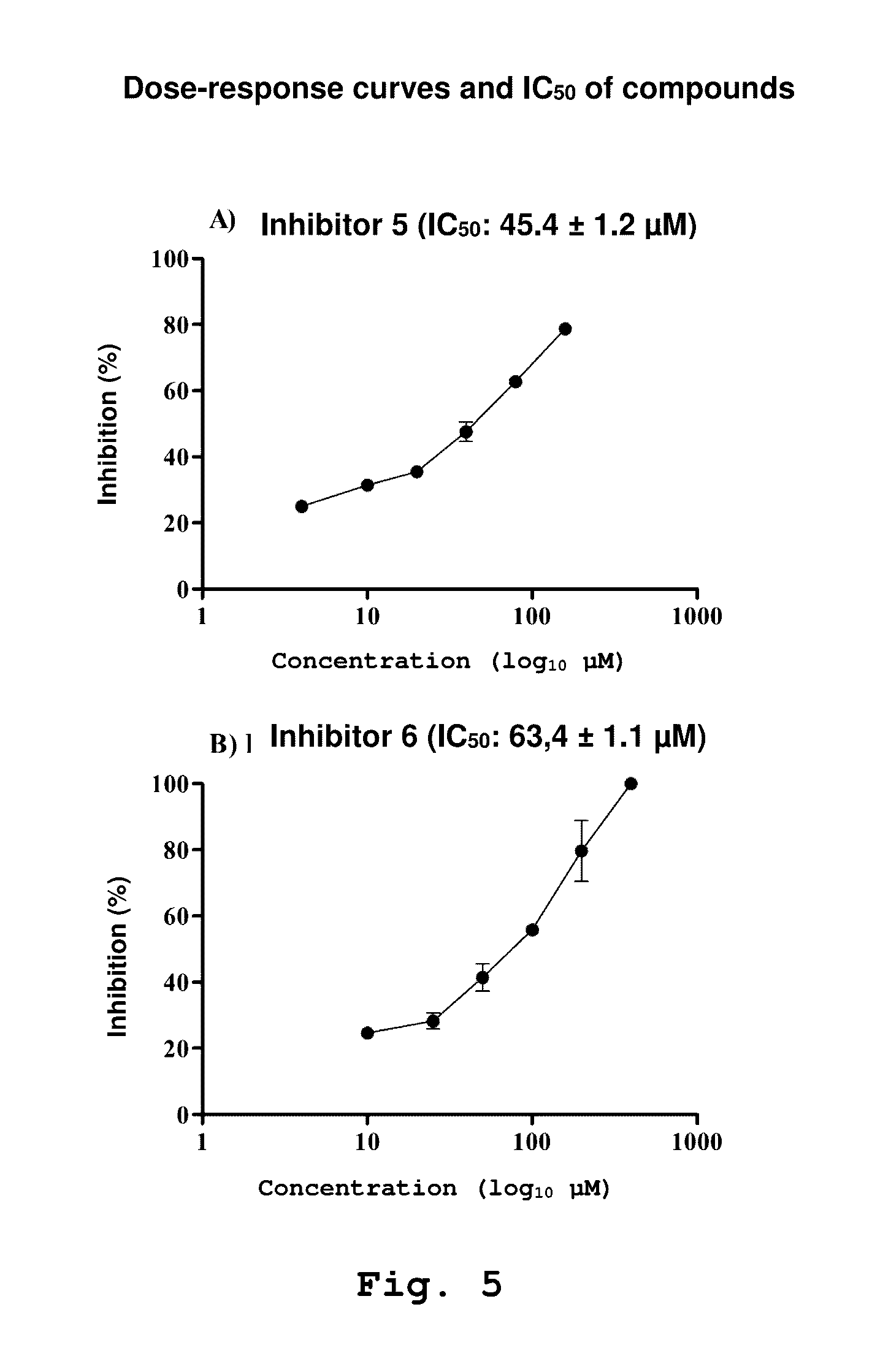
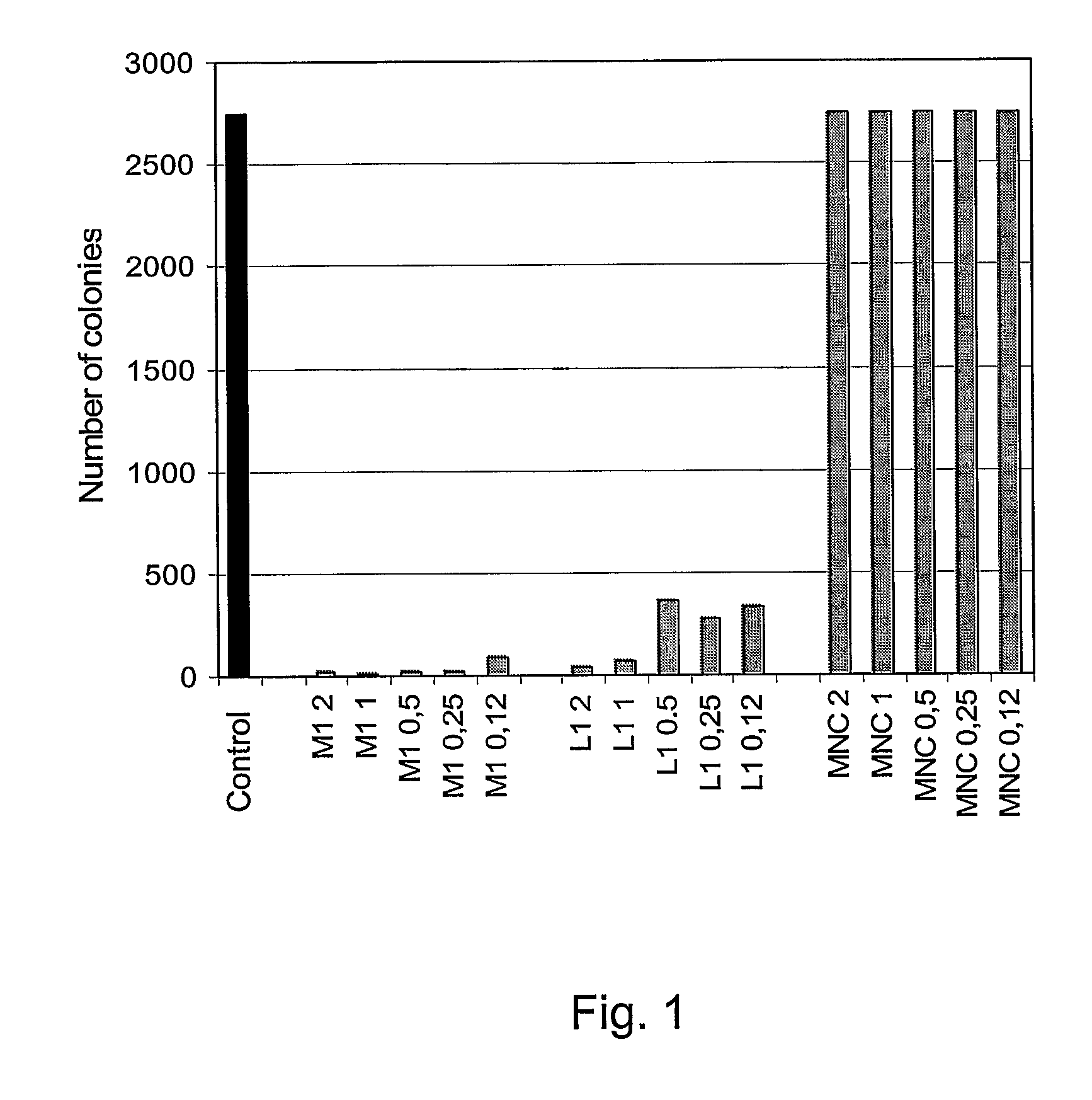
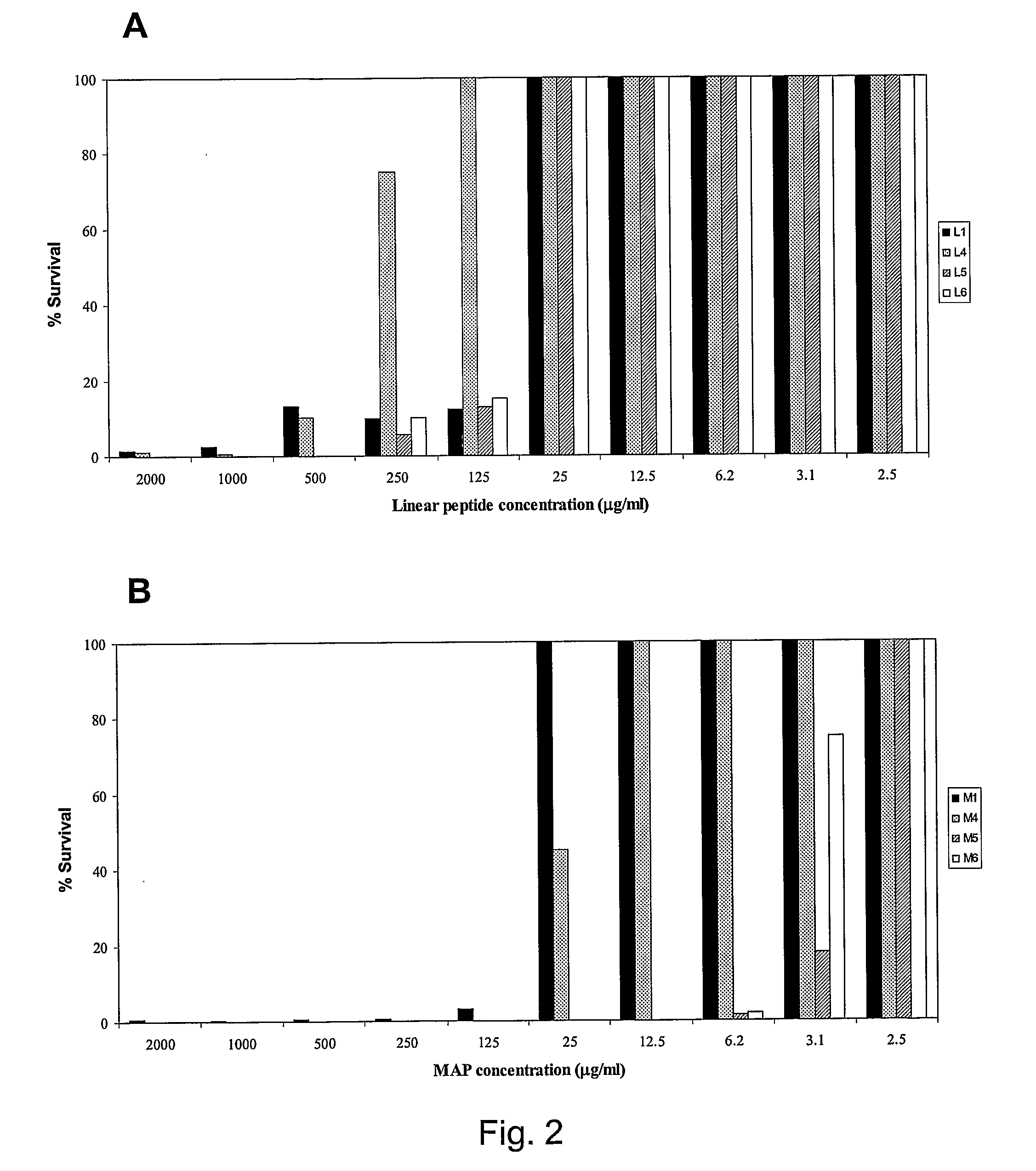
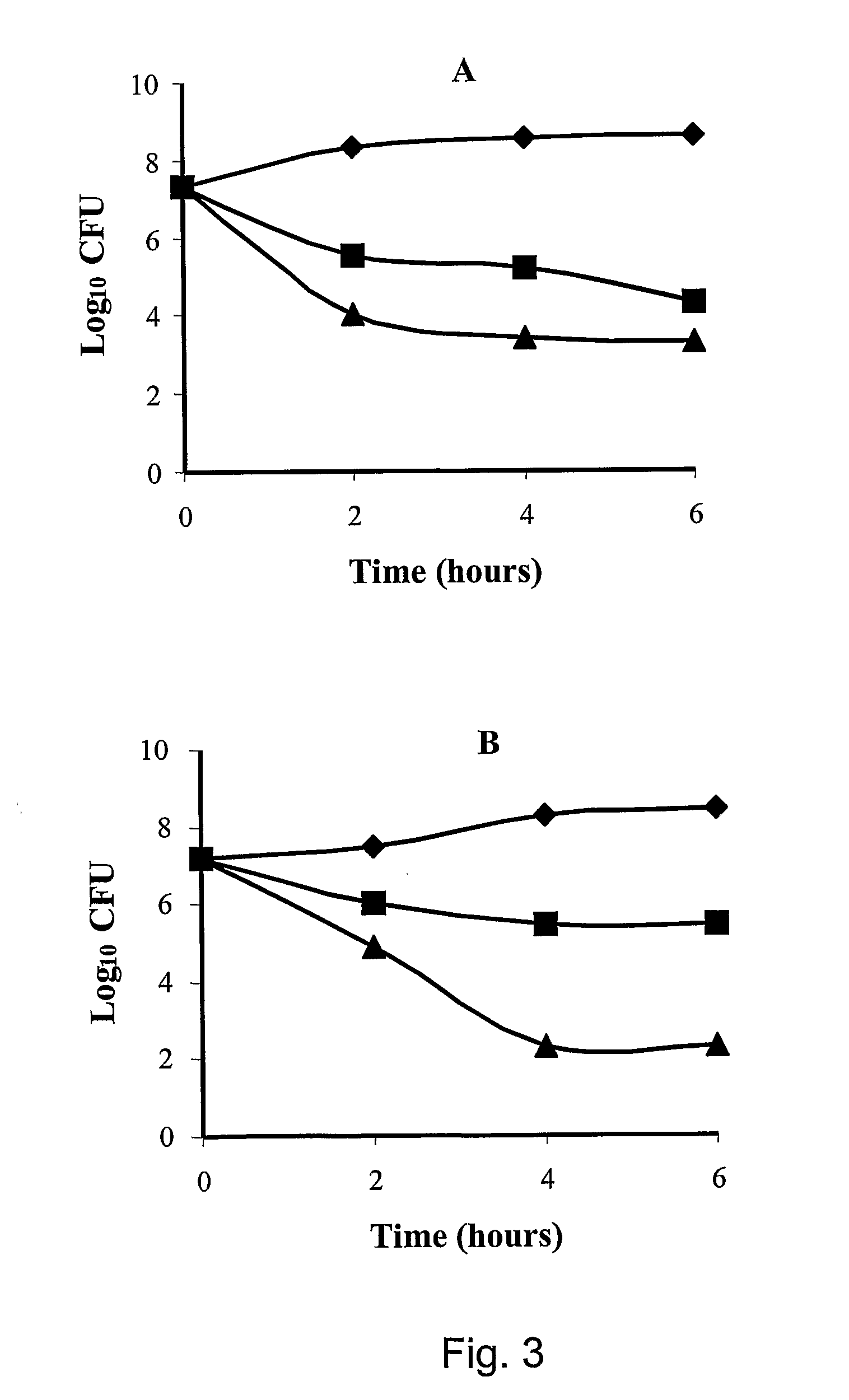
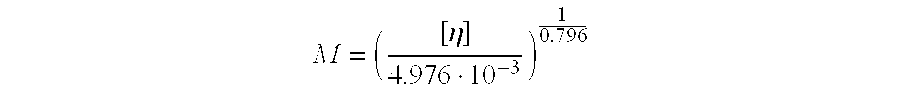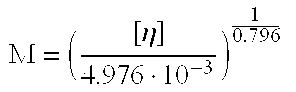Patents
Literature
50 results about "Haemolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Destruction of red blood cells
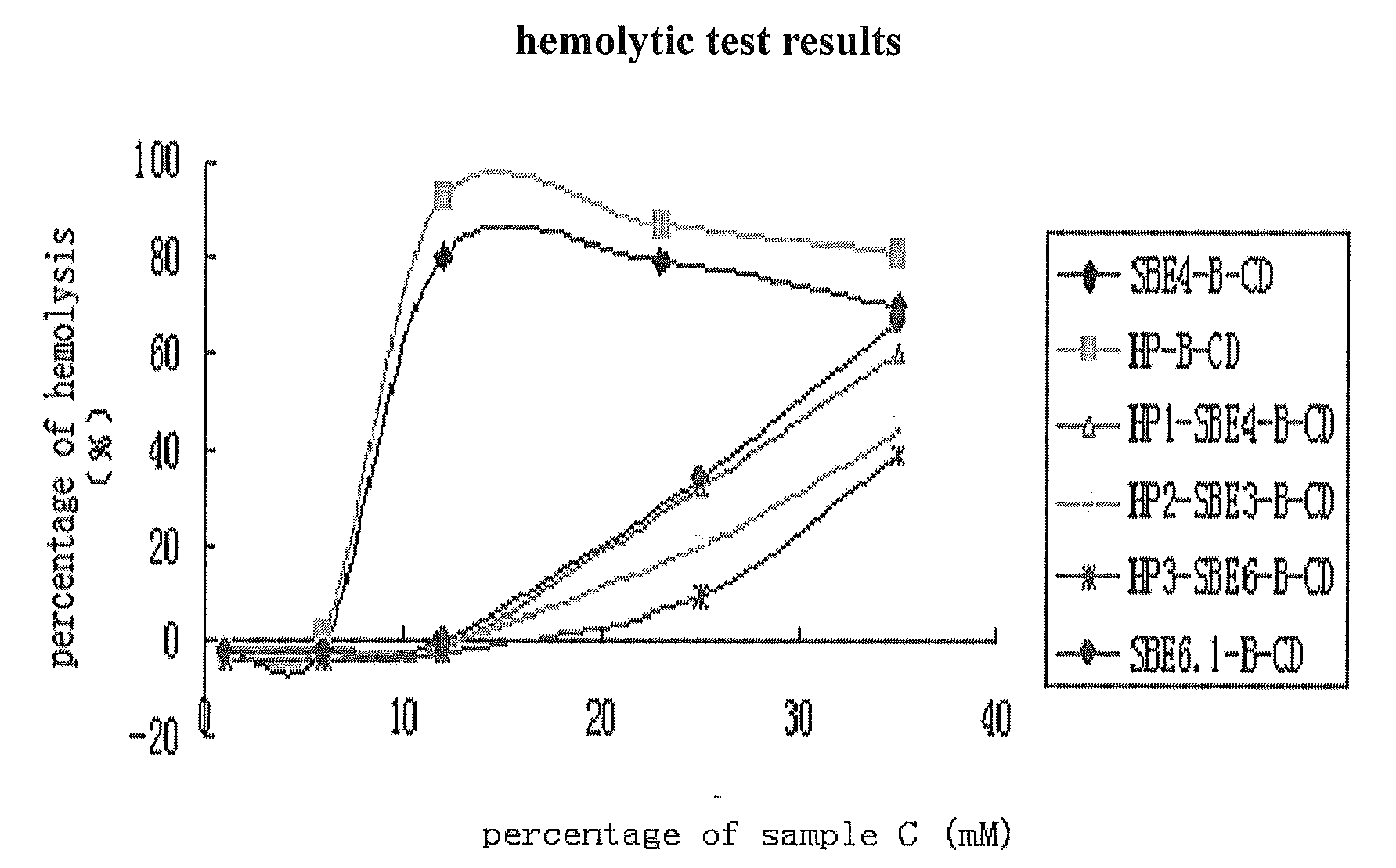
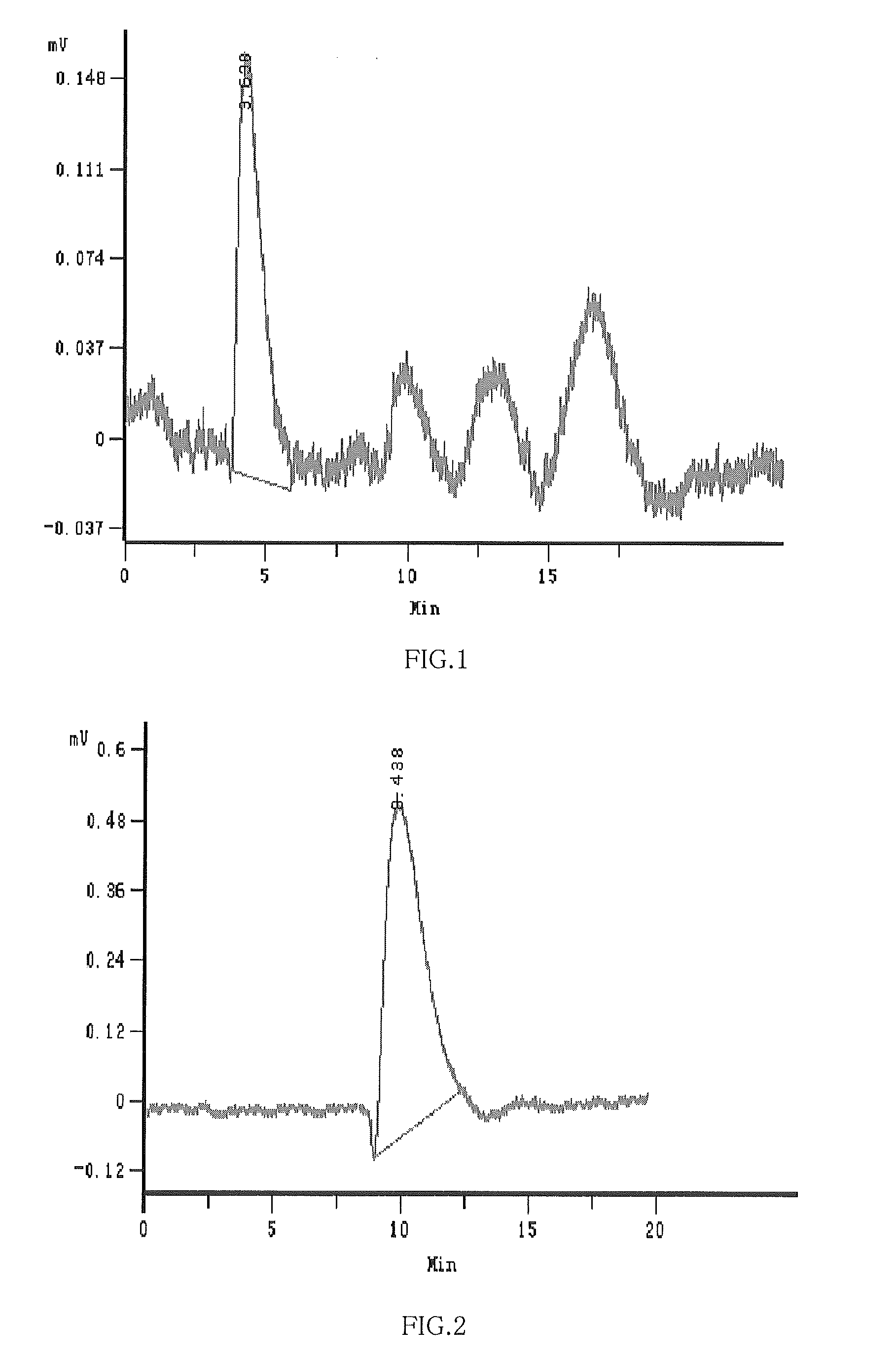
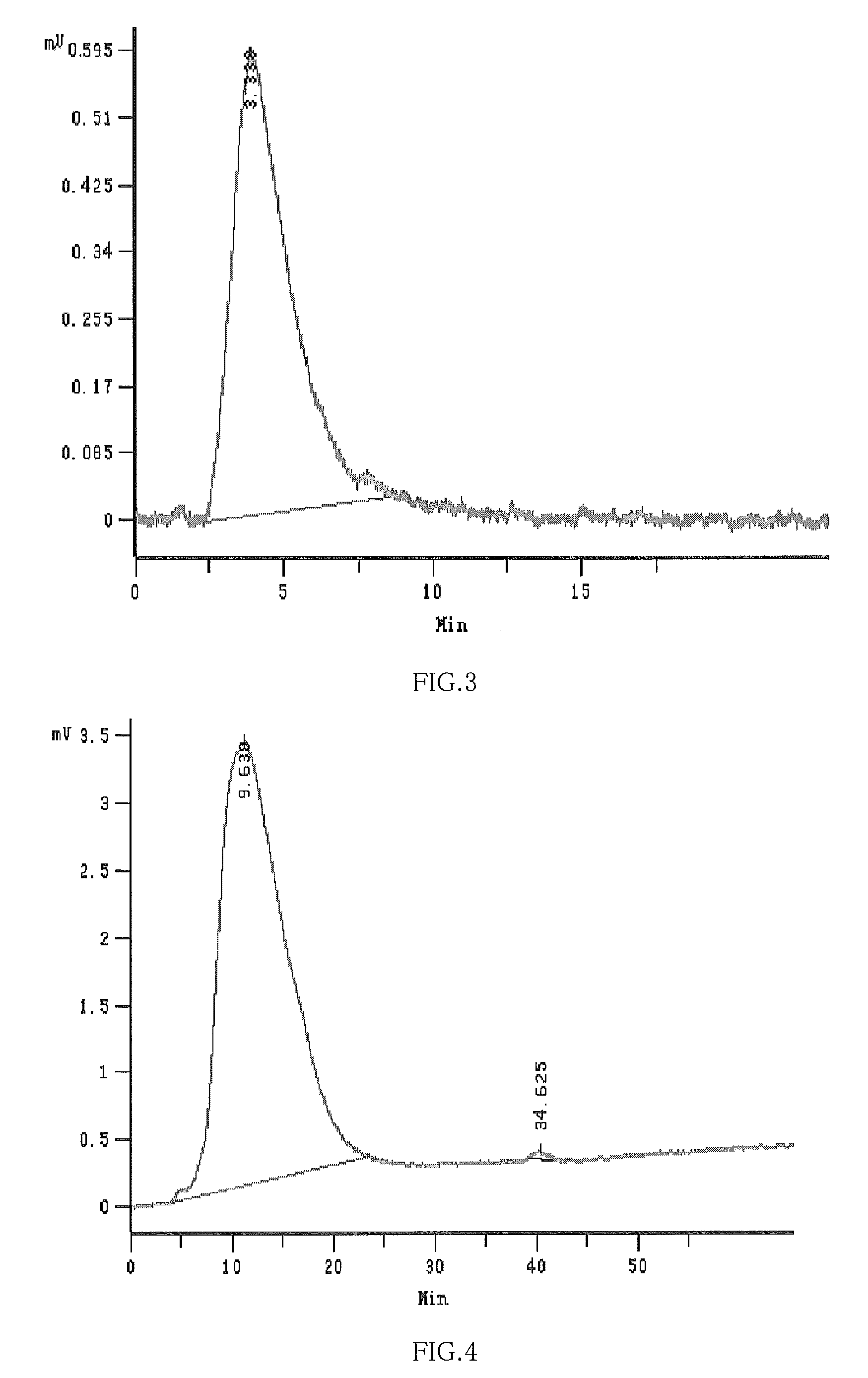
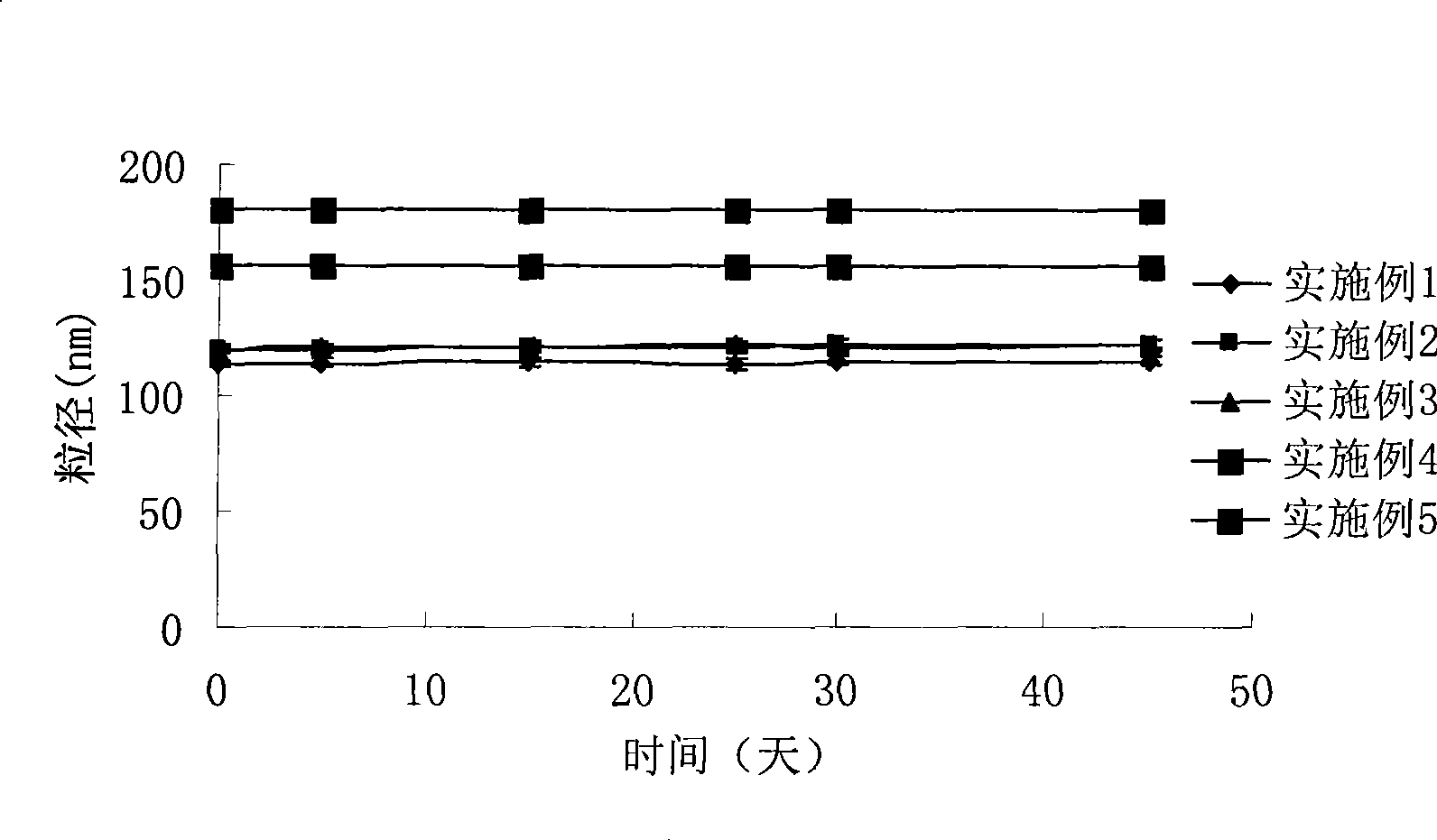
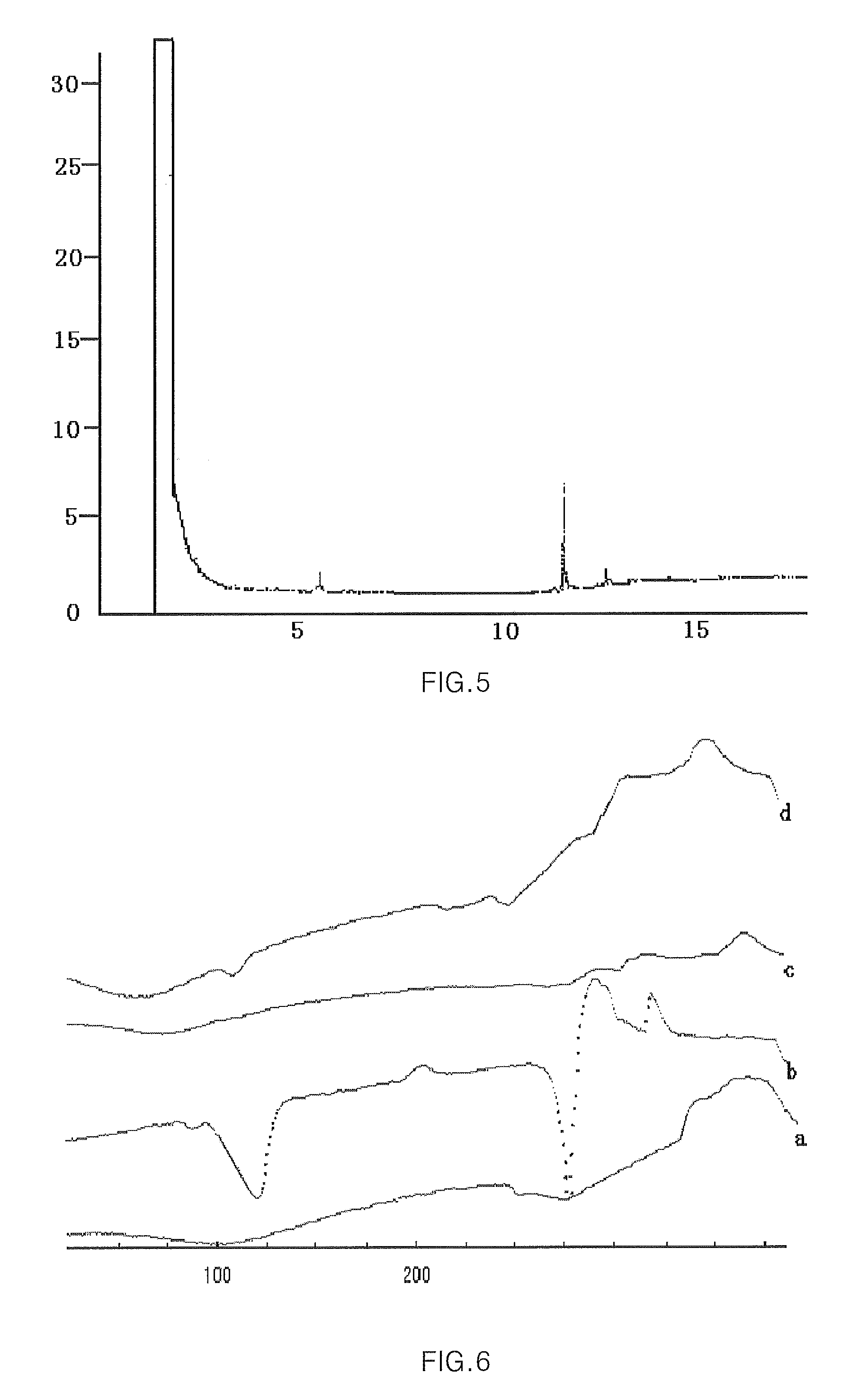
Hydroxypropyl-Sulfobutyl-Beta-Cyclodextrin, the Preparation Method, the Analytical Method, and the Pharmacutical Application Thereof
ActiveUS20090012042A1Little hemolysisLow toxicityBiocideOrganic active ingredientsHaemolysisCyclodextrin
Hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin, the preparation method, analytical method, and the pharmaceutical application thereof. The hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin is a derivate of cyclodextrin which is substituted by hydroxypropyl group and sulfobutyl group: n-(2,3,6-O-2-hydroxypropyl)-m-(2,3,6-O-sulfobutyl)-&bgr;-cyclodextrin. The number of substituent groups per mole cyclodextrin is n hydroxypropyl groups and m sulfobutyl groups. “n” represents the average substituent degree of hydroxypropyl groups; “m” represents the average substituent degree of sulfobutyl groups; “n+m=z” is the gross average substituent degree, in which n is a random integer chosen from 1, 2, 3, 4, 5, 6, 7, 8, 9; m is a random integer chosen from 1, 2, 3, 4, 5, 6, 7, 8, 9; and the gross average substituent degree z is a random integer chosen from 2, 3, 4, 5, 6, 7, 8, 9, 10. The present invention shows low haemolysis and low toxicity.
