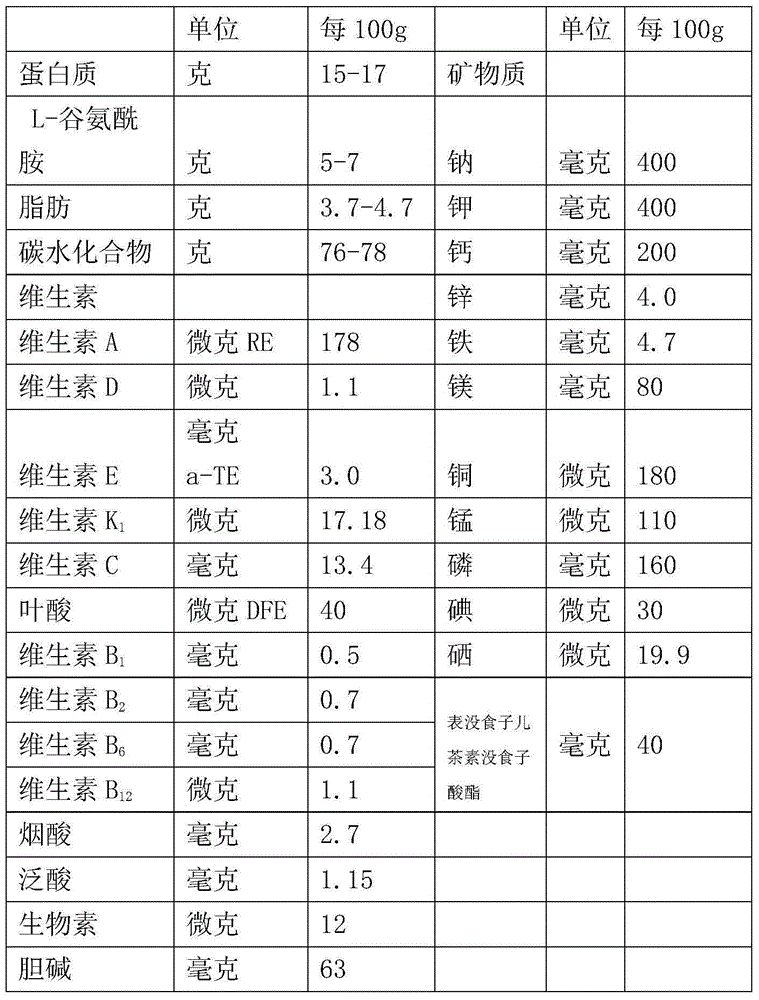
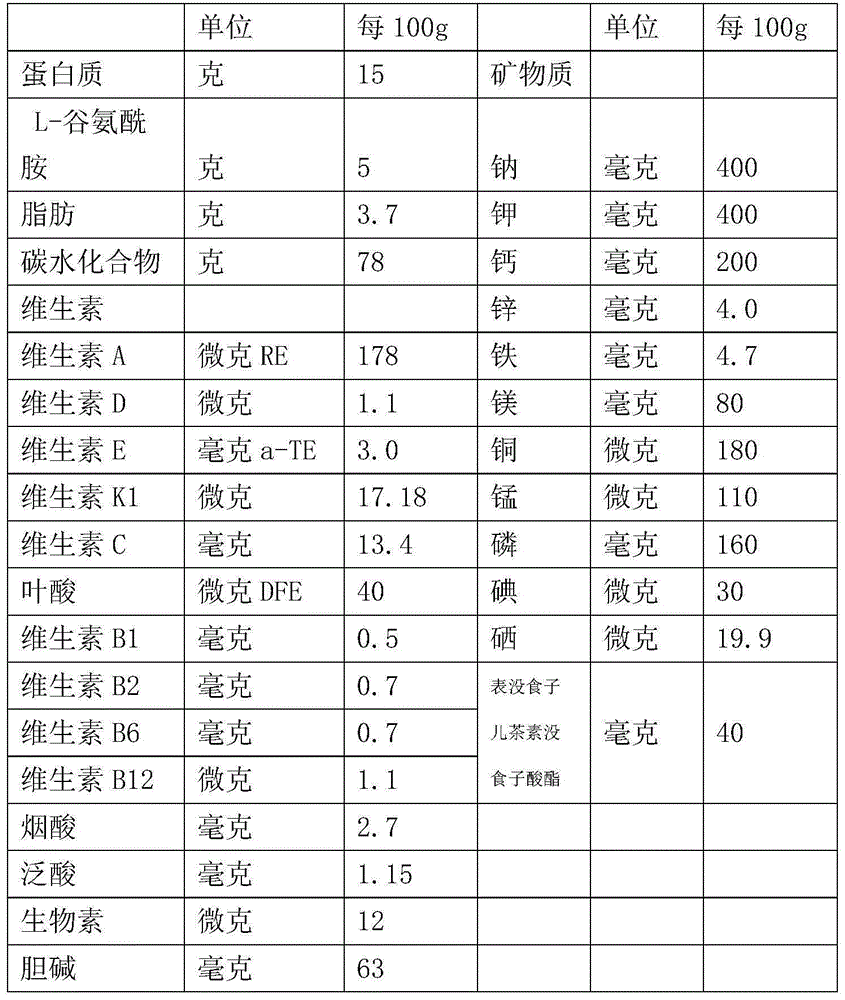
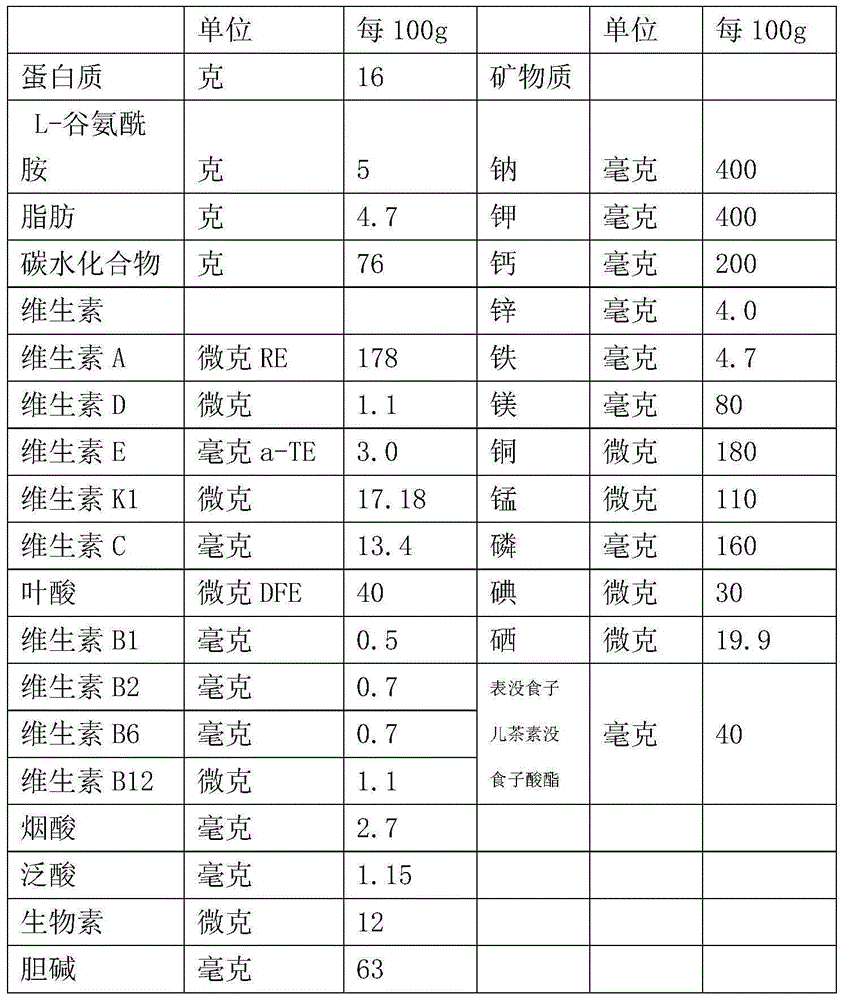
Patents
Literature
518 results about "L-glutamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
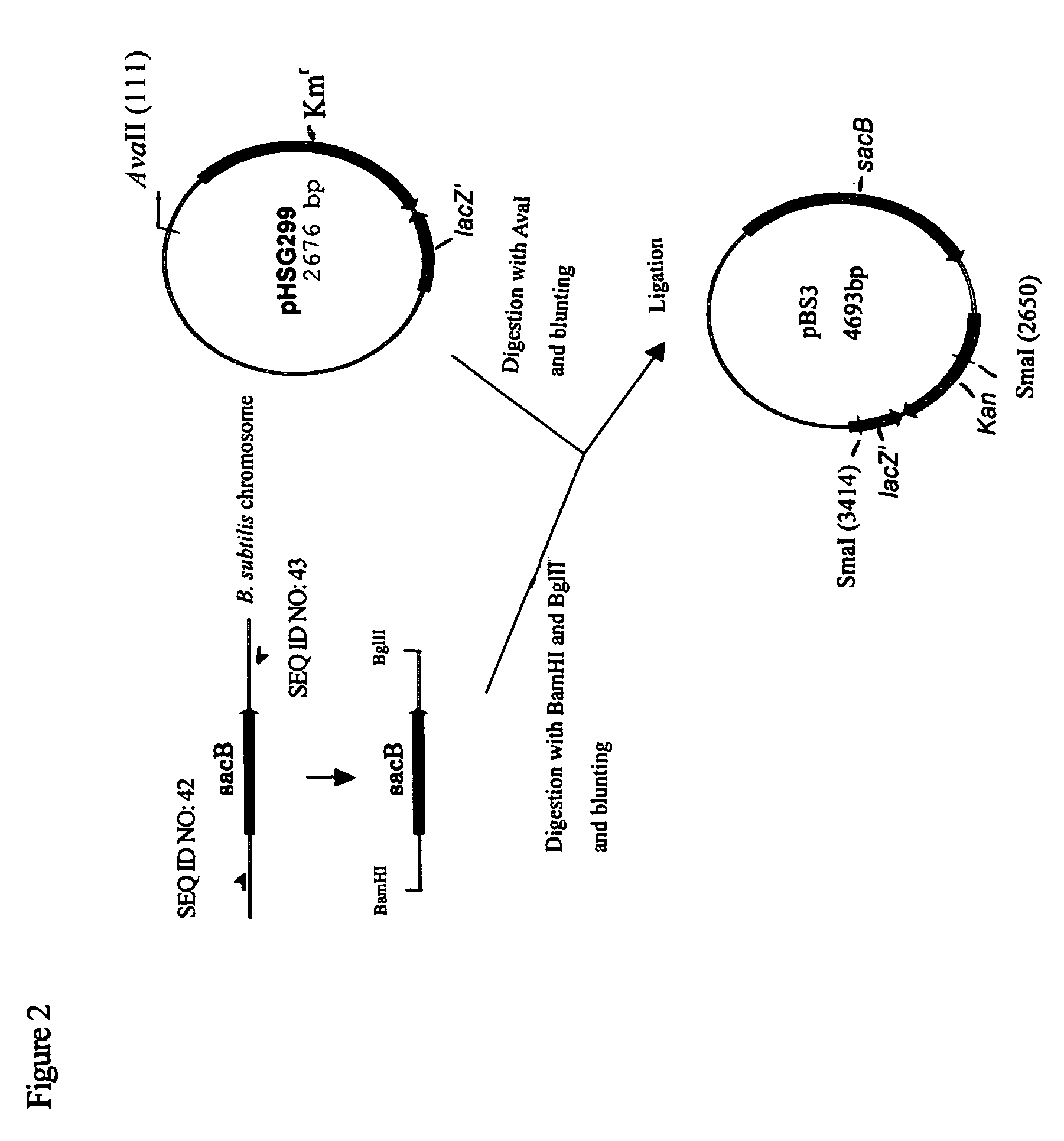
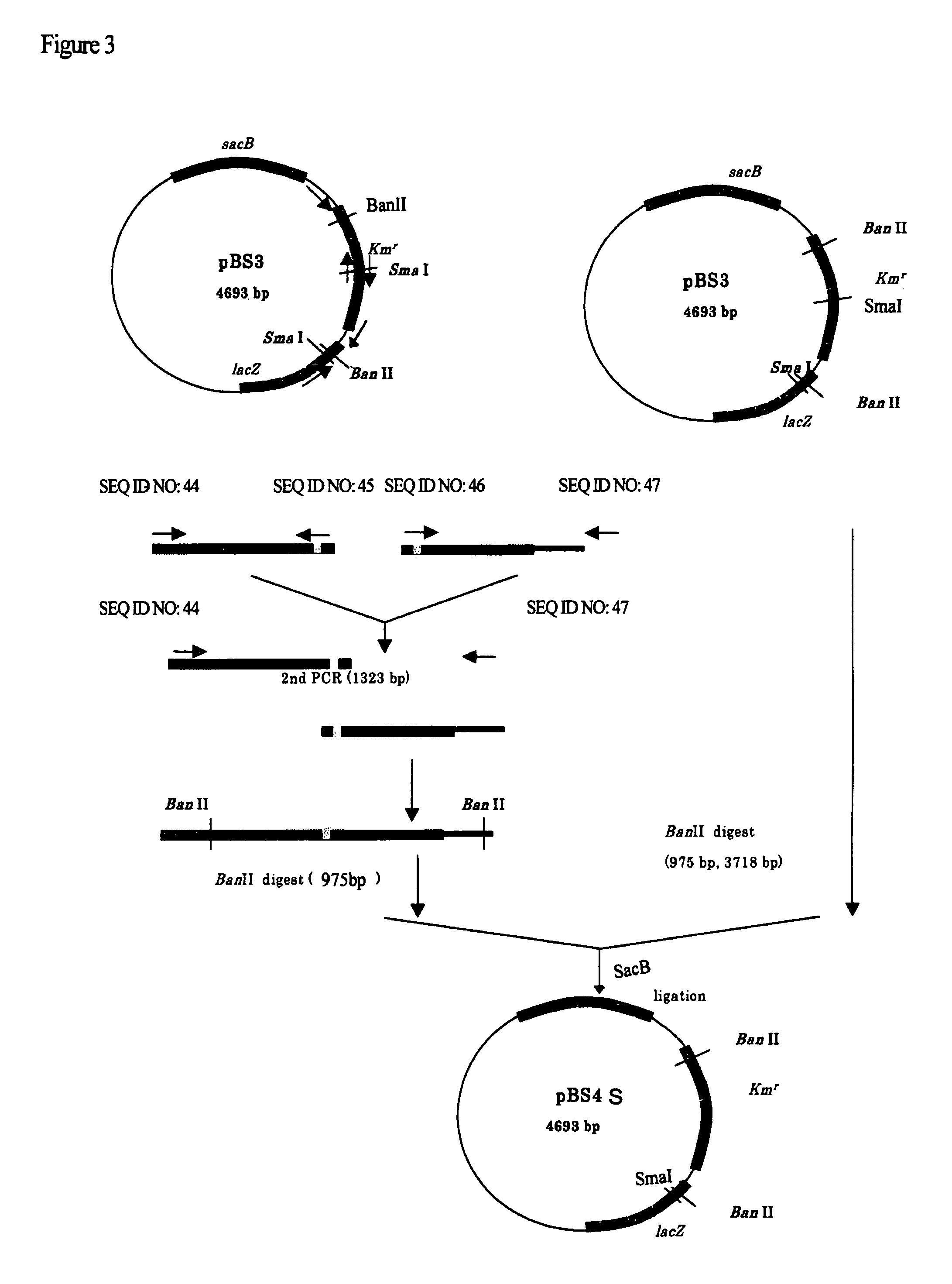
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
Enzyme composition for improving food digestion
InactiveUS20080199448A1Improving food absorptionPromote digestionOrganic active ingredientsPeptide/protein ingredientsProteinase activityAdditive ingredient
An orally administered composition for improving food absorption and digestion contains therapeutically effective dosages of digestive enzymes and L-glutamine as active ingredients. The digestive enzymes include at least one each of a lipase, a protease, and an amylase, and at least a portion of each of these enzymes is enteric coated.
