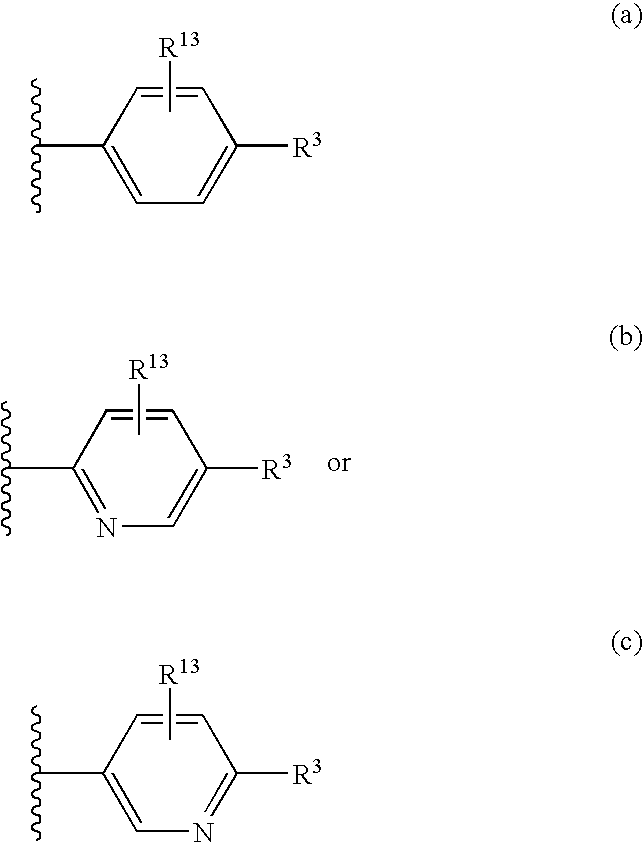
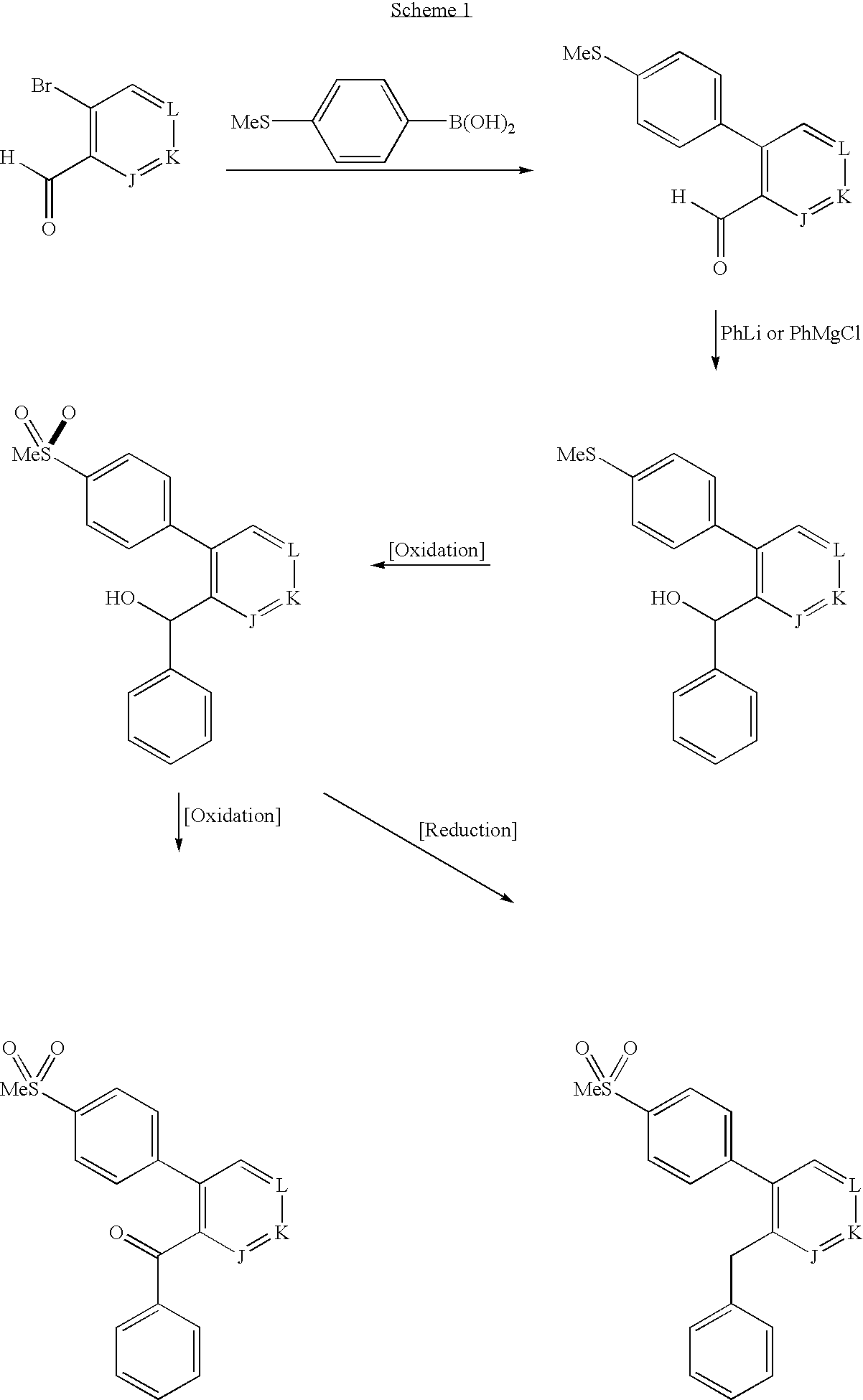
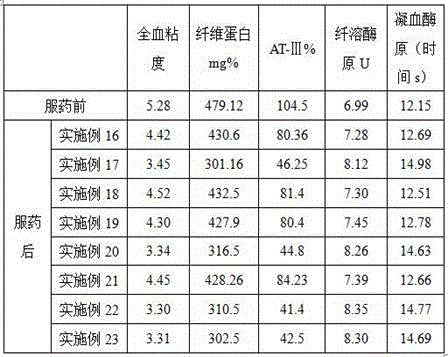
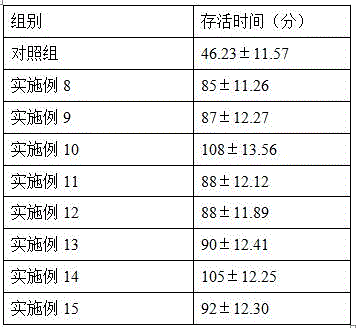
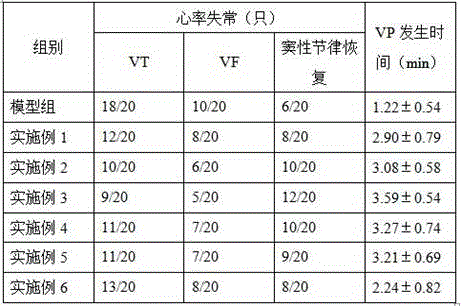
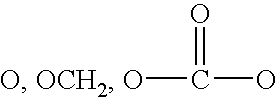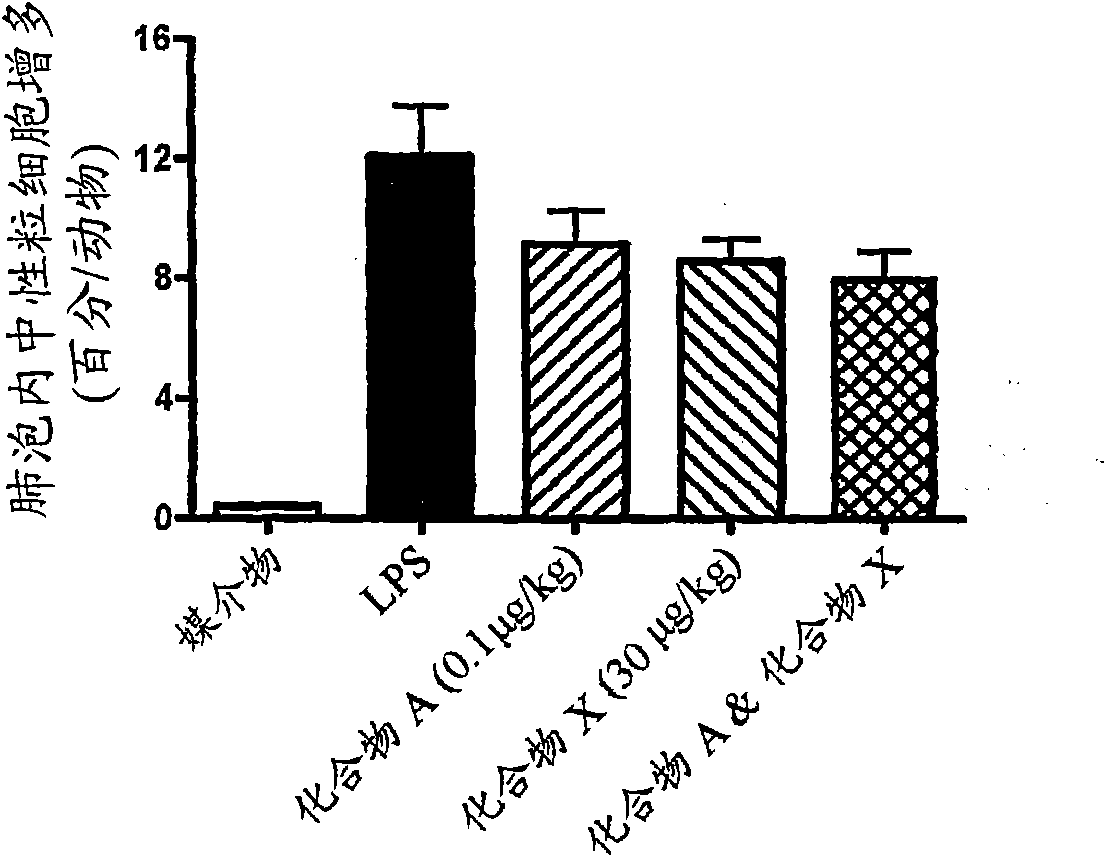Patents
Literature
43 results about "Thromboxanes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Physiologically active compounds found in many organs of the body. They are formed in vivo from the prostaglandin endoperoxides and cause platelet aggregation, contraction of arteries, and other biological effects. Thromboxanes are important mediators of the actions of polyunsaturated fatty acids transformed by cyclooxygenase.
Compositions of stable bioactive metabolites of docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids
InactiveUS20050282781A1Affect rate of absorptionOptimal moisture rangeBiocideNervous disorderMetaboliteBenzopyrone
An invention that adduces cogent evidence to establish that oxygenated dibenzo-α-pyrones (DBPs and their conjugates), the major bioactives of shilajit (Ayurvedic vitalizer), have their origin, at least partly, in EPA and DHA. Earlier research has shown that, in mammals, C-20 PUFAs are metabolized by oxygenases and other enzymes to produce short-lived prostaglandins, leukotrienes and thromboxanes that bind to specific G-protein-coupled receptors and signal cellular responses, e.g., inflammation, vasodilation, blood pressure, pain etc. But never before it was suggested / shown that C20:5n-3 (and C22:6 n-3) PUFAs, e.g., EPA (and DHA), are transformed into stable aromatic metabolites, DBPs, which elicit a large array of bioactivities in the producer organisms and also control the synthesis and metabolism of arachidonate-derived prostaglandins. The major beneficial effects attributed to EPA and DHA are now found to be largely contributed by DBPs and their aminoacyl conjugates and the dibenzo-α-pyrone-chromoproteins (DCPs). Because of the highly unstable nature of EPA and DHA, when administered, they are metabolized into a large array of uncontrolled products, several of which are systemically undesirable. By contrast, DBPs, because of their stability, perform the biological response modifier (BRM) functions in a directed and sustained way. Many of the biological effects of DBPs described in this invention, were earlier attributed to EPA and DHA,—the precursors of DBPs.
Owner:NATREON INC
Composition comprising a combined thromboxane receptor antagonist and thromboxane synthase inhibitor and a COX-2 inhibitor
InactiveUS20060217431A1Inhibit platelet activationPrevents vasoconstrictive actionBiocideNervous disorderDiseaseCOX-2 inhibitor
The invention relates to a pharmaceutical composition comprising a combined thromboxane receptor antagonist and thromboxane synthase inhibitor and a COX-2 inhibitor. In addition a method of treating cyclooxygenase dependent disorders, including inflammation, pain and / or rheumatic diseases, and / or neoplasia is described.
Owner:BOEHRINGER INGELHEIM INT GMBH
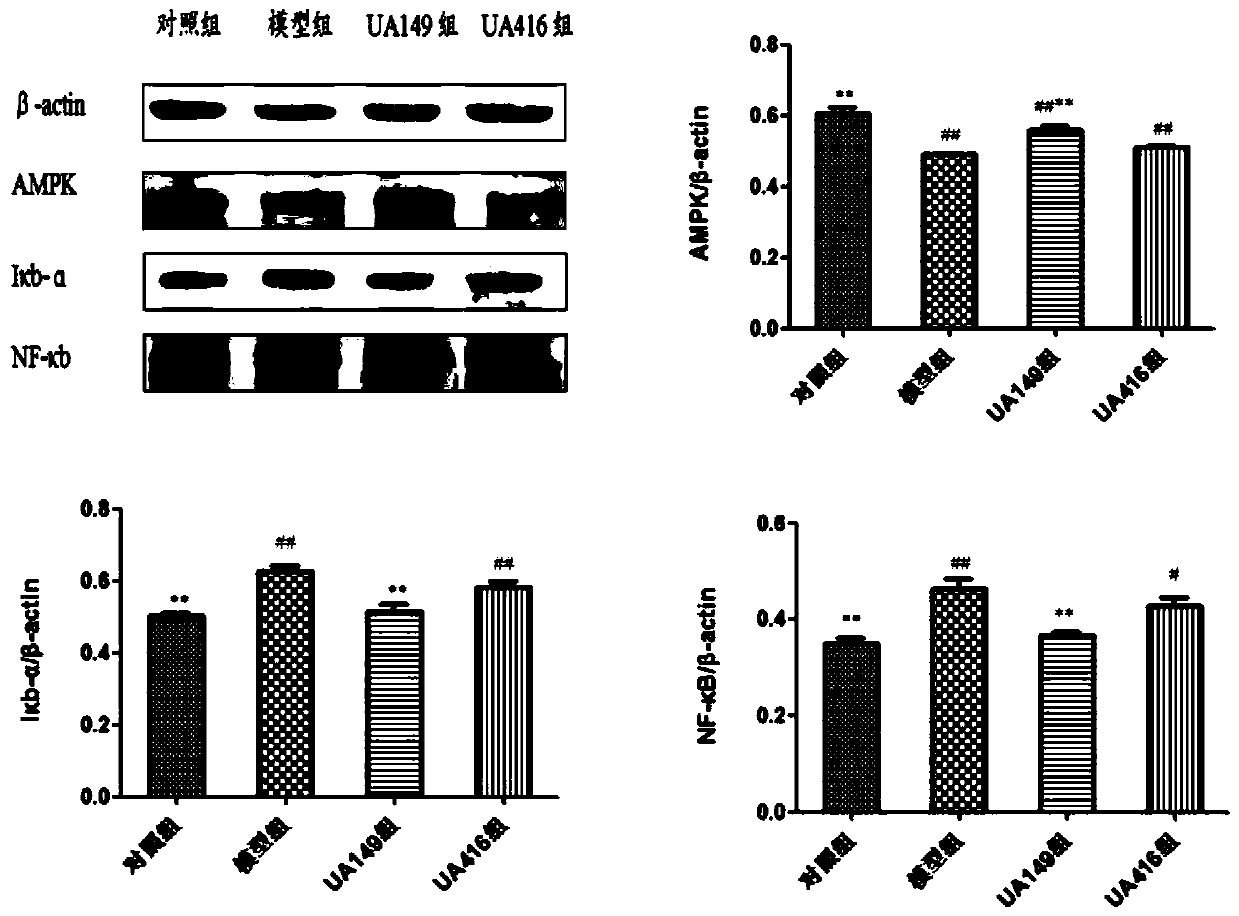
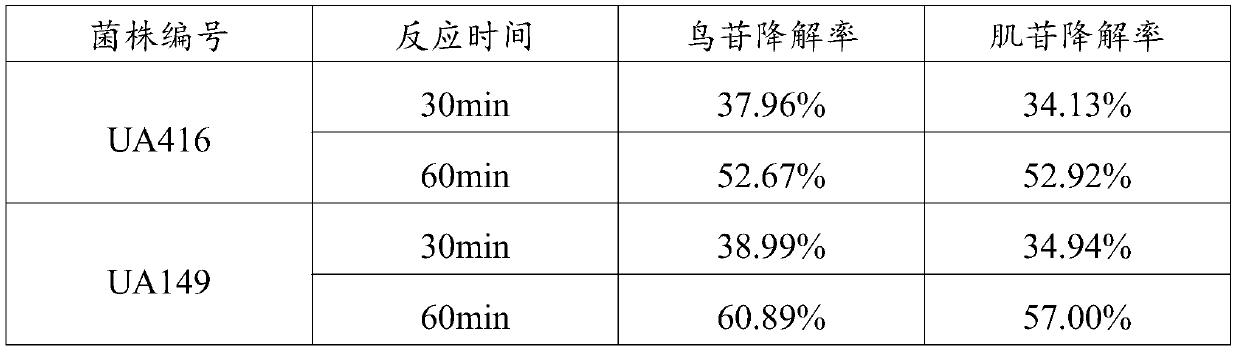
L.plantarum UA149 strain and application thereof
ActiveCN110055199AReduce synthesisLower uric acid levelsBacteriaMicroorganism based processesInflammatory factorsResearch Object
The invention discloses an L.plantarum UA149 strain and an application thereof, and belongs to the field of functional food microorganisms. The L.plantarum UA149 strain is deposited in China Typical Culture Collection Center on November 29, 2018 with the preservation number of CCTCC No: M2018842. The strain can be apply to preparing products with a uric acid reducing function or a gout resisting function. Lactic acid bacteria separated and identified from the surface of fleshy plant leaves are taken as research objects, and a new strain of lactic acid bacteria is screened through a large number of experiments. Hyperuricemia model rats are established by potassium oxazinate combined with fructose water, continuous intragastric administration of the lactobacillus plantarum UA149 strain for 14 days can significantly reduce the level of uric acid; and during gout attack, the release of inflammatory factors thromboxane and leukotriene mediated by neutrophils is reduced, the influx of neutrophils into joints is avoided, and the symptoms of redness, swelling, pain, heat and the like are reduced.
Owner:JILIN MINGZHIYUAN BIOTECH
Nutritional supplement for pregnant females
Preeclampsia and intrauterine growth restriction in a pregnant female mammal are prevented or decreased in severity by administering thereto a combination of a vitamin compound containing B1, Folic Acid (or active form 5-Methyl-Tetrahydrofolate i.e.; Metafolin®), B 6 (Pyridoxine or active form Pyridoxine 5-Phosphate P5P), B 12, Ascorbic Acid, Selenium, Zinc, Co-enzyme Q 10 and N-Acytyl Cysteine, Lycopene, optionally in further combination with Melatonin and / or Vitamin E or / and ASA 81 mg both of later, are cyclooxygenase inhibitors, a PGI.sub.2-mimetic, a thromboxane (TXA.sub.2) inhibitor, a compound possessing TXA.sub.2-agonistic and TXA.sub.2-inhibiting properties, a compound possessing TXA.sub.2-antagonistic and PGI.sub.2-mimetic activities, and a TXA.sub.2 antagonist.
