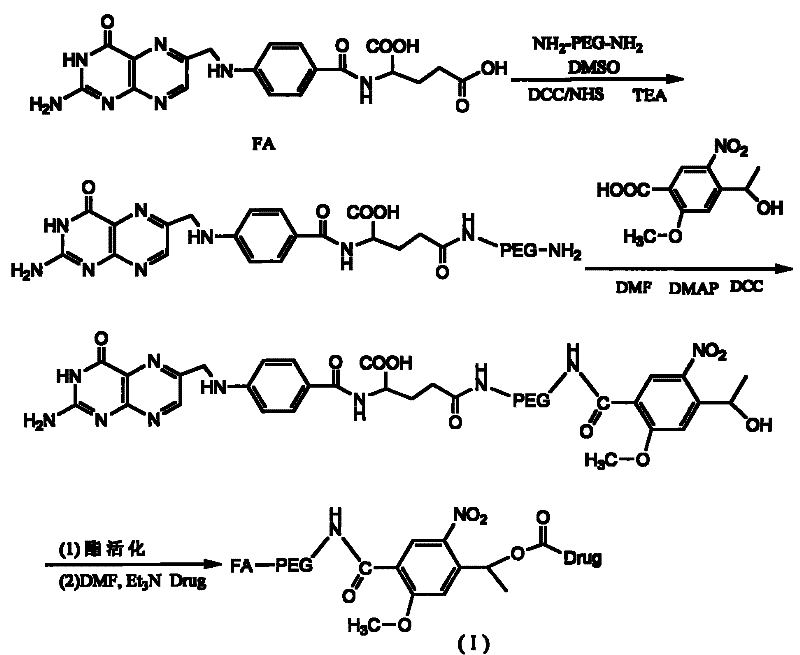
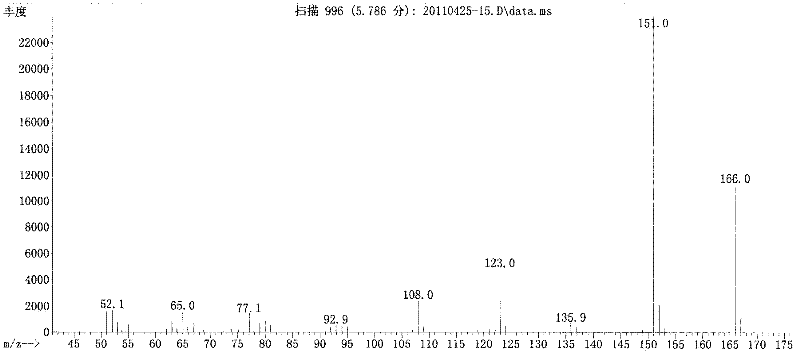
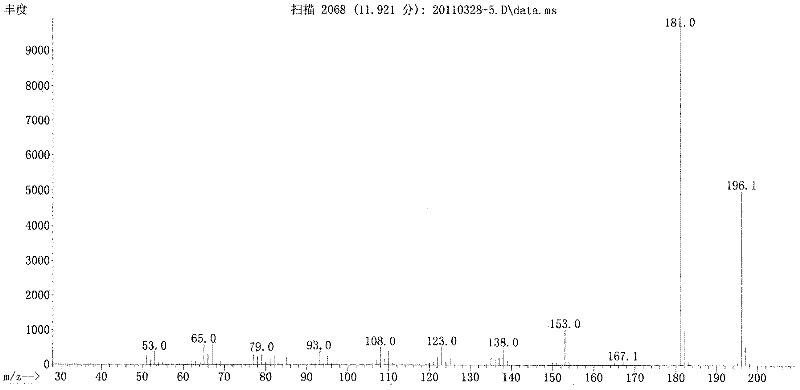
Patents
Literature
438 results about "Nitrobenzoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
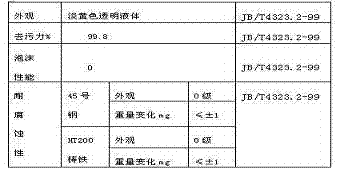
Nitrobenzoic acids are derivatives of benzoic acid. Two are commercially important. They are about ten times more acidic than the parent benzoic acid. Nitrobenzoic acid can be prepared through the oxidation of styrene in boiling nitric acid.
Method for performing supersensitive detection on fungaltoxin of DTNB mark-based gold@silver core-shell nanorod
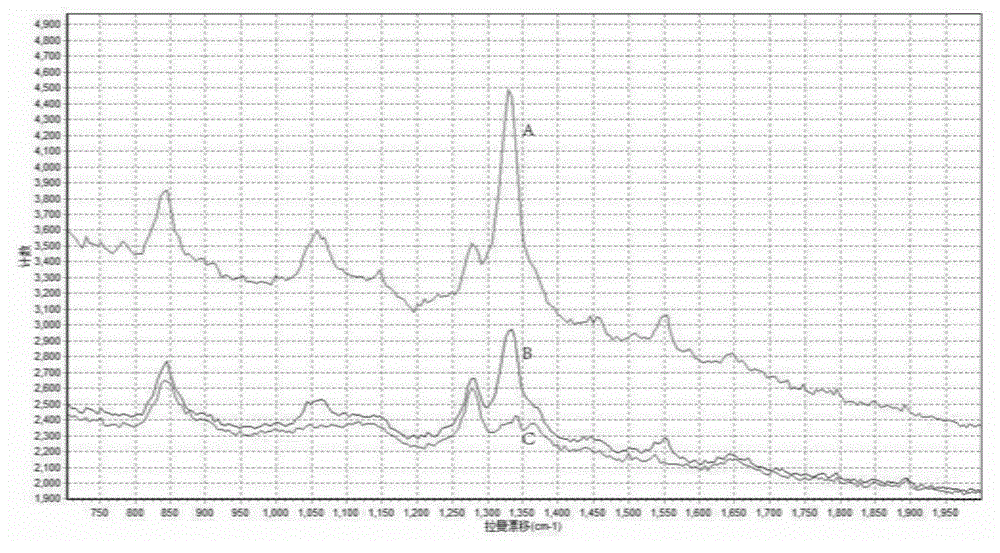
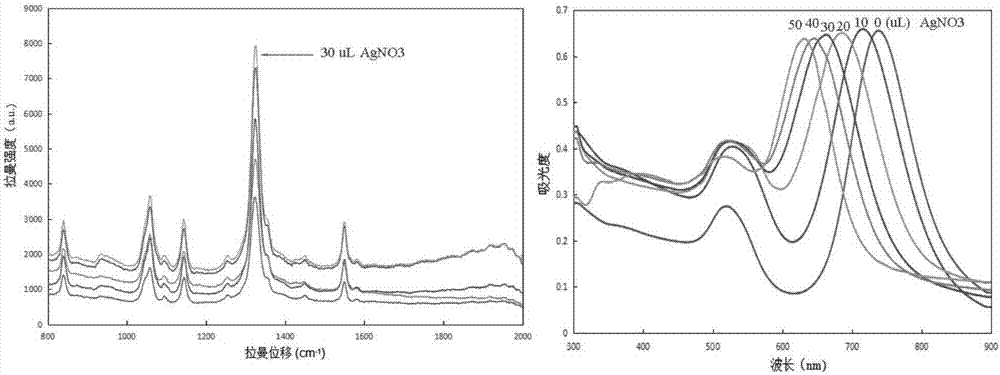
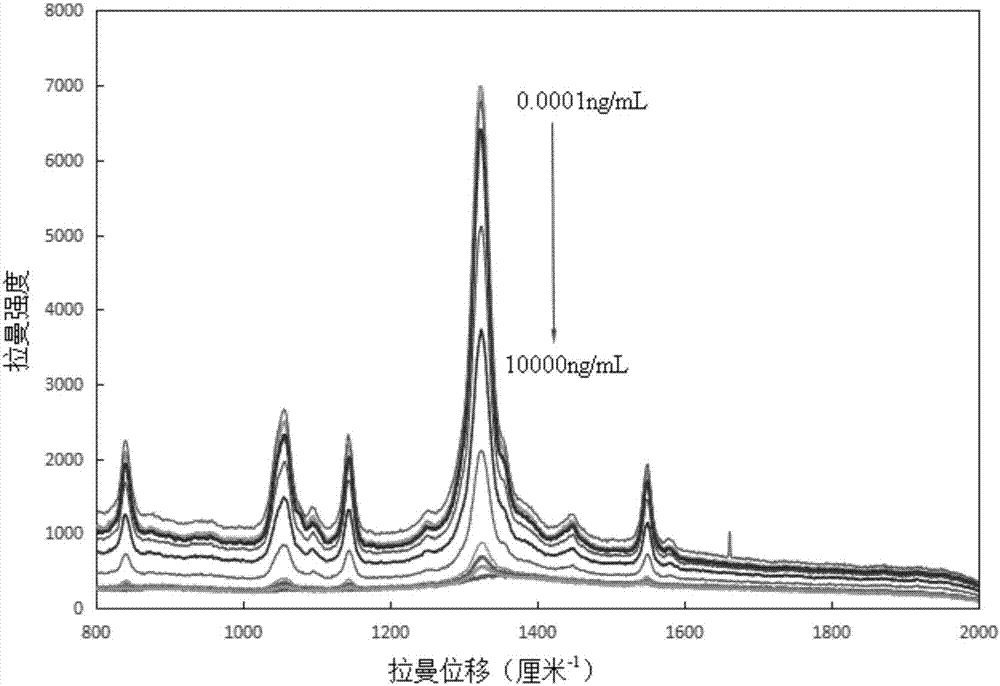
ActiveCN106932376AAvoid interferenceLarge scattering cross sectionPreparing sample for investigationRaman scatteringGold nanorodFood safety
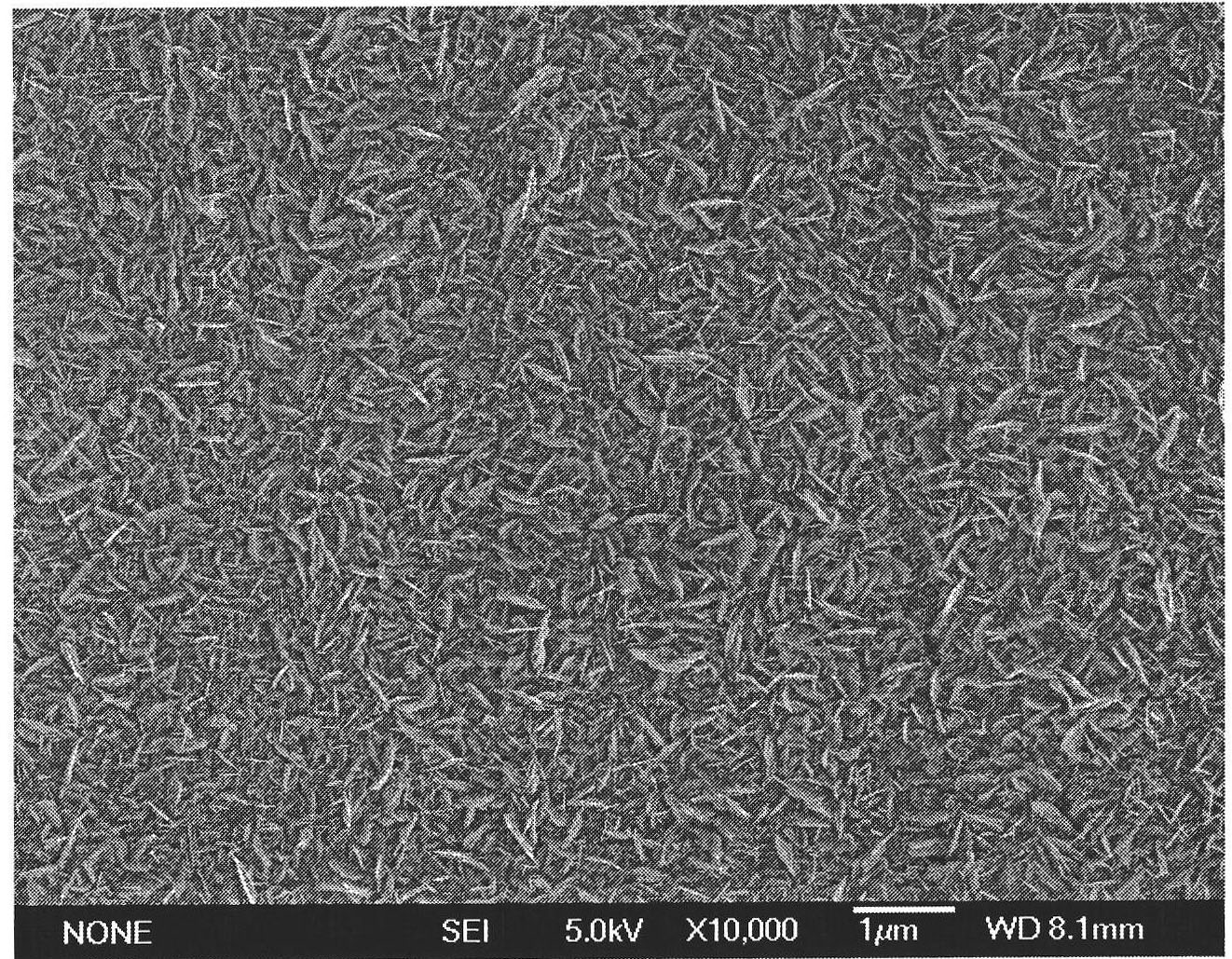
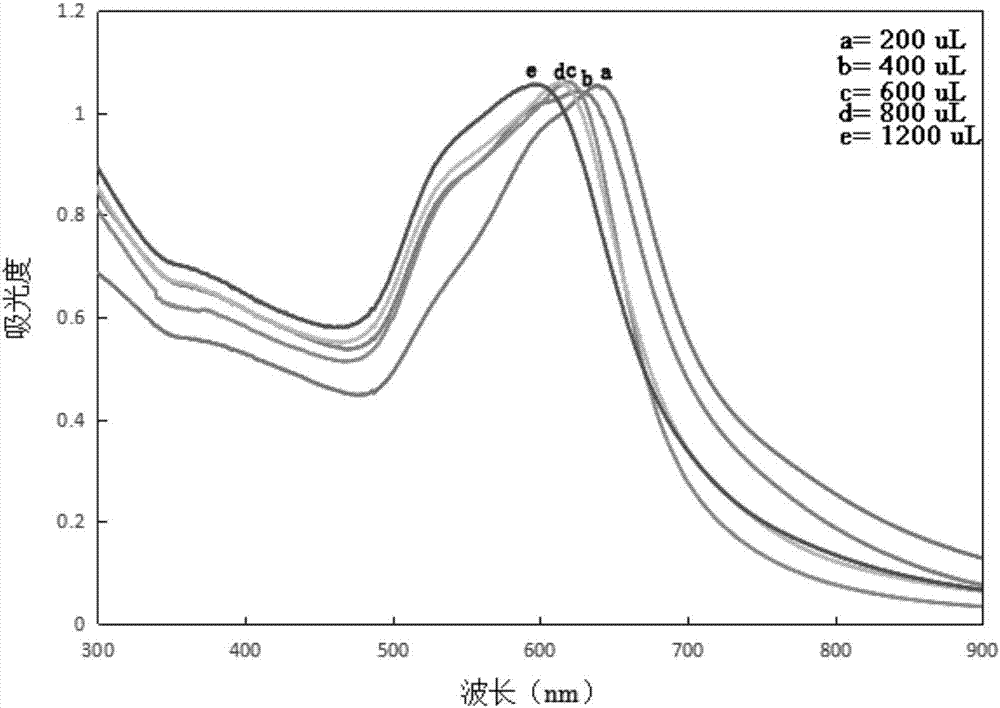
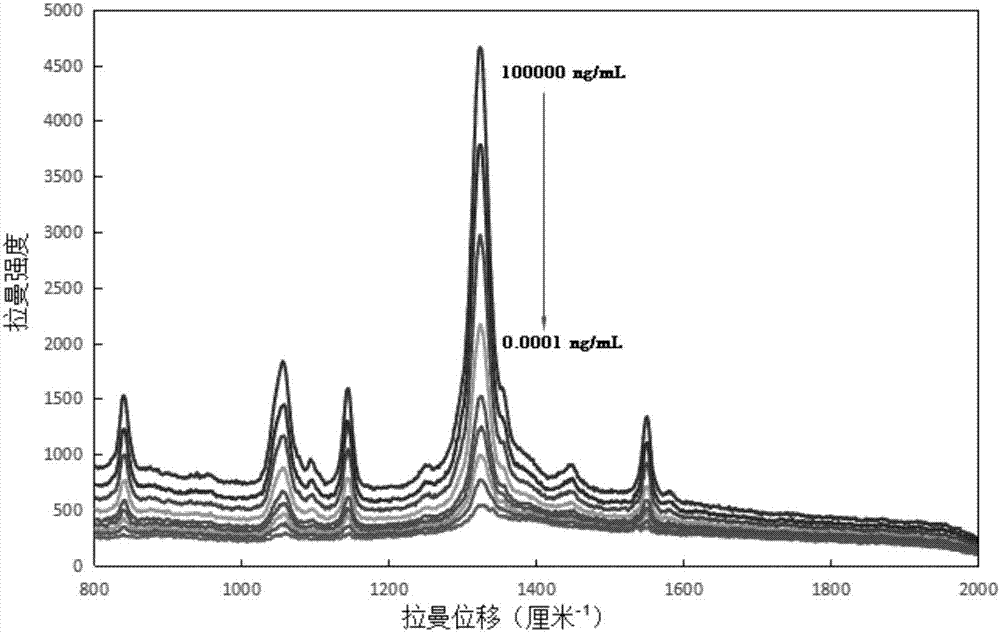
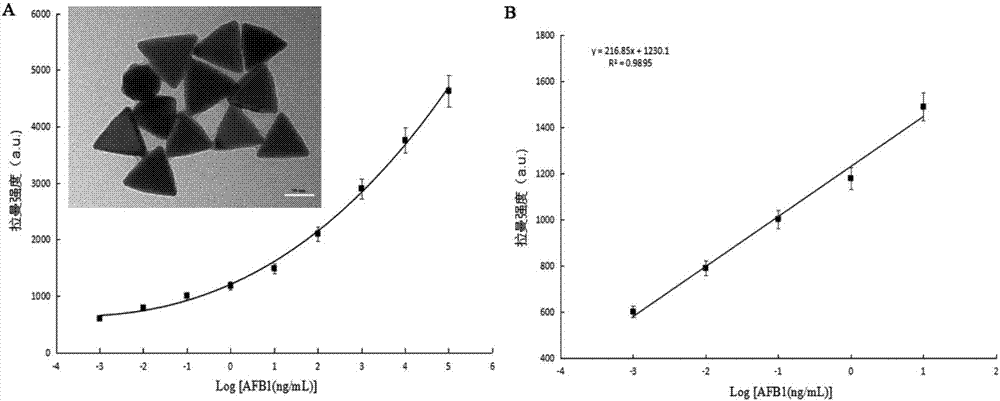
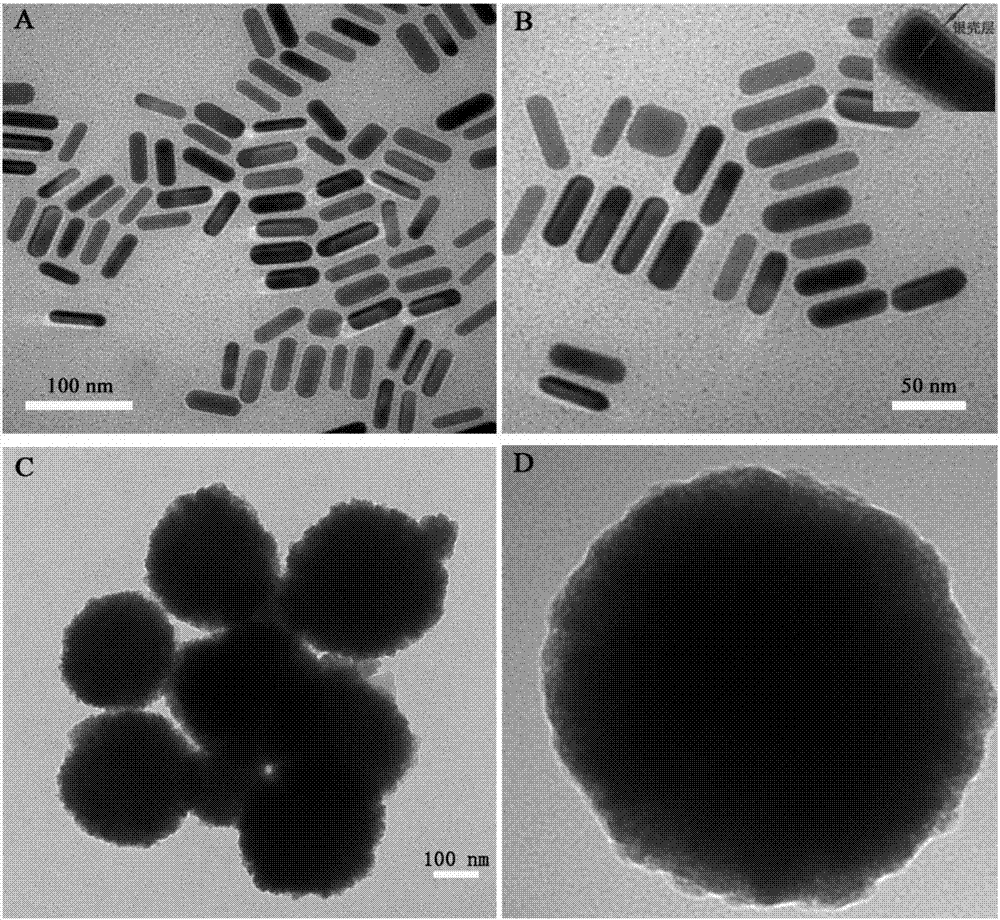
The invention relates to a method for performing supersensitive detection on fungaltoxin of a DTNB mark-based gold@silver core-shell nanorod, and belongs to the technical fields of food safety, environment monitoring and the like. The gold nanorod is prepared by a seed growing method, 5,5'-dithio-bis(2-nitrobenzoic acid) (DTNB) is marked on the gold nanorod, meanwhile, the gold nanorod is coated with a silver shell layer to prepare a gold@DTNB@silver surface Raman enhanced substrate, a superparamagnetic magnetic material chitosan ferroferric oxide is prepared, a fungaltoxin aptamer complementary chain is coupled on the prepared Raman enhanced substrate, a fungaltoxin aptamer chain is coupled on the magnetic material, an enhancer and the magnetic material are combined and a Raman signal of a system is strongest when the fungaltoxin does not exist in the detection system, and the aptamer modified magnetic material is specifically and preferentially combined with the fungaltoxin and the Raman signal of the system is changed after an external magnetic field is separated when the fungaltoxin exists, so that the aim of quantitatively detecting the fungaltoxin is fulfilled.
Owner:中科怡海高新技术发展江苏股份公司
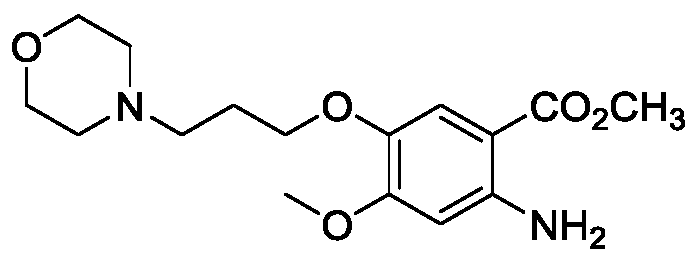
Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline
ActiveCN101570516AReduce pollutionReduce manufacturing costOrganic active ingredientsOrganic chemistry4 FluorophenylalanineNitration
