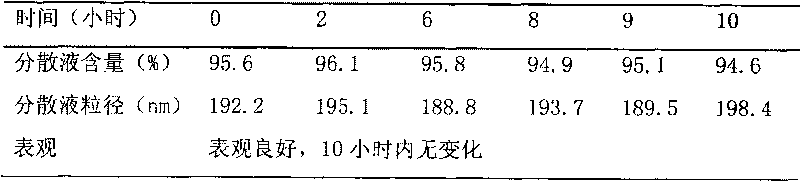
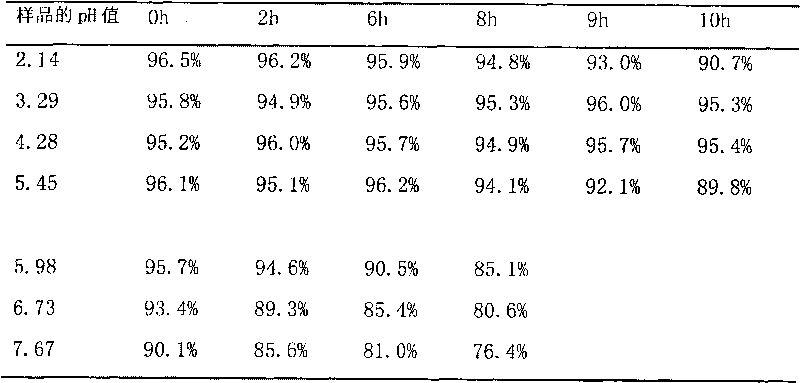
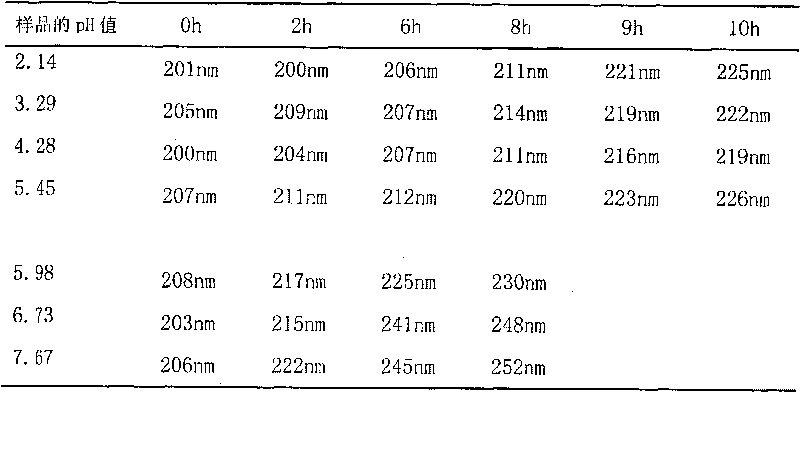
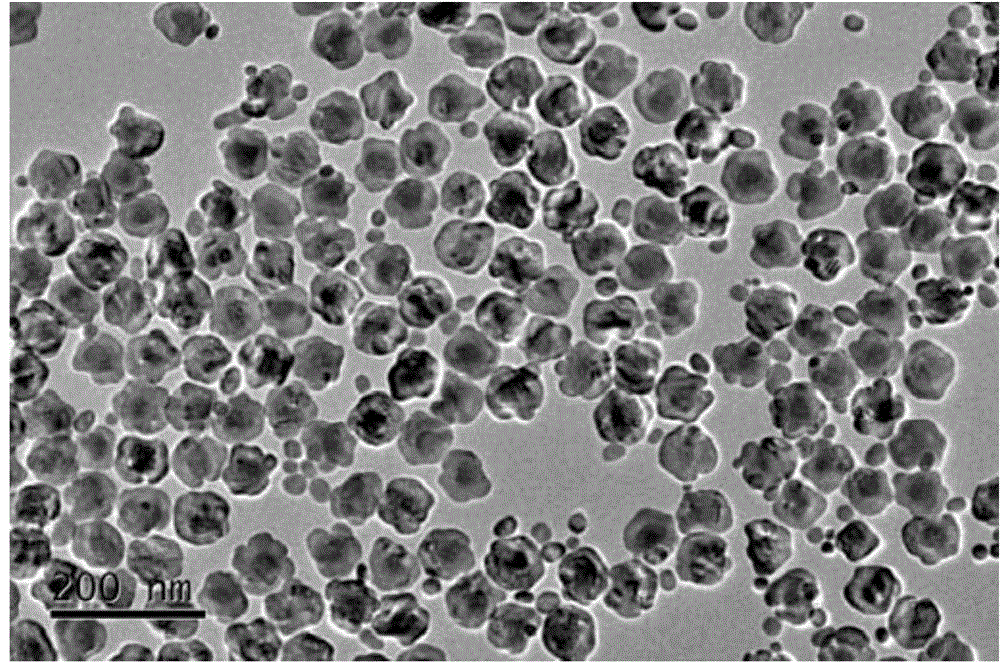
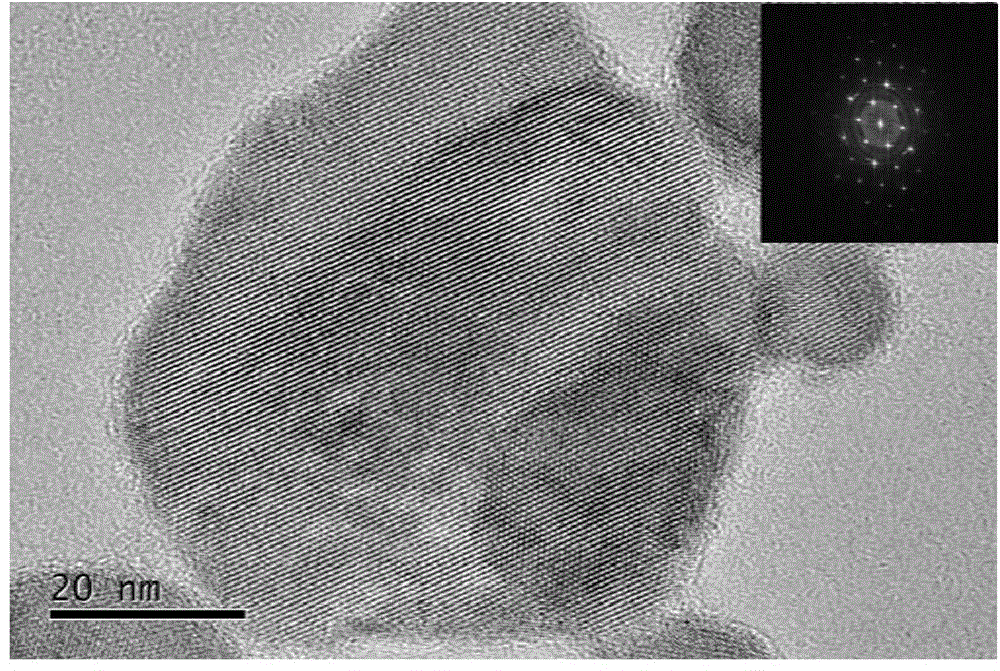
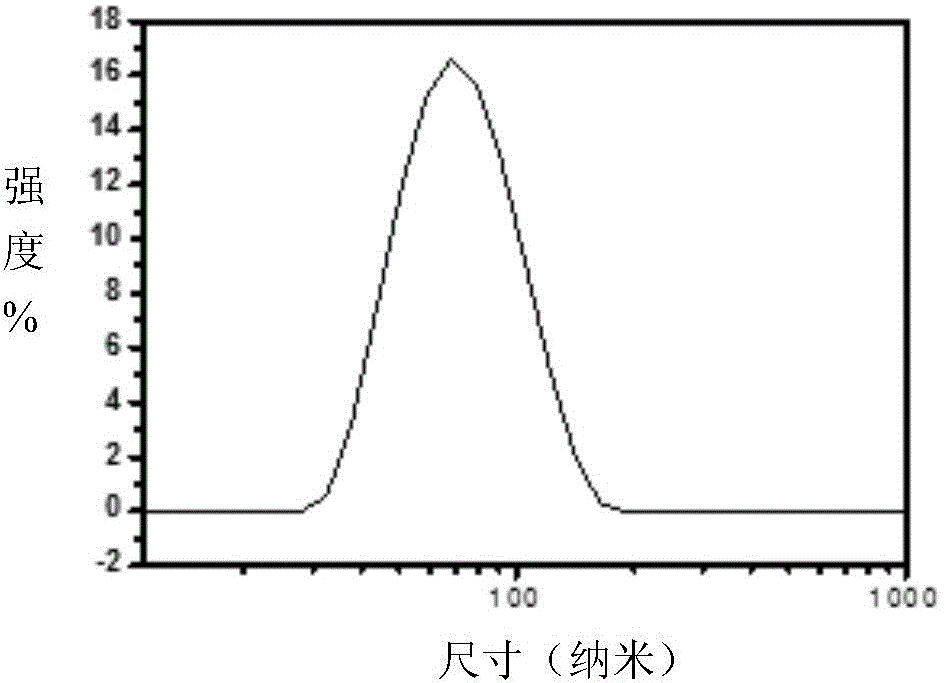
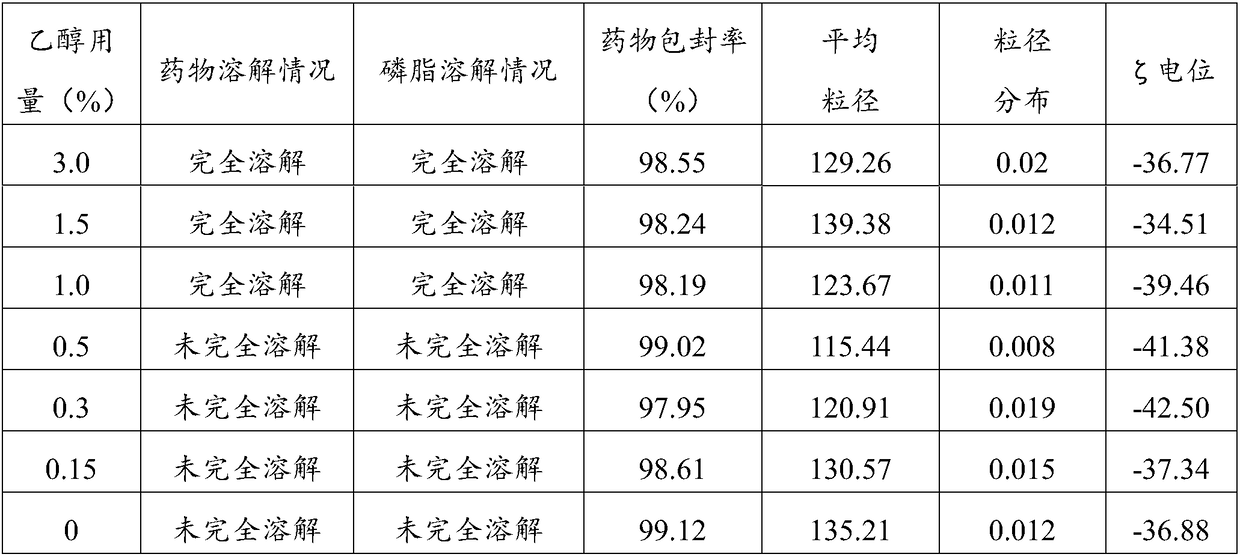
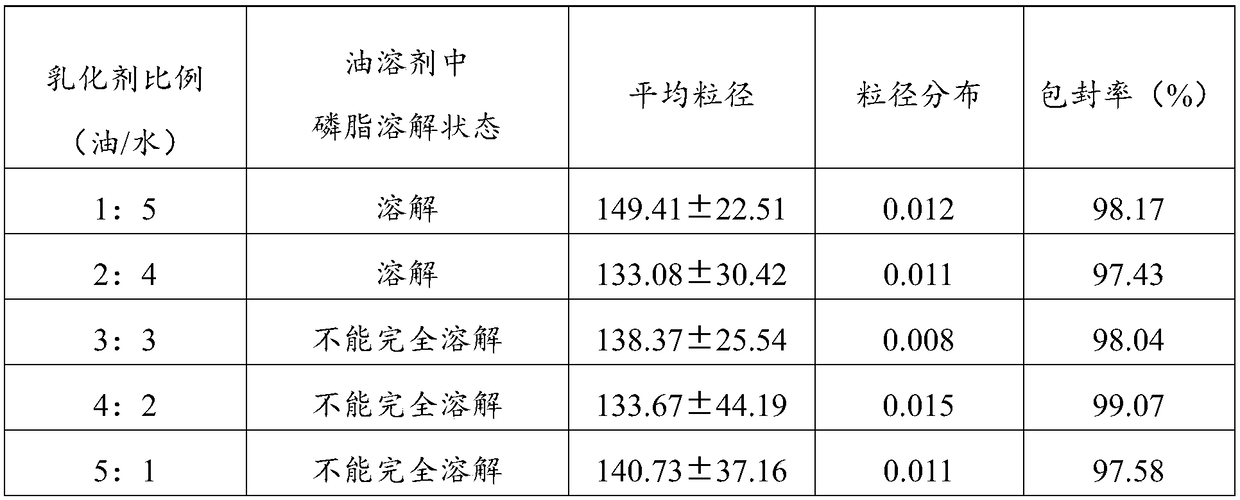
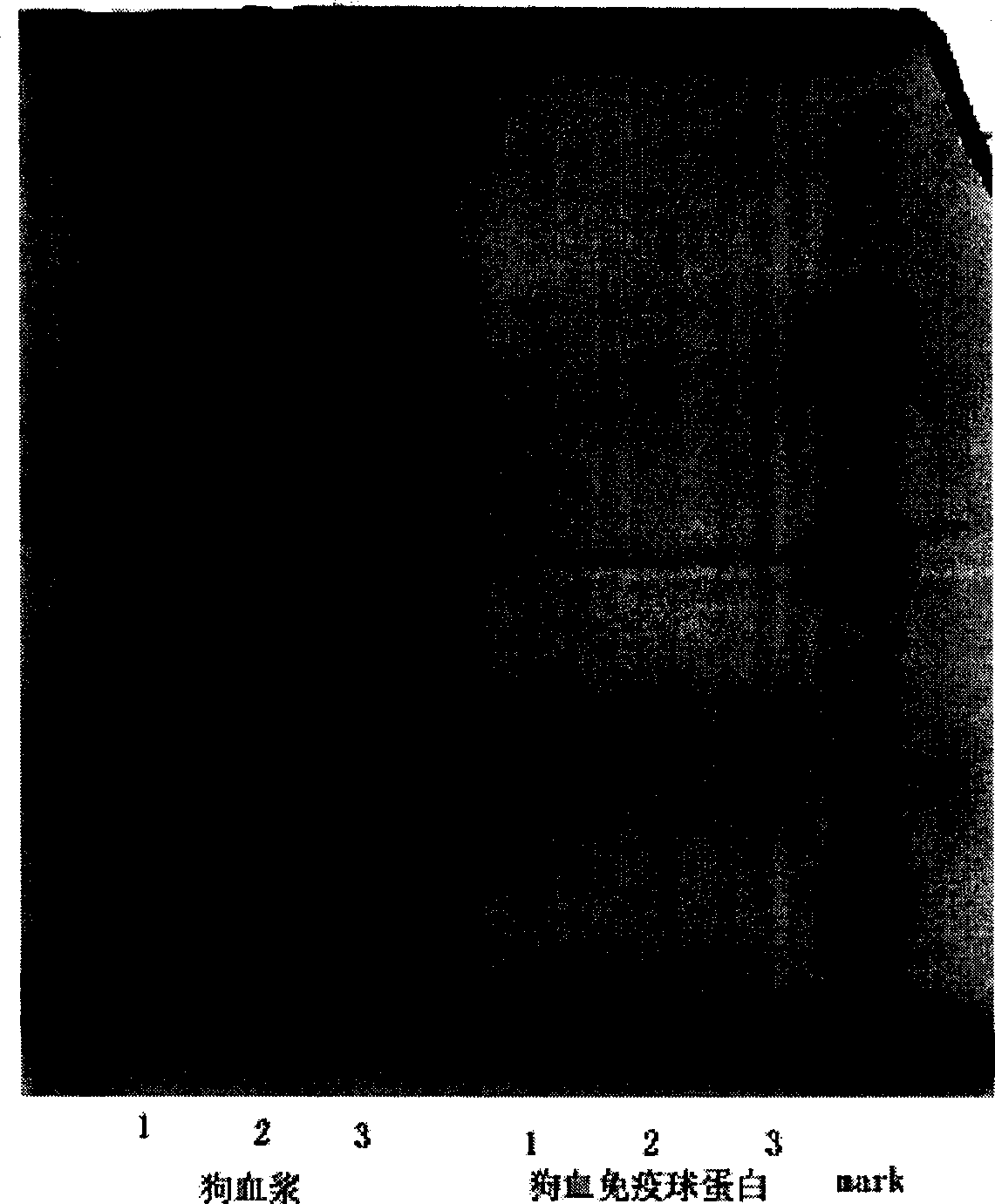Patents
Literature
74 results about "IV injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
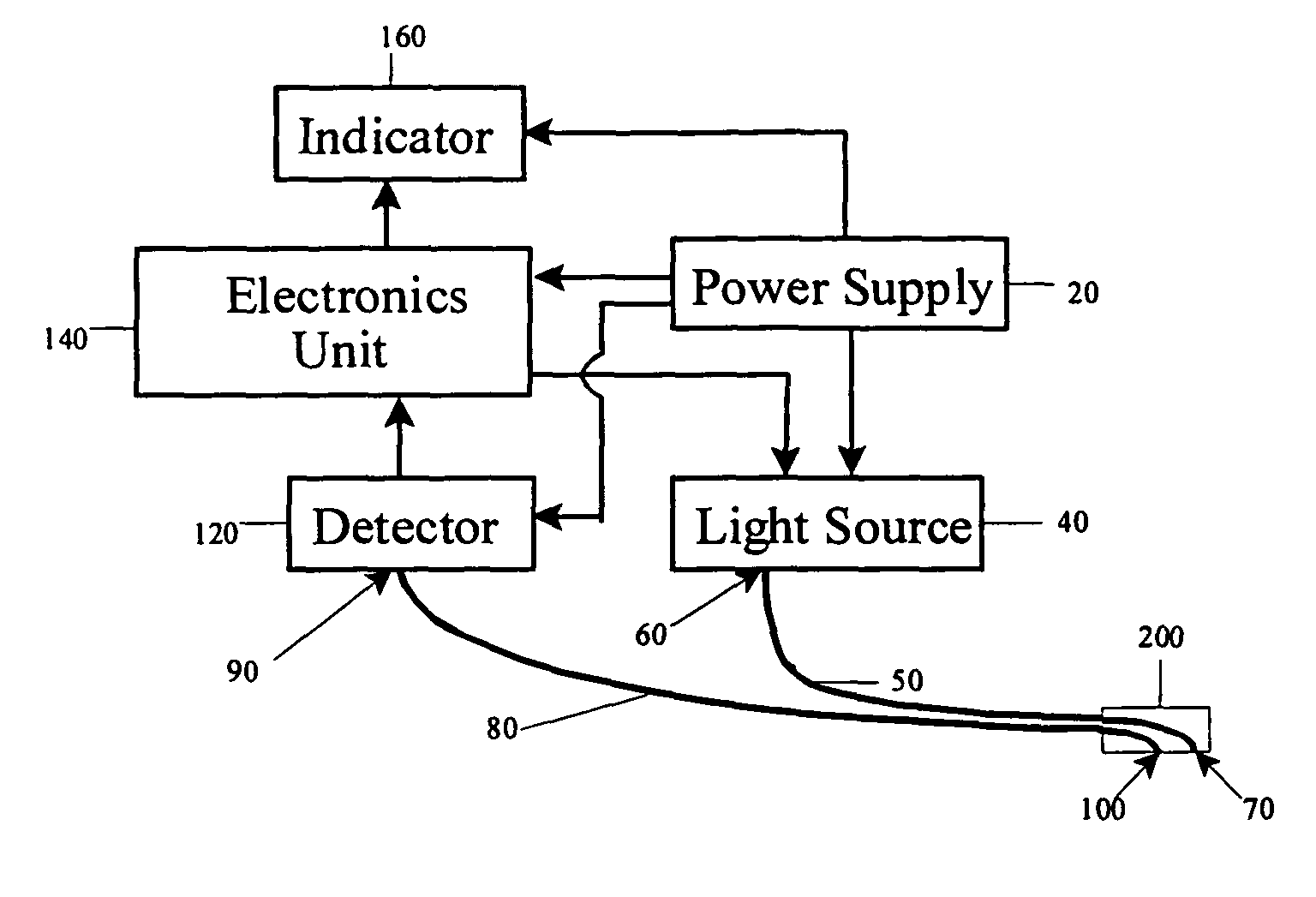
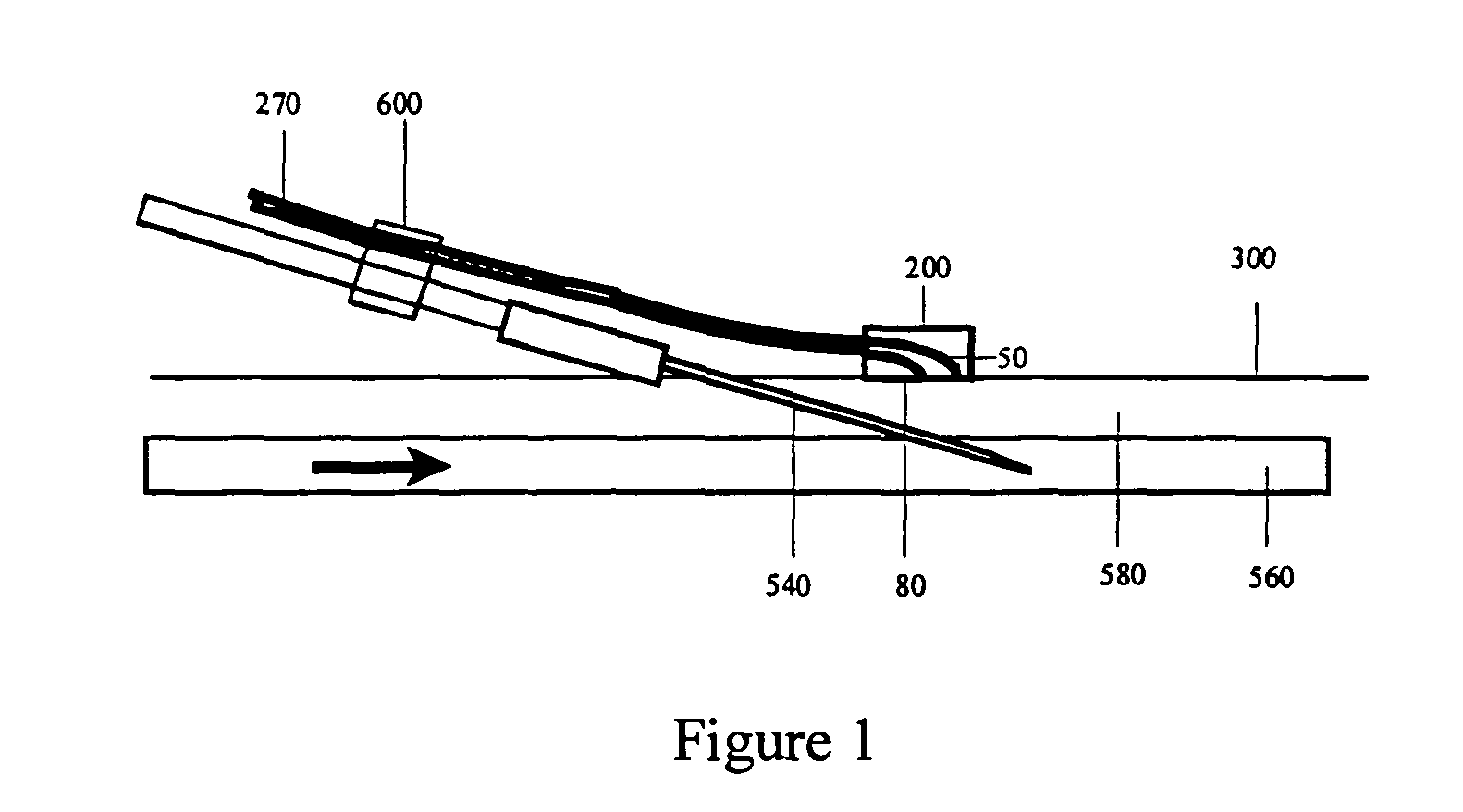
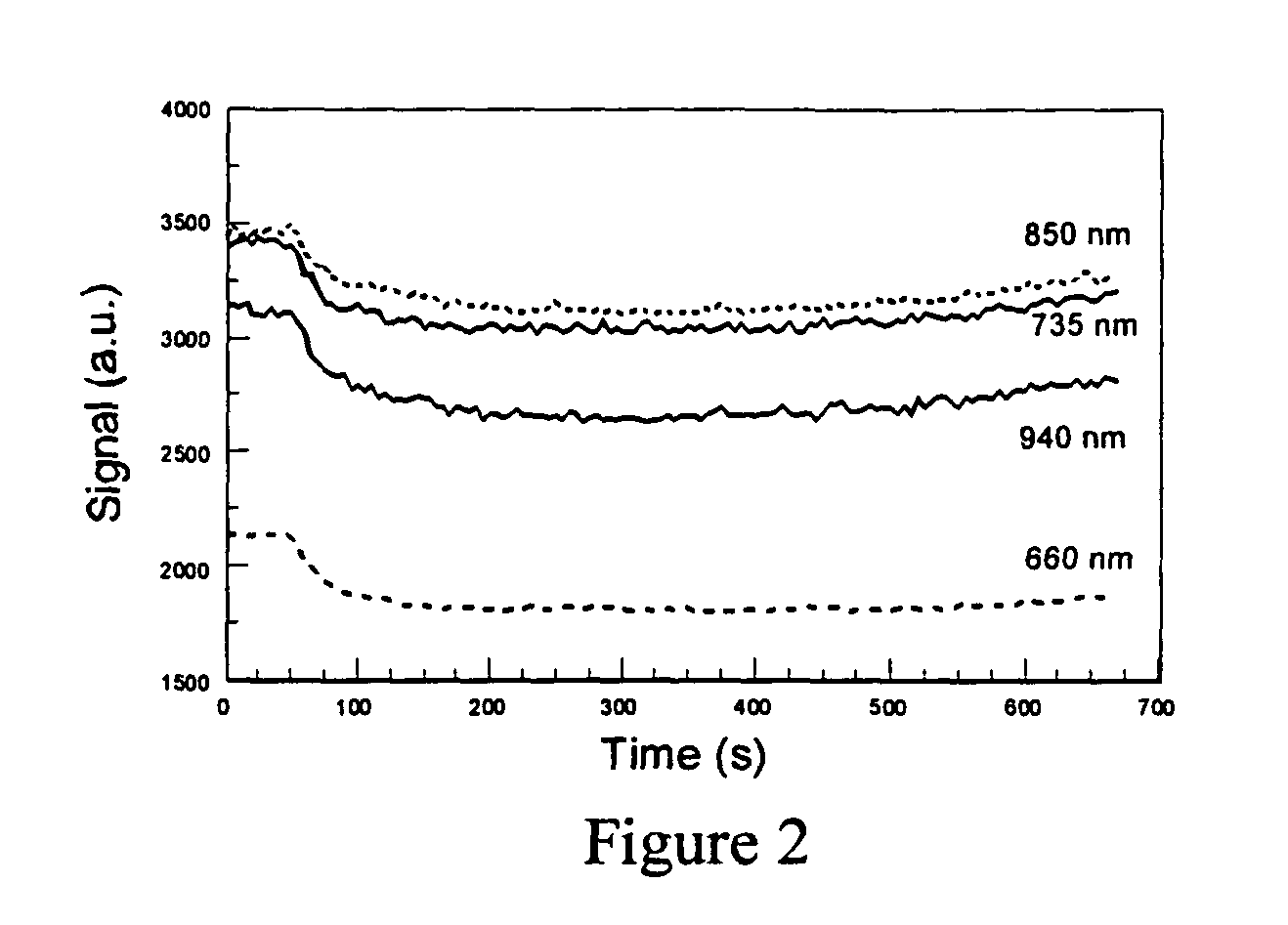
Optical detection of intravenous infiltration
An intravenous infiltration detection apparatus for monitoring intravenous failures, which applies an optical method coupled with fiber optics and algorithms for tissue optics to provide a means for noninvasive detection of intravenous infiltration surround the site of IV injection. In the invention, the tissue surrounding the injection site is exposed to a single-wavelength of electromagnetic radiation, and light is collected with only one detector. Changes in the relative intensity of the radiation reflected, scattered, diffused or otherwise emitted provide a means for monitoring infiltration. The invention provides routine, automated, continuous, and real-time monitoring for patients undergoing IV therapy.
Owner:IVWATCH
Nanocomposite temperature-sensitive gel and preparation method and application thereof
InactiveCN102525882AEasy to prepareSuitable for mass productionGenetic material ingredientsEmulsion deliveryHypodermic injectionLung cancer
The invention relates to a nanocomposite temperature-sensitive gel and a preparation method and an application thereof. The preparation method comprises the following steps of: coating antitumor active substances with a high molecular polymer serving as a carrier material to obtain nanoparticles; and adding a temperature-sensitive high molecular material to obtain the nanocomposite temperature-sensitive gel. The preparation method disclosed by the invention is simple and convenient, is suitable for large-scale production, and is particularly suitable for preparing medicaments or diagnostic reagents having the characteristics of long cycle, biodegradability, slow release, passive targeting, active targeting, active substance conveying function and tumor resistance. An antitumor medicament prepared with the method disclosed by the invention is suitable for ways such as intravenous injection, intramuscular injection, hypodermic injection, intradermal injection, intratumor injection, tumor-side injection, oral administration or transdermal medicament delivery and the like, is applied to treatment and diagnosis of pancreatic cancer, liver cancer, lung cancer, gastric cancer, colorectal cancer, esophageal cancer, prostatic cancer, uterine cancer and ovarian cancer, and has a good application prospect.
