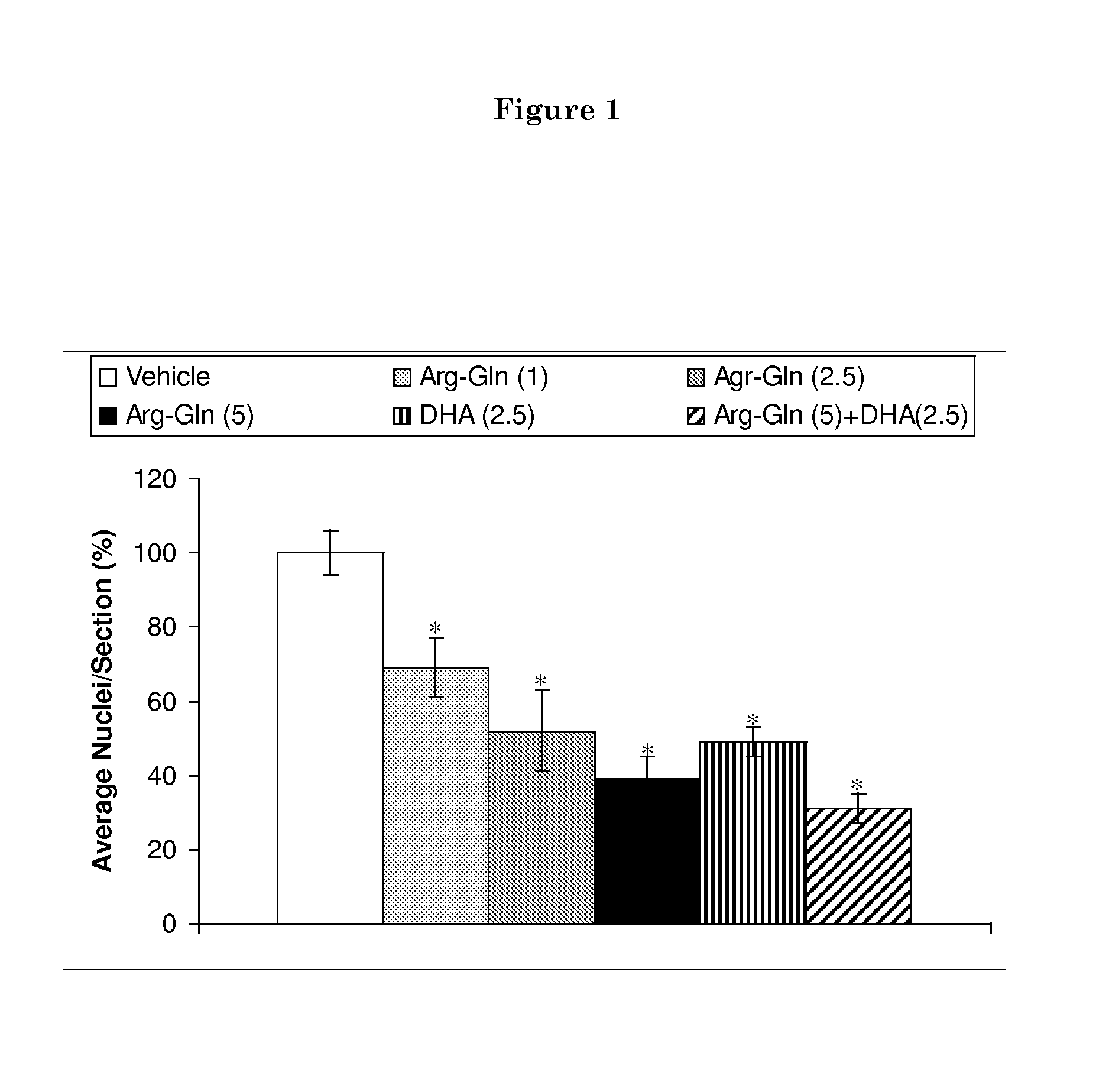
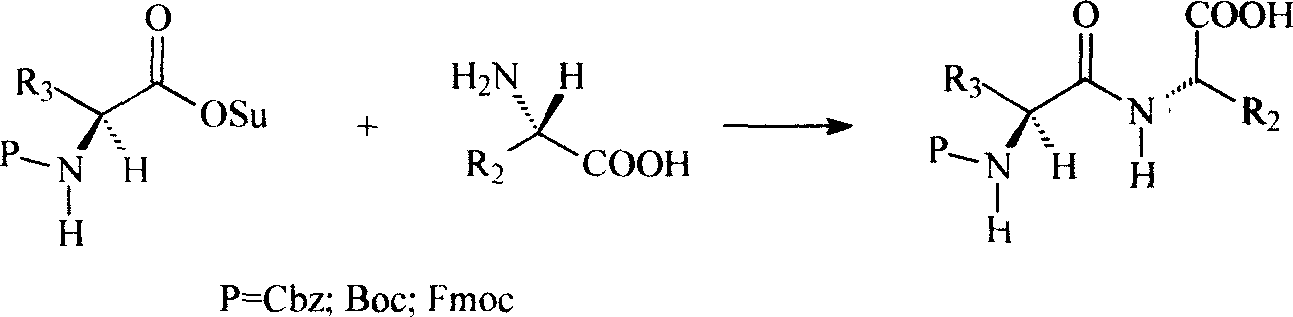
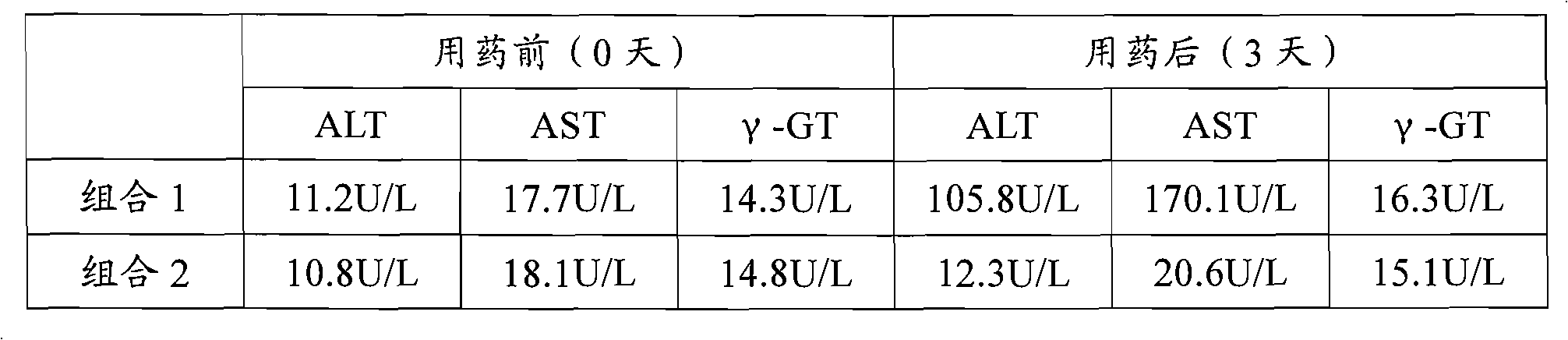
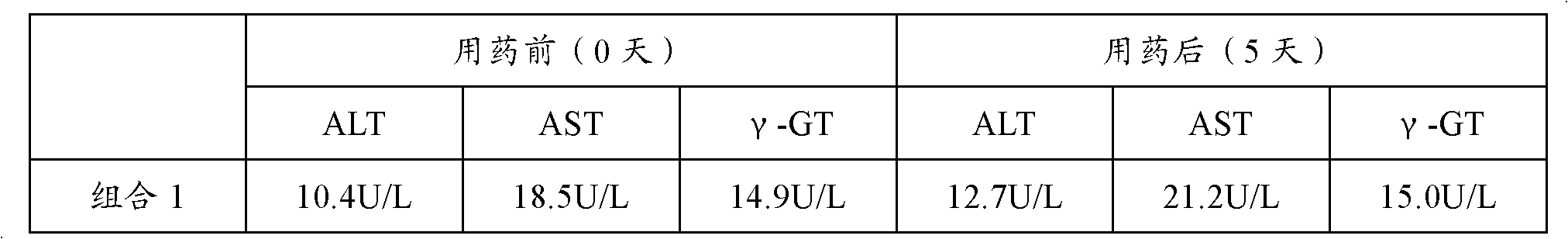
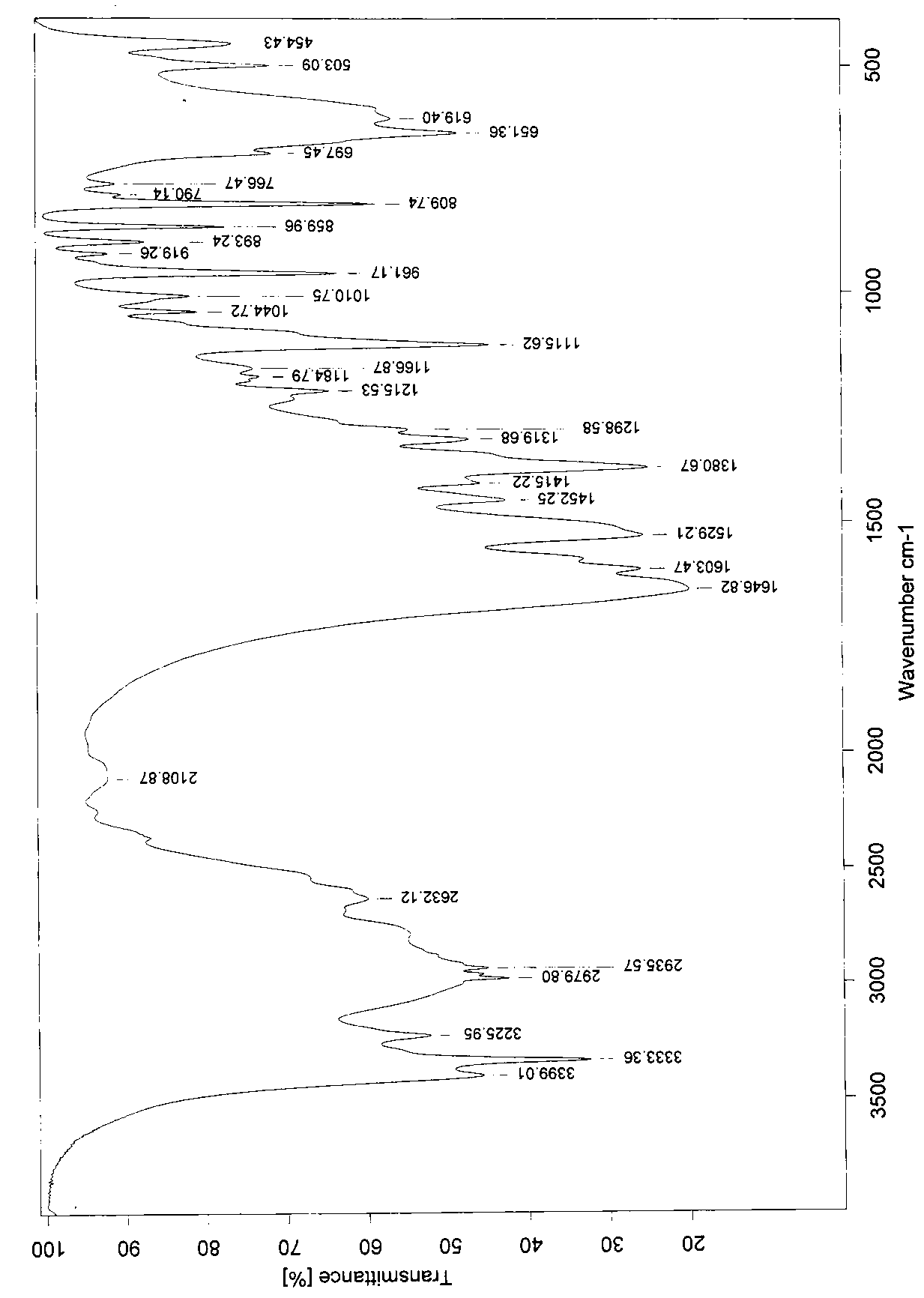
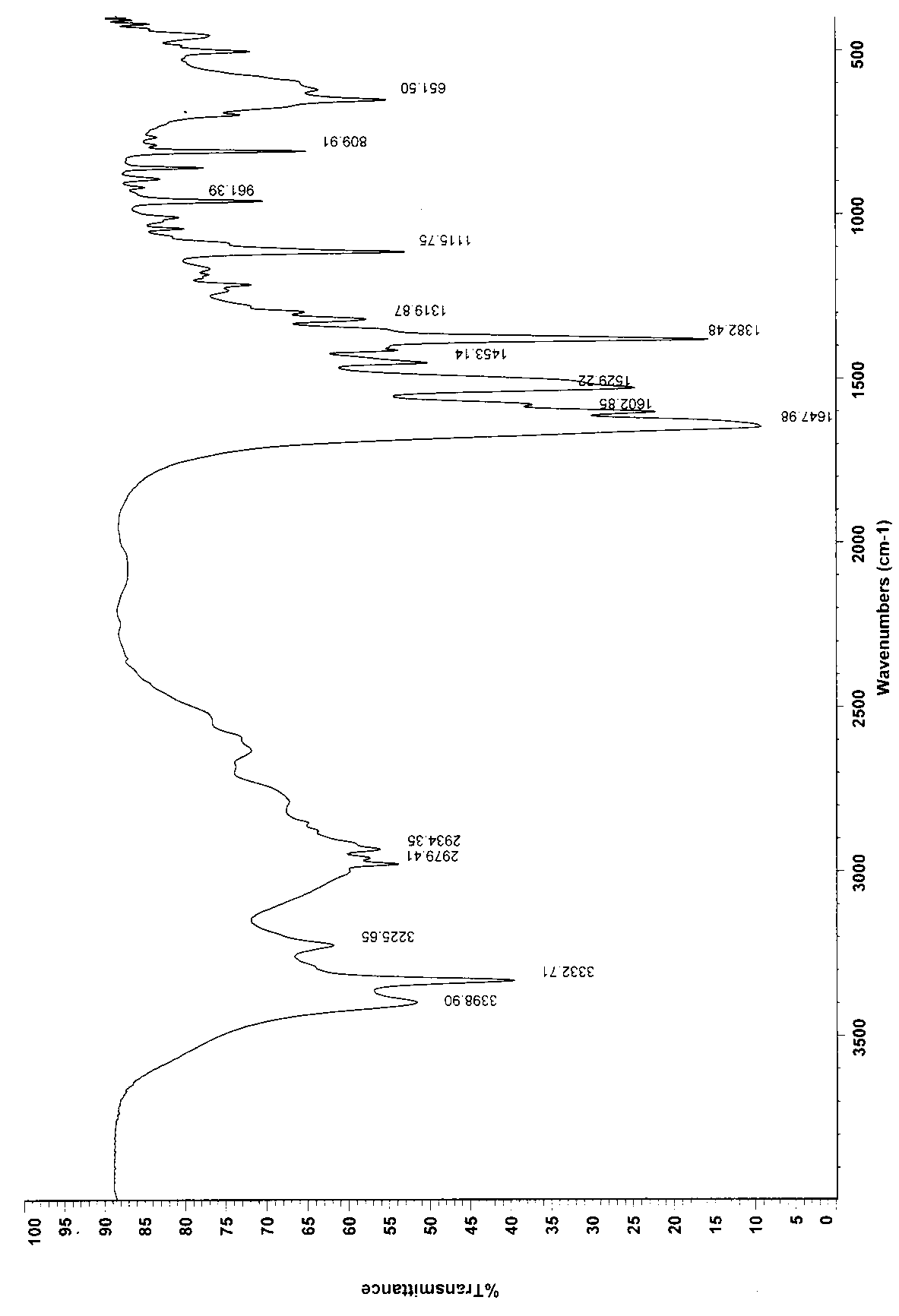
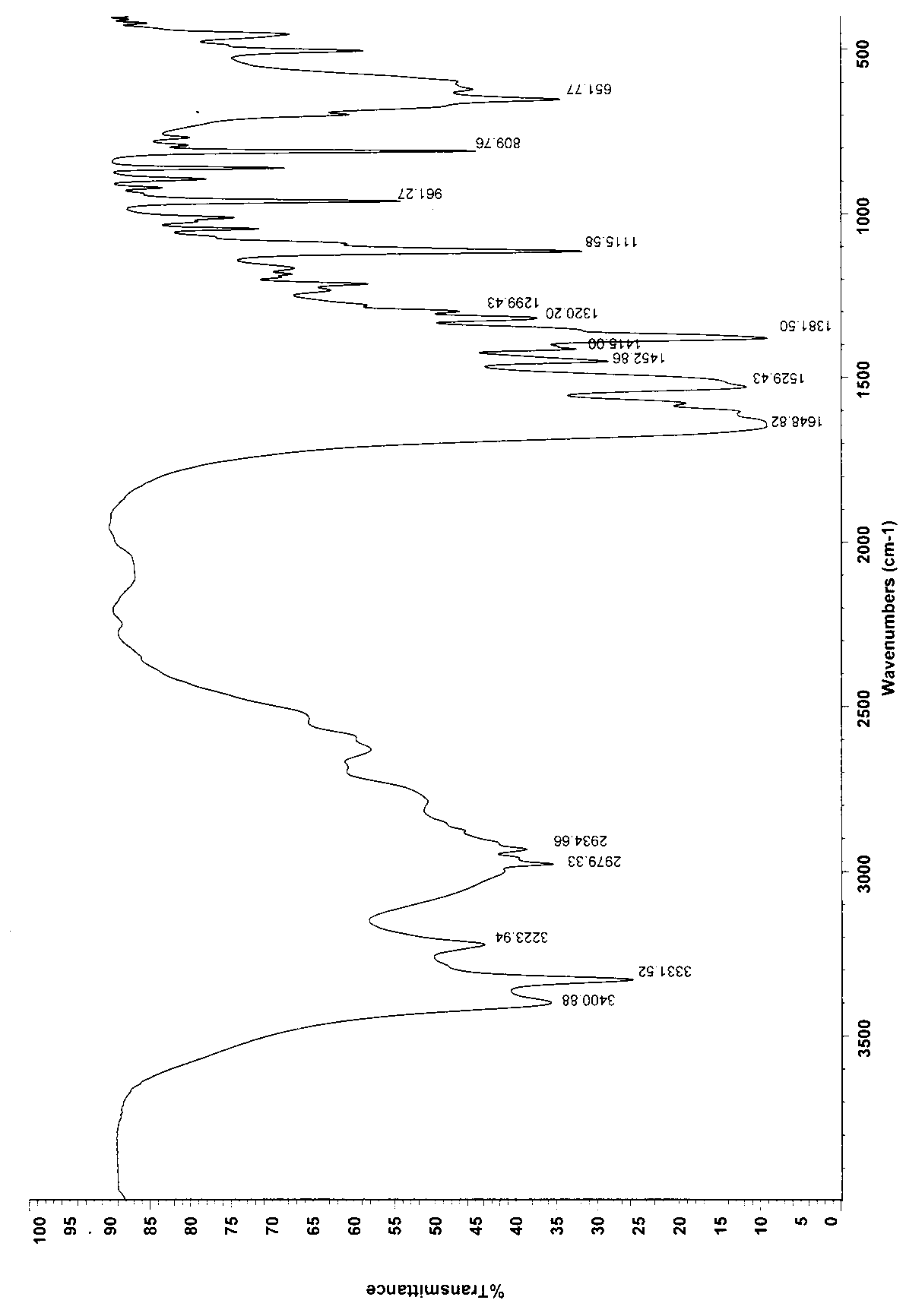
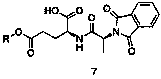
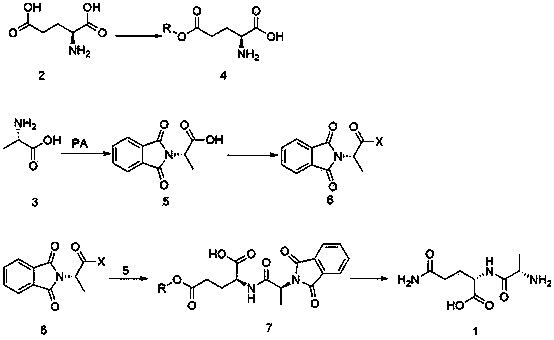
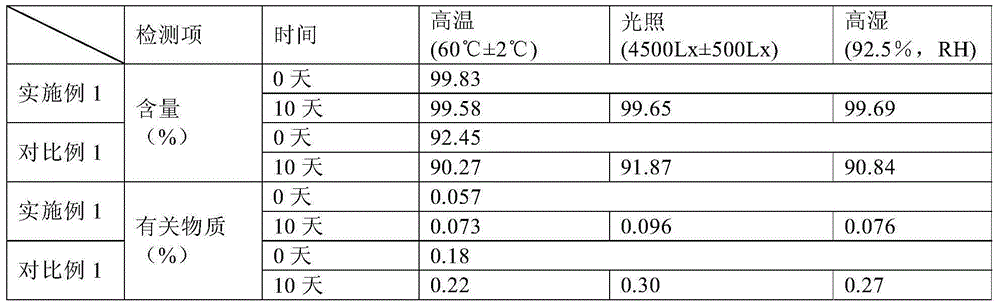
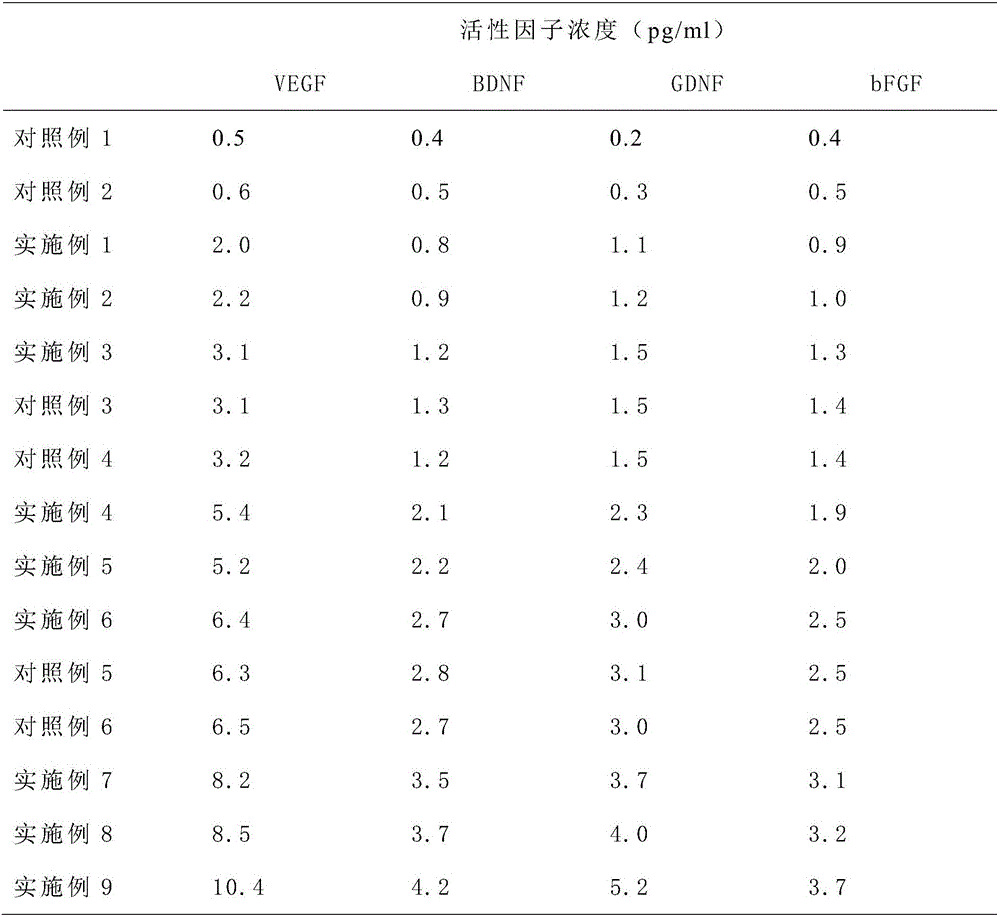
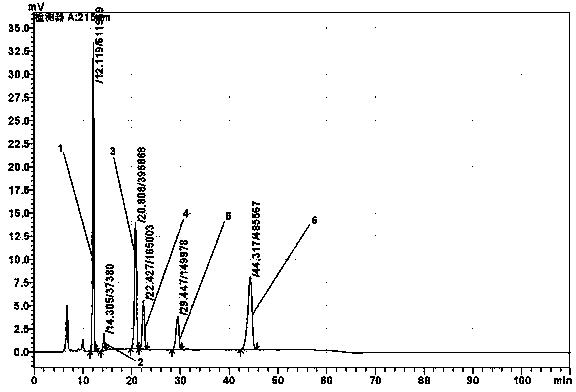
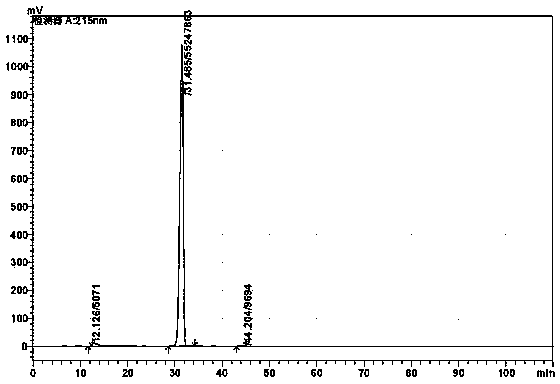
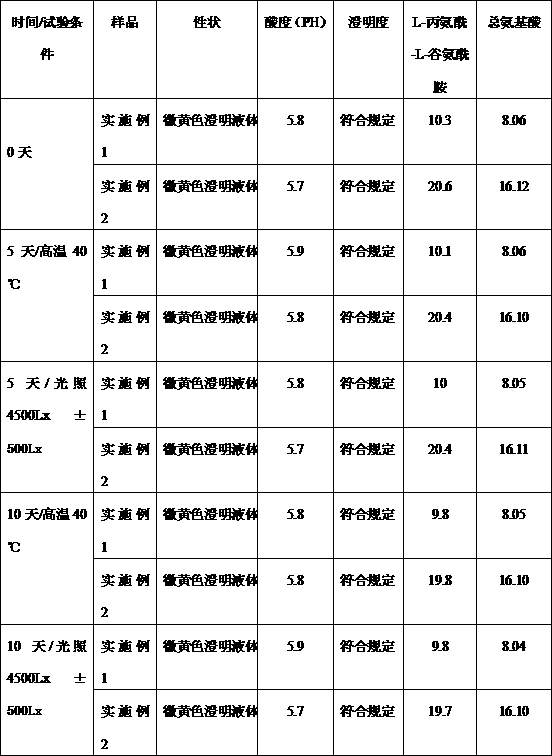
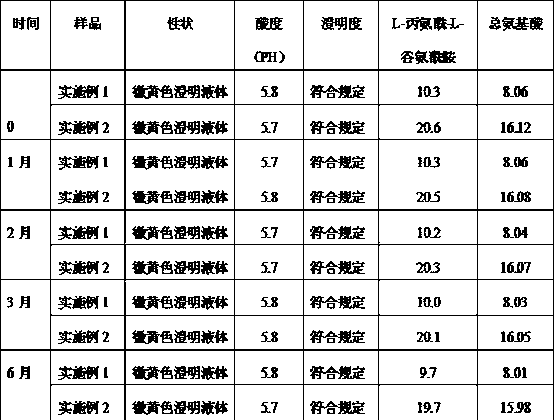
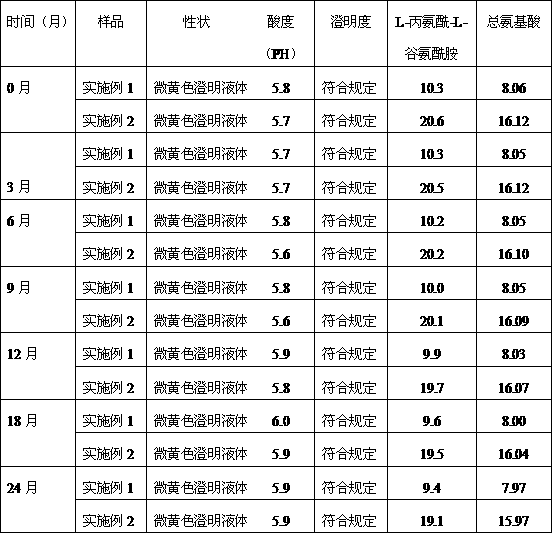
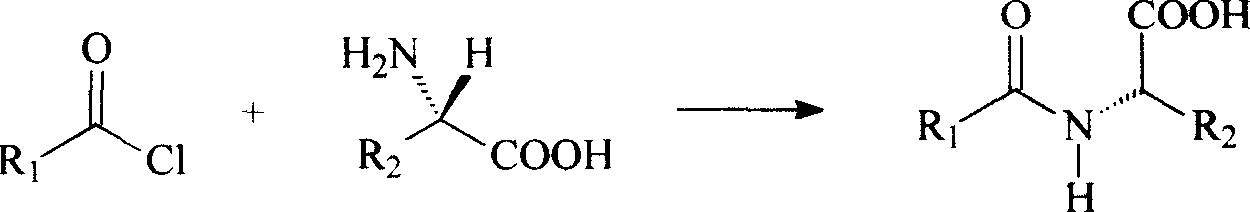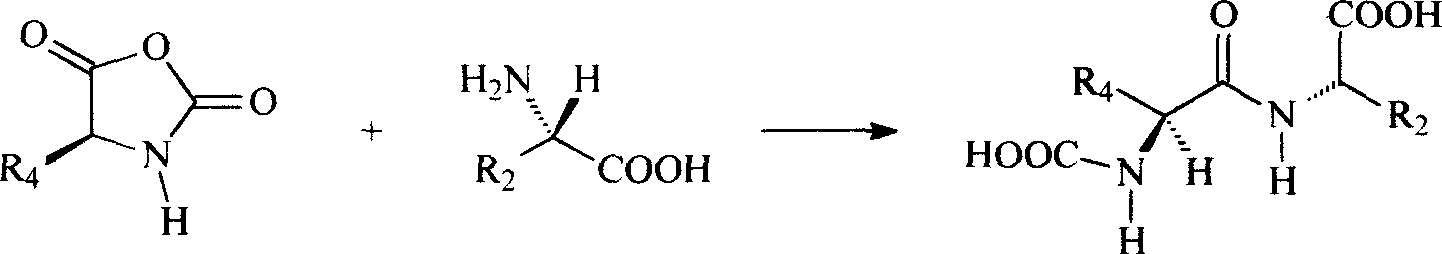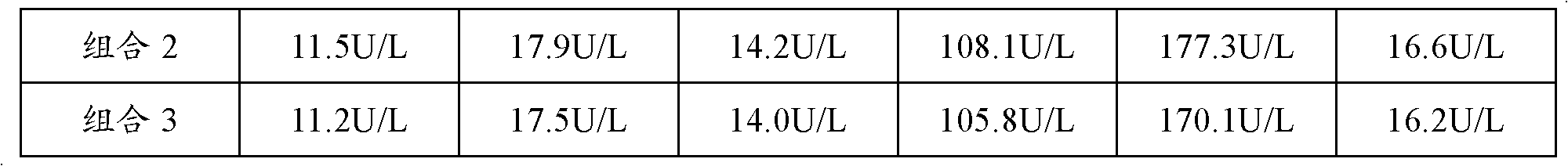Patents
Literature
99 results about "Alanyl glutamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Formulations and methods for nutrient delivery
InactiveUS20110208153A1Promote healthy developmentAvoid developmentMilk preparationEdible oils/fats ingredientsDocosahexaenoic acidNutritional deficiency
The present disclosure provides formulations and methods for delivering water-soluble and lipid-soluble nutrients for preventing or correcting nutrient deficiencies to subjects requiring small-volume nutritional support, such as preterm infants. The formulations may comprise an emulsion of docosahexaenoic acid (DHA) stabilized by a protein emulsifier, such as α-lactalbumin, and may further comprise other valuable nutrients, such as arachidonic acid (ARA), arginine, glutamine, arginyl-glutamine dipeptide and / or alanyl-glutamine dipeptide. The formulation is useful, for example, for correcting nutritional deficiencies by increasing a subject's intake of nutrients such as ω-3 or ω-6 long-chain polyunsaturated acids, proteins, peptides, vitamins, minerals, other fatty acids, and / or essential amino acids. The nutritional