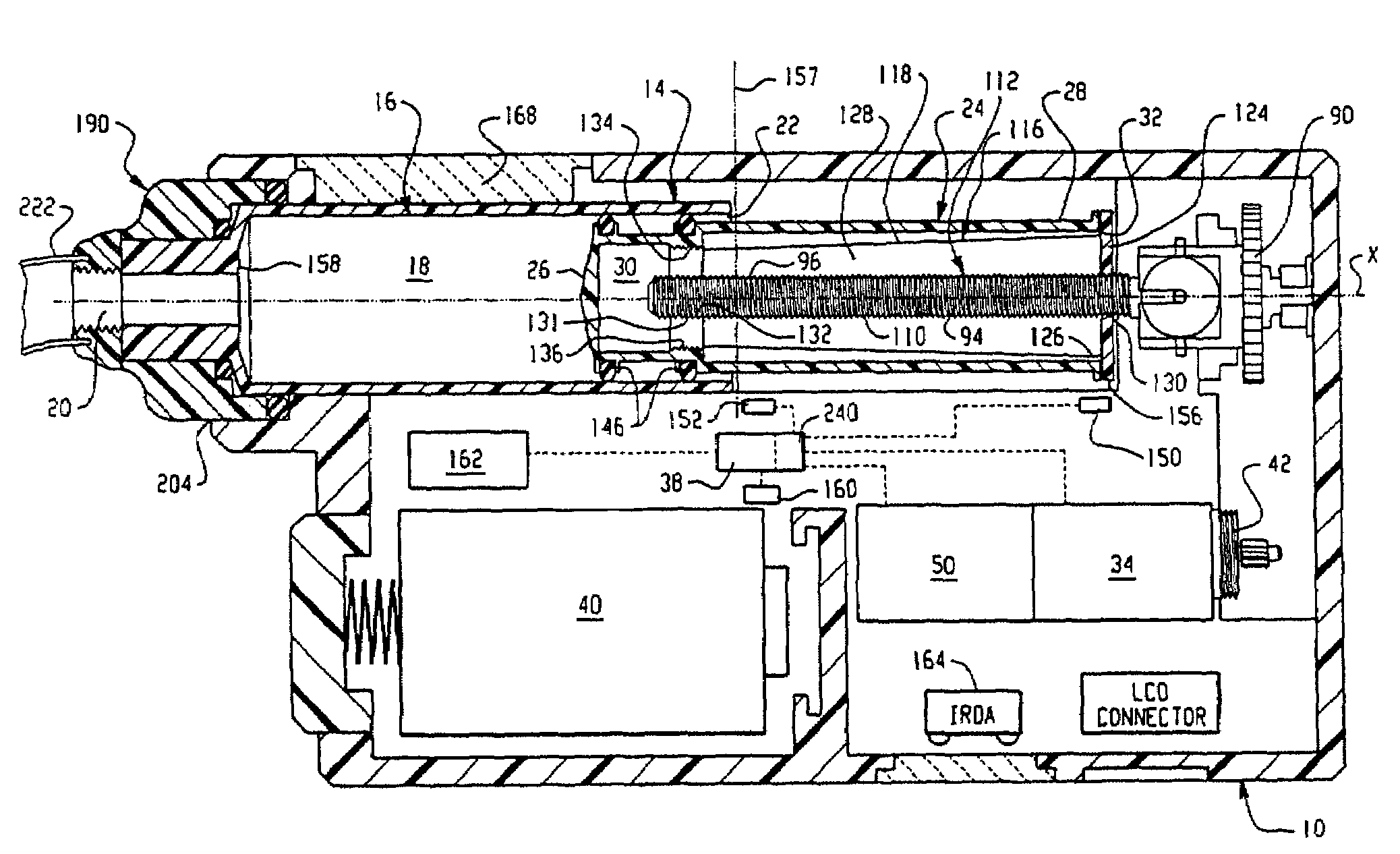
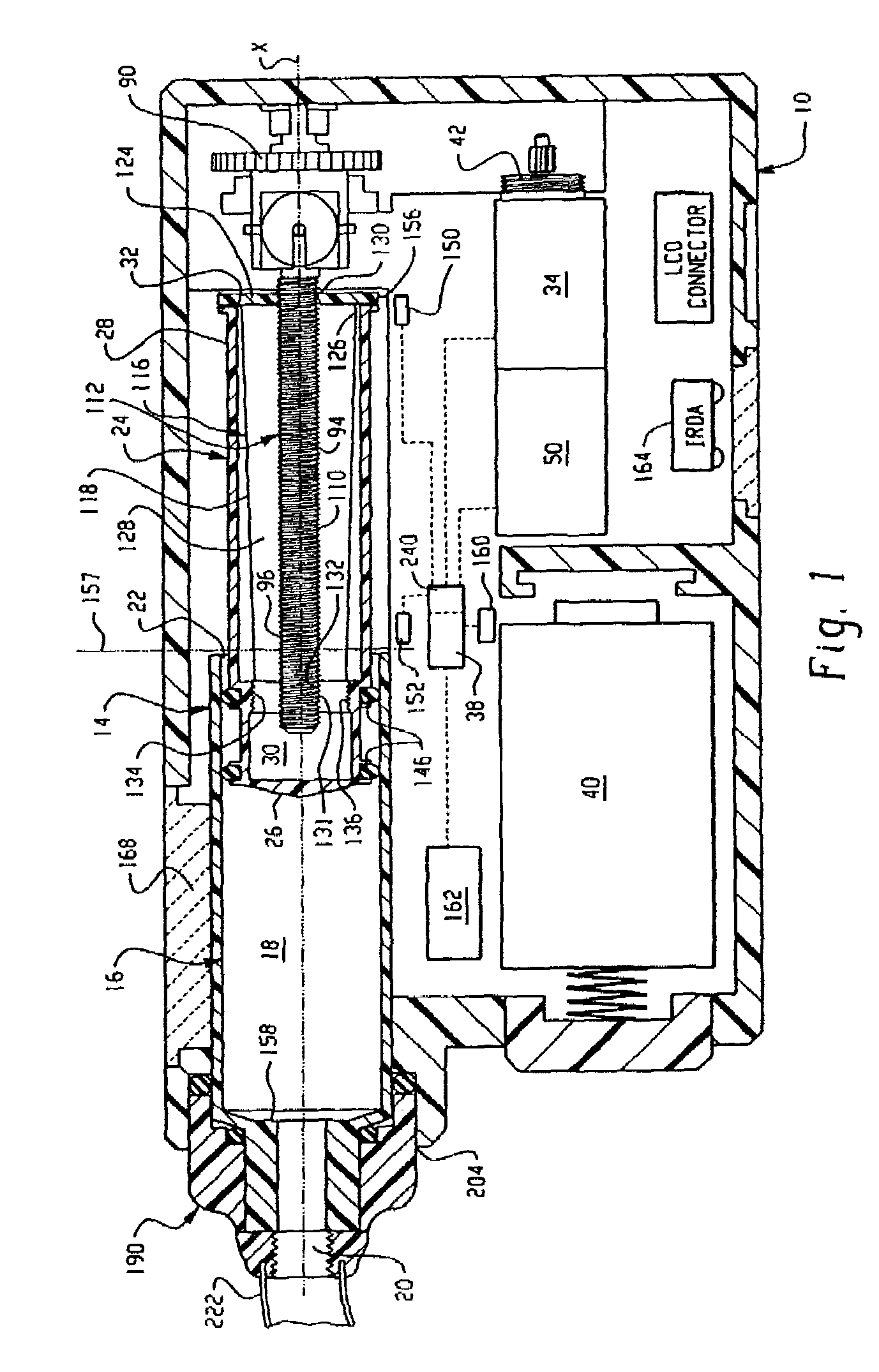
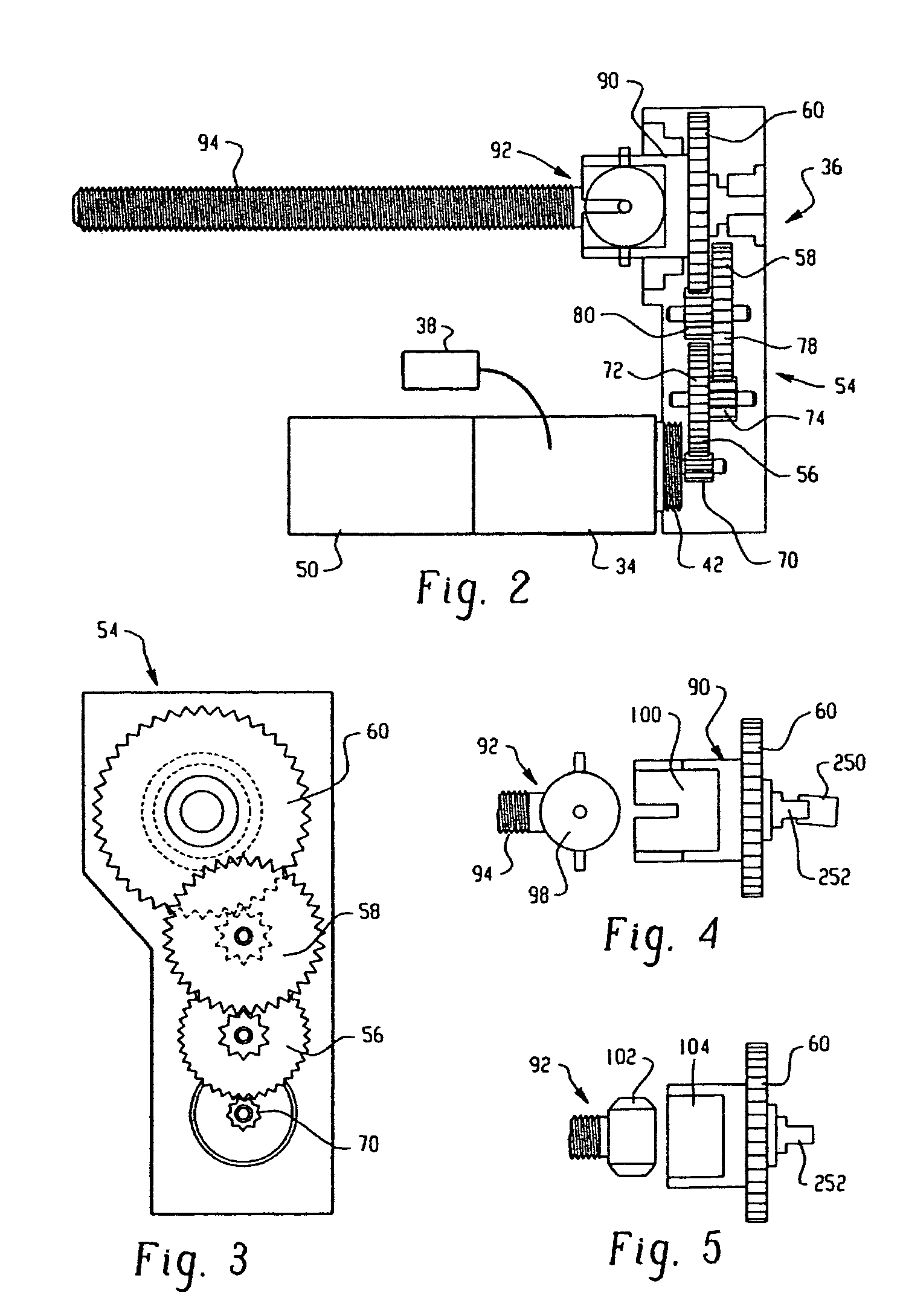
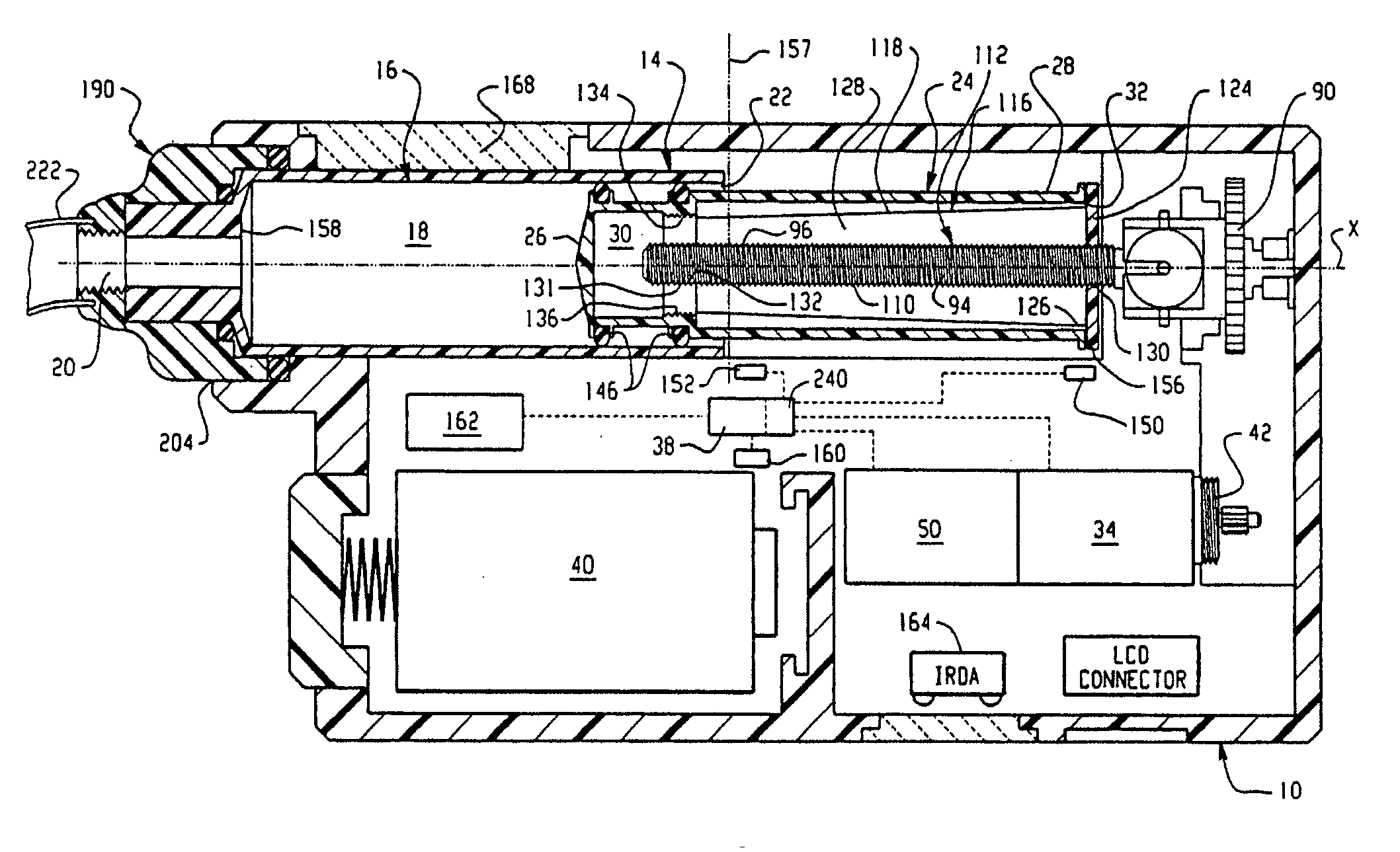
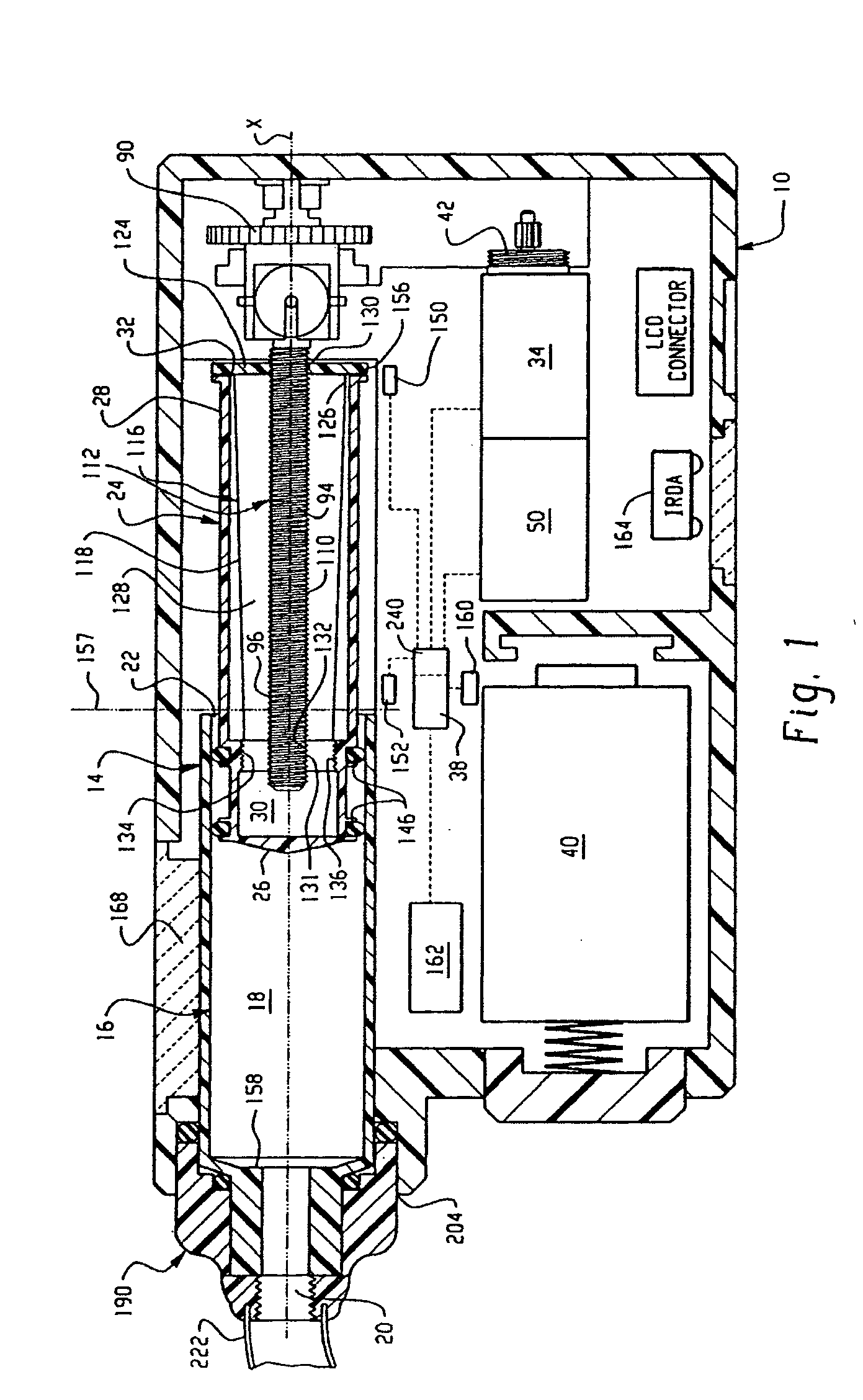
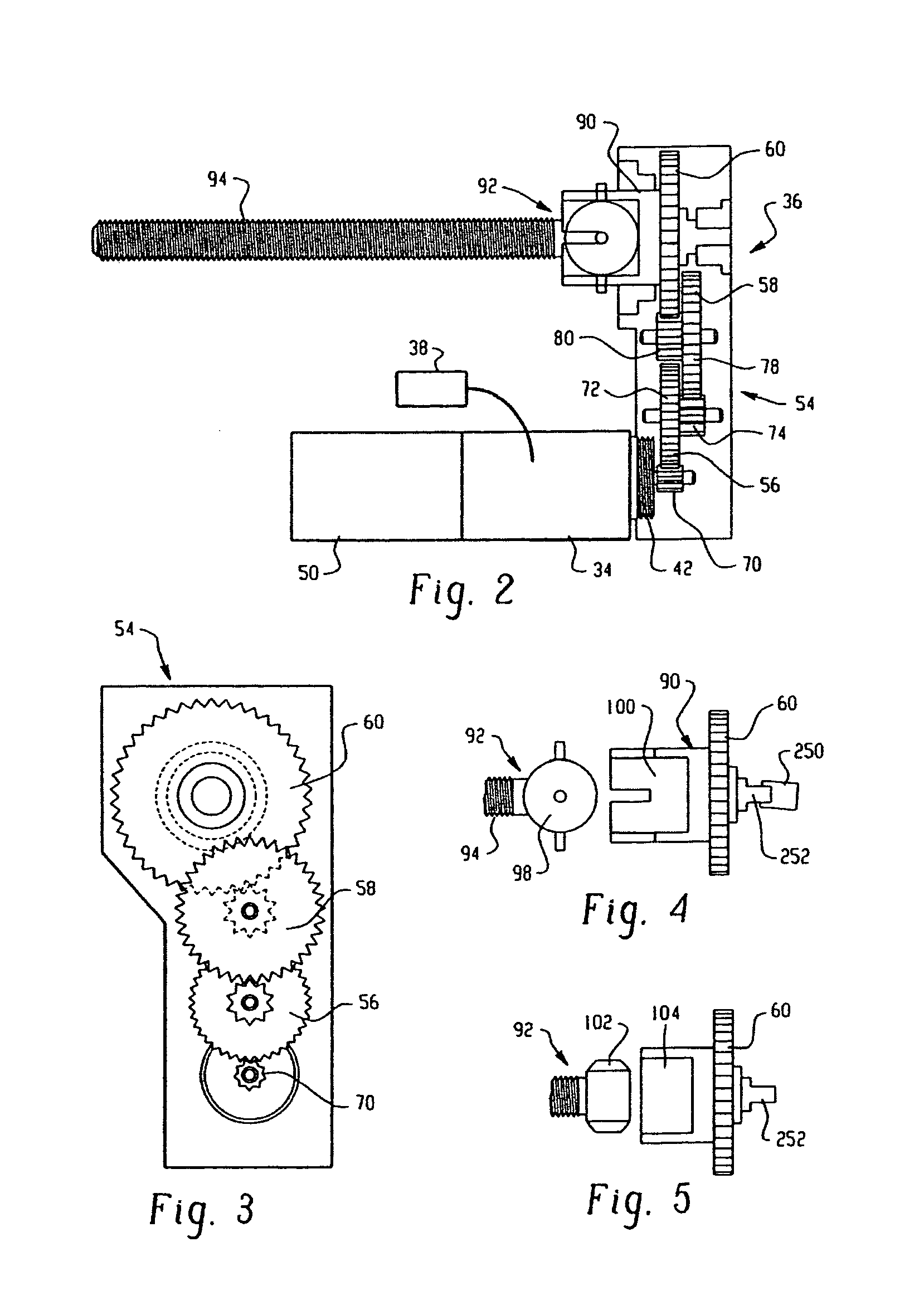
Drive system for an infusion pump
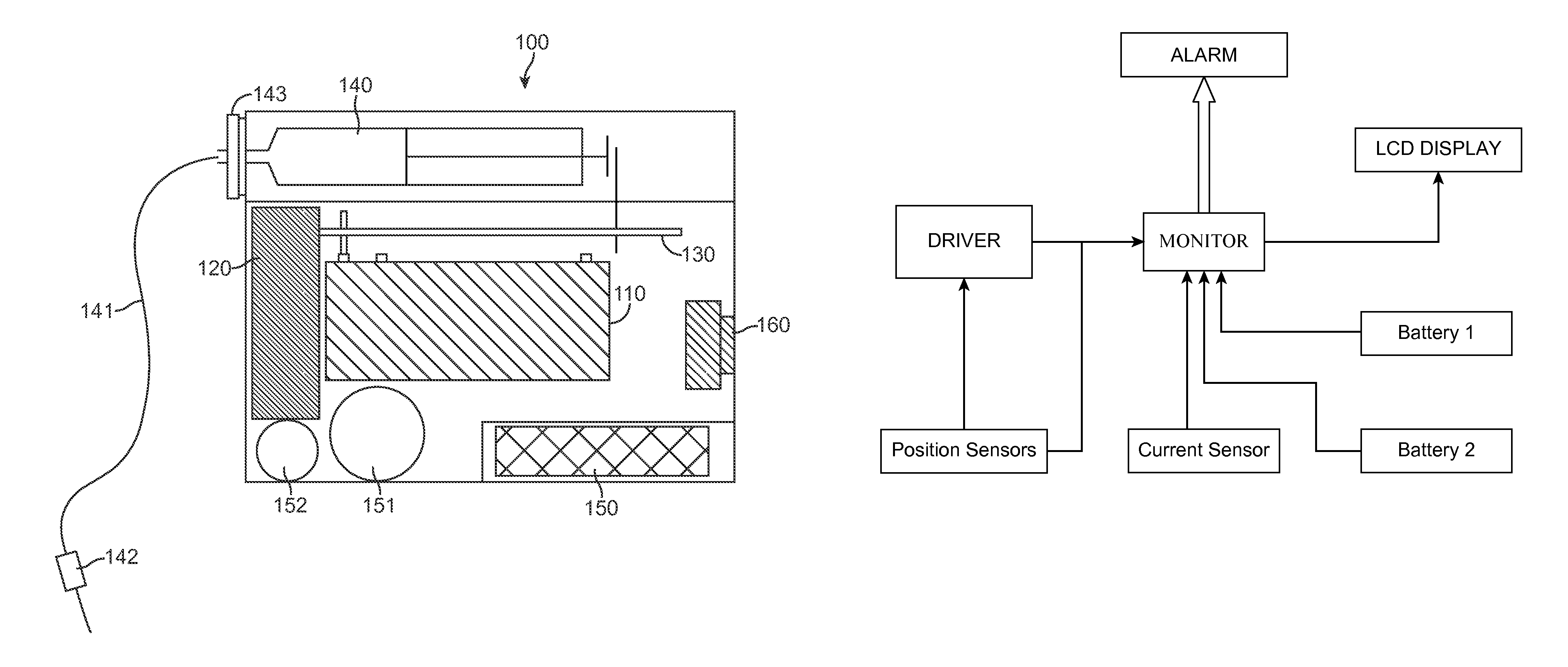
InactiveUS7025226B2Reduce sizeImprove portabilityOpening closed containersPower operated devicesLinear motionProximity sensor
A pump system for an infusion system includes a linear drive (36, 36′) which minimizes the space occupied by the pump components in a portable housing (10, 10′). A motor (34) and a motor drive shaft (42) are arranged in parallel with, and adjacent to a syringe (14, 14′) and lead screw (94, 94′). A gear box (54) connects the drive shaft and lead screw to transfer rotational movements between them. A piston driving member, such as a cone (116) or drive nut (116′) converts the rotational movement of the lead screw into linear motion of a syringe piston (24). Sensors (150, 152) detect when the piston or cone is in a “home” position and in an “end” position, respectively. Optionally, a proximity sensor (170) is used to ensure that the cone and the piston (24) are abutting during dispensing. Alternatively, a clamping member (350) selectively clamps the lead screw (94′) against linear motion in at least a dispensing direction.