Owner:NUTRI-HEALTH SUPPLEMENTS LLC
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
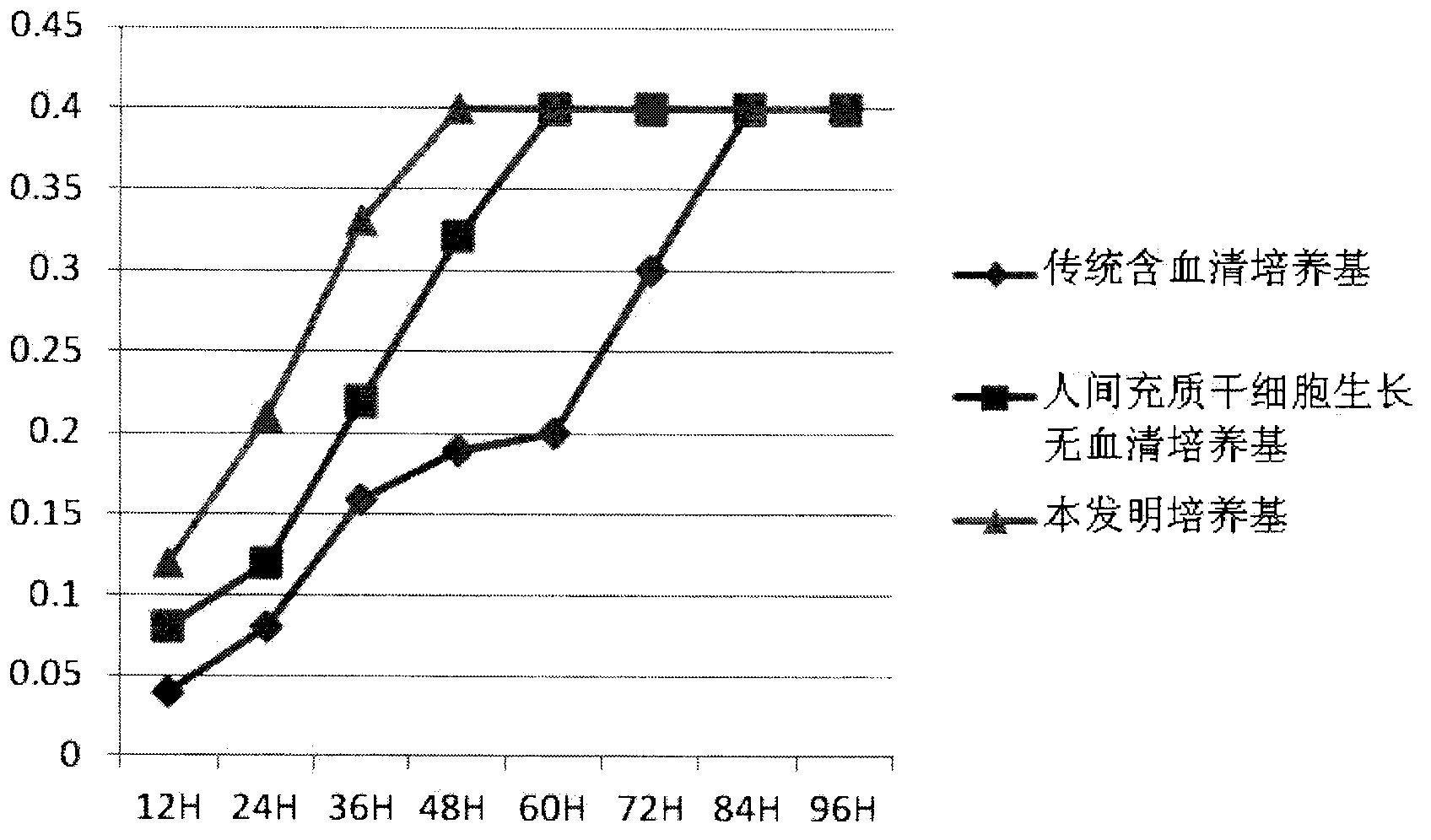
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Serum-free medium for culturing placenta mesenchymal stem cells
ActiveCN103805562AIncrease growth rateMaintain stem cell propertiesSkeletal/connective tissue cellsFibroblast growth factor receptor 2Cell culture media
The invention discloses a serum-free medium for culturing placenta mesenchymal stem cells. The serum-free medium takes a DMEM (Dulbecco Modified Eagle Medium) culture solution as a basis and also contains a fibroblast growth factor receptor 2, growth hormone, insulin, transferrin, glutathione, BMP-4, L-glutamine, sodium pyruvate, non-essential amino acids and beta-mercaptoethanol. According to various serum-free media provided by the invention, growth and proliferation of the placenta mesenchymal stem cells in a serum-free medium system can be effectively promoted, the placenta mesenchymal stem cells have higher growth and proliferation rate in the serum-free medium system compared with a serum cell culture medium, the characteristics of the stem cells are preserved, the serum-free medium has multiple differential potentials, and the stem cells can be directionally induced into fat cells and osteoblasts.
