Patents
Literature
103 results about "Tiotropium bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
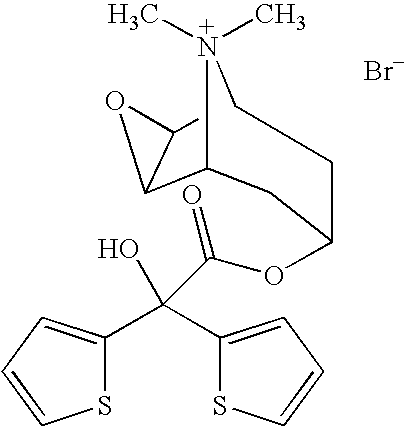
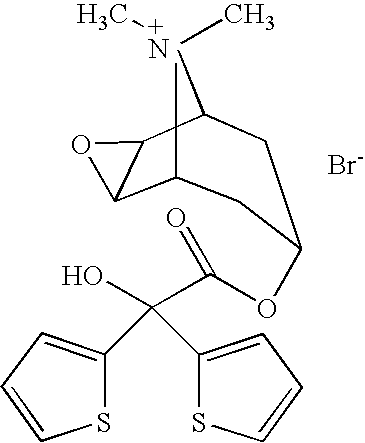
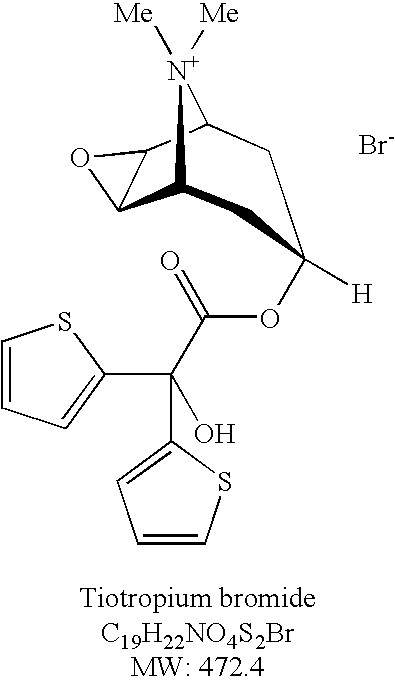
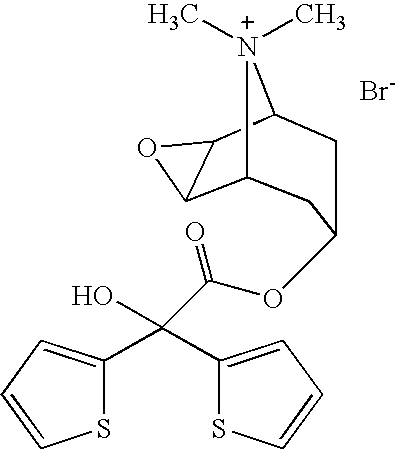
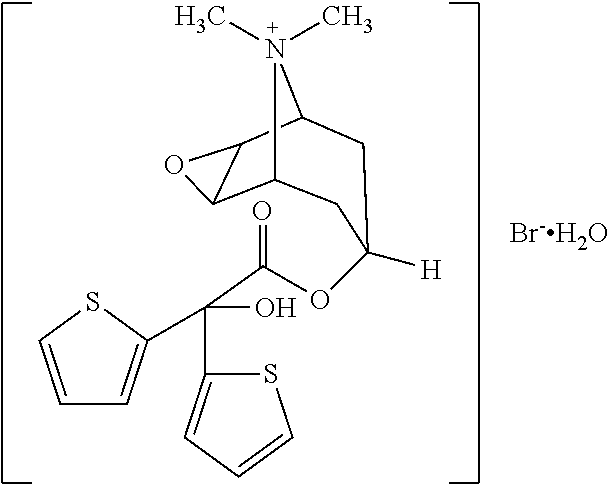
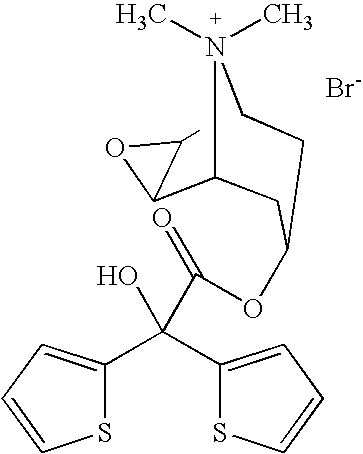
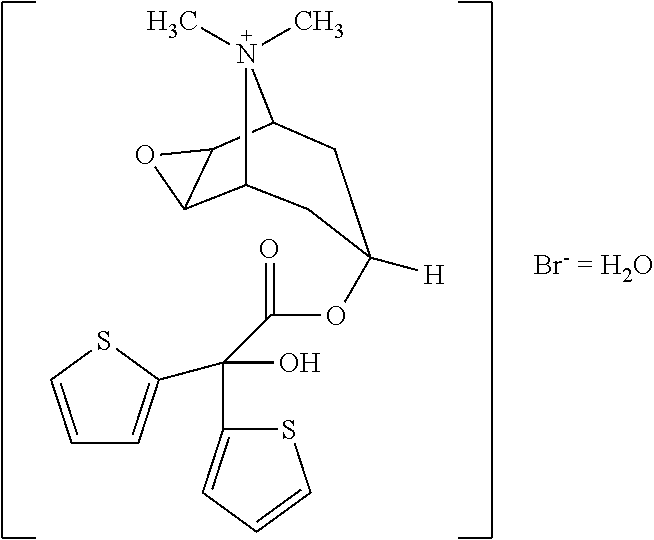
Tiotropium bromide, sold under the brandname Spiriva among others, is a long-acting bronchodilator used in the management of chronic obstructive pulmonary disease (COPD) and asthma. Specifically it is used to try to prevent periods of worsening rather than for those periods themselves. It