Owner:MUENCH MICHAEL V +2
Conductivity-normalized urinary analyte concentration measurement for use in disease diagnosis
The invention relates to a method for the diagnosis of a medical condition in a subject, comprising the steps of measuring the concentration of one or more analytes, relevant for the diagnosis of said medical condition, in a urine sample; measuring the electrical conductivity of said urine sample; obtaining a normalized value for the analyte concentration by dividing said analyte concentration by the electrical conductivity; and determining whether said subject is suffering from said medical condition by means of comparing the normalized value with a pre-determined reference value. The medical condition to be diagnosed can be, for example, an acute cardiac condition for which the relevant urinary analyte can be one or more thromboxanes.
Owner:BIOPREVENTIVE
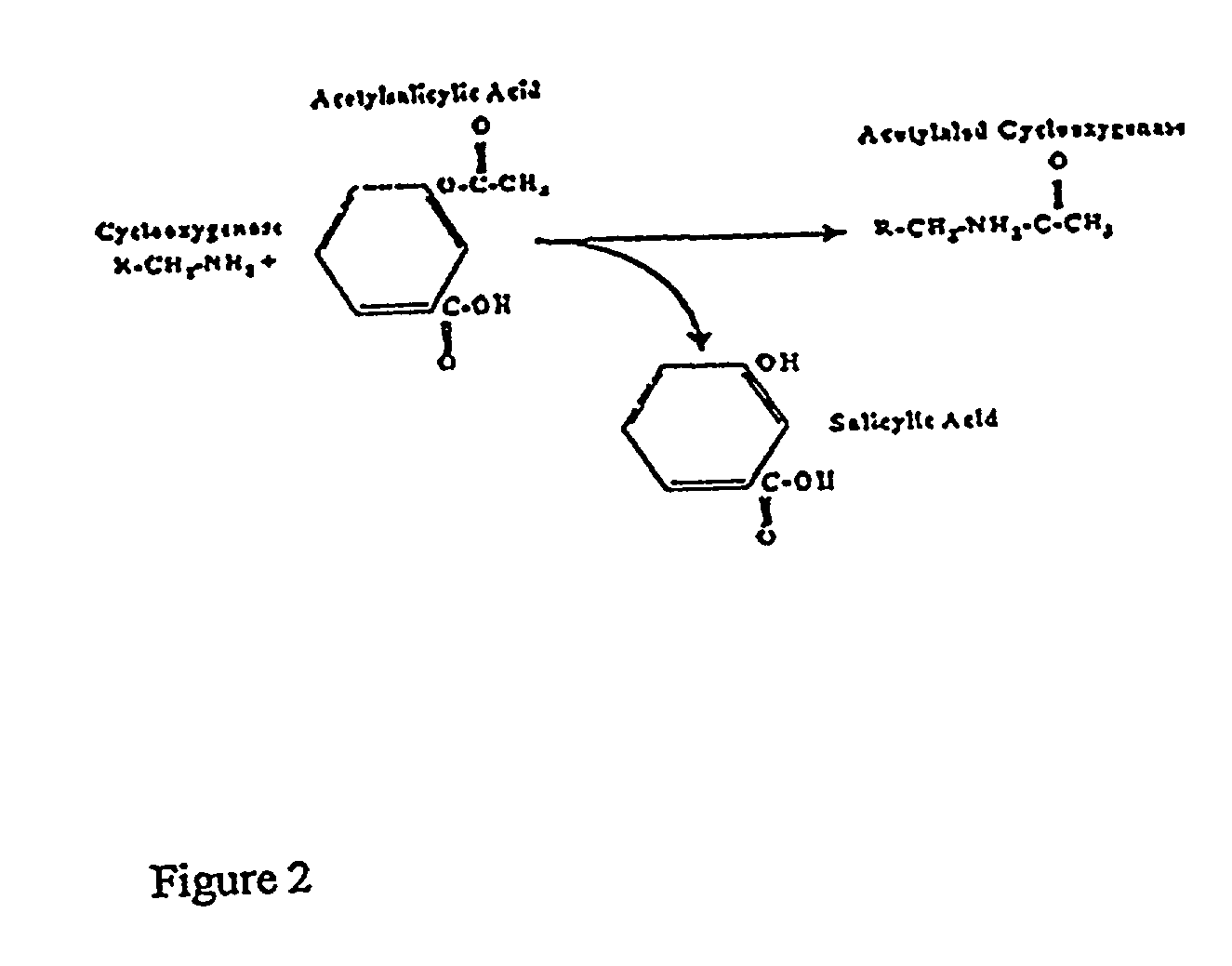
Novel acetysalicylic acid formulations
InactiveUS20100009005A1Reduce riskReduce productionBiocideSalicyclic acid active ingredientsDiseaseSide effect
The invention relates to pharmaceutical compositions of acetylsalicylic acid-based microcapsules to selectively inhibit the COX in the portal vein and / or in the liver to reduce the production of thromboxane. Further, the pharmaceutical composition minimizes COX inhibition in the systemic circulation to optimize the inhibition of platelet aggregation. Certain embodiments also address methods of prevention and / or treatment of these diseases, using these oral compositions such as enhancing the safety of antithrombotic treatments. Other embodiments contemplate oral pharmaceutical compositions that combine acetylsalicylic acid with anti-platelet aggregation drugs, without inducing gastric side effects.
Owner:NEW HAVEN PHARMA
Methods of treating hepatorenal syndrome and hepatic encephalopathy with thromboxane-a2 receptor antagonists
InactiveUS20130197044A1Avoid failureSpeed up the flowBiocideNervous disorderThromboxane A2 receptorNK1 receptor antagonist
The present invention is directed to methods of treating hepatorenal syndrome by administration of a therapeutically effective amount of a thromboxane A2 receptor antagonist to a patient in need thereof. The present invention is also directed to methods of treating hepatic encephalopathy and cerebral edema by administration of a therapeutically effective amount of a thromboxane A2 receptor antagonist to a patient in need thereof.
Owner:CUMBERLAND EMERGING TECH
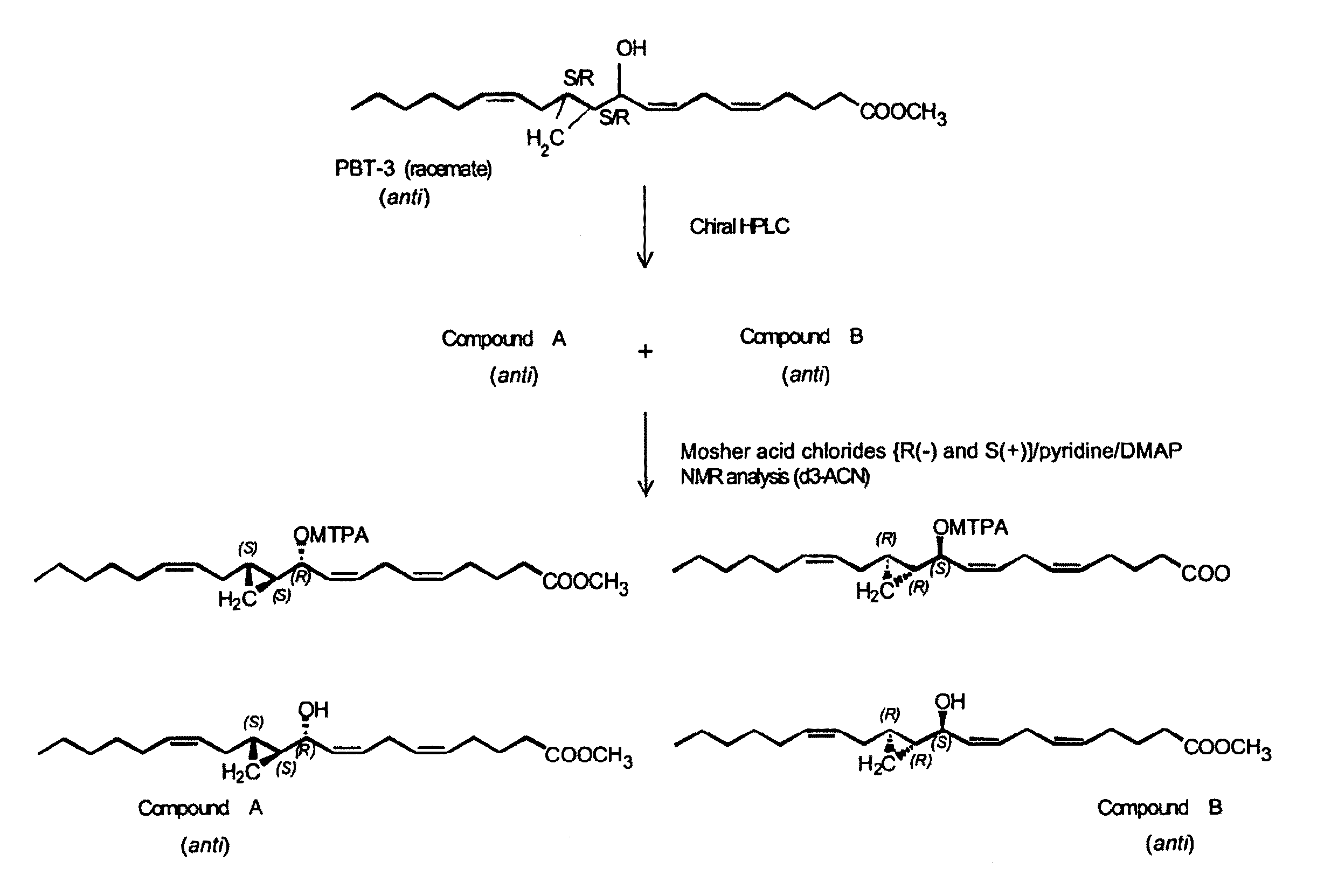
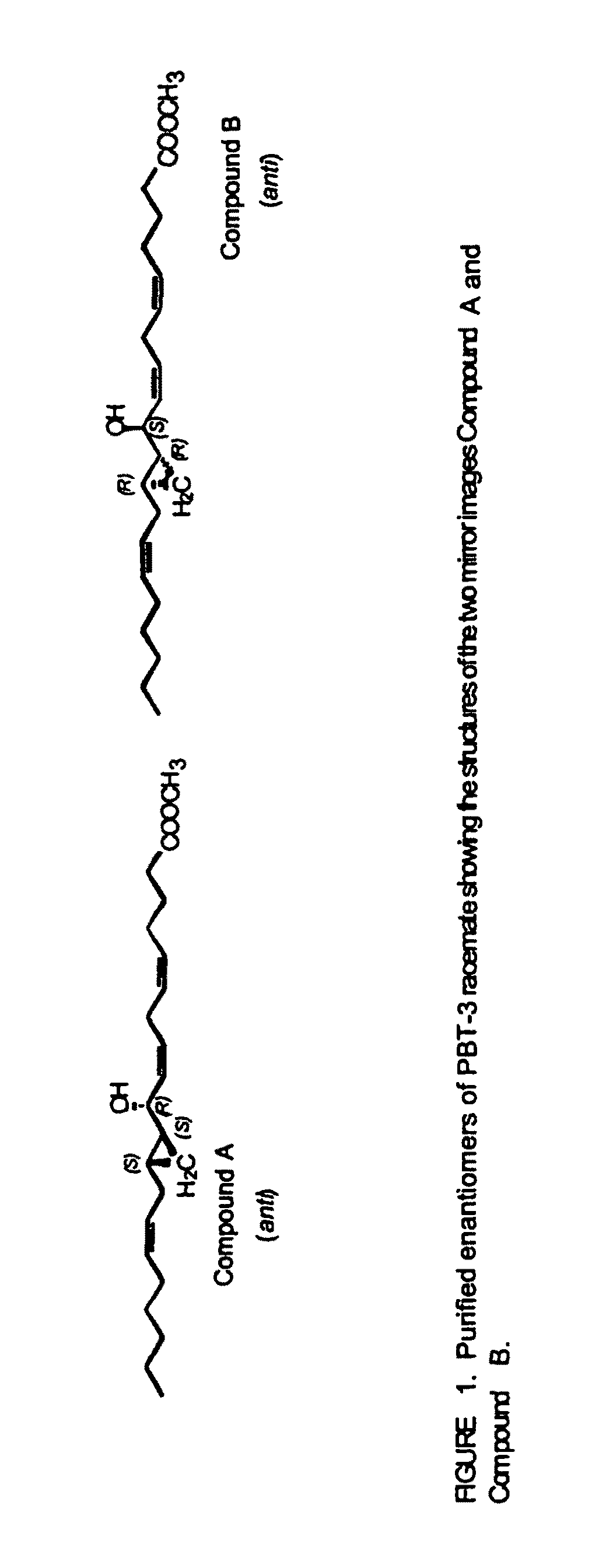
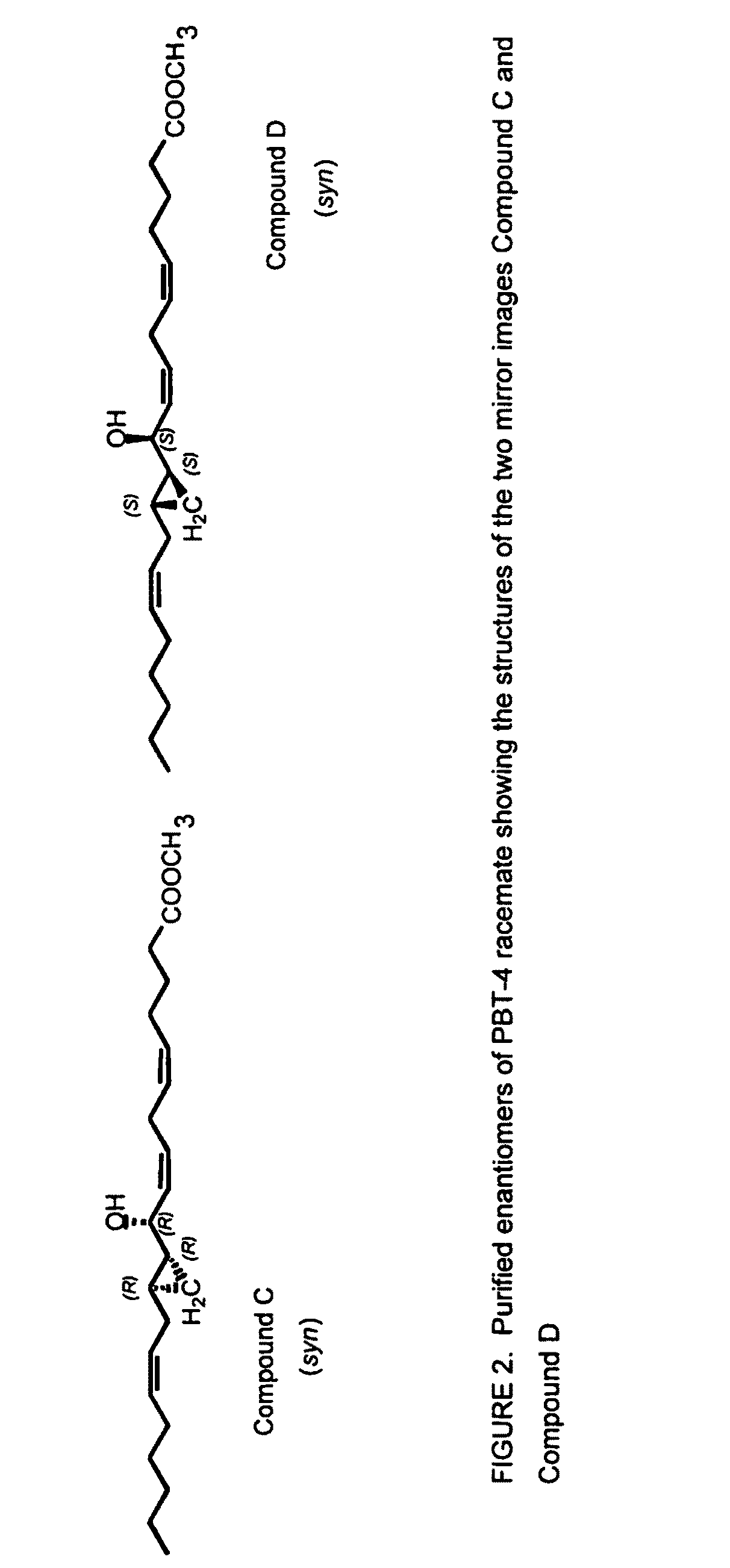
Hepodxilin analog enantiomers
The present invention relates to enantiomeric forms of hepoxilin analogs of Formula I-VIII, pharmaceutical compositions thereof, a method for the separation of said enantiomeric forms of hepoxilin analogs comprising applying said hepoxilin to a chiral phase HPLC column and eluting said hepoxilin with an alkane and alcohol solvent mixture. Said enantiomeric forms of hepoxilin analogs of Formula I-VIII were found to be useful in controlling the biological effects of PPAR mediated transcriptional control for the treatment of diseases such as cancer, thromboxane-mediated diseases and for modulating intracellular calcium concentration.
Owner:CECIL PACE ASCIAK +1
Substituted aryl compounds as novel cyclooxygenase-2 selective inhibitors, compositions and methods of use
InactiveUS20050059665A1Unexpected potential for facilitating wound healingHave antiinflammatory propertiesBiocideSenses disorderHydrolase inhibitorThromboxanes
The invention describes novel substituted aryl compounds that are cyclooxygenase 2 (COX-2) selective inhibitors and novel compositions comprising at least one cyclooxygenase 2 (COX-2) selective inhibitor, and, optionally, at least one compound that donates, transfers or releases nitric oxide, stimulates endogenous synthesis of nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor or is a substrate for nitric oxide synthase, and / or, optionally, at least one therapeutic agent, such as, steroids, nonsterodal anti-inflammatory compounds (NSAID), 5-lipoxygenase (5-LO) inhibitors, leukotriene B4 (LTB4) receptor antagonists, leukotriene A4 (LTA4) hydrolase inhibitors, 5-HT agonists, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, H2 antagonists, antineoplastic agents, antiplatelet agents, thrombin inhibitors, thromboxane inhibitors, decongestants, diuretics, sedating or non-sedating anti-histamines, inducible nitric oxide synthase inhibitors, opioids, analgesics, Helicobacter pylori inhibitors, proton-pump-inhibitors, isoprostane inhibitors, and mixtures thereof. The invention also provides novel kits comprising at least one COX-2 selective inhibitor, and, optionally, at least one nitric oxide donor, and / or, optionally, at least one therapeutic agent. The novel cyclooxygenase 2 selective inhibitors of the invention can be optionally nitrosated and / or nitrosylated. The invention also provides methods for treating inflammation, pain and fever; for treating and / or improving the gastrointestinal properties of COX-2 selective inhibitors; for facilitating wound healing; for treating and / or preventing renal toxicity or other toxicities; for treating and / or preventing other disorders resulting from elevated levels of cyclooxygenase-2; and for improving the cardiovascular profile of COX-2 selective inhibitors.