formulation is suitable for enteral delivery and small-volume delivery via nasogastric tube, intragastric feeding, transpyloric administration and / or any other means of administration that result in the introduction of the nutritional formulation into the digestive tract of a subject.
Owner:MEAD JOHNSON NUTRITION
Preparation method of N(2)-L-alanyl-L-glutamine
InactiveCN101062938ASimple processEasy to operatePeptide preparation methodsBulk chemical productionL-alanyl-l-glutamineDipeptide
The invention discloses a preparing method of N(2)-L-alanyl-L-glutamine, which comprises the following steps: producing precursor acid potassium; producing mixed acid anhydride; producing glutamine sodium salt; producing N(2)-L-alanyl-L-glutamine (ala paddy dipeptide); refining. This invention possesses the advantages of simple craft, convenient operation and little contamination.
Owner:福建三爱药业有限公司
Method for preparing alanyl-glutamine dipeptide through biological enzyme conversion
InactiveCN105274174AHigh activityImprove catalytic conversion effectFermentationTemperature controlReaction rate
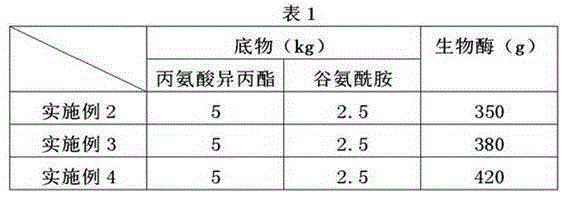
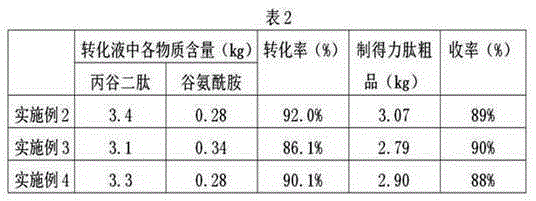
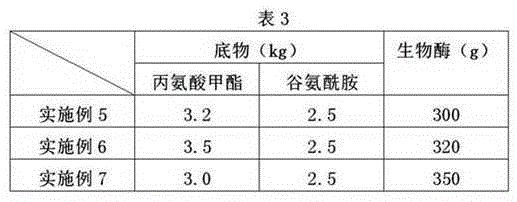
The invention relates to the technical field of the preparation for alanyl-glutamine dipeptide and particularly discloses a method for preparing alanyl-glutamine dipeptide through biological enzyme conversion. The method comprises the following steps: 1) preparing a biological enzyme liquid: dissolving the biological enzyme in a buffered liquid, controlling pH value within the scope of 7.0+ / -2.0, controlling the temperature at 5-10 DEG C, thereby acquiring the biological enzyme liquid, wherein the mass fraction of the ester acyl-containing transferring biological enzyme is 5%-20%; 2) dissolving the ester derivates of substrate L-alanine and glutamine in pure water, and then uniformly