Owner:SUN XIAODONG
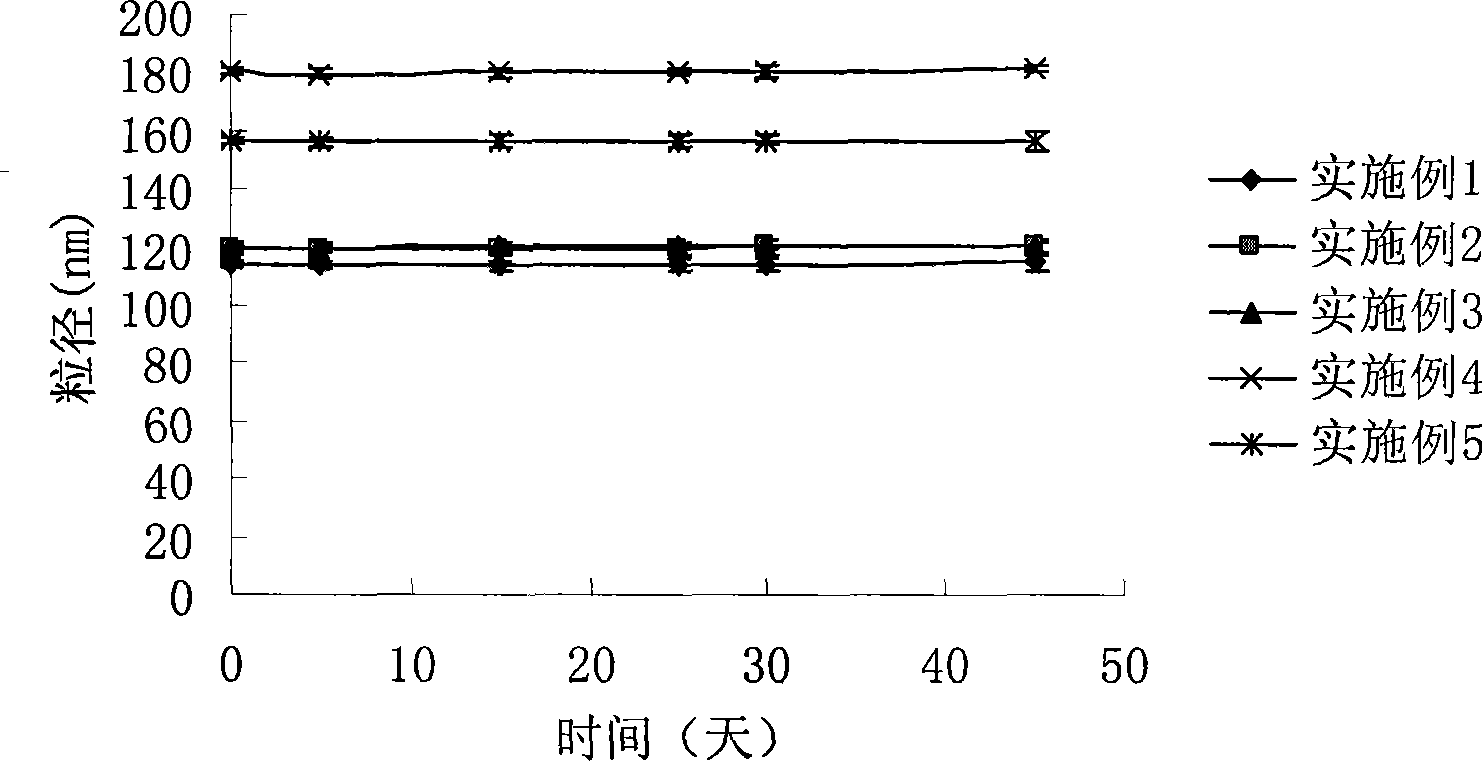
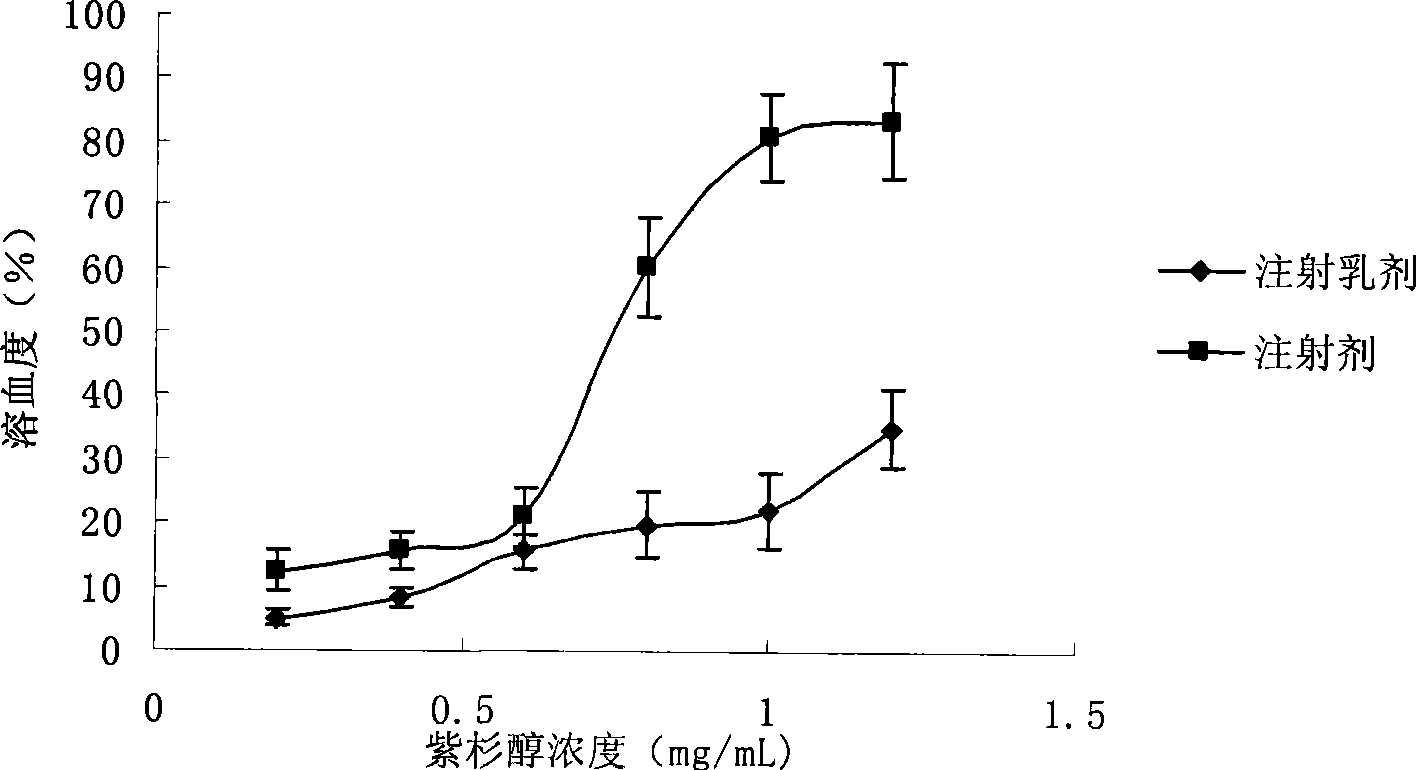
Intravenous injection emulsion of paclitaxel or polyenic taxusol
InactiveCN101433533AReduce allergic reactionsThe preparation process is simple and feasibleOrganic active ingredientsEmulsion deliveryPharmacyHaemolysis
The invention belongs to the field of traditional Chinese medicine pharmacy, in particular to a novel emulsion for intravenous injection of an anti-cancer drug taxol or polyenic taxusol, and a preparation method thereof. The emulsion consists of the taxol or the polyenic taxusol, and oil for injection, emulsion, in particular to glycol laurylhydroxystearate, and other pharmaceutically necessary auxiliary materials. Results via experiments show that the injection emulsion has excellent stability and haemolysis significantly smaller than a commercial taxol injection, and is helpful for improving administration safety and effectiveness of the taxol. The emulsion for intravenous injection can be directly used for intravenous injection, has the advantages of safe administration and small stimulation, can be easily accepted by patients, and can be used as an energy replenisher. The emulsion has the advantages of good stability, large drug loading, convenient use and simple preparation technology method, and is applicable to mass production.
Owner:FUDAN UNIV
Hydroxypropyl-sulfobutyl-beta-cyclodextrin, the preparation method, the analytical method, and the pharmacutical application thereof
ActiveUS8278437B2Little hemolysisLow toxicityOrganic active ingredientsBiocideHaemolysisCyclodextrin
Hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin, the preparation method, analytical method, and the pharmaceutical application thereof. The hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin is a derivate of cyclodextrin which is substituted by hydroxypropyl group and sulfobutyl group: n-(2,3,6-O-2-hydroxypropyl)-m-(2,3,6-O-sulfobutyl)-&bgr;-cyclodextrin. The number of substituent groups per mole cyclodextrin is n hydroxypropyl groups and m sulfobutyl groups. “n” represents the average substituent degree of hydroxypropyl groups; “m” represents the average substituent degree of sulfobutyl groups; “n+m=z” is the gross average substituent degree, in which n is a random integer chosen from 1,2,3,4,5,6,7,8,9; m is a random integer chosen from 1,2,3,4,5,6,7,8,9; and the gross average substituent degree z is a random integer chosen from 2,3,4,5,6,7,8,9,10. The present invention shows low haemolysis and low toxicity.
Owner:SUN XIAODONG
Compound taxol and its derivative docetaxel fat emulsion and preparation method
InactiveCN101006997APro-apoptosisGood anticancer effectOrganic active ingredientsEmulsion deliverySolubilityVegetable oil
The invention relates to complex paclitaxel and its derivates docetaxel intralipid which includes the following ingredients: paclitaxel or docetaxel, vegetable oil, solubilizing agent, lecithin, glycerine, and water for injection at a ratio of 0.5-10:10-100:10-100:10-20:20-25:700-950. The preparing method includes the following steps: stirring with high speed homogenating machine or ultrasonic oscillating to get the protogala; preparing the complex paclitaxel intralipid with high pressure homogenizer. The preparation is intralipid in O / W type which packages paclitaxel or docetaxel into the compound oil phase. The compound oil phase has good solubility for paclitaxel or docetaxel which has prevented the phenomenon of precipitation after diluting the emulsion; the ingredients of compound oil has the function of coordinated antitumous effect; it can also release the injecting irritative response, haemolysis and hypersensitiveness; it has the function of targeting which has increased the drug action.
Owner:董英杰
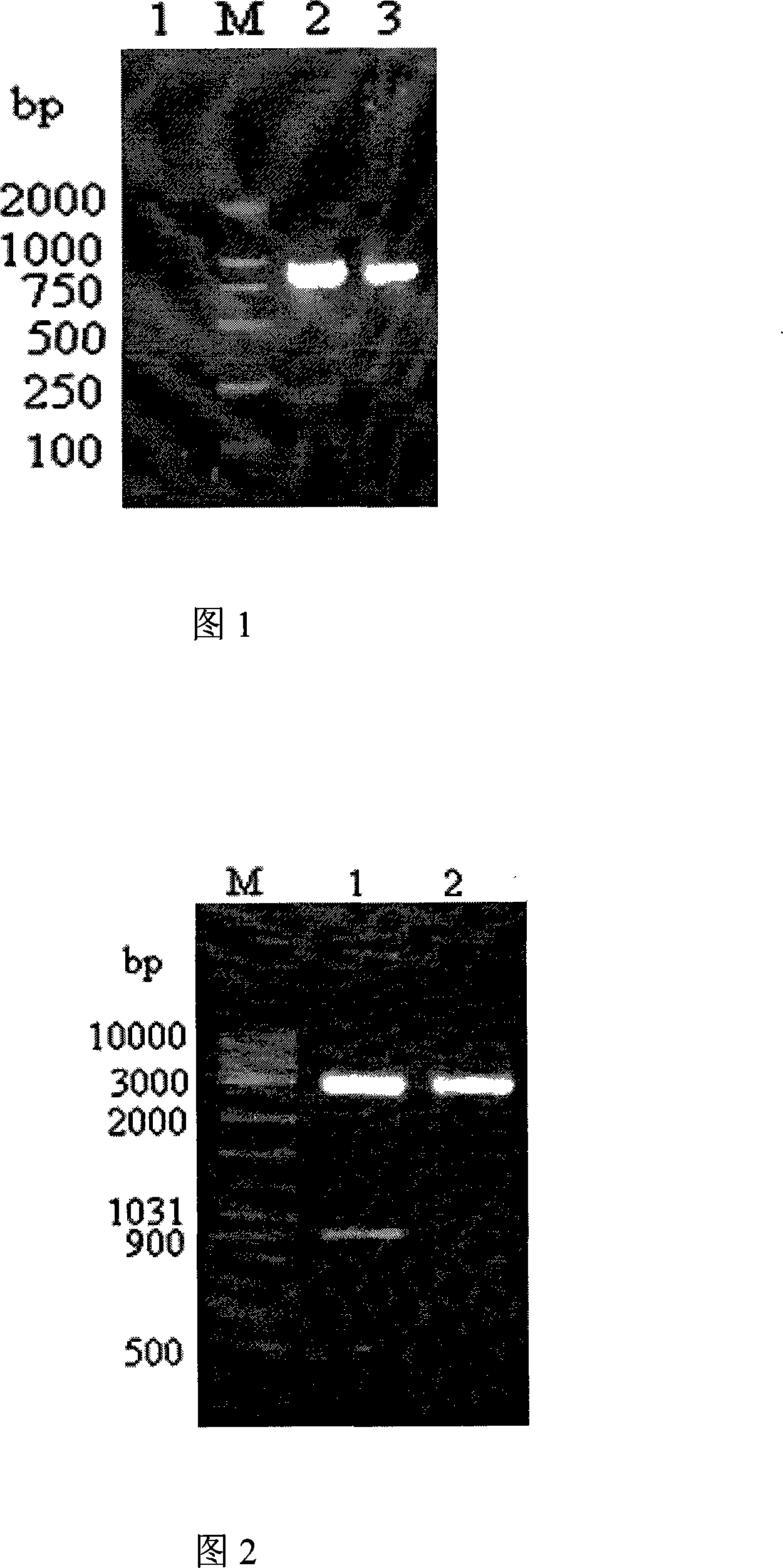
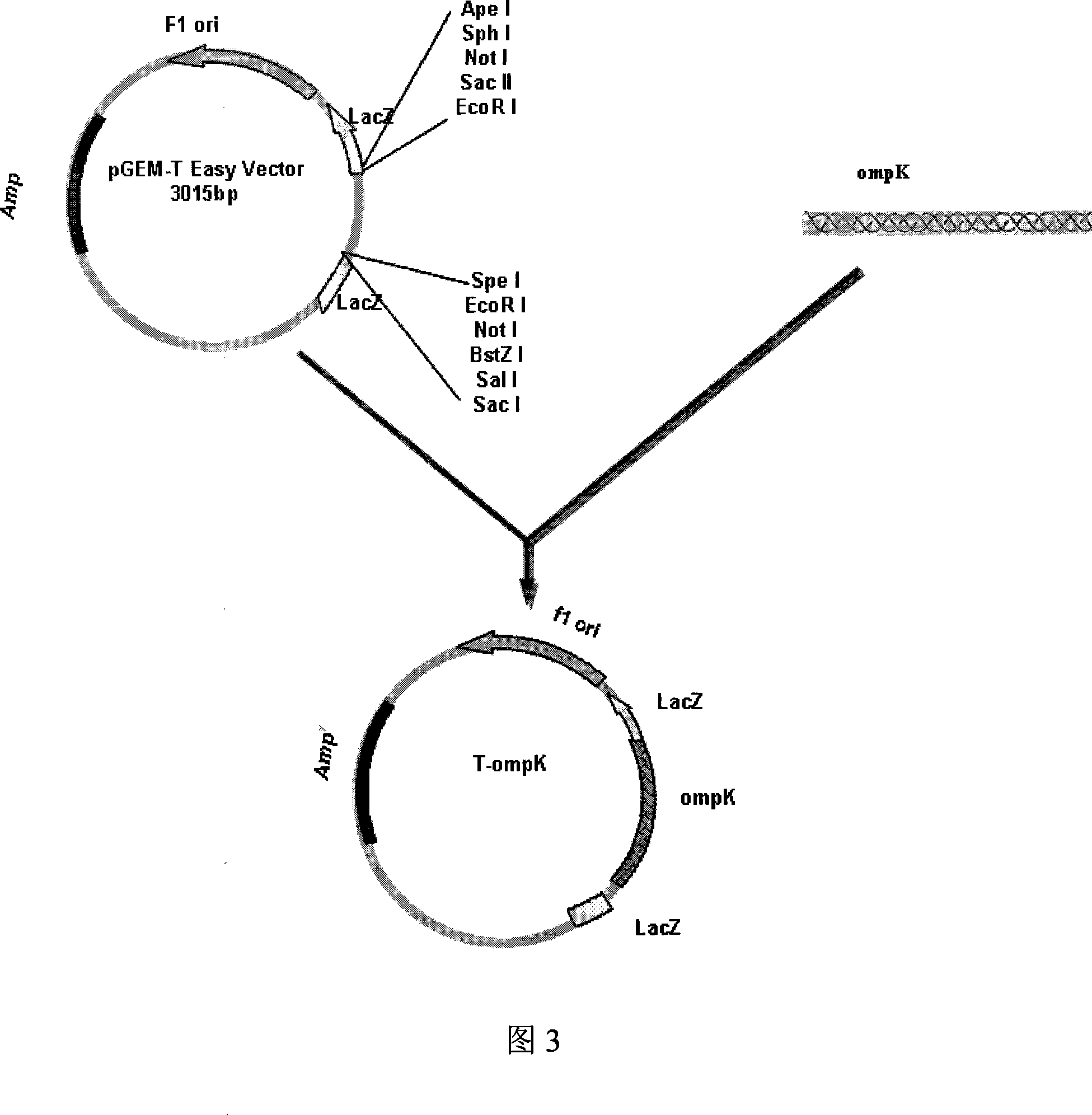
Vibrio parahaemolyticus tunica externa protein ompK subunit vaccine and preparation method thereof
InactiveCN101172157APreserve immunogenicityDefense against invasionAntibacterial agentsDigestive systemEscherichia coliHaemolysis
The invention discloses protein ompK subunit vaccine of assistant haemolysis vibrio extine and a preparation method thereof. The vaccine is PBS solution which converts the recombination protein of the coliform bacteria of recombination prokaryon expression plasmid pET30a-ompK expressed by inducing and after being purified; the concentration of the PBS solution is 0.25-0.5mg / ml. The method has the steps that: firstly, the extraction of an assistant haemolysis vibrio full gene group, the overall length of extine protein ompK DNA and the clone of a mature peptide coded sequence; secondly, the construction of a prokaryon expression plasmid of the ompK mature peptide coded sequence, thirdly, the obtaining way of the recombination ompK protein; fourthly, the detection to the immunity way and the immunity effect of a large yellow croaker with recombination ompK protein. The invention provides the preparation method of the assistant haemolysis vibrio ompK protein subunit vaccine, and simultaneously provides the detection method of the immunity effect of the large yellow croaker with recombination ompK protein, the preparation method is simple, and the usage is convenient.
