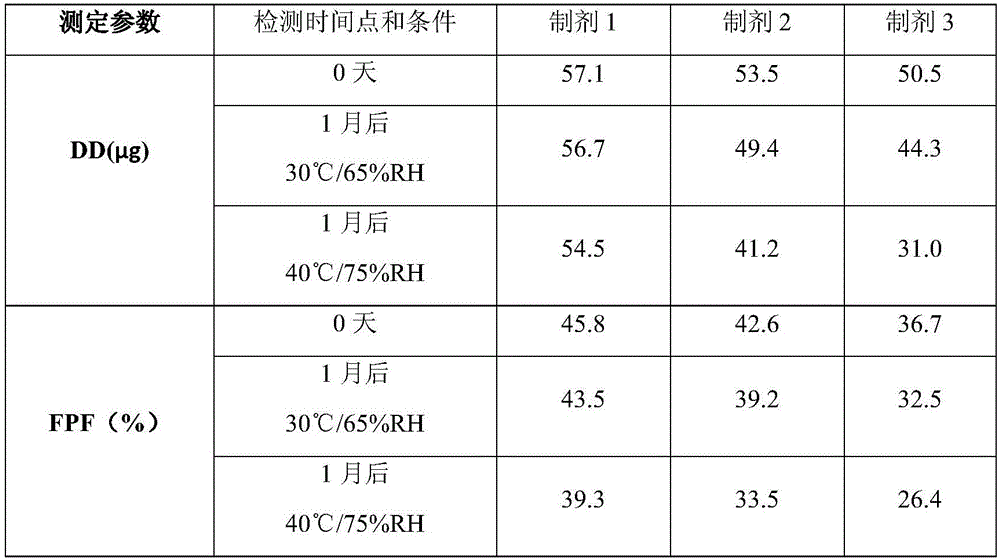
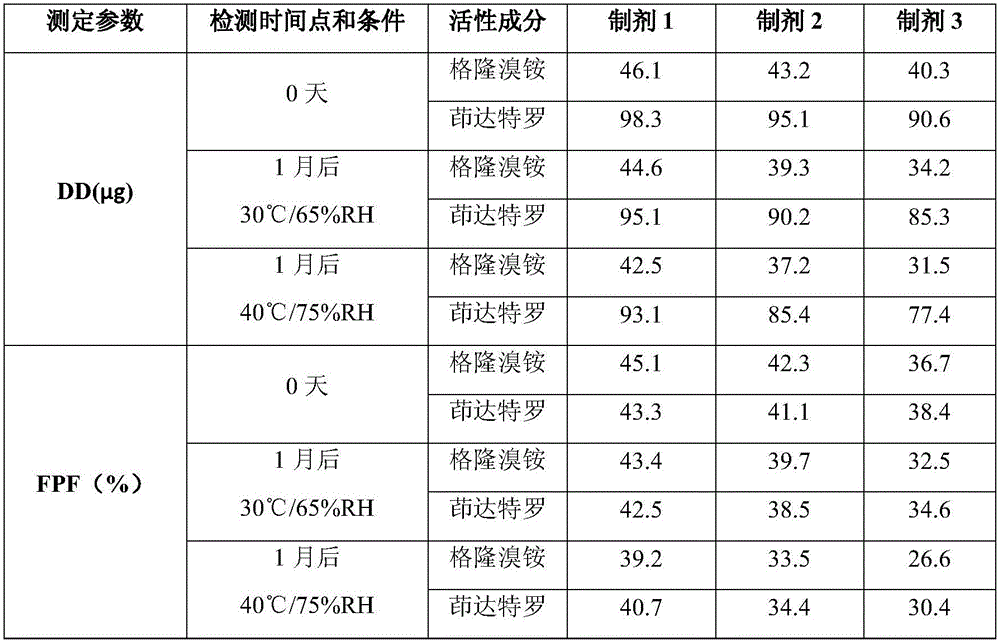
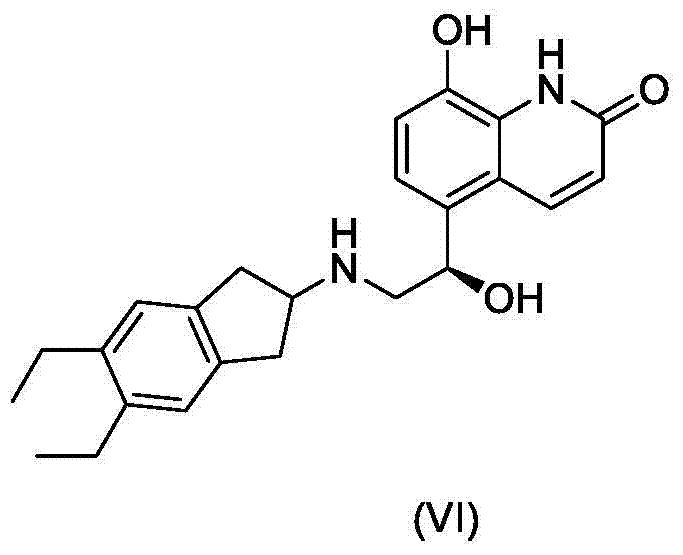
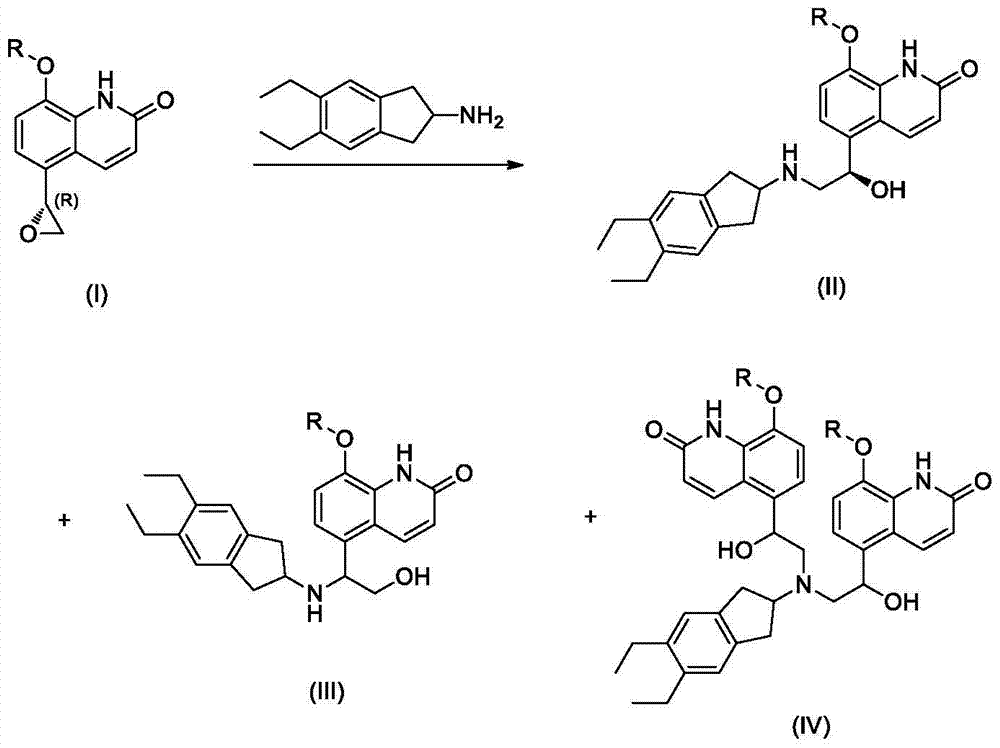
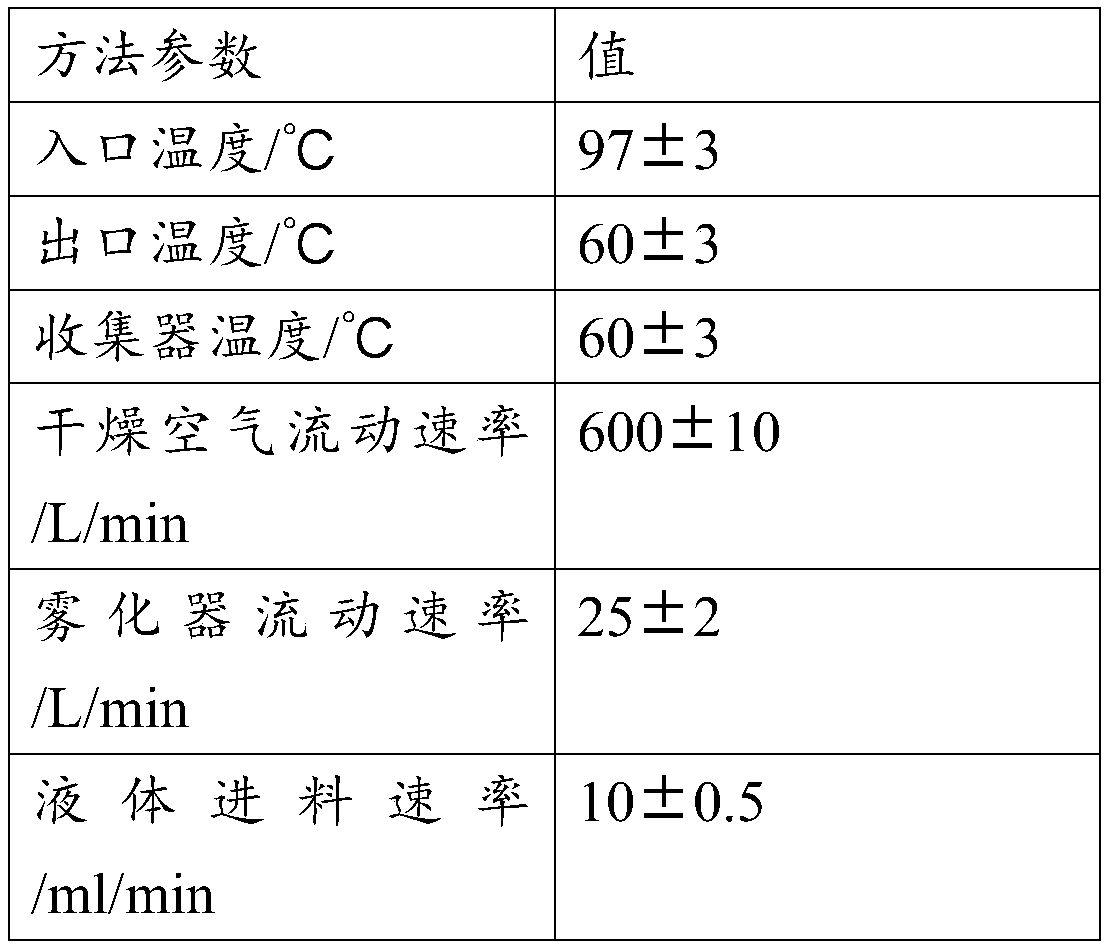
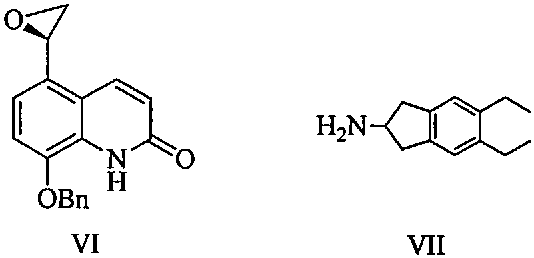
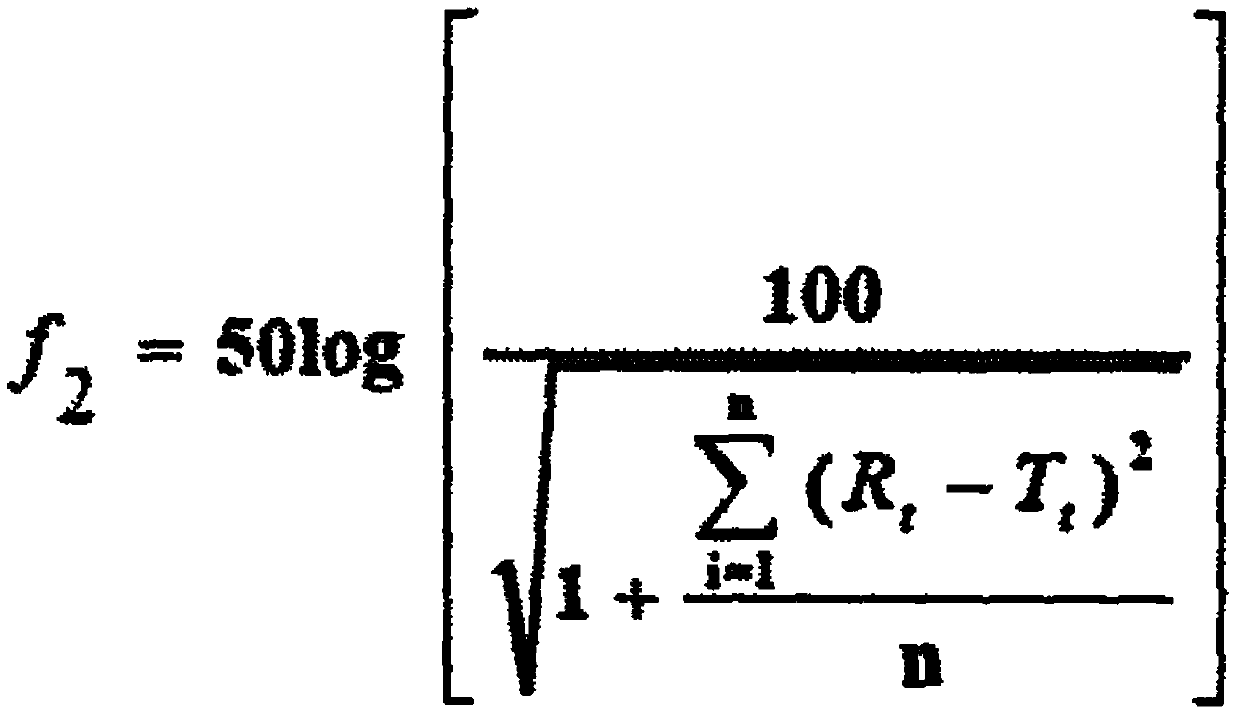Patents
Literature
64 results about "Indacaterol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
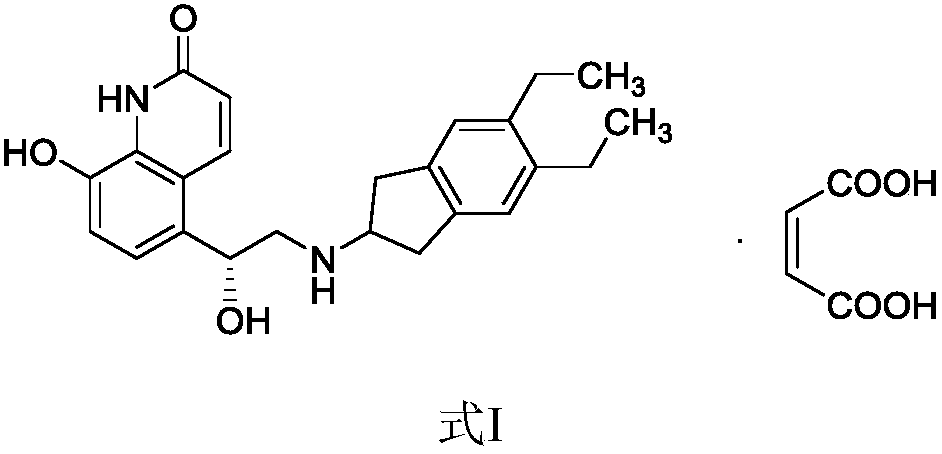
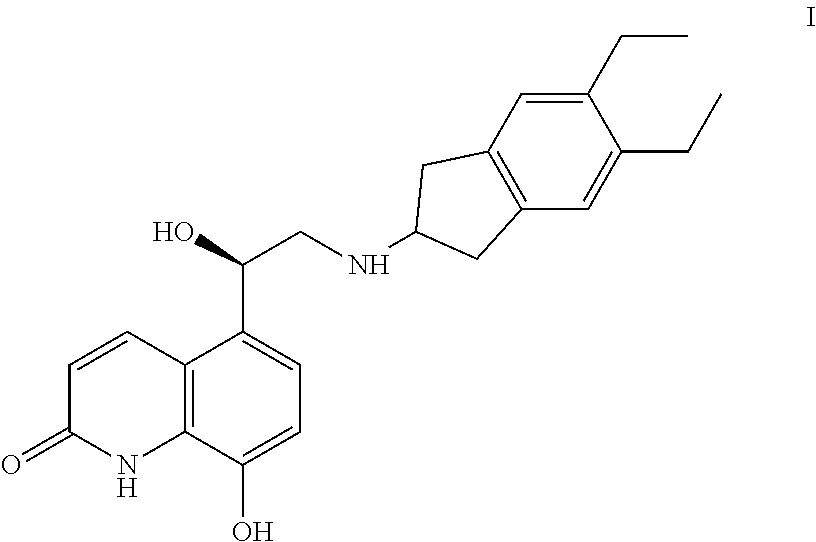
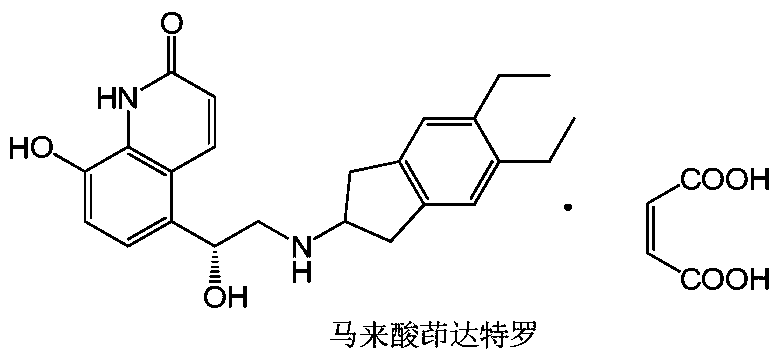
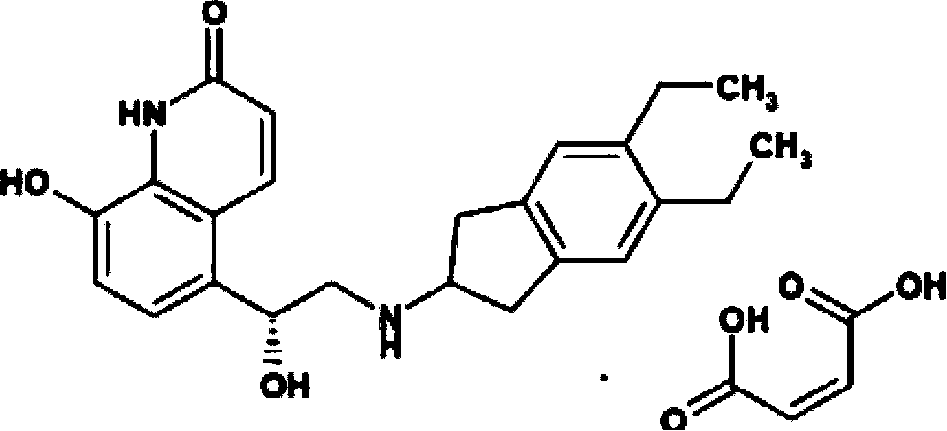
Indacaterol is used to treat trouble breathing and wheezing due to a certain ongoing lung disease (chronic obstructive pulmonary disease-COPD, including chronic bronchitis and/or emphysema). It is used if your breathing problems are not controlled with other medication (such as a quick-relief inhaler).
