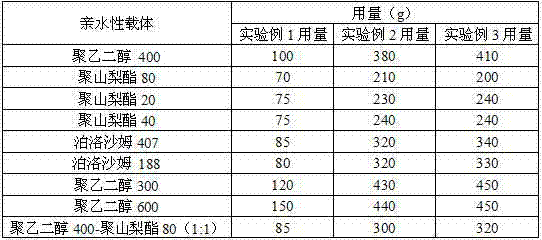
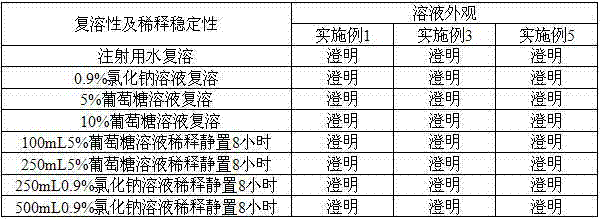
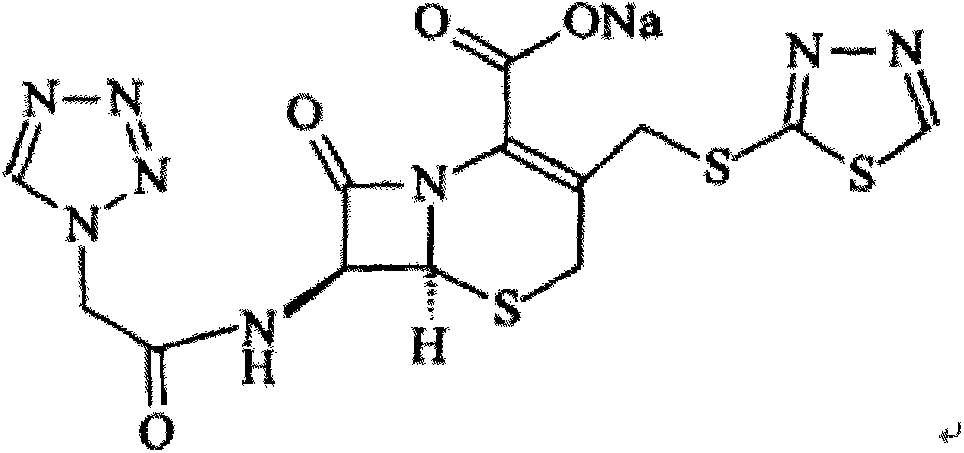
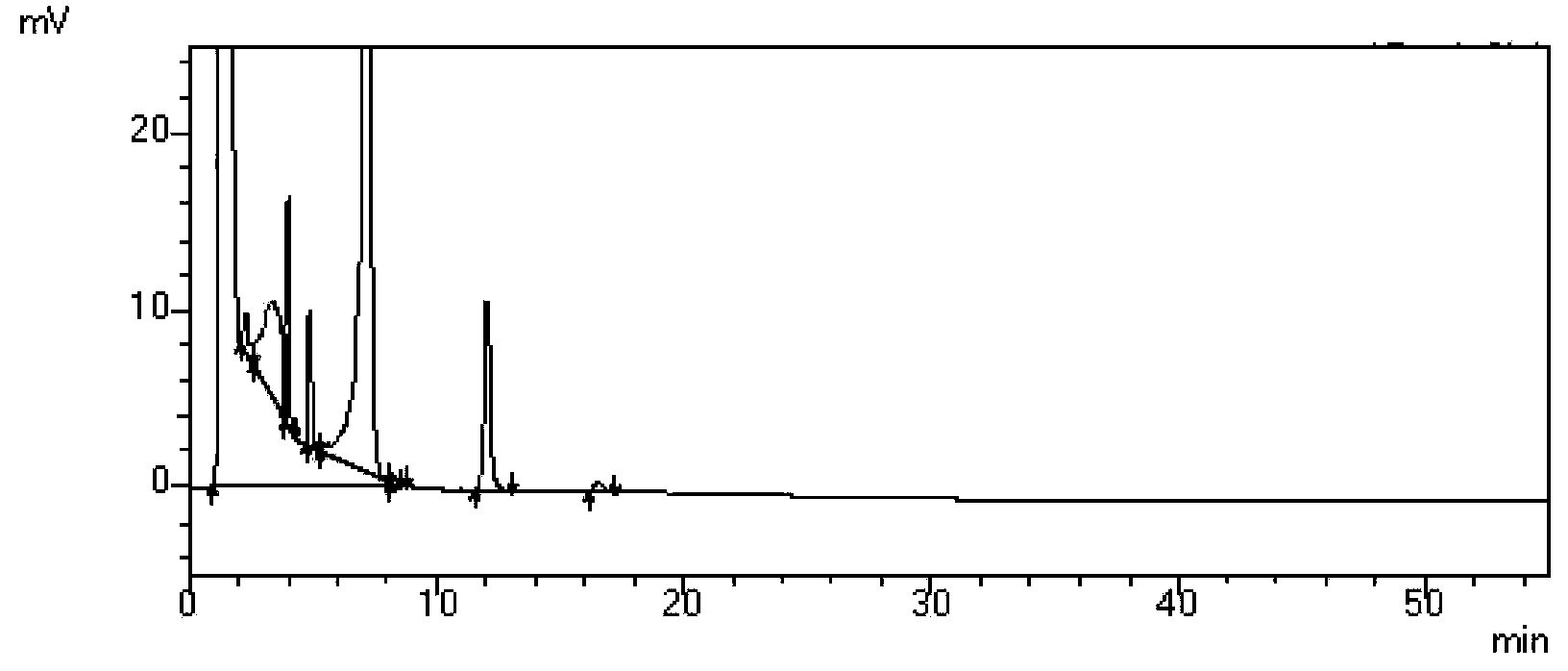
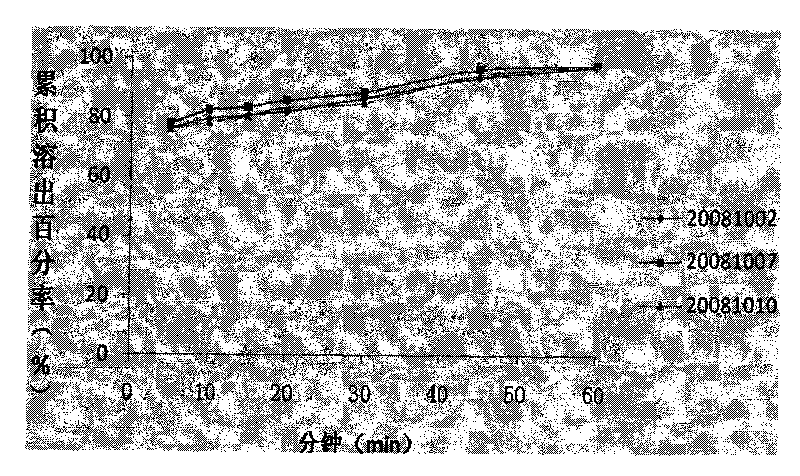
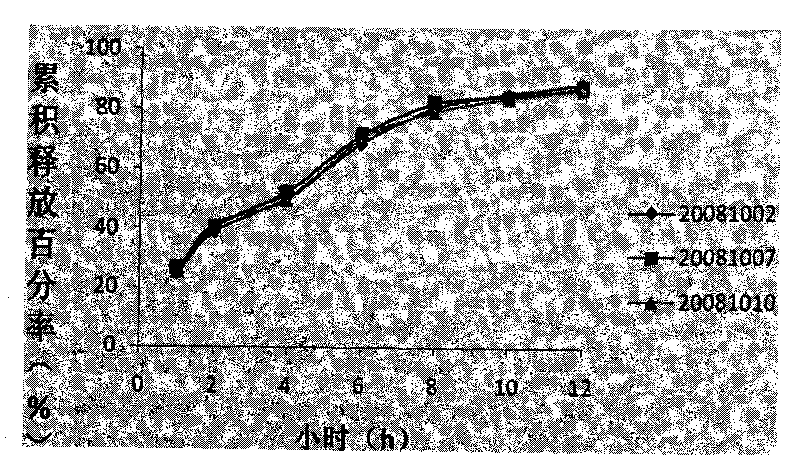
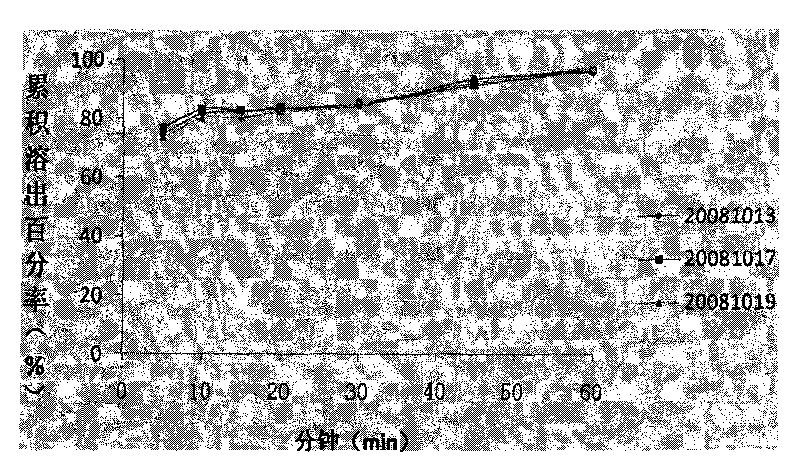
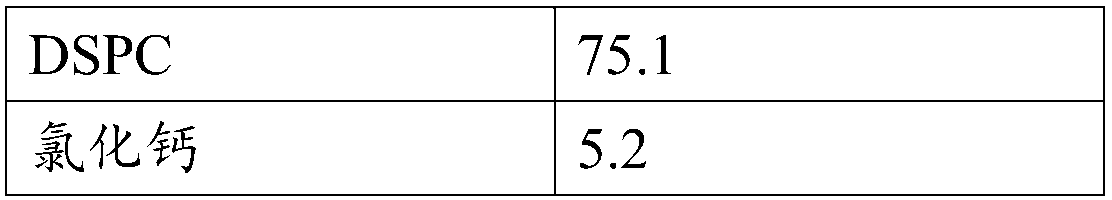
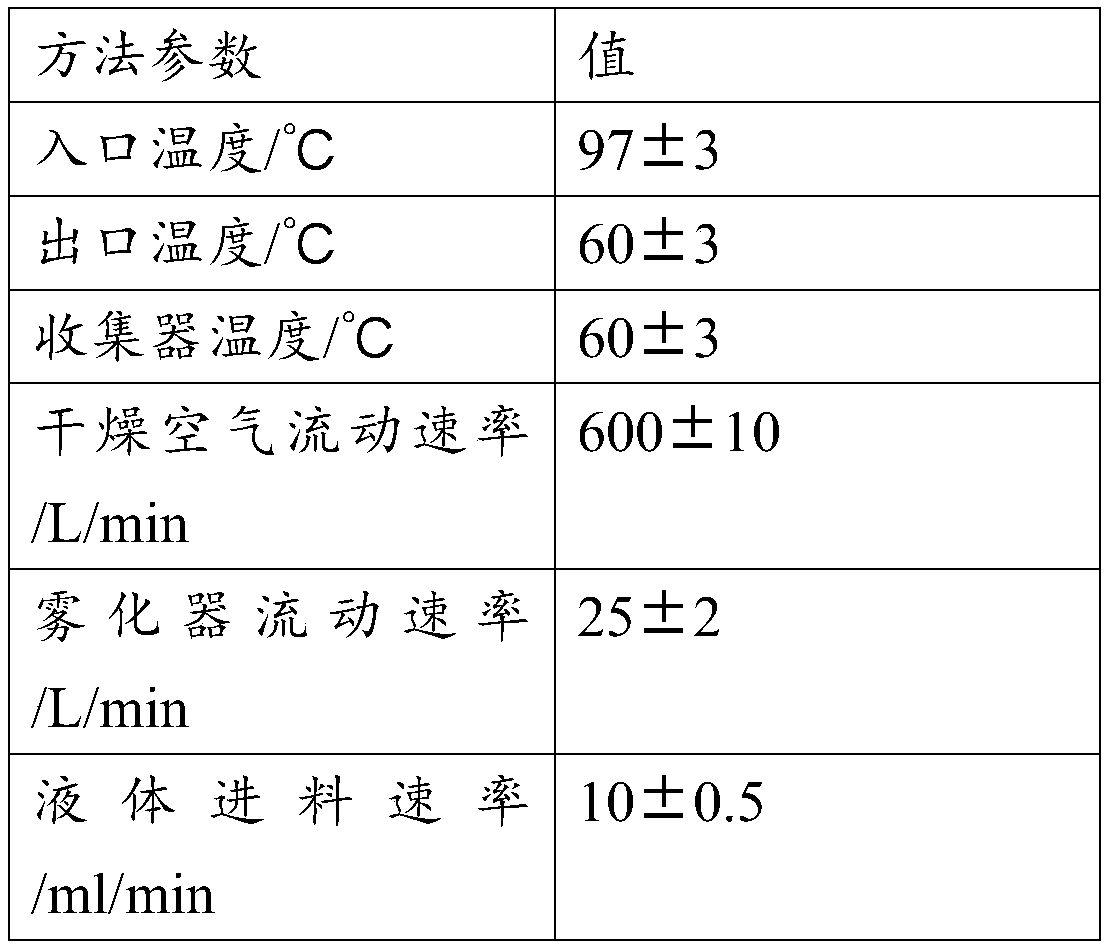
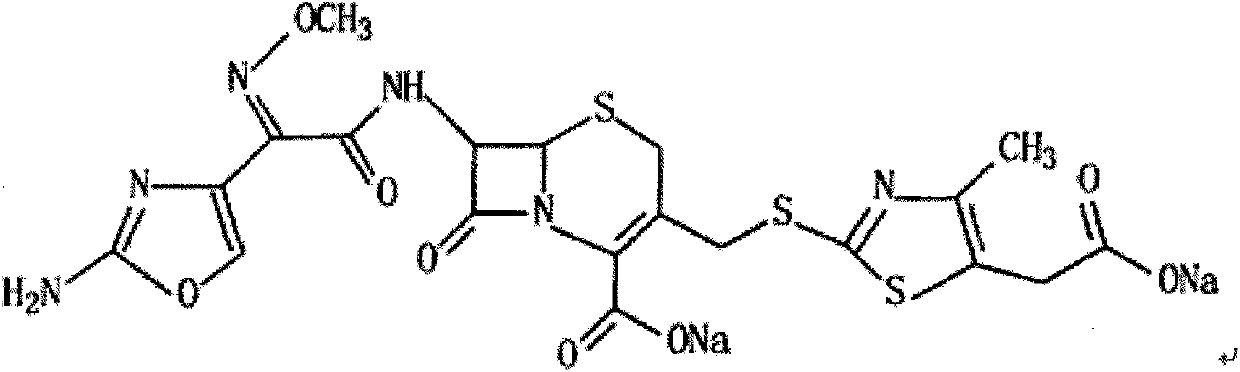
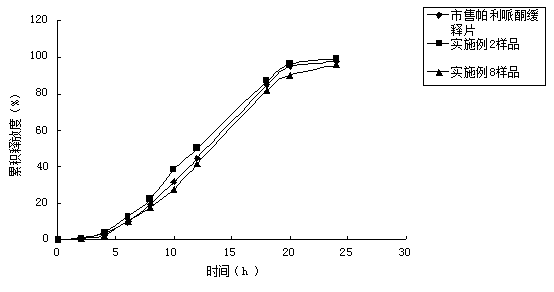
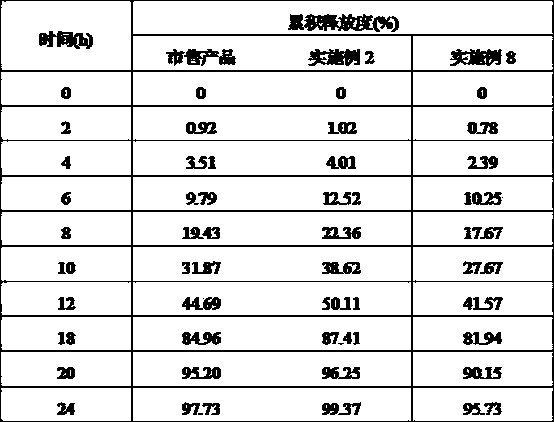
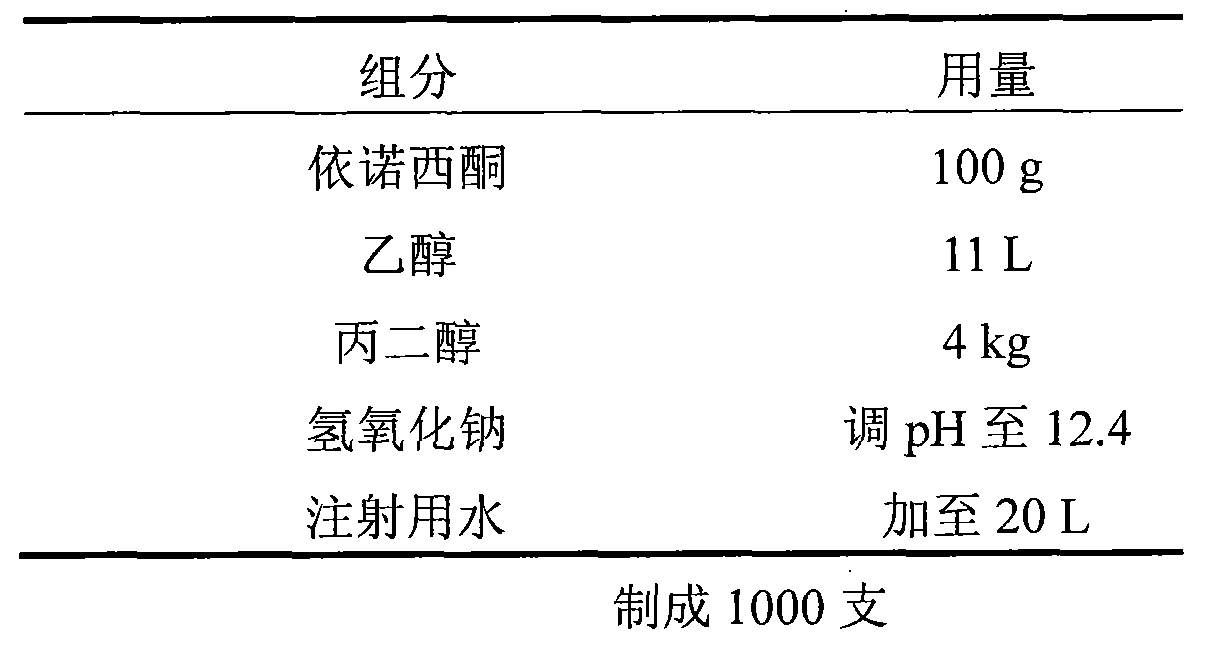
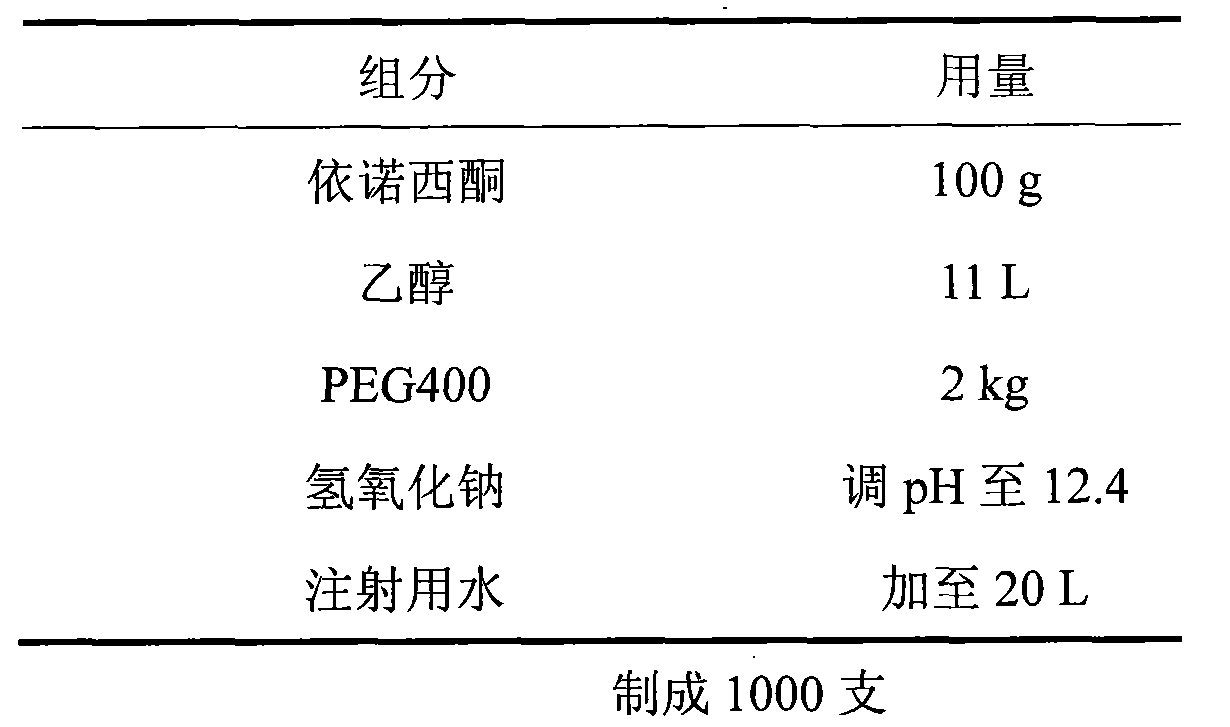
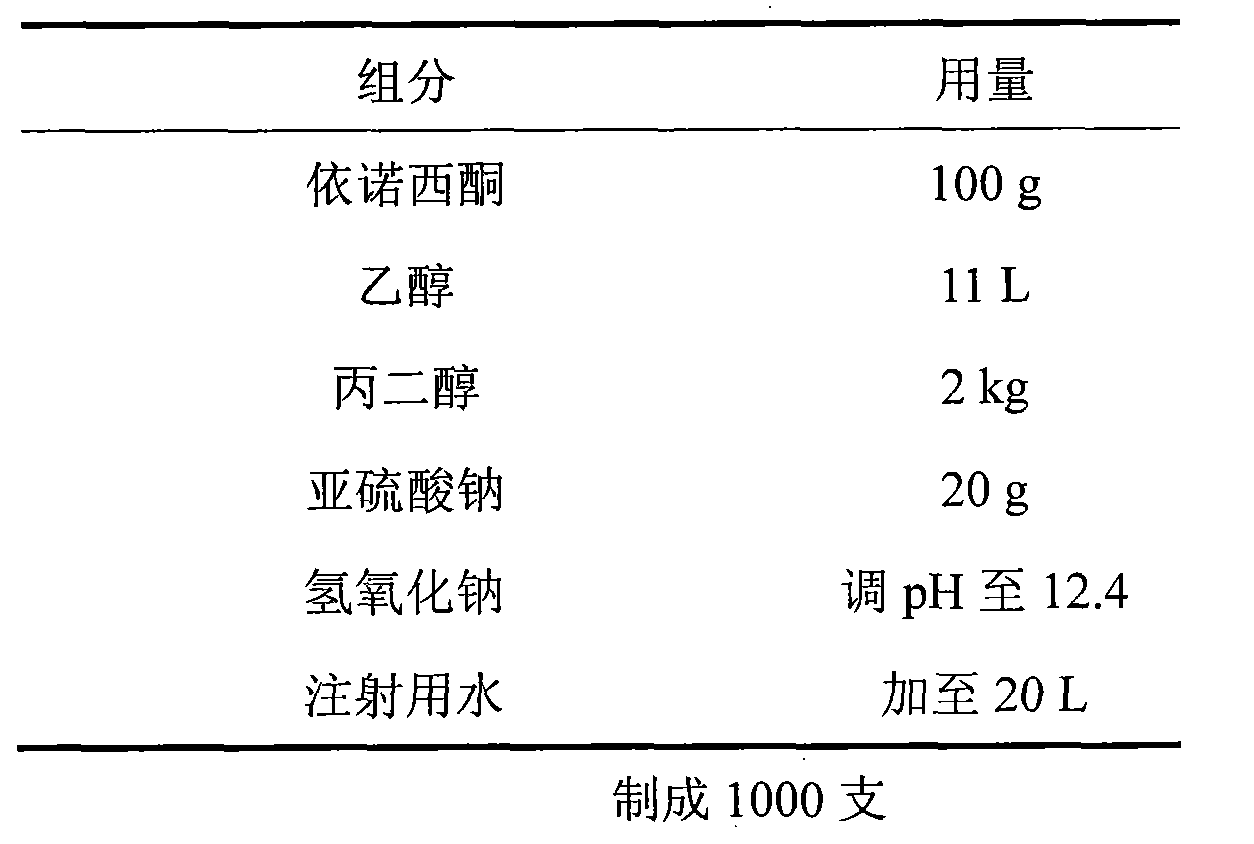
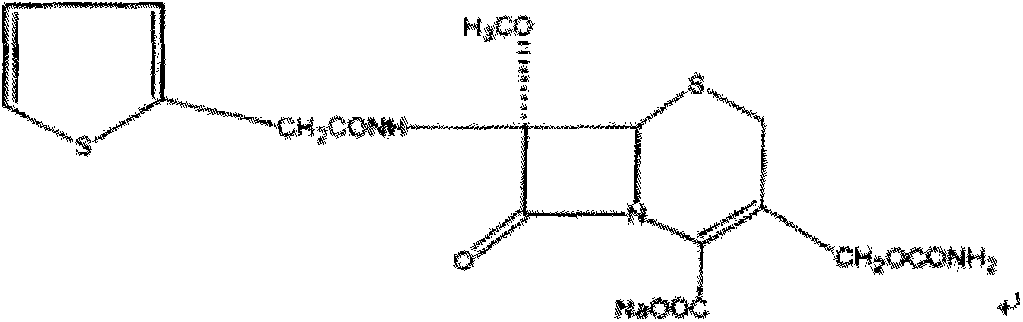
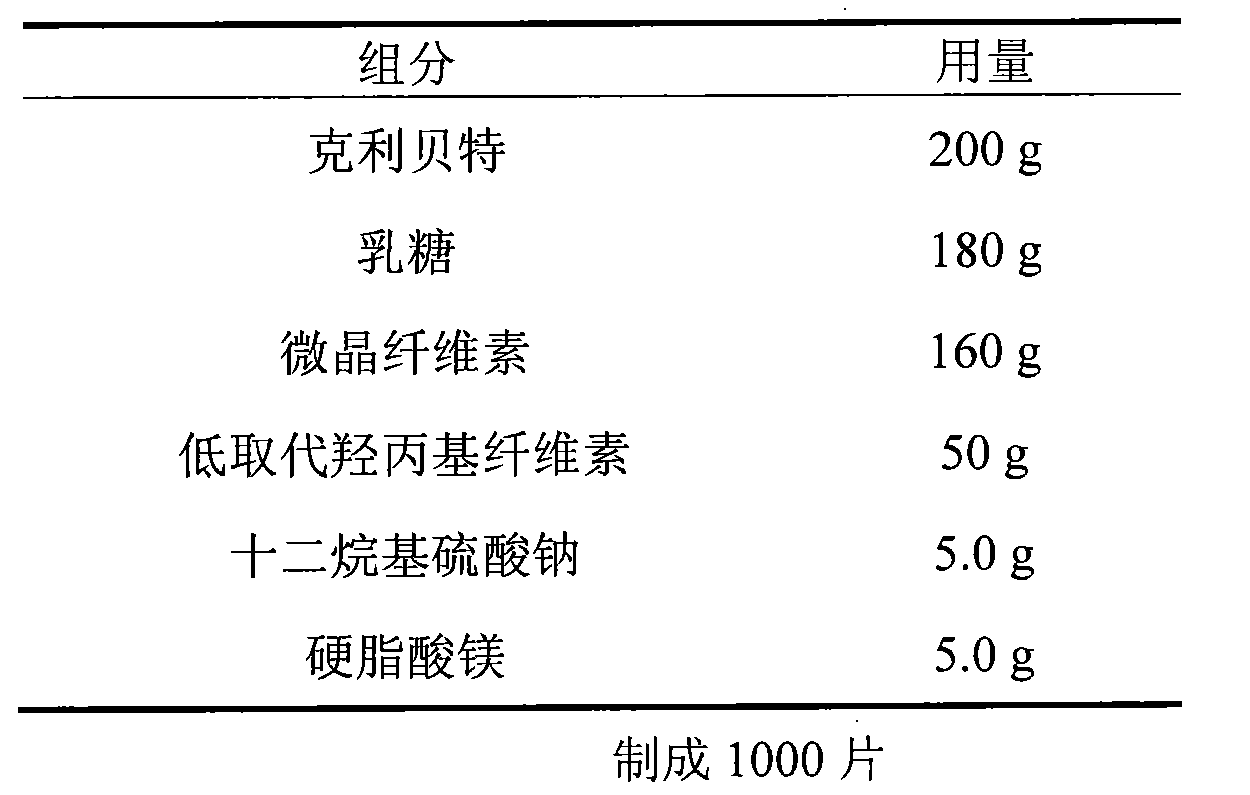
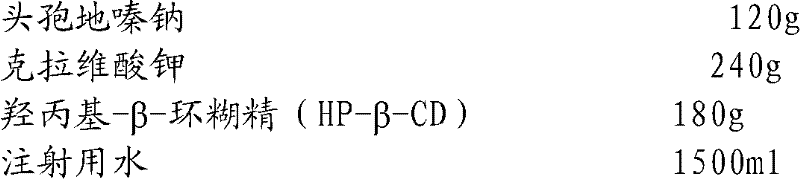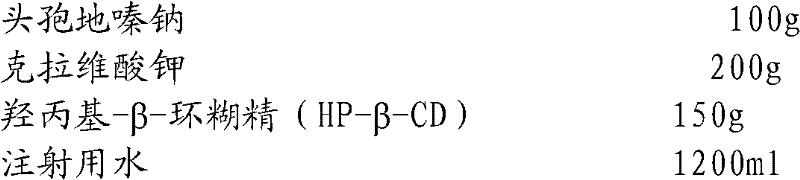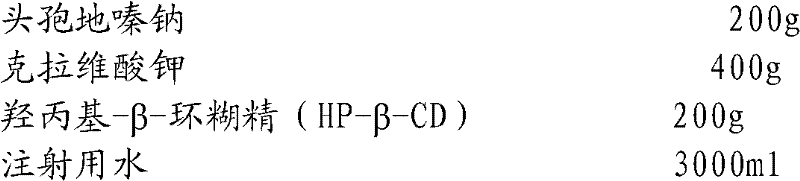Patents
Literature
60results about How to "The prescription process is simple" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bromhexine hydrochloride compound and pharmaceutical composition thereof