Owner:ZHEJIANG UNIV
Hydrophilic polymer-puerarin specific conjugated non-hemolytic conjugate
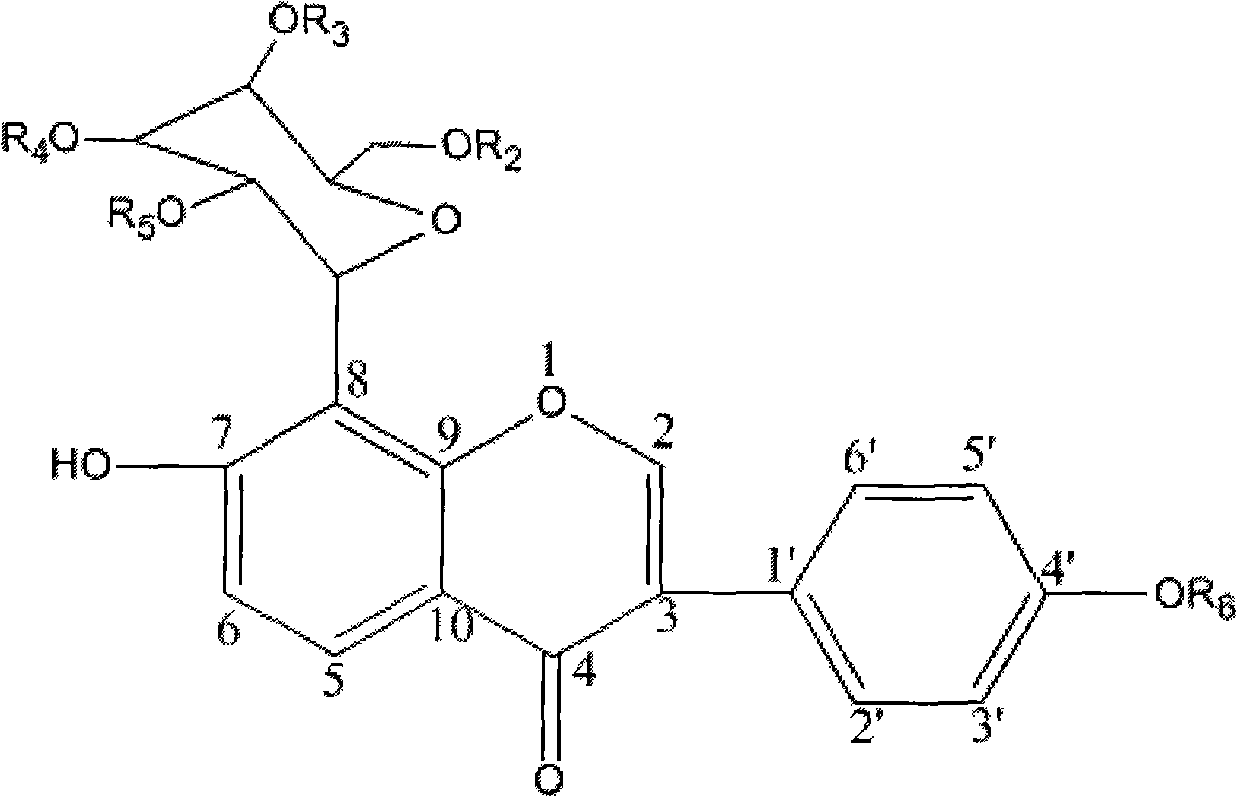
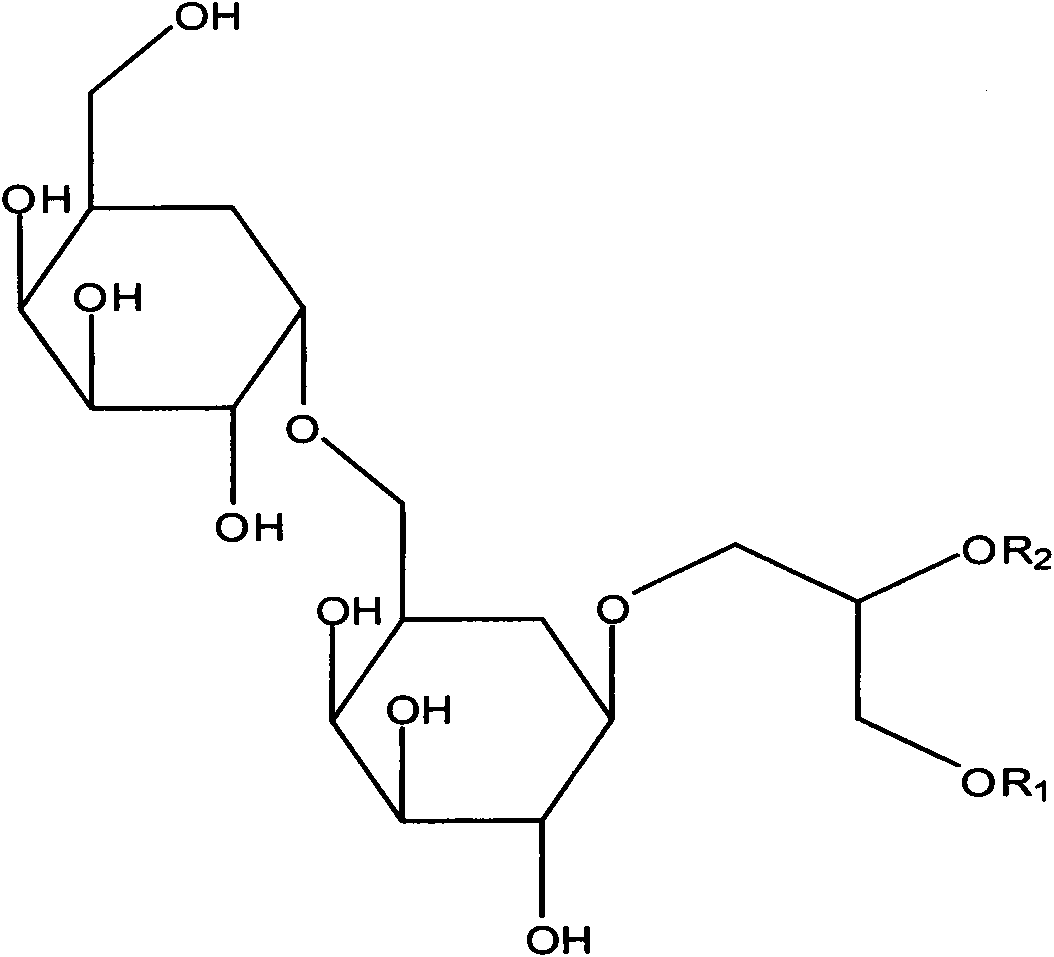
The invention relates to a conjugate shown in formula 1 or pharmaceutically acceptable salt thereof. The conjugate has good water solubility, overcomes the defects of poor water solubility, low bioavailability and the like of puerarin, greatly reduces or eliminates the adverse reactions such as puerarin haemolysis and the like, has simple synthesis method and low preparation cost, and is suitable for industrialized production. In addition, the invention also relates to a pharmaceutical composition based on the conjugate, a preparation method and the application in the medicine aspect.Formula 1.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
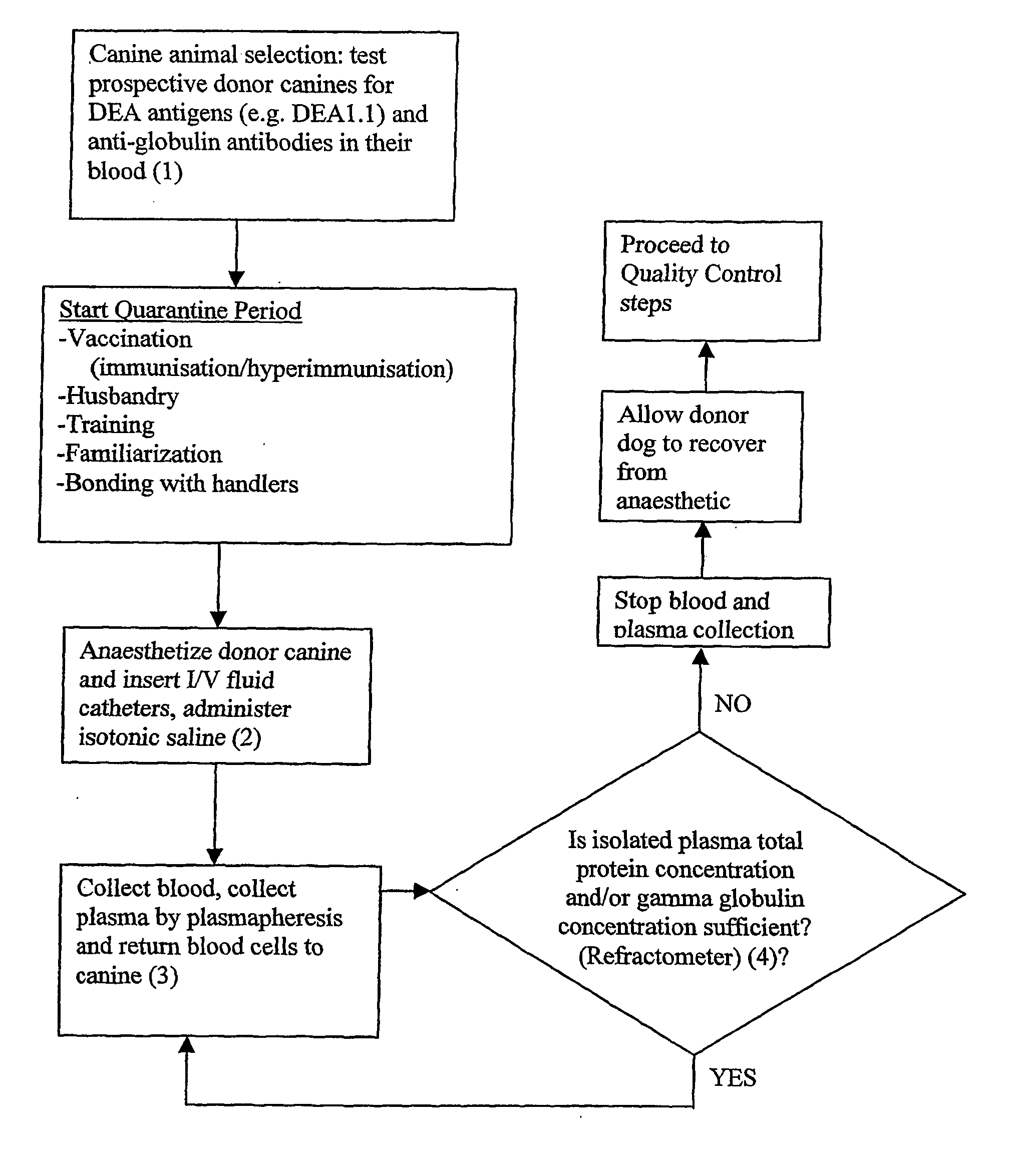
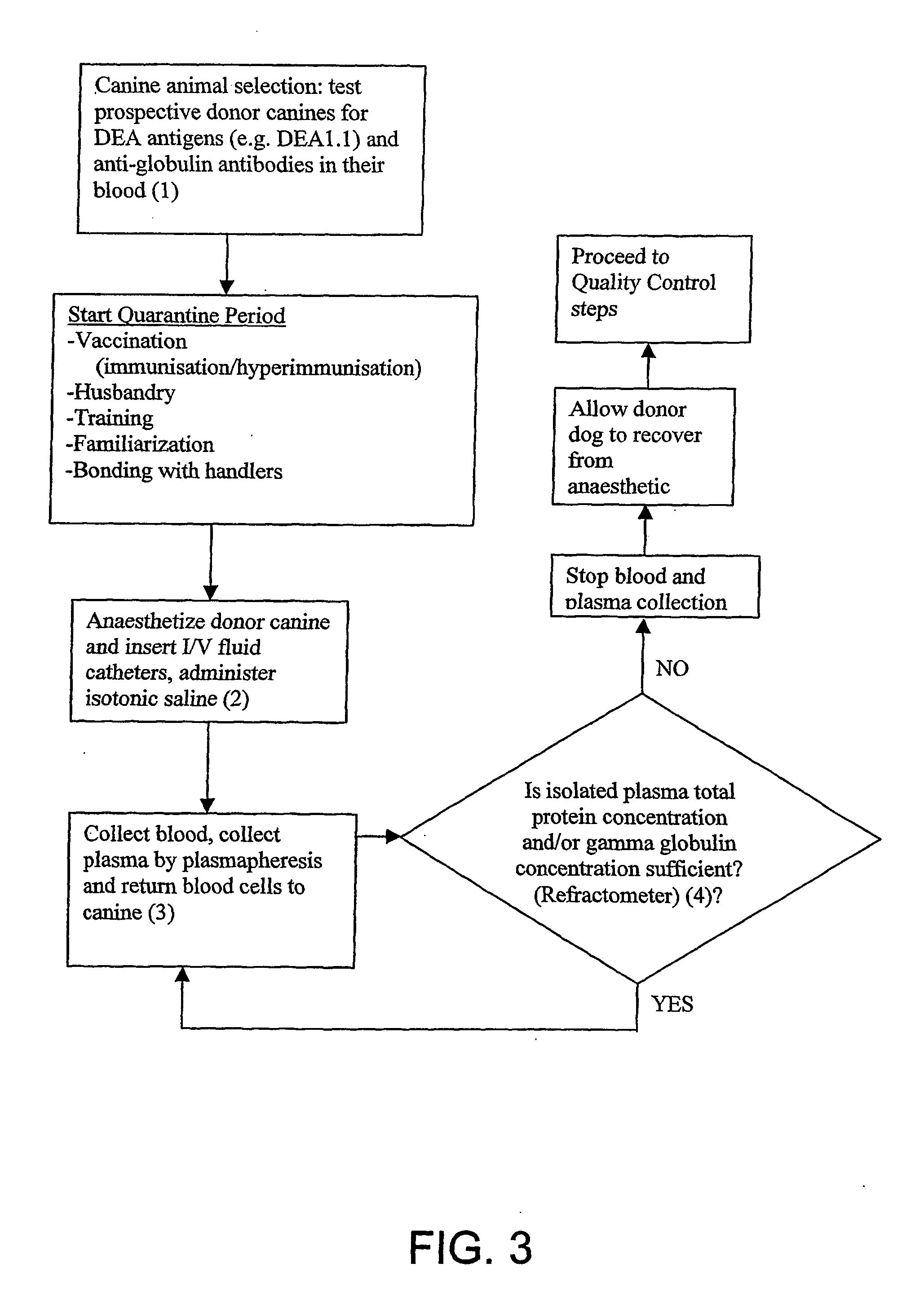
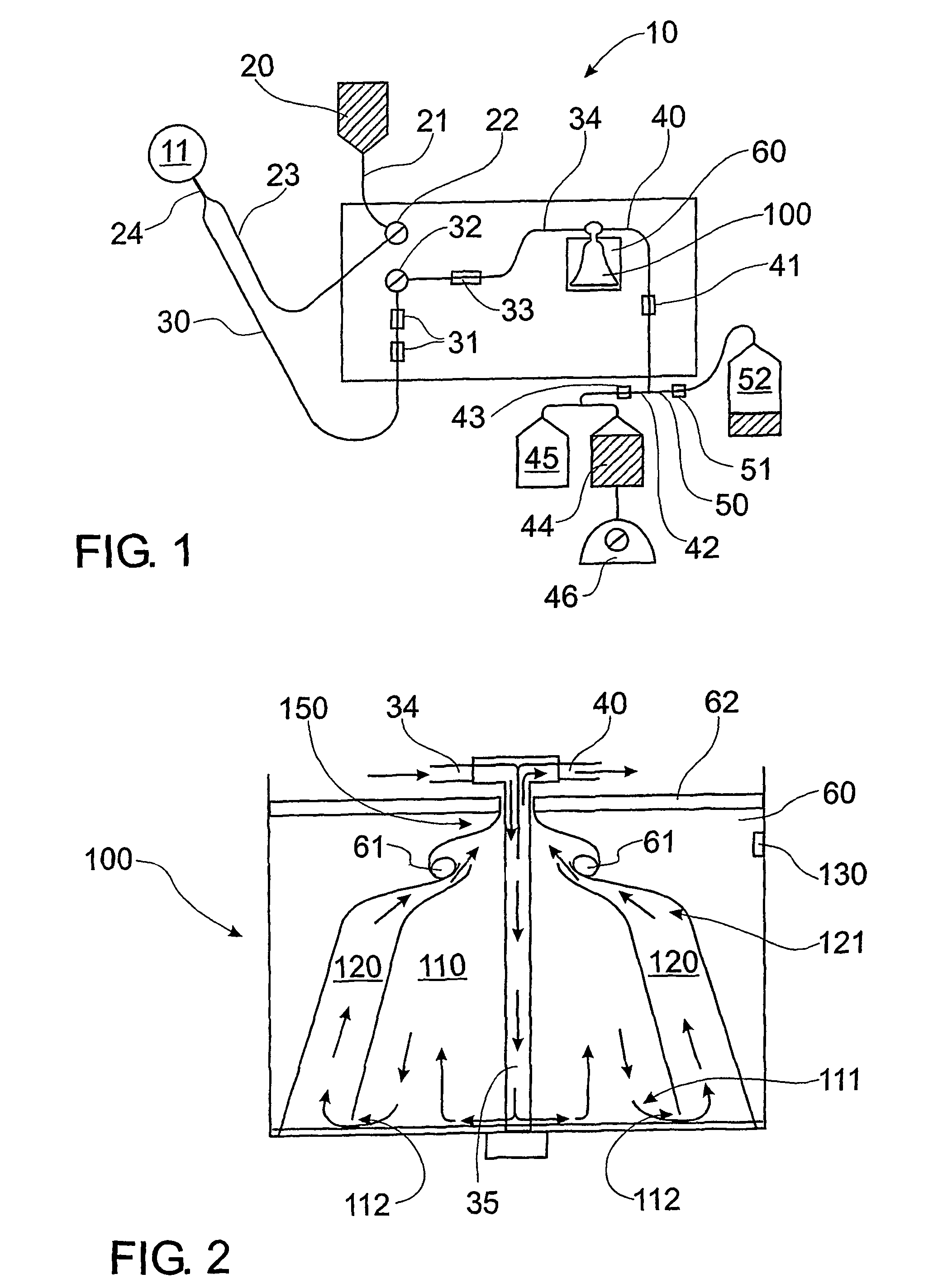
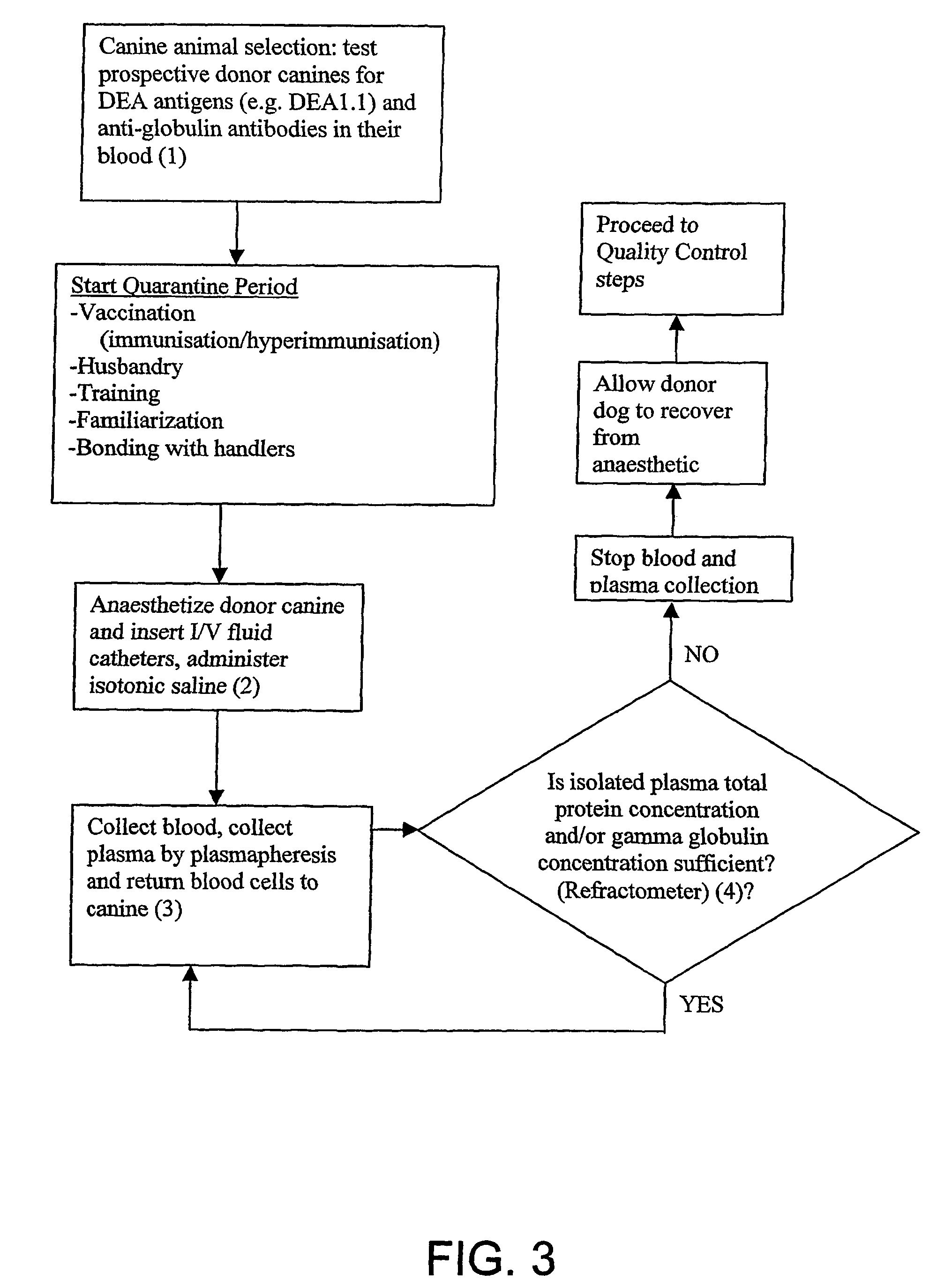