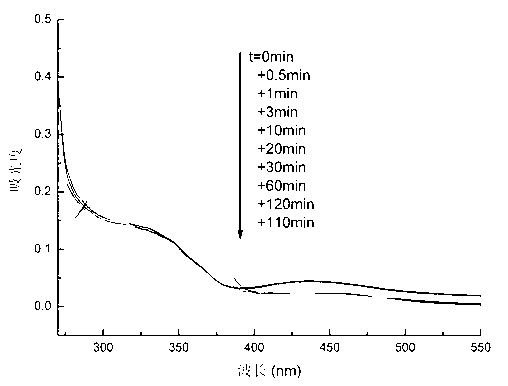
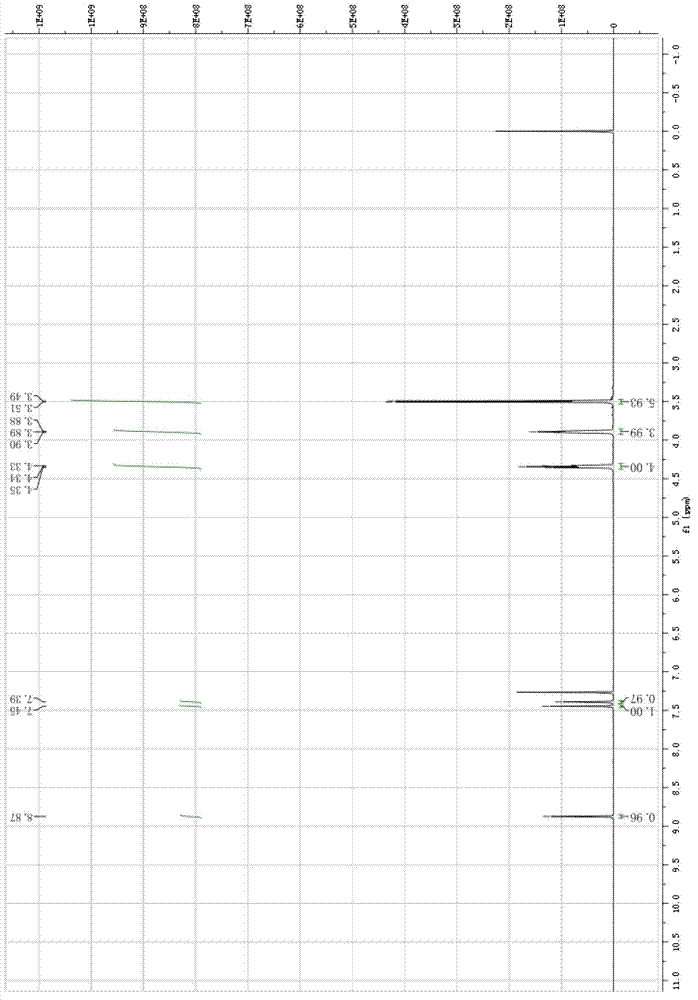
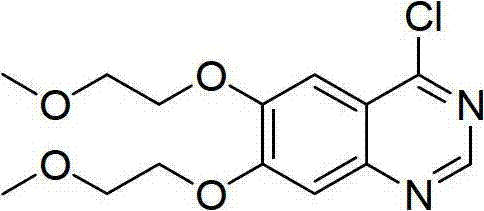
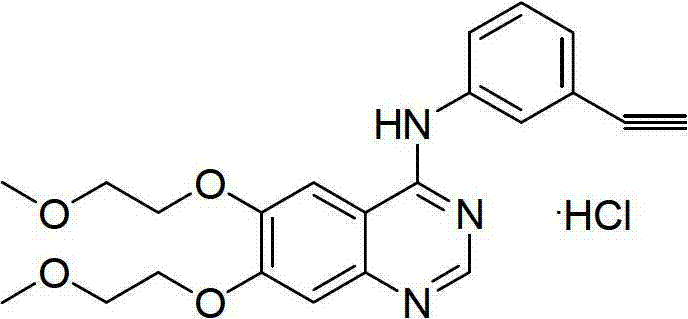
The invention relates to a method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline (I). The method takes 4-methoxy-2-nitrobenzoic acid as initial material which is subjected to reduction and nitration to obtain 4-methoxy-5-nitryl-2-aminobenzoic acid, is then subjected to cyclization to obtain 7-methoxy-6-nitryl quinazoline-4(3H)-ketone, is again subjected to reduction and diazo reaction hydrolysis to obtain 7-methoxy-6-hydroxy quinazoline-4(3H)-ketone, after etherification reaction is carried out, phosphorus oxychloride is used for preparing 7-methoxy-6-[3-(4-morphjolinyl) propoxy]-4-chloroquinazoline, which is subjected to amination to obtain the compound in the formula (I). The initial material adopted by the preparation method is convenient and accessible, the cost is low, the process route is simple and reasonable, the three wastes produced in the process of preparation generates less pollution, therefore the preparation method can prepare final product with high quality and yield in mass production, and is suitable for industrialized production.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
Enzyme inhibition method for detecting carbamates
InactiveCN103323415ALow costSimple methodColor/spectral properties measurementsNitrobenzoic acidFormic acid
The invention relates to the chemical field and in particular to an enzyme inhibition method for detecting carbamates. Ethyl carbamate is oxidized by using an oxidant in the presence of an acetylcholine iodide substrate, a 5,5'-dithio bis-(2-nitrobenzoic acid) (DTNB) developer and an acetylcholin esterase with the activity of more than 200U / g, and then the absorbance variation value of the ethyl carbamate along with time at 3min is detected by spectrophotometry to obtain the inhibition condition of the ethyl carbamate on the acetylcholin esterase so as to obtain the content of the ethyl carbamate. The substrate and the developer used in the invention are low in cost; the inhibition rate is greatly increased since the ethyl carbamate is oxidized by using the oxidant; and the method is rapid, simple, high in sensitivity, mild and easily controlled in reaction conditions, simple in steps and easy to operate and needs less equipment. According to the principle of the method, a rapid kit is developed for the rapid detection of ethyl carbamates in alcoholic beverages and fermented foods.