Owner:NICOX SA
Medicine for treating cardiovascular and cerebrovascular diseases
PendingCN105617355ALower triglyceridesLower total cholesterolOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
The invention provides medicine for treating cardiovascular and cerebrovascular diseases. The medicine is glycoprotein, mixture of polysaccharide and protein, polypeptide or protein. The medicine has the advantages that the triglyceride and total cholesterol contents of rats can be lowered effectively; the anoxia survival time of mice can be prolonged to reach 70-85 minutes; the microcirculation can be improved, the coronary artery can be expanded, the myocardial blood supply can be improved, the blood flow of the brain and the coronary artery can be increased, the heart rate can be reduced, and the myocardial oxygen consumption index can be lowered to improve myocardial blood metabolism, resist thromboxane, increase high-density lipoprotein, resist vasospasm and reduce platelet aggregation, and the total cholesterol and triglyceride of the blood can be lowered; the medicine is safe, efficient and free of side effect.
Owner:徐宝贞
Early diagnosis marker for lung cancer and diagnosis kit and application
The invention relates to an early diagnosis marker for lung cancer and a diagnosis kit and application. Thromboxane can be used as the early diagnosis marker for the lung cancer, and comparison of thromboxane contents of experiment groups and control groups shows that the contents of the thromboxane in the experiment groups and the control groups are very remarkably different, the thromboxane is applicable to early identification and diagnosis of lung cancer and has good popularization and application values, and a novel clinical diagnosis method is provided for early diagnosis of lung cancer.
Owner:深圳瑞科生物科技有限公司
Hepoxilin analog enantiomers
ActiveUS20100210727A1Enhanced chemilluminescenceIncreased cleavageBiocideSenses disorderAlkaneDisease
The present invention relates to enantiomeric forms of hepoxilin analogs of Formula I-VIII, pharmaceutical compositions thereof, a method for the separation of said enantiomeric forms of hepoxilin analogs comprising applying said hepoxilin to a chiral phase HPLC column and eluting said hepoxilin with an alkane and alcohol solvent mixture. Said enantiomeric forms of hepoxilin analogs of Formula I-VIII were found to be useful in controlling the biological effects of PPAR mediated transcriptional control for the treatment of diseases such as cancer, thromboxane-mediated diseases and for modulating intracellular calcium concentration.
Owner:CECIL PACE ASCIAK +1
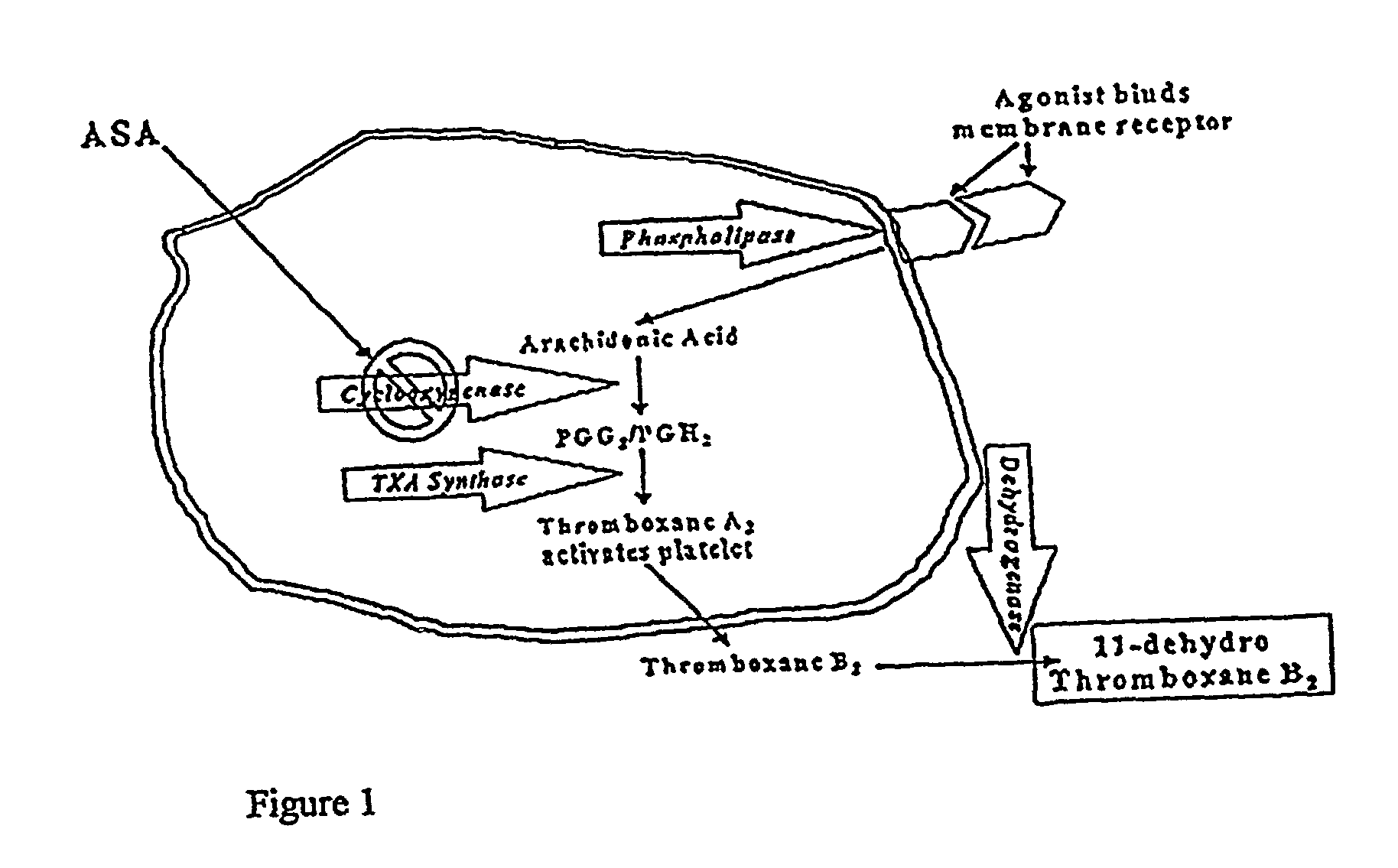
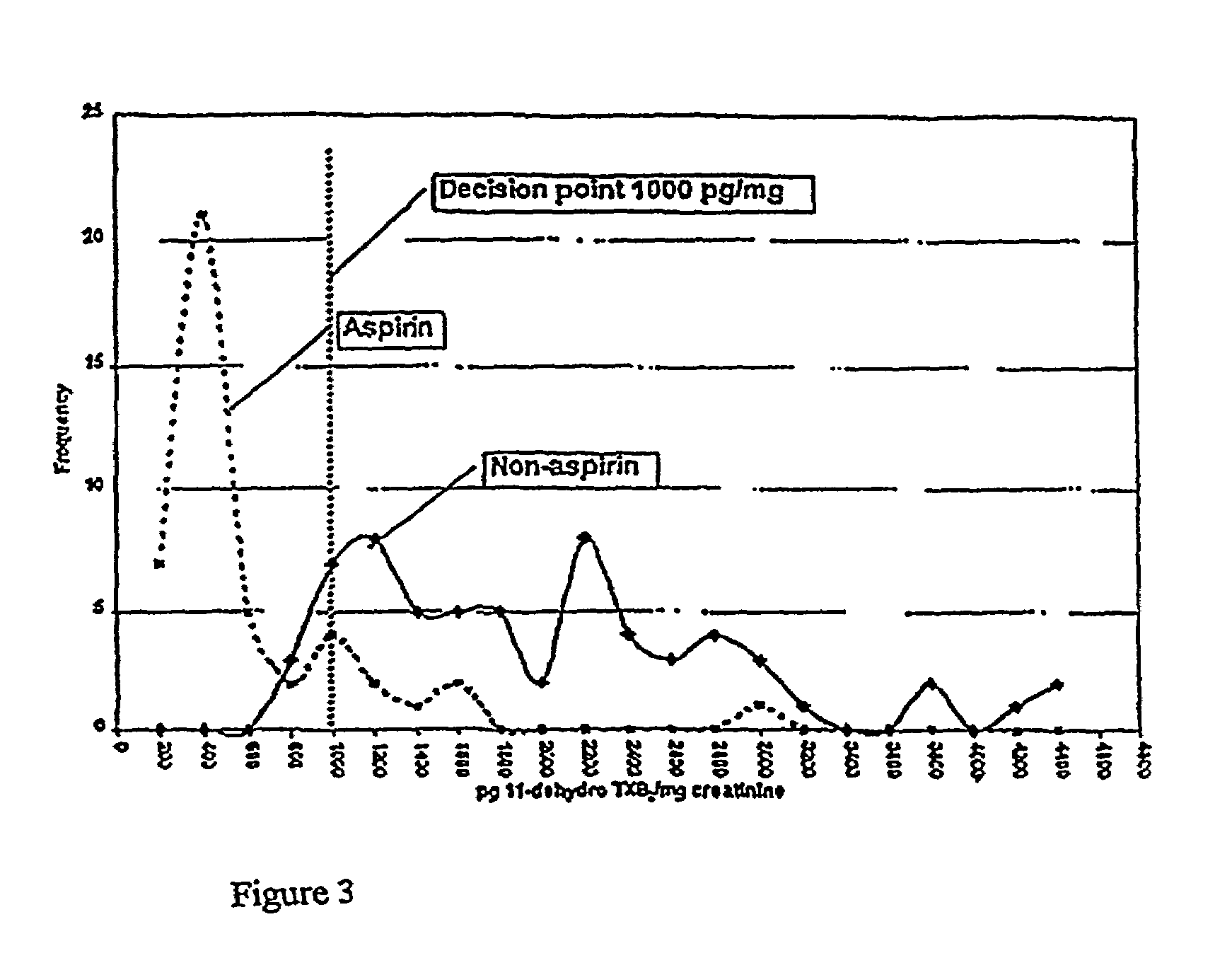
Kits for determination of thromboxane B2 metabolite and optimizing aspirin dosage
Kits are provided that can be used to determine a thromboxane B2 metabolite level and a creatinine level in a biological sample, particularly a human urine sample. This information can be used to optimize aspirin dosage in a patient. The present invention further includes calibrant preparations. In some embodiments, these calibrants comprise urine, particularly human urine that is identified in repeated trials to provide a consistent and reproduceable level of thromboxane B2 metabolite. As such, they function as control preparations that reduce error from intra-assay sampling measurements.
Owner:ESOTERIX
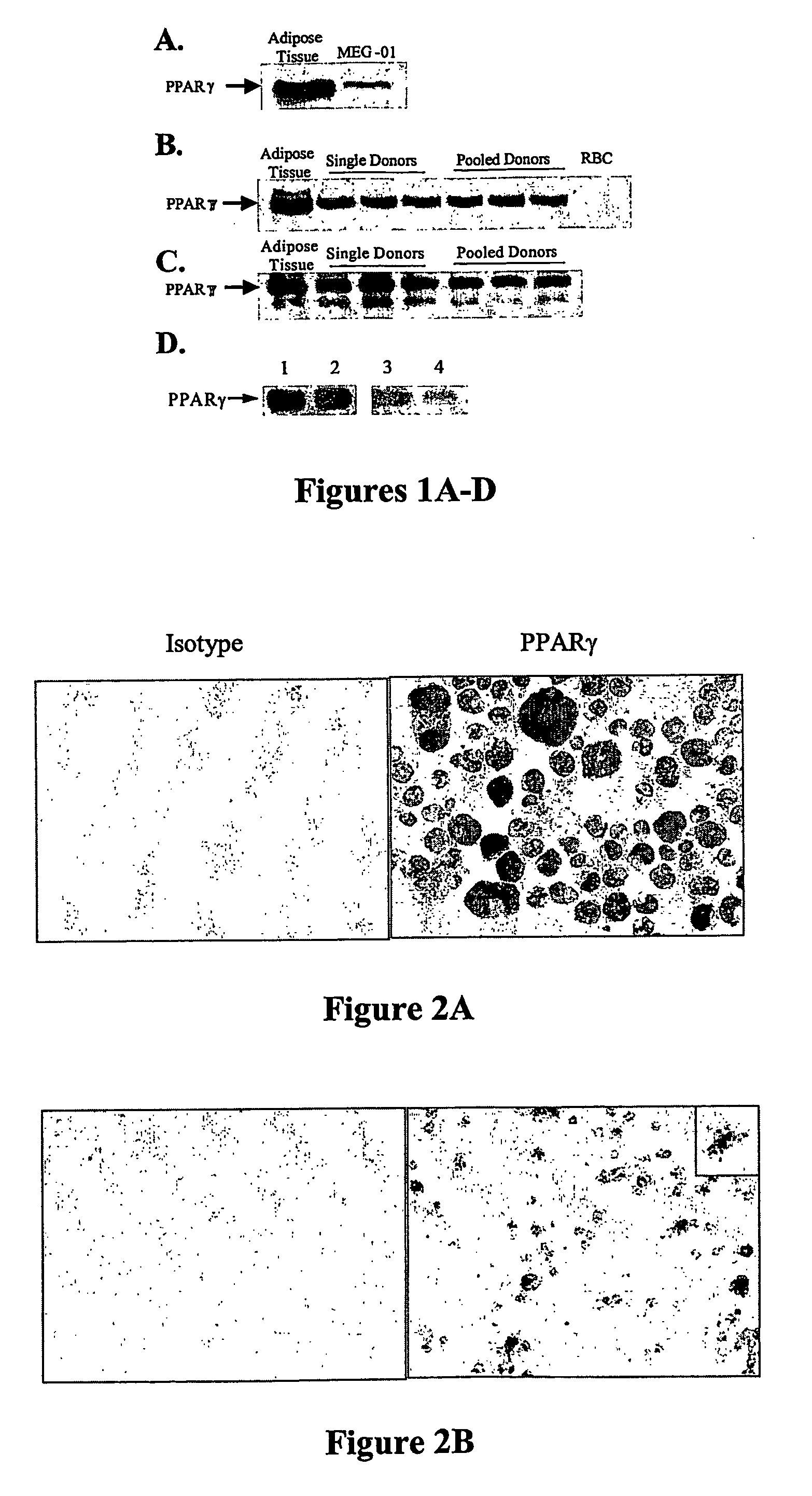
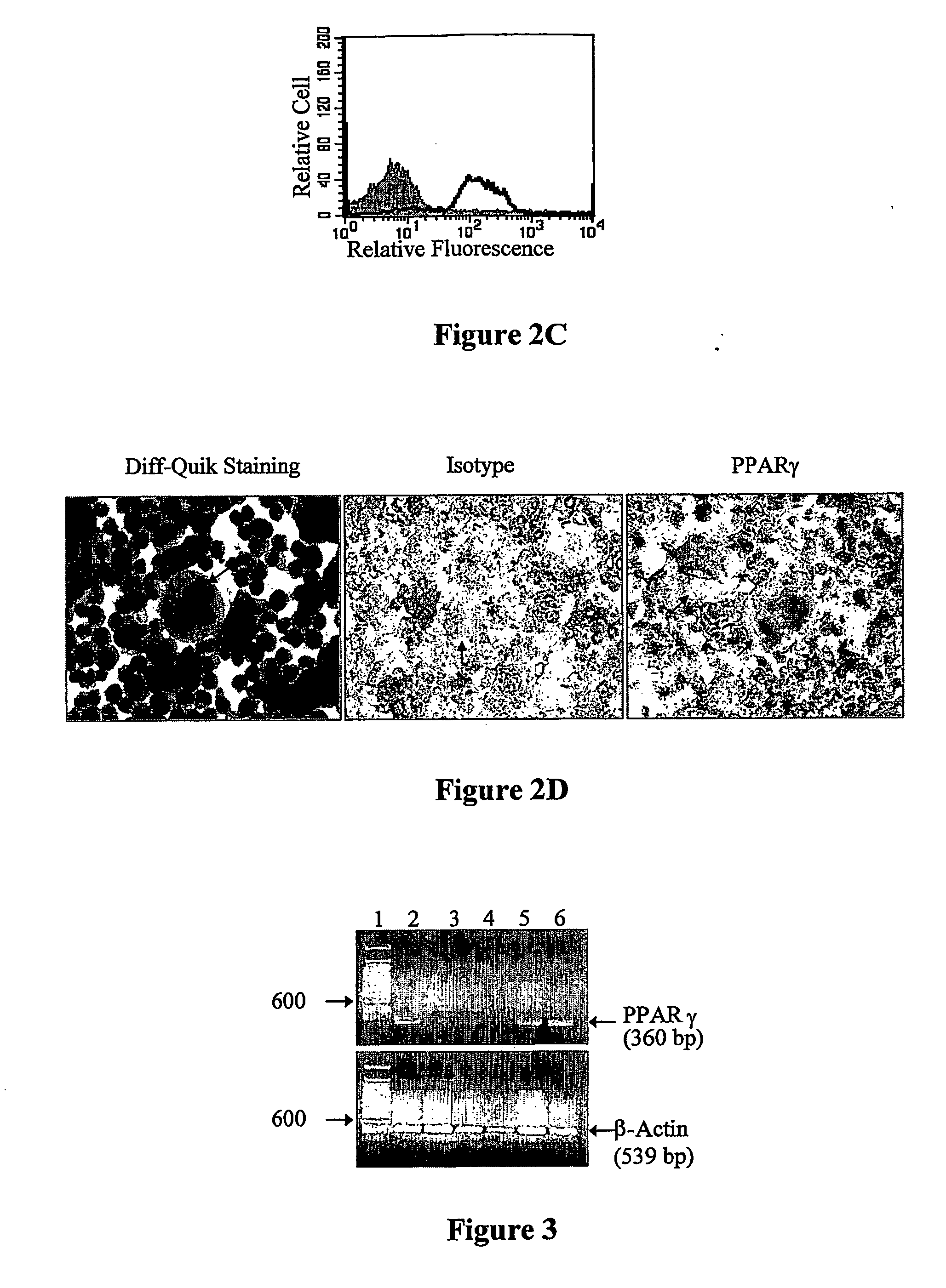
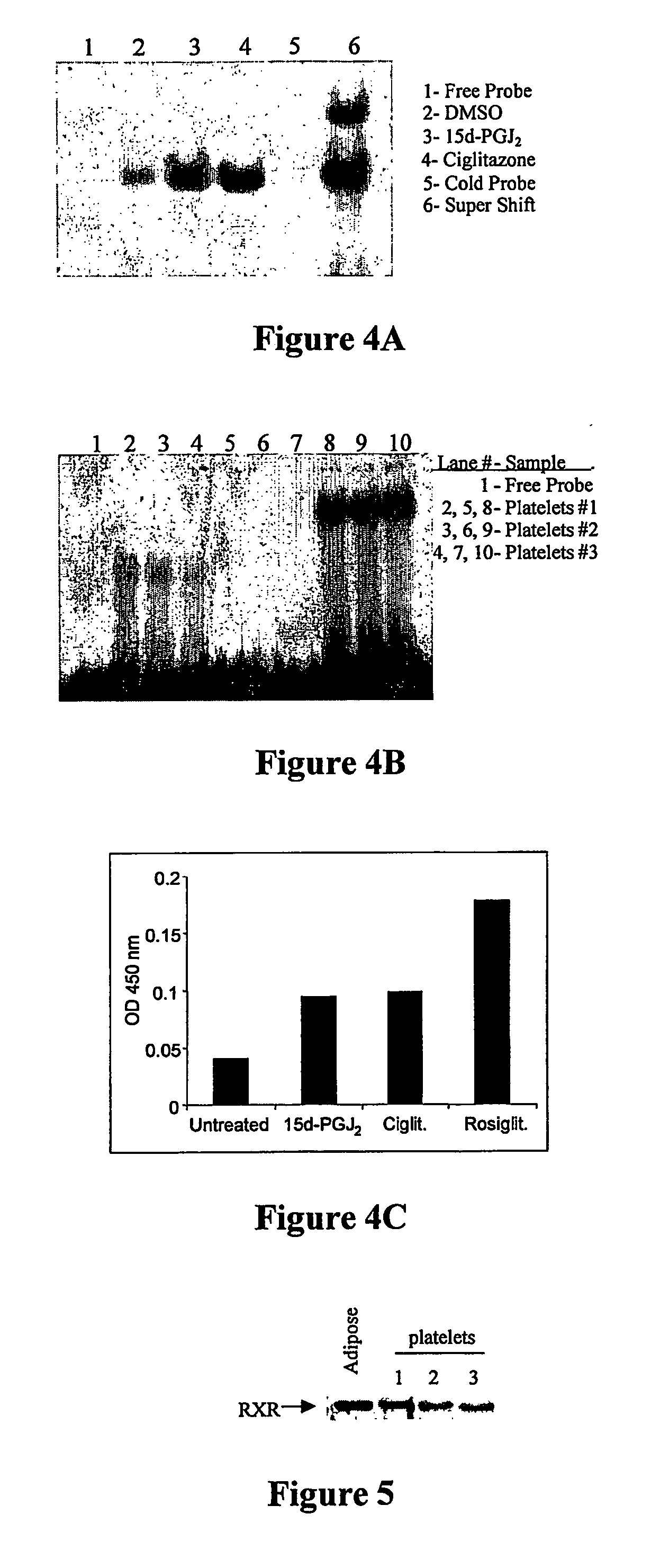
Use of peroxisome proliferator-activated receptor gamma (ppary) and/or retinoic acid receptor (rxr) agonists to inhibit platelet functions
InactiveUS20070135382A1Inhibits platelet aggregationEffectively control levelBiocideElcosanoid active ingredientsWhole blood productThrombus
Methods of inhibiting mammalian platelet release of CD40 ligand, thromboxanes, or prostaglandin E2, or surface expression of CD40 ligand that involve contacting mammalian platelets with an effective amount of a PPARγ agonist, an RXR agonist, or a combination thereof. As a consequence of inhibiting CD40 ligand and thromboxane release, the present invention allows for inhibition of thrombus fon-nation by (or clotting activities of) activated platelets, as well as treating or preventing CD40 ligand-mediated conditions and / or thromboxane-mediated conditions. Use of PPARγ agonist, RXR agonist, and / or inducers of PPARγ agonist in preparing a stored blood product, and for diagnostic testing of patient samples is also disclosed.