ActiveCN103145564AThe prescription process is simpleImprove stabilityOrganic active ingredientsAmino compound purification/separationPharmaceutical SubstancesMedicinal chemistry
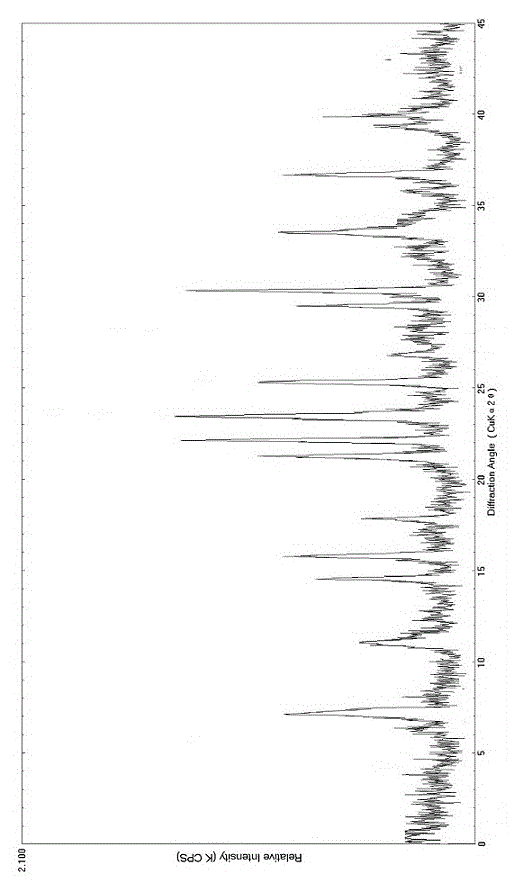
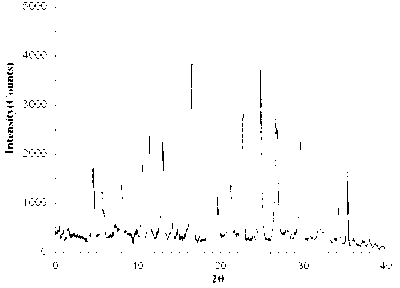
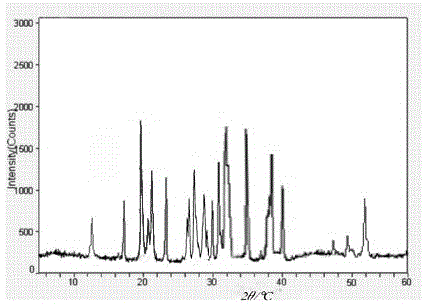
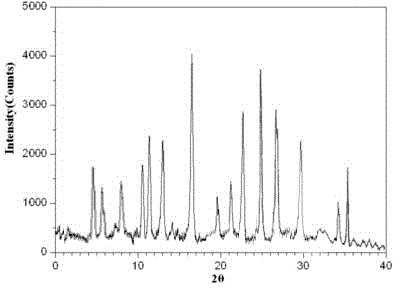
The invention relates to a bromhexine hydrochloride compound. The bromhexine hydrochloride compound is a crystal body and determined by using an X-ray powder diffraction method, and the characteristic peaks of the bromhexine hydrochloride compound are shown in a map in which 2theta plus or minus 0.2 degree is equal to 7.0 degrees, 10.9 degrees, 14.6 degrees, 15.8 degrees, 17.9 degrees, 21.1 degrees, 22.2 degrees, 23.4 degrees, 25.2 degrees, 29.3 degrees, 30.3 degrees, 33.4 degrees, 36.7 degrees and 39.6 degrees. The invention further provides a preparation and a pharmaceutical composition preparation which comprise the bromhexine hydrochloride compound, wherein the preparations can be prepared to form powder injection, freeze-dried powder injection, water injection and tablets. The formulation technology of the powder injection, freeze-dried