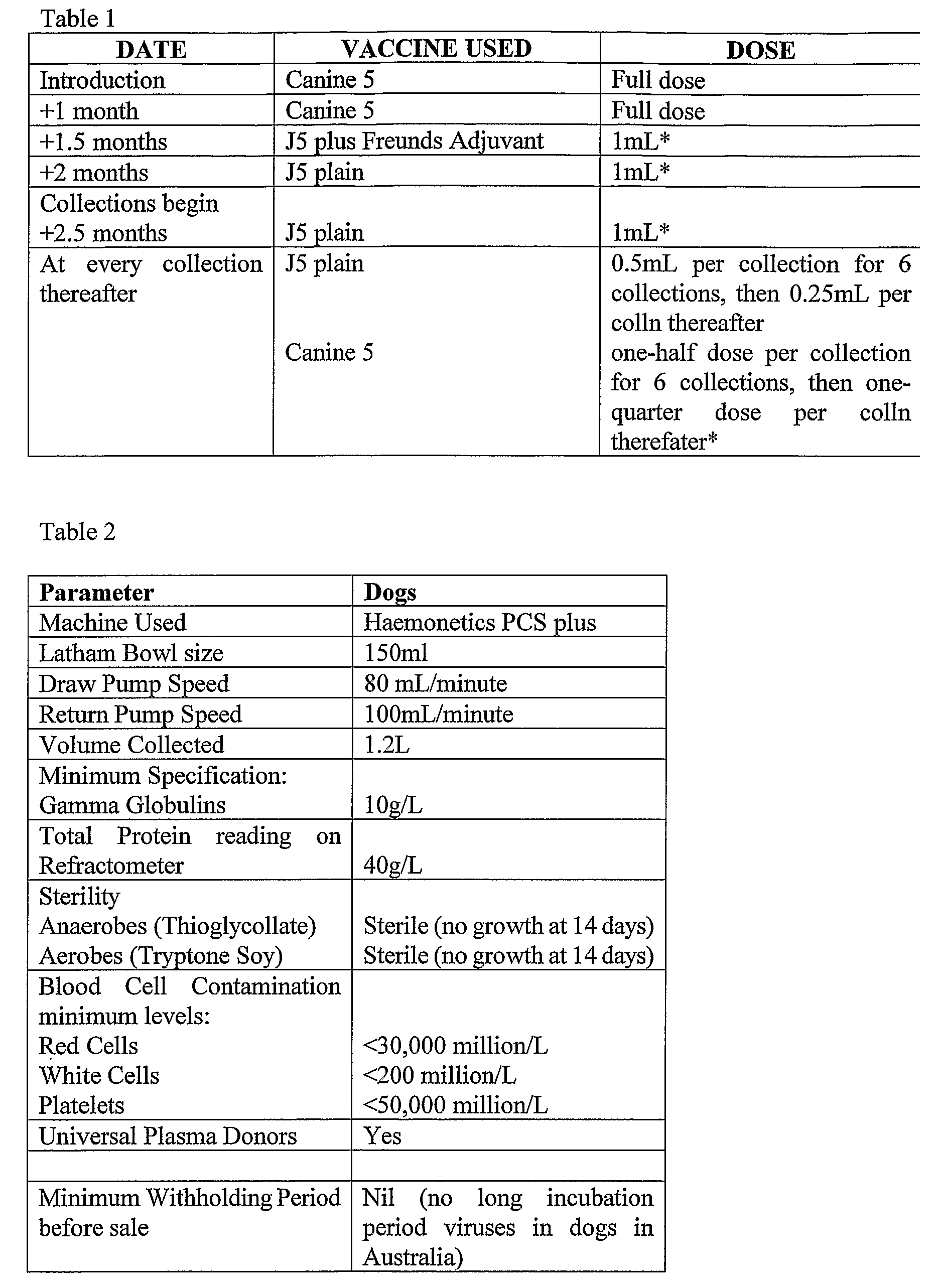
Isolated Plasma And Method For Hyperimmunisation And Plasma Collection
InactiveUS20070248612A1Maintain blood pressureMaintaining pulse rate of the canine animalAnimal cellsOther blood circulation devicesAntigenHaemolysis
The present invention relates to isolated canine animal plasma, a method for isolating canine animal plasma, plasma obtained form an immunised or hyperimmunised canine animal and treating a canine animal with the isolated canine animal plasma. The method includes the step of selecting a canine animal having a blood group compatible with a recipient canine animal having an unmatched blood group, namely, selecting a canine animal for a blood group that does not cause plasma transfusion reaction and / or haemolysis. In one form, the method includes the step of immunising or hyperimmunising a canine animal plasma donor with one or more antigens of a canine animal pathogen. The pathogen is preferably a bacteria or virus.
