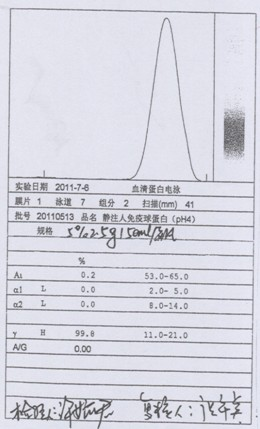
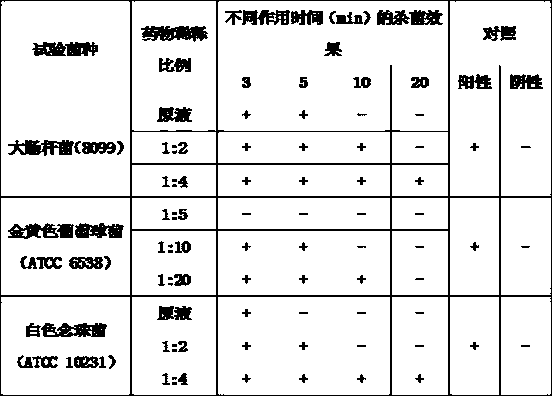
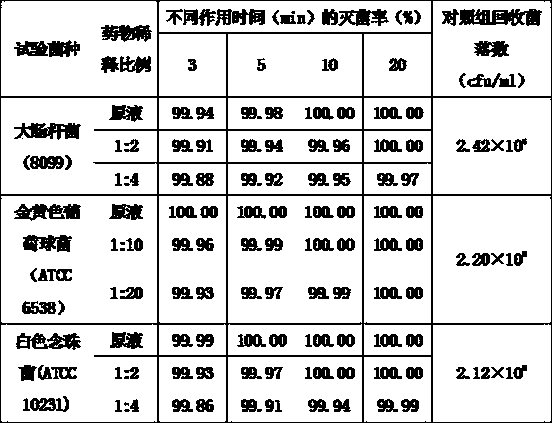
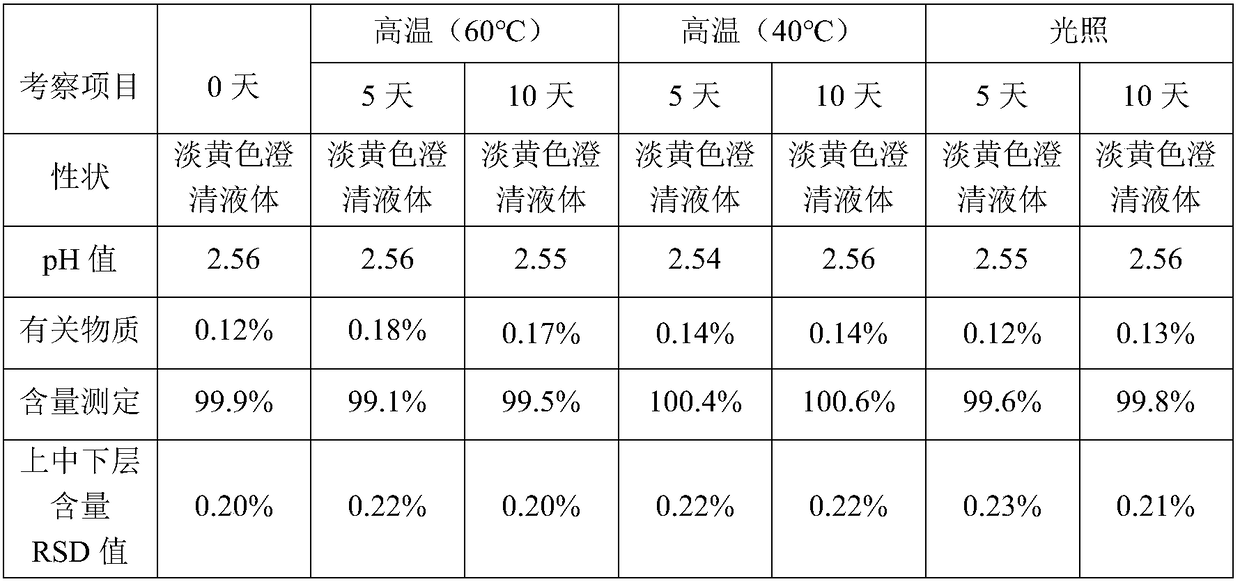
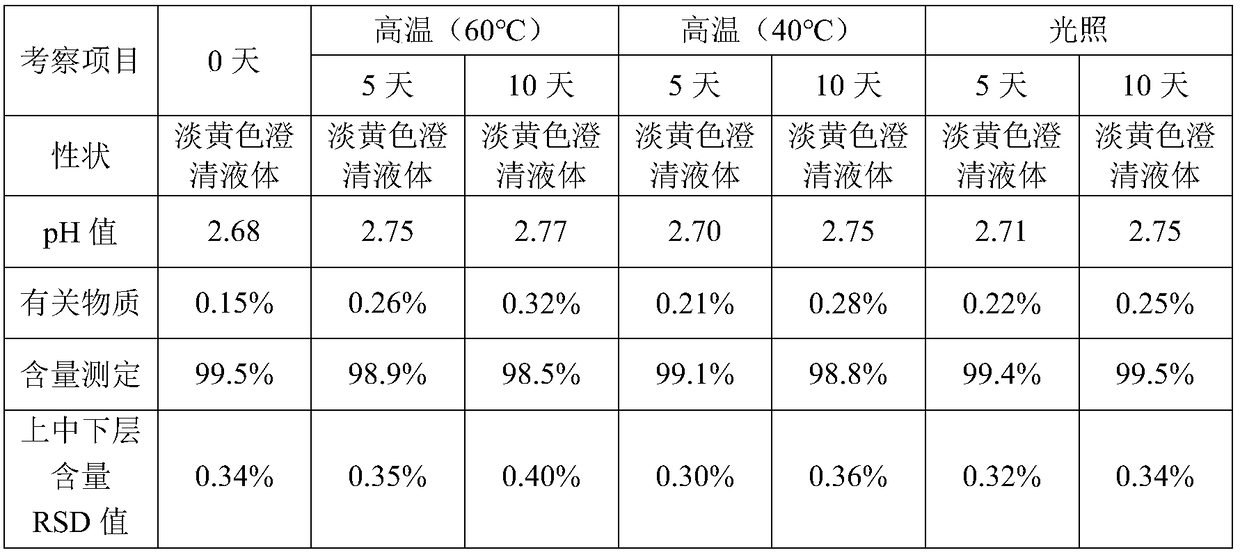
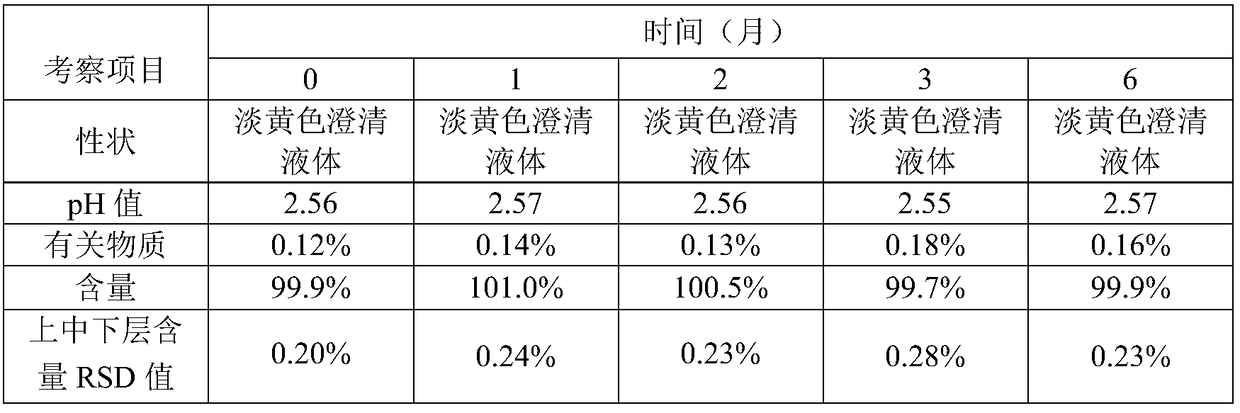
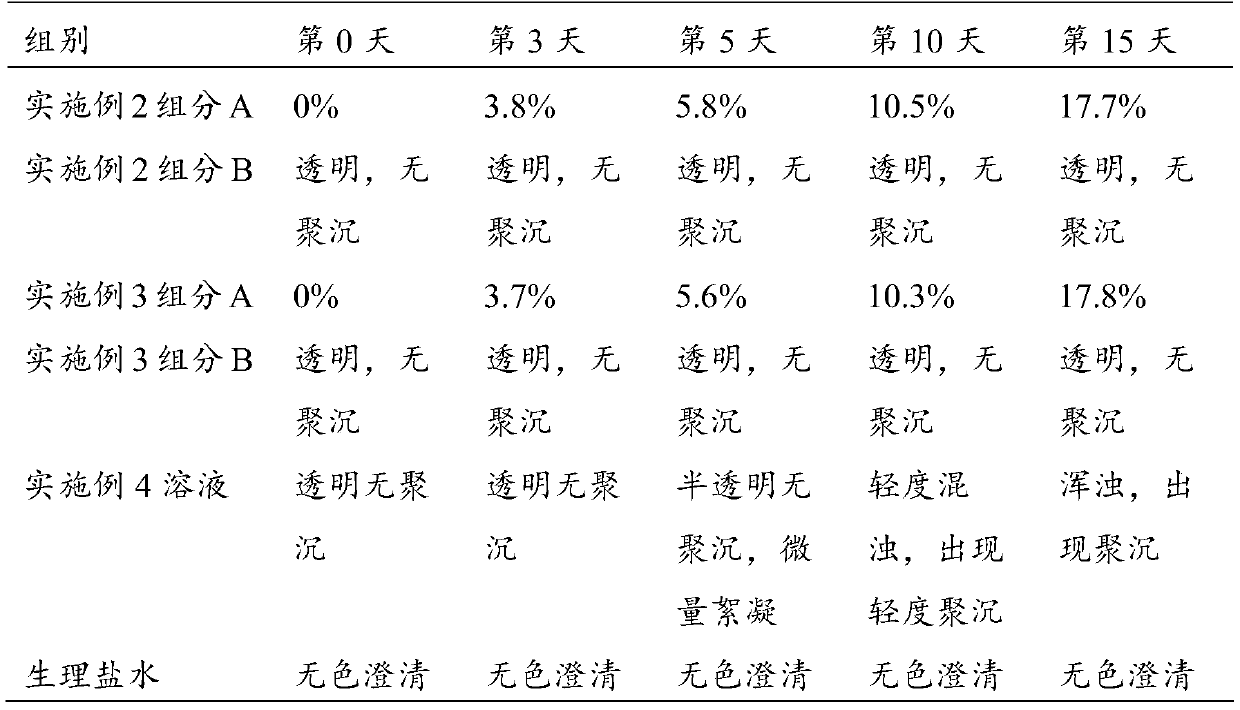
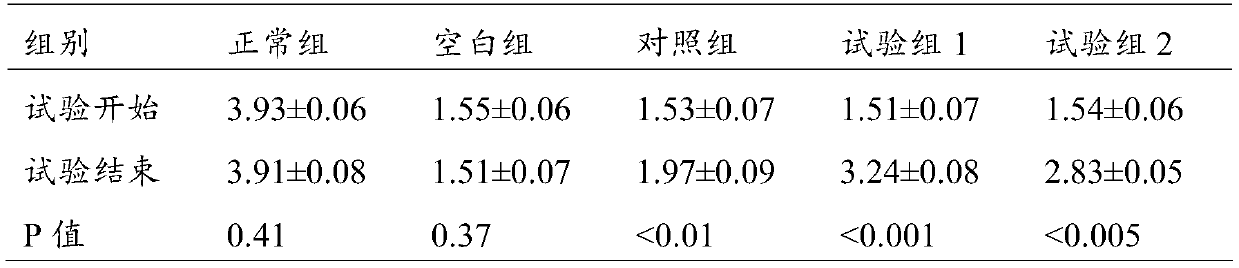
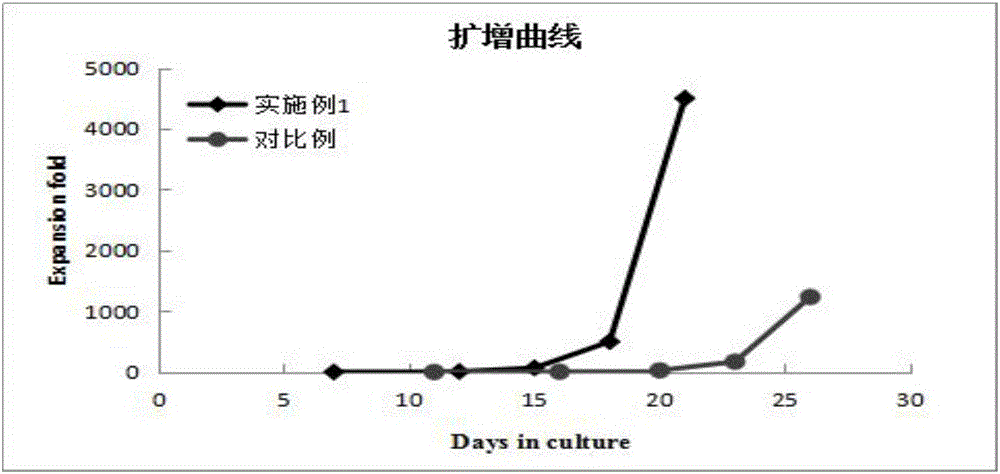
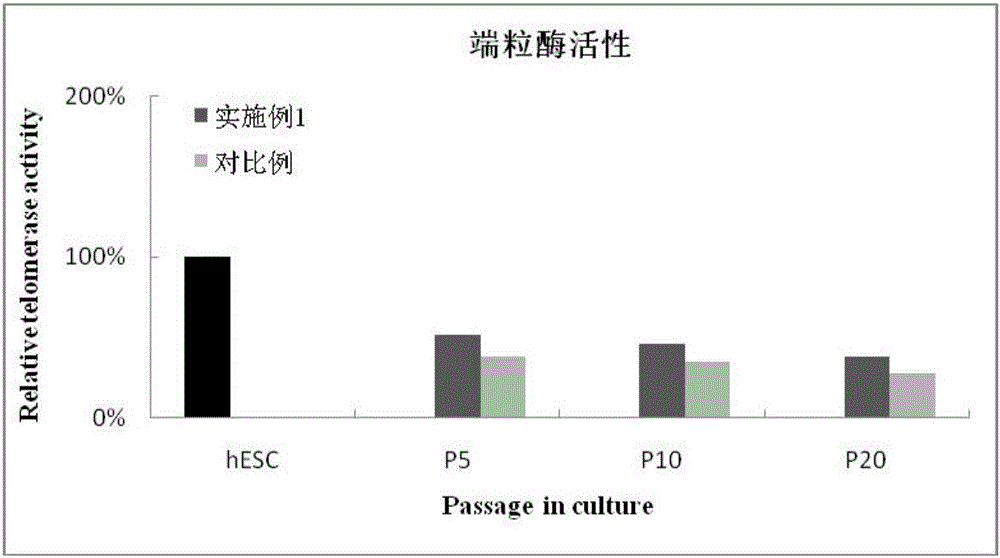
Patents
Literature
230results about How to "High clinical safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of gel containing stem cell exosomes for repairing skin wounds
InactiveCN111420117AImprove application securityHigh clinical safetyCulture processSkeletal/connective tissue cellsMesenchymal stem cellEngineering
The invention relates to a preparation method of a gel containing stem cell exosomes for repairing skin wounds. The method comprises the following steps: 1) primary extraction and culture of human umbilical cord mesenchymal stem cells: 1.1) primary extraction of human umbilical cord mesenchymal stem cells, 1.2) subculture, and 1.3) collection of the culture supernatant; 2) extraction of human umbilical cord mesenchymal stem cell exosomes: 2.1) primary centrifugation, 2.2) secondary centrifugation, 2.3) removal of organelles by centrifugation, 2.4) coarse extraction of exosomes, and 2.5) finalextraction of exosomes; 3) preparation of a gel material: 3.1) preparation of chitosan, 3.2) configuration of beta-glycerol phosphate (beta-GP), and 3.3) preparation of the gel material; and 4) gel loading of the exosomes. The gel containing stem cell exosomes can promote repair of skin wounds, shorten the healing time of wounds and reduce scar formation.
Owner:陕西朗泰生物科技有限公司
Process for preparing human immunoglobulin for intravenous injection
ActiveCN102584934AReduce adverse effectsImprove biological activityPeptide preparation methodsIon exchangeTwo step
The invention relates to a process for preparing human immunoglobulin for intravenous injection, and belongs to the field of biological pharmacy. The precipitate of components II and III in the process for preparing the human immunoglobulin for intravenous injection is treated by a caprylic acid and calcium chloride precipitation method, miscellaneous proteins are removed from the precipitate of the components II and III, and two-step chromatography is performed. Compared with the general caprylic acid precipitation method, the process has the advantages that various blood coagulation factors are effectively removed from a precipitate solution of the components II and III by adding calcium chloride, the stability of the immunoglobulin is improved, and the yield of the product is obviously improved, namely more than 7g of immunoglobulin can be prepared from each liter of blood plasma; upper column chromatographic purification is performed by two ion exchange columns and a chromatographic technology, so that the miscellaneous proteins can be effectively removed, and the purity of the product is over 99.5 percent; and in addition, caprylic acid is added, and viruses are removed and filtered by a DV20 filter element, so that a virus removing effect can be obviously improved, and the safety of clinical medication is improved.
Owner:华润博雅生物制药集团股份有限公司
Process for preparing human serum albumin
ActiveCN103394076ANon-deterministicHigh yieldPeptide/protein ingredientsSerum albuminUltrafiltrationFiltration
The invention discloses a process for preparing human serum albumin. According to the process, a low-temperature ethanol separation method is adopted, and the human serum albumin is prepared from human plasma. The process comprises the steps of dissolving plasma; preparing an ingredient I; preparing an ingredient II and an ingredient III; preparing an ingredient IV; preparing an ingredient V; refining the ingredient V; carrying out ultrafiltration; diluting; carrying out pasteurization; sterilizing and packaging albumen; incubating products; and packaging finished products. The process has the advantages that solid-liquid separation is carried out by adopting a pressure filtration technology, so that the albumin yield which is higher than 29 g / L plasma is increased remarkably, the purity is higher than 98%, and the stability of the products is improved remarkably; Zetaplus deep filter-core filtration is combined with the prolongation of pasteurization time, so that the PKA (Protein Kinase A) level of the products is effectively controlled to be lower than 20IU / ml, and the risks of excessive heat source and virus infection in the products are reduced; and during the process, sodium chloride solutions of two gradient concentrations are used for carrying out ultrafiltration, so that not only can the ethanol residual quantity of the products be controlled to be lower than 0.025%, but also the aluminum residual quantity can be effectively minimized to be lower than 50 micrograms / L.
