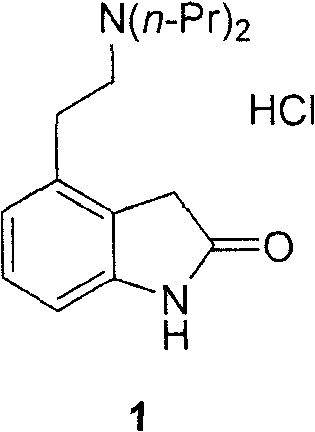
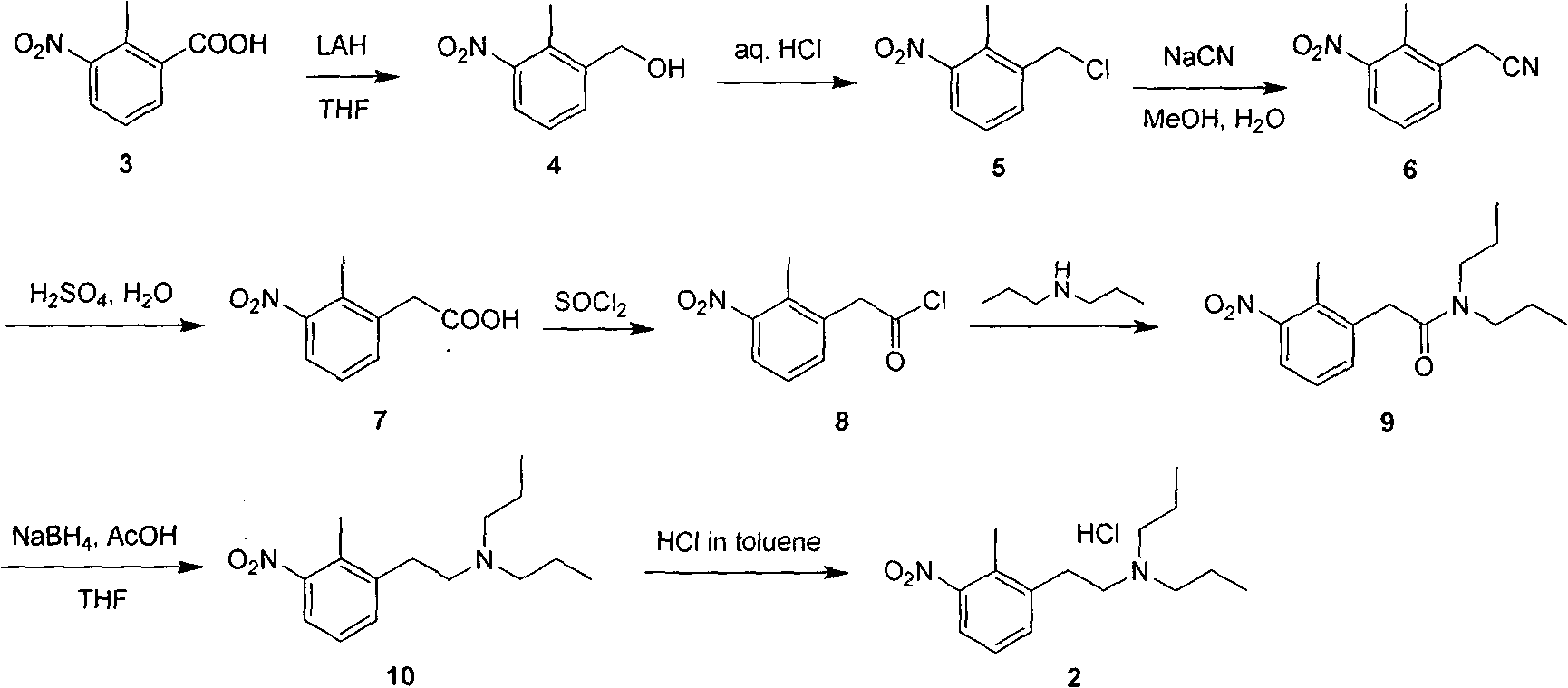
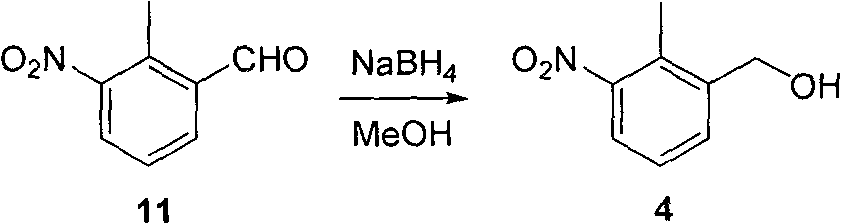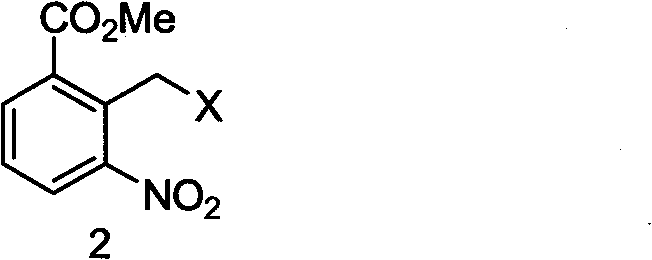Patents
Literature
44 results about "3-nitrobenzoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
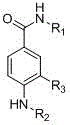
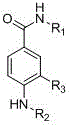
3-Nitrobenzoic acid is an organic compound with the formula C 6 H 4 (NO 2)CO 2 H. It is an aromatic compound and under standard conditions, it is an off-white solid. The two substituents are in a meta position with respect to each other, giving the alternative name of m-Nitrobenzoic acid.
Method for preparing lenalidomide
InactiveCN104311536ASignificant technological progressOptimize the process routeCarbamic acid derivatives preparationOrganic compound preparationDicarbonateL-Glutamin
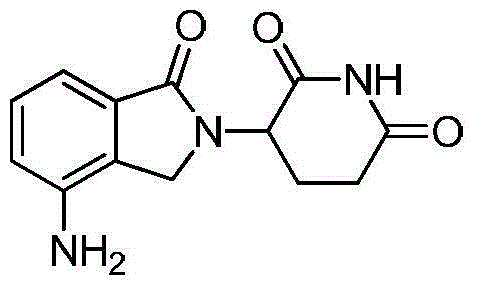
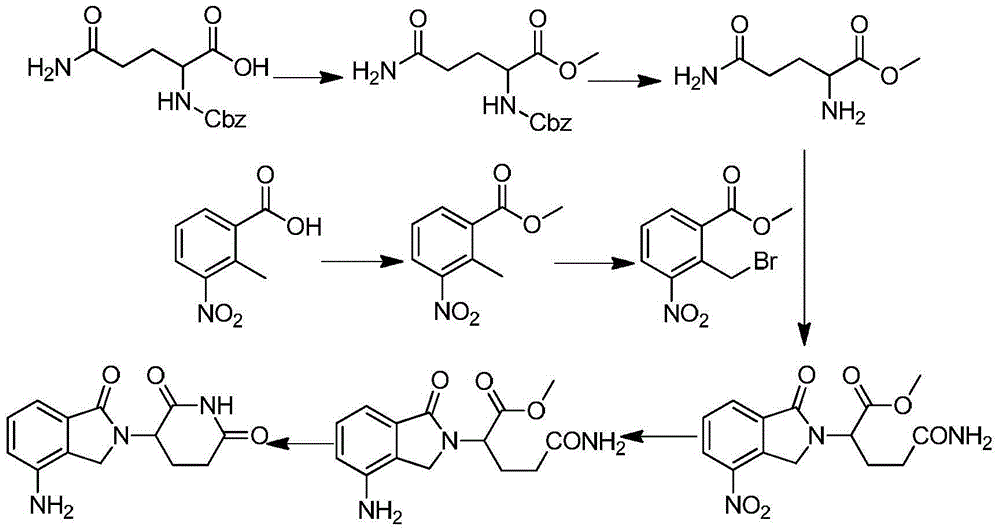
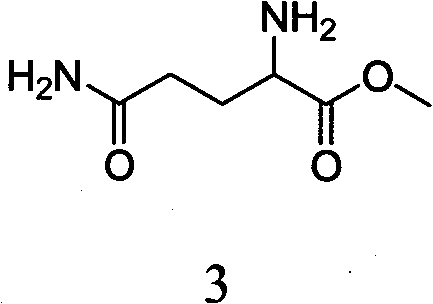
The invention discloses a method for preparing lenalidomide. The method comprises the following steps: firstly, etherifying 2-methyl-3-nitrobenzoic acid to obtain 2-methyl-3-nitrobenzoic acid methyl ester, brominating to obtain 2-brooethyl-3-nitrobenzoic acid methyl ester, reacting L-glutamine and tert-butyl dicarbonate to obtain N-Boc glutamic acid, acquiring 3-amino-2,6-piperidine diketone protected by Boc from N-Boc-glutamic acid in the presence of a condensing agent and a catalyst, further reacting with acid to prepare 3-amino-2,6-piperidine diketone hydrochloride, reacting 3-amino-2,6-piperidine diketone with 2-brooethyl-3-nitrobenzoic acid methyl ester so as to obtain 3-(4-nitryl-1,3 dihydro-1-oxo-2 hydrogen-isobenzazole-2-yl) piperidine-2,6-diketone, and finally reducing, thereby obtaining lenalidomide. The method disclosed by the invention is high in product yield.
Owner:SHANGHAI INST OF TECH
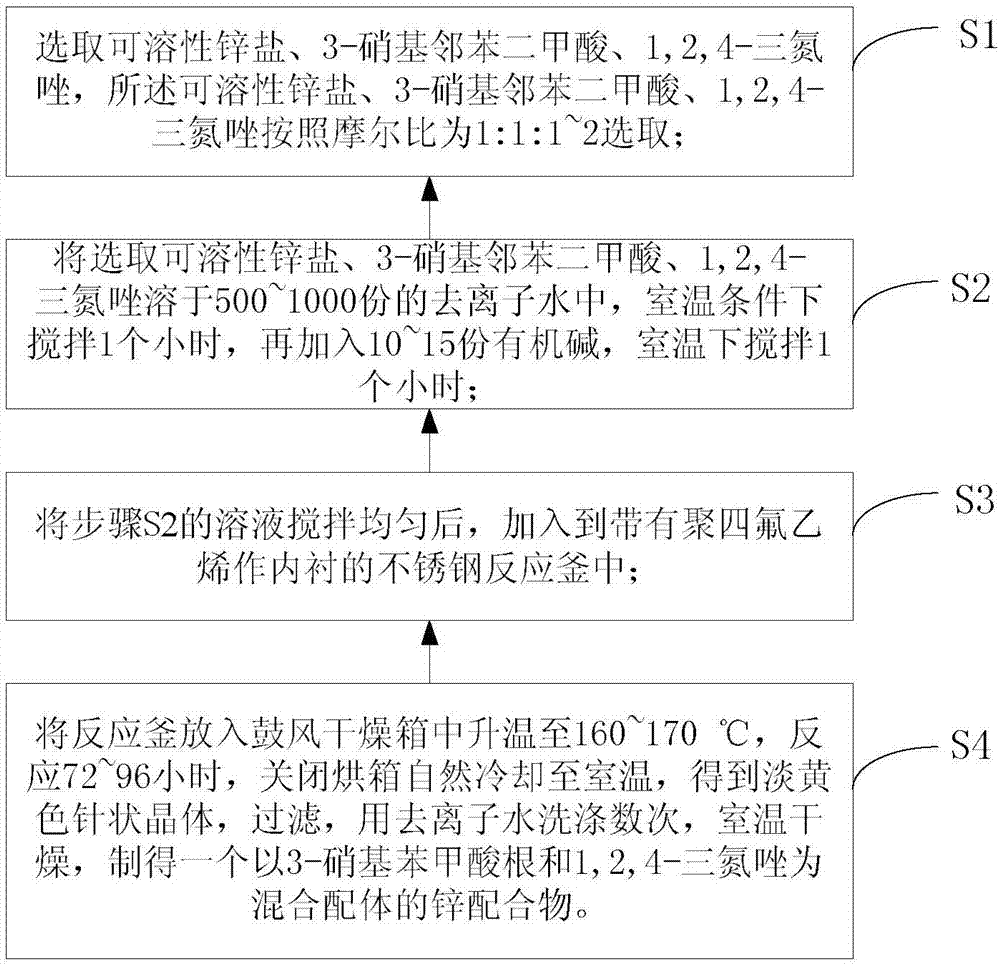
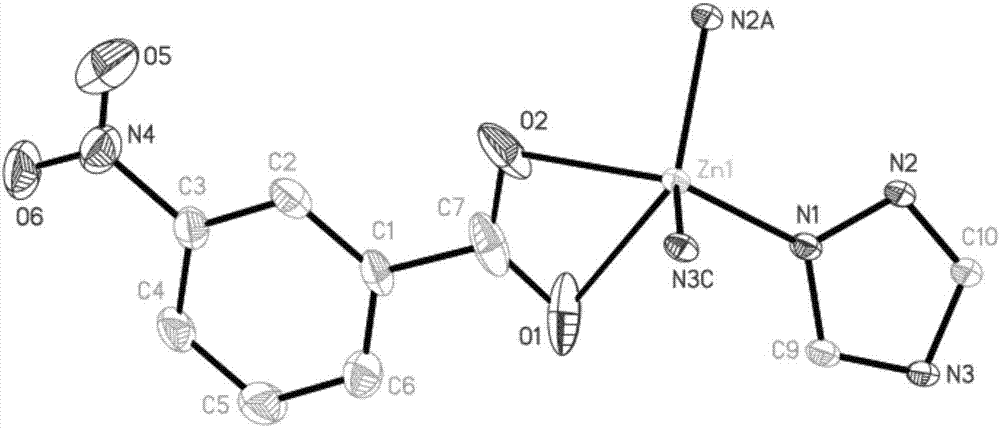
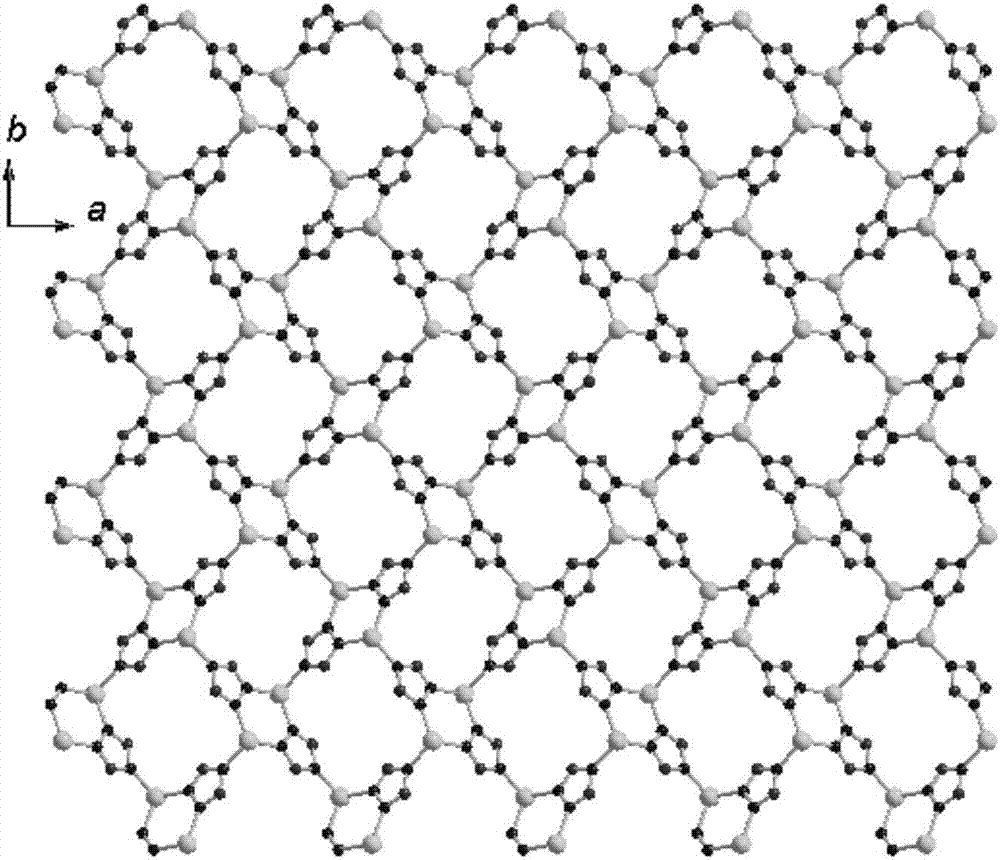
Two-dimensional zinc coordination polymer and preparation method thereof
ActiveCN107880277AGood fluorescence recognition and sensing effectFluorescence/phosphorescenceLuminescent compositionsFluorescenceDecomposition
The invention provides a two-dimensional zinc coordination polymer. The two-dimensional zinc coordination polymer has a two-dimensional layered structure, and is composited from soluble zinc salt, 1,2, 4-triazole and 3-nitrophthalic acid ligand by a hydrothermal reaction, and the chemical formula of the zinc coordination polymer is [Zn(3-NBA)(trz)]n, wherein 3-NBA is an anion of 3-nitrobenzoic acid, trz is an anion of 1,2,4-triazole, and n is the degree of polymerization. The coordination polymer is prepared by steps as follows: soluble zinc salt is added to organic ligand 3-nitrophthalic acid, 3-nitrophthalic acid is subjected to high-temperature hydrothermal reaction, and in-situ decomposition reaction is performed by breaking of C-C bonds; the zinc coordination polymer is of the two-dimensional layered structure and has good fluorescence recognition and sensing effects on nitrobenzene, thereby having important potential application prospect in environmental detection.