Owner:PLASMA VENTURES
Isolated plasma and method for hyperimmunisation and plasma collection
InactiveUS7794720B2Maintain blood pressureMaintaining pulse rate of the canine animalAnimal cellsOther blood circulation devicesAntigenBacteroides
Owner:PLASMA VENTURES
Preparation method and quality control method of heartleaf houttuynia herb injection
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
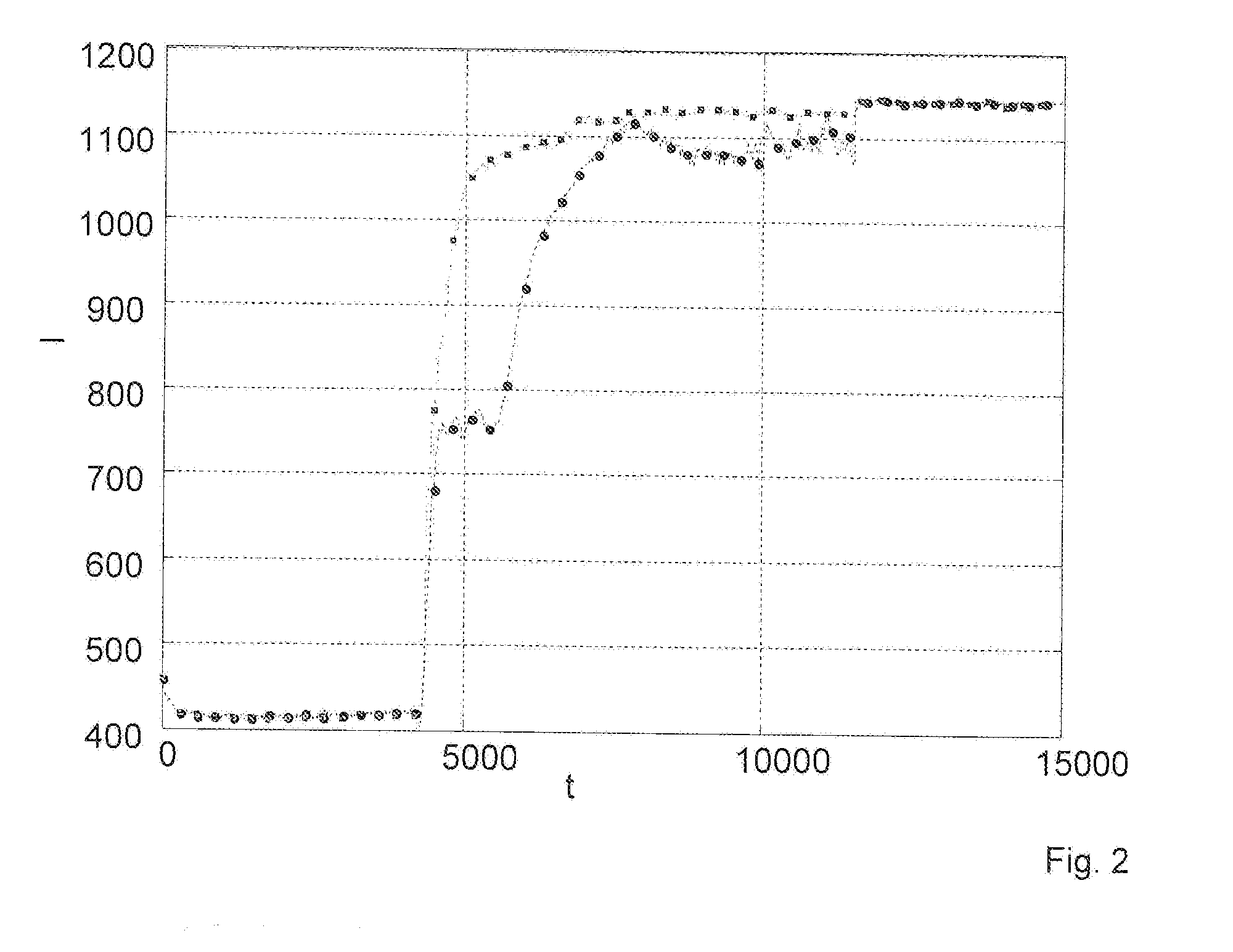
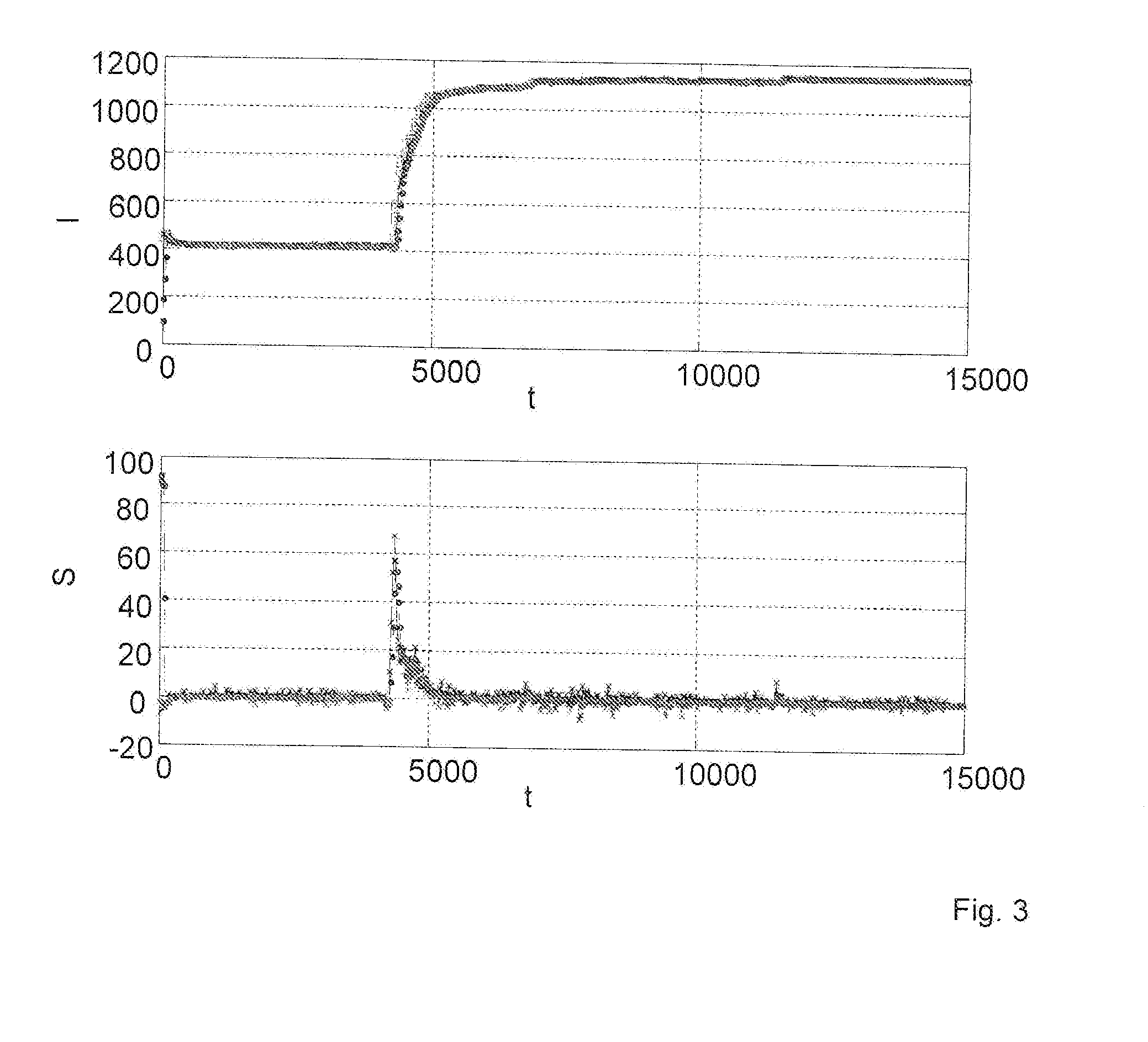
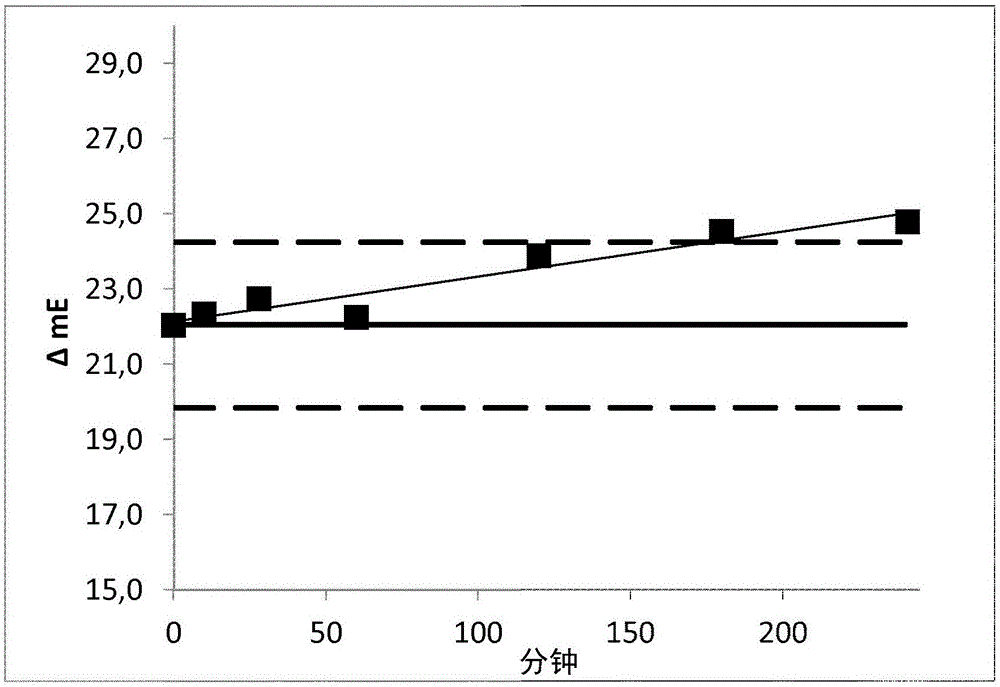
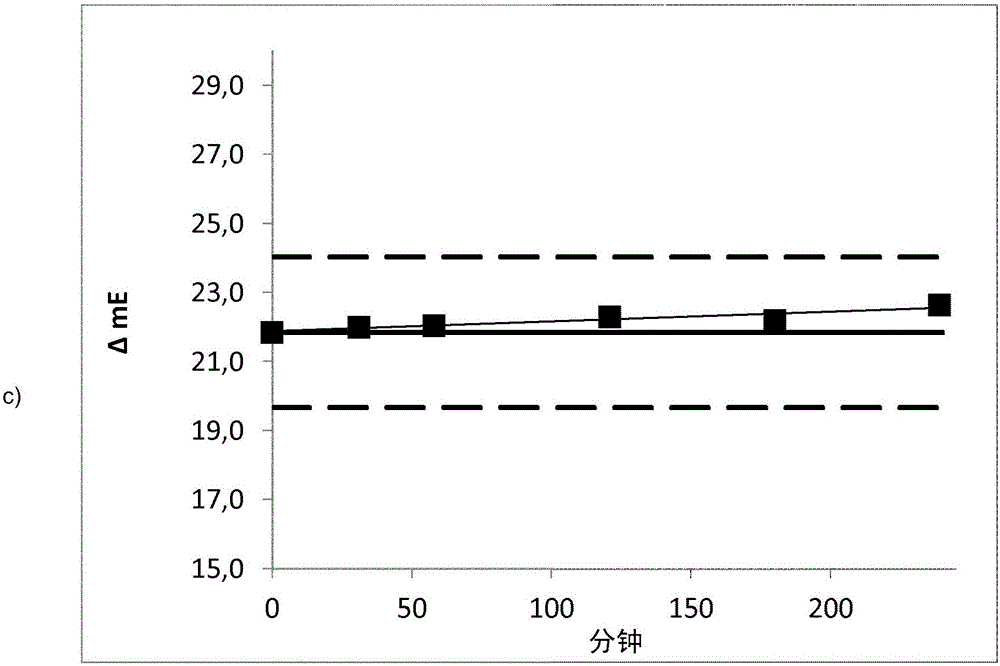
Method for determining the haemolysis of a blood sample and device
ActiveUS20100159500A1Advanced technologyBioreactor/fermenter combinationsBiological substance pretreatmentsHaemolysisMeasurement precision
The invention relates to a method for determining the haemolysis of a blood sample during which the haemolysis progress is determined, wherein the method comprises the steps of irradiating measuring light which is radiated from a measuring light source on the blood sample during haemolysis; detecting measuring light values at several measuring points of time for measuring light transmitted through and / or reflected by the blood sample by a detector device; comparing several of the measuring light values for different measuring points of time and determining a measure for the haemolysis progress by an evaluation device by forming a time-dependent course for the measuring light values from the detected measuring light values, and determining for the time-dependent course at least section-wise a gradient in an assigned measuring curve as a comparative measure for the comparison of the measuring light values at the different measuring points of time; and determining the conclusion of the haemolysis when the gradient within a selectable measurement accuracy, after a measuring period in which the gradient is different from zero, falls to a minimum going down to zero. Another aspect of the invention relates to a device for the determination of the haemolysis of a blood sample.
Owner:ROCHE DIAGNOSTICS OPERATIONS
Application of combination of cnidium lactone and baicalin to preparation of drugs for treating pneumonia
The invention relates to application of combination of cnidium lactone and baicalin to preparation of drugs for treating pneumonia. Rabbit erythrocyte haemolysis test, human pulmonary epithelial cell (A549) injury protection test and a mouse staphylococcus aureus pneumonia model prove that the treatment effect of the combination of cnidium lactone and baicalin is obviously better than the treatment effect of singly using each of the cnidium lactone and baicalin. Conventional antibiotics are abused and the bacterial drug resistance is increasingly enhanced; the combination of cnidium lactone and baicalin has the characteristics of high curative rate and no drug resistance, so that the combination of cnidium lactone and baicalin is capable of enhancing selectivity of application of drugs and has great significance on development of new drugs.
Owner:JILIN UNIV
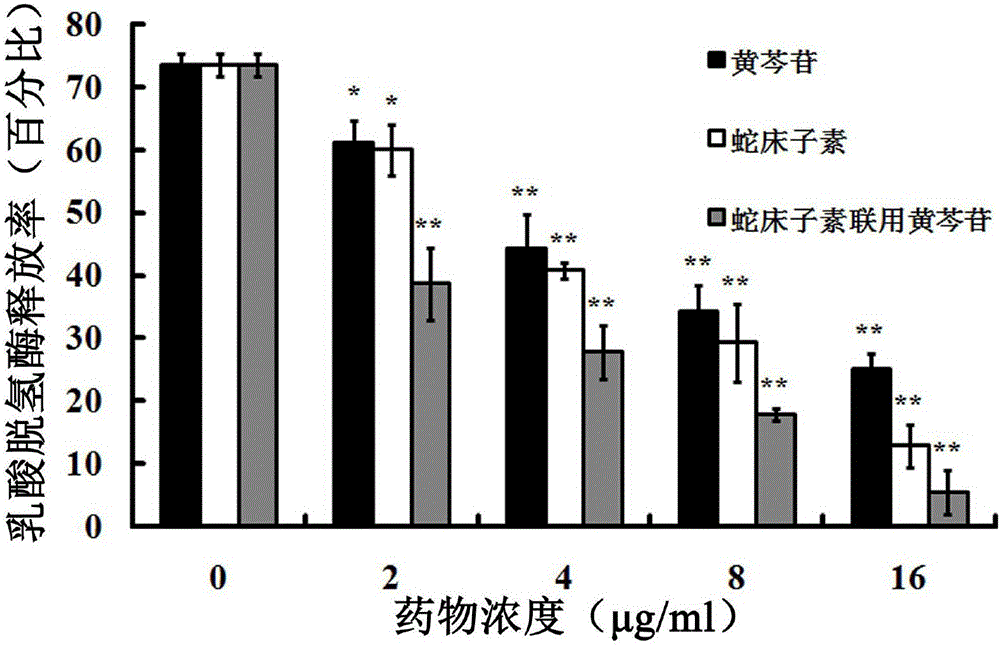
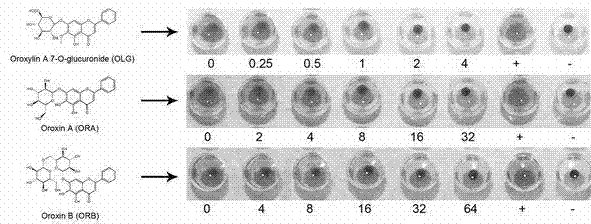
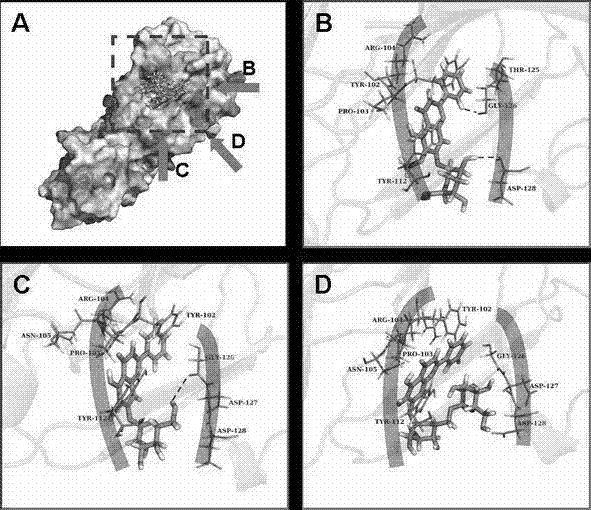
Application of three kinds of oroxin in preparing drug for resisting staphylococcus aureus infection
The invention relates to application of three kinds of oroxin in preparing a drug for resisting staphylococcus aureus infection. The staphylococcus aureus infection resistance of three kinds of oroxin can be proved by virtue of a sheep red blood cell haemolysis test, molecular dynamics simulation and a human pulmonary epithelial cell (A549) injury protective test. Compared with treatment by antibiotics, treatment by using three kinds of oroxin has the characteristics of no drug resistance and high cure rate, so that the three kinds of oroxin can be used in development of new drugs, and has an important meaning to drug target confirmation.
Owner:JILIN UNIV
Antibacterial peptides and analogues thereof
Antibacterial peptides and their multimeric analogues, with a wide range of action and low haemolytic activity are described. In particular, the peptide molecules exhibit a high antimicrobial activity against numerous bacterial species, with reduced cytotoxicity and a low haemolysis rate. The molecules of the invention are advantageously usable as therapeutic agents and coadjutants against infections caused by strains that are resistant to common antibiotics.