Owner:GUANGZHOU INST FOR FOOD INSPECTION(GUANGZHOU INSPECTION CENT FOR WINE & SPIRITS) +1
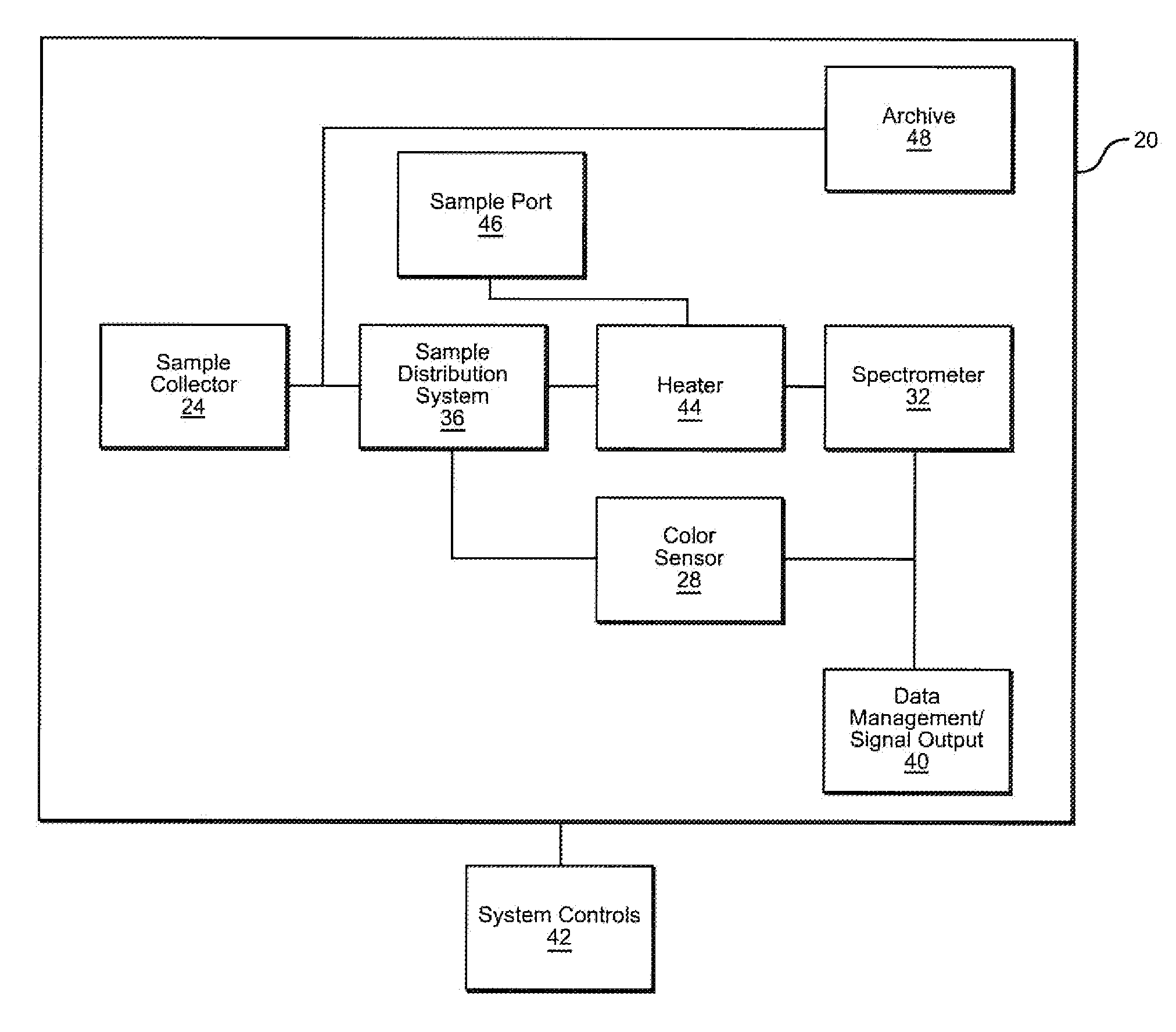
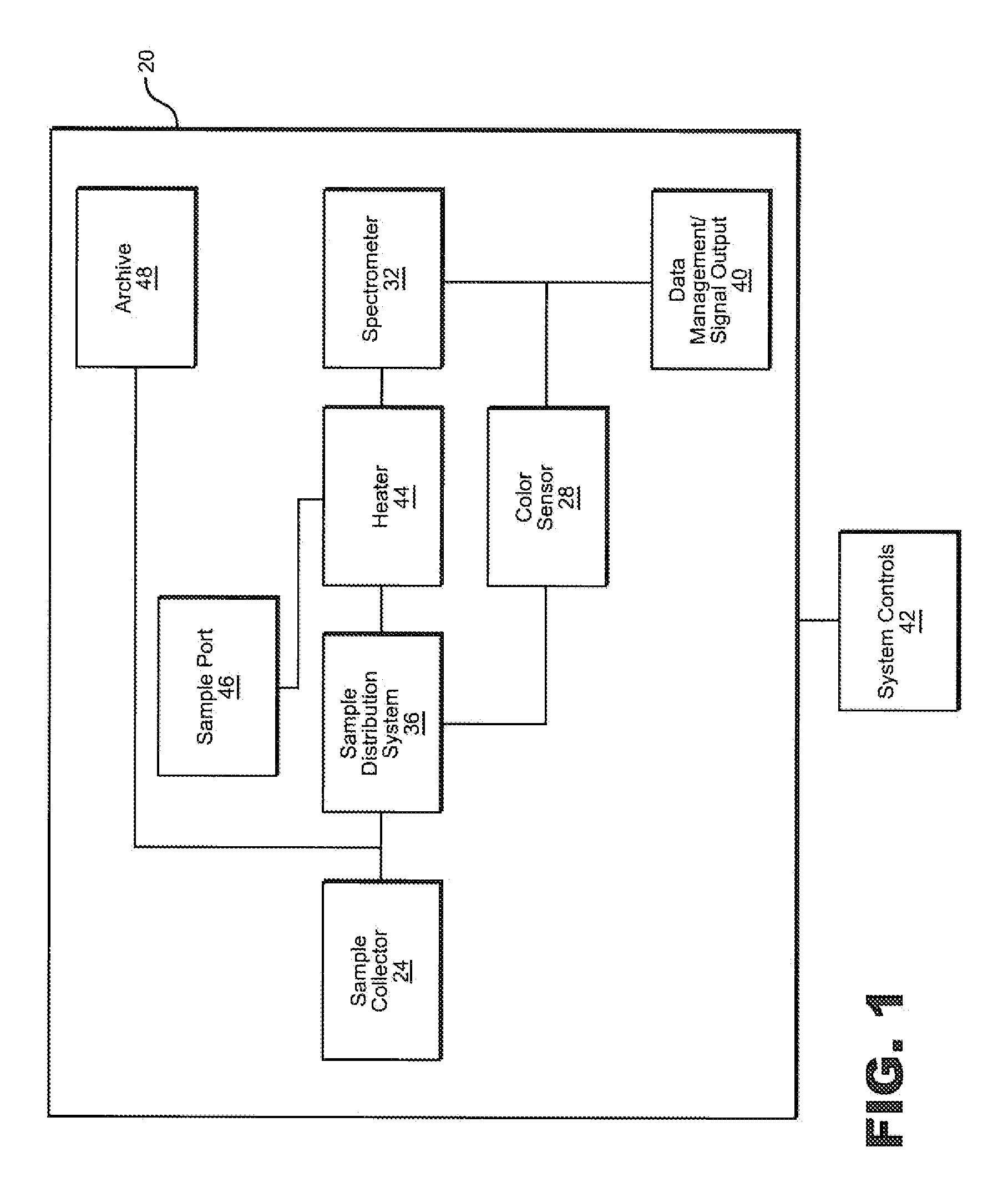
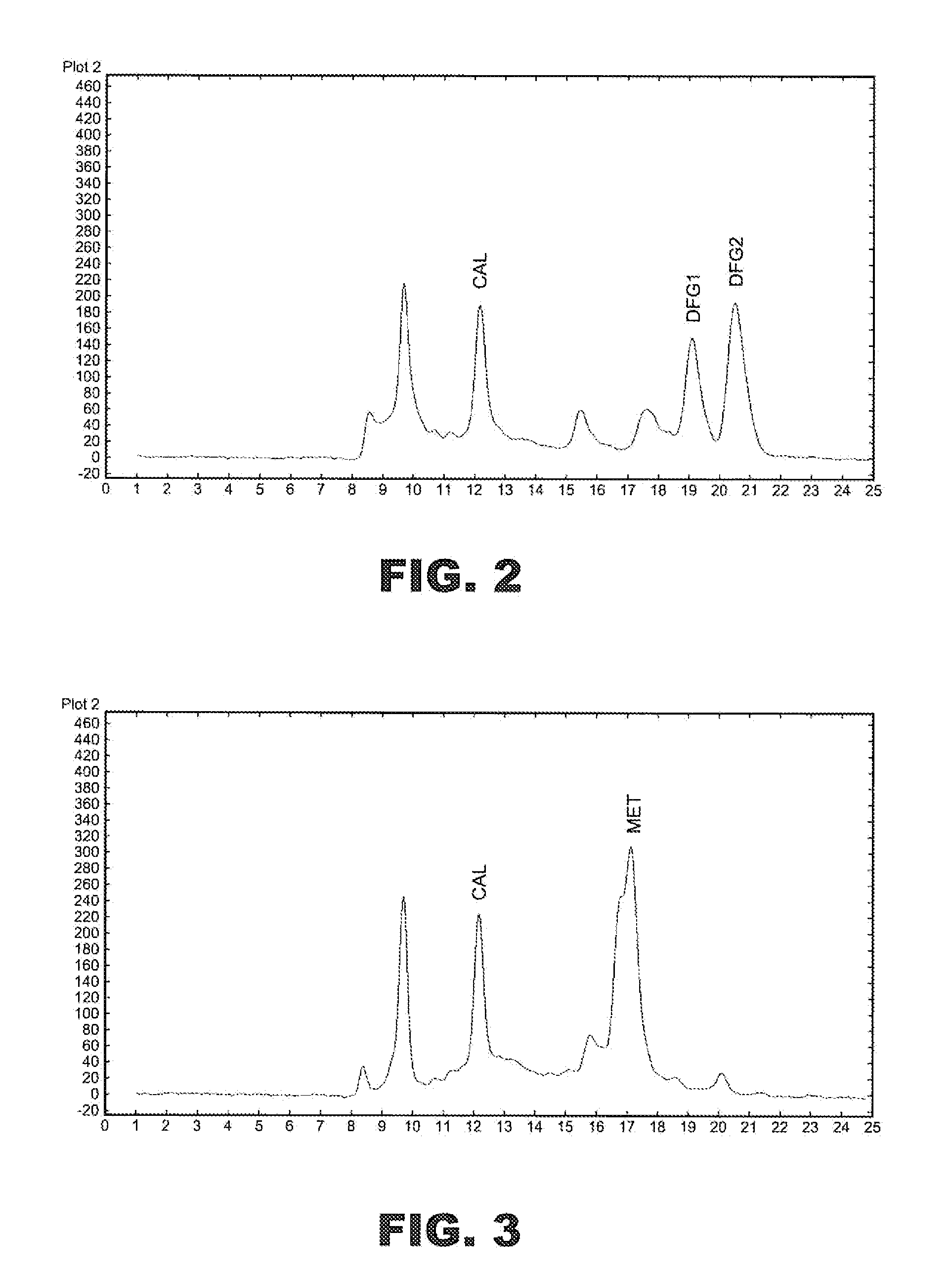
Toxic Material Detection Apparatus and Method
InactiveUS20110045517A1Easy to handleStrong specificityBioreactor/fermenter combinationsBiological substance pretreatmentsReal time analysisToxic material
A toxic material detection apparatus includes a sample collection portion including a sample inlet and a sample concentrator adapted to concentrate an environmental sample on a substrate. A sample distributing system transfers portions of the substrate to a color sensor and an ion mobility spectrometer for simultaneously analysis and toxin detection, particularly cholinesterase inhibitor detection. Optionally, a portion of the substrate may be directed to an archive for possible analysis at a later time. Reagents utilized include the enzyme acetylcholinesterase (AChE), and the reactants acetylthiocholine iodide (ATCI) and 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTB). A data management unit provides for near-real-time analysis of the samples in under 5 minutes. Simultaneous “hits” by both analysis methods indicate the presence of a cholinesterase inhibitor.
Owner:BATTELLE MEMORIAL INST
Water-based cleaning agent
ActiveCN102230182AStrong degreasing abilityNo irritating smellFoam dispersion/preventionWater basedPolyethylene glycol
The invention discloses a water-based cleaning agent, which comprises the following components in percentage by mass: 1 to 3 percent of sodium tripolyphosphate, 2 to 4 percent of disodium hydrogen phosphate, 0.5 to 1 percent of borax, 2 to 4 percent of nitrobenzoic acid, 0.5 to 2 percent of sodium hydroxide, 1 to 4 percent of surfactant, 2 to 5 percent of polyethylene glycol, 13 to 15 percent of triethanolamine, 0.01 to 0.1 percent of defoamer and the balance of water. The water-based cleaning agent is high in cleaning capacity of greasy dirt, avoids generating a large amount of foam in the cleaning process, is suitable for full-automatic cleaning and ultrasonic cleaning, has the good protective effect on cleaning machines, does not contain inflammable and explosive ingredients, is used safely, does not have influence on human bodies and does not pollution on environment.
