Polyene phosphatidyl choline capsule and preparation process thereof
A polyene phosphatidylcholine and capsule technology, which is applied in the directions of capsule delivery, oil/fat/wax inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of unstable polyene phosphatidylcholine stability, High moisture and oxygen permeability, unfavorable easy oxidative hydrolysis and other problems, to achieve the effect of ensuring safety and effectiveness, high degree of automation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
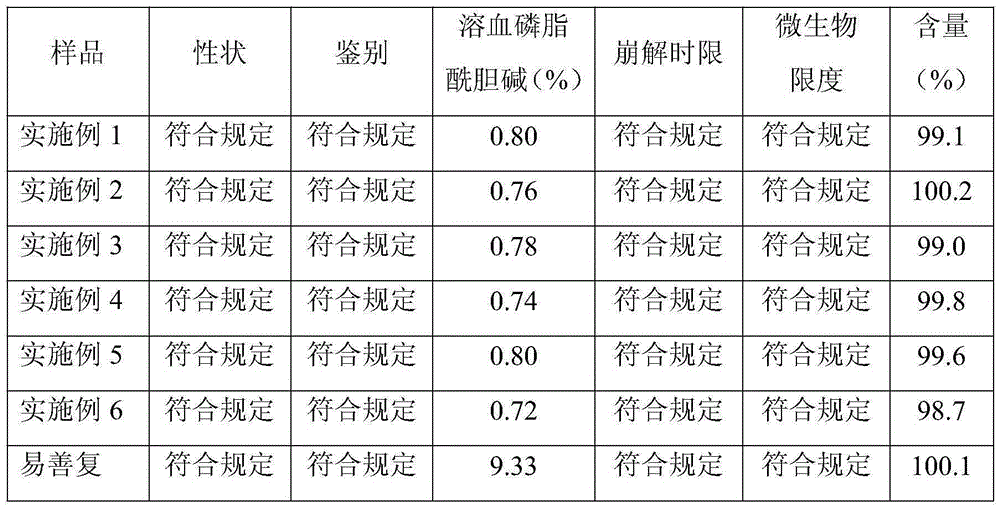
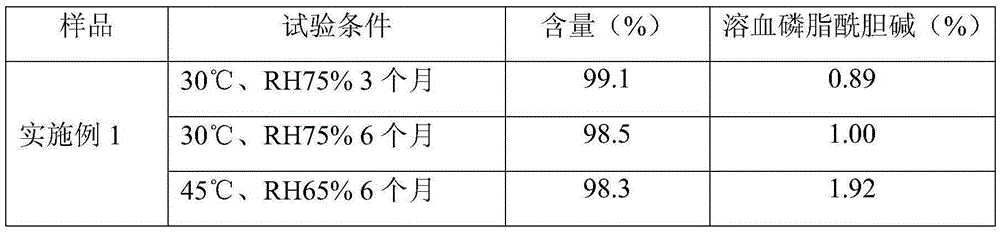
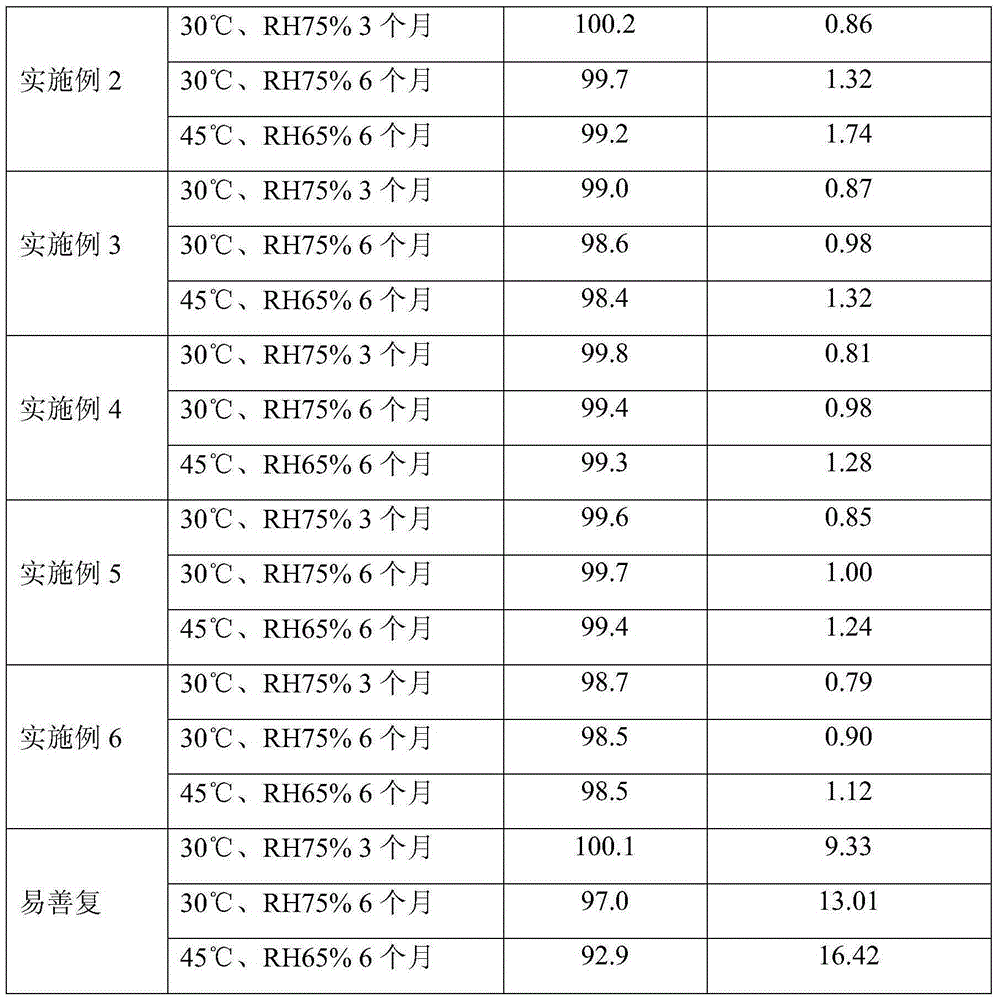
Examples
Embodiment 1
[0031] Prescription (based on 1000 capsules):
[0032] polyene phosphatidylcholine 228g Soybean oil 22.8g hard fat 15g hydrogenated castor oil 12g ethanol 30g。
[0033] Preparation Process:
[0034] (1) the polyene phosphatidylcholine of prescription quantity is mixed with soybean oil, ethanol, for subsequent use;
[0035] (2) In a vacuum state, heat (60-90°C) hydrogenated castor oil and hard fat in the prescribed amount and stir until completely melted, then add the polyene phosphatidylcholine-ethanol-da soybean oil mixture, mix well;
[0036] (3) Transfer the material obtained in the above step (2) to the hopper of the capsule filling machine, and carry out capsule filling under the condition of heat preservation at 50-80°C.
Embodiment 2
[0038] Prescription (based on 1000 capsules):
