Hydrophilic polymer-puerarin specific conjugated non-hemolytic conjugate
A technology of hydrophilic polymers and conjugates, which can be used in drug combinations, organic active ingredients, cardiovascular system diseases, etc., and can solve the problems of reducing the active potency of active ingredients, inapplicability, difficulties in preparation and purification, etc.
Inactive Publication Date: 2010-12-22
ZHEJIANG CHINESE MEDICAL UNIVERSITY
View PDF22 Cites 10 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, this patent not only describes in general terms, but also fails to scientifically evaluate the efficacy of the final product, water solubility, hemolysis, etc., and more seriously, the conjugation of PEG to puerarin is not specific, or the conjugation is carried out by excessive PEG. All hydroxyl groups on puerarin, or conjugation to carboxyl groups on the flavone sugar ring
The former has many by-products due to various incomplete conjugations, which makes preparation and purification difficult, and the quality of the final product is not easy to control, and because the proportion of PEG in the final product is too high, the activity of active ingredients (flavonoids) is greatly reduced. valence, which increases the final dosage; the latter cannot be applied because puerarin just does not have a carboxyl group on the sugar ring.
Especially the former, there is no selectivity for the conjugation of flavonoids, such excessive conjugation will lose or reduce the activity of the drug
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
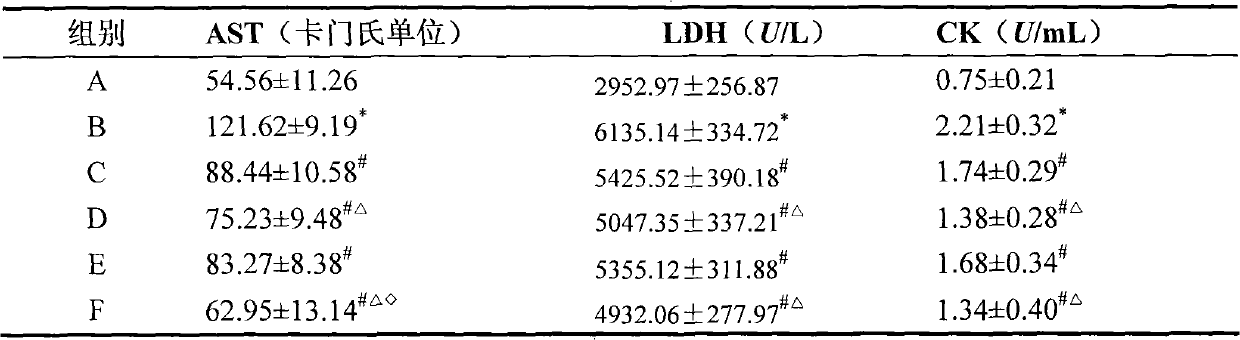
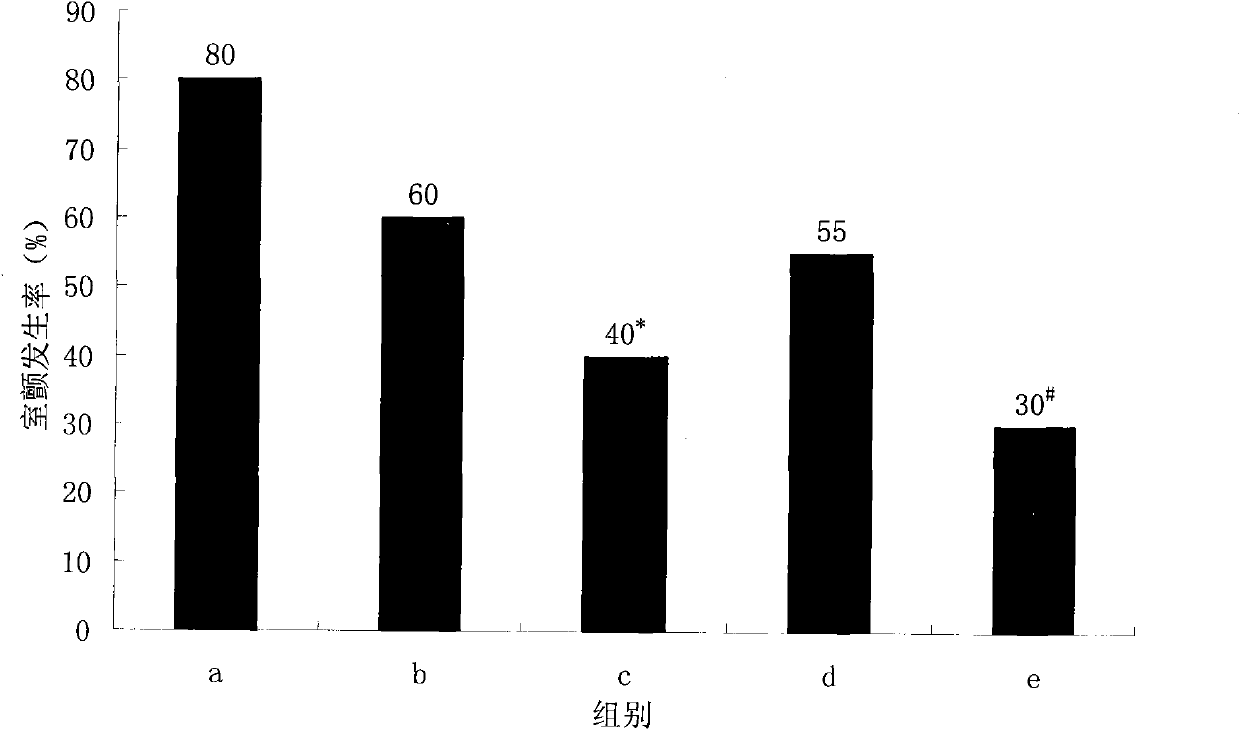
Effect test
Embodiment 1
Embodiment 2
Embodiment 3( comparative example 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Login to View More
Abstract
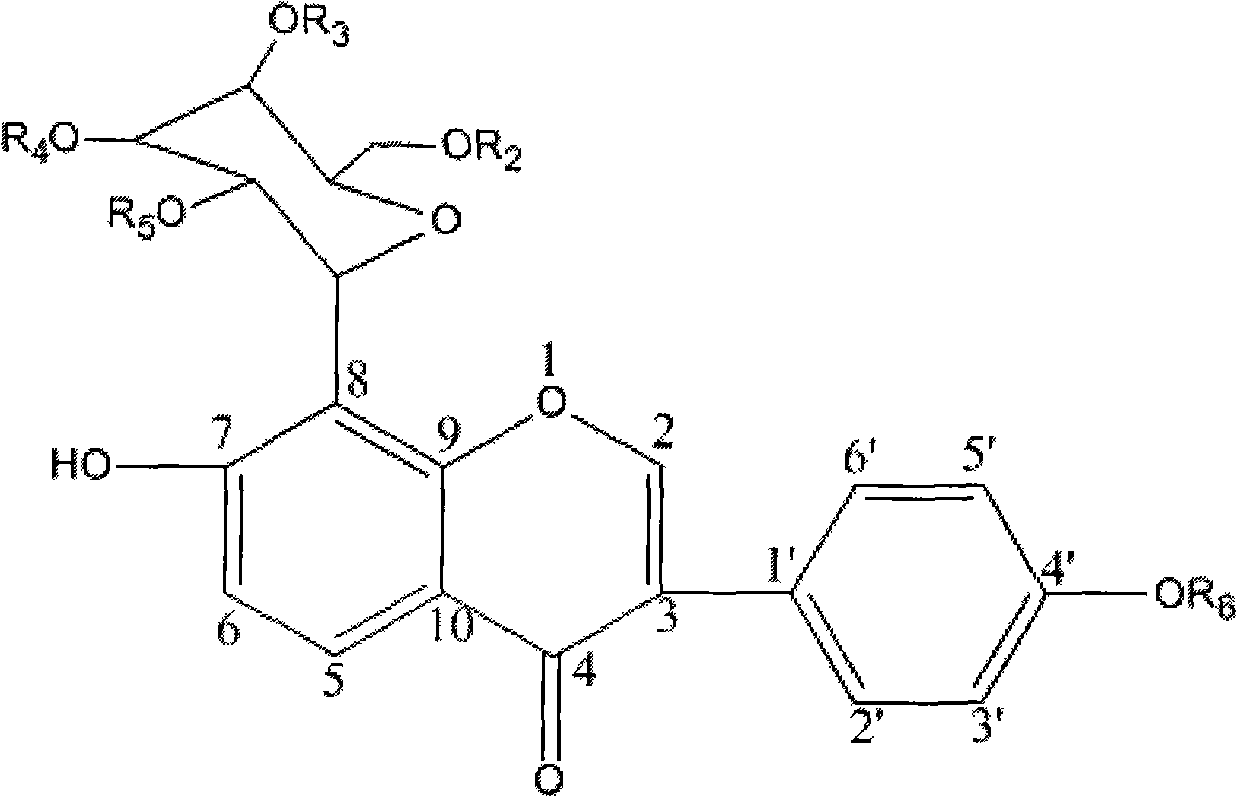