Owner:UNIVERSITY OF ROCHESTER
Thromboxane ligands without blood clotting side effects
InactiveUS7019149B2Potent ocular hypotensive agentsTreatment safetyBiocideElcosanoid active ingredientsAnginaThromboxanes
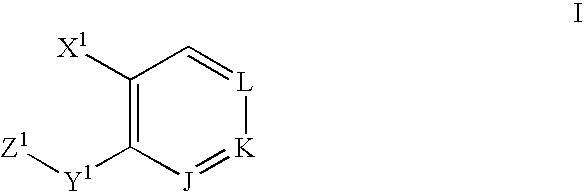
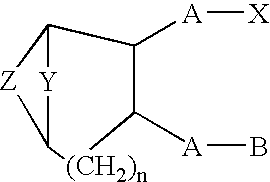
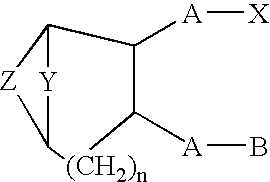
A method of treating ocular hypotension, hypertension, hemorrhage, myocardial ischemia, angina pectoris, coronary contraction, cerebrovascular contraction after subarachnoidal hemorrhage, cerebral hemorrhage and asthma which comprises administering to a mammal suffering therefrom a therapeutically effective amount of a thromboxane ligand which is a compound formula I, wherein Y is (CH2)x; Z is selected from the group consisting of and (CR2)x, x is an integer of 1 or 2; n is 0 or 1; R2 is hydrogen or an alkyl radical of from 1 to 4 carbons; A is an alkylene or alkenylene radical having from two to seven carbon atoms, which radical may be substituted with one or more hydroxy, oxo, alkyloxy or alkylcarboxy groups or said alkylene or alkenylene may have one or more enchained oxa or imino radicals; B is a methyl radical or a cycloalkyl radical having from three to seven carbon atoms, or an aryl radical, selected from the group consisting of hydrocarbyl aryl and heteroaryl radicals wherein the heteroatom is selected from the group consisting of nitrogen, oxygen and sulfur atoms, or substituted derivatives of said methyl, cycloalkyl or aryl radicals wherein said substituent is selected from the group consisting of halo, nitro, amino, thiol, hydroxy, alkyloxy and alkylcarboxy; and X is selected from the group consisting of nitro, cyano, —COOR, —CH2OR1, —C(O)N(R1)2, —CH2N(R1)2—CH═N—OH and —CH2SR1 radicals wherein R is a C1 to C10 alkyl, phenyl or benzyl and R1 is R or hydrogen; or a pharmaceutically acceptable salt thereof.
Owner:ALLERGAN INC
Method for researching pharmacodynamic relationship of various medicinal components of compound thromboxane preparation
InactiveCN107742058ASubstantively innovativeGood practice valueMolecular designSpecial data processing applicationsUniform designMedicine
The present invention discloses a method for researching the pharmacodynamic relationship of various medicinal components of a compound thromboxane preparation. In the method, the mass percentage of each herbal medicine in the formula of the compound thromboxane preparation is taken as a variable, a uniform design method is used to prepare multiple difference samples with different proportions ofmedicinal materials, and at the same time, multiple missing samples of prescription medicinal materials is prepared; through pharmacodynamics research, the pharmacodynamic data of each difference sample and missing sample of the medicinal materials are obtained; and a variety of statistical methods are used to analyze the relationship between medicinal materials and efficacy in multiple samples, and the contribution, primary and secondary effects and interactions of each medicinal material are determined. The present invention discloses for the first time a method for researching the contribution, primary and secondary effects and interactions of each medicinal material in the compound thromboxane preparation, provides a basis for clarifying the compatibility rule of the scientific constituents and screening the optimal proportion of the constituents, and provides more scientific and more complete modern scientific data support for the clinical application of the compound thromboxane preparation.
Owner:SUN YAT SEN UNIV
Novel acetylsalicylic acid formulations
