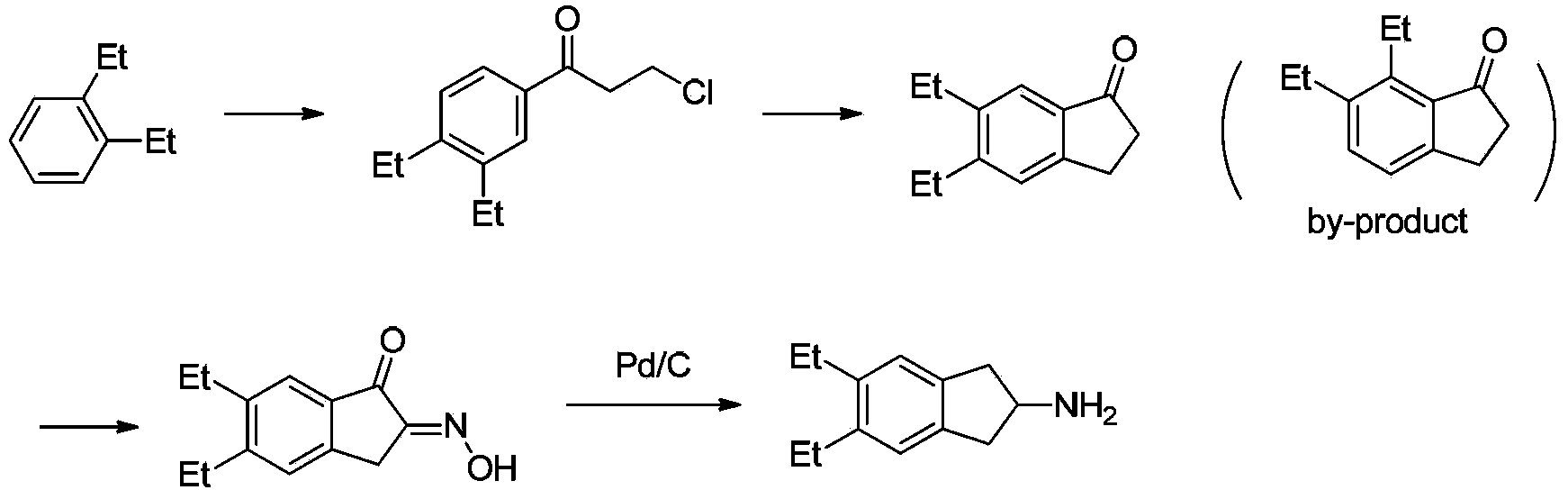
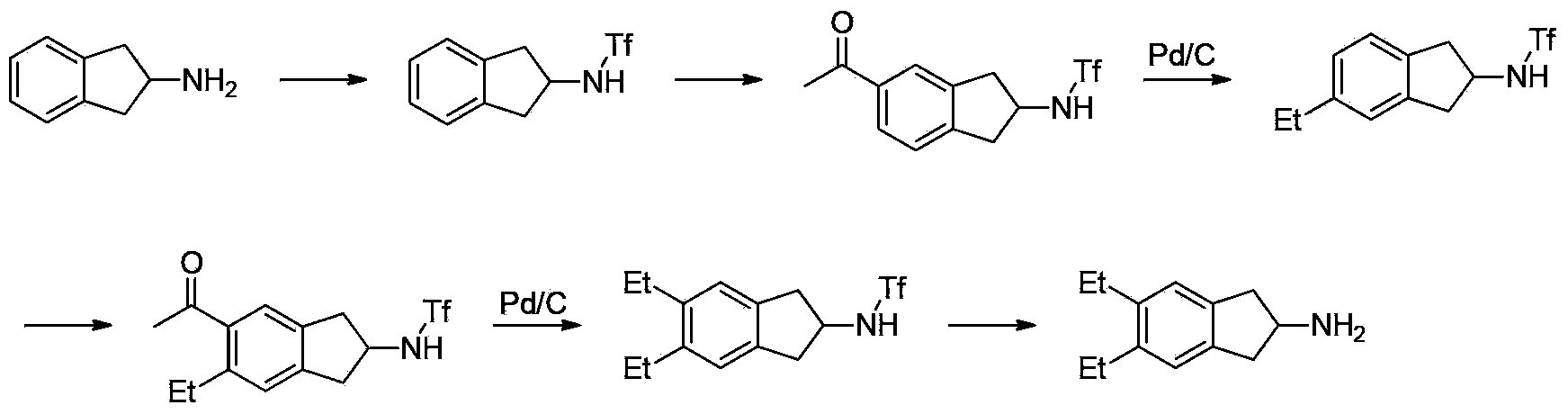
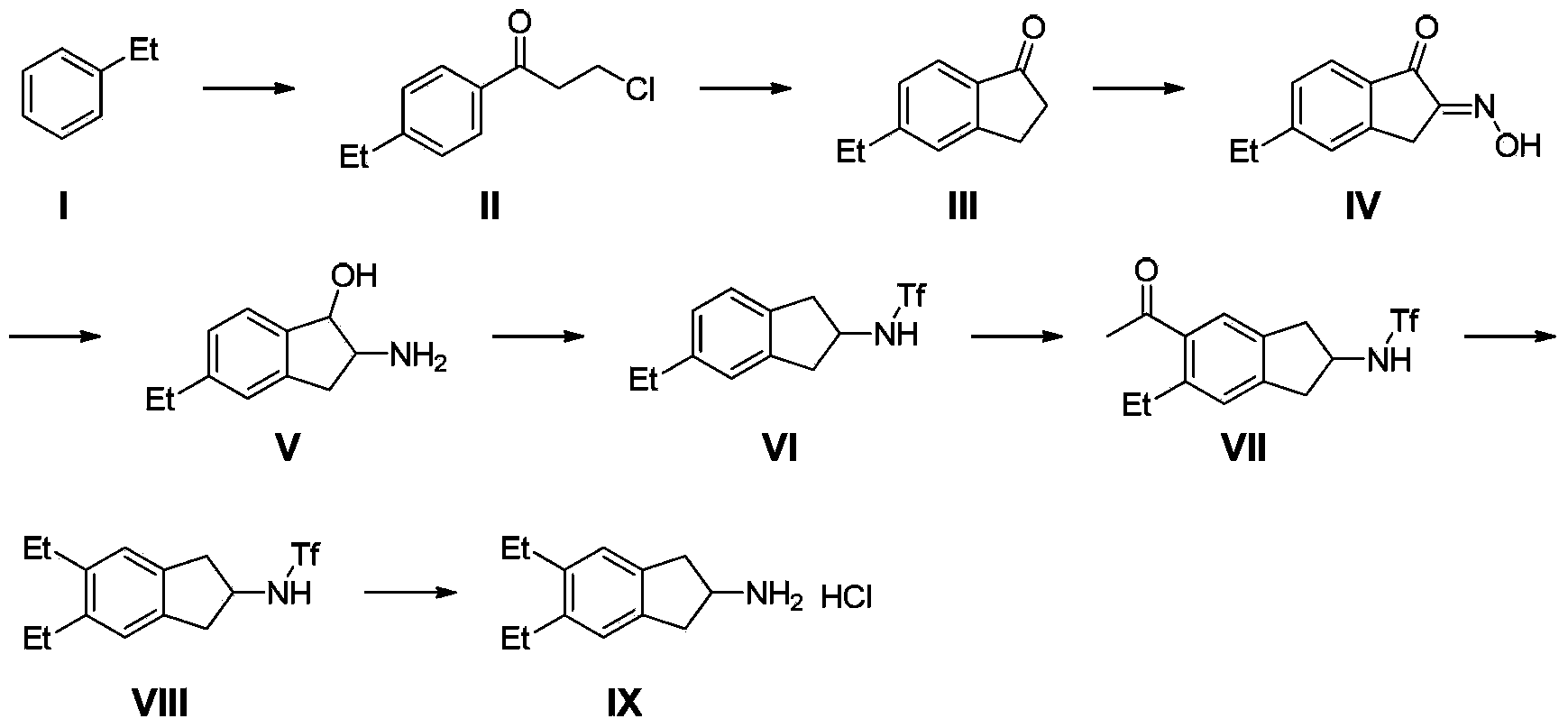
Synthesizing method of indacaterol amino fragment 5,6-diethyl-2,3-dihydro-1H-inden-2-amine hydrochloride
A technology of amine hydrochloride and diethyl, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of poor economy and environmental friendliness, unsuitable for scale-up production, difficult to industrialized production and the like, and achieves low production cost and large scale. Implementation of value and socioeconomic benefits, mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037](1) Put 4.1Kg of zinc chloride and 10L of dichloromethane into the reaction kettle, cool down to about -5°C in an ice bath, add 1.55Kg of 3-chloropropionyl chloride and 1.3Kg of ethylbenzene dropwise in sequence, and then rise to room temperature React for 2 hours. The reaction was processed in a conventional manner to obtain 3.2Kg of 3-chloro-1-(4-ethylphenyl)propan-1-one, with a yield of 92%.