is used by inhalation through the mouth. Onset typically begins within half an hour and lasts for 24 hours.
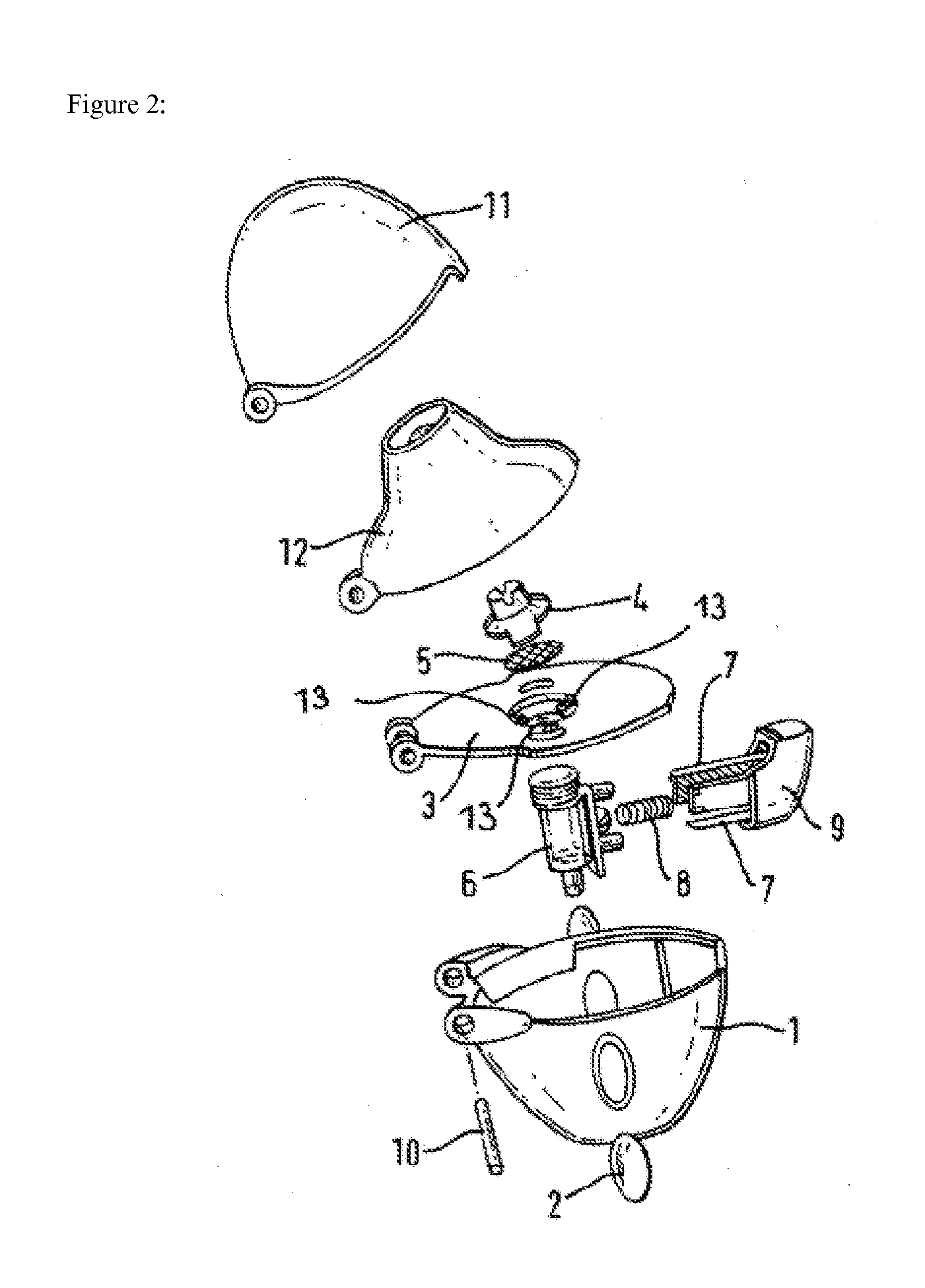
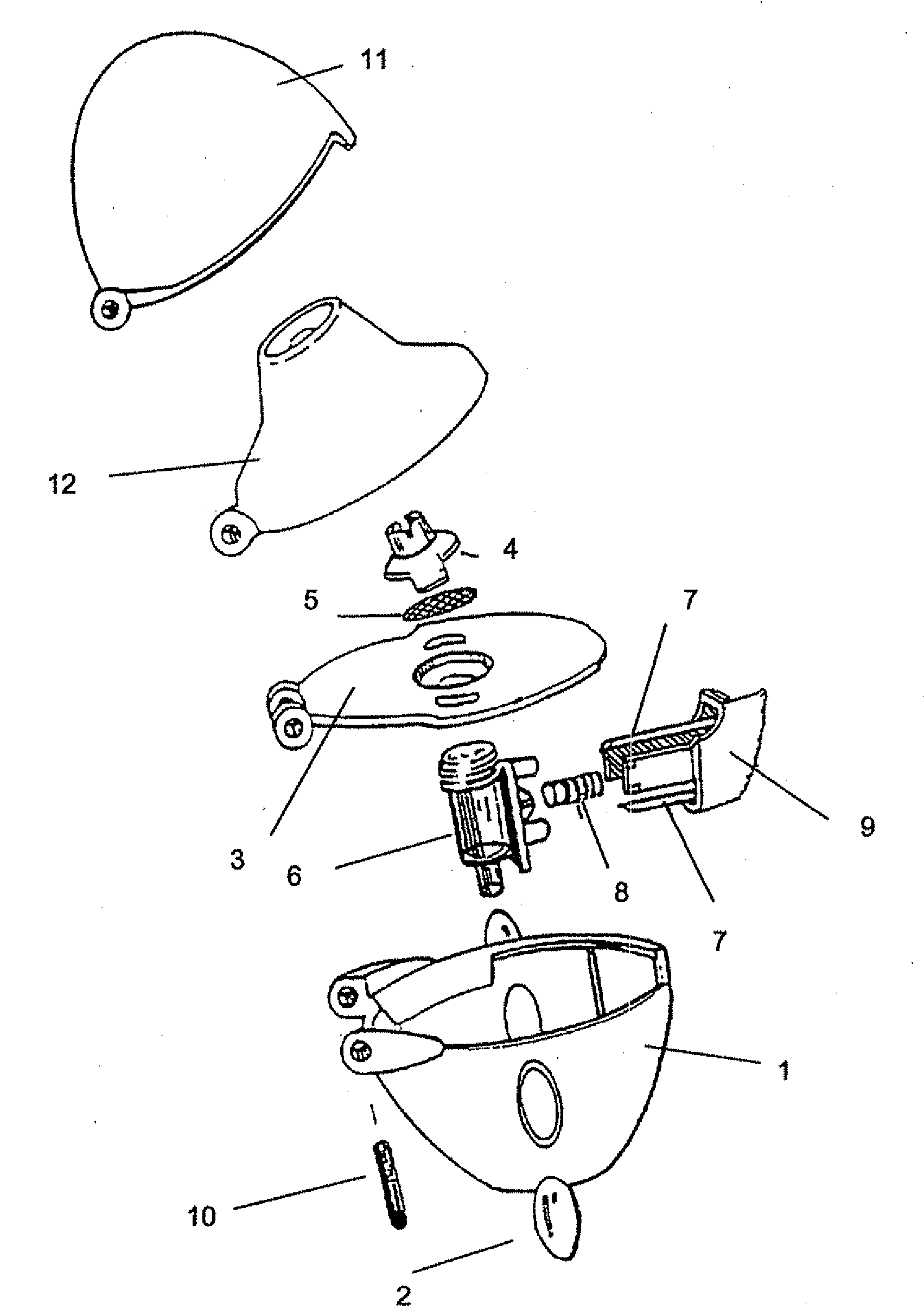
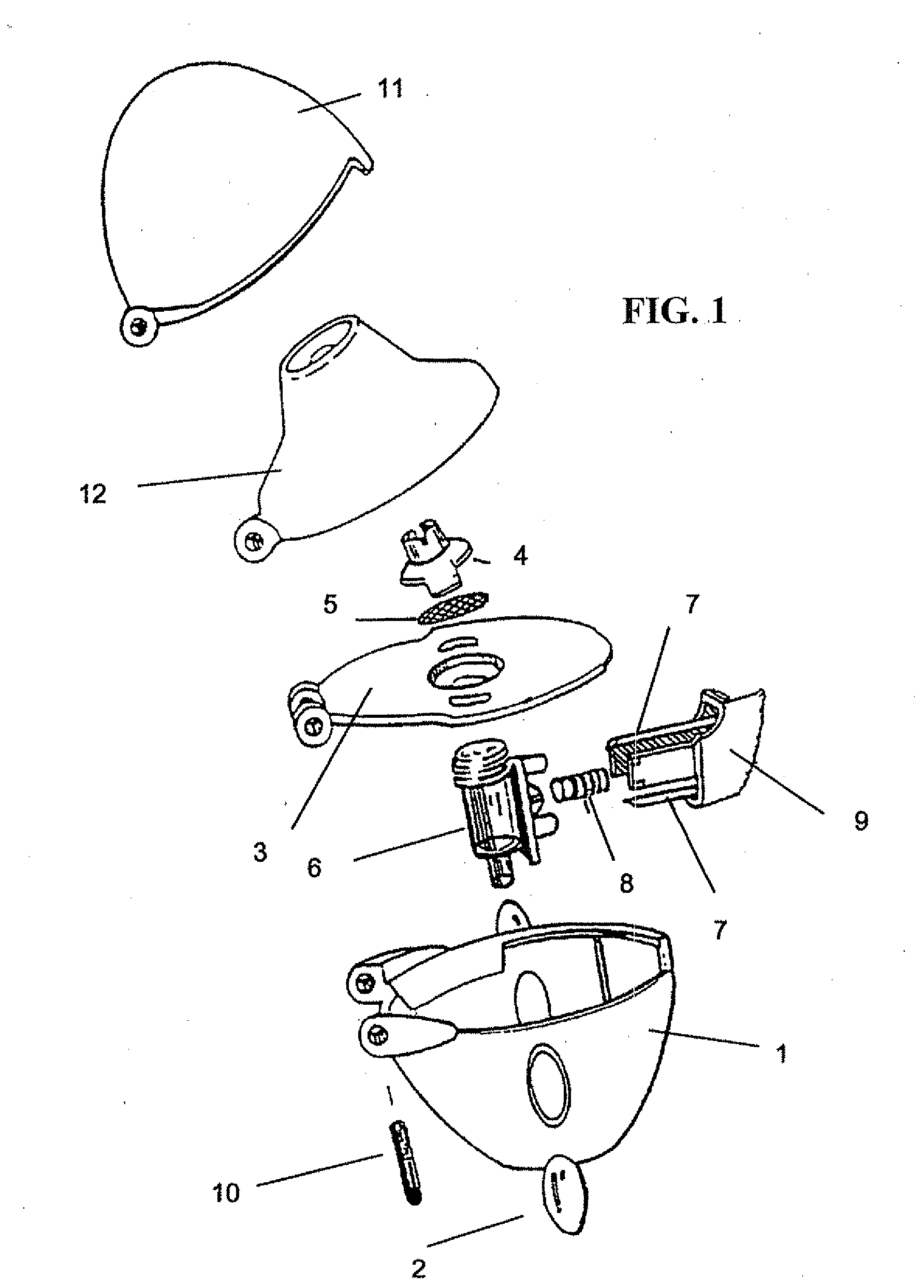
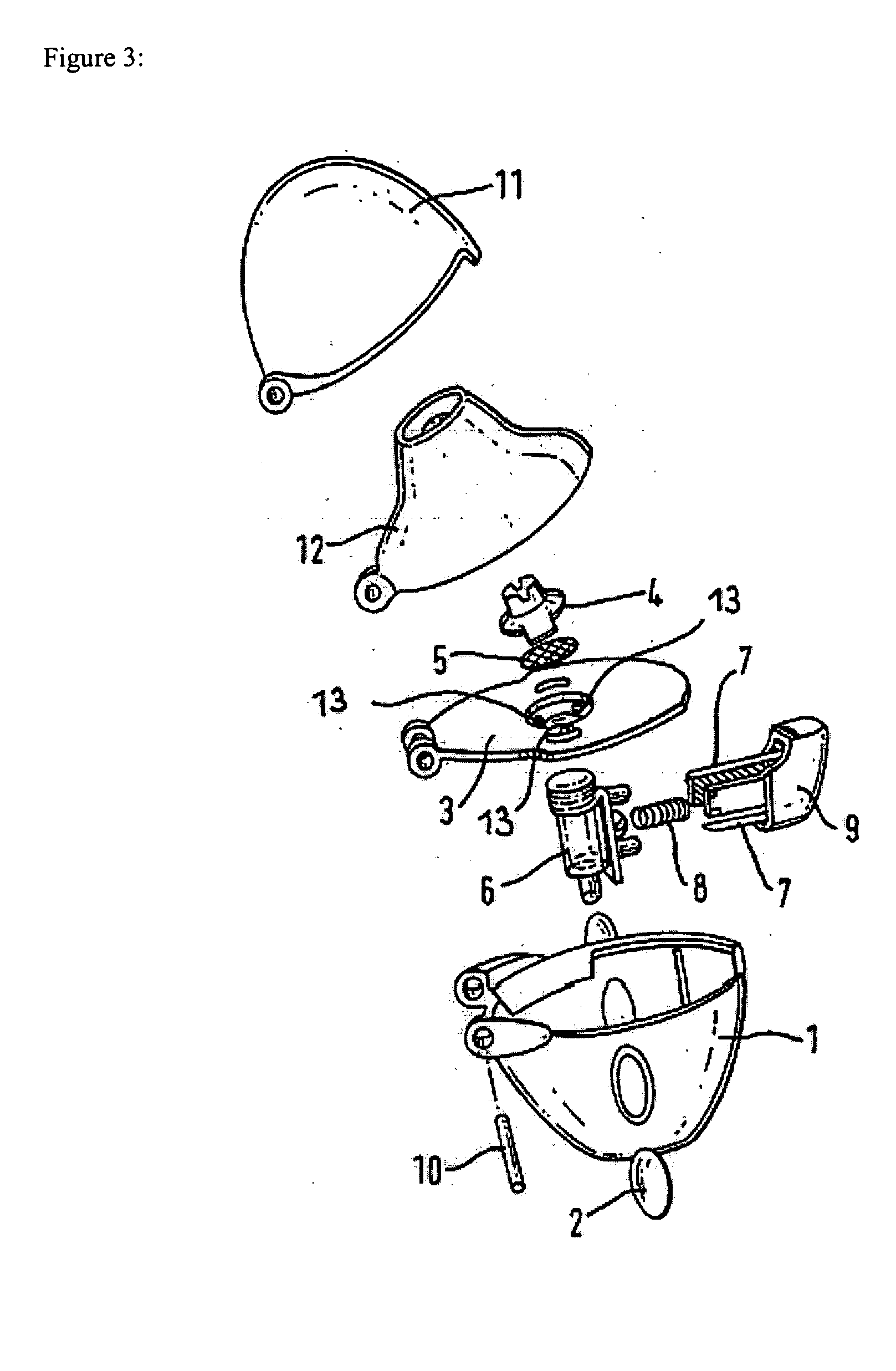
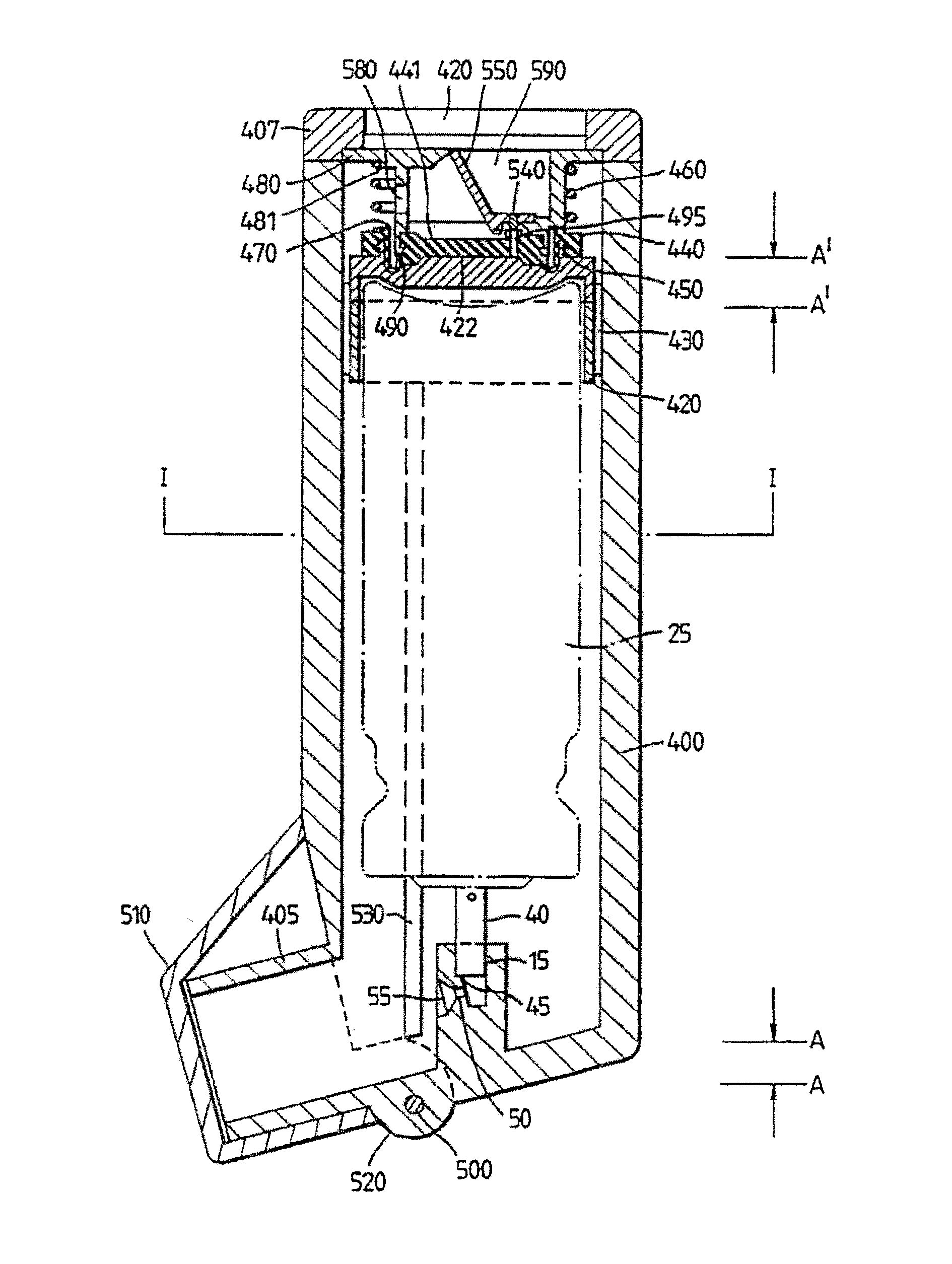
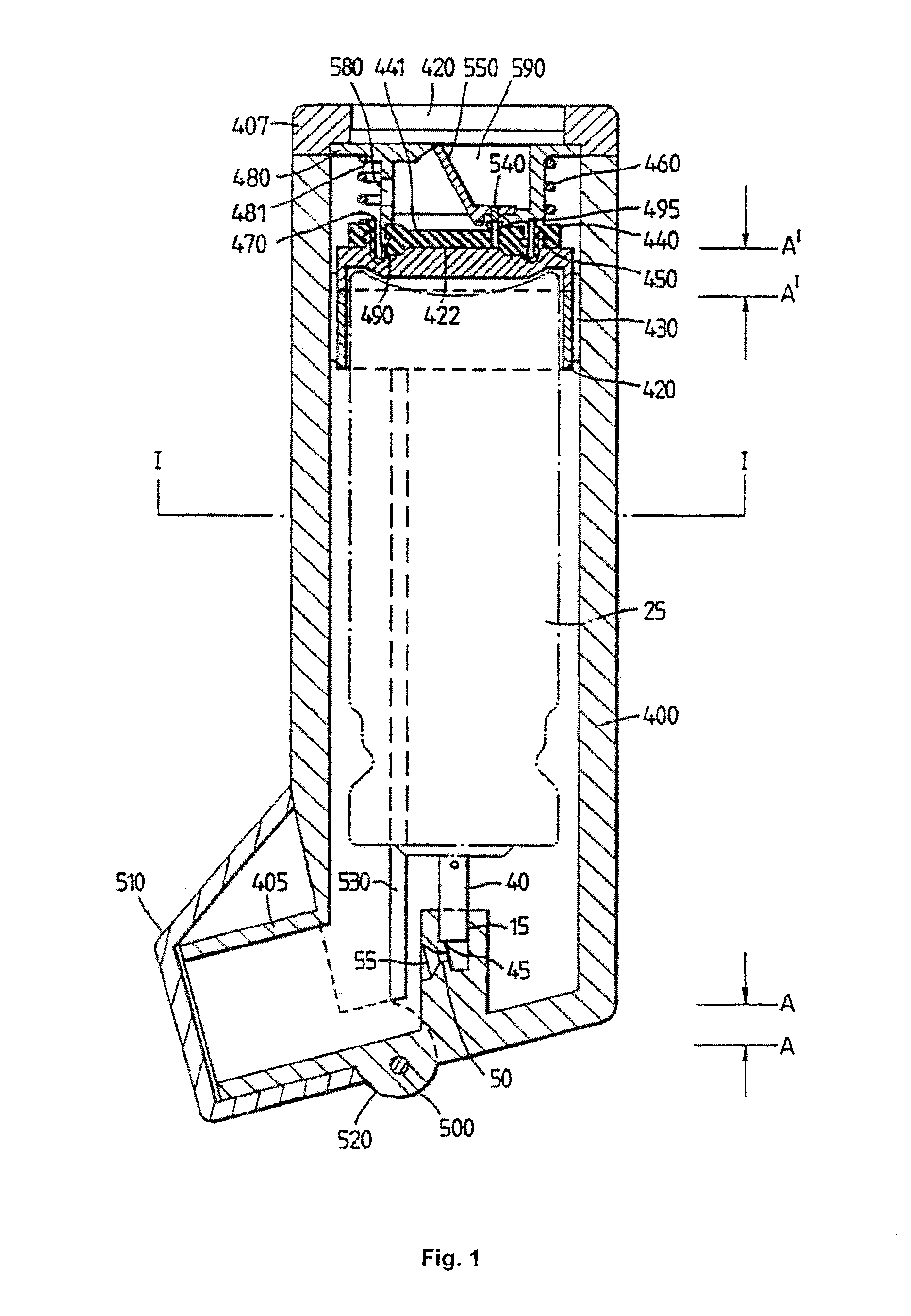
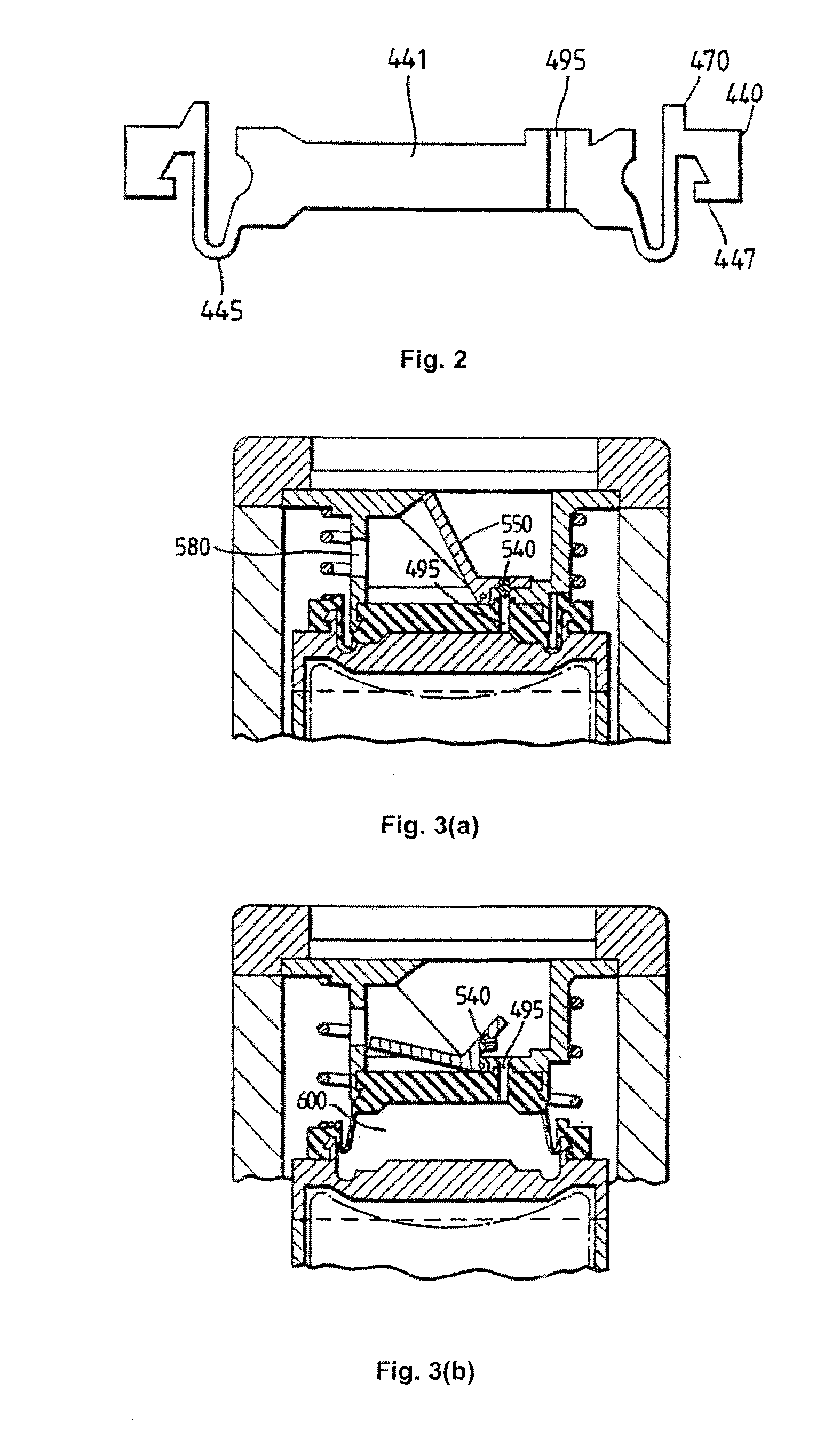
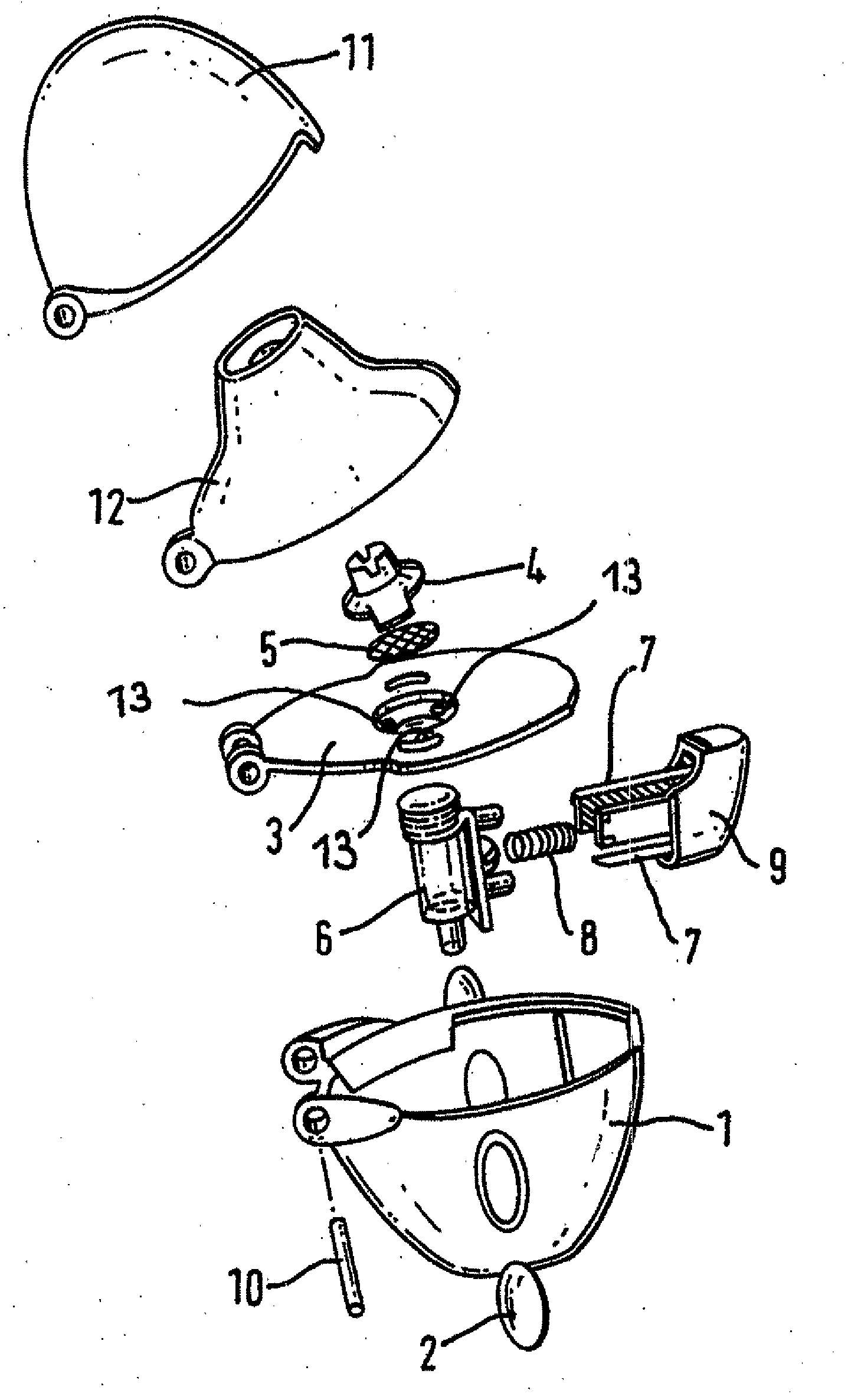
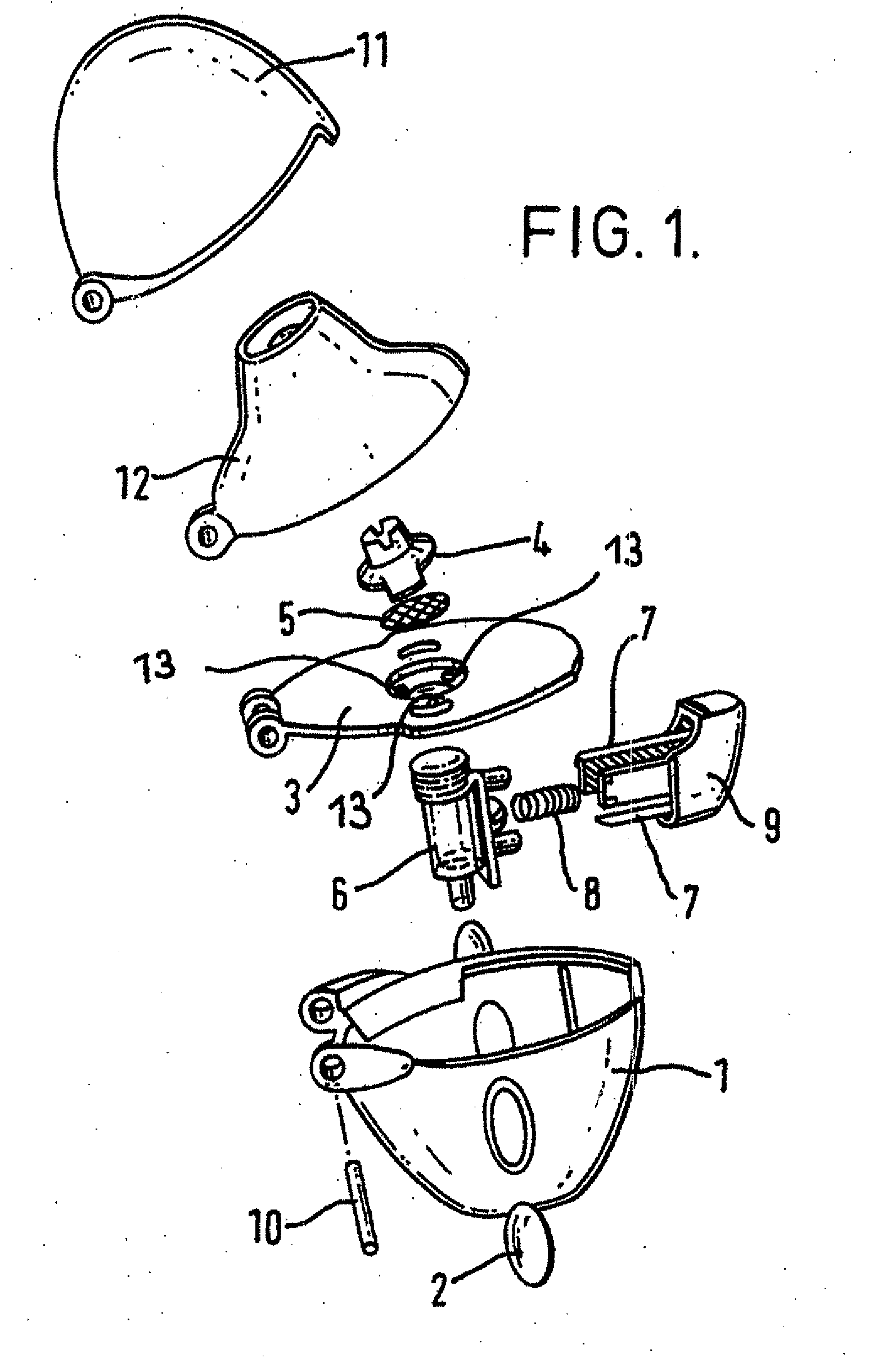
Inhalable tiotropium and container therefor
A medical product suitable for storing and delivering a pre-metered dose of tiotropium, devices containing the same, and methods of using the same.