Owner:SHANGHAI INST OF ONCOLOGY
Preparation method of clindamycin phosphate powder injection
ActiveCN1602889AHigh purityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsHemolysisBlood vessel
The invention relates to a method for preparing clindamycin phosphate powder filling agent, including: adding and dissolve clindamycin phosphate in alcohol solution; adding in activated charcoal to make decolorization, then roughly filtering, finely filtering, and placing to crystallize, filtering to eliminate supernatant and obtaining clindamycin phosphate crystal; then making transfer-solution for the second time, recrystallizing once, filtering and obtaining the recrystallized clindamycin phosphate crystal; drying and crushing, making split charging, capping and packaging in aseptic condition and making it. In the preparing course, it needs no high temperature treatment, need not add in additive, and its powder has good fluidity, high purity, few impurities, high bio-capability and good stability. It has no anaphylaxis and hemolysis and has no stimulation to blood vessels by intravenous injection. It adds a new clinic form of clindamycin phosphate, meeting clinic requirement.
Owner:ZHUHAI EBANG PHARMA
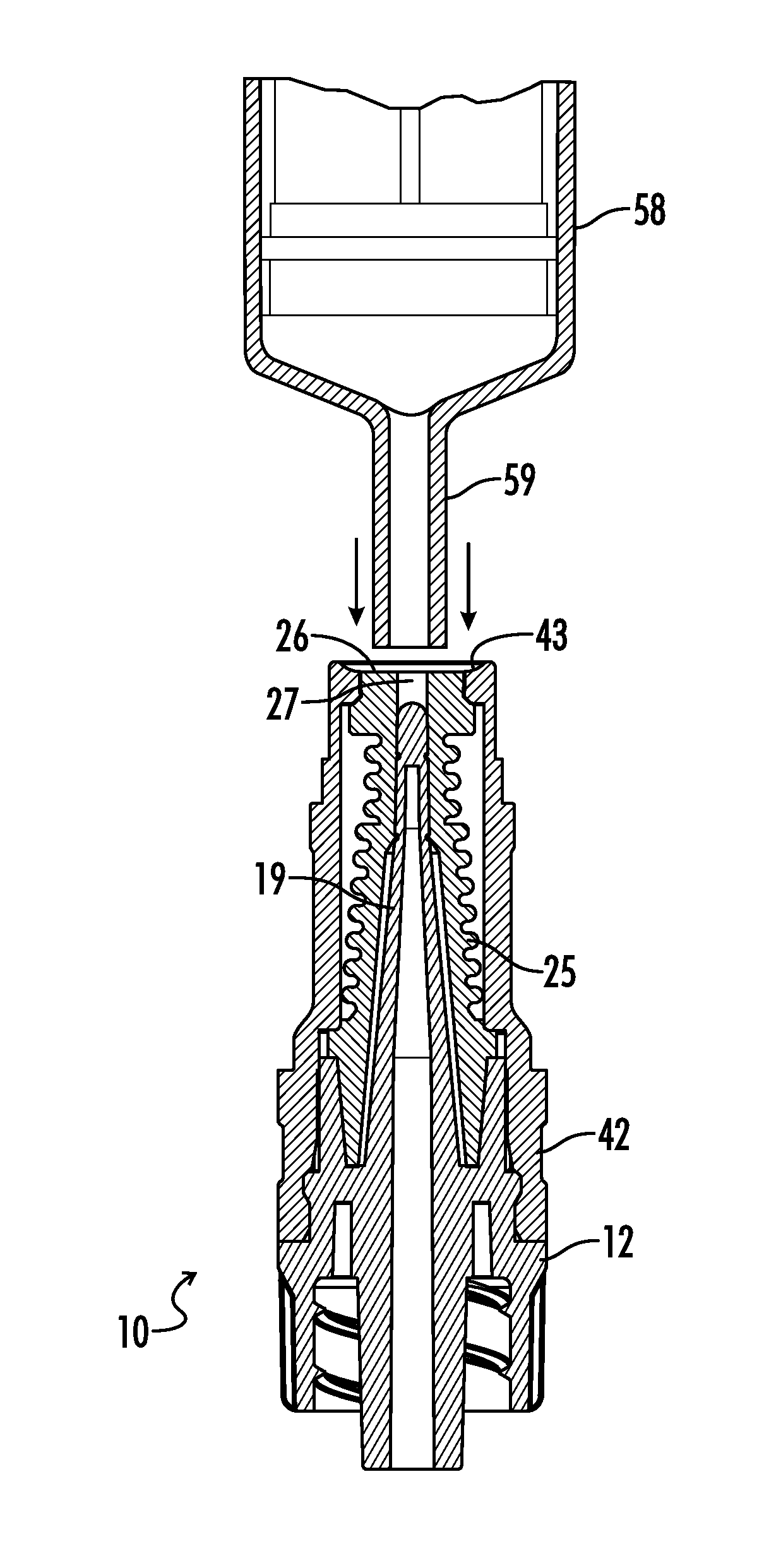
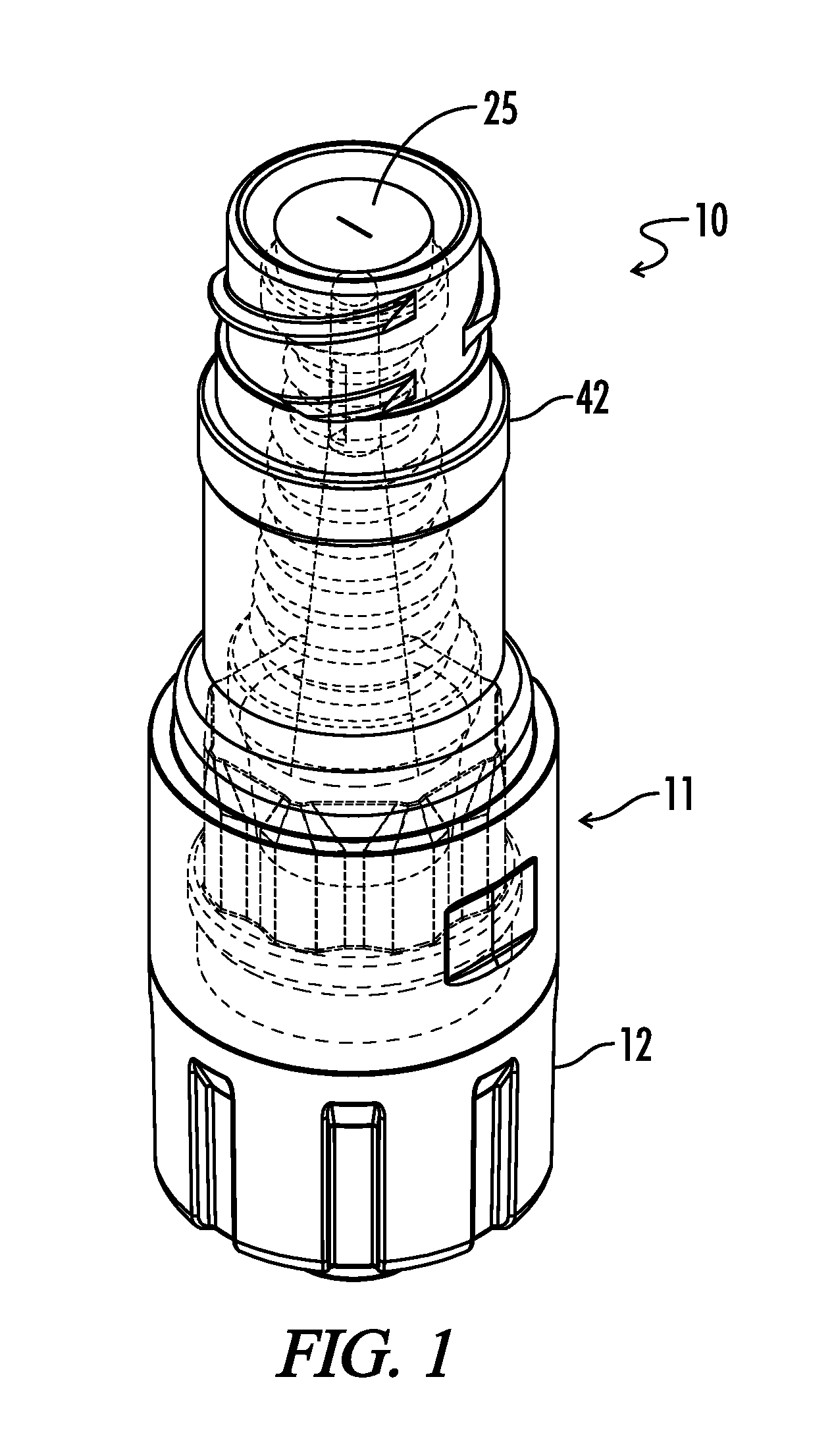
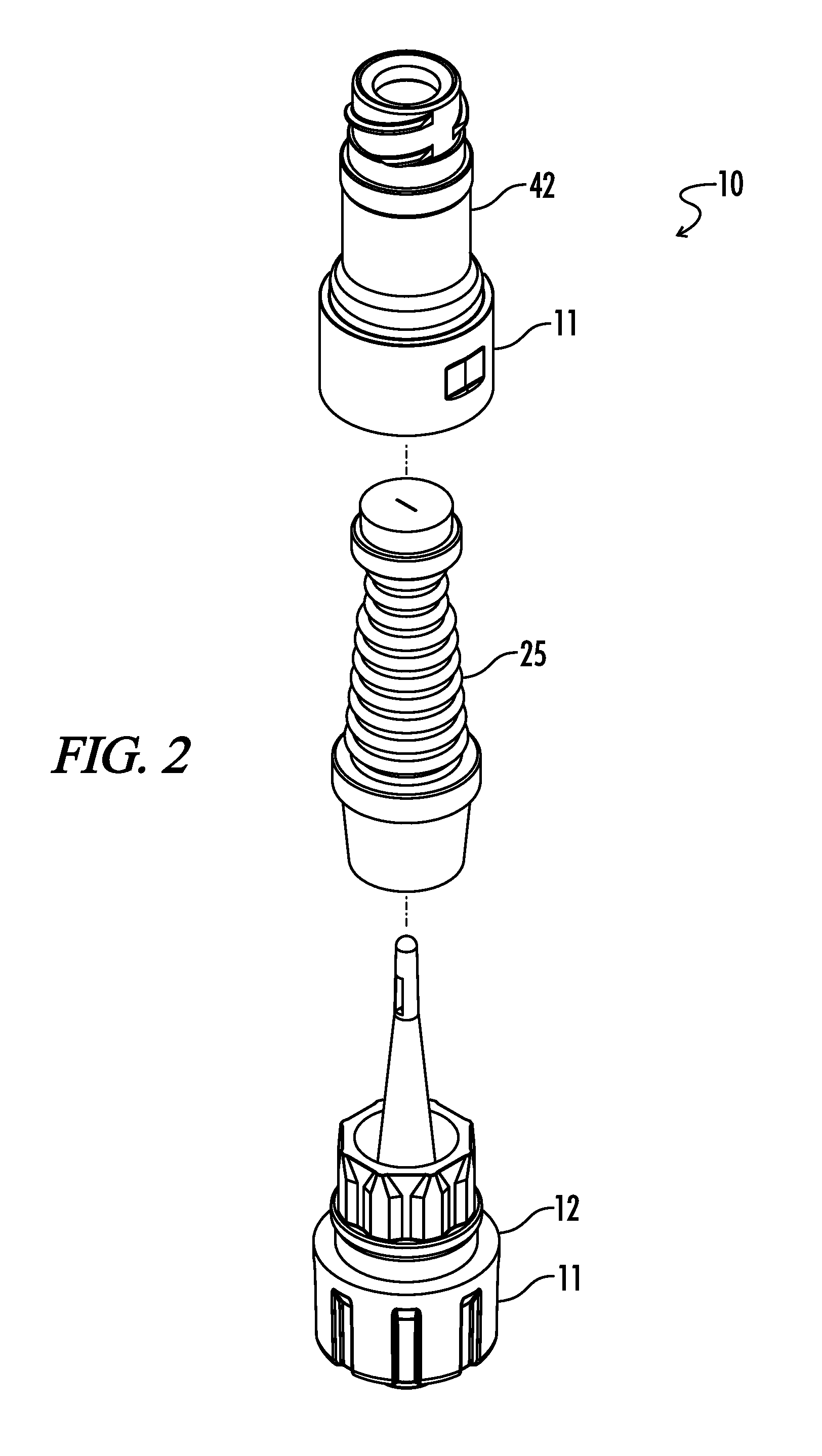
Needleless, Intermittent, Neutral Displacement IV Injection Port
ActiveUS20160129235A1Reduce dead spaceSmall priming volumeInfusion devicesTube connectorsInjection portIV injection
An injection port assembly including a body having first and second mating structures configured to mate with a first connector for a first fluid pathway and a second connector on a device, respectively. A resilient barrier substantially contained within the body and compressible from a first position in which fluid flow between the first and second connectors is blocked to a more compressed second position in which fluid flow between the first and second connectors is permitted. A hollow cannula can be coupled with the first mating structure and disposed within the resilient barrier, the hollow cannula having a distal end configured to extend through the resilient barrier when the resilient barrier is in the second position, the distal end having lateral slots. The resilient barrier can have first and second annular sealing rings positioned above and below the lateral slots respectively.
Owner:FRESENIUS KABI DEUT GMBH
Microemulsion of hypocrellin, and its preparing method
InactiveCN101002757AImprove stabilityRaw materials are cheap and easy to getOrganic active ingredientsAntipyreticActive agentPhosphate
A microemulsion of hypocrelline for venous injection with high stability, target nature and biodegradability and strong photodynamic function is proportionally prepared from hypocrelline, natural oil, surfactant, water-soluble polyol, and water. Its preparing process is also disclosed.
