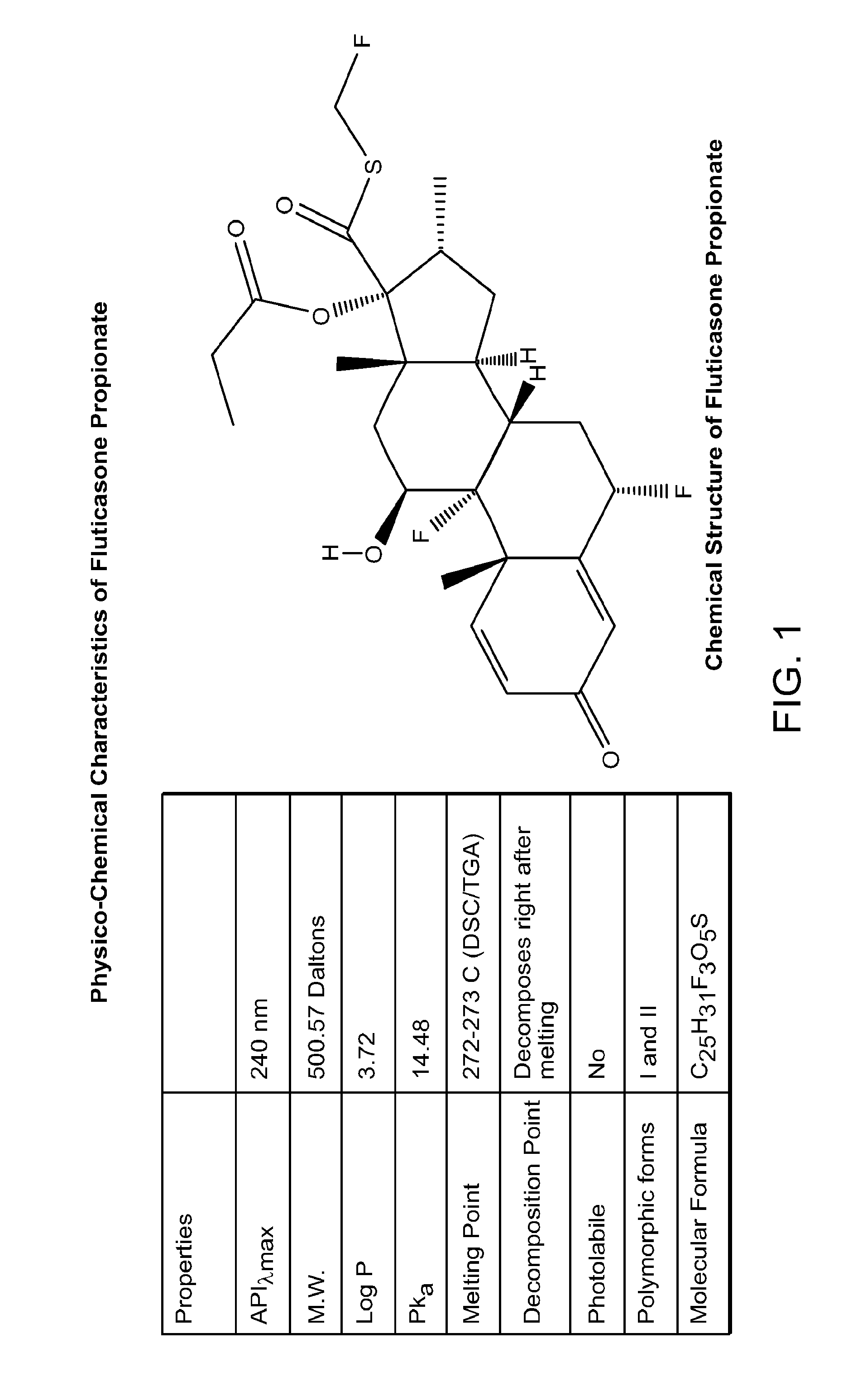
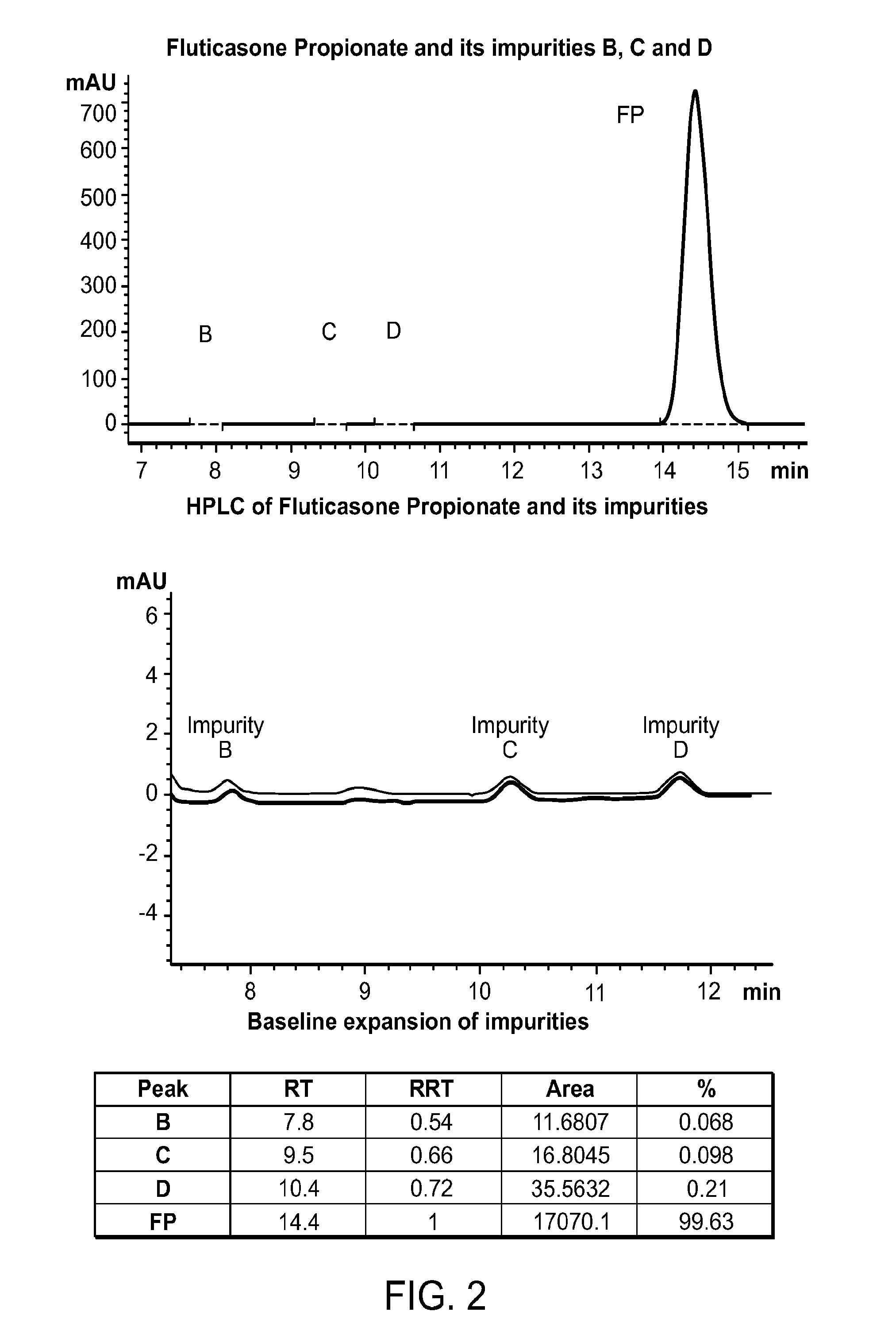
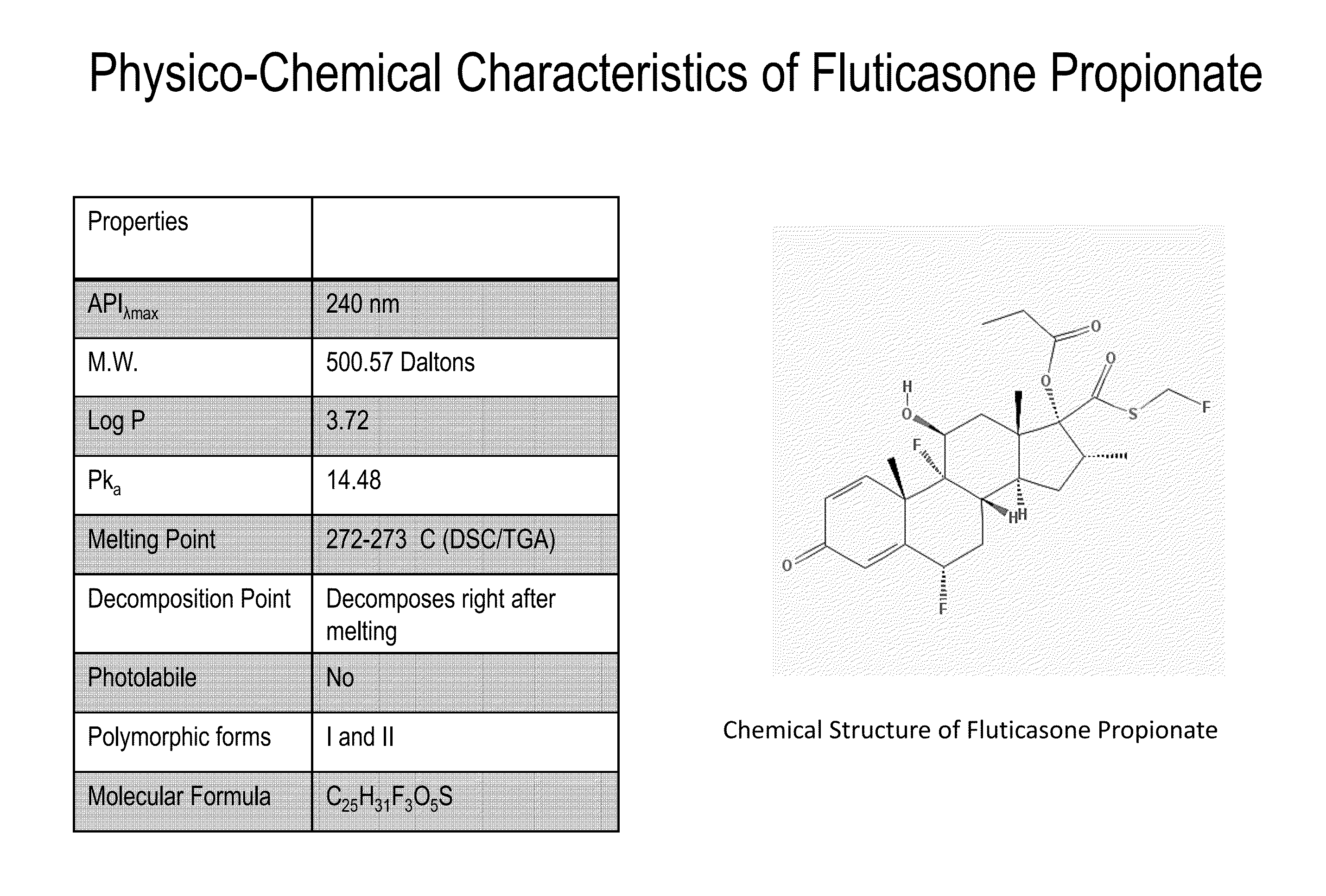
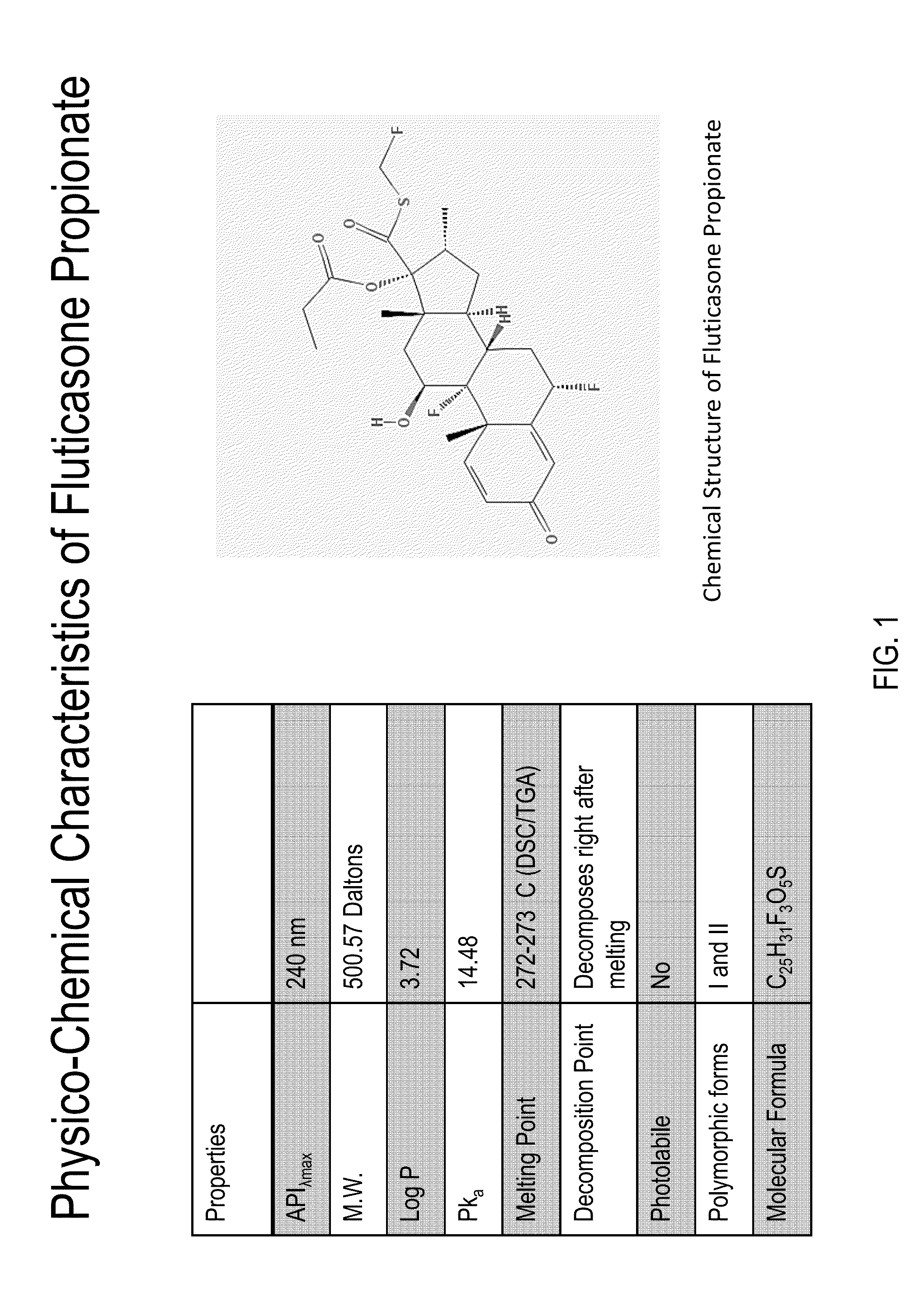
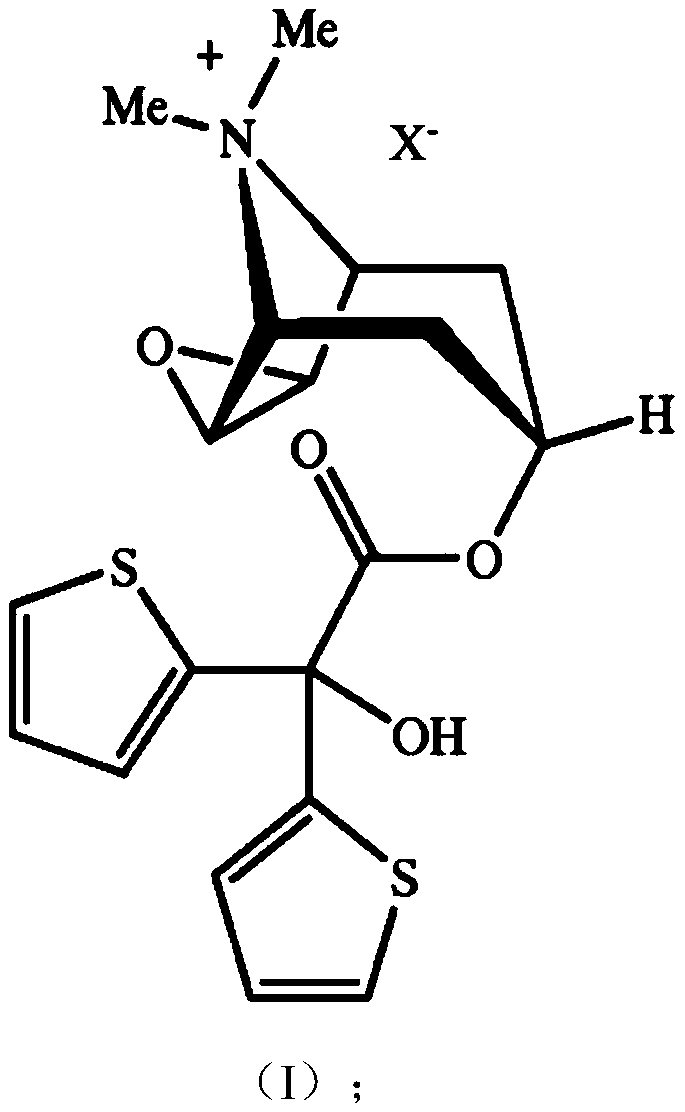