Owner:华润博雅生物制药集团股份有限公司
Kukoline intravenous transfusion preparation
ActiveCN101347408AOvercome the bias that IV route of administration is not possibleSolve the clinical problems prone to anaphylactic shockOrganic active ingredientsAntipyreticClinical efficacyRheumatism
The invention provides a sinomenine injection special for intravenous administration. The sinomenine injection is an injection which comprises 0.005-0.3wt% of sinomenine and an aqueous solvent for injection, or an injection which comprises sterile injection powder or lyophilized injection powder used for preparation just before injection to cause the sinomenine concentration to be 0.005-0.3wt% in the injection and the aqueous solvent for injection. The sinomenine injection of the invention is used for treating rheumatism, chronic pain and other chronic inflammatory diseases. Compared with other existing injection forms, the sinomenine injection special for intravenous injection has lower drug adverse reaction, and the clinical curative effect is obviously improved.
Owner:李蕴麟
Human adipose tissue-derived stromal cell frozen stock solution
InactiveCN104472474AGood stem cell propertiesAvoid pollutionDead animal preservationMotilityStromal cell
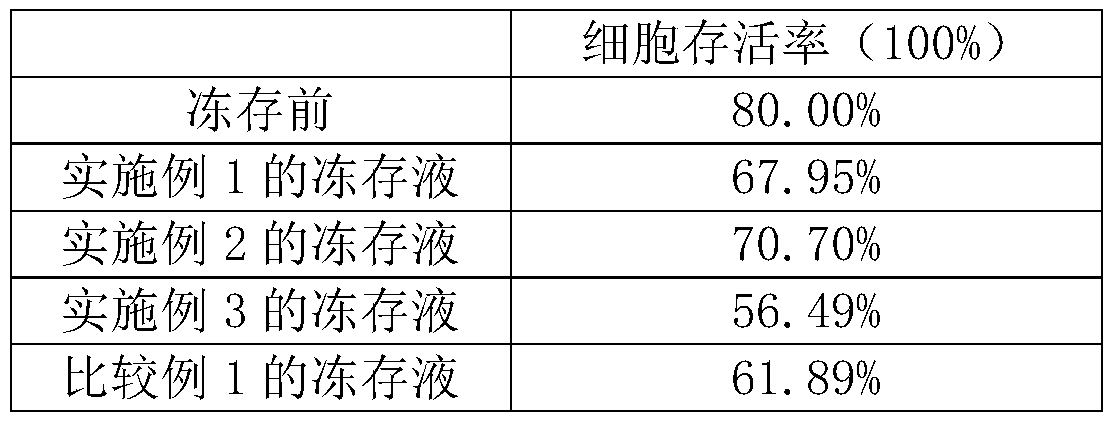
The invention relates to the field of stem cells, discloses a frozen stock solution of stem cells, and in particular discloses a human adipose tissue-derived stromal cell frozen stock solution which consists of human plasma and dimaethyl sulfoxide. Compared with an ordinary cell frozen stock solution, the human adipose tissue-derived stromal cell frozen stock solution disclosed by the invention is relatively high in clinical security as no non-human source serum or protein is provided and the risk that contamination or allergen is introduced is avoided. The experience shows that compared with the ordinary cell frozen stock solution, the human adipose tissue-derived stromal cell frozen stock solution disclosed by the invention is relatively high in motility rate of cells in frozen stock, good stem cell characteristics are maintained, and human fat stem cells can be preserved and applied for a long time.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Traditional Chinese medicine composition for treating acne and preparation method thereof
InactiveCN103432536AIncrease lethalityAchieve sterile growthAntisepticsDermatological disorderViola yedoensisTreatment acne
The invention discloses to a traditional Chinese medicine composition for treating acnes and a preparation method thereof. The traditional Chinese medicine composition for treating the acnes is prepared from the following raw materials in parts by weight: cortex lycii radicis, oldenlandia diffusa, stemona, belvedere fruit, fructus cnidii, liquorice, schizonepeta, divaricate saposhnikovia root, chrysanthemum, salviae miltiorrhizae, cape jasmine, scutellaria baicalensis, honeysuckle, turmeric, paris polyphylla, polygonum perfoliatum, Chinese violet, Chinese angelica and spina gleditsiae. The invention also provides a preparation method of the traditional Chinese medicine composition for treating the acnes. The traditional Chinese medicine composition for treating the acnes has definite curative effect, good safety and no side effect in the aspect of treatment on the acnes; meanwhile, the traditional Chinese medicine composition for treating the acnes is easy to use and low in cost, and the effect of the traditional Chinese medicine composition for treating the acnes is better than that of the prior art.
Owner:唐荣兰 +2
Application of sinomenine or pharmaceutically acceptable salt thereof as medicament for preventing and treating pulmonary interstitial fibrosis
ActiveCN102579445AAvoid generatingAvoid developmentOrganic active ingredientsAntipyreticHydroxyprolineAdjuvant
The invention relates to application of sinomenine as a medicament for preventing and treating pulmonary interstitial fibrosis. The medicament is prepared by sinomenine or pharmaceutically acceptable salt thereof and other adjuvants, wherein sinomenine or pharmaceutically acceptable salt thereof serves as an activating agent. The routes of administration of the medicament include intravenous drip, intramuscular injection, oral administration, transdermal absorption, atomization inhalation and bronchoalveolar lavage. Experiments show that sinomenine does not have obvious difference with the dexamethasone control group in the effect on inhibiting formation of bleomycin-induced pulmonary fibrosis in mice and prove that sinomenine can alleviate pulmonary alveolitis and fibrosis degrees of themice with bleomycin-induced pulmonary fibrosis and the mechanisms are probably realized by inhibiting expressions of TGF (transforming growth factor)-beta1 and alpha-SMA (smooth muscle actin) and thecontent of HYP (hydroxyproline).