Owner:INST OF CHEM CHINESE ACAD OF SCI
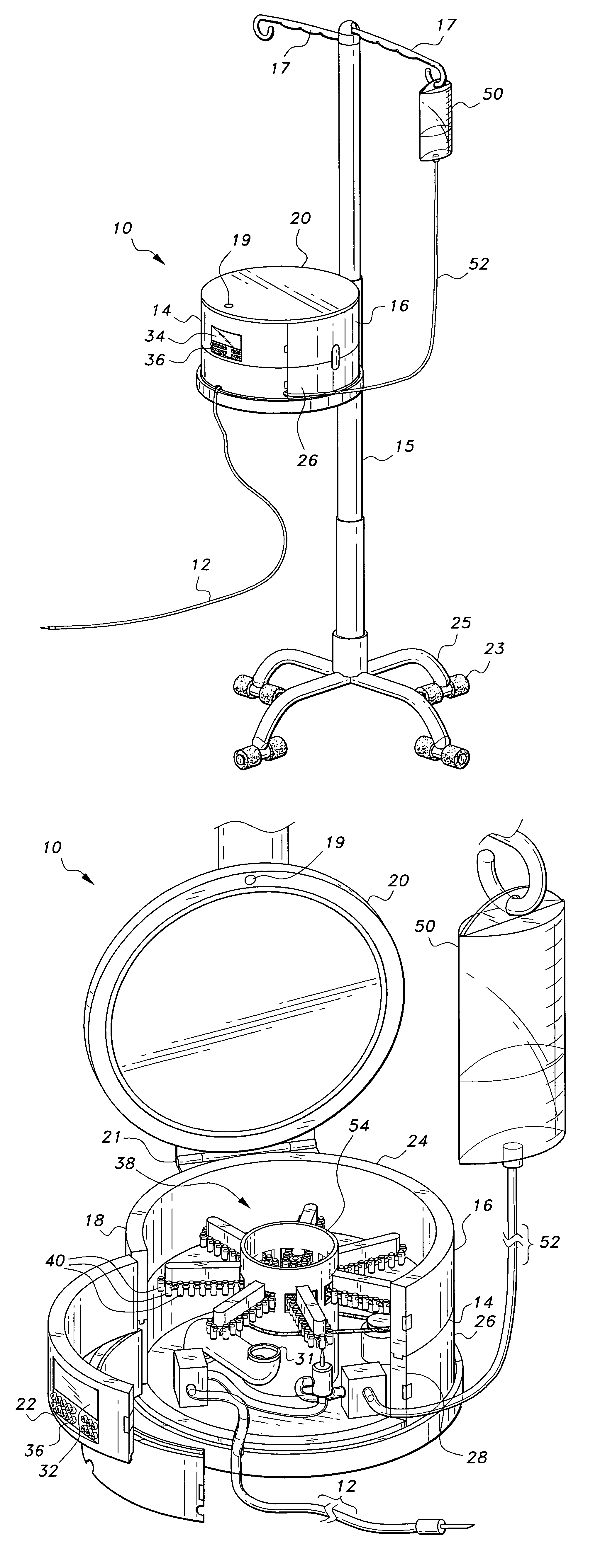
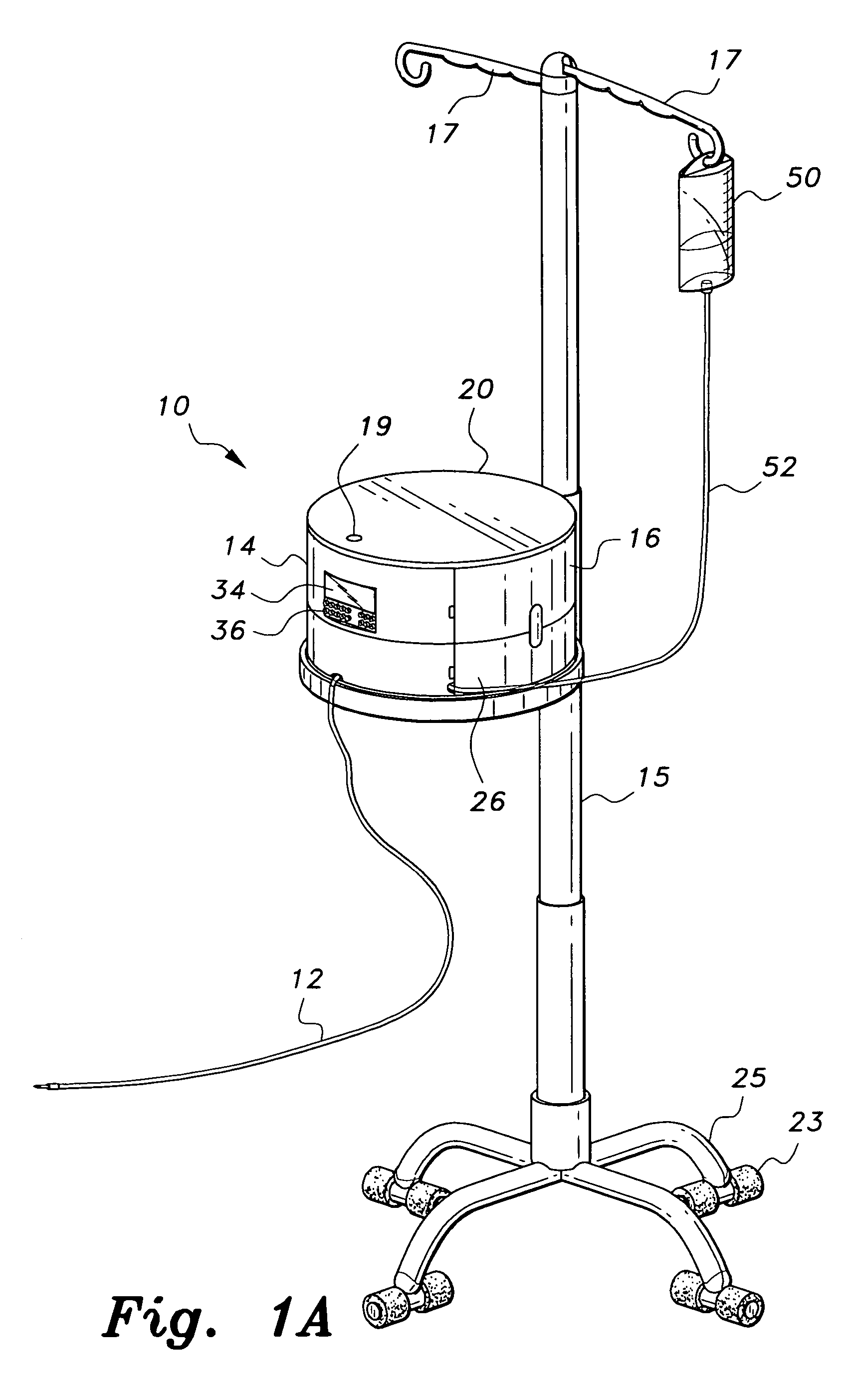
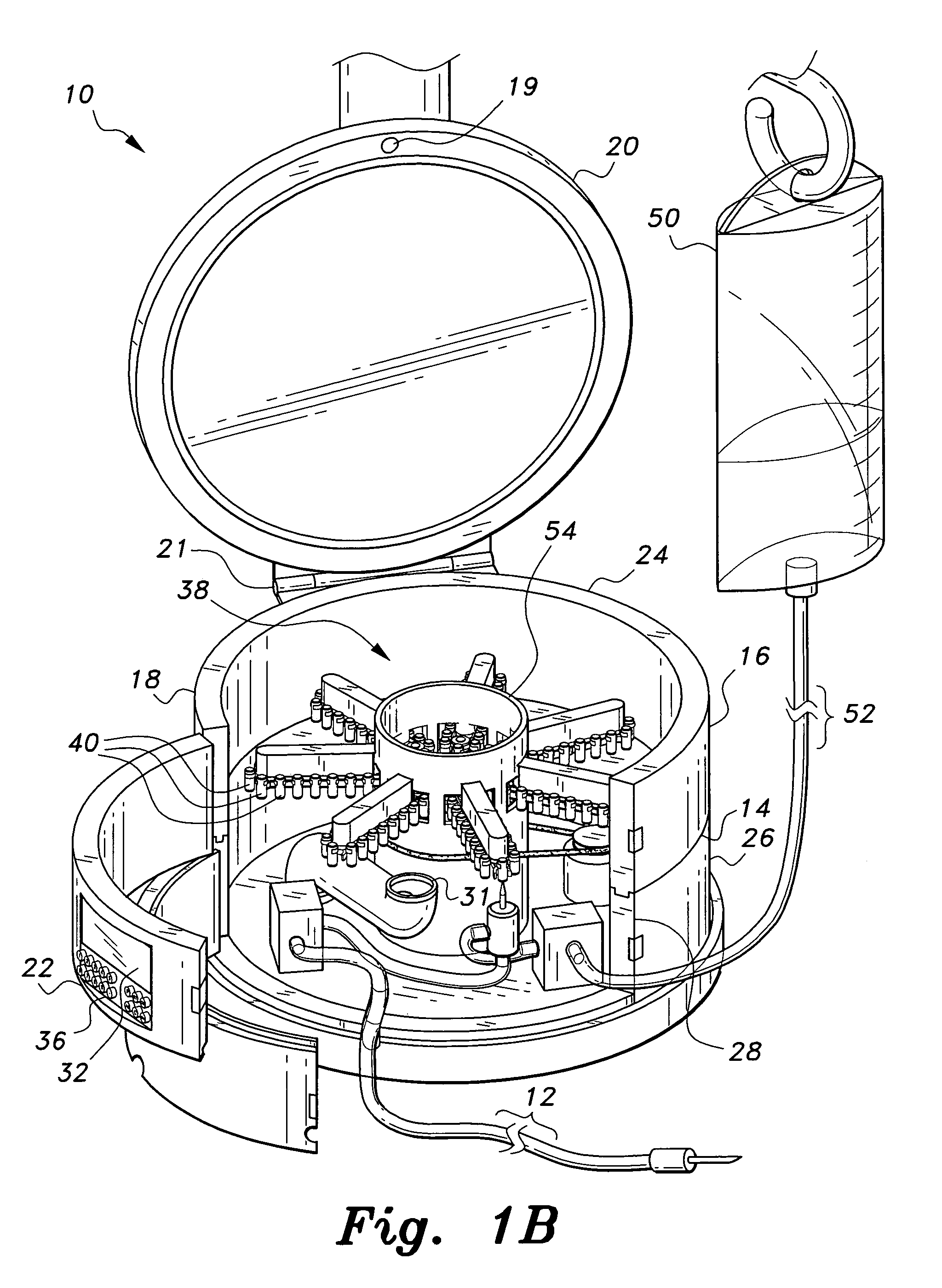
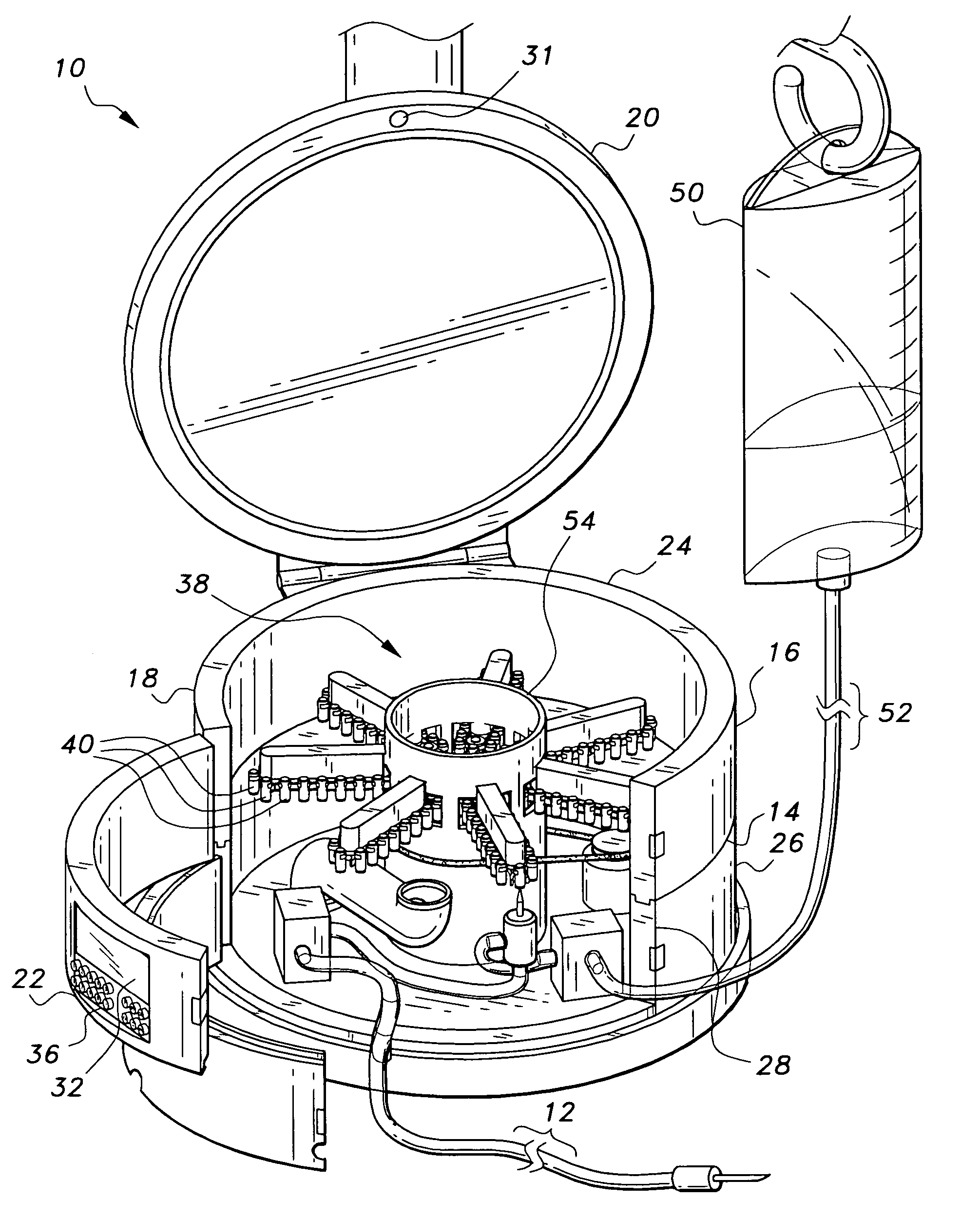
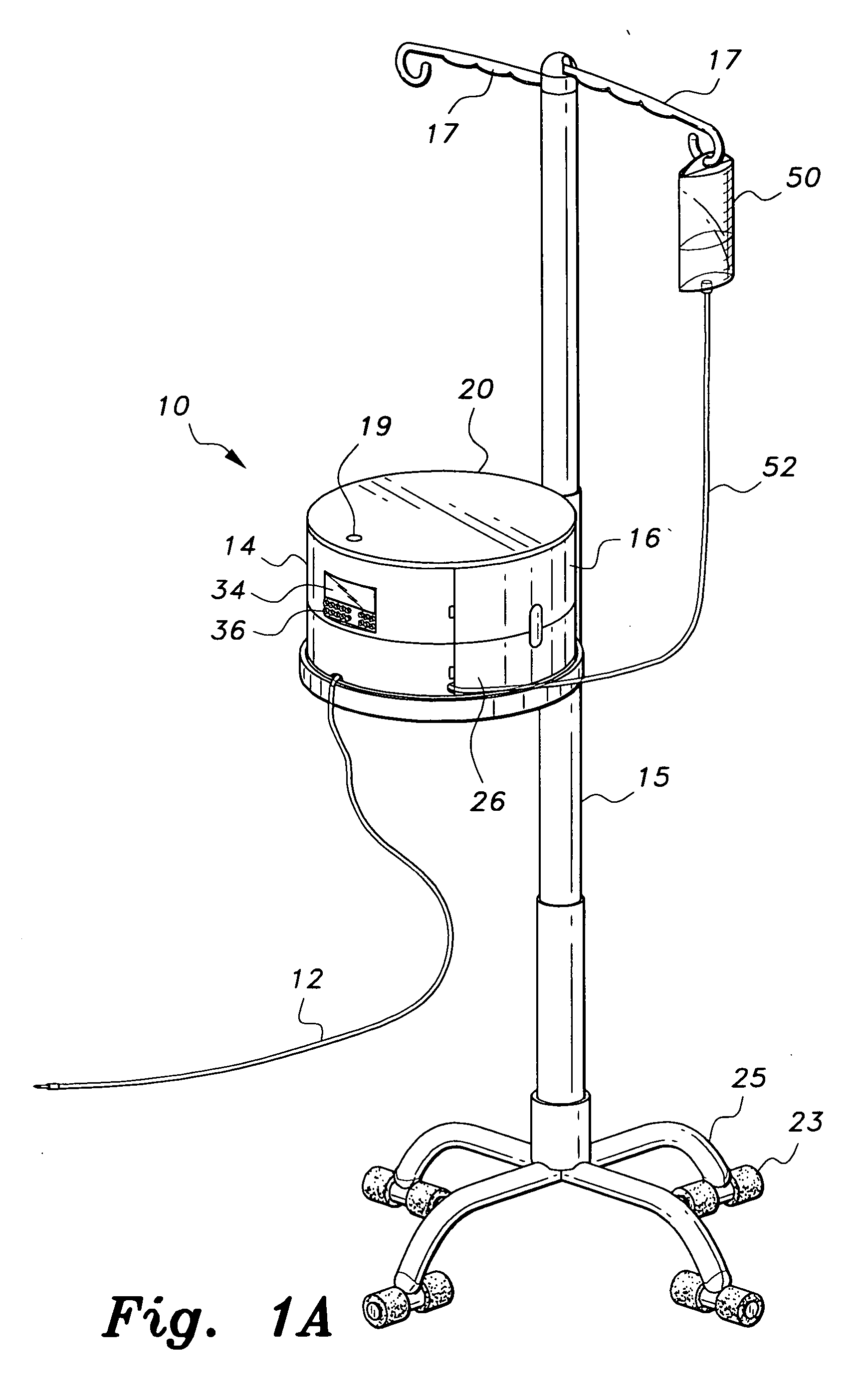
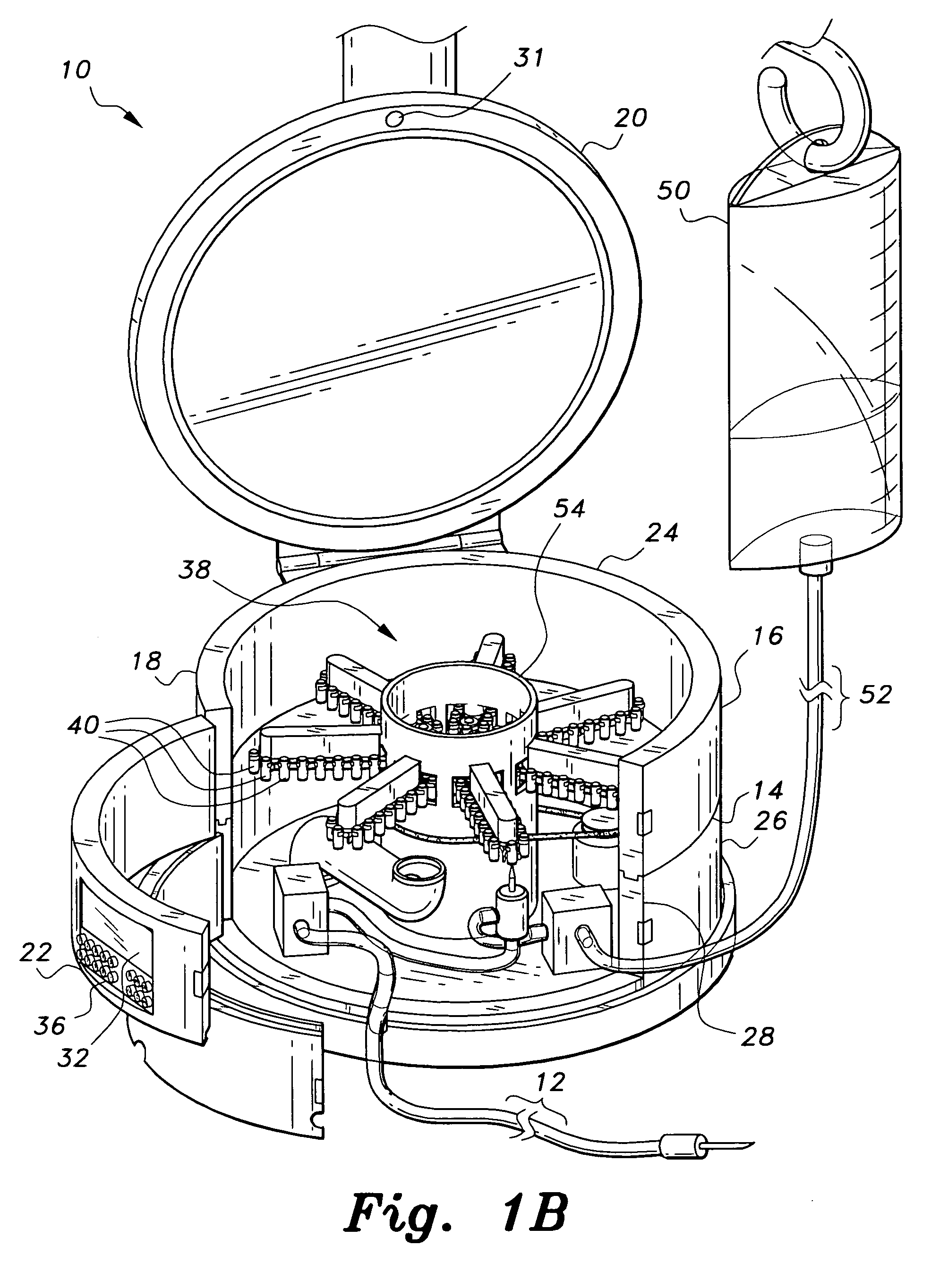
Multiple medication IV pump
The multiple medication IV pump provides automated intravenous delivery of one or more medications. A carousel assembly holds multiple medication vials, and a computerized control circuit operates the carousel to position a selected medication vial proximate to a spiking unit and mixing chamber to deliver the contents of the vial into an IV solution. The spiking unit and mixing chamber comprise a hollow chamber and a hollow spike, or needle, extending from the hollow chamber. The contents of a medication vial spiked onto the spiking unit flow into the mixing chamber. A fluid entry port located near the top of the mixing chamber is connected to a first pump such that a fluid may be pumped into the mixing chamber. A fluid exit port located near the bottom of the mixing chamber is connected to a second pump such that a fluid may be pumped out from the mixing chamber.
Owner:SIMPKINS DALE H
Nanometer capsule of anthracene nucleus anticancer antibiotic with polyethylene glycol-phospholipid
This invention provides an intravenous injection approvable anthracene-nucleus antineoplastic-antibiotic nanomicelle drug, which comprises anthracene-nucleus antineoplastic antibiotic, macrogol derivelized phosphatide those have therapy dose, and findings acceptable in medicine. In the nanomicelle, the macrogol wraps around the drug hydrophobilic nucleuses and forms hydrophilic protective layer, this can provide the drugs from contacting with proteins in blood and identifying and licking up by esoderma system in body, so the drug cycle time in body will be prolonged.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
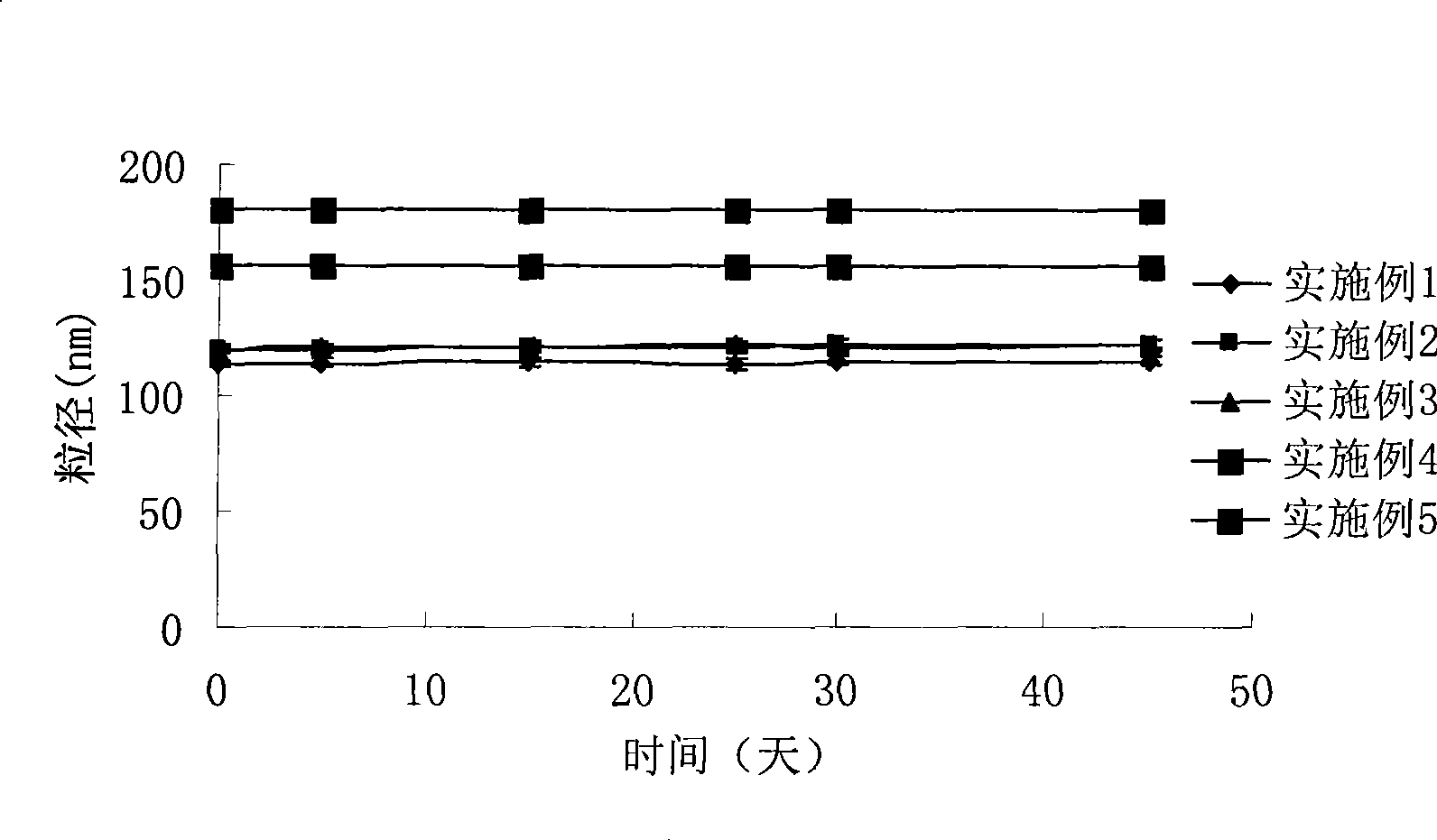
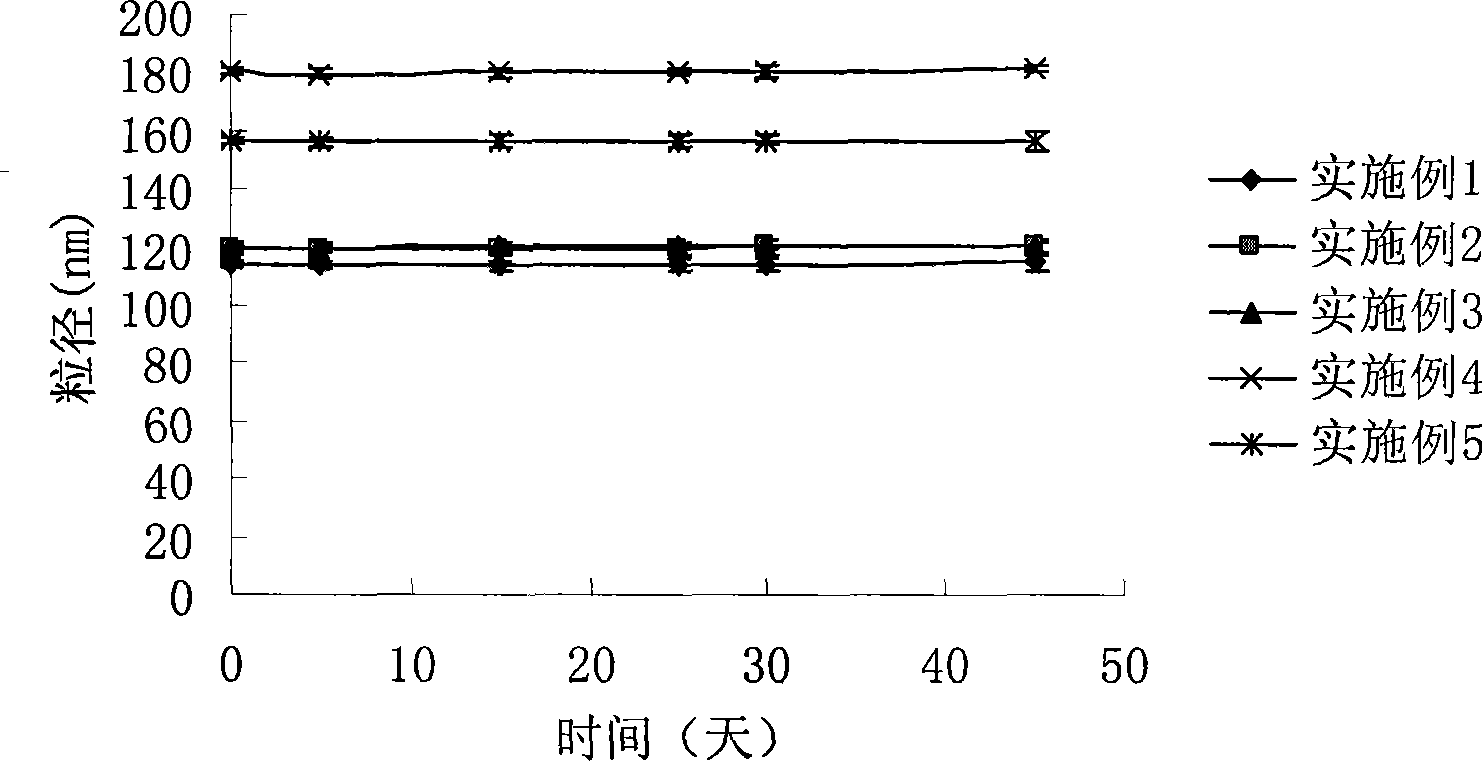
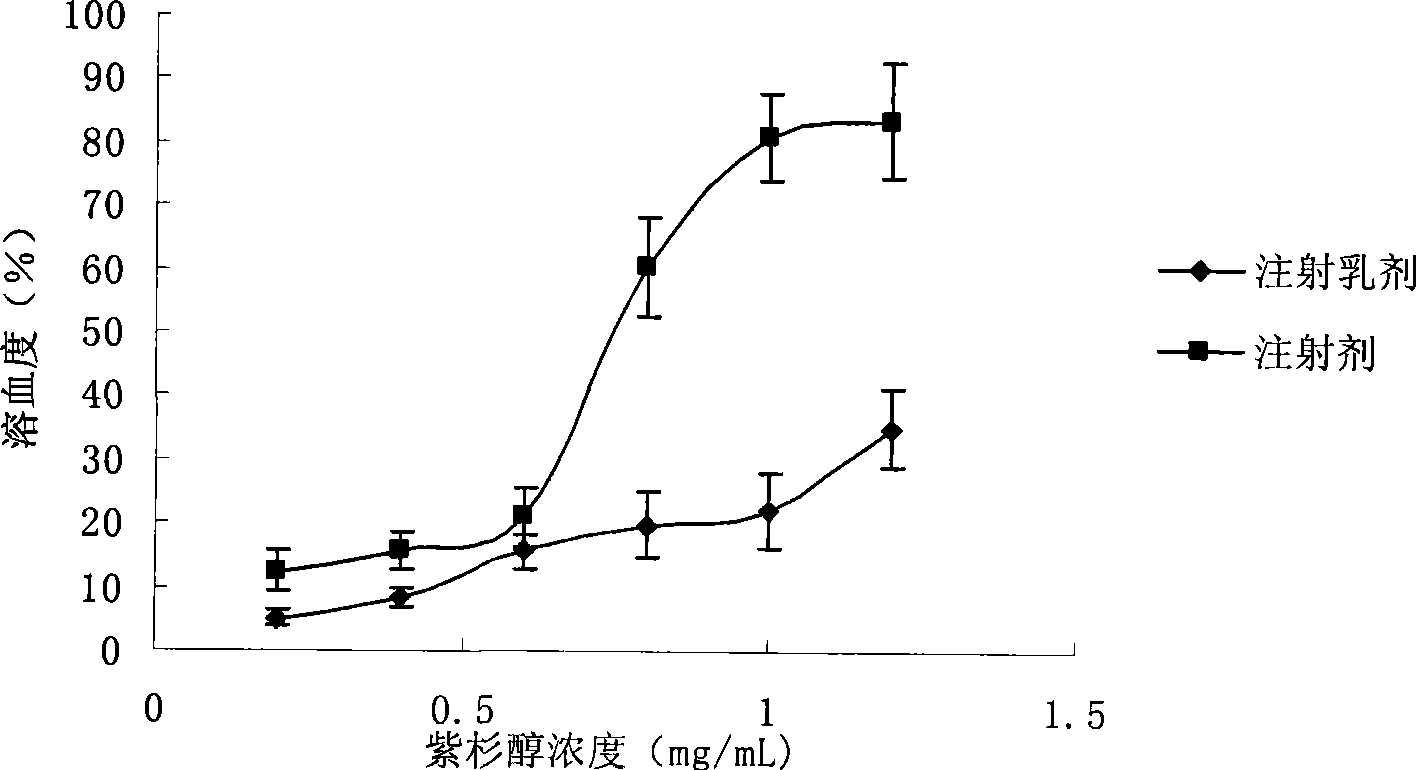
Intravenous injection emulsion of paclitaxel or polyenic taxusol
InactiveCN101433533AReduce allergic reactionsThe preparation process is simple and feasibleOrganic active ingredientsEmulsion deliveryPharmacyHaemolysis
The invention belongs to the field of traditional Chinese medicine pharmacy, in particular to a novel emulsion for intravenous injection of an anti-cancer drug taxol or polyenic taxusol, and a preparation method thereof. The emulsion consists of the taxol or the polyenic taxusol, and oil for injection, emulsion, in particular to glycol laurylhydroxystearate, and other pharmaceutically necessary auxiliary materials. Results via experiments show that the injection emulsion has excellent stability and haemolysis significantly smaller than a commercial taxol injection, and is helpful for improving administration