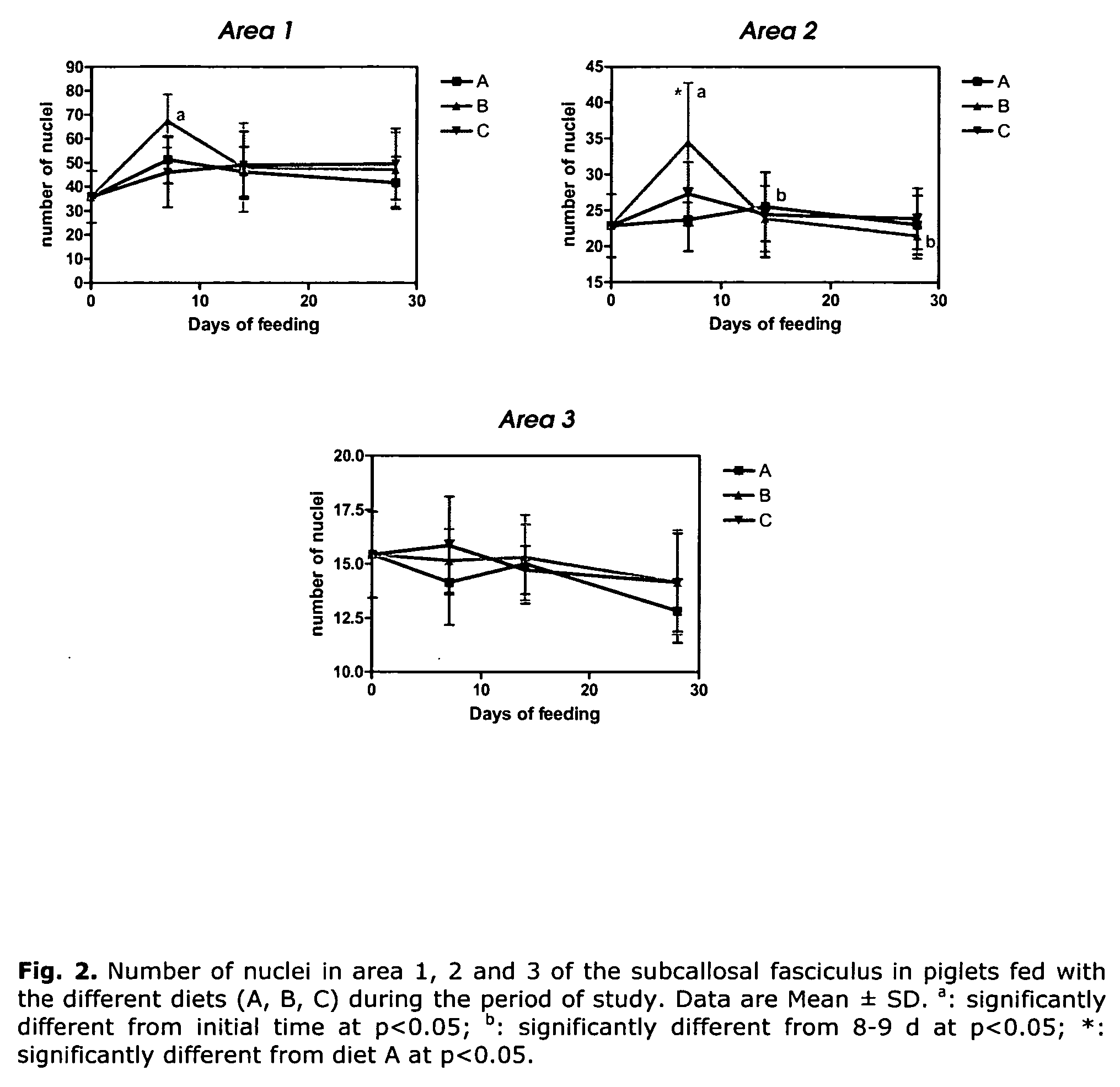
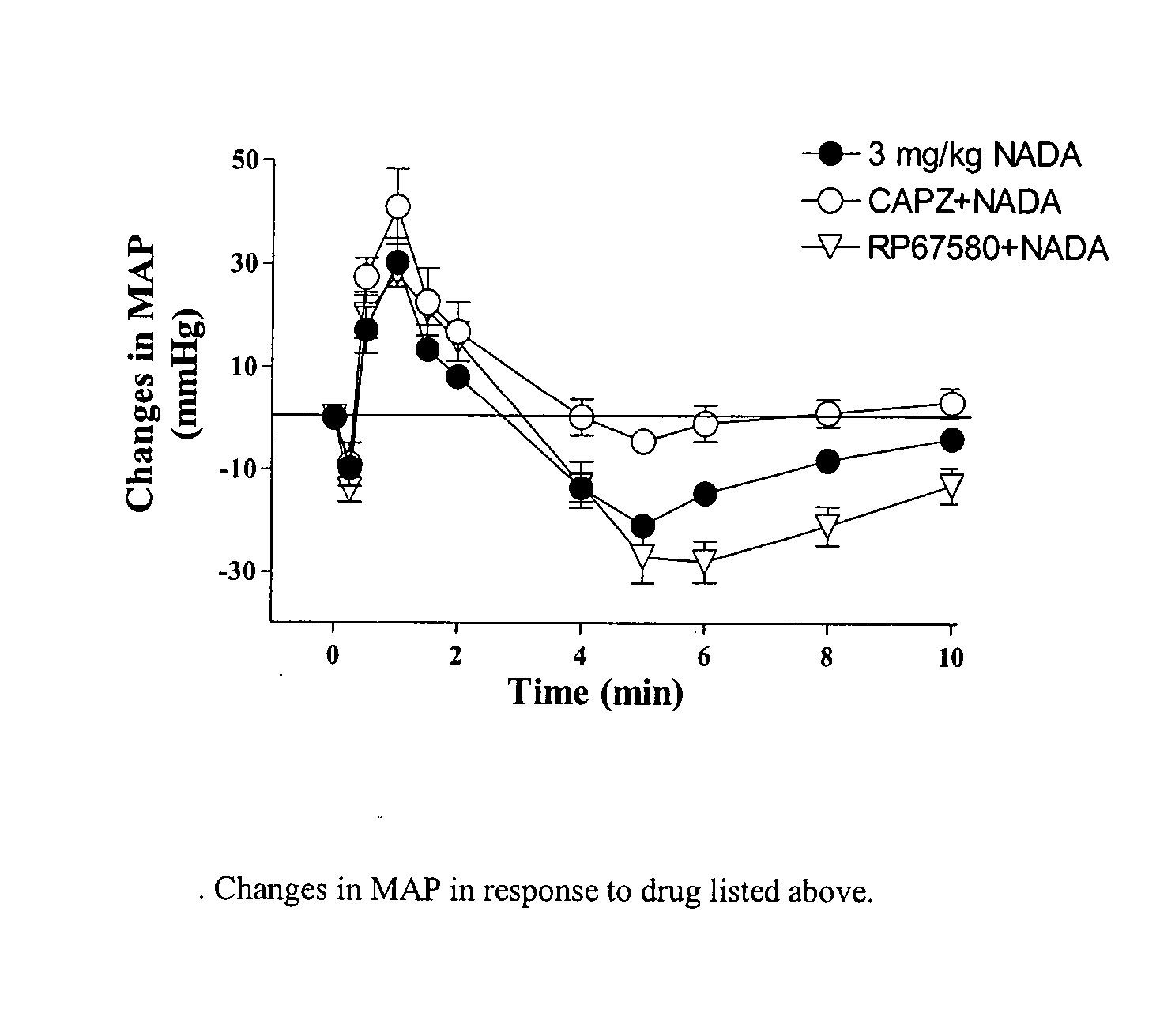
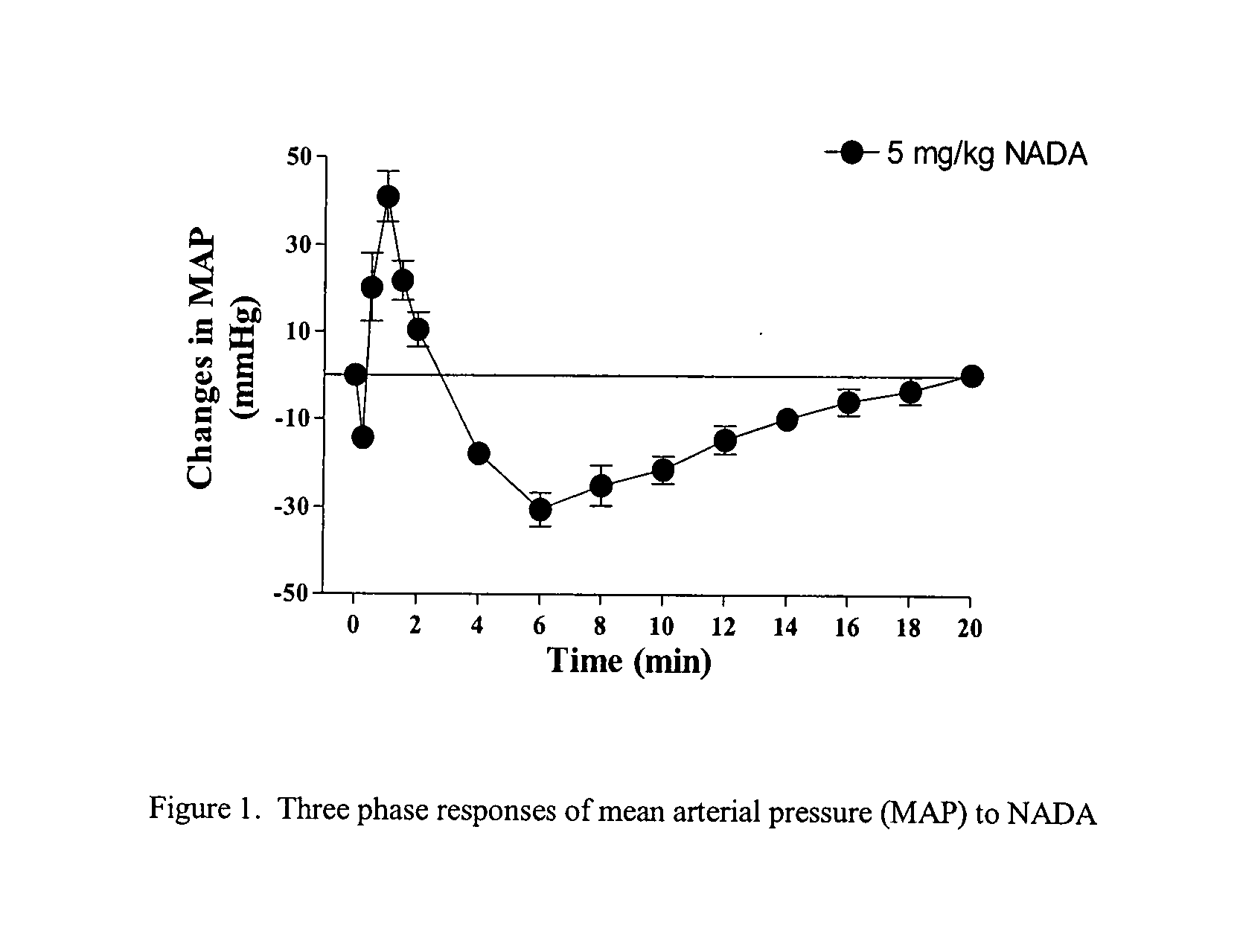
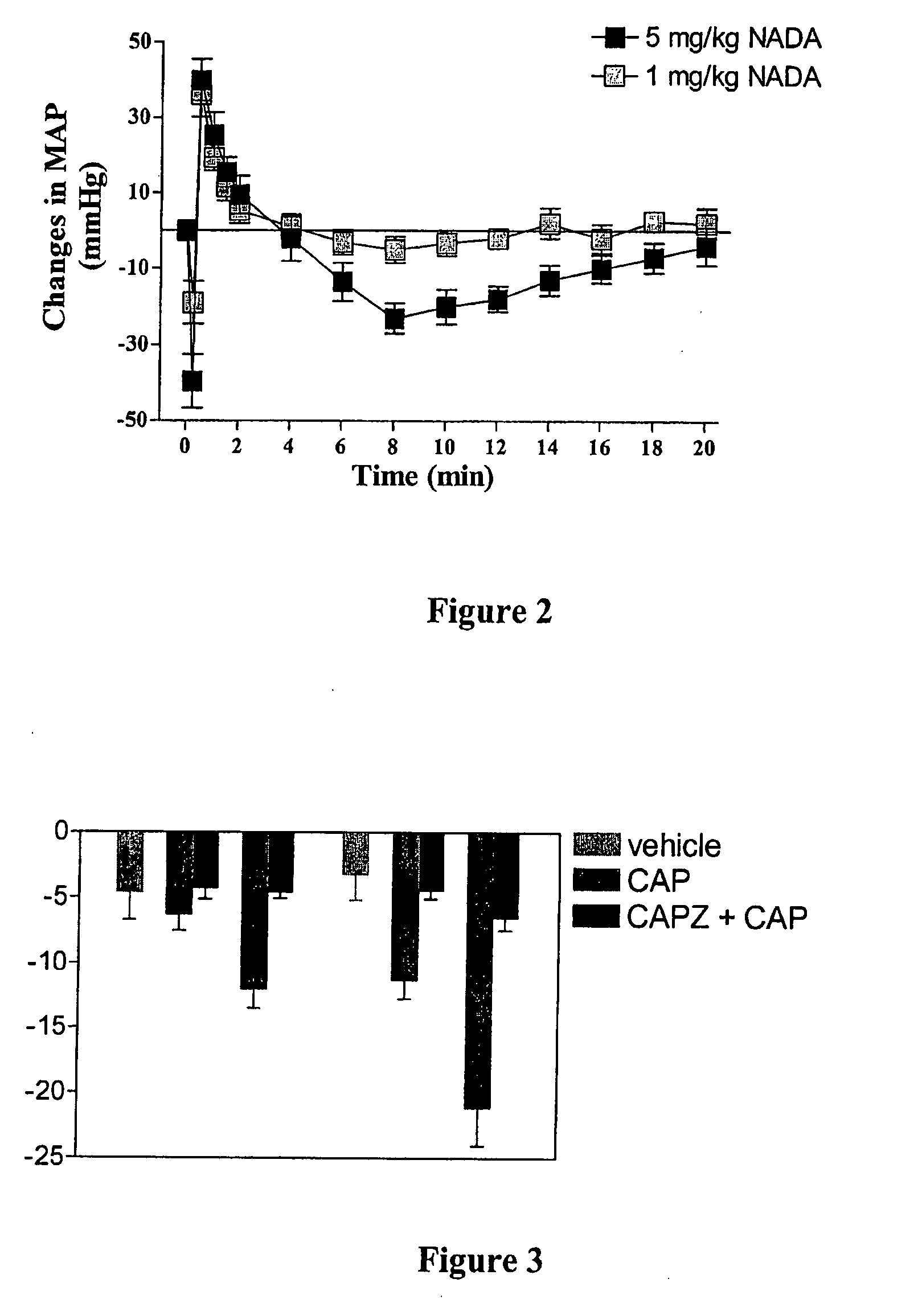
Patents
Literature
691 results about "Arachidonic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
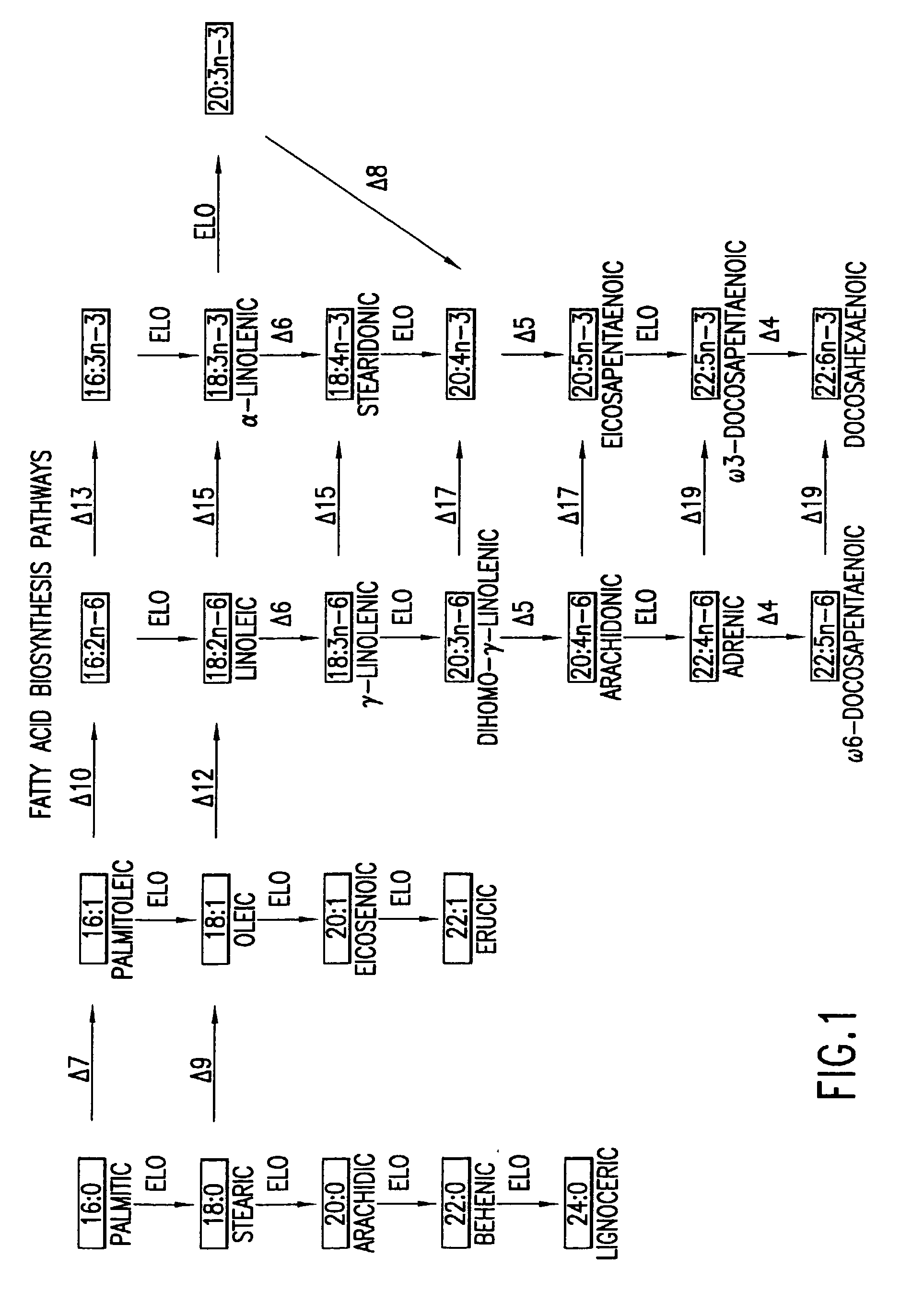
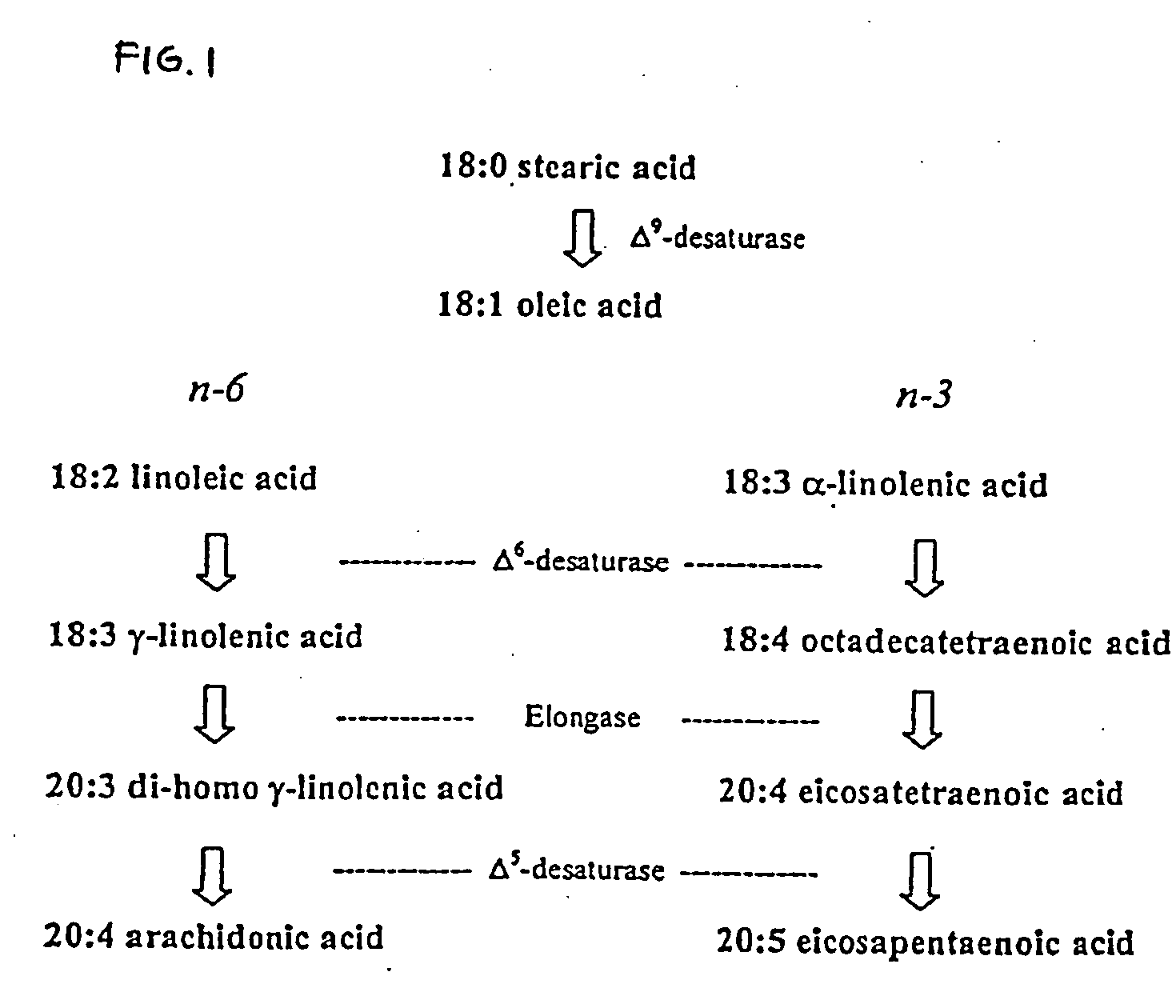
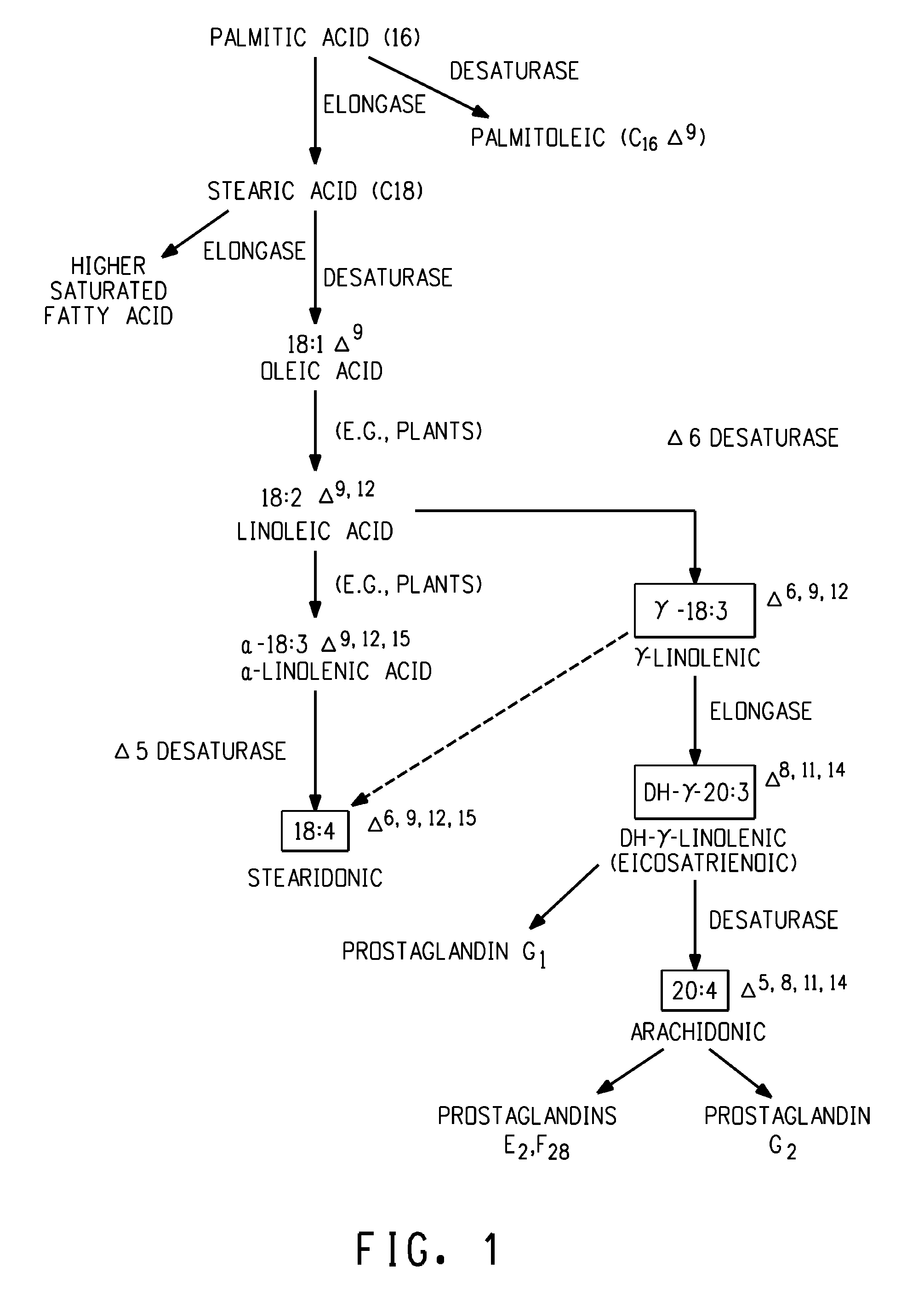
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter.