Owner:北京蓝星清洗有限公司
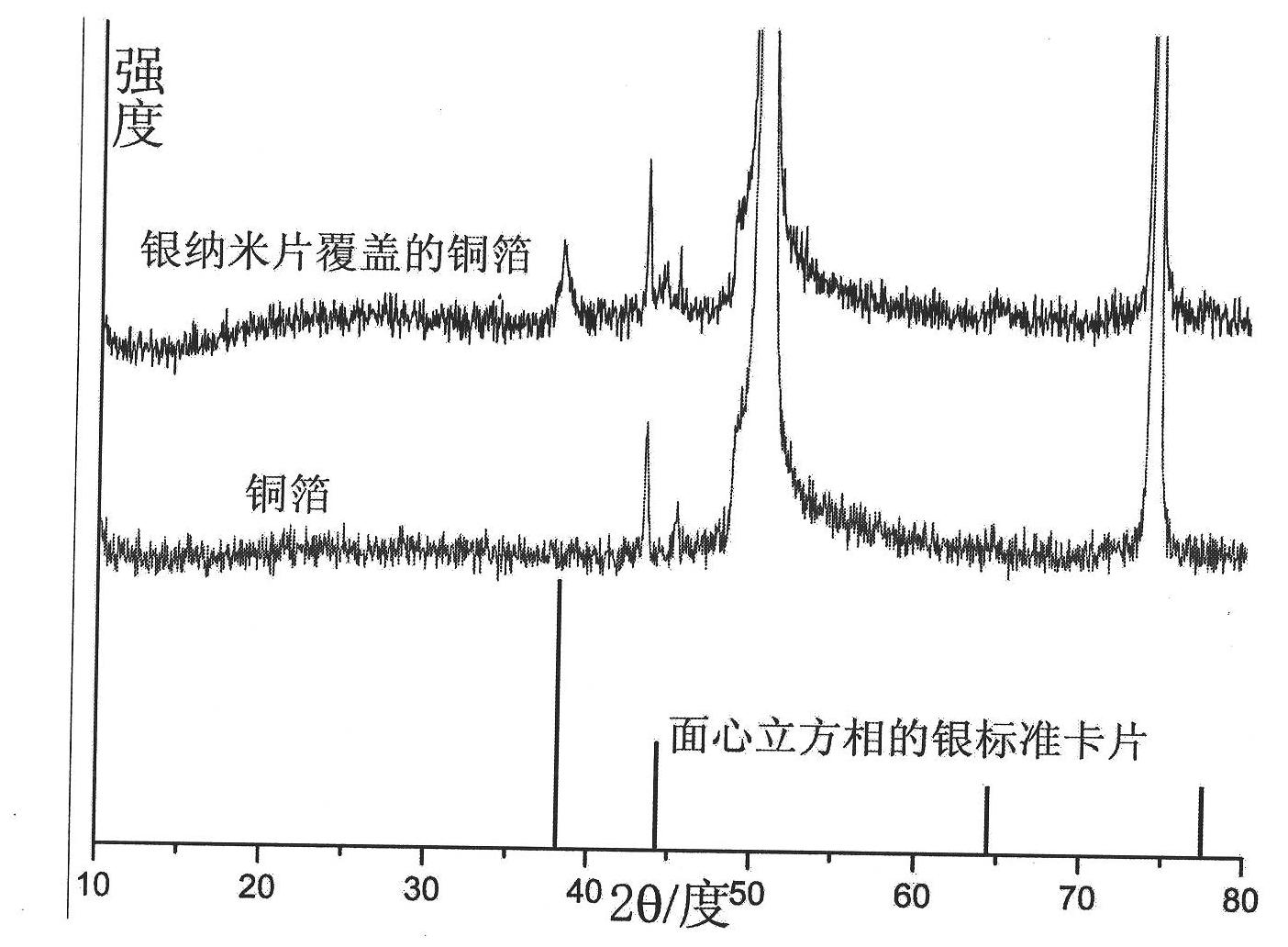
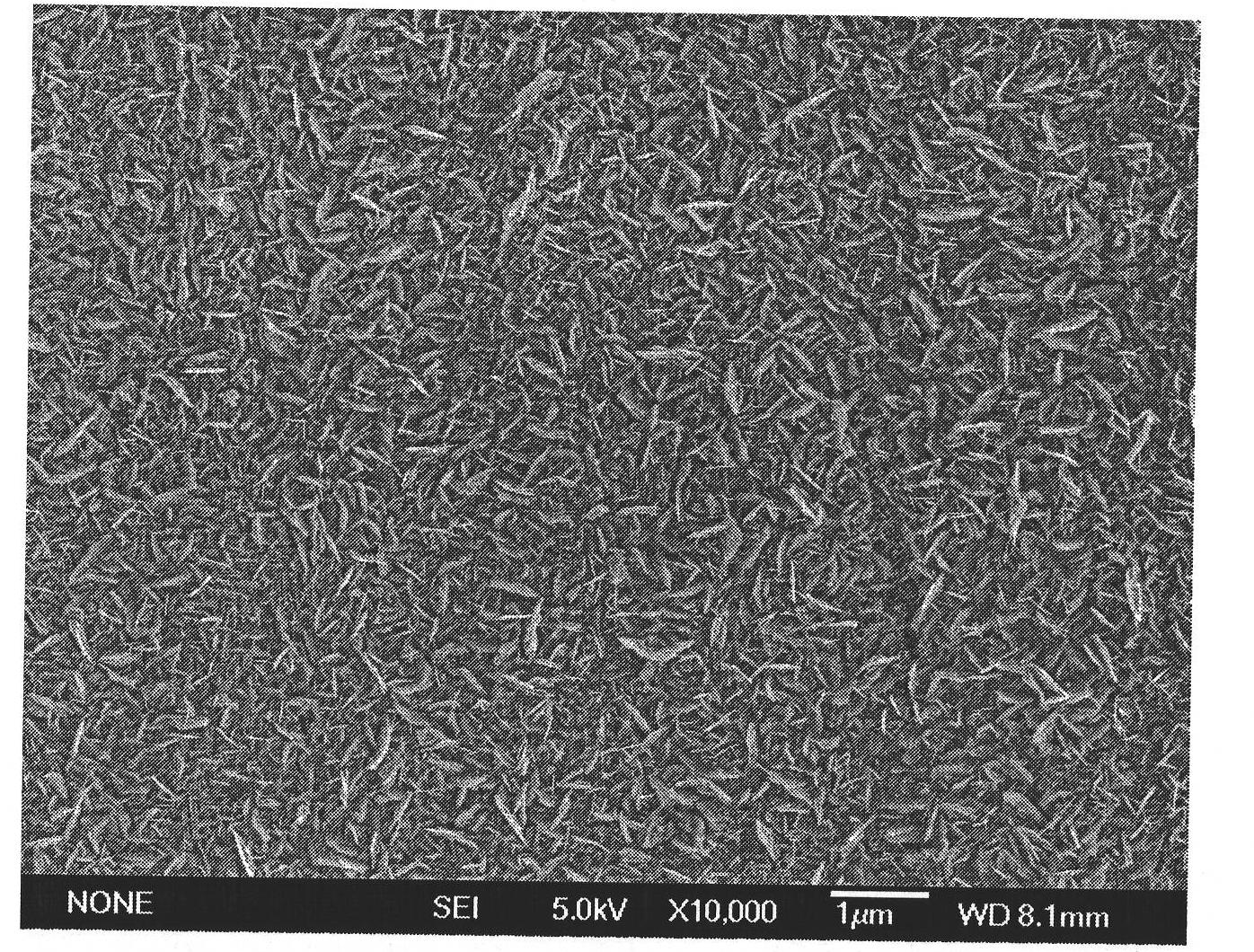
Method for covering silver nano-film on copper material
ActiveCN101781759AUniform sizeImprove conductivityLiquid/solution decomposition chemical coatingSurface-enhanced Raman spectroscopyVolumetric Mass Density
The invention relates to a method for growing a silver nano-film on copper material, which belongs to the field of inorganic nano-material preparation. The method uses the replacement reaction between elemental copper and silver ions, takes nitrobenzoic acid as a control agent, and covers a layer of silver nano-film on the surface of the copper material. The method has the advantages of reasonable design, simple operation, easily controllable reaction, good repeatability and easy large-scale production. The silver nano-film has the advantages of uniform size, high distribution density, and very high conductivity and inoxidability. The method can be used in SERS, electronics industry and other fields.
Owner:SHANDONG UNIV
Surface-enhanced Raman detection method for mycotoxin based on silica-coated gold nanotriangle
InactiveCN106970063AEnhanced Raman signal stabilityHigh yieldRaman scatteringMycotoxinSignalling molecules
The invention provides a surface-enhanced Raman detection method for mycotoxin based on a silica-coated gold nanotriangle. According to the method, a three-step seed induction method is employed for preparation of a gold nanotriangle, and the gold nanotriangle is labeled with a 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB) signal molecule and coated with silica so as to prepare a gold@DTNB@silica surface Raman enhancing agent; a chitosan ferriferrous oxide (CS-Fe3O4) magnetic material is prepared at the same time; an enhanced substrate and the magnetic material are modified with mycotoxin aptamer so as to construct a mycotoxin detection system; when mycotoxin exists, gold@DTNB@silica and CS-Fe3O4 are bonded together via the effect of the specific recognition of the aptamer so as to form a CS-Fe3O4-aptamer-mycotoxin-gold@DTNB@silica detection system; with changes of the concentration of mycotoxin, the Raman signal of the system is not changed after magnetic separation; and thus, quantitative ultra-sensitive detection of mycotoxin in food is realized. The method is applicable to the technical fields of food safety, material chemistry and the like.
Owner:JIANGSU UNIV
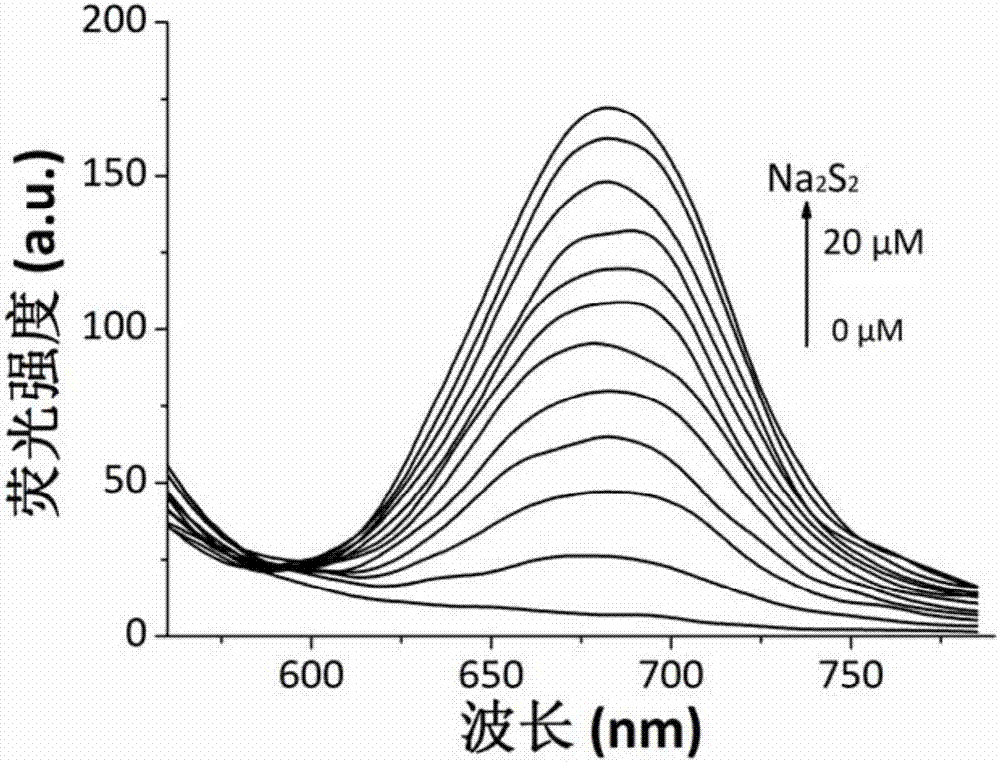
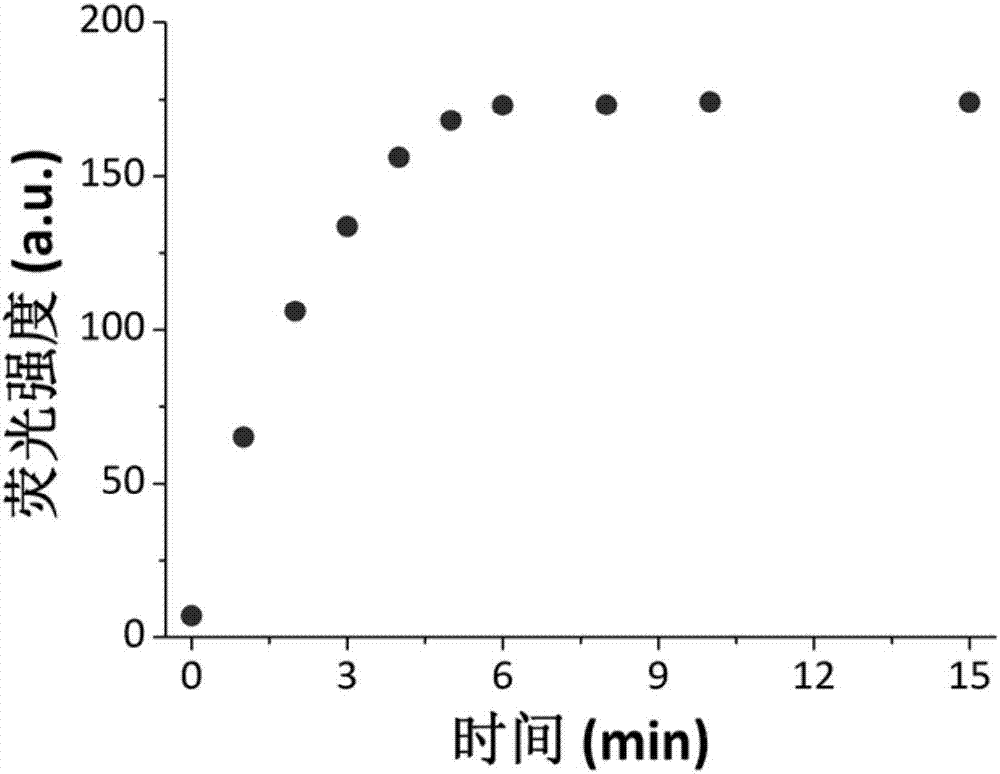
Synthesis and application of near infrared fluorescence probe for detecting hydrogen polysulfide
The invention relates to a synthesis method and application of a novel high-selectivity and high-sensitivity near infrared fluorescence probe for visually detecting hydrogen polysulfide. The method comprises the steps of making benzopyran carbonitrile and p-hydroxy benzaldehyde react to obtain a precursor, and then conducting an esterification reaction with 2-fluoro-5-nitrobenzoic acid to obtain the fluorescence probe. The probe reacts with sodium polysulfide to generate a strengthened fluorescence change, which is that the fluorescence at the 682 nm is strengthened remarkably. Besides, a dynamic experiment result shows that the reaction time of the compound and hydrogen polysulfide is shorter than 5 min, and the interference rejection is strong. Thus, the pyran carbonitrile compound can serve as the application of the near infrared fluorescence probe for detecting hydrogen polysulfide.