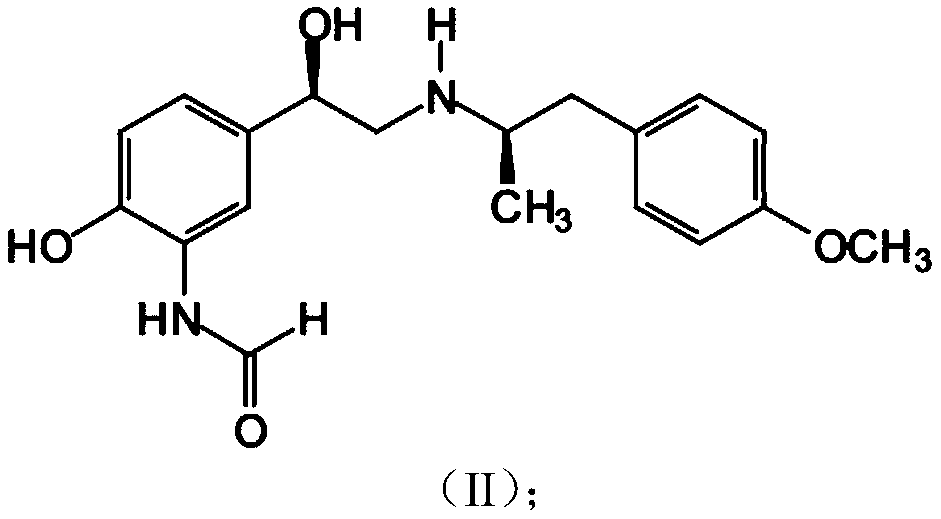
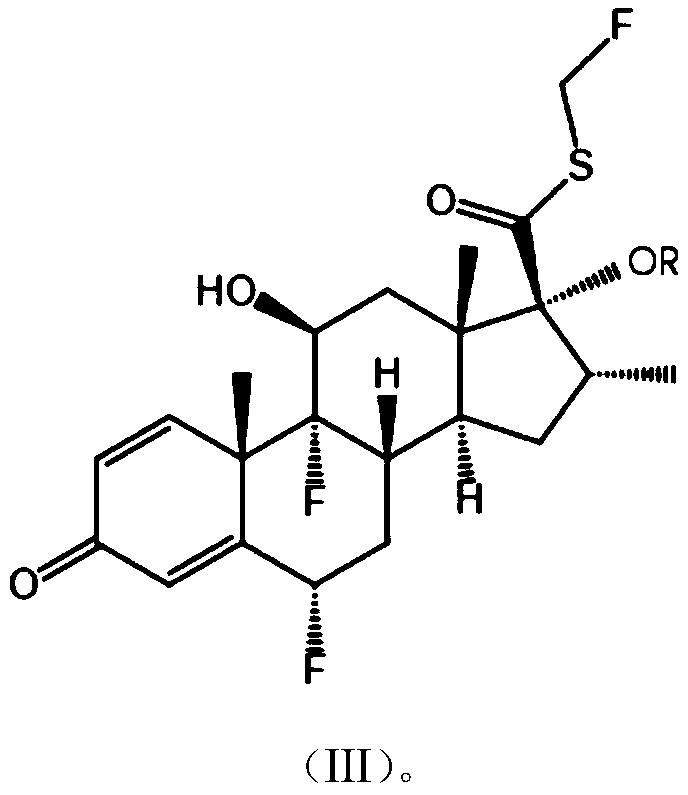
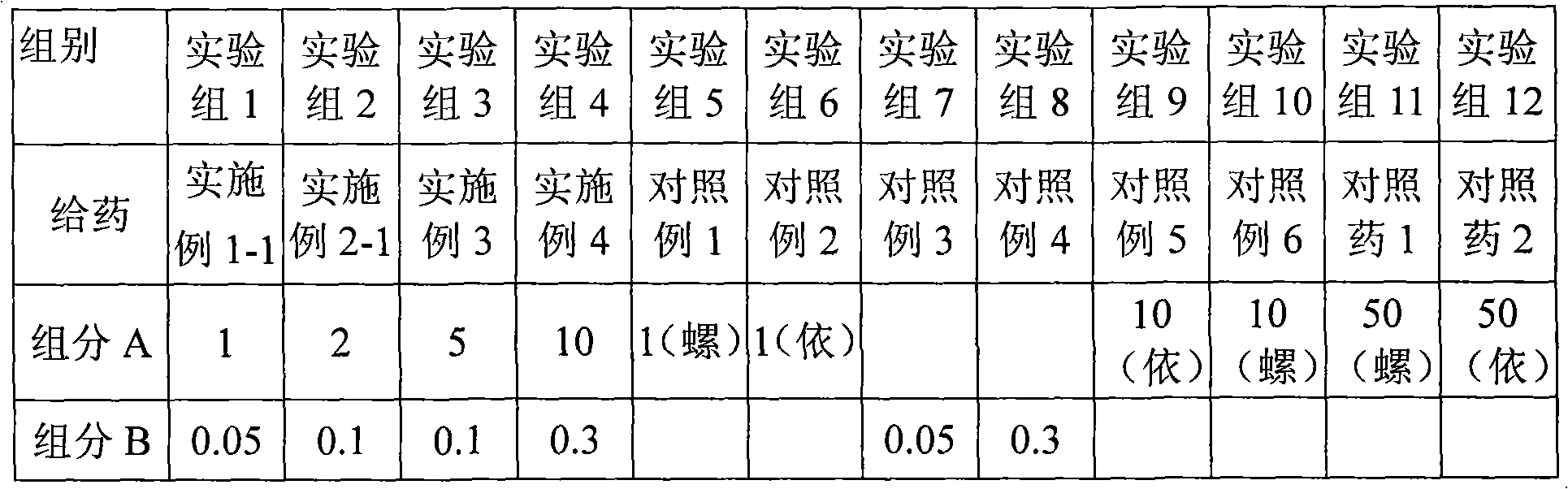
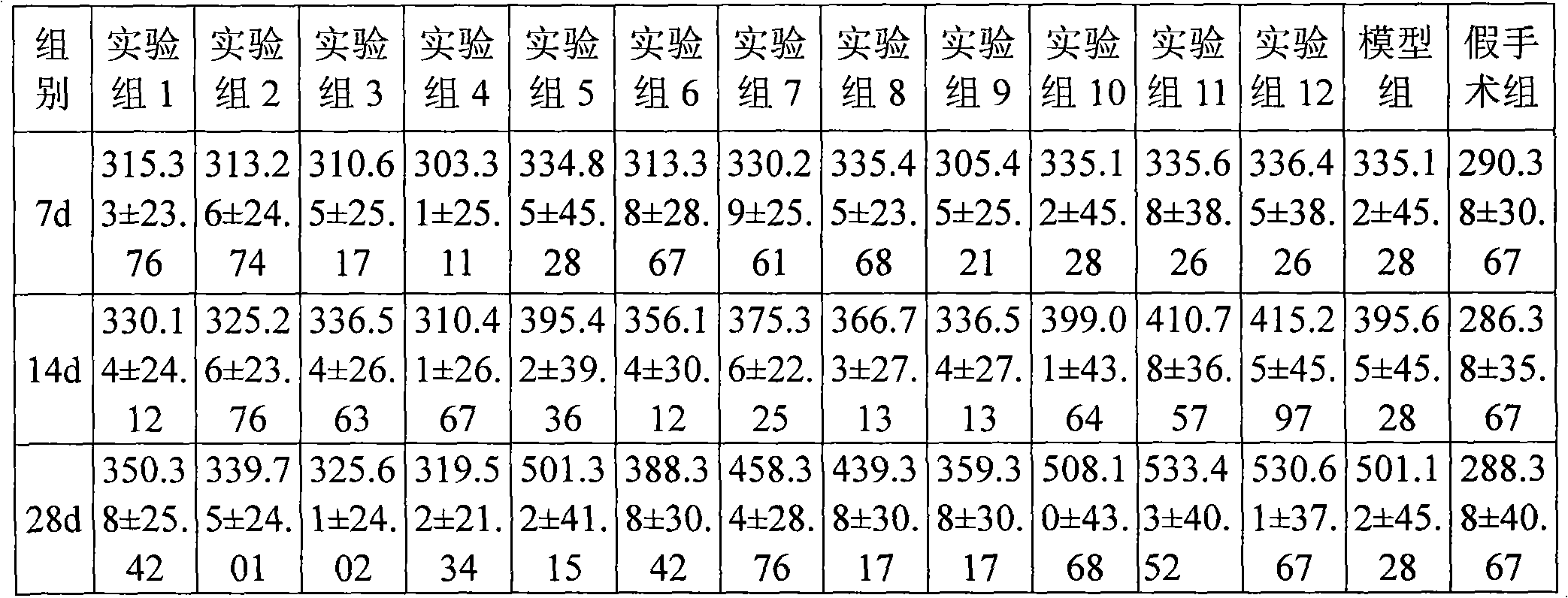
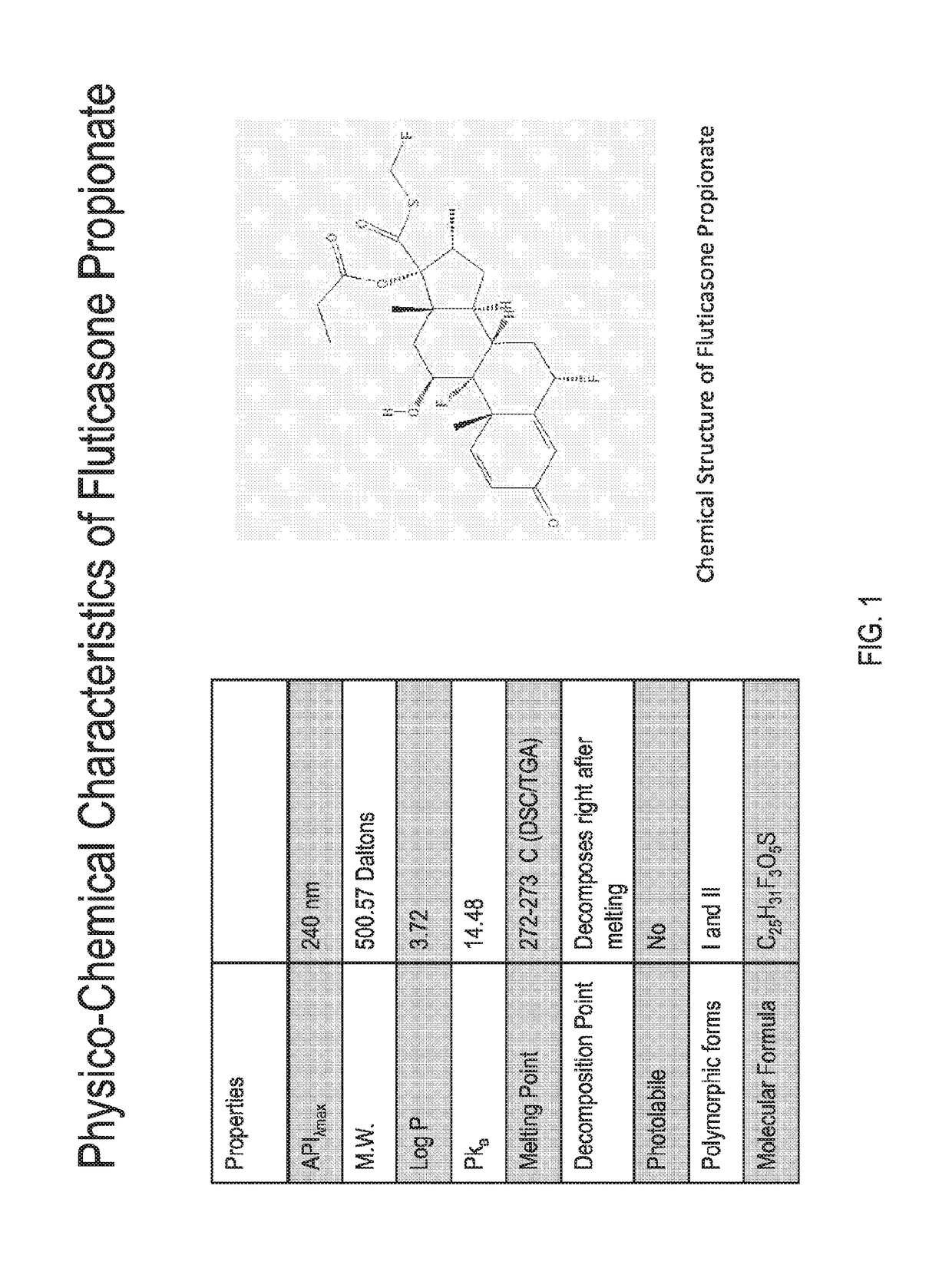