Process for preparing materials for extraction
InactiveUS20060122410A1Improve extraction efficiencyQuality improvementFungiUnicellular algaeArachidonic acid supplementationFermentation
The present invention relates to a process for preparing a biomass, such as from a microbial fermentation, for an extraction process to separate desired chemicals, nutritional products, bioactive components, proteins, carbohydrates, and lipids, from the biomass. Particularly preferred substances to extract include docosahexaenoic acid, docosapentaenoic acid, and arachidonic acid. The present invention also includes extracting the prepared biomass. Biomasses to be treated in accordance with the methods of the invention include plant, animal, and microbial biomass, particularly a microorganism such as Crypthecodinium cohnii and a fungus such as Mortierella alpina.
Owner:MARTEK BIOSCIENCES CORP
Compositions for treatment of diabetic complications
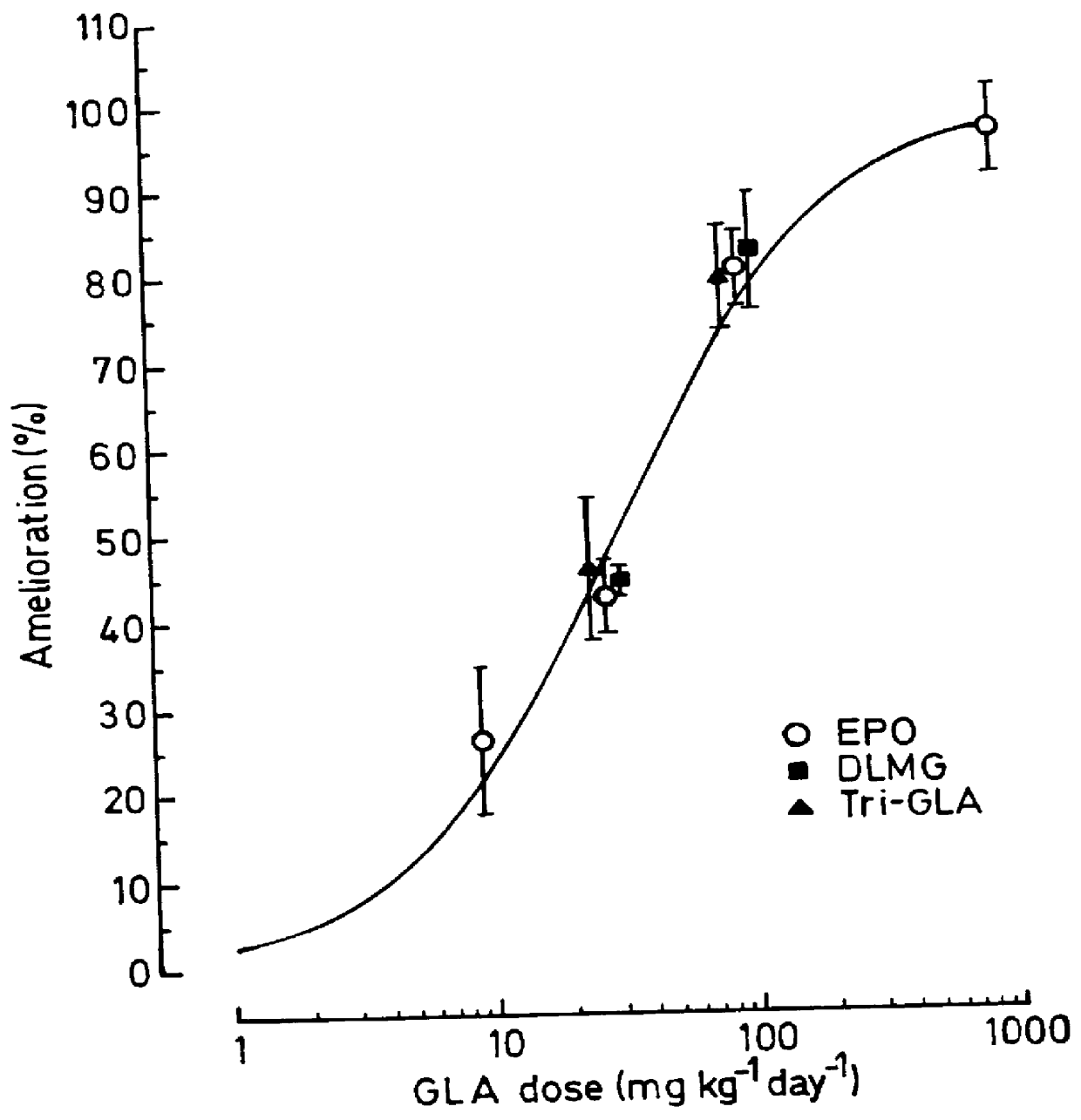
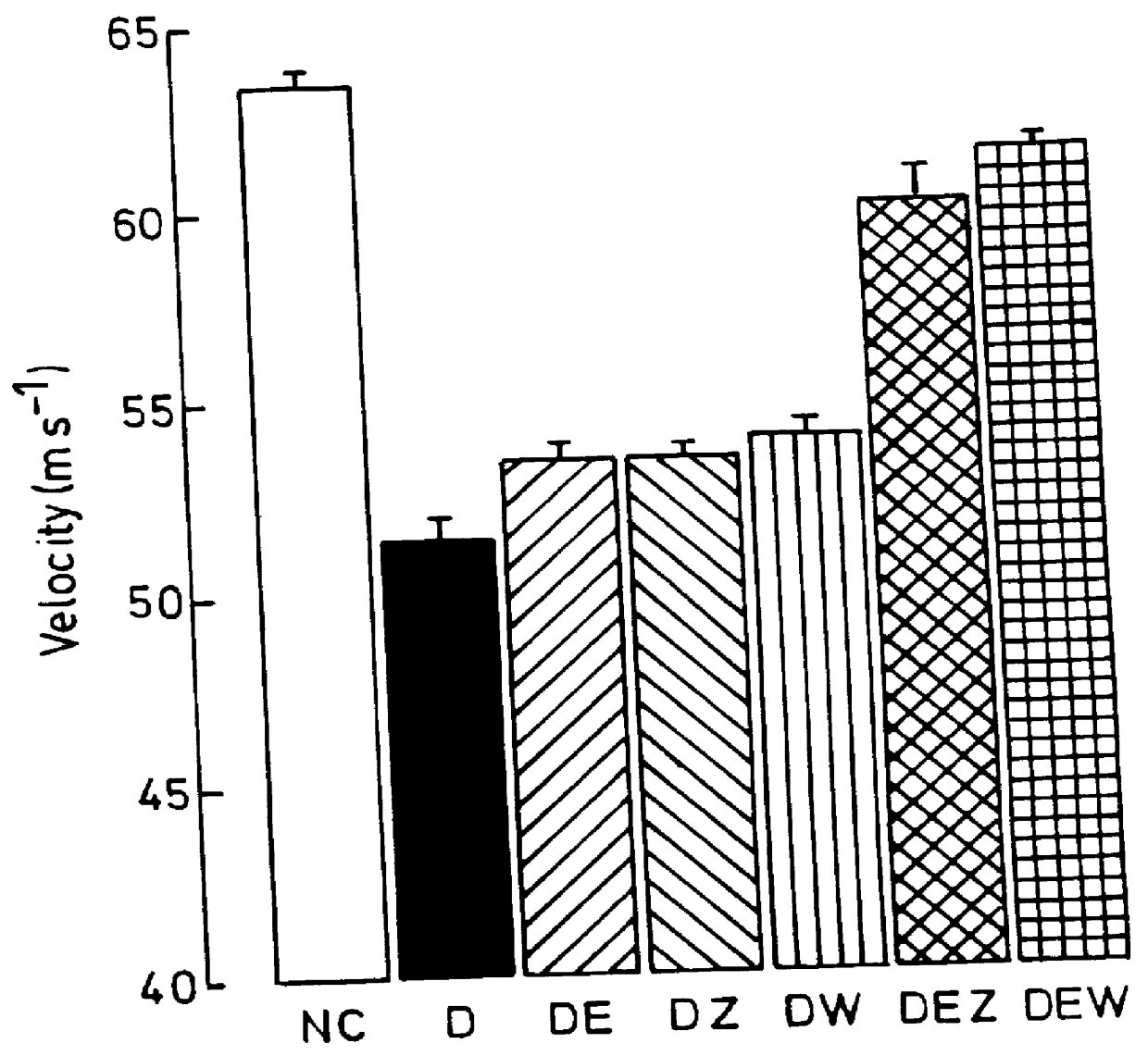
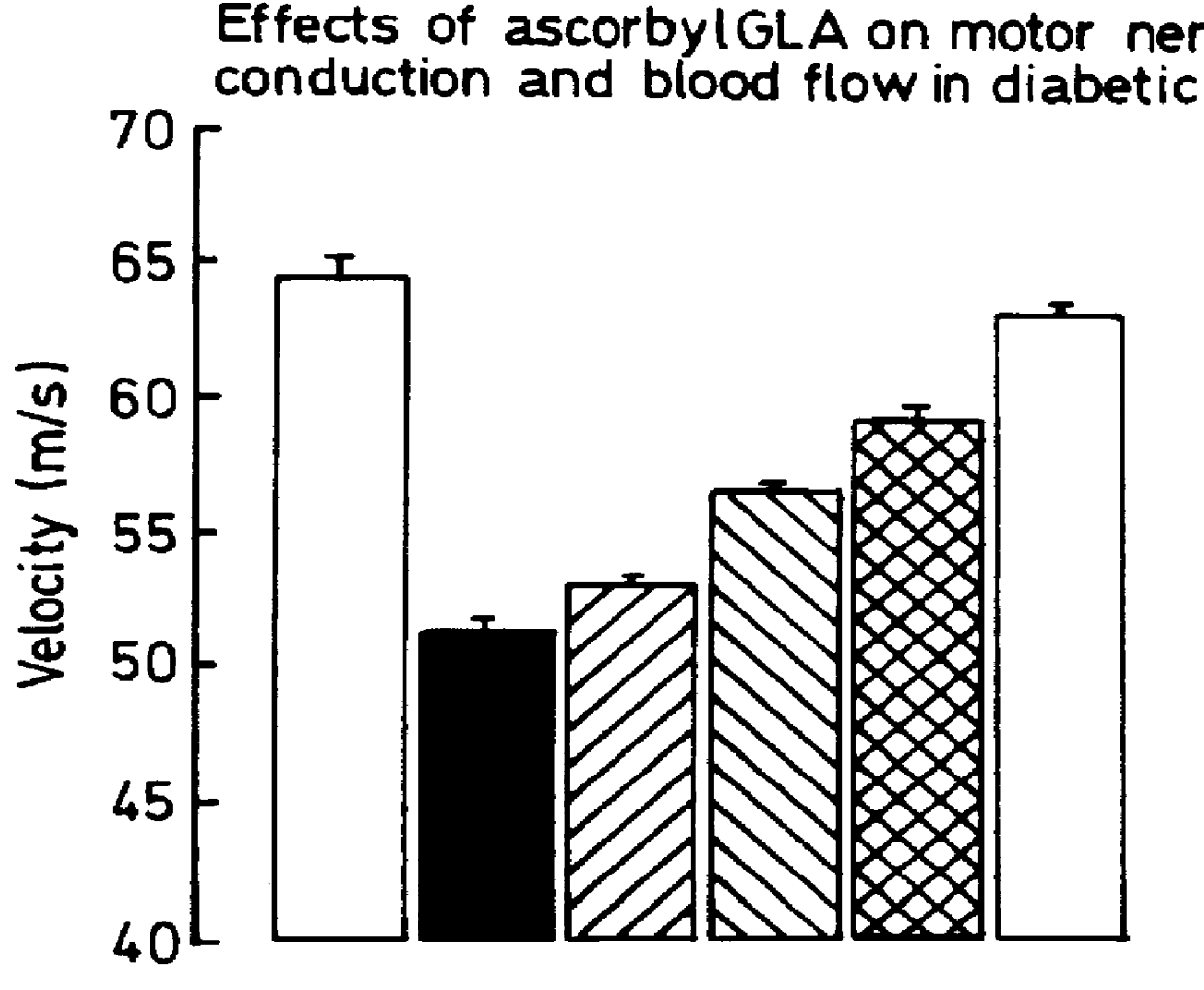
Use of 6-desaturated n-6 fatty acids, especially gammalinolenic acid (GLA), dihomogammalinolenic acid (DGLA) or arachidonic acid (AA), together with a pharmaceutically acceptable material reducing intracellular levels of sorbitol in the body, particularly an aldose reductase inhibitor, in the treatment of (including prophylactic treatment), and in the preparation of medicaments for the treatment of (including prophylactic treatment), the long-term complications of diabetes mellitus. Pharmaceutical compositions of said materials. The ascorbate esters of 6-desaturated n-6 fatty acids (other than GLA or DGLA) per se.