safety and effectiveness of the taxol. The emulsion for intravenous injection can be directly used for intravenous injection, has the advantages of safe administration and small stimulation, can be easily accepted by patients, and can be used as an energy replenisher. The emulsion has the advantages of good stability, large drug loading, convenient use and simple preparation technology method, and is applicable to mass production.
Owner:FUDAN UNIV
Paclitaxel lipid microspheres injection and preparation method thereof
InactiveCN101204373ALess irritatingNot easy to precipitateOrganic active ingredientsSolution deliveryLipid formationPolyoxyethylene castor oil
The invention relates to a paclitaxel lipid microsphere injection and a manufacturing method thereof. In the invention, a paclitaxel lipid microsphere injection that does not contain polyoxyethylene castor oil is prepared. The medicine 90-98 per cent paclitaxel is coated in the oil phase and the oil-water interfacial film of the lipid microsphere. Thus the toxicity and stimulation of paclitaxel in clinic use are largely reduced, and the adverse reaction resulted from the excipient in the existing paclitaxel injection is prevented. With low stimulation, low toxicity and high efficiency, the preparation as a antineoplastic drug is administrated by intravenous injection.
Owner:李时海 +1
Doxofylline venous injection with small volume as well as preparation method and quality control method thereof
ActiveCN101647776AReduce health impactReduce the impactInorganic non-active ingredientsSurface/boundary effectDoxofyllineVein injection
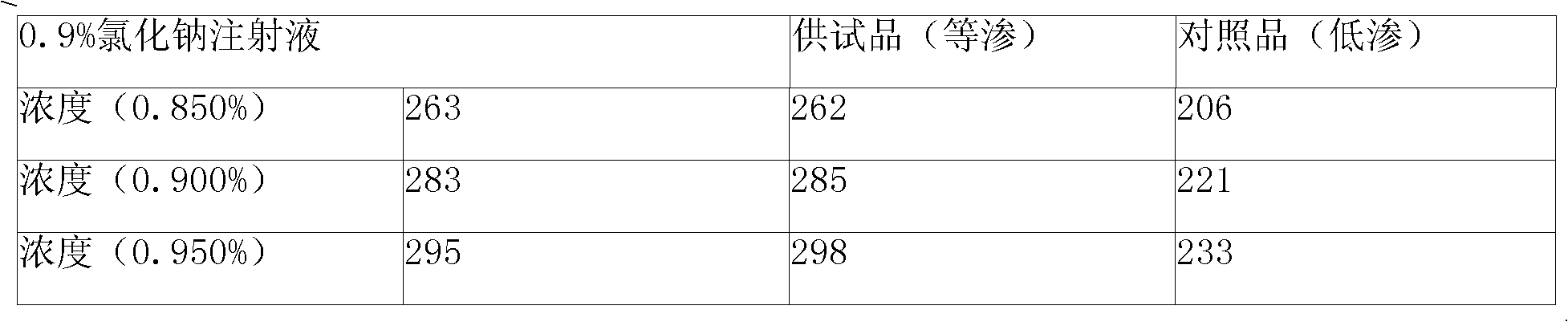
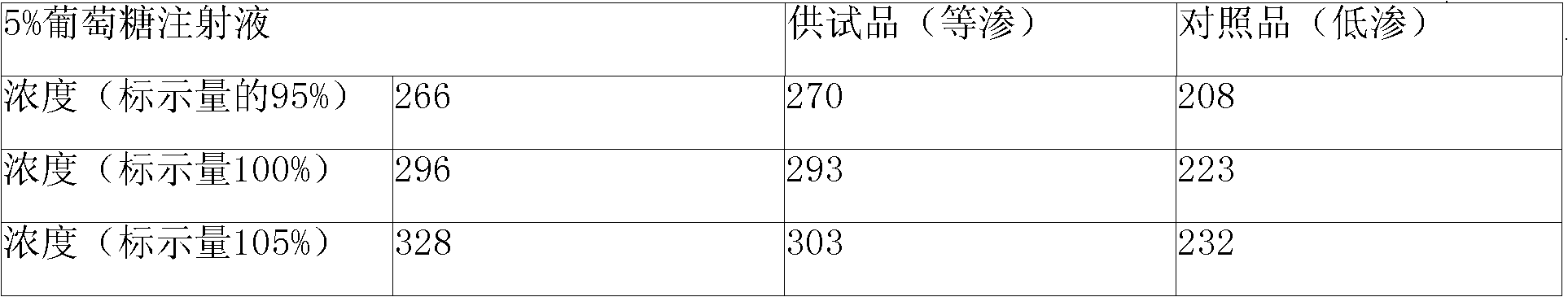
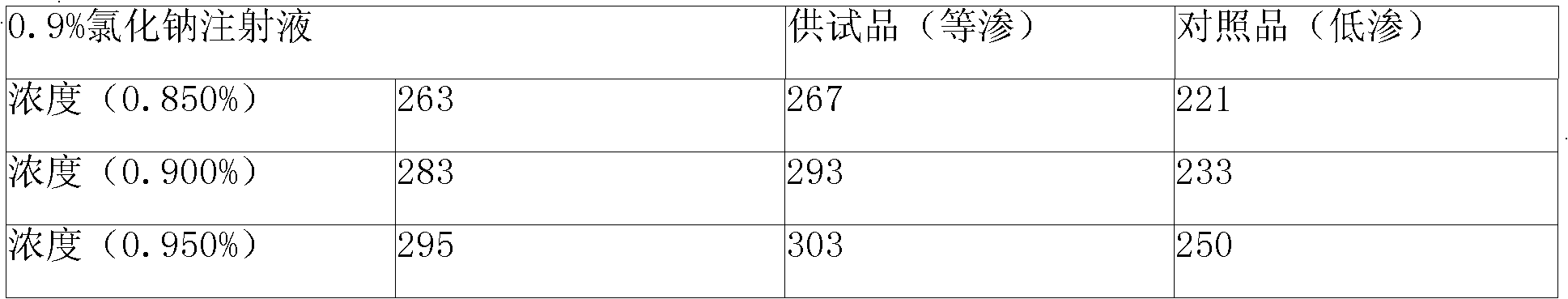
The invention discloses Doxofylline venous injection with a small volume and a preparation method thereof. The preparation unit of the isotonic small volume of doxofylline venous injection is 1-20ml,the weight percentage concentration of doxofylline is 1-4 percent, and the isotonic value of the venous injection is 257-340mosmol / kg. The invention enlarges the optional range of clinical applicationof doxofylline and provides the isotonic doxofylline venous injection with favorable security.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Multiple medication IV pump
The multiple medication IV pump provides automated intravenous delivery of one or more medications. A carousel assembly holds multiple medication vials, and a computerized control circuit operates the carousel to position a selected medication vial proximate to a spiking unit and mixing chamber to deliver the contents of the vial into an IV solution. The spiking unit and mixing chamber comprise a hollow chamber and a hollow spike, or needle, extending from the hollow chamber. The contents of a medication vial spiked onto the spiking unit flow into the mixing chamber. A fluid entry port located near the top of the mixing chamber is connected to a first pump such that a fluid may be pumped into the mixing chamber. A fluid exit port located near the bottom of the mixing chamber is connected to a second pump such that a fluid may be pumped out from the mixing chamber.