Owner:UNIV DEGLI STUDI DI ROMA LA SAPIENZA
Use of chemical compounds that can inhibit the toxic activity of sphingomyelinase d from venoms of loxosceles spiders and pharmaceutical composition comprising said compounds
ActiveUS20160367533A1Improve performanceFavorable cost/benefit ratioOrganic active ingredientsAntinoxious agentsFuranHaemolysis
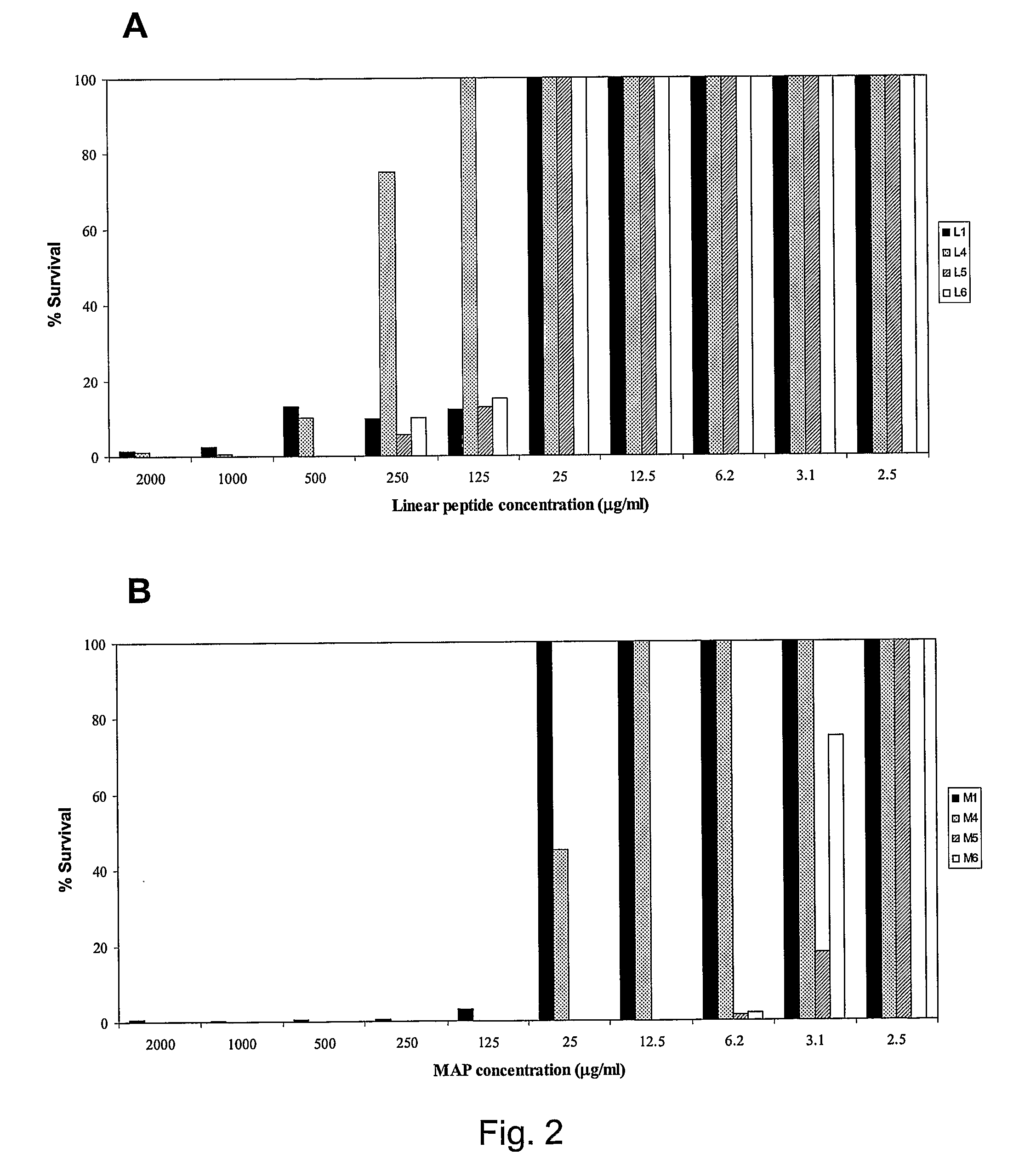
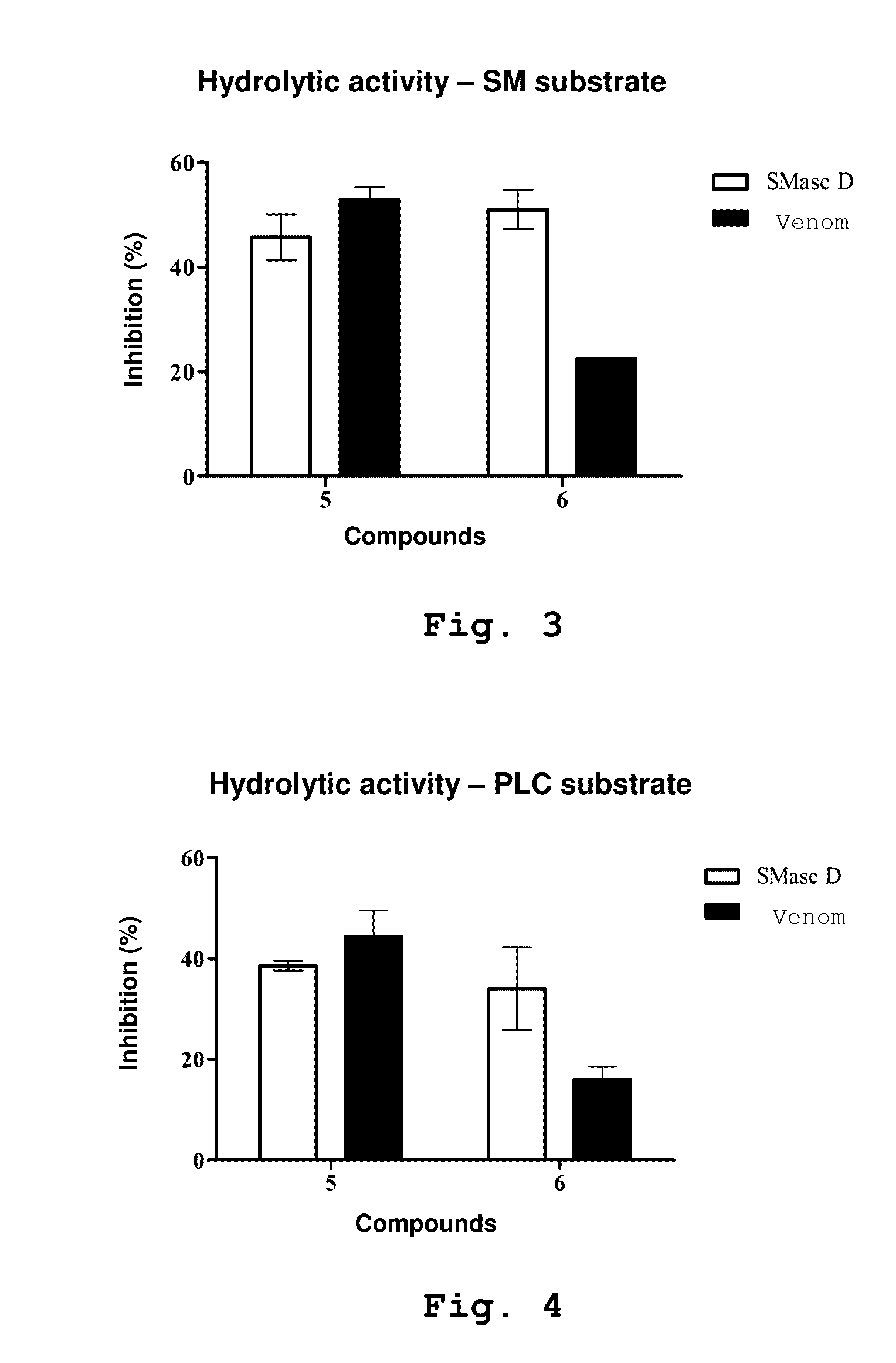
The present invention relates preferably to the use of 4-bromo-N-[(E)-(2-methyl-1H-indol-3-yl)methyleneamino]benzenesulphonamide and 4-methyl-3-oxo-2-(3-pyridylmethylene) benzo[3,4-b]furan-6-yl-4-chlorobenzenesulphonate (compounds 5 and 6, respectively), which are compounds that can inhibit the toxic activity of sphingomyelinase D from Loxosceles venom, controlling the development of cutaneous and systemic loxoscelism; reducing haemolysis; inhibiting the formation of skin lesions; inhibiting skin necrosis; inhibiting intracellular signaling pathways and the production of reactive oxygen species. In addition to the therapeutic potential thereof, said inhibitors can be used to study the activity of sphingomyelinases and phospholipases D. The present invention also relates to a pharmaceutical composition for treating loxoscelism, reducing haemolysis, inhibiting the formation of skin lesions, inhibiting skin necrosis, inhibiting intracellular signaling pathways and the production of reactive oxygen species, comprising said compounds and a pharmaceutically acceptable carrier.
Owner:FUNDACAO BUTANTAN
Antibacterial peptides and analogues thereof
Antibacterial peptides and their multimeric analogues, with a wide range of action and low haemolytic activity are described. In particular, the peptide molecules exhibit a high antimicrobial activity against numerous bacterial species, with reduced cytotoxicity and a low haemolysis rate. The molecules of the invention are advantageously usable as therapeutic agents and coadjutants against infections caused by strains that are resistant to common antibiotics.
Owner:UNIV DEGLI STUDI DI ROMA LA SAPIENZA
Sclera external pressurized biologic composite materials and preparation method
InactiveCN101829365ASoft textureHigh mechanical propertiesSurgeryProsthesisCross-linkChemical reaction
The invention provides sclera external pressurized biologic composite materials which comprise 5-40 percent of pharmaceutical grade hyaluronic acid glucuronic acid, wherein the light transmittance of the pharmaceutical grade hyaluronic acid glucuronic acid is no more than 98 percent, the haemolysis is not detected, streptococcus haemolyticus is not detected, the total amount of the mycete and the saccharomycetes is no more than 10CFU / g, the mechanical tensile strengthen is larger than 3Mpa, the tearing strengthen is larger than 5kN / M, and the materials accord with the biologic safety requirements of three types of national medical devices. The invention takes hyaluronic acid as basic raw materials, carries out chemical crosslinking for more than two times with cross-linking agent to prepare crosslinking hyaluronic acid materials with high intensity, lowers toxic cross-linking agent residues through chemical reaction and is sterilized to prepare biologic materials which accord with three types of national medical devices. In the invention, through a composite crosslinking method, the sandwich type ultrahigh intensity crosslinking hyaluronic acid materials with higher surface rigidity and better interior elasticity are prepared, and the contradiction between the biocompatibility and the mechanical strength can be efficiently solved.
Owner:HANGZHOU FIRST PEOPLES HOSPITAL
Micro-RNA biomarkers for haemolysis and methods of using same
InactiveUS20170159125A1Health-index calculationMicrobiological testing/measurementHaemolysisMedicine
The present disclosure relates to micro-RNAs associated with haemolysis and methods of using them. One disclosed method is a procedure for identifying biological samples in which haemolysis is present by assessing the levels of expression of miRNA in the blood or another biological fluid. Also described is a method for identifying individuals at risk of having a disease or disorder associated with haemolysis.
Owner:BIOMIRNA HLDG
Strain, bacterium agent and application
ActiveCN109679879AExcellent probiotic effectToleratedBacteriaMicroorganism based processesPectinasePathogenic microorganism
The invention discloses a strain, a bacterium agent and application and relates to the technical field of microorganisms. The strain is bacillus subtilis; the strain is preserved in China Center for Type Culture Collection (CCTCC), the preservation number is CCTCC NO: M 2018398 and the preservation date is June 25, 2018. The strain provided by the invention can be used for inhibiting 5 types of pathogenic microorganisms and is sensitive to 15 types of antibiotics; the strain has a non-specific adhesion potential, has tolerance to acid and bile salt, has the property of producing pectinase andcellulase and also has a haemolysis-free property; the strain provided by the invention has an excellent probiotic effect, can be used for controlling and regulating the balance of intestinal flora ofanimals and is used for preparing an animal feed additive to protect the immune system and gastrointestinal health of the animals.
Owner:SHIHEZI UNIVERSITY
A kind of preparation method of Houttuynia cordata injection
ActiveCN102266420AReduce dosageClarify and refine productivityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsHaemolysisIrritation
The invention discloses heartleaf houttuynia herb injection and a preparation method thereof. In the heartleaf houttuynia herb injection prepared by the method, production links and production parameters are defined and refined, and the using amount of polysorbate 80 is reduced on the basis of the legal departmental standard. Different batches of the prepared heartleaf houttuynia herb injection are stable and uniform, the effect of cell membrane cracking caused by the polysorbate 80 is reduced, irritation, haemolysis and histamine release are reduced, and the safety of administration is improved.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD +1
Application of apigenin on preparation of medicine for treating pneumonia
The invention relates to an application of apigenin on preparation of a medicine for treating pneumonia. A therapeutical effect of the apigenin on staphylococcus aureus infection is proved by a rabbit red blood cell haemolysis test, a protection test of human pulmonary epithelial cells (A549) damage and a mice staphylococcus aureus pneumonia model. Compared with an antibiotic therapy, a therapy applying the apigenin has the characteristics of no drug tolerance and high cure rate.
Owner:SHANDONG JINZHUJI PHARM CO LTD +1
Saponin compound, preparation method for same, and application thereof in immunologic adjuvant preparation
InactiveCN102382154ALow hemolyticImprove securityOrganic active ingredientsSugar derivativesHaemolysisCryopreservation
The invention provides a saponin compound, which comprises psammosilene tunicoides saponin B and psammosilene tunicoides saponin C which are extracted and separated from traditional Chinese medicine psammosilene tunicoides. The saponin compound is capable of inducing organism to generate Th1 type and Th2 type immune response and inducing the organism to generate stronger cellular immunity and humoral immunity reaction on vaccines as compared with alumina gel adjuvant, thereby being capable of serving as immunologic adjuvant of various vaccines to achieve higher immunity effect. The saponin compound is low in haemolysis, fine in safety, simple and convenient in preparation method, easily controllable in quality, convenient in use, capable of realizing cryopreservation, thereby having higher clinical application value.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
Preparation method for digalactosyl diacylglycerol (DGDG) and application thereof
The invention relates to a preparation method for medicinal natural surfactant, namely, digalactosyl diacylglycerol (DGDG) and applications thereof as a pharmaceutical carrier. The preparation process is as follows: oat bran is extracted by acetone; leaching liquor is absorbed by silicon gel after being concentrated; eluent with different polarities are used for eluting; and refined DGDG is obtained from the final eluent. Or the oat bran can be degreased by supercritical carbon dioxide, and then extracted by acetone; leaching liquor is absorbed by silicon gel after being concentrated; and refined DGDG can be obtained after being eluted by eluent with different polarities. The hydrophile-lipophile balance value of the DGDG is 7.00 to 8.15, and the critical micelle concentration (CMC) thereof is 2*10 g / L. The raw materials of the method are easy to be obtained, and the obtained DGDG is thin-layer chromatographically pure without haemolysis and acrimony. The DGDG can be taken as a pharmaceutical carrier as two amphipathic properties thereof can self-assemble to form the property of bilayer vesicles. Galactose residues in DGDG molecules can be taken as ligands which can specifically distinguish galactose accepters on parenchymal hepatic cells so as to realize active hepatic targeting mediated by ligands.
Owner:SHENYANG PHARMA UNIVERSITY
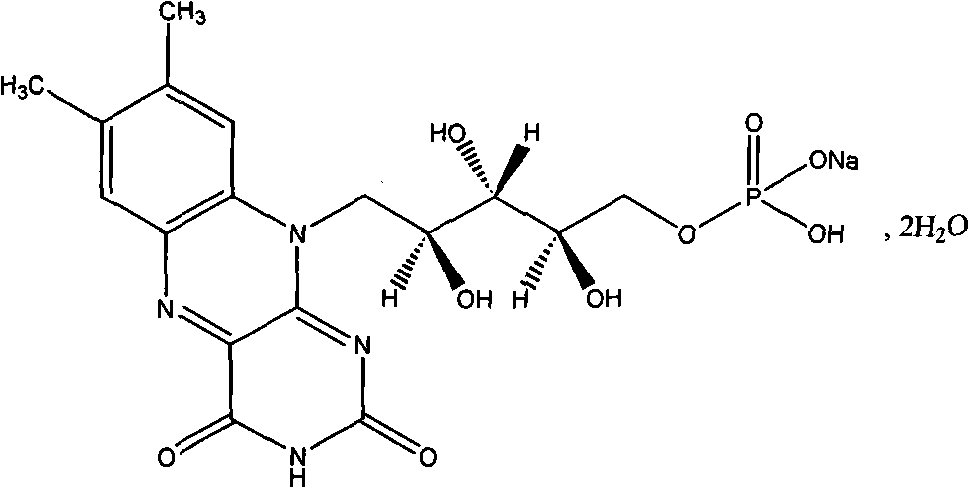
Riboflavin sodium phosphate freeze-dried powder injection and preparation method thereof
ActiveCN101874787AMeet the requirements for intravenous drug useReduce the risk of adverse reactionsOrganic active ingredientsPowder deliveryHaemolysisFreeze-drying
The invention relates to a riboflavin sodium phosphate freeze-dried powder injection and a preparation method thereof. The injection is characterized by comprising a riboflavin sodium phosphate dehydrate, mannitol and / or ammonia water. The injection is sensitive to light, so a brown amoxicillin bottle is used as the inner package of the injection. The riboflavin sodium phosphate freeze-dried powder injection has the following advantages: (1) single and clear auxiliary materials in the formula can meet the requirement of industrialized batch production; (2) medicinal injection grade raw and auxiliary materials accord with the medication requirement of human intravenous injection, so that potential adverse reaction risk in clinical application can be greatly reduced; (3) long-term stability tests prove that the preparation can be preserved for 2 years at the temperature below 30 DEG C, so that stability of the medicament in circulation and safety of clinical medication can be ensured; and (4) special safety tests, such as irritability, haemolysis, vascular stimulation and the like, prove that haemolysis, agglutination, stimulation and anaphylaxis reactions are not found.