The invention relates to pharmaceutical compositions of acetylsalicylic acid-based microcapsules to selectively inhibit the COX in the portal vein and / or in the liver to reduce the production of thromboxane. Further, the pharmaceutical composition minimizes COX inhibition in the systemic circulation to optimize the inhibition of platelet aggregation. Certain embodiments also address methods of prevention and / or treatment of these diseases, using these oral compositions such as enhancing the safety of antithrombotic treatments. Other embodiments contemplate oral pharmaceutical compositions that combine acetylsalicylic acid with anti-platelet aggregation drugs, without inducing gastric side effects.
Owner:FLAMEL TECHNOLOGIES
Treatment of preeclampsia, toxemia and preterm labor with combination of progestational agent and a nitric oxide synthase substrate and/or donor
Preeclampsia and preterm labor in a pregnant female mammal are treated by administering thereto a combination of a progestin and a nitric oxide synthase substrate, a nitric oxide donor or both, optionally in further combination with one or more of a cyclooxygenase inhibitor, a PGI2-mimetic, a thromboxane (TXA2) inhibitor, a compound possessing TXA2-agonistic and TXA2-inhibiting properties, a compound possessing TXA2-antagonistic and PGI2-memetic activities, and a TXA2 antagonist.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Kit for detecting metabolism of aspirin and high reactivity of platelets and detection method
The invention belongs to the technical field of biomedicine and relates to a chemiluminiscence detection kit for detecting the metabolism of aspirin and the high reactivity of platelets and a detection method. The kit comprises an 11-dehydro-thromboxane B2 monoclonal antibody, an 11-dehydro-thromboxane B2 competitive product, magnetic particles and a luminous substrate; and whether a patient has drug resistance or resistance to aspirin can be detected through the detection kit. The detection method has the advantages of high sensitivity, high accuracy and little possibility of being influencedby biochemical indexes. The detection method provides a scientific reference basis for clinical administration and can be applied to clinical medication guidance. With the detection method adopted, the problem of the failure or poor effect of the antiplatelet aggregation treatment of a clinical patient caused by aspirin resistance of the clinical patient can be solved.
Owner:湖南菲思特精准医疗科技有限公司
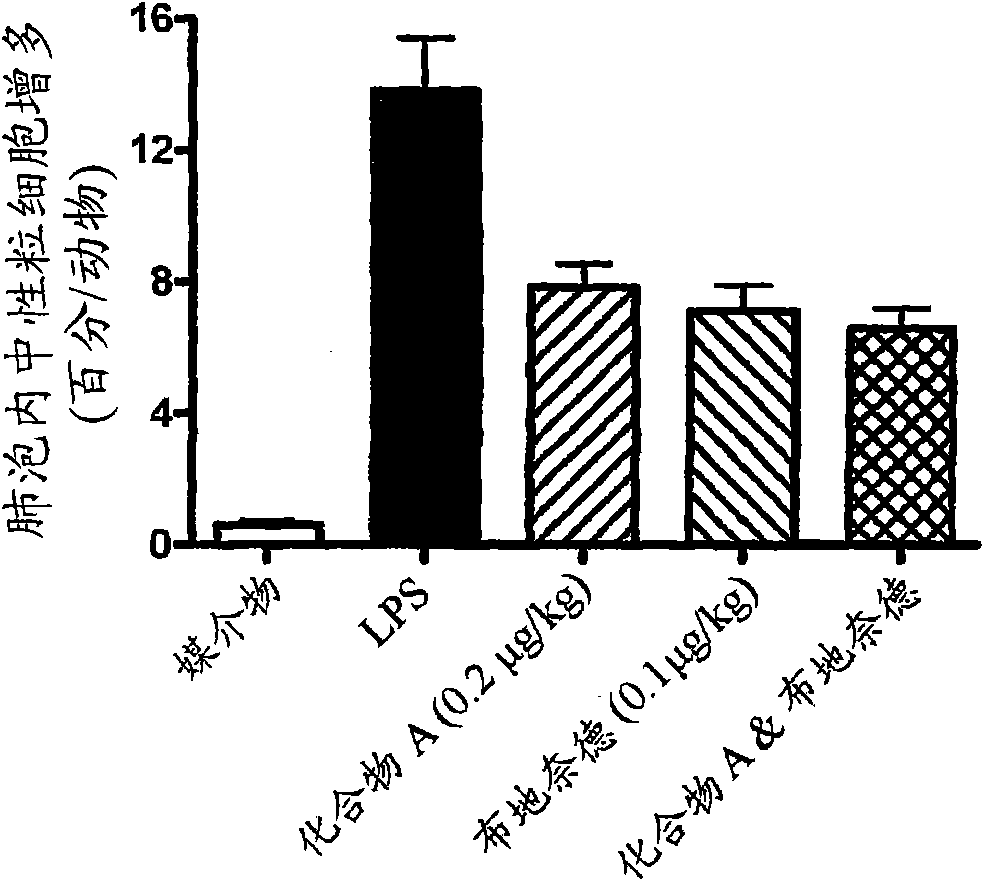
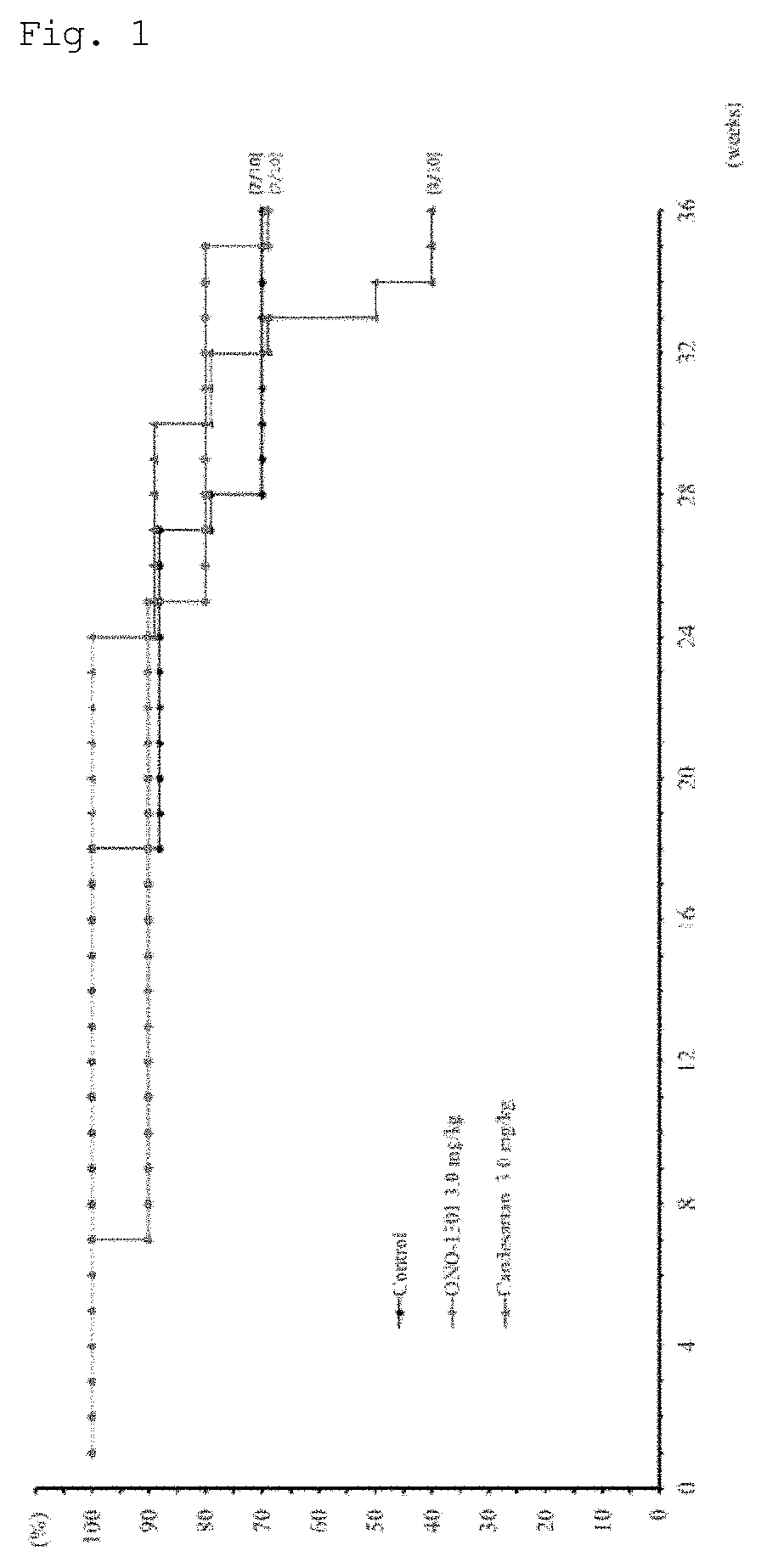
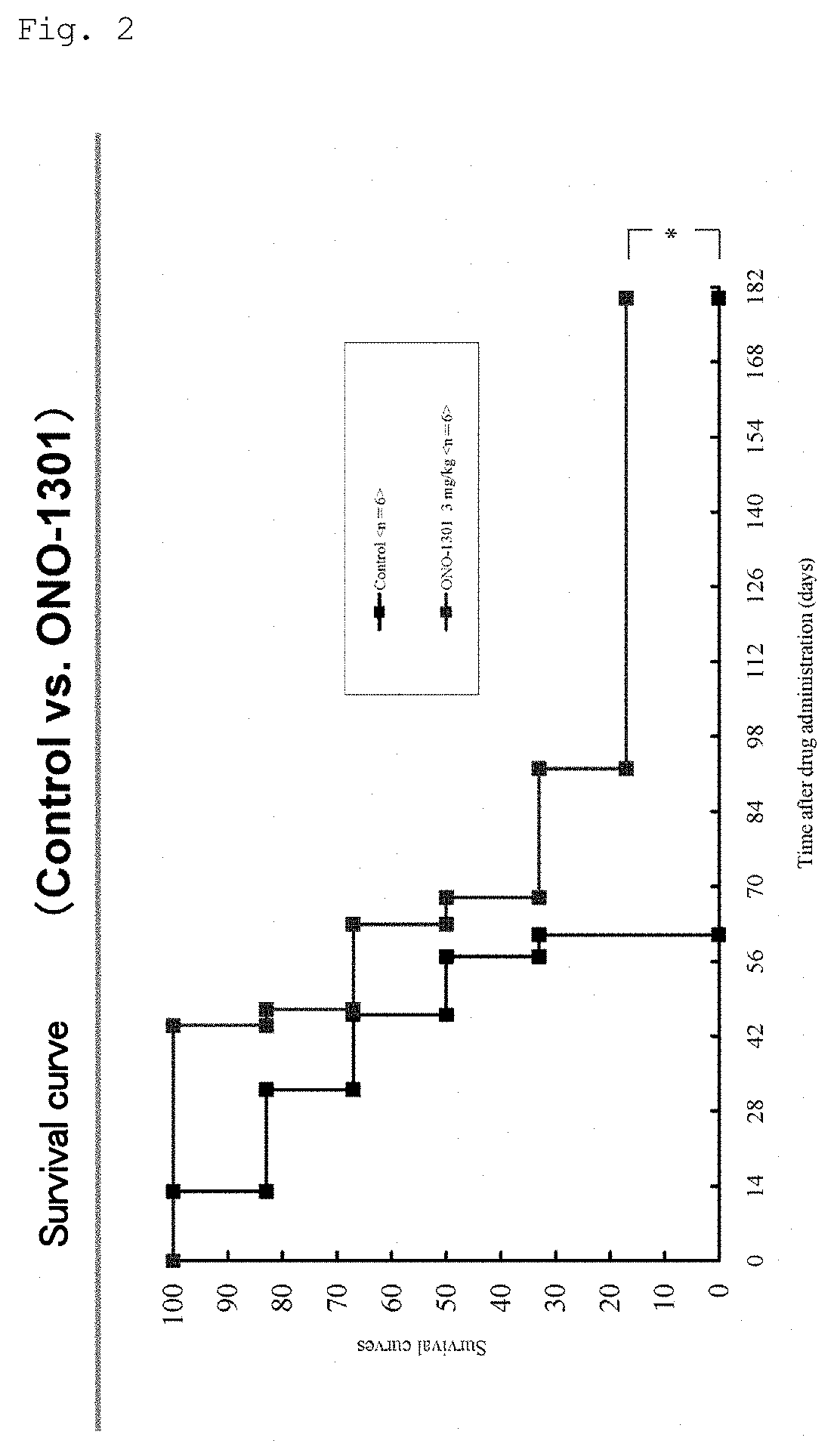
Compositions and Methods of Treating Muscular Dystrophy with Thromboxane-A2 Receptor Antagonists
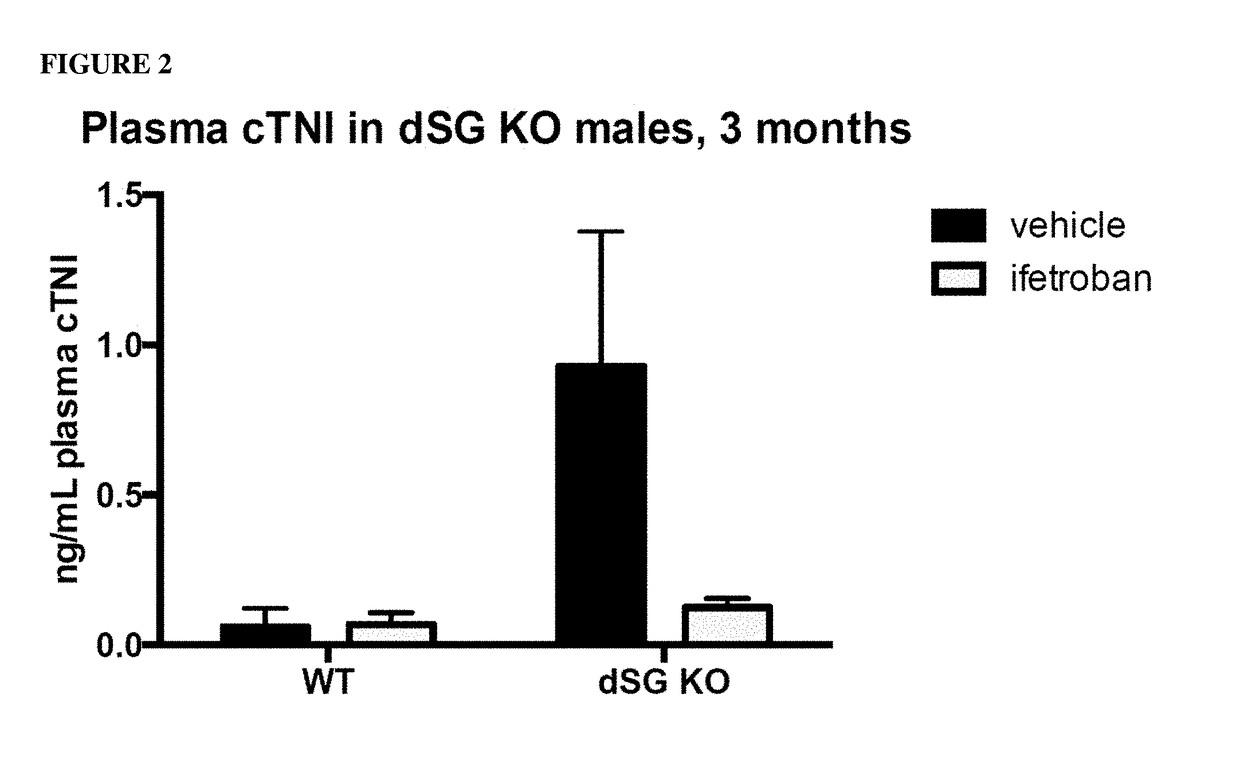
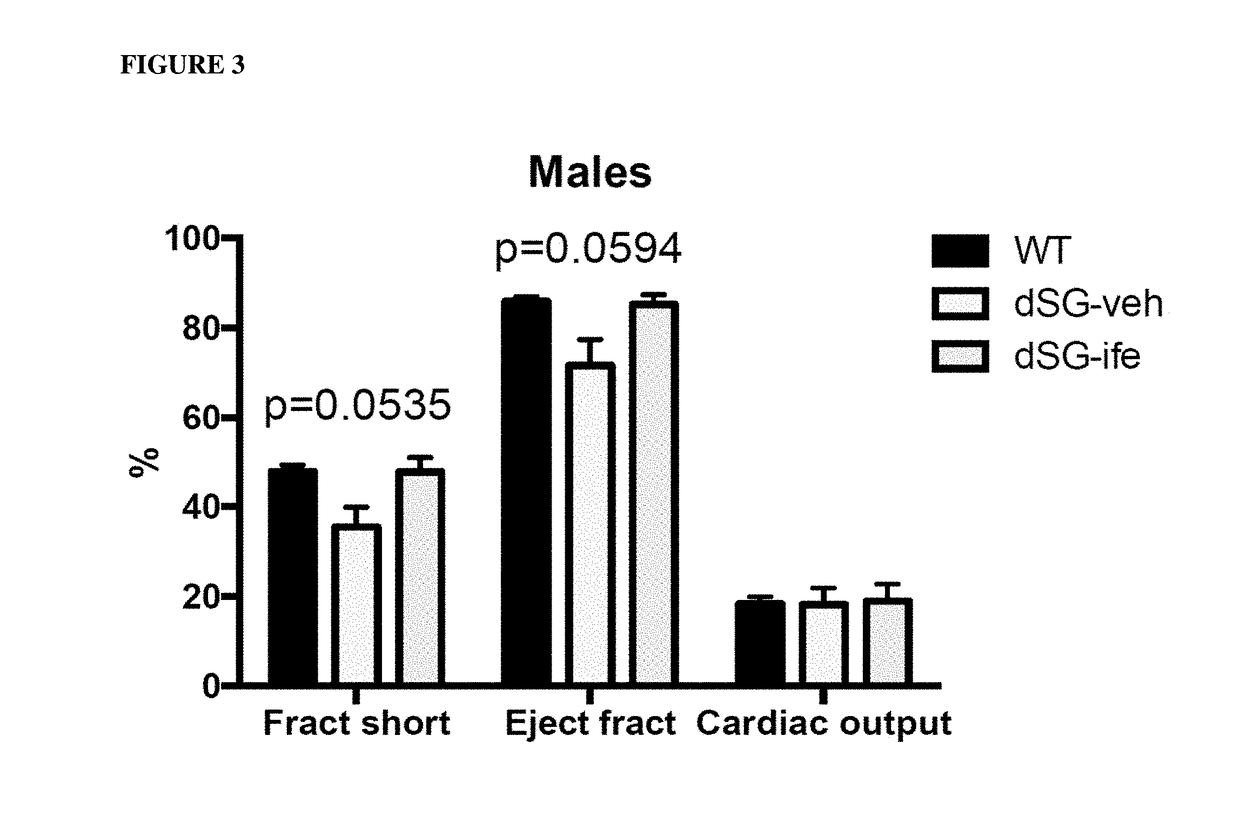
ActiveUS20170340614A1Exact fitImproved ventricular functionOrganic active ingredientsDigestive systemThromboxane A2 receptorMuscular dystrophy
The present invention is directed to methods of treating and / or ameliorating muscular dystrophy and / or treating cardiomyopathy in muscular dystrophy patients by administration of a therapeutically effective amount of a thromboxane A2 receptor antagonist.
Owner:CUMBERLAND PHARM INC +1
Method of protecting erythricytes, in particular for improvement of blood cytopenia
InactiveUS7153839B2Altered penetrabilityAltered structureBiocideMicroorganismsMetaboliteRed blood cell
The present invention concerns a compound consisting of RNA, in particular RNA extracted from yeast, a pharmaceutical composition comprising such RNA and a method for the treatment of inflammatory and inflammatory-related disorders comprising administering to a patient in need of such treatment a pharmaceutical composition comprising an amount effective to ameliorate the symptoms of inflammation or inflammatory-related disorder of ribonucleic acid and a pharmaceutically acceptable vehicle, carrier, or diluent. The exogenous yeast RNA used in the present invention has a pronounced membrane-stabilizing action in a wide range of concentrations. At the same time, yeast RNA normalizes metabolism of arachidonic acid and levels of its key metabolites, thromboxane and leukotriene. Its anti-inflammatory action is accompanied by normalization of the activity of NO-synthetase and anti-oxidant activity.