Owner:SIMPKINS DALE H
2-methoxyestradiol nanosuspension frozen powder and preparation method thereof
InactiveCN101612131AImprove stabilityEasy to transportOrganic active ingredientsPowder deliverySolubilityHalf-life
The invention relates to a 2-methoxyestradiol nanosuspension frozen powder and a preparation method thereof, which effectively solves the problems of poor solubility, short half-life period, low oral bioavailability, difficulty for common injection to achieve high drug loading and targeting effect existing in 2-methoxyestradiol. The 2-methoxyestradiol nanosuspension frozen powder prepared by the invention comprises the following components by weight ratio: 1 part of 2-methoxyestradiol, 1-50 parts of surface active agent and 3-30 parts of cryoprotector. The preparation of the 2-methoxyestradiol nanosuspension frozen powder can be realized by the following method: 1. preparing primary nanosuspension by using a precipitation method or a dispersion method; 2. preparing nanosuspension by using the method of reducing the particle diameter including a high pressure homogenizer method and an ultrasound comminution method; 3. preparing the frozen powder; and 4. re-dissolving the frozen powder by water for injection when in use. The frozen powder for intravenous injection prepared by the invention has fine stability and is favor of transporting and storing drugs.
Owner:ZHENGZHOU UNIV
Temozolomide intravenous injection fat emulsion and preparation method thereof
InactiveCN103405385ASolve the problem of low solubilityGood treatment effectOrganic active ingredientsEmulsion deliveryFat emulsionMedicine
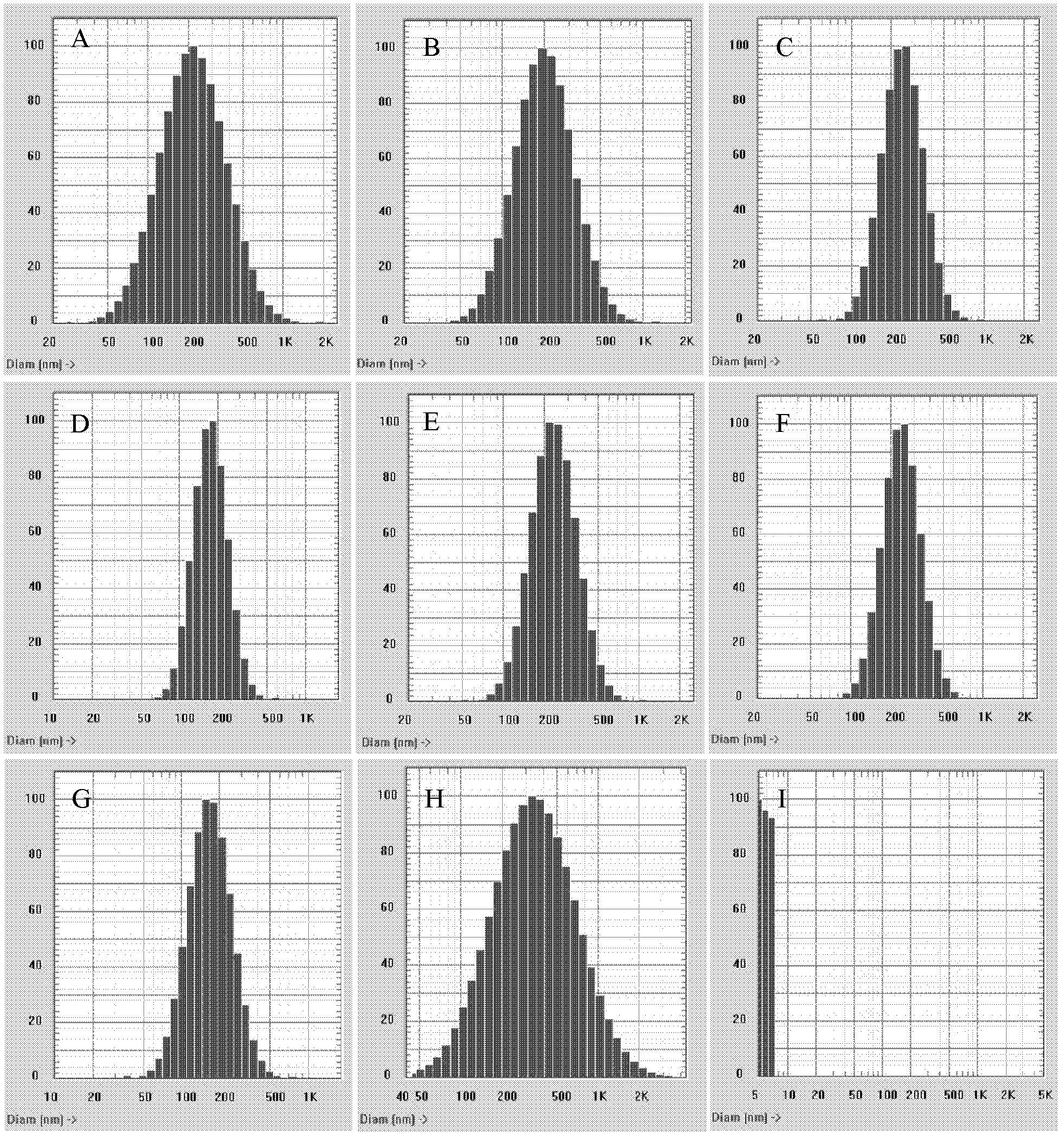
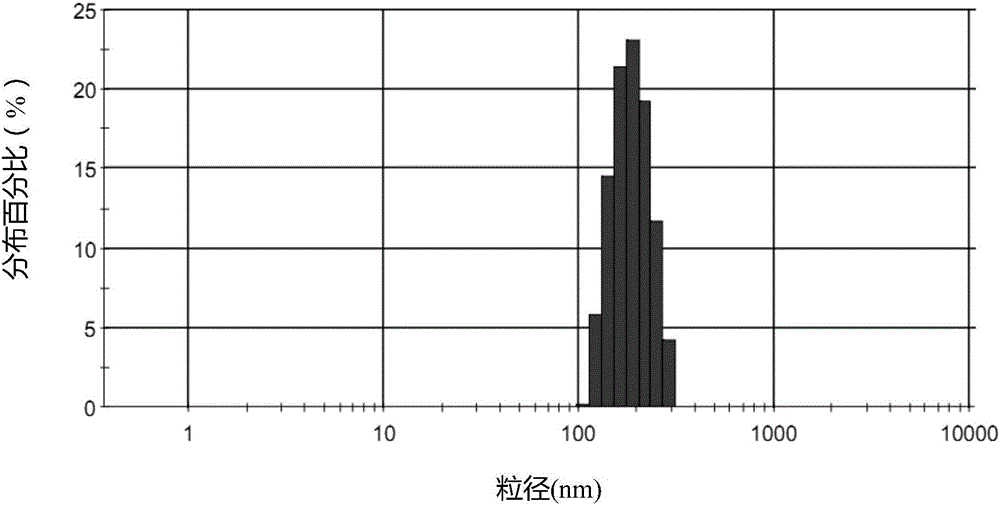
The invention discloses a temozolomide intravenous injection fat emulsion which comprises the following components in percentage by weight: 0.01-5% of temozolomide, 5-30% of oil for injection, 0.2-5% of emulgator, 0.2-3% of stabilizer, 1-5% of isoosmotic adjusting agent and the balance of water for injection. The prepared temozolomide intravenous injection fat emulsion is uniform in appearance, narrower in distribution, high in drug loading capacity (1 mg / ml), stable in quality, used for intravenous injection, simple and practical in production process, high in controllability, and liable to large-scale industrial production; the average grain diameter is about 200 nm, the Zeta electric potential is about -40 mV, and the pH is 5.0-9.0; the temozolomide intravenous injection fat emulsion has excellent biocompatibility, can provide energy for an organism, and can realize therapeutic action to various tumours.