Owner:李蕴麟
Periodontal ligament stem cell cryoprotectant and cryopreservation method thereof
InactiveCN107114357AImprove survival ratePrevent shrinkageDead animal preservationSurface markerSerum free media
The invention belongs to the field of cell cryopreservation and particularly relates to a periodontal ligament stem cell cryoprotectant and a cryopreservation method thereof. The cryoprotectant disclosed by the invention is prepared from glycerinum, mesenchymal steam cell serum-free medium and human serum albumin. The cryoprotectant disclosed by the invention can effectively solve the technical defects that an existing cryoprotectant has low use effect and a survival rate of post-resuscitation periodontal ligament stem cells is low. The periodontal ligament stem cell cryoprotectant disclosed by the invention can keep activity of cryopreserved cells; furthermore, the survival rate of the post-resuscitation periodontal ligament stem cells is obviously improved, and expression of surface markers of the stem cells and differentiation potential of the stem cells are not affected.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Cell cryoprotectant and cryopreservation method
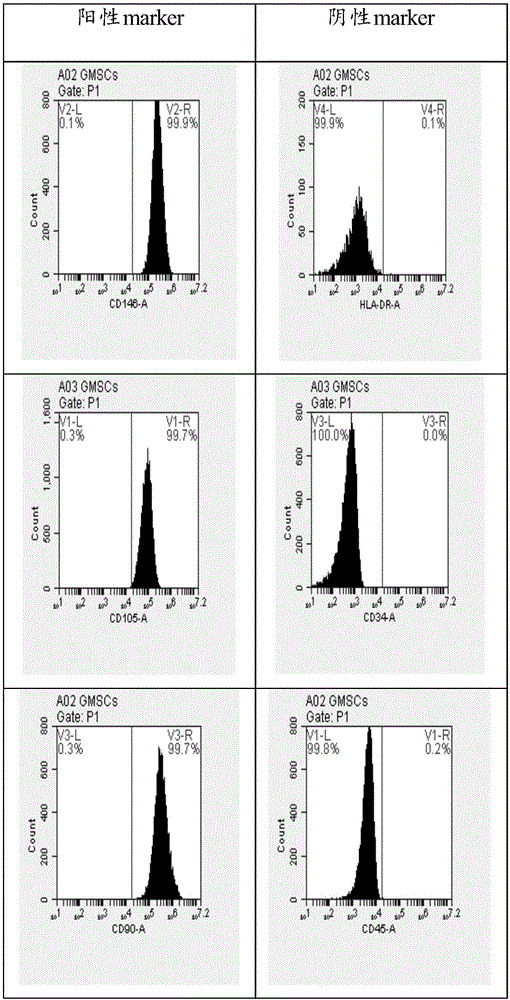
The invention relates to the field of cells, in particular to a cell cryoprotectant and a cryopreservation method. The cell cryoprotectant is prepared from DMSO, human albumin and a serum-free medium. The cryprotectant does not contain animal serum, the risks of introducing contamination and allergens are avoided, and the higher clinical safety is achieved compared with a conventional cell cryoprotectant. Meanwhile, the GMSCs cryoprotectant can well keep the activity of cryopreserved cells.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
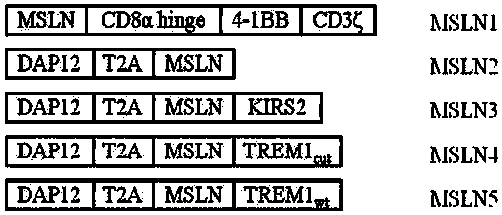
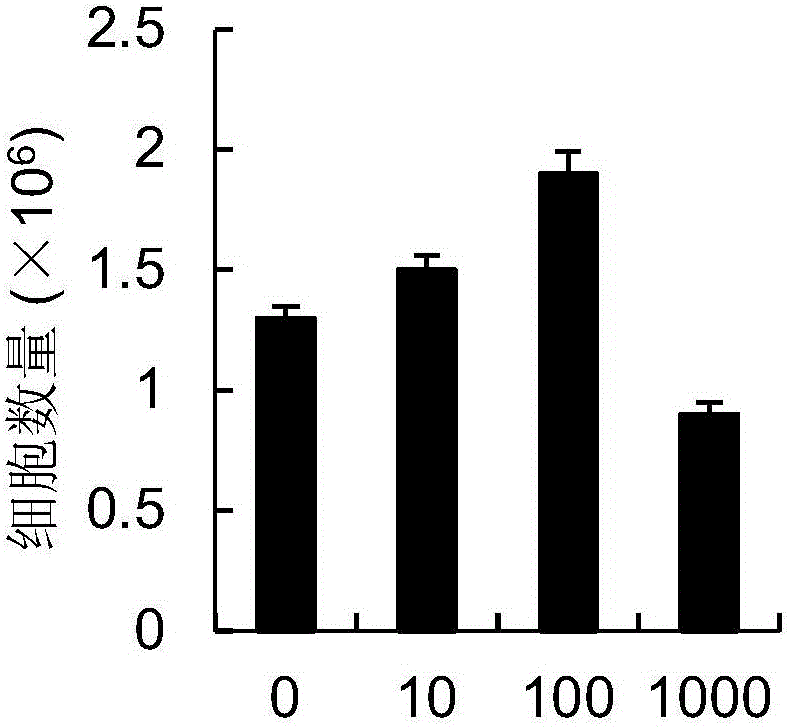
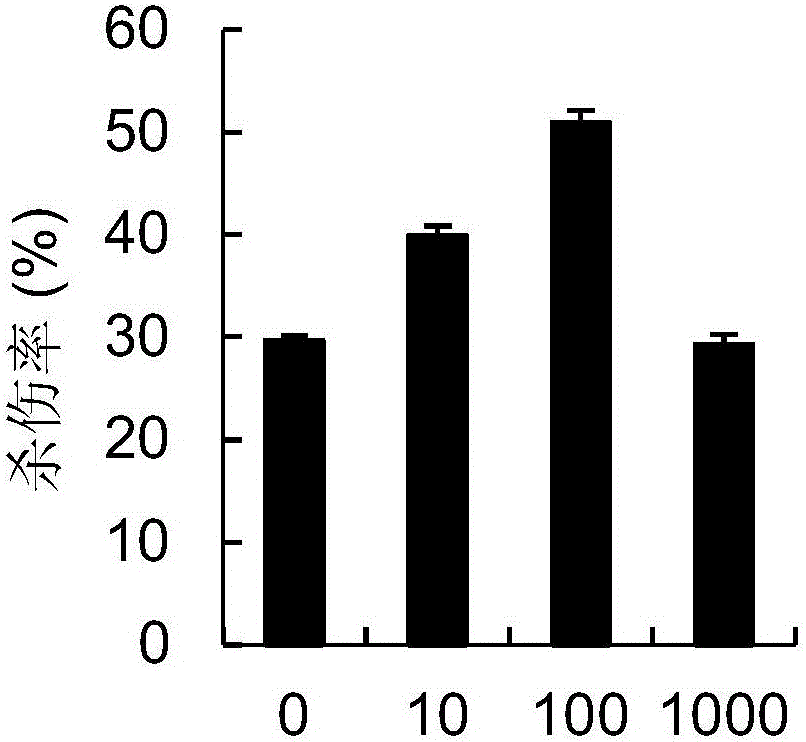
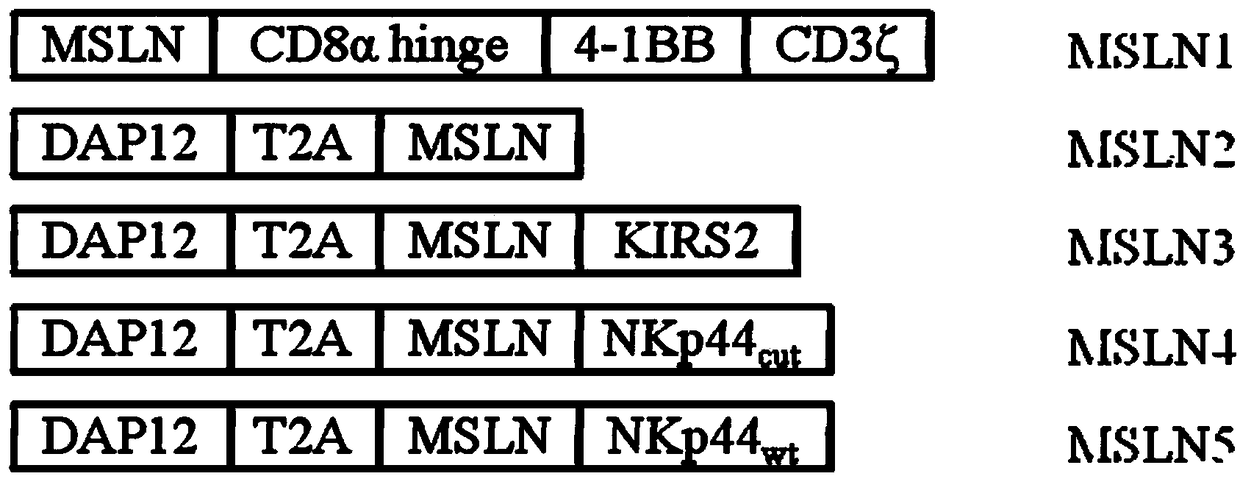
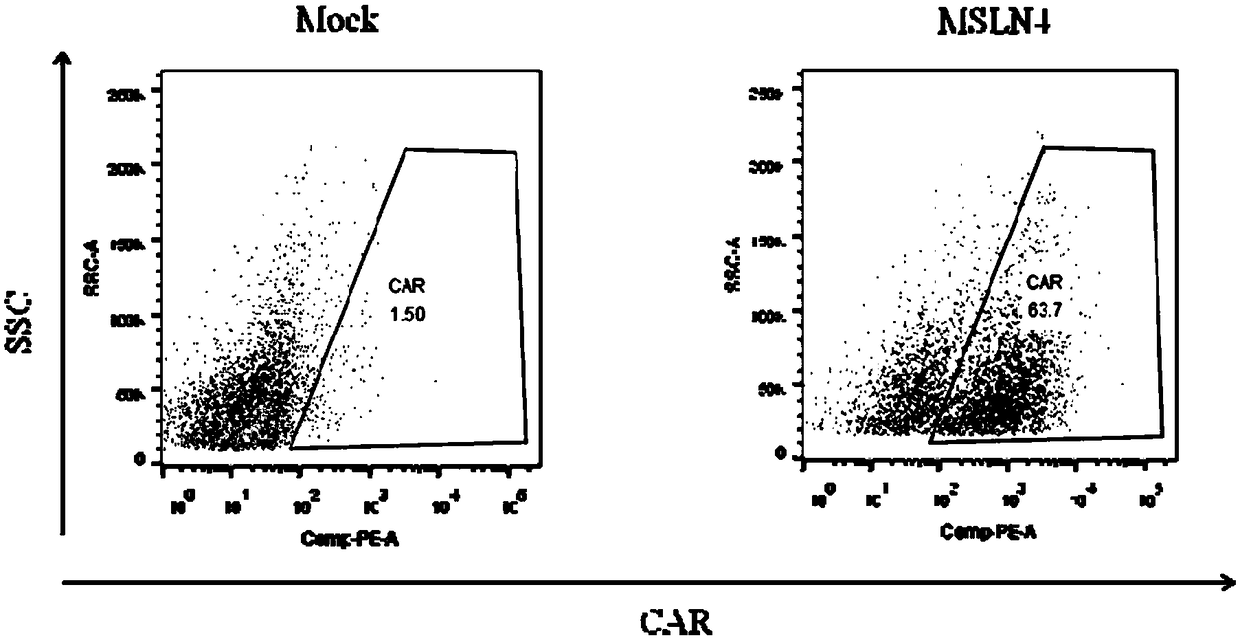
Chimeric antigen receptor carrying truncated or non-truncated myeloid cell triggered receptor signal structure
ActiveCN108752482AEnsure the safety of clinical applicationHigh clinical safetyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorAntigen stimulation
Owner:NANJING CART MEDICAL TECH LTD
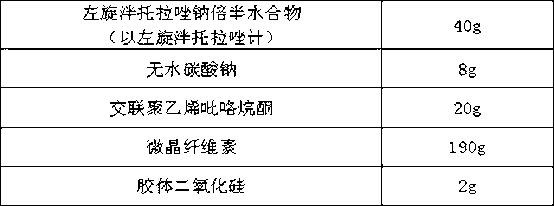
Levo-pantoprazole salt aquo-complex enteric-coated tablet and preparation method thereof
InactiveCN103211778AOne-sided bright and tidyAccurate measurementOrganic active ingredientsDigestive systemMedicineIsolation layer
The invention discloses a levo-pantoprazole salt aquo-complex enteric-coated tablet which comprises a tablet core, an isolation layer and an enteric-coated layer. The tablet is characterized in that the tablet core comprises a levo-pantoprazole salt aquo-complex, a shaping agent, a pH conditioning agent, a disintegrating agent and a lubricant; the levo-pantoprazole salt aquo-complex is 15-25% of the total weight of the tablet core by the weight of levo-pantoprazole; the shaping agent is 60-75% of the total weight of the tablet core; the disintegrating agent is 5-10% of the total weight of the tablet core; the pH conditioning agent is 3-7% of the total weight of the tablet core; the lubricant is 0.5-1.5% of the total weight of the tablet core; and the percentage is of percentage by weight. The enteric-coated tablet of the levo-pantoprazole or the salt of the levo-pantoprazole is convenient to carry over, transport and take; the enteric-coated tablet is high in process operability and is applicable to industrial production; and compared with a racemate preparation, the enteric-coated tablet reduces the dosage and avoids adverse effects of dextroisomer, thereby being better in clinical security.