Owner:BIOCELL LAB
Combinations of beta- 2 -adrenoceptor agonistic benzothiazolone
The invention provides a pharmaceutical product comprising a first active ingredient which is N-[2-(Diethylamino)ethyl]-N-(2-{[2-(4-hydroxy-2-oxo-2,3-dihydro-1,3-benzothiazol-7-yl)ethyl]amino}ethyl)-3-[2-(1-naphthyl)ethoxy]propan amide or a salt thereof, and a second active ingredient selected from: a non-steroidal Glucocorticoid Receptor (GR Receptor) Agonist; an antioxidant; a CCR1 antagonist; achemokine antagonist (not CCR1); a corticosteroid; a CRTh2 antagonist; a DP1 antagonist; an Histone Deacetylase Inducer; an IKK2 inhibitor; a COX inhibitor; a lipoxygenase inhibitor; a leukotriene receptor antagonist; an MPO inhibitor; a muscarinic antagonist which is Aclidinium bromide, Glycopyrrolate, Oxitropium bromide, Pirenzepine, telenzepine, Tiotropium bromide,3(R)-(2-hydroxy-2,2-dithien-2-ylacetoxy)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane bromide,3(R)-1-phenethyl-3-(9H-xanthene-9-carbonyloxy)-1-azoniabicyclo[2.2.2]octane bromide or (3R)-3-[(2S)-2-cyclopentyl-2-hydroxy-2-thien-2-ylacetoxy]-1-(2-phenoxyethyl)-1-azoniabicyclo[2.2.2]actane bromide; a p38 inhibitor; a PDE inhibitor; a PPARy agonist; a protease inhibitor; a Statin; a thromboxane antagonist; a vasodilator; or, an ENAC blocker (Epithelial Sodium-channel blocker); and its use in the treatment of respiratory disease.
Owner:ASTRAZENECA AB
Compositions and methods of treating muscular dystrophy with thromboxane-A2 receptor antagonists
ActiveUS10064845B2Function increaseOrganic active ingredientsDigestive systemDuchenne muscular dystrophyMuscular dystrophy
The present invention is directed to methods of treating and / or ameliorating muscular dystrophy and / or treating cardiomyopathy in muscular dystrophy patients by administration of a therapeutically effective amount of a thromboxane A2 receptor antagonist.
Owner:CUMBERLAND PHARM INC +1
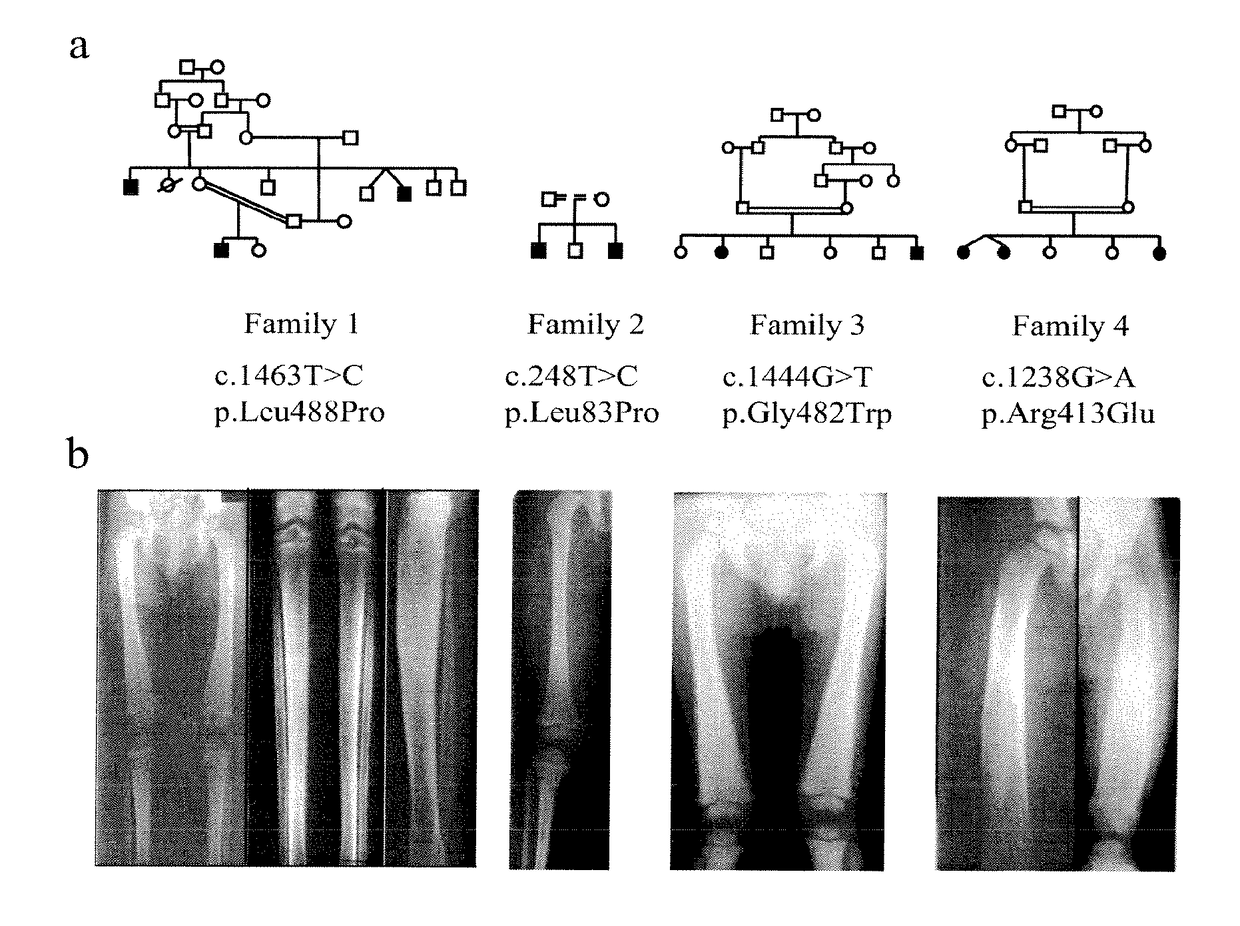
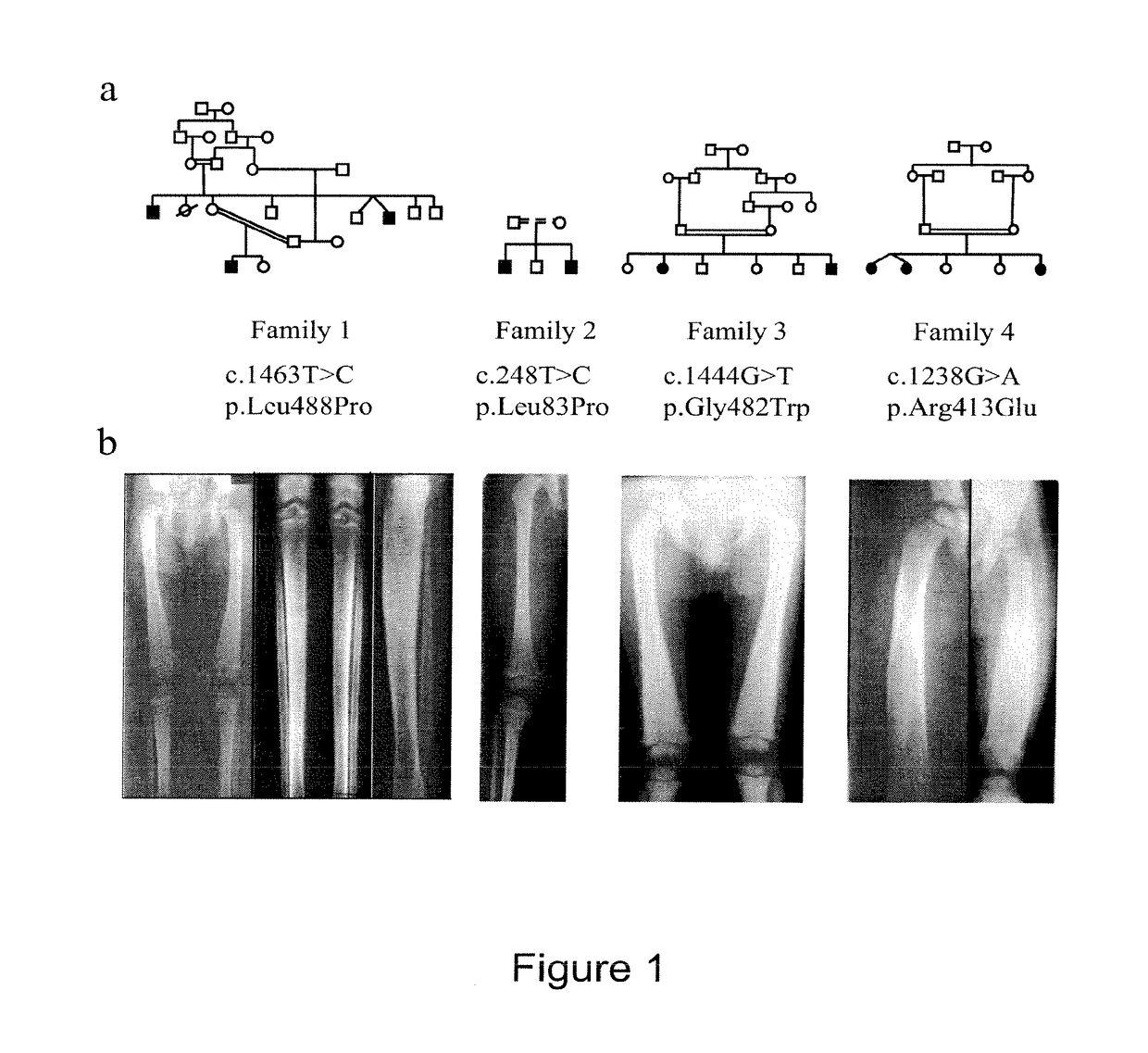
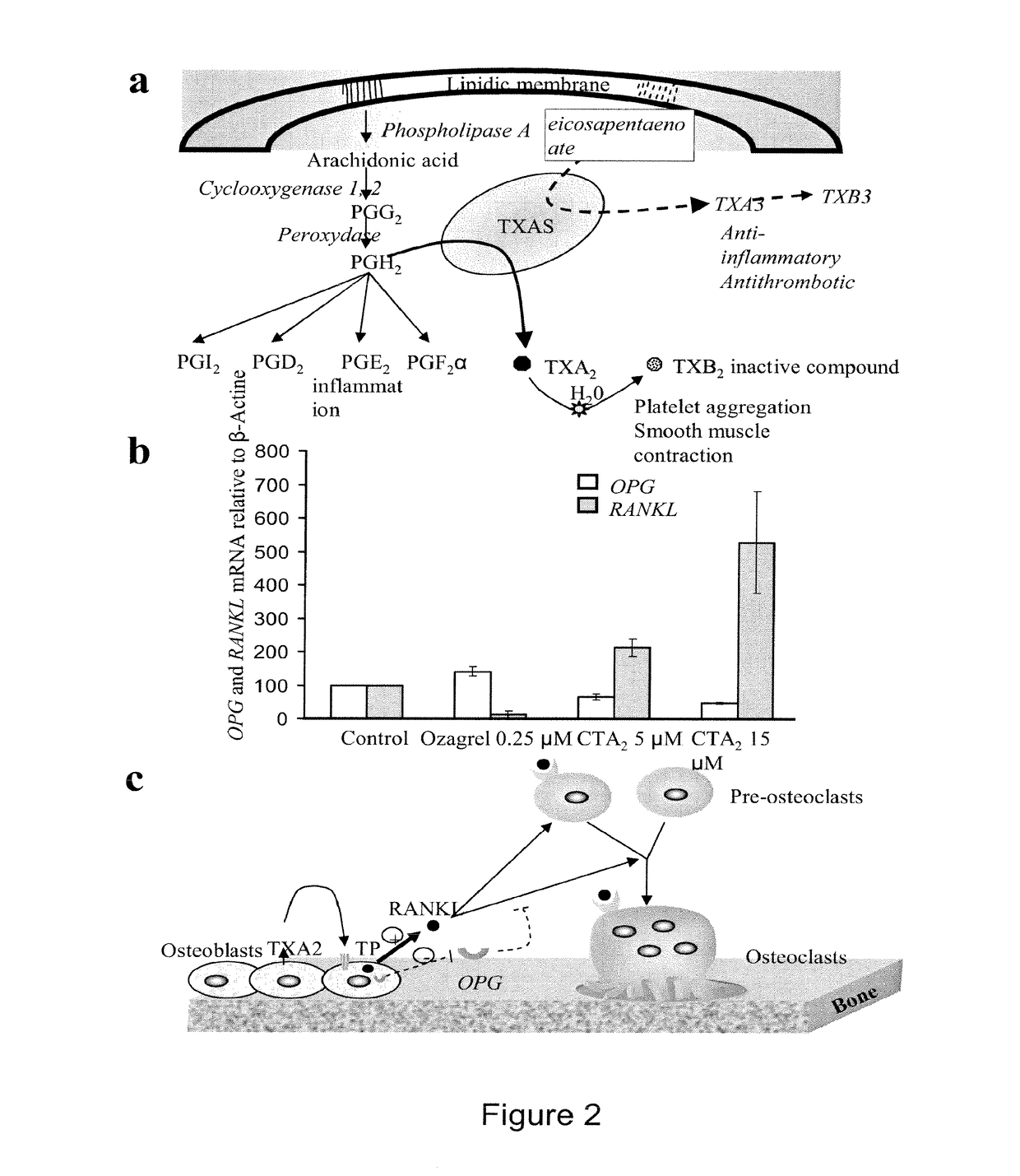
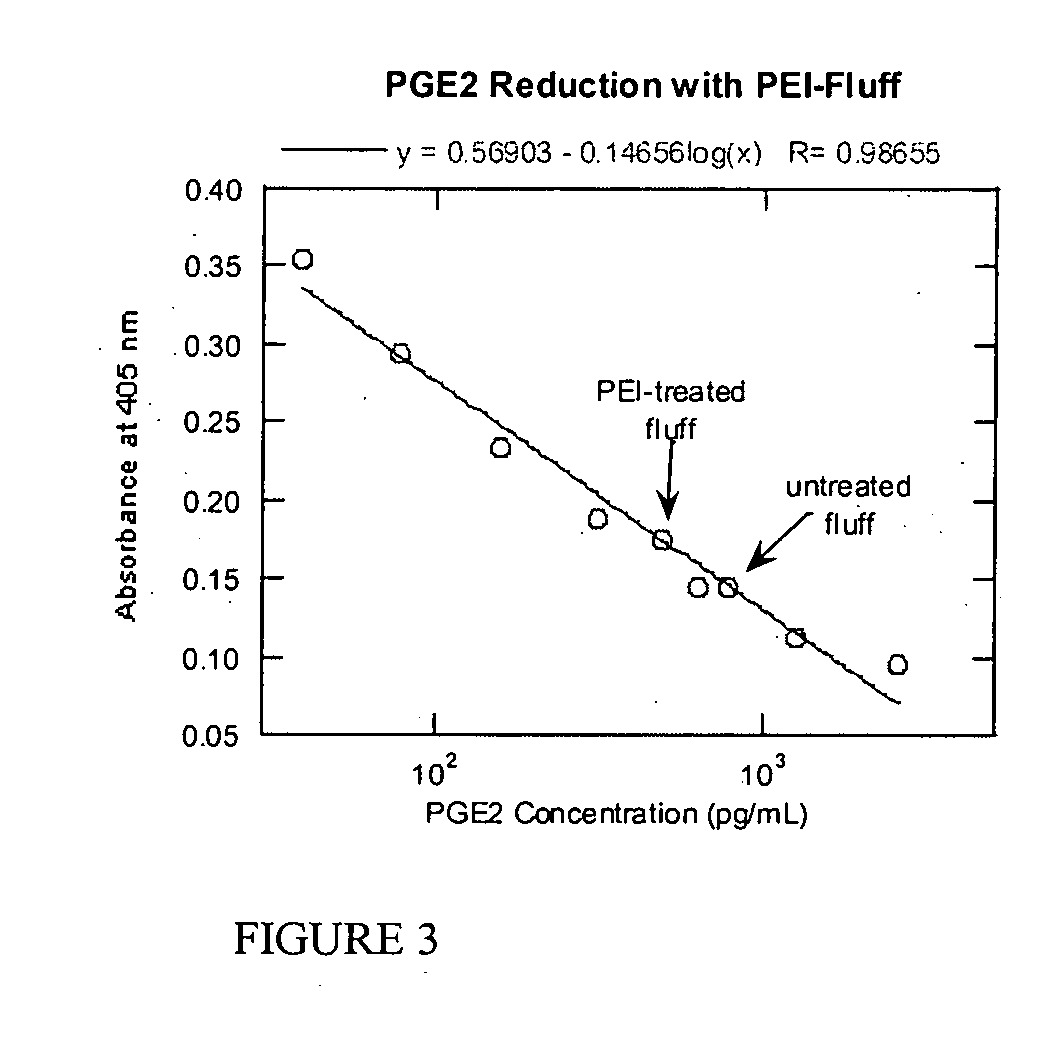
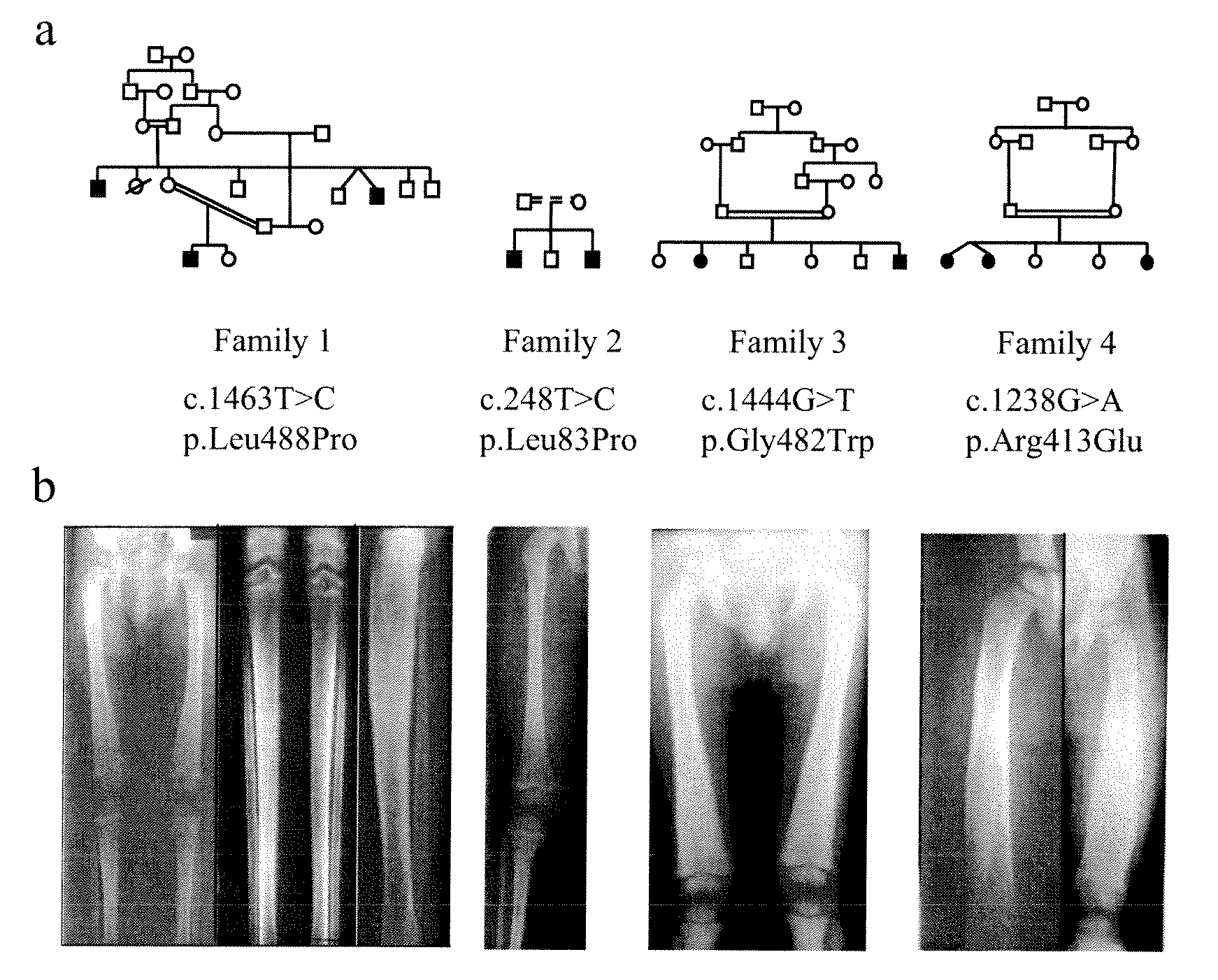
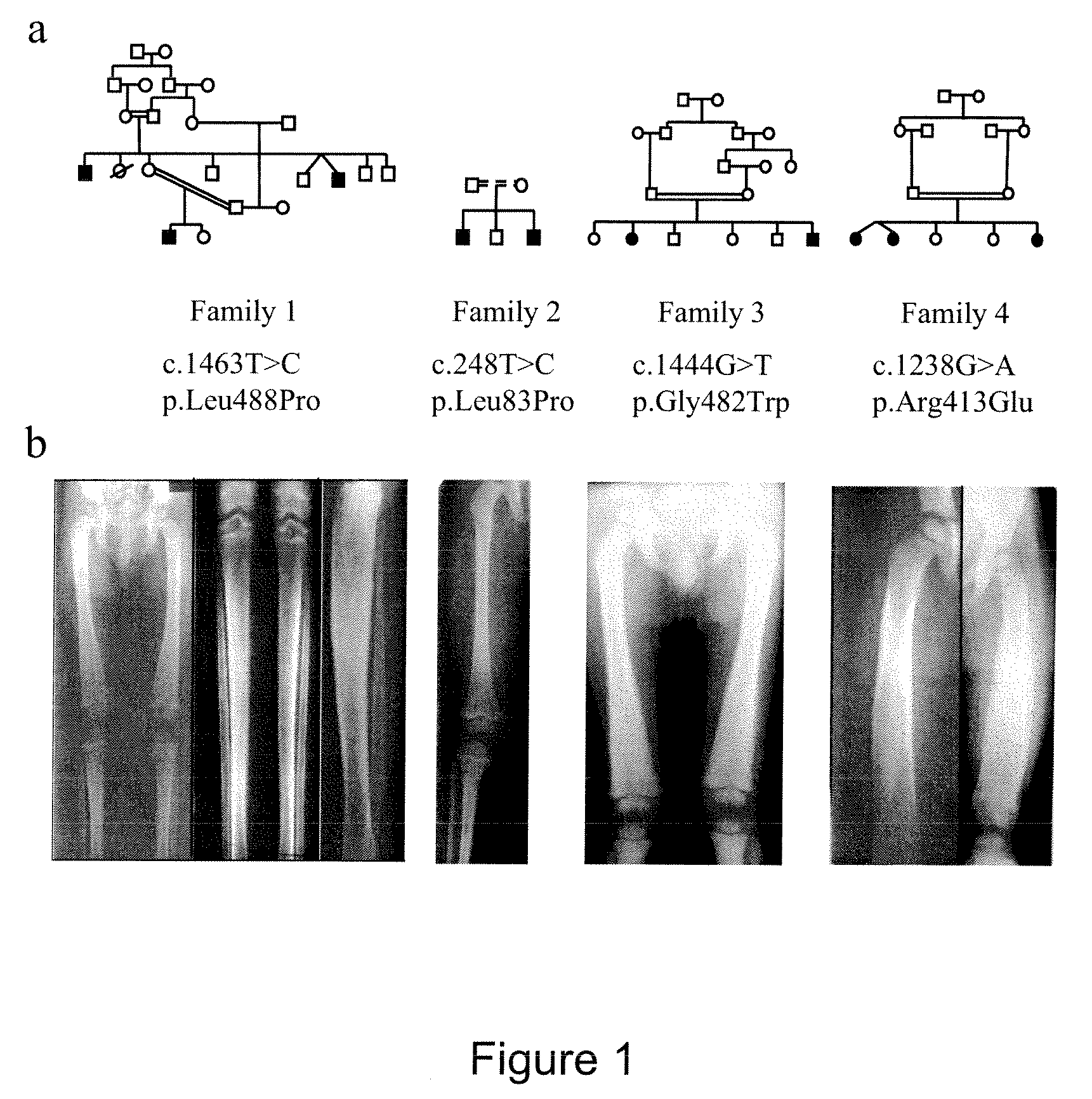
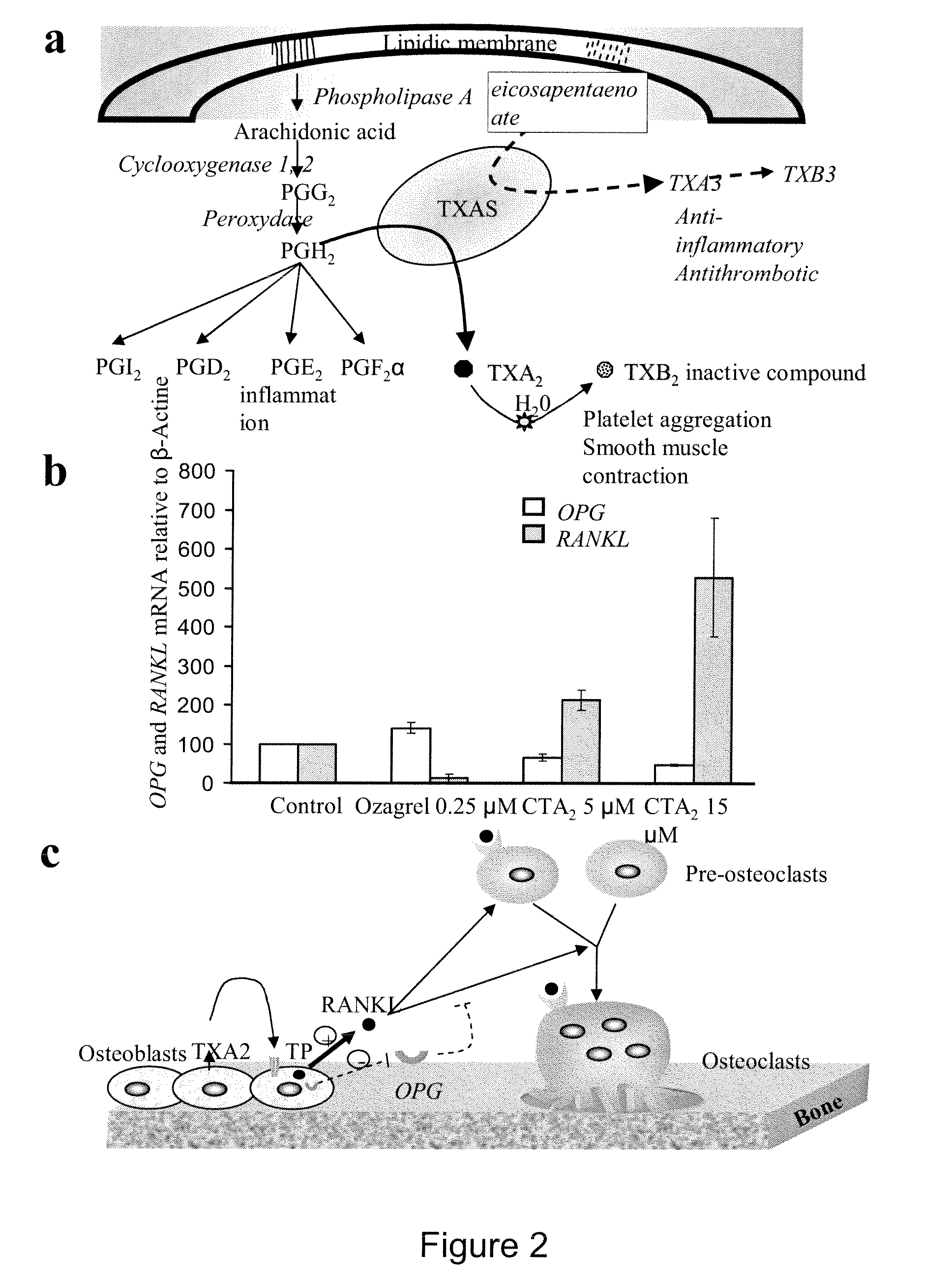
Methods for the treatment and diagnosis of bone mineral density related diseases
ActiveUS9834820B2Simple processAvoid side effectsOrganic active ingredientsPeptide/protein ingredientsGhosal hematodiaphyseal dysplasiaBone density
Described herein are methods of the treatment and diagnosis of bone mineral density related disorders. More particularly, described herein are methods of diagnosing or predicting a bone mineral density related disease, or a risk of a bone mineral density related disease, in a subject, which method comprises detecting a mutation in the TBXAS1 gene, wherein the presence of such a mutation is indicative of a bone mineral density related disease or of a risk of a bone mineral density related disease. Also described are compounds such as a thromboxane synthase (TXAS) encoding polynucleotide, a TXAS, thromboxane A2 or an analog thereof for treating or preventing a disease associated with an increased bone mineral density (e.g., Ghosal hematodiaphyseal dysplasia syndrome). Additional aspects describe an inhibitor of TBXAS1 gene expression or a thromboxane inhibitor for treating or preventing a disease associated with a decreased bone mineral density (e.g., osteoporosis).