powder injection, water injection and tablets of the bromhexine hydrochloride compound provided by the invention is simple in process, the stability of the bromhexine hydrochloride compound is obviously improved, and the safety and effectiveness of medication are improved.
Owner:湖北美林药业有限公司
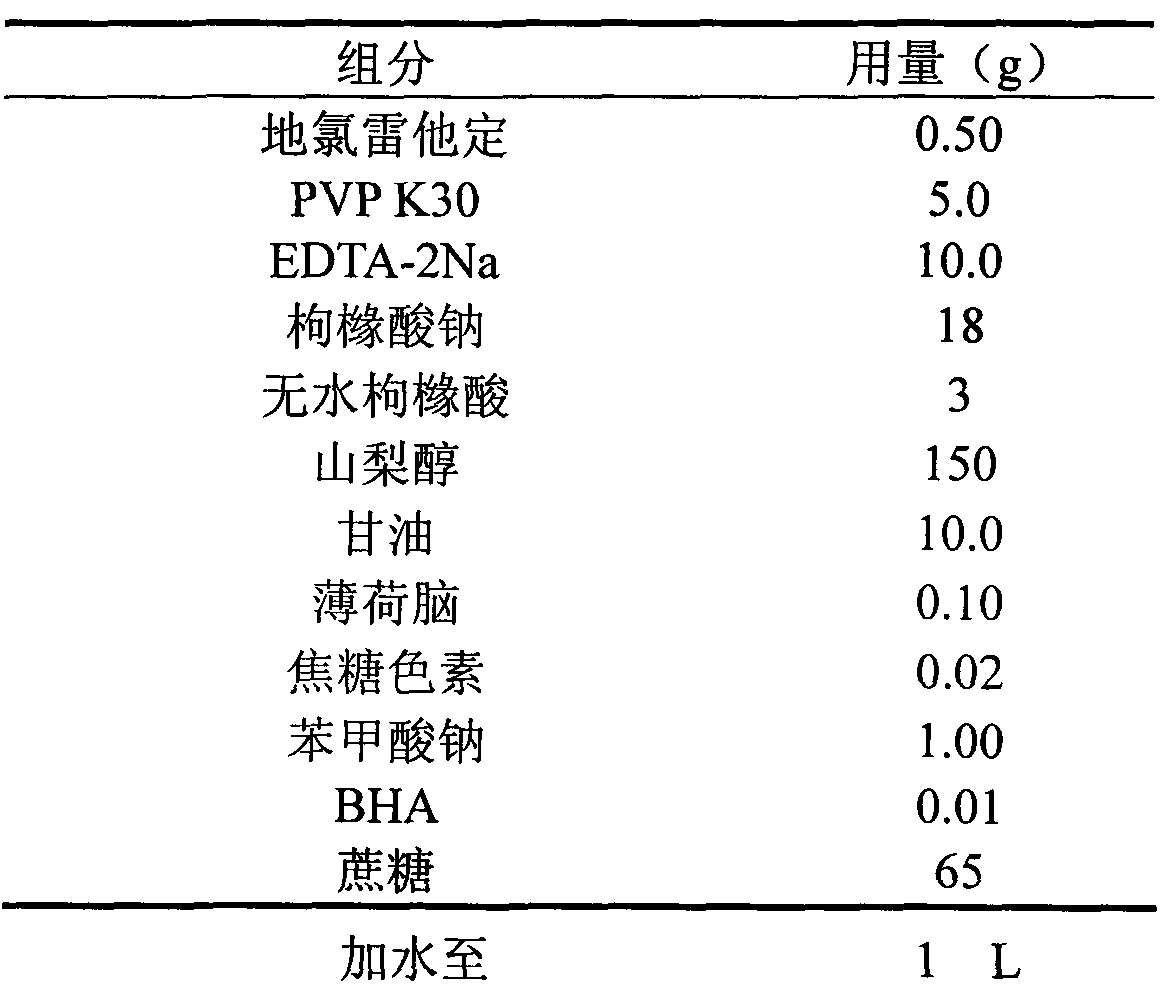
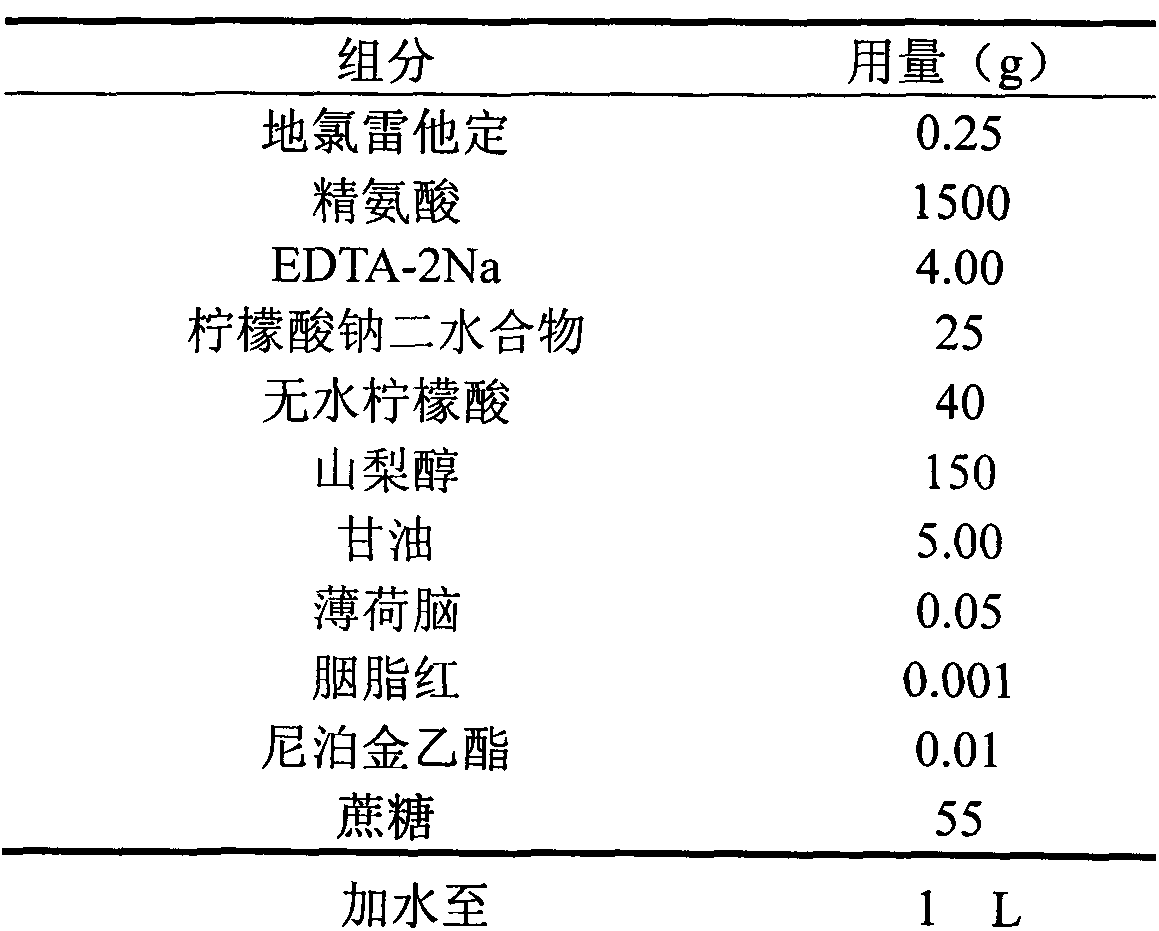
Desloratadine syrup and preparation method thereof
InactiveCN104095807AEasy to take medicineThe prescription process is simpleOrganic active ingredientsPharmaceutical delivery mechanismDesloratadineAntioxidant
Belonging to the technical field of medicine, the invention in particular relates to an antiallergic medicine syrup and a preparation method thereof. The medicinal composition contains desloratadine, a cosolvent, an antioxidant, a pH regulator and other pharmaceutically acceptable excipients. The syrup provided by the invention solves the difficult drug taking problem of patients with weak swallowing ability, and ensures simple process, controllable quality and improved stability, thus bettering meeting clinical needs.