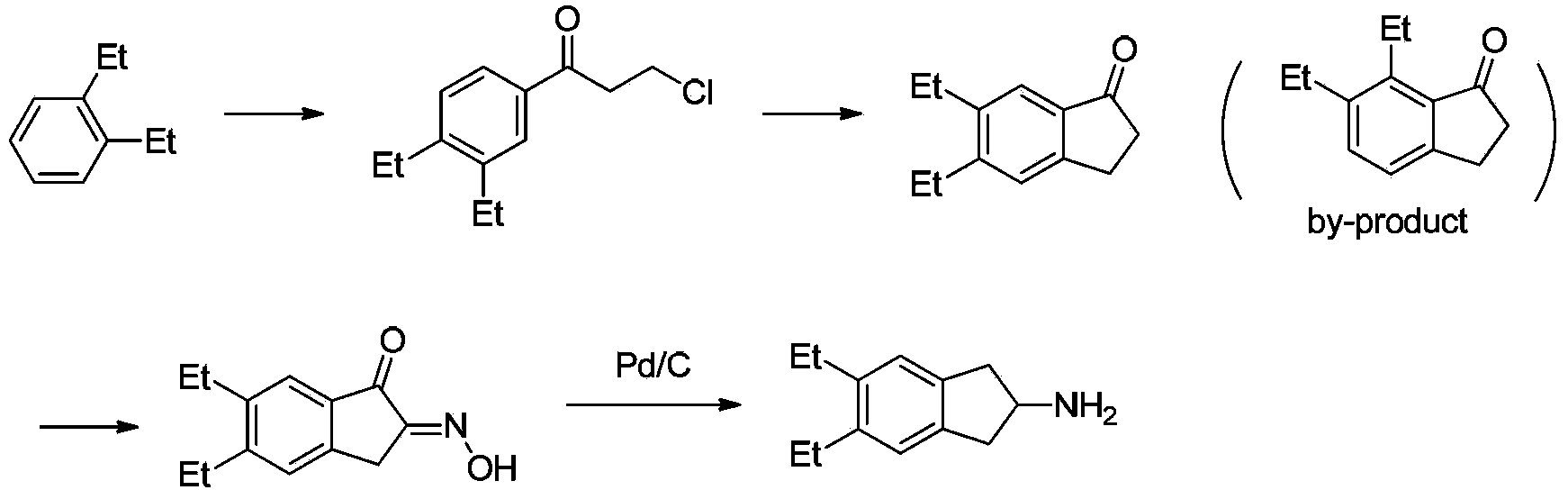
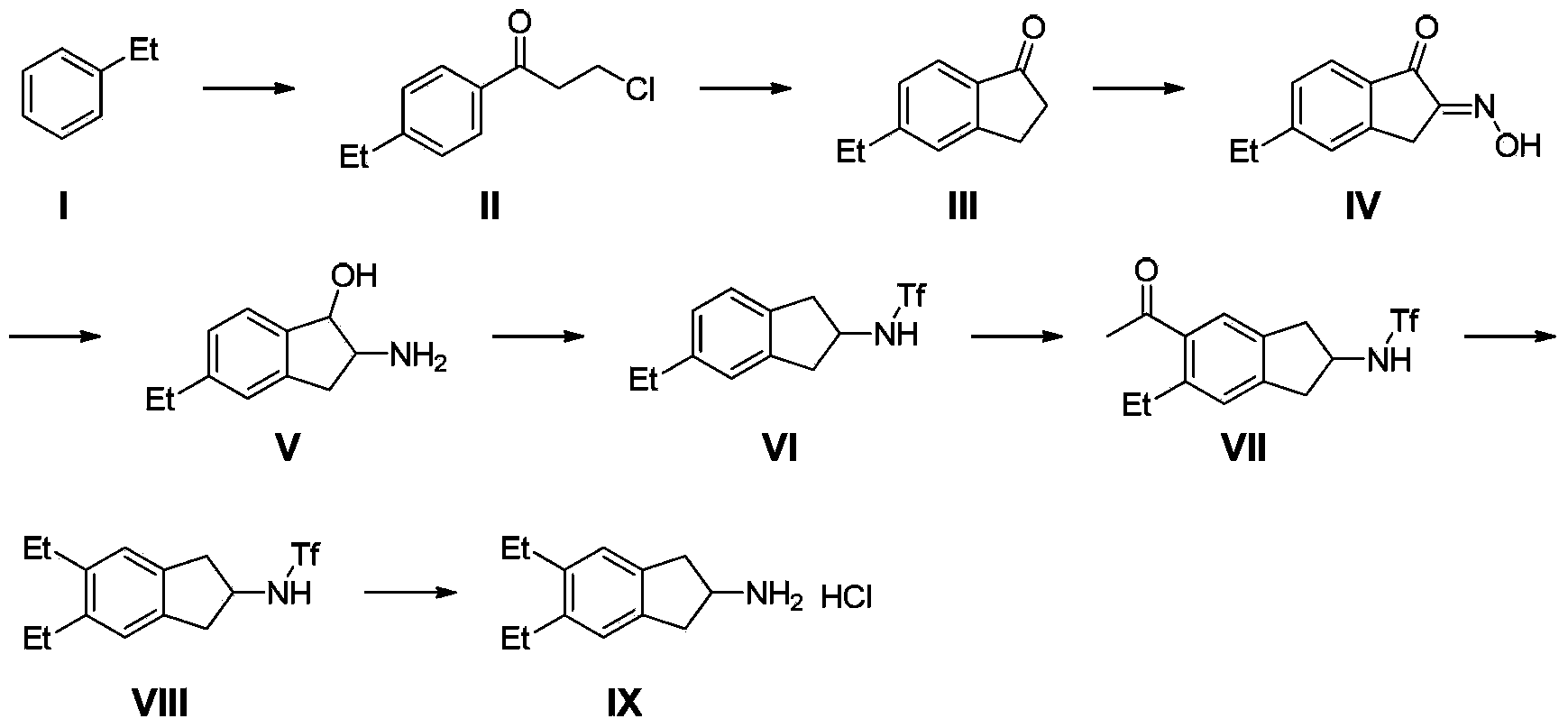
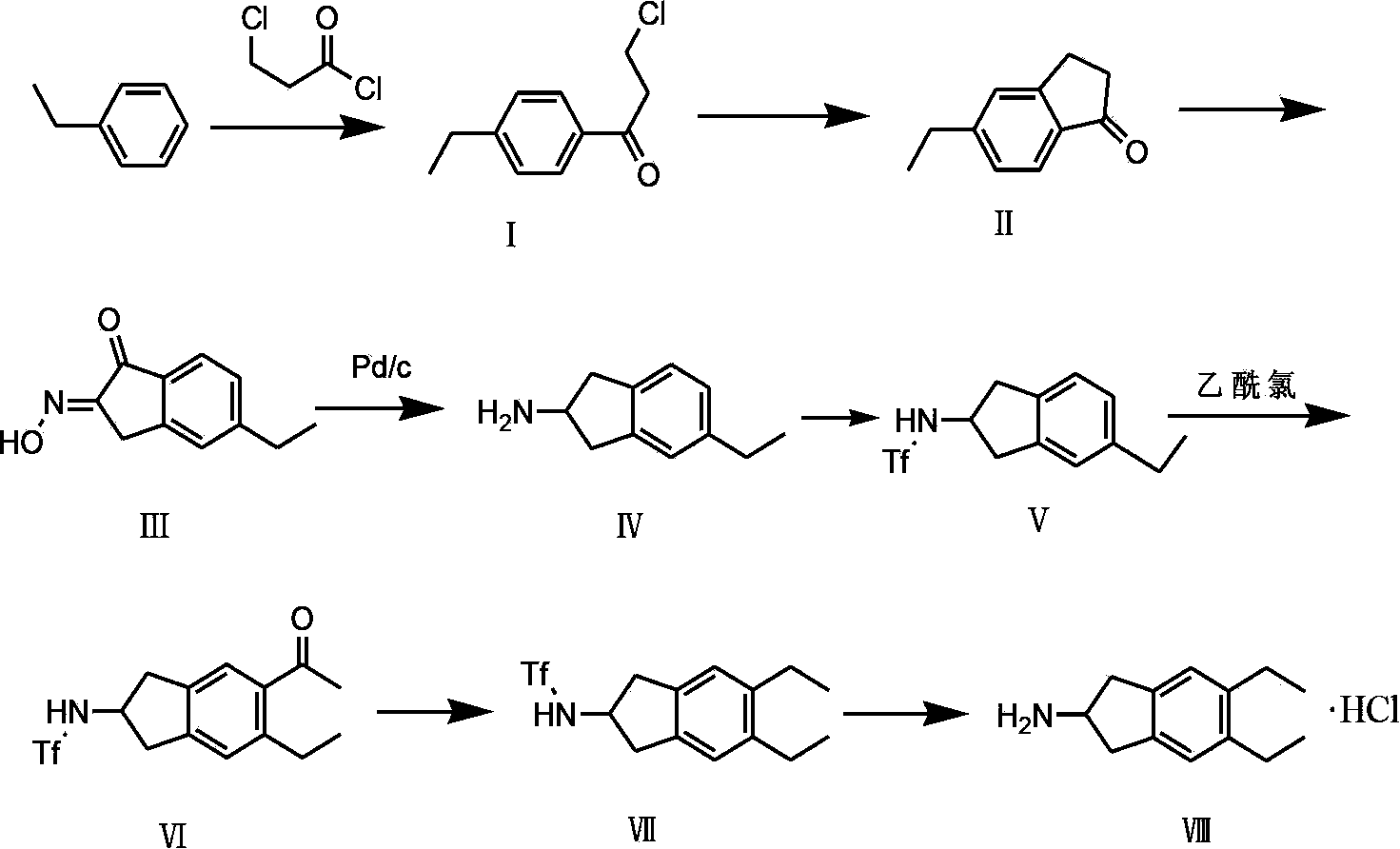
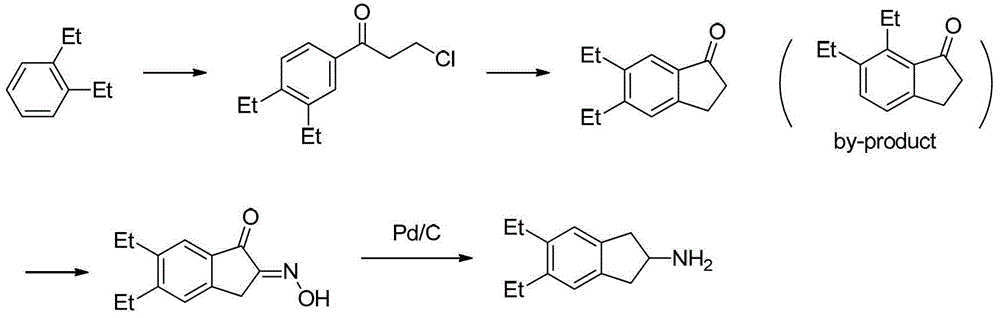
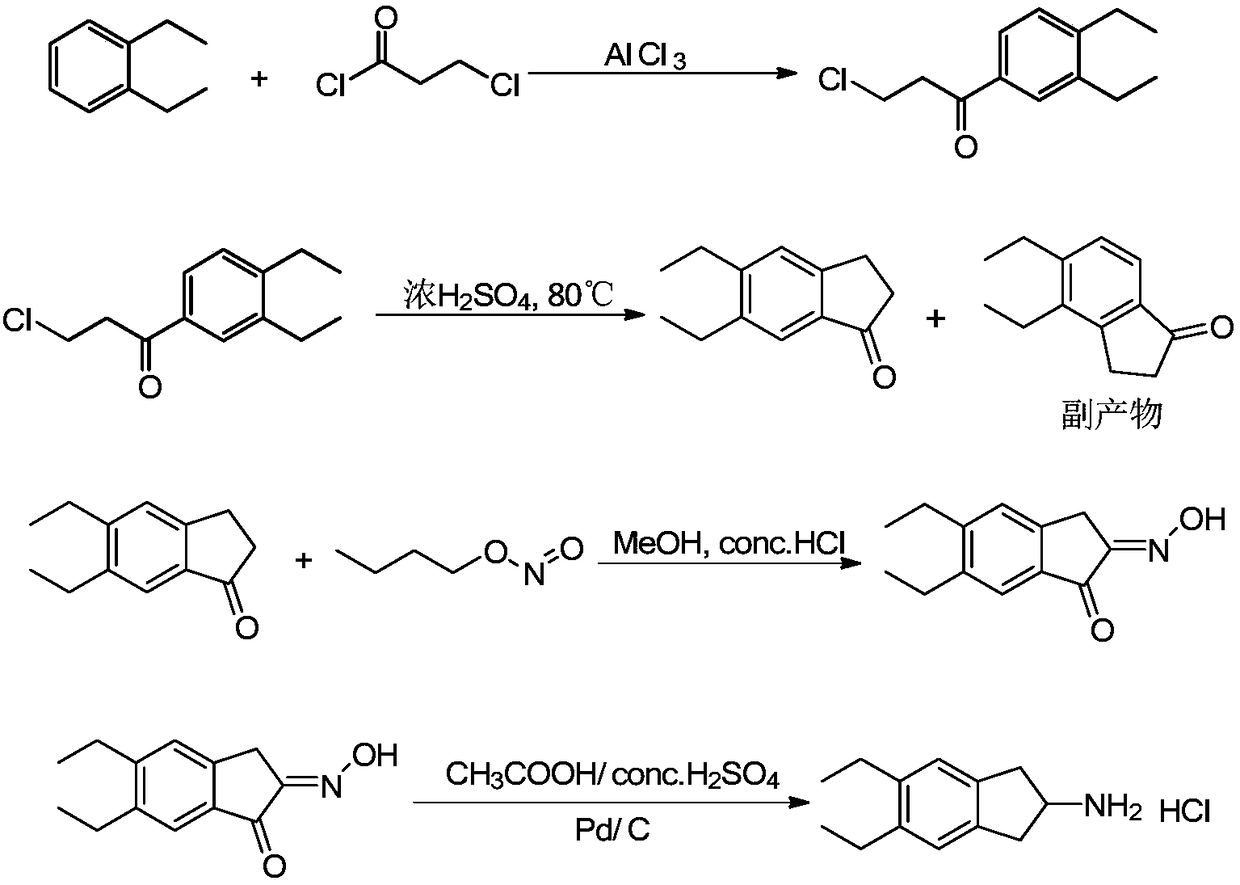
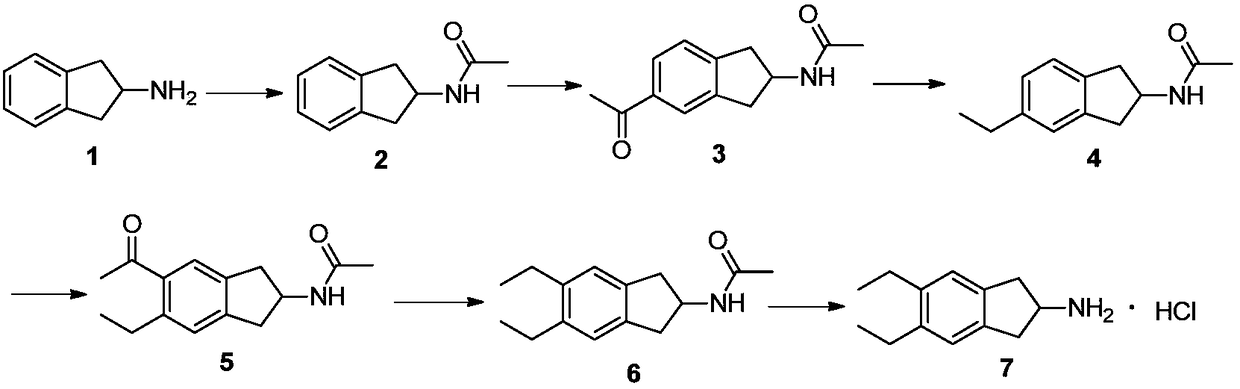
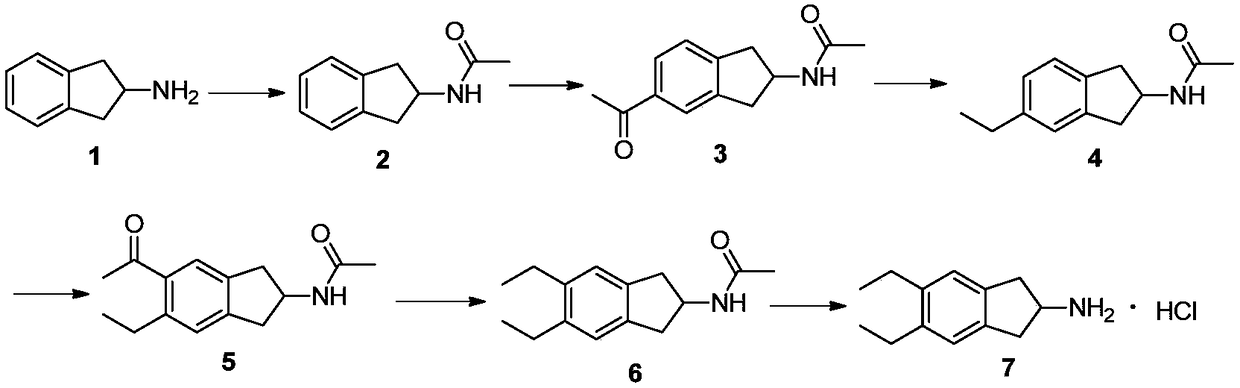
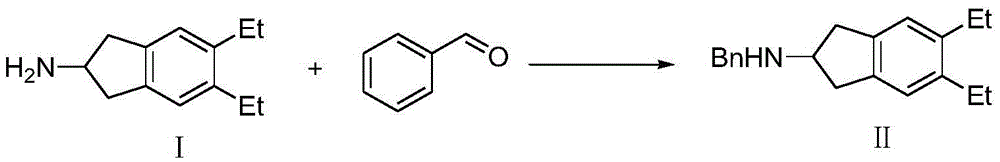
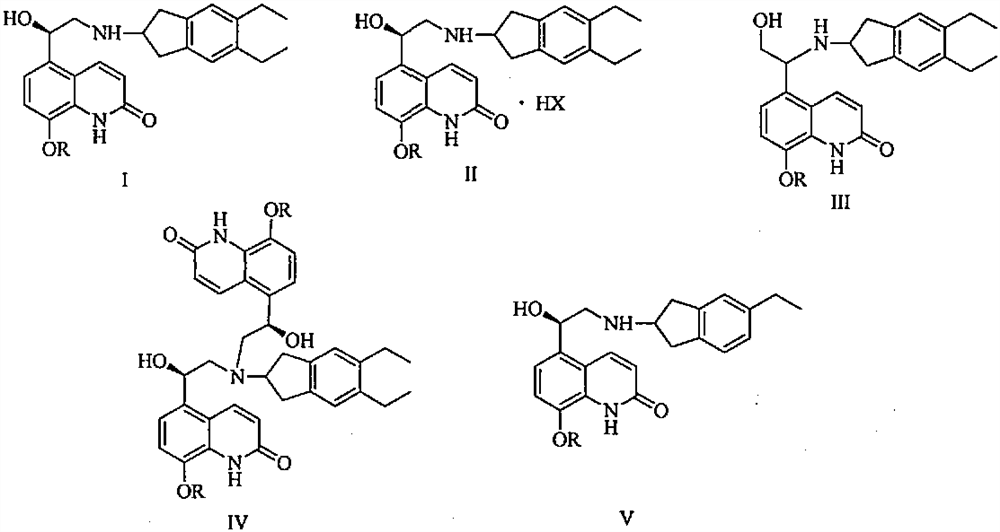
Synthesizing method of indacaterol amino fragment 5,6-diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride
ActiveCN103360264AAvoid bringing inAvoid expensiveOrganic compound preparationAmino compound preparationIndacaterolHydrochloride
The invention discloses a synthesizing method of an indacaterol amino fragment 5,6-diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride. According to the invention, ethylbenzene I is adopted as a raw material, and a Friedel-Crafts reaction is carried out, such that a compound II is obtained; the compound II is subjected to cyclization, such that ethylindene III is obtained; ethylindene III is subjected to alpha-site oximation reaction, such that a compound IV is obtained; the compound IV is subjected to RaneyNi reduction, such that a compound V is obtained; the compound V is processed with a Tf-protection and sodium borohydride reduction one-pot method, such that a compound VI is obtained; the compound VI is subjected to a Friedel-Crafts reaction, such that a compound VII is obtained; the compound VII is reduced, such that a compound VIII is obtained; and the compound VIII is subjected to protection removing and hydrochloride salt formation, such that a final product compound IX is obtained, and the 5,6-diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride is obtained. The method provided by the invention is simple and convenient, and has low cost. The content of the product is higher than 99%. The method also has the advantages of low environmental pollution and suitability for industrialized production.
Owner:武汉恒和达生物医药有限公司
Dry powder inhalation medicine composition and preparation method thereof
InactiveCN105982880AAvoid stimulationImproving In Vitro Assay ParametersPowder deliveryPharmaceutical non-active ingredientsIndacaterolAdditive ingredient
The invention provides a dry powder inhalation medicine composition and a preparation method thereof. The composition is prepared from a coating agent with a specific particle size characteristic, lactose monohydrate with a specific particle size characteristic for a carrier and a micronized medicinal active ingredient, wherein the coating agent is an inhaling magnesium stearate or a mixture of the inhaling magnesium stearate and micronized lactose monohydrate; and the medicinal active ingredient is selected from at least one of glycopyrronium bromide, umeclidinium, indacaterol, formoterol, vilanterol, fluticasone and pharmaceutically available salt of the active ingredients. The preparation method comprises the following steps of sufficiently mixing and coating the coating agent and the lactose monohydrate, and uniformly mixing with the micronized medicinal active ingredients.