[0038] (2) Add 2kg of 3-chloro-1-(4-ethylphenyl)propan-1-one in batches to a reaction kettle filled with 10L of concentrated sulfuric acid, raise the temperature to 100°C after the addition, and react for 1.5 hours. Pour into ice water for extraction, and treat according to conventional methods to obtain 1.5Kg of 5-ethyl-2,3-dihydro-1H-inden-1-one. The yield is 92%.
[0039] (3) Dissolve 900g of 5-ethyl-2,3-dihydro-1H-inden-1-one methanol in 4.5L of methanol, add 700g of n-butyl nitrite dropwise, react at 40°C for 30 minutes, then add dropwise Concentrated hydrochloric acid 5...
Embodiment 2
[0046] (1) Put 4.0Kg of aluminum trichloride and 10L of dichloromethane into the reaction kettle, cool down to about -5°C in an ice bath, add 1.55Kg of 3-chloropropionyl chloride and 1.3Kg of ethylbenzene dropwise in sequence, and then rise to React at room temperature for 2 hours. The reaction was processed in a conventional manner to obtain 3.4Kg of 3-chloro-1-(4-ethylphenyl)propan-1-one, with a yield of 98%.
[0047] (2) Add 2kg of 3-chloro-1-(4-ethylphenyl)propan-1-one in batches to a reaction kettle filled with 5L of concentrated sulfuric acid and 5L of 1,2-dichloroethane. Afterwards, the temperature was raised to reflux for 1.5 hours, poured into ice water for extraction, and treated according to conventional methods to obtain 1.43 Kg of 5-ethyl-2,3-dihydro-1H-inden-1-one. Yield 88%.
[0048] (3) Dissolve 900g of 5-ethyl-2,3-dihydro-1H-inden-1-one methanol in 4.5L of methanol, add 700g of n-butyl nitrite dropwise, react at 40°C for 30 minutes, then add dropwise Concen...
Embodiment 3
[0055] (1) Put 7.8Kg of tin tetrachloride and 10L of carbon disulfide into the reaction kettle, cool down to about -5°C in an ice bath, add 1.55Kg of 3-chloropropionyl chloride and 1.3Kg of ethylbenzene dropwise in sequence, and then rise to room temperature for reaction 2 hours. The reaction was processed in a conventional manner to obtain 2.78Kg of 3-chloro-1-(4-ethylphenyl)propan-1-one, with a yield of 80%.
[0056] (2) Add 2kg of 3-chloro-1-(4-ethylphenyl)propan-1-one in batches to a reaction kettle filled with 5kg of polyphosphoric acid and 20L of 1,2-dichloroethane, add After completion, the temperature was raised to reflux for 6 hours, poured into ice water for extraction, and treated according to conventional methods to obtain 1.2Kg of 5-ethyl-2,3-dihydro-1H-inden-1-one. Yield 74%.
[0057] (3) Dissolve 900g of 5-ethyl-2,3-dihydro-1H-inden-1-one methanol in 4.5L of methanol, add 700g of n-butyl nitrite dropwise, react at 40°C for 30 minutes, then add dropwise Concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com