Owner:HAINAN LEVTEC PHARMA
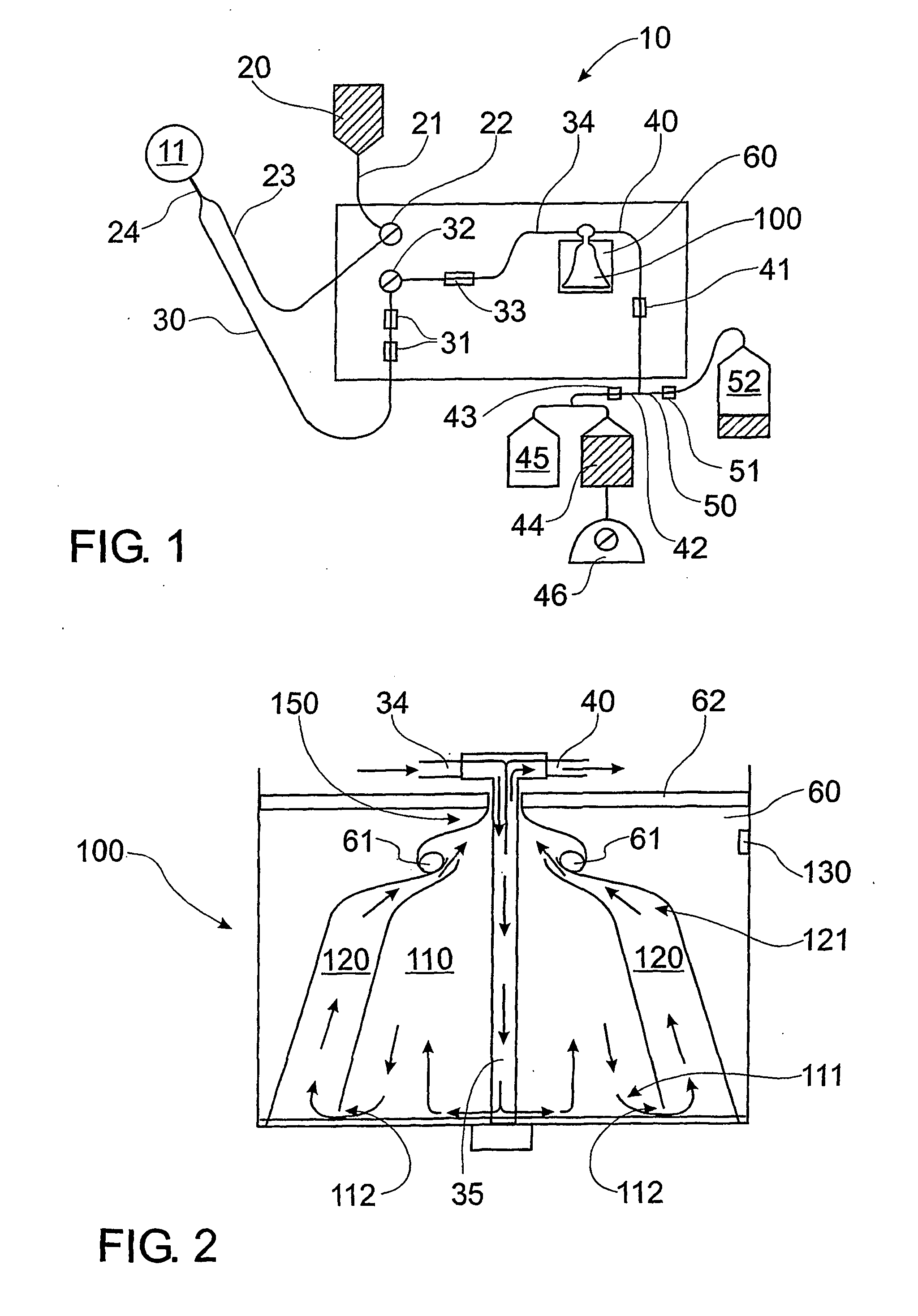
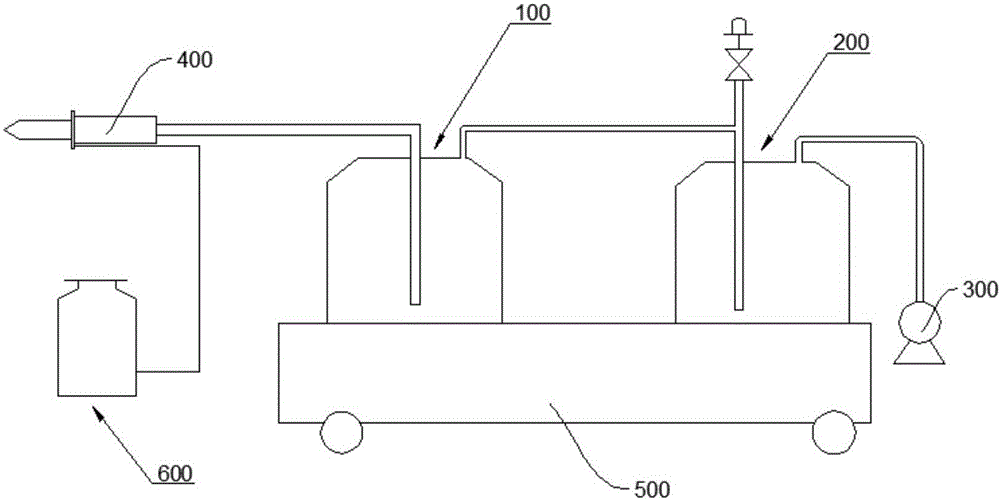
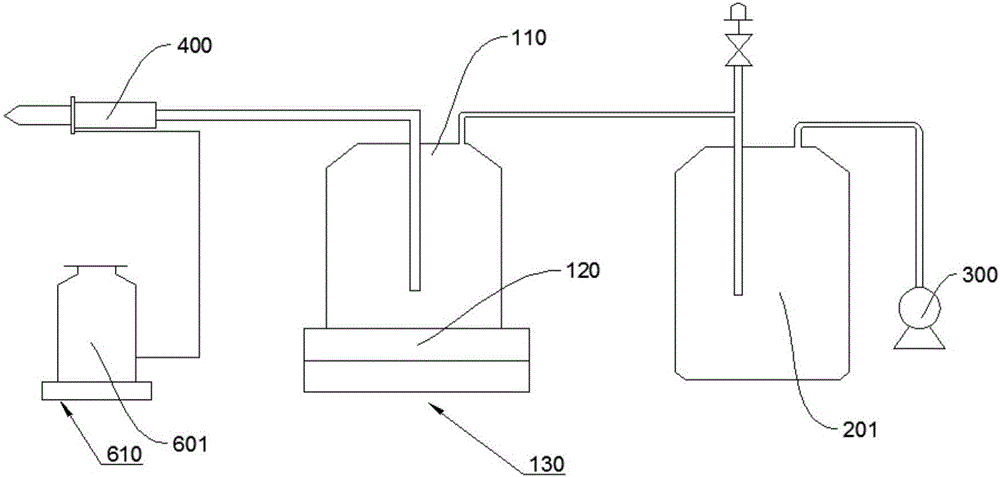
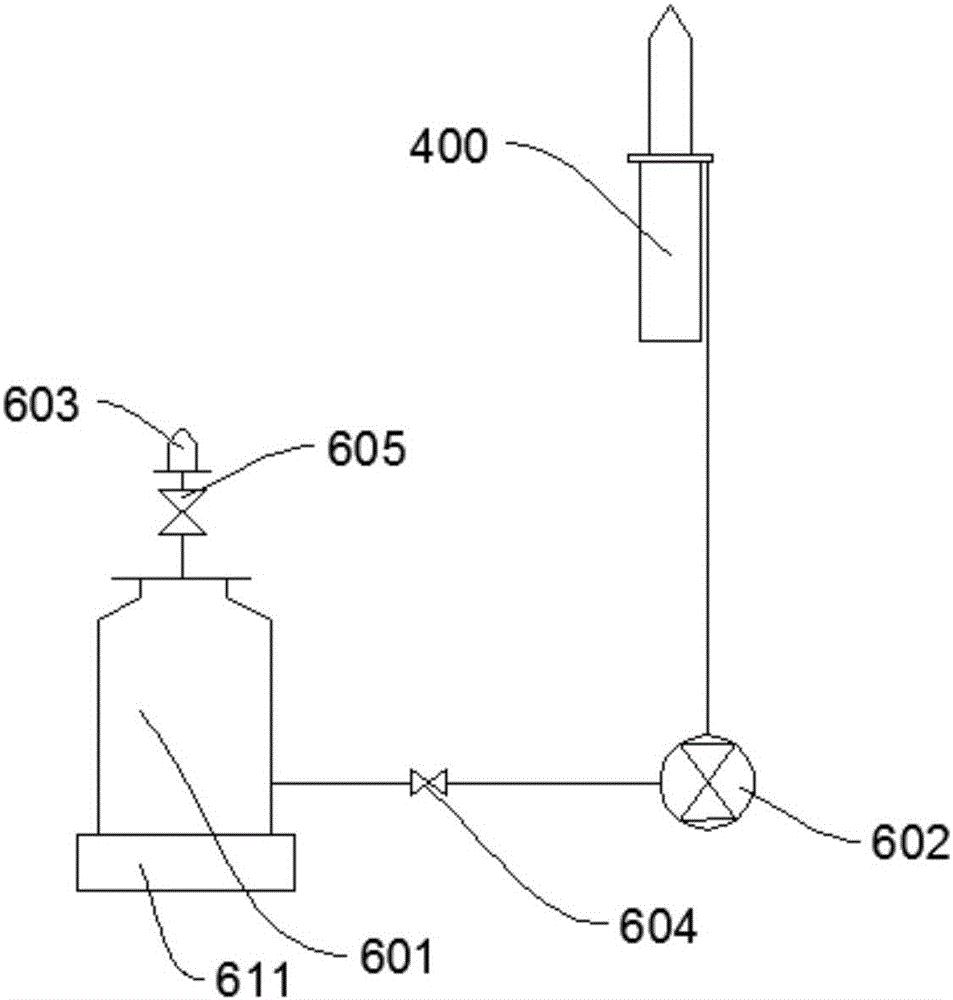
Vacuum blood drawing device
ActiveCN106035591APrevent solidificationAvoid pollutionBlood collection/stirring apparatusHaemolysisMagnetic stirrer
The invention relates to a vacuum blood drawing device for slaughtering. The vacuum blood drawing device comprises a blood drawing system and an anticoagulant adding system, wherein the blood drawing system comprises a blood collecting tank and a negative pressure generator connected with the blood collecting tank; and the anticoagulant adding system comprises an anticoagulant tank, wherein the anticoagulant tank is connected with the blood collecting tank. The blood drawing system also comprises a vacuum buffering tank, a magnetic stirrer and a weight collecting device which is used for collecting the weight of the blood collecting tank, wherein the vacuum buffering tank comprises a tank body which is provided with a blood foam inlet pipe and a vacuumizing pipe; a rotor of the magnetic stirrer is arranged in the blood collecting tank; and the vacuum buffering tank is connected with the negative pressure generator and the blood collecting tank respectively. The vacuum blood drawing device disclosed by the invention has the advantages of preventing blood from solidifying, controlling haemolysis, shortening blood drawing time and preventing microbiological pollution.
Owner:HANBANG MEDICAL SCI & TECH HARBIN CITY
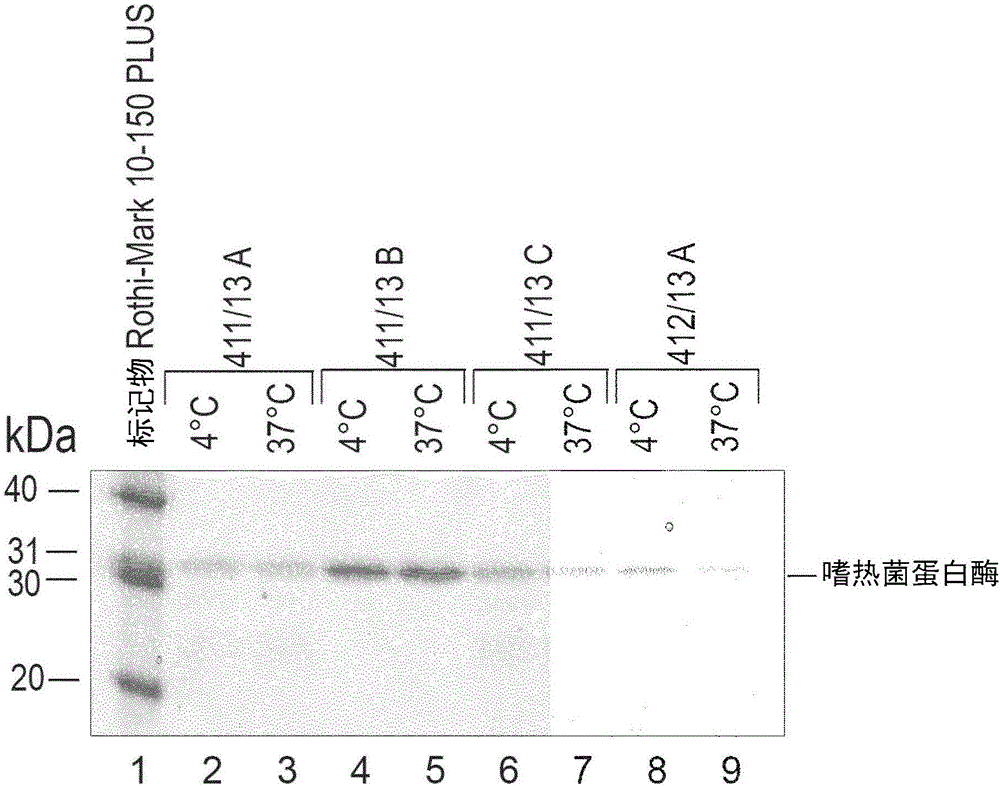
Enzymatic determination of hba1c
ActiveCN106461682AMicrobiological testing/measurementBiological material analysisHaemolysisGlycated haemoglobin
The present invention relates to a method for determining the amount of glycated haemoglobin (HbA1c), in which -if required -the erythrocytes in a sample are haemolysed, the haemoglobin that is then released -if required -is contacted with a proteolytic agent and the glycated haemoglobin degradation products obtained in this way or otherwise are quantified. In order to provide such a process and reagents employable therein that has / have the property of sufficient stability of the chemical compounds that are essential to the reaction, the provision of the requisite proteolytic agent in the form of an inactivated protease is proposed, which is then only reactivated in situ. In particular embodiments of the invention, for the stabilization of the haemoglobin which is unfolded at a very low pH in the range from 1 to 3, at least one suitable stabilizer should be present in the haemolysis solution, and, in the embodiments of the invention in which a leuco dye is used in connection with the determination of the amount of HbA1c, it is proposed in accordance with the invention that the latter be stabilized with particular phosphine compounds and / or thio compounds.
Owner:DIASYS DIAGNOSTIC SYST
pH-sensitive polymer
The invention relates to a pH-sensitive polymer which is a (meth)acrylate copolymer composed of 20 to 65% by weight acrylic and / or methacrylic acid units and 80 to 35% by weight units of C1- to C18-alkyl esters of (meth)acrylic acid, characterized in that it has a molecular weight in the range from 1 000 to 50 000 g / mol, and brings about at least 60% haemolysis at pH 5.5, and less than 5% haemolysis at pH 7.4, in a concentration of 150 μg / ml in a cytotoxicity test with human red blood cells. The invention further relates to the use of the pH-sensitive polymer as carrier for pharmaceutically effective biomolecules or active pharmaceutical ingredients and as ingredient of corresponding dosage forms.
Owner:EVONIK OPERATIONS GMBH +1
Preparation method of globin peptide iron chelate
InactiveCN109868301ASimple processIncrease productivityPeptide preparation methodsFermentationHaemolysisIron salts
The invention relates to a preparation method of a globin peptide iron chelate. The preparation method comprises main steps of preparing porcine blood corpuscle liquid, performing haemolysis, performing enzymolysis, performing centrifuging, performing ultrafiltration, performing chelating, and performing spray drying so as to obtain the globin peptide iron chelate. According to the method disclosed by the invention, porcine blood which is low in price and broad in source is used as a protein peptide source, inorganic iron salt is used as an iron source, iron elements and protein peptide are chelated, the technological process is simple, the cost is low, and the method is suitable for large-scale industrial production. The prepared product namely the globin peptide iron chelate is high in content of small and medium molecule peptide, and high in the content of chelating-state iron ions, and is a novel efficient iron supplement for feeds.
Owner:陈石良
Application of amentoflavone in research and development of medicines for treating pneumonia
The invention relates to application of amentoflavone in research and development of medicines for treating pneumonia. Rabbit erythrocyte haemolysis tests, human pulmonary epithelial cell (A549) injury protection tests and a mouse streptococcus pneumonia model prove that the amentoflavone has a treatment effect on infection of streptococcus pneumonia. Compared with treatment with antibiotics, the amentoflavone for treatment has the characteristics of no drug resistance and high protection performance.
Owner:JILIN UNIV
Injection prepared from heartleaf houttuynia herb and preparation method thereof
The invention discloses an injection. On the basis of the conventional production process, tent-planted heartleaf houttuynia herb consisting of an aboveground part and an underground part in a specific ratio is taken as a raw material, so that the quality of products of different batches is stable and uniform; and the dosage of polysorbate 80 is reduced, the cell membrane cracking action, irritation, haemolysis and histamine release (sensitization) caused by the polysorbate 80 are lowered, and the administration safety is improved.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
Micro-RNA biomarkers for haemolysis and methods of using same
InactiveUS20150232939A1High sensitivityStrong specificityHealth-index calculationMicrobiological testing/measurementHaemolysisMedicine
The present disclosure relates to micro-RNAs associated with haemolysis and methods of using them. One disclosed method is a procedure for identifying biological samples in which haemolysis is present by assessing the levels of expression of miRNA in the blood or another biological fluid. Also described is a method for identifying individuals at risk of having a disease or disorder associated with haemolysis.
Owner:BIOMIRNA HLDG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com