Owner:SICHUAN HAISCO PHARMA CO LTD
Indacaterol intermediate salt and preparation method thereof
InactiveCN107021921AEasy to operateLow costGroup 4/14 element organic compoundsBulk chemical productionIndacaterolInorganic chemistry
The invention relates to an indacaterol intermediate salt and a preparation method thereof, and the preparation method of the indacaterol intermediate salt has the advantages of simple operation, low cost, good reproducibility and easy realization, and is suitable for industrial production.
Owner:SHANGHAI FANGNAN PHARMA
Liquor containing indacaterol maleate and inhalation spray of liqour
The invention relates to liquor containing indacaterol maleate. The content of the indacaterol maleate is 2.0 mug / ml-15 mug / ml; while being used, content can be released in the forms of mists by virtue of pressure of a manual pump, ultrasonic spraying, an air compressor, and the like, and can be used for lung inhalation administration. The invention specifically relates to inhalation spray containing the indacaterol maleate.
Owner:熊妲妮
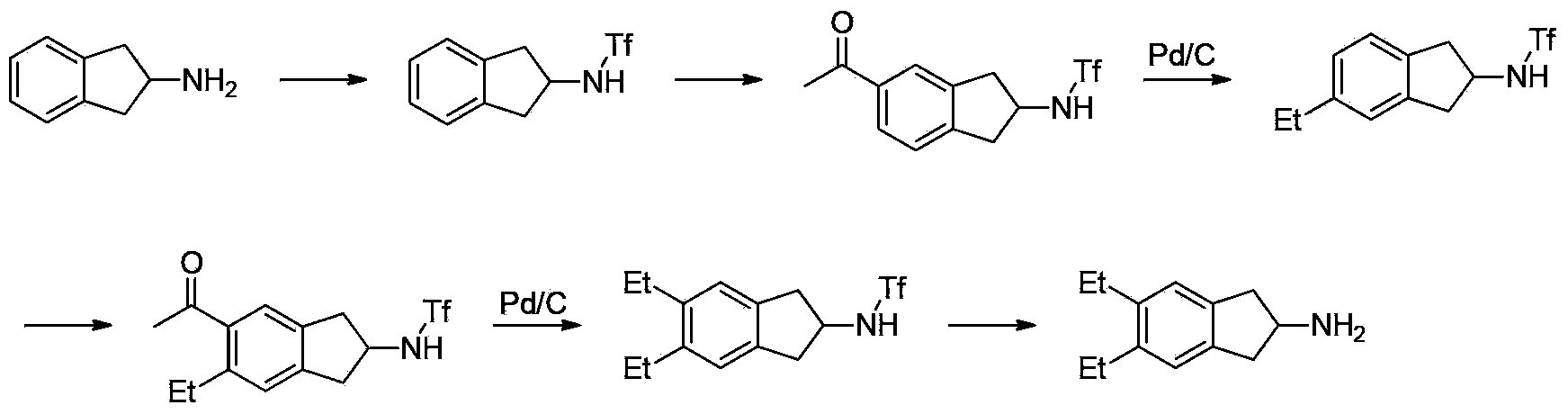
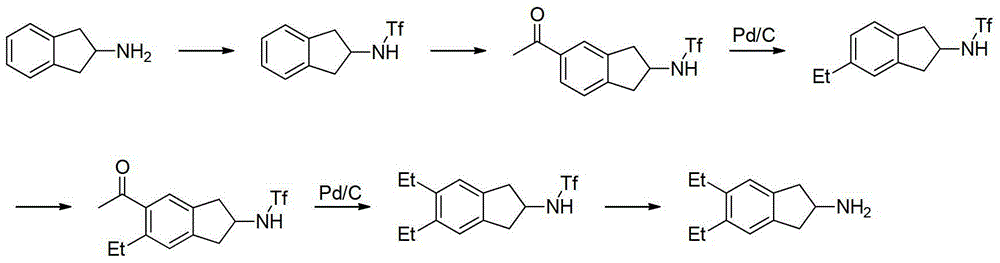
Preparation method of 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride
InactiveCN103539677AEase of industrial implementationOrganic compound preparationAmino compound preparationIndacaterolN-butyl nitrite
The invention belongs to the technical field of synthesis of a medical immediate, and particularly relates to a synthesis method of a key intermediate 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride of indacaterol. The method comprises the following steps: by taking ethylbenzene as a raw material, preparing a compound I by propionyl chloride; preparing a compound II from the compound I by cyclization reaction; preparing a compound III by reaction of the compound II and butyl nitrite; preparing a compound IV from the compound III by palladium hydrogen reduction; finally preparing a compound V from the compound IV under protection of trifluoroacetyl; preparing a compound VI from the compound V by amino acetylation reaction; preparing a compound VII from the compound VI by reduction; carrying out deprotection, hydrolysis and acidification on the compound VII, so as to obtain the final product compound VIII, namely the 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride. The method disclosed by the invention is simple and convenient to operate, reasonable in reaction flow, low in cost, good in product quality, free of pollution to environment, and applicable to industrial production; the content is greater than 99%.
Owner:湖北万知化工医药股份有限公司
Pharmaceutical Composition
Described herein is a pharmaceutical composition that includes a beta2-agonist selected from indacaterol and formoterol in combination with a corticosteroid selected from fluticasone and ciclesonide, and, optionally, one or more pharmaceutically acceptable excipients.
Owner:CIPLA LTD
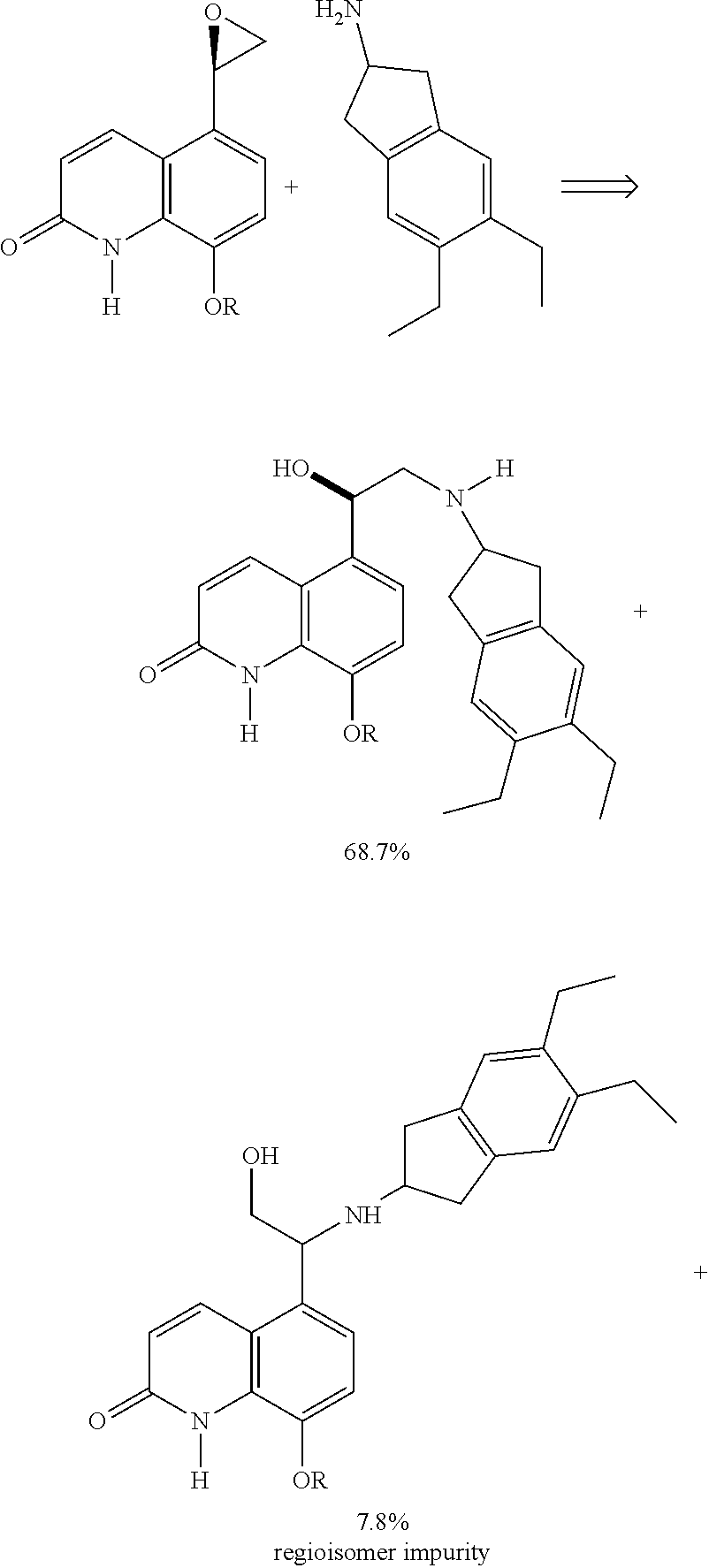
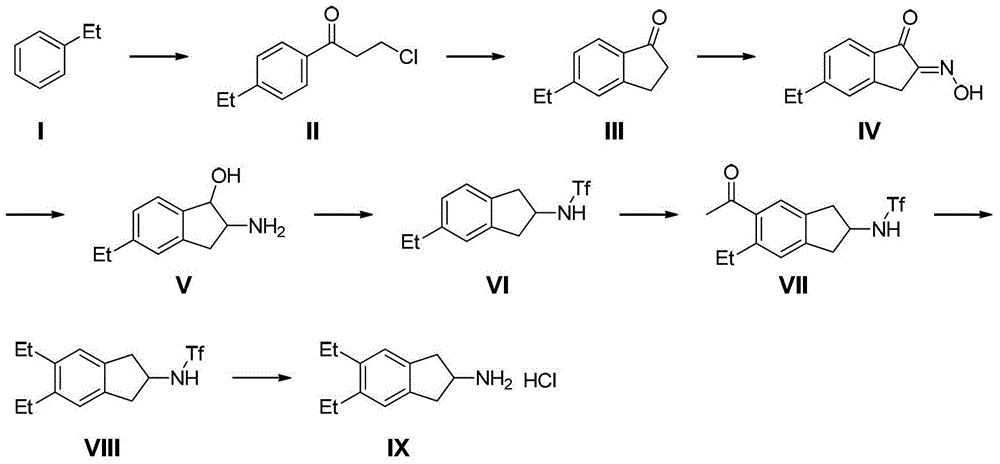
Methods for the preparation of indacaterol and pharmaceutically acceptable salts thereof
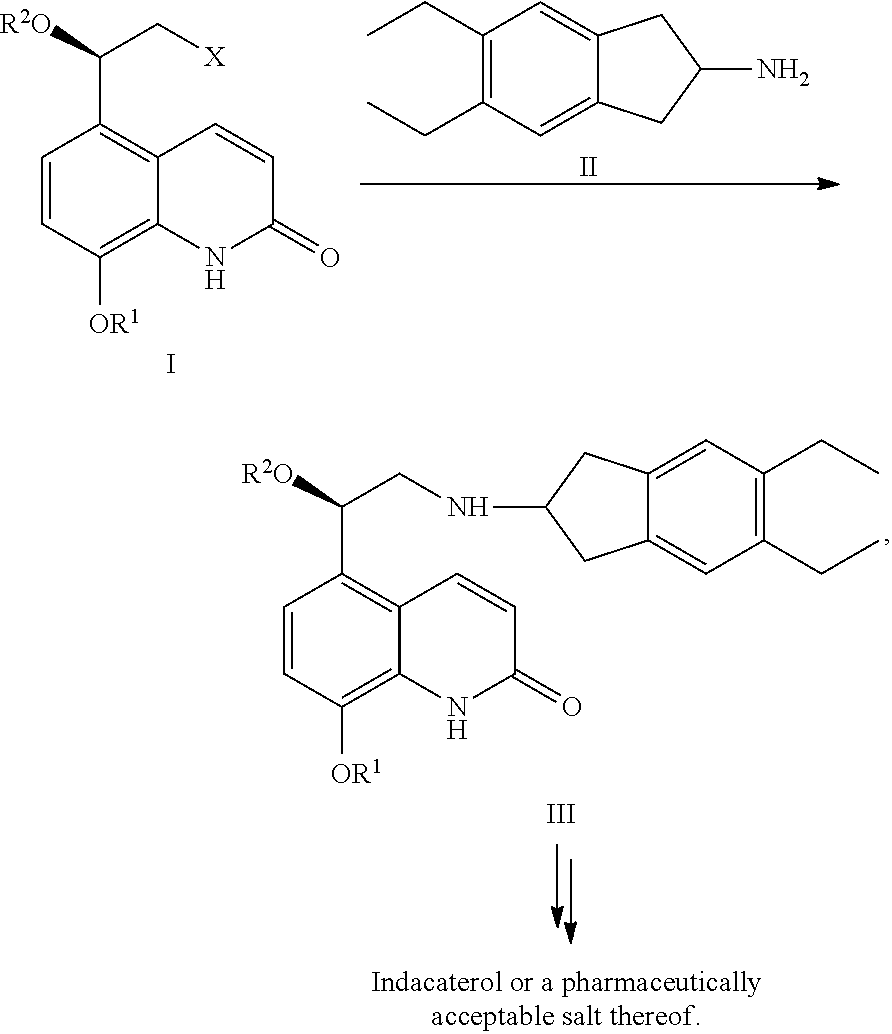
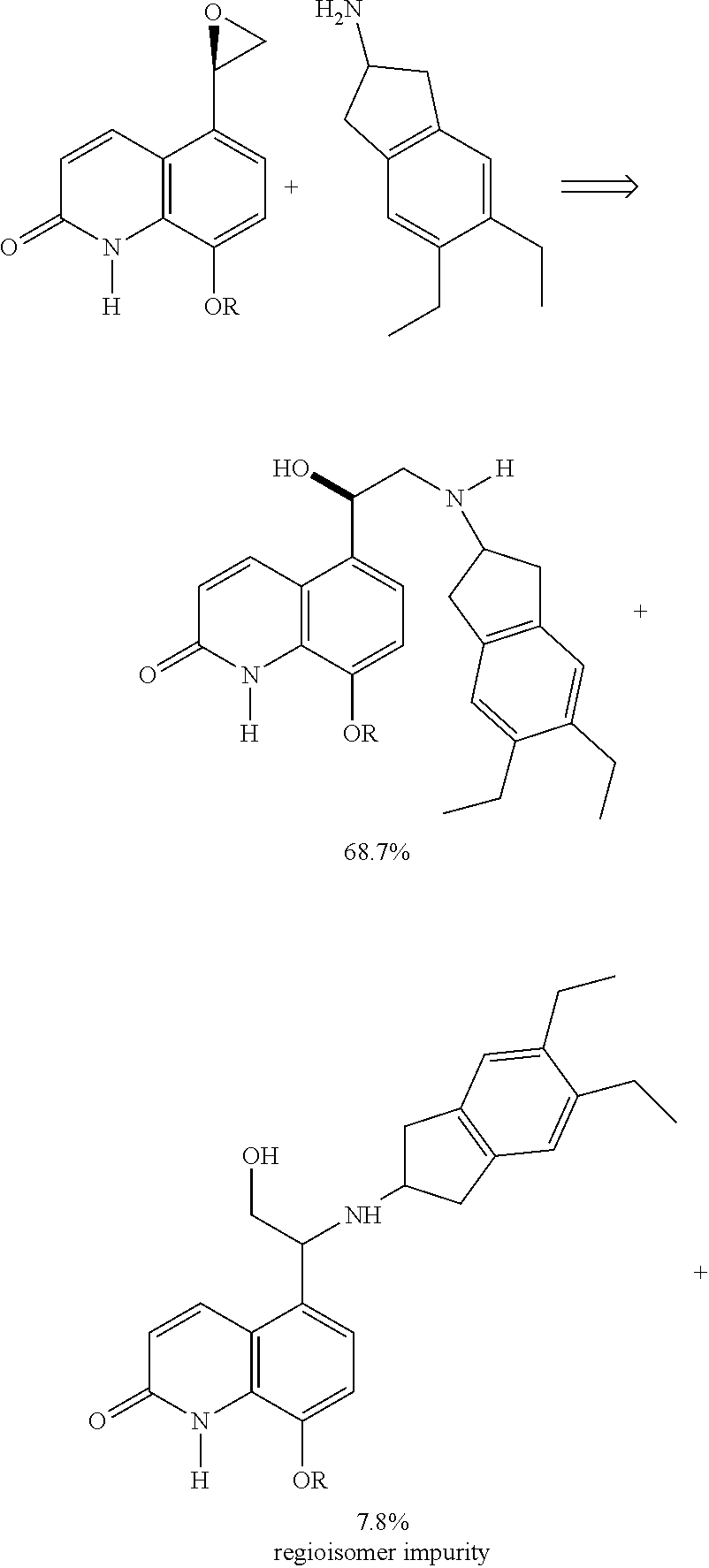
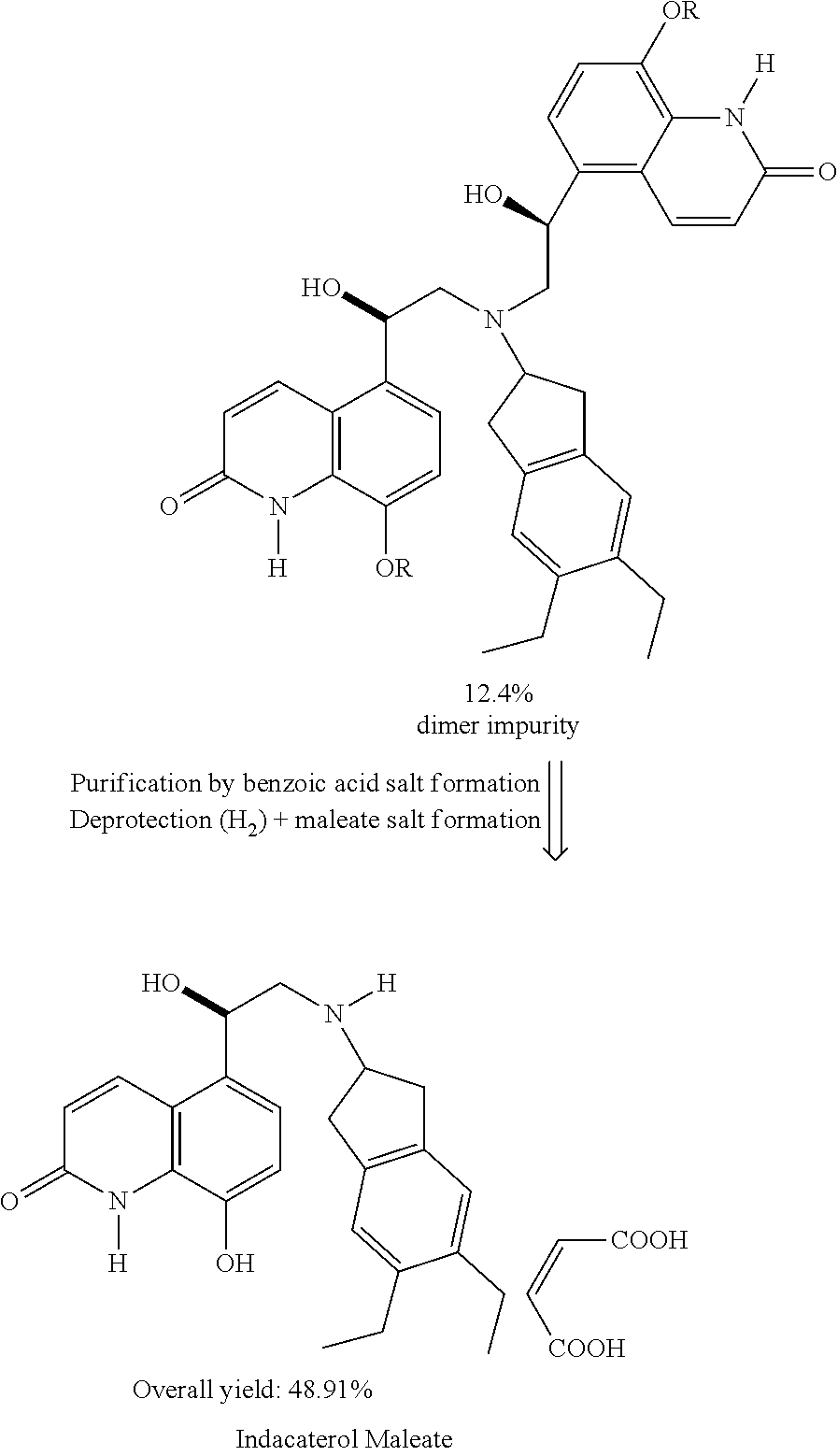
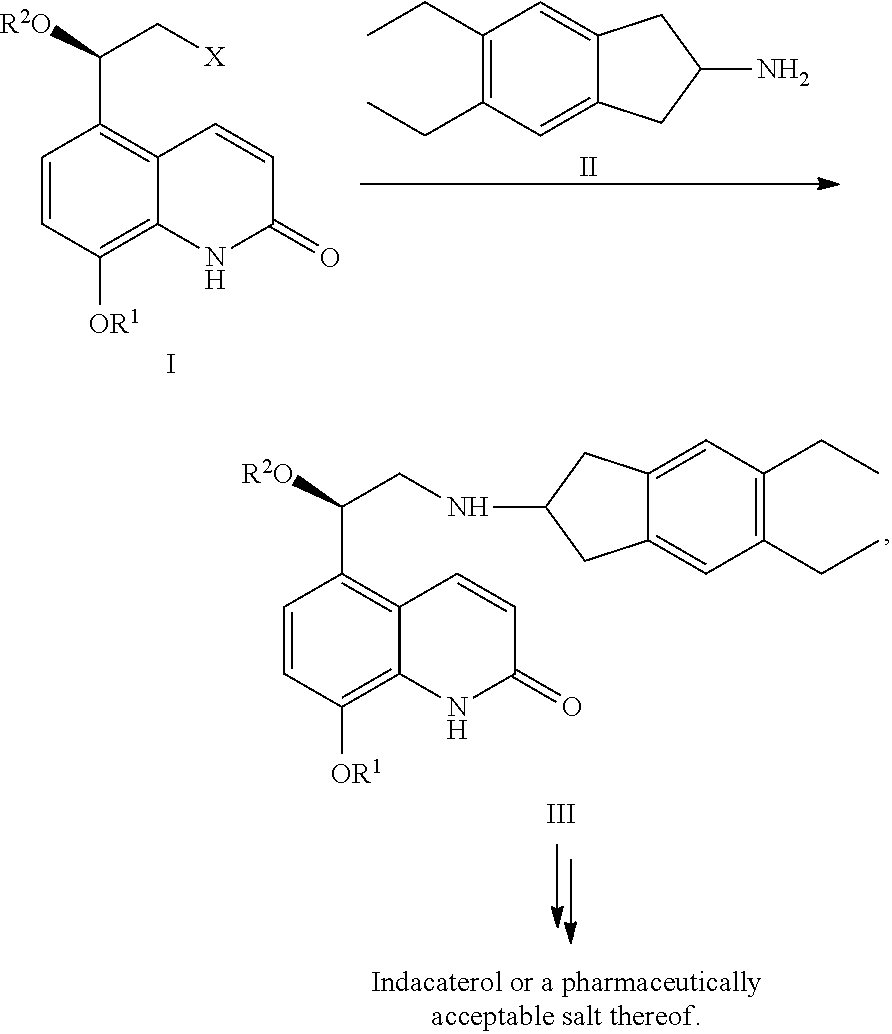
ActiveUS20150225346A1Improve purification effectInhibition formationOrganic chemistry methodsRespiratory disorderIndacaterolCombinatorial chemistry
The invention relates to new and improved processes for the preparation of Indacaterol and pharmaceutically acceptable salts thereof as well as intermediates for the preparation of Indacaterol. The new process avoids the use of the epoxide compound known in the art and the impurities associated therewith and results in a higher yield.
