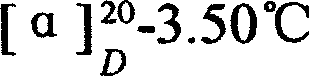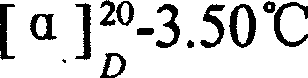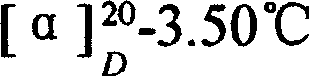Preparation of alanyl glutamine dipeptide compound
A technology of alanyl glutamine and peptide compounds, which is applied in the field of preparation of alanyl glutamine dipeptide compounds, can solve problems such as restrictions on popularization and application, high price, and difficulty in obtaining halogenated propionyl halides. The effect of simple process and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] 1. Preparation of N-(α-chloropropionyl)-L-glutamine
[0008] Add 16.0g (0.11 mol) of L-glutamine to 100ml of water and 50ml of toluene at room temperature and mix them. After cooling to 3°C±1°C, add 22ml (0.11mol) of 5N sodium hydroxide aqueous solution to make - Glutamine dissolved. Then, maintaining the above temperature, dropwise added 14.0 g of α-chloropropionyl chloride (0.11 mol), 30 ml of toluene and 25 ml of 5N sodium hydroxide aqueous solution into the above solution within 2 hours. Adjust the pH value to 9-11 with 5N sodium hydroxide, react at a temperature of 10°C for 2 hours, and separate the organic layer. 20 g of sodium chloride was added to the mixed solution after separating the organic layer at room temperature. Use concentrated hydrochloric acid to adjust the pH value to 2.5 and stir for 30 minutes, then use concentrated hydrochloric acid to adjust the pH value to 1.0, and keep it at room temperature for 4 hours. After filtering and drying the precip...
Embodiment 2
[0012] 1. Preparation of N-(α-chloropropionyl)-L-glutamine
[0013] Add 16.0g (0.11 mol) of L-glutamine to 50ml of water and 25ml of toluene at room temperature and mix. After cooling to 4°C±1°C, add 22ml (0.11mol) of 5N sodium hydroxide aqueous solution to make L - Glutamine dissolved. Then keep the above temperature, dropwise add 28.0g α-chloropropionyl chloride (0.22 mol), 60ml toluene and 50ml 5N sodium hydroxide aqueous solution to the above solution within 4 hours. Adjust the pH value to 9-11 with sodium hydroxide, react at a temperature of 15° C. for 2 hours, and separate the organic layer. 20 g of sodium chloride was added to the mixed solution after separating the organic layer at room temperature. Use concentrated hydrochloric acid to adjust the pH value to 2.5 and stir for 30 minutes, then use concentrated hydrochloric acid to adjust the pH value to 1.0, and keep it at room temperature for 4 hours. After filtering and drying the precipitated crystals, 20.0 g of N-...
Embodiment 3
[0017] 1. Preparation of N-(α-bromopropionyl)-L-glutamine
[0018] Add 16.0g (0.11 mol) of L-glutamine to 100ml of water and 50ml of toluene at room temperature and mix them. After cooling to 3°C±1°C, add 22ml (0.11mol) of 5N sodium hydroxide aqueous solution to make - Glutamine dissolved. Then keep the above temperature, dropwise add aqueous solution containing 17.8g α-bromopropionyl chloride (0.11 mol), 30ml toluene and 25ml sodium hydroxide to the above solution within 2 hours. Adjust the pH value to 9-11 with sodium hydroxide, react at 10°C for 2 hours, and separate the organic layer. 20 g of sodium chloride was added to the mixed solution after separating the organic layer at room temperature. Use concentrated hydrochloric acid to adjust the pH value to 2.5 and stir for 30 minutes, then use concentrated hydrochloric acid to adjust the pH value to 1.0, and keep it at room temperature for 4 hours. After filtering and drying the precipitated crystals, 25.8 g of N-(α-bromop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com