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
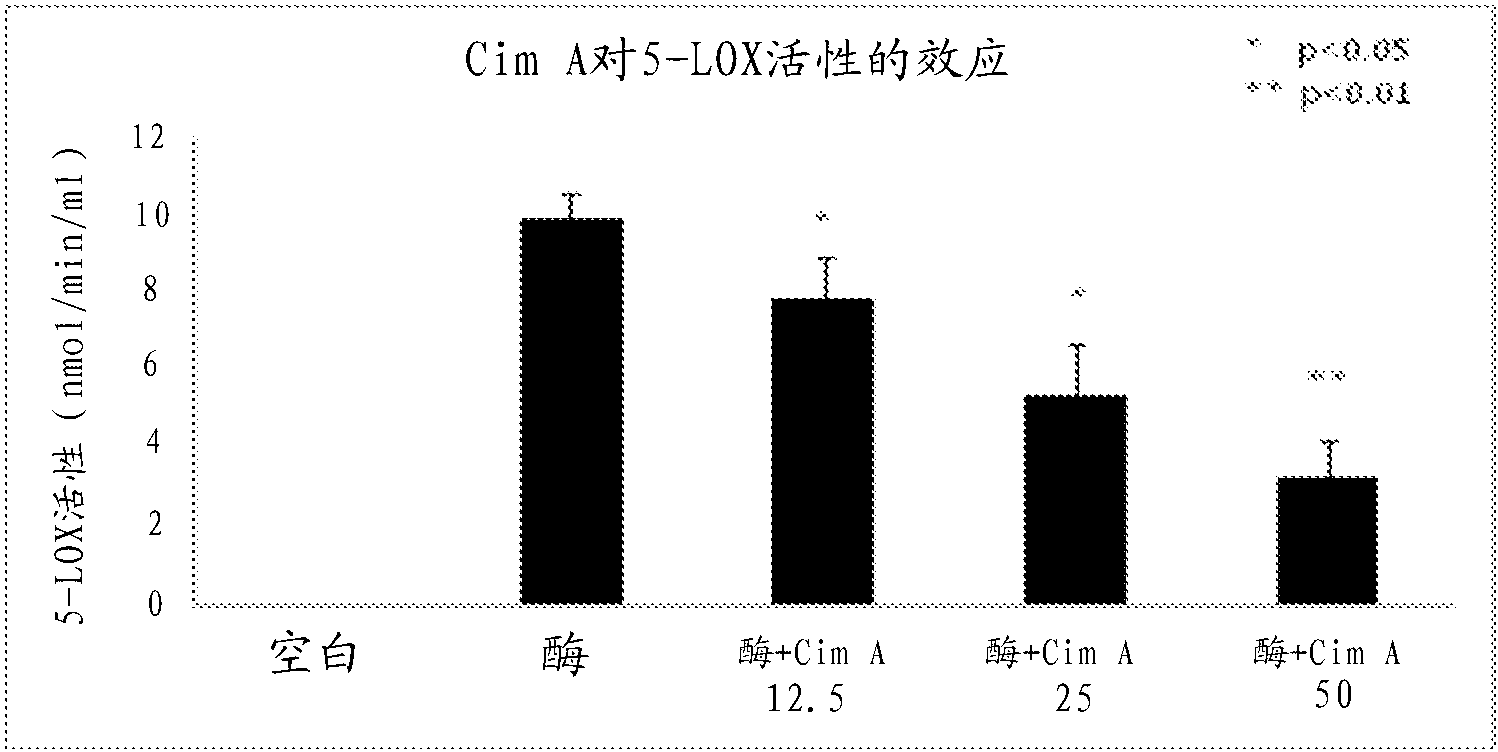
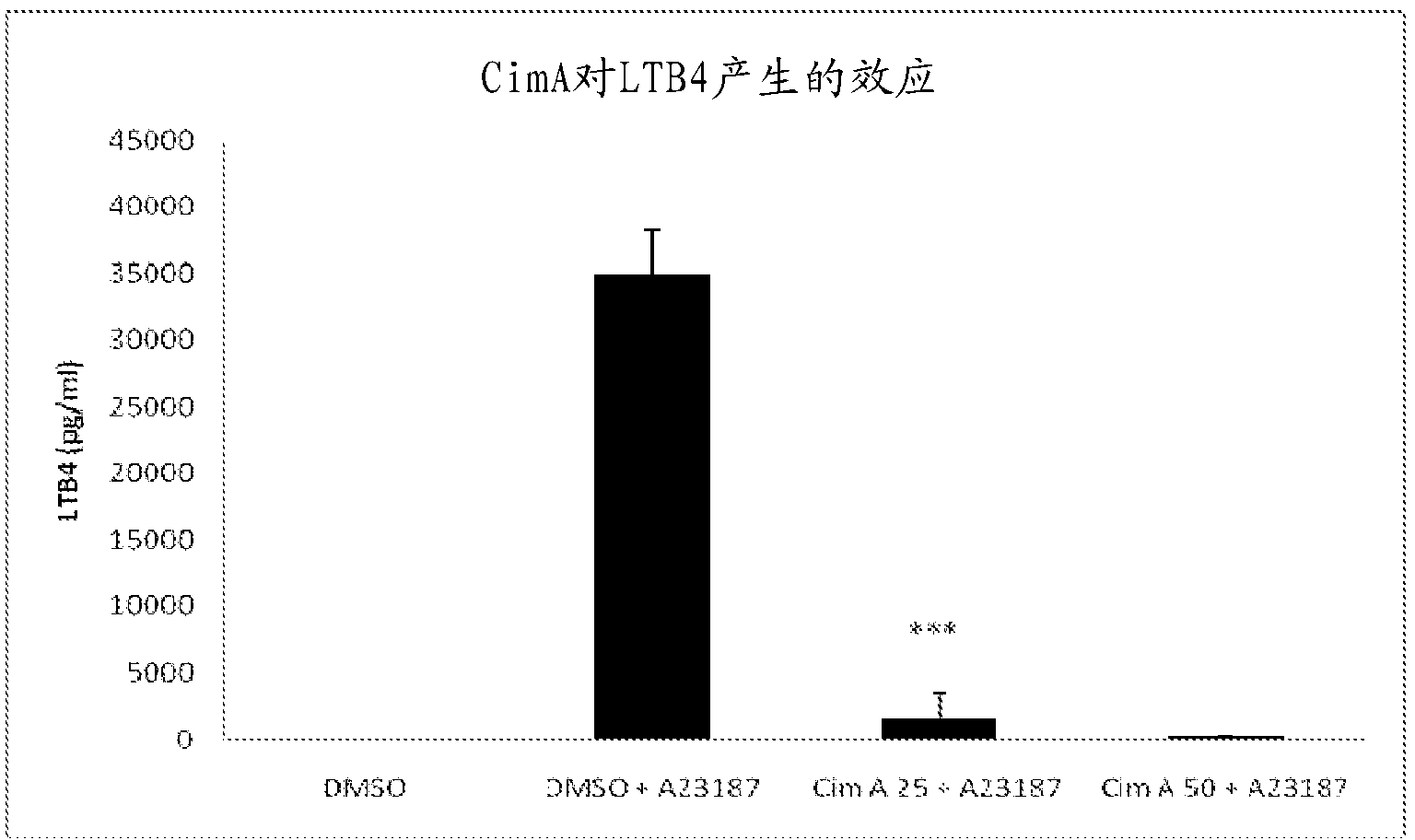
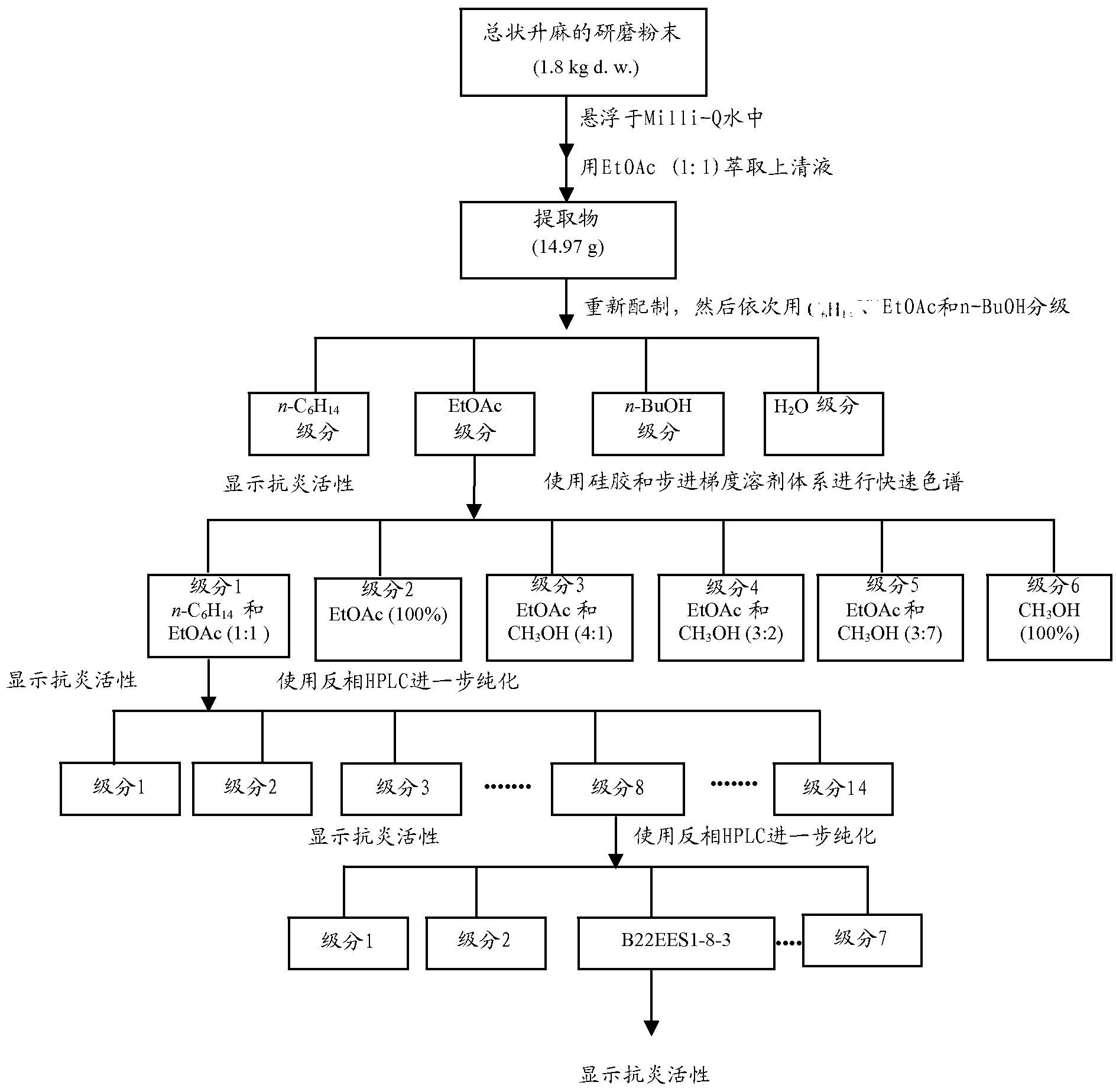
Uses of cimiracemate and related compounds for treating inflammation and modulating immune responses
Owner:BAGI RES +1
Medicinal Composition for Treating Intractable Heart Disease
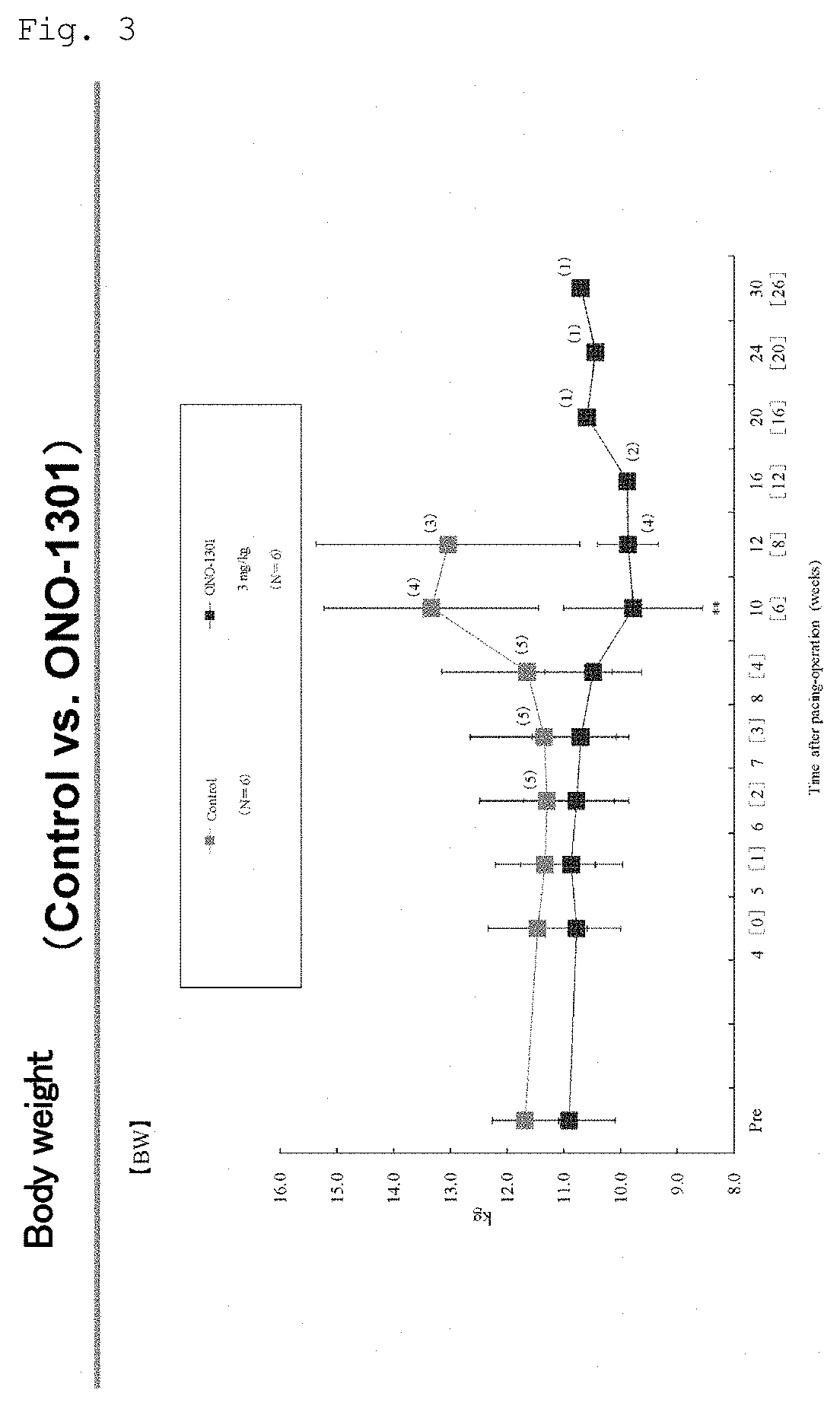
InactiveUS20190343841A1Excellent administration complianceImprove complianceOrganic chemistryEster active ingredientsHMG-CoA reductaseFibrosis
The present invention provides a pharmaceutical composition for use in treating an intractable heart tissue fibrosis disease accompanied by chronic heart failure. The pharmaceutical composition for use in treating an intractable heart tissue fibrosis disease accompanied by chronic heart failure comprises, as an active ingredient, at least one member selected from the group consisting of protease inhibitors, thromboxane A2 synthase inhibitors, thromboxane A2 synthase antagonists, phosphodiesterase (PDE) inhibitors, tyrosine kinase inhibitors, HMG-CoA reductase inhibitors, and antifibrotic agents. (The pharmaceutical composition includes biodegradable polymer-encapsulated, long-acting preparations thereof.)