Owner:SCOTIA HLDG
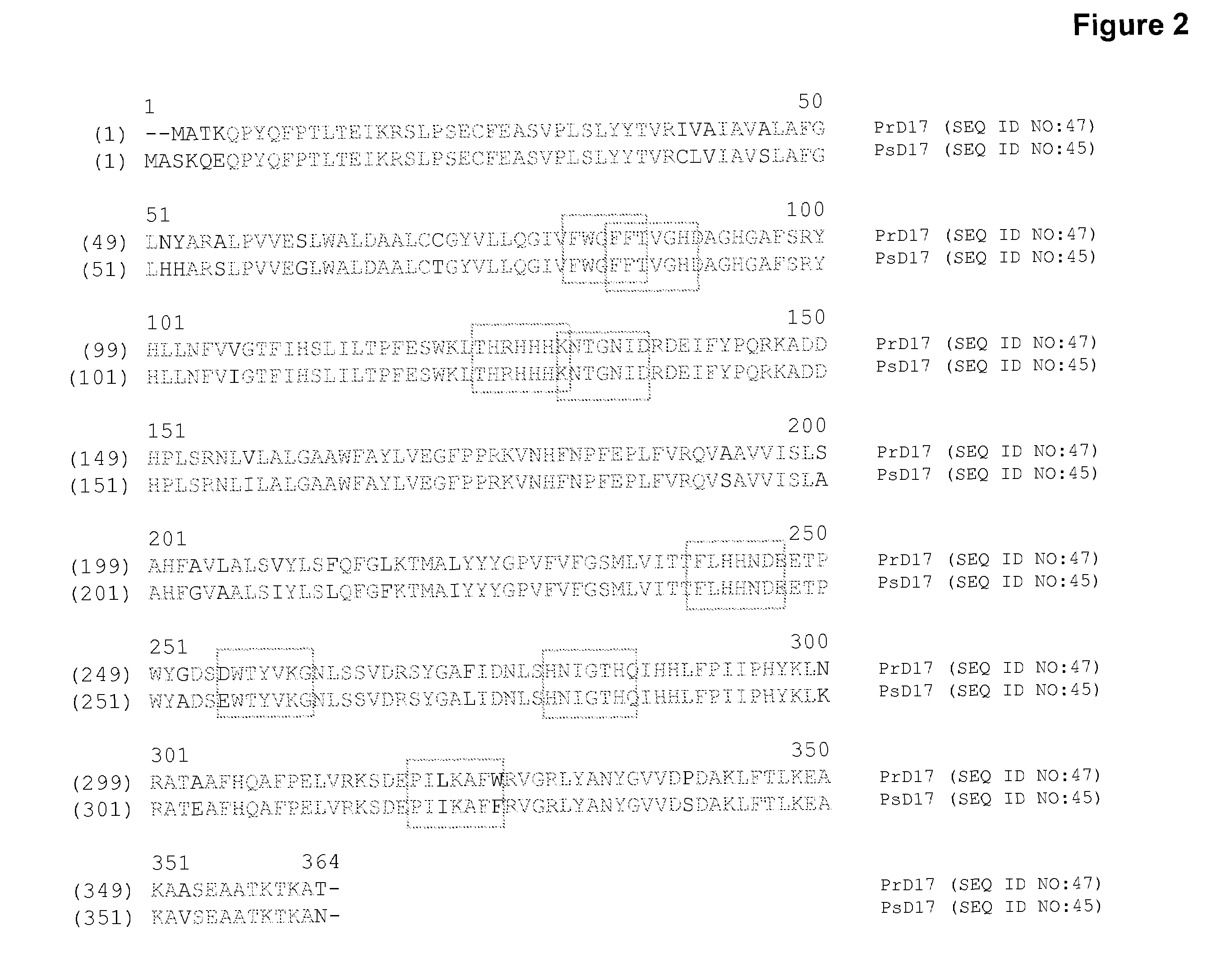
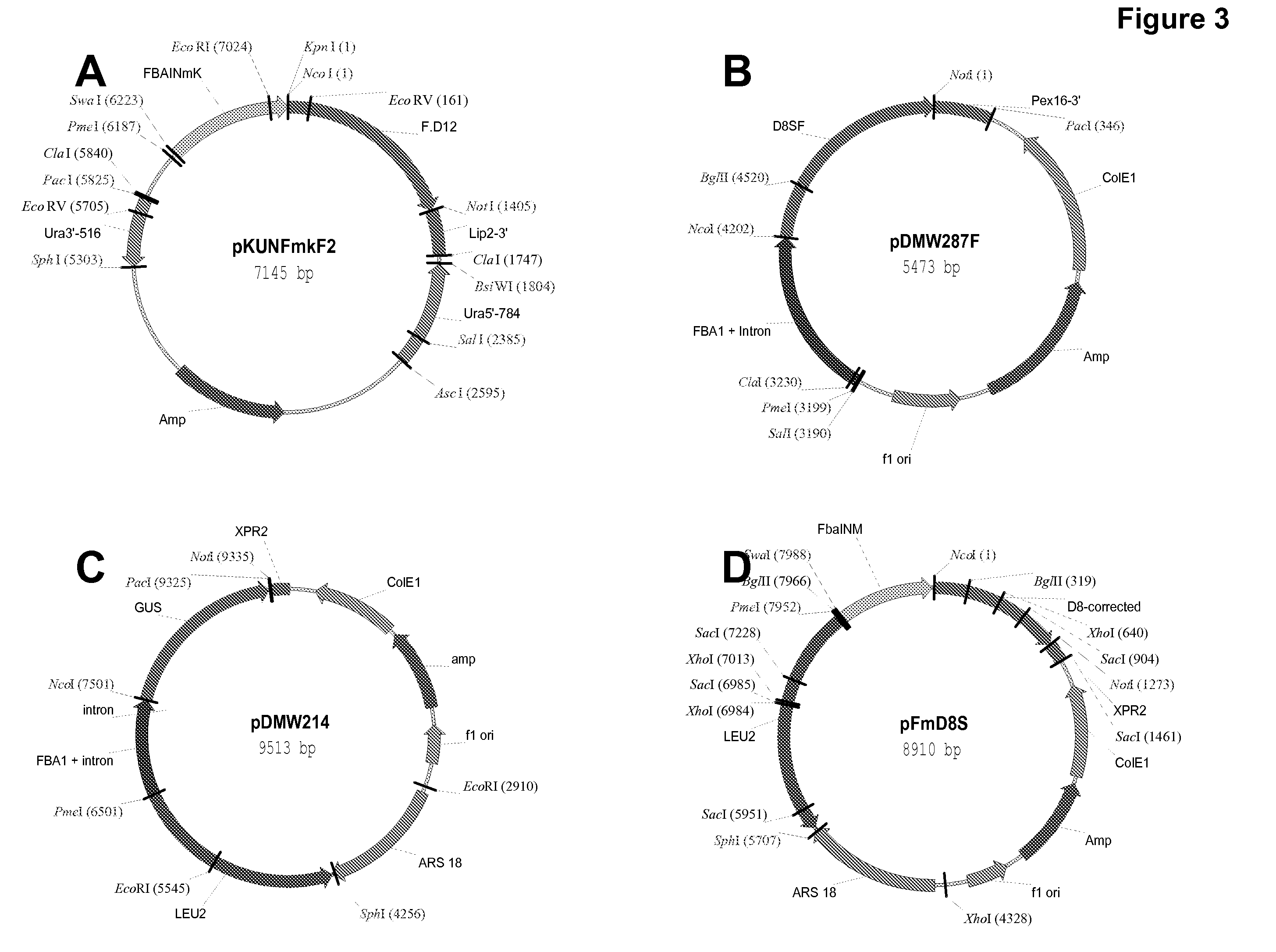
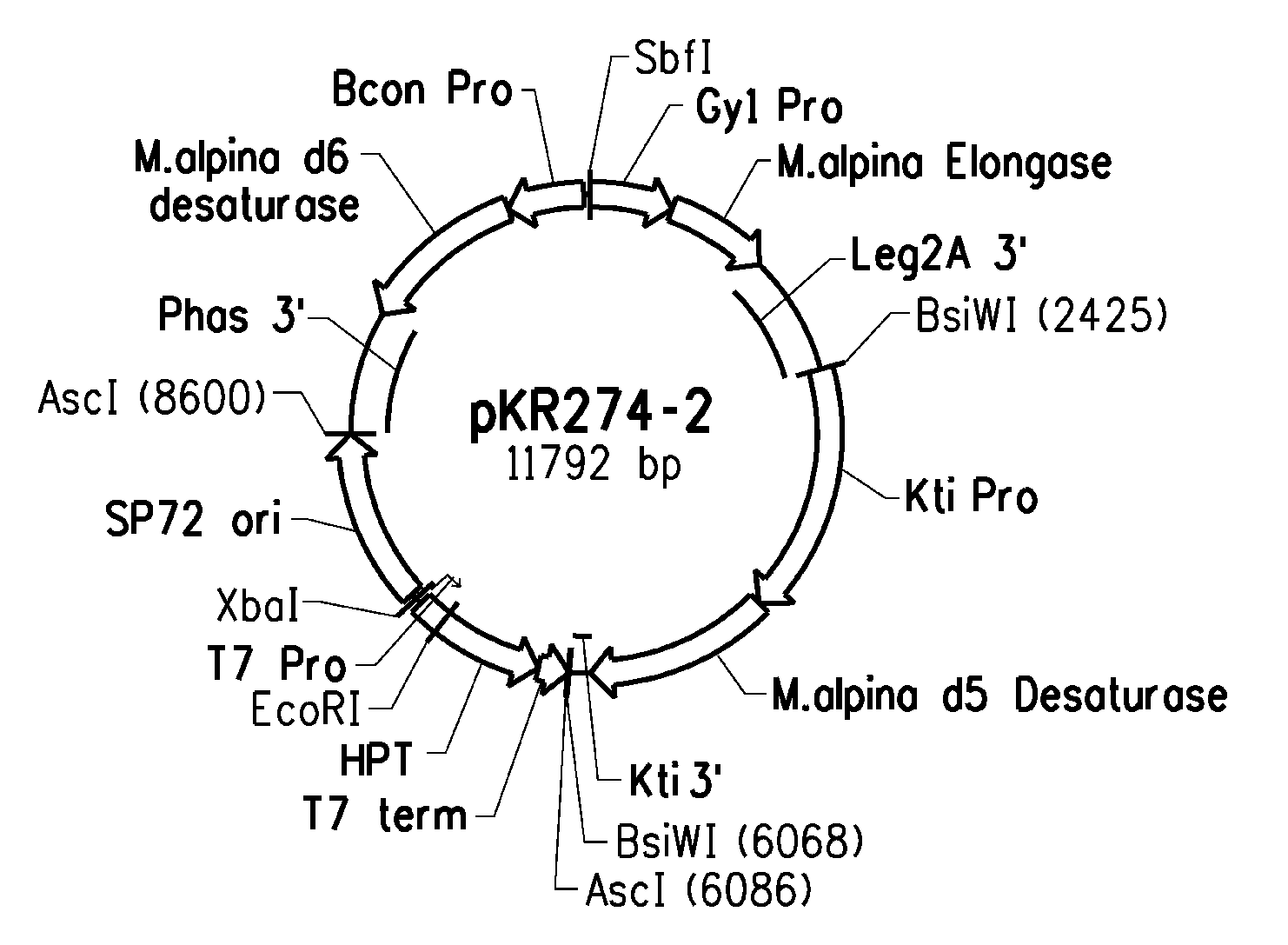
High arachidonic acid producing strains of Yarrowia lipolytica
Engineered strains of the oleaginous yeast Yarrowia lipolytica capable of producing greater than 10% arachidonic acid (ARA, an ω-6 polyunsaturated fatty acid) in the total oil fraction are described. These strains comprise various chimeric genes expressing heterologous desaturases, elongases and acyltransferases, and optionally comprise various native desaturase and acyltransferase knockouts to enable synthesis and high accumulation of ARA. Production host cells are claimed, as are methods for producing ARA within said host cells.
Owner:DUPONT US HLDG LLC
Infant formulas for early brain development
Disclosed are infant formulas comprising fat, protein, carbohydrate, vitamins, and minerals, including on an as-fed basis, at least about 5 mg / L of gangliosides, at least about 150 mg / L of phospholipids, at least about 70 mg / L of total sialic acid with at least about 2.5% as lipid-bound sialic acid, at least about 0.13% docosahexaenoic acid by weight of total fatty acids, and at least about 0.25% arachidonic acid by weight of total fatty acids. Also disclosed are methods of accelerating brain development, neural migration, and cognitive development in an infant by administering the infant formulas during the first 2-4 months of life, preferably as a sole source of nutrition.
Owner:ABBOTT LAB INC
Compositions and methods for transient receptor potential vanilloid (TRPV) channel mediated treatments
InactiveUS20080051454A1Increasing TRPV1-responsesCompound screeningBiocideScreening methodArachidonic acid supplementation
The present inventions relate to therapeutic compositions comprising, and methods utilizing, arachidonic acid derivatives and analogs for treatment of patients demonstrating symptoms of pathological conditions. Specifically, the inventions relate to therapeutic compositions for activating transient receptor potential vanilloid-1 channels (TRPV1). Additionally, therapeutic compositions are provided for increasing TRPV1-type responses. These pathological conditions include, but are limited to, hypertension, in particular salt induced hypertension, and cardiovascular complications, including myocardial infarction, kidney dysfunction, diabetes, and inflammation. Further, the inventions relate to drug screening methods for providing additional therapeutic compounds.
Owner:MICHIGAN STATE UNIV
Lipid composition for infant formula and method of preparation
InactiveUS6034130AImprove stabilityBiocideFatty acid esterificationDocosahexaenoic acidLipid composition
Synthetic lipid composition in which the content and the distribution of the fatty acids are similar to those of human milk fat, containing less than 2% by weight of free fatty acids, in which palmitic acid is predominantly at the 2-position of the triacylglycerols and the arachidonic and docosahexaenoic acids are distributed between the 1-, 2- and 3-positions and in particular predominantly at the 2-position of the triacylglycerols.
