Aprepitant intravenous injection emulsion as well as preparation method and application thereof
A technology of aprepitant and water for injection, applied in the field of pharmacy, can solve problems such as injection site pain and neuritis, achieve good fluidity, good stability, and improve the safety of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
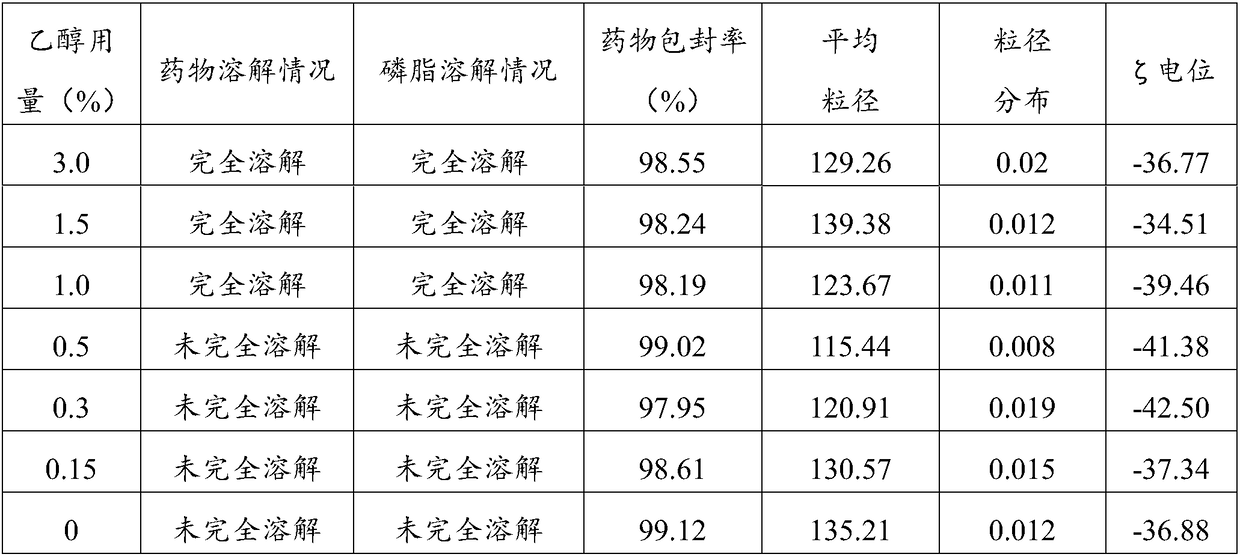
[0041] In the aprepitant intravenous emulsion of this embodiment, every 1000 mL of the emulsion contains: 1.3 g of aprepitant, 100.0 g of refined soybean oil, 12 g of refined egg yolk lecithin, 2.5 g of oleic acid, an appropriate amount of 0.1N NaOH, and 25 g of glycerin , dilute to 1000mL with water for injection. Wherein, the proportion of refined soybean oil as the oil phase is 10%.
[0042] The preparation method of the present embodiment aprepitant intravenous emulsion is:
[0043] (1) Weigh the prescribed amount of refined soybean oil, refined egg yolk lecithin and oleic acid, stir to dissolve the emulsifier, add the prescribed amount of aprepitant, and heat in a water bath to 70°C as the drug-loaded oil phase for use;
[0044] (2) Weigh the prescribed amount of glycerin and add it to 800mL water for injection, and heat it in a water bath to 70°C as the water phase;
[0045] (3) Slowly add the oil phase to the water phase, keep the temperature at 70°C, shear at 8,000 r...
Embodiment 2
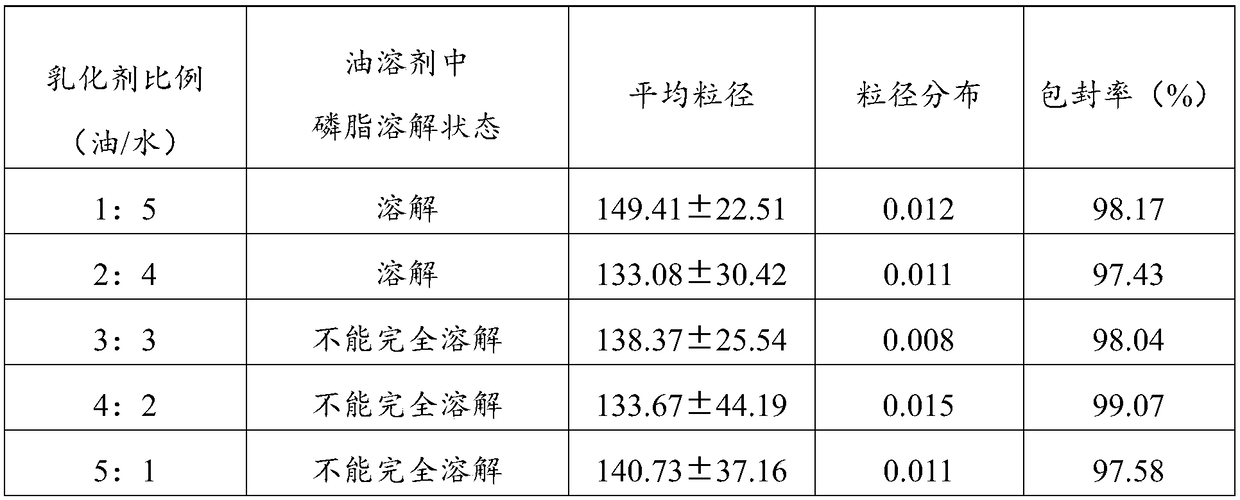
[0050] In the aprepitant intravenous emulsion of this embodiment, every 1000mL of the emulsion contains: 13g of aprepitant, 150g of refined olive oil, 50g of refined egg yolk lecithin, 2.5g of oleic acid, appropriate amount of 0.1N NaOH, 25g of glycerin, injection Dilute to 1000mL with water. Wherein, the proportion of refined olive oil as the oil phase is 15%.