Owner:安徽锦圣新材料有限公司
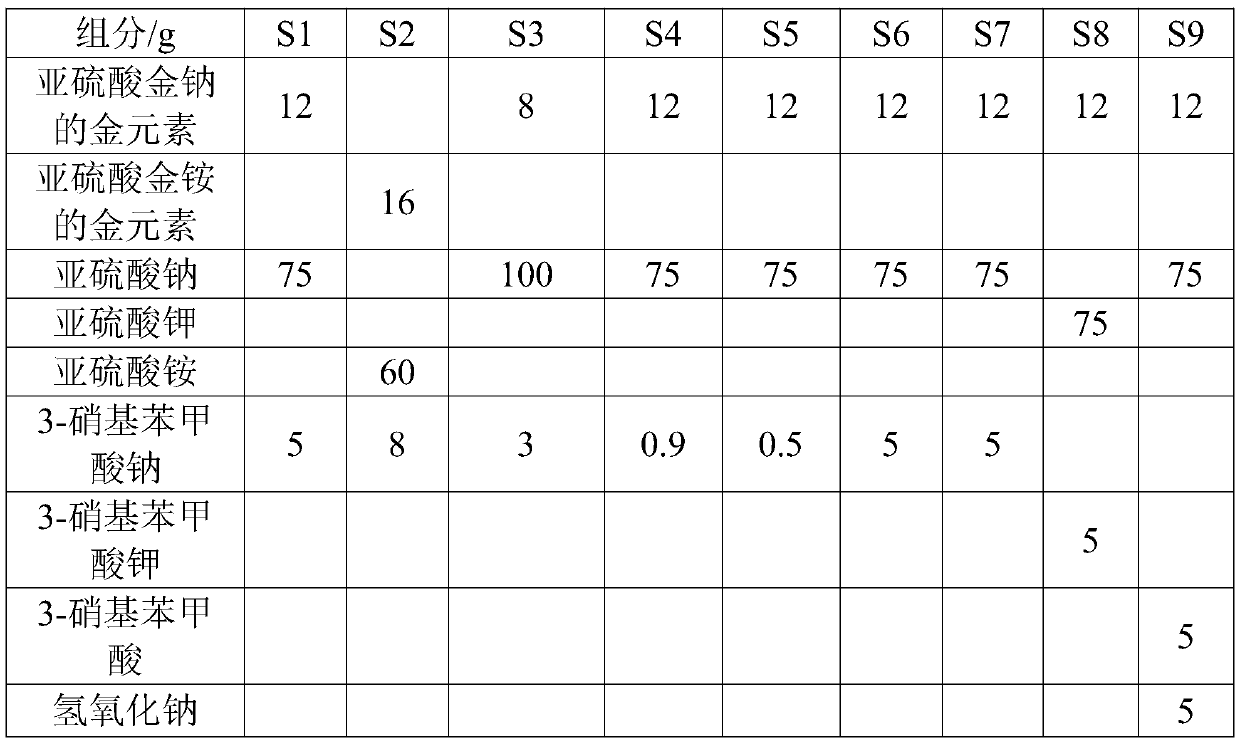
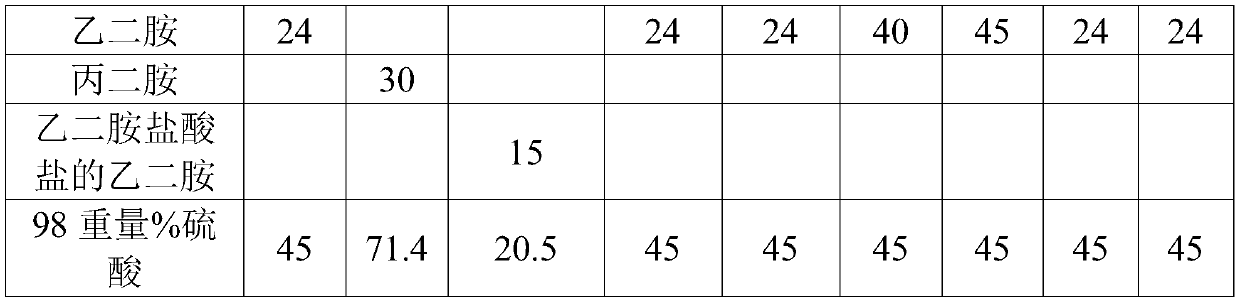
Non-cyanide gold plating bath and preparation method and application thereof
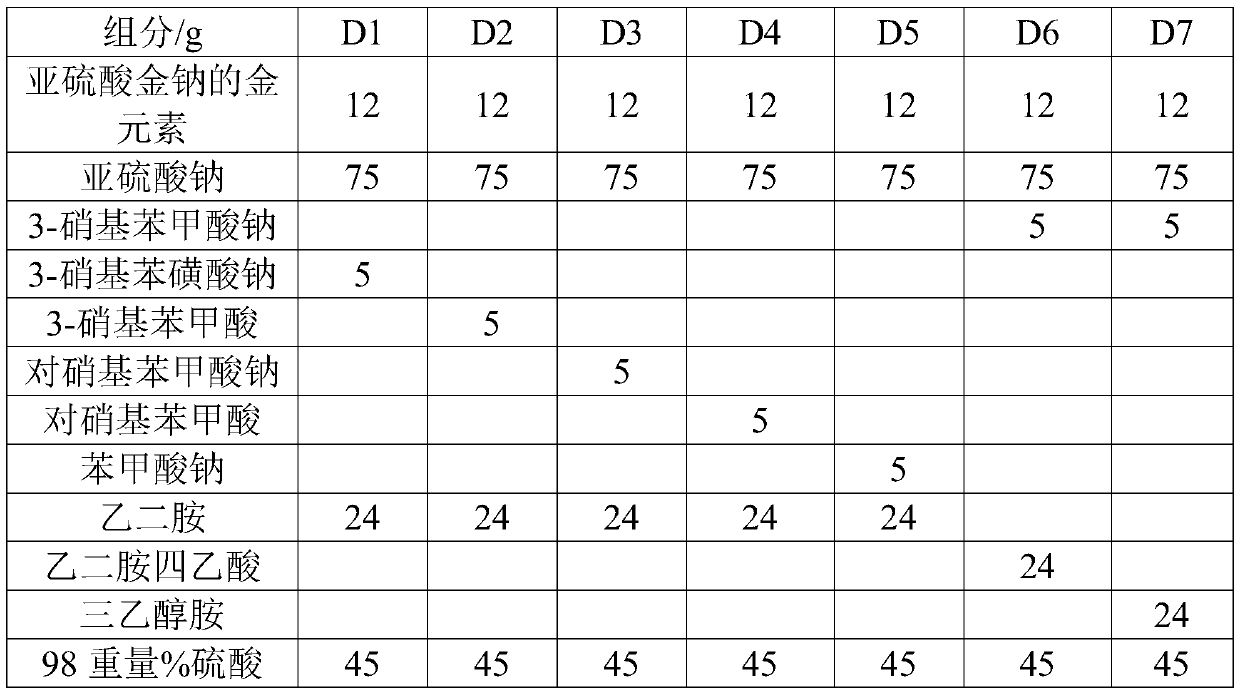
The invention relates to the field of gold plating baths, in particular to a non-cyanide gold plating bath and a preparation method and application thereof. The gold plating bath comprises a sulphurous acid gold salt, sulfite, 3-nitrobenzoic acid salt, organic amine and acid. The non-cyanide gold bath has the advantages that the plating bath stability is good, and the current efficiency stabilitywithin a certain current density range is high.
Owner:SHENZHEN UNITED BLUEOCEAN TECH DEV
Synthetic method of (R)-2-methyl-4-nitro-1-butanol
InactiveCN103483201AIncrease splitLow costOrganic chemistryOrganic compound preparationTrimethylsilyl trifluoromethanesulfonatePyrrolidine
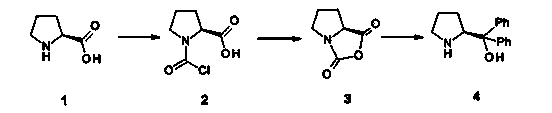
The invention discloses a synthetic method of (R)-2-methyl-4-nitro-1-butanol. The method comprises the following steps that firstly, (S)-alpha, alpha-diphenyl-pyrrolidinemethanol is reacted with trimethylsilyl trifluoromethanesulfonate under the catalyzing action of triethylamine to obtain (S)-2-(1, 1-diphenyl-1-trimethylsilanolate) methyl pyrrolidine, and secondly, (S)-2-(1, 1-diphenyl-1- trimethylsilanolate) methyl pyrrolidine is reacted with acetaldehyde, 3-nitrobenzoic acid and nitro ethylene in a mixing mode to obtain (S)-2-methyl-4-nitro n-butanal, and the (S)-2-methyl-4-nitro n-butanal is reduced to obtain (R)-2-methyl-4-nitro-1-butanol. By means of the method, the (R)-2-methyl-4-nitro-1-butanol can be obtained directly instead of racemate, the racemate is prevented from being resolved, cost is saved, and the yield is 50%.
Owner:CHEMFUTURE PHARMATECH JIANGSU
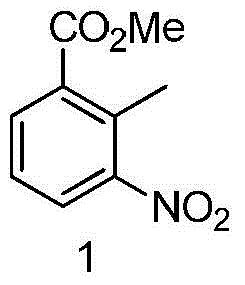
Clean production method to oxidize 3-nitro-o-xylene into 2-methyl-3-nitrobenzoic acid
InactiveCN109824517ABig pollutionEasy to handleOrganic chemistryOrganic compound preparationOrganic solventMethyl group
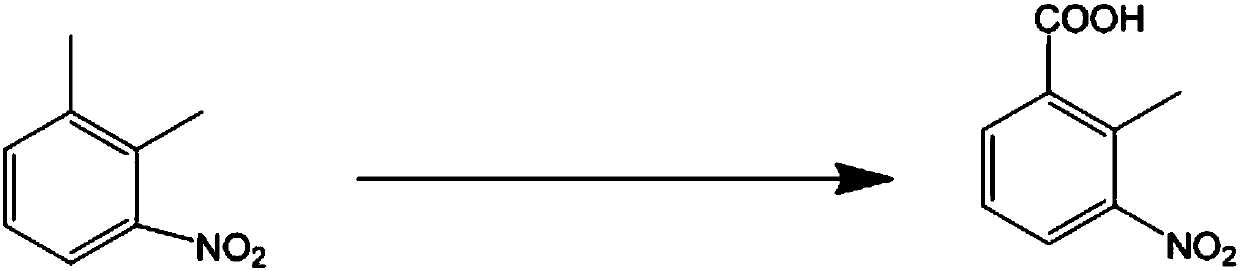
The invention discloses a clean production method to oxidize 3-nitro-o-xylene into 2-methyl-3-nitrobenzoic acid. The clean production method comprises the steps of adding 3-nitro-o-xylene with an acidinto an organic solvent under the action of a catalyst, adding an oxidant, and stirring to allow reacting to obtain 2-methyl-3-nitrobenzoic acid, wherein the oxidant is hydrogen peroxide, and the catalyst is a transition metal compound. The hydrogen peroxide is used herein as a clean oxidant, so that the oxidants, such as potassium permanganate, causing high environmental pollution are avoided. The reaction system is mild; post-reaction treatment is simple and practical; reaction selectivity is good; few byproducts are produced; the target product is easy to purify; both the yield and purityare high; the cost is low; the clean production method has a good industrial application prospect.