Owner:CRYSTAL PHARMA SA
Methods for the preparation of indacaterol and pharmaceutically acceptable salts thereof
ActiveUS9475772B2Improve purification effectInhibition formationOrganic chemistry methodsCarboxylic acid salt preparationIndacaterolCombinatorial chemistry
The invention relates to new and improved processes for the preparation of Indacaterol and pharmaceutically acceptable salts thereof as well as intermediates for the preparation of Indacaterol. The new process avoids the use of the epoxide compound known in the art and the impurities associated therewith and results in a higher yield.
Owner:CRYSTAL PHARMA SA
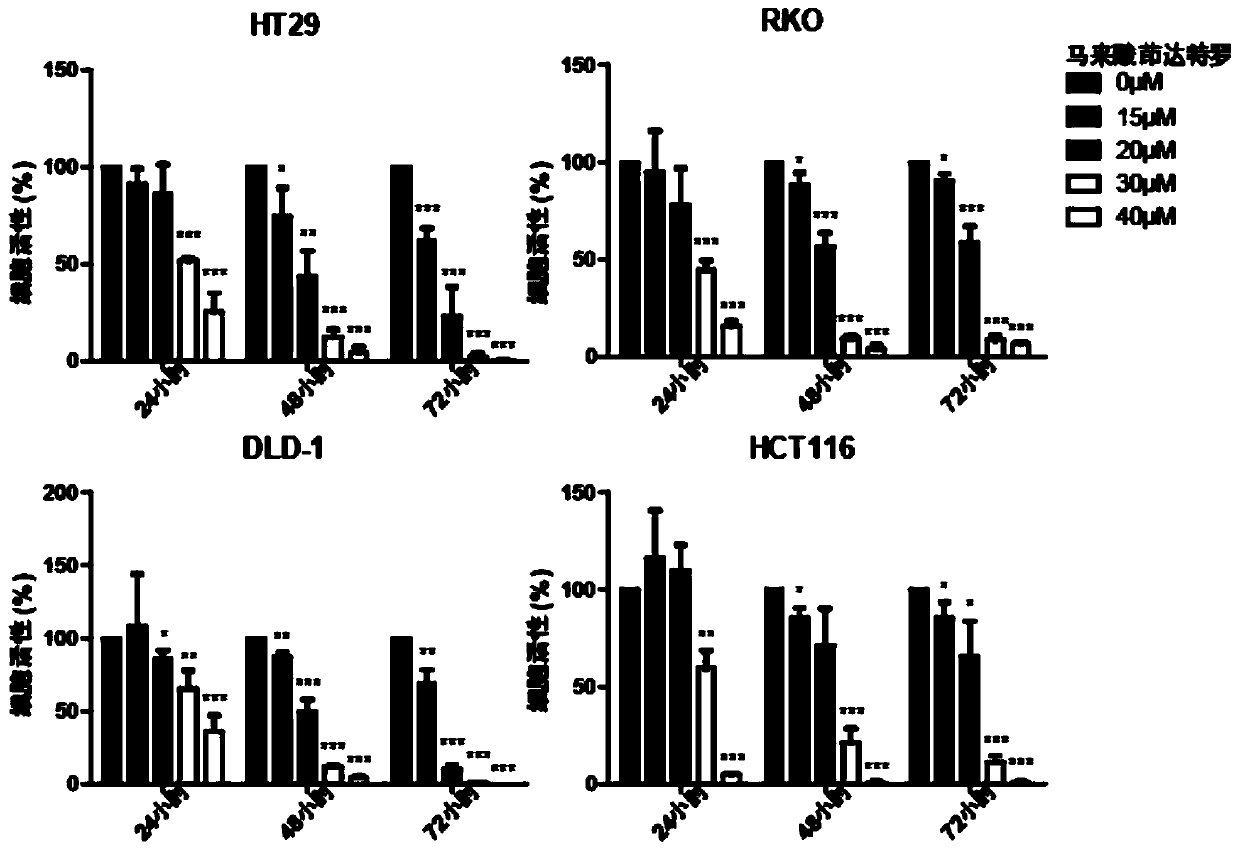
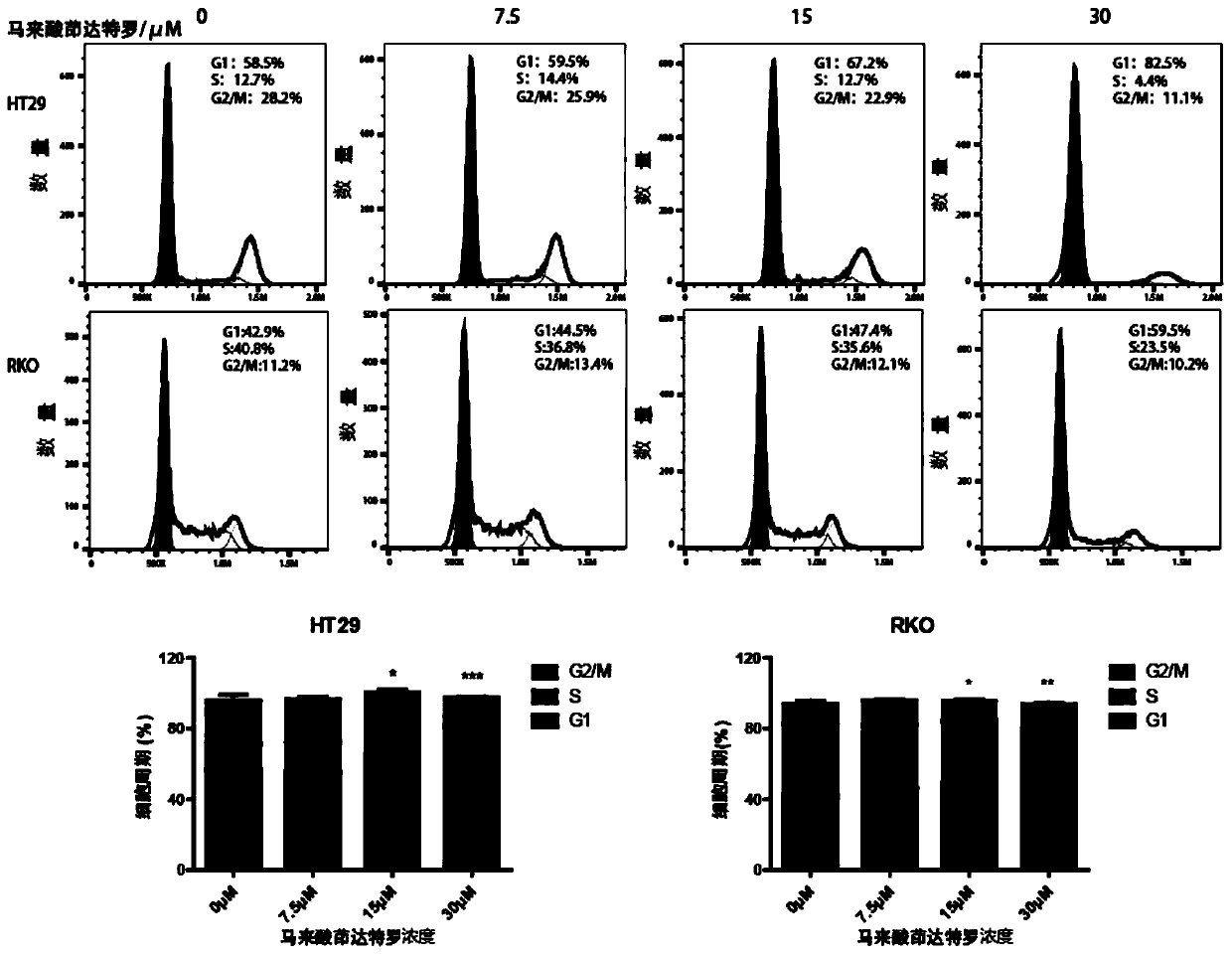
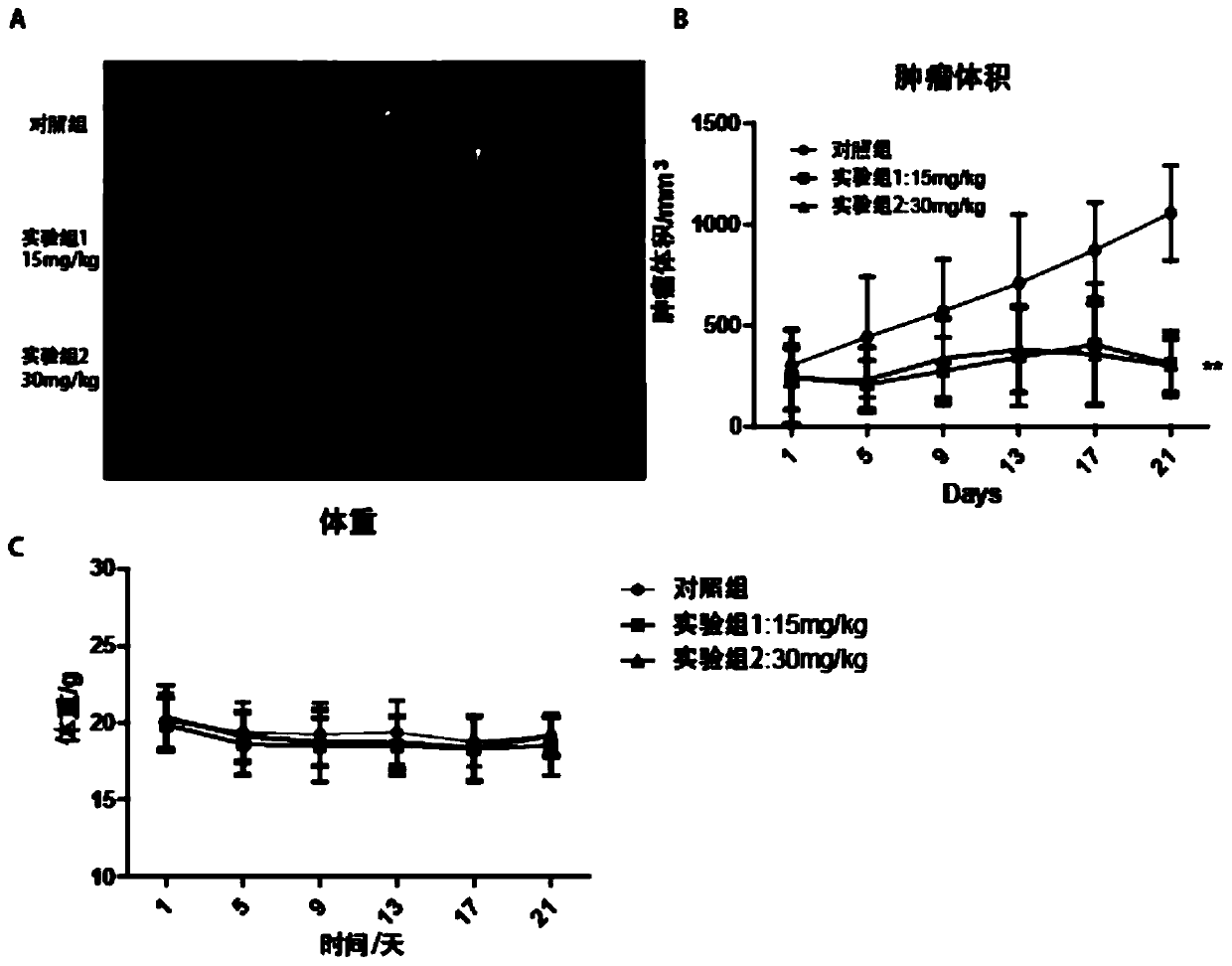
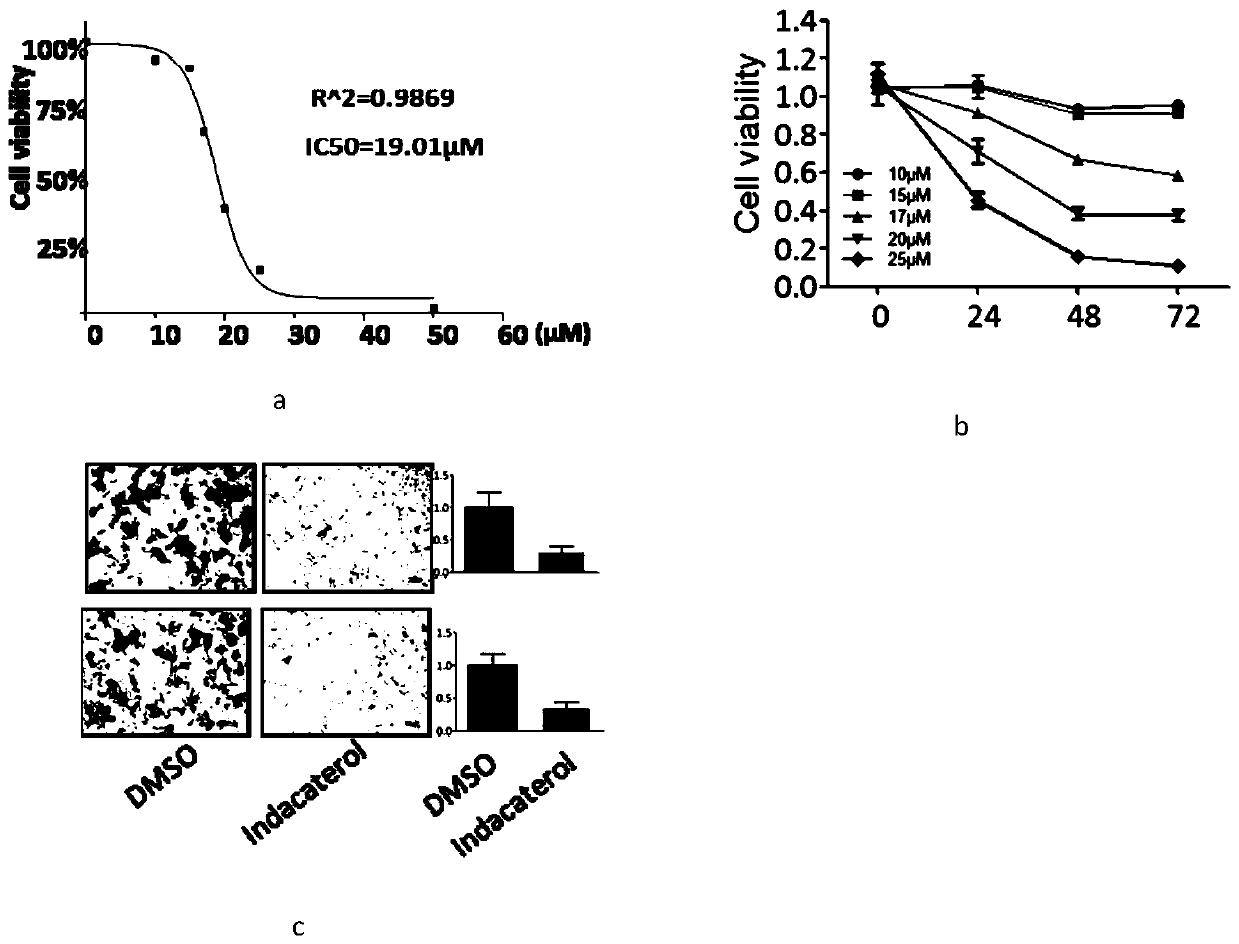
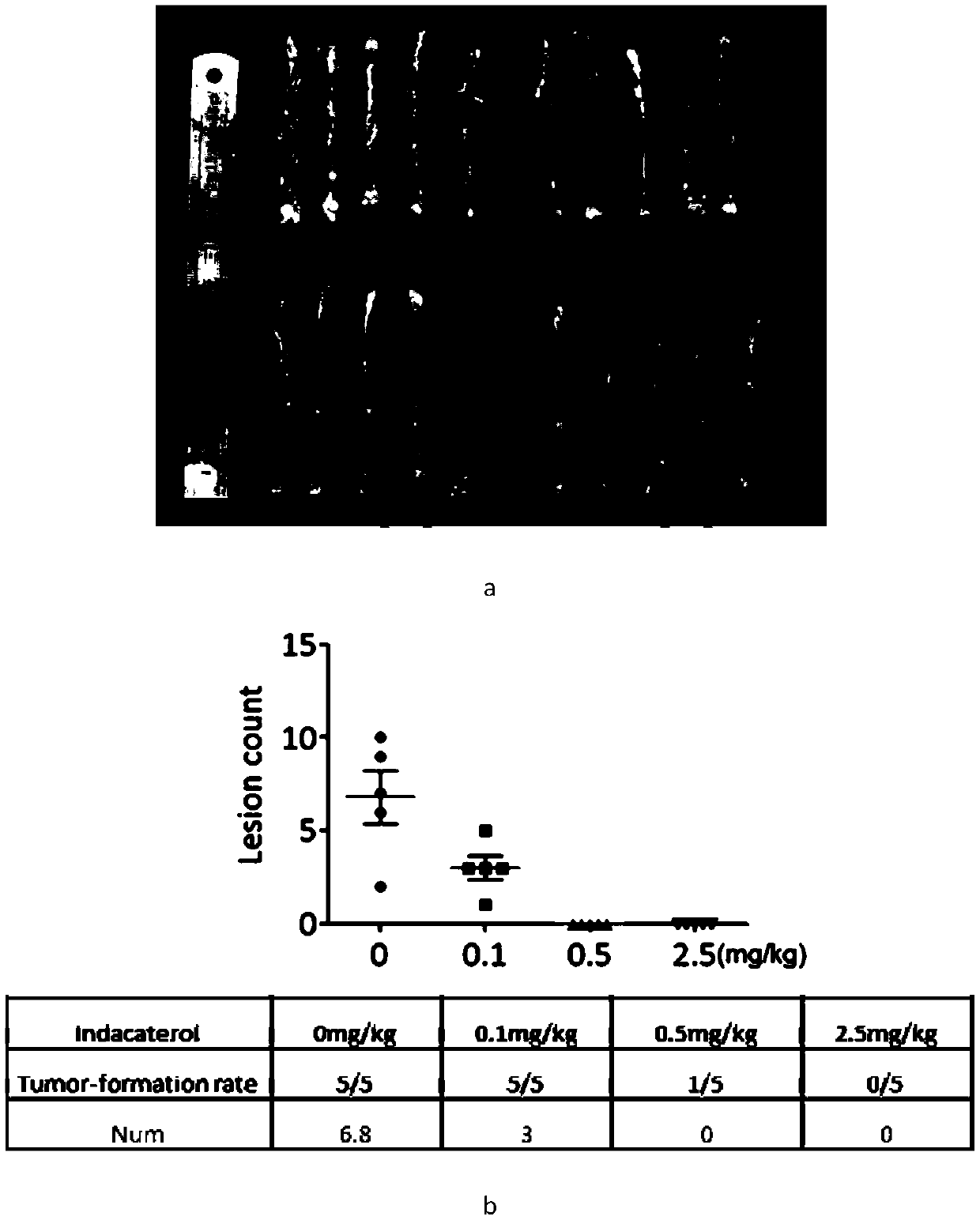
Application of indacaterol maleate to preparation of tumor resisting medicines
InactiveCN110013481AGrowth inhibitionAntiproliferative activityOrganic active ingredientsAntineoplastic agentsIndacaterolSide effect
The invention discloses an application of indacaterol maleate to preparation of tumor resisting medicines. According to the application of the invention, the situation that the indacaterol maleate canrestrain the growth of colorectal cancer is found for the first time. The indacaterol maleate can restrain the reproductive activity of colorectal cancer cells, block the cell cycle of the colorectalcancer, cause G1-period blocking to restrain tumor growth of in vivo colorectal cancer. Besides, the effect of the indacaterol maleate for restraining the cell proliferation activity of the colorectal cancer is more significant along with the increase of the concentration of the indacaterol maleate and prolonging of medication time, and the indacaterol maleate has concentration dependence on thecycle checking effect and in vivo growth of colorectal cancer cells. Besides, the indacaterol maleate is a medicine which is approved by FDA for sale, is lower in cost than developing new medicines, and does not have any obvious toxic or side effects, and when the indacaterol maleate is used for auxiliary treatment of the colorectal cancer, a new medicine source can be provided for auxiliary treatment of cancer.