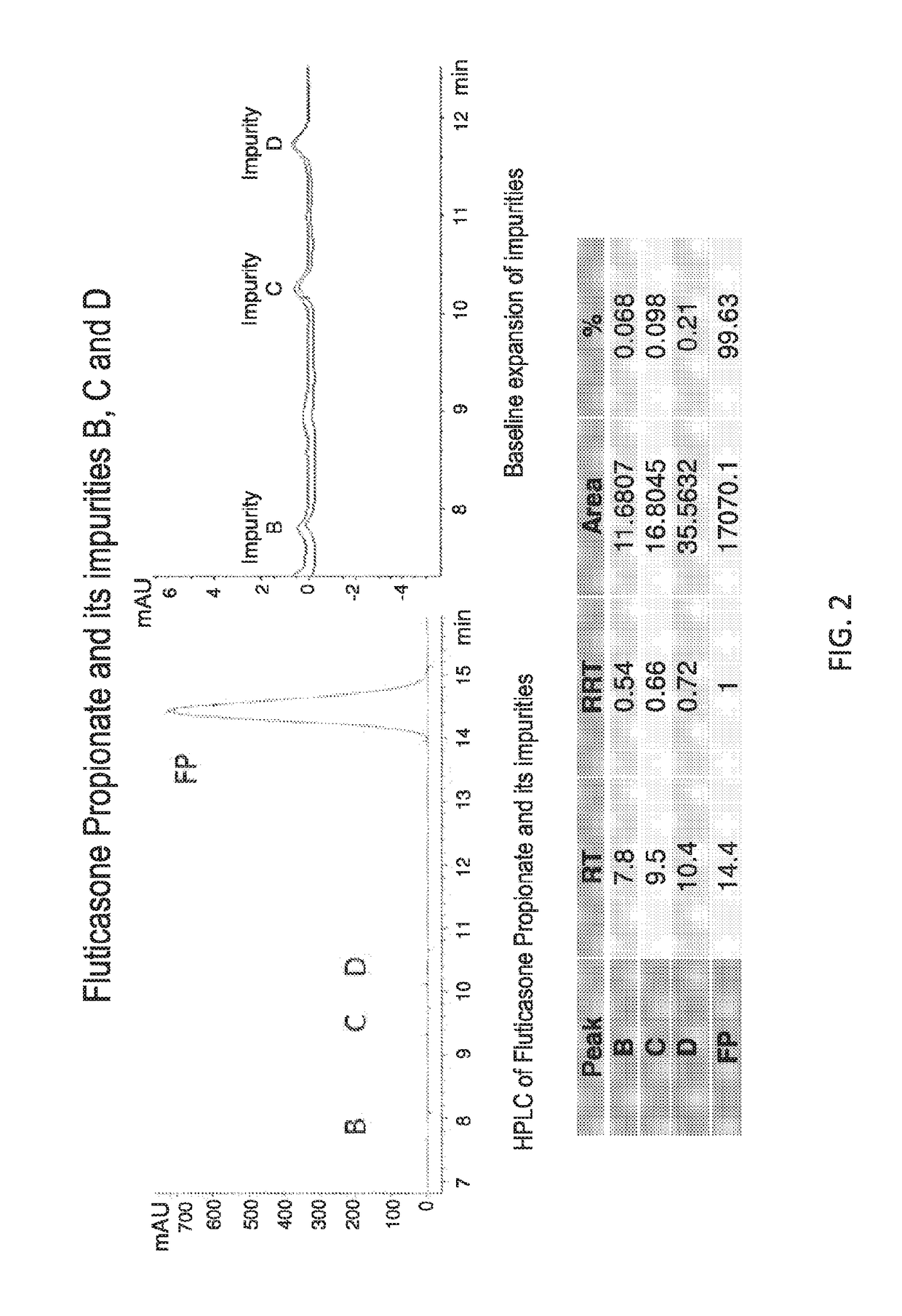
Patents
Literature
84 results about "Fluticasone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a variety of skin conditions (such as eczema, psoriasis, rash).