Owner:SHANDONG UNIV
Camptothecin medicament injection solution and injection and preparation method thereof
InactiveCN101708156AGood dispersionSolve problems that are difficult to make into injectionsOrganic active ingredientsEmulsion deliverySolubilityMedication injection
The invention relates to the technical field of medicaments, in particular to a camptothecin medicament injection solution and an injection and a preparation method thereof. The invention provides a camptothecin medicament injection which is good in stability, high in curative effect and low in toxicity and consists of the camptothecin medicament injection solution and a disperse medium, wherein the camptothecin medicament injection solution consists of a medicament active component, a stabilizing agent, a pH value regulator and an injection solvent. The medicament active component of the camptothecin medicament injection solution is a camptothecin medicament mainly existing in the form of a lactonic ring, which can improve the curative activity of the medicament and reduce toxic side effects; and the disperse medium is an injection emulsion, which can improve the dispersion degree of the camptothecin medicament and solve the problem that the camptothecin medicament is difficult to be prepared into the injection due to poor water solubility. The camptothecin medicament injection solution and the injection emulsion are packed and then stored respectively, and are mixed uniformly before use for intravenous administration, so the stability of the medicaments stored for a long time can be improved. The invention provides an intravenous injection which has low toxicity and is save and convenient to store for camptothecin medicaments.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Targeting antitumor drug using magnetic micro-nano material to destroy tumor blood vessels under driving of alternating magnetic field or radio frequency
ActiveCN105641695AHas a therapeutic effectAvoid harmPowder deliveryEnergy modified materialsMicro nanoTumor target
The present invention discloses a novel use of a magnetic micro-nano material. The new use is application of the magnetic micro-nano material in manufacture of a targeting tumor treatment drug, the magnetic micro-nano material has all the following properties: (1) the magnetic micro-nano material is magnetic, sol that the magnetic micro-nano material can produce mechanical destructive effects on tumor blood vessel endothelial cells under driving of an alternating magnetic field or radio frequency; (2) the magnetic micro-nano material has a hydrophilic surface; (3) the magnetic micro-nano material has certain electronegativity on surface; and (4) the particle size range of the magnetic micro-nano material is 10-500nm, so that the magnetic micro-nano material has rigidity (namely, is not easy to deform). The magnetic micro-nano material can be specifically gathered and retained in tumor blood vessel parts by EPR effect after intravenous injection, and the mechanical destructive effects produced under driving of the alternating magnetic field or radio frequency can act on the tumor blood vessel endothelial cells highly selectively to further destroy tumor blood vessels to lead to tumor rapid necrosis and achieve the efficient tumor targeting treatment purpose.
Owner:赤峰福纳康生物技术有限公司 +1
Novel route for administering hairy holly injection and preparation process thereof
The invention relates to the novel administration methods of traditional medicinal injection for treating coronary atherosclerotic heart disease, thrombolysis in myocardial infarction, center syrup liquid chorioretintis and children's pneumonia, and the process for its preparation. The invention is prepared from pubescent holly root, freeze-dried powder, germ-free powder, 5% glucose injection, 5% sodium chloride injection through extracting with water, decompression concentrating, alcohol depositing, filtrating, adjusting pH, filtrating, charging water for injection into the filter liquor. A novel administration method of intra-vascular injection is provided.
Owner:FUKANGREN BIO PHARMA
Preparation for injection of diclofenac sodium capable of being used for intravenous injection and preparation thereof
The invention provides an injection formulation of diclofenac sodium for intravenous injection and the preparation method, which is characterized in that the diclofenac sodium is included successfully with the inclusion material of propyl-Beta-cyclodextrin. The injection formulation has the advantages of improving the solubility of diclofenac sodium in the solution and reducing the adverse reaction in intravenous injection. The injection formulation exists in the form of injection, frozen powder, sterile powder and large-capacity infusion. The invention also describes the main technical parameters of adopting propyl- Beta -cyclodextrin to include the diclofenac sodium, such as inclusion method, the concentration of propyl-Beta-cyclodextrin, solution temperature in inclusion and inclusion time.
Owner:FUKANGREN BIO PHARMA
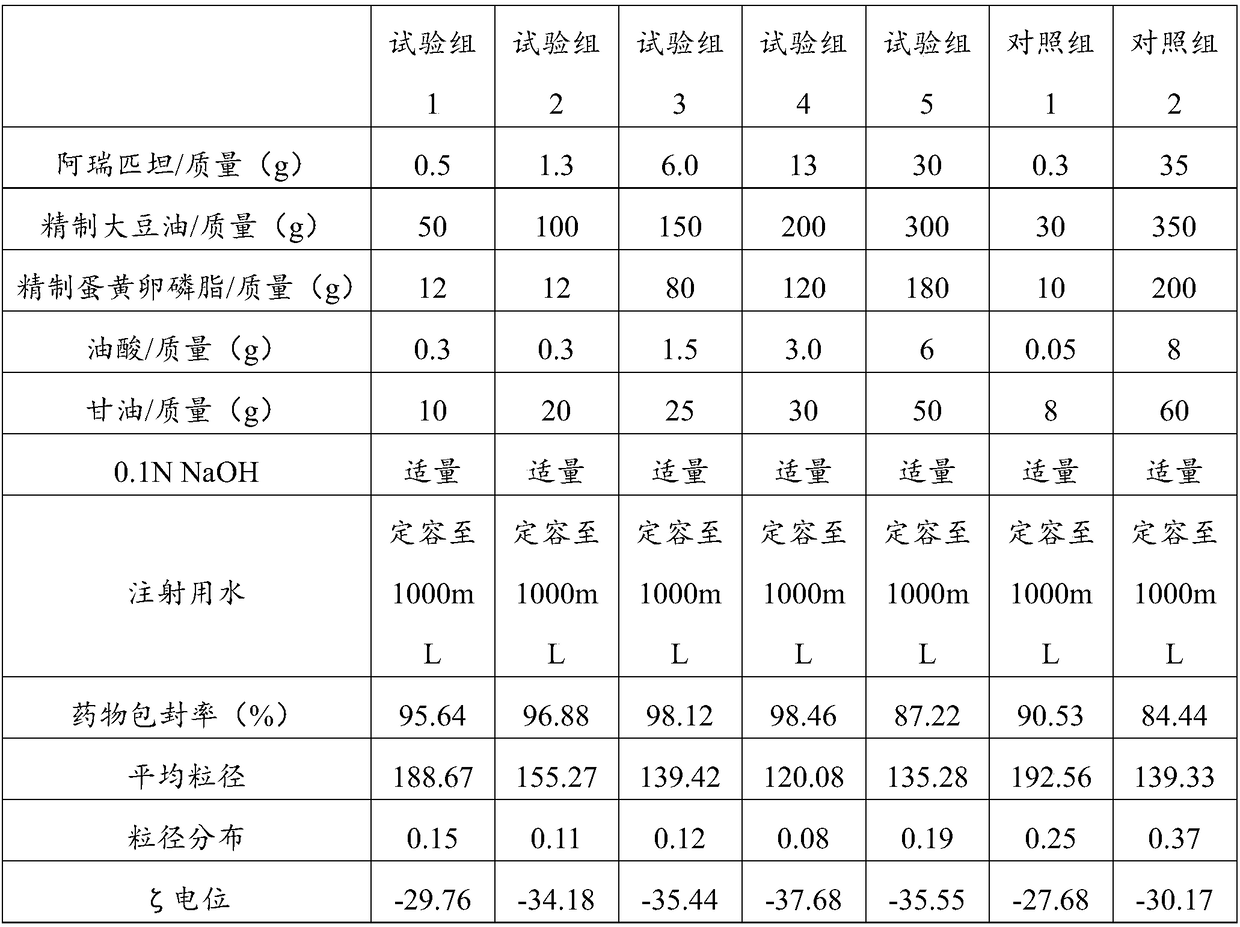
Aprepitant intravenous injection emulsion as well as preparation method and application thereof
PendingCN109364023AImprove liquidityNo hanging phenomenonOrganic active ingredientsDigestive systemHigh dosesMarketed products
The invention relates to aprepitant intravenous injection emulsion as well as a preparation method and an application thereof, and belongs to the technical field of pharmaceutics. The aprepitant intravenous injection emulsion is prepared from components in percentage by mass as follows: 0.05%-3% of aprepitant, 5%-30% of an oil phase solvent, 1.2%-18% of an emulsifier, 0.03%-0.6% of a stabilizer and 1%-5% of an isoosmotic adjusting agent, the injection emulsion further contains a pH regulating agent and the balance of water for injection, and pH value of the injection emulsion is 5.5-8.0. The aprepitant intravenous injection emulsion contains no low-carbon chain alcohol such as ethanol and the like, meanwhile, the quality requirement for a marketed product can be met by technological innovation and adjustment, and clinical use safety of the aprepitant intravenous injection emulsion is greatly improved; direct intravenous injection is utilized during clinical using, and dilution is not needed; the aprepitant intravenous injection emulsion can be used for treating acute and tardive nausea and vomiting caused by high-dose cis-platinum combined high-dose sensitizing cancer chemotherapy,and nausea and vomiting in early stage and middle stage of tumor chemotherapy are reduced.
Owner:GUANGZHOU HANFANG PHARMA
Immunoglobulin for dog intravenous injection, its preparing method and the formulation thereof
ActiveCN1654072ASimple processLow costMammal material medical ingredientsAntibody ingredientsDextrose salineBlood plasma
The present invention relates to immunoglobulin for intravenous injection of dog, and its preparation process and preparations. Through low temperature ethanol process or rivanol combined low temperature ethanol process, blood plasma or serum from health dog is separated to prepare immunoglobulin with pH 3.0-5.4 and purity over 95 % for intravenous injection of dog. The said immunoglobulin is further compounded into immunoglobulin injection with protein content of 1-15 % and containing one or two kinds of protecting agents of 5-15 % maltose, 5-10 % glucose and 3-10 % glucose salt solution.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
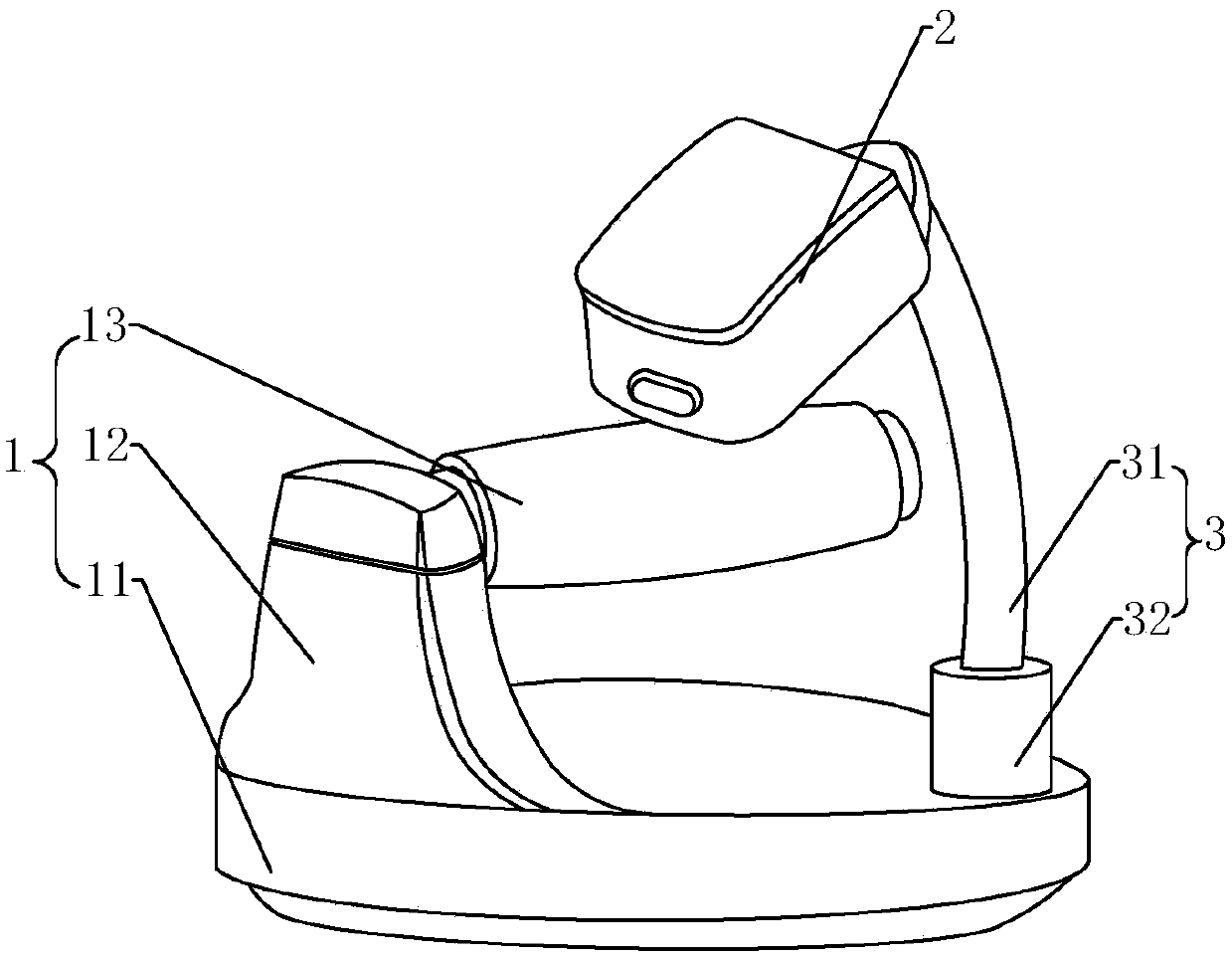
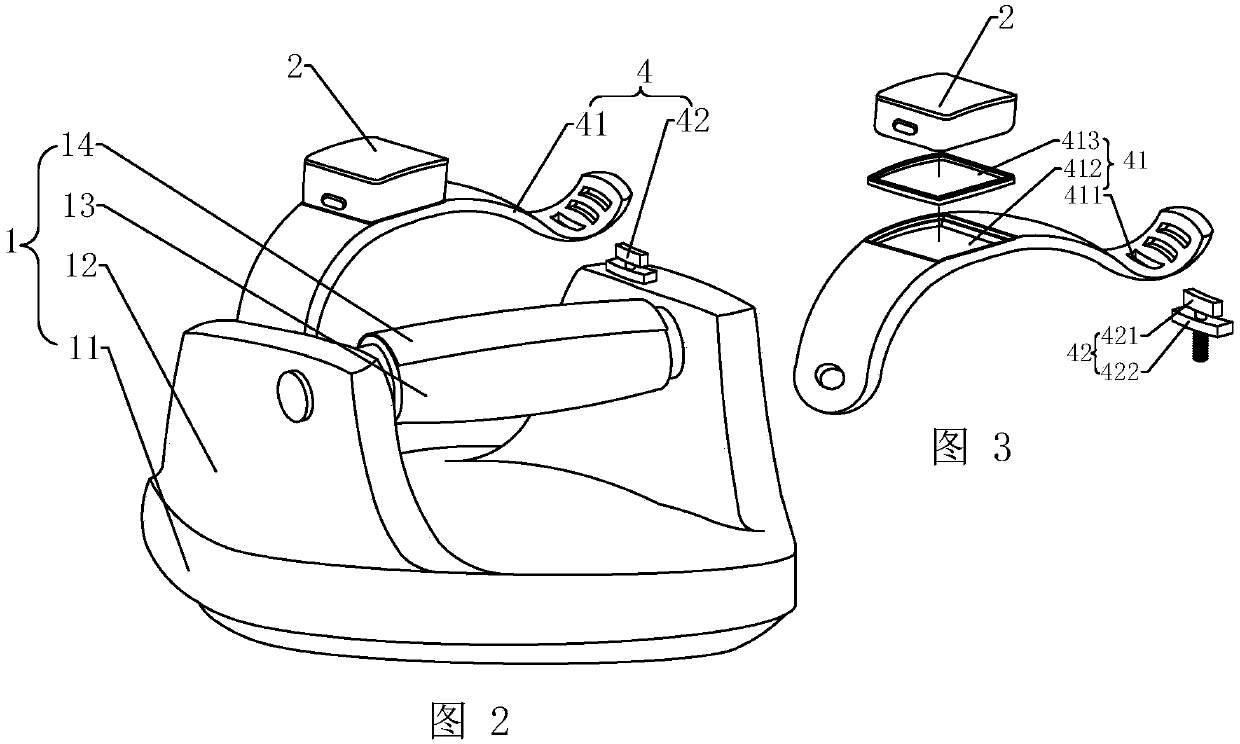
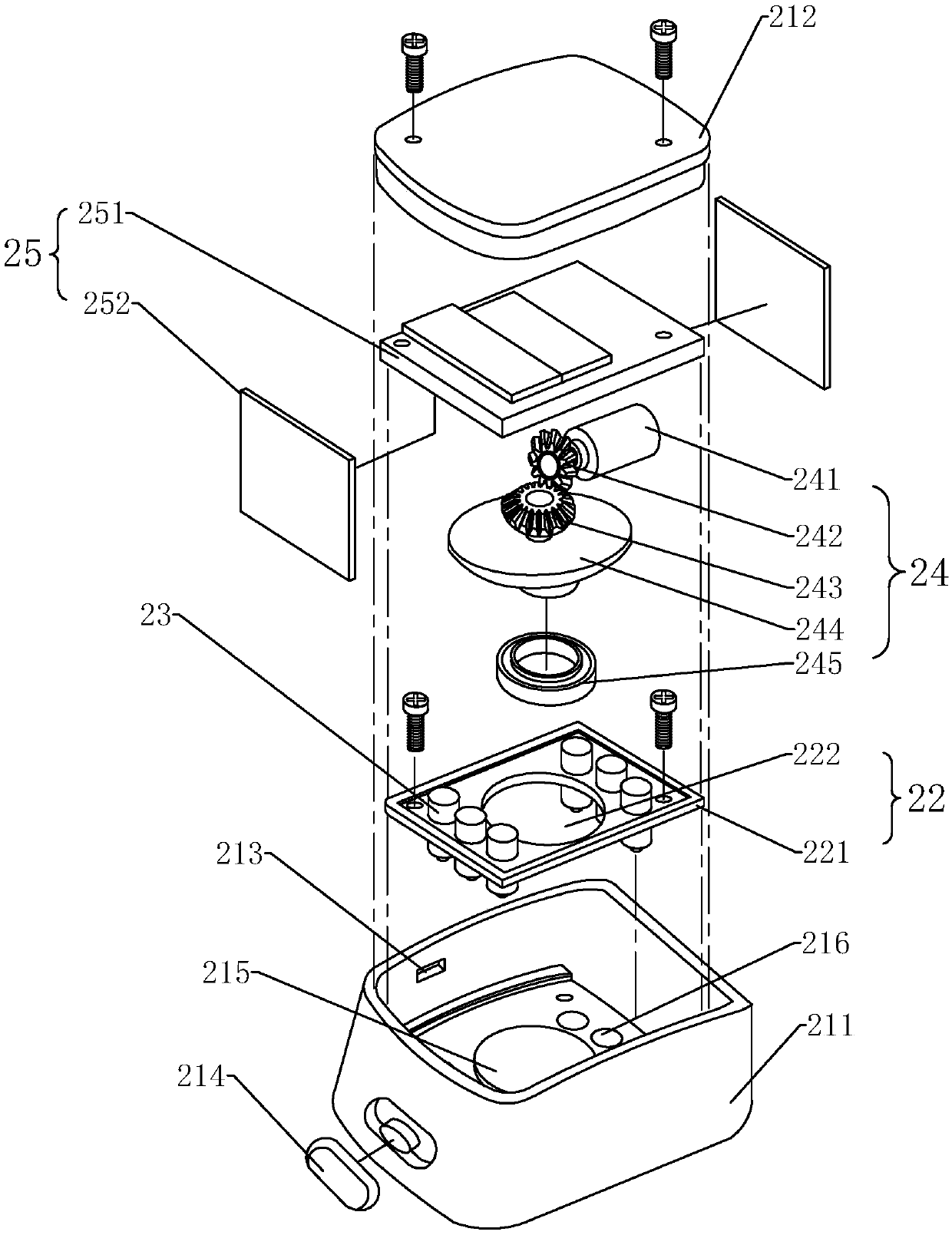
Medical intravenous injection auxiliary device
The invention provides a medical intravenous injection auxiliary device. The medical intravenous injection auxiliary device comprises a handle supporting device and a skin irritation device mounted onthe hand supporting device through a connecting part. The hand supporting device comprises a supporting seat. The supporting seat stretches upwards to form a supporting plate, and a hand supporting rod is horizontally mounted at the upper end of the supporting plate. The skin irritation device is in a cuboid shape and internally comprises multiple massage parts making contact with skin to generate irritation, and multiple massage holes allowing massage heads of the massage parts to stretch out are formed in the skin irritation device. The connecting part comprises a fixing pipe mounted on thesupporting seat, and an adjusting pipe with one end rotationally connected into the fixing pipe, or comprises an elastic connecting band and a lock knob matched with the connecting band. The skin irritation interference device at least comprises a body, a supporting body, a power part and the massage parts. The device makes contact with the skin at certain rhythm and actions to generate an irritation interference effect, the skin is in slight point contact intermittently, a patient is relaxed, the attention of the patient to needle inserting portions is dispersed, and therefore the pain senseis relieved.