dropping the biological enzyme liquid into a reaction liquor, and meanwhile, adding acid liquor or alkali liquor, controlling the pH of the reaction liquor at 5-10, reacting under the temperature controlled at 15-25 DEG C, sampling and detecting the production of the alanyl-glutamine dipeptide during the reaction process, stopping reaction when the content of the alanyl-glutamine dipeptide is highest, and separating the acquired converted liquid, thereby acquiring the alanyl-glutamine dipeptide. According to the method provided by the invention, the enzyme activity is high, the reaction rate of the materials is higher, the method has the advantages of simple technique control, easy control on quality index and low cost, and the method is more fit for industrial production.
Owner:JING JING PHARMA
Preparation of alanyl glutamine dipeptide compound
Production of alanyl glutamine dipeptide compound is carried out by adding L-glutamine into mixed liquid of methylbenzene and water, cooling, adding into sodium hydrate, dissolving L-glutamine, dripping into solution with alpha - halopropacyl halide, methylbenzene and sodium hydrate, regulating pH, reacting, separating organic layer, adding sodium chloride into solution with organic layer separation, regulating pH, adding into concentrated chlorhydric acid, regulating pH, laying aside, filtering, separating out crystal, drying crystal, reacting with ammonia water, controlling temperature and pressure, cooling, de-pressuring, concentrating, adding water, dripping into methyl alcohol, laying aside, filtering, separating out L-alanyl-L-glutamate crude products, purifying and obtaining refined products. It achieves simple process and low cost.
Owner:上海依福瑞实业有限公司
Nú¿2ú®-L-alanyl-L-glutamine injection and its preparation method
InactiveCN1602850AImprove stabilityEasy to transportPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLL-alanyl-l-glutamine