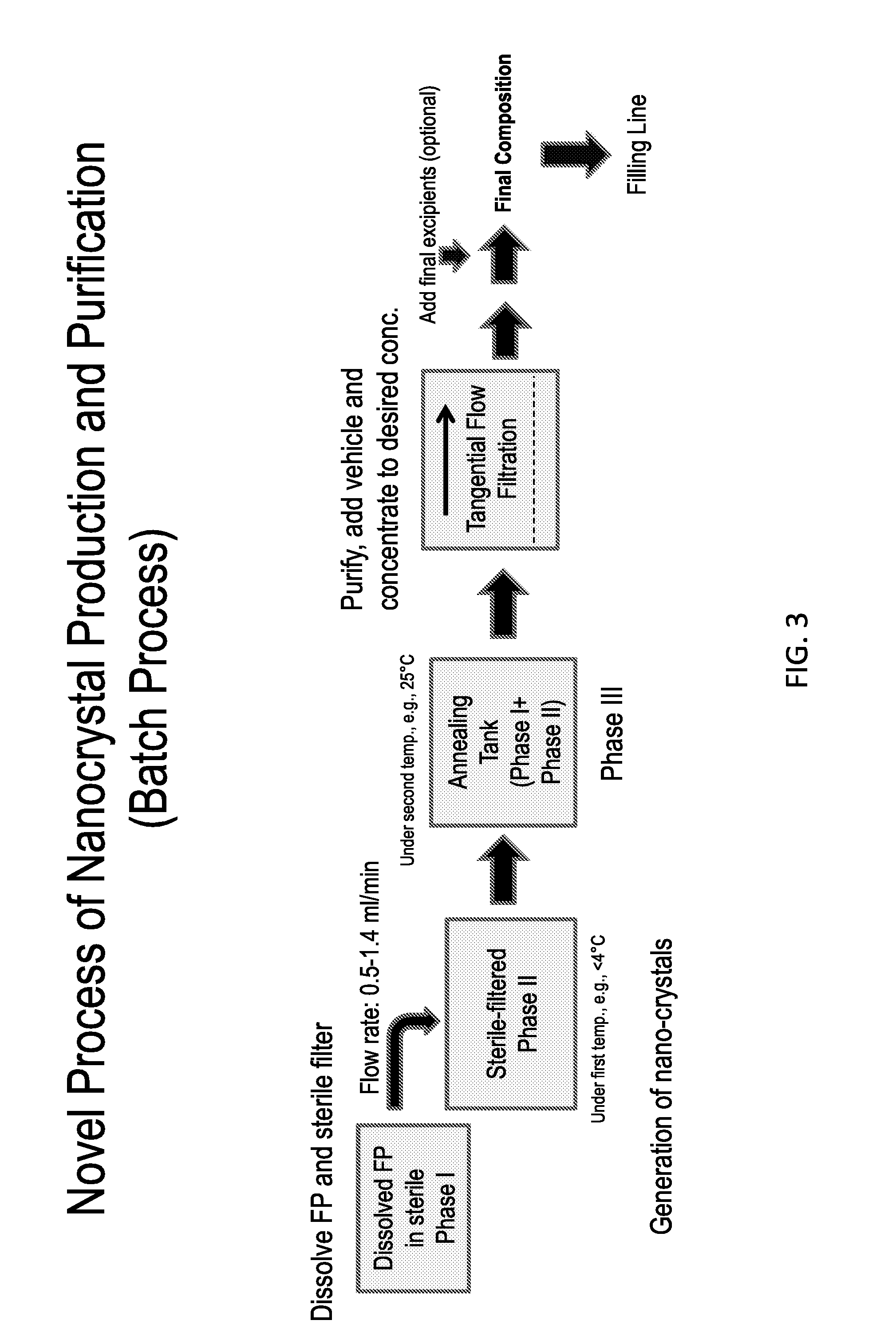
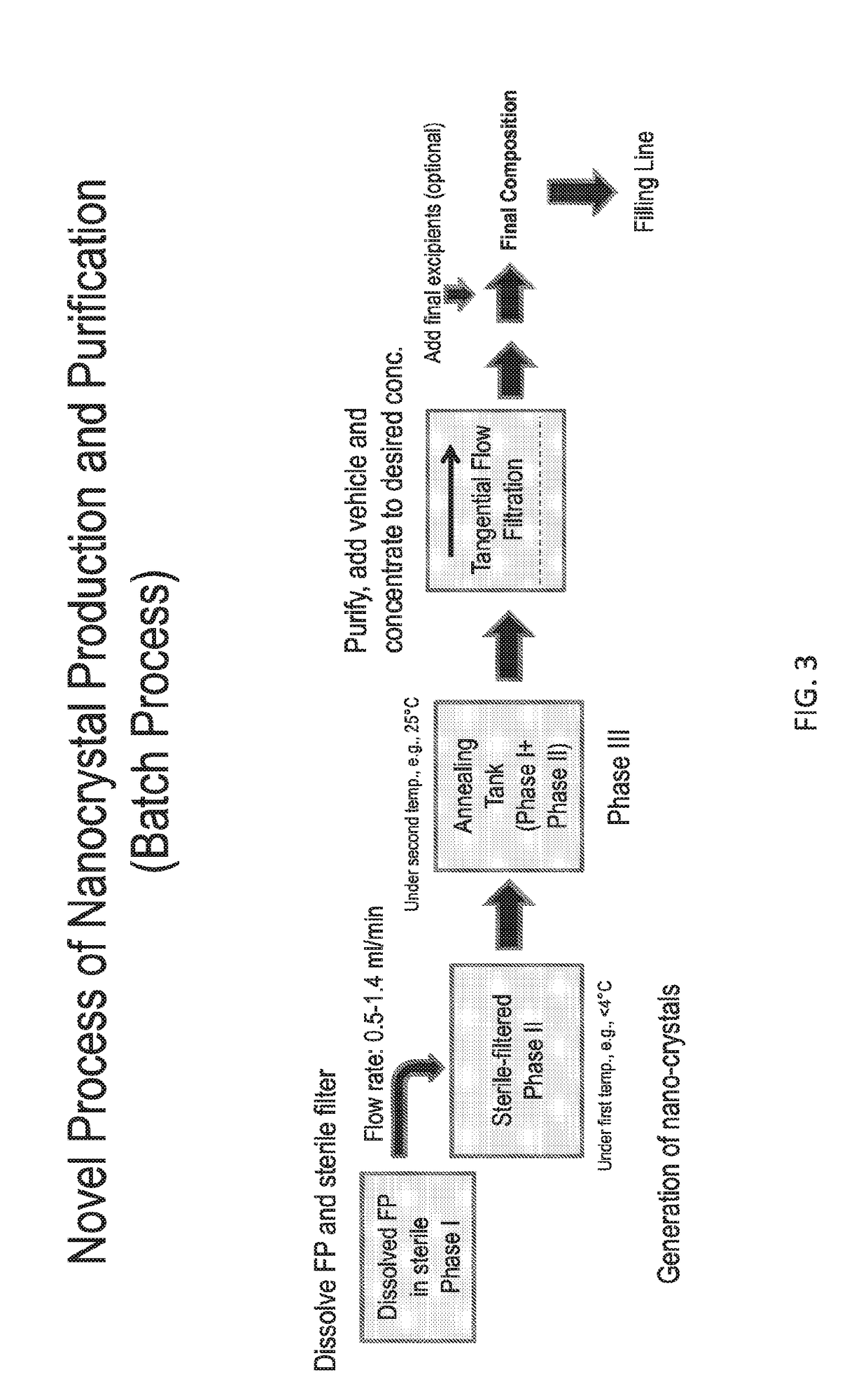
Preparations of Hydrophobic Therapeutic Agents, Methods of Manufacture and Use Thereof
ActiveUS20130303502A1Increase concentrationMaintaining osmolalityOrganic active ingredientsOrganic chemistry methodsDiseaseRespiratory disease
The present invention further provides method of preparing nanocrystals of a hydrophobic therapeutic agent such as fluticasone, pharmaceutical compositions (e.g., topical or intranasal compositions) thereof and methods for treating and / or preventing the signs and / or symptoms of disorders such as blepharitis, meibomian gland dysfunction or skin inflammation or a respiratory disease (e.g., asthma).
Owner:NICOX OPHTHALMICS
Ophthalmic formulations of fluticasone and methods of use
InactiveUS20110105450A1Relieve signRelieve symptomsSenses disorderInorganic non-active ingredientsOphthalmologyAllergic conjunctivitis
The present invention provides ophthalmic formulations of fluticasone that provide a comfortable formulation when instilled in the eye and is effective in the treatment of allergic conjunctivitis and / or allergic conjunctivitis. The invention further provides methods of treating allergic conjunctivitis and / or allergic conjunctivitis in a subject in need of such treatment by topical application of the fluticasone formulations of the invention directly to the eye.