Owner:JINAN UNIVERSITY
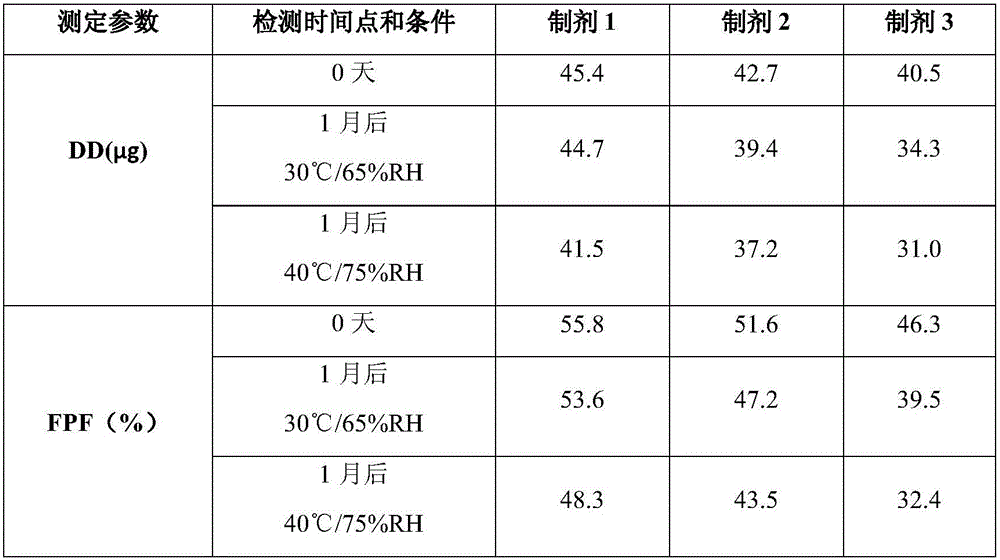
Preparation process of compound dry powder inhaler for glucocorticoids and beta2 receptor agonists
ActiveCN108771761AImprove FPFImprove stabilityPowder deliveryOrganic active ingredientsIndacaterolGlucocorticoid
The invention relates to a preparation process of a compound dry powder inhaler for glucocorticoids and beta2 receptor agonists. The process comprises the following steps: (a) performing micronizationon glucocorticoids and beta2 receptor agonists; (b) mixing the micronized product in the step (a) with a drug carrier, or mixing the micronized product in the step (a) with a drug carrier and an additive; and (c) adding the composition in the step (b) into a sample handling room for handling, thereby obtaining the pharmaceutical composition of the dry powder inhaler. The sample handling room is an environment of which the humidity is 45-75% or an environment of which the pressure is not higher than 500Pa; the handling time is 2-72 hours; the glucocorticoids are mometasone furoate or pharmaceutically acceptable salts thereof; and the beta2 receptor agonists are indacaterol or pharmaceutically acceptable salts thereof. The process disclosed by the invention is simple, convenient to operateand low in production cost.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Synthesizing method of indacaterol amino fragment 5,6-diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride
ActiveCN103360264BAvoid bringing inAvoid expensiveOrganic compound preparationAmino compound preparationIndacaterolHydrochloride
Owner:武汉恒和达生物医药有限公司
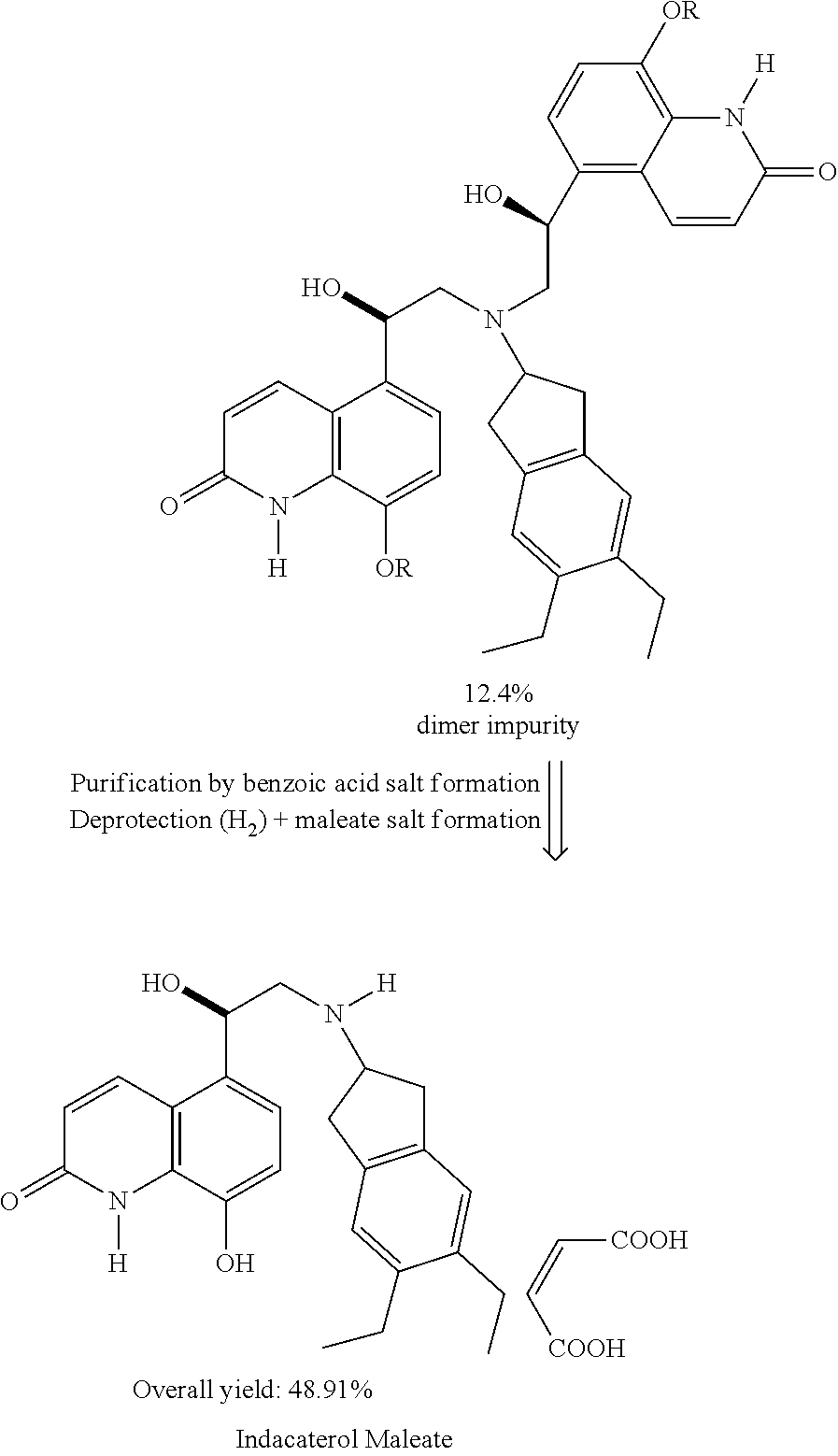
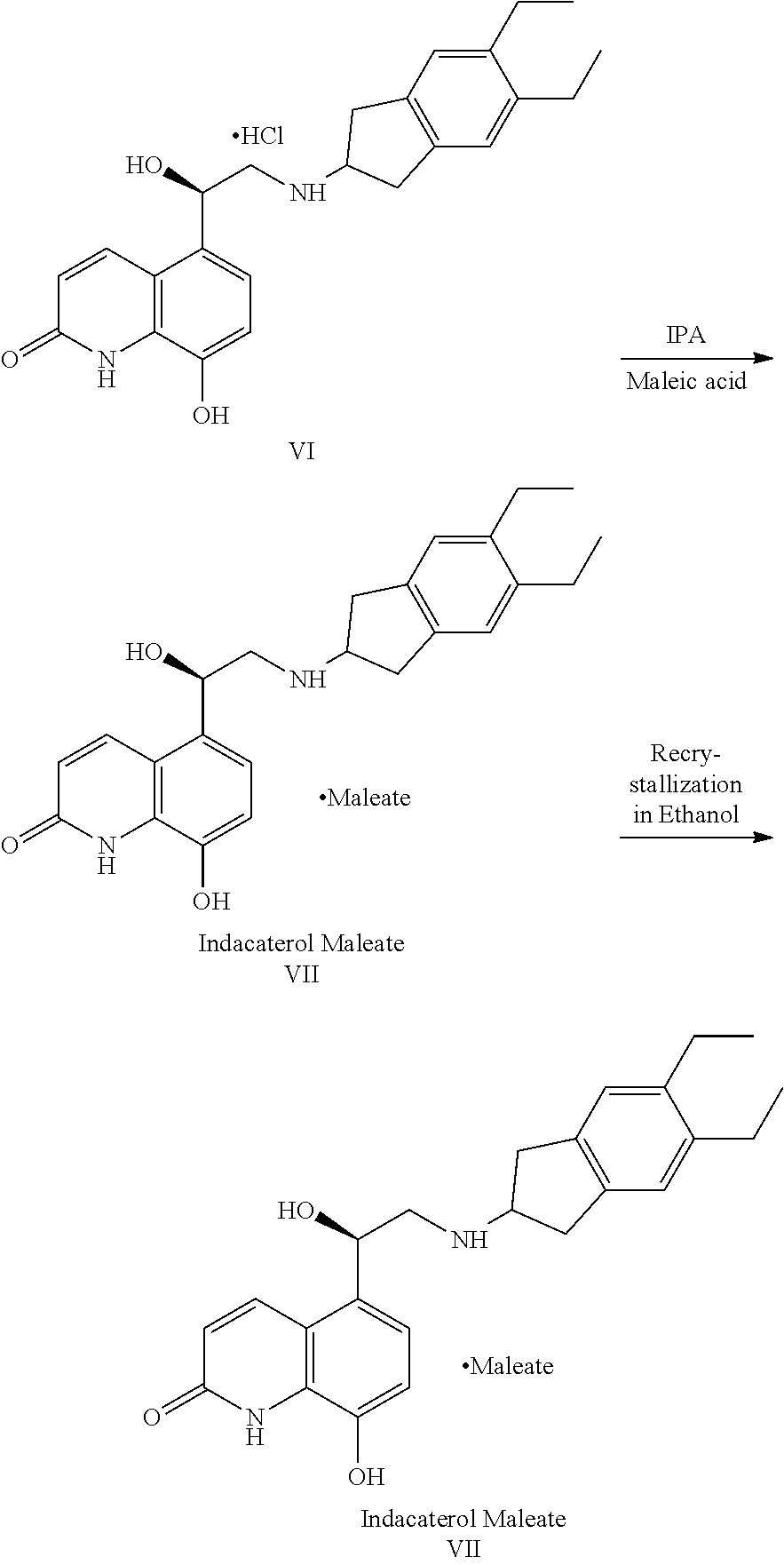
Preparation method of indacaterol maleate
InactiveCN108409650AThe reaction steps are simpleHigh yieldCarboxylic acid salt preparationBulk chemical productionIndacaterolOxazolidine
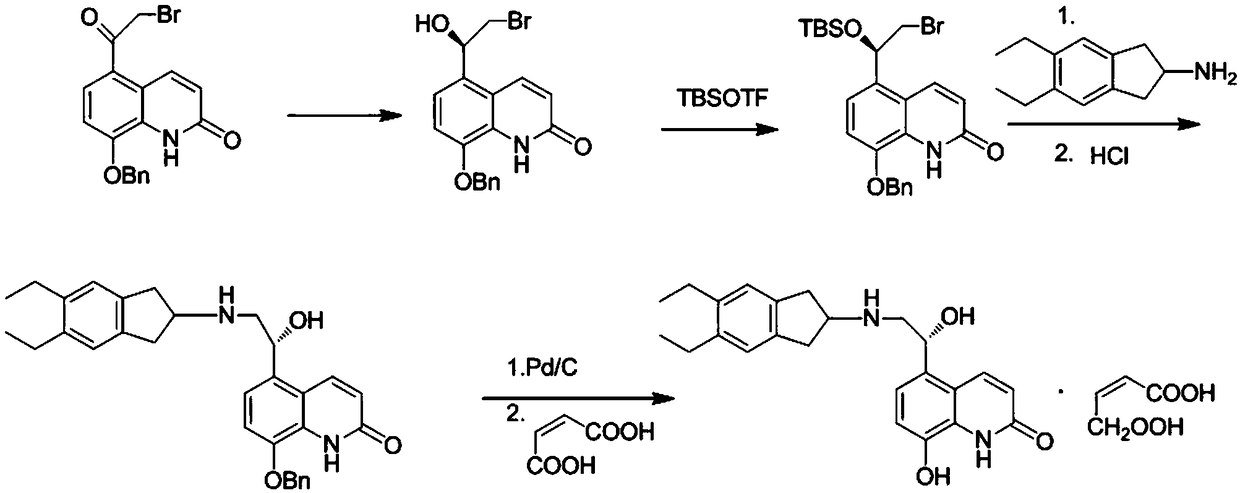
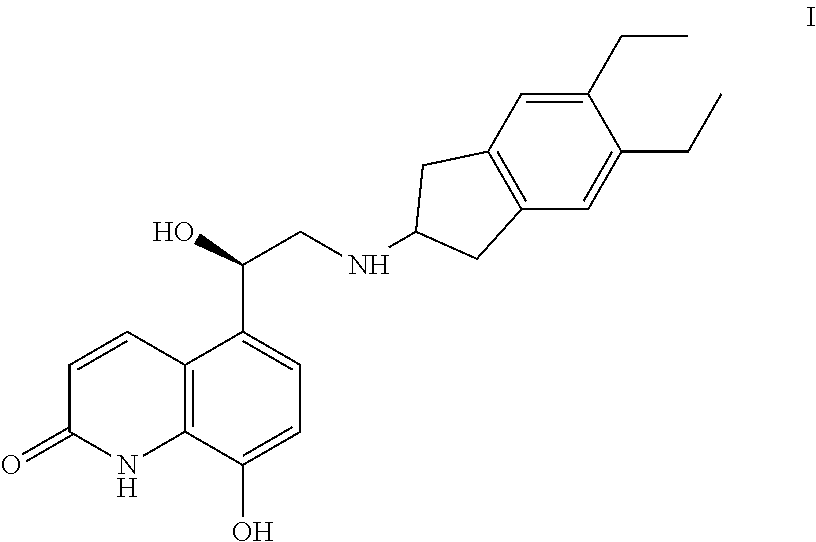
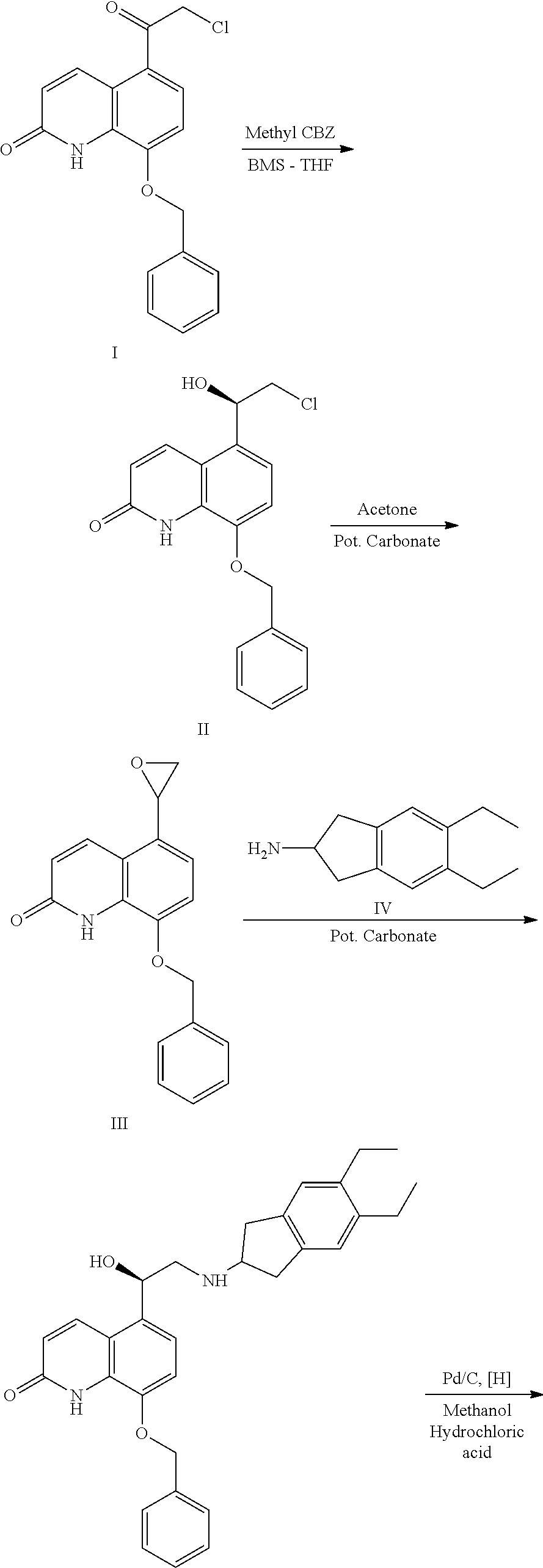
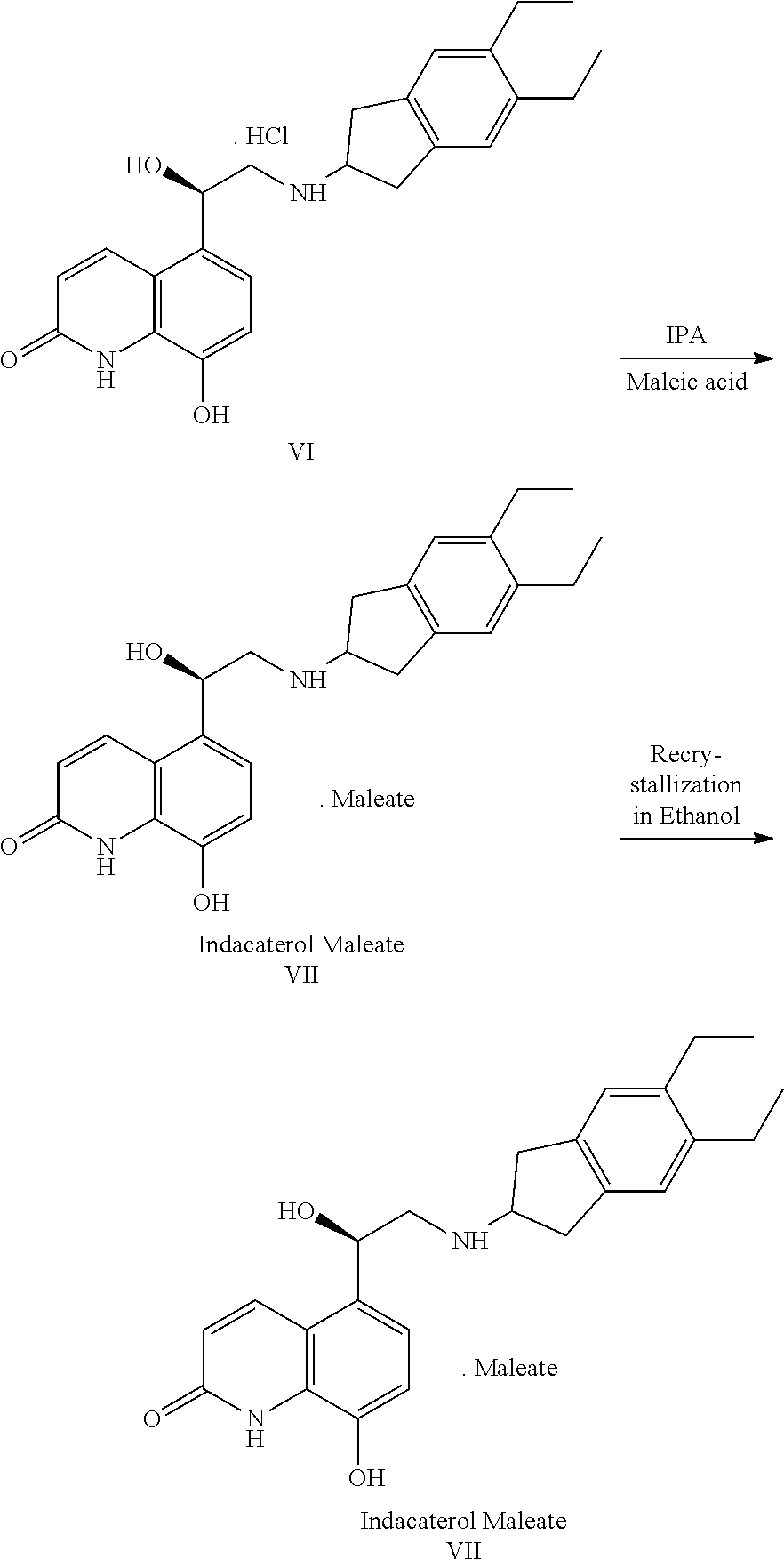
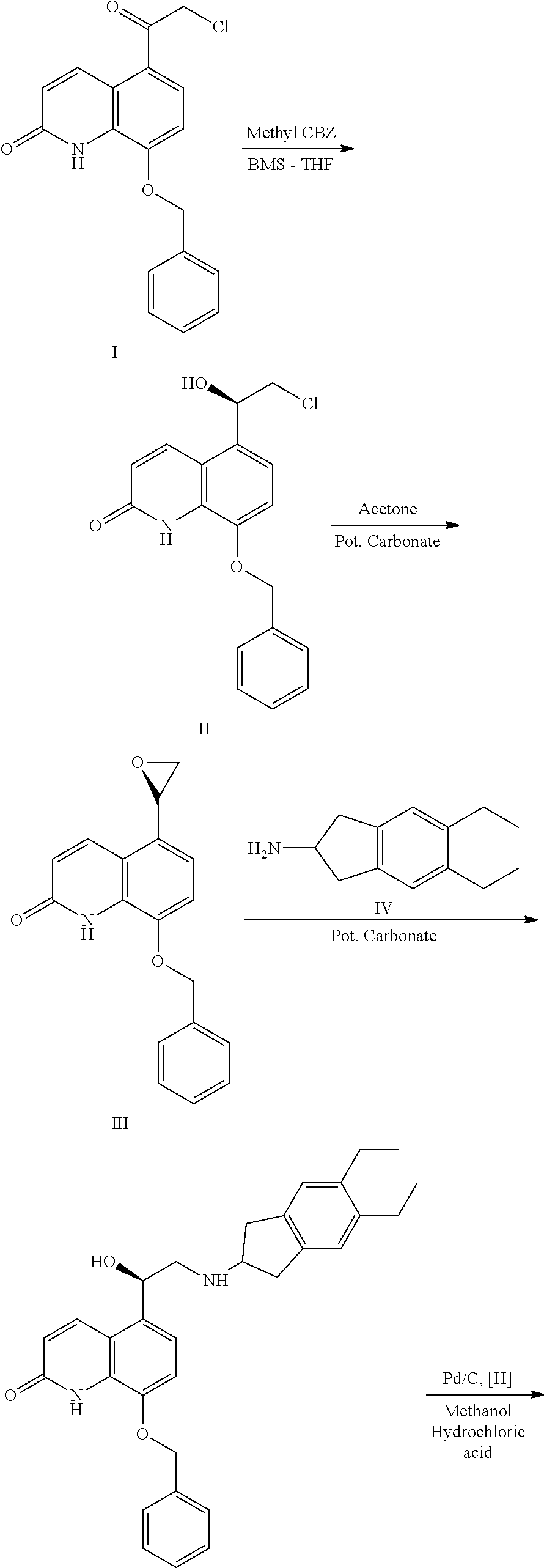
The invention relates to a preparation method of indacaterol maleate, in particular, the indacaterol maleate is prepared from 8-benzyloxy-5-(2-bromoacetyl)-2-hydroxyquinoline, (R)-2-methyl-CBS-oxazolidine borane, tert-butyldimethylsilyl trifluoromethanesulfonate, 5,6-diethyl-2,3-dihydro-1H-2-amine hydrochloride, palladium / carbon and maleic acid as raw materials through four-step reaction of chiralreduction reaction, TBS hydroxyl protection reaction, condensation reaction and hydrogenation reduction reaction, and the preparation method of the indacaterol maleate provided by the invention is high in yield, low in cost, environmentally friendly, easy to operate, and suitable for industrial production.
