Dry powder inhalation medicine composition and preparation method thereof
A technology of dry powder inhalation and composition, which is applied in the field of medicine, can solve the problems of alveolar irritation and side effects, achieve strong hygroscopicity, increase the proportion of fine particles, and avoid stimulation and other side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
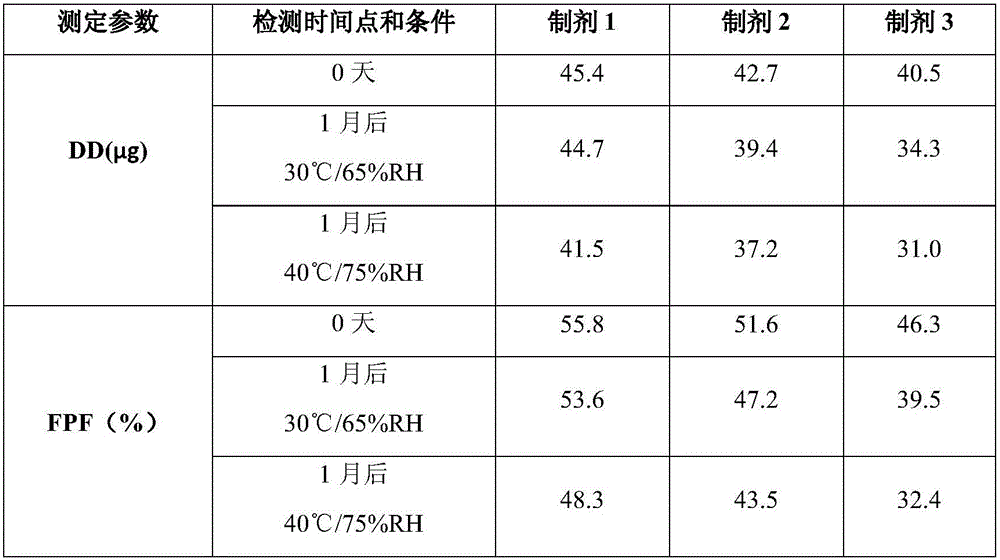
[0024] 1. Mix 0.5 g of magnesium stearate for inhalation with 249 g of lactose monohydrate as a carrier in a three-dimensional mixer at a speed of 150 rpm for 4 hours, and 0.5 g of micronized glycopyrronium bromide (equivalent to 0.63 glycopyrronium bromide) g) adding to the above powder, using three-dimensional mixing to continue mixing for 2 hours to obtain inhalable dry powder 1;
[0025] 2. Add 0.5g of magnesium stearate for inhalation, 249g of lactose monohydrate for carrier, and 0.5g of micronized glycopyrronium bromide (equivalent to 0.63g of glycopyrronium bromide) into a three-dimensional mixer and mix at a speed of 150 rpm After 6 hours, the inhalable dry powder 2 was obtained;
[0026] 3. Add 249 g of lactose monohydrate as carrier and 0.5 g of micronized glycopyrronium bromide (equivalent to 0.63 g of glycopyrronium bromide) into a three-dimensional mixer and mix for 6 hours at a speed of 150 rpm to obtain inhalable dry powder 3 .
[0027] Use the above dry powde...
Embodiment 2
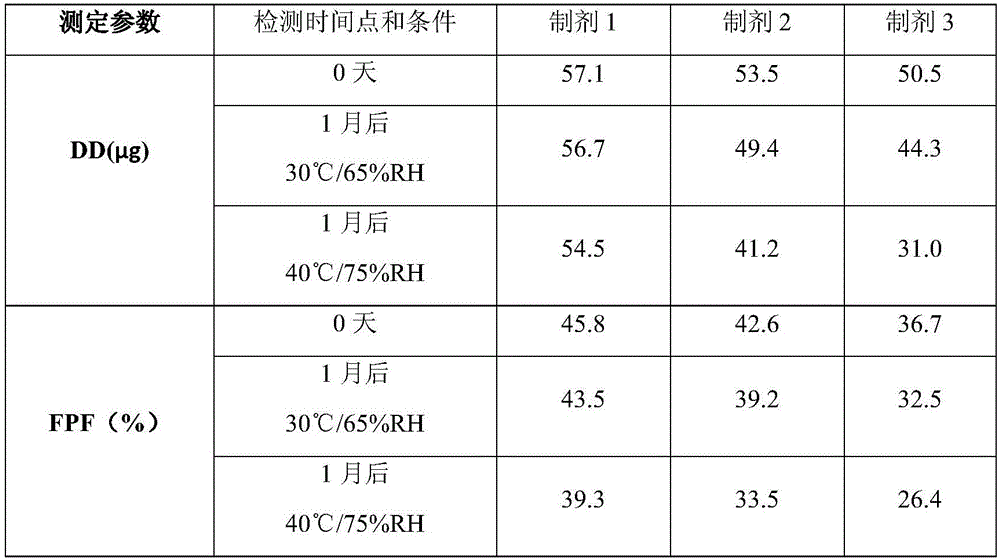
[0035] 1. 1.5 g of magnesium stearate for inhalation and 248 g of lactose monohydrate as carriers were mixed in a three-dimensional mixer for 4 hours at a speed of 150 rpm, and 0.625 g of micronized umeclidinium (equivalent to 0.75 umeclidinium bromide) g) adding to the above powder, using three-dimensional mixing to continue mixing for 2 hours to obtain inhalable dry powder 1;
[0036] 2. Add 1.5g of magnesium stearate for inhalation, 248g of lactose monohydrate for carrier, and 0.625g of micronized umeclidinium (equivalent to 0.75g of umeclidinium bromide) into a three-dimensional mixer and mix at a speed of 150 rpm After 6 hours, the inhalable dry powder 2 was obtained;
[0037] 3. Add 249 g of lactose monohydrate and 0.625 g of micronized umeclidinium bromide (equivalent to 0.75 g of umeclidinium bromide) into a three-dimensional mixer and mix for 6 hours at a speed of 150 rpm to obtain inhalable dry powder 3. .