Owner:BOEHRINGER INGELHEIM INT GMBH
Tiotropium containing HFC solution formulations
This invention relates to tiotropium containing stable pharmaceutical solution formulations suitable for aerosol administration. More particularly, this invention relates to tiotropium containing stable pharmaceutical solution formulations suitable for aerosol administration wherein either an inorganic acid or an organic acid is added to the aerosol solution formulation which contains a tiotropium salt, preferably tiotropium bromide in solution with an environmentally safe hydrofluorocarbon (HFC) as a propellant, together with an organic compound as a cosolvent. The acid provides stability against degradation or decomposition of the medicament resulting largely from interaction of the medicament with the cosolvent and / or water present in the solution formulation.
Owner:BOEHRINGER INGELHEIM PHARM KG
Novel tiotropium salts, process for the preparation and pharmaceutical compositions thereof
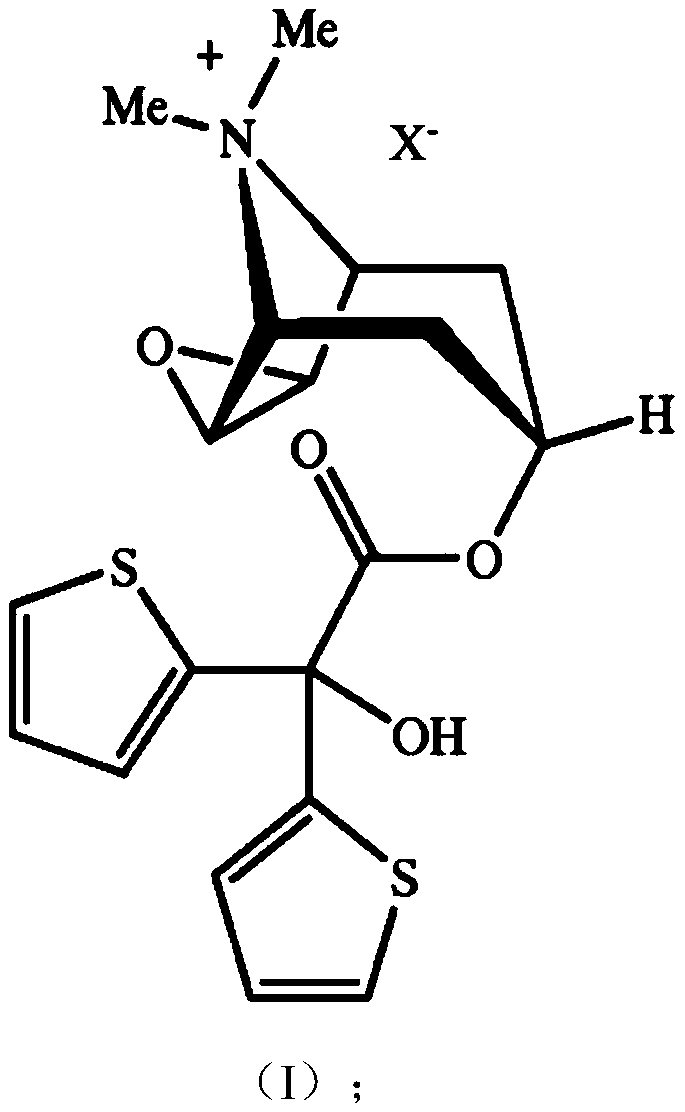
The invention relates to new tiotropium salts, processes for preparing them, pharmaceutical formulations containing them and their use for preparing a medicament for the treatment of respiratory complaints, particularly for the treatment of COPD (chronic obstructive pulmonary disease) and asthma.
Owner:BOEHRINGER INGELHEIM INT GMBH
Combination of a PDE4 inhibitor and tiotropium or derivative thereof for treating obstructive airways and other inflammatory diseases
InactiveUS20050107420A1Easy to controlInhibition is effectiveBiocideAnimal repellantsTiotropium bromidePDE4 Inhibitors
The present invention relates to a combination of therapeutic agents useful in the treatment of obstructive airways and other inflammatory diseases comprising (I) a PDEIV inhibitor that is therapeutically effective in the treatment of said diseases when administered by inhalation; together with (II) an anti-cholinergic agent comprising a member selected