The invention provides a N(2)-L-alanyl-glutamine for injection and its preparing method, it has mixed N(2)-L-alanyl-glutamine and mannite to make N(2)-L-alanyl-glutamine whose mess percentage density is 50-100%. The invention has increased the stability of medicine greatly, convenient for transportation and storage, suitable for sicker in catabolism and hypermetabolism condition who needs glutamine.
Owner:灵康药业集团股份有限公司
Large-scale preparation method of mesenchymal stem cell factors
ActiveCN106754639AAvoid inactivationEasy to separate and purifyBioreactor/fermenter combinationsBiological substance pretreatmentsSingle sampleL-alanyl-l-glutamine
The invention relates to a large-scale preparation method of mesenchymal stem cell factors. The large-scale preparation method comprises three steps of invitro culture of mesenchymal stem cells, induction and secretion of the mesenchymal stem cell factors and separation and purification of the mesenchymal stem cell factors, wherein an induction medium required by the step of the induction and secretion of the mesenchymal stem cell factors is prepared from a mixed medium of a DMEM (Dulbecco's Modified Eagle Medium), a PBS buffer solution and an L-alanyl-L-glutamine aqueous solution of which the volume ratio is (30 to 80):(20 to 60):(0.2 to 1.5) and an inducer. According to the large-scale preparation method of the mesenchymal stem cell factors, a single sample source is adopted to culture on a large scale, so that the uniformity of batch products is guaranteed, and the induction medium is adopted in the step of the induction and secretion of the mesenchymal stem cell factors, so that a large quantity of cell factors are stimulated to secrete, a formula is reasonable and effective, the conditions of the whole preparation process are stable and reasonable, and the large-scale preparation method is suitable for large-scale production.