Owner:TAIZHOU UNIV
Low silicon content coolant liquid applicable to aluminum radiator of heavy-duty commercial vehicle engine
ActiveCN101602936AImprove heat transfer and cooling performanceMachines/enginesHeat-exchange elementsWater jacketAdipate
The invention relates to low silicon content coolant liquid applicable to an aluminum radiator of a heavy-duty commercial vehicle engine, and belongs to coolant liquid. The coolant liquid comprises the following raw materials in portion by weight: 36 to 52 portions of terylene grade glycol, 1.0 to 1.5 portions of mannite, 0.3 to 0.7 portion of phenylformic acid, 0.7 to 0.9 portion of adipate, 0.1 to 0.2 portion of methylbenzotriazole, 0.05 to 0.1 portion of p-nitrobenzoic acid, 0.001 to 0.004 portion of polyether, 0.3 to 0.7 portion of potassium phosphate, 0.2 to 0.25 portion of sodium nitrite, 0.25 to 0.3 portion of sodium nitrate, 0.5 to 1.0 portion of borax, 0.007 to 0.02 portion of sodium silicate, 0.45 to 0.68 portion of sodium hydroxide, and distilled water for fixing the volume to 100 portions. The coolant liquid contributes to improvement of heat exchange and cooling performance of a water jacket of the engine, is more adapted to an aluminum water tank radiator, is adapted to sedans and light vehicles, and is more adapted to heavy-duty commercial vehicles.
Owner:CHANGCHUN YONGCHANG PETROCHEM
Polymethylmethacrylate composite material for 3D printing and preparation method thereof
The invention discloses a polymethylmethacrylate composite material for 3D printing and a preparation method thereof. The preparation method comprises the following steps: dissolving acrylic acid in ethyl acetate, adding zaodiisobutyronitrile, placing at room temperature, then sequentially adding paranitrobenzoic acid and isobutyl cyanacetate, stirring at room temperature, subsequently adding polymethylmethacrylate particles and continuously stirring at room temperature to obtain the polymethylmethacrylate composite material for 3D printing. The polymethylmethacrylate composite material comprises 30-60 percent of polymethylmethacrylate, 10-30 percent of isobutyl cyanoacetate, 1-10 percent of crylic acid, 20-40 percent of ethyl acetate, 1-2 percent of zaodiisobutyronitrile, and 1-10 percent of paranitrobenzoic acid. The composite material prepared according to the preparation method can be used for 3D printing within the temperature range of 10-35 DEG C, and the polymethylmethacrylate composite material for 3D printing and the preparation method thereof are easily popularized and applied due to simple preparation process, easy availability of the raw materials and low production cost.
Owner:TAICANG BIQI NEW MATERIAL RES & DEV
Process for producing m-dimethylamine benzoic acid
InactiveCN1418863AOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidSolvent
The method for producing m-dimethylaminobenzoic acid includes the following steps: in the presence of catalyst and methyl alcohol solvent making raw material m-nitrobenzoic acid and hydrogen gas produce reduction reaction to obtain intermediate product, making said intermediate product continuously implement ankylation reaction with formaldehyde to obtain crude product, making said crude product undergo the processes of filtering, washing and purification so as to obtain the invented product m-dimethylaminobenzoic acid. Its catalyst uses active carbon as carrier, and its effective component is selected from palladium, platinum, rhodium or their mixture and selected from iron, cobalt, nickel, copper zinc or their mixture. Said catalyst is long in service life, and the methyl alcohol can berecovered for reuse.
Owner:CHINA PETROLEUM & CHEM CORP +1
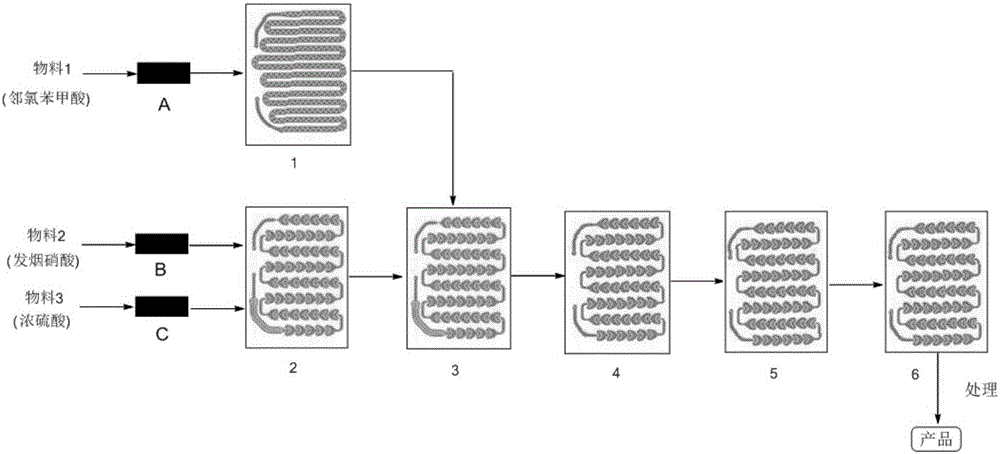
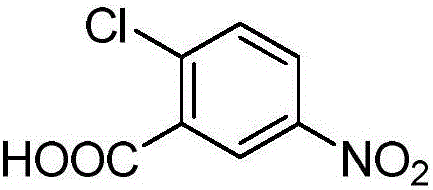
Method for synthesizing 2-chloro-5-nitrobenzoic acid through microchannel reactor
InactiveCN106674016AAvoid infringementAvoid corrosionNitro compound preparationIntrinsic safetyReaction temperature
The invention provides a method for synthesizing 2-chloro-5-nitrobenzoic acid through a microchannel reactor. The method comprises the steps of dissolving a raw material o-chlorobenzoic acid into concentrated sulfuric acid to obtain a material 1, and entering a preheating module; adopting fuming nitric acid and the concentrated sulfuric acid as a material 2 and a material 3, and entering another preheating module; preheating the material 1, the material 2 and the material 3, entering a reaction module group for reacting, collecting effluent reaction liquid, processing to obtain a crude product, and refining to obtain the product 2-chloro-5-nitrobenzoic acid. According to the method provided by the invention, the reaction time is shortened to a few minutes to a few seconds, the production energy consumption is reduced, and the reaction efficiency is remarkably improved. High-efficient mass and heat transfer efficiency ensures the reaction temperature to maintain within a setting range, the possibilities of temperature out of control, temperature runaway and even sharp reaction overflow or explosion caused by over-high local concentration do not exist, the intrinsic safety problem of the nitration reaction is solved fundamentally, and the reaction yield and the product purity are remarkably improved.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
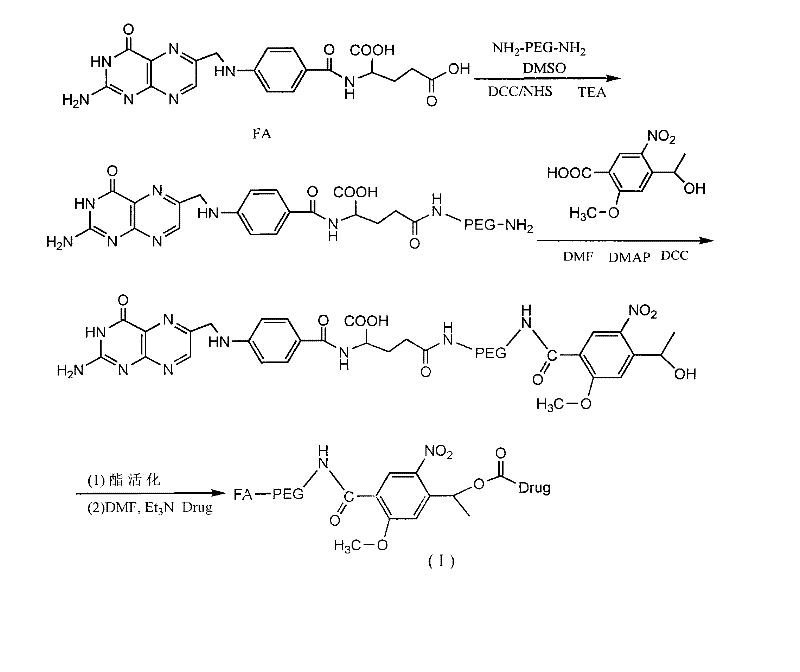
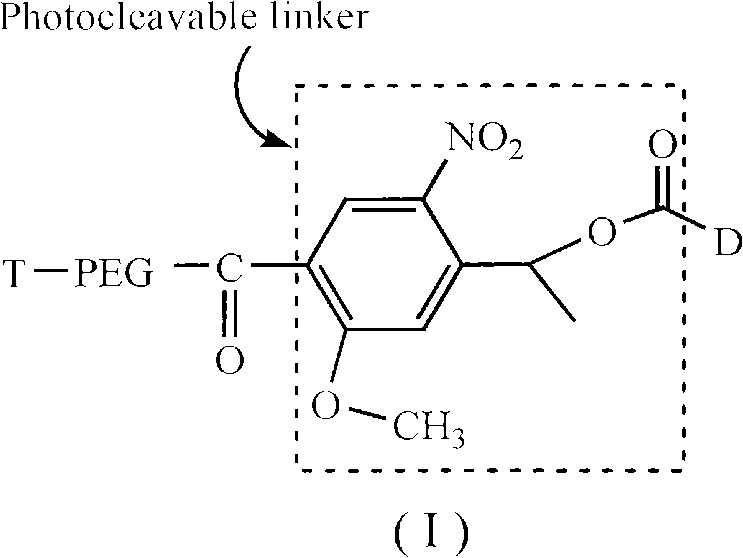
Long-circulating target photosensitive antitumor medicine conjugate and preparation method thereof
InactiveCN102631681AAchieve therapeutic effectExtend cycle timeEnergy modified materialsPharmaceutical non-active ingredientsPolyethylene glycolPolymer
The invention belongs to the technical field of polymer drugs, and particularly relates to a long-circulating target photosensitive antitumor drug conjugate and a preparation method of the long-circulating target photosensitive antitumor drug conjugate. The preparation method comprises the following steps: firstly, carrying out amidation reaction to a single-end amino polyethylene glycol amino with ligand functionalization and 4-(1-ethoxy)-2- methoxy group-5-nitrobenzoic acid containing a photosensitive group; then carrying out esterification reaction with an esterifying agent; and finally coupling with the antitumor drug containing the amino to obtain the long-circulating target photosensitive antitumor drug conjugate. According to the conjugate prepared by the preparation method, the circulating time of the drug in the body can be prolonged, tumor tissues are accelerated to adsorb drug, and the conjugate has the target to quicken the enrichment speed of the antitumor drug in the tumor tissues. The conjugate is fractured by the photostimulation of specific wavelength to release original drug and quickly achieve treatment concentration, and 'time / space' controllable effective treatment is obtained. Meanwhile, the preparation method provides a simple and effective path for preparing the target controllable photosensitive biological polymer drug.
Owner:YANCHENG INST OF TECH
Method for preparing material of Nano carbon tube grafted by azobenzene in light responsibility
The present invention discloses a preparation method of photoresponsibility azobenzene graft carbon nano tube material, belonging to preparation technology of optical, electric and energy-transducing material. Said preparation process includes the following steps: adding multiwall or single-wall carbon nano tube into mixed acid formed from nitric acid and sulfuric acid to make acidification, dispersing acidified carbon nano tube in the SOCl2 solution to make reaction to obtain carbon nano tube with acyl chloride function group; mixing nitrodracylic acid and zinc powder in sodium hydroxide to obtain para-dihydroxyazobenzene, then mixing it and sulphoxide dichloride in chloroform to obtain p-4,4'-amide linear alkylamine azobenzene, adding p-4,4'-amide linear alkylamine azobenzene and nano tube with acyl chloride function group into tetrahydrofuran, heating and reacting for above 96h, dewatering, evaporating to obtain paste-like material, dispersing it in chloroform, filtering and drying so as to obtain the invented product.
Owner:TIANJIN UNIV
Method for preparing acetosyringone and vanillyl ethyl ketone by oxidizing lignin
InactiveCN102295547AEfficient use ofHarm reductionCarbonyl compound preparation by oxidationGas liquid chromatographicNitrobenzene
The invention discloses a method for preparing acetovanillone and acetosyringone (AS) through oxidation of lignin by using an oxidizing agent. According to the method, an oxidizing agent reacts with the lignin in an alkaline solution; the resulting reaction solution is subjected to acidification, extraction and concentration to obtain the crude product after completing the reaction; the treatments of recrystallization and rectification are performed to obtain the acetovanillone and the AS. The oxidizing agent is the one selected from p-nitrobenzoic acid, 3,5-dinitrobenzoic acid, 3-nitrosalicylic acid, 5-nitrosalicylic acid or 3,5-dinitrosalicylic acid. According to the present invention, the oxidizing agent has low toxicity, such that the harm to the environment can be reduced; the post-treatment steps are simplified, and the uses of the organic solvents are reduced, such that the secondary pollution to the environment is reduced; the yield is relatively high, the purities of the products are respectively 97.3% and 98.2% through the gas chromatography analysis; the two important chemical raw materials of guaiacol and lilac alcohol can be synchronously obtained when preparing the acetovanillone and the AS.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Preparation method and applications of hyperbranched azo polymer
InactiveCN103193967AHigh thermo-optic coefficientLow driving powerTenebresent compositionsBenzoic acidPolymer science
The invention relates to a preparation method and applications of a hyperbranched azo polymer, belonging to the organic synthesis field. The preparation method comprises the steps: firstly, performing nitroreduction and carboxyl acylation by taking p-nitrobenzoic acid as a raw material, to obtain an A2 type monomer azo-bi(benzoyl chloride) with an azobenzene structure, and then reacting the A2 type monomer azo phthaloyl dichloride with a B3 type monomer glycerol according to certain mole ratio to obtain the hyperbranched azo polymer. The materials of the obtained hyperbranched azo polymer has higher thermo-optical coefficient which is more than ten times of that of inorganic materials such as borosilicate glass, zinc silicate glass, silica glass and the like, and the material provides a possibility for developing a novel digital thermo-optical switch with low driving power.