Owner:TRIVIDIA HEALTHCARE SYST LLC
Drive system for an infusion pump
InactiveUS20050051580A1Small sizeImprove portabilityOpening closed containersPower operated devicesLinear motionMotor drive
A pump system for an infusion system includes a linear drive (36, 36′) which minimizes the space occupied by the pump components in a portable housing (10, 10′). A motor (34) and a motor drive shaft (42) are arranged in parallel with, and adjacent to a syringe (14, 14′) and lead screw (94, 94′). A gear box (54) connects the drive shaft and lead screw to transfer rotational movements between them. A piston driving member, such as a cone (116) or drive nut (116′) converts the rotational movement of the lead screw into linear motion of a syringe piston (24). Sensors (150, 152) detect when the piston or cone is in a “home” position and in an “end” position, respectively. Optionally, a proximity sensor (170) is used to ensure that the cone and the piston (24) are abutting during dispensing. Alternatively, a clamping member (350) selectively clamps the lead screw (94′) against linear motion in at least a dispensing direction.
Owner:TRIVIDIA HEALTHCARE SYST LLC
Infusion apparatus
ActiveUS20100280430A1Reduce need for interventionReduce riskMedical devicesPressure infusionEngineeringExpansion chamber
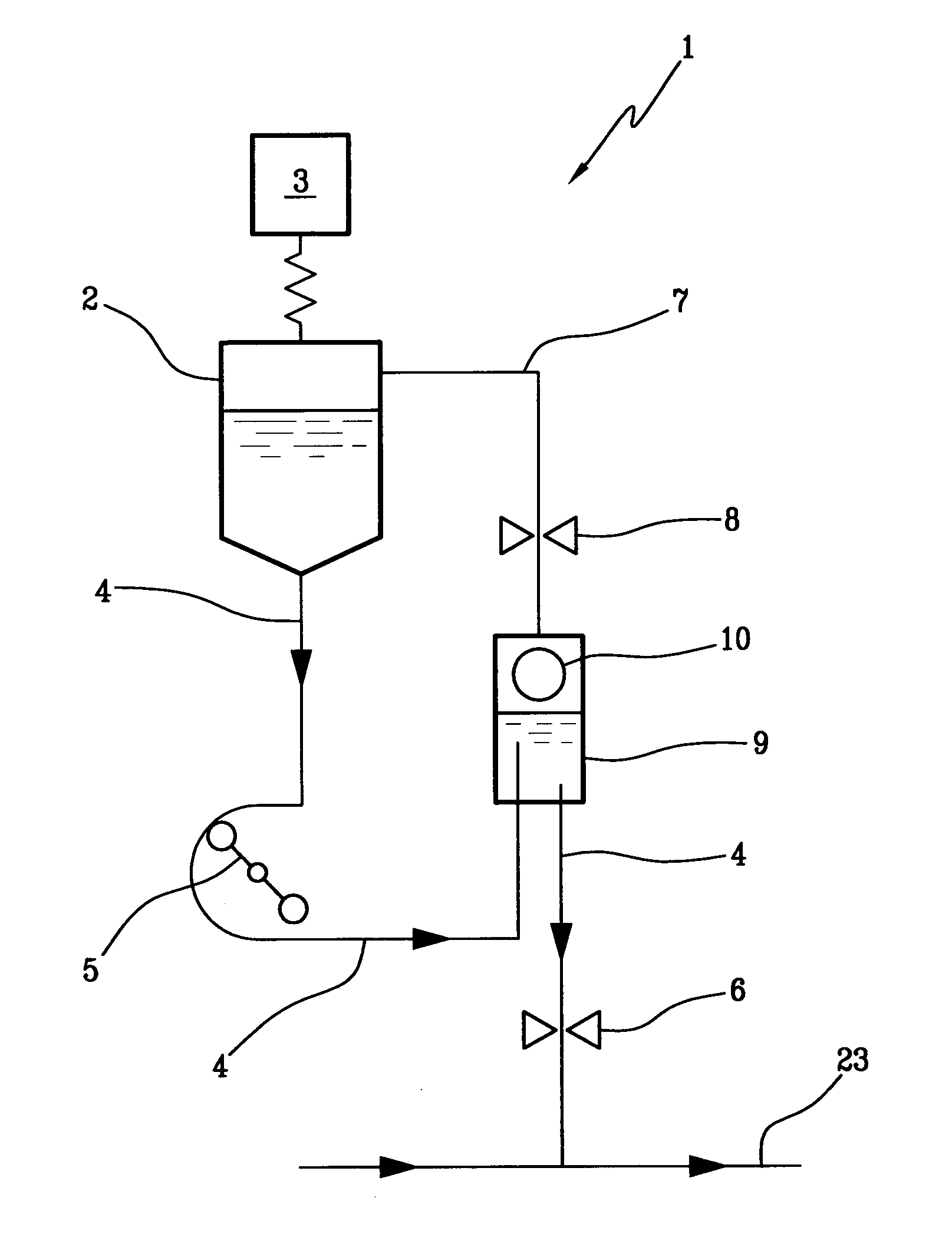
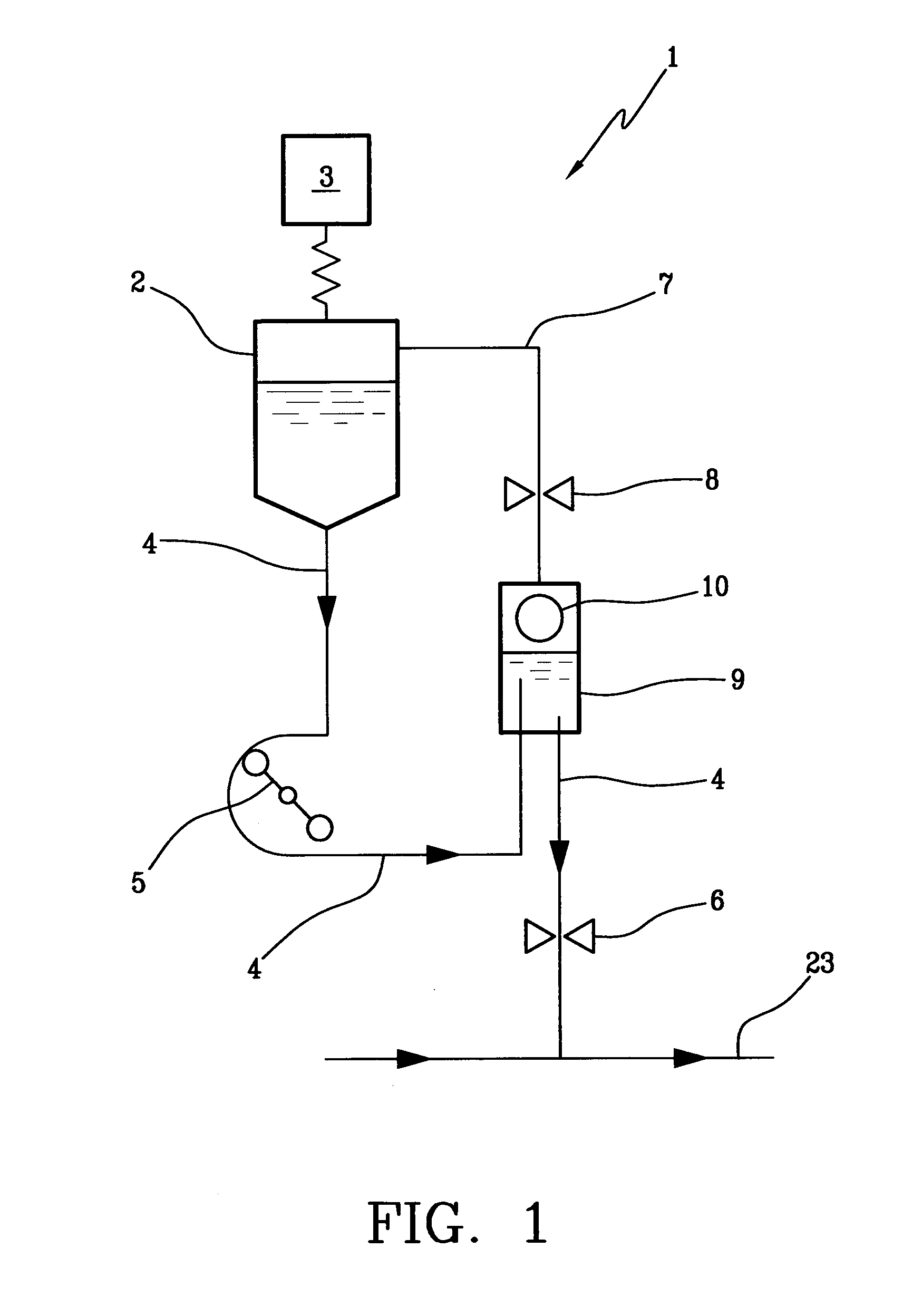
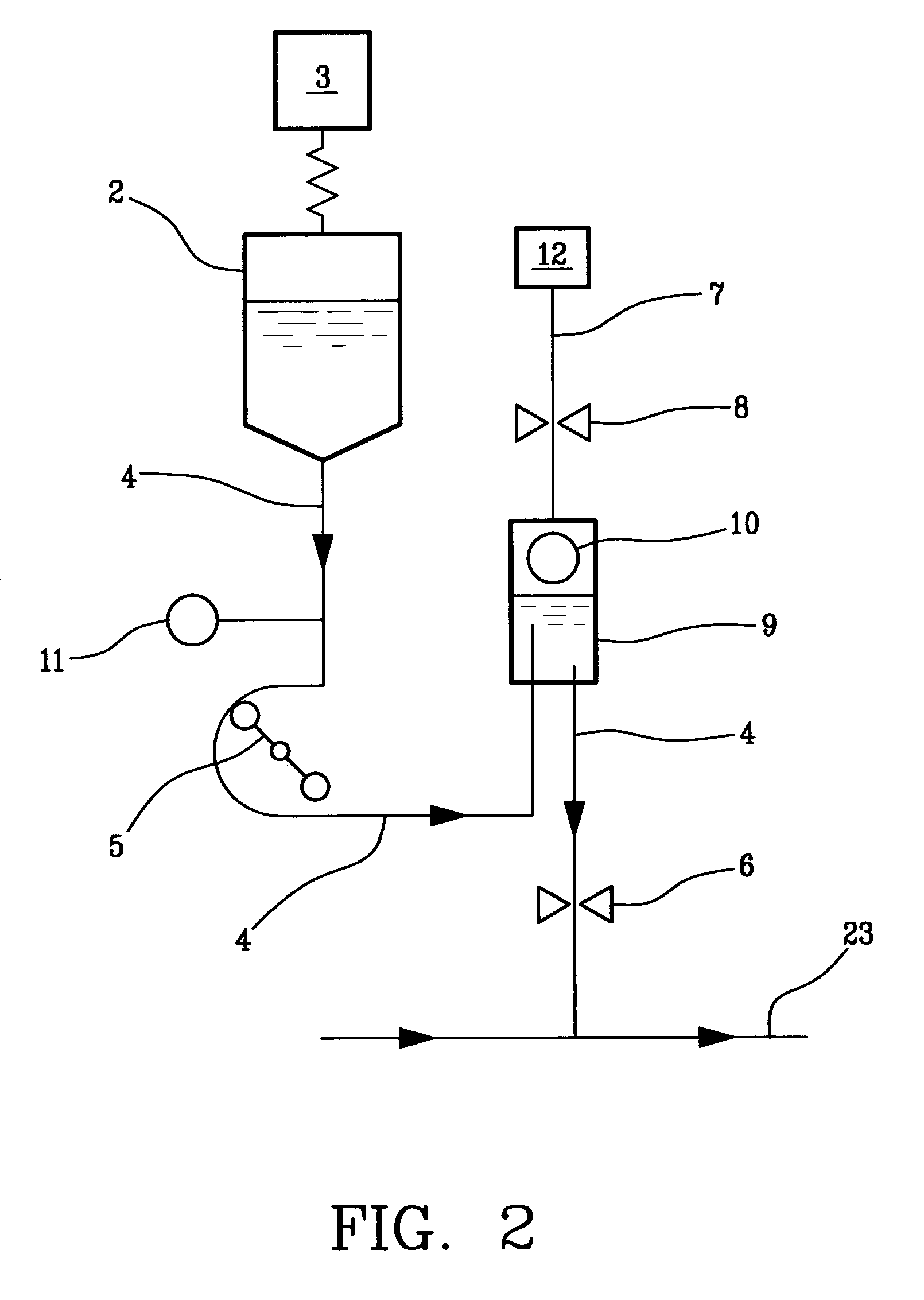
In an infusion apparatus, an infusion line (4) connects a container (2) of an infusion fluid to an extracorporeal blood circuit (23). A first valve (6) closes the infusion line downstream of an infusion pump (5). An expansion chamber (9), provided with a pressure sensor (10), is arranged between the infusion pump and the first valve. A second valve (8) closes a vent line (7) of the expansion chamber. The processor closes the first valve when the container is emptied. After replacement with a new and full container, the processor restarts the pump and selectively opens the first valve or the second valve according to the increase in pressure measured in the expansion chamber.
Owner:GAMBRO LUNDIA AB
Dual microcontroller-based liquid infusion system
Owner:AMRITA VISHWA VIDYAPEETHAM
Method and System for Programming an Infusion Device
InactiveUS20110098674A1Function is disabledError detection/correctionDrug and medicationsTime segmentInfusion solution