Owner:ACIEX THERAPEUTICS INC
Lyophilized Cake Formulations
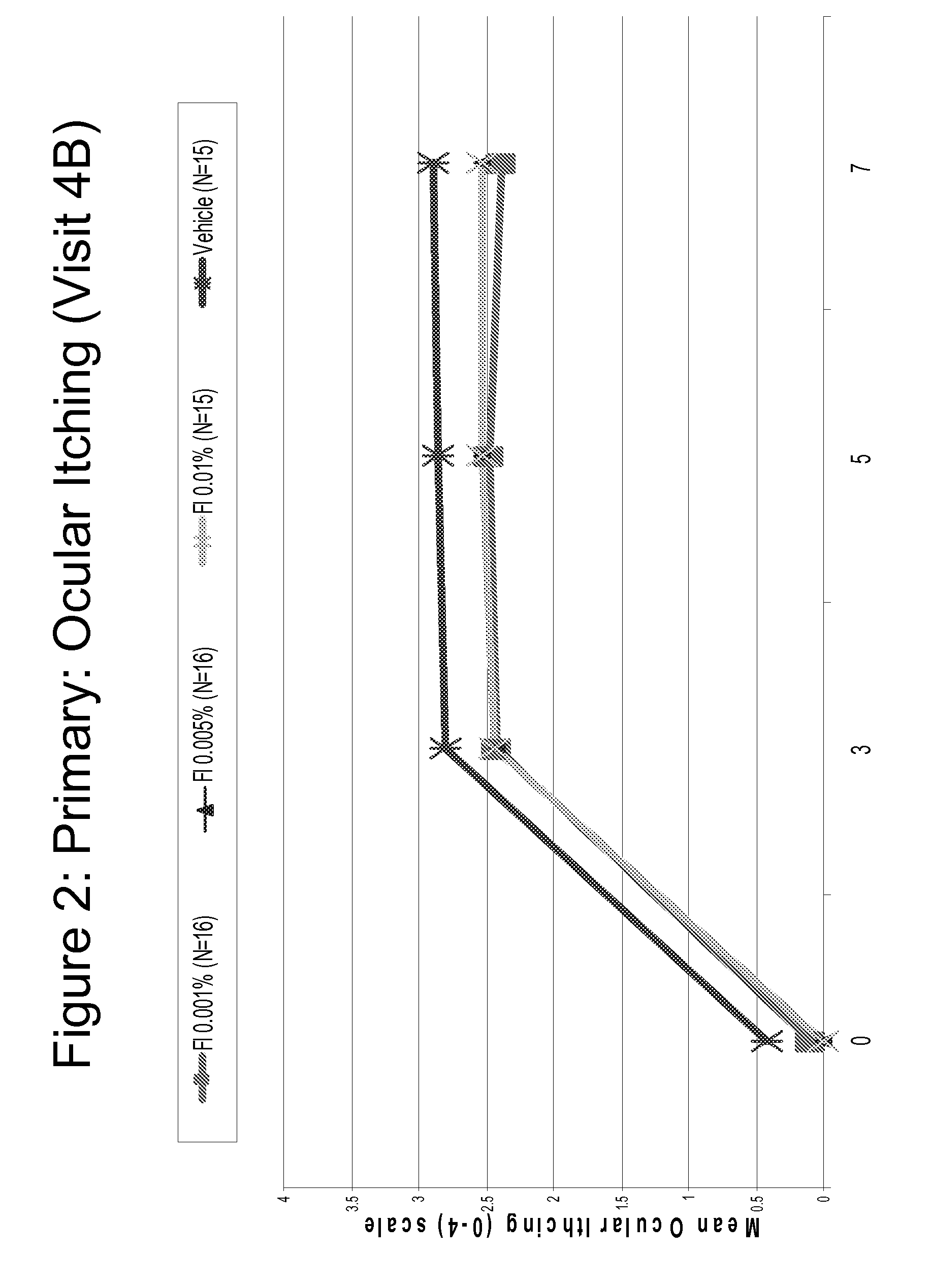
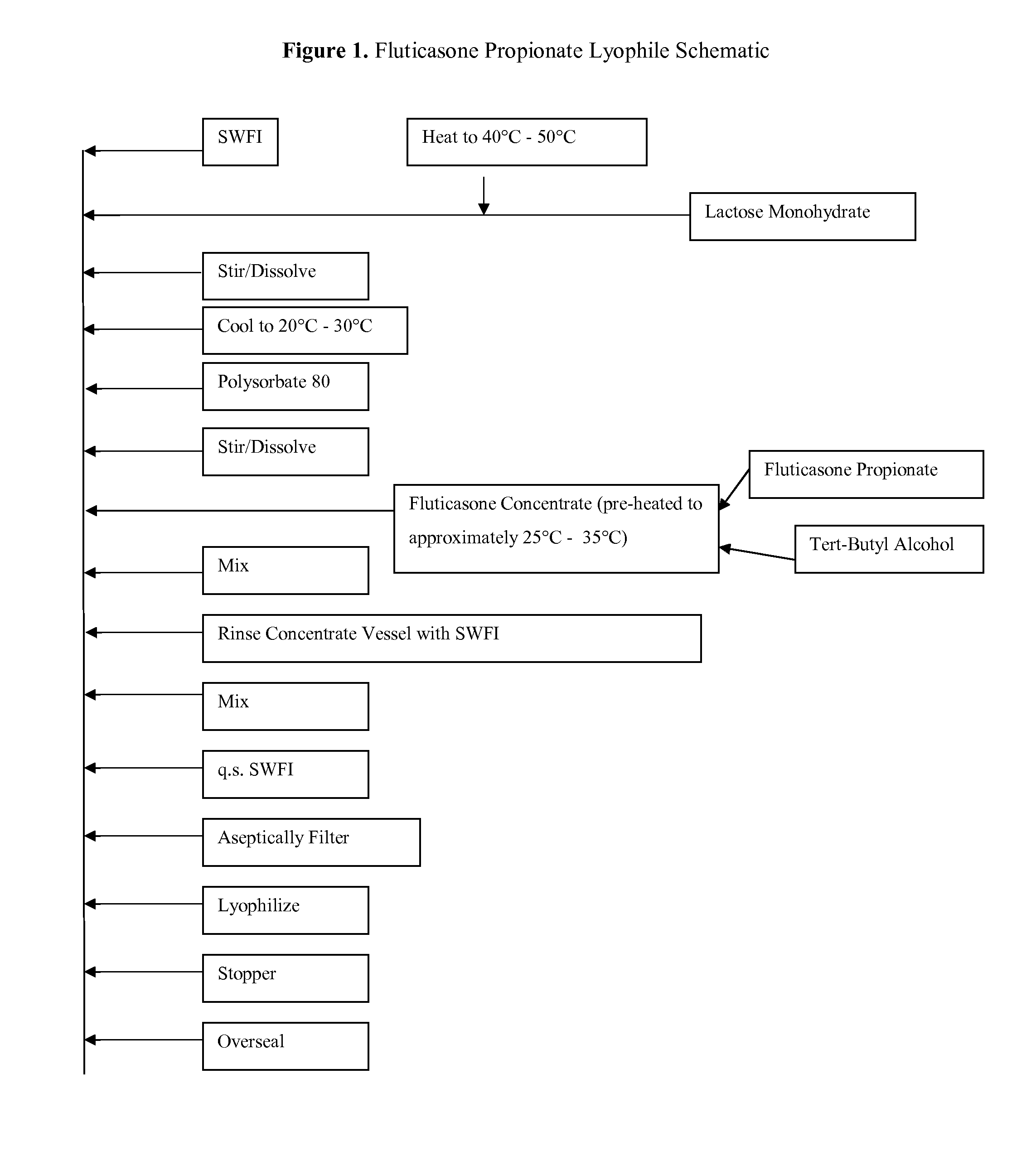
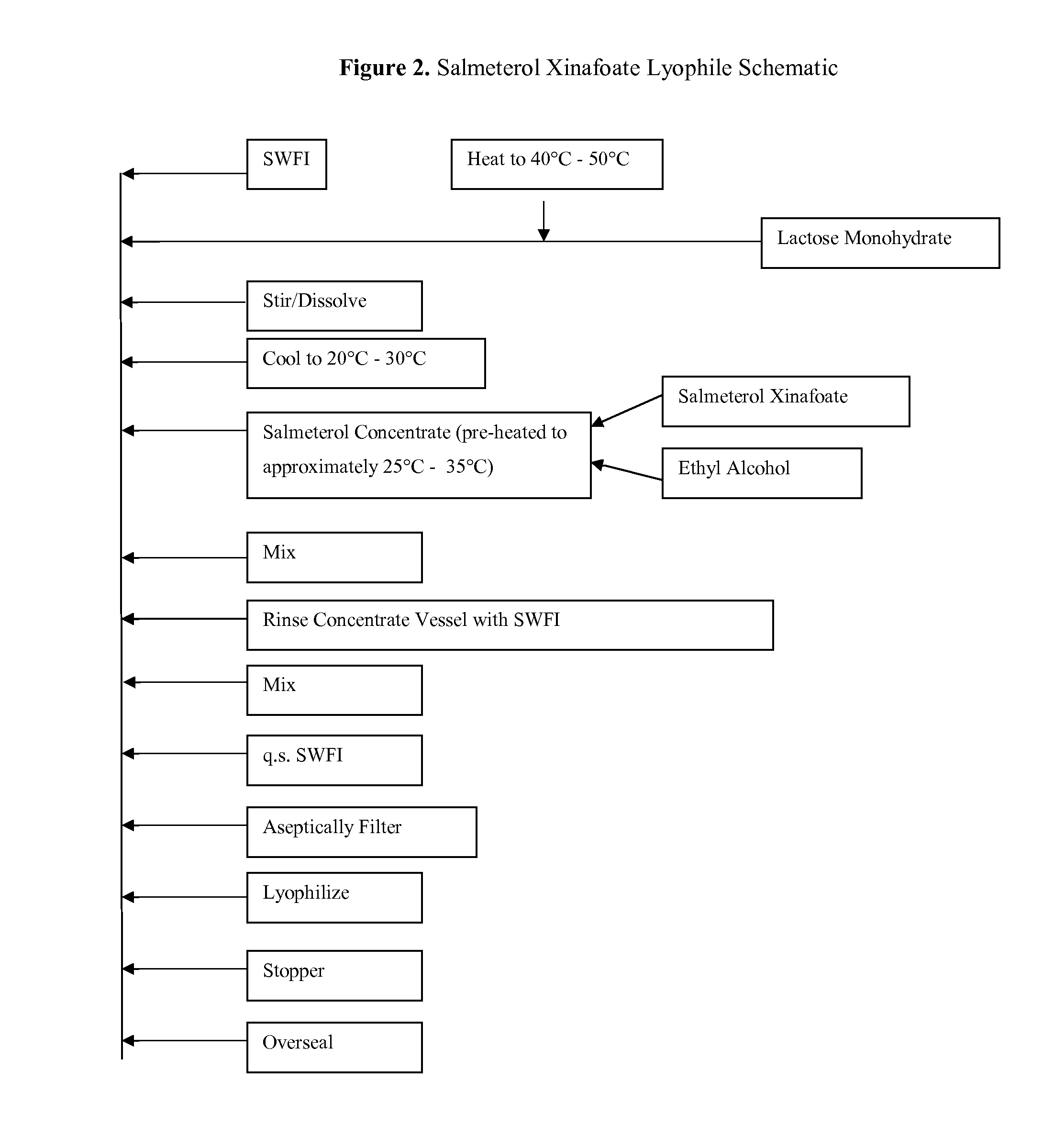
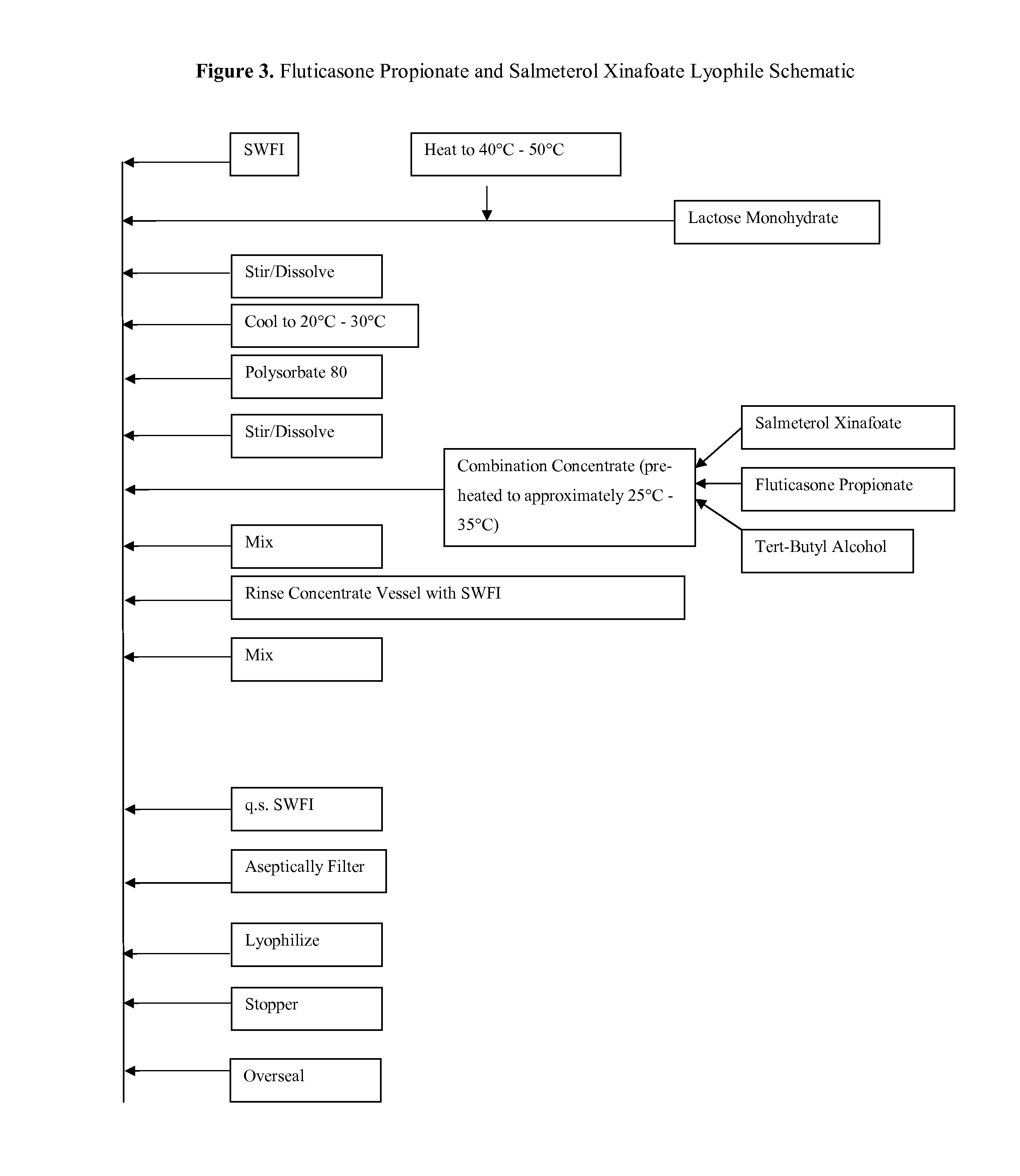
Provided herein are lyophilized cake forms of fluticasone, salmeterol, or a pharmaceutically acceptable salt or a combination thereof which provides room temperature stability for an extended period of time. Upon reconstitution with an acceptable solvent (e.g., a carrier or diluent), the reconstituted pharmaceutical or cosmetic formulation provides a sterile, non-suspension form suitable for parenteral injectable administration, including subcutaneous injection.