Owner:北京银丰鼎诚生物工程技术有限公司
Pharmaceutical composition of alanyl glutamine and compound amino acid
InactiveCN102429902AImprove securityAvoid quality impactOrganic active ingredientsPowder deliveryFreeze-dryingAmino Acid Injection
The invention relates to a pharmaceutical composition of alanyl glutamine aseptic frozen-dried powder for injection and compound amino acid injection, particularly a combined application package, comprising alanyl glutamine aseptic frozen-dried powder for injection, water for injection, and compound amino acid injection. When the pharmaceutical composition is used, the alanyl glutamine aseptic frozen-dried powder for injection is dissolved with water for injection, and then is mixed with compatible amino acid injection or transfusion containing amino acid, and intravenous infusion is used. The combined application package disclosed herein avoids the influence to quality caused by quality discrepancy of different factories when the combination is used, and greatly increases the safety of application of patients.
Owner:HAINAN LINGKANG PHARMA CO LTD
Pharmaceutical composition of alanyl glutamine injection and compound amino acid injection
InactiveCN102631665AProne to decompositionLittle side effectsOrganic active ingredientsDipeptide ingredientsAmino Acid InjectionPharmacology
The invention relates to a pharmaceutical composition of an alanyl glutamine injection and a compound amino acid injection, in particular to combination application package. The pharmaceutical composition comprises the alanyl glutamine injection and the compound amino acid injection. When the pharmaceutical composition is in use, the alanyl glutamine injection and the compound amino acid injection are jointly utilized and are intravenously dripped. The combination application package greatly facilitates the requirement of clinical application.
Owner:灵康药业集团股份有限公司
Preparation method of L-alanyl-L-glutamine
The invention relates to a preparation method of L-alanyl-L-glutamine; the preparation method comprises the following steps of: a) adding a solvent to a reaction container; stirring the solvent; and then adding N-phthaloyl-L-alanyl-L-glutamine to the reaction container; putting the reaction container in an ice bath; dripping a short-chain amine solution into a solution for clarification; removing the ice bath; adding the rest short-chain amine solution to the solution; after finishing dripping, carrying out room temperature reaction on the solution; decompressing and concentrating the solution to remove excessive short-chain amine in a water bath until the pH value of the solution is 6-7; standing for crystallization; filtering crystals; and drying filter cakes at a normal pressure so as to obtain a crude product; and b) adding water to the crude product at room temperature; dissolving the crude product in the water; dripping short-chain alcohol into the water for crystallization and purification during stirring, so as to obtain the L-alanyl-L-glutamine. Through the adoption of the preparation method of the L-alanyl-L-glutamine provided by the invention, hypertoxic hydrazine hydrate is avoided; and the preparation method of the L-alanyl-L-glutamine provided by the invention has the advantages of greater safety in operation, high product purity, high optical rotation and low impurity content.
Owner:山东诚汇双达药业有限公司
Liquid feed capable of improving immunologic function and antioxidative function of live pigs and preparation method thereof
InactiveCN106212948AEnhance immune functionImprove growth functionFood processingAnimal feeding stuffWeight gainingAnimal science
The invention discloses liquid feed capable of improving the immunologic function and antioxidative function of live pigs and a preparation method thereof. According to the liquid feed, wheat middling is taken as a main raw material and cooperates with tomato meal, broccoli residues, red beans, iron glycine, a lotus leaf fermentation substance and the like, therefore, the immunologic function of the live pigs can be improved, the ill getting rate is decreased, growth and weight gain of the pigs are accelerated, the oxidation resistance of the pigs is enhanced, and the nutrition and mouthfeel of port are improved; the wheat middling can replace usage of the raw materials such as soybean meal and corn, and therefore the feed cost is reduced; lily seeds, green plum pulp and dendrobe flowers are cooperatively used, therefore, the growth mechanism of the pigs can be improved, and growth and development can be promoted; tributyrin, alanyl glutamine dipeptide and calcium propionate are cooperatively used, therefore, the palatability of the feed can be improved, and the nutritional value of the feed can be increased.
Owner:MAANSHAN WUGU POULTRY IND SPECIALIZED COOP
New preparation method of L-alanyl-L-glutamine
The invention provides a new preparation method of L-alanyl-L-glutamine. The new preparation method comprises the following steps of: reacting phthalic anhydride with L-alanyl so as to prepare phthalic anhydride-L-alanyl, and acylating and halogenating the phthalic anhydride-L-alanyl with a halogenated reagent so as to synthesize phthalic anhydride-L-alanyl halogen; carrying out esterification reaction on L-glutamic acid and alcohol under the action of acid catalysis to obtain L-glutamic acid monoester; condensing the L-glutamic acid monoester and the phthalic anhydride-L-alanyl halogen under the action of alkali to obtain phthalic anhydride-L-alanyl-L-glutamic acid monoester which is an important intermediate for synthesizing the L-alanyl-L-glutamine; adding the phthalic anhydride-L-alanyl-L-glutamic acid monoester to ammonium hydroxide for ammonolysis and deprotection, and finally crystallizing in alcohol to obtain L-alanyl-L-glutamine. The new preparation method has the advantages that reaction raw materials are easily available, L-glutamic acid and L-alanyl are low in price, the product purity and the product yield are high, the production cost can be effectively reduced, and large-scale production is realized.
Owner:CONSCI PHARMA
Protein-free, hydrolysate-free and serum-free culture medium and preparation method thereof
ActiveCN104911143AReduce manufacturing costSimplified purification stepsArtificial cell constructsVertebrate cellsDisodium EdetateHydrolysate
The invention relates to a protein-free, hydrolysate-free and serum-free culture medium and a preparation method thereof. The culture medium contains the following substances: manganese gluconate, calcium citrate malate, disodium edetate, progesterone, alanyl-glutamine,glycerophosphate, fructose, vitamin C, vitamin E, sodium selenite, hydroxypropyl-beta-cyclodextrin, cholesterol, malic acid, oxaloacetic acid, vitamin B12, lipoic acid, ferrous sulfate, ferric citrate, uric acid, taurine, reduced glutathione, zinc sulfate, copper sulfate and ferric nitrate. The protein-free, hydrolysate-free and serum-free culture medium has the advantages that the cost is low, the difference among different batches of products is small, the purification step of down-stream products is simplified, and potential safety hazards are reduced.
Owner:SICHUAN BAINUOJI TECH CO LTD
Synthesis method of N-(2)-L-alanyl-L-glutamine
Belonging to preparation of immune dipeptide compounds, the invention discloses a synthesis method of N(2)-L-alanyl-L-glutamine. The preparation steps include: taking D-2-chloropropanoyl chloride and L-glutamine as raw materials, conducting condensation to obtain N-(D-2-propionyl chloride)-L-glutamine, then performing ammoniation to obtain N(2)-L-alanyl-L-glutamine, and carrying out refining so as to obtain a refined product.
Owner:QINGDAO VLAND BIOTECH INC
N-(2)-L-alanyl-L-glutamine compound prepared by adopting particle crystal form optimization technique and preparation thereof
ActiveCN105085612AHigh purityHigh yieldDipeptide ingredientsMetabolism disorderL-alanyl-l-glutamineFreeze-drying
The invention discloses a method for preparing an N-(2)-L-alanyl-L-glutamine compound. The N-(2)-L-alanyl-L-glutamine compound is prepared by adopting a particle process crystal product molecule assembly and form optimization technique. The compound has the characteristics of high purity, high yield, low hygroscopicity and good stability. Meanwhile, the invention also discloses a pharmaceutical composition prepared from the alanyl glutamine prepared by the method. The pharmaceutical composition is prepared through the steps of preparing the alanyl glutamine into a 10-30% aqueous solution, adsorbing the aqueous solution with medicinal activated charcoal according to the ratio (w / v) of 0.1-0.5%, then, carrying out big-dish freeze-drying, and carrying out aseptic packaging. The whole preparation process is simple in operation and does not need any excipient, and the product has better stability compared with the products in the past.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
Secretion inducing medium for large-scale preparation of human umbilical cord mesenchymal stem cell factors
The invention discloses a secretion inducing medium for large-scale preparation of human umbilical cord mesenchymal stem cell factors. The secretion inducing medium is mainly prepared by dissolving DMEM (Dulbecco's modified eagle medium), L-alanyl-L-glutamine, 4-2-ethoxyl-1-piperazine ethanesulfonic acid, D-glucose and L-ascorbic acid inside phosphate buffer, wherein the concentrations of the DMEM, the L-alanyl-L-glutamine, the 4-2-ethoxyl-1-piperazine ethanesulfonic acid, the D-glucose and the L-ascorbic acid inside the secretion inducing medium are, respectively, 400-600 ml / L, 1-3 mmol / L, 10-20 mu mol / L and 10-100 mu mol / L. The secretion inducing medium for large-scale preparation of the human umbilical cord mesenchymal stem cell factors is applicable to large-scale cultivation of human umbilical cord mesenchymal stem cells and can efficiency induce secretion of active factors.
Owner:北京银丰鼎诚生物工程技术有限公司
Intestine nutrition peptide for piglings
InactiveCN1792218AAvoid Weaning StressIncrease daily weight gainFood processingAnimal feeding stuffRapeseedOligopeptide
An intestinal nutrition peptide as an additive of feed for piglet is prepared from one or more of oligopeptide, plycyl-L-glutamine, alanyl glutamine and iron glycinate. It can prevent the delactation stress of piglet and can be used to replace to plasma albumin powder. Said oligopeptide is prepared from soybean protein and rapeseed protein through sterilizing, enzymolyzing, deactivating enzyme and ultra-filtration.
