Novel methods for preparing cobalt oxide, nickel oxide and copper oxide
A technology of copper oxide and nickel oxide, applied in the field of preparation, can solve the problem of difficulty in obtaining a product with uniform particle size distribution, such as tricobalt tetroxide, and achieve the effects of low cost, cost reduction and product cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
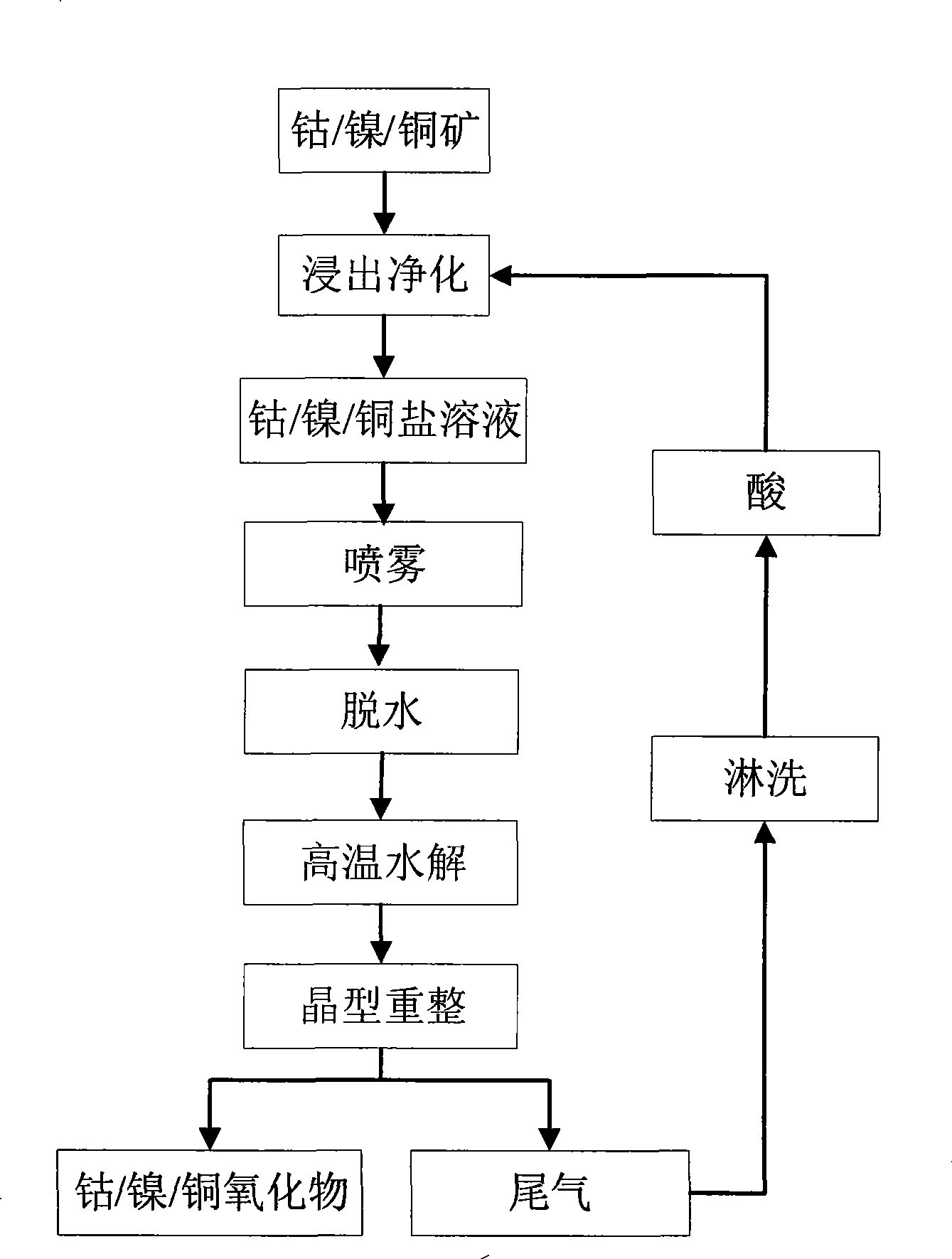
[0024] Embodiment 1, with reference to figure 1 and 2 .