Owner:章毅 +10
Pharmaceutical formulations of antineoplastic agents and processes of making and using the same
In its several embodiments, this invention discloses a pharmaceutical formulation comprising at least one antineoplastic agent or a pharmaceutically acceptable salt thereof, and at least one dissolution enhancing agent sufficient to substantially dissolve said at least one antineoplastic agent in at least one aqueous diluent, wherein said dissolution enhancing agent is urea, L-histidine, L-threonine, L-asparagine, L-serine, L-glutamine or mixtures thereof; a lyophilized powder comprising said pharmaceutical formulation, and articles of manufacture thereof.
Owner:MERCK SHARP & DOHME LLC
Dietary Supplement Cognitive Support System
The present invention relates to a nutritional supplement composition, comprising a therapeutically effective amounts of Vitamin C, Vitamin D3, Thiamin, Riboflavin, Niacin, Vitamin B6, Folic acid, Vitamin B12, Pantothenic acid, Calcium, Magnesium, Zinc, Chromium, Sugar, Protein, Acetyl-L-Carnitine, Dimethylaminoethanol complex, Phosphatidylserine complex, L-Glutamine, N-Acetyl-L-Tyrosine, L-Phenylalanine, Taurine, Methionine, Valine, Isoleucine, 5 Hydroxytryptophan, L-Taurine, N-Acetyl-Tyrosine, N-Acetyl-L-Cysteine, Alpha Lipoic Acid, Alpha Glycerylphosphoricholine complex, Bacopa Monnieri extract, Gingko Biloba extract, Passion flower, Lemon Balm, Gotu Kola, Ashwagandha, Choline Bitartrate complex, Panax Ginseng extract, Turmeric, Organic freeze dried fruit juice blends (concord grape, red raspberry, pineapple, cranberry, acai, pomegranate, acerola cherry, bilberry, lingonberry, black currant, aronia, sour cherry, black raspberry), Organic freeze dried greens blends (barley grass, broccoli, beet, carrot, alfalfa, oat), and Protein digestive enzyme blends (Protease 4.5, peptidase, bromelain, protease 6.0, protease 3.0, L planatrum, B bifidum) in a mixture to provide optimal cognitive function.
Owner:FANTZ DAVID R
Cryopreservation solution of tissue engineering products and application method thereof
InactiveCN101720753AResuscitation is easy to useLow toxicityDead animal preservationWater bathsSucrose
A cryopreservation solution of tissue engineering products uses DMEM culture solution as a basic solvent which is added with vitamin B, vitamin C, chondroitin sulfate, beta-integrin, cromolyn sodium, cytochalasin B, L-glutamine, bovine serum albumin, fetal bovine serum, trehalose, sodium carbonate, polysucrose-70, and dimethyl sulfoxide added during freezing storage. The cryopreservation solution provided by the invention has little toxicity to cells and long storage time, and can be stored for six months under 80 DEG C below zero, and 12 to 18 months in liquid nitrogen; and the cell viability of the resuscitated cells can be over 60%. The stored tissues can be simply and conveniently used after being resuscitated for only three to five min in water bath under the temperature of 37 DEG C and being washed by sterilized saline water. The invention can be widely used, and is suitable for tissue engineering skin, tissue engineering cornea, tissue engineering blood vessel, tissue engineering nerve and the like, and also is applicable to the cryopreservation of normal tissues.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY +1
SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells)
ActiveCN103555665AClear ingredientsAvoid heterogeneous contaminationSkeletal/connective tissue cellsSodium bicarbonateSerum free media
The invention relates to an SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells). Based on volume, the SFM comprises the following components: 10.2 grams per liter of alpha-MEM (alpha-minimum essential medium), 2.4 grams per liter of sodium bicarbonate, 1 to 5 millimoles of L-glutamine, 50 to 300 milligrams per liter of poloxamer 188, 2 to 8 grams per liter of recombinant human albumin, 10 to 20 milligrams per liter of recombinant human transferrin, 2 to 10 milligrams per liter of recombinant human insulin, 1 to 5 millimoles per liter of Hepes, 50 nanomoles of beta-mercaptoethanol, 0.1 to 1 milligram per liter of lipid, 1 to 5 milligrams per liter of trace element, 0.1 to 5 milligrams per liter of glutathione, 0.5 to 5 milligrams per liter of para-aminobenzoic acid, 1 to 50 nanograms per milliliter of hydrocortisone, 20 to 50 milligrams per liter of vitamin PP, 5 to 50 milligrams per liter of vitamin C, 2 to 10mu M of compound shown in a formula I, 5 to 20mu M of compound shown in a formula II, 10 to 20 nanograms per milliliter of progestin, 1 to 10 milligrams per liter of putrescine, 1 to 10 international units per liter of heparin, 1 to 10 nanograms per milliliter of EGF (epidermal growth factor), 1 to 10 nanograms per milliliter of b-FGF (b-fibroblast growth factor), 1 to 10 nanograms per milliliter of HGF (hepatocyte growth factor) and 1 to 10 nanograms per milliliter of VEGF (vascular endothelial growth factor). The SFM for culturing the MSCs is a BPS-SFM which has determinate chemical components and is free of animal-derived substances.
Owner:BEIJING DONGFANG HUAHUI BIOMEDICAL TECH
Food product and process for manufacturing same
This invention is concerned with packaged food products which contain specific combinations of functional additives aimed at addressing specific health indicators, in particular flatulence, gastro-intestinal health, stress and immune system responsiveness, in pet animals. There is provided a commercially packaged mammal pet food product that includes a manufactured, shelf-life stable food substrate and a combination of functional additives. The functional additives include at least one non-palatable plant-based remedy and / or dietary fiber source that are present to strengthen and / or maintain a specified health indicator of a mammal pet animal. The food product is portioned and packaged with the functional additives being present in predetermined concentrations and amounts sufficient to be effective in achieving said indications on regular feeding of the pet animal with said food product. The food substrate is present in a proportion sufficient to mask the flavor and / or odor of the non-palatable additive and is made-up of a unique combination of materials that are able to be processed at lower temperatures to preserve the natural botanical functional additive's activity. Functional additives intended to address dietary flatulence problems include a combination of Yucca extract, charcoal and salts of zinc, such as zinc acetate. Functional additives to promote or maintain gastro-intestinal health include a combination of L-glutamine, D-glucosamine sulphate, sugar beet pulp, slippery elm. Functional additives to strengthen or maintain a pet animal's natural body defenses include a combination of vitamin E, vitamin B complex, primrose oil vitamin C and Marigold meal. Functional additives to promote or maintain reduction of stress and / or improved behavior of a pet animal include a combination of Valerian root extract, Kava root extract, vitamin B complex and magnesium salt.