Owner:AVENTIS PHARMA HAINAN
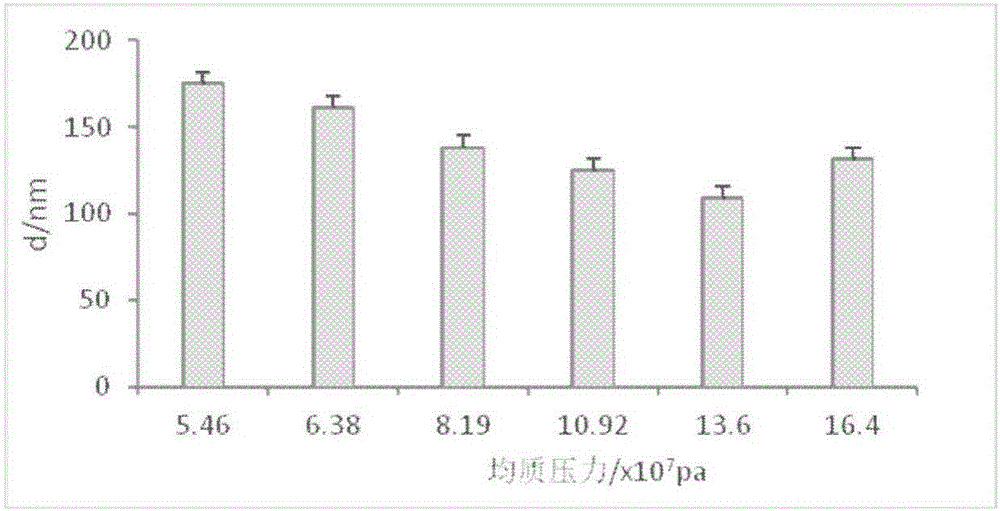
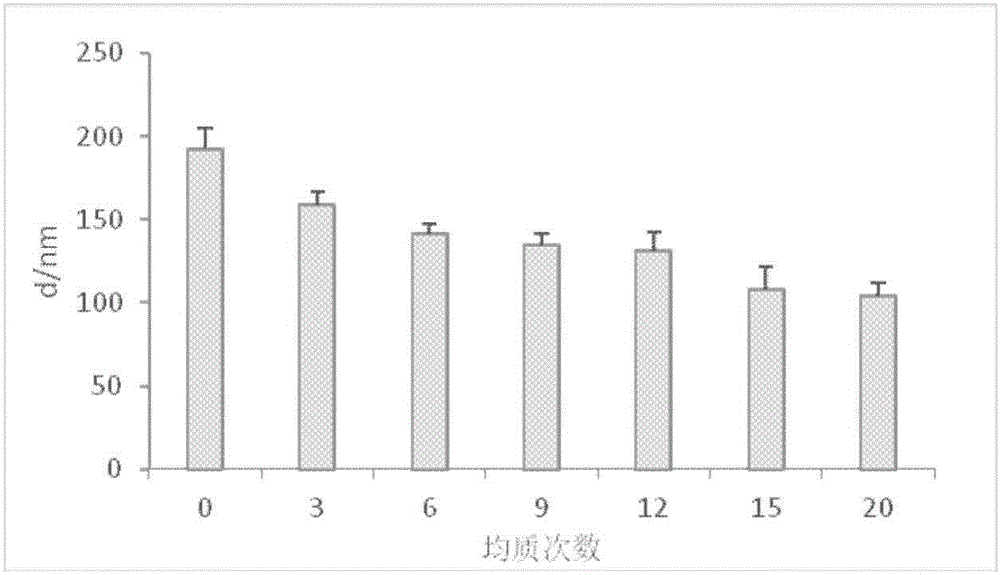
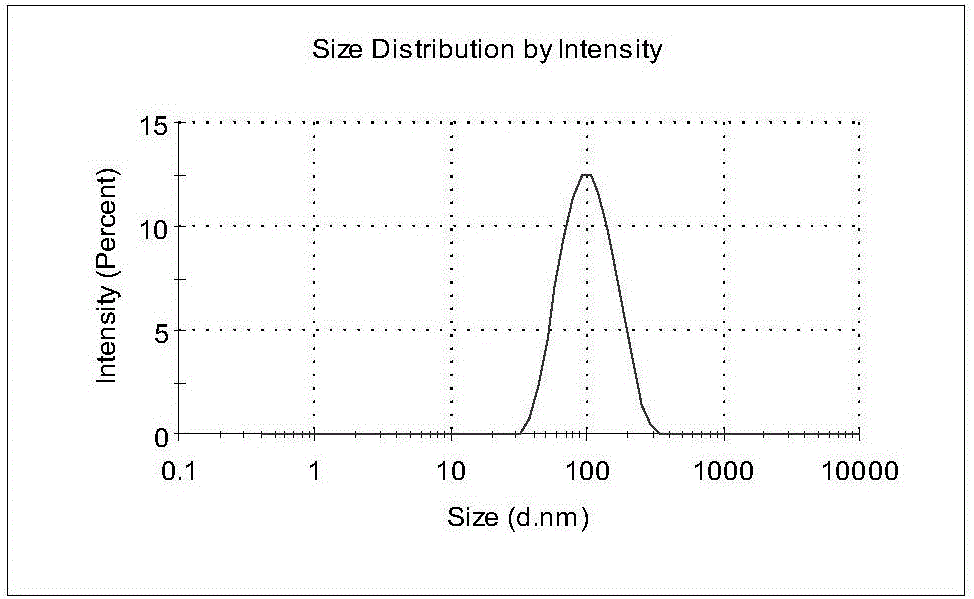
Cefodizime sodium composition and preparation method thereof
InactiveCN102258521AImprove stabilityStable recipe processAntibacterial agentsOrganic active ingredientsCefodizime SodiumFreeze-drying
The invention relates to the field of drug synthesis and preparation thereof, relates to a preparation method of an antibacterial drug, in particular to a stable cefodizime sodium composition preparation and a preparation method thereof. The present invention directly dissolves sterile cefodizime sodium in water, adds sterile potassium clavulanate to dissolve it completely, and obtains an aqueous solution of cefodizime sodium / clavulanate potassium, and adds hydroxypropyl-β to the aqueous solution. -Cyclodextrin (HP-β-CD) inclusion, sub-package, and freeze-drying to obtain Cefodizime Sodium / Clavulanate Potassium for Injection. The preparation method provided by the invention is simple, and the cefodizime sodium / clavulanic acid potassium salt is clathrated with hydroxypropyl-β-cyclodextrin, which increases the stability of the sterile cefodizime sodium drug and reduces its toxic and side effects , improve drug availability, and the preparation process is simple, suitable for industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD +1
Curcumin compound injection and intravenous injection preparation thereof
InactiveCN102225048AComply with clinical drug requirementsHigh drug loadingAntipyreticAnalgesicsSolventAdverse effect
The invention relates to the technical field of medicine, and particularly relates to a curcumin compound injection and an intravenous injection preparation thereof. The curcumin compound injection mainly comprises a curcumin compound, a solvent for injection and a small amount of pH regulator. The curcumin compound intravenous injection preparation is used only by mixing the curcumin compound injection and an emulsifier in a volume ratio of (1:5)-(1:250). After the curcumin compound injection and the emulsifier are mixed, the drug loading rate of the curcumin compound in the intravenous injection preparation is up to 1.5 mg / ml, and no crystal is separated out within 18 hours. The curcumin compound injection provided by the invention has a simple and safe preparation method and good stability, and is convenient for storage and transportation. The curcumin compound intravenous injection preparation has good stability due to the use of the emulsifier as a carrier solvent, and has no toxic and adverse effects. The invention solves the problem that the curcumin compound can not be prepared into the intravenous injection preparation and applied in clinics for a long period of time.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Curcumenol nanosuspension, and preparation method and application thereof
ActiveCN106344508ASmall particle sizeImprove solubilityOrganic active ingredientsPowder deliveryDissolutionHigh pressure
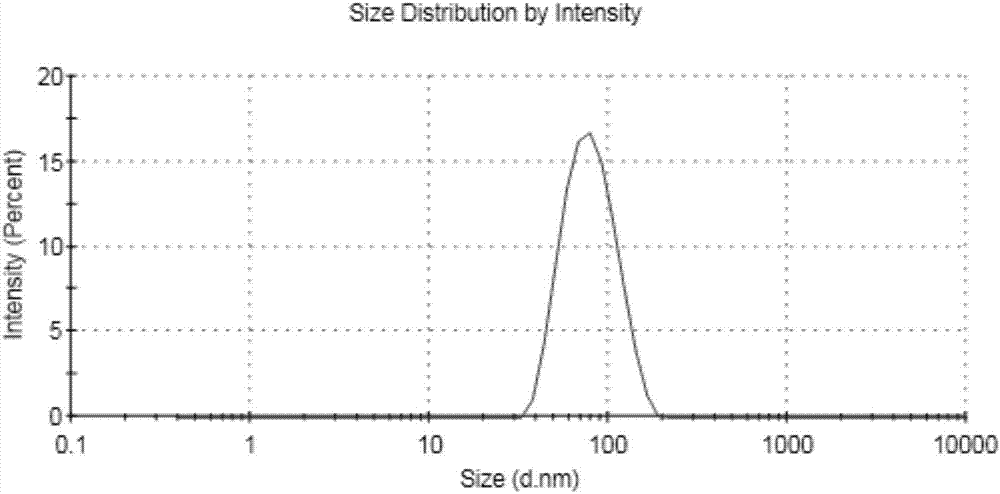
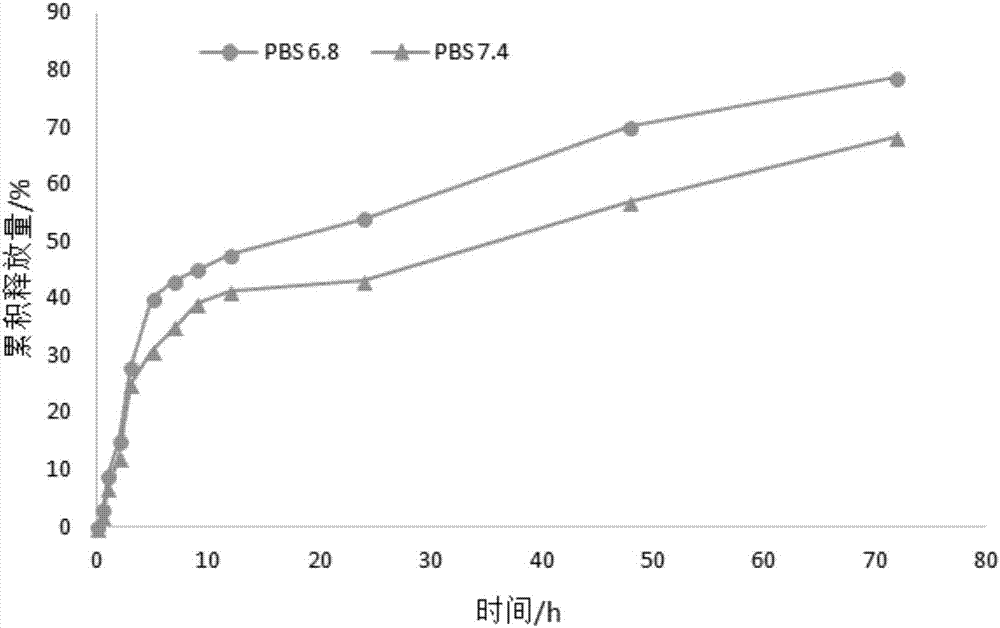
The invention relates to a curcumenol nanosuspension, and a preparation method and application thereof, and belongs to the field of pharmaceutical preparations. According to the invention, the curcumenol nanosuspension is prepared by adopting a precipitation method in combination with high-speed shearing and high-pressure homogenization. The prescription is optimized by using particle size and polydispersity index (PDI) as indicators, the form of the preparation is inspected by means of a transmission electron microscope, and the dissolution of the drug is inspected by means of in-vitro release experiments. The curcumenol nanosuspension provided by the invention is characterized in that the curcumenol nanosuspension comprises curcumenol, lecithin and PVP (polyvinyl pyrrolidone), wherein the mass ratio of the curcumenol to the lecithin to the PVP is 1:(0.4-0.6):(0.25-4); the particle size of drug in the suspension is 50-500 nm; and the curcumenol concentration in the suspension is 0.25-1 mg / ml. The nanosuspension preparation method provided by the invention is simple, controllable and suitable for industrial production, and improves the drug dissolution.
Owner:LIAONING UNIVERSITY
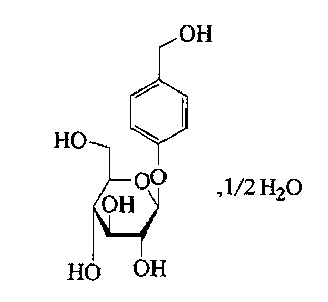
Gastrodine compound and pharmaceutical composition thereof
ActiveCN103224539AThe prescription process is simpleImprove stabilityOrganic active ingredientsNervous disorderCaplet Dosage FormPharmaceutical drug
The invention relates to a gastrodine compound and a pharmaceutical composition thereof. The gastrodine compound is a crystal. The X-ray powder diffraction determines that the characteristic peaks are displayed when 2theta+ / -0.2 degree is 4.5 degrees, 5.7 degrees, 7.9 degrees, 10.3 degrees, 11.2 degrees, 13.0 degrees, 16.4 degrees, 19.6 degrees, 21.2 degrees, 22.7 degrees, 24.8 degrees, 26.7 degrees, 29.6 degrees, 34.1 degrees and 35.3 degrees. The gastrodine compound has very high stability, thereby greatly enhancing the medicine safety. The invention also relates to a pharmaceutical composition preparation containing the gastrodine compound. The composition preparation can be a freeze-dried powder injection, injection, tablet or capsule. The gastrodine freeze-dried powder injection, injection, tablet or capsule preparation provided by the invention has the advantages of simple prescription technique, obviously higher stability, and enhances the medicine safety and effectiveness.