Owner:LITHERA
Dry powder inhaler
This invention provides a dry powder inhaler comprising: a dry powder medicament comprising fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of salmeterol per actuation is less than 50 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose. A method of treating a patient includes administering to a patient a dry powder medicament having fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of salmeterol per actuation is less than 50 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose.
Owner:TEVA BRANDED PHARMA PROD R & D
Nasal pharmaceutical formulations and methods of using the same
ActiveUS20040208830A1Improve bioavailabilityImprove efficacyPowder deliveryOrganic active ingredientsDrugFluticasone
Nasal pharmaceutical formulations comprising a drug substance having a specific particle size distribution profile are disclosed herein. Such profile provides increased bioavailability, increased efficacy or prolonged therapeutic effect of the drug substance when administered intranasally. The formulations of the present invention may comprise one or more corticosteroids having a specific particle size distribution profile. In a preferred embodiment, the corticosteroid is fluticasone or a pharmaceutically acceptable derivative thereof for the treatment of one or more symptoms of rhinitis. Preferably, the drug substance is fluticasone propionate. The formulations herein may be provided as an aqueous suspension suitable for inhalation via the intranasal route.
Owner:MYLAN SPECIALTY
Pharmaceutical formulation with enhanced solubility for the delivery of corticosteroids
InactiveUS20080081070A1Improve solubilityPrecision releaseOrganic active ingredientsBiocidePorositySolubility
Formulations have been developed to improve the solubility of corticosteroids such as fluticasone proprionate in a composition designed to achieve localized release of the drug in the small intestine and / or colon. In one embodiment, solid dispersions of fluticasone are prepared wherein the drug is blended with or coated onto a highly water soluble substrate such as nonpareil (sugar beads) then coated with a layer of polymer soluble in small intestinal fluid, then coated with an enteric coating. The inner polymer layer controls release of the drug, and the enteric coating, a pH sensitive polymer that is broken down in the ileum and colon, controls localized release of drug at various sites within the gastrointestinal tract. The multilayer pharmaceutical composition can be in the form of pellets, tablets compressed from pellets or pellets packed into capsules. The release profile of the drug can be manipulated by (1) altering size or shape (i.e., surface area) and solubility of the inert substrate; (2) the ratio of drug to polymer, the polymer composition and solubility, the porosity of the polymer; (3) the drug form (i.e., free base or salt, or which salt); and the thickness and / or surface area of the drug / polymer and / or enteric coating. In a preferred embodiment, the composition is administered orally. This may also be packaged to provide for an escalating or tapering dosage.
Owner:AURIGA LAB +1
Nasal pharmaceutical formulations and methods of using the same
InactiveUS20060051299A1Improve bioavailabilityGood curative effectPowder deliveryOrganic active ingredientsFluticasone propionateCurative effect
Nasal pharmaceutical formulations comprising a drug substance having a specific particle size distribution profile are disclosed herein. Such profile provides increased bioavailability, increased efficacy or prolonged therapeutic effect of the drug substance when administered intranasally. The formulations of the present invention may comprise one or more corticosteroids having a specific particle size distribution profile. In a preferred embodiment, the corticosteroid is fluticasone or a pharmaceutically acceptable derivative thereof for the treatment of one or more symptoms of rhinitis. Preferably, the drug substance is fluticasone propionate. The formulations herein may be provided as an aqueous suspension suitable for inhalation via the intranasal route.
Owner:MYLAN SPECIALTY
Fluticasone lotion having improved vasoconstrictor activity
InactiveUS7300669B2Cosmetic preparationsOrganic active ingredientsFluticasone propionateSafety profile
A fluticasone lotion having improved vasoconstrictor and anti-inflammatory activity and higher than expected potency. The fluticasone lotion contains 0.05 weight percent fluticasone propionate and an oil-in-water vehicle that includes excipients. The fluticasone lotion is unexpectedly efficacious while exhibiting an improved safety profile.
Owner:FOUGERA PHARM INC
Process for preparing fluticasone diproprionate superfine particles and product thereof
Owner:CHINA NAT ACAD NANOTECH & ENG
Synthesizing process of fluorine propionate ticasone
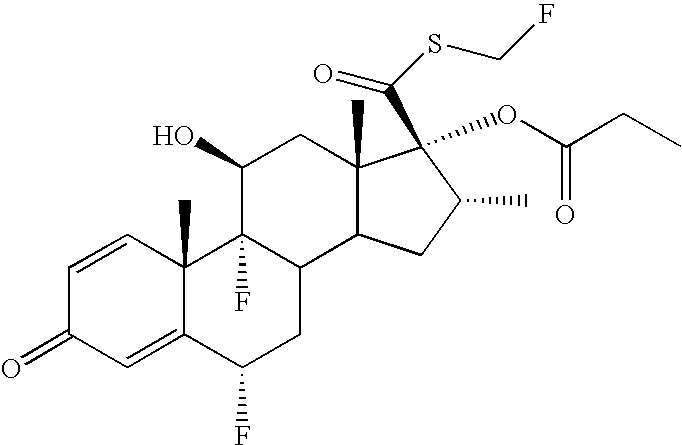
The present invention relates to new technological process of converting carboxylic acid into thiocarboxylic acid, and is especially synthesis process of fluticasone acetate. The present invention prepares fluticasone acetate with medicinal level flumethasone as initial material and through one of six technological paths, and has the advantages of short reaction path, high yield, simple reaction condition, high product purity, low cost, etc.
Owner:江苏新海康制药有限公司
Inhalation Drug Combinations
InactiveUS20070122351A1Less systemic exposureEliminate side effectsPowder deliveryBiocideDiseaseFluticasone propionate
A method for treating respiratory disorders by administrating by inhalation an effective amount of a β2-receptor agonist, an acceptable amount of a corticosteriod, and HFA 134a, to a patient in need thereof, is disclosed. Preferably, the β2-receptor agonist is salmeterol or a physiologically acceptable salt thereof, and the corticosteriod is fluticasone propionate or a solvate thereof. The combination of salmeterol, fluticasone proprionate, and HFA 134a may lower the risk of cardiac arrhythmias, sudden death, or hypercorticism that are sometimes associated with the simultaneous administration of a β2-receptor agonist and an anti-inflammatory corticosteroid.
Owner:GLAXO GROUP LTD
Transdermal formulations of fluticasone
InactiveUS20160081915A1Improve efficiencyAntibacterial agentsOrganic active ingredientsIonic strengthGlucocorticoid
The present invention generally relates to the transdermal delivery of fluticasone and other glucocorticoids. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment, for example, a high ionic strength environment. One set of embodiments provides a composition for transdermal delivery comprising fluticasone and / or a salt thereof, and optionally, a nitric oxide donor. Other glucocorticoids can also be used in some cases. Such compositions may be used to facilitate the delivery of effective amounts of fluticasone or other glucocorticoids systemically or to deeper tissues, rather than only locally or superficially, and in some cases, the effects of fluticasone or other glucocorticoids may be prolonged for unexpectedly long periods of time, e.g., days or weeks.
Owner:STRATEGIC SCI & TECH
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
ActiveUS20150337006A1Modifies release timeExtend posting timeOrganic active ingredientsSynthetic resin layered productsDiseaseRespiratory disease
The present invention further provides method of preparing nanocrystals or microcrystals of a hydrophobic therapeutic agent such as fluticasone or triamcinolone, pharmaceutical compositions (e.g., topical or intranasal compositions) thereof and methods for treating and / or preventing the signs and / or symptoms of disorders such as blepharitis, meibomian gland dysfunction or skin inflammation or a respiratory disease (e.g., asthma).
Owner:NICOX OPHTHALMICS
Dry powder inhalation medicine composition and preparation method thereof
InactiveCN105982880AAvoid stimulationImproving In Vitro Assay ParametersPowder deliveryPharmaceutical non-active ingredientsIndacaterolAdditive ingredient
The invention provides a dry powder inhalation medicine composition and a preparation method thereof. The composition is prepared from a coating agent with a specific particle size characteristic, lactose monohydrate with a specific particle size characteristic for a carrier and a micronized medicinal active ingredient, wherein the coating agent is an inhaling magnesium stearate or a mixture of the inhaling magnesium stearate and micronized lactose monohydrate; and the medicinal active ingredient is selected from at least one of glycopyrronium bromide, umeclidinium, indacaterol, formoterol, vilanterol, fluticasone and pharmaceutically available salt of the active ingredients. The preparation method comprises the following steps of sufficiently mixing and coating the coating agent and the lactose monohydrate, and uniformly mixing with the micronized medicinal active ingredients.
Owner:SICHUAN HAISCO PHARMA CO LTD
Veramyst medicinal preparation and preparation method
InactiveCN101474192AImprove efficiencyGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismMedicineDosage form
The invention provides a single preparation and compound preparation using furoic acid fluticasone as main constituent. The weight ratio of furoic acid fluticasone in pharmaceuticals is 0.0001-50%. The pharmaceuticals can be made into jellies, supensoid agent, liquor and pulvis, and the like, which are administrated in a percutaneous way. The preparation is suitable for anaphylactic rhinitis, asthma and stubborn skin disease. The invention has the advantages of high healing rate, high safety and high promotional value.
Owner:崔晓廷
Inhalation Drug Combinations
InactiveUS20070122352A1Good curative effectReduce systemic side effectsHalogenated hydrocarbon active ingredientsBiocideDiseaseFluticasone propionate
A method for treating respiratory disorders by administrating by inhalation an effective amount of a β2-receptor agonist, an acceptable amount of a corticosteroid, and HFA 134a, to a patient in need thereof, is disclosed. Preferably, the β2-receptor agonist is salmeterol or a physiologically acceptable salt thereof, and the corticosteroid is fluticasone propionate or a solvate thereor. The combination of salmeterol, fluticasone proprionate, and HFA 134a may lower the risk of cardiac arrhythmias, sudden death, or hypercorticism that are sometimes associated with the simultaneous administration of a β2-receptor agonist and an anti-inflammatory corticosteroid.