A method of programming an infusion device includes receiving an infusion rate for a time period, wherein the time period overlaps with a predefined start of a predefined period of the infusion device. The time period is converted into (1) a first converted time period extending from a start of the time period to the predefined start of the predefined period, and (2) a second converted time period extending from the predefined start of the predefined period to an end of the time period. The infusion device is programmed with the infusion rate for the first converted time period and the second converted time period.
Owner:MEDTRONIC MIMIMED INC
Sensor-Augmented Medication Infusion System
InactiveUS20110098638A1Function is disabledDigital data processing detailsFlow control using electric meansMedication infusionPhysical medicine and rehabilitation
A sensor-augmented medication infusion system includes a sensor attached to a body of a user to detect an analyte level of the user. An infusion device is adapted to be carried by the user that includes a drive mechanism operatively coupled to a reservoir containing a fluid to infuse the fluid into the body of the user, a processor operatively coupled to the drive mechanism to control the drive mechanism, a communication receiver operatively coupled to the processor to receive data from the sensor corresponding to the analyte level of the user, and a display screen operatively coupled to the processor to display the data corresponding to the analyte level of the user, wherein the infusion device includes a user-selectable function to disable the infusion device from infusing the fluid into the body of the user while continuing to display the data corresponding to the analyte level of the user on the display screen.
Owner:MEDTRONIC MIMIMED INC
Variable flow control device, system and method
InactiveUS20130138075A1Easy to controlImprove securityMedical devicesPressure infusionEngineeringInfusion pump
A device, system and method are provided for controlling the rate of infusion of fluids during infusion therapy using non-electric infusion devices. Rotation of a flow regulator dial causes an orifice connected to the inlet to modify its position relative to a particular one or more orifices or groove portions, the characteristics of which provide a certain flow rate characteristic. The regulator allows for the infusion pump to infuse at a rate that may be varied during use by the user.