Owner:沈阳双鼎制药有限公司
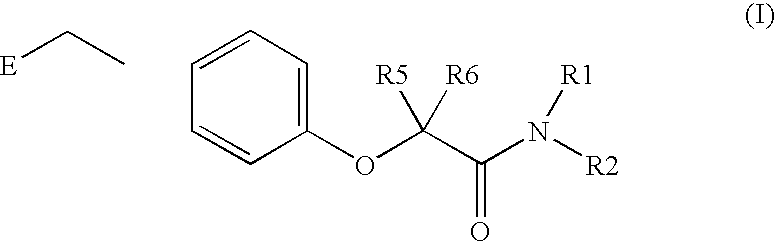
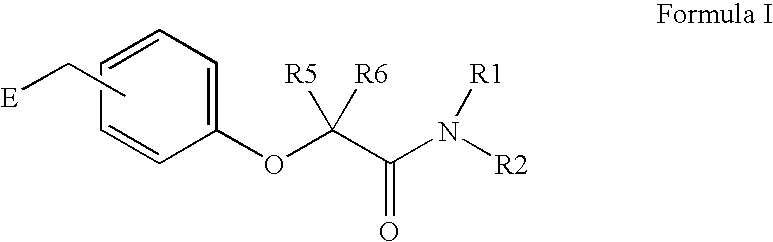
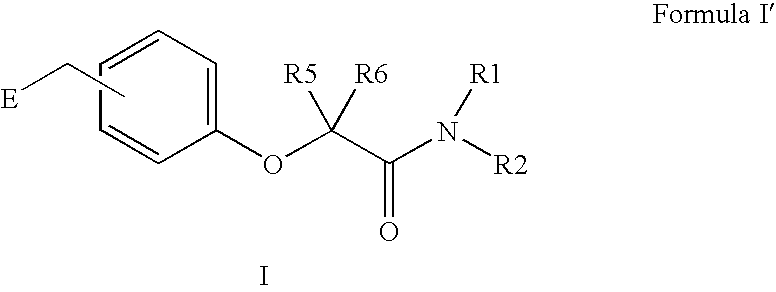
Amide linker peroxisome proliferator activated receptor modulators
InactiveUS20060111406A1High clinical safetyFavorable efficacy profileBiocideOrganic chemistryPeroxisome proliferator-activated receptorStructural formula
The present invention is directed to compounds, compositions, and use of compounds the structural Formula (I).
Owner:ELI LILLY & CO
Patient support and/or transport means and magnetic resonance system
InactiveUS8798717B2Improve connection securityHigh clinical safetyOperating tablesStretcherResonanceEngineering
A patient support and / or transport device is proposed. The patient support and / or transport device comprises a receptacle in particular for a push-in patient support plate. At least one metal detection device is provided on the receptacle adjacent to the patient support plate when the patient support plate is pushed in.
Owner:SIEMENS HEALTHCARE GMBH
Remote controlled modicine reliesing electronic capsule capable of partly degradation
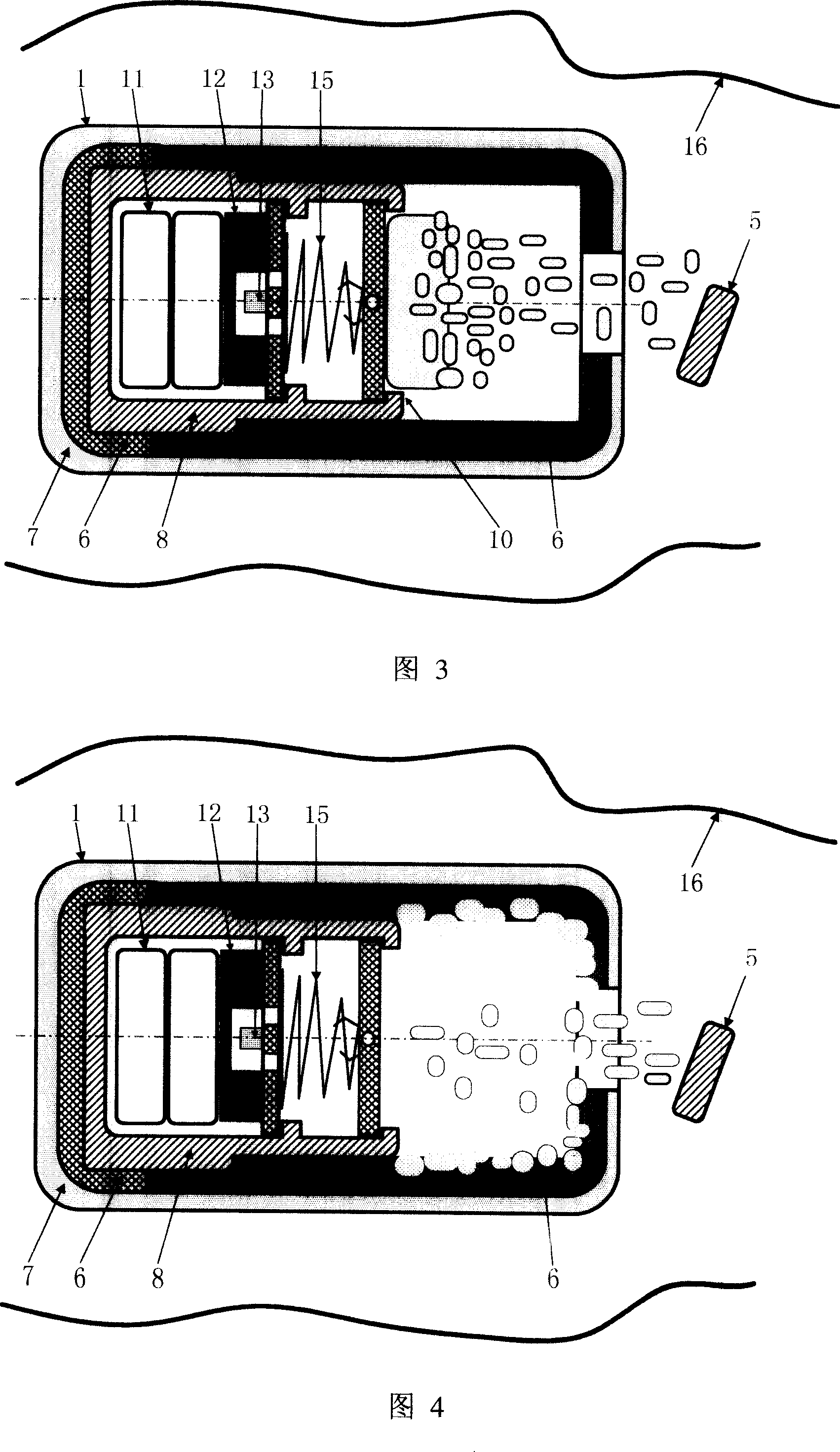
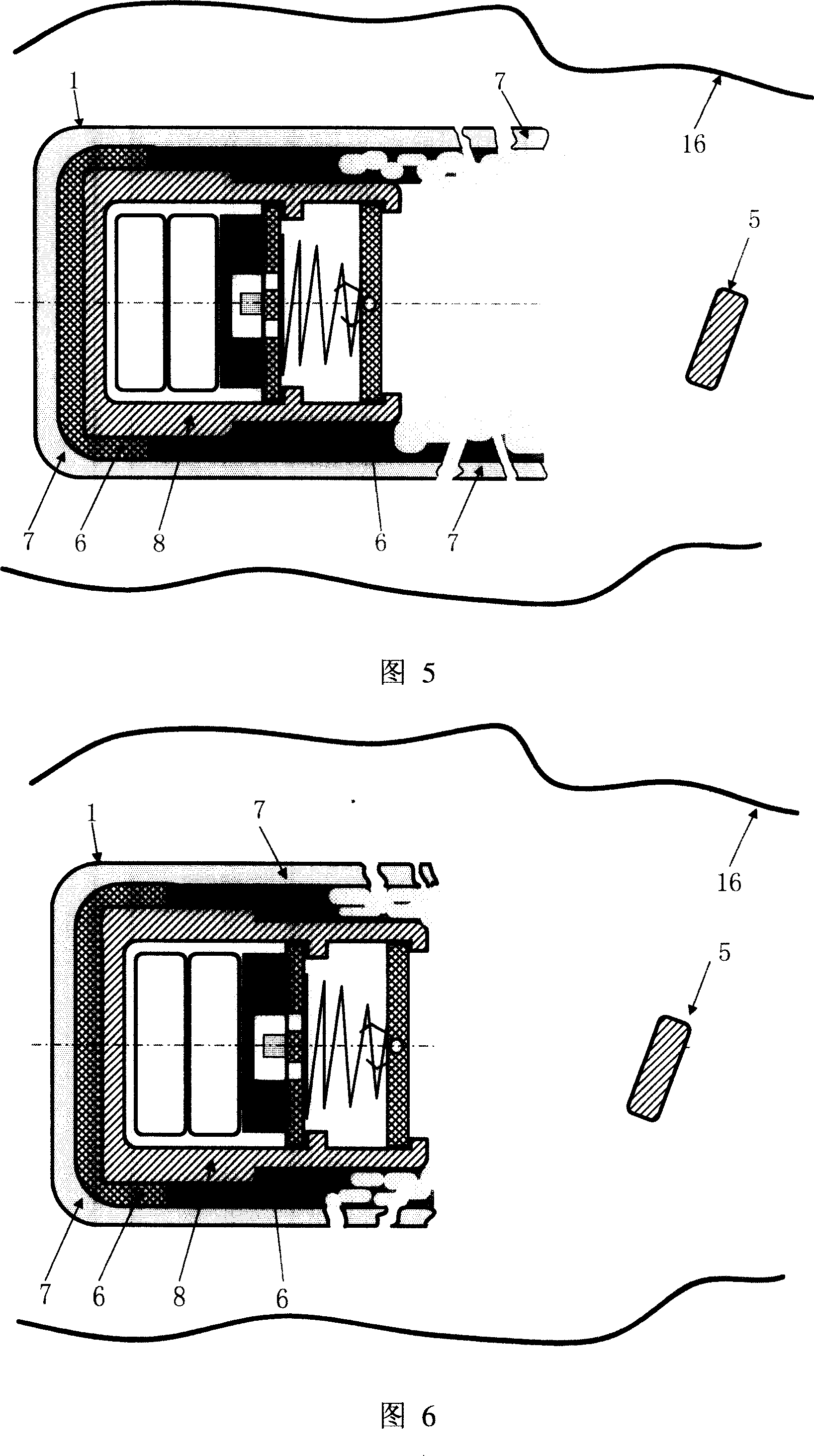
InactiveCN100998906AHigh clinical safetySimple structureMedical devicesDiagnostic recording/measuringRemote controlDrug release
A remotely controlled slow-releasing electronic capsule able to be partially degradated for releasing medicine, food or making substance in digestive tract is composed of casing, remotely controlled driver, slow-releasing channel of medicine and a medicine tablet core with a conic or cylindrical boss at its one end. Under the drive of said driver, said boss on medicine tablet can move forward to open the medicine releasing channel for slowly releasing the medicine. Said casing consists of the scaffold able to be degradated and a coated layer.
