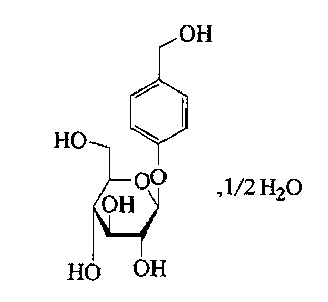
Gastrodine compound and pharmaceutical composition thereof
A technology of gastrodin and its compounds, which is applied in the field of gastrodin compounds and their pharmaceutical compositions, can solve problems related to unstable substances, complex processes, unstable pH value of gastrodin compounds, etc., and achieve improved safety and effectiveness, stable Enhanced performance and simple prescription process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
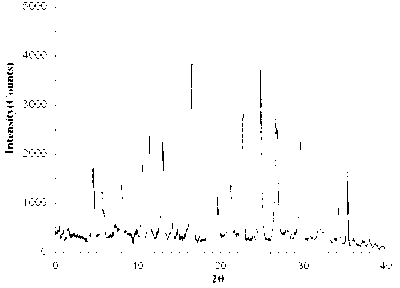
Image
Examples
Embodiment 1
[0050] 20 ℃, under the stirring speed of 150 rpm, gastrodin (commercially available gastrodin raw material, Jiangsu Hanstone Pharmaceutical Co., Ltd.) was dissolved in ethanol and methanol with a volume ratio of 1:8 at a volume ratio of 2:1. In the mixed solution; maintain 20°C, add gastrodin weight 1.0% activated carbon, stir at 150 rpm for 25 minutes, filter to remove carbon, filter the filtrate through a 0.22μm filter membrane; 25°C, at a stirring speed of 100 rpm , while stirring, slowly and uniformly add dropwise at a speed of 40ml / min a mixed solution of chloroform and ether whose volume ratio is 7 times that of ethanol and methanol mixture and whose volume ratio is 4:1; at the same time, the temperature is lowered to 8°C at a speed of 0.4°C / min, and the stirring is stopped. , cooling down to 2°C at a speed of 0.1°C / min and standing for crystal growth for 10 hours, then filtering; washing the filter cake obtained by filtering twice with a mixed solution of chloroform and ...
Embodiment 2
[0053] 25 ℃, under the stirring speed of 200 rev / mins, gastrodin (commercially available gastrodin raw material, Jiangsu Hanstone Pharmaceutical Co., Ltd.) was dissolved in ethanol and methanol with a volume ratio of 1:10 in a volume ratio of 2:1 In the mixed solution; maintain 25°C, add 1.0% gastrodin weight activated carbon, stir at 200 rpm for 25 minutes, filter to remove carbon, and filter the filtrate through a 0.22μm filter membrane; 30°C, at a stirring speed of 150 rpm , while stirring, slowly and uniformly add dropwise at a speed of 50ml / min a mixed solution of chloroform and ether whose volume ratio is 9 times that of ethanol and methanol mixture and whose volume ratio is 4:1; at the same time, the temperature is lowered to 10°C at a speed of 0.6°C / min, and the stirring is stopped , lower the temperature to 4°C at a rate of 0.2°C / min and let the crystal grow for 10 hours, filter; wash the filter cake obtained by filtering in 4 twice with a mixed solution of chloroform ...
Embodiment 3
[0055] prescription:
[0056] The gastrodin compound 100g that embodiment 1 makes
[0057] Mannitol 60g
[0058] Water for injection 2000ml
[0059] Make 1000 pieces
[0060] Process:
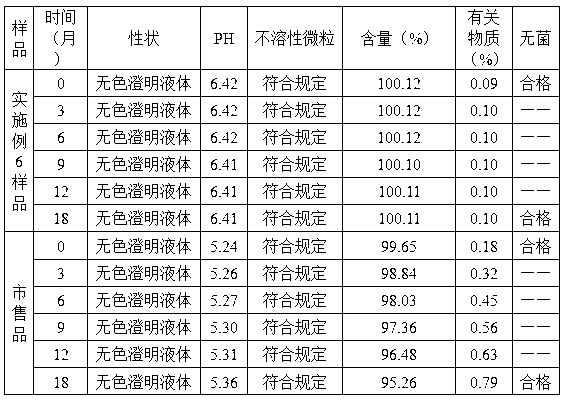
[0061] 1. Add the prescribed amount of gastrodin into 70% of the amount of water for injection, stir to dissolve at 30°C, add water for injection to 80%, then add mannitol according to the prescribed amount, stir to dissolve, add water for injection to the full amount, and stir evenly.
[0062] 2. Add 0.15% activated carbon to 1, stir for 20 minutes, decarbonize by filtration, and sterilize by filtration with a 0.22 μm filter membrane to detect intermediates.
[0063] 3. Freeze-drying:
[0064] ① Pre-freezing: Put the filtrate in 2 into a half-packed and stoppered container and place it in a pre-cooled freezer at -25°C, keep it for 1 hour, then cool it down to -35°C at a speed of 0.2°C / min, and keep it warm for 2 hours;
[0065] ②Sublimation: Turn on the vacuum device, control the vacuum ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com