Owner:BIO HEALTH FRONTIERS
Device for introducing medicine into an infusion container
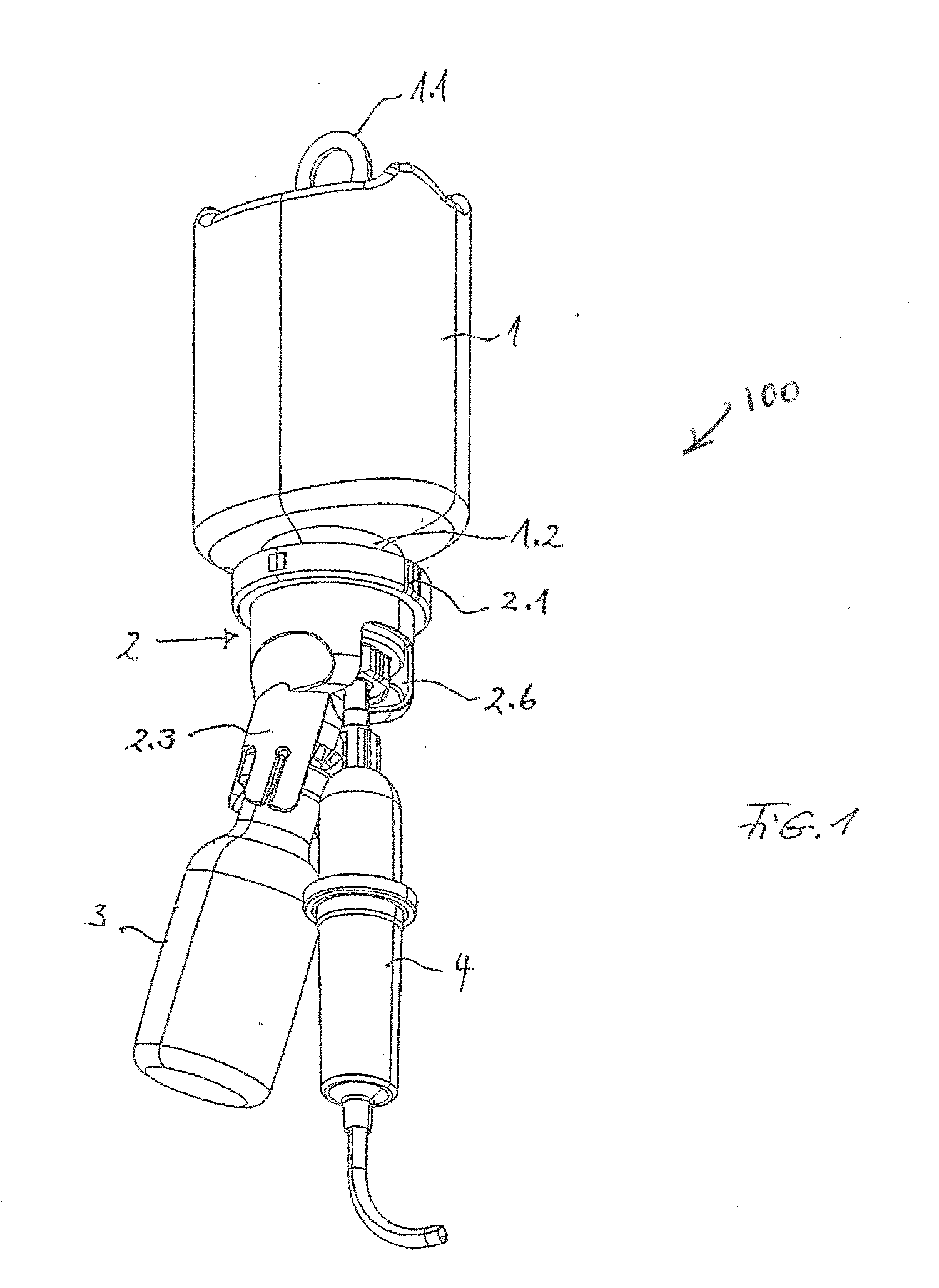
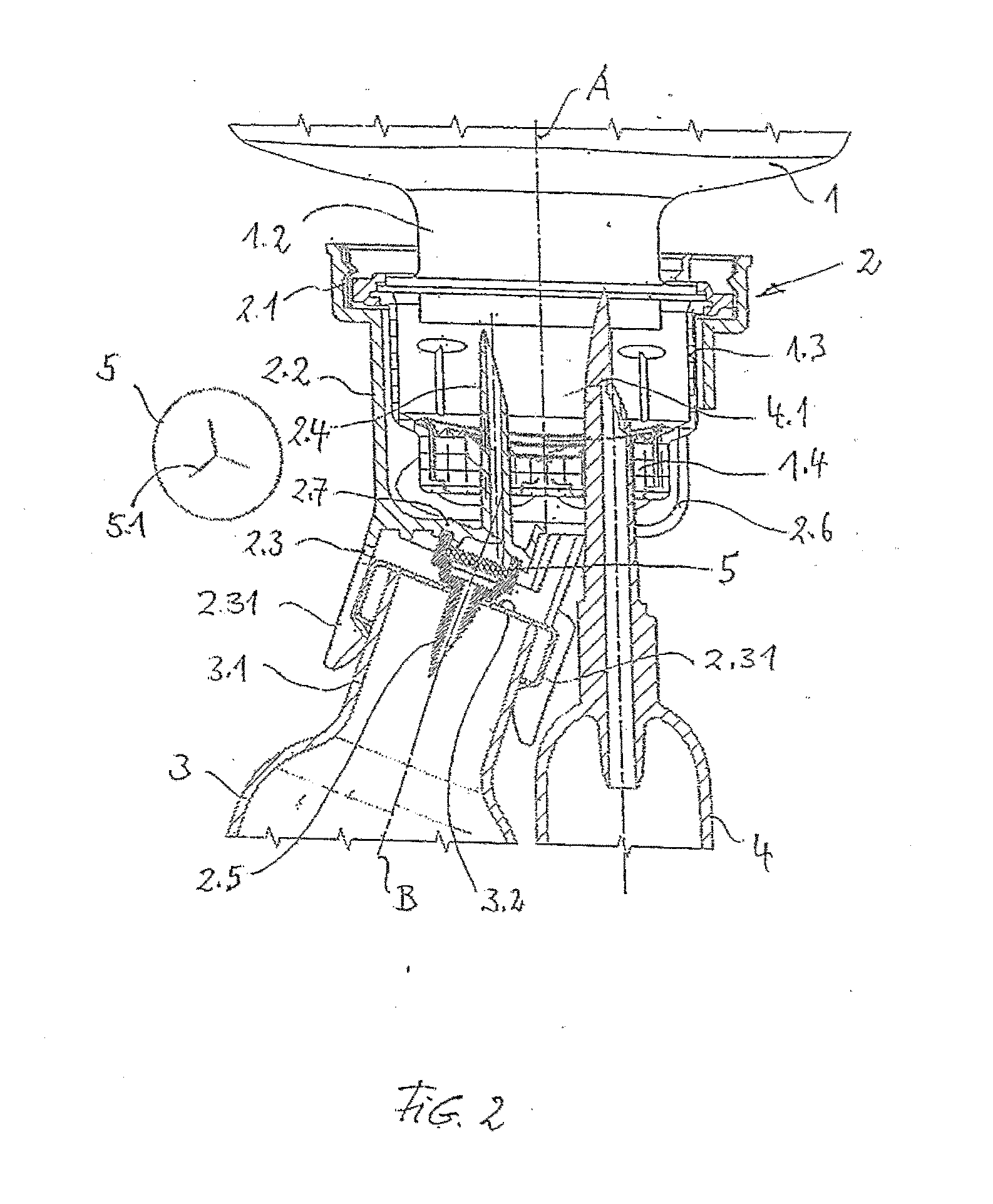
A device for adding a medicine to an infusion solution in an infusion container having a removal opening provided with a seal area. A transfer cap, which has a first hollow spike for piercing the seal area, is connected to the removal opening of the infusion container. A receiving means Dora medicine container is formed at the transfer cap, which has a second hollow spike for piercing a seal at the medicine container. A valve is arranged between the first and second hollow spikes in the transfer cap, where the valve interrupts the connection between the first and second hollow spikes and can be moved into the open position by the action of a force.