Owner:南京法恩化学有限公司
Novel Process for Preparation of Indacaterol or Its Pharmaceutically Acceptable Salts
A novel process the for preparation of Indacaterol or its pharmaceutically acceptable salts and novel intermediates employed in the preparation thereof that is economically significant for large scale.
Owner:RAO DAVULURI RAMAMOHAN MR
Indacaterol solid dispersion and pharmaceutical composition containing same
InactiveCN103830194AImprove solubilityDissolution rate is fastPowder deliveryOrganic active ingredientsIndacaterolSolubility
The invention provides an indacaterol solid dispersion and a pharmaceutical composition containing the same. The indacaterol solid dispersion comprises (1) indacaterol, (2) one or more of crosslinked polyvinylpyrrolidone, lactose, polyethylene glycol and microcrystalline cellulose; (3) sodium dodecyl sulfate. The pharmaceutical composition comprises the indacaterol solid dispersion and a pharmaceutically acceptable carrier. By making the indacaterol into a solid dispersion, the drug solubility is improved, the dissolution speed of the drug is increased, and the dissolution rate of the pharmaceutical composition containing the solid dispersion is greatly improved in a dissolving medium; moreover, in a grinding process, the premixing of the drug and partial accessories is realized, and the problems in the content uniformity of a small-dose preparation are remarkably reduced.
Owner:熊妲妮
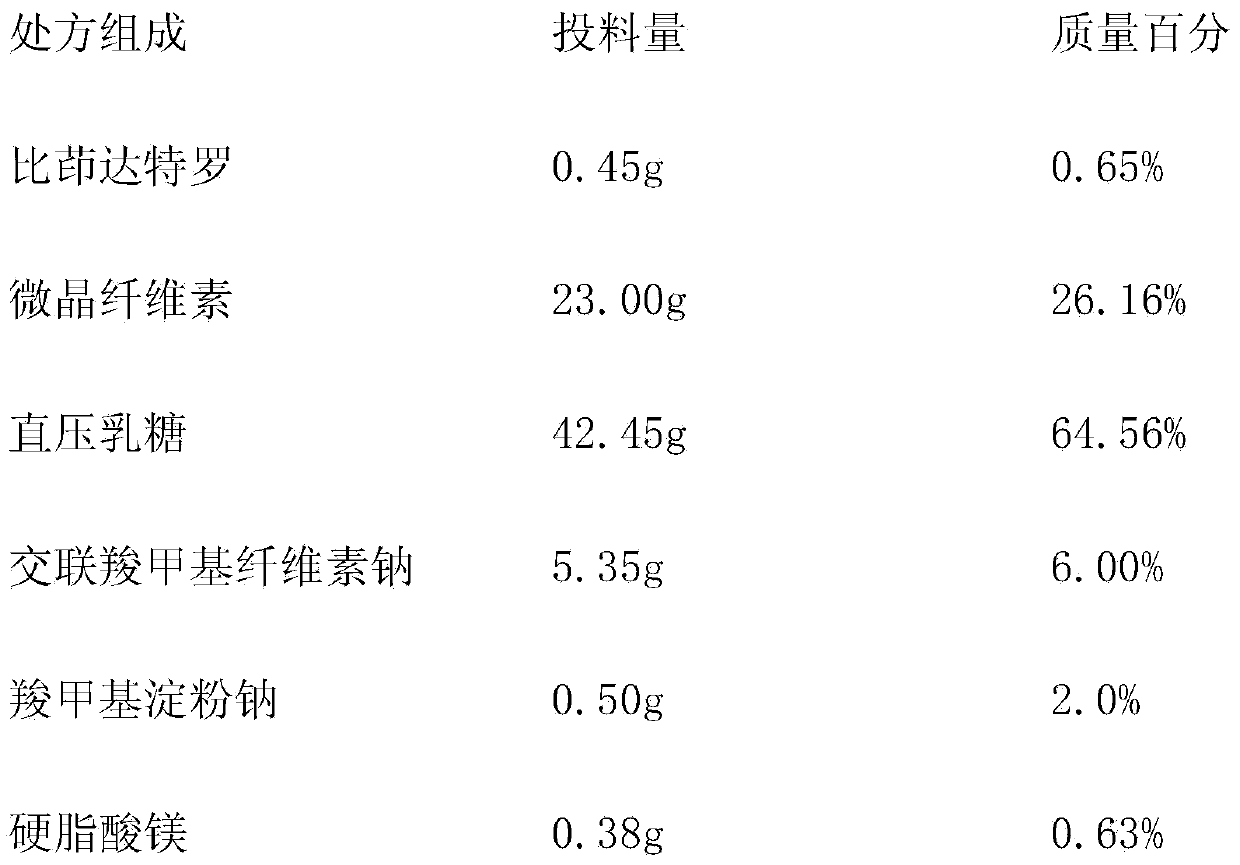
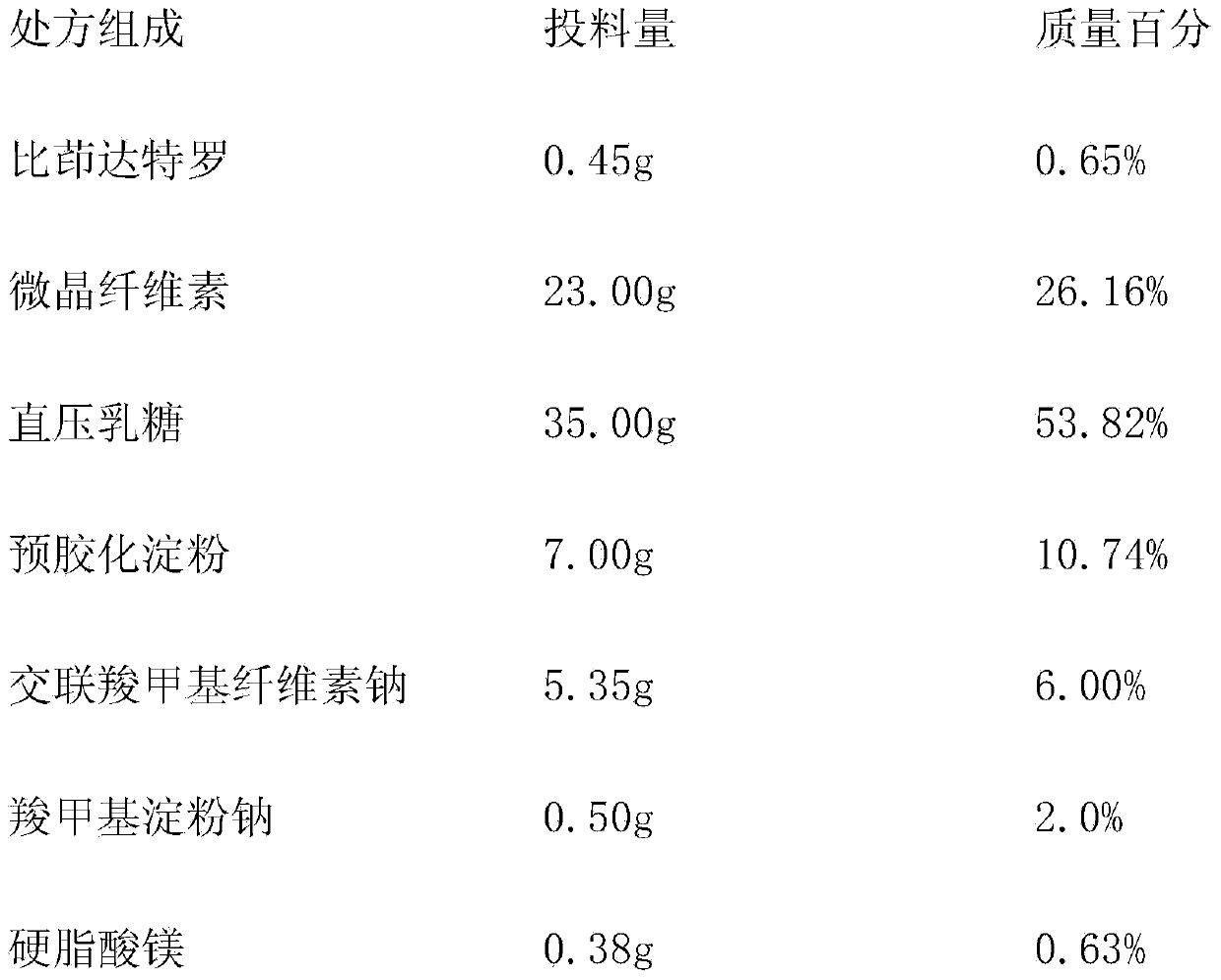
Indacaterol tablet and preparation method thereof
InactiveCN103830195AContent uniformity meets the requirementsGood lookingOrganic active ingredientsPill deliveryIndacaterolTreatment effect
The invention provides an indacaterol tablet and a preparation method thereof. According to the method, the indacaterol tablet is prepared in a manner that the powder is directly pressed to form the tablet. Thus, the production process is simple; the production cycle is shortened; and the equipment cost and the operating cost are lowered. The prepared indacaterol tablet is good in appearance, smooth in technological process, good in operability as well as high in dissolubility and has the qualified content uniformity; and the good bioavailability of the indacaterol tablet in a human body is realized. Thus, a good treatment effect is obtained.