Owner:李昊洋
Intravenous injection microemulsion preparation of teniposide
This invention relates to a formulation and preparation method of micro emulsion of Teniposide for intravenous injection. The micro emulsion comprises active components and auxiliary materials, wherein the active components are Teniposide or mixture of Teniposide, paclitaxel, and etoposide; and auxiliary materials is composed of vegetable oil, emulsifying agent or assistant emulsifier, and organic solvent. The micro emulsion for intravenous injection is obtained by preparing active components and auxiliary materials to pre-concentration liquid and adding to 5% glucose solution for clinical use. The invention has the advatages of convenient adminstration, high bioavailability, and reduced toxic anaphylaxis.
Owner:PEKING UNIV
Fatty emulsion injection of seal oil, method for preparation and the use in manufacturing intravenous injection
The present invention relates to a seal oil based lipid emulsion injection, and the main ingredients of which contains 190-210 g / l of refined OMEGA3 seal oil, 11-13 g / l of refined lecithin, 24-26 g / l of glycerol for injection, and balance amount of water. The preparation of the seal oil based lipid emulsion injection comprising the steps of stir and dispersion, high-pressure homogenization, vacuum filtration, antisepsis and encapsulation. The OMEGA3 seal oil lipid based emulsion injections have high content of energy, and thus are highly absorbable to human body, and can not only provide human body with caloricity, but also supply the fatty acids necessary for human body, and can enhance body's immunizing ability, reduce the content of cholesterol, adjust the blood concentration and then be used for anti-inflammation, in particular, it is highly useful for a postoperative patient to restore his strength.
Owner:LIU WEI +5
Intravenous baclofen formulations and treatment methods
ActiveUS20160213631A1Rapid attainmentAccurate and precise dose titrationOrganic active ingredientsNervous disorderIV injectionAnesthesia
An intravenous baclofen solution is disclosed, along with methods of dosing and treatment therewith.
Owner:RGT UNIV OF MINNESOTA +1
Lipid microballoon injection solution for progestational hormone drug and preparation method thereof
ActiveCN106074383ANon-irritatingImprove complianceOrganic active ingredientsEmulsion deliveryLipid formationIrritation
The invention discloses a lipid microballoon injection solution for a progestational hormone drug and a preparation method thereof. The lipid microballoon injection solution for the progestational hormone drug comprises progestational hormone drug progesterone as a primary drug and pharmaceutically acceptable drug excipient, wherein the drug excipient comprises oil phase, emulsifier, osmotic pressure adjusting agent, stabilizer, pH value modifier and injection water. The lipid microballoon injection solution for the progestational hormone drug is stable in quality, free from injection irritation, high in safety, applicable to local intramuscular injection and intravenous injection, and beneficial to promotion of compliance of clinical medication of patient and enriches clinical medication selection.
Owner:WUHAN CONFORM PHARMA CO LTD
Compound emulsion of docetaxel and volatil Chinese medicine oil for intravenous injection and its prepn process
InactiveCN1931158ASmall toxicityImprove anti-tumor effectOrganic active ingredientsEmulsion deliveryEmulsionDocetaxel
The present invention provides one kind of compound emulsion of docetaxel and volatile Chinese medicine oil for intravenous injection and its preparation process. Docetaxel is first dissolved in volatile Chinese medicine oil, and the obtained solution is then prepared into the compound emulsion together with emulsifier, osmotic regulator, tocophenol, pH regulator and water for injection. Compounding docetaxel with volatile Chinese medicine oil can reduce the untoward reaction, dissolve docetaxel, raise curative effect and raise the clinical medicine safety. The preparation process is simple and feasible and suitable for industrial production.