Owner:OSAKA UNIV +1
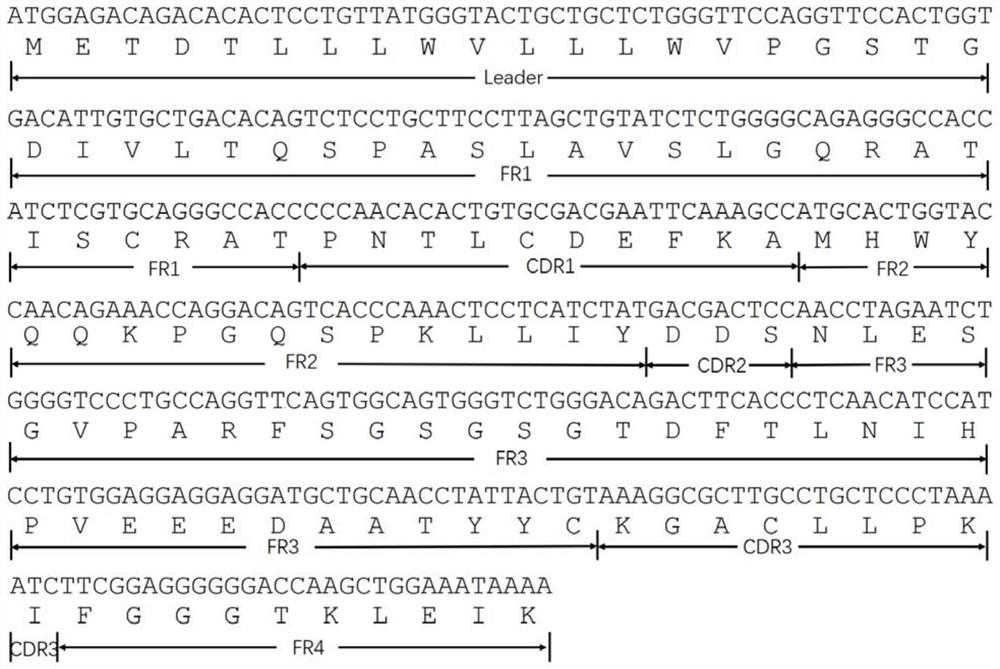
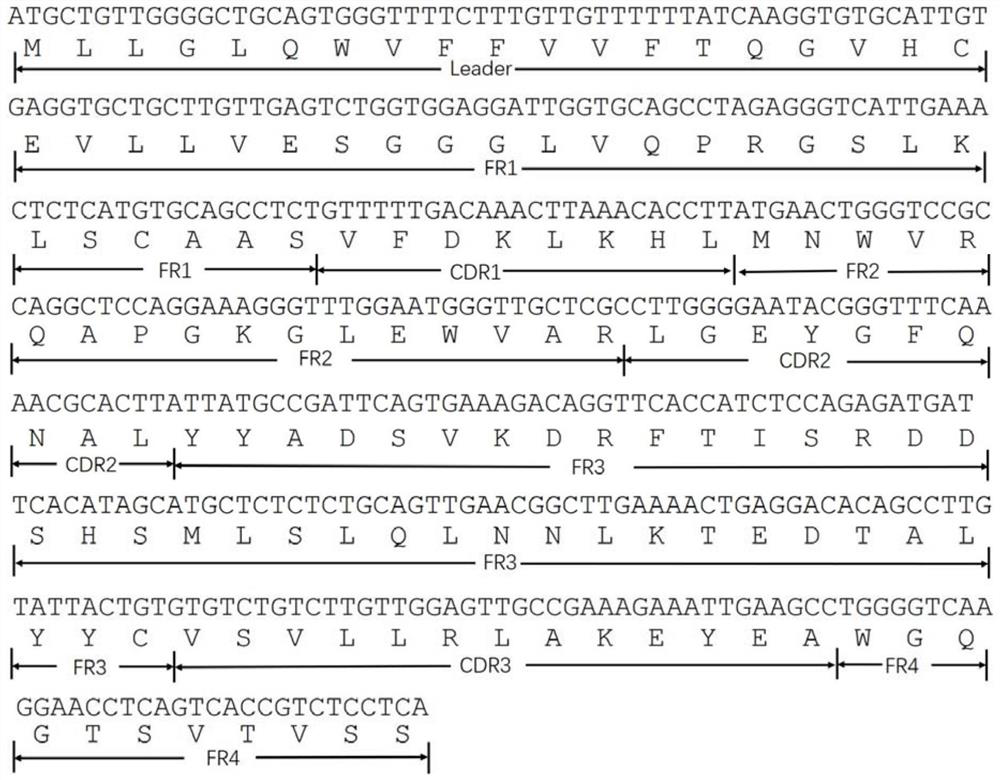
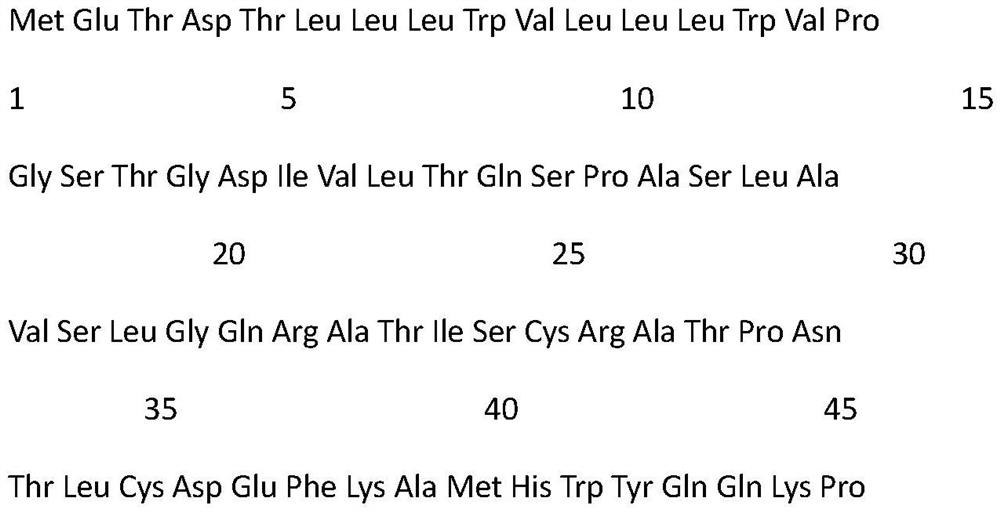
Chemiluminiscence detection method and chemiluminiscence detection kit for aspirin metabolism and platelet high reactivity
ActiveCN111735809AReduce healingReduce foulingChemiluminescene/bioluminescenceAntiendomysial antibodiesAnti platelet
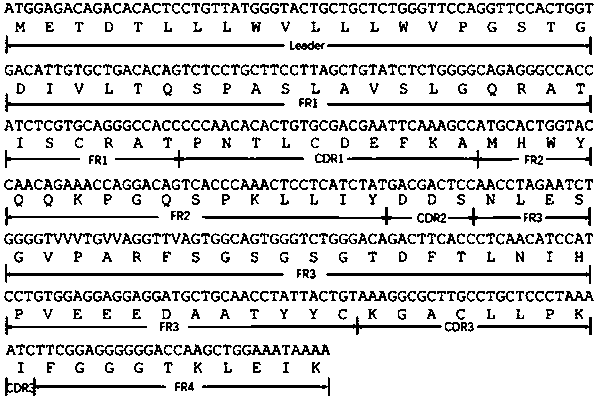
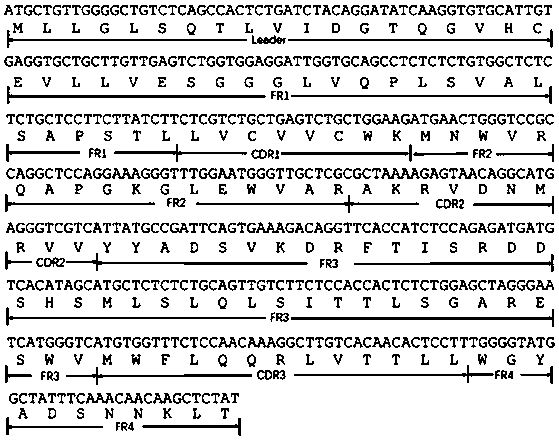
The invention discloses a chemiluminiscence detection method and a chemiluminiscence detection kit for aspirin metabolism and platelet high reactivity. The kit comprises a monoclonal antibody combinedwith 11-dehydrothromboxane B2, a competitive product of 11-dehydrothromboxane B2, magnetic particles and a luminous substrate, wherein the monoclonal antibody is a mouse monoclonal antibody, the amino acid sequence of a light chain variable region of the mouse monoclonal antibody is shown as SEQ ID NO: 2 in a sequence table, and the amino acid sequence of a heavy chain variable region of the mouse monoclonal antibody is shown as SEQ ID NO: 4 in the sequence table. According to the present invention, with the application of the aspirin metabolism and platelet high-reactivity chemiluminiscencedetection method and the detection kit, whether the patient has aspirin drug resistance or resistance can be rapidly detected; the kit and the detection method provided by the invention have the advantages that the sensitivity is high, the accuracy is good, the kit and the detection method are not easily influenced by biochemical indexes, and the problem that the anti-platelet aggregation treatment of a clinical patient fails or the effect is poor due to aspirin resistance is solved.
Owner:湖南远璟生物技术有限公司
Compositions and Methods For Delivery Of Polyunsaturated Fatty Acid Derivatives And Analogs
ActiveUS20180280318A1Improve bioavailabilityLong exposure timeHydroxy compound active ingredientsAntipyreticMetaboliteAntioxidant
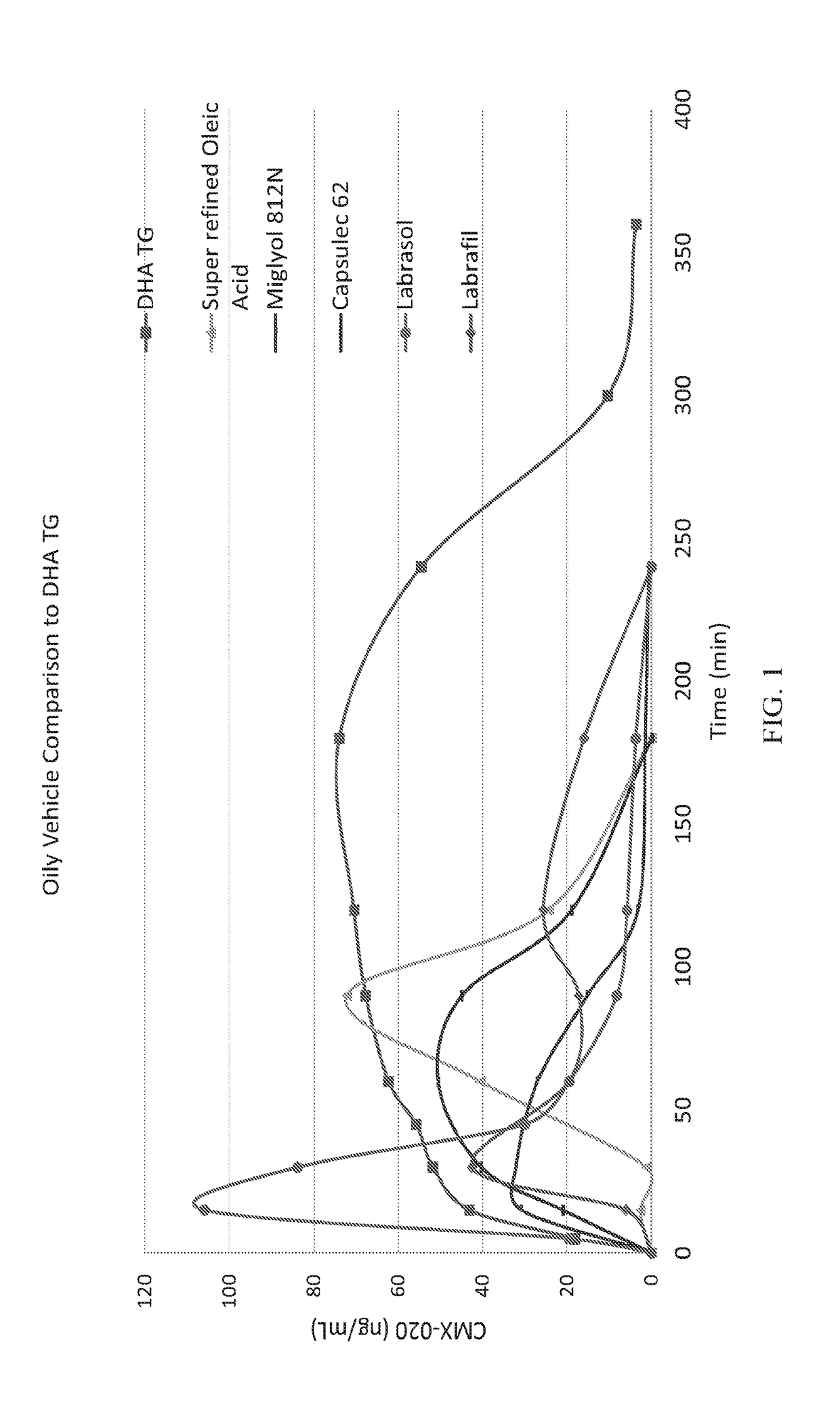
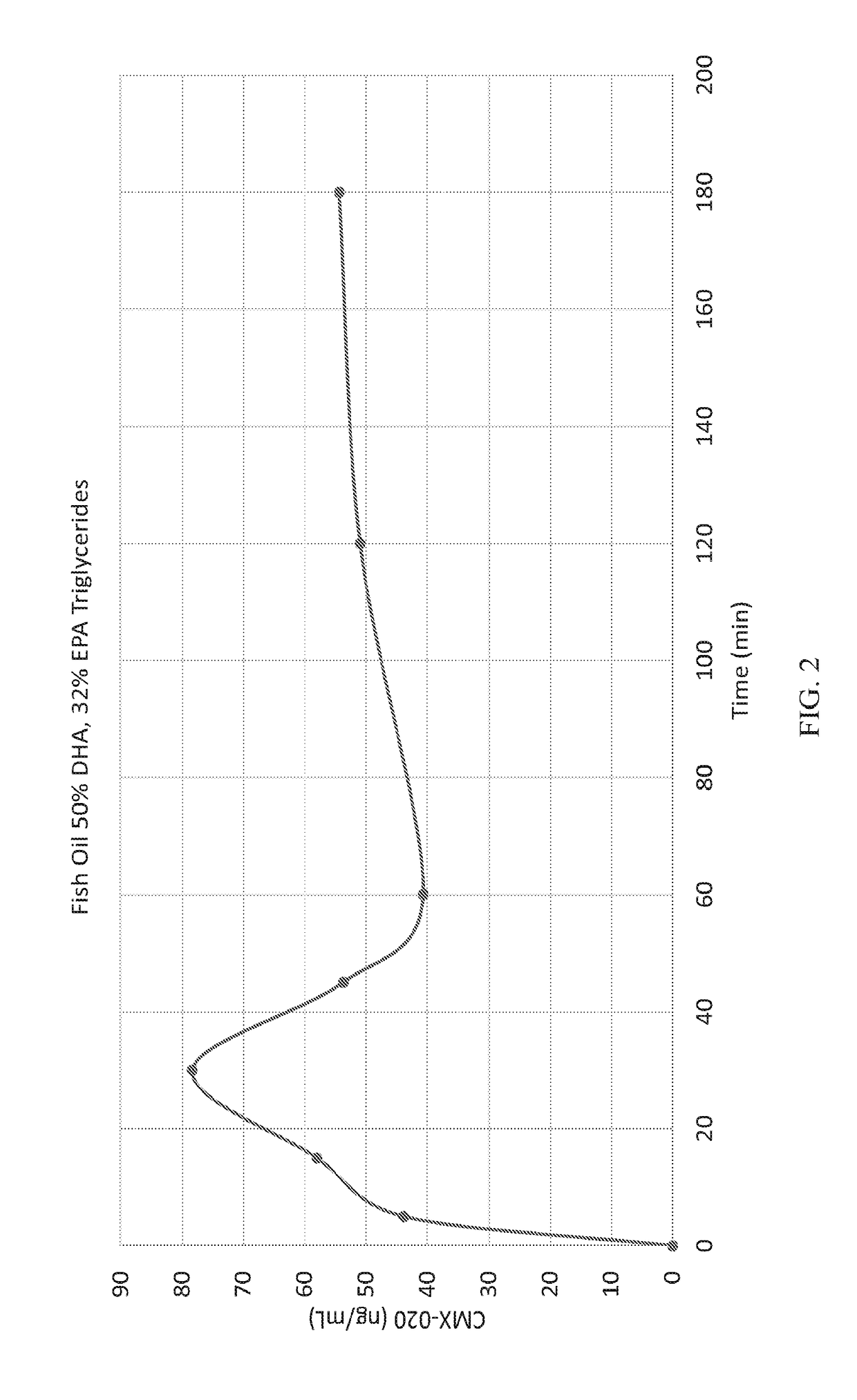
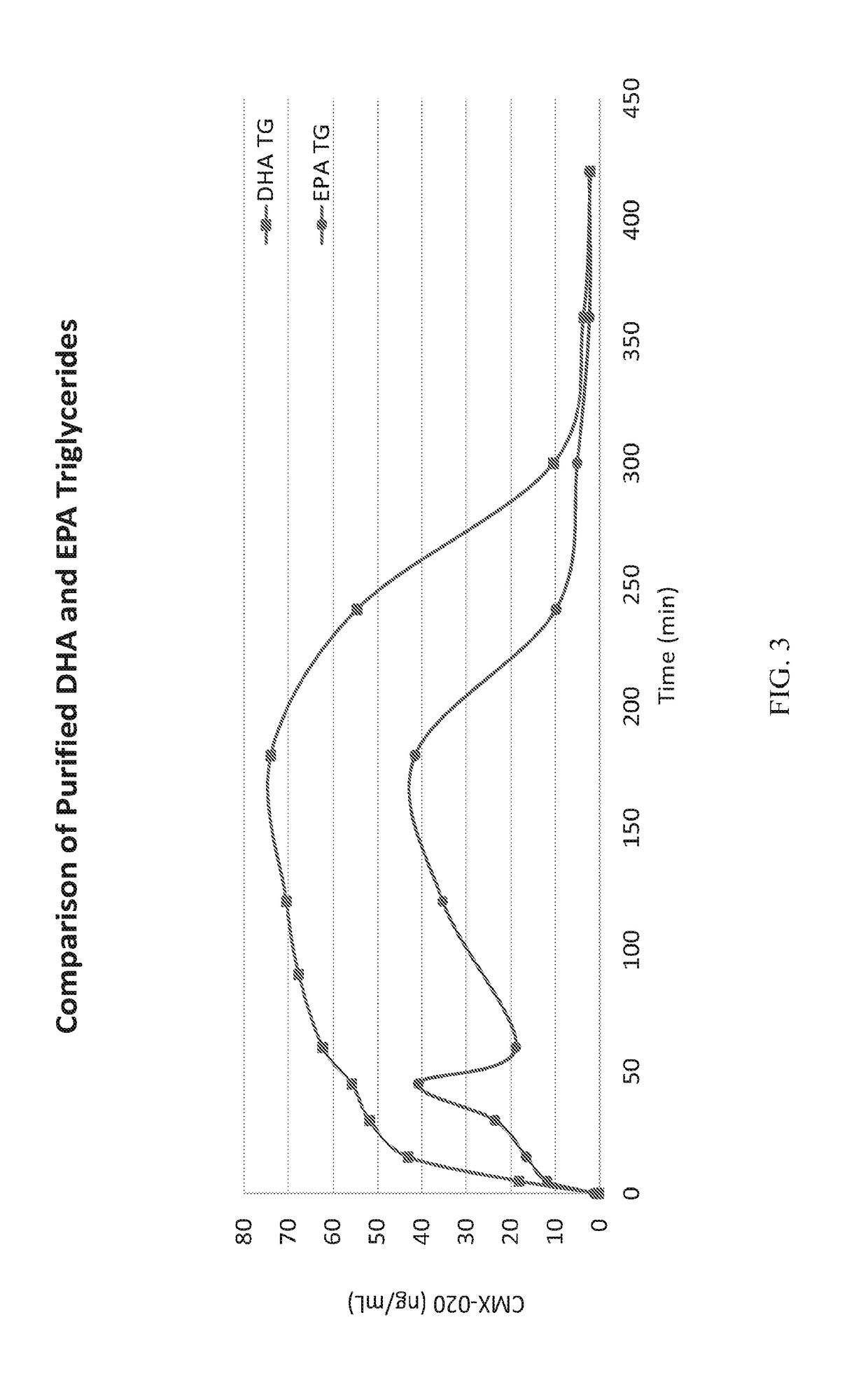
The present invention provides a system enabling the oral delivery of therapeutics derived from polyunsaturated fatty acids (PUFAs), their metabolites and derivatives, including, eicosanoids, prostaglandins, prostacyclins, leukotrienes, resolvins, endocannabinoids, thromboxanes, epoxyeicosa-trienoic acids (EETs), hydroxyeicostetraenoic acids (HETEs), and CMX-020. The delivery system includes a vehicle comprising a purified docosahexaenoic acid (DHA) in triglyceride or ester form; a purified eicosapentaenoic acid (EPA) in triglyceride or ester form; a combination of DHA, EPA in either triglyceride or ester forms; or a modified DHA, EPA, or omega-3 fatty acid analog; and optionally, an antioxidant, a surfactant, a solubilizer, a stabilizer, a lubricant, or a pH / tonicity adjustment agent.
Owner:CYTOMETIX INC D B A CMXTWENTY
Absorption of pain-causing agents
There is provided a pain relieving substance made of an eicosanoid-absorbent substance and a carrier. The eicosanoid-absorbent substance may be an eicosanoid enzyme, antibody and / or acid moiety binder. The eicosanoids may be prostaglantins, thromboxanes, leukotrienes, lipoxins and hydroxyl and hydroperoxy fatty acids and mixtures thereof. The eicosanoid-absorbent substance may be delivered in a liquid carrier such as a gel or lotion or may be delivered by a solid carrier such as a tampon, sponge, etc.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Methods for the Treatment and Diagnosis of Bone Mineral Density Related Diseases
InactiveUS20100284991A1Decreased bone mineral densitySimple processBiocidePeptide/protein ingredientsGhosal hematodiaphyseal dysplasiaBone density
The present invention relates to methods of the treatment and diagnosis of bone mineral density related disorders. More particularly, the present invention relates to a method of diagnosing or predicting a bone mineral density related disease, or a risk of a bone mineral density related disease, in a subject, which method comprises detecting a mutation in the TBXAS1 gene, wherein the presence of said mutation is indicative of a bone mineral density related disease or of a risk of a bone mineral density related disease. The invention also relates to a compound selected in the group consisting of a thromboxane synthase (TXAS) encoding polynucleotide, a TXAS, thromboxane A2 or an analog thereof for treating or preventing a disease associated with an increased bone mineral density (e.g., Ghosal hematodiaphyseal dysplasia syndrome). The invention also relates to a compound selected from the group consisting of an inhibitor of TBXAS1 gene expression or a thromboxane inhibitor for treating or preventing a disease associated with a decreased bone mineral density (e.g., osteoporosis).
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com