Owner:ZHEJIANG UNIV
Anti-stress puffed compound feed for grass carps and preparation method of anti-stress puffed compound feed
ActiveCN104489396AWell mixedGood for healthClimate change adaptationAnimal feeding stuffAnimal sciencePhytase
The invention discloses an anti-stress puffed compound feed for grass carps. The anti-stress puffed compound feed is prepared from the following raw materials in parts by weight: 1-4 parts of fish meal, 10-25 parts of soybean meal, 20-35 parts of rapeseed meal, 5-15 parts of cottonseed meal, 5-13 parts of DDGS, 0-10 parts of rice bran, 15-25 parts of wheat, 0.2 part of choline chloride, 1.2-2 parts of monocalcium phosphate, 0.5 part of a multi-mineral-substance pre-mixture, 1-2 parts of soybean oil, 0.15 part of a multi-vitamin pre-mixture, 0.01-0.03 part of alanyl-glutamine, 0.02 part of bile acid, 0.02-0.1 part of high temperature resisting phytase, 0.1-0.3 part of radix ophiopogonis, 0.1-0.3 part of eclipta and 0.1-0.3 part of astragalus membranaceus. The invention provides the puffed compound feed for the grass carps, and the puffed compound feed has the advantages of satisfying the rapid growth demands of the grass carps, improving the high-temperature stress resistance of the grass carps, reducing economic loss caused by poor transportation resistance of the grass carps and promoting continuous development and healthy cultivation of the grass carps.
Owner:GUANGZHOU PANYU DACHUAN FEED
Refining method of alanyl-glutamine crude drug
The invention relates to a refining method of an alanyl-glutamine crude drug. The method comprises the following processes: adding an alanyl-glutamine crude product to water, stirring until the alanyl-glutamine crude product is completely dissolved; adding alkali to adjust the pH value, adding active carbon, stirring for realizing absorption; filtering and decolorizing to obtain clear material liquid, then adding a polar solvent, crystallizing and filtering; and drying to obtain an alanyl-glutamine refined product. By adopting the refining method, alanyl-glutamine impurities and the content of bacterial endotoxin are effectively controlled; the target of intravenous administration is achieved; and the safety of alanyl-glutamine intravenous preparations is also improved.
Owner:SHANDONG QIDU PHARMA
Nú¿2ú®-L-alanyl-L-glutamine injection and its preparation method
InactiveCN100428931CImprove stabilityEasy to transportOrganic active ingredientsPowder deliveryPharmaceutical drugHypermetabolism
The invention provides a N(2)-L-alanyl-glutamine for injection and its preparing method, it has mixed N(2)-L-alanyl-glutamine and mannite to make N(2)-L-alanyl-glutamine whose mess percentage density is 50-100%. The invention has increased the stability of medicine greatly, convenient for transportation and storage, suitable for sicker in catabolism and hypermetabolism condition who needs glutamine.
Owner:灵康药业集团股份有限公司
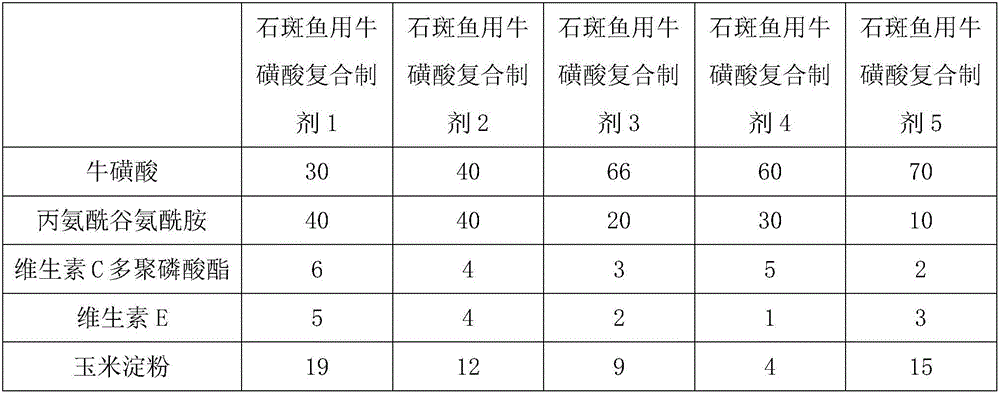
Taurine compound preparation for groupers and application thereof
ActiveCN106616037APromote proliferationConducive to survivalClimate change adaptationAccessory food factorsVitamin CTaurine
The present invention discloses a taurine compound preparation for groupers and an application thereof. The taurine compound preparation is composed of the following raw materials in parts by weight: 30-70 parts of taurine, 10-40 parts of alanyl-glutamine, 2-6 parts of vitamin C polyphosphate, 1-5 parts of vitamin E and 4-19 parts of corn starch. The taurine compound preparation for the groupers has the advantages of promoting the growth and feed conversion efficiency of the groupers, reducing the ratio of liver weight and body weight, reducing the deposition of body fat and liver fat of the groupers, can significantly improve the activity and total antioxidant capacity of superoxide dismutase, catalase, serum antioxidant enzymes and glutathione peroxidase in liver and plasma of saddletail groupers, and at the same time significantly reduces the malondialdehyde content.
Owner:JIMEI UNIV
Pharmaceutical composition for treating acute cerebral hemorrhage and application thereof
The invention relates to a pharmaceutical composition for treating acute cerebral hemorrhage. The pharmaceutical composition comprises a vitamin B6 injection, a concentrated solution of N(2)-L-alanyl-glutamine injection concentration, a compound amino acid (15AA)-containing injection or a compound amino acid (18AA)-containing injection, which are placed in a three-chamber co-extrusion infusion bag. Pharmacodynamic experiments show that the pharmaceutical composition has an excellent effect of treating acute cerebral hemorrhage of rats and rabbits. The pharmaceutical composition has effects ofpreventing further bleeding, decreasing intracranial pressure, controlling cerebral oedema, maintaining vital functions, preventing and curing complications and the like; the pharmaceutical composition has excellent curative effects of treating endogenous acute cerebral hemorrhage and exogenous acute cerebral hemorrhage, and is an ideal medicine for treating the acute cerebral hemorrhage.
Owner:张海峰
Breeding method for paracobitis variegatus fries and incubator used for hatching oosperms of paracobitis variegatus
ActiveCN105123560AHigh activityIncrease the number ofClimate change adaptationAnimal feeding stuffAnimal ForagingVitamin B6 synthesis
The invention discloses a breeding method for paracobitis variegatus fries and an incubator used for hatching oosperms of the the paracobitis variegates. The breeding method comprises parent fish selection, cultivation before spawning induction, artificial spawning induction, management after spawning induction, artificial dry-method fertilization, oosperm incubation, and management of paracobitis variegatus fries. Paracobitis variegatus fries can be obtained after the above process. In the step of cultivation before spawning induction, feeding spawning induction forage 5 to 7 days before a breeding season, and the spawning induction forage comprises fish meal, casein, soybean meal, soybean phosphatide, starch, unsaturated fatty acid, vitamin E, vitamin B5, vitamin B6, alanyl-glutamine, selenium powder and DOM. In the step of artificial spawning induction, injecting composite oxytocic to parent fishes after feeding the spawning induction forage after the breeding season to induct the parent fishes to lay eggs and ejaculate. The incubator comprises a box body and a cover body, the cover body has a double-layer structure, a filtering medium is filled in the interlayer, the filtering medium forms a plurality of diversion channels after passing through holes and extends to 2 to 5 cm above the liquid level in the box body.
Owner:SHENNONGJIA FORESTRY DISTRICT JIAYUAN TECH CULTIVATION CO LTD
Liquid amino acid and vitamin additive capable of reducing weaning stress of piglets as well as preparation method and application of additive
InactiveCN107996857AReduce feeding stressPromote growthAccessory food factorsWeight gainingDipeptide
The invention relates to a liquid amino acid and vitamin additive capable of reducing weaning stress of piglets as well as a preparation method and an application of the additive and belongs to the feed field. The liquid amino acid and vitamin additive comprises alanyl-glutamine dipeptide, sodium butyrate, decavitamin, compound amino acid, xylooligosaccharide, an emulsifier and a preservative. Theliquid amino acid and vitamin additive can increase the nutrient substance digestion rate of piglets, regulate micro-ecological balance of intestinal tracts, improve body anabolism of key nutrient substances, enhance protein synthesis, promote growth performance and increase the nursing survival rate. The preparation method of the liquid amino acid and vitamin additive comprises the steps as follows: an aqueous phase solution and an oil phase solution are prepared, respectively, the aqueous phase and the oil phase solution are mixed, and the liquid amino acid and vitamin additive is prepared.The preparation method is simple and easy to operate. After the drink is used for preparing piglet feed, the feed intake of weaned piglets can be increased, the daily weight gain can be increased, diarrhea rate after weaning can be decreased, and weaning stress of the weaned piglets can be relieved.