Owner:EFFEM FOODS
Complete medium and human amnion-derived mesenchymal stem cell culture method
The invention discloses a complete medium and a human amnion-derived mesenchymal stem cell (hAMSCs) culture method. The complete medium is prepared by adding 3 to 10 percent of autologous umbilical cord blood serum into low-sugar Dulbecco minimum essential medium solution according to a volume ratio. The culture method comprises: (1) separation; (2) primary culture; and (3) subculture. The methodusing the complete medium in the hAMSCs culture has the advantages that: the risk of using fetal calf serum is avoided; although the need of adding L-glutamine, non-essential amino acid, 2-mercapitoethanol, pyruvic acid and the like is obviated, the high proliferation properties and phenotypic characteristics of the hAMSCs and expression of multilineage differentiation marker genes sand proteins of some stem cells can still be retained; and in subculture, the wall adherence fastness of the hAMSCs is much lower than that in fetal bovine serum (FBS) culture, the digestion time is reduced obviously, and the damage of trypsinization to cells and loss of cells are reduced.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Composite functional amino acid feed additive and application
InactiveCN104351478AImprove conversion efficiencyImprove securityAnimal feeding stuffDiseaseArginine
The invention discloses a composite functional amino acid feed additive and an application. The composite functional amino acid feed additive is prepared from the raw materials of L-glutamic acid, L-glutamine, glycine, L-arginine and L-cysteine at a certain weight ratio; the preparation method comprises the following steps: step A, respectively weighing the L-glutamic acid, the L-glutamine, the glycine, the L-arginine and the L-cysteine; step B, sequentially feeding the weighed amino acids into a mixer and then uniformly mixing to obtain a composite amino acid feed additive. The invention also discloses the application of the composite amino acid feed additive to livestock and aquatic livestock feeds. The composite functional amino acid feed additive is reasonable in formula, convenient to use, high in safety, free of resistance to drugs and capable of improving animal intestinal health, promoting growth, improving feed conversion efficiency and reducing incidence of diseases, so the composite functional amino acid feed additive can be used for effectively replacing an antibacterial growth promoting agent.
Owner:WUHAN POLYTECHNIC UNIVERSITY
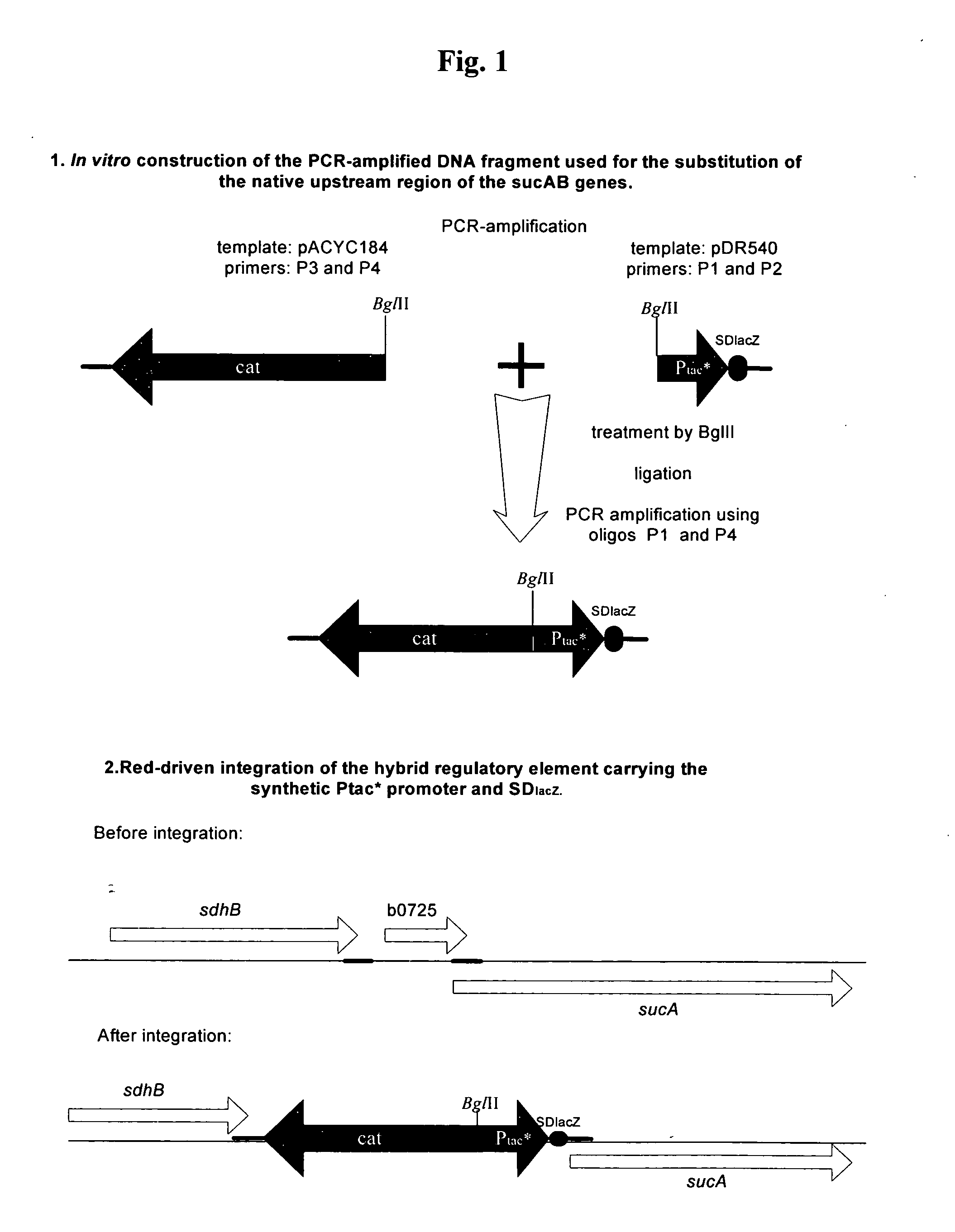
Method for producing an L-amino acid using a bacterium with an optimized level of gene expression
A method is provided for obtaining an L-amino acid or nucleic acid-producing bacterium belonging to the genus Escherichia with an optimized level of expression of the gene which influences the distribution of carbon flow, such as the sucAB genes, comprising introducing into the chromosome of the bacterium a set of in vitro constructed DNA fragments which contain regulatory elements for gene expression instead of the native elements of the regulatory region of the gene, and selecting the colonies with increased L-amino acid productivity. Also, a method is provided for producing an L-amino acid, such as L-glutamic acid, L-proline, L-arginine, L-glutamine, L-leucine, using the bacterium with an optimized level of expression of the sucAB gene.
Owner:AJINOMOTO CO INC
Cell culture medium containing growth factors and L-glutamine
InactiveUS6838284B2Shorten the timeQuick distinctionMicroorganismsCulture processCulture cellCell culture media
The invention relates to a culture medium for culturing cells, in particular human cells in a process for tissue engineering bone. The medium comprises glucose, a mineral, a vitamin, a growth factor and L-glutamine, wherein the L-glutamine is present in a concentration of at least 300 mg / L.
Owner:OCTOPLUS SCI
Nutrient compositions and methods for sustenance and promotion of positive metabolic energy levels in a targeted manner
Nutrient compositions and methods that sustain and promote positive metabolic energy levels in a targeted manner are disclosed. Methods utilize endogenous energy stores (fat oxidation), increase use of those stores (increasing transport rate), increase available energy (increasing the ability to perform ADP to ATP phosphorylation,) as well as decrease catabolism and increase protein synthesis. Compositions are also disclosed, and include Mono- or Dicreatine-HMB salt; Putrescine Dihydrochloride; Alanine; L-Glutamine, which may be combined with Alanine in a 1:2 to 2:1 molecular ratio; Trimethylglycine; and Guanidinopropionic Acid.