Owner:湖北美林药业有限公司
Preparation method of nocathiacin freeze-dried powder injection
ActiveCN103040770AImprove solubilityReduce dosageAntibacterial agentsPowder deliveryBiotechnologyFreeze-drying
The invention provides a preparation method of nocathiacin freeze-dried powder injection. The method comprises the following steps of: (1) preparing liquor from nocathiacin, hydrophilic carriers, a pH regulator, an excipient and injection water, removing bacteria, and filtering and filling the liquor; and (2) performing freeze drying on the nocathiacin liquor. According to the freeze-dried powder injection prepared by the method, on the premise of enhancing the dissolvability of the nocathiacin, the consumption of the hydrophilic carriers such as a solubilizer and a cosolvent is reduced as much as possible; and the formulation and preparation are simple, the method is suitable for industrialized production, and the nocathiacin freeze-dried powder injection is high in stability and re-dissolution.
Owner:NANJING BIOTICA PHARMA
Ceftezole sodium agent and preparation method thereof
InactiveCN103271878AImprove stabilityThe prescription process is simpleAntibacterial agentsPowder deliveryCeftezole SodiumArginine
The invention relates to a ceftezole sodium agent and preparation method thereof, especially relates to a ceftezole sodium injection for treating microbe infection, and preferably relates to a freeze-drying powder injection. The mezlocilin sodium for injection mainly comprises the following components: ceftezole, and accessory sorbitol and arginine. The solvent in the injection of the present invention is injection water; the excipient in the freeze-drying powder injection is sorbitol, and sodium hydroxide or hydrochloric acid is used for adjusting pH valve.
Owner:张宏民
Stable busulfan injection
ActiveCN103446045AImprove stabilityConvenient for clinical operationOrganic active ingredientsPharmaceutical delivery mechanismChemical compositionPEG 400
The invention provides a busulfan injection which is simple in prescription and stable in quality. The injection is prepared by the followings method: (1), dissolving busulfan into a mixed solvent of N, N-dimethyl acetamide and polyethylene glycol 400 in a volume ratio of (17-57):(83-43) to obtain a busulfan solution with concentration of 1mg / mL-7.5mg / mL; and (2), filtering and filling in a sterile manner, wherein in the dissolving process of the step (1), the temperature of the solution is 4 DEG C-25 DEG C, or / and inert gases are charged in an operation process until the solution is saturated. Not only is stability of the busulfan injection obtained by the preparation method disclosed by the invention improved, but also the safety of the injection after long-time storage is effectively ensured. Meanwhile, other substances or other chemical components are not introduced in the preparation process, so that the medication safety is ensured, and the drug cost is also lowered.
Owner:SICHUAN CREDIT PHARMA +1
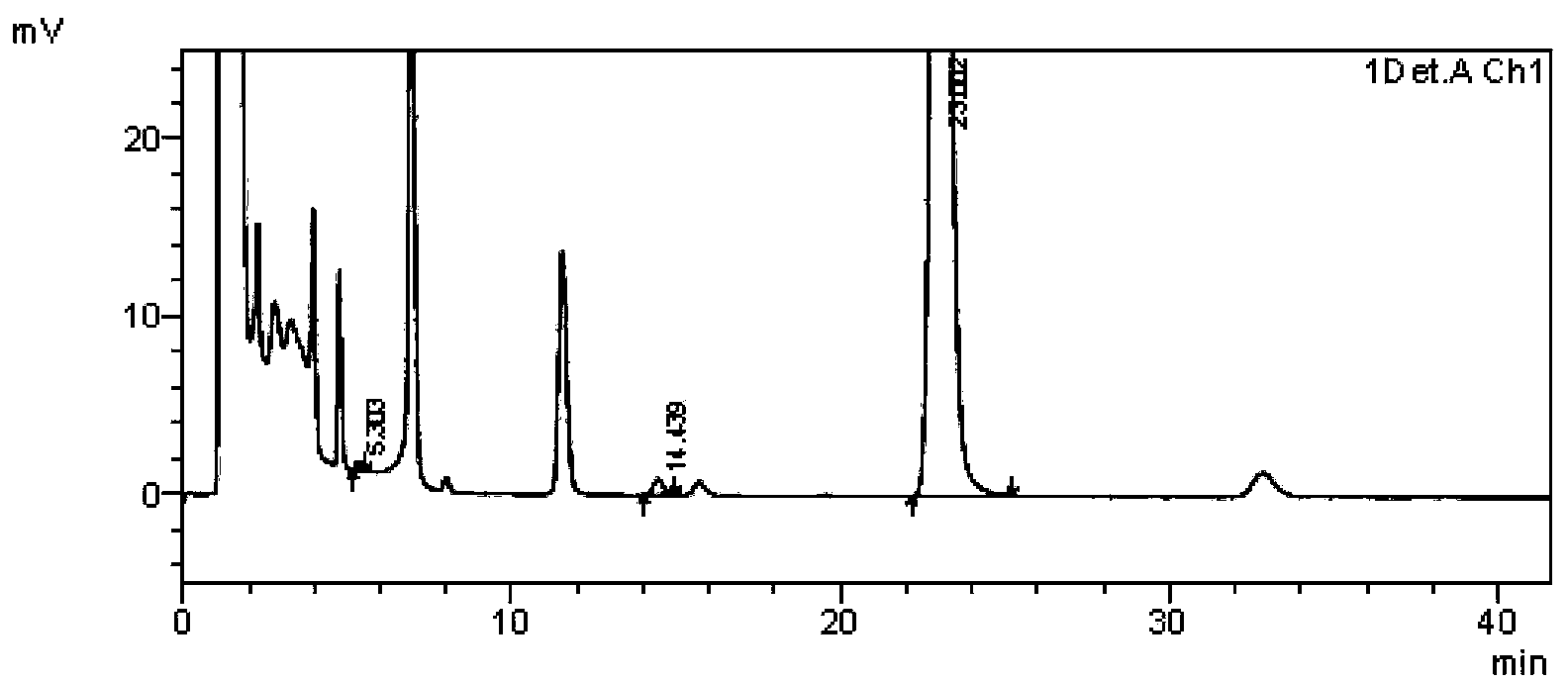
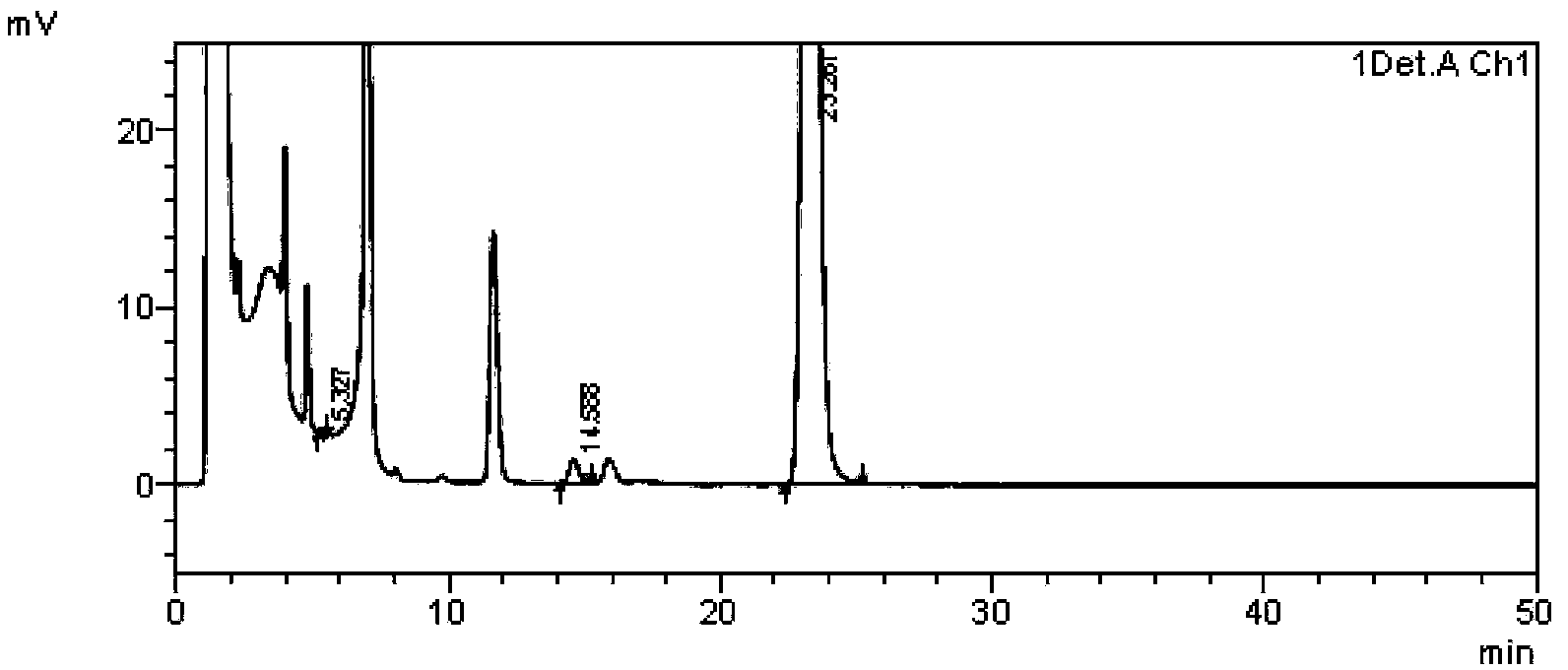
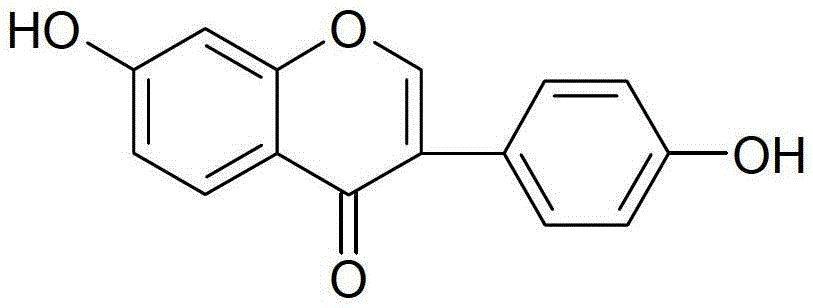
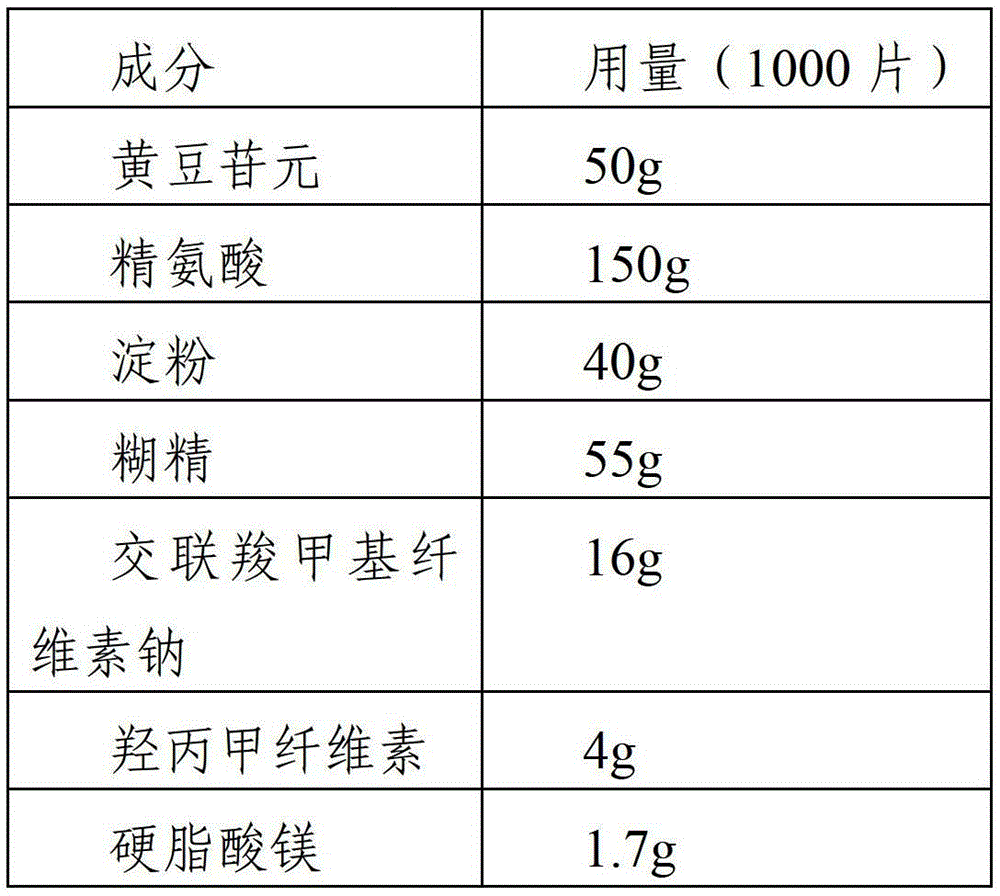
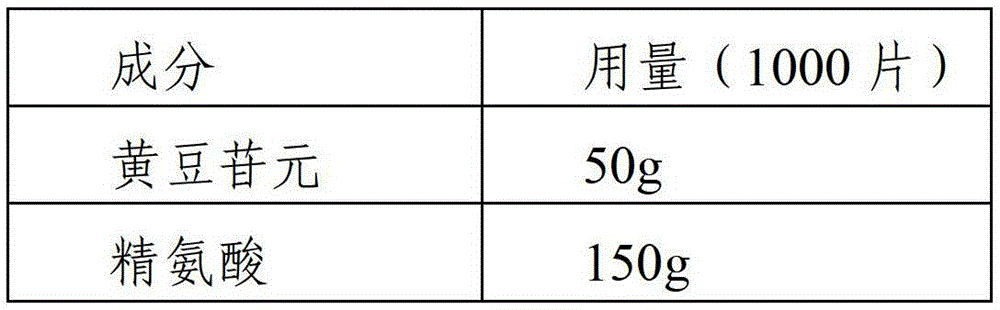
Daidzein-containing tablet composition and preparation method thereof
ActiveCN104095822AHigh dissolution rateImprove stabilityOrganic active ingredientsSenses disorderMedicineArginine
A provided daidzein-containing tablet composition comprises daidzein and arginine with the weight ratio of 1:2.5-4. The tablet composition comprises, in parts by weight, 16 parts of daidzein, 40-64 parts of arginine, 5-7 parts of a disintegrating agent, 14-38 parts of a diluent, 0.2-1.0 parts of a lubricant and 0.9-1.6 parts of an adhesive. By employing the above technical scheme, the water-soluble daidzein-containing tablet composition containing daidzein and arginine with the weight ratio of 1:2.5-4 is provided. The invention further provides a specific prescription and a preparation method. An added weak base enables daidzein to relatively well form a relatively stable easily-soluble salt, and thus improvement of dissolution rate is facilitated, and absorption by human body is facilitated. The technology is simple and the prescription quality is reliable, and batch production of the daidzein tablet can be realized.
