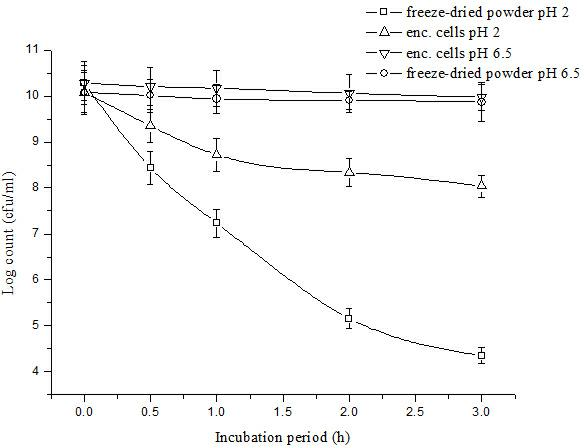
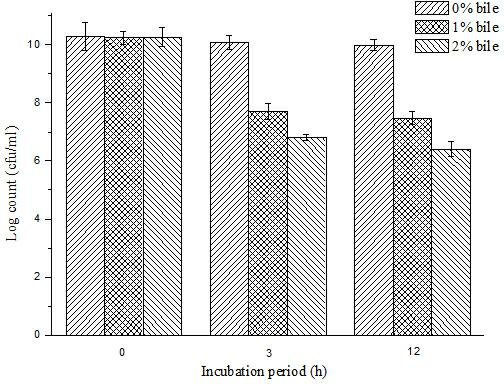
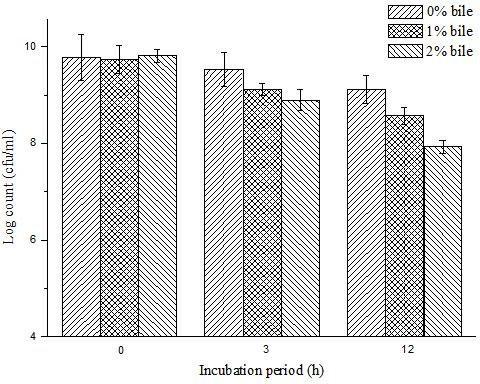
Bifidobacterium microcapsule and preparing method thereof
The technology of bifidobacteria and microcapsules is applied in the field of preparation of probiotic products, which can solve the problems of intolerance to gastric acid and the decrease of viable bacteria content, and achieve the effects of short storage period, simple preparation method and good storage stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Step 1: Under the temperature condition of 37°C, anaerobically culture the activated bifidobacterium in the liquid medium for 24 hours, collect the bacteria by centrifugation, wash with 0.85% normal saline to prepare the bacteria suspension;
[0047] Step 2: Prepare a protective agent, add 1.23 g of animal-derived collagen, 11.50 g of trehalose, and 4.65 mL of glycerin to each 1000 mL of protective agent, and the rest is distilled water;
[0048] Step 3: Mix the sterilized protective agent and bacterial suspension evenly at a volume ratio of 2:1, let stand for 30 minutes, pre-freeze at -20°C for 2 hours, and then store at -50°C under vacuum The temperature is 50 millitorr and dried in a vacuum freeze-drying oven for 48 hours to prepare freeze-dried bacteria powder;
[0049]Step 4: Add 0.1 g of freeze-dried bacteria powder to the sodium alginate solution with a mass fraction of 1%, the mass ratio of freeze-dried bacteria powder to sodium alginate is 1:3, and mix well to ...
Embodiment 2
[0053] Step 1: Under the temperature condition of 37°C, anaerobically culture the activated bifidobacterium in the liquid medium for 24 hours, collect the bacteria by centrifugation, wash with 0.85% normal saline to prepare the bacteria suspension;
[0054] Step 2: prepare a protective agent, add 1.23 g of human-like collagen, 11.50 g of trehalose, and 4.65 mL of glycerin per 1000 mL of protective agent, and the rest is distilled water;
[0055] Step 3: Mix the sterilized protective agent and bacterial suspension evenly at a volume ratio of 2:1, let stand for 30 minutes, pre-freeze at -20°C for 2 hours, and then store at -50°C under vacuum The temperature is 75 millitorr and dried in a vacuum freeze-drying oven for 48 hours to prepare freeze-dried bacteria powder;
[0056] Step 4: Add 0.1 g of freeze-dried bacteria powder to the sodium alginate solution with a mass fraction of 1.5%, the mass ratio of freeze-dried bacteria powder to sodium alginate is 1:4, and mix well to obtai...
Embodiment 3
[0060] Step 1: Under the temperature condition of 37°C, anaerobically culture the activated bifidobacterium in the liquid medium for 24 hours, collect the bacteria by centrifugation, wash with 0.85% normal saline to prepare the bacteria suspension;
[0061] Step 2: prepare a protective agent, add 1.23 g of human-like collagen, 11.50 g of sucrose, and 4.65 mL of glycerin per 1000 mL of protective agent, and the rest is distilled water;
[0062] Step 3: Mix the sterilized protective agent and bacterial suspension evenly at a volume ratio of 2:1, let stand for 30 minutes, pre-freeze at -20°C for 2 hours, and then store at -50°C under vacuum The temperature is 100 millitorr and dried in a vacuum freeze-drying oven for 48 hours to prepare freeze-dried bacteria powder;
[0063] Step 4: Add 0.1 g of freeze-dried bacteria powder to the sodium alginate solution with a mass fraction of 2%, the mass ratio of freeze-dried bacteria powder to sodium alginate is 1:5, and mix well to obtain t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com