Owner:NESTEC SA
Nutrition bar
InactiveUS20050181019A1Stable and goodExtended shelf lifeBiocideHeavy metal active ingredientsRice proteinIngested food
A nutrition bar comprising about 10% wt or more of soy and / or rice protein, at least one transition metal or transition metal compound, and about 2% wt or more of a humectant, and wherein the at least one transition metal or transition metal compound is in a substantially water insoluble form at 20° C. or the nutrition bar has an Aw of 0.45 or less or about 1% wt or more of the soy and / or rice protein is in the form of nuggets and the humectant is selected from polyols. The bars have elevated levels of soy and / or rice protein, yet do not suffer unacceptable from a deterioration in taste or other organoleptic properties over time. In other aspects, a nutrition bar or other food which incorporates pro-oxidants and / or polyunsaturated fatty acids or their sources in encapsulated form, especially as microcapsules. The pro-oxidants may be metal salts such as copper, manganese, iron and / or zinc salts. Sources of omega-3 fatty acids include fish oil. Processes for preparing the polyunsaturated fatty acid capsules are also disclosed. The polyunsaturated fatty acid capsules / microcapsules are prepared by forming an emulsion of the unsaturated fatty acid with a carrier, spray drying the emulsion to form a powder and encapsulating powder, especially with a fluid bed. The invention is especially useful for encapsulating polyunsaturated fatty acids, or oil sources thereof, most preferably omega-3 and omega-6 fatty acids, such as arachidonic acid, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), lineoleic acid, linolenic acid (alpha linolenic acid), and gamma-linolenic acids, fish oil, and oil sources of C18:2 and C18:3 fatty acids such as canola oil, soybean oil or blends thereof.
Owner:SLIM FAST FOODS COMPANY A DIV OF CONOPCO
A kind of infant formula milk powder that does not get angry and its preparation process
ActiveCN102283289ANot ediblePromote digestion and absorptionMilk preparationVegetable oilFructooligosaccharide
The invention relates to an anti-inflaming infant formula milk powder and a preparation process thereof. The anti-inflaming infant formula milk powder comprises the following components in percentage by weight: 22%-50% of lactose, 10%-20% of goat milk whey protein concentrate, 9.8%-20% of structural grease 1,3-dioleoyl 2-palmitoyl triglyceride, 10%-13.5% of non-fat goat milk powder, 8%-10% of vegetable oil, 2%-8% of beta-casein, 0.8%-1.6% of fructooligosaccharides, 0.5%-1.5% of mineral premix, 0.3%-1% of galactooligosaccharide, 0.2%-1% of immunoglobulin G, 0.2%-0.5% of lactulose, 0.10%-0.45% of arachidonic acid, 0.1%-0.6% of