Owner:CHONGQING UNIV
Cell cryopreservation liquid
InactiveCN109792985ALow cytotoxicityHigh clinical safetyDead animal preservationCell expansionGlycerol
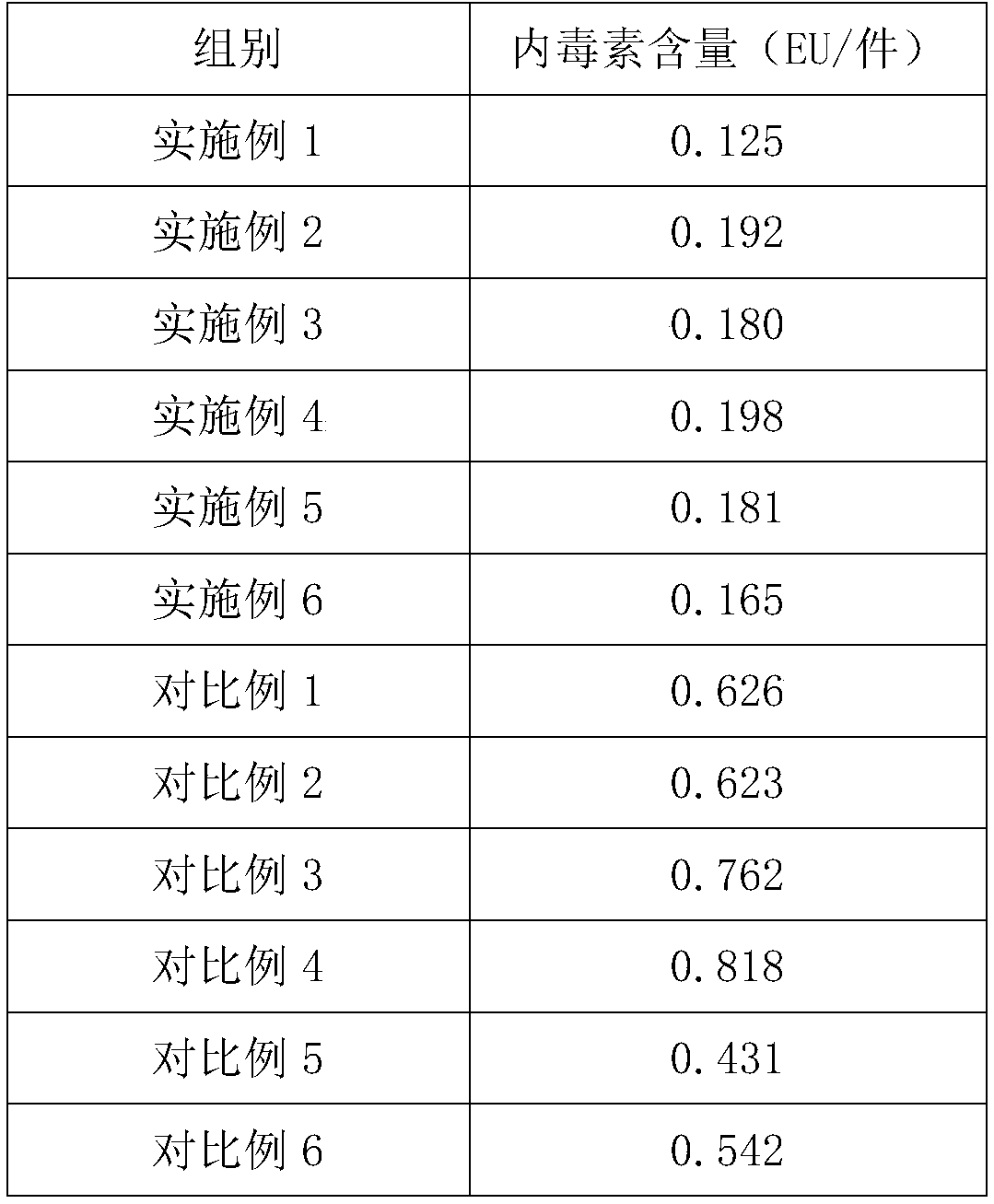
The invention discloses a cell cryopreservation liquid, which is characterized in that the cell cryopreservation liquid comprises, by volume, the following components: 5-10 v / v% of glycerol; 15-20 v / v% of a human serum albumin solution; 10-15v / v% of a 18AA compound amino acid solution; 15-54 v / v% a dextran-40 glucose solution; 1-42.5v / v% of a mixed sugar electrolyte solution. The cell cryopreservation liquid does not contain dimethyl sulfoxide (DMSO) and has low cytotoxicity; the human serum albumin is used for replacing components such as serum, plasma and the like, thereby having higher clinical safety; the cell cryopreservation liquid can be directly prepared by using commercially available injection liquid, and has the advantages of simple and convenient operation and low cost; the additive components are clear and convenient for scientific research and analysis; the safety is high, and the damage to cells is small; the cell expansion ability is good after cryopreservation resuscitation; the cell cryopreservation liquid can be used for replacing frozen liquid containing dimethyl sulfoxide (DMSO).
Owner:EAST CHINA UNIV OF SCI & TECH
Artificial biological dura mater and preparation method thereof
ActiveCN108815578AImprove mechanical propertiesImprove stitching effectTissue regenerationProsthesisMass ratioBiocompatibility Testing
The invention belongs to the technical field of bio-based materials, and particularly relates to an artificial biological dura mater and a preparation method thereof. The artificial biological dura mater is prepared from the following raw materials of bacterial cellulose and collagen according to a mass ratio of (0.1 to 3):10; any crosslinking agent is not added, so that the adverse effect to therepair process of the dura mater by the crosslinking agent is reduced, and the clinical use safety of the dura mater product is improved. The artificial biological dura mater has the advantages that the artificial biological dura mater is prepared by an electrostatic spinning method; the pH (potential of hydrogen), temperature and other technology conditions are strictly controlled, so that the good biocompatibility and mechanical property are realized, the requirement of operation suture in the clinical use process is met, and the leakage of cerebrospinal fluid is effectively prevented.
Owner:NKD PHARMA CO LTD
Plasma filtering device for treatment of acute cerebral infarction and method
InactiveCN106540344AReduce extracorporeal circulationRelief of clinical symptomsOther blood circulation devicesHaemofiltrationInflammatory factorsFiltration membrane
The invention provides a novel plasma filter device for treatment of acute cerebral infarction and filter method of the novel plasma filter device. The device comprises a shell and a filter element. The shell comprises an upper shell body and a lower shell body connected with the upper shell body. The upper shell body and the lower shell body form an accommodating space. The filter element is arranged in the accommodating space and comprises first filter films, a second filter film and a third filter film. The filter element is formed in the mode that the first filter film, the second filter film, the first filter film, the first filter film and the third filter film are arranged sequentially in the plasma flowing direction, wherein the first filter films are DELP films with a lipidosome and fibrinogen affinity adsorption function, the second filter film is an EKSP depth filtering film capable of adsorbing inflammatory factors and free radicals, and the third filter film is a 0.2 micrometer-thick film used for filtering out various granular substances with the hole diameter being greater than 0.2 micrometer. The novel plasma filter device for treatment of acute cerebral infarction and the filter method of the novel plasma filter device have the advantages that the plasma filter device can be used for filtering the plasma of an acute cerebral infarction patient.
Owner:SHANGHAI JIANGXIA BLOOD TECH
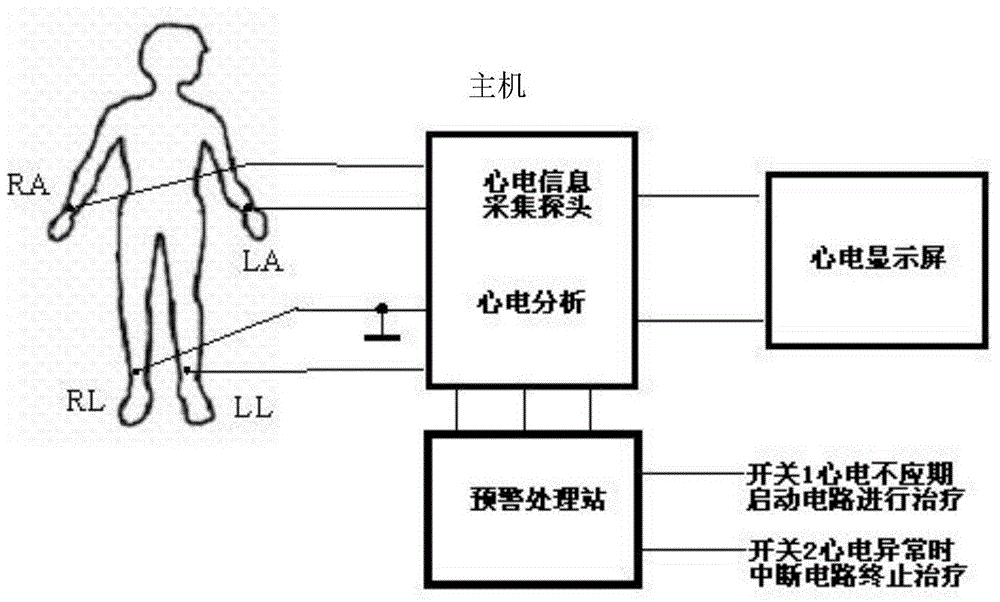
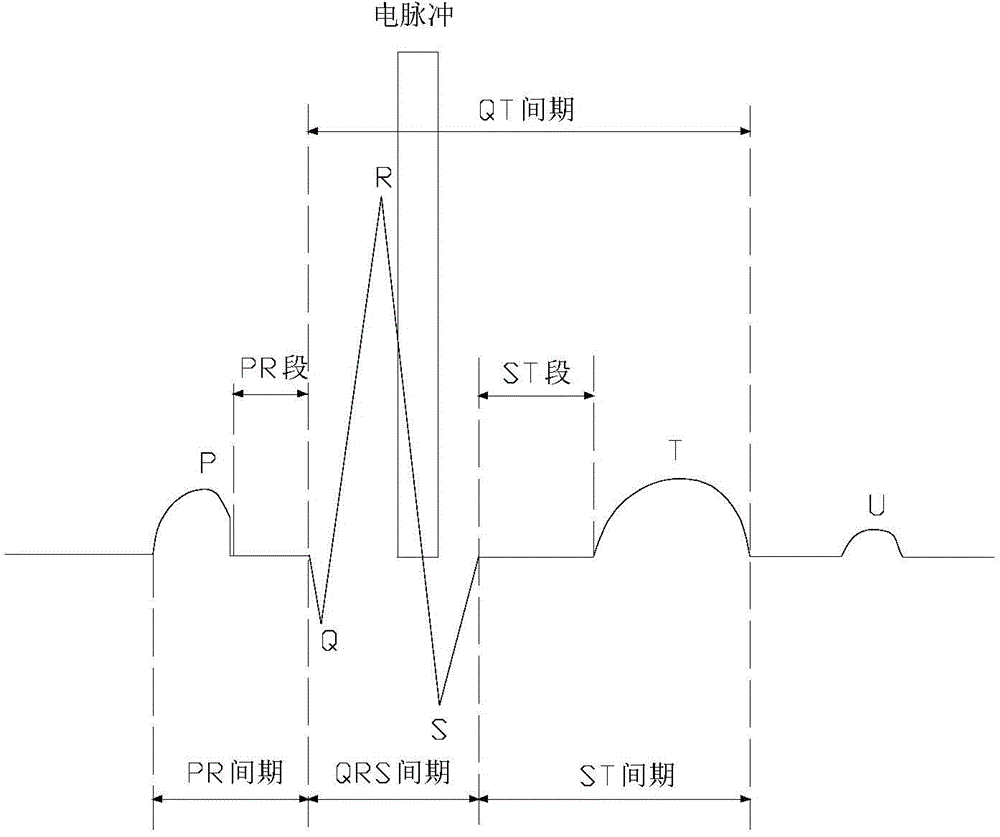
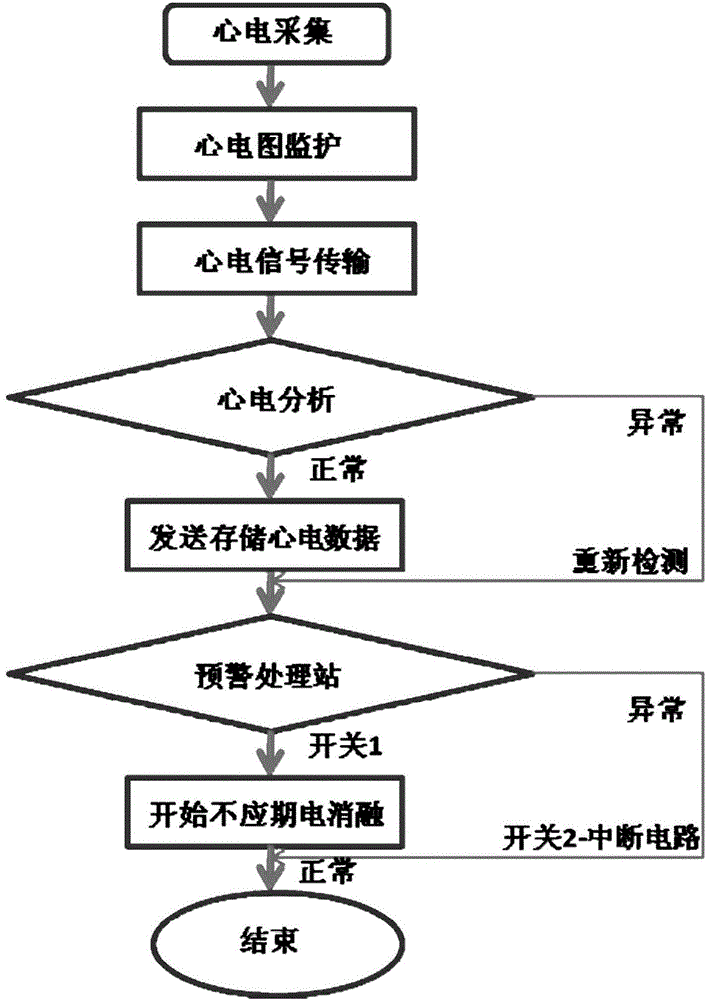
Electrocardiogram monitoring and automatic protection device for electrical ablation of tumors
InactiveCN104783894AAvoid arrhythmiaConvenient real-time monitoringSurgical instruments for heatingReal time acquisitionElectricity
The invention discloses an electrocardiogram monitoring and automatic protection device for electrical ablation of tumors. The electrocardiogram monitoring and automatic protection device comprises an electrocardiogram information collection probe, a host, an electrocardiogram display screen and an early warning processing station, wherein the electrocardiogram information collection probe, the electrocardiogram display screen and the early warning processing station are all connected with the host so that signal transmission can be conducted. The electrocardiogram information collection probe collects electrocardiogram data of a patient in real time and transmits the electrocardiogram data to the host, the host conducts electrocardiogram analysis on a received electrocardiogram signal and then transmits the electrocardiogram signal to the electrocardiogram display screen and the early warning processing station, and the electrocardiogram display screen receives the signal and displays an electrocardiogram waveform. The early warning processing station processes the received signal, and according to the processing result, two switches arranged on the early warning processing station act, wherein if electrocardiogram detection is normal, the first switch is closed, an ablation power source is switched on, and an electric pulse is controlled to be released in the electrocardiogram refractory period; if abnormal electrocardiogram activities are detected, the second switch is closed, the ablation power source is switched off, and electrical ablation treatment is terminated.