Owner:B BRAUN MELSUNGEN AG
Predictive infusion device operations and related methods and systems
ActiveUS20150165117A1Well formedDrug and medicationsMedical devicesEngineeringPhysiological condition
Infusion systems, infusion devices, and related operating methods are provided. An exemplary method of operating an infusion device capable of delivering fluid to a user involves determining a current value for a physiological condition of the user influenced by the fluid violates a first threshold value, determining a predicted value for the physiological condition of the user violates a second threshold value, and automatically altering operation of the infusion device to modify delivery of the fluid to the user after determining the predicted value violates the second threshold value when the current value violates the first threshold value.
Owner:MEDTRONIC MIMIMED INC
Guided user help system for an ambulatory infusion system
An ambulatory infusion pump can include a guided user help system that allows for a user to correct an error with the pump without needing to summon a home healthcare aide or other medical professional. When an error occurs with the pump, the user can select an option to receive help with the error. The help screen can display a possible solution for correcting the error that the user can follow. Additional help screens can display additional possible solutions if prior possible solution prove ineffective at correcting the problem.
Owner:SMITHS MEDICAL ASD INC
Reservoir compartment adapter for infusion device
The present invention provides a reservoir compartment adapter for use with a fluid delivery device. The adapter includes a first end adapted for coupling with a fluid delivery device, a second end adapted for coupling with a connector, and a structure between the first end and the second end including an interior space for receiving the reservoir, wherein an extended reservoir compartment for accommodating the reservoir is adapted to be formed when the first end is coupled to the fluid delivery device, and the reservoir is adapted to be secured in the extended reservoir compartment when the connector is coupled to the second end. The adapter allows a user of a delivery device accommodating reservoirs of a certain size to manage periods where increased medication dosage is needed without the burden of carrying a larger delivery device for accommodating reservoirs filled with the increased dosage.
Owner:MEDTRONIC MIMIMED INC
Infusion flow guidewire system
ActiveUS20080312671A1Highly deliverableSubstantial thrombectomy/fibrinolytic infusion effectivenessCannulasGuide wiresThrombusHigh pressure
An infusion flow guidewire system including a delivery sheath and an infusion flow guidewire. The minimal cross section flexible infusion flow guidewire includes a nitinol hypotube, a flexible tip and distally located rearwardly directed orifices for infusion of fibrinolytics and for introduction of high pressure fluids for maceration and rearwardly directed flow of thrombus debris located in tortuous small sized vessels. Apparatus, some of which is removably attachable, is provided for grasping members of the invention for rotational torquing and for longitudinal actuation along and within the vasculature.
Owner:BOSTON SCI LTD
User interface improvements for medical devices
ActiveUS20090177991A1Easy to navigateMaintaining the infusion parametersDrug and medicationsSurgeryEmergency medicineHuman–computer interaction