[0039] polyene phosphatidylcholine 228g Soybean oil 228g hard fat 70g
[0040] hydrogenated castor oil 2g。
[0041] Preparation Process:
[0042] (1) the polyene phosphatidylcholine of prescription quantity is mixed with soybean oil, for subsequent use;
[0043] (2) Under vacuum, heat (60-90°C) hydrogenated castor oil and hard fat in the prescribed amount and stir until completely melted, then add the polyene phosphatidylcholine-soybean oil mixture obtained in the above step (1) ,well mixed;
[0044] (3) Transfer the material obtained in the above step (2) to the hopper of the capsule filling machine, and carry out capsule filling under the condition of heat preservation at 50-80°C.
Embodiment 3
[0046] Prescription (based on 1000 capsules):
[0047] polyene phosphatidylcholine 228g Soybean oil 45.6g hard fat 20g hydrogenated castor oil 6g ethanol 10g。
[0048] Preparation Process:
[0049] (1) the polyene phosphatidylcholine of prescription quantity is mixed with soybean oil, ethanol, for subsequent use;
[0050] (2) In a vacuum state, heat (60-90°C) hydrogenated castor oil and hard fat in the prescribed amount and stir until completely melted, then add the polyene phosphatidylcholine-ethanol-da soybean oil mixture, mix well;
[0051](3) Transfer the material obtained in the above step (2) to the hopper of the capsule filling machine, and carry out capsule filling under the condition of heat preservation at 50-80°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com