Preparation process of compound dry powder inhaler for glucocorticoids and beta2 receptor agonists
A technology for glucocorticoid and dry powder inhaler, which is applied to the preparation of glucocorticoid and β2 receptor agonist compound dry powder inhaler composition, and the preparation field of indacaterol and mometasone compound dry powder inhaler composition. Solve the problems of reducing FPF value and preparation stability, the fluidity of preparation composition, and preparation stability, etc., to achieve the effect of improving the stability of FPF and preparation, convenient operation, and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Get the indacaterol maleate and mometasone furoate of 10g respectively, carry out jet milling, feeding nozzle pressure 1.1MPa, pulverizing nozzle pressure is 1.1MPa, obtains the indacaterol maleate of micronization (D10= 0.35 μm, D50=1.2 μm, D90=2.0 μm), mometasone furoate (D10=0.23 μm, D50=1.1 μm, D90=1.9 μm).
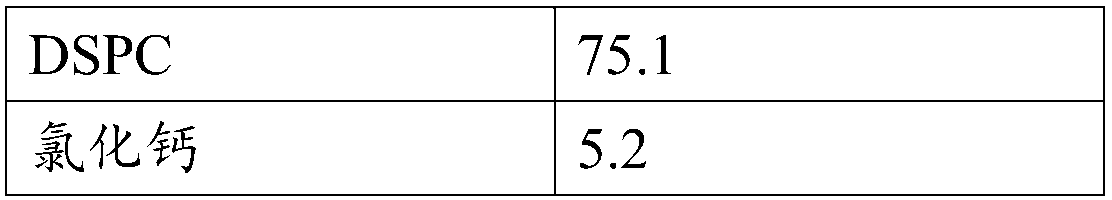
[0041] Ingredients: indacaterol maleate 0.8g (micronized), mometasone furoate 0.6g (micronized), lactose 90g and mannitol 10g (the particle size after mixing lactose and mannitol is D10=1.2μm, D50 = 35 μm, D90 = 150 μm).
[0042] Process 1:
[0043] Mix indacaterol maleate, mometasone furoate, lactose and mannitol, put the mixed sample in a wet granulator, the vertical rotation speed is 10r / s, finish mixing after 10min, let it stand for 1 hour, and the obtained sample Place in a sample processor with 75% humidity for 2 hours. The dry powder is filled into size 3 capsules in 25 mg aliquots.
[0044] Process 2:
[0045] Mix indacaterol maleate, mometasone furo...
Embodiment 2
[0058] Get 10g of indacaterol acetate and mometasone furoate, carry out airflow pulverization respectively, adjust feed nozzle pressure 0.5MPa, pulverize nozzle pressure and be 0.5MPa, obtain micronized indacaterol acetate (D10=0.70 μ m, D50=2.50 μm, D90=3.20 μm), mometasone furoate (D10=0.84 μm, D50=2.30 μm, D90=3.80 μm).
[0059] Ingredients: indacaterol acetate 0.6g (micronized), mometasone furoate 0.3g (micronized), lactose monohydrate 16g, lactose 63.9g.
[0060] Process 1:
[0061] Mix indacaterol acetate, mometasone furoate, lactose monohydrate and lactose (the particle size after mixing lactose monohydrate and lactose is D10=0.8 μm, D50=20 μm, D90=101 μm), and the mixed sample is In the high-speed shearing machine, the vertical rotation speed is 18r / s, the mixing is completed after 10min, and the sample is left to stand for 1 hour, and the obtained sample is put into a sample processor with a pressure of 500Pa for 24 hours. The dry powder is filled into size 3 capsul...
Embodiment 3
[0072] Process 1:
[0073] Take 10g of indacaterol maleate and mometasone furoate monohydrate respectively, carry out airflow pulverization respectively, adjust the feeding nozzle pressure to 0.5MPa, and crush the nozzle pressure to 0.4MPa to obtain micronized indacaterol maleate Terol (D10=1.1 μm, D50=3.8 μm, D90=5.5 μm) and mometasone furoate monohydrate (D10=0.58 μm, D50=1.5 μm, D90=3.2 μm).
[0074] Indacaterol maleate (0.6g), mometasone furoate monohydrate (1.0g) and lactose monohydrate (80g, D10=2.2μm, D50=53μm, D90=98μm) were mixed, and the mixed sample In the mixer, the vertical rotation speed is 4r / s, and the mixing is completed after 16min, and left to stand for 1 hour, and the obtained sample is put into a sample processor with a humidity of 58% for 72 hours. The dry powder is filled into size 3 gelatin capsules in 25 mg aliquots.
[0075] Process 2:
[0076] Take 10 g of indacaterol maleate and mometasone furoate monohydrate respectively, and carry out jet milli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com