Owner:JIANGSU YONGAN CHEM CO LTD
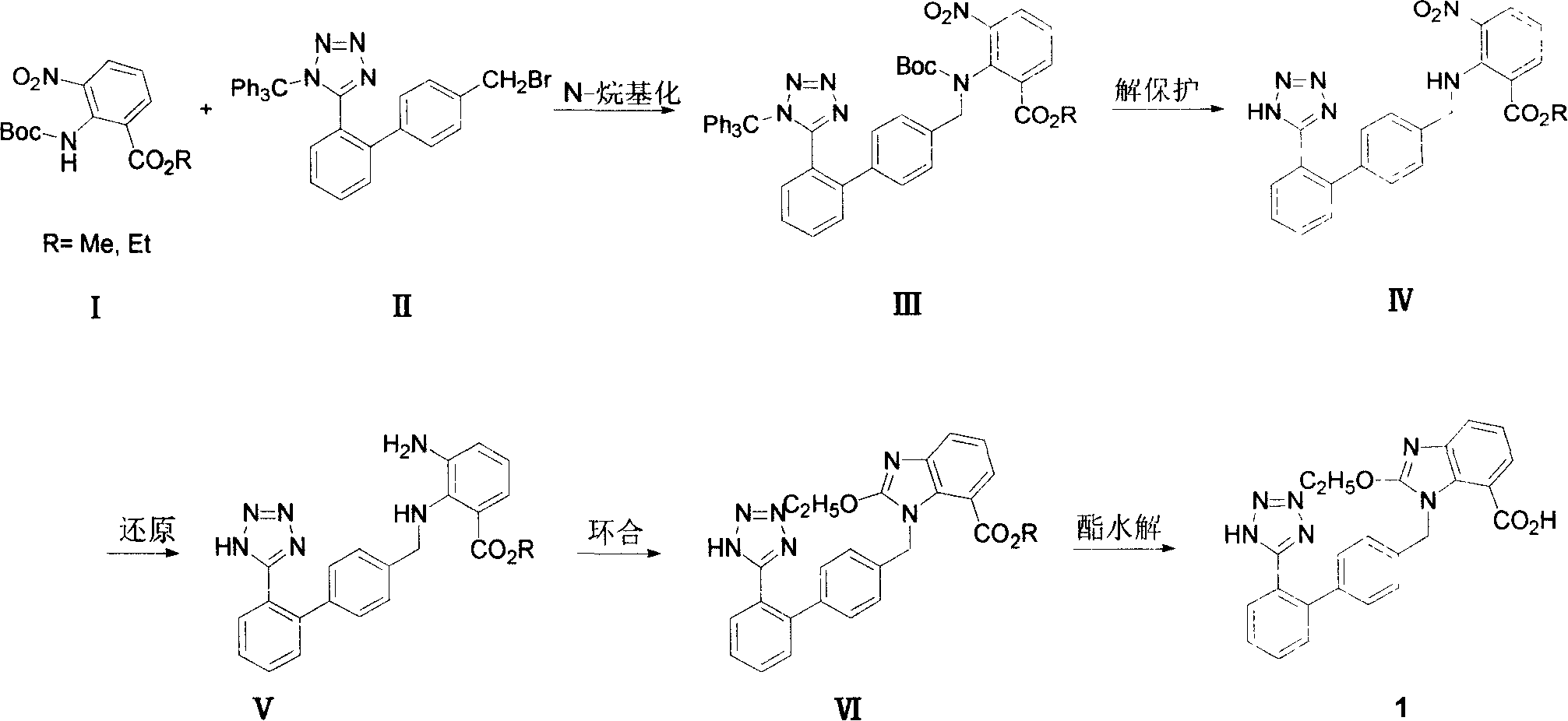
Method for preparing candesartan cilexetil
ActiveCN101781286AReduce usageHigh yieldOrganic chemistryBulk chemical productionCandesartanTetrazole
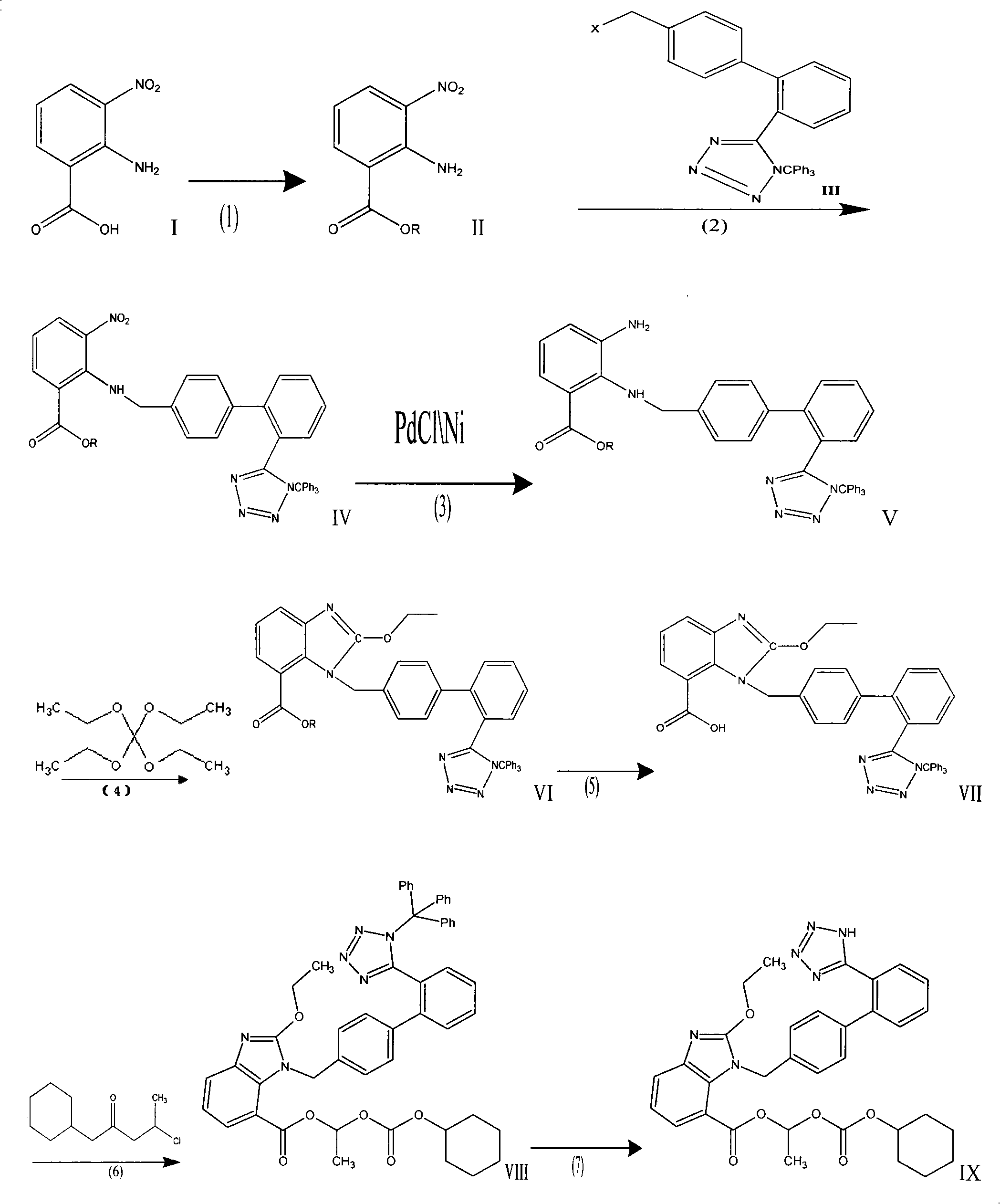
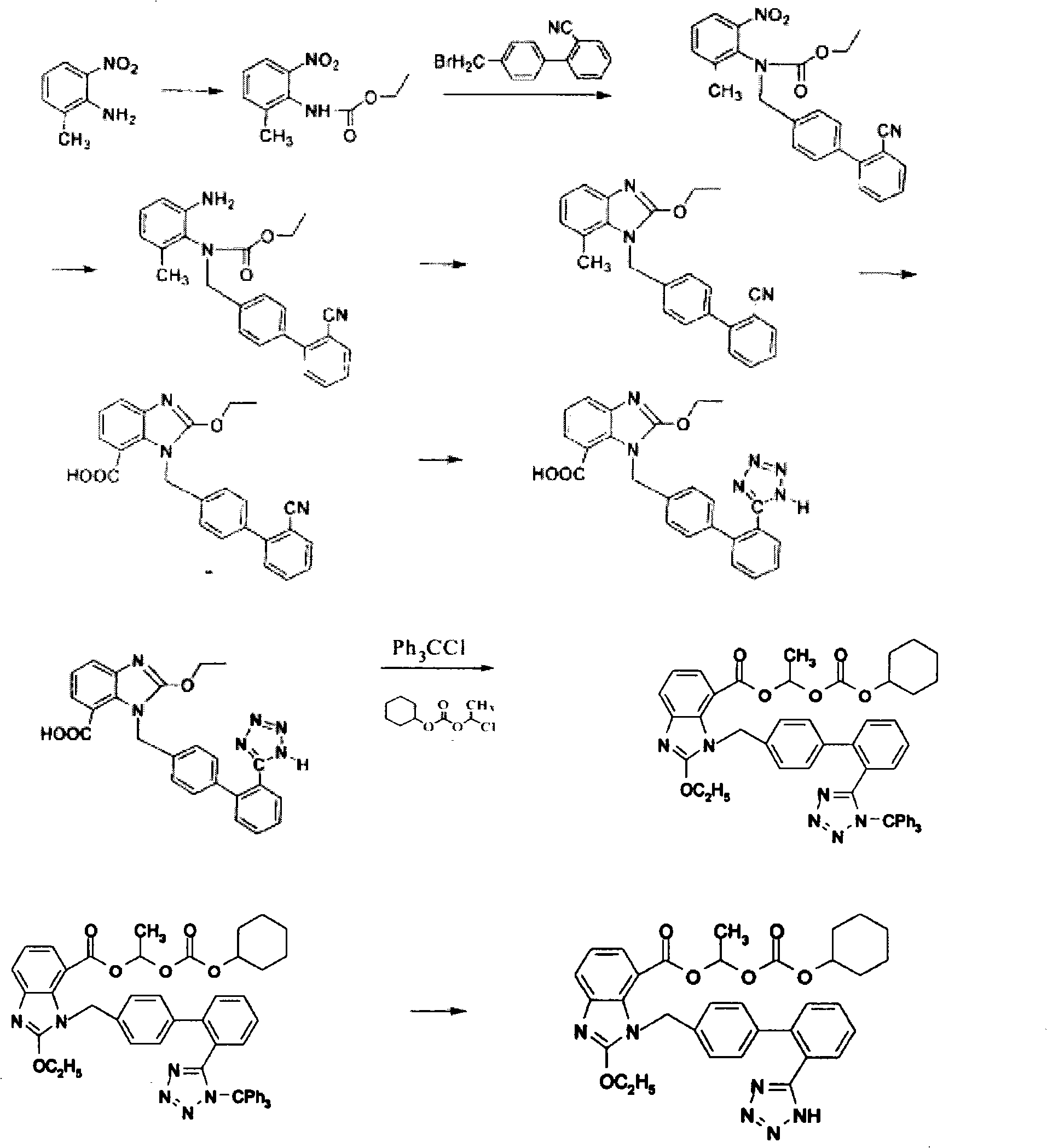
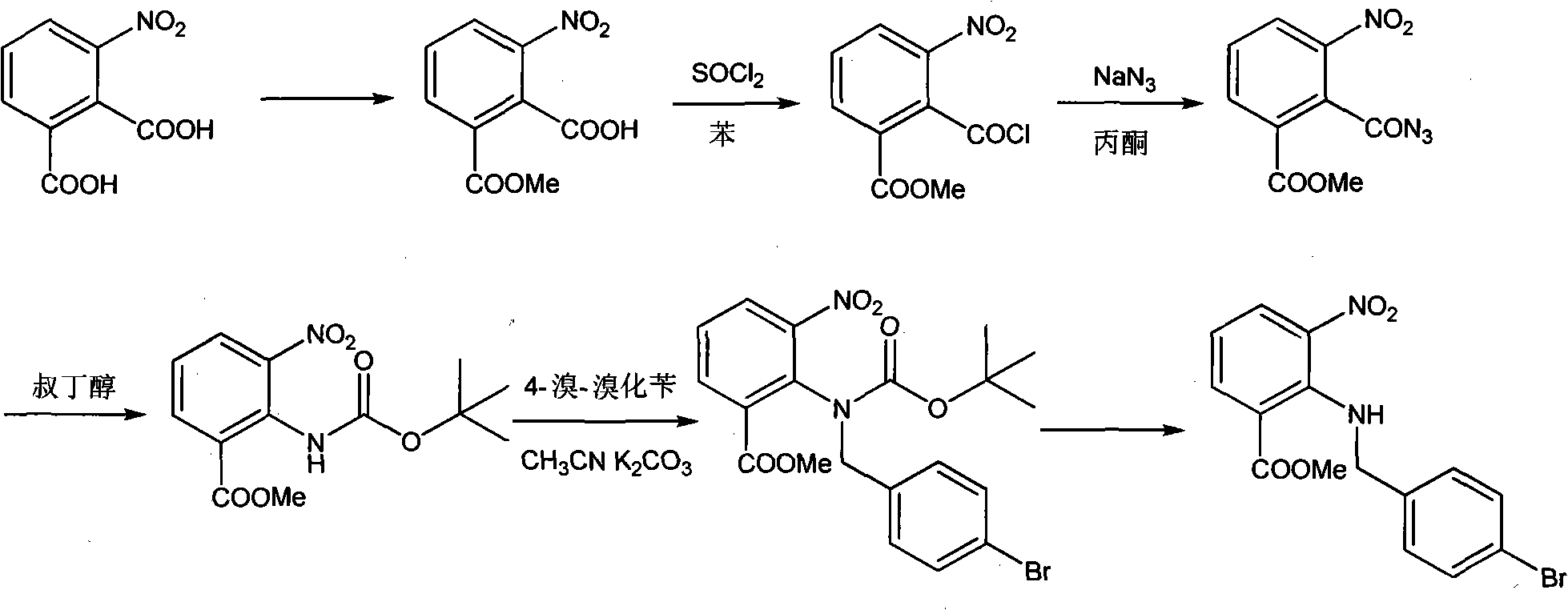
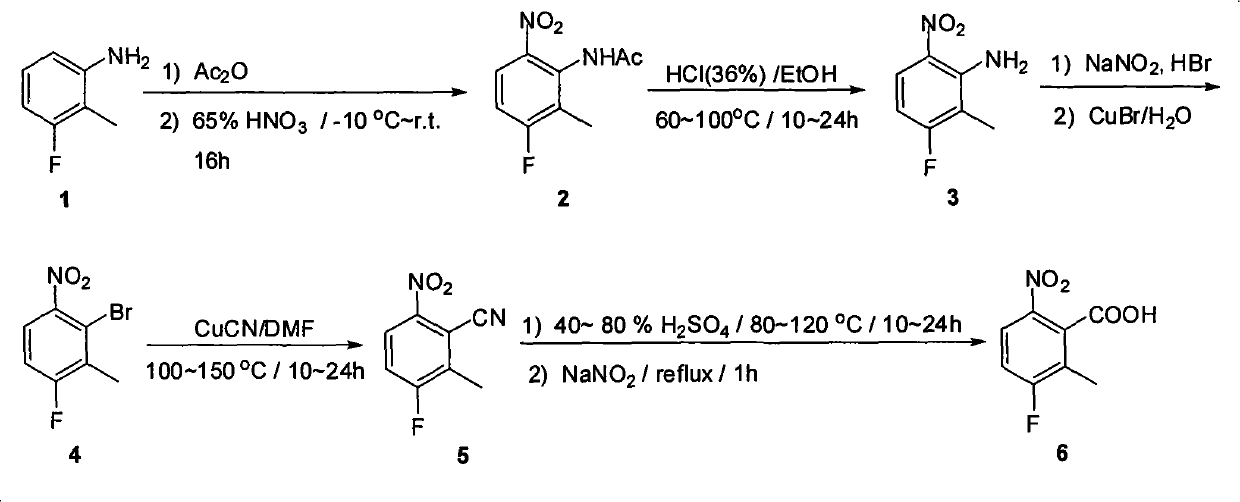
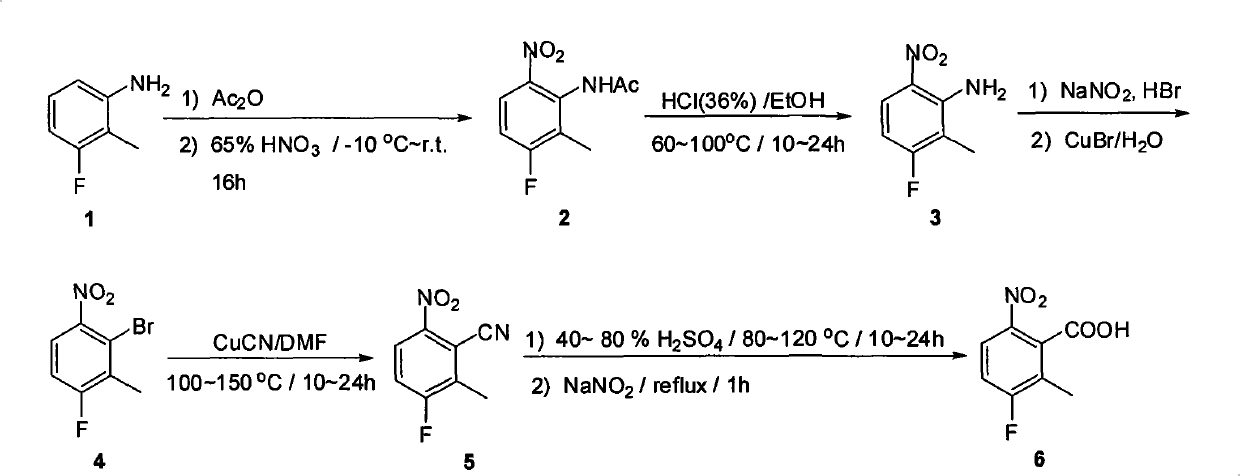
The invention provides a method for preparing candesartan cilexetil, which can solve the problems of longer reaction route, easy remaining of toxic substances in medicines and lower total yield existing in the prior art. In the method, the candesartan cilexetil is finally prepared by using 2-amino-3-nitrobenzoic acid as an initial raw material through esterification reaction, N-alkylation reaction, nitro reduction reaction, cyclization reaction, hydrolysis reaction, esterification reaction and tetrazole protecting group deprotection reaction. The synthetic method has simple steps and high yield, greatly reduces the participation and generation of toxic products in the reaction process, reduces the release of waste and is beneficial to clean production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
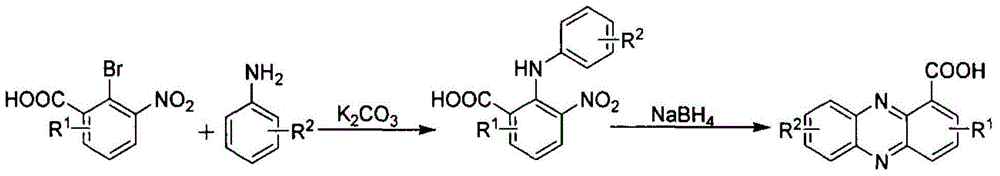
Preparation method for phenazine-1-carboxylic acid
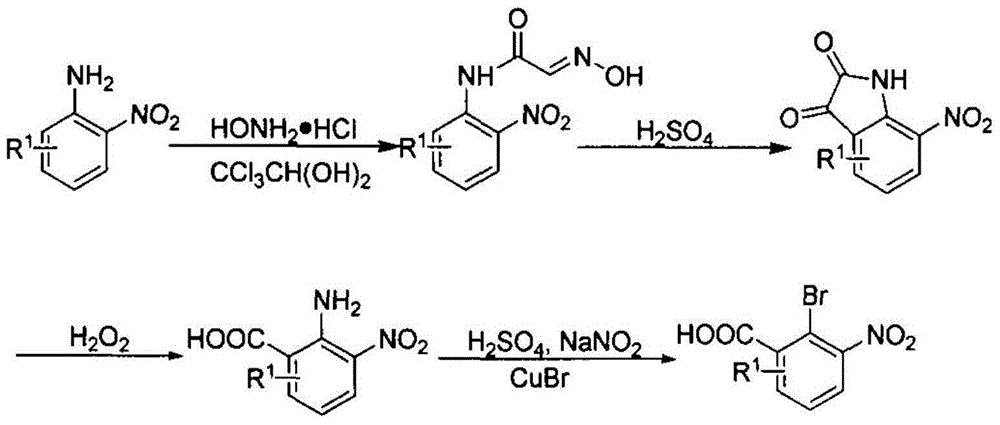
ActiveCN104829544AReaction raw materials are readily availableHigh yieldOrganic chemistrySandmeyer reactionHydroxylamine
The invention relates to a preparation method for phenazine-1-carboxylic acid. The preparation method comprises the following steps: reacting aniline with chloral hydrate and hydroxylamine to produce alpha-oximidoacetanilide, treaing alpha-oximidoacetanilide with concentrated sulfuric acid to obtain isatin, reacting isatin with hydrogen peroxide so as to obtain 2-amino-3-nitrobenzoic acid and then carrying out Sandmeyer reaction to prepare 2-bromo-3-nitrobenzoic acid; and subjecting prepared 2-bromo-3-nitrobenzoic acid and aniline to Jourdan-Ullmann reaction so as to obtain substituted diphenylamine and carrying out ring closure to prepare phenazine-1-carboxylic acid. Compared with the prior art, the preparation method provided by the invention has the advantages of easy availability of raw materials, easy control of reaction, mild reaction conditions, easy post-treatment, high overall yield, as high as 32 to 47%, and suitability for industrial production; and compared with conventional method for production of shenqinmycin from ferment powder, the method provided by the invention enables cost to be greatly reduced.
