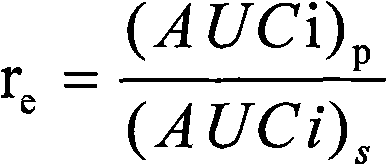2-methoxyestradiol nanosuspension frozen powder and preparation method thereof
A technology of methoxyestradiol and nanosuspension, applied in the field of medicine, can solve the problems of difficult to achieve high drug loading and targeting effect, low oral bioavailability, poor water solubility, etc., and achieve good long-term stability , Conducive to long-term storage and transportation, the effect of high drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 600mg of poloxamer 188, add it to 20ml of phosphate buffered saline (PBS buffer), heat at a slight temperature (30-40°C) to dissolve it completely, add micronized 2-methoxyestradiol under magnetic stirring Diol raw material 150mg, 10000rpm internal homogenizer pre-emulsification for 5min to obtain the primary suspension; the primary suspension was subjected to high-pressure emulsification, the conditions were 9k psi cycle 3 times, 12k psi cycle 3 times, 18k psi Cycle 20 times to obtain a milky white nanosuspension, dissolve 1000mg trehalose in the nanosuspension, sterilize with Co-60, sub-package in vials, pre-freeze at -80°C for 10 hours, and then freeze-dry for 12 hours to obtain freeze-dried powder. The lyophilized powder was reconstituted with water for injection, the average particle size was 320nm, and the Zeta potential was -25.68mV.
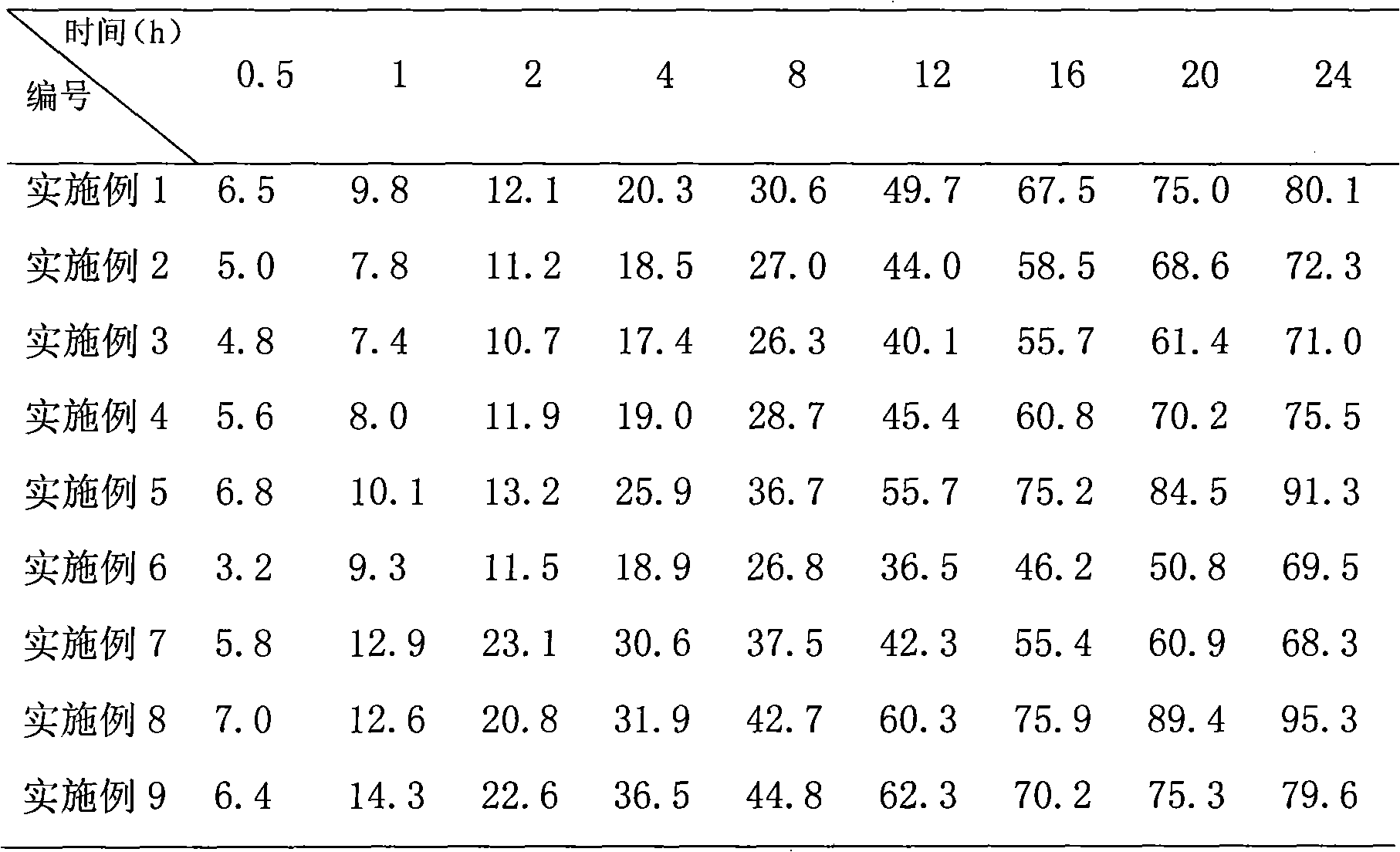
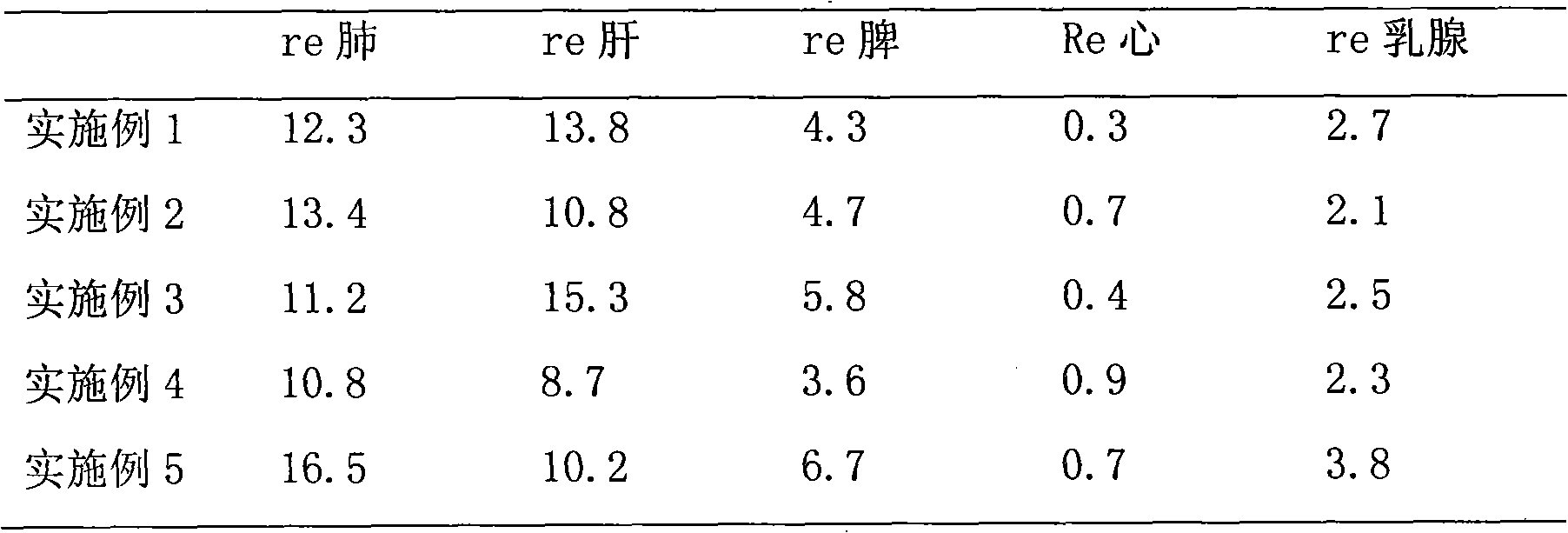
[0031] The in vitro release results of the product of this example are shown in Table 1, and the results show that the pre...
Embodiment 2