[0038] Use the above dry powder for the preparation of fo...
Embodiment 3
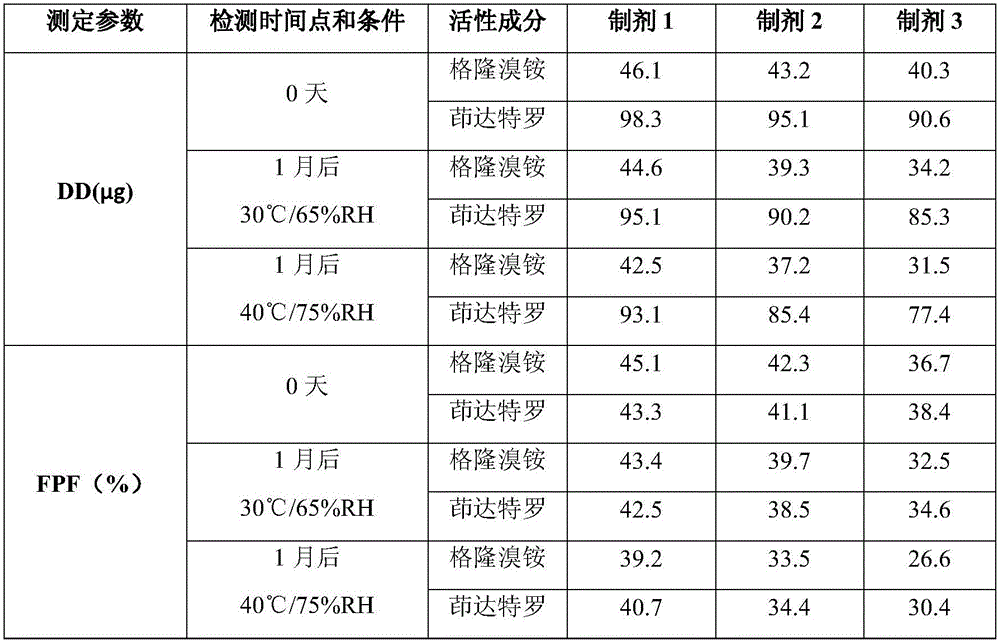
[0046] 1. Mix 3.0 g of magnesium stearate for inhalation, 24.5 g of micronized lactose monohydrate and 220.5 g of lactose monohydrate for carrier in a three-dimensional mixer at a speed of 150 rpm for 4 hours, and mix 0.5 g of micronized glycopyrronium (equivalent to 0.63g of glycopyrronium bromide), 1.10g of micronized indacaterol (equivalent to 1.43g of indacaterol maleate) were added to the above powder, and three-dimensional mixing was used to continue mixing for 2 hours to obtain inhalable dry powder 1;
[0047] 2. 3.0 g of magnesium stearate for inhalation, 245 g of lactose monohydrate for carrier, 0.5 g of micronized glycopyrronium bromide (equivalent to 0.63 g of glycopyrronium bromide), and 1.10 g of micronized indacaterol (equivalent to Indacaterol maleate 1.43g) was added together in a three-dimensional mixer and mixed for 6 hours at a speed of 150 rpm to obtain inhalable dry powder 2;
[0048] 3. The carrier is 248g of lactose monohydrate, 0.5g of micronized glyco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com