Owner:SELLO AZUL
Method and media for single cell serum-free culture of CHO cells
InactiveUS20060115901A1Cell density can not be enhancedGenetically modified cellsCulture processSerum freeAntioxidant
The present invention relates to methods and media for single cell serum free culture of CHO cells. Generally, the invention relates to a method of culturing CHO cells at a cell density of less than 100 cells / ml in a serum-free cell culture medium. The medium is sufficient to support the growth of a single CHO cell and comprises a basal medium sufficient to support the growth of CHO cells and a supplemental medium, wherein the combined basal medium and supplemental medium comprise an antioxidant, L-glutamine, iron, ethanolamine, and albumin; and insulin, wherein the insulin may be present in either the basal medium or the supplemental medium, or both. Optionally, the medium may contain EGF or IGF.
Owner:AMGEN INC
Oral amino acid composition
InactiveUS20050032898A1Reduce the amount requiredEfficient reductionOrganic active ingredientsBiocideGlutethimideArginine
Amino acid compositions which contain 10 to 40 parts by weight of L-arginine, 10 to 40 parts by weight of L-glutamine, 5 to 20 parts by weight of L-valine, 8 to 30 parts by weight of L-isoleucine, and 10 to 35 parts by weight of L-leucine may be taken orally and are effective for reducing body fat without dietary restrictions and exercise.
Owner:AJINOMOTO CO INC
L-Amino Acid-Producing Bacterium and a Method for Producing L-Amino Acid
ActiveUS20070254345A1Improved ability to produce L-amino acidsEfficient productionBacteriaFermentationArginineL-Glutamin
Coryneform bacteria are described that have an ability to produce L-amino acids and are modified so that acetyl-CoA hydrolase activity is decreased. The bacteria are used to produce L-amino acids generated by a biosynthetic pathway in which pyruvic acid is an intermediate, such as L-glutamic acid, L-arginine, L-glutamine, L-proline, L-alanine, L-valine, and L-lysine.
Owner:AJINOMOTO CO INC
High-yield gastrodia elata planting method
InactiveCN103782744APromote growthQuality improvementHorticultureFertilizer mixturesLeaf fallL-glutamine
The invention discloses a high-yield gastrodia elata planting method and relates to a gastrodia elata planting method. The method includes the steps: 1) mycelia culturing: A. preparing leaves, branches and sticks; B. culturing mycobacteria; C. culturing mycelia; 2) land selection and land preparation; 3) plantation: parallelly placing seed gastrodia elata in furrows of adjacent Chinese scholar tree sticks and adjacent mycelia, allowing the seed gastrodia elata to approach to the mycelia as close as possible, wetting the Chinese scholar tree sticks, the mycelia, Chinese scholar tree fallen leaves and the seed gastrodia elata with armillaria mellea nutrient solution, and filling gaps with humus soil; 4) management: spraying gastrodia elata growth medium composed of manganese sulfate, potassium nitrate, calcium nitrate, monopotassium phosphate, citric acid rare earth, L-glutamine and water during growing of the seed gastrodia elata; 5) harvesting. Yield and quality of gastrodia elata can be effectively improved by the high-yield gastrodia elata planting method.
Owner:CHENGDU ZUIHUAGU FORESTRY DEV
Serum-free adipose tissue-derived mesenchymal stem cell culture medium
ActiveCN103255103AAvoid exogenous contaminationAvoid the influence of cultivationSkeletal/connective tissue cellsPenicillinCuticle
The invention relates to a serum-free adipose tissue-derived mesenchymal stem cell culture medium, which consists of a basic culture medium and added ingredients, wherein the basic culture medium is DMEM-LG, and the added ingredients and the content of each added ingredient are shown as follows: 5 to 20ng / mL of alkaline fiberblast growth factors, 5 to 20ng / mL of epidermal growth factors, 100U / mL of penicillin, 100 micrograms / mL of streptomycin, 50 to 200 micrograms / mL of heparin, 2 to 8mM of L-glutamine, 100 to 300 microM of 2-mercaptoethanol, 500 to 2000U / mL of leukaemia inhibitory factors and 0.5 to 2mM of sodium pyruvate. The serum-free adipose tissue-derived mesenchymal stem cell culture medium does not contain the serum, so that the inter-batch difference and the influence of the serum component on the cell culture can be avoided; the exogenous pollution of the serum and the toxicity of the serum on the cells can be avoided; and the ingredients are clear, so that the research of the psychological regulation mechanism of the cells can be facilitated.
Owner:冯文峰
Serum-free medium of stem cell
ActiveCN104805054APromote proliferationPromote differentiationSkeletal/connective tissue cellsSerum free mediaVitamin C
The invention discloses a serum-free medium of a stem cell, which comprises a basal medium and an additive, wherein the basal medium is DMEM / F12 (Dulbecco Modified Eagle Medium / F12); the additive comprises 2-15%(v / v) of serum replacement, 20-100ug / ml vitamin C, 0.5-10ng / ml stem cell growth factor, 5-20ng / ml human platelet-derived growth factor and 1-5mmol / ml L-glutamine. The serum-free medium of the stem cell for cell culture has the advantages of short cell cycle, strong multiplication capacity, good cell uniformity and high purity, effectively inhibits differentiation of the stem cell and adherence growth of an endothelial cell, ensures purity and dryness of the stem cell and is free from ingredients of animal origin such as fetal calf serum, and the stem cell obtained by the medium is suitable for clinical application.
Owner:钜威细胞(厦门)医学科技有限公司
Animal source-free and serum-free culture medium of lymphocyte
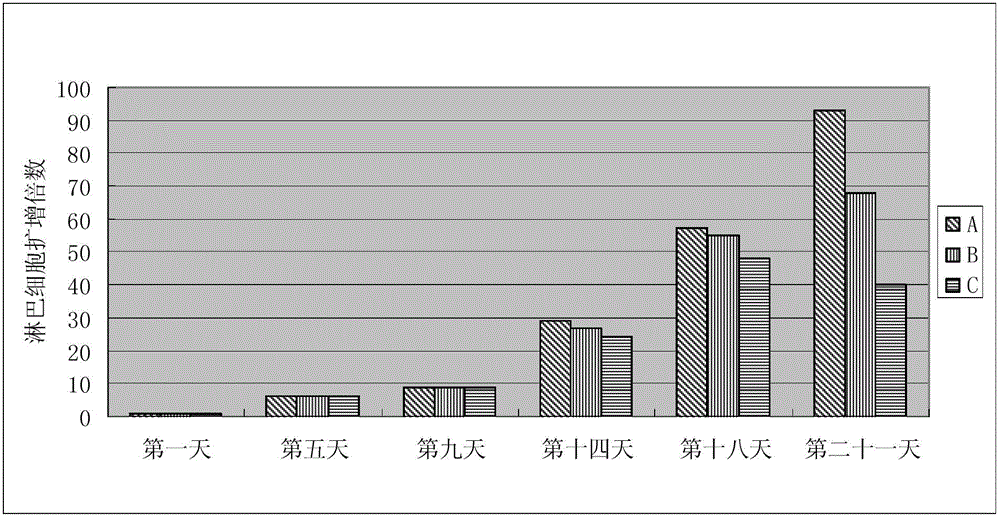
InactiveCN103146648AGood amplification factorEnhance cell viabilityBlood/immune system cellsCell phenotypeSodium bicarbonate
The invention relates to the biological field and discloses an animal source-free and serum-free culture medium of lymphocyte. The culture medium disclosed by the invention essentially consists of IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, human transferrin, human serum albumin, 2-mercaptoethanol, N-acetyl-cysteine, lipid, amino acid, vitamin, microelement, ferric citrate, hydrocortisone, cholamine and non-essential amino acid. The serum-free culture medium disclosed by the invention has the advantages of clear chemical components, no animal source, no serum, safe and ideal culture cells; the instability caused by the doping of animal components and batches is avoided; the result of culturing lymphocyte shows that the total number of the cells and the cell phenotypes are normal; and the serum-free culture medium disclosed by the invention has a good industrial application prospect.