[0051] The preparation method of the present embodiment aprepitant intravenous emulsion is:
[0052] (1) Weigh the prescribed amount of refined olive oil, refined egg yolk lecithin and oleic acid, stir to dissolve the emulsifier, add the prescribed amount of aprepitant, and heat to 75°C in a water bath as the drug-loaded oil phase;
[0053] (2) Weigh the prescribed amount of glycerin and add it to 700mL water for injection, and heat it in a water bath to 75°C as the water phase;
[0054] (3) Slowly add the oil phase to the water phase, maintain the temperature at 75°C, shear at 15,000 rpm for 5 minutes at high speed...
Embodiment 3
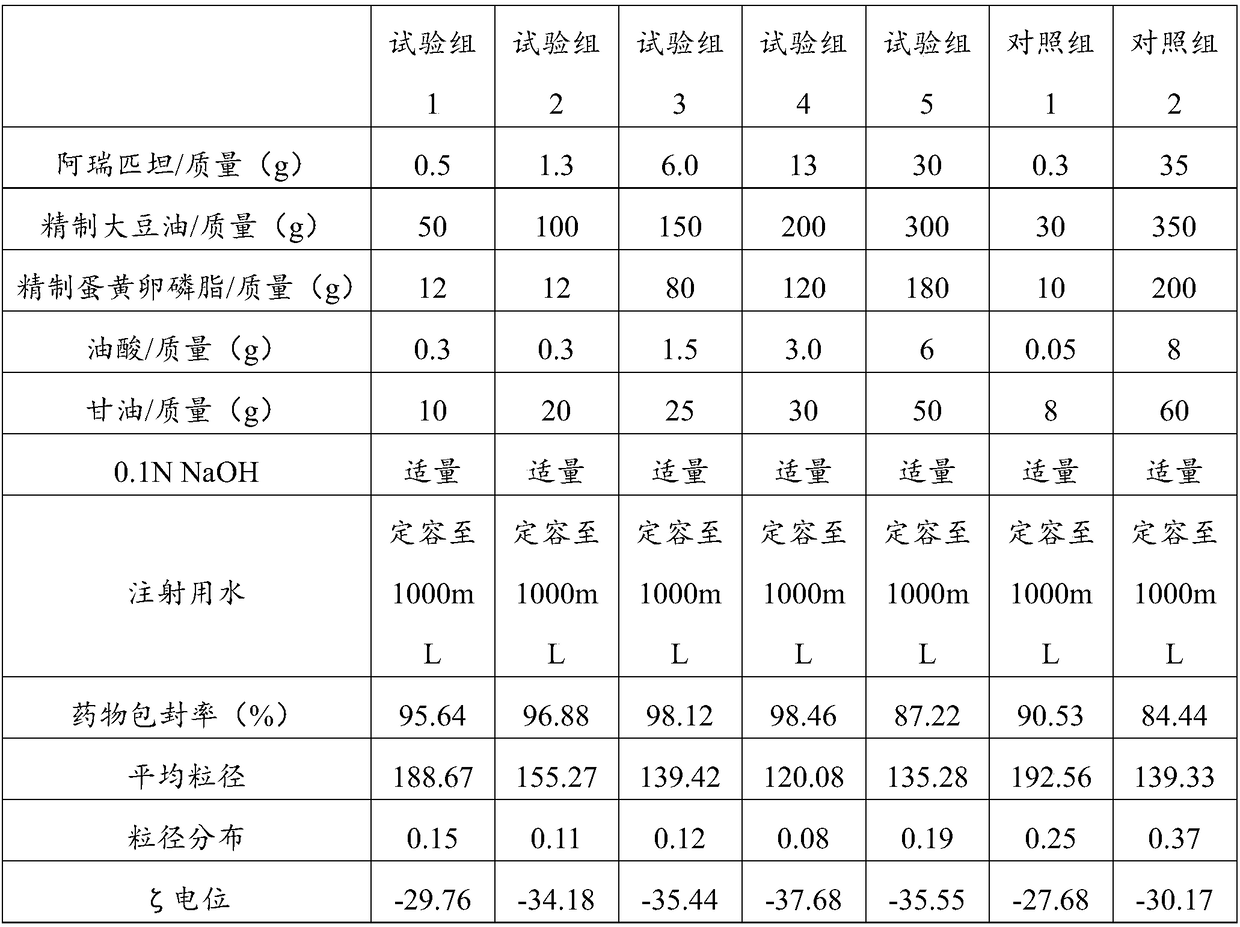
[0059] In the aprepitant intravenous injection emulsion of this embodiment, every 1000mL emulsion contains: 20g of aprepitant, 100g of refined soybean oil, 100g of refined olive oil, 100g of egg yolk phosphatidylcholine, 5g of sodium oleate, 0.1N NaOH Appropriate amount, glycerin 25g, water for injection and dilute to 1000mL. Wherein, the proportion of refined soybean oil and refined olive oil as the oil phase is 20%.
[0060] The preparation method of the present embodiment aprepitant intravenous emulsion is:
[0061] (1) Weigh the prescribed amount of refined olive oil, refined soybean oil, and egg yolk phosphatidylcholine, stir to dissolve the emulsifier, add the prescribed amount of aprepitant, and heat in a water bath to 75°C as the drug-loaded oil phase;
[0062] (2) Weigh the prescribed amount of glycerin and sodium oleate, add it to 800mL water for injection, and heat it in a water bath to 75°C as the water phase;
[0063] (3) Slowly add the oil phase to the water ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Zeta potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com