Owner:NANCHANG AONONG BIOLOGICAL SCI & TECH +2
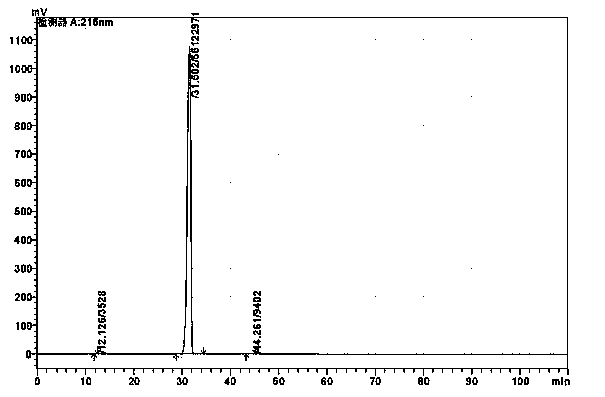
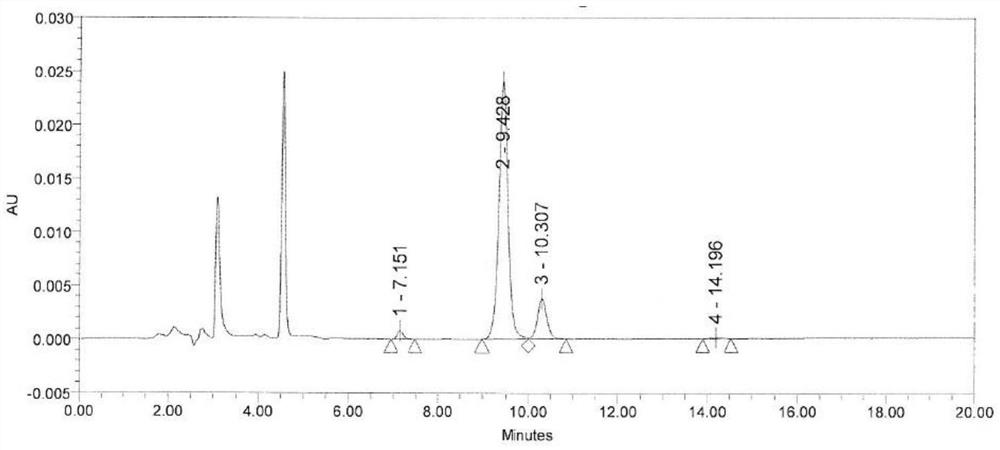
Method for detecting alanyl glutamine impurities in compound amino acid injection
ActiveCN111812259ARapid determinationDon't worry about interferenceComponent separationOrganic solventFluid phase
The invention belongs to the technical field of medicinal chemistry detection, and particularly relates to a method for detecting alanyl glutamine impurities in a compound amino acid injection. The detection method provided by the invention comprises the following steps: preparing an L-pyroglutamic acid reference substance solution; preparing a compound amino acid injection (containing alanyl glutamine) test solution by adopting cation exchange resin; determining high performance liquid chromatography conditions, detecting the L-pyroglutamic acid reference substance solution and the compound amino acid injection test solution by adopting high performance liquid chromatography, and calculating the content of alanyl glutamine impurities in the test solution by using a correction factor method. The method can accurately detect the content of the alanyl glutamine impurities in the complex sample, does not involve the use of an organic solvent in the experiment process, only uses the pyroglutamic acid with low price as a reference substance, and greatly saves the daily detection cost.
Owner:费森尤斯卡比华瑞制药有限公司
Dental pulp mesenchymal stem cell culture medium and application method thereof
ActiveCN114107187APromote rapid proliferationHigh purityCulture processSkeletal/connective tissue cellsStem cell cultureLysis buffer
The invention provides a dental pulp mesenchymal stem cell culture medium, which is prepared from the following components in percentage by volume: 5 to 20 percent of platelet lysis buffer, 0.05 to 0.3 percent of epidermal growth factor solution, 0.05 to 0.3 percent of basic fibroblast growth factor solution, 0.1 to 1 percent of L-alanyl-L-glutamine additive and a cell basal culture medium. According to the dental pulp mesenchymal stem cell culture medium, the contents of the components are added and limited, so that the dental pulp mesenchymal stem cells can be promoted to rapidly proliferate from the aspects of supplying cell nutrition, preventing bacterial pollution and promoting cell growth.
Owner:GUANGDONG PROCAPZOOM BIOSCIENCES CO LTD
N(2)-L-alanyl-L-glutamine/compound amino acid injection (18AA-V) pharmaceutical composite preparation
ActiveCN103071145AGood water solubilityImprove bioavailabilityOrganic active ingredientsDipeptide ingredientsPharmaceutical drugAmino Acid Injection
The invention relates to the technical field of medicines and discloses an N(2)-L-alanyl-L-glutamine / compound amino acid injection (18AA-V) pharmaceutical composite preparation which is prepared by mixing N(2)-L-alanyl-L-glutamine and compound amino acid injection (18AA-V). The pharmaceutical composite preparation has the advantages of definite components, stable quality, high bioavailability, less adverse reactions and the like and has a good application prospect in clinical practice.
Owner:ZHEJIANG CHANGDIAN PHARMA
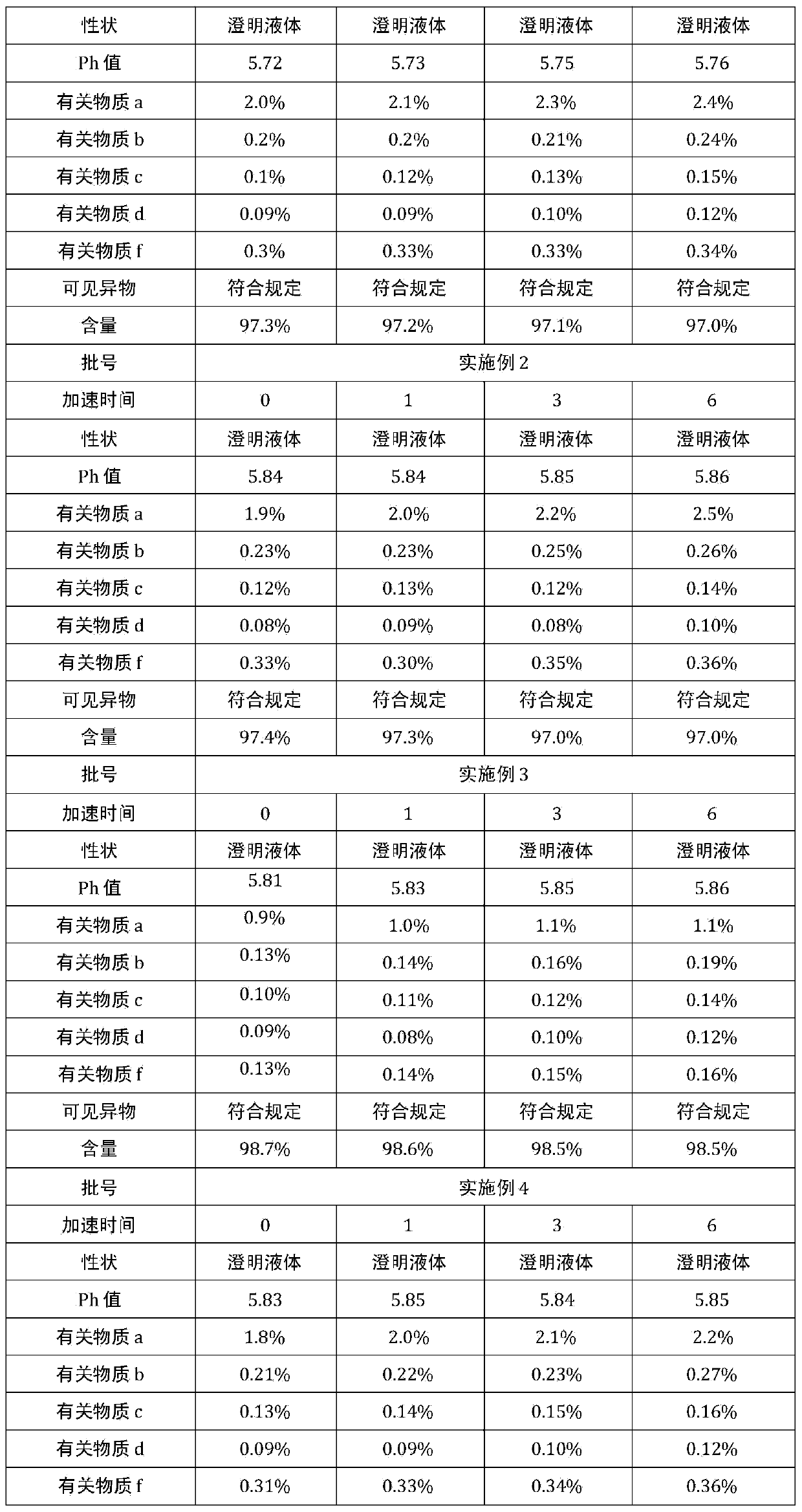
Preparation method of alanyl-glutamine freeze-dried powder
ActiveCN103610652AGood precisionIncrease production capacityPowder deliveryDipeptide ingredientsTemperature controlFreeze-drying
The invention discloses a preparation method of alanyl-glutamine freeze-dried powder. By improving and regulating the conventional preparation method, adjusting a component adding sequence, controlling temperature more precisely, increasing a refined filtration layer, and the like, finished product quality is more stable, and precision is improved. And moreover, the preparation method further can be adapted to different-scale production, and is wider in scope of application.
Owner:HAINAN GENERAL & KANGLI PHARMA
Method for preparing alanyl-glutamine injection
ActiveCN103720649AImprove stabilityDipeptide ingredientsMetabolism disorderActivated carbonInjections water
The invention belongs to the technical field of medicine preparation, and in particular relates to a method for preparing an alanyl-glutamine injection. The method comprises the following steps: (1) adding 30 percent by prescription of injection water and alanyl-glutamine by prescription into a concentrated preparation tank, stirring until the raw materials are completely dissolved, adding wet injection activated carbon, stirring and adsorbing, pumping into a diluted preparation tank, washing the concentrated preparation tank in a fractional manner with 10-60 percent by prescription of injection water, and drawing all the washing water into the diluted preparation tank; (2) filling injection water to 90 percent of the prescription amount after the liquid medicine in the concentrated preparation tank is drawn, regulating the pH value to 5.5-6.3 with a pH value regulating agent, adding the wet injection activated carbon, filling the injection water to full dose, circulating the drawing action for 30-40 minutes; (3) filling, sterilizing and sub-packaging. Compared with the prior art, the alanyl-glutamine injection prepared by the method is safe, reliable and good in stability.