Owner:JIANGSU UNIV
Preparation method for 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline
The invention discloses a preparation method for 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline. The process flow of the method is as follows: A, 3,4-bis(2-methoxyethoxy)benzaldehyde undergoes nitration to produce 4,5-bis(2-methoxyethoxy)-2-nitrobenzaldehyde; B, oxidation is carried out so as to obtain 4,5-bis(2-methoxyethoxy)-2-nitrobenzoic acid; C, esterification is carried out so as to obtain 4,5-bis(2-methoxyethoxy)-2-nitrobenzoate; D, reduction is carried out so as to obtain 4,5-bis(2-methoxyethoxy)-2-aminobenzoate; E, cyclization is carried out so as to obtain 6,7-bis(2-methoxyethoxy)-4(3H)-quinazolinone; and F, chlorination is carried out so as to obtain 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline. The preparation method provided in the invention has the advantages of a concise and practical synthetic route, high yield and a good industrial application prospect.
Owner:ZHEJIANG SCI-TECH UNIV
Environment-friendly chemical nickel stripper and deplating method thereof
The invention discloses an environment-friendly chemical nickel stripper and a deplating method thereof. Preferably, m-nitrobenzoic acid serves as an oxidizing agent for stripping a nickel-plated layer on an iron substrate; and since nitrate is generally stable in nature and low in toxicity, no waste gas or waste water is generated when the stripper provided by the invention is used, and the stripper does not corrode devices and has high affinity with the environment. Meanwhile, the stripper is further matched with diethyl dithiocarbamate and dodecyl sulfonate and combined with the deplating method thereof, the rate of stripping the nickel-plated layer on the iron substrate can be effectively increased, the nickel-plated layer can be stripped in a short time, and the production period is shortened. Compared with the prior art, the environment-friendly chemical nickel striper and the deplating method thereof have the advantages of being free of corrosion, poison gas and pollution, high in efficiency, environmentally friendly, easy and convenient to maintain and operate and the like.
Owner:HUIZHOU CITY HIROMI CHEM CO LTD
Preparation method of highly selective 3-methyl-2-nitrobenzoic acid
InactiveCN106496038AEasy to cleanReduce pollutionOrganic compound preparationCarboxylic acid esters preparationNitrationMedicinal chemistry
The invention discloses a preparation method of 3-methyl-2-nitrobenzoic acid. 3-methylbenzoic acid alkyl ester is used as raw material in nitrification, two-grade selectivity and high yield are realized, and the amount of waste acid was reduced. In nitrification product, only 3-methyl 2-nitrobenzoic acid alkyl ester and 3-methyl-4-nitrobenzoic acid alkyl ester are hydrolyzed after separation to obtain 3-methyl 2-Nitrobenzoic acid and 3-methyl-4-nitrobenzoic acid. The process is simple, and suitable for industrial production.
Owner:安徽安生生物化工科技有限责任公司
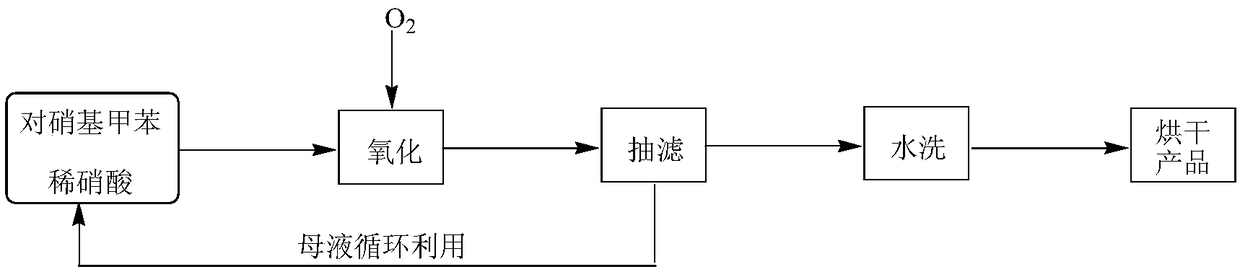
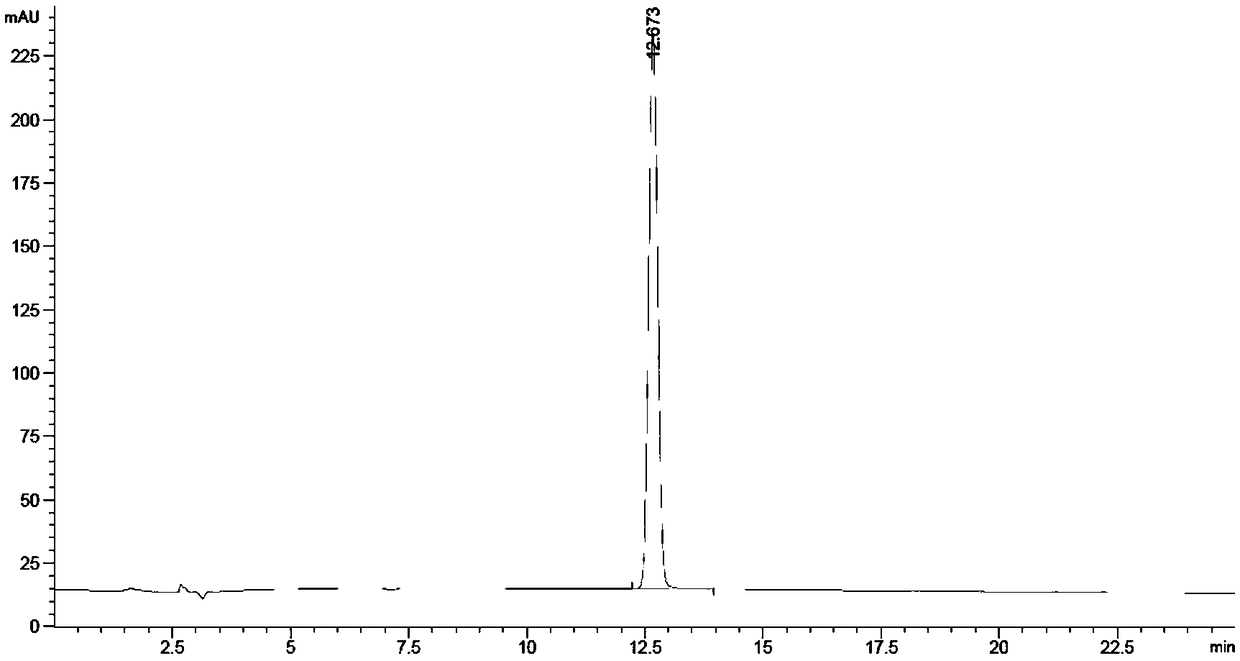
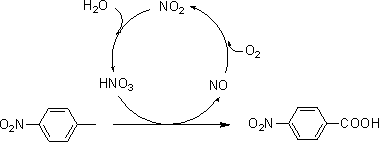
Method for synthesizing p-nitrobenzoic acid
InactiveCN109232260ANo cloggingNo pollution in the processOrganic chemistryOrganic compound preparationP-nitrobenzoic acidP-nitrotoluene
The invention relates to the field of production of chemical engineering products, in particular to a method for synthesizing p-nitrobenzoic acid. The method comprises the following steps of using p-nitrotoluene as the raw material, using diluted nitric acid as a reaction medium and a catalyst, using oxygen as an oxidant, and oxidizing in a high-pressure kettle, so as to prepare the p-nitrobenzoicacid; cooling, separating crystal form solid in the reaction liquid, sucking and filtering, so as to obtain a mother liquid and filter cake; spraying the filter cake by water, and drying, so as to obtain a light yellow product, namely p-nitrobenzoic acid. The method for synthesizing the p-nitrobenzoic acid has the advantages that the production purity is high, the preparation is simple, the costis low, the pollution is avoided, and the green effect is realized.
Owner:浙江优创材料科技股份有限公司
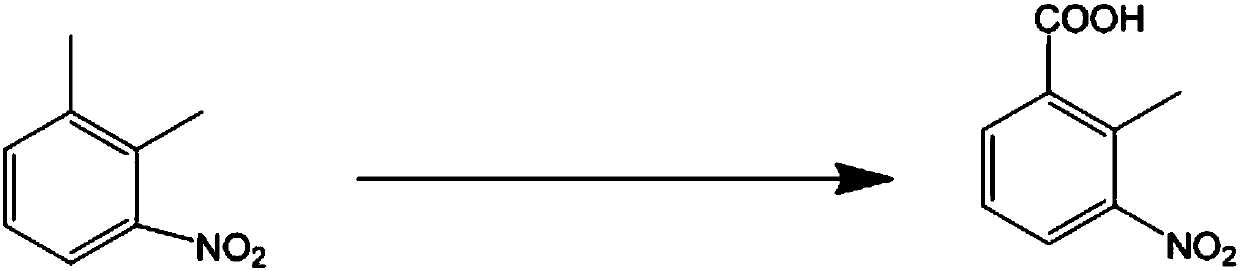
Clean production method to oxidize 3-nitro-o-xylene into 2-methyl-3-nitrobenzoic acid
InactiveCN109824517ABig pollutionEasy to handleOrganic chemistryOrganic compound preparationOrganic solventMethyl group
The invention discloses a clean production method to oxidize 3-nitro-o-xylene into 2-methyl-3-nitrobenzoic acid. The clean production method comprises the steps of adding 3-nitro-o-xylene with an acidinto an organic solvent under the action of a catalyst, adding an oxidant, and stirring to allow reacting to obtain 2-methyl-3-nitrobenzoic acid, wherein the oxidant is hydrogen peroxide, and the catalyst is a transition metal compound. The hydrogen peroxide is used herein as a clean oxidant, so that the oxidants, such as potassium permanganate, causing high environmental pollution are avoided. The reaction system is mild; post-reaction treatment is simple and practical; reaction selectivity is good; few byproducts are produced; the target product is easy to purify; both the yield and purityare high; the cost is low; the clean production method has a good industrial application prospect.
Owner:JIANGSU YONGAN CHEM CO LTD
Method for synthesizing 3-methyl-4-nitrobenzoic acid by using stepped heating method and indirect electrosynthesis method
The invention discloses a method for synthesizing 3-methyl-4-nitrobenzoic acid by using a stepped heating method and an indirect electrosynthesis method. The method is implemented by taking 2,4-dimethylnitrobenzene and chromium sulfate as main raw materials through the following steps of: firstly, carrying out electrolytic oxidation on chromium sulfate by using an electrolytic process so as to obtain chromium trioxide; then, selectively oxidizing 2,4-dimethylnitrobenzene into a 3-methyl-4-nitrobenzoic acid by using chromium trioxide through using the stepped heating method. According to the invention, through adopting the stepped heating method, the oxidation reaction rate of 2,4-dimethylnitrobenzene is controlled, and the selectivity and conversion rate of reaction are remarkably improved. The conversion rate of chromium trioxide obtained by using an electrolytic oxidation method is 85-95%, and the conversion rate of 3-methyl-4-nitrobenzoic acid obtained by using the stepped heating method can reach 65-86%. According to the invention, the problem that in the process of reaction, the reaction rate declines due to the consumption of acids is solved; through carrying out electrolytic oxidation on chromium sulfate produced after reaction, chromium trioxide is circularly produced, thereby not only avoiding the environment pollution, but also greatly saving the cost.