Owner:黄成安
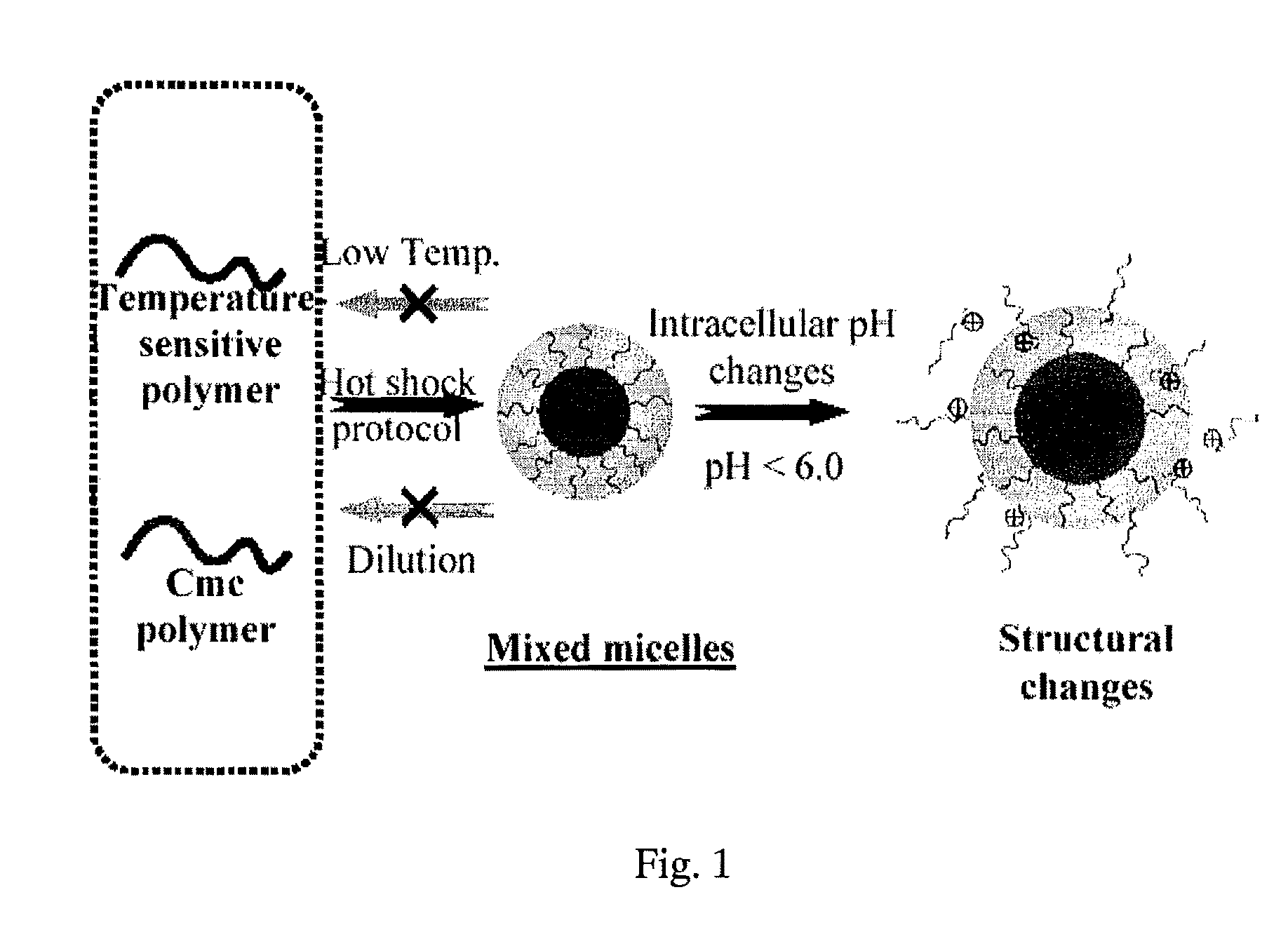
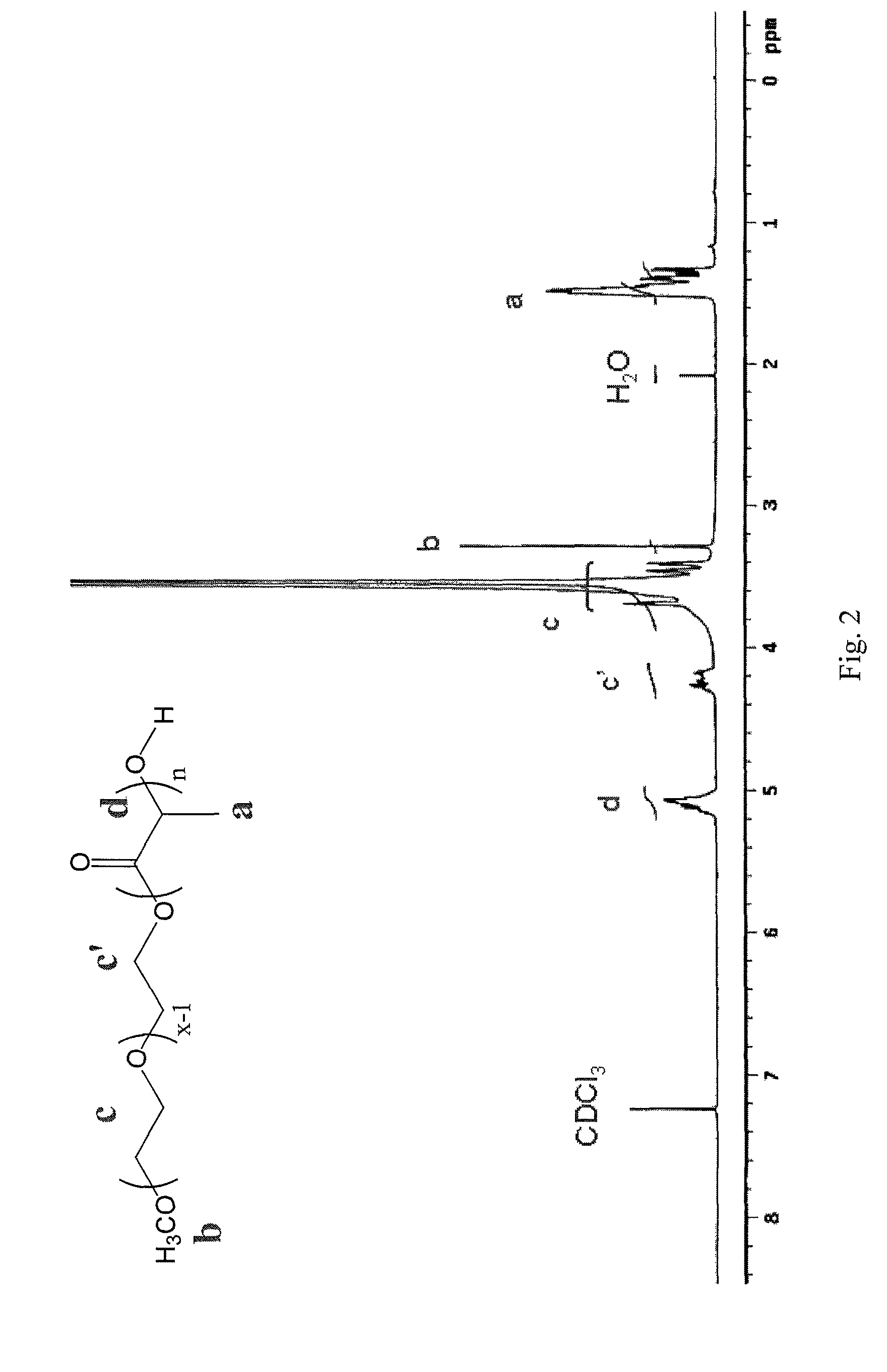
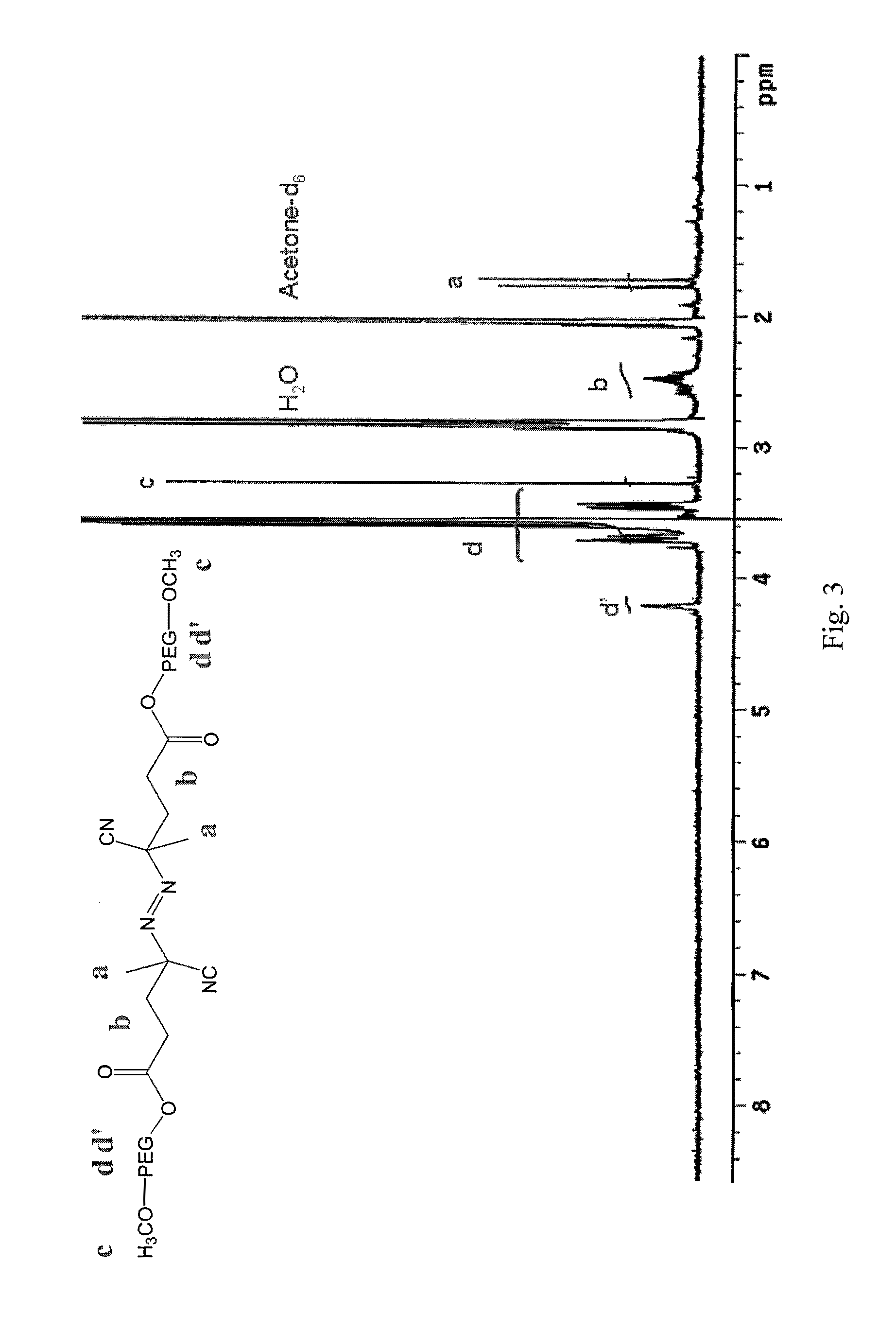
Stable micelles formed with diblock copolymers of critical micelle concentration copolymer and temperature-sensitive copolymer
InactiveUS8299178B2Small sizeImprove stabilityPowder deliveryBiocideCritical micelle concentrationMixed micelle
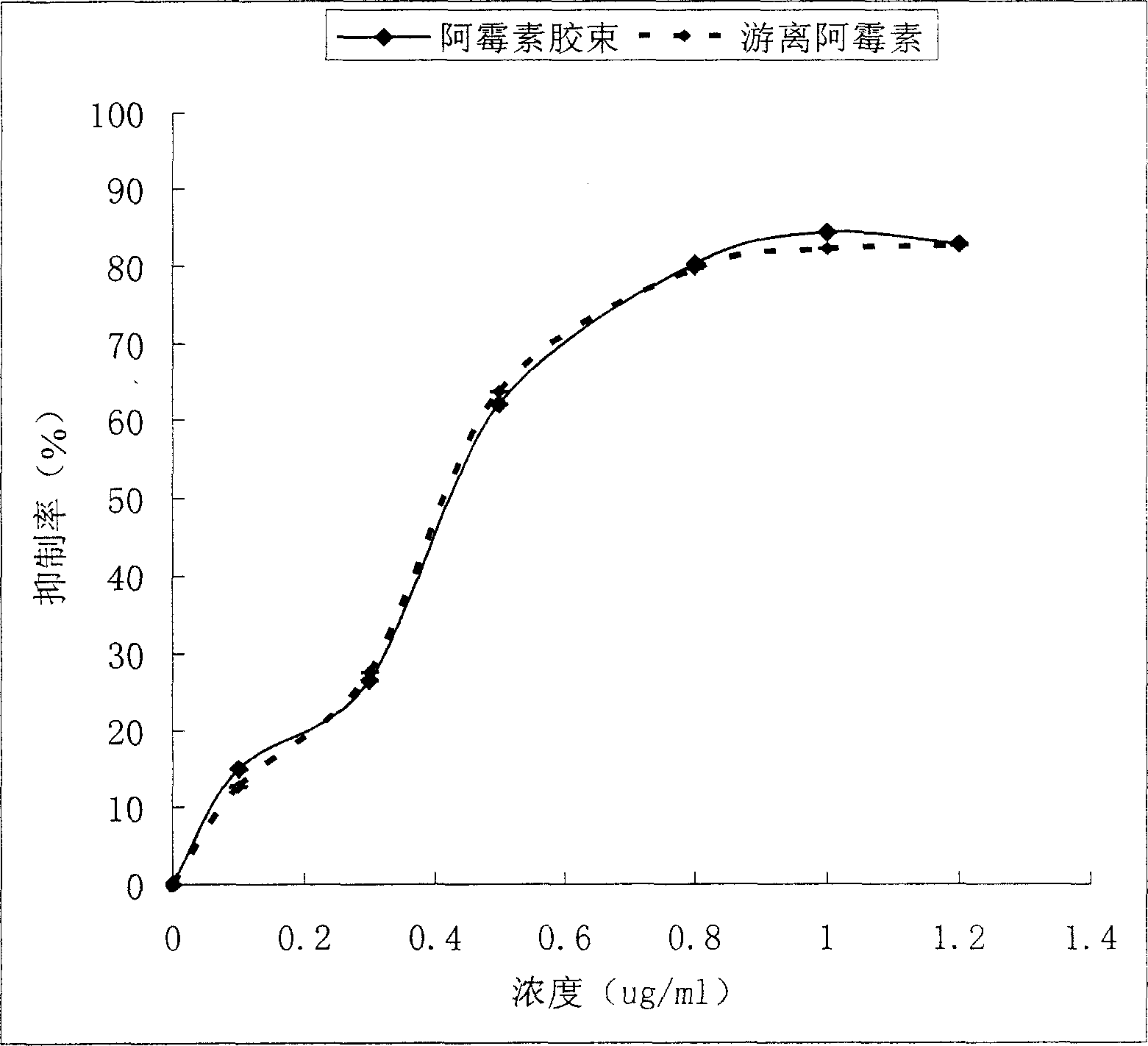
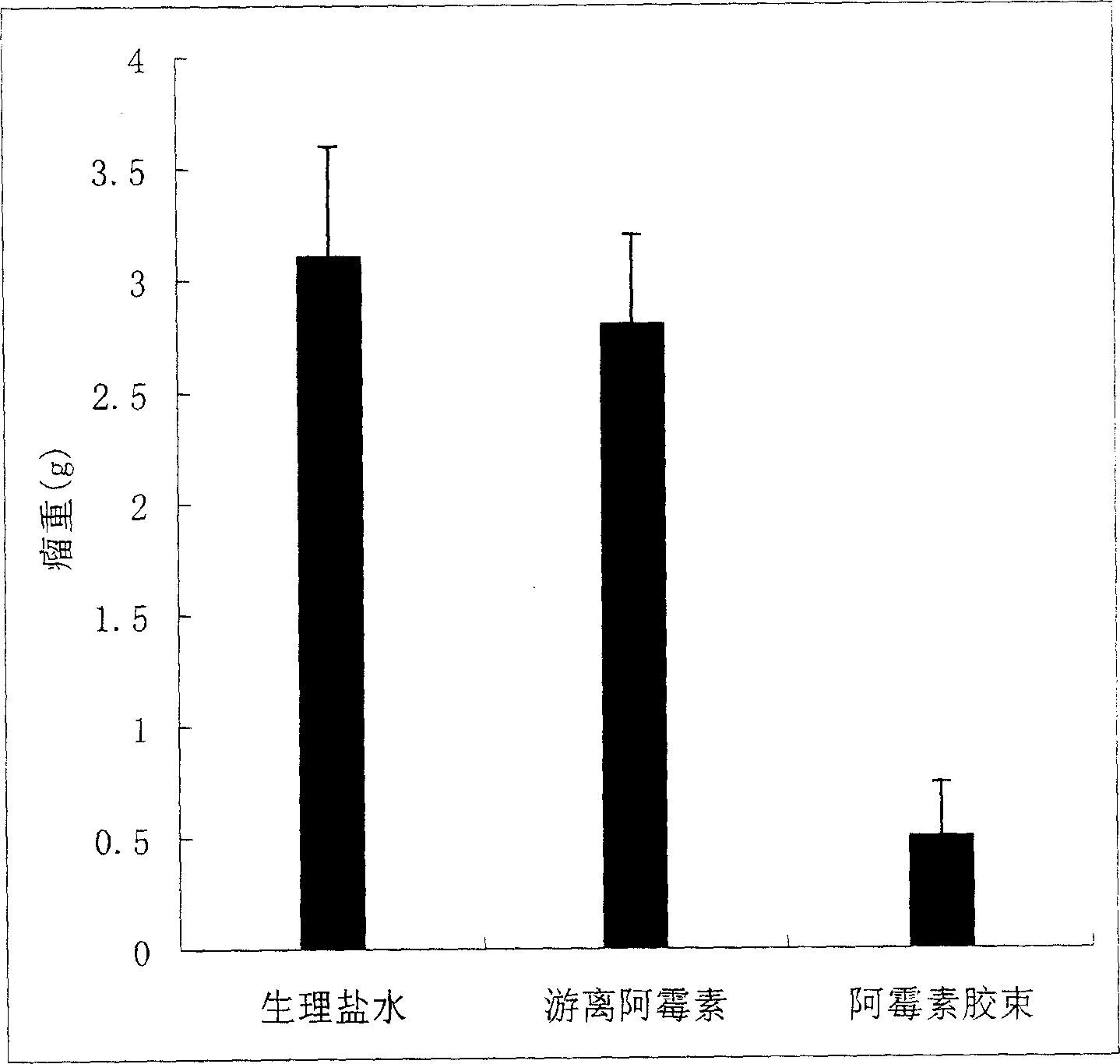
A novel class of mixed micelles formed with critical micelle concentration (Cmc) character's diblock copolymer, and temperature-sensitive character's diblock copolymer were disclosed. The mixed micelles possess complementary effects in adjusting external temperature shift (storage vs. body temperature) and concentration change (dilution after intravenous injection). The mixed micelles of the present invention can serve as a potential injectable drug delivery system for anticancer drugs, such as doxorubicin and many others.
Owner:NATIONAL TSING HUA UNIVERSITY
Vitexin injection and oral administration preparation thereof
InactiveCN1919206AQuality is easy to controlQuality improvementOrganic active ingredientsMetabolism disorderTreatment effectCoronary heart disease
The invention relates to a Vitexin injection or oral administration preparation for treating cardiovascular diseases and process for preparation, wherein each 1000ml of the Vitexin injection comprises Vitexin 0.1-100g, each 1000 dosage units of the oral administration preparation comprises Vitexin 0.1-100g, and medicinal supplementary materials comprises diluent, flow aid, auxiliary solvent, excipient and osmoregulation agent.
Owner:合肥七星医药科技有限公司
Tripterine nanosuspension and preparation method thereof
ActiveCN106309364AEasy to achieve passive targetingSimple prescriptionPowder deliveryOrganic active ingredientsIn vivoSolvent
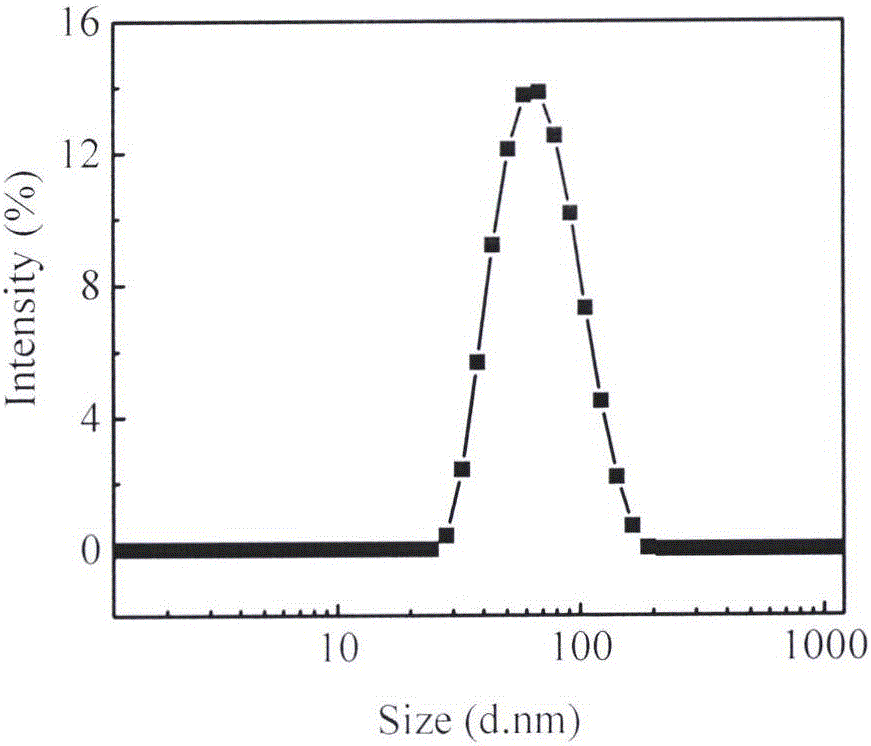
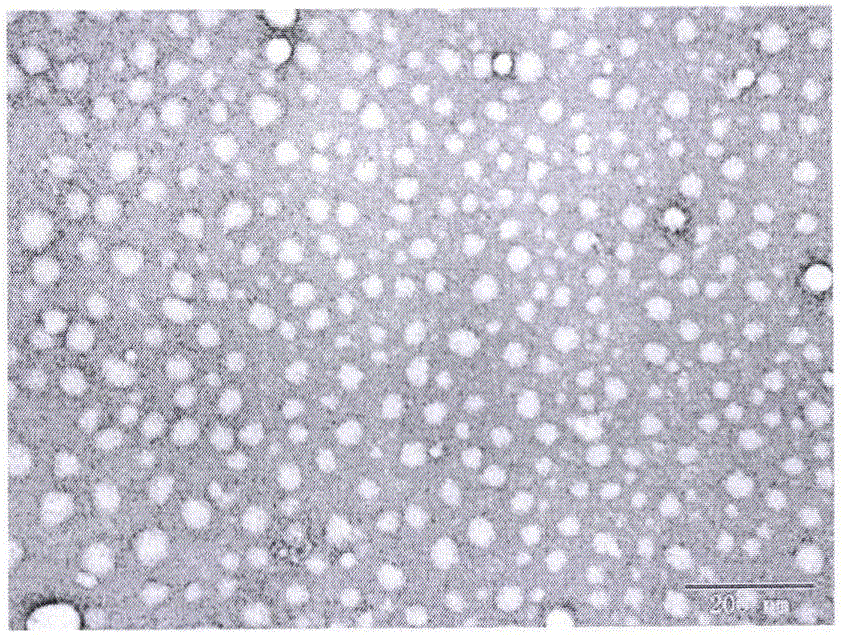
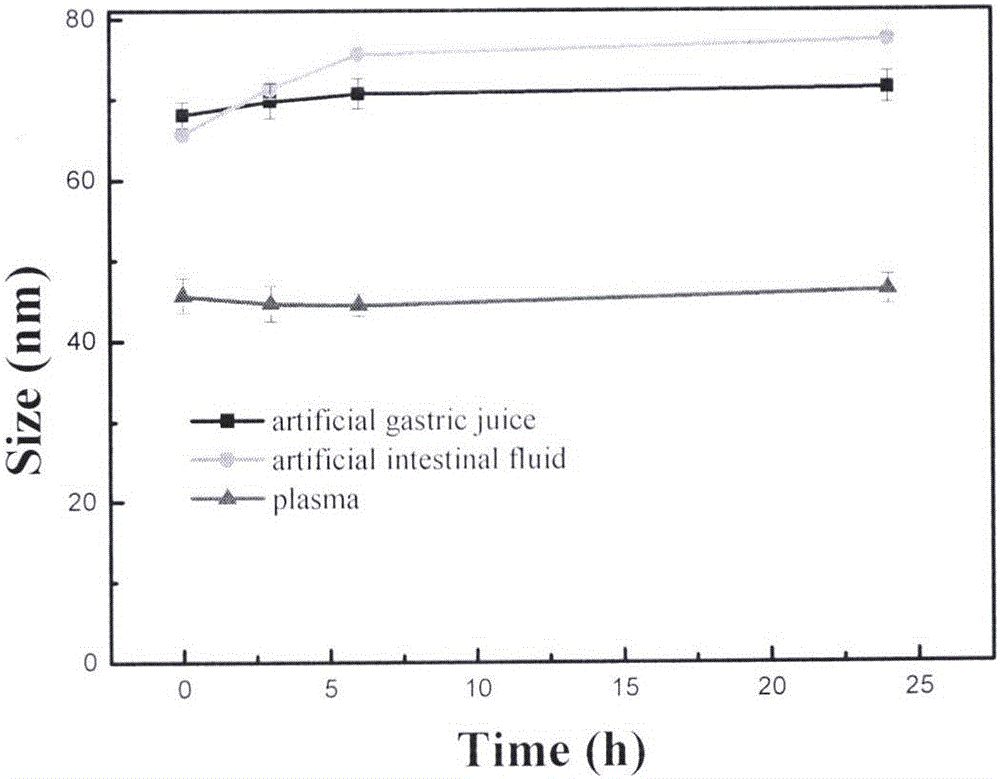
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of tripterine nanosuspension and application thereof, which are used for preparation of amphipathic stabilizers including mPEG-PCL, mPEG-DSPE, Bianze78, SPC, Tween80, BSA, TPGS and the like. The tripterine nanosuspension is prepared by adopting a solvent precipitation-ultrasonic injection method, and the formulation composition is as follows: the combination ratio of tripterine to the stabilizer is 1:(0.1-1) (weight ratio); the maximal drug loading capacity can reach 85%, the minimum grain size can reach 67.1nm, the polydispersity is good; the nanosuspension is steady in both gastrointestinal fluid and plasma, can be used by oral administration and intravenous injection; the nanosuspension has a good sustained-release effect in vitro, and burst release cannot happen; the nanosuspension is remarkable in tumor cell inhibition ratio in vitro in comparison with a raw medicine liquor; and in vivo pharmacological experiment also shows excellent anti-tumor efficacy, thus having wide application and development prospect.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Prulifloxacin water-soluble salt and its injection formulation
InactiveCN1557314AGood water solubilityRapid therapeutic effectAntibacterial agentsOrganic active ingredientsWater solubleIV injection
The present invention aims at providing one kind of water soluble Prulifloxacin salt for muscular injection or intravenous injection and its proper injection form. The water solution of these soluble salts is easy to release Prulifloxacin salt, and the injection of Prulifloxacin salt has the same antiseptic effect as orally taken Prulifloxacin preparation. In addition, the injected medicine enters blood directly to produce treating effect and thus has faster effect and less medicine consumption compared with available orally taken form.
Owner:魏雪纹 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com