Owner:SHANGHAI TAIHE INT TRADE CO LTD +1
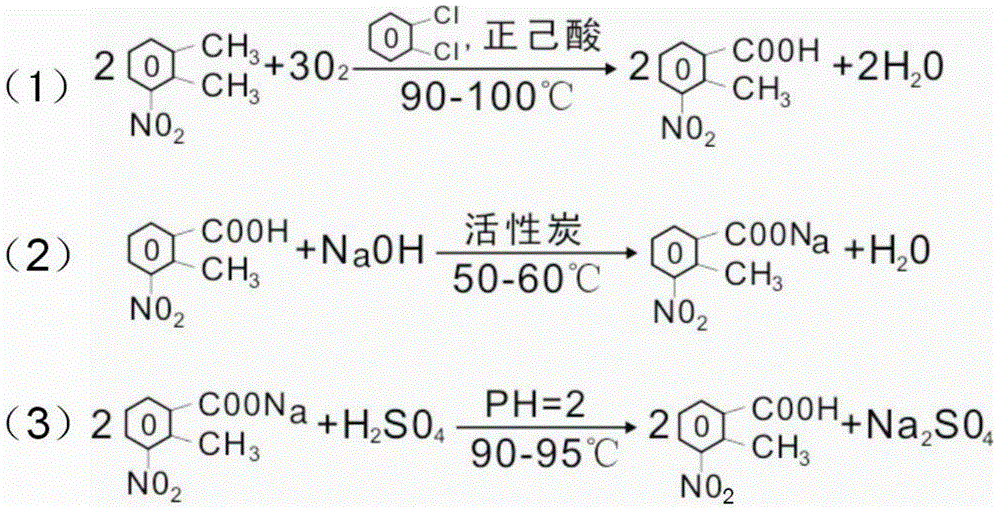
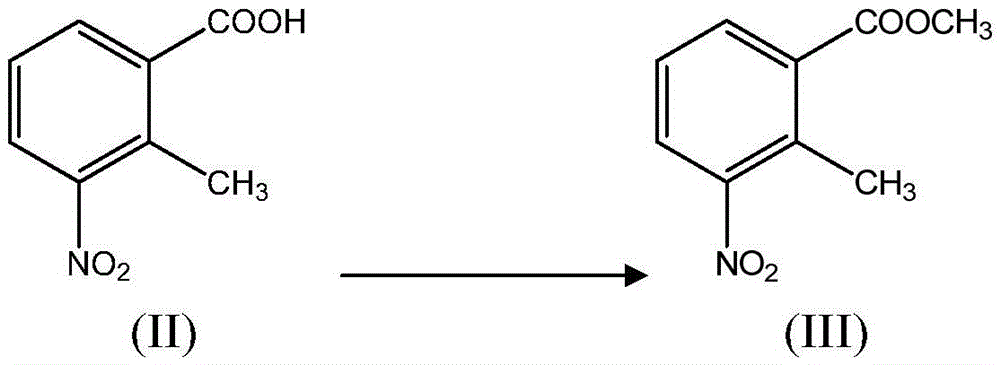
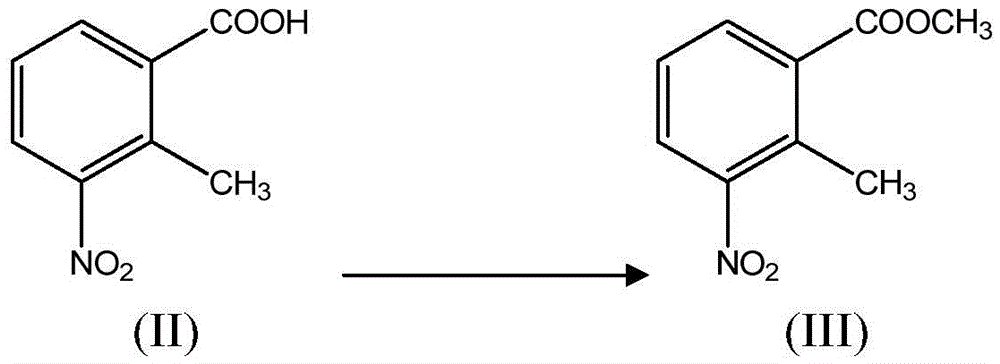
New 2-methyl-3-nitrobenzoic acid preparation method
ActiveCN105130820ALow costReduce riskOrganic chemistryOrganic compound preparationFiltrationMethyl group
The present invention relates to a new 2-methyl-3-nitrobenzoic acid preparation method, which is characterized by comprising: adding 3-nitro-o-xylene, an organic solvent and a catalyst to a reactor, and introducing oxygen gas to carry out oxidation, wherein the oxidation temperature is 90-100 DEG C, and the reaction ending point is achieved when the mass concentration of the 3-nitro-o-xylene in the reactor is less than 1%; carrying out cooling filtration to obtain a crude product, wherein the mother liquor is recovered and recycled; and sequentially performing conventional alkalization method, active carbon decoloration and acidification to obtain the 2-methyl-3-nitrobenzoic acid finished product. According to the present invention, the oxygen in the air is used to replace nitric acid to oxidize the 3-nitro-o-xylene into the 2-methyl-3-nitrobenzoic acid, the oxygen is used to oxidize the 3-nitro-o-xylene, the reaction is the normal pressure reaction, the mother liquor is applied, the yield is up to 80%, and the method is the clean production method with characteristics of low risk, low pollution and low raw material cost.
Owner:黄石市利福达医药化工有限公司
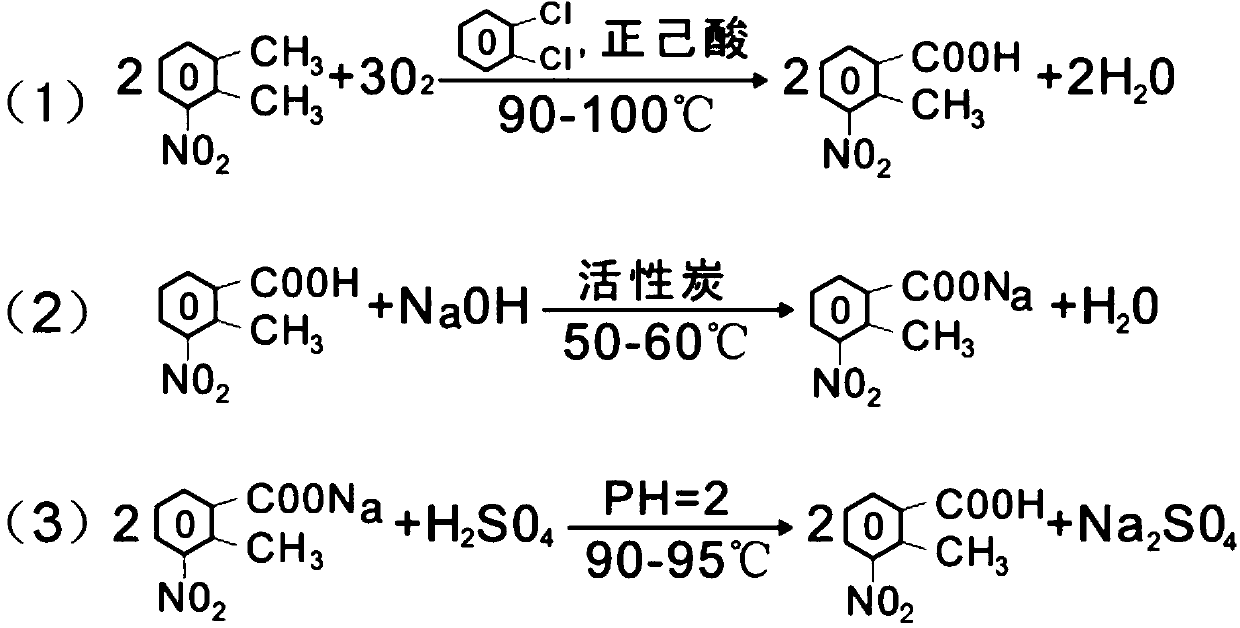
New preparation method of 2-methyl-3-nitrobenzoic acid
ActiveCN105130820BLow costReduce riskOrganic chemistryOrganic compound preparationO-XyleneOrganic solvent
The present invention relates to a kind of new preparation method of 2-methyl-3-nitrobenzoic acid, it is characterized in that carry out by following steps: add 3-nitro-o-xylene, organic solvent and catalyst in reactor, pass into oxygen Oxidation, the oxidation temperature is 90-100 °C, when the mass concentration of 3-nitro-o-xylene in the reactor is less than 1% as the reaction end point, the crude product is obtained by cooling and filtering, and the mother liquor is recovered and recycled; the crude product is sequentially subjected to conventional alkalization methods, The finished product of 2-methyl-3-nitrobenzoic acid is obtained by activated carbon decolorization and acidification. The method of the present invention replaces nitric acid oxidation 3-nitro-o-xylene with oxygen in the air to become 2-methyl-3-nitrobenzoic acid, and adopts oxygen to oxidize 3-nitro-o-xylene, reacts at normal pressure, and applies mechanically to the mother liquor. The yield is up to 80%, and the invention is a clean production method with little risk, little pollution and low cost of raw materials.
Owner:黄石市利福达医药化工有限公司
Method for preparing 2-(substituted phenyl) methylamino-3-nitrobenzene methyl formate by one-pot method
ActiveCN101880241AReduce pollutionReduce lossesCarboxylic acid nitrile preparationOrganic compound preparationNitrobenzeneMethyl group
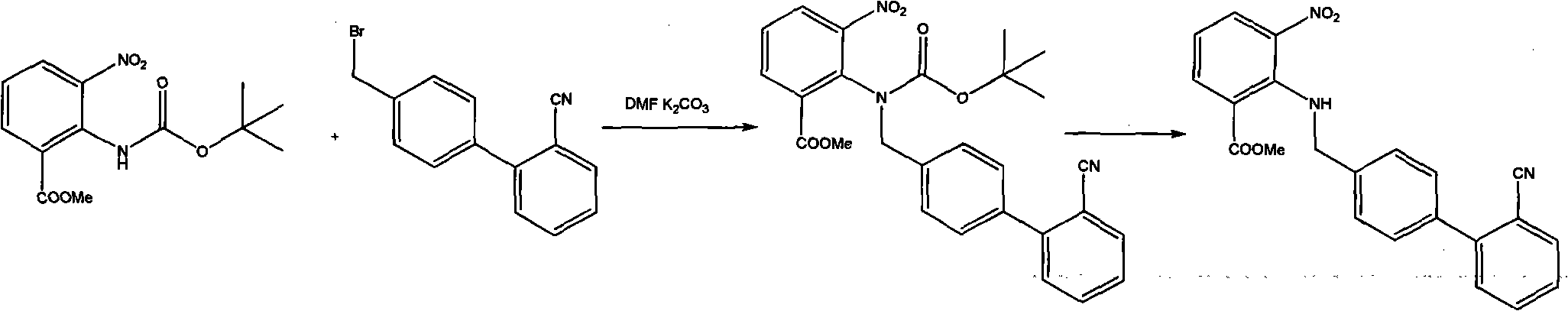
The invention provides a method for preparing candesartan cilexetil intermediate products of 2-(substituted phenyl) methylamino-3-nitrobenzene methyl formate by a one-pot method. The candesartan cilexetil intermediate products are 2-(4-bromophenyl) methylamino-3-nitrobenzene methyl formate and 2-[[(2'-cyano xenyl-4-pyridyl) methyl] amino]-3-nitrobenzene methyl formate. The process comprises the step of using 3-nitryl-2-carboxyphenyl methyl formate as raw materials to obtain target products through continuous reaction process. The extraction and the purification are not needed, the work intensity is reduced, the operation environment is improved, the environment pollution is reduced, the raw material and product loss is reduced, the industrial production cost is greatly reduced, and the yield is improved.