Owner:GLAXO GROUP LTD
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
ActiveUS20150126483A1Maintaining osmolalityMaintain purityOrganic active ingredientsOrganic chemistry methodsDiseaseRespiratory disease
Owner:NICOX OPHTHALMICS
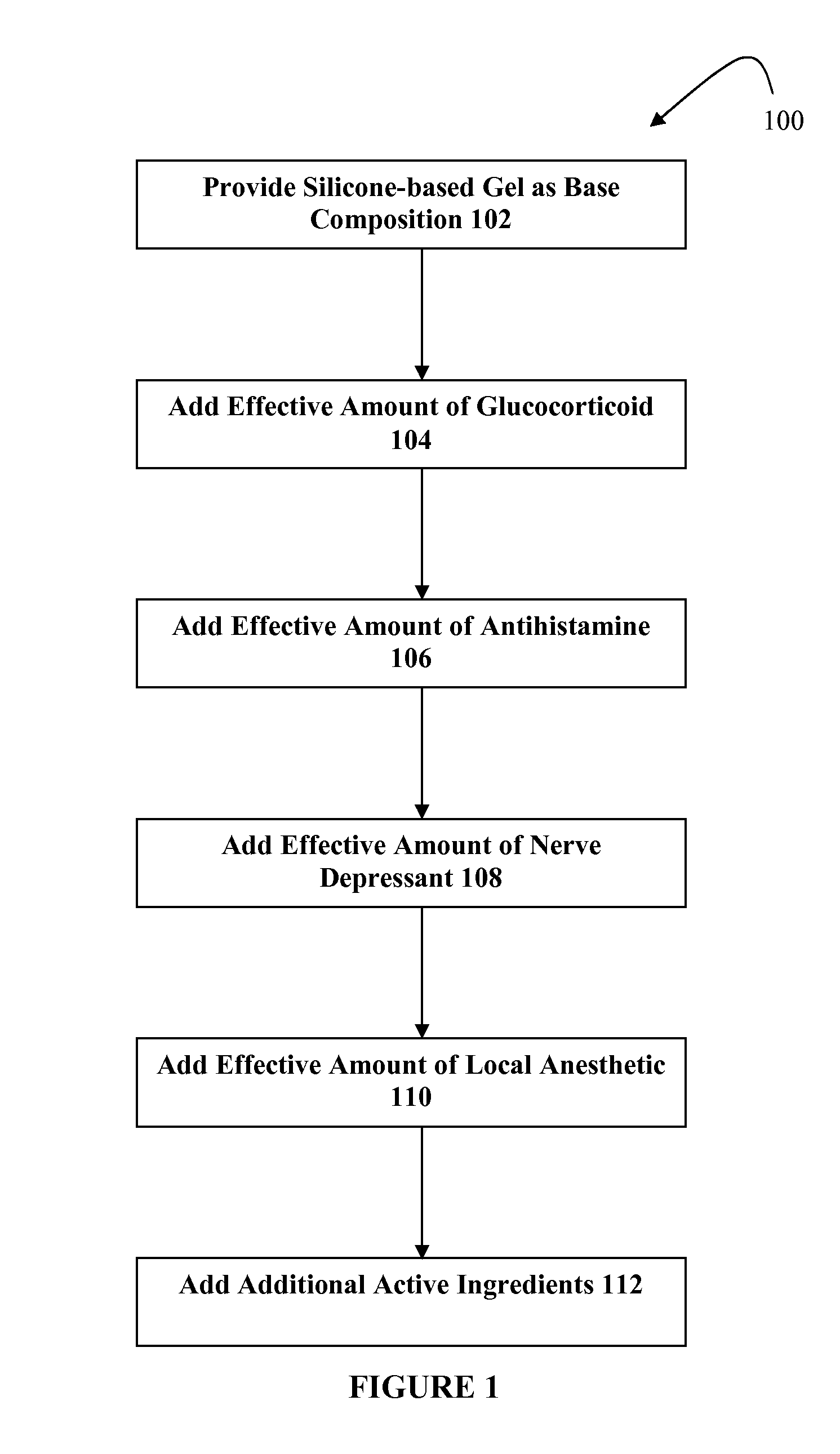
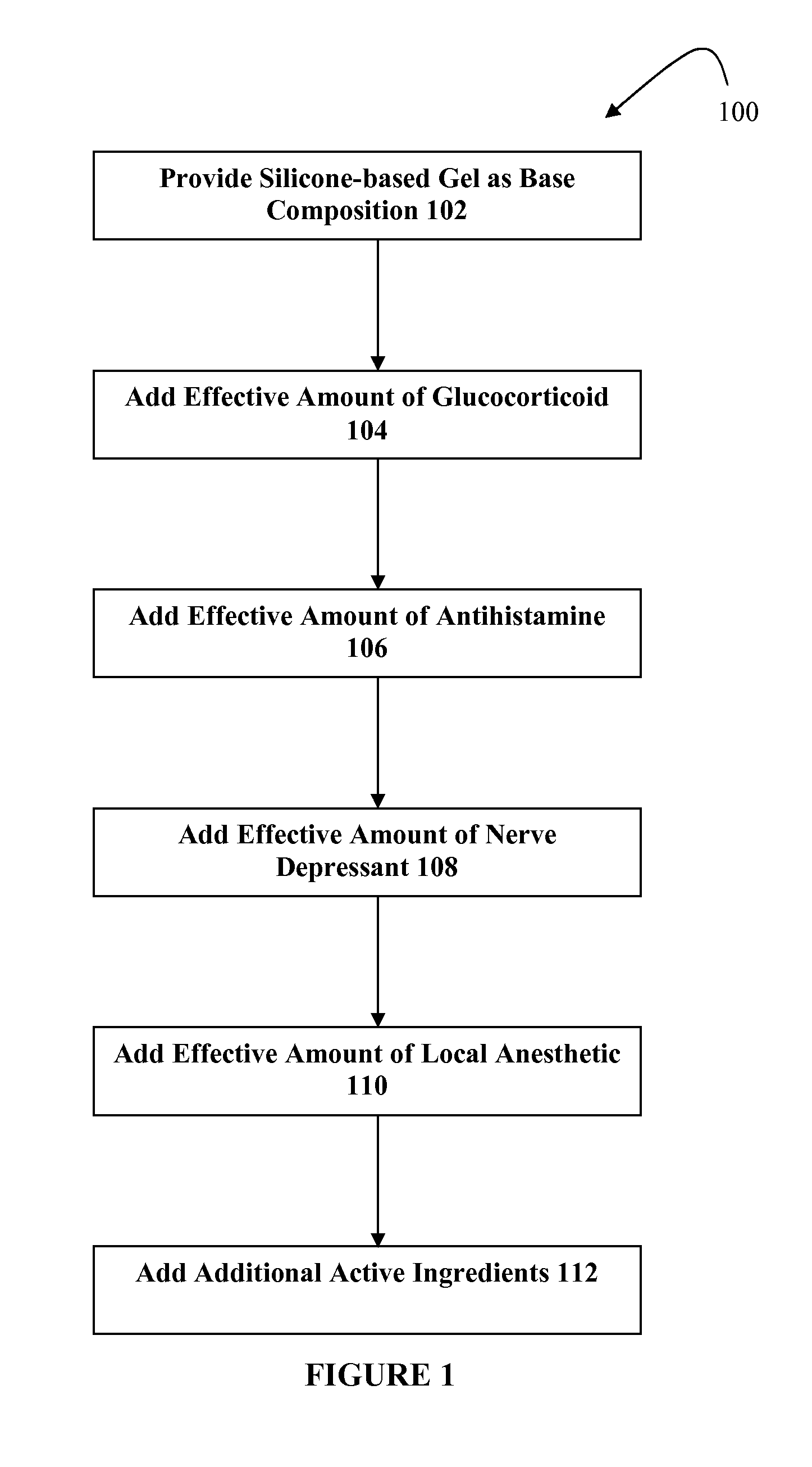
Silicone-based composition for skin treatment
The present embodiments may relate to topically delivered compounded medications for the treatment of scar tissue, skin disorders, and / or other ailments. In one aspect, a transdermal cream or gel may provide for the effective administration of multiple medications simultaneously. Preferably, a silicone-based gel may be provided as a base composition and may have a non-zero percentage of silicone or silicone variant. The silicone-based gel may comprise cyclopentasiloxane, polysilicone-11, dimethicone, and C30-45 alkyl cetearyl dimethicone crosspolymer, and include several active ingredients, such as glucocorticoids, antihistamines, and nerve depressants. The silicone-based gel may include a combination of fluticasone, loratadine, and gabapentin. The concentrations of fluticasone and loratadine may be relatively low, while that of gabapentin moderately high. The silicone-based gel may also have one or more local anesthetics, such as prilocaine and / or lidocaine. The silicone-based gel may include additional active ingredients, such as NSAIDs, anticonvulsants, antidepressants, muscle relaxants, and / or other active ingredients.
Owner:CMPD LICENSING
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
ActiveUS8765725B2Maintaining osmolalityMaintain purityBiocideOrganic chemistry methodsDiseaseNanocrystal
The present invention further provides method of preparing nanocrystals of a hydrophobic therapeutic agent such as fluticasone, pharmaceutical compositions (e.g., topical or intranasal compositions) thereof and methods for treating and / or preventing the signs and / or symptoms of disorders such as blepharitis, meibomian gland dysfunction or skin inflammation or a respiratory disease (e.g., asthma).
Owner:NICOX OPHTHALMICS
Dry powder inhaler
This invention provides a dry powder inhaler comprising: a dry powder medicament comprising fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of fluticasone propionate per actuation is less than 100 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose. A method of treating a patient includes administering to a patient a dry powder medicament having fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of fluticasone propionate per actuation is less than 100 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose.
Owner:TEVA BRANDED PHARMA PROD R & D
Pharmaceutical composition preparation
InactiveCN111467498AReduce exacerbationsImprove lung functionRespiratory disorderHeterocyclic compound active ingredientsAnticholinergic DrugsGlucocorticoid
The invention provides a pharmaceutical composition preparation. The pharmaceutical composition preparation comprises an anticholinergic drug tiotropium or a hydrate thereof, a beta 2-receptor agonistarformoterol or a salt thereof, and an inhaled glucocorticoid fluticasone or an ester derivative thereof; tiotropium bromide acts on a muscarinic receptor on bronchial smooth muscles, the cholinergiceffect of acetylcholine released by the tail ends of parasympathetic nerves can be inhibited, and muscular tension is blocked; the arformoterol acts on a beta 2-receptor on an airway smooth muscle cell membrane, so that the release of degranulation and media of mast cells and basophils is reduced, the permeability of capillaries is reduced, and swinging of airway epithelial cilia is increased; the fluticasone is an effective anti-inflammatory drug, and when the three drugs are combined for use, the three mechanisms jointly exert the bronchus relaxing effect and the anti-inflammatory effect; the application range of the triple therapy is wider on the important clinical indexes of reducing acute exacerbation of patients suffering from chronic obstructive pulmonary disease, reducing total-cause mortality rate, improving the lung function of the patients suffering from chronic obstructive pulmonary disease, improving the living quality and the like.