Owner:HANGZHOUREADY BIOLOGICAL TECH CO LTD
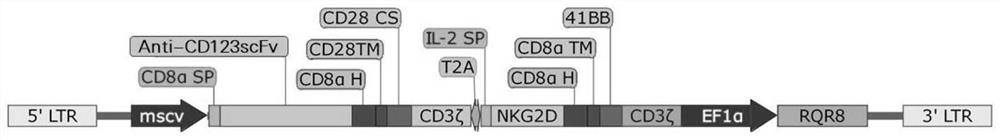
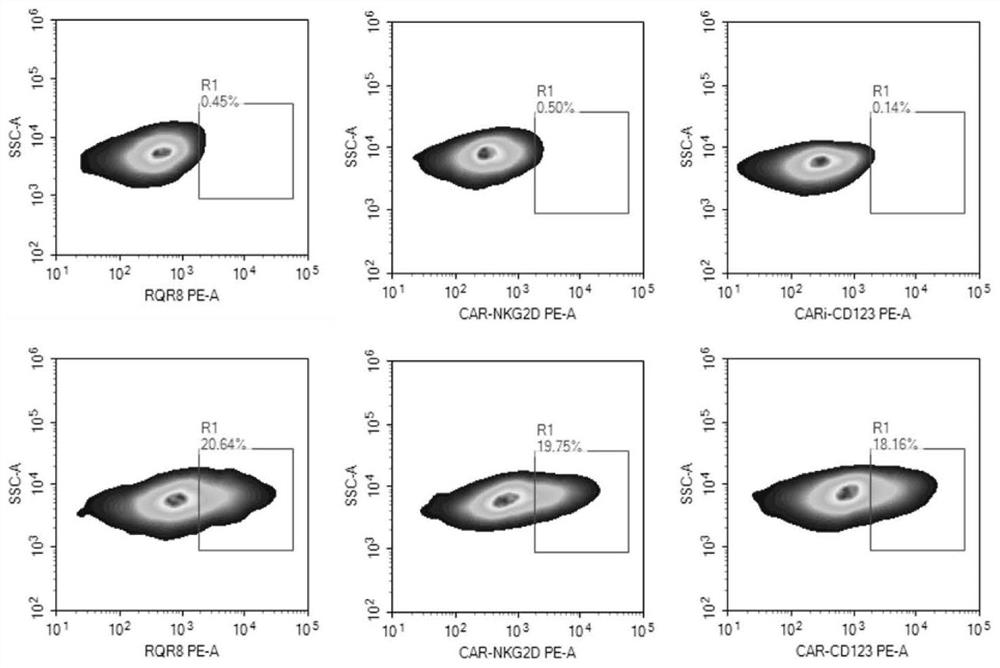
Bispecific chimeric antigen receptor targeting CD123 and NKG2D ligands and application of bispecific chimeric antigen receptor
PendingCN111995688AEnhance specific killing effectOvercoming immunosuppressionVirusesMicroorganism based processesSingle-Chain AntibodiesNkg2d ligands
The invention discloses a bispecific chimeric antigen receptor targeting CD123 and NKG2D ligands and the application of the bispecific chimeric antigen receptor, and particularly discloses a bispecific chimeric antigen receptor (CAR) amino acid construct or a functional variant thereof, wherein the bispecific chimeric antigen receptor (CAR) amino acid construct can simultaneously expresses an anti-CD123 single-chain antibody and a natural NKG2D extracellular fragment and can target CD123 and NKG2DL; the bispecific chimeric antigen receptor has a parallel connection mode and a series connectionmode; and the invention discloses a CAR (chimeric antigen receptor), a construct structure thereof, a nucleotide sequence of the construct, a recombinant expression vector containing the nucleotide sequence and a construction mode and application of the corresponding expression vector. The CAR structure endows T cells with higher and lasting multiplication capacity and high anti-tumor capacity; and according to the bispecific chimeric antigen receptor, multiple infusions of single-target CAR-T cells can be avoided, so that not only is the harm to a patient reduced, but also the economic pressure of the patient can be reduced, and the bispecific chimeric antigen receptor has relatively great clinical research and application values.
Owner:金鑫
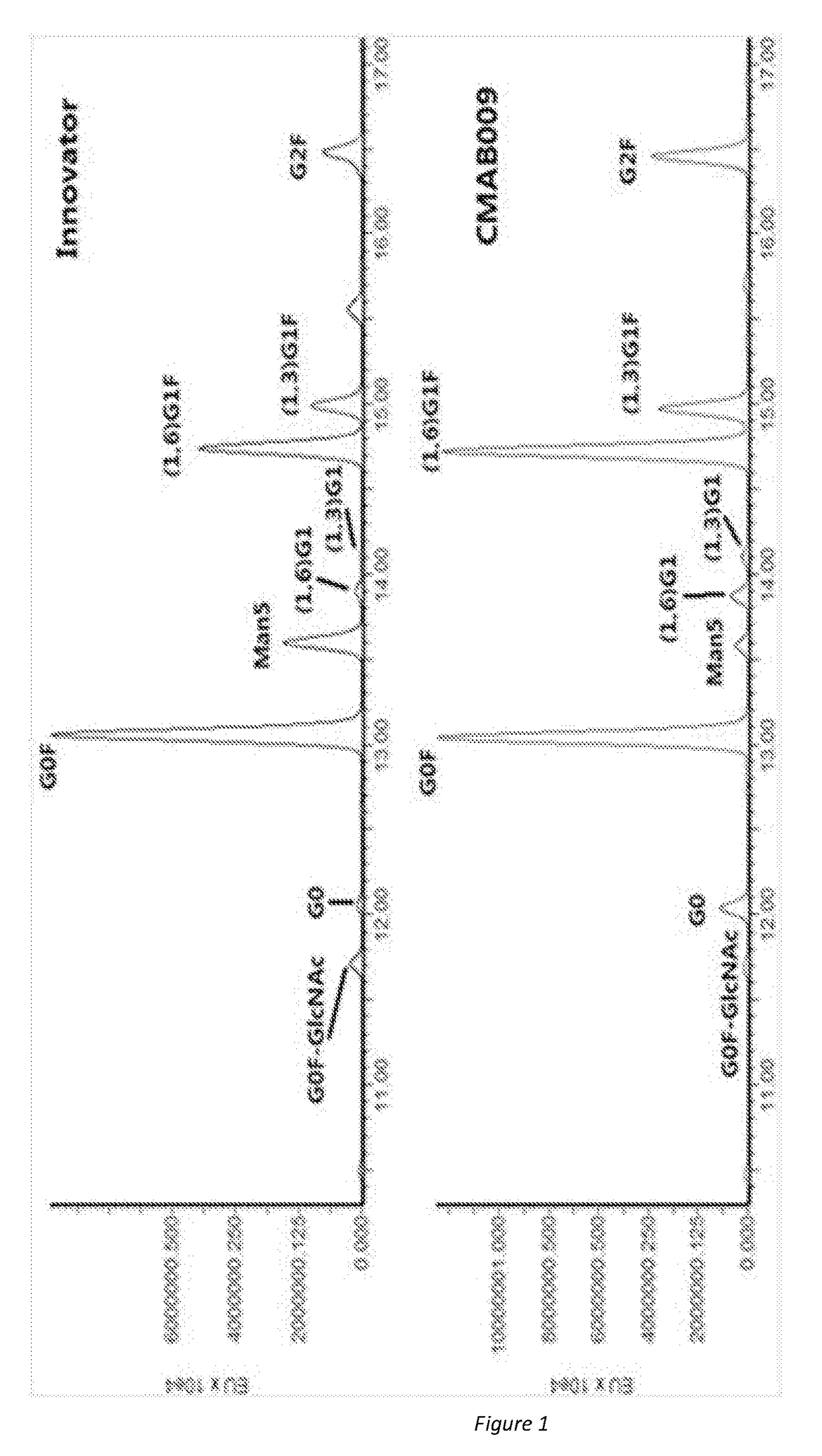
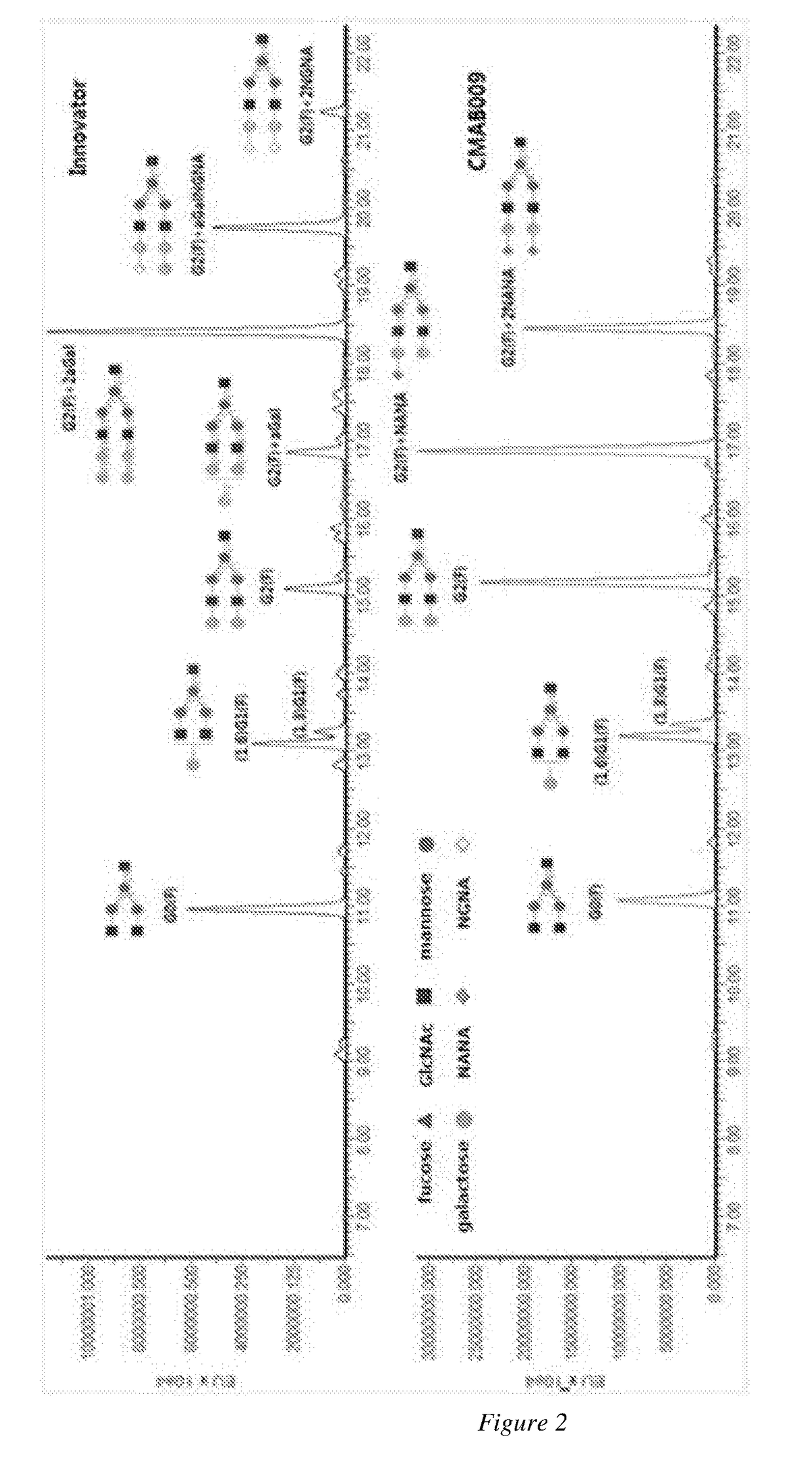
A novel Anti-egfr monoclonal antibody, method of making, and use thereof
ActiveUS20180030139A1Reduce the differenceHigh drug safetyPolypeptide with localisation/targeting motifOrganic active ingredientsSerum igeHeavy chain
The present invention provides a method for producing an anti-EGFR monoclonal antibody and the applications thereof. The method comprises the steps of: designing and synthesizing the light chain and heavy chain according to the codons preferred by Chinese hamster, transfecting GS knockout host cells CHO-CR-GS− / −, culturing cells using serum-free technology, isolating and purifying the antibody, and obtaining the low immunogenicity CMAB009 antibody.