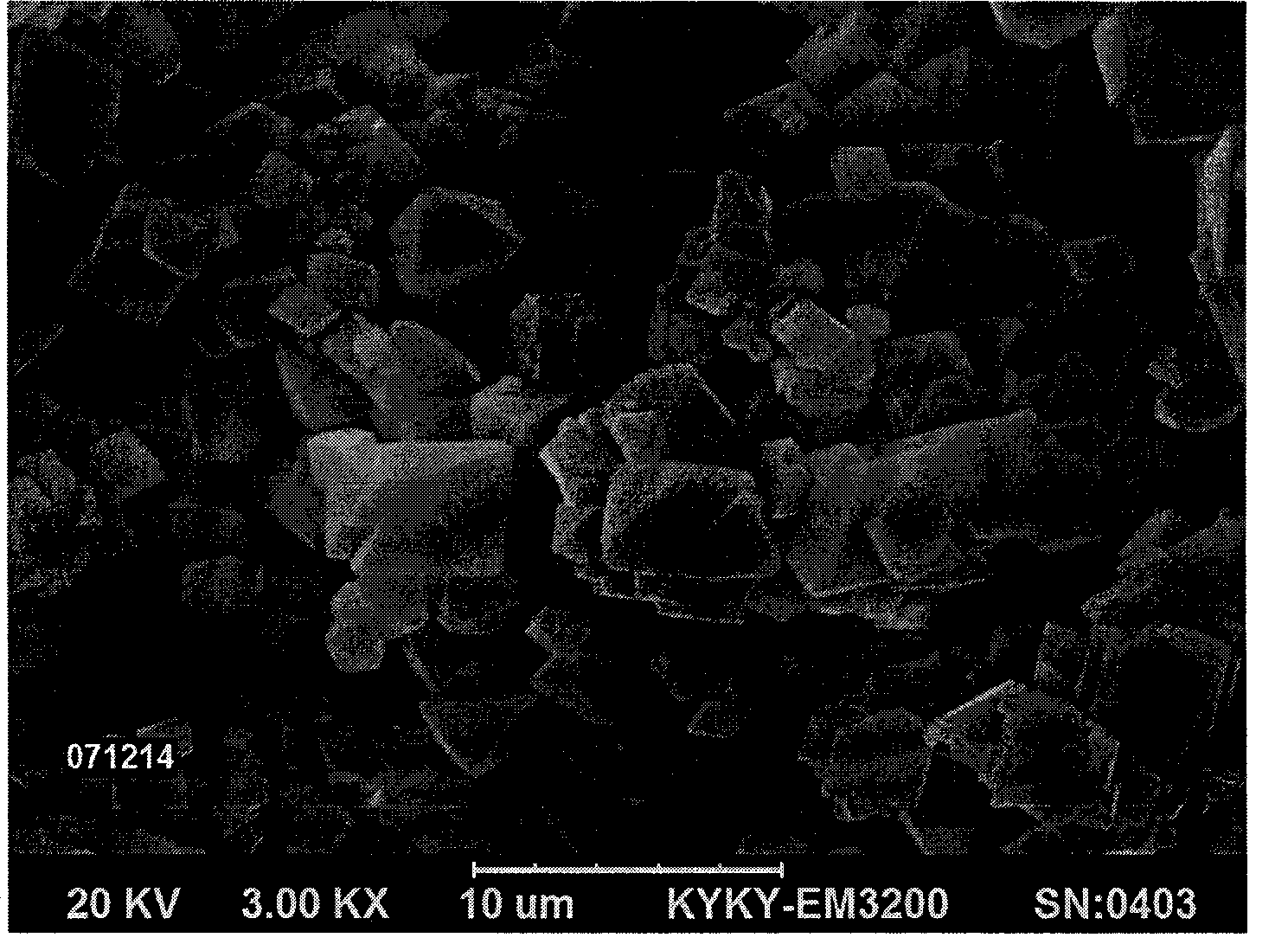
[0025] The cobalt-containing ore is used as a raw material, and after leaching and purification, a high-purity cobalt-containing 80-150 g / L cobalt chloride solution is obtained. The above cobalt chloride solution is pressure sprayed under the pressure of 0.2-5 MPa to obtain cobalt chloride mist droplets. Put the mist droplets obtained by spraying under air or oxygen atmosphere at 500-750°C for 10-40 seconds for partial dehydration to remove most of the water in the solution, followed by high temperature at 1000-1200°C The hydrolysis reaction is carried out for 20-80 seconds under high temperature, that is, the hydrolysis reaction occurs between the chloride salt of cobalt and water (and oxygen), so that it is converted from chloride salt to metal oxide, and then carried out at a high temperature of 800-950°C for 30-100 second crystal form reformation reaction, after crystal form reformation, polyhedral cobalt oxide i...
Embodiment 2
[0029] Embodiment 2, with reference to figure 1 and 3 .
[0030] The nickel-containing ore is used as a raw material, and after leaching and purification, a high-purity nickel chloride solution containing 80-150 g / L of cobalt is obtained. The above nickel chloride solution is subjected to pressure spraying under a pressure of 0.6-5 MPa to obtain nickel chloride mist droplets. Put the mist droplets obtained by spraying under air or oxygen atmosphere at 500-750°C for 10-40 seconds for partial dehydration to remove most of the water in the solution, followed by high temperature at 1000-1200°C The hydrolysis reaction is carried out for 20-80 seconds under high temperature, that is, the hydrolysis reaction of nickel chloride salt and water (and oxygen) makes it convert from chloride salt to metal oxide, and then 30-100 seconds at 800-950°C second crystal form reformation reaction, after crystal form reformation to obtain polyhedral nickel oxide, and release tail gas containing w...
Embodiment 3
[0033] Embodiment 3, with reference to figure 1 and 4 .
[0034]The copper-containing ore is used as a raw material, and after leaching and stilling, a high-purity copper chloride solution containing 80-150 g / L of cobalt is obtained. The above copper chloride solution is pressure sprayed under a pressure of 0.8-4 MPa to obtain copper chloride mist droplets. Put the mist droplets obtained by spraying under air or oxygen atmosphere at 500-750°C for 10-40 seconds for partial dehydration to remove most of the water in the solution, followed by high temperature at 1000-1200°C The hydrolysis reaction is carried out for 20-80 seconds under high temperature, that is, the hydrolysis reaction of copper chloride salt and water (and oxygen) converts it from chloride salt to metal oxide, and then it is carried out at a high temperature of 800-950°C for 30-100 Second crystal form reformation reaction, after crystal form reformation, copper oxide with polyhedral morphology is obtained, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com