Owner:BEIJING JING MENG STEM CELL TECH
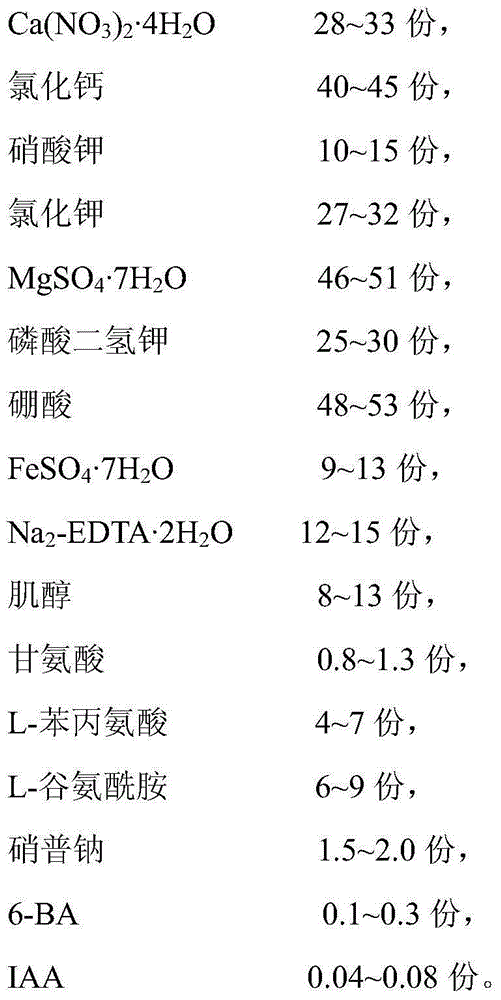
Foliar nutrient special for tea-oil trees and application thereof
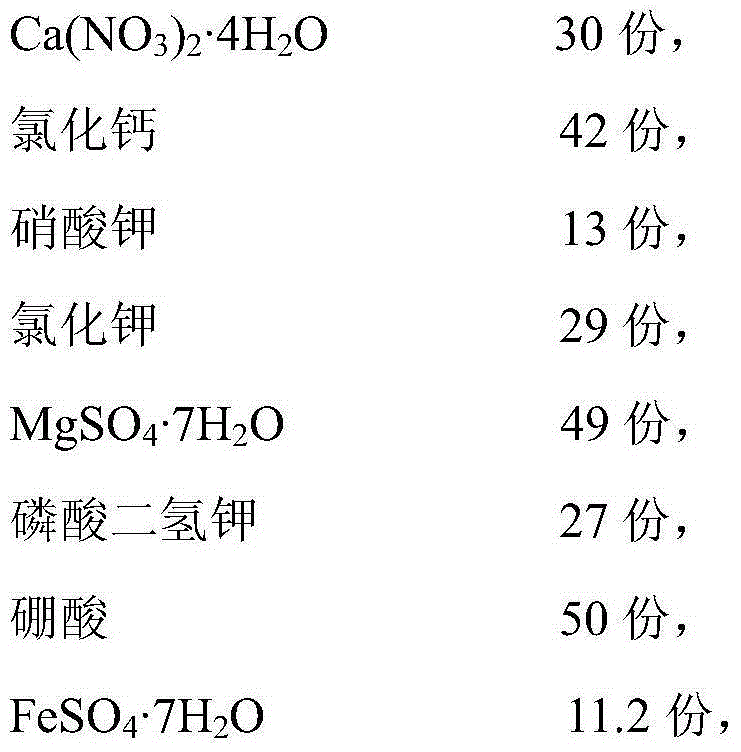
ActiveCN105036926ARich in nutrientsPrevent and reduce sheddingFertilizer mixturesCamellia oleiferaMonopotassium phosphate
The invention discloses a foliar nutrient special for tea-oil trees and an application thereof. The effective components of the foliar nutrient special for the tea-oil trees comprise calcium chloride, potassium nitrate, potassium chloride, MgSO4.7H2O, monopotassium phosphate, boric acid, FeSO4.7H2O, Na2-EDTA.2H2O, inositol, glycine, L-phenylalanine, L-glutamine, sodium nitroprusside, 6-BA and IAA. Before fruit of the tea-oil trees matures, the foliar nutrient is applied to the leaf surfaces of tea-oil trees, drop of immature fruit of the tea-oil trees can be effectively reduced and prevented, and the tea-oil tree yield is greatly improved.
Owner:HUNAN TIANHUA OIL TEA CASMELLIA TECH CO LTD
Preparation of alanyl glutamine dipeptide compound
Production of alanyl glutamine dipeptide compound is carried out by adding L-glutamine into mixed liquid of methylbenzene and water, cooling, adding into sodium hydrate, dissolving L-glutamine, dripping into solution with alpha - halopropacyl halide, methylbenzene and sodium hydrate, regulating pH, reacting, separating organic layer, adding sodium chloride into solution with organic layer separation, regulating pH, adding into concentrated chlorhydric acid, regulating pH, laying aside, filtering, separating out crystal, drying crystal, reacting with ammonia water, controlling temperature and pressure, cooling, de-pressuring, concentrating, adding water, dripping into methyl alcohol, laying aside, filtering, separating out L-alanyl-L-glutamate crude products, purifying and obtaining refined products. It achieves simple process and low cost.
Owner:上海依福瑞实业有限公司
Frozen stock solution of neural stem cells and application method of frozen stock solution
ActiveCN104542576AEffective removal of toxic effectsRemove toxic effectsDead animal preservationL-glutamineStock solution
The invention discloses a frozen stock solution of neural stem cells and an application method of the frozen stock solution. The frozen stock solution contains B-27 Supplement without retinoic acid, L-Glutamine, EGF, FGF and vitamin E in Neurobasal-A medium. The frozen stock solution is low in cost; the problem of low thawing rate after existing neural stem cells are frozen is critically solved; and the frozen thawing rate of the neural stem cells is improved.