Owner:SHANGHAI BIOMABS PHARMA
Loratadine syrup and preparation method thereof
InactiveCN109498569AUniform contentStable contentOrganic active ingredientsDispersion deliveryGlycerolSolvent
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a loratadine syrup and a preparation method thereof. The loratadine syrup, in terms of 1000ml,comprises the following components: 0.9-1.1g of loratadine, 650-750g of a sweetener, 80-120ml of propylene glycol, 40-60ml of glycerol, 0.9-1.1g of ethylenediaminetetraacetic acid disodium salt, 15-25g of an acidity regulator, 2-4g of a preservative, and water added until the total amount is 1000ml. The loratadine syrup prepared by the method provided by the invention can meet a requirement of uniform dispersion of bulk drugs; the effective components of the loratadine syrup can be uniformly dispersed in the syrup, the contents of the upper layer, the middle layer and the lower layer of the syrup are uniform, and the quality is uniform; and the loratadine syrup produced by the preparation method provided by the invention does not contain an ethanol solvent, has no ethanol residue, and hasgood clinical safety. The loratadine syrup prepared by the method provided by the invention has stable quality and a shelf life of more than 36 months.
Owner:HUBEI KANGYUAN PHARMA
Chinese medicinal composition for treating dysmenorrhea and preparation method thereof
ActiveCN101375983AGood clinical efficacyActive ingredient clearSexual disorderPlant ingredientsMedicinal herbsClinical efficacy
The invention relates to a Chinese medicinal composition for treating dysmenorrhea and a preparation method thereof, and the Chinese medicinal composition is prepared from Cyperus rotundus and Ligusticum chuanxiong. The invention has the advantages of easily accessible raw materials, good therapeutic effect, definite active ingredients, stable quality, simple process, low time and labor consumption and high extraction yield.
Owner:SHANDONG BUCHANG SHENZHOU PHARMA
Cefoperazone sodium and sulbactam sodium composition
InactiveCN101143146AReduce contentReduce manufacturing costAntibacterial agentsPharmaceutical delivery mechanismInfected patientCurative effect
The invention provides a combination of cefoperazone sodium and sulbactam sodium. The combination consists of the cefoperazone sodium and the sulbactam sodium, and the weight ratio of which is 3 to 1. The combination of cefoperazone sodium and sulbactam sodium of the invention has the comparative curative effect at the aspect of antibacterial effect with a compound preparation of 1 to 1 or 2 to 1 of the cefoperazone sodium / the sulbactam sodium in markets. The invention reduces the corresponding content of the sulbactam sodium in the combination, so the invention has wider clinical application range and can reduce the production cost of the drug. Compared with the prior compound preparation of the cefoperazone sodium / the sulbactam sodium, the invention is characterized by being fit for the anti-infection remedy of the patient with the renal dysfunction and the remedy of the seriously infected patient.
Owner:CHENGDU BOAOTONG TECH
Composition injection of glycerol and fructose and preparation method thereof
ActiveCN101810629AReasonable prescriptionImprove medication safetySenses disorderHydroxy compound active ingredientsFructoseAdditive ingredient
The invention relates to a composition injection of glycerol and fructose and a preparation method thereof. The composition injection of the glycerol and the fructose comprises the following main ingredients: 25-40 parts of glycerol, 3-8 parts of fructose, 3-13 parts of maltitol and 80-120 parts of water for injection. The composition injection of glycerol and fructose is prepared according to the following steps: (1) liquid preparation: dissolving the main materials and auxiliary materials into proper amount of water for injection to prepare solution, respectively stirring evenly, mixing the prepared solution evenly by stirring, and using a pH regulator to regulate pH to be 4 to 5, wherein hydrochloric acid or sodium hydroxide is preferred as the pH regulator, and filtering to obtain filtrate; (2) sterilization: carrying out high-temperature sterilization on the filtrate in the step (1), and taking proper amount of active carbon for high-temperature sterilization; and (3) decolorization: adding the medical active carbon after high-temperature sterilization into the filtrate after high-temperature sterilization, decolorizing and filtering.
Owner:罗诚
Method for enhancing the activity of CIK cells, CIK cells, and preparation method and application thereof
ActiveCN105925526ARaise the ratioEnhance killing activityMammal material medical ingredientsBlood/immune system cellsPeripheral blood mononuclear cellA-DNA
The present invention relates to the field of cell engineering, and discloses a method for enhancing the activity of CIK cells. The method includes the culture of CIK cells in the presence of a DNA demethylation drug. The invention also discloses CIK cells and a preparation method thereof. The preparation method includes culturing peripheral blood mononuclear cells in the presence of cytokines for 9 to 11 days, and then culturing in the presence of a DNA demethylation drug. The invention also discloses the application of the CIK cells in the preparation of the preparation for the treatment of tumors. In addition, the invention also discloses the application of the DNA demethylation drug as a stimulating factor in enhancing the activity of CIK cells. The invention by the use of DNA demethylation drug significantly enhances the ratio of utility cells in CIK cells, significantly increase the killing activity of CIK cells on tumor cells, also can promote the proliferation activity of CIK cells, and has good clinical safety, no toxic or side effects, considerable overall anti-tumor effect, and great value in clinical application.
Owner:GENERAL HOSPITAL OF PLA
CAR (chimeric antigen receptor) carrying truncated or non-truncated natural cytotoxic receptor signal structure and application of CAR
ActiveCN108822216AEnsure the safety of clinical applicationHigh clinical safetyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyReceptorAntigen receptors
The invention discloses a CAR (chimeric antigen receptor). The CAR comprises an antigen binding structural domain (scfv) and a signal conducting domain, wherein the signal conducting domain comprisesa first conducting domain and a second conducting domain, and the antigen binding structural domain is connected in series between the first conducting domain and the second conducting domain. When the CAR structure is stimulated by antigen, the level of secreted cytokines is extremely low, and safety of clinical application can be well ensured and is higher; killing capacity of antigen positive tumor cells in vitro is higher, and anti-tumor activity is better.