from the group consisting of tiotropium and derivatives thereof that is therapeutically effective in the treatment of said diseases when administered by inhalation; as well as to a method of treating said obstructive airways and other inflammatory diseases comprising administering to said mammal by inhalation a therapeutically effective amount of said combination of therapeutic agents; and a pharmaceutical composition comprising a pharmaceutically acceptable carrier together with said combination of therapeutic agents; and a package containing a pharmaceutical composition for insertion into a device capable of simultaneous or sequential delivery of said pharmaceutical composition in the form of an aerosol or dry powder dispersion to said mammal, where said device is a metered dose inhaler or a dry powder inhaler. It is preferred that said anti-cholinergic agent component be tiotropium bromide.
Owner:BOEHRINGER INGELHEIM PHARM KG
Novel Powderous Medicaments Comprising Tiotropium and Salmeterol and Lactose as Carrier
The invention relates to stable preparations in powder form for inhalation comprising a tiotropium salt and salmeterol xinafoate, process for the production thereof, and the use thereof for manufacturing a medicament for the treatment of respiratory disorders, especially for the treatment of COPD (chronic obstructive pulmonary disease) and asthma.
Owner:BOEHRINGER INGELHEIM INT GMBH
Crystalline anti-cholinergic tiotropium crystal
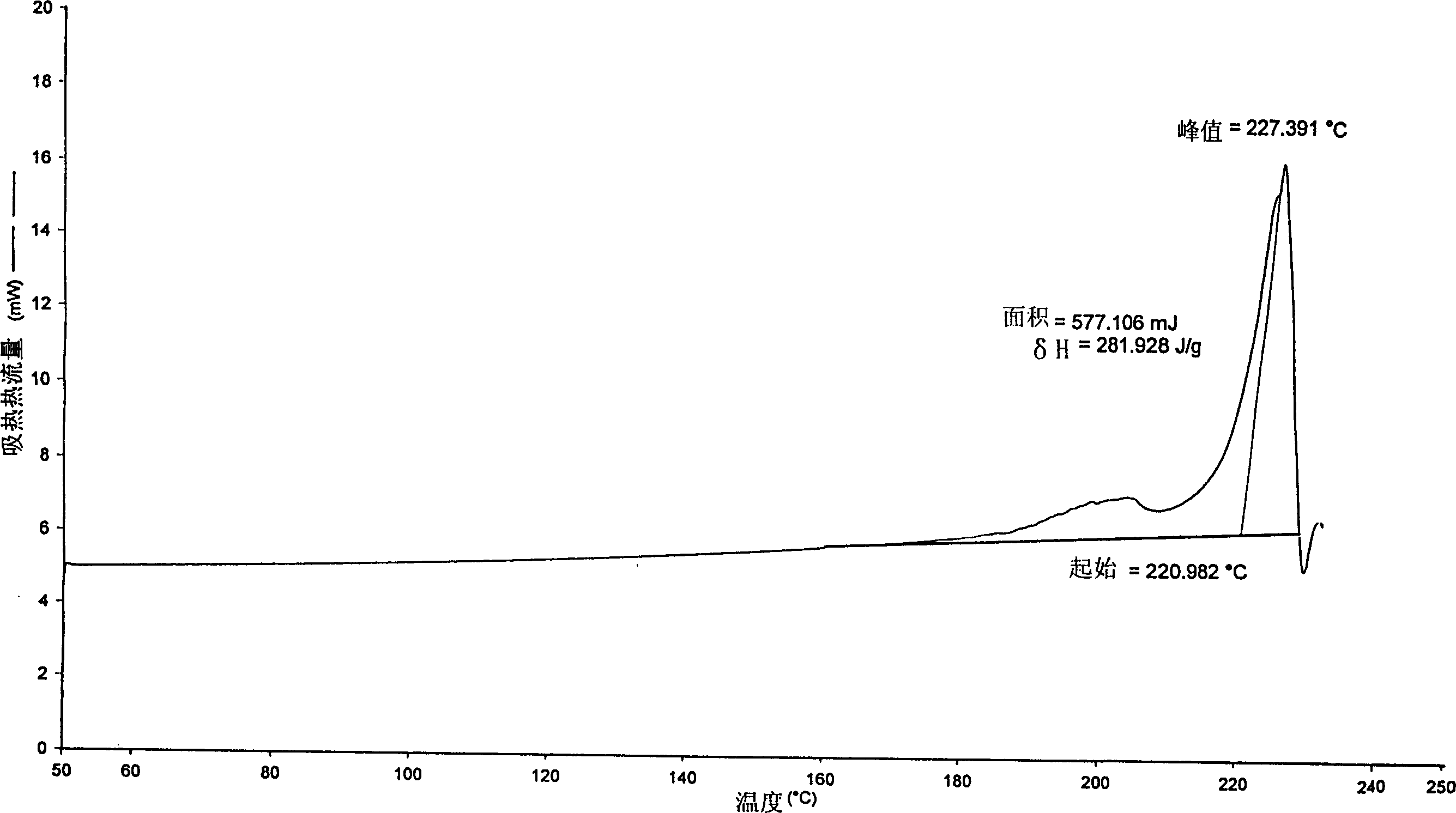
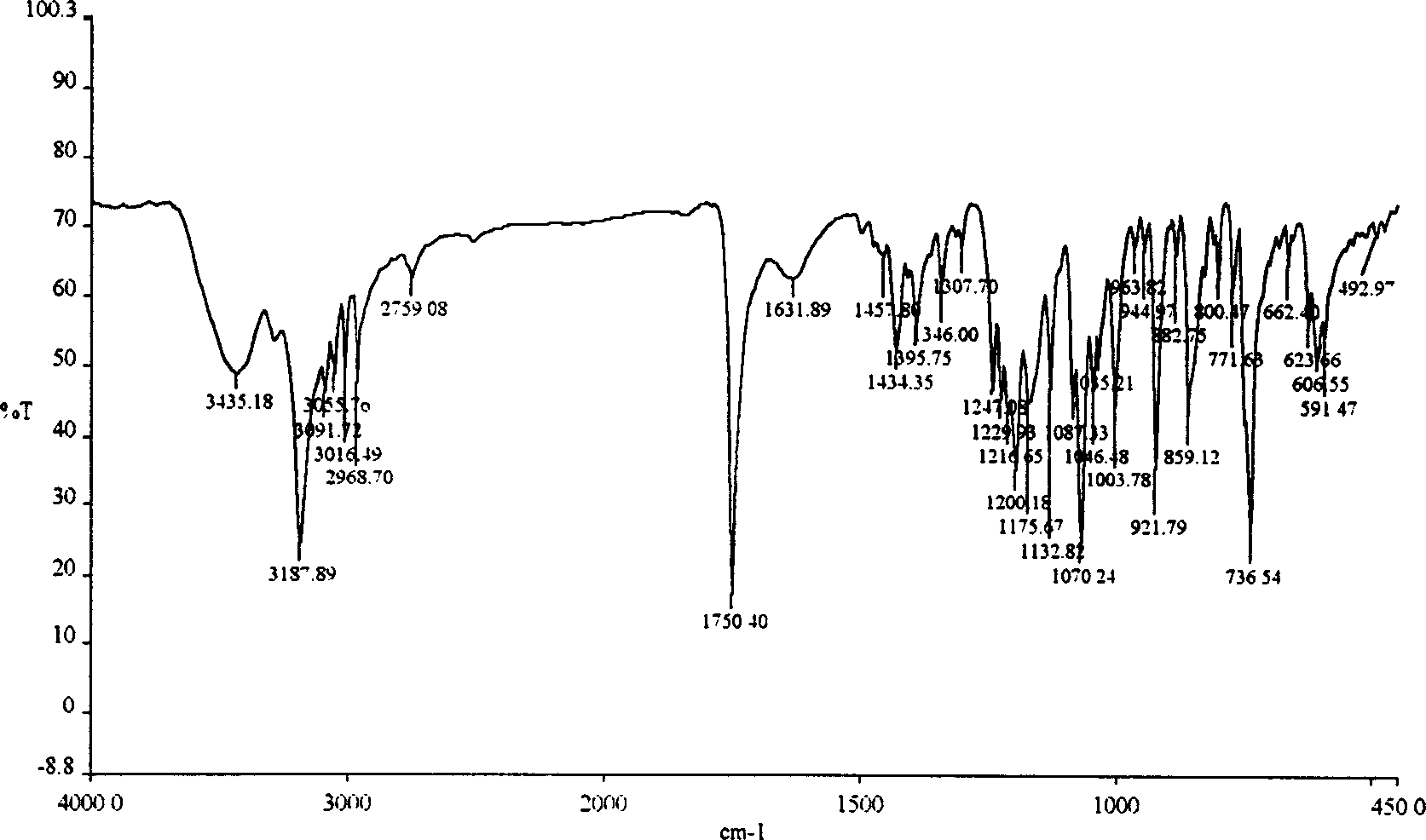
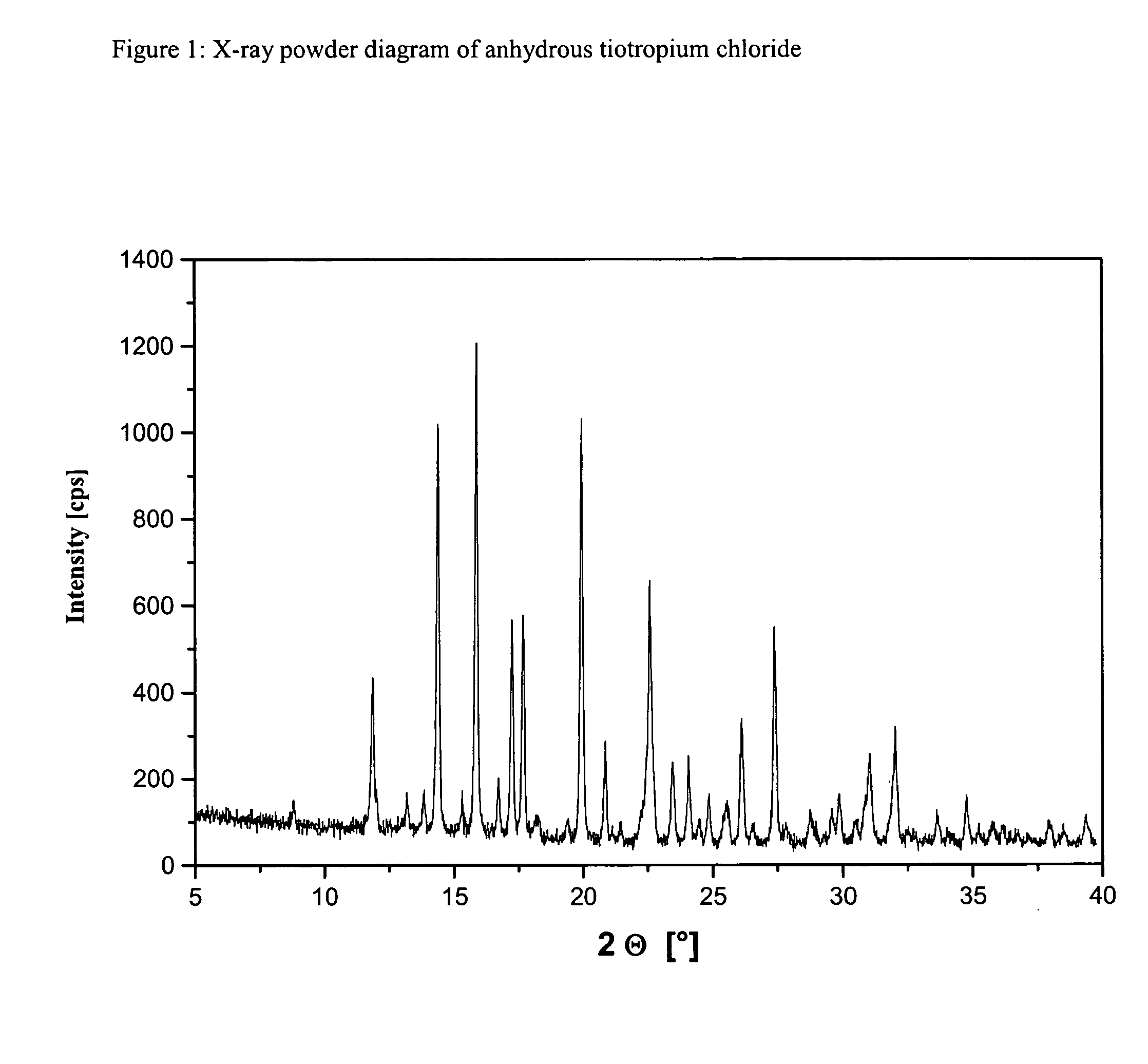
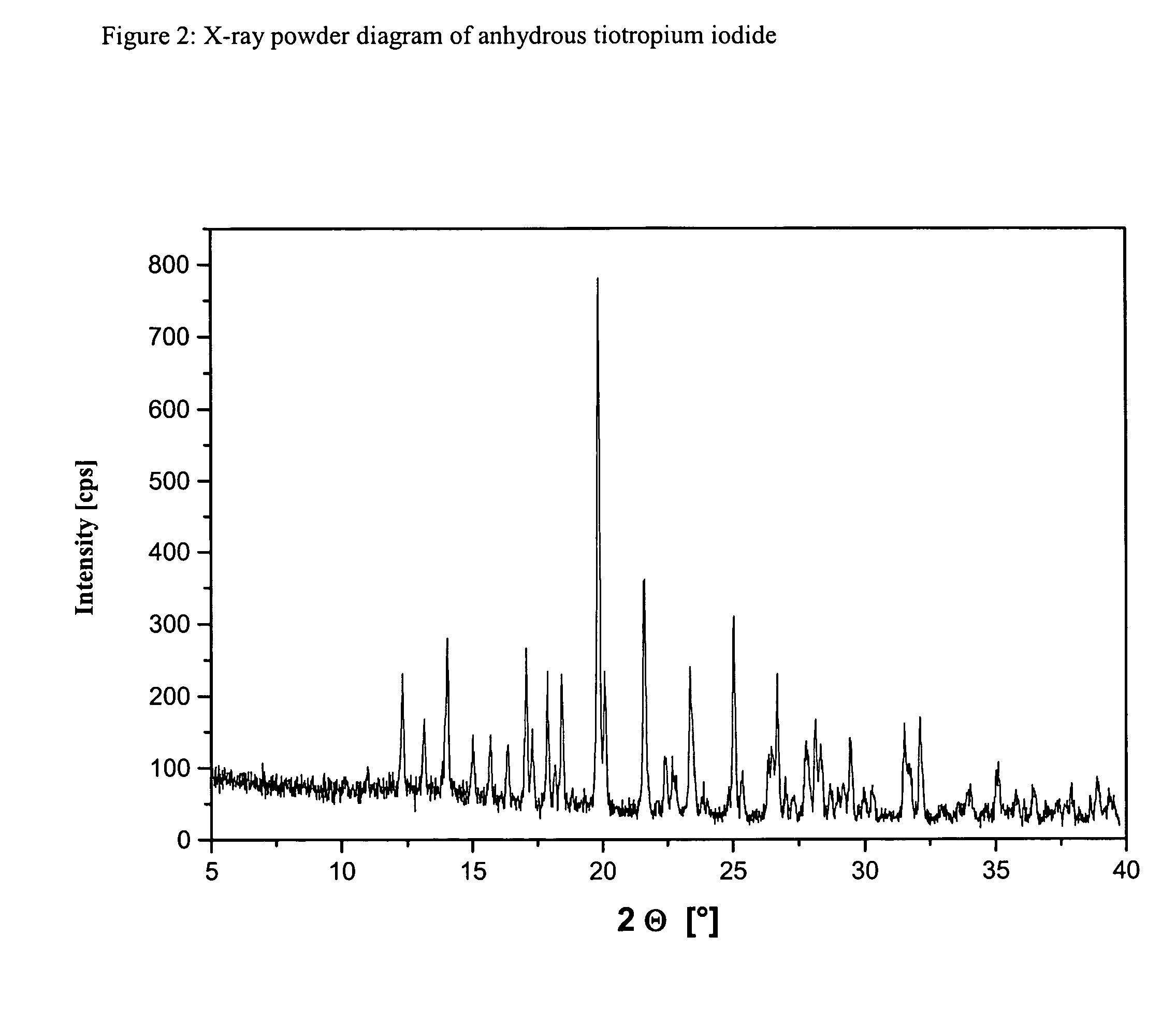
ActiveCN1634921ANo significant difference in formulation qualityOrganic active ingredientsOrganic chemistryAnticholinergic agentsTiotropium bromide
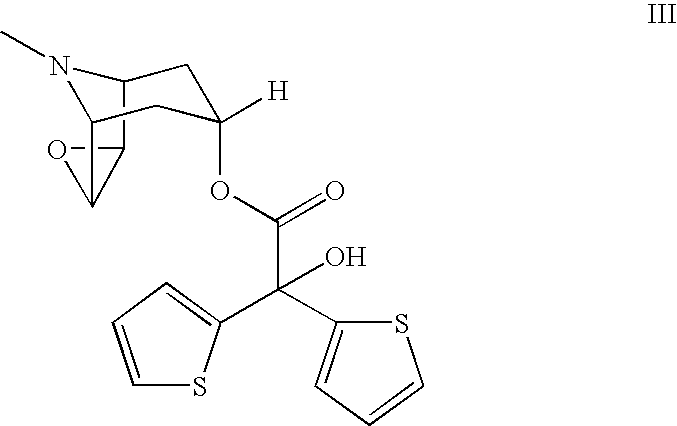
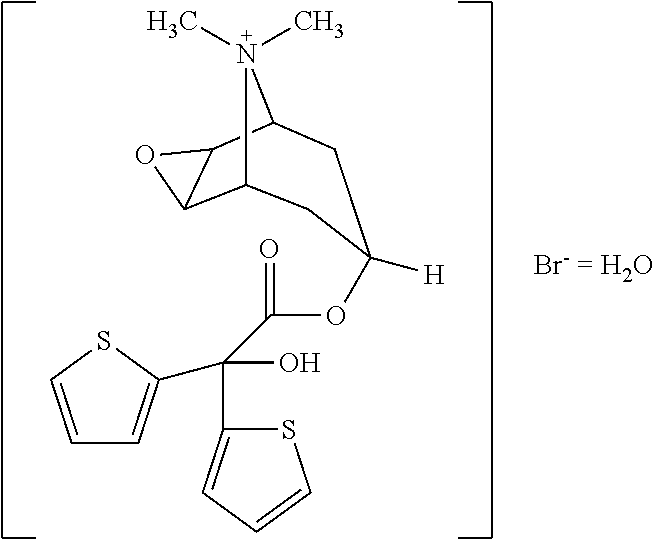