docosahexaenoic acid, 0.06%-0.12% of vitamin premix, 0.04%-0.06% of lactoferrin, and 0.01%-0.06% of nucleotide. The invention also includes the preparation process ofthe infant formula milk powder. The nutrition constituents and the functions of the infant formula milk powder are close to those of breast milk, the infant formula milk powder is easy to assimilate,and infants do not get inflamed after eating the milk powder.
Owner:AUSNUTRIA DAIRY CHINA
Elongase genes and uses thereof
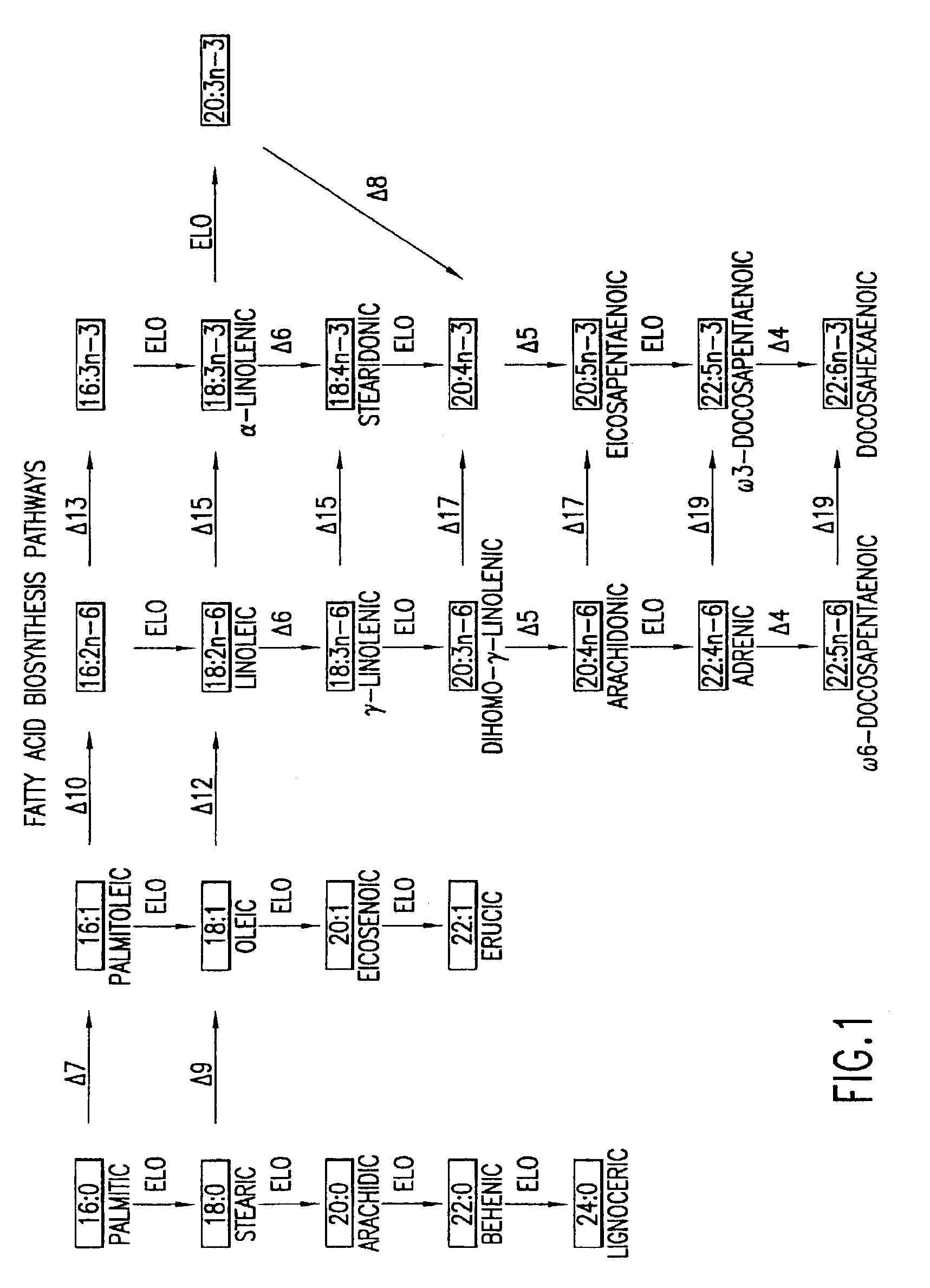
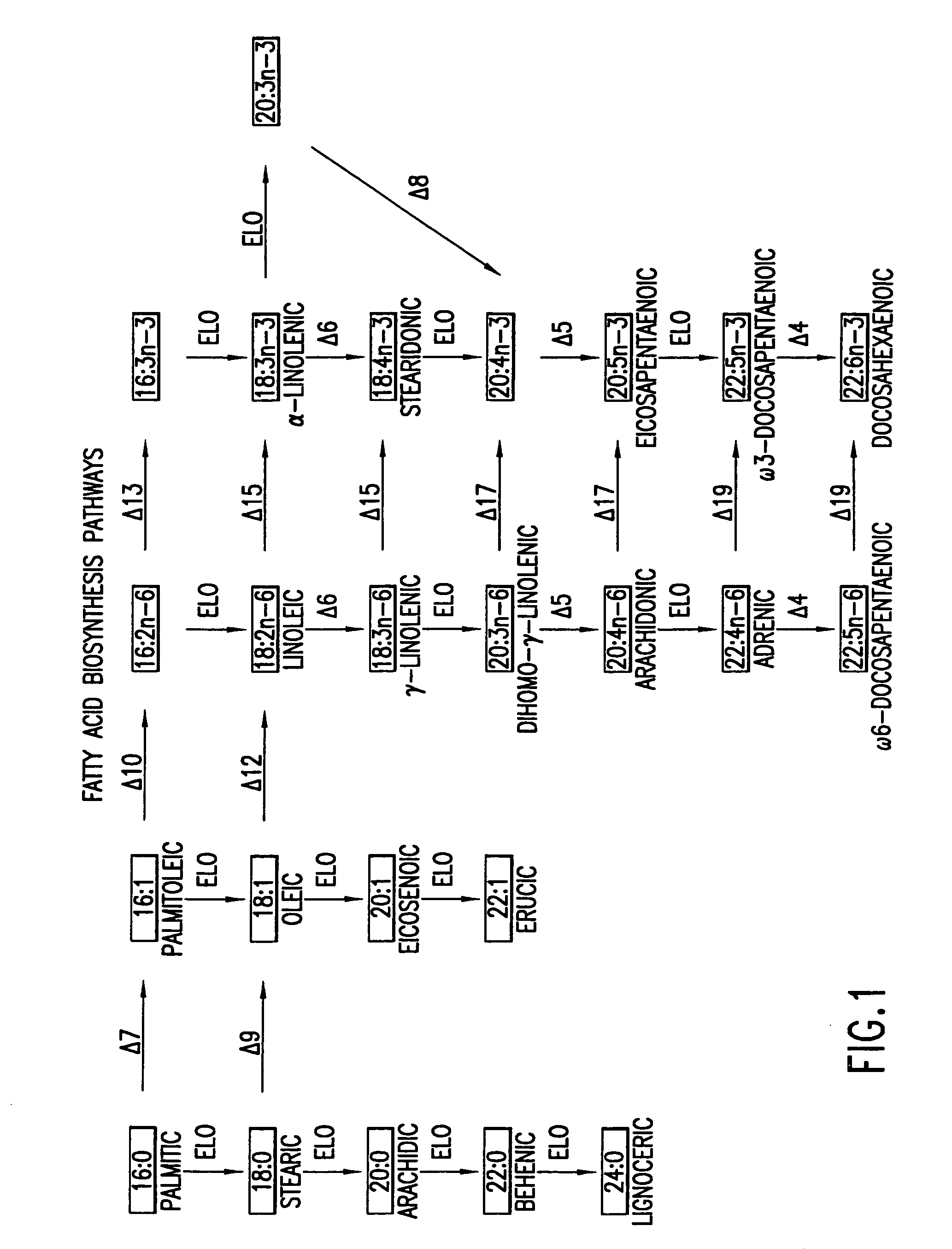
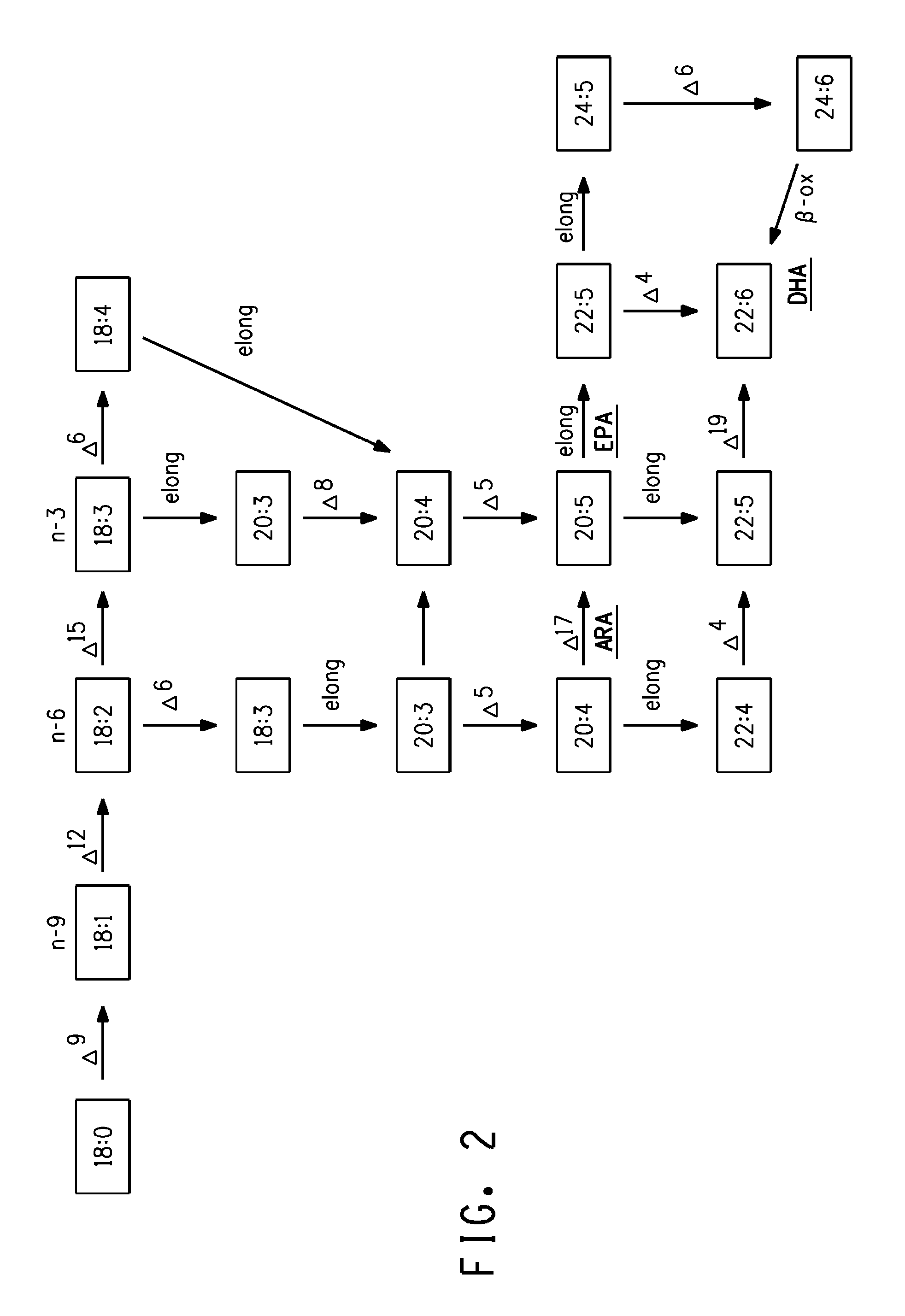
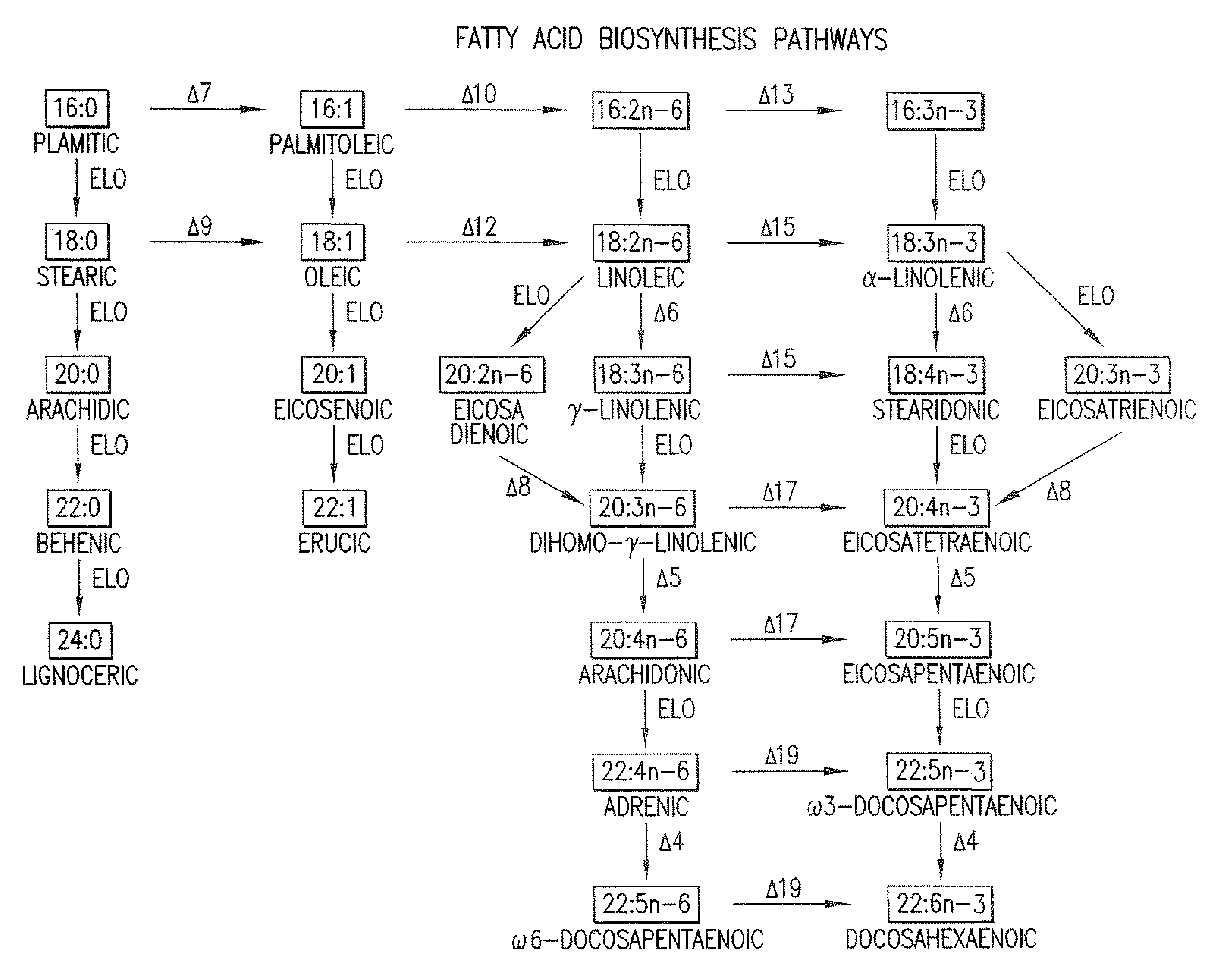
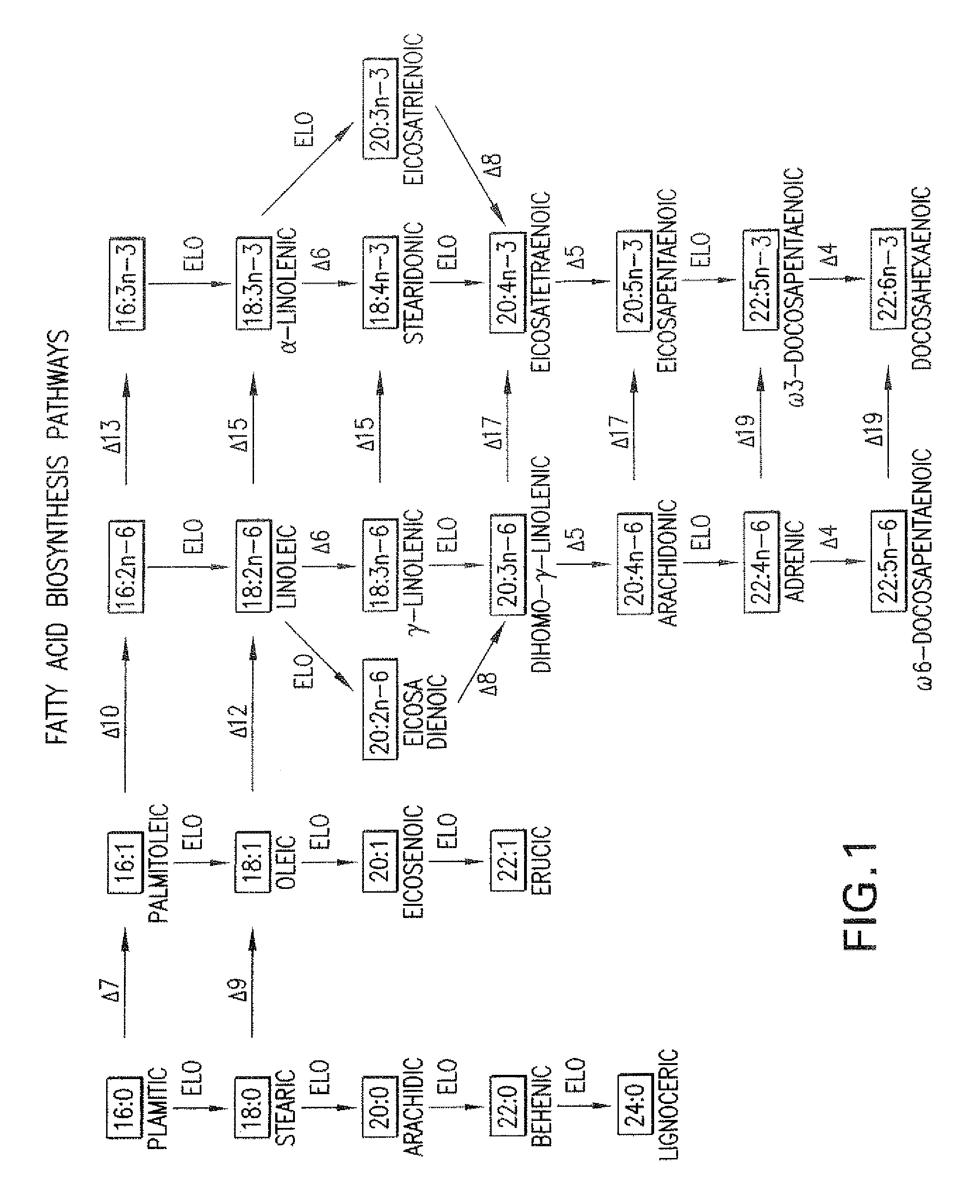
The subject invention relates to the identification of several genes involved in the elongation of polyunsaturated acids (i.e., "elongases") and to uses thereof. At least two of these genes are also involved in the elongation of monounsaturated fatty acids. In particular, elongase is utilized in the conversion of gamma linolenic acid (GLA) to dihomogamma linolenic acid (DGLA) and in the conversion of AA to adrenic acid (ADA), or eicosapentaenoic acid (EPA) to omega3-docosapentaenoic acid (DPA). DGLA may be utilized in the production of polyunsaturated fatty acids, such as arachidonic acid (AA), docosahexaenoic acid (DHA), EPA, adrenic acid, omega6-docosapentaenoic acid or omega3-docosapentaenoic acid which may be added to pharmaceutical compositions, nutritional compositions, animal feeds, as well as other products such as cosmetics.
Owner:ABBOTT LAB INC
Methods of treating senile dementia and Alzheimer's diseases using docosahexaenoic acid and arachidonic acid compositions
InactiveUS20050027004A1Lower triglyceride levelsCorrecting lipid imbalanceBiocideNervous disorderMicrobial oilMedicine
A method of treating a neurological disorder comprises administering to a person affected from such a disorder a microbial oil comprising DHA, a microbial oil comprising ARA or a combination of DHA and ARA oils in an amount sufficient to elevate the levels of circulating DHA and / or ARA in the person's blood to at least normal levels.
Owner:MARTEK BIOSCIENCES CORP
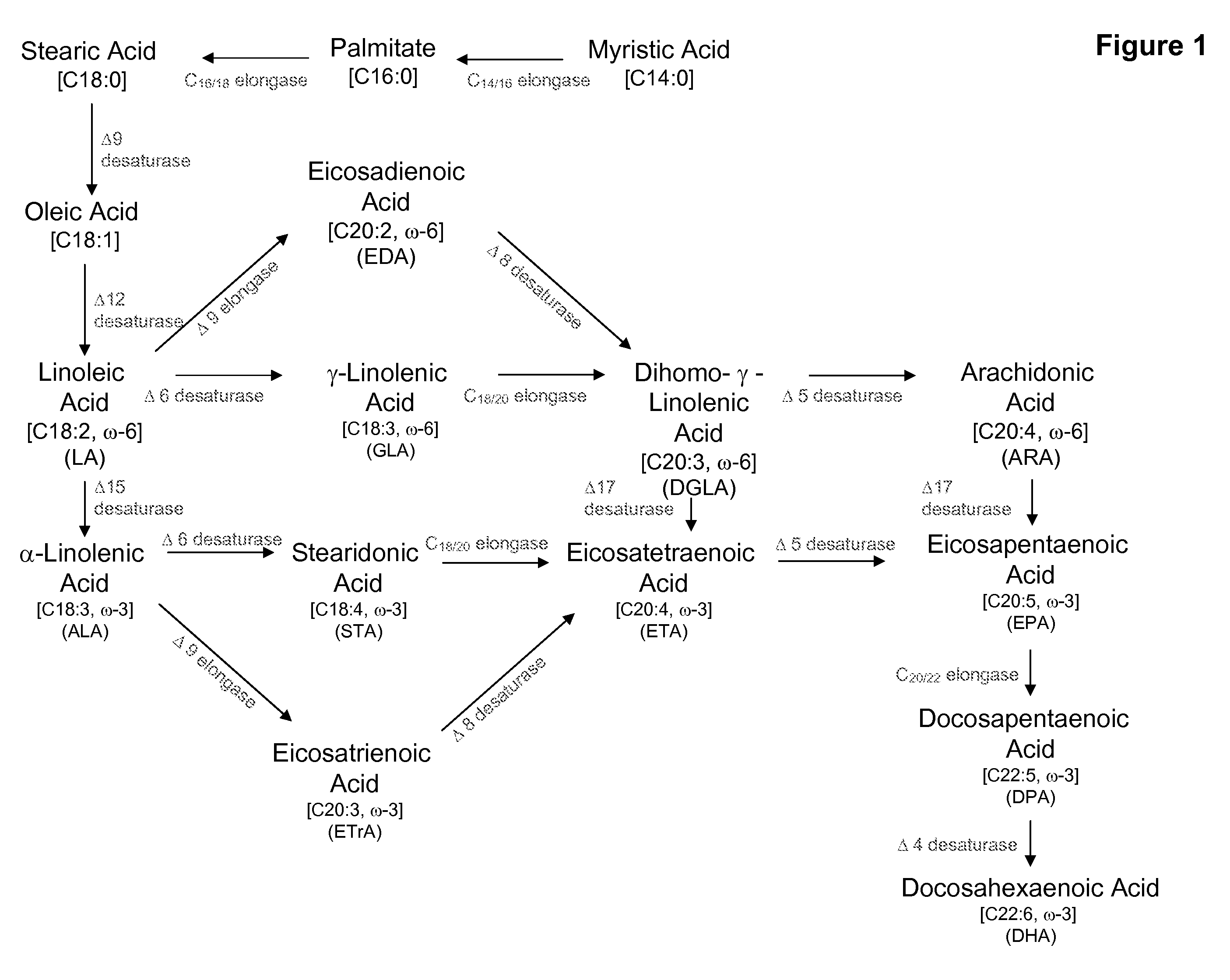
Delta17 desaturase and its use in making polyunsaturated fatty acids
The present invention relates to Δ17 desaturases, which have the ability to convert ω-6 fatty acids into their ω-3 counterparts (i.e., conversion of arachidonic acid [20:4, ARA] to eicosapentaenoic acid [20:5, EPA]). Isolated nucleic acid fragments and recombinant constructs comprising such fragments encoding Δ17 desaturases along with a method of making long-chain polyunsaturated fatty acids (PUFAs) using these Δ17 desaturases in oleaginous yeast are disclosed.