Owner:王兆霖
Fluticasone furoate in the treatment of COPD
ActiveUS20170189424A1Reduce probabilityOrganic active ingredientsPowder deliveryFluticasone propionateEosinophil
The present invention relates to pharmaceutical products comprising fluticasone furoate for use in the treatment of COPD patients, particularly a subgroup of COPD patients that through analysis have been identified as possessing an eosinophil blood count of≧150 cells / W. The present invention is further directed to methods for treating a patient with COPD which methods include identifying a patient that will respond to treatment and administering a pharmaceutical product of the present invention comprising fluticasone furoate to said patient.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Combination therapy comprising a thiazole and a corticosteroid to treat skin conditions
InactiveUS20190255023A1Preferable effectReduce inflammation and itchinessOrganic active ingredientsAerosol deliveryFluocinoloneFluocinonide
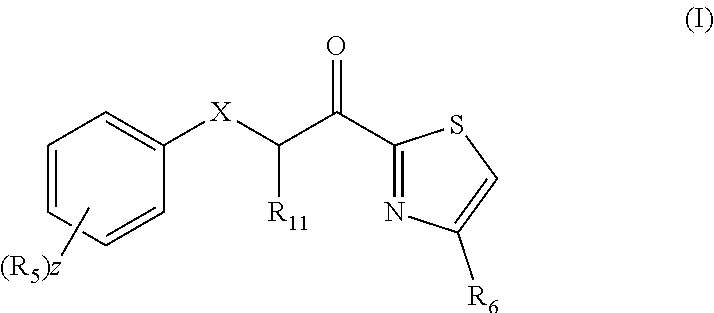
A pharmaceutical composition comprising: (A) at least one compound of formula (I): wherein X is O or S, preferably O R6 is H, C1-6alkyl, —(CH2)pCOOH, —(CH2)pCOOC1-6alkyl, —(CH2)pCONH2, —(CH2)pCONHC1-6alkyl, —(CH2)pCON(C1-6alkyl)2, R11 is H or C1-6 alkyl; each R5 is —OC1-10alkyl, —SC1-10alkyl, —C1-12alkyl, or OAr2; wherein Ar2 is phenyl, optionally substituted with one or more halo; each p is 0 to 3; each z is 1 to 2; or a pharmaceutically acceptable salt, or a hydrate or solvate thereof; and (B) one or more corticosteroid partners, preferably selected from the group consisting of betamethasone, clobetasol, halometasone, dexamethasone, fluocortolone, desoximetasone, diflorasone, fluocinonide, flurandrenolide, halobetasol, amcinonide, halocinonide, triamcinolone, hydrocortisone, aclometasone, fluticasone, mometasone, clocortolone, fluocinolone, desonide, prednisone, prednisolone, and prednicarbate or a pharmaceutically acceptable salt, or a hydrate or solvate thereof, especially betamethasone or a pharmaceutically acceptable salt, or a hydrate or solvate thereof.
Owner:AVEXXIN
Separate type water suspension medicament of fluticasone propionate containing auxiliary materials for treating skin disease
InactiveCN102526066AReduce wasteEasy to useOrganic active ingredientsSolution deliveryDiseaseFluticasone propionate
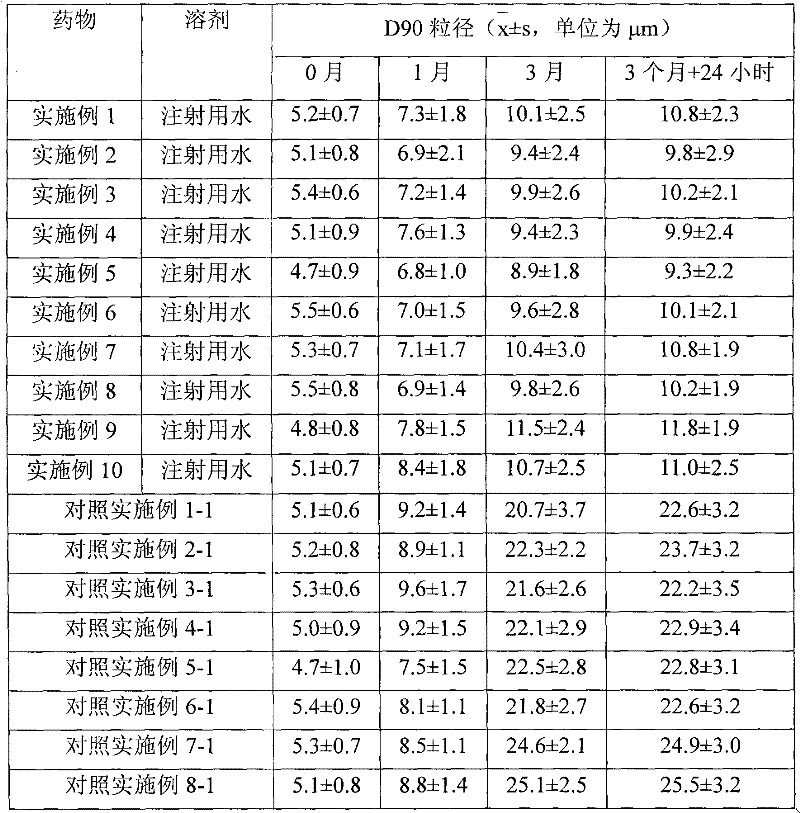
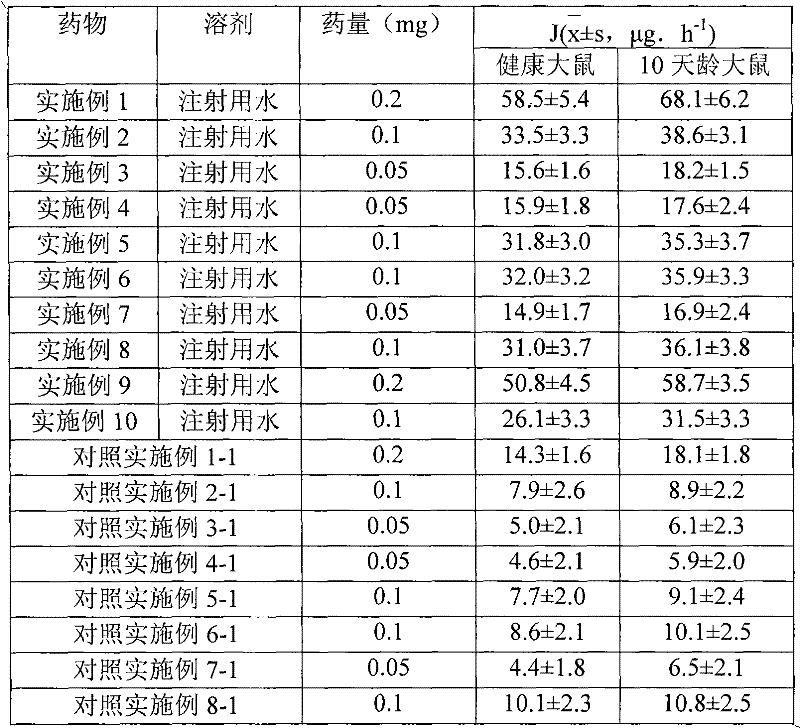
The invention relates to a separate type water suspension medicament of fluticasone propionate containing auxiliary materials for treating skin disease, composed of separately packed fluticasone propionate and separately packed water, wherein the fluticasone propionate contains one or more kinds of pharmaceutical auxiliary materials for skin, is insoluble in water and has a D90 particle size of 0.1-10mu m.
Owner:TIANJIN JINYAO GRP
Inhalation medicinal composition prepared from eplerenone and glucocorticoid serving as active ingredients
ActiveCN102370984AInhibit compensatoryLittle side effectsOrganic active ingredientsRespiratory disorderFlunisolideGlucocorticoid
The invention discloses an inhalation medicinal composition prepared from eplerenone and glucocorticoid serving as active ingredients. The inhalation medicinal composition consists of eplerenone, glucocorticoid and one or more carriers suitable for inhalation administration. The glucocorticoid is one or more of ciclesonide, desonide, fluticasone propopnate, mometasone furoate, beclomethasone dipropionate, flunisolide, and triamcinolone acetonide and medicinal salts or esters thereof; the inhalation medicinal composition is preferably prepared into aerosol powder which consists of eplerenone, glucocorticoid and carrier micro powder; the average grain diameter of the eplerenone and glucocorticoid micro powder is 0.5 to 1.0 mu m; and the average grain diameter of the carrier micro powder is 20 to 45 mu m.
Owner:TIANJIN JINYAO GRP
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
ActiveUS20180022775A1Maintaining osmolalityMaintain purityOrganic active ingredientsSenses disorderDiseaseRespiratory disease
The present invention further provides method of preparing nanocrystals of a hydrophobic therapeutic agent such as fluticasone or triamcinolone, pharmaceutical compositions (e.g., topical or intranasal compositions) thereof and methods for treating and / or preventing the signs and / or symptoms of disorders such as blepharitis, meibomian gland dysfunction or skin inflammation or a respiratory disease (e.g., asthma).
Owner:NICOX OPHTHALMICS
Fluticasone furoate liposome suspension and preparation method thereof
ActiveCN111789816AHigh encapsulation efficiencySimple production processOrganic active ingredientsDispersion deliverySterolPhospholipid
The invention provides a fluticasone furoate liposome and a preparation method thereof. The fluticasone furoate liposome comprises the components of phospholipid, sterol and fluticasone furoate, wherein the fluticasone furoate accounts for 0.01%-1%, the phospholipid accounts for 0.065%-0.196%, and the sterol accounts for 0.035%-0.104%. A preparation method of the fluticasone furoate liposome comprises the steps of (1) performing mixing and injecting; and (2) performing filtering and concentrating. The method is simple to operate, stable in technology and suitable for mass production.
Owner:SHANGHAI ANOVENT PHARMA CO LTD
Silicone-based composition for skin treatment
The present embodiments may relate to topically delivered compounded medications for the treatment of scar tissue, skin disorders, and / or other ailments. In one aspect, a transdermal cream or gel may provide for the effective administration of multiple medications simultaneously. Preferably, a silicone-based gel may be provided as a base composition and may have a non-zero percentage of silicone or silicone variant. The silicone-based gel may comprise cyclopentasiloxane, polysilicone-11, dimethicone, and C30-45 alkyl cetearyl dimethicone crosspolymer, and include several active ingredients, such as glucocorticoids, antihistamines, and nerve depressants. The silicone-based gel may include a combination of fluticasone, loratadine, and gabapentin. The concentrations of fluticasone and loratadine may be relatively low, while that of gabapentin moderately high. The silicone-based gel may also have one or more local anesthetics, such as prilocaine and / or lidocaine. The silicone-based gel may include additional active ingredients, such as NSAIDs, anticonvulsants, antidepressants, muscle relaxants, and / or other active ingredients.
Owner:CMPD LICENSING
Pharmaceutical Compositions Comprising Ebastine and Fluticasone
A pharmaceutical composition comprises at least one antihistamine, at least one corticosteroid, and at least one pharmaceutical excipient, wherein the at least one antihistamine comprises ebastine or its pharmaceutically acceptable salt, solvate, ester or physiologically functional derivative thereof, and wherein the at least one corticosteroid comprises fluticasone or its pharmaceutically acceptable ester thereof.
Owner:CIPLA LTD
Dry powder inhaler
This invention provides a dry powder inhaler comprising: a dry powder medicament comprising fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of fluticasone propionate per actuation is less than 100 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose. A method of treating a patient includes administering to a patient a dry powder medicament having fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of fluticasone propionate per actuation is less than 100 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose.
Owner:TEVA BRANDED PHARMA PROD R & D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com