Owner:山西国润制药有限公司
Doxorubicin hydrochloride self-assembled polymer nanoparticle and preparation method thereof
ActiveCN107126426AThe prescription process is simpleImprove securityOrganic active ingredientsPharmaceutical non-active ingredientsRelease timeTherapeutic effect
The invention discloses a doxorubicin hydrochloride self-assembled polymer nanoparticle which is prepared from doxorubicin hydrochloride and an amphipathic high-molecular polymer PLGA (Polylactic-co-Glycolic Acid), wherein the PLGA is formed by two monomers, namely lactic acid (LA) and glycolic acid (GA). The preparation method comprises the following steps: 1) dissolving a drug doxorubicin hydrochloride into a polar organic solvent A so as to obtain a doxorubicin hydrochloride solution; 2) dissolving the PLGA into a polar organic solvent B so as to obtain a PLGA solution; 3) uniformly mixing the doxorubicin hydrochloride solution with the PLGA solution so as to obtain an eutectic solution, dripping the eutectic solution into an aqueous phase under the stirring condition, continuously stirring, and volatilizing the organic solvent; and 4) centrifuging the completely volatilized solution at a high speed by using a centrifugal machine, and collecting the precipitate, thereby obtaining the doxorubicin hydrochloride self-assembled polymer nanoparticle. The prepared product can achieve the effects of reducing the drug toxicity, reducing the administration frequency, improving the drug stability, delaying in-vivo release time of the drug, reducing adverse reactions of the drug, improving the bioavailability and efficacy of the drug and achieving excellent treatment effects.
Owner:LIAONING UNIVERSITY
Resveratrol injection solution and intravenous injection
InactiveCN102188370AHigh drug loadingSimple and fast operationAntibacterial agentsHydroxy compound active ingredientsEmulsionSide effect
The invention relates to the technical field of medicament, in particular relates to a resveratrol injection solution and an intravenous injection thereof, wherein the resveratrol injection solution mainly comprises resveratrol, an injection solvent and a small amount of pH conditioning agent. The resveratrol intravenous injection is prepared by evenly mixing the resveratrol injection solution and the emulsion according to the volume ratio being 1:5 to 1:250 when in use. After the resveratrol injection solution and the emulsion are mixed, the drug loading rate of the resveratrol in the intravenous injection can achieve 1.5mg / ml, and the resveratrol can not be crystallized and separated out within 18 hours. The preparation method of the resveratrol injection solution is simple and safe, the solution has good stability and is convenient to store and transport, the resveratrol intravenous injection has good stability and no toxic and side effects by taking the emulsion as a carrier solution medium. According to the invention, the problem that the resveratrol can not be prepared into the intravenous injection and applied clinically for a long term is solved.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Carbazochrome sodium sulfonate compound and medical composition thereof
ActiveCN103145603AThe prescription process is simpleImprove stabilityOrganic active ingredientsOrganic chemistryCarbazochrome Sodium SulfonateChemical compound
The invention relates to a carbazochrome sodium sulfonate compound which is a crystal. The characteristic peak in a map through X-ray powder diffraction determination is 12.5 degrees, 17.4 degrees, 19.8 degrees, 21.0 degrees, 21.5 degrees, 23.4 degrees, 26.2 degrees, 26.8 degrees, 27.7 degrees, 29.0 degrees, 3.01 degrees, 31.6 degrees, 32.1 degrees, 35.0 degrees, 38.5 degrees, 40.3 degrees and 51.9 degrees at 2theta+ / -0.2 degrees. The invention further provides a medical composition preparation containing the carbazochrome sodium sulfonate compound. The medical composition preparation is a freeze-dried powder injection, a water injection and a troche. The compound provided by the invention can be prepared in various medical forms and is extremely high in stability. The carbazochrome sodium sulfonate freeze-dried powder, water injection and troche preparations provided by the invention are simple in formulation and technology, the stability is remarkably improved, and the pharmaceutical safety and effectiveness are improved.
Owner:HUNAN WUZHOUTONG PHARMA
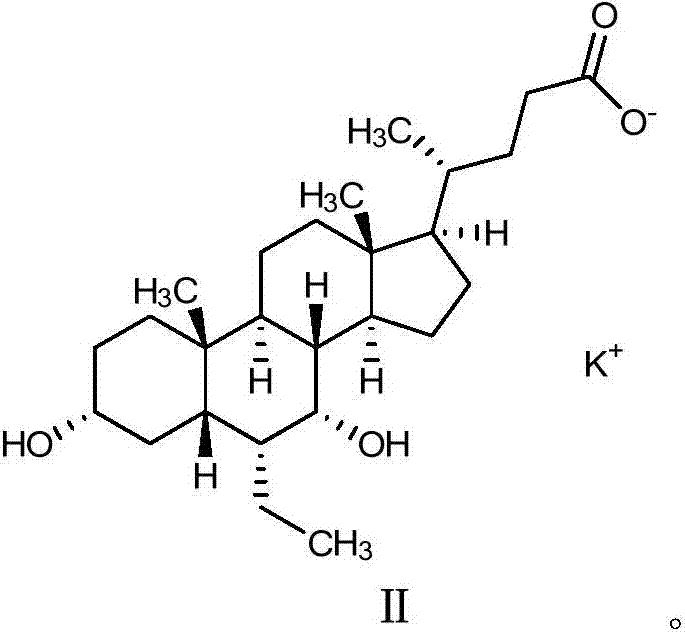
Obeticholic acid salts and pharmaceutical composition thereof
PendingCN107188917AImprove stabilityThe prescription process is simpleOrganic active ingredientsMetabolism disorderSolubilityMagnesium salt
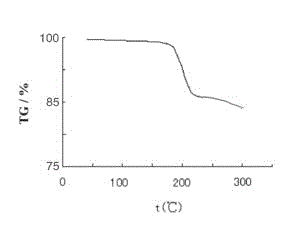
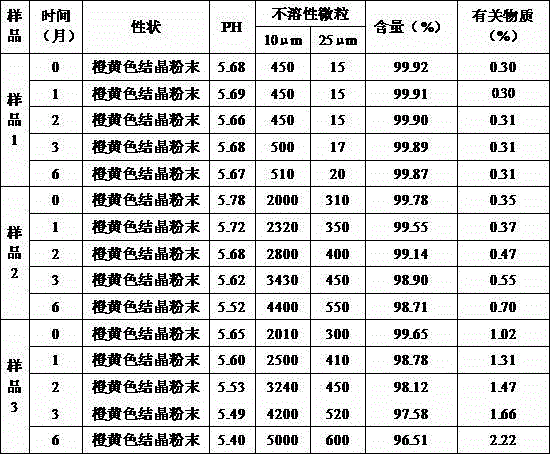
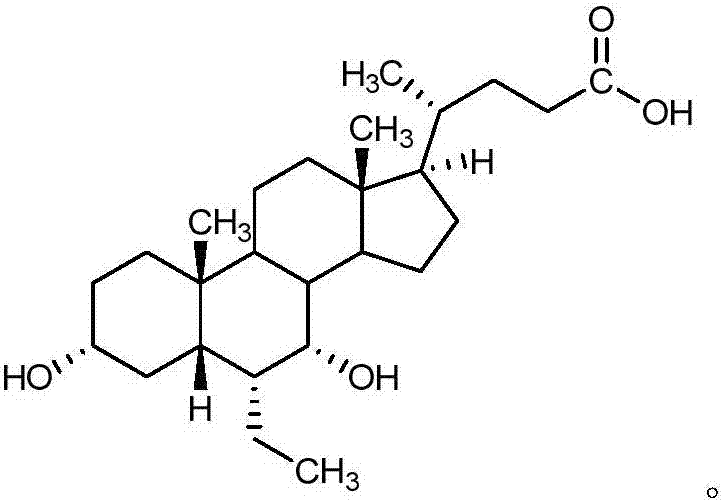
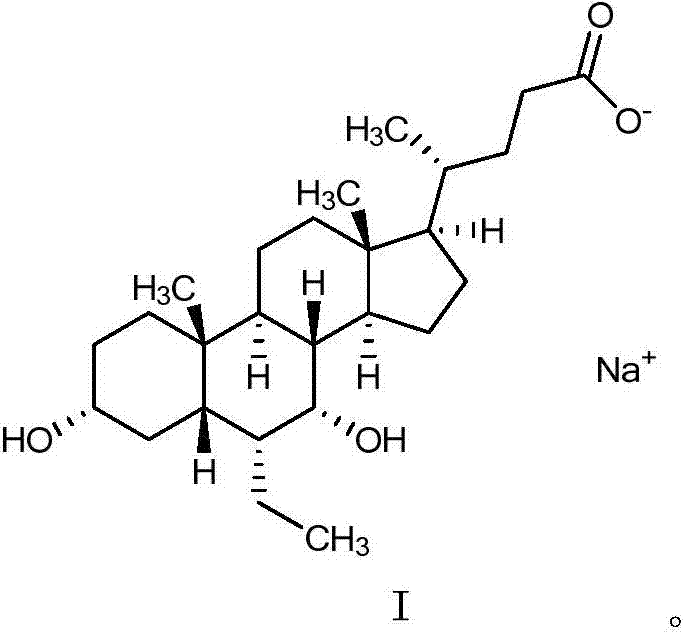
The invention belongs to the field of medicine chemistry and relates to obeticholic acid salts and a pharmaceutical composition thereof. Particularly, obeticholic acid salts includes obeticholic acid sodium salt, obeticholic acid potassium salt, obeticholic acid magnesium salt, obeticholic acid calcium salt and obeticholic acid ammonium salt. The invention further relates to a preparing method of obeticholic acid sodium salt, obeticholic acid potassium salt, obeticholic acid magnesium salt, obeticholic acid calcium salt and obeticholic acid ammonium salt, a pharmaceutical composition including the salts and medical application of the salts. The obeticholic acid salts have the advantages of being high in purity, stability and solubility property, show a good pharmacokinetics property and are suitable for being prepared into medicine products for use.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
A gastrodin compound and its pharmaceutical composition
ActiveCN103224539BThe prescription process is simpleImprove stabilityOrganic active ingredientsNervous disorderDrug utilisationCaplet Dosage Form
The invention relates to a gastrodine compound and a pharmaceutical composition thereof. The gastrodine compound is a crystal. The X-ray powder diffraction determines that the characteristic peaks are displayed when 2theta+ / -0.2 degree is 4.5 degrees, 5.7 degrees, 7.9 degrees, 10.3 degrees, 11.2 degrees, 13.0 degrees, 16.4 degrees, 19.6 degrees, 21.2 degrees, 22.7 degrees, 24.8 degrees, 26.7 degrees, 29.6 degrees, 34.1 degrees and 35.3 degrees. The gastrodine compound has very high stability, thereby greatly enhancing the medicine safety. The invention also relates to a pharmaceutical composition preparation containing the gastrodine compound. The composition preparation can be a freeze-dried powder injection, injection, tablet or capsule. The gastrodine freeze-dried powder injection, injection, tablet or capsule preparation provided by the invention has the advantages of simple prescription technique, obviously higher stability, and enhances the medicine safety and effectiveness.
Owner:湖北美林药业有限公司
Compound sustained-release tablet of cetirizine and pseudoephedrine and preparation method thereof
ActiveCN101708178AGood reproducibilitySimple processOrganic active ingredientsRespiratory disorderSustained Release TabletPseudoephedrine
The invention discloses a compound sustained-release tablet of cetirizine and pseudoephedrine and a preparation method thereof. The tablet comprises cetirizine or pharmaceutically acceptable cetirizine salt and pseudoephedrine or pharmaceutically acceptable pseudoephedrine salt. The preparation method comprises the steps of: preparing the pseudoephedrine or the pharmaceutically acceptable pseudoephedrine salt into a sustained-release tablet core; uniformly dispersing the cetirizine or the pharmaceutically acceptable cetirizine salt in a coating solution to coat the surface of the tablet core. Two active materials with different doses are prepared into the compound sustained-release tablet by a coating method. The preparation method solves the problems of the quick release of the cetirizine and the sustained release of the pseudoephedrine, has convenient operation and easy quality control, and is suitable for industrial production. In the tablet, more than 85% of the cetirizine is dissolved within 30 minutes, 90% of the cetirizine is dissolved out within 1 hour, and the pseudoephedrine releases medicaments in a sustained mode within 12 hours or 24 hours. The tablet is taken once or twice a day, and can reduce the administration time, better stabilize the concentration of blood medicaments and reduce adverse effect.
Owner:YANGTZE RIVER PHARM GRP CO LTD +1
Preparation process of compound dry powder inhaler for glucocorticoids and beta2 receptor agonists
ActiveCN108771761AImprove FPFImprove stabilityPowder deliveryOrganic active ingredientsIndacaterolGlucocorticoid
The invention relates to a preparation process of a compound dry powder inhaler for glucocorticoids and beta2 receptor agonists. The process comprises the following steps: (a) performing micronizationon glucocorticoids and beta2 receptor agonists; (b) mixing the micronized product in the step (a) with a drug carrier, or mixing the micronized product in the step (a) with a drug carrier and an additive; and (c) adding the composition in the step (b) into a sample handling room for handling, thereby obtaining the pharmaceutical composition of the dry powder inhaler. The sample handling room is an environment of which the humidity is 45-75% or an environment of which the pressure is not higher than 500Pa; the handling time is 2-72 hours; the glucocorticoids are mometasone furoate or pharmaceutically acceptable salts thereof; and the beta2 receptor agonists are indacaterol or pharmaceutically acceptable salts thereof. The process disclosed by the invention is simple, convenient to operateand low in production cost.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Cefodizime sodium injection and preparation method thereof
InactiveCN103271880AImprove stabilityThe prescription process is simpleAntibacterial agentsPowder deliveryCefodizime SodiumFreeze-drying
The invention relates to a cefodizime sodium and preparation method thereof, especially relates to a cefodizime sodium injection for treating microbe infection, and preferably relates to a freeze-drying powder injection. The cefodizime sodium injection of the present invention is mainly composed of active component cefodizime sodium and accessories such as mannitol and citric acid. The solvent in the injection of the present invention is injection water; the excipient in the freeze-drying powder injection is mannitol, and sodium hydroxide or hydrochloric acid is used for adjusting pH valve.