Owner:ZHEJIANG MENOVO PHARMA
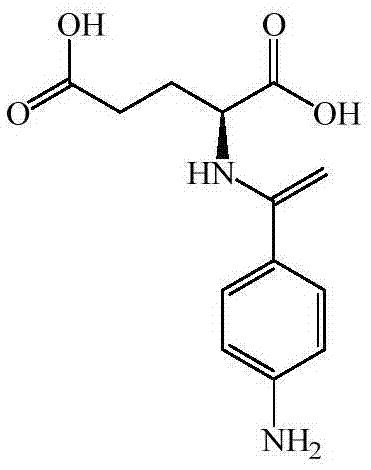
Preparation method of N (4-aminobenzoyl)-L-glutamic acid
ActiveCN105439895AFacilitates controlled absorption processingMild responseOrganic compound preparationCarboxylic acid amides preparationP-nitrobenzoic acidSodium Glutamate
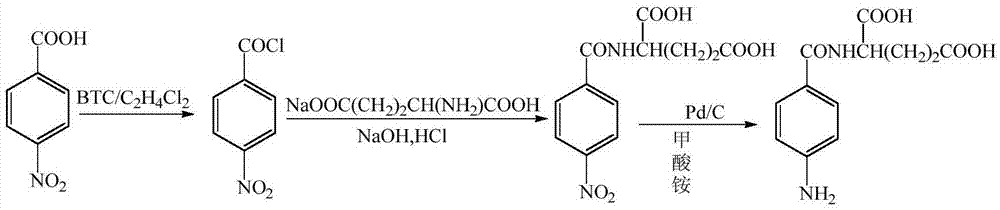
The invention provides a preparation method of N (4-aminobenzoyl)-L-glutamic acid. According to the method, p-nitrobenzoic acid is taken as a raw material, BTC / C2H4Cl1 is taken as an acylating chlorination agent, DMF (dimethyl formamide) is added to serve as an initiator, and p-nitrobenzoyl chloride is prepared through reaction at a reflux temperature; p-nitrobenzoyl chloride and sodium glutamate have condensation, and N-(4-nitrobenzoyl)-L-glutamic acid is prepared; N-(4-nitrobenzoyl)-L-glutamic acid is reduced by Pd / C / HCO2NH4, and N (4-aminobenzoyl)-L-glutamic acid is prepared. The preparation method has mild reaction conditions and is simple in process, easy to operate and suitable for industrial production; few three wastes are generated, and a product has high purity and high yield.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
Near-infrared light controlled gene editing method
ActiveCN109971790AImprove targetingStrong penetrating powerStable introduction of DNALight irradiationIn vivo
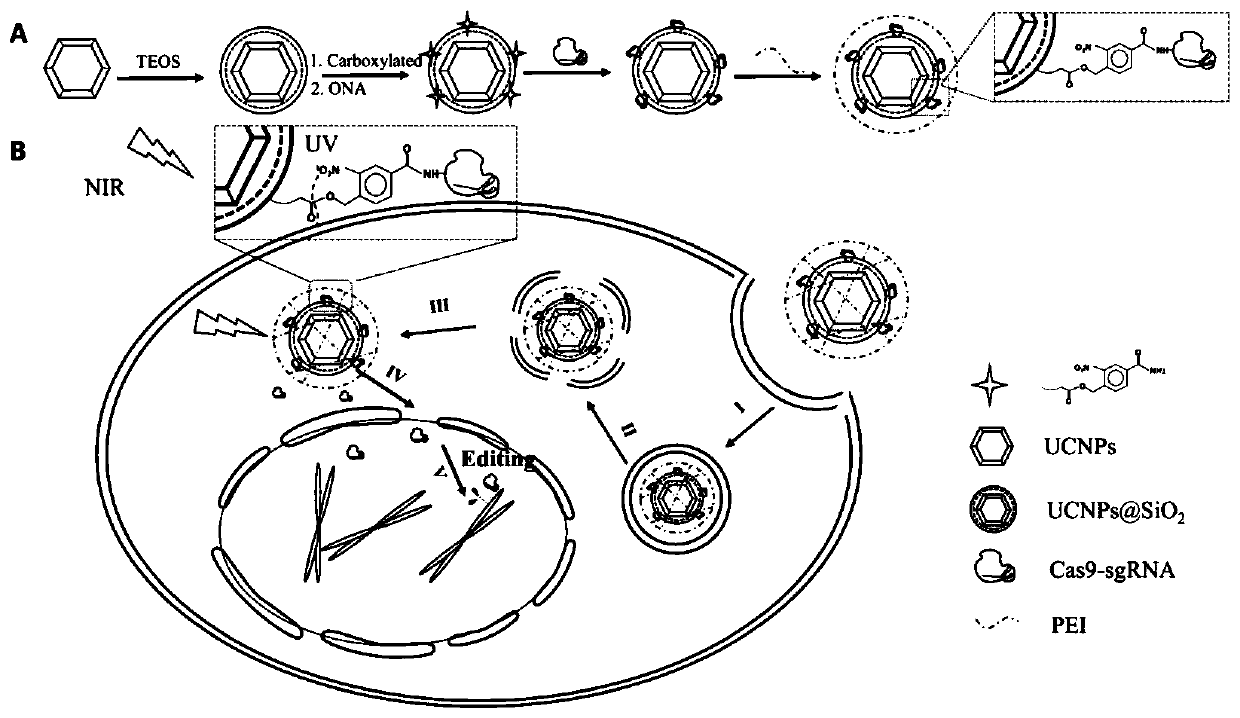
The invention discloses a near-infrared light controlled gene editing method. A UCNPs-Cas9 complex is obtained by covalent connection of carboxylated UCNPs@SiO2 with a CRISPR / Cas9 system under the action of 4-hydroxymethyl-3-nitrobenzoic acid, and after coating with PEI, nanoparticles are subjected to endocytosis; then, under infrared light irradiation, up-conversion nanoparticles emit ultravioletlight to disconnect Cas9 protein with the up-conversion nanoparticles, so that the protein is released to enter the cell nucleus to realize gene editing. Due to strong tissue penetration of infraredlight, the near-infrared light controlled gene editing method is evidently advantageous in in-vivo application.
Owner:NANJING UNIV
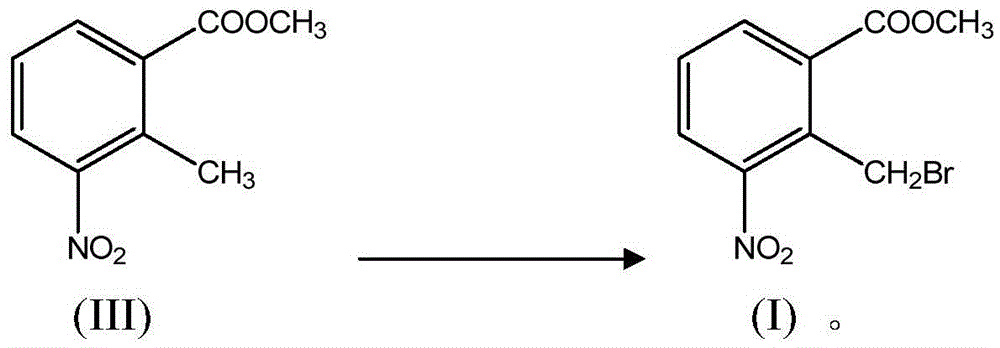
Method for preparing N-(2-methyl-3-nitro)-N-propyl-1-propylamin hydrochloride
The invention relates to a novel method for synthesizing N-(2-methyl-3-nitro)-N-propyl-1-propylamin hydrochloride only by three-step reactions, which comprises the steps of: taking 2-methyl-3-nitrobenzoic acid as a raw material, preparing epoxy propionic ether through Darzons condensation, then hydrolyzing and decarboxylating the epoxy propionic ether to obtain 2-methyl-3-nitrophenylacetaldehyde,and finally obtaining the N-(2-methyl-3-nitro)-N-propyl-1-propylamin hydrochloride by reducing ammoniation and salification. Compared with the prior method, the method has the advantages that: the method has a short route, thereby reducing working procedures and reducing production period; and the method avoids the use of virulent cyanidums, reduces the production cost for materials and three wastes and the like.
Owner:JIANGSU SKYRUN PHARMA CO LTD
High performance liquid chromatography method for 3-nitrophthalic acid
InactiveCN109324142ARapid determinationAccurate measurementComponent separationPhosphoric acidLength wave
The invention discloses a high performance liquid chromatography method for 3-nitrophthalic acid, comprising the following steps of: 1) preparing 2-Methyl-3-nitrobenzoic acid into a sample solution having a concentration of 0.4 g / L; 2) injecting 10-40 [mu]L of the sample solution into a liquid chromatography; 3) using a C18 chromatographic column, taking a solution composed of methanol and sulfuric acid as a mobile phase or taking a solution composed of methanol and phosphoric acid as a mobile phase, wherein the flow rate is 0.8-1.2 mL / min, the detection wavelength is 254 nm, and the sensitivity is 0.2 aufs. According to the high performance liquid chromatography method, the content of 3-nitrophthalic acid, 2-Methyl-3-nitrobenzoic acid and other impurities can be quickly and accurately determined, and the analysis time can be effectively shortened. The high performance liquid chromatography method has the advantages of simple and convenient operation, accurate and reliable result, therepeatability, the special property of separating different interferences, and is suitable for popularization.
Owner:JIANGSU YONGAN CHEM CO LTD
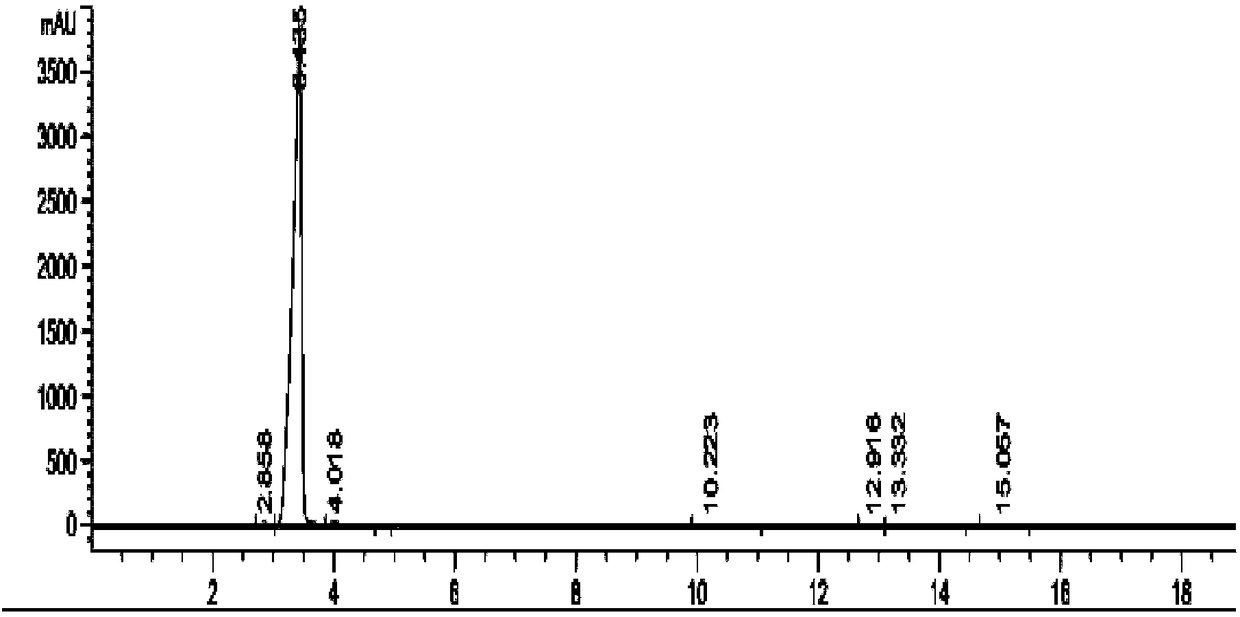
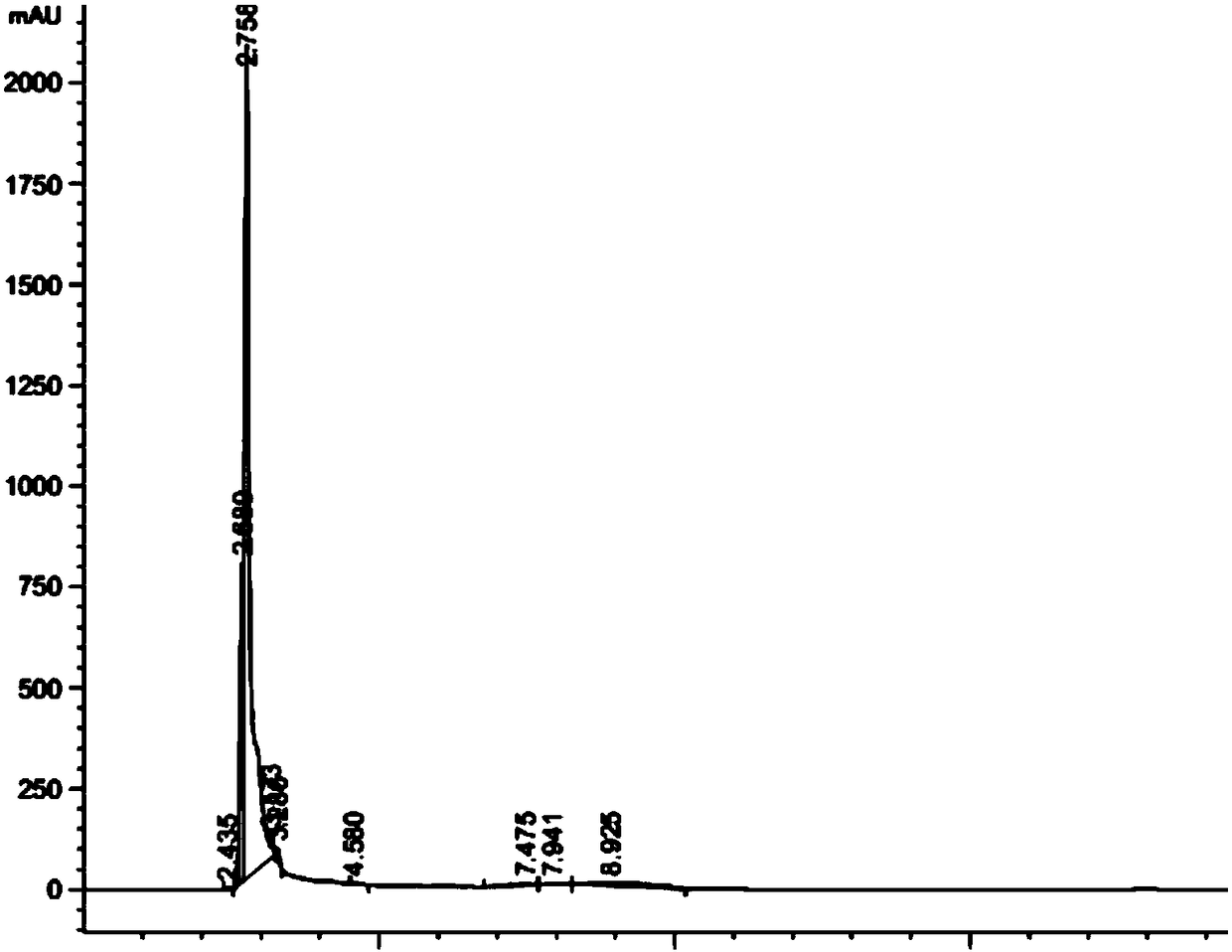