The invention relates to crystal unhydrous (1R,2R,4S,5S,7S)-7-[2-hydroxy-2,2-bis(2-thienyl) acetoxy]-9,9-dimethyl-3-oxa-9-azo cation tricyclo octane [3.3.1.02.4] nonane bromide, clinic use of tiotropium bromide anhydrous crystal as anti-cholinergic medicines, and preparation process of tiotropium bromide anhydrous crystal.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
InactiveUS20070293460A1Adequate levelShortness of breathRespiratorsBiocideObstructive Pulmonary DiseasesCombination therapy
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and an aqueous solution comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium. A pharmaceutical composition is also described for the treatment of respiratory conditions and diseases comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer.
Owner:CMPD LICENSING
Tiotropium aerosol inhalant and its preparation method
InactiveCN1557308APromote absorptionImprove complianceOrganic active ingredientsAerosol deliveryIrritationSodium cromoglicate
Owner:SHANGHAI PUKANG PHARMA +2
Tiotropium containing powder formulation for inhalation
ActiveUS20040136919A1Improve homogeneityImprove uniformityPowder deliveryMedical devicesDiseaseTiotropium bromide
The invention relates to powdered preparations containing tiotropium for inhalation, processes for preparing them as well as their use in preparing a pharmaceutical composition for the treatment of respiratory complaints, particularly for the treatment of COPD (chronic obstructive pulmonary disease) and asthma.
Owner:BOEHRINGER INGELHEIM PHARM KG
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and a fluid comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic in a pharmaceutically acceptable vehicle, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium in a an aqueous solution, suspension or emulsion suitable for administration with the nebulizer.
Owner:RICHIES PHARMACY & MEDICAL SUPPLY
Process for the preparation of tiotropium bromide
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
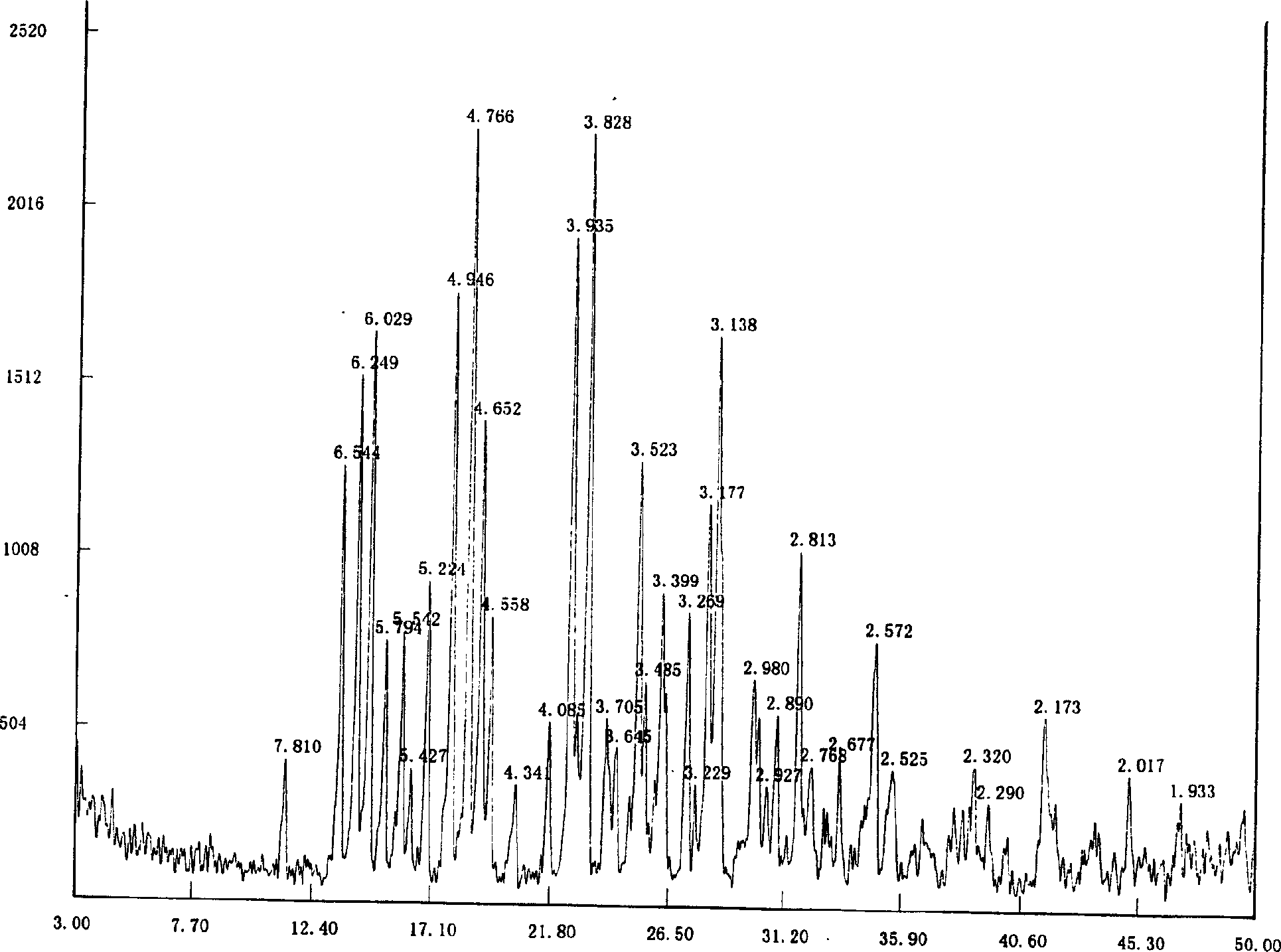
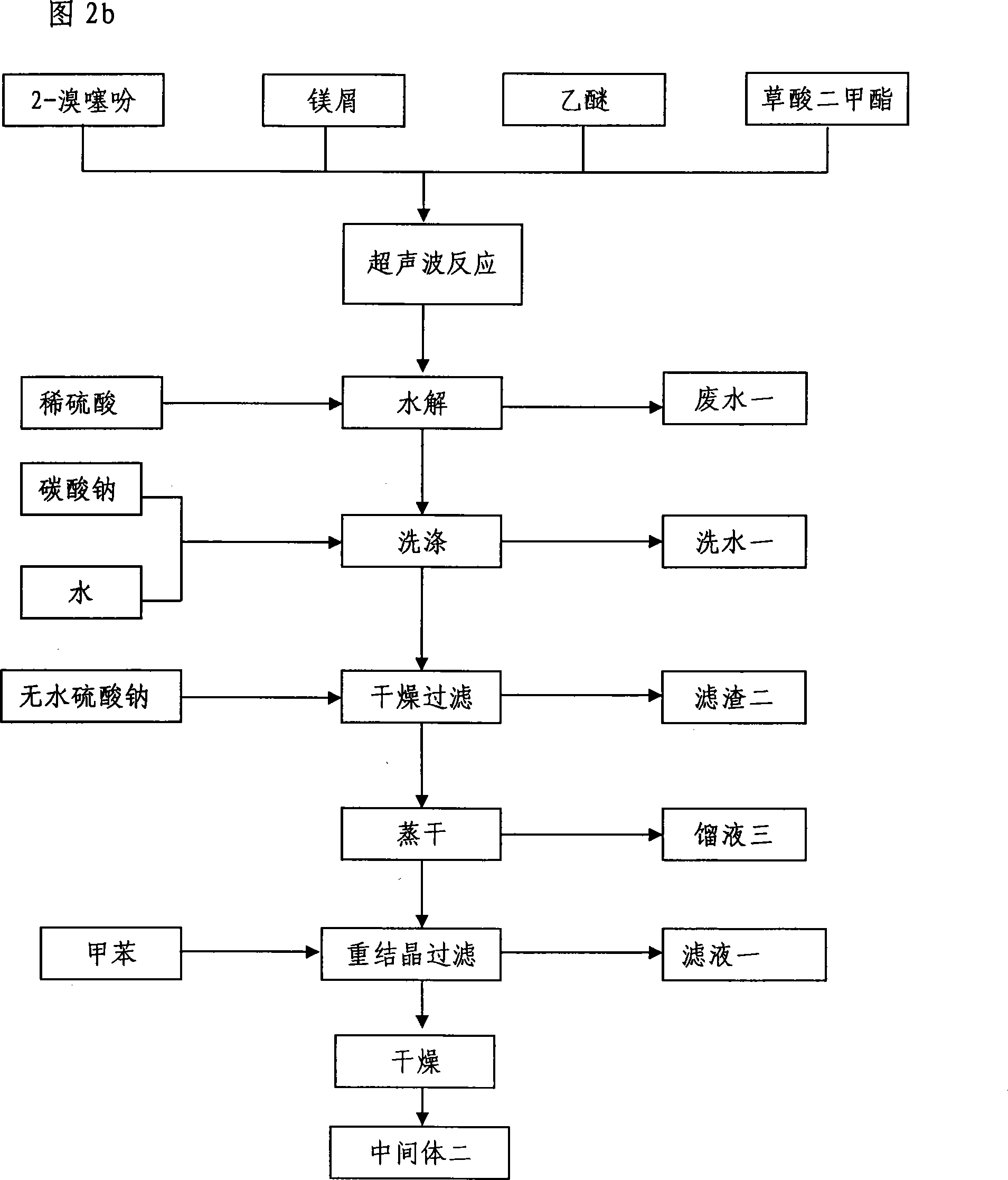