Owner:CENT SOUTH UNIV +1
Storage solution capable of storing human mesenchymal stem cell at 4 DEG C
InactiveCN108651442AMeet short-distance transportation needsConvenient for clinical operationDead animal preservationHuman bodySodium lactate
The invention relates to a storage solution capable of storing a human mesenchymal stem cell at 4 DEG C, the storage solution comprises the ingredients of human albumin, trehalose, sodium chloride, glucose, sodium lactate ringer's injection, heparin, L-glutamine, EDTA (Ethylene Diamine Tetraacetic Acid), a phosphate buffer solution, sodium selenite, an essential amino acid, and a non-essential amino acid. The provided formula guarantees that more than 80% of the vitality and more than 70% of the adhesion rate of the cell are kept after 48 hours in the storage and transportation, the requirement on the short-distance transportation of the fresh mesenchymal stem cell is met, the storage solution uses the clinical medicine having clear compositions, is safe to the human body, can be directlyinjected when recovered to the room temperature, and greatly facilitates the clinical application of the mesenchymal stem cell after the short-distance transportation.
Owner:广东佰鸿干细胞再生医学有限公司
Method for preparing lenalidomide
InactiveCN104311536ASignificant technological progressOptimize the process routeCarbamic acid derivatives preparationOrganic compound preparationDicarbonateL-Glutamin
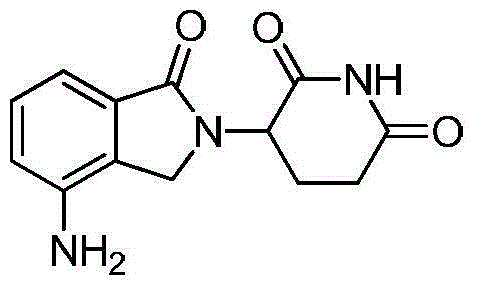
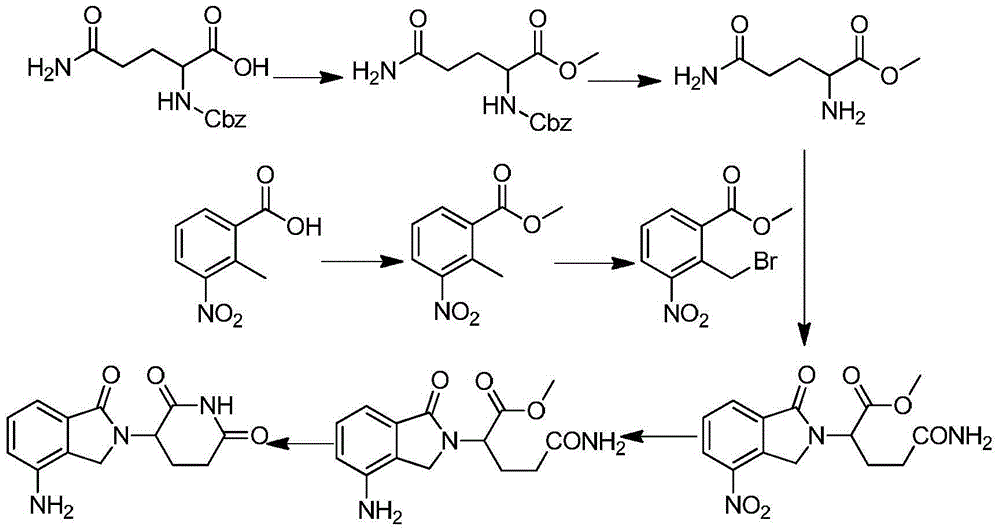
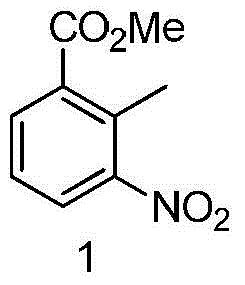
The invention discloses a method for preparing lenalidomide. The method comprises the following steps: firstly, etherifying 2-methyl-3-nitrobenzoic acid to obtain 2-methyl-3-nitrobenzoic acid methyl ester, brominating to obtain 2-brooethyl-3-nitrobenzoic acid methyl ester, reacting L-glutamine and tert-butyl dicarbonate to obtain N-Boc glutamic acid, acquiring 3-amino-2,6-piperidine diketone protected by Boc from N-Boc-glutamic acid in the presence of a condensing agent and a catalyst, further reacting with acid to prepare 3-amino-2,6-piperidine diketone hydrochloride, reacting 3-amino-2,6-piperidine diketone with 2-brooethyl-3-nitrobenzoic acid methyl ester so as to obtain 3-(4-nitryl-1,3 dihydro-1-oxo-2 hydrogen-isobenzazole-2-yl) piperidine-2,6-diketone, and finally reducing, thereby obtaining lenalidomide. The method disclosed by the invention is high in product yield.
Owner:SHANGHAI INST OF TECH
Low-fat low-osmosis low-residue type enteral nutritional powder for patients with inflammatory bowel diseases
ActiveCN105054025AImprove tolerancePromote recoveryVitamin food ingredientsInorganic compound food ingredientsWhey protein powderL-Glutamin
The invention provides a low-fat low-osmosis low-residue type enteral nutritional powder for patients with inflammatory bowel diseases. The powder is prepared from protein, fat, carbohydrate, vitamin complex, minerals and epigallocatechin gallate, and does not contain gluten, wherein the protein is supplied by whey protein powder and L-glutamine; the fat is supplied in a mode of long-chain fatty acid and medium-chain fatty acid, and a ratio of (omega-6) to medium-chain fatty acid to (omega-3) to (omega-9) is 40% to 40% to 10% to 10%; and the carbohydrate is maltodextrin. The enteral nutritional powder is suitable for people who are relatively poor in gastroenteric function and easily have diarrhea or people requiring low-fat low-residue diet, such as patients with inflammatory bowel diseases, pancreatitis patients who are in transition of initial oral diet, heart failure patients, patients who initially use enteral nutrition after fasting, patients with cholecystitis or gall-stone, patients subjected to cholecystectomy, obesity people, patients in preparation of bowel surgery, patients after gastrointestinal surgery and the like. The enteral nutritional powder can be used for supplying balanced nutrition support, is easy to brew and store, is convenient to control dosage and osmotic pressure, and can be used for improving the living quality of patients.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Preparation of N(2)-L-alanyl-L-glutamine
The invention discloses a method for preparing N(2)-L-alanyl-L-glutamine, belonging to a method for preparing dipeptide containing four amino acids at most. The method comprises the following steps: (1) inorganic base is added into the solution of toluene and water which contains L-glutamine; then the mixture of D-alpha-chloropropionyl chloride and toluene is added and the pH value of reaction solution is controlled between 9.5 and 10.5 by the inorganic base; the reaction temperature is maintained between 0-5 DEG C; the reaction solution is quiescent so that a toluene layer is separated; sodium chloride is added into the remaining solution at room temperature and N(2)-D-alpha-chloropropionyl-L-glutamine is obtained after filtration and drying; ammonia is added into the N (2)-D-alpha-chloropropionyl-L-glutamine, and concentrated solution after concentration under reduced pressure is transferred to a crystallizer; methanol is added and a crude product is obtained after filtration and drying; a refined product of the N (2)-L-alanyl-L-glutamine is obtained by refining.
Owner:TIANJIN TIANCHENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com