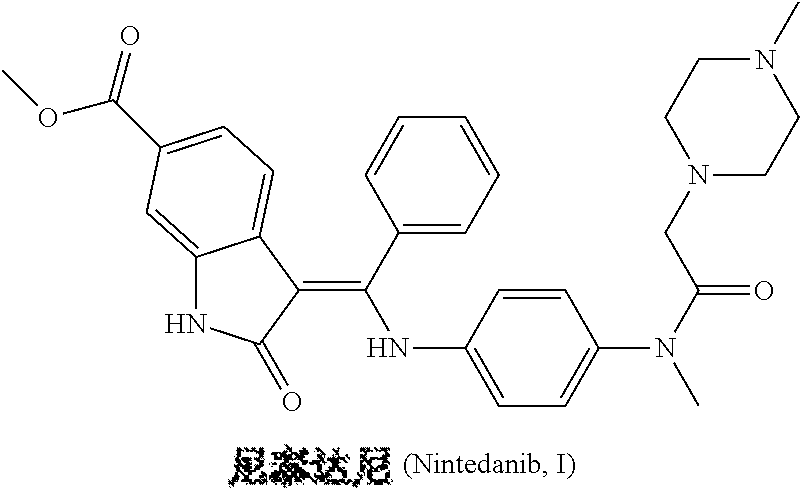
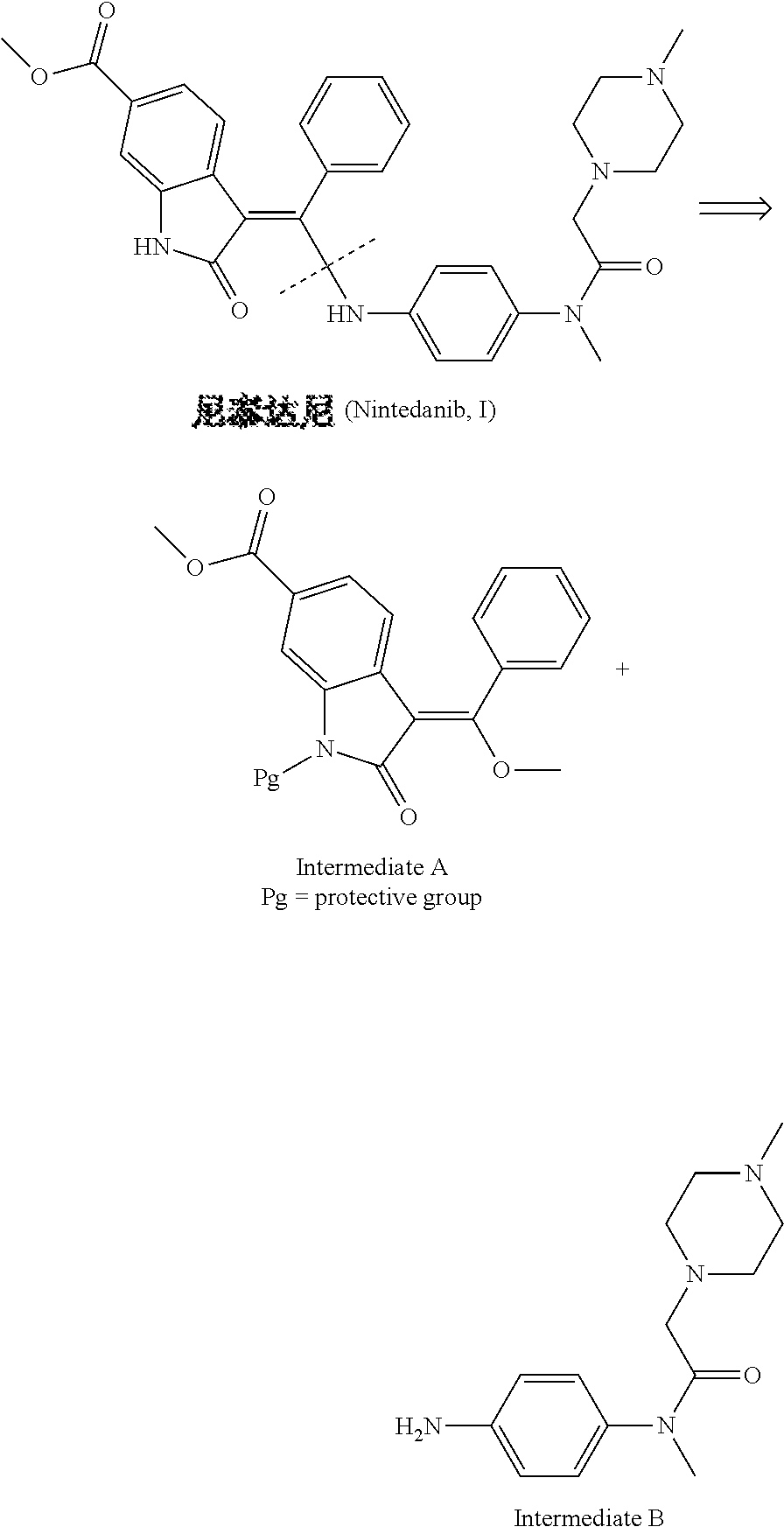
Preparation method of nintedanib
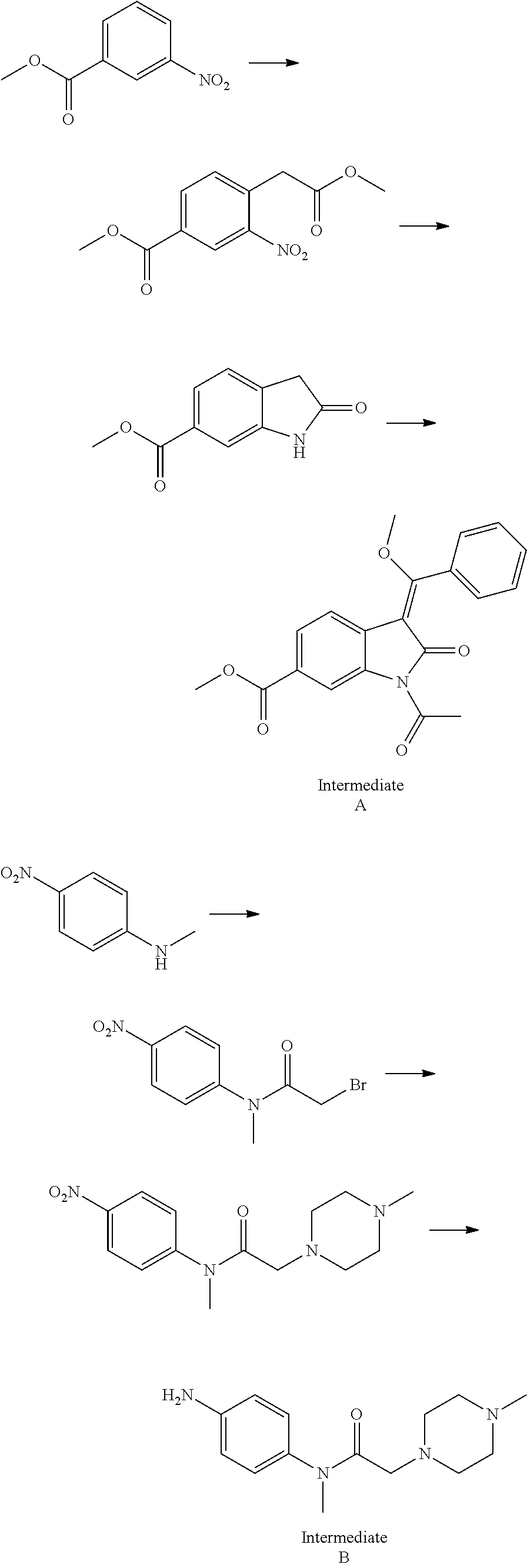
Disclosed is a preparation method of nintedanib (I), comprising the following steps: carrying out a condensation reaction on 4-(R acetate-2-yl)-3-nitrobenzoate (II) and trimethyl orthobenzoate to obtain (E)-4-[(2-methoxybenzylidene) R acetate-2-yl]-3-nitrobenzoate (III); carrying out a substitution reaction on the compound (EI) and N-(4-aminophenyl)-N-methyl-2-(4-methyl piperazine-1-yl) acetamide (IV) under the action of an acid-binding agent to generate (Z)-4-{[2-(N-methyl-2-(4-methyl piperazine-1-yl) acetamido-aniline) benzylidene] R acetate-2-yl}-3-nitrobenzoate (V); and sequentially carrying out reduction reactions and cyc-lization reactions on the compound (V) to prepare the nintedanib (I). The preparation method has an easily obtained raw material and a simple process, is economical and environmentally friendly, and is suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH
Preparation method of N-(4-aminobenzoyl)-L-glutamic acid
InactiveCN109053482AEasy to recycleHas a pungent smellOrganic compound preparationOrganic chemistry methodsBenzoic acidNitro compound
The invention relates to the technical field of medicinal intermediates, and discloses N-(4-aminobenzoyl)-L-glutamic acid. The N-(4-aminobenzoyl)-L-glutamic acid comprises the following raw materialsin parts by weight: 5-10 parts of nitrobenzoic acid, 10-15 parts of sulfoxide chloride, 2-4 parts of nitrobenzoyl chloride, 8-10 parts of ammonia water, 5-10 parts of ethanol, 2-3 parts of water, 2-4parts of glutamic acid and 8-10 parts of aminobenzoyl. According to the N-(4-aminobenzoyl)-L-glutamic acid, the nitrobenzoic acid and the sulfoxide chloride are placed in a reaction container and heated and the nitrobenzoic acid reacts with the sulfoxide chloride; the nitrobenzoic acid is in a form of a light yellow crystal, o-nitrobenzoic acid and p-nitrobenzoic acid are prepared by oxidizing corresponding nitrotoluene, m-nitrobenzoic acid is prepared by directly nitrating benzoic acid, and the nitrobenzoic acid is used as a chemical raw material and an analytical reagent for dyes, medicinesand pesticides; the sulfoxide chloride is used as a chlorinating agent for synthesis or replacement of organic substances such as alcoholic hydroxyl, acid anhydride, organic sulfonic acid and a nitrocompound; and by the preparation method, the purposes of simple operation, high yield and relatively low cost are achieved.
Owner:ABA CHEM SHANGHAI
Lenalidomide intermediate preparation method
ActiveCN105523939ALow toxicityGood for healthOrganic chemistryOrganic compound preparationKetoneCombinatorial chemistry
The present invention provides a lenalidomide intermediate preparation method, namely a preparation process of important intermediate 2-bromomethyl-3-nitro-methyl benzoate of 3-(4-amino-1-oxo-1,3-dihydro- 2H- isoindolyl-2-yl) piperidine-2,6-dione (namely lenalidomide).
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Preparation method of candesartan cilexetil
InactiveCN105272969AEasy to purifyMild reaction conditionsOrganic chemistryBulk chemical productionCandesartanTetrazole
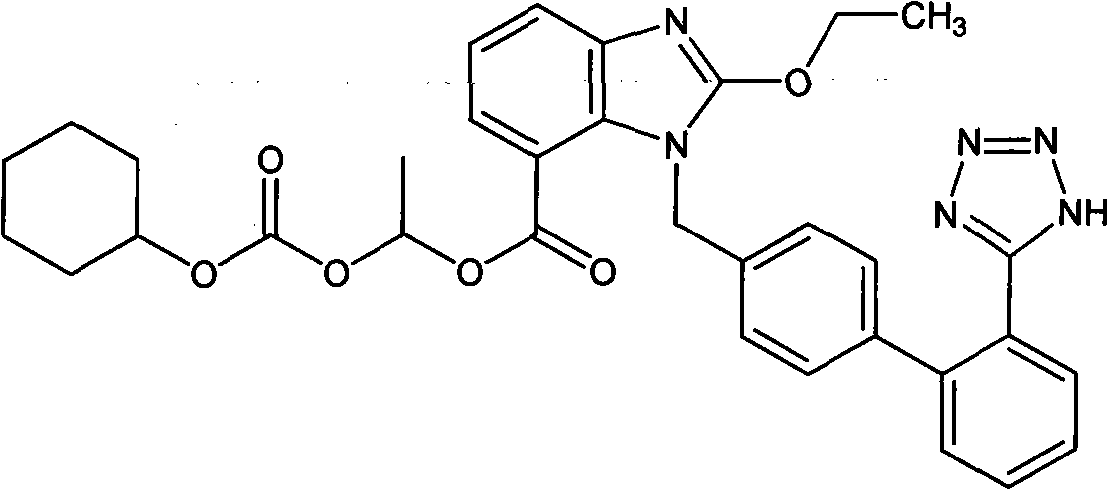
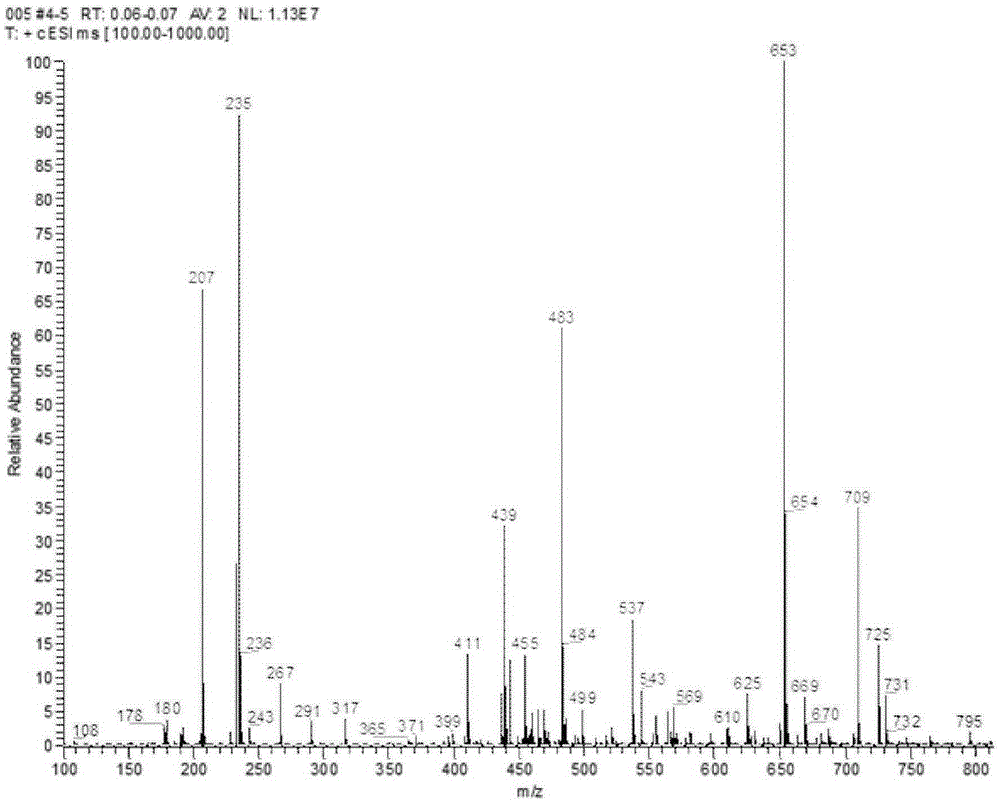
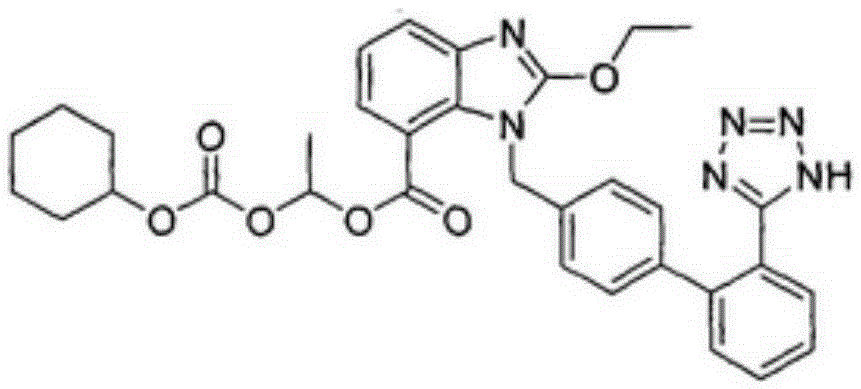
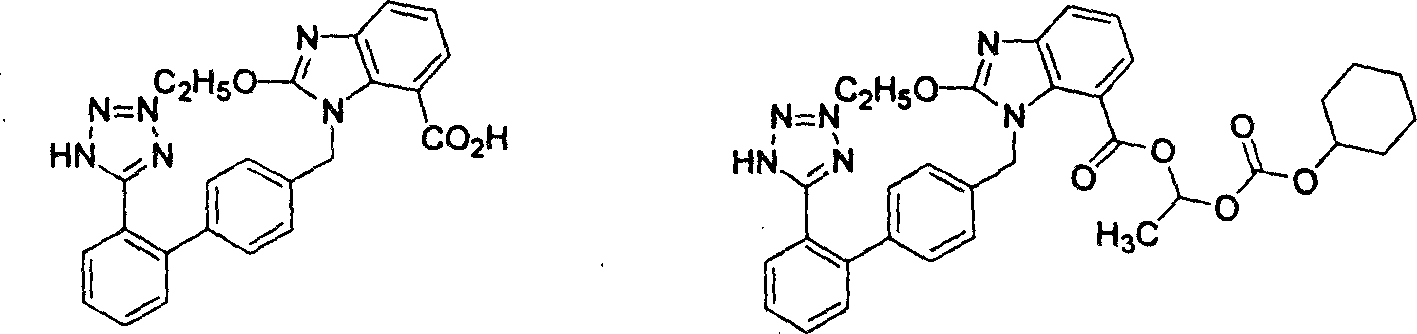
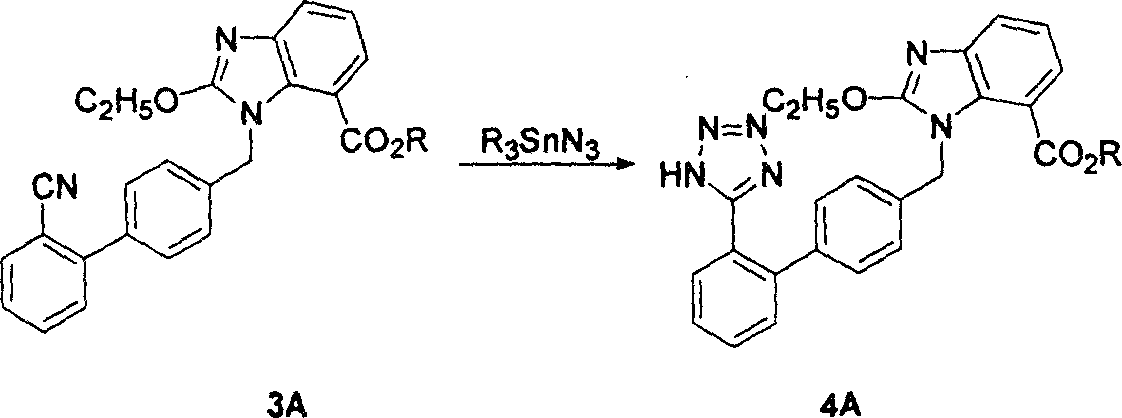
The invention relates to a preparation method of candesartan cilexetil. 2-(tert-butoxycarbonyl((2-(1-trityl-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)amino)-3-nitrobenzoic acid-1-(((cyclohexyloxy)carbonyl)oxy)ethyl ester is used as an initial reactant, a tetrazole protecting group has a deprotection reaction through alcoholysis, 2-(tert-butoxycarbonyl((2-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)amino)-3-nitrobenzoic acid-1-(((cyclohexyloxy)carbonyl)oxy)ethyl ester is obtained, a tetrazole ring is directly introduced, raw materials which are expensive and have dangerous toxic properties, such as trialkyl tin azide or trialkyl tin chloride, organic zinc or organic palladium are avoided in the follow-up process, reaction conditions are mild, and the safety of the preparation process is improved; meanwhile, the preparation method has simple technological steps, products are easy to purify, the candesartan cilexetil can be synthesized on a large scale, and the industrial development prospect is brighter.