Owner:熊妲妮
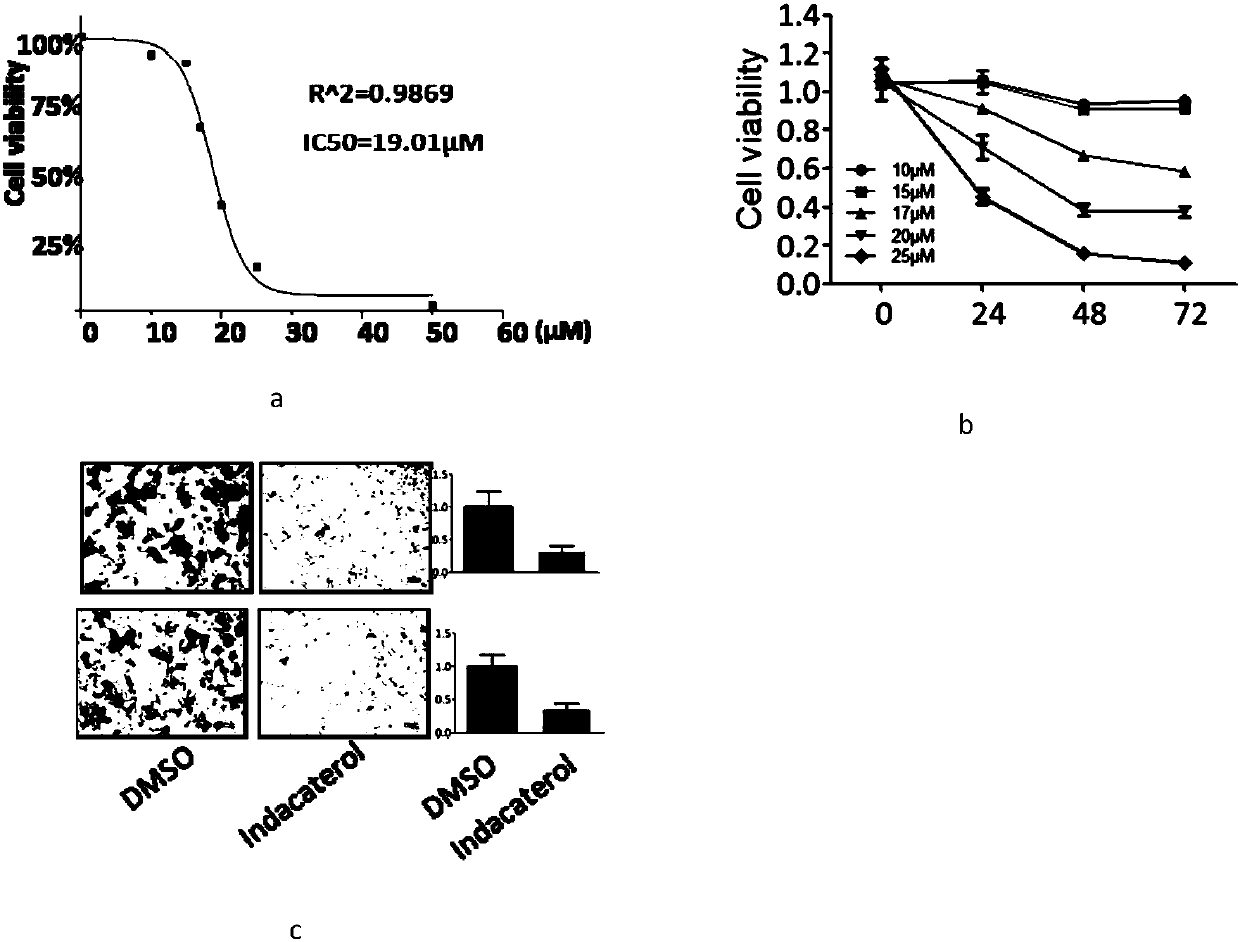
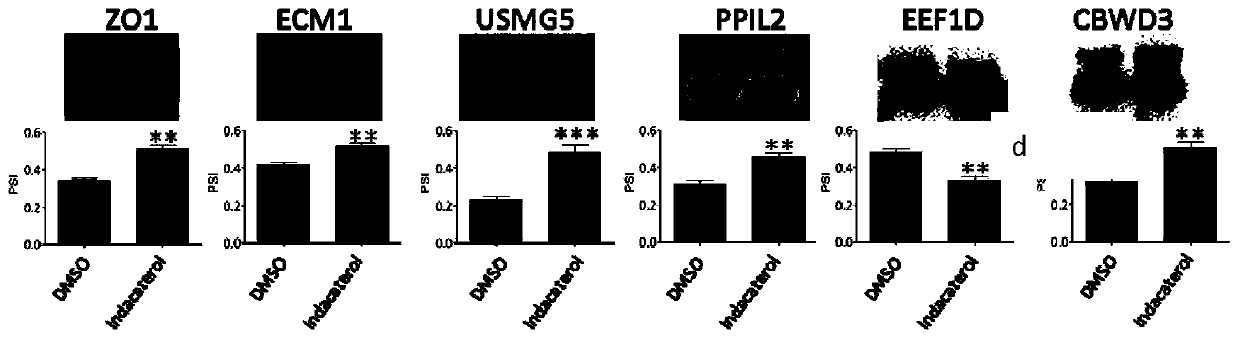
Application of indacaterol in treatment of colorectal cancer
ActiveCN107582550AAchieve inhibitionOrganic active ingredientsAntineoplastic agentsIndacaterolColorectal tumor
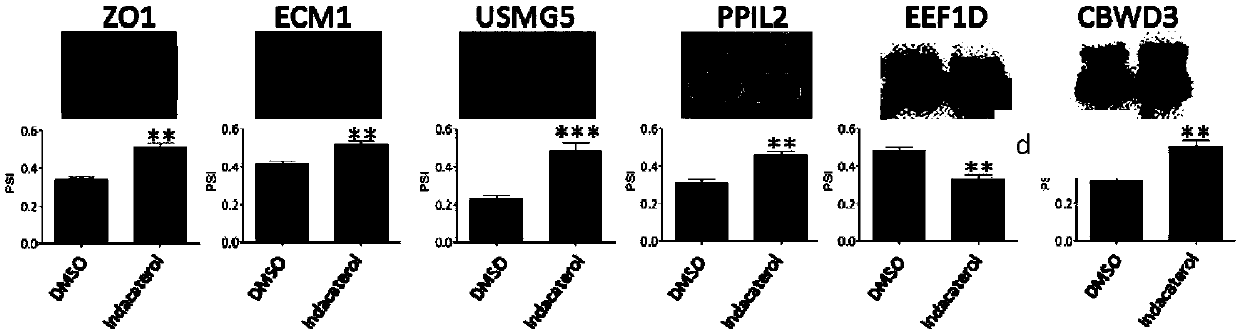
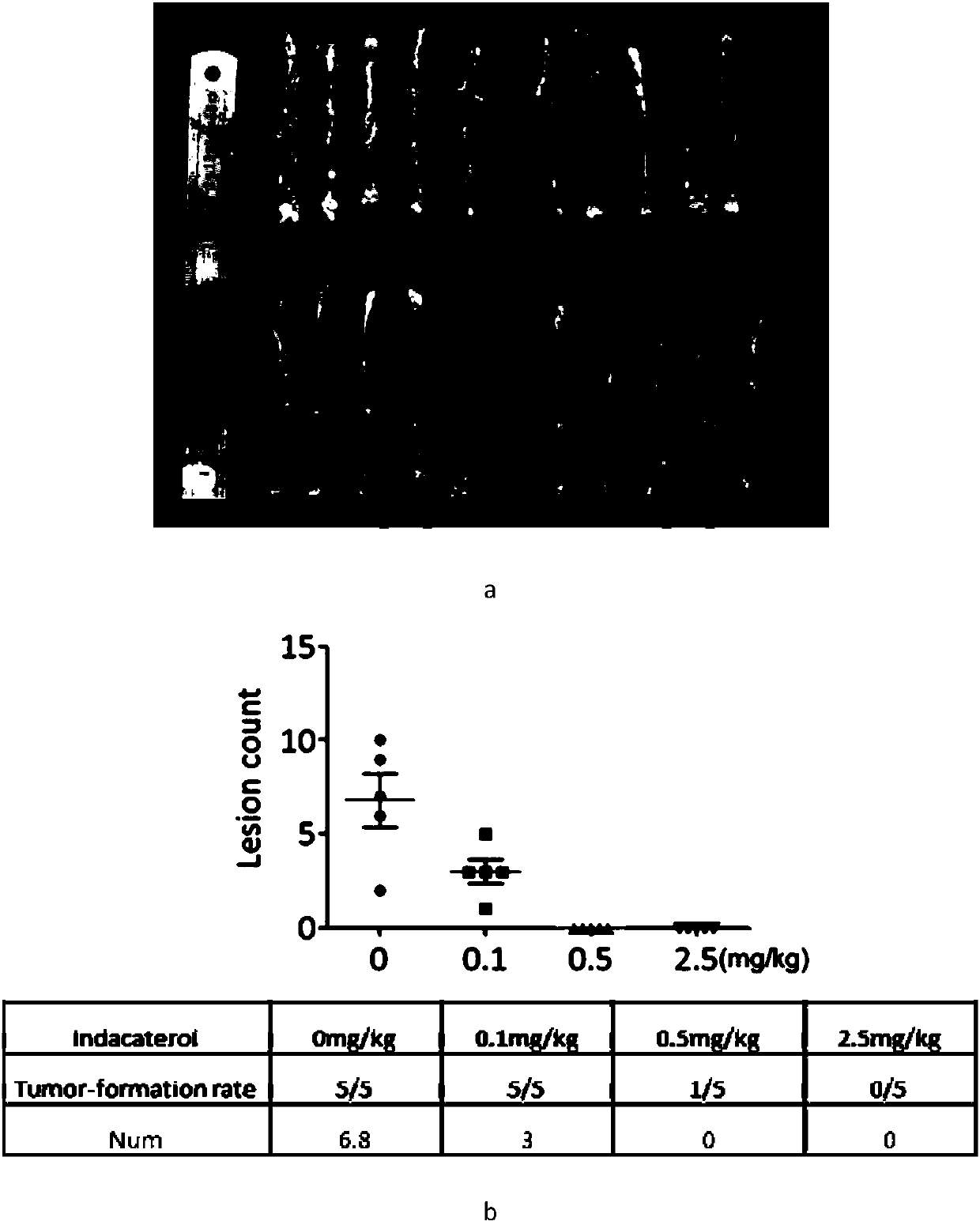
The invention discloses an application of indacaterol in the treatment of colorectal cancer, and belongs to the field of molecular biology, in particular, tumor treatment. Specifically, indacaterol selected from disclosed drugs can treat colorectal cancer. The results of cell experiments and animal experiments show that indacaterol can inhibit the proliferation, invasion and migration of colorectal tumor cell lines, and can obviously reduce the size of colorectal tumors of a mouse model. At the same time, for the first time, people find that indacaterol corrects the alternative splicing of tumor cells to achieve the effects mentioned above.
Owner:ZHEJIANG UNIV
Preparation method for indacaterol maleate
ActiveCN108250140AHigh optical purityShort stepsOrganic chemistryBulk chemical productionIndacaterolDrugs synthesis
The invention specifically relates to a preparation method for indacaterol maleate, belonging to the field of drug synthesis. The preparation method is simple and short in steps, easy to operate, safe, friendly to environment and fee of an alkaline solution; after a heating reaction, a coupled product in the step 1) can be obtained, and the amount of produced regioisomers, dimers and other by-products is small; the yield of the product indacaterol maleate is high, as high as 93%, and the HPLC purity of the product indacaterol maleate is more than 97.8%; post-treatment is simple, and industrialproduction of indacaterol maleate is facilitated; and the product indacaterol maleate is high in optical purity.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Indacaterol and roflumilast-containing pharmaceutical combined product
The invention relates to a combination of inhaled / oral roflumilast or its pharmaceutically acceptable salt combined with inhaled indacaterol or its pharmaceutically acceptable salt, and the pharmaceutical combined product is used for simultaneous, successive or respective drug administration, is used for treatment or prevention of respiratory tract disease or its symptoms, and is especially used for treatment of obstruction or inflammation-accompanying diseases such as chronic obstructive pulmonary disease (COPD) or asthma.
Owner:QINGDAO CITY CHENGYANG DISTRICT PEOPLES HOSPITAL
Indacaterol maleate intermediate, and preparation method and application thereof
ActiveCN109721534AGood removal effectEasy to prepareOrganic chemistryBulk chemical productionIndacaterolQuinoline
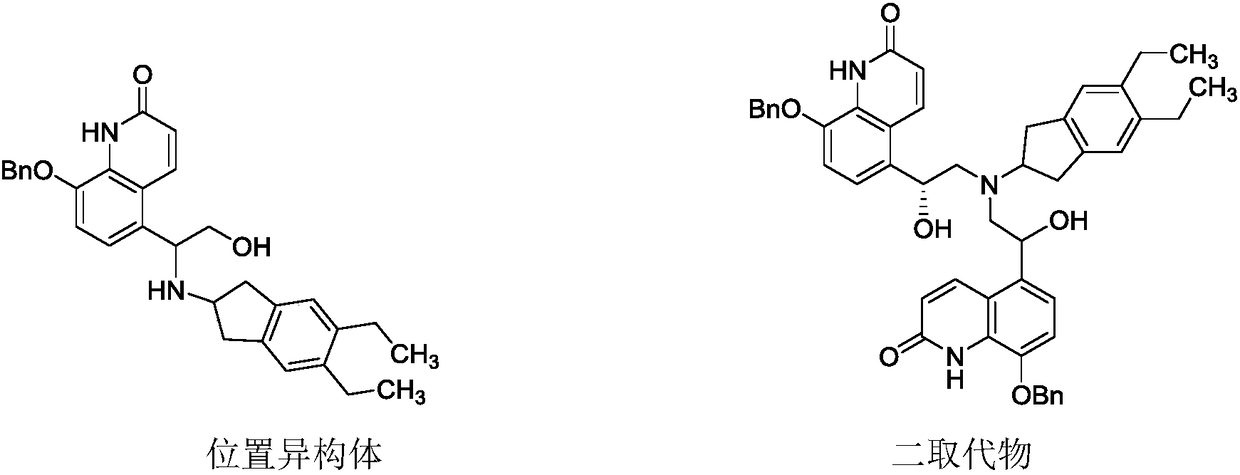
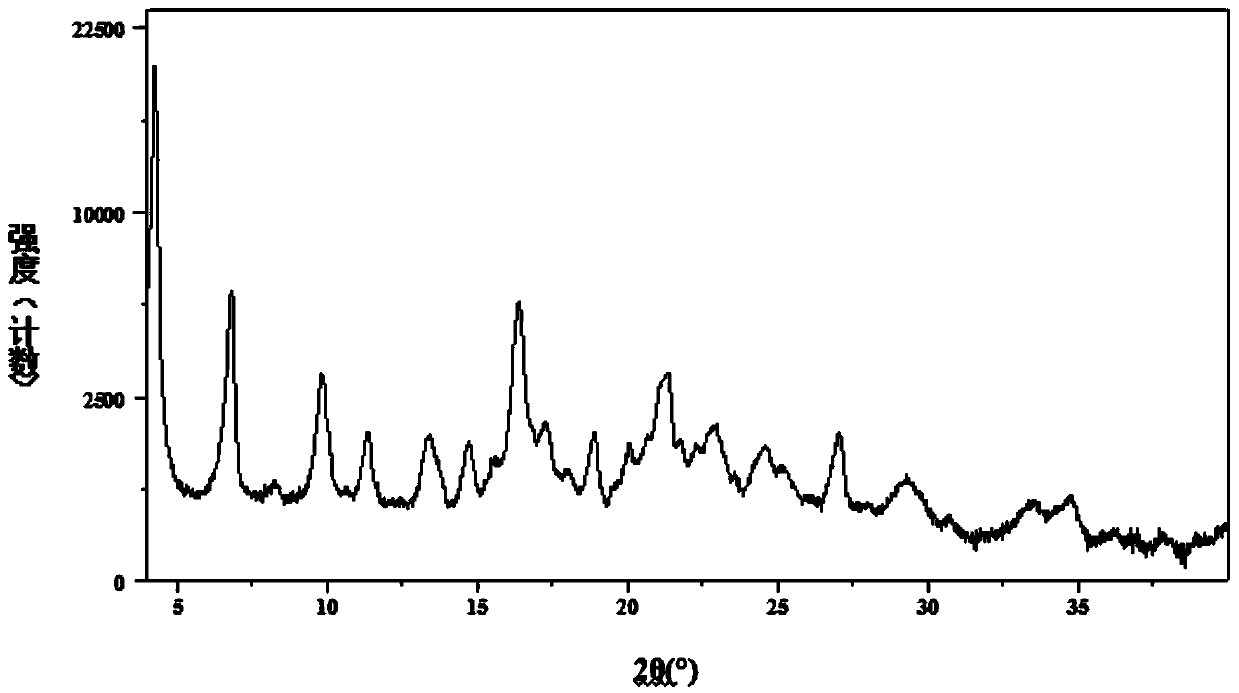
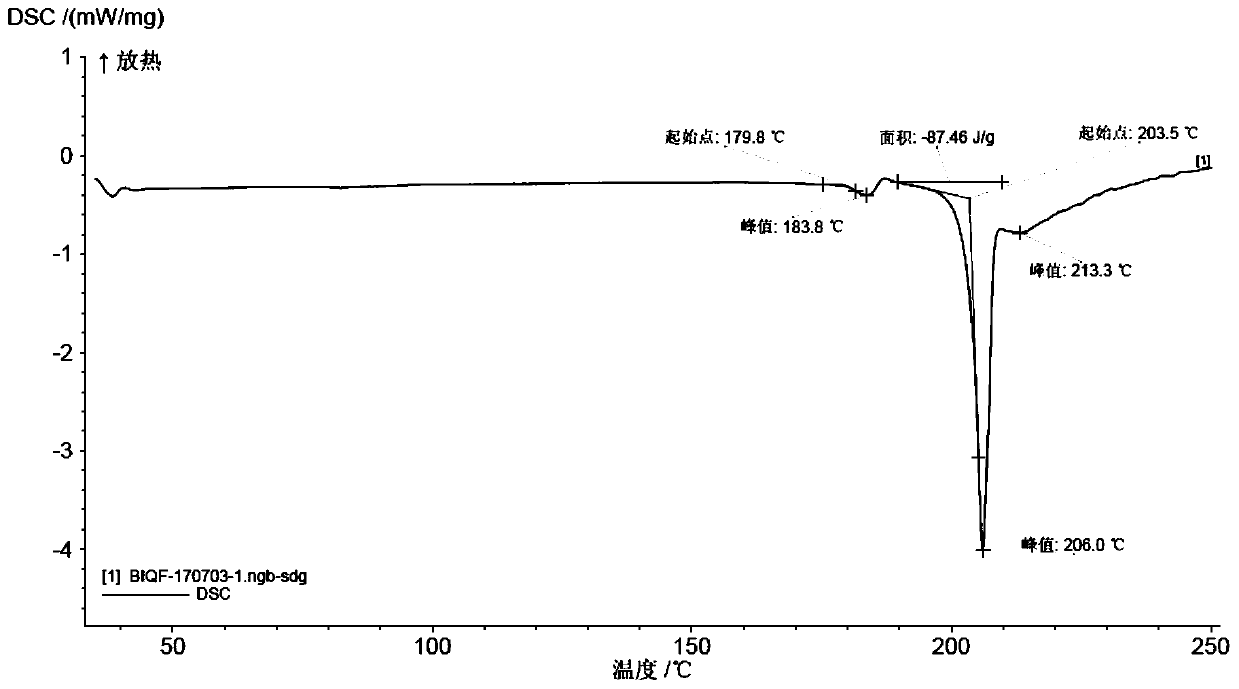
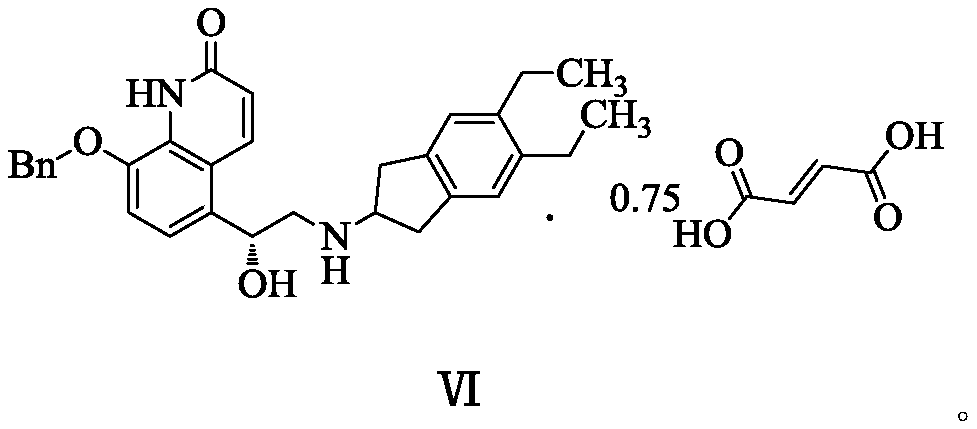
The invention relates to a novel salt form of an indacaterol maleate intermediate namely (R)-8-(benzyloxy)-5-[2-[(5,6-diethyl-2,3-dihydro-1H-indene-2-yl)amino]-1-hydroxyethyl]quinoline-2(1H)-one and acrystal form thereof. The salt form and the crystal form thereof provided by the invention have good impurity removal effect and thermodynamic stability and simple and convenient preparation method,facilitate operation and storage, and are applicable to industrial application. The invention also relates to preparation methods for the salt form and the crystal form of the salt form, and an application of the salt form and the crystal form of the salt form in preparation of indacaterol maleate.