The invention relates to a conjugate shown in formula 1 or pharmaceutically acceptable salt thereof. The conjugate has good water solubility, overcomes the defects of poor water solubility, low bioavailability and the like of puerarin, greatly reduces or eliminates the adverse reactions such as puerarin haemolysis and the like, has simple synthesis method and low preparation cost, and is suitable for industrialized production. In addition, the invention also relates to a pharmaceutical composition based on the conjugate, a preparation method and the application in the medicine aspect.Formula 1.
Description
technical field The invention belongs to the field of medicinal chemistry, in particular, the invention relates to a conjugate formed by specifically conjugating biodegradable puerarin and its derivatives with polyethylene glycol through the 7-position phenolic hydroxyl group. In addition, the present application also relates to the pharmaceutical composition based on the conjugate, the preparation method and the application as a medicine. Background technique Puerarin (Puerarin, hereinafter referred to as Pur, chemical name 7,4'-dihydroxy-β-C-D-glucosyl isoflavone) is produced from the roots of leguminous plants Pueraria lobata and Pueraria thomsonii. An extracted isoflavone glycoside, which has the functions of myocardial protection, renal protection, brain tissue protection, anti-oxidation, anti-inflammation, anti-atherosclerosis, anti-arrhythmia, anti-ischemia-reperfusion injury, anti-alcohol central inhibition, anti-cancer It has important pharmacological activities in...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K47/48A61K47/34A61K31/7048A61P9/00A61P3/10A61P9/10A61P27/16A61K47/60
Inventor 丁志山蒋福升余美荣刘霞李伟平金波高承贤陈铌铍吕圭源
Owner ZHEJIANG CHINESE MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com