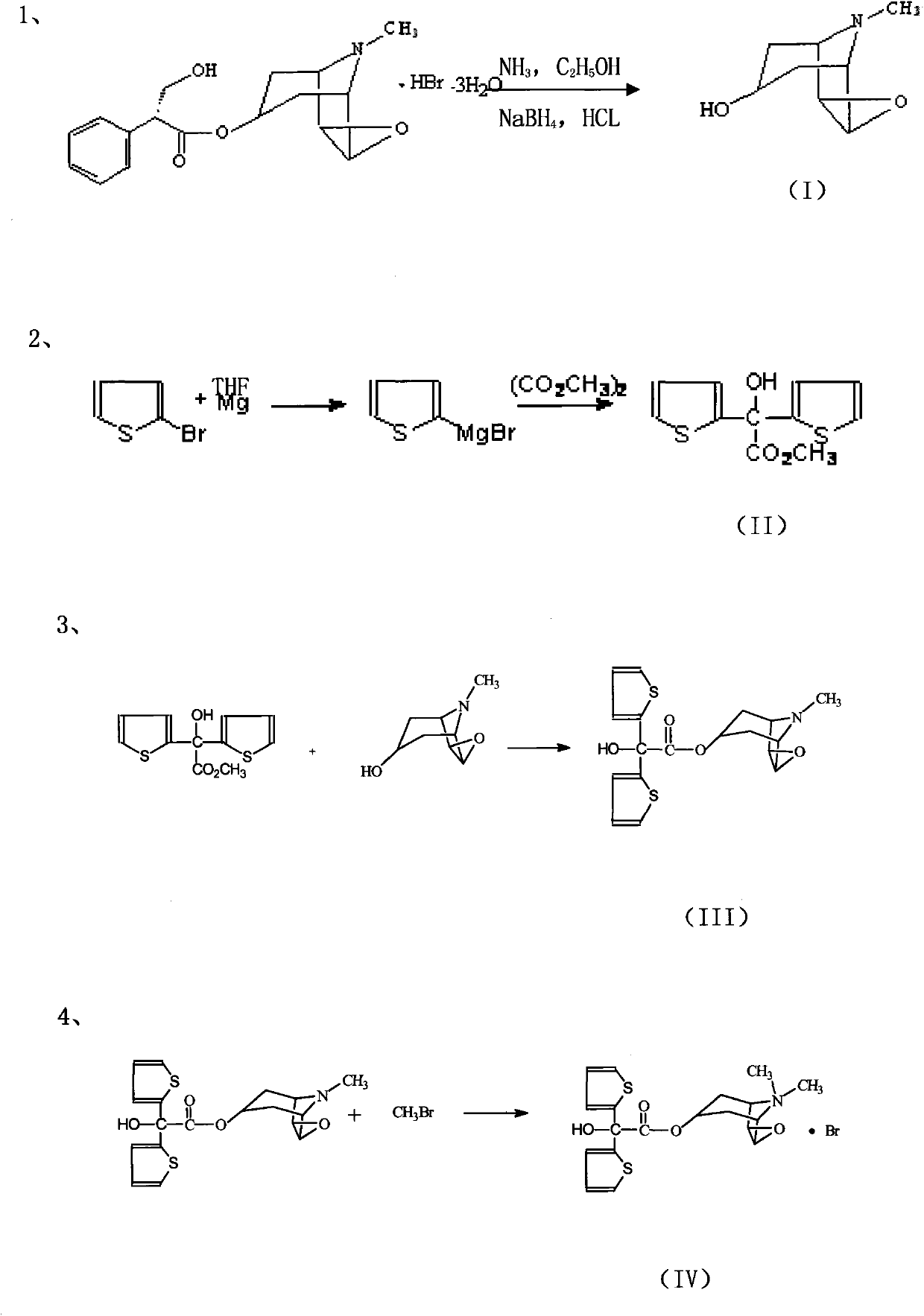
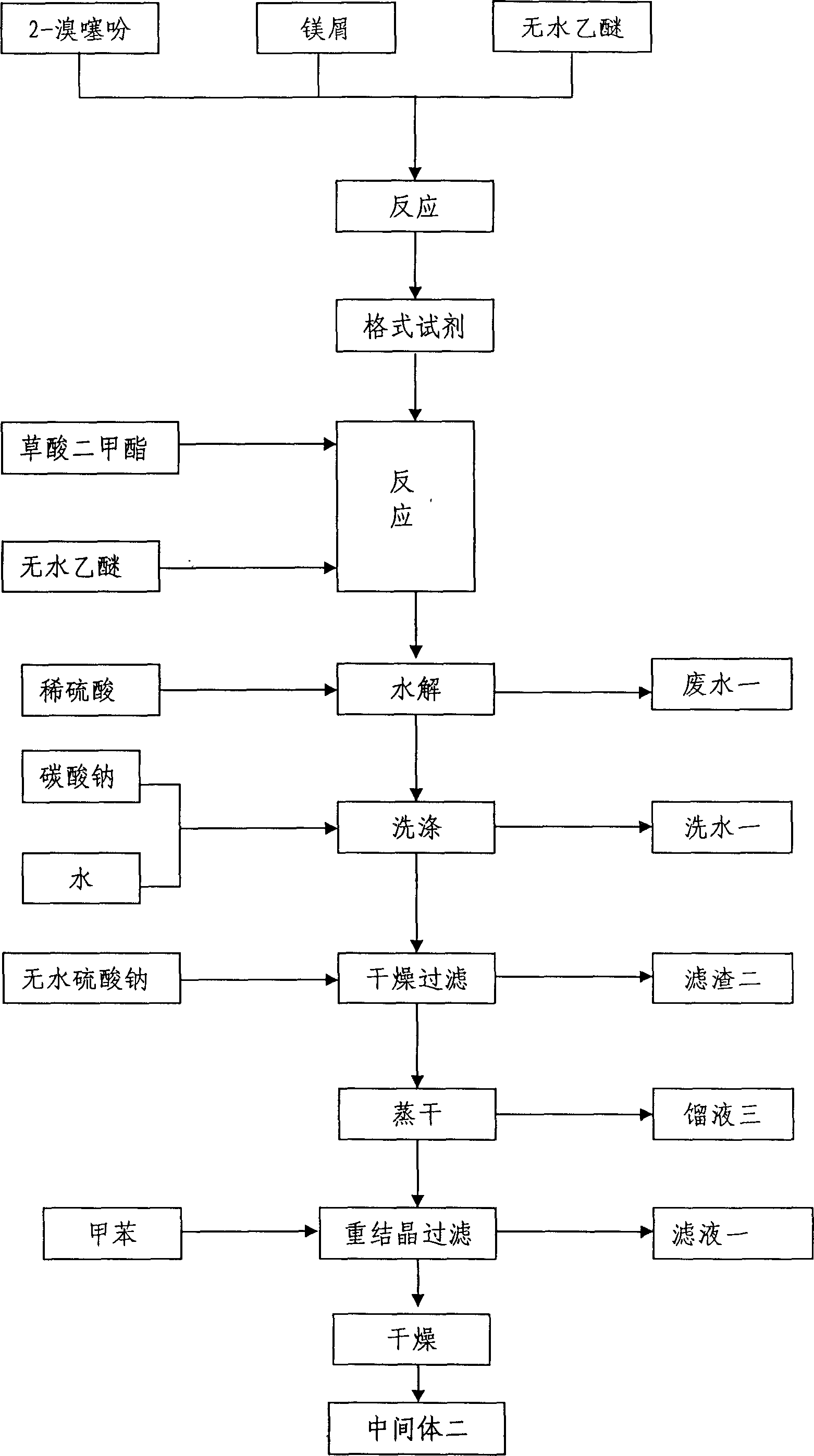
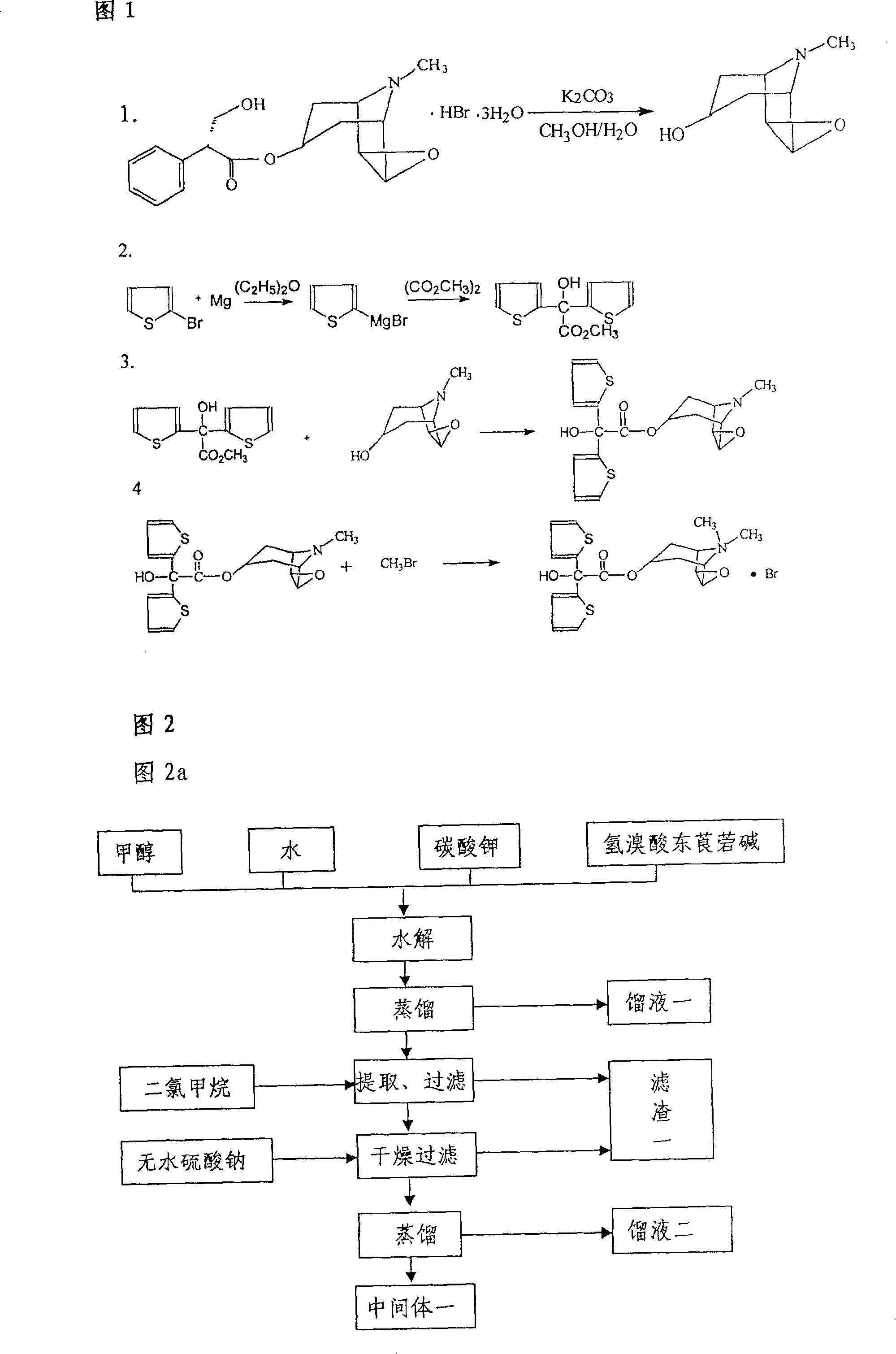
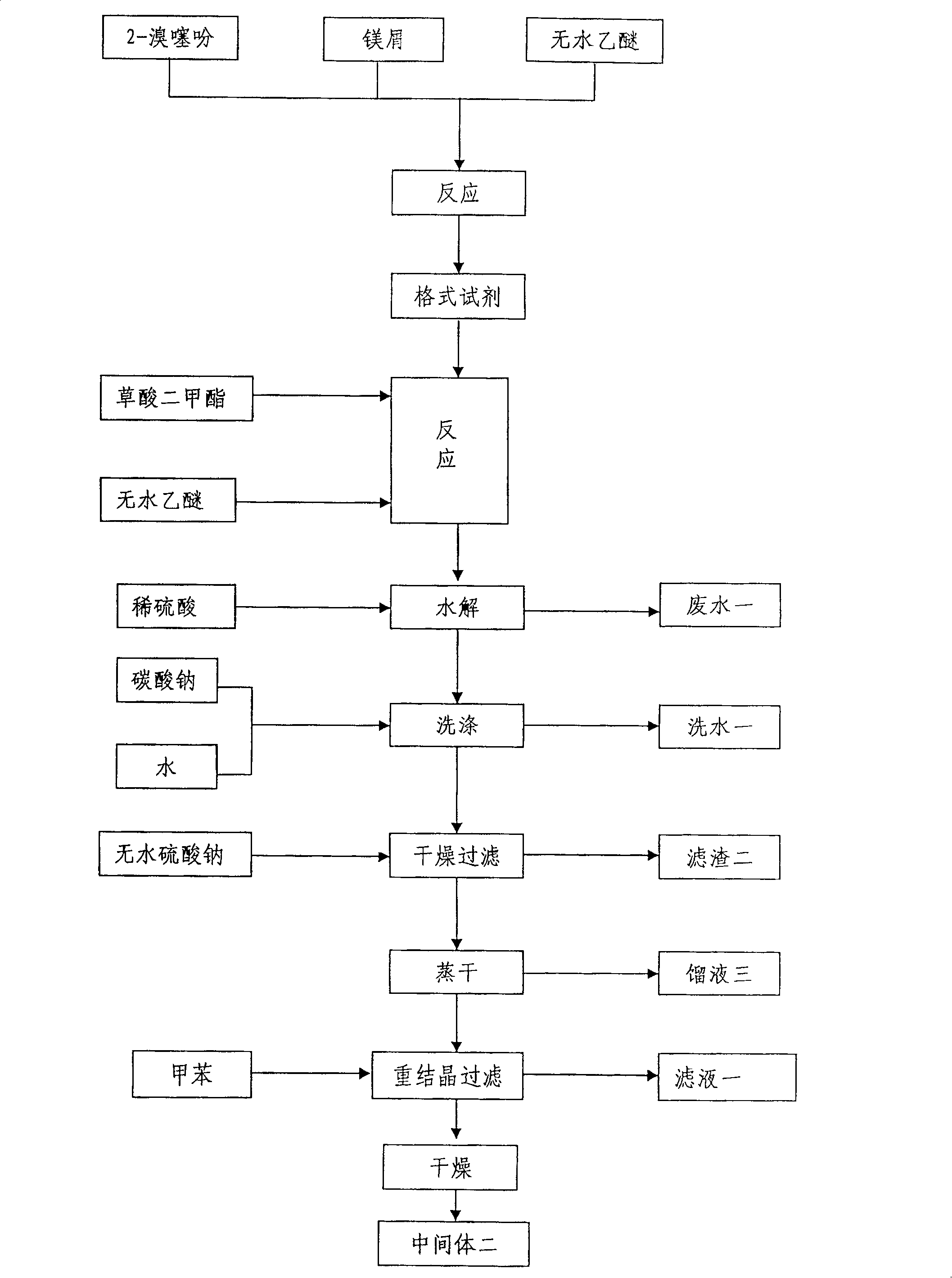
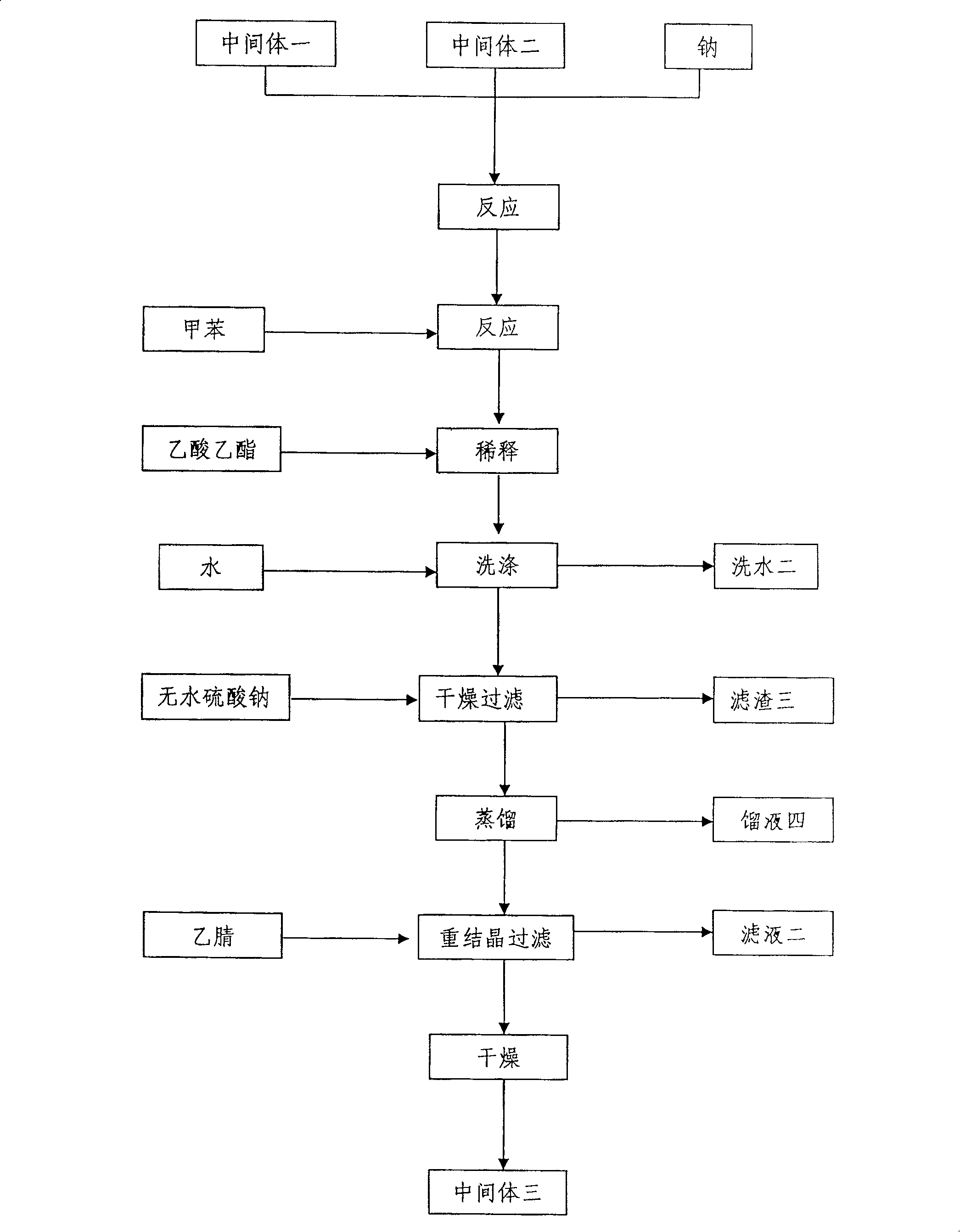
Method for preparing tiotropium bromide
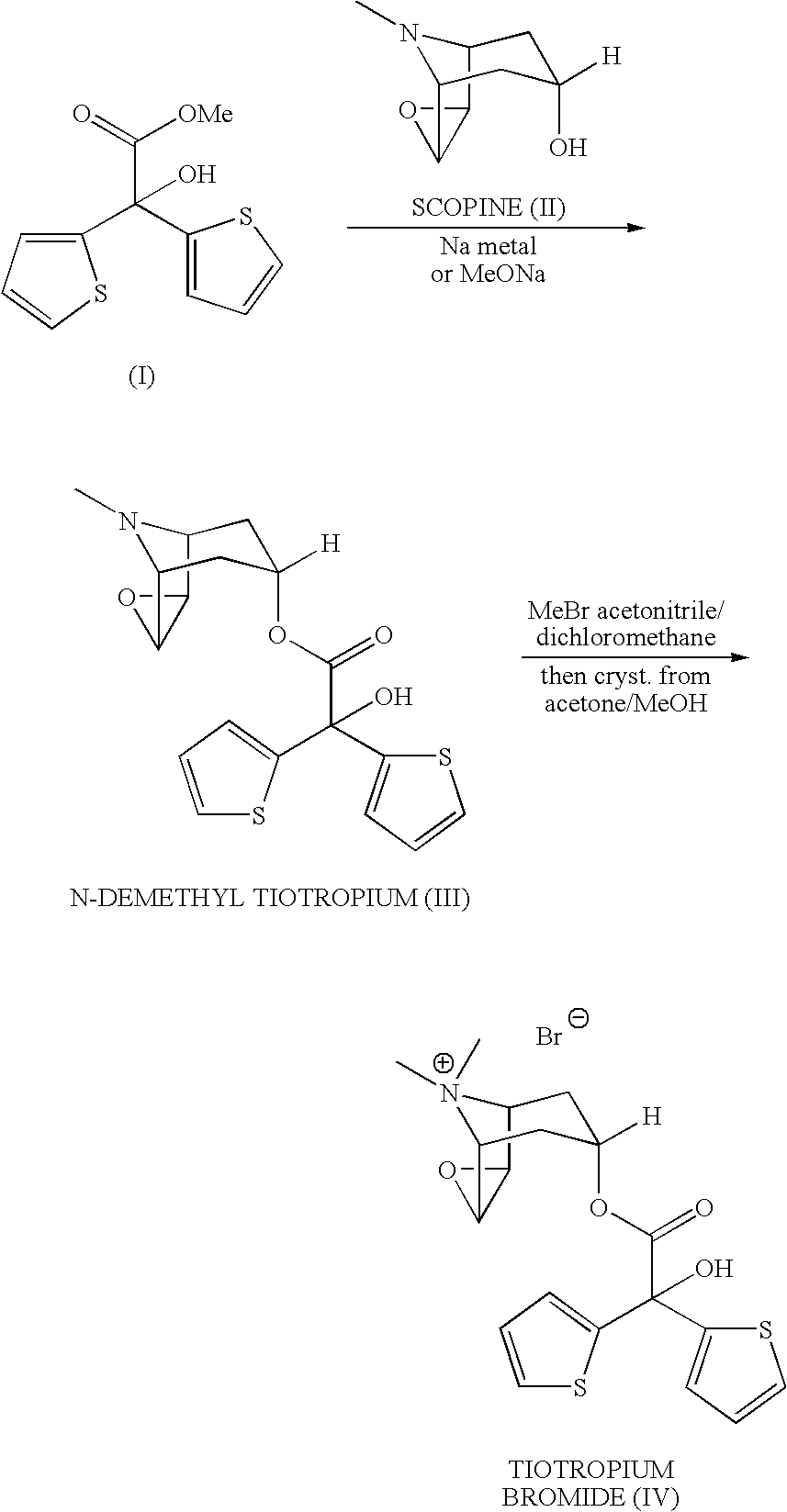
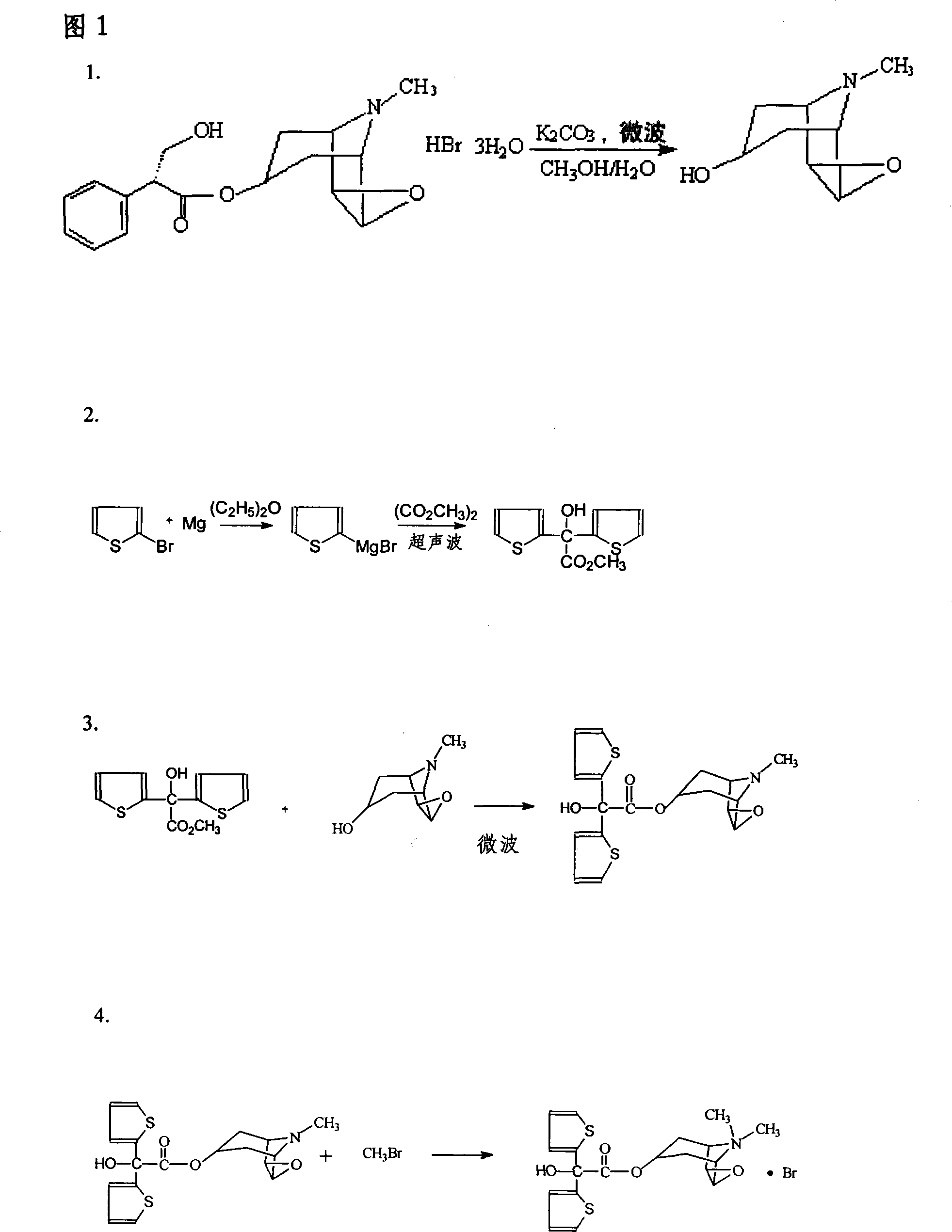
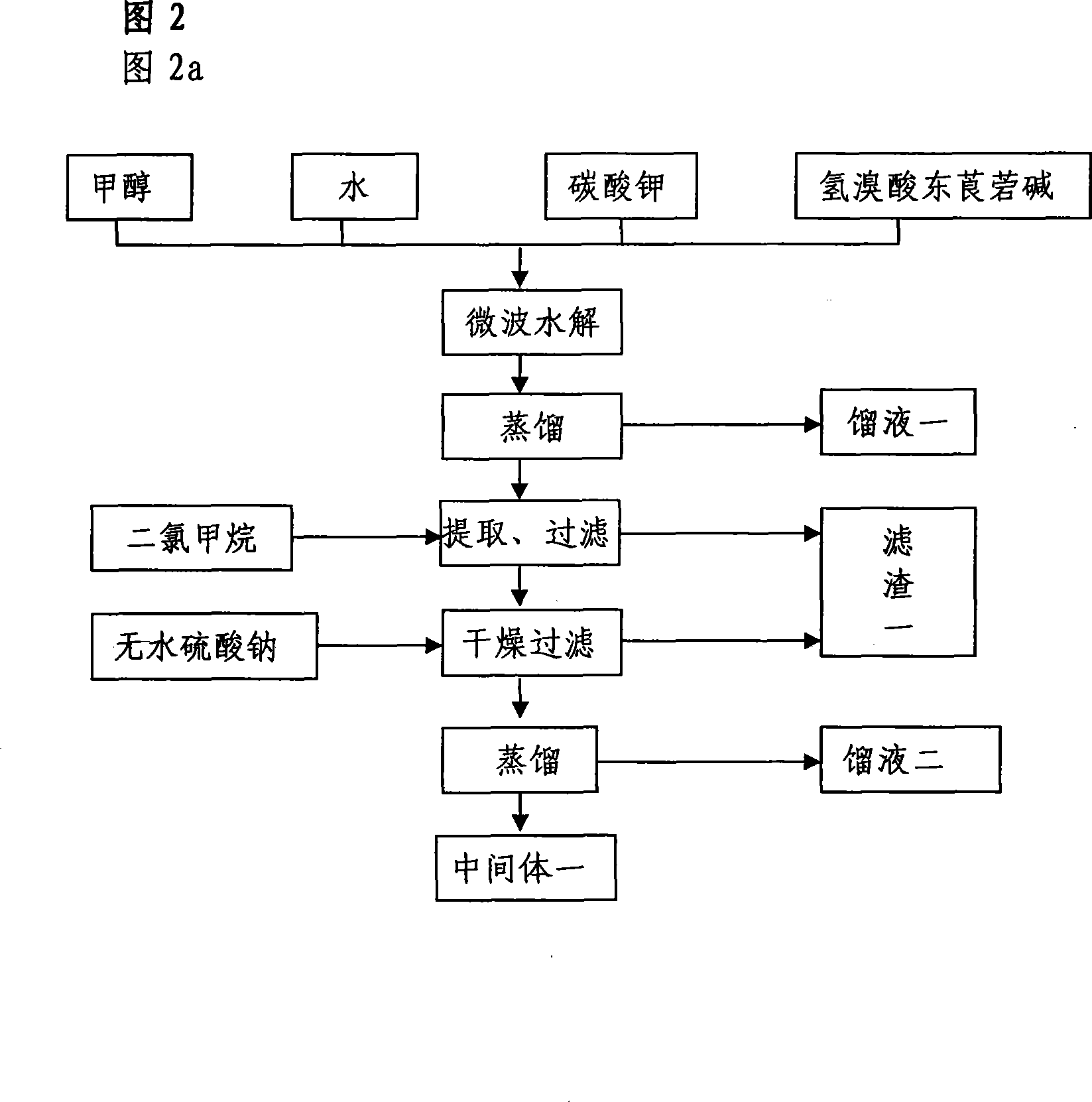
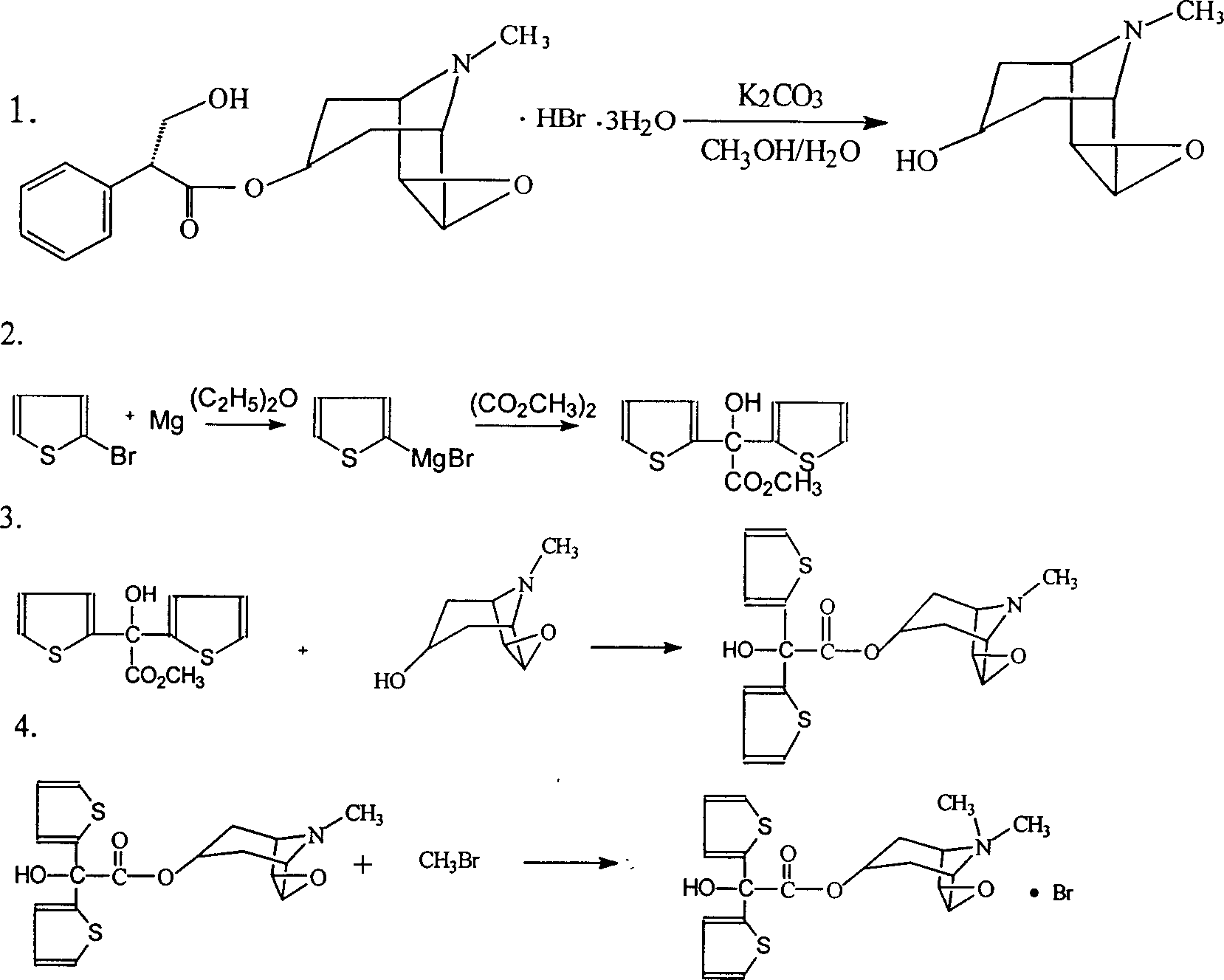
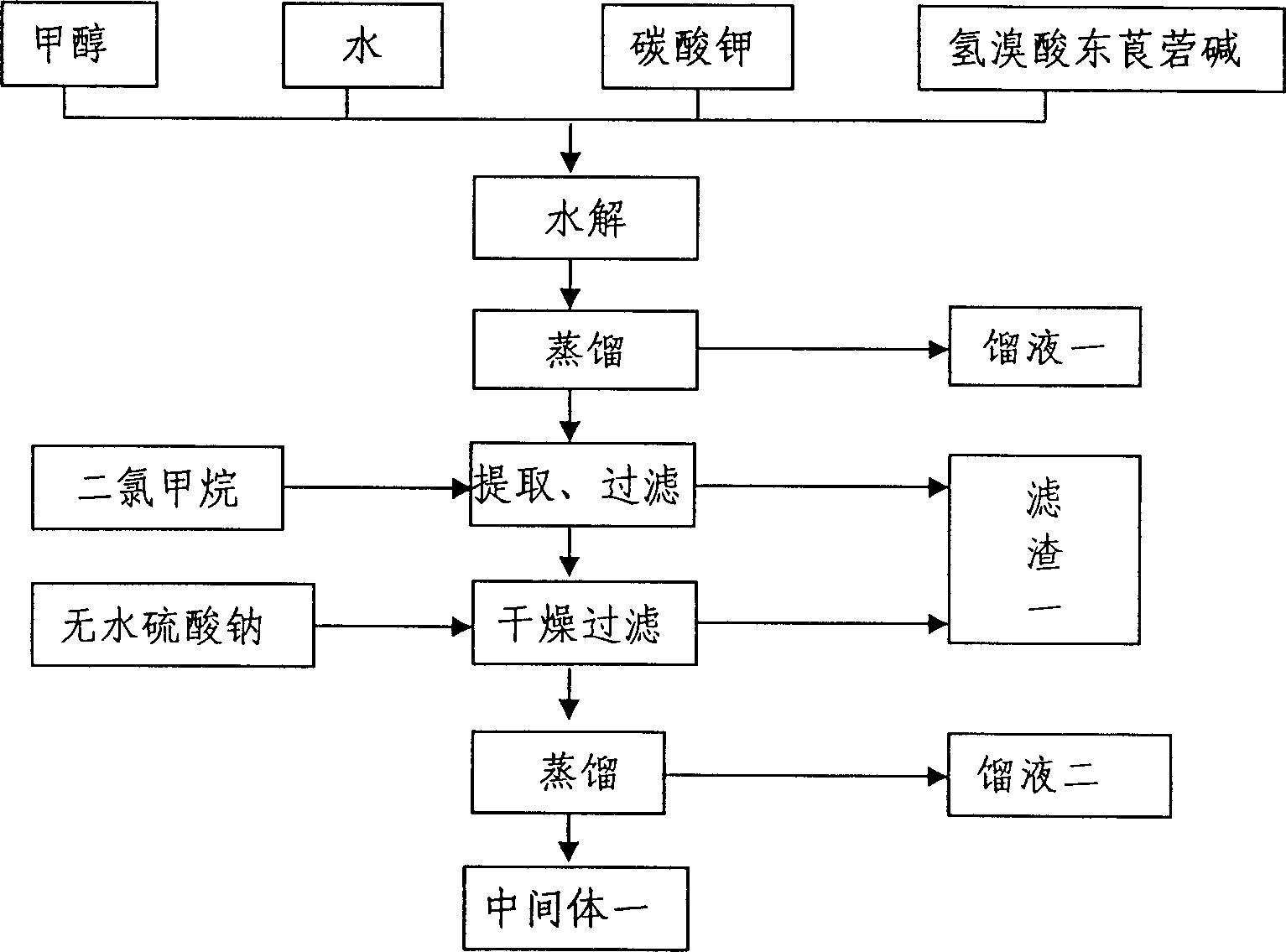
The invention relates to the application of microwave and ultrasonic technologies in synthesizing tiotropium bromide anhydride, in particular to a method for preparing the refined product of a finished product, namely, tiotropium bromide anhydride through crystallization and recrystallization processes by using a crude product of tiotropium bromide prepared through bromization reaction after hydrolytic reaction of scopolamine hydrobromide under microwave function, substitution and coupling reaction of bromothiophene, magnesium, and oxalic acid dimethyl ester under the microwave function, and condensation reaction of scopine and thiophen methyl glycollate under the microwave function. Using the invention to prepare tiotropium bromide anhydride, not only the reaction time is short, the side reaction is less, and the yield rate is high in the room temperature condition, but also the product quality is stable, controllable, safe and effective.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Medical product containing tiotropium
InactiveUS20090038612A1Good moisturizing effectEasy dosePowder deliveryOrganic active ingredientsDiseaseTiotropium bromide
Owner:BOEHRINGER INGELHEIM INT GMBH
Capsules Containing Inhalable Tiotropium
The invention relates to capsules for inhalation (inhalettes) made from specific capsule materials with a reduced moisture content, which contain the active substance tiotropium in the form of powdered preparations and are characterised by increased stability.