Owner:SICHUAN HAISCO PHARMA CO LTD
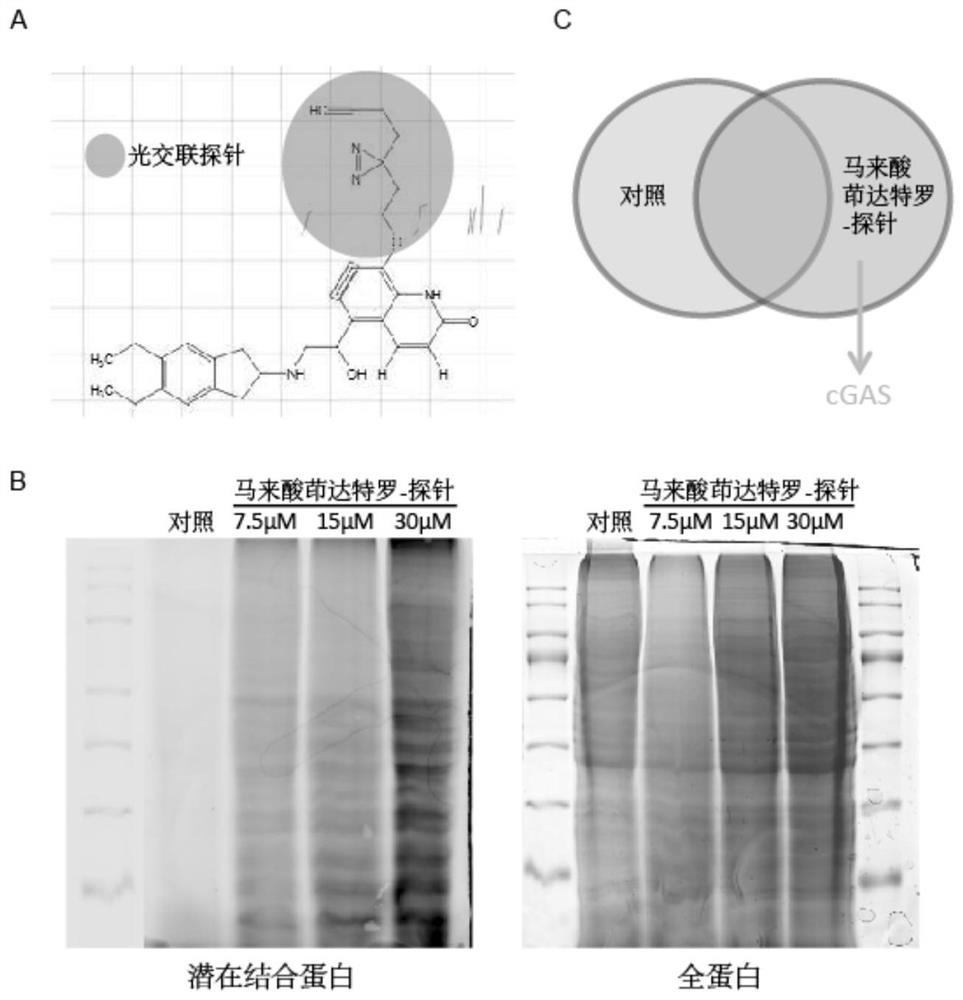
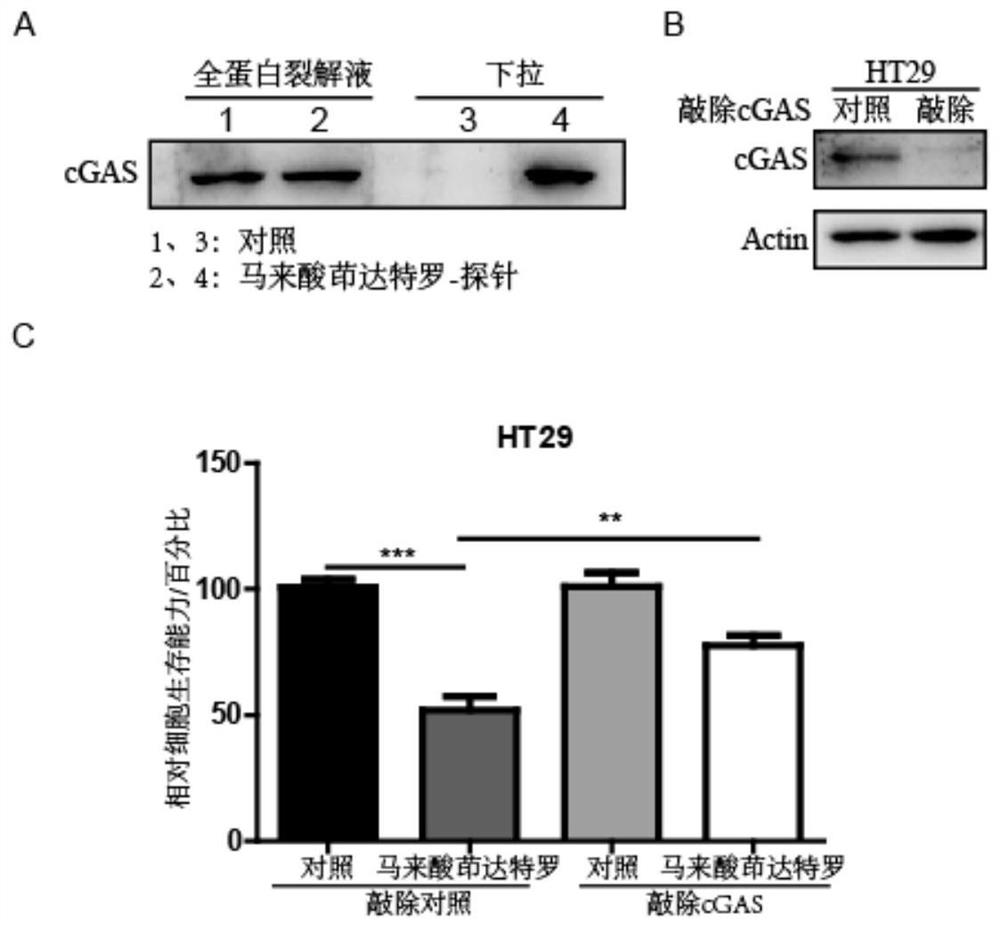
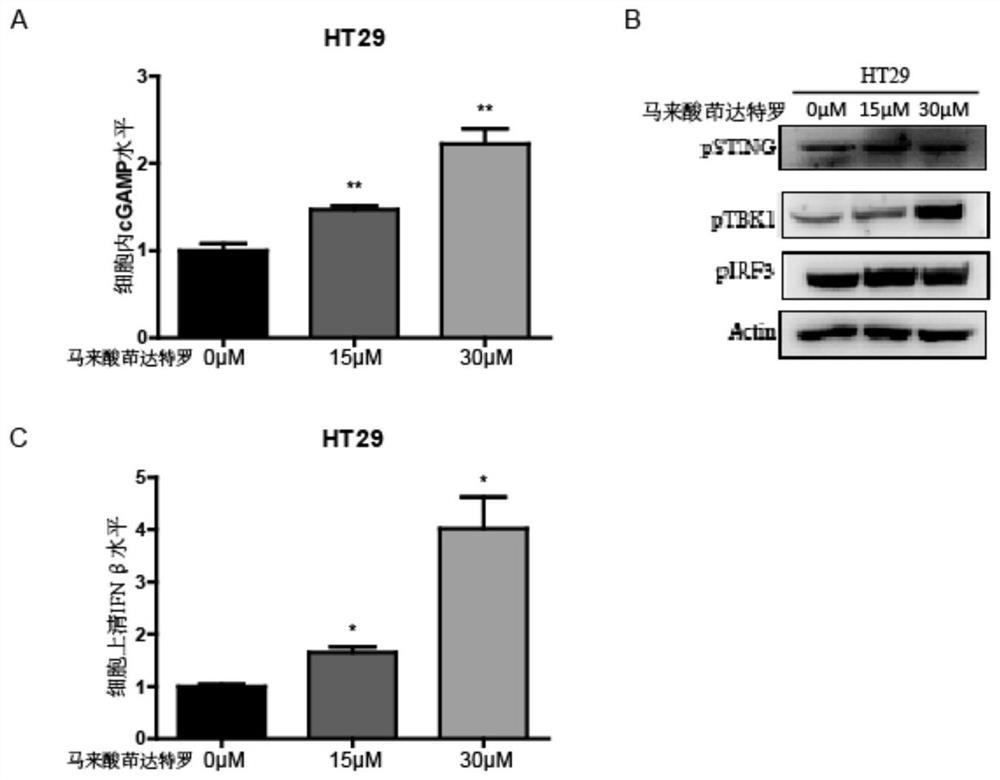
Application of indacaterol maleate as cGAS-STING pathway targeted agonist
PendingCN113876772ALow costImprove securityOrganic active ingredientsAntineoplastic agentsIndacaterolDisease
The invention discloses application of indacaterol maleate as a cGAS-STING pathway targeted agonist. In-vitro level verification, biotin photoaffinity labeling, conjugated protein omics, bioinformatics and molecular biology results show that the indacaterol maleate can activate a cGAS-STING pathway in a targeted manner and inhibit cancer cell proliferation and has a good treatment prospect in adjuvant therapy of colorectal cancer related diseases.
Owner:JINAN UNIVERSITY
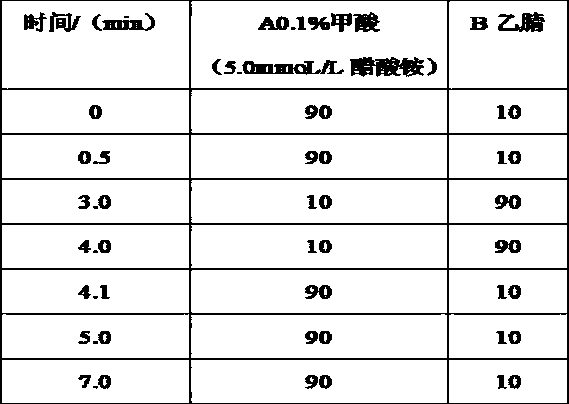
Method for determining salmeterol, indacaterol and olodaterol in pork
InactiveCN108982712ASolve detection gapsHigh ion responseComponent separationIndacaterolSpectrometer
The invention provides a method for determining salmeterol, indacaterol and olodaterol in pork. The method includes the following steps: step 1, preparing samples; step 2, extracting to-be-detected samples; step 3, determining detection conditions of a liquid chromatography-tandem mass spectrometer and performing detection; step 4, drawing standard working curves; step 5, according to liquid chromatograms of to-be-detected solutions in the S2, calculating the contents of the olodaterol, the indacaterol and the salmeterol in the pork. The method has the advantages that the salmeterol, the indacaterol and the olodaterol in the pork are simultaneously determined for the first time, and the blank in current detection of the salmeterol, the indacaterol and the olodaterol in the pork is filled up; ion response values are high, sensitivity is high, detection limits are low, qualitativeness, quantitativeness, accuracy, rapidness, high efficiency and sensitivity are truly realized, and the method can serve as a reliable measure to detect the three drugs in the pork.
Owner:食药环检验研究院(山东)集团有限公司
Process for preparation of indacaterol or its pharmaceutically acceptable salts
A novel process the for preparation of Indacaterol or its pharmaceutically acceptable salts and novel intermediates employed in the preparation thereof that is economically significant for large scale.
Owner:RAO DAVULURI RAMAMOHAN MR
Preparation method of indacaterol intermediate
InactiveCN108752220AHigh yieldEasy to operateOrganic compound preparationCarboxylic acid amides preparationIndacaterolColumn chromatography
The invention provides a preparation method of an indacaterol intermediate. According to the preparation method provided by the invention, low-price raw materials are adopted, the operation is simpleand feasible, the reaction selectivity is good, complicated operations such as column chromatography are not required, the yield of a final prepared product is as high as 92.6%, and the total yield ofa whole route is as high as 71.2%, so that the preparation method is very suitable for industrial production.
Owner:成都伊诺达博医药科技有限公司
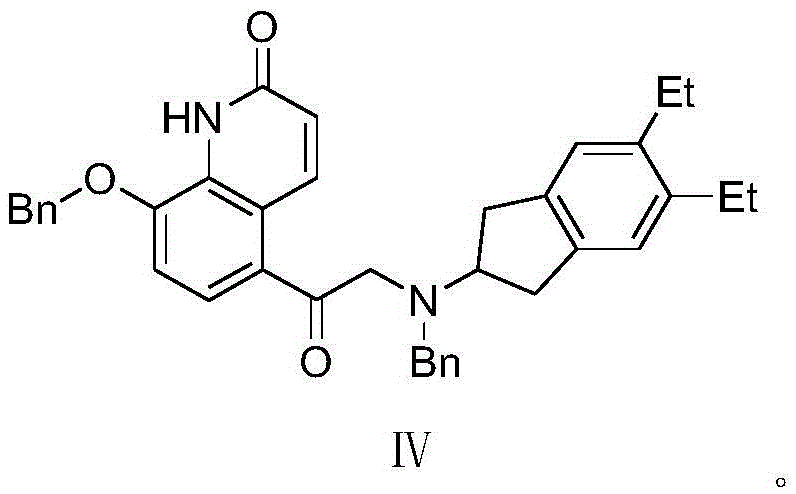
The synthetic method of indacaterol intermediate and indacaterol
ActiveCN104379566BAvoid it happening againEasy to operateOrganic chemistryIndacaterolSynthesis methods
Disclosed is an intermediate for synthesizing indacaterol, having a structure represented by formula IV. Also disclosed are a method for synthesizing the indacaterol intermediate and a method for synthesizing indacaterol by using the intermediate. During synthesis of indacaterol, the intermediate of formula IV is reduced to obtain a chiral or racemic compound, which is then debenzylated to obtain indacaterol or a raceme thereof. By using the compound of formula IV as an intermediate for synthesizing indacaterol, the present invention provides a new route for synthesizing indacaterol, and avoids various byproducts produced in the process of synthesizing indacaterol using conventional methods.
Owner:SHANGHAI VIWIT PHARMA CO LTD
Improved preparation process of indacaterol maleate
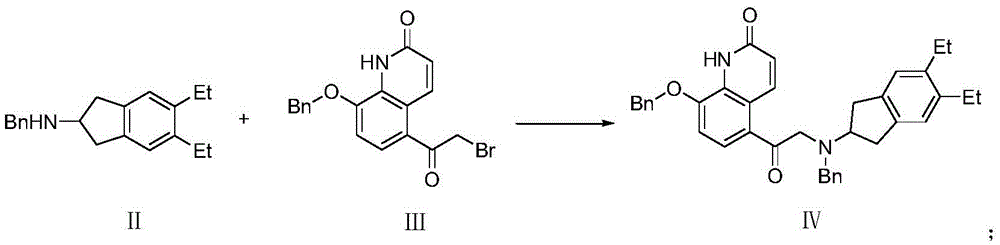
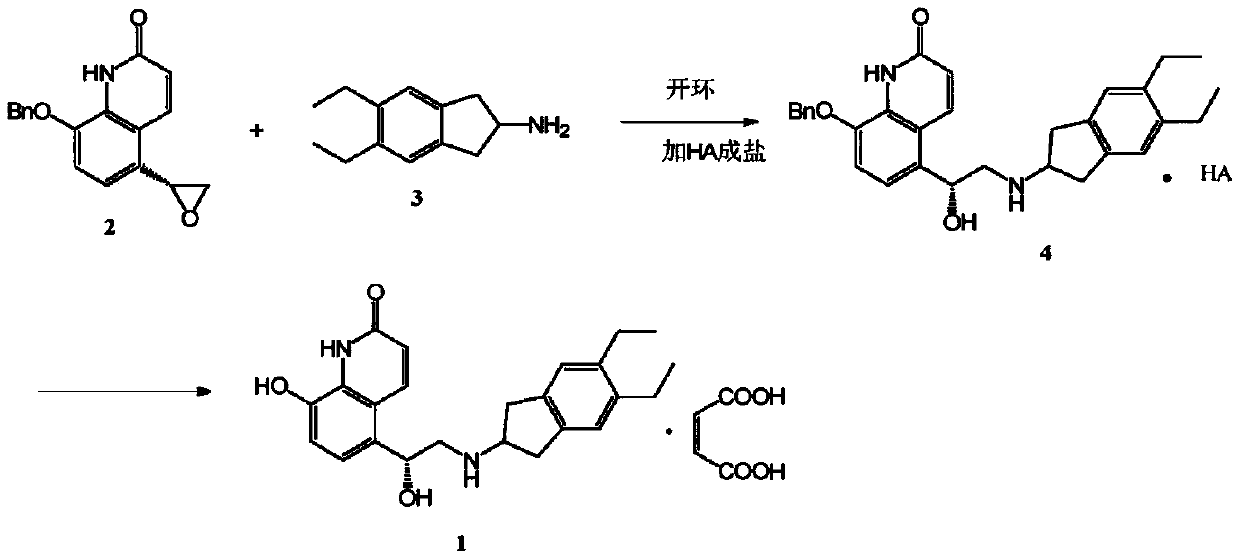
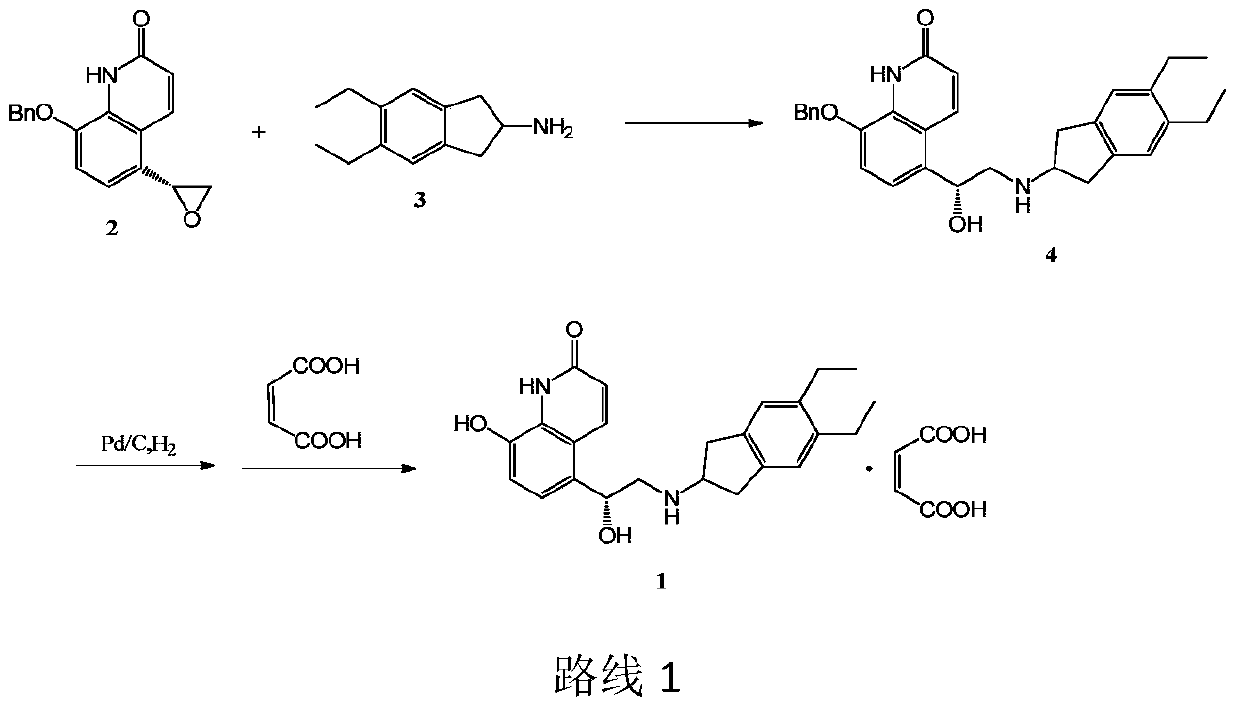
The invention discloses an improved indacaterol maleate preparation technology. The technology comprises the following steps: 1, carrying out a ring opening reaction on a compound 2 and a compound 3 in a solvent to obtain a compound 4, carrying out salt formation on the compound 4 and an organic acid HA in a second solvent to obtain a compound 4.HA salt, and carrying out crystallization treatment to precipitate the compound 4.HA salt; and 2, carrying out catalytic hydrogenation debenzylation on the compound 4.HA salt in the presence of a catalyst, and carrying out salt formation on the compound 4.HA and maleic acid to obtain indacaterol maleate. The improved indacaterol maleate preparation technology greatly shortens the reaction time, and the obtained indacaterol maleate product has high yield and purity, so the integral technology has the advantages of simple operation and suitableness for industrialization.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Application of indacaterol in the treatment of colorectal cancer
ActiveCN107582550BAchieve inhibitionOrganic active ingredientsAntineoplastic agentsIndacaterolColorectal tumor
Owner:ZHEJIANG UNIV
Preparation method of indacaterol intermediate diethylbenzene
InactiveCN103896714AHigh yieldRaw materials are easy to getHydrocarbonsHydrocarbon preparationIndacaterolAcetophenone
The invention provides a preparation method of an intermediate diethylbenzene. The preparation method comprises the steps: reacting diethyl acetophenone and hydrazine hydrate, and making a compound react with alkaline to obtain the diethylbenzene. The preparation method provided by the invention has the advantages of availability of raw materials, simplicity and convenience in operation, good quality, high yield and low cost, and is suitable for large-scale industrialized production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of indacaterol and salt thereof
ActiveCN111808021ACost of protectionQuality assuranceOrganic compound preparationOrganic chemistry methodsM-chlorobenzoic acidBenzoic acid
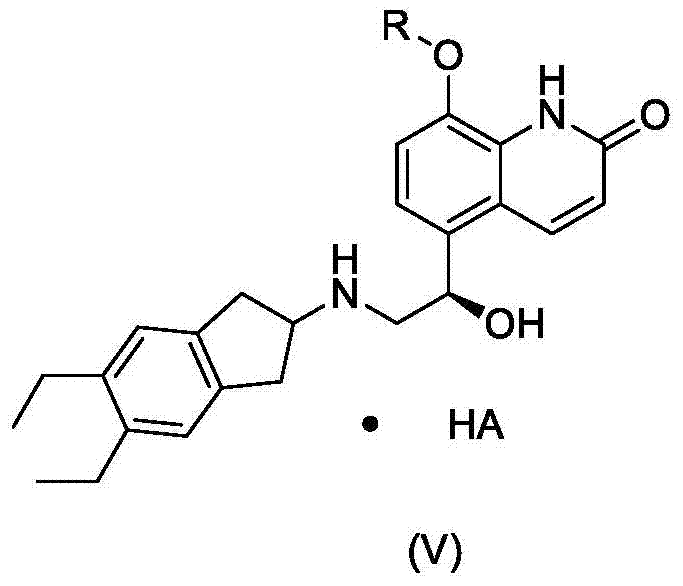
The invention relates to a preparation method of indacaterol and a salt thereof. The preparation method comprises the following steps: in a solvent, reacting a mixture containing a compound as shown in a formula I with m-chlorobenzoic acid to obtain a compound as shown in a formula II-1; and subjecting the obtained compound as shown in the formula II-1 to reacting and conversion to obtain indacaterol and the salt thereof. The preparation method of indacaterol and the salt thereof has the advantages of higher yield, high purity, easiness in refining, simple and convenient operation and suitability for industrialization.
Owner:SHANGHAI ANOVENT PHARMA CO LTD
Inhalable preparation containing indacaterol maleate and glycopyrronium bromide solution
The present invention discloses a liquid, propellant-free pharmaceutical formulation and a method of administering the pharmaceutical formulation by nebulizing the pharmaceutical formulation in an inhaler. The liquid, propellant-free pharmaceutical preparation includes: (a) an active substance selected from glycopyrronium bromide and indacaterol maleate; (b) a solvent; (c) a pharmaceutically acceptable preservative, any Optionally include a pharmaceutically acceptable stabilizer, a pharmaceutically acceptable solubilizer, a pharmaceutically acceptable co-solvent or other pharmaceutically acceptable additives.
Owner:GUANGZHOU ANOVENT PHARM CO LTD
Indacaterol maleate liquid preparation and preparation method thereof
The invention relates to an indacaterol maleate liquid preparation which comprises an active ingredient, a diluent and a solvent, wherein the active ingredient is indacaterol maleate, per 1,000 granules of the liquid preparation contains 0.3-0.8g of indacaterol maleate and 150-300g of diluent. The invention prepares indacaterol maleate into a liquid preparation, solves the poor homogeneity problem of indacaterol maleate in other preparations, and provides an indacaterol maleate liquid preparation with good homogeneity.
Owner:QINGDAO CITY CHENGYANG DISTRICT PEOPLES HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com