Owner:CISEN PHARMA
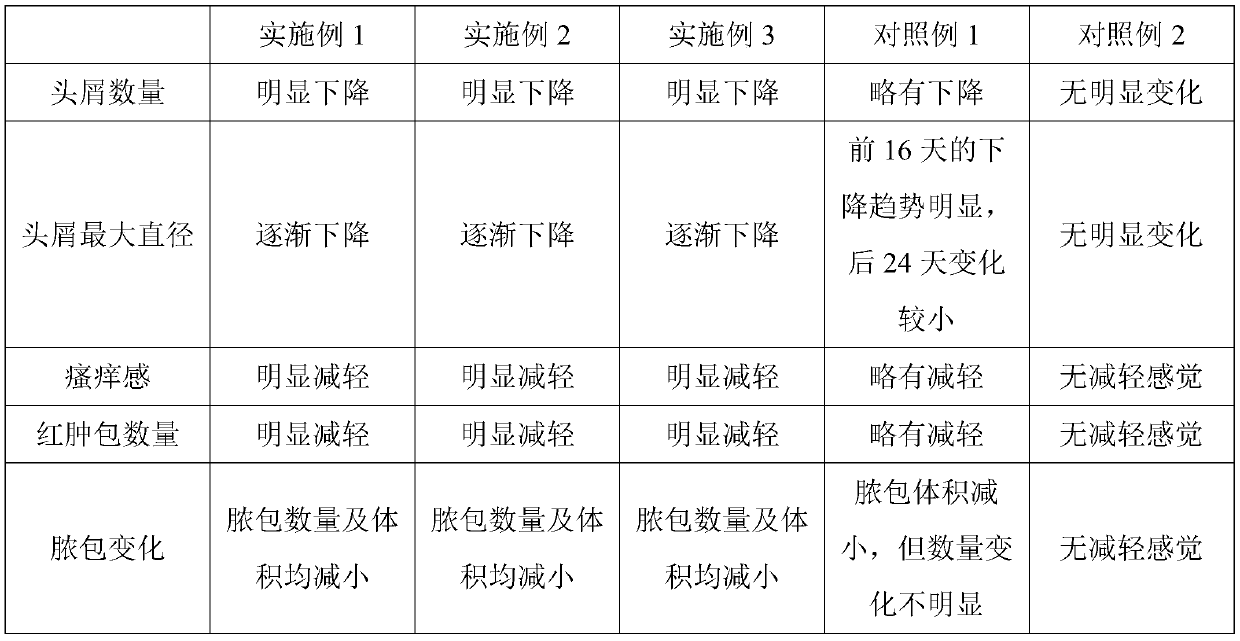
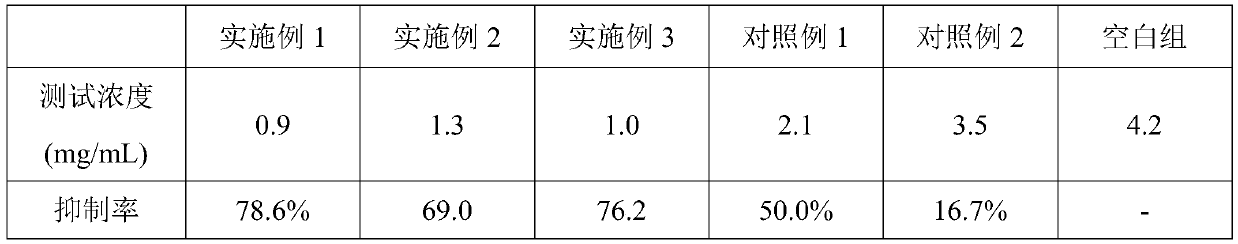
Dandruff-removing itch-relieving composition and preparation method thereof
PendingCN110169925ALowers histamine levelsReduce itching and rednessCosmetic preparationsHair cosmeticsIrritationShampoo Product
The invention provides a dandruff-removing itch-relieving composition and a preparation method and application method thereof. The dandruff-removing itch-relieving composition is prepared from the following raw materials in parts by weight: 4-10 parts of dihydrogen oat alkaloid, 4-8 parts of alanyl glutamine, 5-30 parts of a thickener, 10-25 parts of organic alcohol, 5-10 parts of a surfactant, and 20-60 parts of a dispersing agent. The used raw materials are clear in component, and have little irritation to scalp cells; the dandruff-removing itch-relieving composition containing the dihydrogen oat alkaloid and the alanyl glutamine has a relatively good sterilization effect and anti-allergy effect, nourishes the scalp, slows down the abnormal rapid defluvium of stratum corneum cells of thescalp, and has an obvious itch-relieving effect; in addition, the composition is transparent liquid in appearance, and can be directly added into existing shampoo under room temperature to prepare dandruff-removing itch-relieving shampoo; development of new products, especially transparent shampoo products, by manufactures is convenient; the problems that existing dandruff-removing itch-relievingfunctional components cannot be directly added and / or preparation of the transparent shampoo is difficult are effectively solved.
Owner:甄旭东
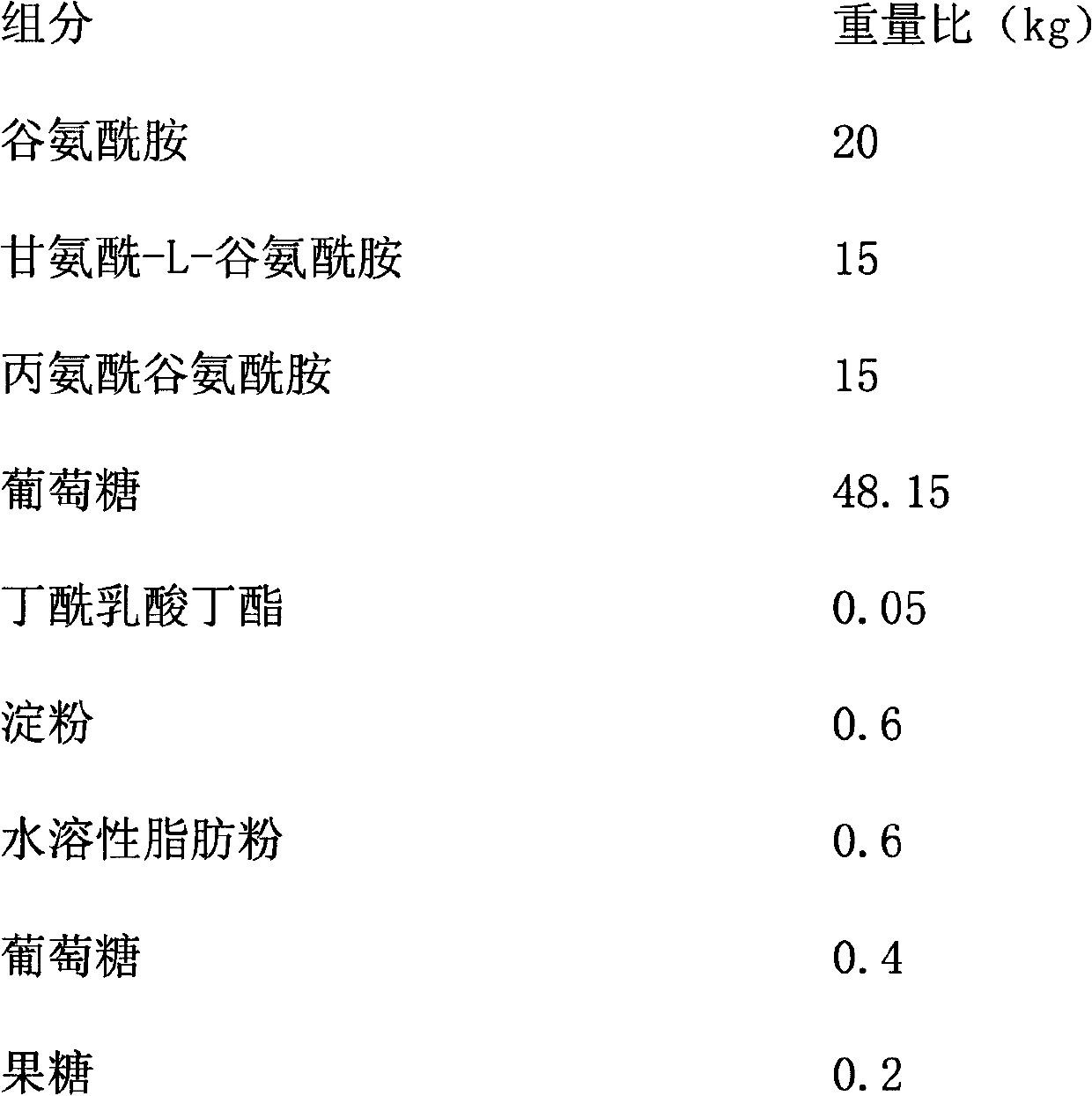
Calf colostrum material
The invention discloses a calf colostrum material, belonging to foster nursing feeds for early-stage weaning of calves. The colostrum material is easy to prepare, after the colostrum material is used, early-stage weaning of the calves is accelerated, daily gain is improved, growth and development of the calves are promoted, and the intestine absorptivity of the newly born calves is greater than 90%. The calf colostrum material is characterized by being composed of glutamine, glycyl-L-glutamine, alanyl-glutamine, glucose, butyl butyryllactate, starch, water-soluble fatty powder and fructose. The calf colostrum material has the beneficial effects of improving the intestinal function, enhancing the activity of digestive enzyme, improving the immunity, obviously reducing weaning stress of the calves, reducing diarrhea rate of the calves, improving the utilization rate of the feed, and improving the daily gain before and after weaning of the calves; the calf colostrum material can completely replace whey mist, has obvious economic benefit and social benefit, and a broad market prospect.
Owner:魏婷
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com