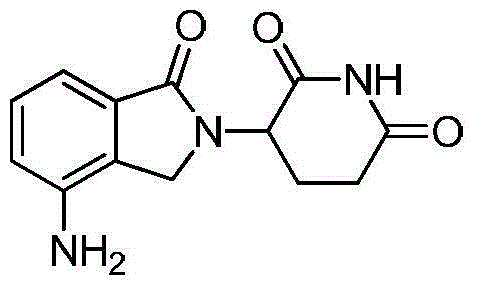
Method for preparing lenalidomide
A technology of lenalidomide and reflux temperature, applied in the field of lenalidomide and preparation of lenalidomide, can solve the problems of low total yield, long steps, difficult industrialization, etc., and achieves short reaction steps, simple operation, and high reaction high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
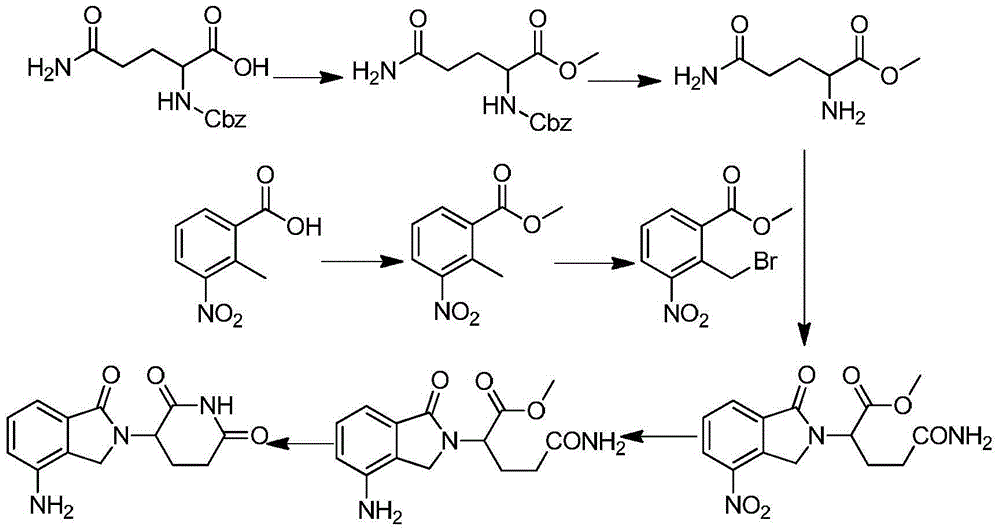
Embodiment 1
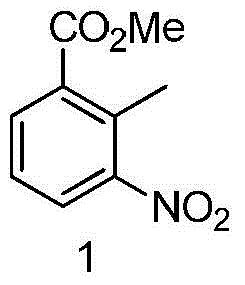
[0035] (1) Preparation of 2-methyl-3-nitrobenzoic acid methyl ester
[0036] 30g of 2-methyl-3-nitrobenzoic acid was dissolved in 100mL of anhydrous methanol, and 15mL of thionyl chloride was added dropwise while keeping the temperature below 0°C. After the dropwise addition was completed, it was heated to reflux for 5 hours. Evaporate the methanol and pour it into ice water, adjust the solution to neutral, filter the precipitated solid, wash with distilled water until the filtrate is clear and colorless, and dry the filter cake to obtain 31 g, with a yield of 96%.
[0037] 1 HNMR (500MHz, CDCl 3 )δ: 2.6(s, 3H), 3.95(s, 3H), 7.35(m, 1H), 7.90(m, 2H)
[0038] (2) Preparation of 2-bromomethyl-3-nitrobenzoic acid methyl ester
[0039] Dissolve 20 g of methyl 2-methyl-3-nitrobenzoate in 250 mL of carbon tetrachloride, add 22 g of N-bromosuccinimide (NBS), 2.5 g of benzoyl peroxide (BPO), and heat to 75 The reaction was stirred at °C for 8 hours. After cooling, extract three t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com