Owner:张宏民
Pharmaceutical composition of palipeddone
InactiveCN103385857AEasy to take medicineThe prescription process is simpleOrganic active ingredientsNervous disorderPharmacyPharmaceutical drug
The invention discloses a pharmaceutical composition of palipeddone, belongs to medicine field, and relates to an antipsychotic pharmaceutical composition, wherein the pharmaceutical composition comprises palipeddone active component, controlled-release auxiliary material and other acceptable auxiliary material in pharmacy. The pharmaceutical composition provided by the invention solves the problem of difficult medicine taking by mental patients, and has advantages of simple technology, low cost and easy industrialization, and can better satisfy clinic needs.
Owner:BEIJING VENTUREPHARM BIOTECH
Gel gent of Chiense forest frog bactericidal peptide and its preparation process
ActiveCN100333789CStir wellGood treatment effectPeptide/protein ingredientsAntisepticsDiseaseCarboxymethyl cellulose
A tree frog's antibacterial peptide gel for treating the dermatopathy caused by aeruginose pseudomonads and the infection of burn and scald is prepared from antibacterial peptide for tree frog, carboxymethyl cellulose sodium glycerine, sodium dodecyl sulfonate, ethylester of nipagin and water through preparing transparent gel matrix and adding antibacterial peptide of tree frog.
Owner:TONGHUA KANGYUAN BIOLOGICAL TECH
A stable busulfan injection
ActiveCN103446045BThe prescription process is simpleStable storageOrganic active ingredientsPharmaceutical delivery mechanismPEG 400Chemistry
The invention provides a busulfan injection which is simple in prescription and stable in quality. The injection is prepared by the followings method: (1), dissolving busulfan into a mixed solvent of N, N-dimethyl acetamide and polyethylene glycol 400 in a volume ratio of (17-57):(83-43) to obtain a busulfan solution with concentration of 1mg / mL-7.5mg / mL; and (2), filtering and filling in a sterile manner, wherein in the dissolving process of the step (1), the temperature of the solution is 4 DEG C-25 DEG C, or / and inert gases are charged in an operation process until the solution is saturated. Not only is stability of the busulfan injection obtained by the preparation method disclosed by the invention improved, but also the safety of the injection after long-time storage is effectively ensured. Meanwhile, other substances or other chemical components are not introduced in the preparation process, so that the medication safety is ensured, and the drug cost is also lowered.
Owner:SICHUAN CREDIT PHARMA +1
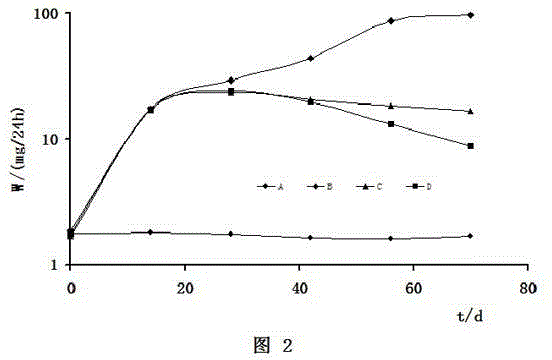
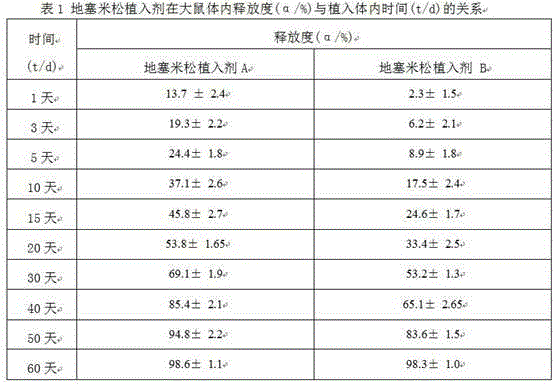
Preparation method of dexamethasone implant for kidney
ActiveCN105560161APromote meltingEasy extrusionOrganic active ingredientsPharmaceutical delivery mechanismDexamethasone acetateEngineering
The present invention relates to a preparation method, uses and a use method of a dexamethasone implant for kidney, wherein the dexamethasone implant comprises dexamethasone or dexamethasone acetate, a degradable polymer material, and a water soluble auxiliary material. The preparation method comprises: crushing various materials, mixing, carrying out micro-spheroidization, carrying out mold pressing molding, and heating for a certain time at a proper temperature to prepare the cylindrical implant with a diameter of 0.2-0.9 mm and a length of 0.8-4 mm. According to the present invention, the implant has characteristics of smooth surface and uniform drug release in vivo, wherein the time for releasing 90% of the drug is 1 month to 1 year; and the implant can be implanted into the renal sac through a drug implanting needle so as to treat nephrotic syndrome, nephritis and other chronic kidney diseases.
Owner:ANHUI ZHONGREN TECH +2
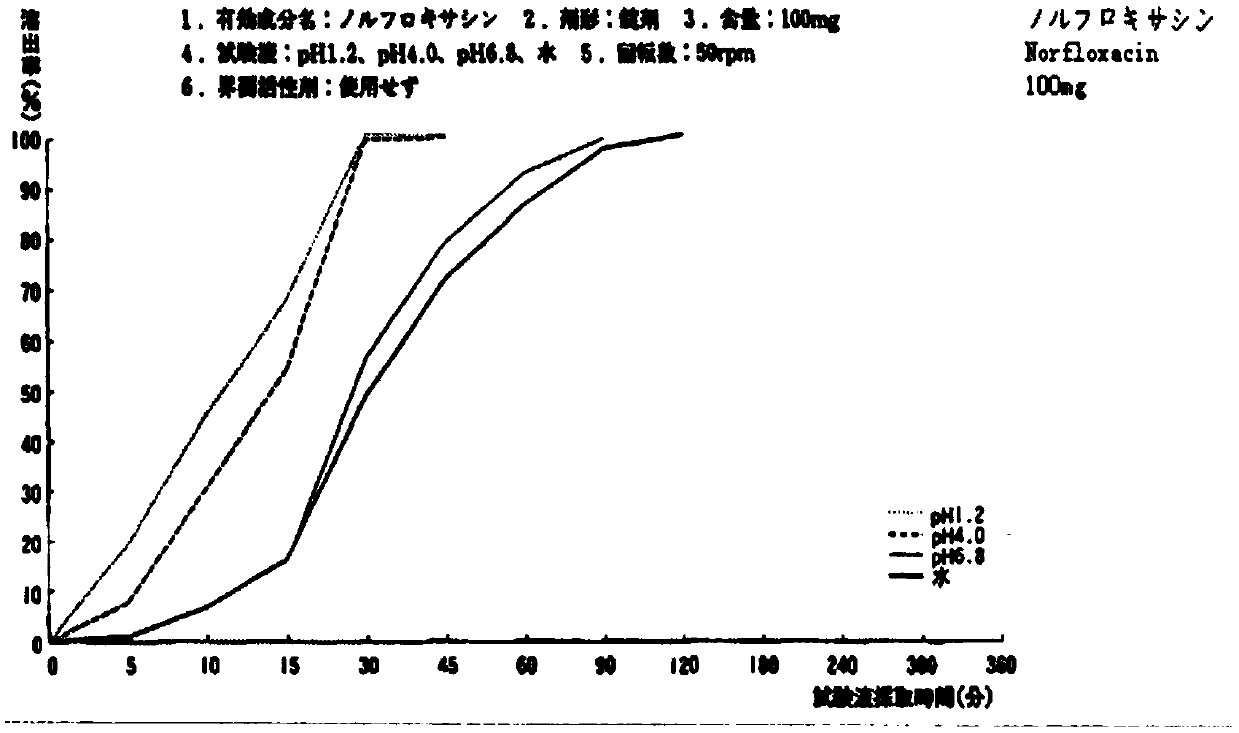
Norfloxacin composition
InactiveCN107661305APromote dissolutionAddressing Poor Dissolution ProblemsAntibacterial agentsOrganic active ingredientsCross-linkFiller Excipient
The invention relates to a norfloxacin composition and belongs to the technical field of pharmacy. According to the technical scheme provided by the invention, the norfloxacin composition is preparedfrom norfloxacin, lactose, cross-linked sodium carboxymethyl cellulose and other pharmaceutically acceptable auxiliary materials. The pharmaceutically acceptable auxiliary materials comprise a fillingagent and a lubricant and the granularity of the norfloxacin is distributed in a range that D90 is greater than or equal to 200mu m and less than or equal to 300mu m. The invention provides the stable norfloxacin composition with a dissolution behavior which is the same as that of an original research.
Owner:迪沙药业集团(天津)药物研究有限公司 +1
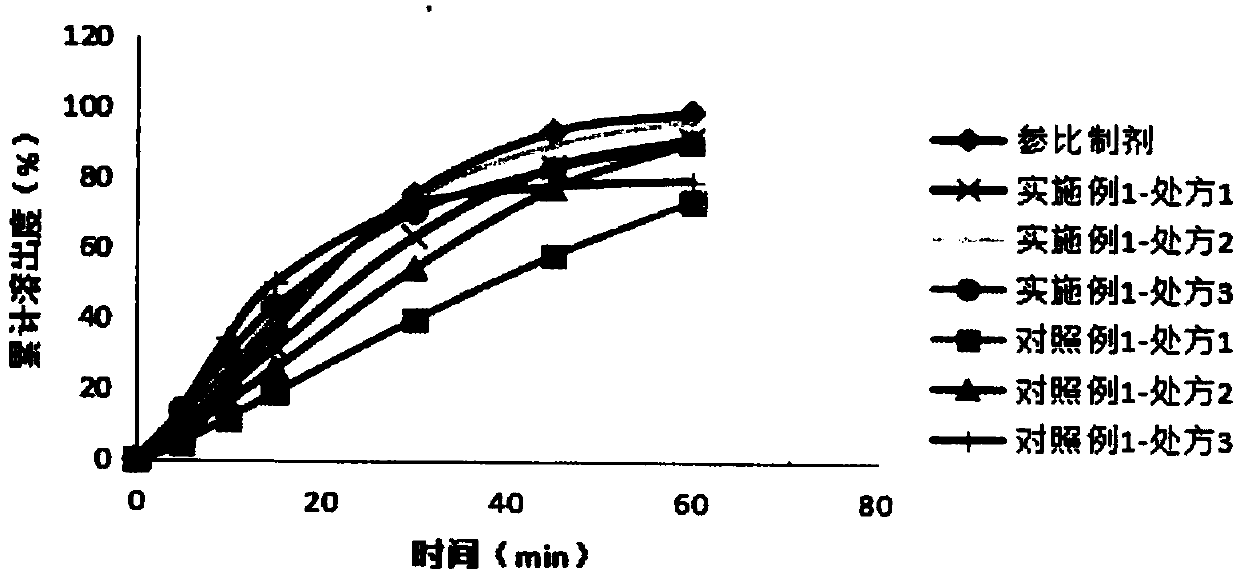
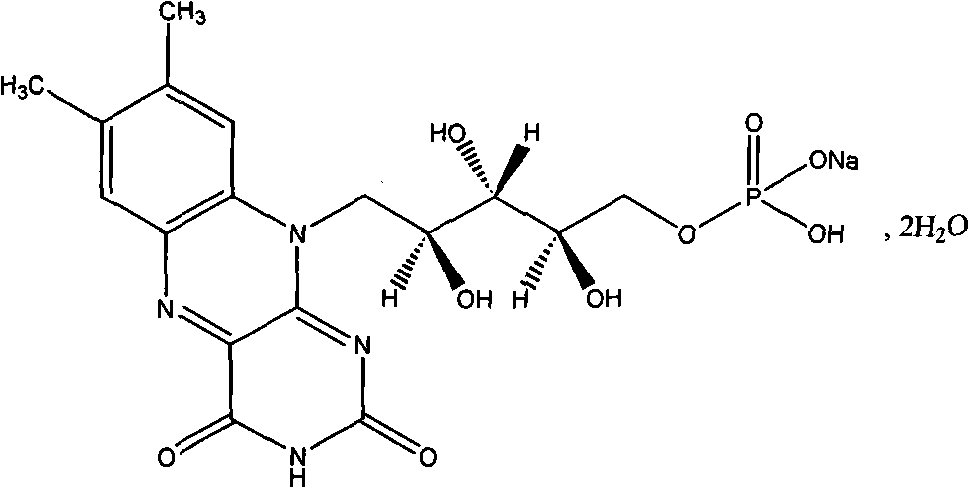
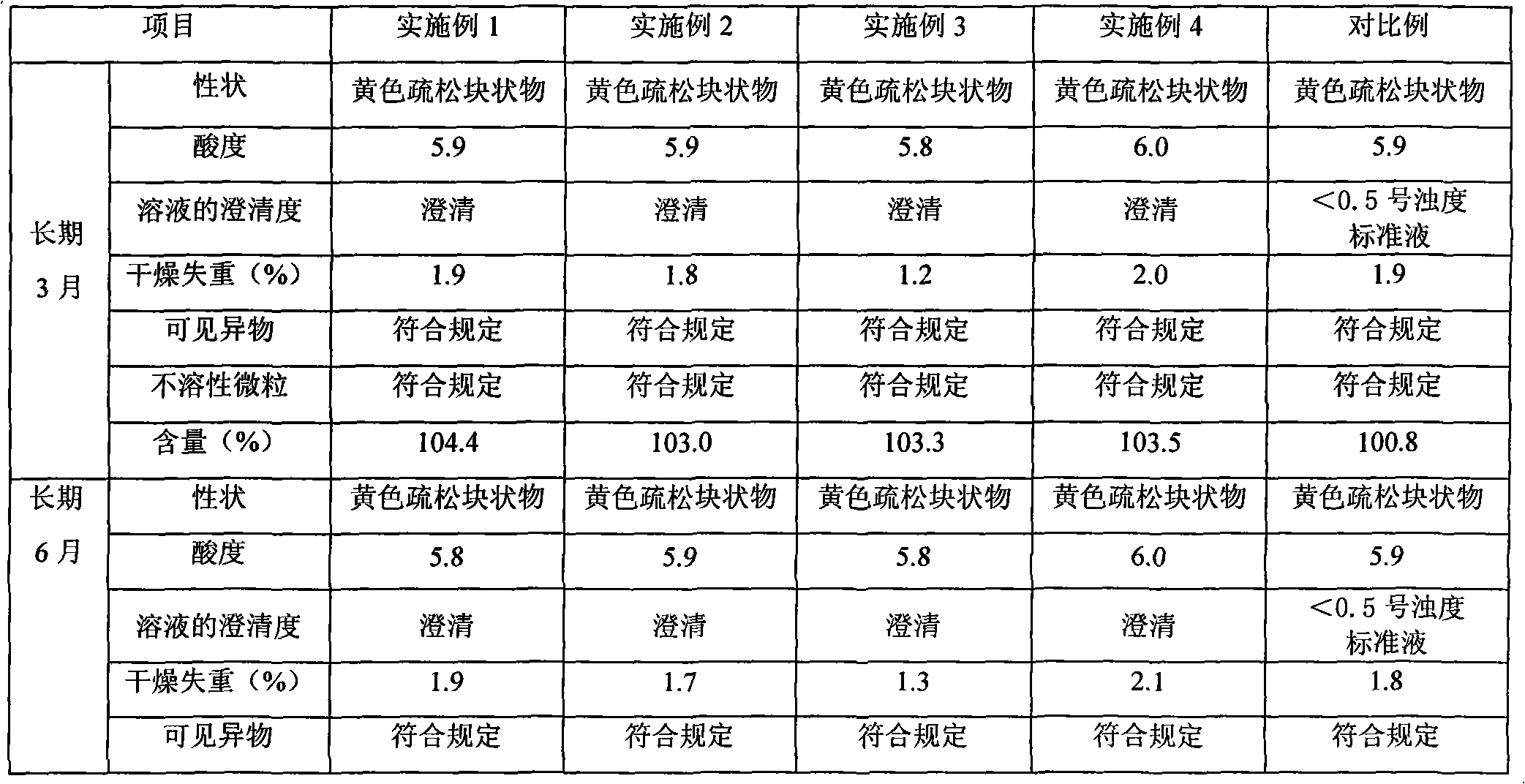
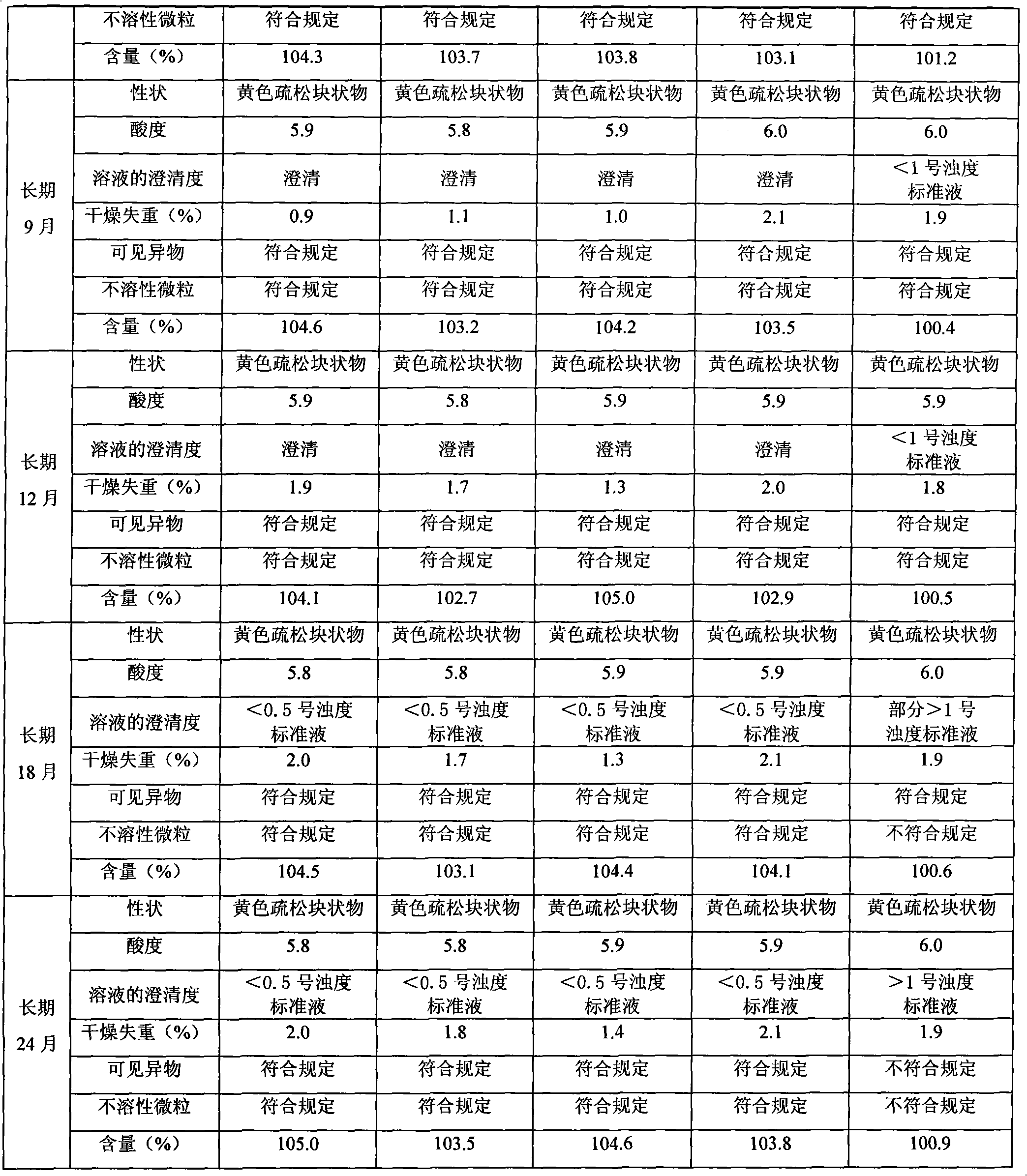
Riboflavin sodium phosphate freeze-dried powder injection and preparation method thereof
ActiveCN101874787AMeet the requirements for intravenous drug useReduce the risk of adverse reactionsOrganic active ingredientsPowder deliveryHaemolysisFreeze-drying