A method and system is disclosed for operating a medical device with or without a cassette in place. A method is disclosed for adding additional VTBI to an ongoing infusion without stopping the infusion and with maintaining the infusion parameters. A method and system is disclosed for changing the CCA without having to interrupt or completely stop an ongoing infusion. Quick titration buttons are provided to allow improved navigation between various delivery display screens.
Owner:ICU MEDICAL INC
Method and device for monitoring the flow speed of an infusion solution
Method and device for monitoring the flow speed of an infusion solution, such as with hemofiltration or hemodiafiltration. The device has a tube for conducting an infusion solution from a source of infusion solution to a pump device in the form of a metering pump, such as a peristaltic pump or a ceramic pump, and on to an infusion device, such as a drip chamber. The flow speed is determined by the metering pump. The flow speed is monitored separately in that the pressure across a restriction device in the tube is measured. The restriction device can be a separately arranged restriction valve. Alternatively the restriction device is arranged by the tube having a small internal diameter of the order of 0.5 mm in size. The pressure measuring device only needs to measure the absolute pressure with respect to the atmosphere. The hydrostatic pressure due to the infusion bag being hung above the pump and the pressure meter is subtracted in a calculation device.
Owner:GAMBRO LUNDIA AB
Control of a drug infusion device
PendingUS20190096518A1Detecting faulty hardware by power-on testDrug and medicationsWork flowMedication identifier
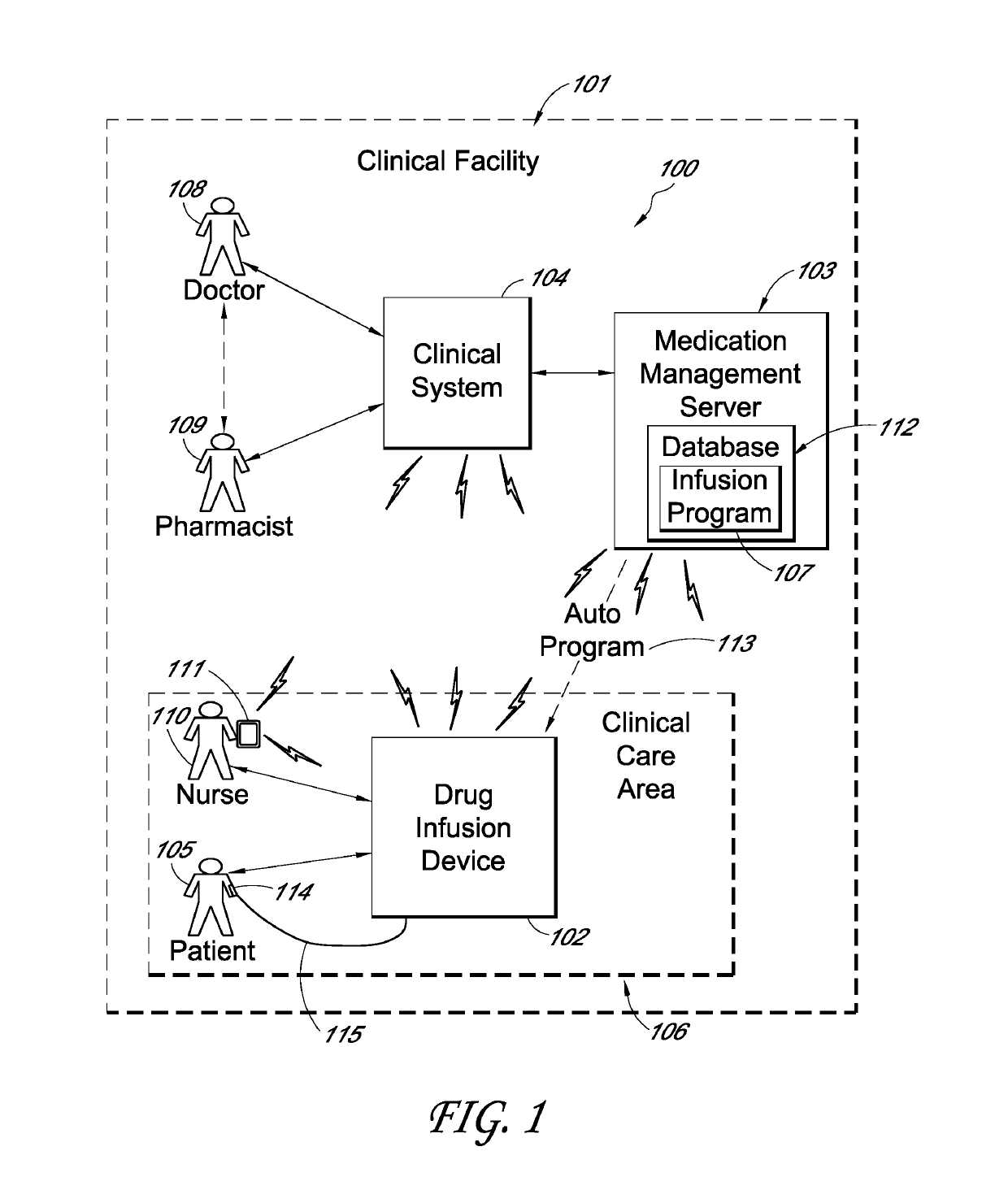
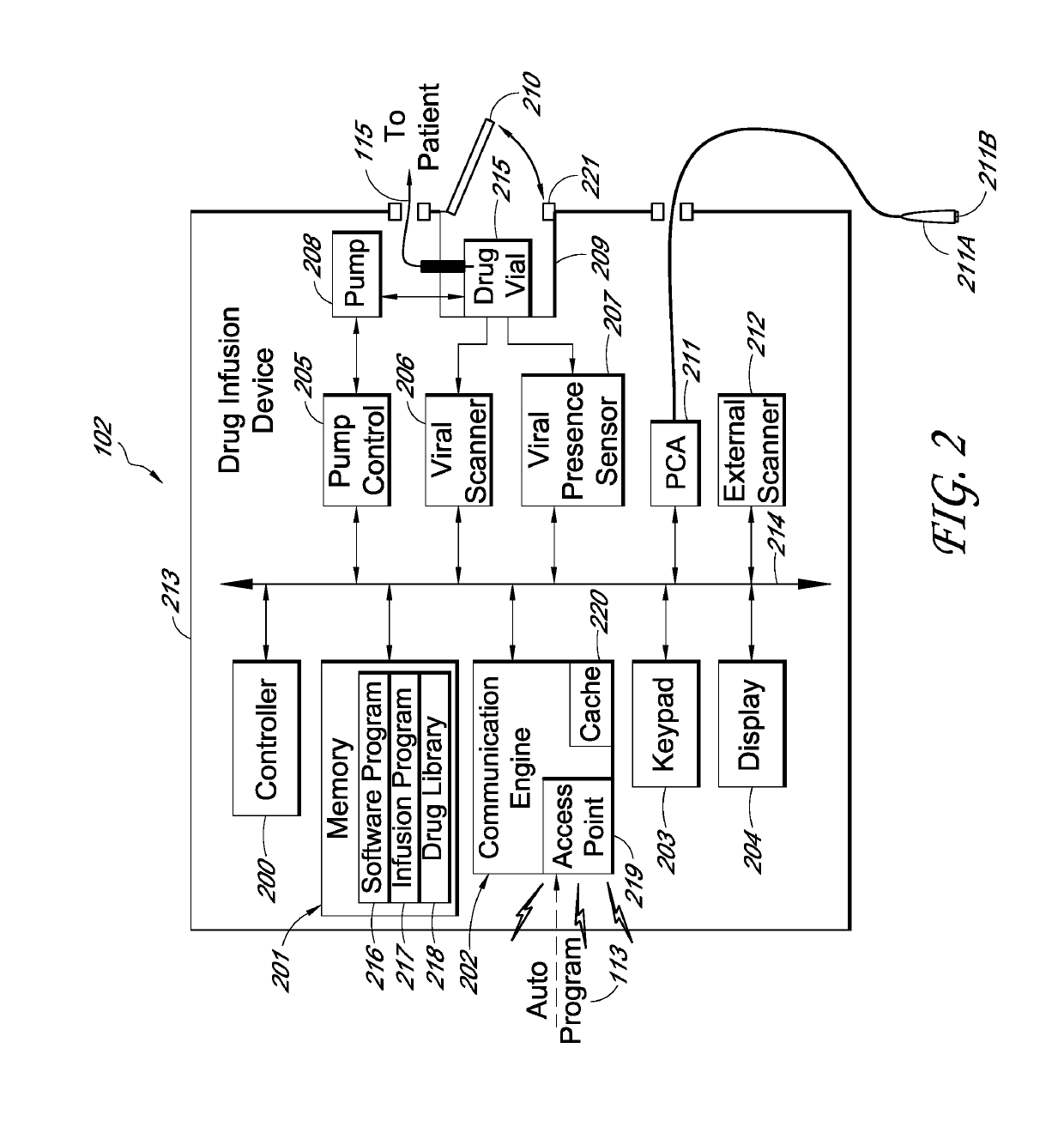
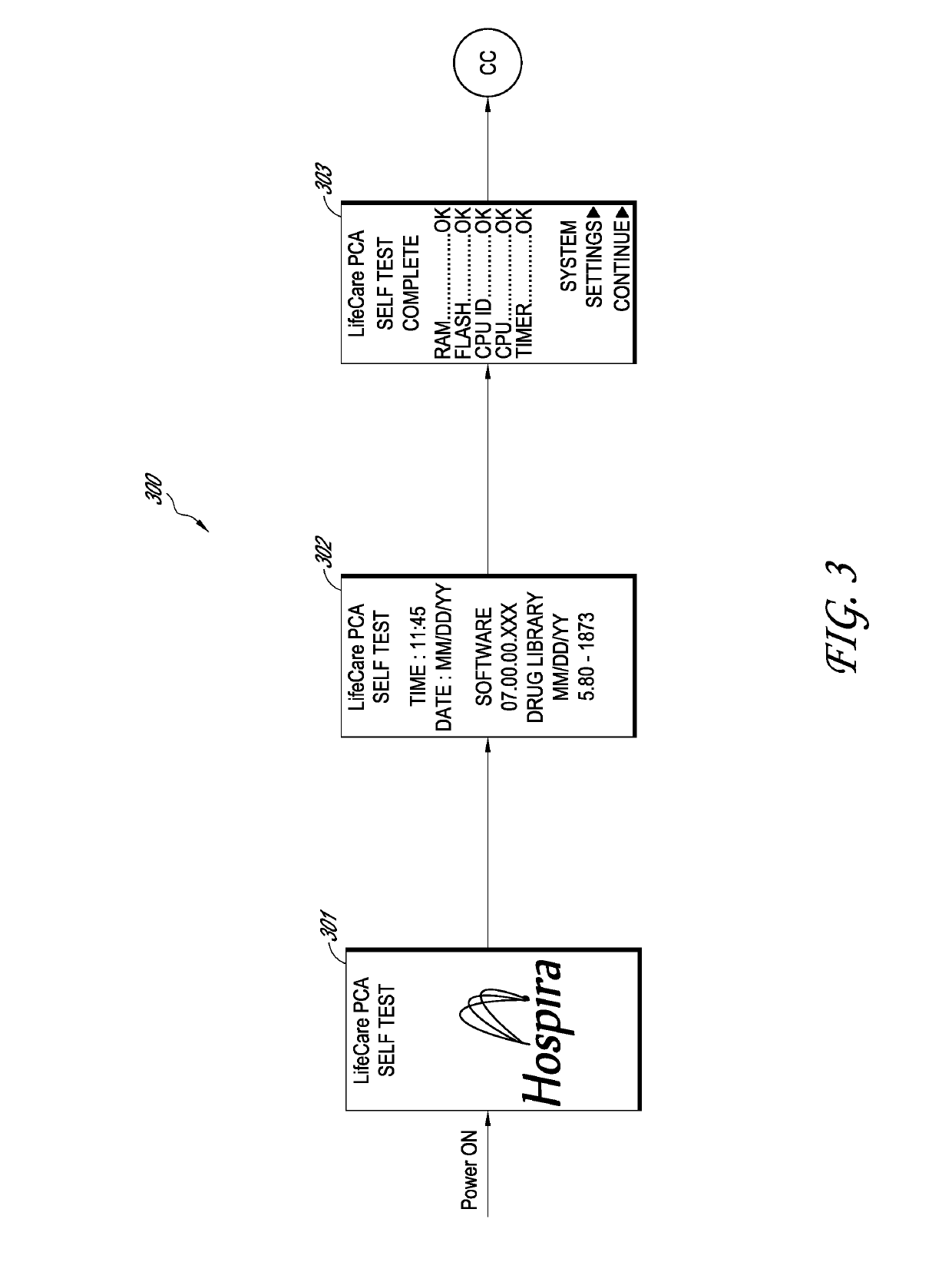
A drug infusion device determines whether an infusion program is stored within a memory. If so, a user is prompted to confirm usage of the program and a drug identifier, and to review the configuration of the device. If the program is not in the memory, the drug infusion device is enabled to receive an auto program with which it can configure itself. When auto program receipt is enabled, the drug infusion device waits for the auto program or for a selection for manual entry of the infusion program. A menu screen is displayed to indicate that the drug infusion device is waiting for the auto program, but the menu screen is not displayed if the auto program is already received. Therefore, a waiting for auto program menu screen may be avoided in many cases, reducing device setup time. In some embodiments, removal of a drug vial resets the device to a point after a power-on self-test and thus provides an easy method for resetting the device to a point that avoids unnecessary steps and expands a window of opportunity for receiving an auto program compared to prior work flows.
Owner:ICU MEDICAL INC
Breviscapine infusion preparation and its preparing method
InactiveCN1425385AInfusion stabilityOvercome the disadvantages of poor clarityOrganic active ingredientsCardiovascular disorderMedicineArginine
The Breviscapine infusion preparation is compounded with Breviscapine as main component, glucose or sodium chloride, propylene glycol, L-arginine, sodium bisulphate, EDTA-2Na and water for injection through certain technological process. The preparation is suitable for great dosage application clinically to avoid cross infection caused by intermediate links. In addition, the present invention is clear and stable.
Owner:上海博泰医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com