Owner:ZHEJIANG MENOVO PHARMA
Method for preparing lenalidomide
The invention discloses a method for preparing lenalidomide (3-(7-amino-3-oxo-1H-isoindole-2-base) piperidine-2,6-diketone). Two intermediates of the lenalidomide are 2-halomethyl-3-nitro-benzoic acid methyl ester and 3-aminopiperidine-2,6-diketone. The invention discloses methods for preparing the two intermediates and preparing the lenalidomide by using the two intermediates. The method has novel process, short procedures, high reaction yield, low production cost, and larger implementation value and social and economic benefits.
Owner:SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD
Alcohol-based power fuel with automobile cleaning function and preparation process thereof
InactiveCN108485730AEasy layeringSolve the low temperature operation performanceLiquid carbonaceous fuelsFuel additivesEnvironmental resistanceBenzoxazole
The invention discloses an alcohol-based power fuel with an automobile cleaning function and a preparation process thereof. The alcohol-based power fuel comprises, by weight, the following components:(1) alcohol group: 86.37-95.39%, (2) one of dimethyl carbonate, hydrogen peroxide or composite raw material formula: 1-2%, (3) methyl tert-butyl ether: 2-7%, (4) benzene, 3-nitrobenzoic acid and diethylene glycol dibenzoate compound: 1-3%o, (5) one of isopropanol, ethanol or urea: 1-2%, (6) one of 1-phenyl-5-sulfydryl tetrazole, benzoxazole and pyrazolone complex agent and corrosion inhibition complex agent: 0.3-2%, (7) 2, 6- butylated hydroxytoluene: 0.3-0.6%. The formula is an alcohol-based environment-friendly new energy, and reduces the dependence degree of the automobile on petroleum products and the emission of automobile tail gas. According to the invention, raw materials such as corrosion resistance, anti-swelling agent and the like in the formula of methanol or ethanol gasoline or automobile cleaner are improved, and the fuel optimized to solve the problems of methanol gasoline or automobile cleaner in the prior art.
Owner:丁志强 +1
Function board for adsorbing nitrobenzene and preparation method thereof
InactiveCN106423092AImprove adsorption capacityFast adsorptionOther chemical processesWater contaminantsN dimethylformamideFreeze-drying
The invention discloses a function board for adsorbing nitrobenzene and a preparation method thereof. The method comprises the steps that 4-hydroxybutyraldehyde, 4-acetamido-3-nitrobenzoic acid, N,N-dimethylformamide, polypropylene and dibenzothiazyl disulfide are adopted for preparing a mixed solution B; polyacrylonitrile, N,N-dimethylformamide, Na2HPO4 and Mg(NO3)2 are adopted for preparing a mixed solution D; acetonyl acetate, chloroform and ferric chloride are adopted for preparing a mixed solution F; the mixed solution, B, the mixed solution D, the mixed solution F, methyl isobutyrate, polyethylene glycol and chloroform are adopted for preparing a mixed solution H; distilled water is sprayed into a container containing liquid nitrogen to prepare ice-ball particles, and the ice-ball particles with the particle size being 50-100 micrometers are placed into a cavity of a mold to be compacted; then, the mixed solution H is poured into the mold and subjected to freezing and sizing in liquid nitrogen for 6 h, and the function board for adsorbing nitrobenzene is obtained after chloroform and ice-ball particles are removed through vacuum freeze drying.
Owner:北京益净环保设备科技有限公司
Electrolytic solution of aluminum electrolytic capacitor, and preparation method of electrolytic solution
ActiveCN109448993AExtended service lifeImprove stabilityElectrolytic capacitorsElectrolysisPolyethylene glycol
The invention relates to the technical field of electrolytic solutions for electrolytic capacitors, in particular to an electrolytic solution of an aluminum electrolytic capacitor, and a preparation method of the electrolytic solution. The electrolytic solution of the aluminum electrolytic capacitor is prepared from the following raw materials in parts by weight: 45-50 parts of ethanediol, 0.5-1.5parts of 4-chloro-3-nitrobenzoic acid, 10-15 parts of polyethylene glycol, 0.5-2.5 parts of ammonium hypophosphite, 0.5-1.5 parts of 1H-1,2,3-triazole, 6-9 parts of citric acid, 0.1-1 part of p-toluenesulfonyl isocyanate, 0.5-1.5 parts of ammonium hydrogen azelate, 3-3.5 parts of ammonium sebacate and 0.1-0.8 parts of poly alpha olefin. The electrolytic solution of the aluminum electrolytic capacitor provided by the invention is resistant to high temperature, has high flash voltage and conductivity, is long in service life and can be widely applied to the fields of large-screen color TVs, automobile electronics, frequency conversion technologies, energy-saving lamps, communication electronics and the like.
Owner:广州金立电子有限公司
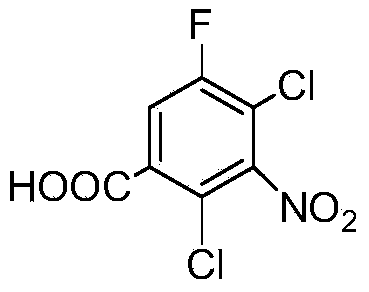
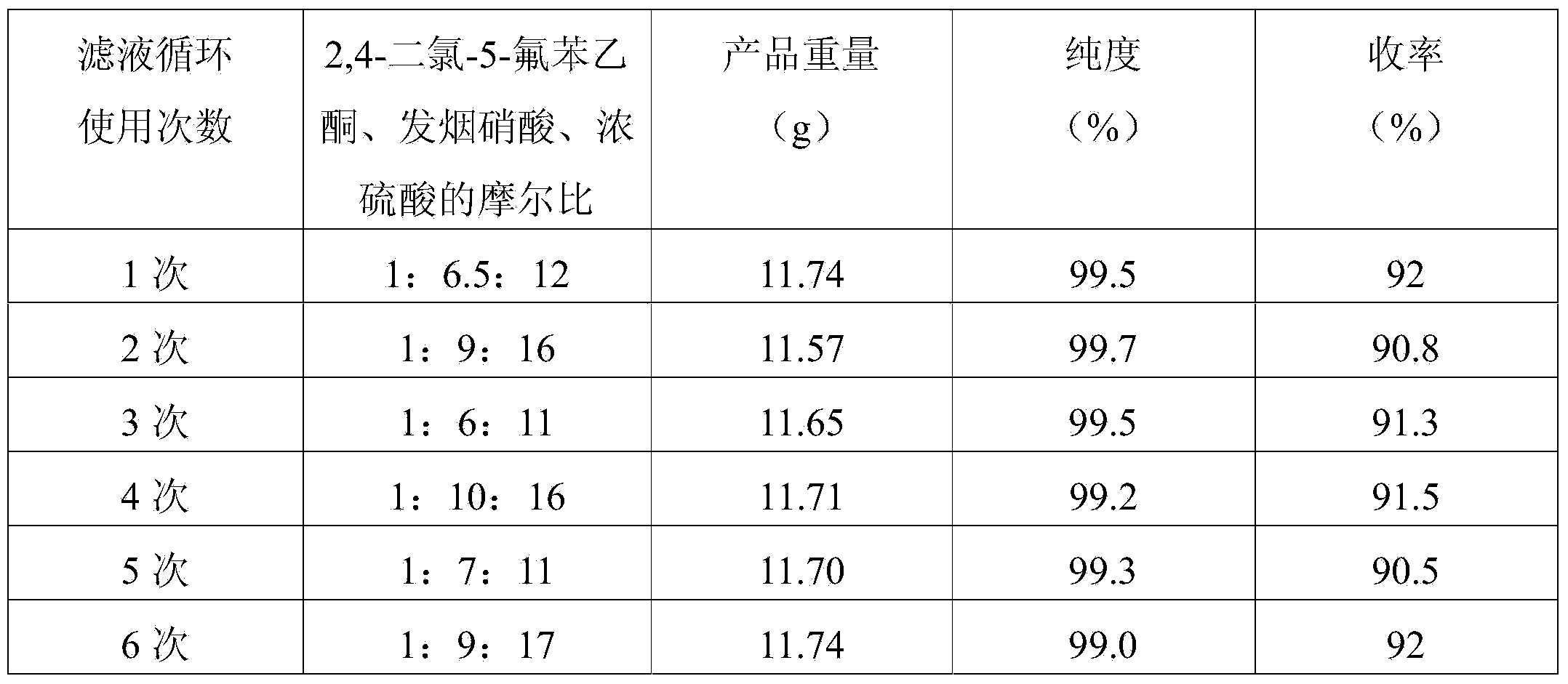
Preparation method of 2, 4-dichloro-5-fluoro-3-nitrobenzoic acid
ActiveCN103922942AHigh purityHigh yieldNitro compound preparationChemical synthesis3-nitrobenzoic acid
The invention relates to a preparation method of 2, 4-dichloro-5-fluoro-3-nitrobenzoic acid and belongs to the technical field of chemical engineering, and particularly belongs to the field of chemical synthesis. The preparation method of 2, 4-dichloro-5-fluoro-3-nitrobenzoic acid comprises the following step: carrying out a reaction on 2, 4-dichloro-5-fluoroacetophenone and fuming nitric acid in concentrated sulfuric acid, wherein the molar ratio of 2, 4-dichloro-5-fluoroacetophenone, fuming nitric acid and concentrated sulfuric acid is 1:(6-15):(11-25). The product prepared by the method provided by the invention is high in purity and yield, and the method is less in three waste, simple to operate and beneficial for industrialization.
Owner:FUXIN RUIGUANG FLUORINE CHEM
Method for preparation of o-nitrobenzoic acid by catalytic oxidation of o-nitrotoluene
InactiveCN104311425AHigh yieldMild reaction conditionsOrganic chemistryOrganic compound preparationCatalytic oxidationHigh pressure
The invention relates to a method for preparation of o-nitrobenzoic acid by catalytic oxidation, and the method comprises the following steps: adding a solvent, o-nitrotoluene, catalyst MnO2 and RuO4 into a high pressure reactor, introducing oxygen, reacting 5 to 15 hours at 100-150 DEG C under 0.1-2MPa, and after the reaction, filtering the catalyst, extracting, washing with water, and distilling to recover the solvent to obtain the o-nitrobenzoic acid. The filtered catalyst can be recycled.
Owner:王晓伟
Method for preparing candestartan
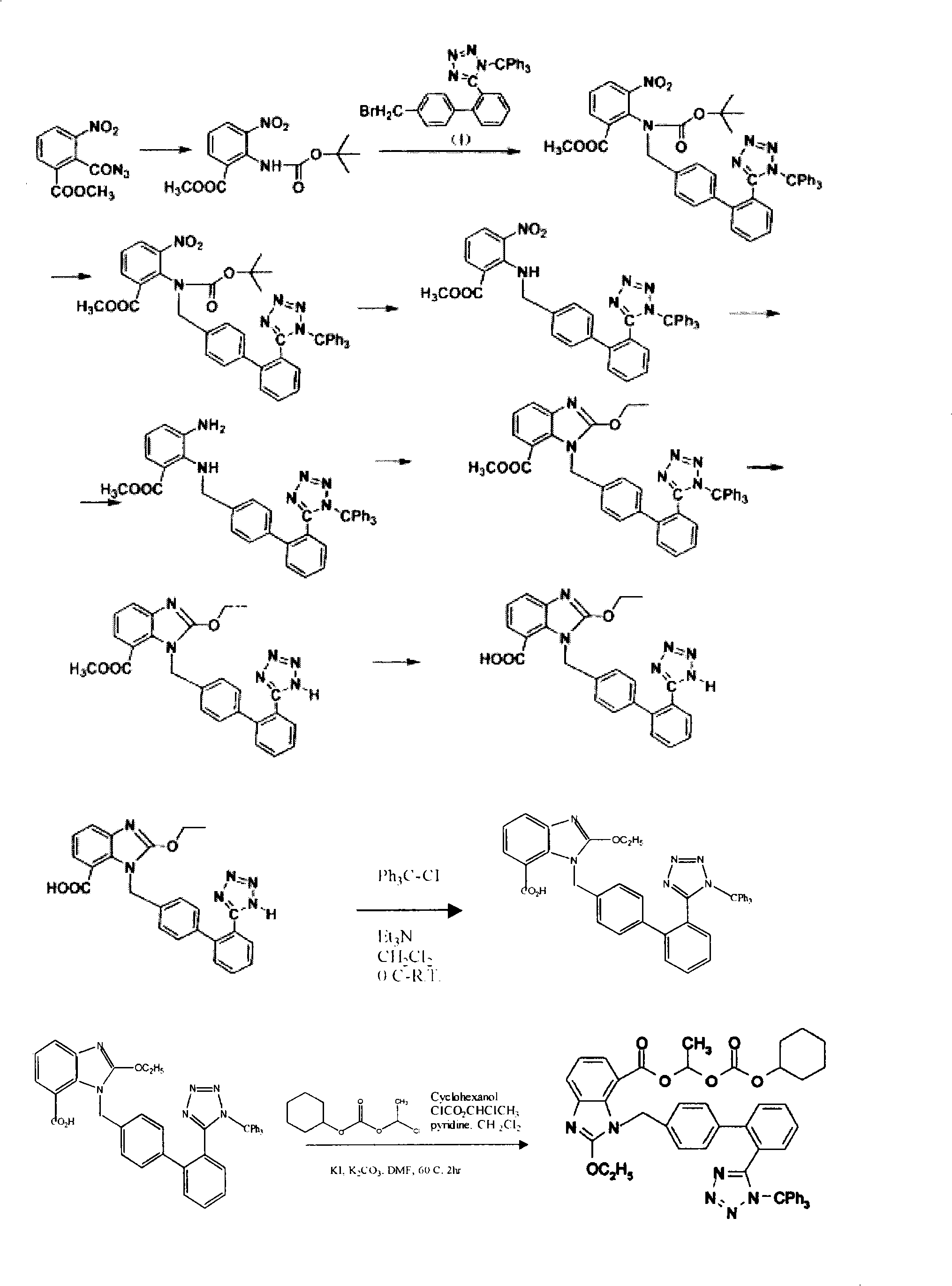
ActiveCN100344625CRaw materials are easy to getSimple operation processOrganic chemistryTert-Butyloxycarbonyl protecting groupOxygen
The invention relates to a method for synthesizing ridge sha tan, which uses the 2-tert-butyl ketonic oxygen amido-3-nitro benzoate (I) and N-(trityl )-5-(4'-morphine methylbiphenyll-2-group) tetrazolium (II) as raw material to do N-alkanisation reaction, protecting-released reaction, reduction reaction, ring-closed reaction and ester hydrolytic reaction. <0The protecting-released reaction can slough trityl and tert-butyl ketonic oxygen protect group in the lower aliphatic alcohol organic mixing solution.