The invention relates to a riboflavin sodium phosphate freeze-dried powder injection and a preparation method thereof. The injection is characterized by comprising a riboflavin sodium phosphate dehydrate, mannitol and / or ammonia water. The injection is sensitive to light, so a brown amoxicillin bottle is used as the inner package of the injection. The riboflavin sodium phosphate freeze-dried powder injection has the following advantages: (1) single and clear auxiliary materials in the formula can meet the requirement of industrialized batch production; (2) medicinal injection grade raw and auxiliary materials accord with the medication requirement of human intravenous injection, so that potential adverse reaction risk in clinical application can be greatly reduced; (3) long-term stability tests prove that the preparation can be preserved for 2 years at the temperature below 30 DEG C, so that stability of the medicament in circulation and safety of clinical medication can be ensured; and (4) special safety tests, such as irritability, haemolysis, vascular stimulation and the like, prove that haemolysis, agglutination, stimulation and anaphylaxis reactions are not found.
Owner:HAINAN LEVTEC PHARMA
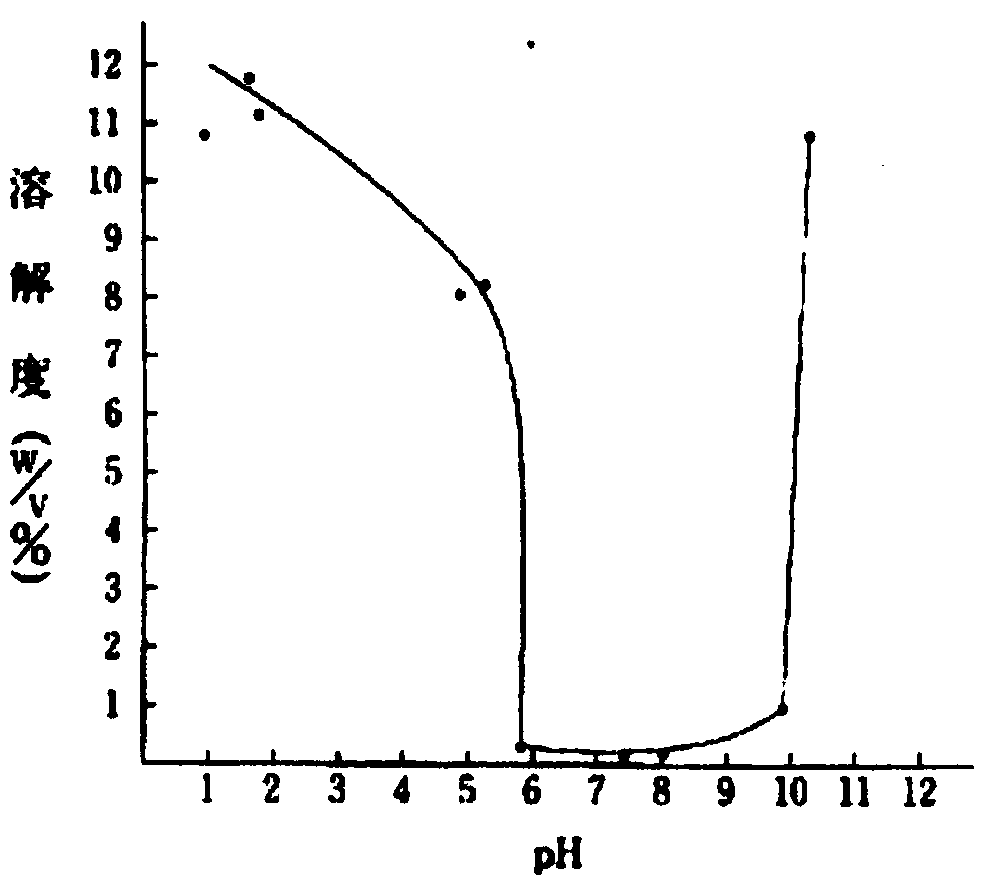
Pharmaceutical composition containing enoximone and preparation method thereof
InactiveCN101548944ASolve the problem of hydrophobicityThe prescription process is simpleOrganic active ingredientsPharmaceutical delivery mechanismPharmaceutical formulationFirst aid
The invention discloses a liquid pharmaceutical composition containing enoximone and used for injection, and the liquid pharmaceutical composition also includes a solution stabilizer and a pH regulator accepted on pharmacology. The liquid pharmaceutical composition has good stability, is used for first aid treatment of the severe heart failure and is applied when the effects of other therapeutic methods are not satisfying.
Owner:BEIJING D VENTUREPHARM TECH DEV
Cefoxitin sodium agent and preparation method thereof
InactiveCN103271877AImprove stabilityThe prescription process is simpleAntibacterial agentsPowder deliveryAdditive ingredientFreeze-drying
The invention relates to a cefoxitin sodium agent and preparation method thereof, especially relates to a cefoxitin sodium injection for treating microbe infection, preferably relates to a freeze-drying powder injection. The cefoxitin sodium injection of the present invention is mainly composed of cefoxitin and accessory mannitol. The solvent in the injection of the present invention is injection water; the excipient in the freeze-drying powder injection is mannitol, and sodium hydroxide or hydrochloric acid are used for adjusting pH valve.
Owner:张宏民
A kind of preparation method of nocafloxacin freeze-dried powder injection
ActiveCN103040770BImprove solubilityReduce dosageAntibacterial agentsPowder deliveryFreeze-dryingOrganic chemistry
Owner:NANJING BIOTICA PHARMA
Medicinal composition for treating chicken necrotic enteritis
InactiveCN110404006AGood curative effectImprove the immunityAntipyreticAnalgesicsCallicarpa nudifloraEpimedium
The invention provides a medicinal composition for treating chicken necrotic enteritis, wherein the medicinal composition comprises the main components in parts by weight: 10 to 100 parts of callicarpa nudiflora, 10 to 80 parts of fructus trichosanthis, 10 to 50 parts of spina date seed, 30 parts of longspur epimedium, and 1-30 of benzoin. The invention discloses a preparation method of the granule of the medicinal composition. The medicinal composition for treating chicken necrotic enteritis has good curative effect against clostridium perfringens, and can significantly enhance the resistanceof the chicken and improve immunity. The process is simple and convenient for industrial production. The prescription has the advantages of pure natural performance, non-polluting, and no drug residue, and the efficiency is 100%.
Owner:湖南宇山玉月农业科技有限公司
Oral solid pharmaceutical composition containing clinofibrate
InactiveCN101548942ASolve the problem of hydrophobicityThe prescription process is simpleOrganic active ingredientsPill deliveryActive componentAdditive ingredient
The invention discloses an oral solid pharmaceutical composition containing clinofibrate. The grain size of the active component of the oral solid pharmaceutical composition is controlled below 30 mum, and the oral solid pharmaceutical composition includes surface-active agent. The oral solid pharmaceutical composition is high in dissolution rate and good in absorptivity and is used for curing hyperlipemia.
Owner:BEIJING D VENTUREPHARM TECH DEV
A kind of preparation method of dexamethasone implant for kidney
ActiveCN105560161BPromote meltingEasy extrusionOrganic active ingredientsPharmaceutical delivery mechanismDexamethasone acetateDrug release
The present invention relates to a preparation method, uses and a use method of a dexamethasone implant for kidney, wherein the dexamethasone implant comprises dexamethasone or dexamethasone acetate, a degradable polymer material, and a water soluble auxiliary material. The preparation method comprises: crushing various materials, mixing, carrying out micro-spheroidization, carrying out mold pressing molding, and heating for a certain time at a proper temperature to prepare the cylindrical implant with a diameter of 0.2-0.9 mm and a length of 0.8-4 mm. According to the present invention, the implant has characteristics of smooth surface and uniform drug release in vivo, wherein the time for releasing 90% of the drug is 1 month to 1 year; and the implant can be implanted into the renal sac through a drug implanting needle so as to treat nephrotic syndrome, nephritis and other chronic kidney diseases.
Owner:ANHUI ZHONGREN TECH +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com