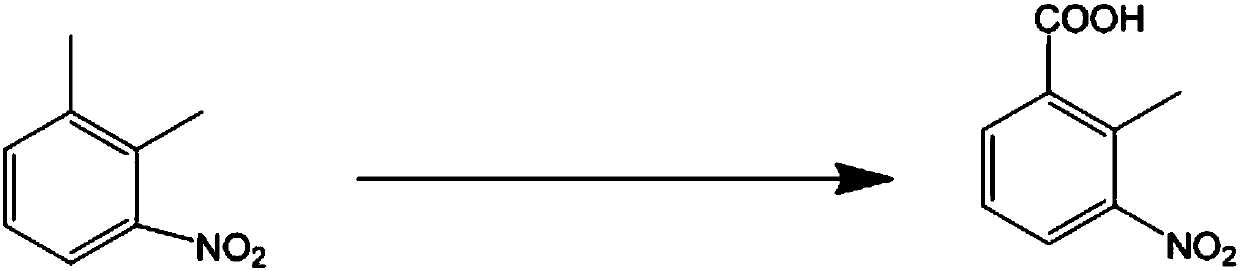
Clean production method to oxidize 3-nitro-o-xylene into 2-methyl-3-nitrobenzoic acid
A technology of nitro-o-xylene and nitrobenzoic acid is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc. Ecological hazards, nitric acid wastewater pollution and other problems, to achieve the effect of simple and practical post-reaction treatment, less by-products, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 500ml three-necked flask, add 20g 3-nitro-o-xylene (0.132mol), 0.164g cobalt acetate (0.00066mol), then add 76g (0.66mol) n-hexanoic acid, slowly add 9.87g (0.29mol) hydrogen peroxide, Slowly increase the temperature to 60 degrees Celsius, keep the temperature for 5 hours, and detect the reaction by HPLC. When the raw material 3-nitro-o-xylene remains below 2%, 34g (0.85mol) sodium hydroxide aqueous solution is added to the reaction system, and the water layer is separated. Adjust the pH value to about 2 with 32.8 g (0.90 mol) hydrochloric acid, and then obtain 19.11 g of the 2-methyl-3-nitrobenzoic acid product with a yield of 80%.
Embodiment 2
[0027] In a 500ml three-necked flask, add 20g 3-nitro-o-xylene (0.132mol), 0.1211g manganese acetate (0.0007mol), 0.0249g cobalt acetate (0.0001mol), and then add 76g (0.66mol) n-hexanoic acid, slowly dropping Add 9.87g (0.29mol) hydrogen peroxide, slowly increase the temperature to 60 degrees Celsius, keep the temperature for 12 hours, and detect the reaction by HPLC. When the raw material 3-nitro-o-xylene remains below 2%, add 34g (0.85mol) hydrogen to the reaction system Separate the aqueous solution of sodium oxide, adjust the pH of the aqueous layer to about 2 with 32.8g (0.90mol) hydrochloric acid, and then filter with suction to obtain 20.78g of the 2-methyl-3-nitrobenzoic acid product with a yield of 87% .
Embodiment 3
[0029] In a 500ml three-necked flask, add 20g 3-nitro-o-xylene (0.132mol), 0.169g iron nitrate (0.0007mol), 0.0727 cobalt nitrate (0.00025mol), then add 42g (0.70mol) of acetic acid, and slowly add 22.44 dropwise. g (0.66mol) hydrogen peroxide, slowly increase the temperature to 80 degrees Celsius, keep it for 5 hours, HPLC detects the reaction, when the raw material 3-nitro-o-xylene remaining less than 2%, add 34g (0.85mol) of hydrogen to the reaction system The sodium aqueous solution was separated, the pH of the aqueous layer was adjusted to about 2 with 32.8 mol (0.90 mol) hydrochloric acid, and 19.58 g of the 2-methyl-3-nitrobenzoic acid was obtained by suction filtration, with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com