Owner:HUNAN UNIV OF SCI & TECH
Electroreduction preparation method of vandetanib and analog intermediates
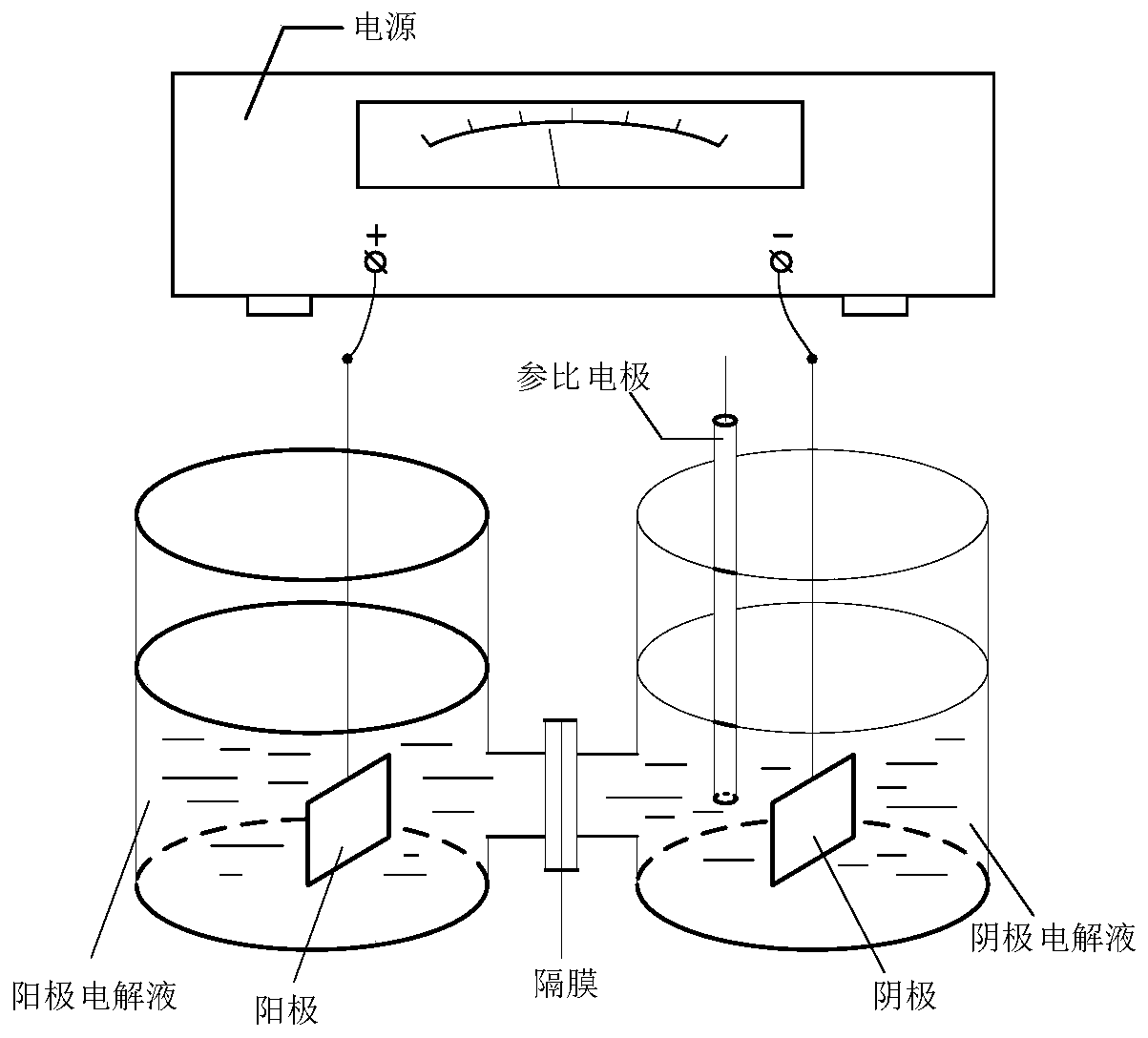
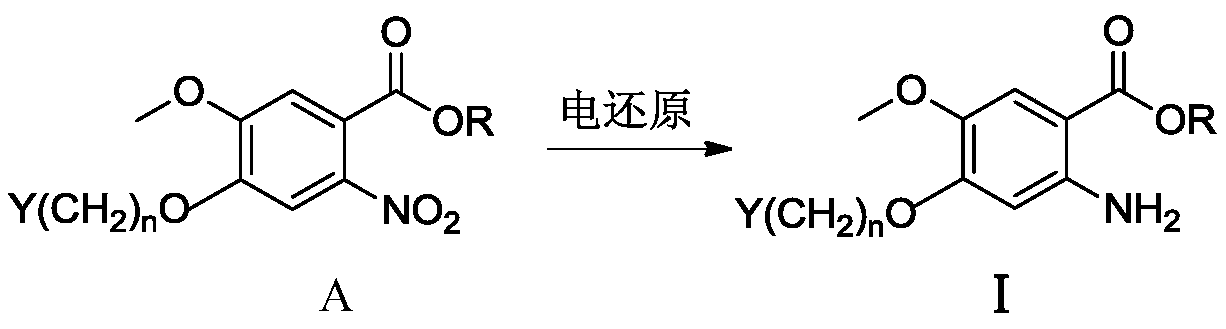
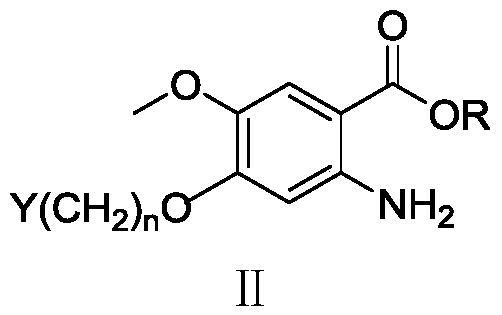
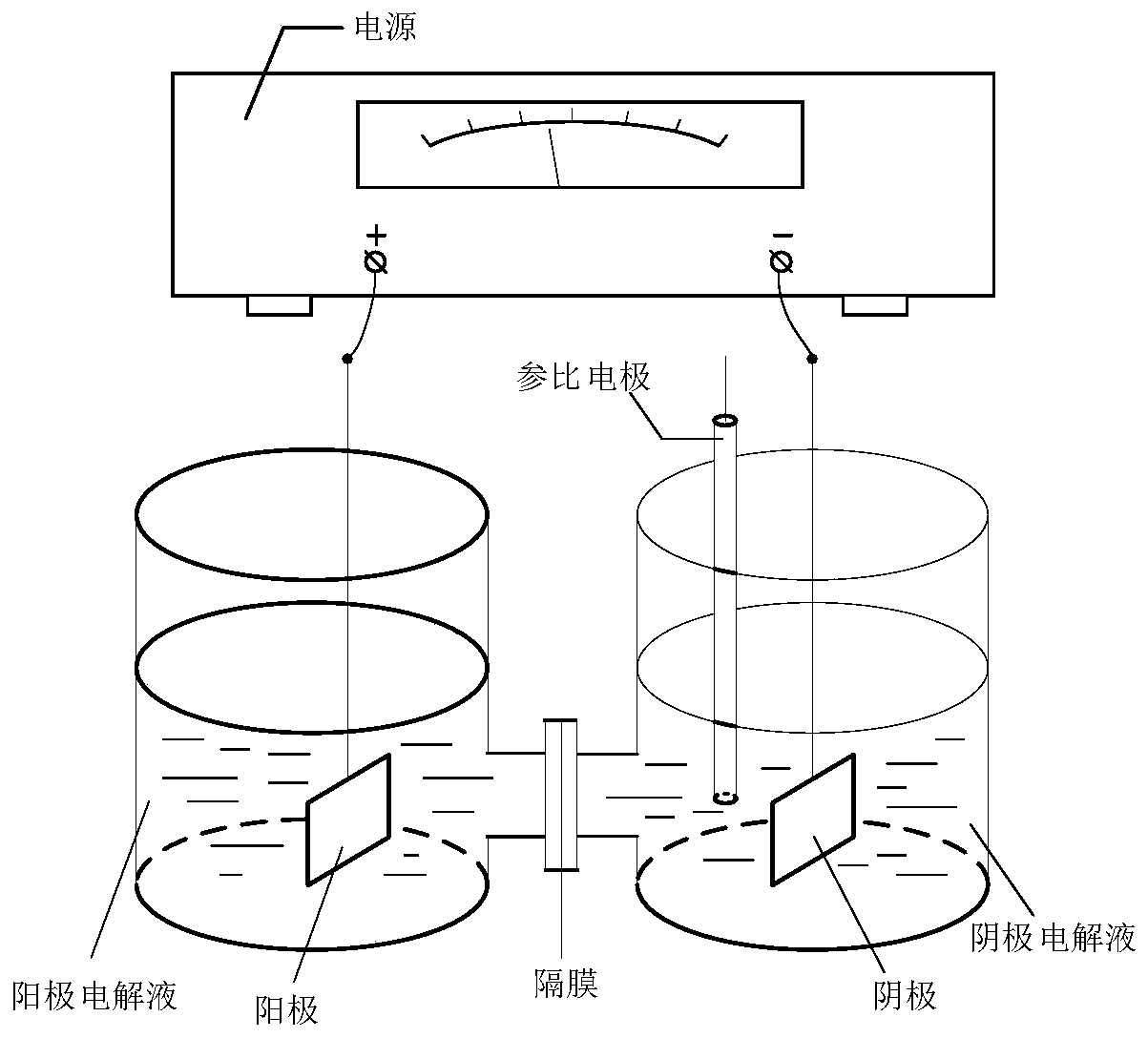
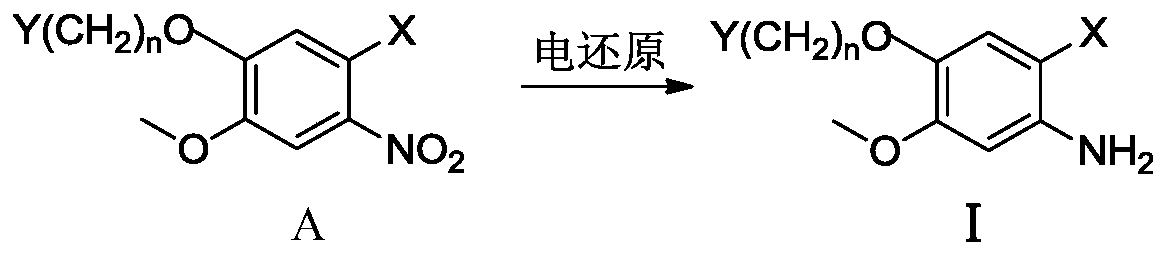
ActiveCN110629246AControl conversion rateLow selectivityElectrolysis componentsElectrolytic organic productionHydrogenAcid derivative
The invention relates to an electroreduction preparation method of 2-amino-5-methoxybenzoic acid derivatives represented by a formula I shown in the specification. A preparation reaction is shown in the specification, wherein n is selected from 1, 2 or 3; R is selected from hydrogen, methyl, ethyl or benzyl; Y is selected from C6H5, HO, Cl, Br, mesyloxy, p-toluenesulfonyloxy or M is selected fromCH or N; and W is selected from CH2, O, S, NH, HOCH, BocN, MeN, EtN, C6H5N, 4-ClC6H4N or 4-HOC6H4N. The electroreduction preparation method for the 2-amino-5-methoxybenzoic acid derivatives represented by the formula provided by the invention is characterized by comprising the step of performing constant-current or constant-voltage electrolysis in a diaphragm electrolytic cell by using an acid solution of a 5-methoxy-2-nitrobenzoic acid derivative as a catholyte and an acidic solution as an anolyte, wherein the voltage of a cathode working electrode is 1.00-2.50 V relative to that of a reference electrode, the current density of constant-current electrolysis is 25.0-250.0 mA / cm<2>, and the electrolysis temperature is 15-80 DEG C.
Owner:HUNAN UNIV +1
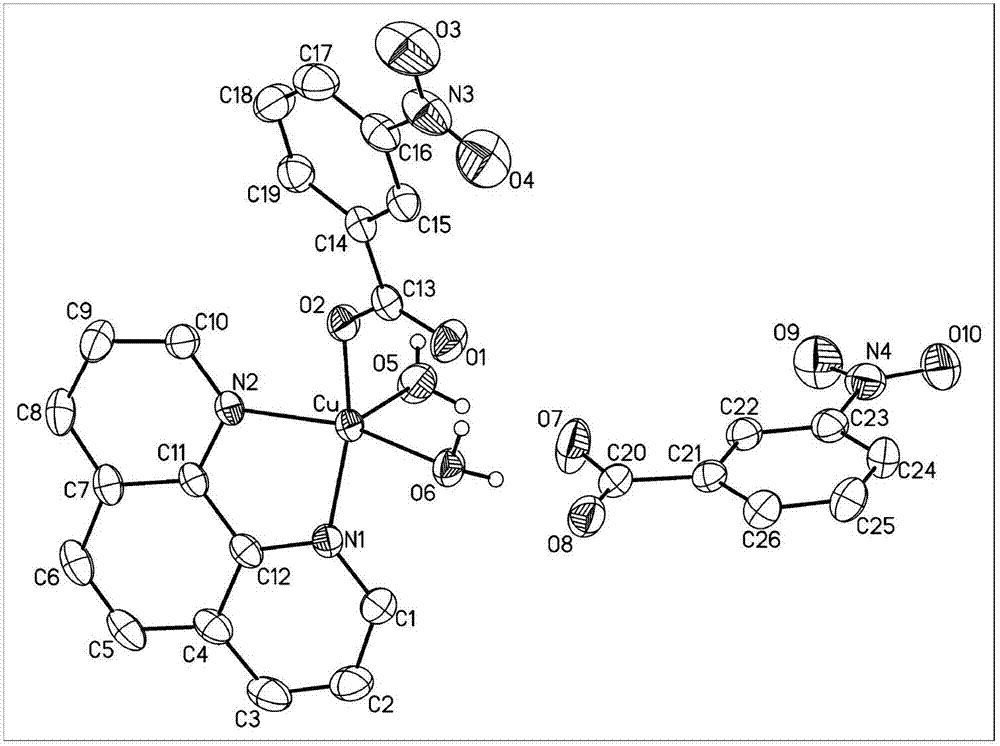
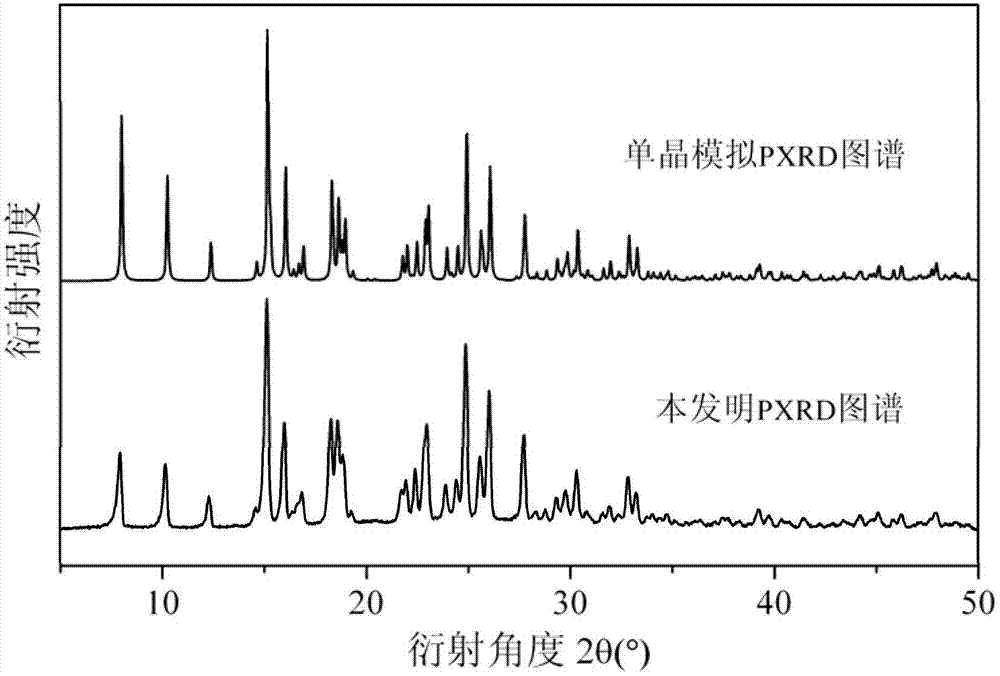
Copper complex ferroelectric functional material and preparation method thereof
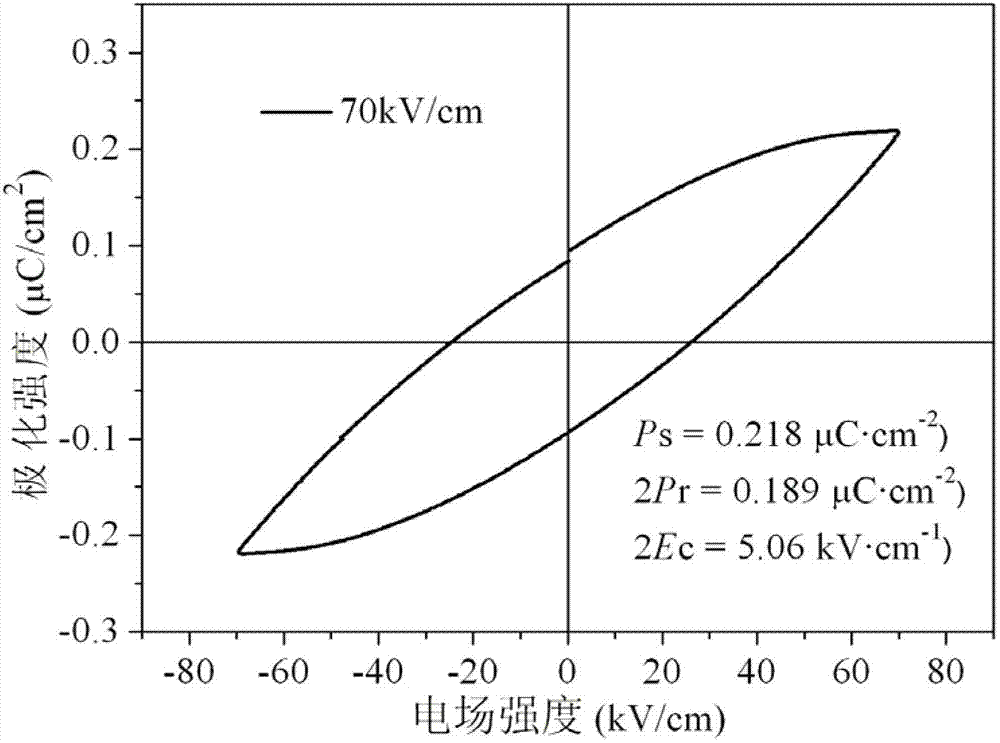
InactiveCN102924487AStrong ferroelectric featuresShort processCopper organic compoundsPhenanthrolineUltimate tensile strength
The invention discloses a copper complex ferroelectric functional material and a preparation method thereof. A molecular formula of a copper complex is [Cu(phen)(H2O)2L]L, wherein L is nitrobenzoic acid; phen is phenanthroline; the ferroelectric functional material is dark blue monoclinic powder crystals and belongs to a P21 space group crystal structure, and the purity is not less than 99 percent; a cell parameter of the ferroelectric functional material is that: beta is 97.75(3) degrees; and ferroelectric characteristic parameters of the ferroelectric functional material are respectively that: Ps is 0.218mu C / cm<2>, 2Pr is 0.189mu C / cm<2>, and 2Ec is 5.06kV / cm. The saturated polarization Ps of the ferroelectric functional material is 0.218mu C.cm<-2>. The preparation method for the copper complex has the advantages that the flow and the process are simple, equipment requirements are low, cheap copper salt, phenanthroline and nitrobenzoic acid are used as raw materials, the pollution is avoided, the cost is low, the industrialization is easy to realize, and the like.