Owner:DUPONT US HLDG LLC
Elongase genes and uses thereof
The subject invention relates to the identification of four genes involved in the elongation of polyunsaturated acids (i.e., “elongases”) and to uses thereof. Two of these genes are also involved in the elongation of monounsaturated fatty acids. In particular, elongase is utilized in the conversion of gamma linolenic acid (GLA) to dihomogamma linolenic acid (DGLA) and in the conversion of DGLA or 20:4n-3 to eicosapentaenoic acid (EPA). DGLA may be utilized in the production of polyunsaturated fatty acids, such as arachidonic acid (AA), docosahexaenoic acid (DHA), EPA, adrenic acid, ω6-docosapentaenoic acid or ω3-docosapentaenoic acid which may be added to pharmaceutical compositions, nutritional compositions, animal feeds, as well as other products such as cosmetics.
Owner:ABBOTT LAB INC
Elongase genes and uses thereof
The subject invention relates to the identification of several genes involved in the elongation of polyunsaturated acids (i.e., "elongases") and to uses thereof. At least two of these genes are also involved in the elongation of monounsaturated fatty acids. In particular, elongase is utilized in the conversion of gamma linolenic acid (GLA) to dihomogamma linolenic acid (DGLA) and in the conversion of AA to adrenic acid (ADA), or eicosapentaenoic acid (EPA) to omega3-docosapentaenoic acid (DPA). DGLA may be utilized in the production of polyunsaturated fatty acids, such as arachidonic acid (AA), docosahexaenoic acid (DHA), EPA, adrenic acid, omega6-docosapentaenoic acid or omega3-docosapentaenoic acid which may be added to pharmaceutical compositions, nutritional compositions, animal feeds, as well as other products such as cosmetics.
Owner:ABBOTT LAB INC
Marchantiales-Derived Unsaturated Fatty Acid Synthetase Genes And Use Of The Same
InactiveUS20080057495A1ImmunoglobulinsOxidoreductasesUnsaturated fatty acid synthesisEicosapentaenoic acid
A Δ5 fatty acid desaturase gene, a Δ6 fatty acid desaturase gene, and a Δ6 fatty-acid-chain elongase gene are isolated from a single species of Marchantiales. By introducing these genes into higher plants, transformed plants which can produce arachidonic acid and eicosapentaenoic acid (EPA) are obtained.
Owner:SUNTORY HLDG LTD
Formula milk powder for promoting absorption of fatty acid and calcium and preparation method thereof
The invention discloses a formula milk powder for promoting the absorption of fatty acid and calcium and a preparation method thereof. Raw cow milk, lactose, 1,3-Dioleoyl 2-palmitoyl triglyceride and demineralized whey powder as main materials are added with concentrated whey albumen powder, Alpha-lactalbumin powder, oligosaccharide, walnut oil, casein phosphopeptide, docosahexaenoic acid, arachidonic acid, nucleotide, lutein, inositol, carnitine and the like as well as vitamins, mineral substances and other nutrients needed for strengthening infants, and fat humanization, protein humanization and carbohydrate humanization are realized. The powdery product is produced by the processes of blending, homogenization, concentration, spray-drying, packaging and the like. According to the physiological characteristics and nutritional demand of the infants, the invention reinforces the calcium, the 1,3-Dioleoyl 2-palmitoyl triglyceride, other nutrient ingredients and the like, and aiming at the oversea clinical test conclusion of the 1,3-Dioleoyl 2-palmitoyl triglyceride, the final test conclusion of comparison with breast milk and infant formula milk powder sold on the market in the process of a clinical feeding test is that the feeding result of the designed formula approximates the feeding result of the breast milk and is better than the feeding result of an infant formula milk powder group sold on the market.
Owner:HEILONGJIANG FEIHE DAIRY
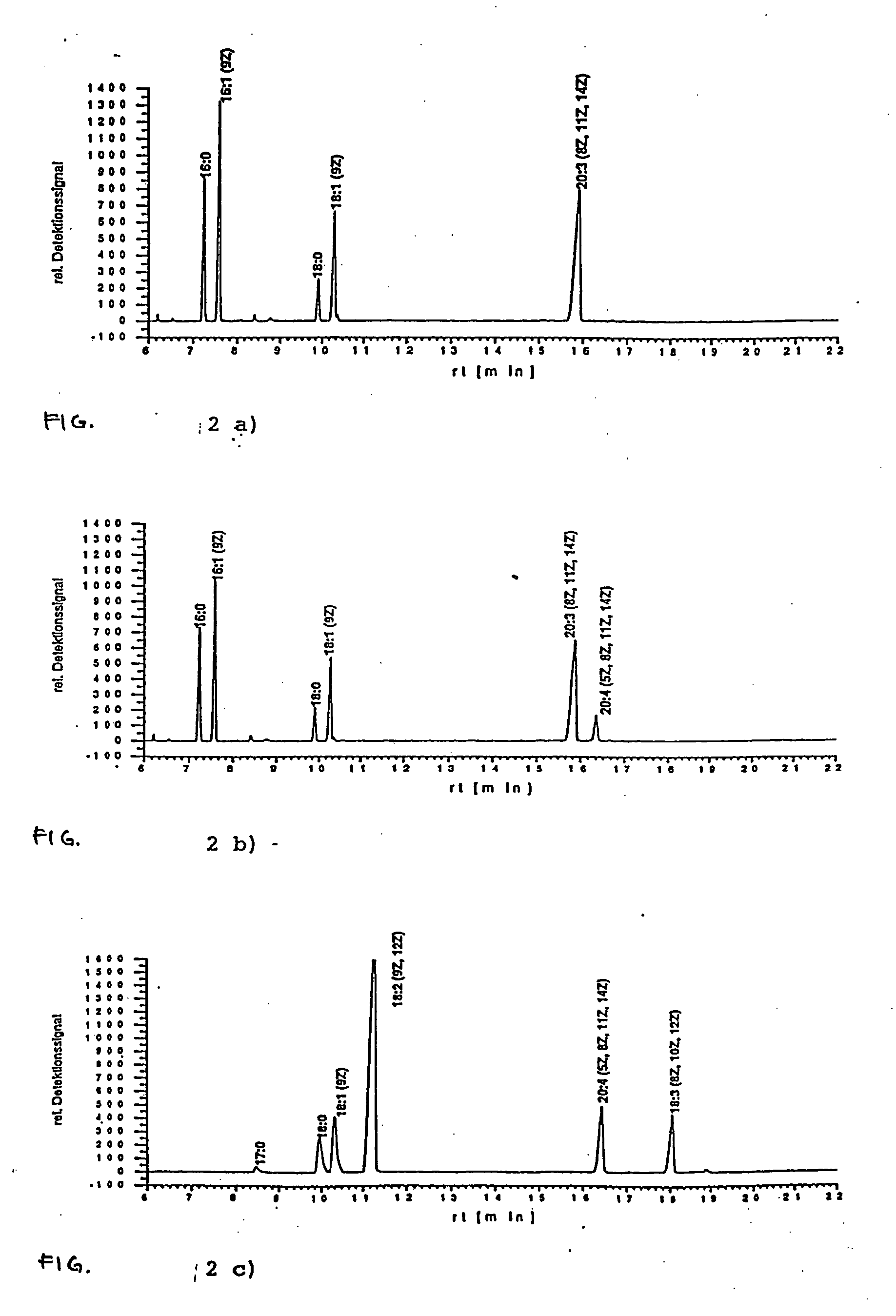
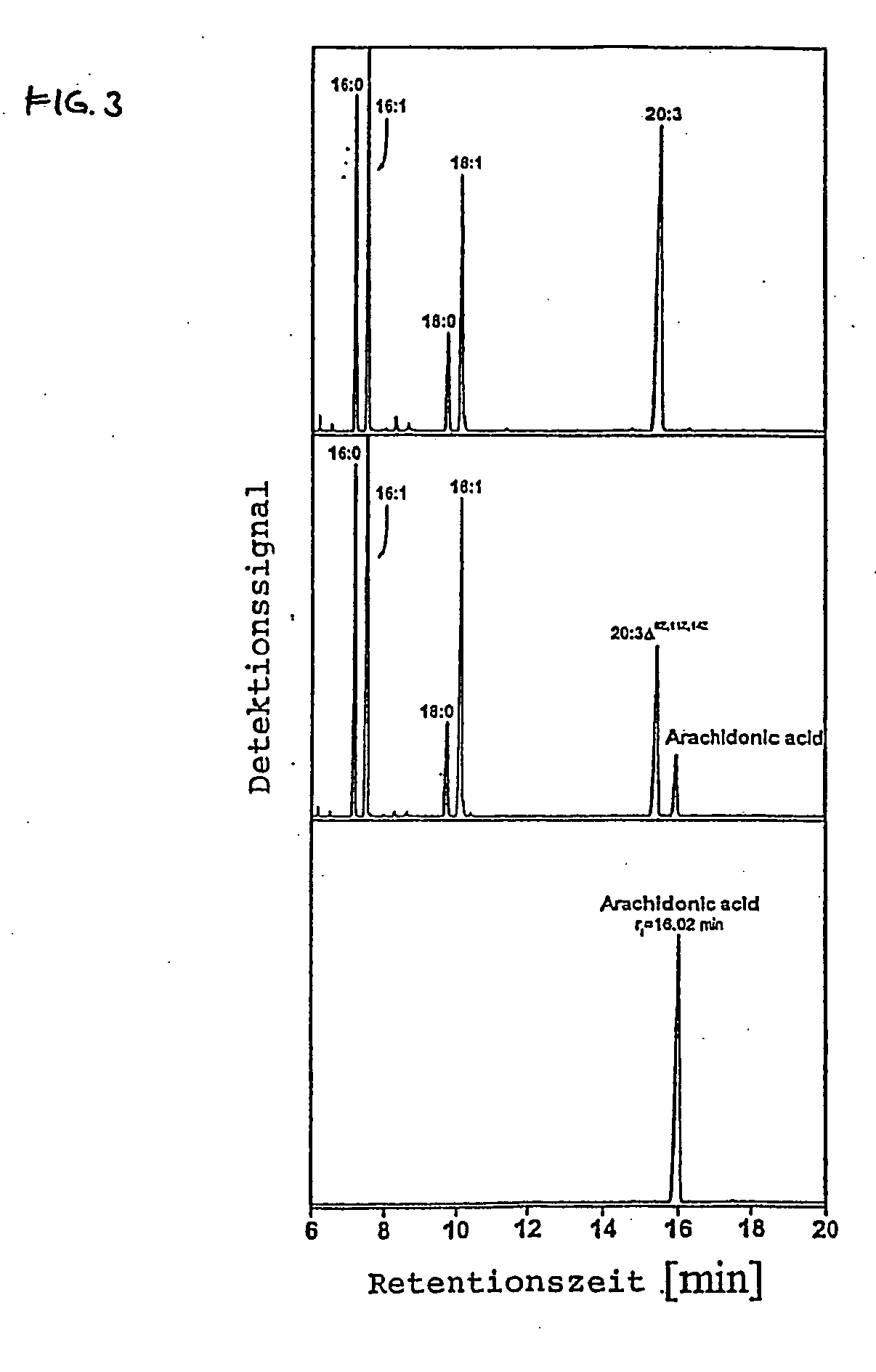
Method for producing arachidonic acid in transgenic organisms
InactiveUS20050089879A1Increase contentSugar derivativesMicrobiological testing/measurementPlant cellA-DNA
The invention relates to a method for the production of arachidonic acid in transgenic organisms, especially in transgenic plants and yeasts. The invention also relates to DNA sequences coding for a protein with enzymatic activity of a Δ5-desaturase from Phytophthera megasperma. The invention further relates to transgenic plants and plant cell and transgenic yeasts containing a nucleic acid molecule comprising a DNA sequence according to the present invention and having, on the basis thereof, an enhanced arachidonic acid synthesis in comparison with wild-type cells. The invention also relates to harvest products and propagating material of transgenic plants.
Owner:INST FUR PFLANZENGENETIK & KULTURPFLANZENFORSCHUNG
Production of Very Long Chain Polyunsaturated Fatty Acids in Oil Seed Plants
Oilseed plants which have been transformed to produce at least 8.0% arachidonic acid (ARA) as well as uses of oils and seeds obtained from such transformed plants in a variety of food and feed applications are described.