Owner:BOEHRINGER INGELHEIM PHARM KG
Tiotropium bromide anhydride and preparation method thereof
ActiveCN101768158AQuality improvementHigh yieldOrganic chemistryFiltration separationGrignard reagentDimethyl oxalate
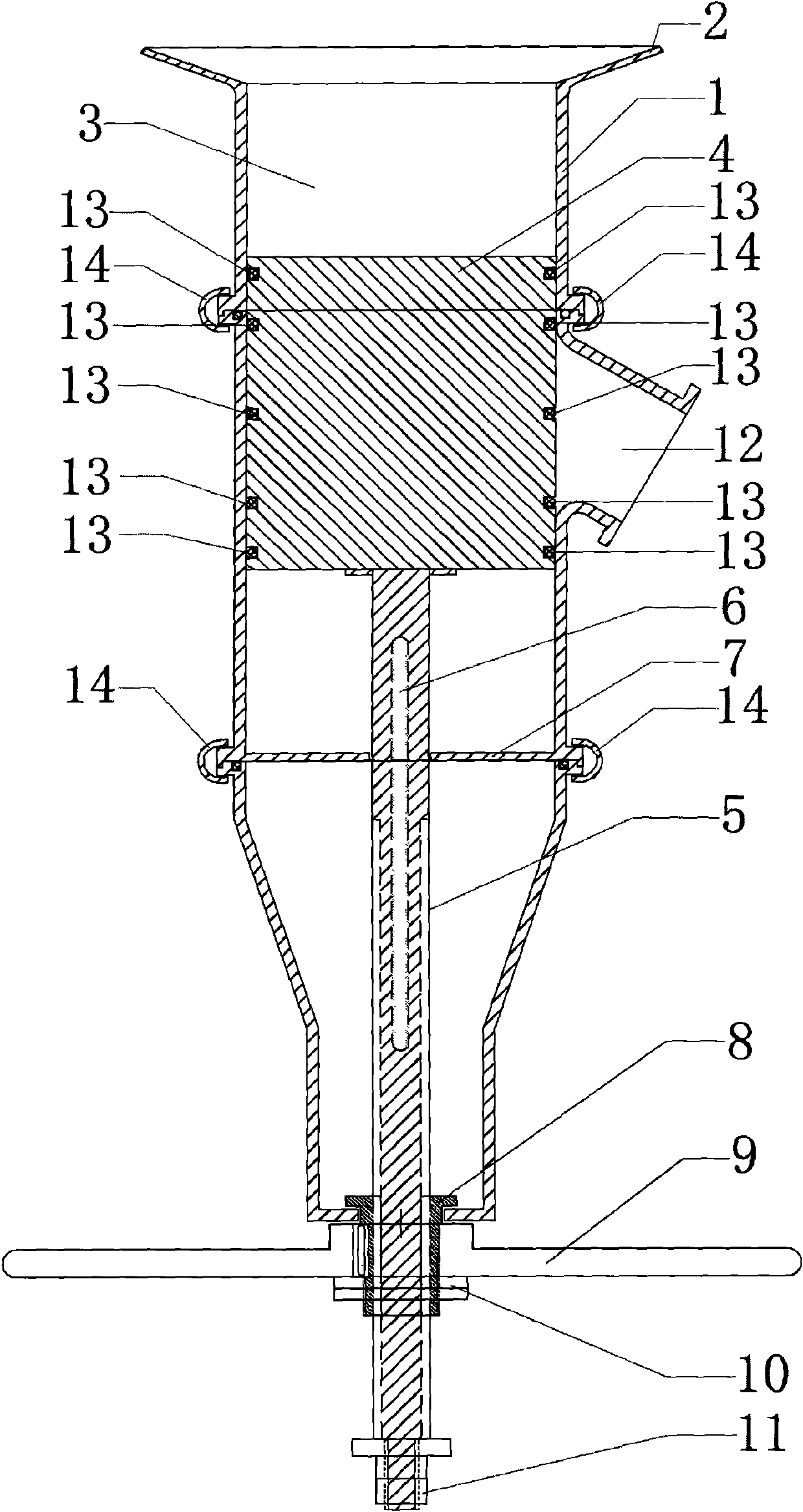
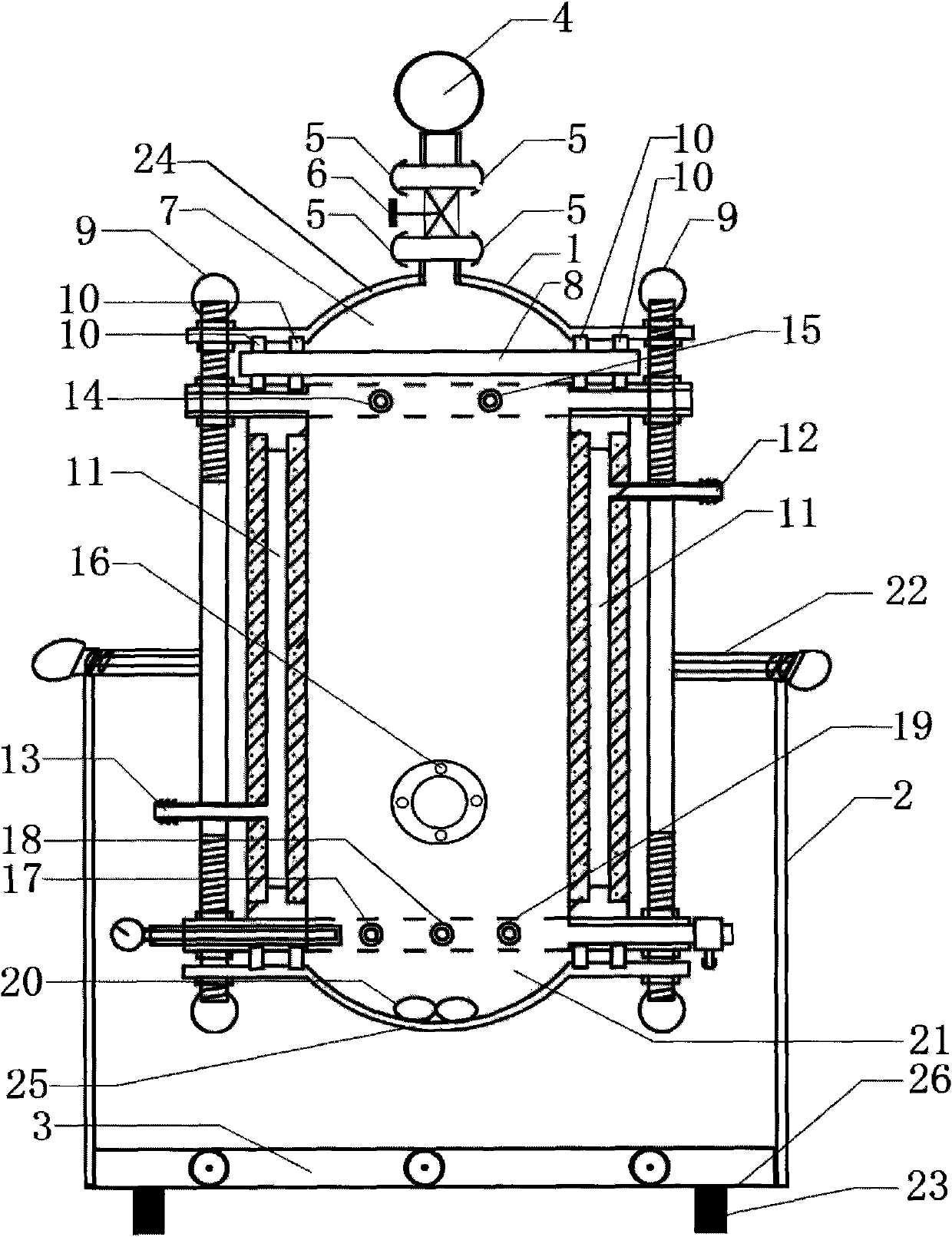
The invention discloses a method for preparing tiotropium bromide anhydride, which comprises the steps of: under the anhydrous and anaerobic condition, preparing an intermediate I by taking a special kettle bottom valve and a multi-functional reaction kettle as a reactor, taking a special filter as refining equipment and taking hydrobromic scopolamine as an initial raw material through reduction hydrogenation reaction; preparing an intermediate II by performing addition reaction of Grignard reagent and dimethyl oxalate in tetrahydrofuran, wherein the Grignard reagent is prepared from bromothiophene by taking tetrahydrofuran as a medium through iodine catalysis; preparing an intermediate III by performing ester exchange condensation reaction of the intermediate I and the intermediate II under the action of sodium; preparing an intermediate IV from the intermediate III through methylation bromination reaction; and preparing the tiotropium bromide anhydride by performing the activated carbon decoloration and recrystallization on the intermediate IV by using aqueous solution and acetonitrile methanol isopropyl ether solution. Compared with those in the prior art, reaction products of the method have the advantages of good quality, high yield, controlled reaction process, safe production and good environmental protection effect.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Preparation process of thiatro bromoaminium anhydrous compound
An anhydrous tiotropium bromide is prepared from scopolamine hydrobromate. Its preparing process is also disclosed.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Process for preparing new tiotropium salts, new tiotropium salts as such and pharmaceutical compositions thereof
The invention relates to a process for preparing new tiotropium salts, these new tiotropium salts as such, pharmaceutical formulations containing them and their use for preparing a medicament for the treatment of respiratory complaints, particularly for the treatment of COPD (chronic obstructive pulmonary disease) and asthma.
Owner:BOEHRINGER INGELHEIM INT GMBH
Medicine composition with tiotropium bromide and formoterol, application of medicine composition and preparation
InactiveCN105125542AEnhance diastolic abilityReduce omissionAntipyreticAnalgesicsTiotropium bromideObstructive Pulmonary Diseases
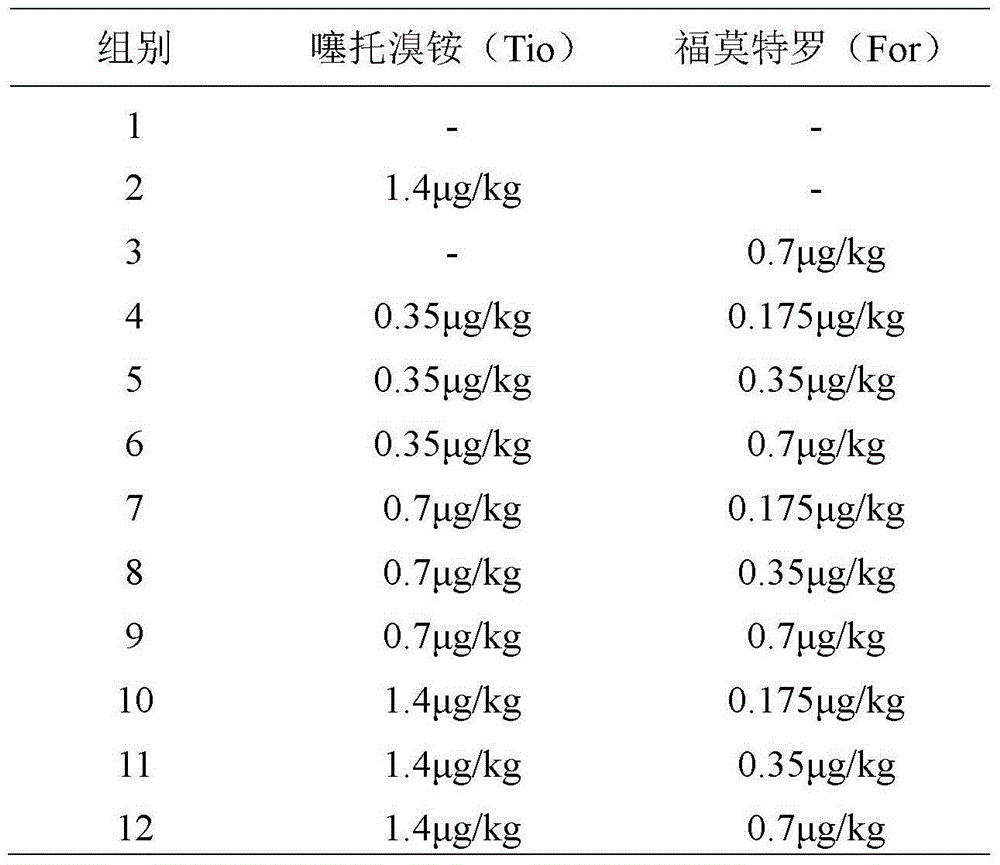
The invention relates to the technical field of medicine preparations, in particular to a medicine composition with tiotropium bromide and formoterol, application of the medicine composition and a preparation. The medicine composition comprises the tiotropium bromide or pharmaceutically acceptable salt of the tiotropium bromide and the formoterol or pharmaceutically acceptable salt of the formoterol. The medicine composition with the tiotropium bromide and the formoterol is applied to preparing medicine for treating respiratory and lung inflammatory diseases. The medicine composition is preferably a tiotropium bromide and formoterol composition with synergistic additive effects. The medicine composition, the application and the preparation have the advantages that as proved by pharmacodynamic experiments, the synergistic additive effects can be realized by the tiotropium bromide and the formoterol which are jointly applied, and accordingly bronchiectasis can be forcefully and quickly realized; the medicine composition and the preparation are used for treating the respiratory and lung inflammatory diseases, and preferably used for treating dyspnea such as bronchial asthma and chronic obstructive pulmonary diseases (COPD) due to bronchial constriction.
Owner:HANGZHOU ZIJIN PHARMA TECH
Inhaler comprising a tiotropium-containing-composition
This invention relates to a pressurised metered dose inhaler comprising a canister, wherein the canister contains a formulation comprising a tiotropium salt and an HFA propellant, wherein the inhaler is an inhalation-actuated inhaler.
Owner:TEVA BRANDED PHARMA PROD R & D
Preparation process of thiatro bromoaminium anhydrous compound
Owner:HONGYI SCI & TECH CO LTD NANCHANG
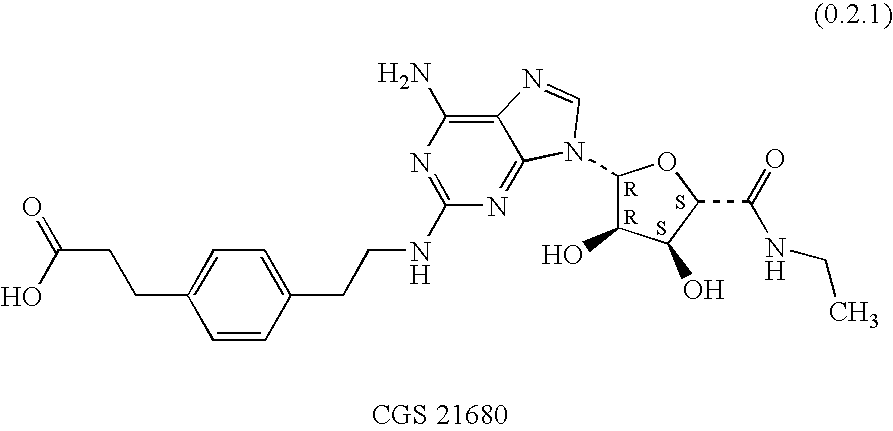
Combination of an adenosine A2A-receptor agonist and tiotropium or a derivative thereof for treating obstructive airways and other inflammatory diseases
InactiveUS20050250730A1Easy to controlInhibition is effectiveBiocidePowder deliveryDiseaseAnticholinergic agents
A combination of therapeutic agents useful in the treatment of obstructive airways and other inflammatory diseases comprising (i) an adenosine A2A receptor agonist; and (ii) an anti-cholinergic agent, preferably comprising a member selected from the group consisting of tiotropium and derivatives thereof; the combination being therapeutically effective in the treatment of the diseases when administered by inhalation; as well as to a method of treating the obstructive airways and other inflammatory diseases comprising administering separately, simultaneously or sequentially to the mammal by inhalation a therapeutically effective amount of the combination of therapeutic agents; as well as to a pharmaceutical composition comprising a pharmaceutically acceptable carrier together with the combination of therapeutic agents; as well as to a product containing the compounds of the combination for separate, simultaneous or sequential administration by inhalation to a mammal for the treatment of obstructive airways and other inflammatory diseases. It is preferred that the anti-cholinergic agent component be tiotropium bromide.
Owner:BOEHRINGER INGELHEIM PHARM KG
Tiotropium inhalation solution for nebulization
ActiveUS9757365B2Improve complianceQuality improvementDispersion deliveryAerosol deliveryDiseaseTiotropium bromide
Owner:GLENMARK SPECIALTY
Pharmaceutical composition preparation
InactiveCN111467498AReduce exacerbationsImprove lung functionRespiratory disorderHeterocyclic compound active ingredientsAnticholinergic DrugsGlucocorticoid
The invention provides a pharmaceutical composition preparation. The pharmaceutical composition preparation comprises an anticholinergic drug tiotropium or a hydrate thereof, a beta 2-receptor agonistarformoterol or a salt thereof, and an inhaled glucocorticoid fluticasone or an ester derivative thereof; tiotropium bromide acts on a muscarinic receptor on bronchial smooth muscles, the cholinergiceffect of acetylcholine released by the tail ends of parasympathetic nerves can be inhibited, and muscular tension is blocked; the arformoterol acts on a beta 2-receptor on an airway smooth muscle cell membrane, so that the release of degranulation and media of mast cells and basophils is reduced, the permeability of capillaries is reduced, and swinging of airway epithelial cilia is increased; the fluticasone is an effective anti-inflammatory drug, and when the three drugs are combined for use, the three mechanisms jointly exert the bronchus relaxing effect and the anti-inflammatory effect; the application range of the triple therapy is wider on the important clinical indexes of reducing acute exacerbation of patients suffering from chronic obstructive pulmonary disease, reducing total-cause mortality rate, improving the lung function of the patients suffering from chronic obstructive pulmonary disease, improving the living quality and the like.