Owner:NANJING CART MEDICAL TECH LTD
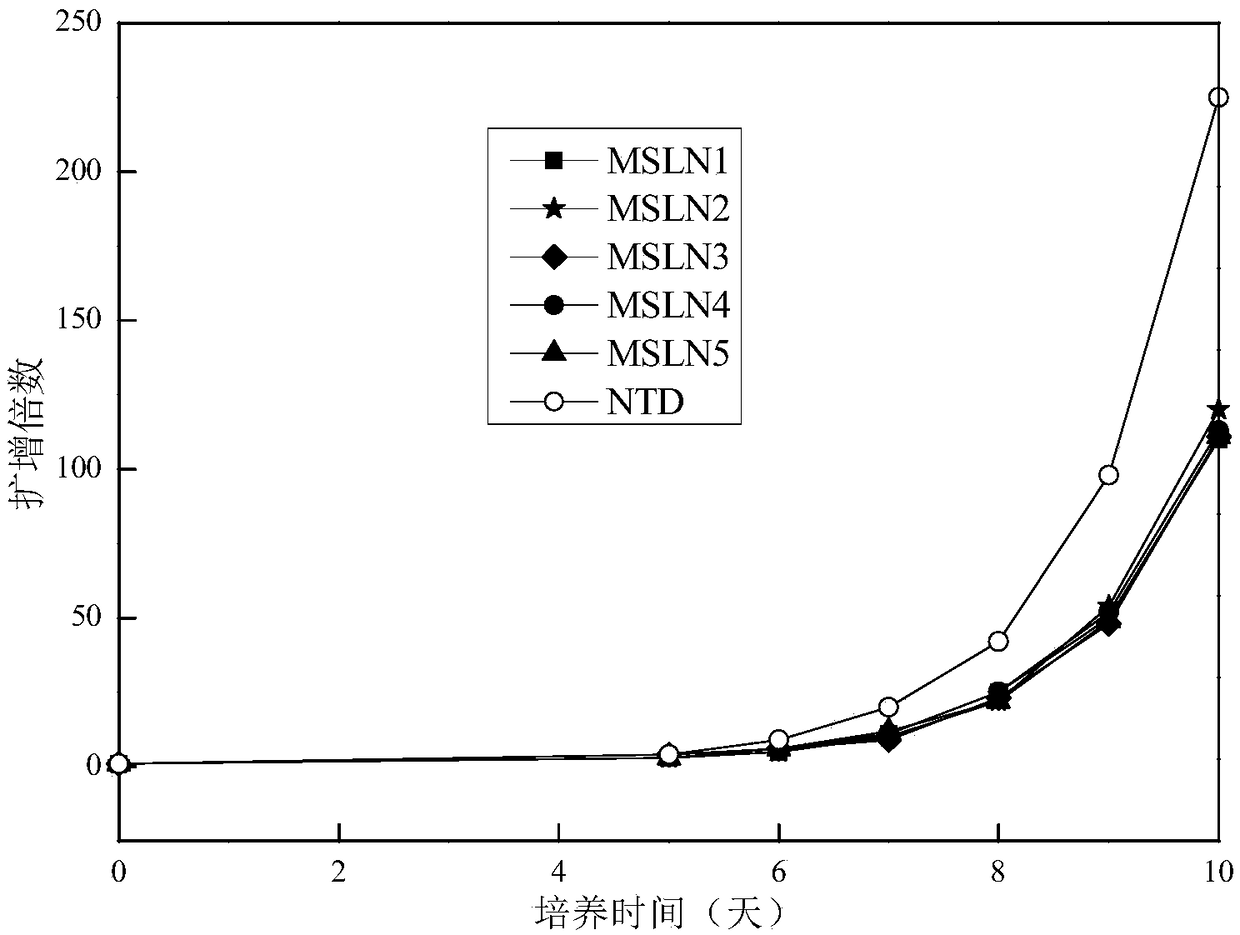
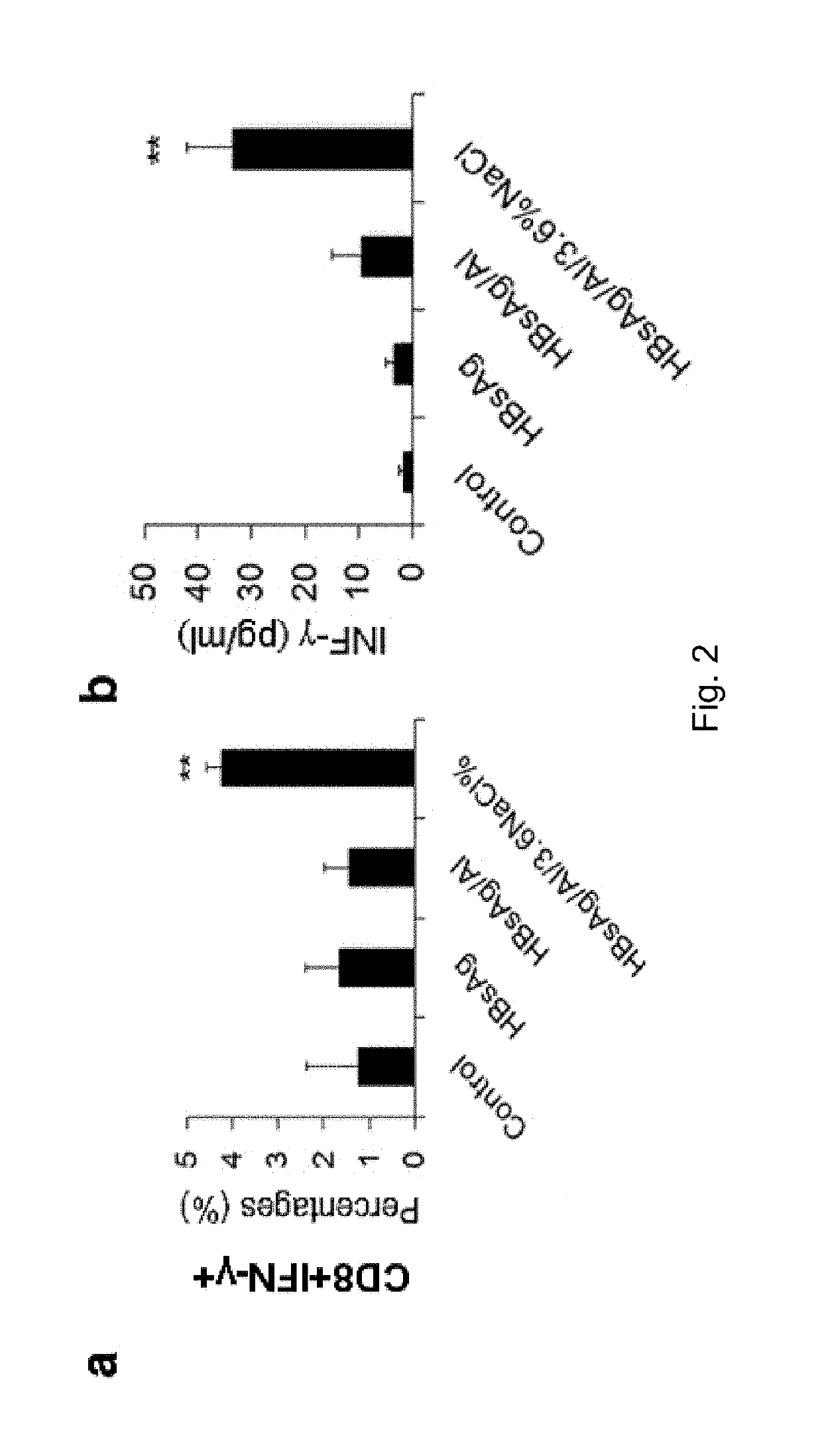
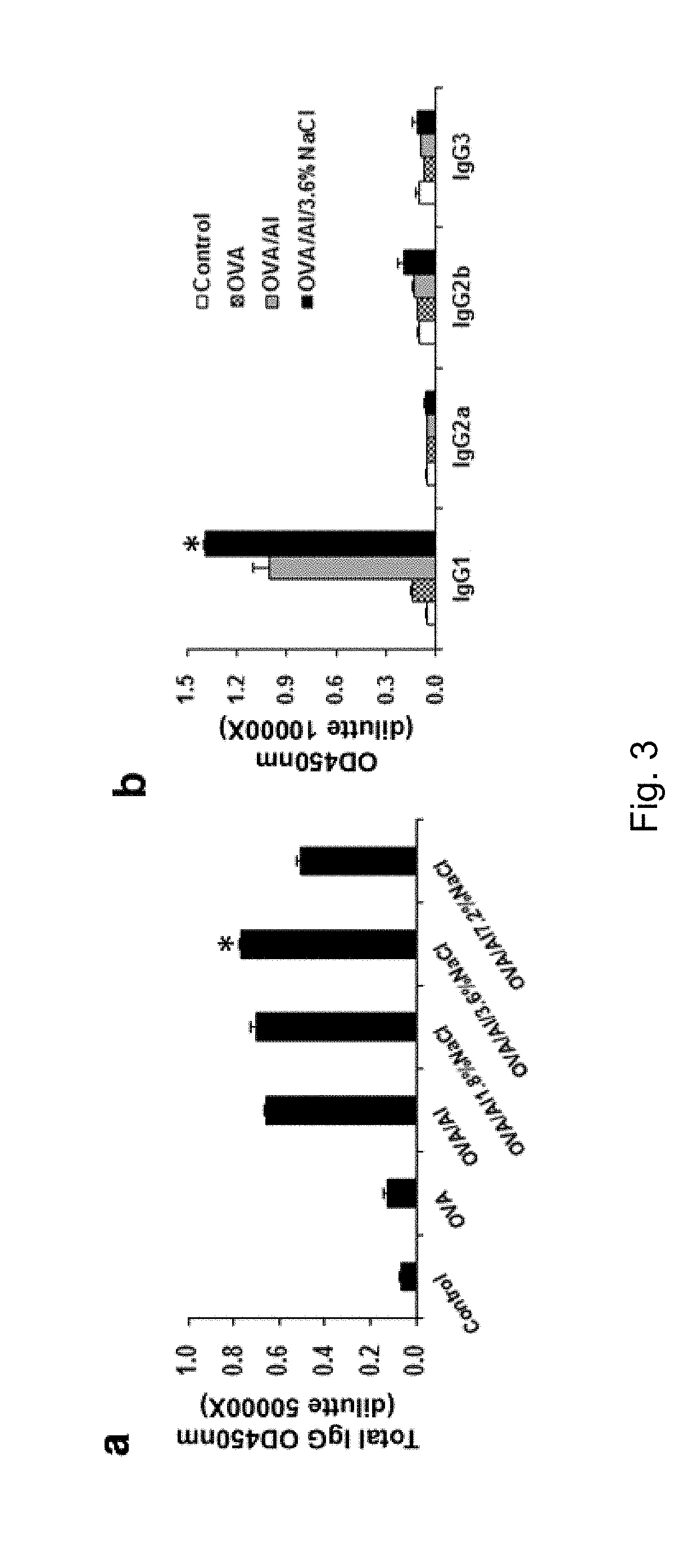
ALHYDROGEL-SODIUM CHLORIDE COMPOUND IMMUNOLOGIC ADJUVANT, PREPARATION METHOD AND USE THEREOF (As Amended)
ActiveUS20190134191A1Strong immune activityHigh clinical safetyBacterial antigen ingredientsViral antigen ingredientsChloride sodiumMedicinal chemistry
The invention belongs to the field of biological medicine, in particular refers to an alhydrogel-sodium chloride compound immunologic adjuvant, preparation method and use thereof. The technical problem to be solved by the invention is to provide a well-behaved and novel immunologic adjuvant. The technical solution for solving the technical problem of the invention is to provide the use of sodium chloride in preparing immunologic adjuvant and the alhydrogel-sodium chloride compound immunologic adjuvant obtained on the basis thereof. The compound immunologic adjuvant mainly includes alhydrogel and sodium chloride. The alhydrogel-sodium chloride compound immunologic adjuvant of the invention is an excellent compound immunologic adjuvant, which can be used for various antigens, and provides a new and effective choice for the development and application of vaccines due to the advantages of simple and convenient use, low cost, strong immune activity, high clinical safety and the like.
Owner:SICHUAN UNIV
Serum-free medium and preparation method thereof
ActiveCN112961825AThe source of the ingredients is clearGood batch-to-batch repeatabilityCulture processSkeletal/connective tissue cellsUmbilical cordCulture mediums
The invention belongs to the technical field of cell culture, and particularly relates to a serum-free medium and a preparation method thereof. According to the serum-free medium disclosed by the invention, animal-derived components such as serum and platelet lysis buffer are replaced by an additive of serum free culture. The serum-free medium has the advantages of clear source of ingredients, good repeatability between batches and high clinical safety, can provide various substance components required by rapid growth and proliferation of mesenchymal stem cells, can keep the characteristics of the stem cells, can meet the requirements of in-vitro culture of mesenchymal stem cells from different sources such as fat, bone marrow, umbilical cord, umbilical cord blood and placenta.
Owner:JIANGSU PURECELL BIOMEDICAL TECH CO LTD
Compounded ophthalmic preparation and preparation method thereof
ActiveCN111202746AImprove visual fatigue and dry eye syndromeImprove securityOrganic active ingredientsSenses disorderSodium hyaluronateChemistry
The invention discloses a compounded ophthalmic preparation which comprises a solid component A and a component B, wherein the component B is used as a buffer isotonic solution; the component A comprises a chondroitin sulfate cross-linking modified carboxylated beta-cyclodextrin carrier as well as components, namely sodium hyaluronate, trehalose, glycine betaine and alanyl glutamine matrix which are carried by the carrier; and the component B as the buffer isotonic solution comprises povidone, taurine, glycerinum and / or a sodium chloride isotonic agent, an antibacterial agent, a pH value adjusting agent and injection water. The preparation disclosed by the invention is used for alleviating asthenopia and relieving xerophthalmia symptoms, has very high stability and can be preserved for a long time without a preservative.
Owner:AFFILIATED HOSPITAL OF WEIFANG MEDICAL UNIV
Method for preparing high-purity menstrual-blood-derived stem cells
PendingCN106011052AReduce the risk of contaminationFast amplificationCell dissociation methodsDead animal preservationChemistryAntibody
The invention provides a method for preparing high-purity menstrual-blood-derived stem cells. Menstrual blood is collected by means of a menstrual blood collection sleeve; middle layer mononuclear cells are collected; a magnetic bead buffering solution is added, an FcR reagent is added after being fully and evenly mixed, then a cross-linking anti-human-antibody micro magnetic bead reagent is added after incubation, and incubation is conducted after uniform mixing; then a magnetic bead buffering solution is added, and after being washed and centrifuged, the mixture is resuspended in the magnetic bead buffering solution; the mixture is added into a separation column placed on a magnetic separation frame in advance, and then the column is washed by means of the magnetic bead buffering solution; the separation column is taken out, 1.5-2 ml of magnetic bead buffering solution is added, and cell suspension is collected; the obtained suspension is cultured by means of a menstrual blood stem cell primary culture medium, and the half of suspension is replaced every 2-3 days; after culture is conducted for 5-7 days, adherent cells are digested and collected, and multiplication culture continues by means of a menstrual blood stem cell subculture medium; the obtained cells are digested, and a cryopreservation solution is added for cryopreservation. The menstrual-blood-derived stem cells obtained through the method are high in purity, short in growth period, high in self-renewal capacity and low in contamination rate.
Owner:浙江奥比特生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com