[0033] Weigh 600mg of Poloxamer 188 and 300mg of Tween80, add it into 20ml of phosphate buffered saline (PBS buffer), heat at a slight temperature (30-40°C) to dissolve it completely, add micronized 2-formazol under magnetic stirring Oxyestradiol bulk drug 150mg, 10000rpm in-cut homogenizer pre-emulsification for 5min to obtain the primary suspension; the primary suspension was subjected to high-pressure emulsification, the conditions were 9k psi cycle 3 times, 12k psi cycle 3 times , 18k psi cycled 20 times to obtain a milky white nanosuspension. Dissolve 1000mg of mannitol in the nanosuspension, sterilize it with Co-60, divide it into vials, pre-freeze at -20°C for 10 hours, then freeze-dry it for 12 hours and take it out , to obtain freeze-dried powder. The lyophilized powder was reconstituted with water for injection, the average particle size was 290nm, and the zeta potential was -33.8mV.
[0034] The in vitro release results of the product of this example are shown in T...
Embodiment 3
[0036]Weigh 300mg of poloxamer 407 and add it to 20ml of phosphate buffered saline (PBS buffer), heat at a slight temperature (30-40°C) to dissolve completely, weigh 200mg of 2-methoxyestradiol raw material, soybean egg Add 900 mg of phospholipids into 8 ml of dichloromethane, heat slightly to dissolve completely, add the oil phase dropwise to the water phase under magnetic stirring, pre-emulsify with a 10,000 rpm internal cutting homogenizer for 5 min, and pour it into the rotary film evaporator. In the round bottom flask, keep the temperature of the water bath at 40 ° C, start the vacuum pump, vacuum rotary evaporation for 4 hours, form the primary suspension, and carry out high-pressure emulsification of the primary suspension under the conditions of 9k psi cycle 3 times, 12k psi cycle 3 times, 18k psi cycled 20 times to obtain a milky white nanosuspension. Dissolve 300mg trehalose and 700mg mannitol in the nanosuspension, sterilize it with Co-60, use vials for aliquot packa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com