Owner:王兆霖
Inhalable tiotropium and container therefor
A medical product suitable for storing and delivering a pre-metered dose of tiotropium, devices containing the same, and methods of using the same.
Owner:BOEHRINGER INGELHEIM PHARM KG
Tiotropium inhalation solution for nebulization
ActiveUS9987260B2Improve complianceQuality improvementDispersion deliveryInorganic non-active ingredientsDiseaseTiotropium bromide
Owner:GLENMARK SPECIALTY +1
Tiotropium Containing Powder Formulation For Inhalation
The invention relates to powdered preparations containing tiotropium for inhalation, processes for preparing them as well as their use in preparing a pharmaceutical composition for the treatment of respiratory complaints, particularly for the treatment of COPD (chronic obstructive pulmonary disease) and asthma.
Owner:BOEHRINGER INGELHEIM PHARMA KG
Tiotropium inhalation solution for nebulization
ActiveUS20160339003A1Improve the quality of lifeImproving user complianceDispersion deliveryAerosol deliveryDiseaseTiotropium bromide
The present invention relates to a sterile pharmaceutical composition comprising tiotropium or a pharmaceutically acceptable salt thereof, for inhalation via nebulization to a subject (e.g. a human). The invention also relates to a process for preparing the pharmaceutical composition and its use in the treatment of respiratory diseases such as chronic obstructive pulmonary disease (COPD) in a subject.
Owner:GLENMARK SPECIALTY
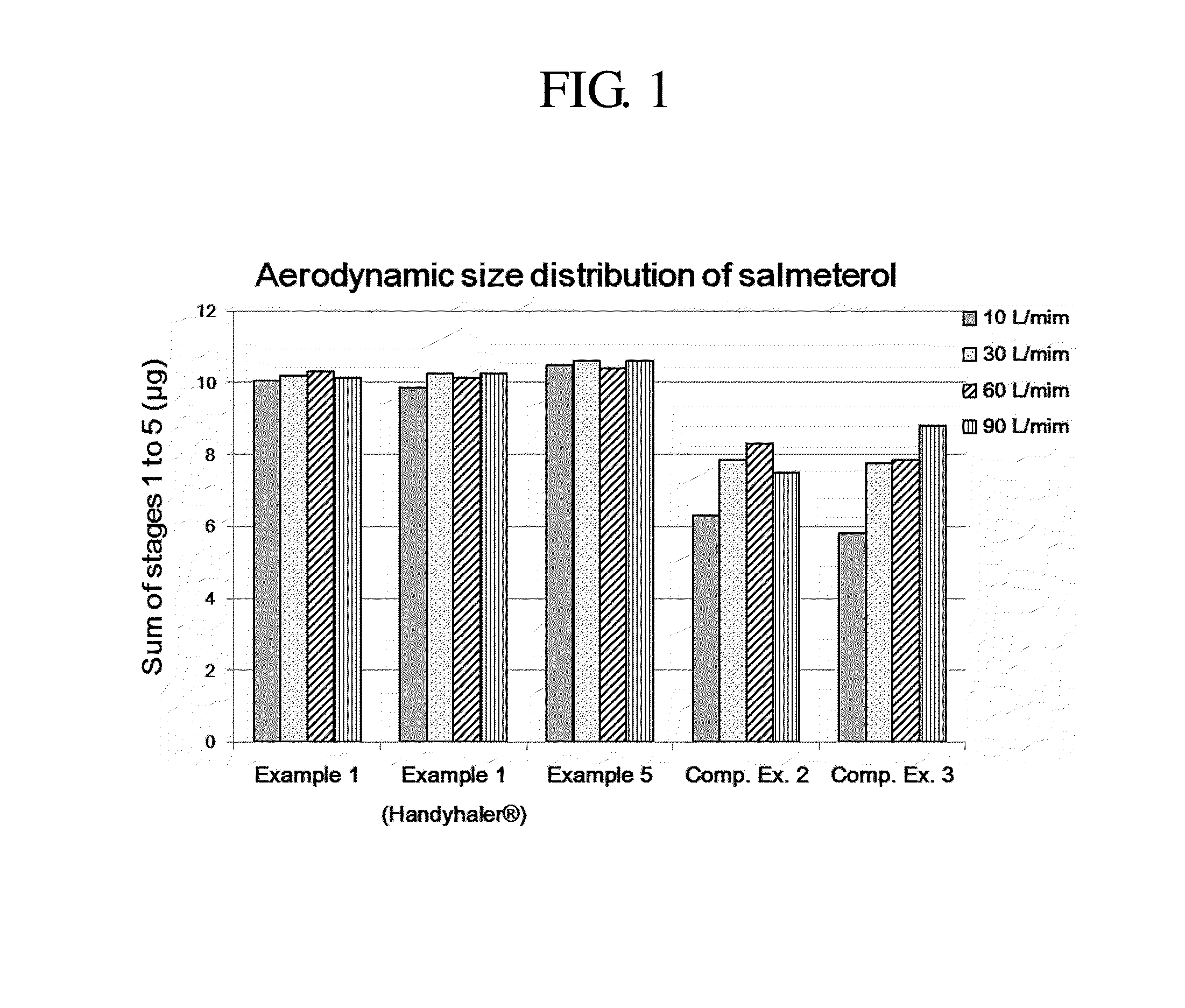
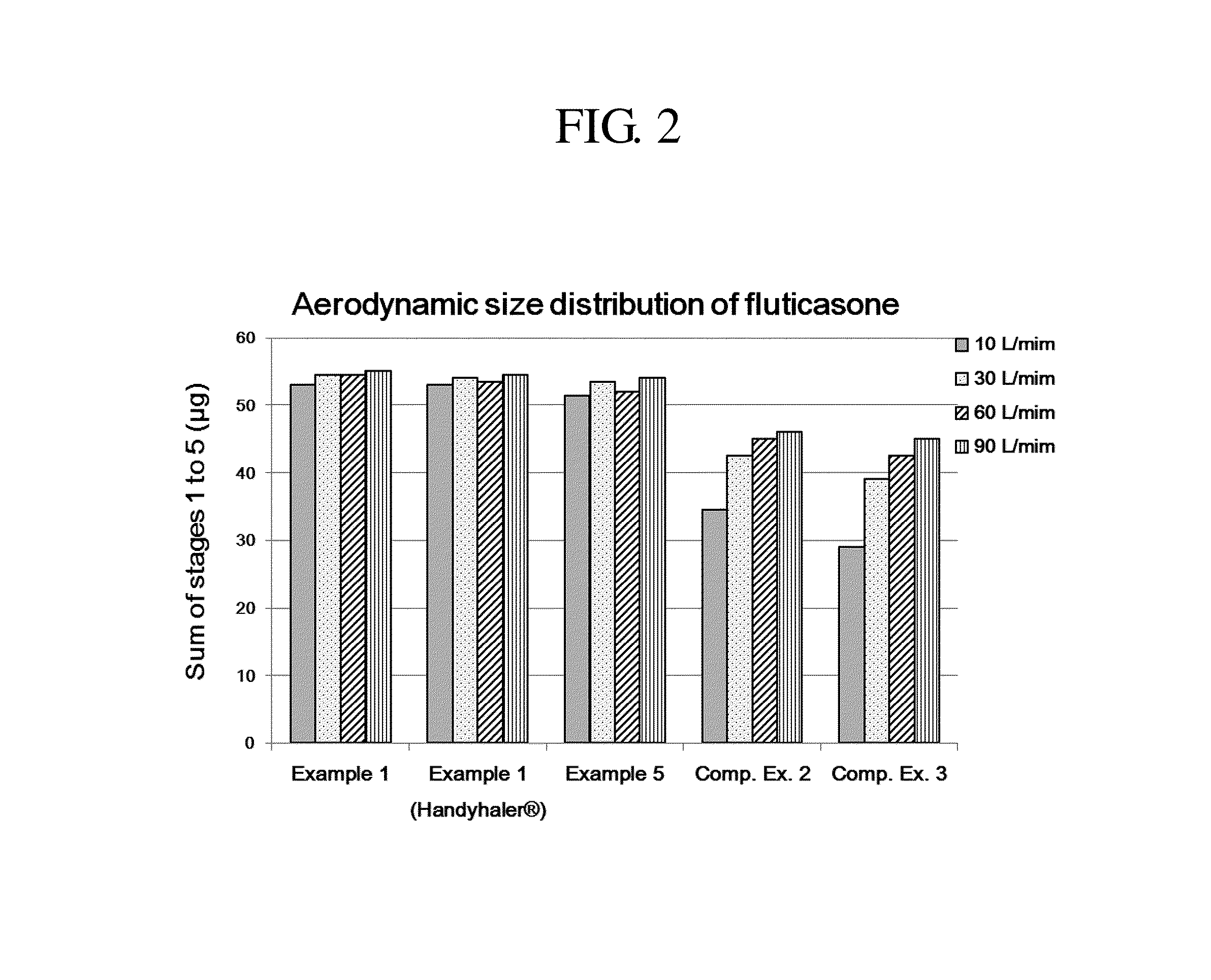
Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same
InactiveUS20150157566A1Good treatment complianceImprove convenienceBiocidePowder deliveryDiseaseRespiratory disease
Provided is a dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, as pharmaceutically active ingredients, and a carrier, and an inhalation formulation comprising same. The inventive dry powder inhalation formulation having good content uniformity and showing small changes in the aerodynamic size distribution in accordance with the flow rate changes can effectively deliver said pharmaceutically active ingredients to a target site upon administration, and thus can be useful in the prevention or treatment of respiratory diseases, particularly asthma and COPD.
Owner:HANMI PHARMA
Tiotropium bromide respirable dry powder composition
ActiveCN101422457AReduce dosageBiodegradablePowder deliveryOrganic active ingredientsTiotropium bromideObstructive Pulmonary Diseases
The invention provides a Tiotropium Bromide dry powder compound used for suction, and a preparation method thereof. The dry powder compound used for suction contains micronized Tiotropium Bromide, a micro-particle vector part, a granule vector part modified by phospholipid, and a coarse particle vector part. The dry powder compound can be used for preparing medicaments for curing chronic obstructive pulmonary disease and asthma.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
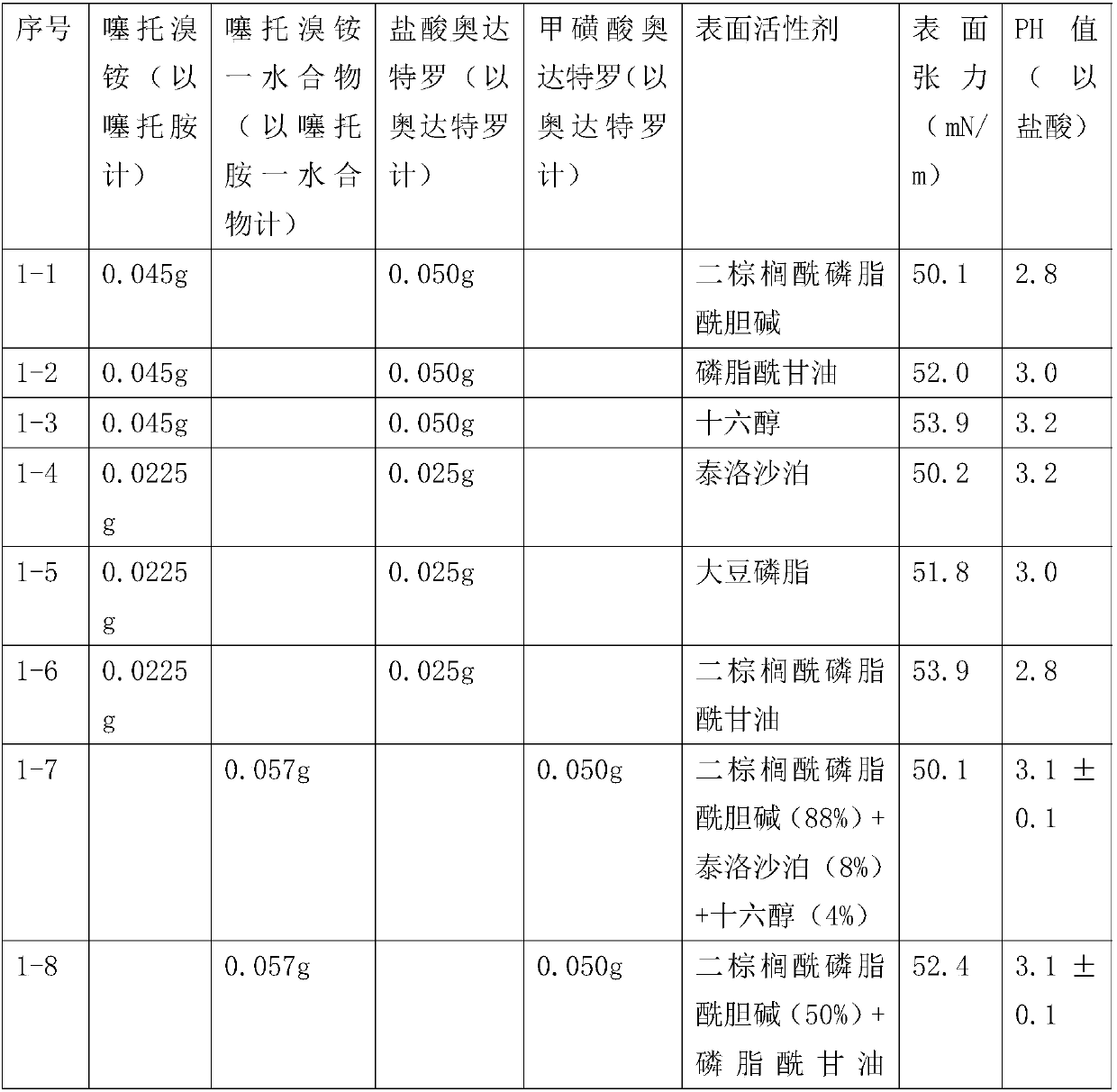
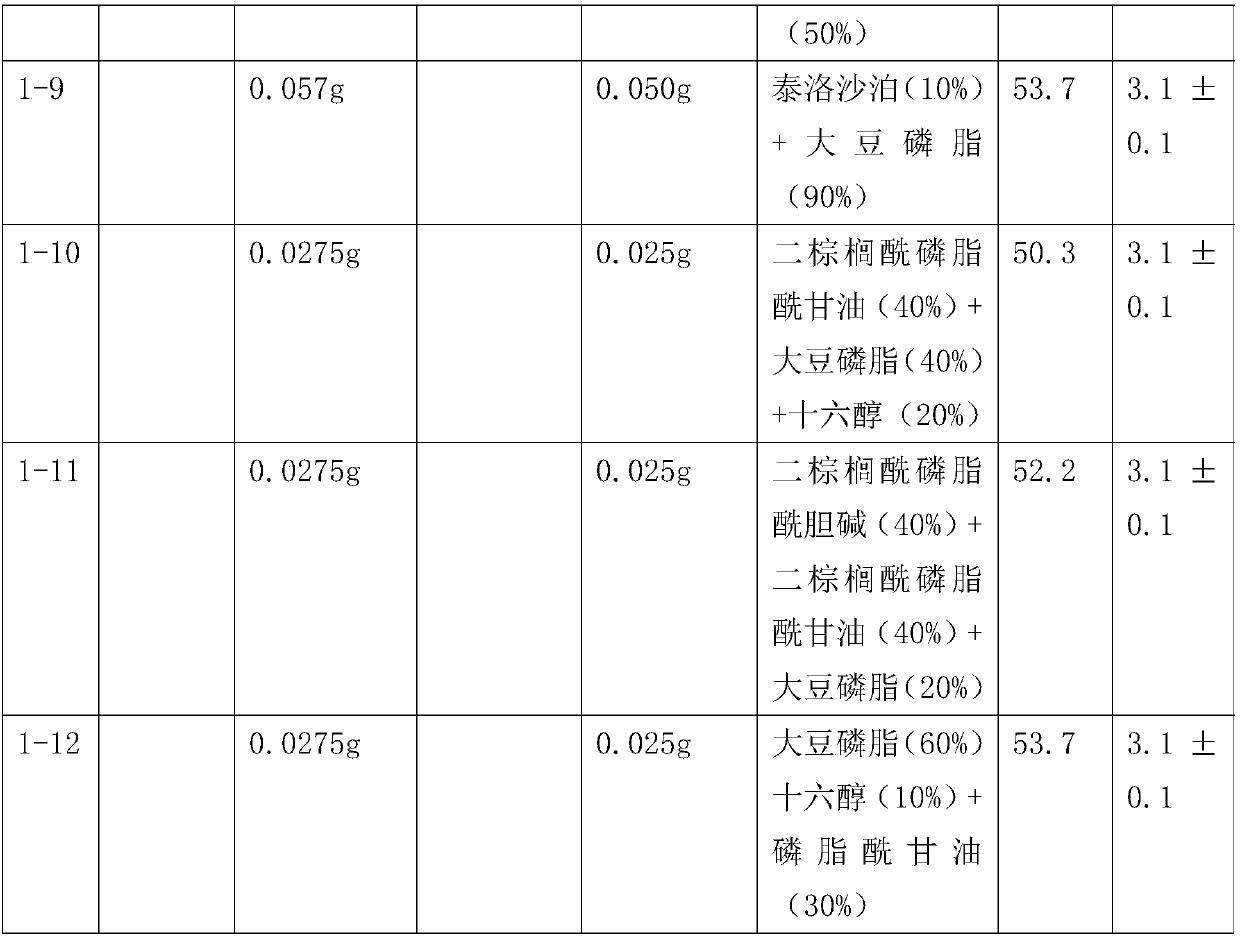
Tiotropium bromide olodaterol spray containing surfactant
The invention relates to a medicine tiotropium bromide olodaterol spray for treating respiratory diseases, especially asthma, chronic obstructive pneumonia, trachitis and the like. An inhalable surfactant enables the spray particle size, spray time and spray speed of the composition to be more suitable by adjusting the surface tension of the tiotropium bromide olodaterol spray pharmaceutical composition without propellants.
Owner:TIANJIN JINYAO GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com