Owner:NINGBO UNIV
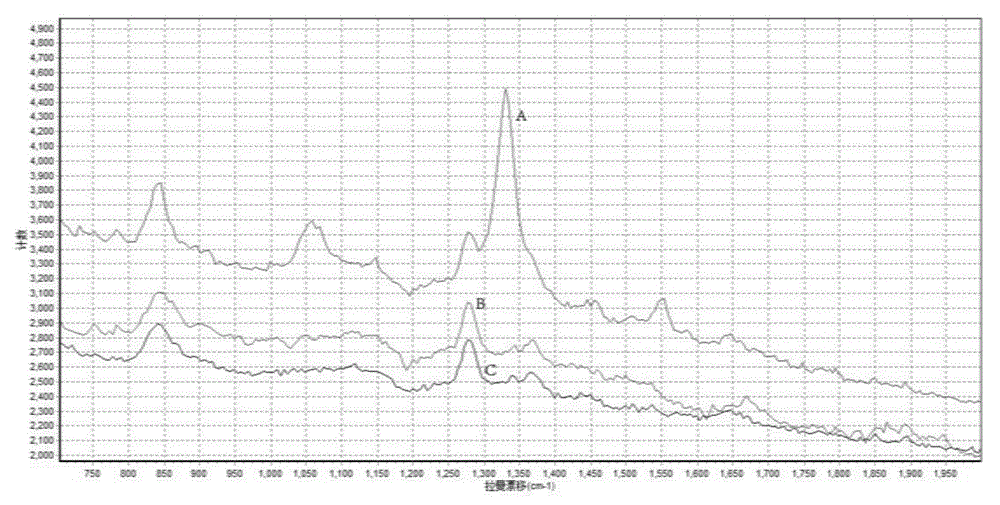
SERS-based newcastle disease virus detection kit and detection method thereof
ActiveCN105092559AStrong specificityRaman scatteringImmune complex depositionNewcastle disease virus NDV
The invention discloses an SERS-based newcastle disease virus detection kit and a detection method thereof, and belongs to the field of spectroscopy and molecular diagnosis. The SERS-based newcastle disease virus detection kit comprises a raman probe solution and a nitrocellulose film; the raman probe solution is a solution obtained by coupling nano gold, 3,3'-dithio di(6-nitrobenzoic acid) di-succinic acid imido ester complex and a newcastle disease antibody. The detection method comprises the following steps: dropping a newcastle disease virus on the nitrocellulose film, incubating the nitrocellulose film and the raman probe coupled with the antibody after closing and washing to form an immune complex with a 'solid phase antigen-labeled antibody' half sandwich structure on the nitrocellulose film, and finally performing raman spectrum detection on the immune complex so as to identify an enhanced raman molecular signal of a material to be identified with high specificity. The detection kit and the detection method which are disclosed by the invention are high in specificity, and the detection limit of the newcastle disease virus is 10<3>PFU / mL, and the sensitivity is high.
Owner:SOUTH CHINA AGRI UNIV
Electroreduction preparation method of anticancer drug gefitinib and analogue intermediate thereof
ActiveCN110747489ALow selectivityHigh purityElectrolysis componentsElectrolytic organic productionBenzoic acidElectrolytic agent
The invention relates to an electroreduction preparation method of a 2-amino-4-methoxybenzoic acid derivative represented by a formula I, wherein the preparation reaction is defined in the specification, n is selected from 1, 2 and 3, X is selected from CO2H, CO2Me, CO2Et, CO2Bn, CONH2 and CN, Bn is benzyl, Y is selected from HO, Cl, Br, MsO, TsO and a group defined in the specification, M is selected from CH and N, and W is selected from CH2, O, S, NH, HOCH, MeN and EtN. The electroreduction preparation method is characterized in that in a diaphragm electrolytic cell, an acidic solution of a4-methoxy-2-nitrobenzoic acid derivative or a mixed solution of an inorganic ammonium salt, an organic solvent and water is used as a cathode electrolytic solution; relative to a reference electrode,the voltage of a cathode working electrode is 1.00-2.50 V; an anode electrolytic solution is an acidic solution, constant-current or constant-voltage electrolysis is carried out, the current density of the constant current is 20.0-250.0 mA / cm<2>, and the electrolysis temperature is 20-80 DEG C.
Owner:HUNAN UNIV +1
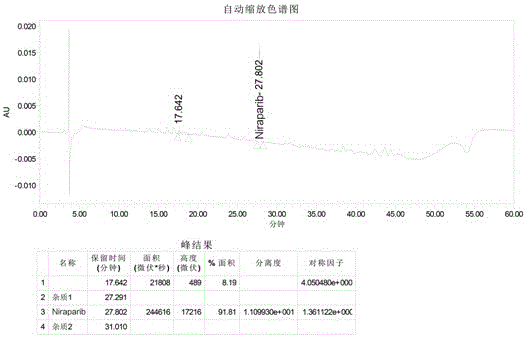
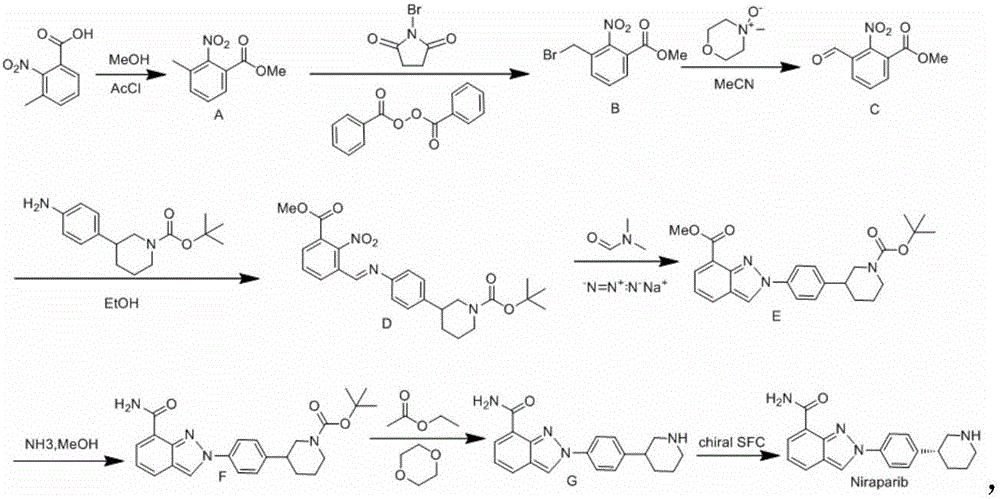
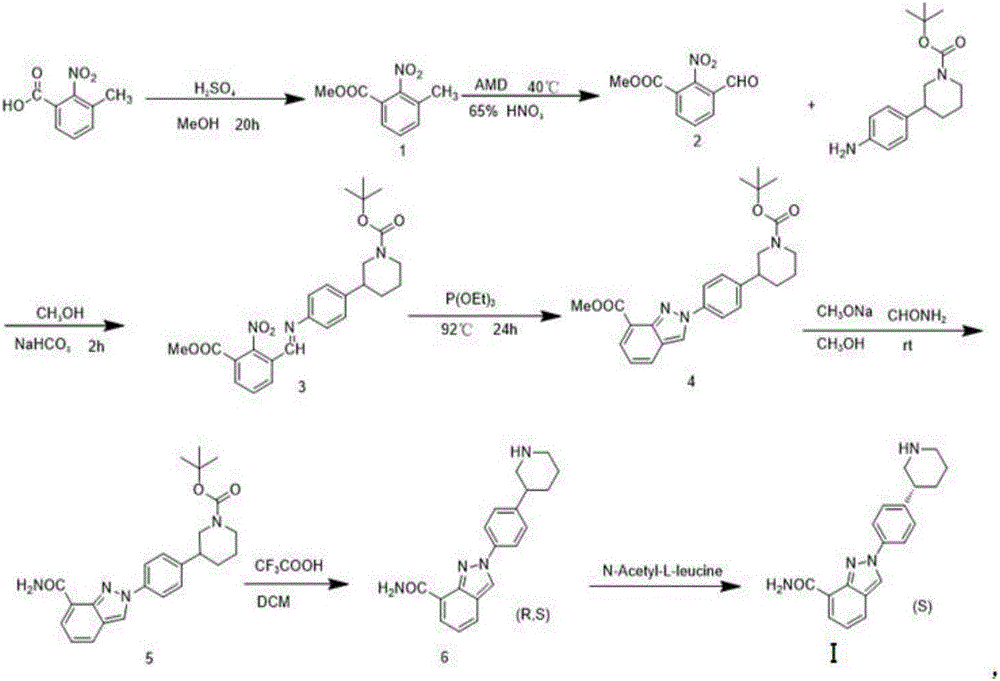
Niraparib synthesis method
The invention discloses a Niraparib synthesis method. The method comprises that through esterification, 3-methyl hydroformylation, 3-schiff base reaction, cyclization, amidation, BOC removal and chiral resolution, optically pure Niraparib with purity of 91% or more is prepared from 3-methyl-2-nitrobenzoic acid. The method has the advantages of simple process, high efficiency, easy operation and less equipment requirement, and is a method suitable for industrial production.
Owner:SHAANXI UNIV OF SCI & TECH
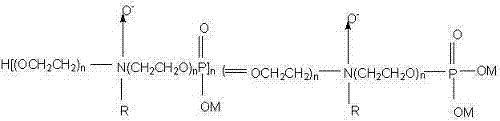
Graphenic carbon particle dispersions and methods of making same
Dispersions of graphenic carbon particles are produced using a polymeric dispersant. The polymeric dispersant includes an anchor block comprising glycidyl (meth)acrylate, 3,4-epoxycyclohexylmethyl(meth)acrylate, 2-(3,4-epoxycyclohexyl)ethyl(meth)acrylate, allyl glycidyl ether and mixtures thereof, reacted with a carboxylic acid comprising 3-hydroxy-2-naphthoic acid, para-nitrobenzoic acid, hexanoic acid, 2-ethyl hexanoic acid, decanoic acid and / or undecanoic acid. The polymeric dispersant also includes at least one tail block comprising at least one (meth)acrylic acid alkyl ester.
Owner:PPG IND OHIO INC
Formula of metal machining liquid
InactiveCN102093928AImprove cooling effectImprove the lubrication effectLubricant compositionPentaerythritolMetal machining
The invention discloses a formula of metal machining liquid, comprising the following components in percentage by weight: 40-50% of pentaerythritol oleate, 5-10% of lauryl sodium sulfate, 3-8% of dodecyl polyoxyethylene ether sodium sulfate, 1-3% of emulsified silicon oil, 0.5-2% of sodium silicate, 0.5-1% of nitrobenzoic acid and the balance of water. The metal machining liquid has the characteristics of good cooling property, good lubricating property, antirust property, oil removing and cleaning function and anti-corrosion function and is easy to dilute.
Owner:江晨
3,5-diaminobenzoic acid preparation method
ActiveCN101362705AShort process routeHigh purityOrganic compound preparationAmino-carboxyl compound preparationHydrogenReaction temperature
The invention relates to a preparation method of 3, 5-diaminobenzoic acid, which is characterized in that: the 3, 5-dinitrobenzoic acid is taken as raw material, carbinol or ethanol as a solvent, and added with a catalyst; a reduction reaction is carried out between the 3, 5-dinitrobenzoic and hydrogen of theoretical reacting weight for 2-10 hours under the reaction pressure of 0.1 MPa to 5MPa as well as the reaction temperature of 20 DEG C to 150 DEG C, thereby obtaining crude product; the crude product is separated and removed off the solvent to obtain the 3, 5-diaminobenzoic acid product, wherein, the dosage ratio of materials is calculated on the basis of 1mol of 3, 5-dinitrobenzoic acid, the dosage of the carbinol or ethanol is 200ml to 1000ml, and the dosage of the catalyst is 0.5 percent to 10 percent of the weight of the 3, 5-dinitrobenzoic acid; the catalyst is either Raney nickel or an active carbon carrier. The method has short technological line, product purity up to 95 percent and yield rate over 96 percent and causes no 'three wastes' (waste gas, waste water and waste residues) pollution and the catalyst can be recovered for later use.
Owner:内蒙古利元科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline](https://images-eureka.patsnap.com/patent_img/ab0c394b-e0e0-4d69-a4b9-9f77c6cd947e/A20091010360500051.PNG)
![Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline](https://images-eureka.patsnap.com/patent_img/ab0c394b-e0e0-4d69-a4b9-9f77c6cd947e/A20091010360500091.PNG)
![Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline](https://images-eureka.patsnap.com/patent_img/ab0c394b-e0e0-4d69-a4b9-9f77c6cd947e/A20091010360500101.PNG)