Owner:CORTEVA AGRISCIENCE LLC
Nutritional composition for supporting brain development and function of children
InactiveUS20120171178A1Contributes to orImprove abilitiesBiocideNervous disorderBrain developmentBiology
The present invention relates to a nutritional composition, in particular directed to children of 3-6 years, said nutritional composition comprising a protein source, a source of available carbohydrates, a lipid source, at least one probiotic microorganism, and prebiotics, wherein said lipid source comprises DHA (docosahexaenoic acid) and / or ARA (arachidonic acid). The nutritional composition improves cognitive performance, in particular memory, learning comprehension, alertness, attention, concentration, processing speed, conceptual thinking, abstract thinking, verbal abilities, language comprehension, psychomotor skills, curiosity, and confident interaction with the environment. Preferably, the composition comprises one, a combination of several or all selected of the group of DHA, ARA, LA, ALA, choline, iron, iodine and folic acid.
Owner:NESTEC SA
Infant nutrition immunity formula milk powder and method of preparing the same
InactiveCN101278688APromote complexationPromote absorptionMilk preparationMilk preservationFluidized bed dryingNervous system
The invention relates to an infant nutrition immunity formula powdered milk and a production method thereof, wherein, the production method of the infant nutrition immunity formula powdered milk comprises the following steps: milk which is raw material, is preprocessed, the mixed solution of docosahexaenoic acid and arachidonic acid is prepared, ingredients are mixed, preheating, homogenizing and heating are carried out for sterilizing, decompression is carried out for concentrating, and spray-drying under negative pressure and low temperature and drying on a fluidized bed are carried out, then cooling, packing and inspecting are carried out. The infant nutrition immunity formula powdered milk manufactured by the method of the invention mainly comprises casein phosphoeptide, vitamin D3, lactoferrin, nucleotide, linoleic acid, linolenic acid, the docosahexaenoic acid, the arachidonic acid, fructo-oligosaccharide and galacto-oligosaccharide, which has the advantage of reasonable trophic structure and also ensures that various nutrient substance to be absorbed and deposited to the utmost extend, so as to promote the development of brain and nervous system, improve active immunity, improve intestinal tract micro-ecological environment and strengthen the absorption of calcium, iron and zinc as well as effectively maintain every active nutrient component during the production process.
Owner:AUSTRALIA DIVINE DAIRY
Long chain fatty acids for reducing off-taste of non-nutritive sweeteners
InactiveUS20080226790A1Food ingredient as taste affecting agentFood preparationArachidic acidAdipic acid
Aspects of the invention relate to beverage compositions, including, for example, concentrated and ready-to-drink formulations sweetened with at least one non-nutritive sweetener and further including a long chain fatty acid compound in an amount sufficient to reduce the off-note taste of the non-nutritive sweetener. In certain embodiments, the long chain fatty acid may be one or more of the following: lauric acid, myristic acid, palmitic acid, stearic acid, arachidic acid, behenic acid, oleic acid, linoleic acid, alpha-linolenic acid, arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, erucic acid, adipic acid, and palmitic acid. In certain exemplary embodiments, a plurality of different long chain fatty acids are utilized. In another embodiments, the long chain fatty acids comprise both natural and synthetic fatty acids.
Owner:CONCENTRATE MFG OF IRELAND
Elongase Gene and Uses Thereof
Owner:ABBOTT LAB INC
Compositions of stable bioactive metabolites of docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids
InactiveUS20050282781A1Affect rate of absorptionOptimal moisture rangeBiocideNervous disorderMetaboliteBenzopyrone
An invention that adduces cogent evidence to establish that oxygenated dibenzo-α-pyrones (DBPs and their conjugates), the major bioactives of shilajit (Ayurvedic vitalizer), have their origin, at least partly, in EPA and DHA. Earlier research has shown that, in mammals, C-20 PUFAs are metabolized by oxygenases and other enzymes to produce short-lived prostaglandins, leukotrienes and thromboxanes that bind to specific G-protein-coupled receptors and signal cellular responses, e.g., inflammation, vasodilation, blood pressure, pain etc. But never before it was suggested / shown that C20:5n-3 (and C22:6 n-3) PUFAs, e.g., EPA (and DHA), are transformed into stable aromatic metabolites, DBPs, which elicit a large array of bioactivities in the producer organisms and also control the synthesis and metabolism of arachidonate-derived prostaglandins. The major beneficial effects attributed to EPA and DHA are now found to be largely contributed by DBPs and their aminoacyl conjugates and the dibenzo-α-pyrone-chromoproteins (DCPs). Because of the highly unstable nature of EPA and DHA, when administered, they are metabolized into a large array of uncontrolled products, several of which are systemically undesirable. By contrast, DBPs, because of their stability, perform the biological response modifier (BRM) functions in a directed and sustained way. Many of the biological effects of DBPs described in this invention, were earlier attributed to EPA and DHA,—the precursors of DBPs.
Owner:NATREON INC
Reelin deficiency or dysfunction and methods related thereto
InactiveUS20090215896A1High activityImprove the level ofBiocideSenses disorderNervous systemSupplement use
A method of measuring Reelin as a biomarker, to non-destructively assess or predict DHA levels in the brain and in other, currently inaccessible or difficult-to-access, key components of the central nervous system (CNS) is described. Also described is a method to prevent, delay the onset of, or treat Reelin deficiency or dysfunction and / or a disease or condition associated with Reelin deficiency or dysfunction, comprising administering to a patient diagnosed with or suspected of having a Reelin deficiency or dysfunction an amount of a PUFA, and particularly an omega-3 PUFA, and more particularly, docosahexaenoic acid (DHA) or a precursor or source thereof, to compensate for the effects of Reelin deficiency or dysfunction in the patient. Also described is a method to prevent or reduce developmental defects or disorders associated with Reelin dysfunction or deficiency through the supplemental use of polyunsaturated fatty acids (PUFAs—unsaturated fatty acids having two or more double bonds), and particularly highly unsaturated fatty acids (HUFAs—unsaturated fatty acids having three or more double bonds), and more particularly a HUFA selected from arachidonic acid (ARA), eicosapentacnoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA), and even more particularly omega-3 HUFAs, and more particularly DHA, to: compensate for reduced fatty acid binding protein or function thereof in the patient; compensate for reduced brain lipid binding protein or function thereof in the patient; improve the activity of fatty acid binding proteins in the patient; increase the expression of brain lipid binding proteins (BLBPs) in the patient; improve at least one parameter of the mechanism of action of brain lipid binding proteins in the patient; overcome a deficiency of DHA in central nervous system (CNS) structures and improve the resulting function thereof; increase the incorporation of functional DHA and other PUFAs into the phospholipid membranes of glial cells and neurons in the patient; increase the level of Reelin and / or improve the activity of Reelin in the patient; and / or improve at least one symptom of a disease or condition associated with Reelin deficiency or dysfunction.
Owner:MARTEK BIOSCIENCES CORP
Formulations and methods for nutrient delivery
InactiveUS20110208153A1Promote healthy developmentAvoid developmentMilk preparationEdible oils/fats ingredientsDocosahexaenoic acidNutritional deficiency
The present disclosure provides formulations and methods for delivering water-soluble and lipid-soluble nutrients for preventing or correcting nutrient deficiencies to subjects requiring small-volume nutritional support, such as preterm infants. The formulations may comprise an emulsion of docosahexaenoic acid (DHA) stabilized by a protein emulsifier, such as α-lactalbumin, and may further comprise other valuable nutrients, such as arachidonic acid (ARA), arginine, glutamine, arginyl-glutamine dipeptide and / or alanyl-glutamine dipeptide. The formulation is useful, for example, for correcting nutritional deficiencies by increasing a subject's intake of nutrients such as ω-3 or ω-6 long-chain polyunsaturated acids, proteins, peptides, vitamins, minerals, other fatty acids, and / or essential amino acids. The nutritional formulation is suitable for enteral delivery and small-volume delivery via nasogastric tube, intragastric feeding, transpyloric administration and / or any other means of administration that result in the introduction of the nutritional formulation into the digestive tract of a subject.
Owner:MEAD JOHNSON NUTRITION
Separation method of silkworm pupa oil polyunsaturated fatty acid by gradient freezing crystallization
InactiveCN101892119APrevent oxidationHigh yieldFatty acids production/refiningFatty-oils/fats productionPolyunsaturated fatty acidPupa
The invention discloses a separation method of silkworm pupa oil polyunsaturated fatty acid by gradient freezing crystallization, comprising the following steps of preprocessing silkworm pupas, extracting silkworm pupa oil with ultrasonic waves, preparing silkworm pupa oil mixed fatty acid, preparing a urea ethanol solution, and conducting urea inclusion treatment and then gradient freezing crystallization. Compared with the traditional separation methods of the silkworm pupa oil polyunsaturated fatty acid, the problem of fatty acid oxidation caused by overhigh temperature can be avoided because the whole operation process is conducted under low temperature all along. The silkworm pupa oil polyunsaturated fatty acid prepared by the invention contains 96.5% of polyunsaturated fatty acid (including 4.2% of linoleic acid, 91.3% of alpha-linoleic acid, and 1.0% of arachidonic acid), and can be served as principal components of relative drugs or health food. The invention has the advantages of simple processing step, high product yield, excellent quality, high purity of the polyunsaturated fatty acid and the like, and can be used for preparing the polyunsaturated fatty acid from silkworm pupa oil.
Owner:SHAANXI NORMAL UNIV
Infant formula goat milk powder added with structural oil and preparation method thereof
ActiveCN102283288APromote growth and developmentPromote fitnessMilk preparationDocosahexaenoic acidVegetable oil
The invention discloses infant formula goat milk powder added with structural grease and a preparation method thereof. The infant formula goat milk powder is prepared from the following components: 20 to 40 percent of lactose, 16 to 22 percent of edible vegetable oil, 3.2 to 9.6 percent of structural grease (1,3-dioleic acid and 2-palmitic acid triglyceride), 16 to 22 percent of concentrated goatwhey protein, 11 to 15 percent of degreased goat milk powder, 2 to 6 percent of glucose syrup solid, 1.0 to 1.5 percent of fructooligosaccharide, 0.5 to 1.0 percent of galactooligosaccharide, 0.15 to0.50 percent of arachidonic acid, 0.12 to 0.48 percent of docosahexaenoic acid, 0.6 to 1.2 percent of mineral premix, 0.08 to 0.12 percent of vitamin premix, and 0.01 to 0.05 percent of nucleotide. The invention also discloses the preparation method of the infant formula goat milk powder. According to the infant formula goat milk powder, the goat smell is effectively eliminated, growth and development of infants are promoted, absorption and utilization rate of calcium are improved, and bone toughness of the infants is promoted.
Owner:HYPROCA NUTRITION CO LTD
Method for transforming fatty acid ethyl ester into glyceride
ActiveCN101818176AImprove reaction efficiencySolve the wrapping effectMicroorganism based processesFermentationDistillationGlycerol
The invention discloses a method for transforming fatty acid ethyl ester into glyceride, which comprises the following steps of: mixing the fatty acid ethyl ester and glycerol in a material tank; making the material pass through a glycerol separator by using a pump to separate free glycerin; then putting the material in a reactor in which immobilized lipase is filled; and making the material passthrough a packed tower to remove ethanol; making the material finally flow back to the material tank for performing 6 to 300h circular reaction; then carrying out molecular distillation on the reaction product to remove the unconverted reactant so as to obtain glyceride products. The method can improve the stability and bioavailability of the products, and has high application value for transforming functional fatty acids such as ethyl ester fish oil, algae oil, conjugated linoleic acid, arachidonic acid and the like into glyceride products.
Owner:XINGYE ZHOUSHAN +1
Use of arachidonic acid and/or docosahexanoic acid for the treatment of dyspraxia
InactiveUS6184251B1Avoid conversionEnsure stabilityBiocideNervous disorderDocosahexaenoic acidGastroenterology
This invention relates to the administration of arachidonic acid or docosahexaenoic acid or precursors thereof to children or pregnant women for the treatment or prevention of dyspraxia.
Owner:SCARISTA
Infant formula milk powder with the fatty acid structure adjusted
InactiveCN101061819AAvoid exposureDelayed Post OxidationMilk preparationWhey manufactureDocosahexaenoic acidVegetable oil
The invention provides an infant milk powder with adjusted structure of aliphatic acid, which comprises the following raw materials (by weight portion in tons): dry matter of fresh milk 276, desalinized whey powder 450, structured fatty oil 105, lactose 50, fructooligosaccharide 20, alpha-lactalbumin 10, docosahexaenoic acid 5, arachidonic acid 6, nucleic acid 0. 42, taurine 0. 45, choline 0. 42, L-carnitine 0. 06, beta-carotene 0. 012, composite vitamin 2. 5, composite microelement 3. 5 and balancing refined vegetable oil.
Owner:SANLU GROUP
Microalgal feeds containing arachidonic acid and their production and use
InactiveUS7396548B2Improve stabilityEasy to oxidizeClimate change adaptationAnimal feeding stuffAquatic animalAquatic product
An animal feed with a high level of arachidonic acid is produced from microalgae, and fed to aquatic animals grown in aquaculture. The arachidonic acid-rich microalgae are fed directly to the aquatic animals, or processed to produce an oil that can be used as a human nutritive supplement. The arachidonic acid-rich microalgae can be combined with long chain omega-3 fatty acids to provide a source of nutrition for humans and animals. The animal feed and nutritional supplements are free of animal byproducts.
Owner:INTERVET INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com