Owner:LINHAI TIANYU PHARMA
Synthesis method of 2 - methyl -3 - fluoride - 6 -nitrobenzoic acid
ActiveCN101870653BEasy to prepareSuitable for large-scale industrial synthesisOrganic chemistryOrganic compound preparationSynthesis methodsNitration
The invention relates to a synthesis method of important medicine midbody 2 - methyl -3 - fluoride - 6 -nitrobenzoic acid. The invention provides a novel synthesis route, which can rapidly and conveniently prepare an important medicine midbody from the cheap and facile raw materials. The process not only has high yield, but also is applicable to the mass production. The process method is started from 2 - methyl -3-fluoroaniline, crucial midbody N-(2-Methyl -3-- fluoride -6-nitrophenyl) acetamide is selectively generated through the nitration reaction, and the acetyl is hydrolyzed to obtain the 2 - methyl -3-- fluoride -6-nitroaniline; then the amidocyanogen has diazo reaction to generate 2 - bromo -3-- methyl -4-- F - nitrobenzene; bromide generates corresponding cyano compound under the effect of cuprous cyanide; and finally the 2 - methyl -3 - fluoride - 6 -nitrobenzoic acid is obtained under the effect of the sulfuric acid and the sodium nitrite.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
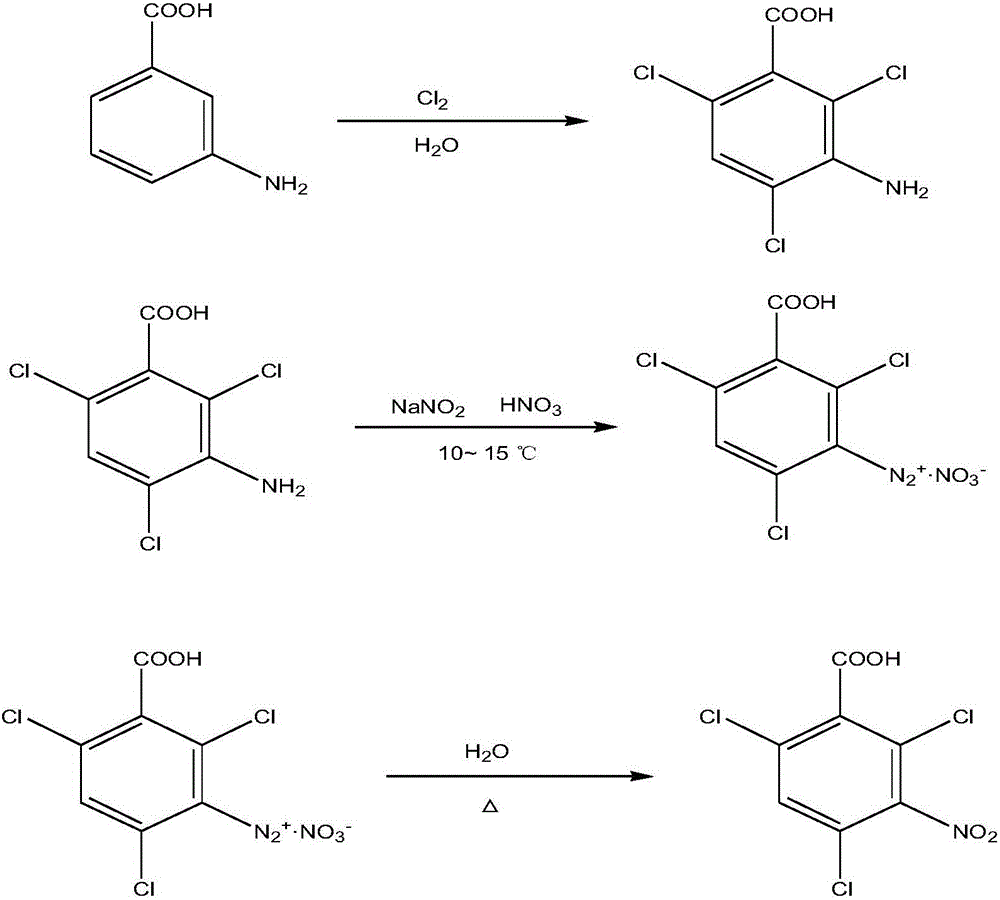
Synthetic method of 2,4,6-trichloro-3-nitrobenzoic acid
InactiveCN105777548AOrganic compound preparationAmino-carboxyl compound preparationHydrolysisAminobenzoic acid
The invention relates to a synthetic method of a compound and in particular relates to a synthetic method of 2,4,6-trichloro-3-nitrobenzoic acid. The method is characterized by taking m-aminobenzoic acid as a raw material and synthesizing 2,4,6-trichloro-3-nitrobenzoic acid through three steps of chlorination, diazotization and hydrolysis. The synthetic method has the advantages of mild reaction conditions and high yield.
Owner:CHANGZHOU UNIV
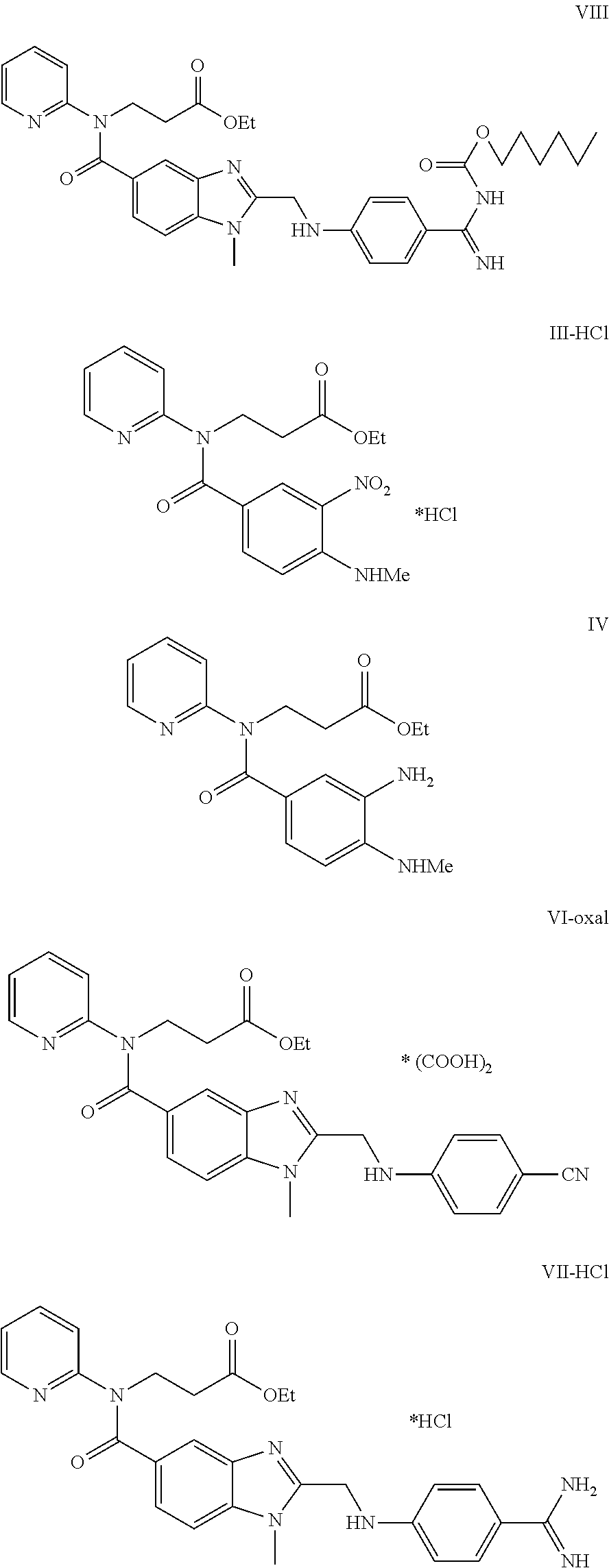
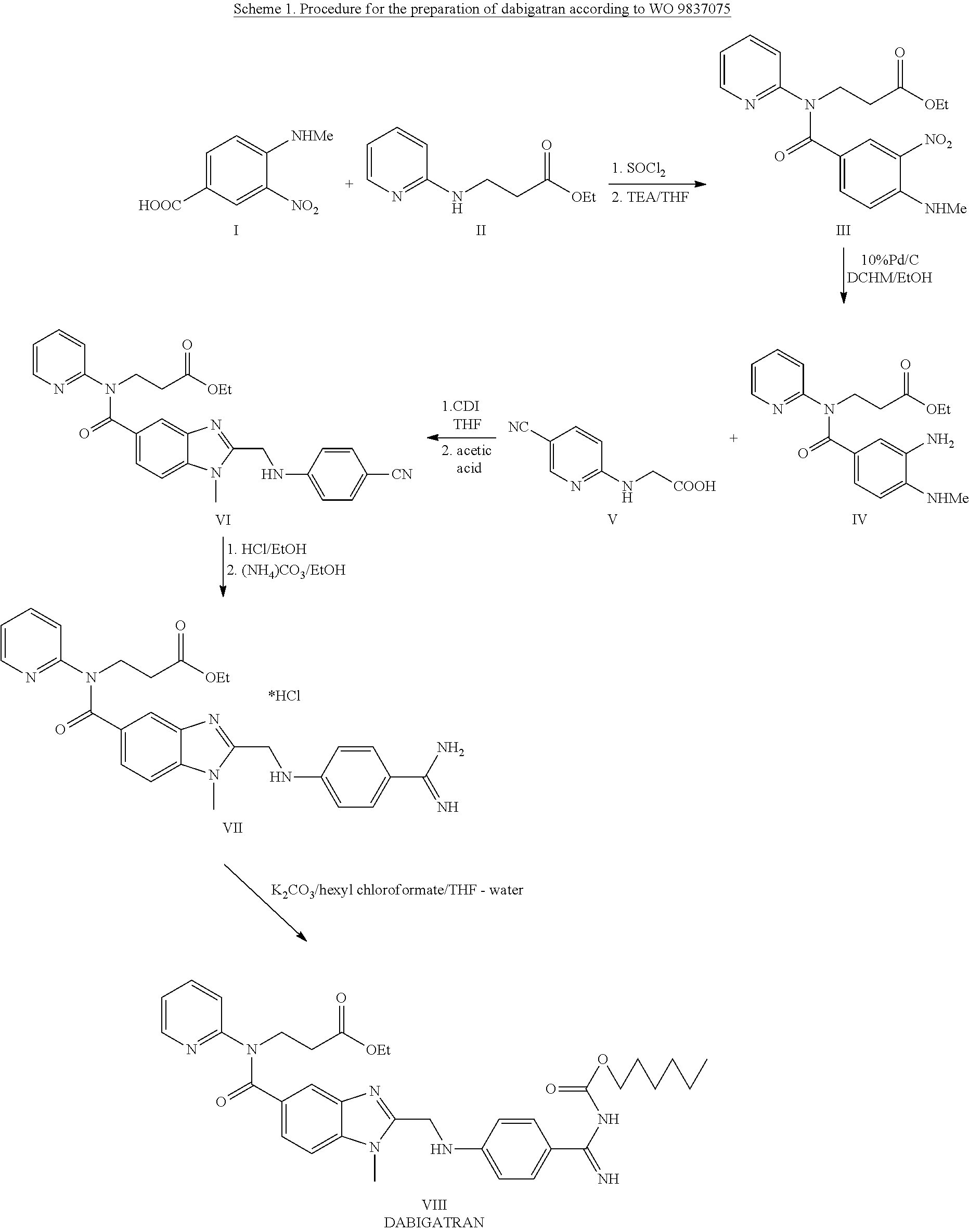
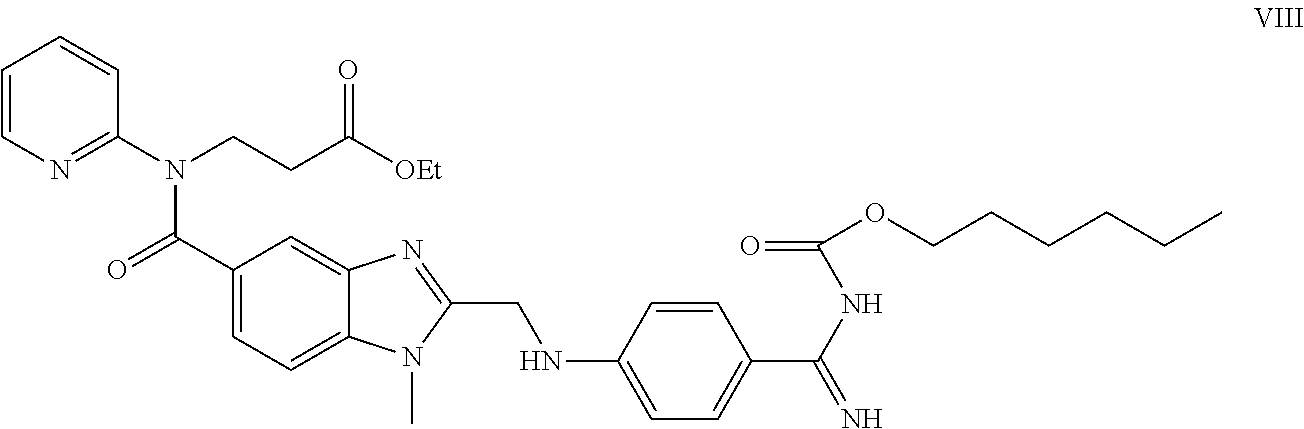
Method for the preparation of dabigatran
Owner:ZENTIVA AS
Function board for adsorbing parathion-methyl, and preparation method of function board
InactiveCN106334528AImprove adsorption capacityFast adsorptionOther chemical processesWater contaminantsParathion methylFreeze-drying
The invention discloses a function board for adsorbing parathion-methyl, and a preparation method of function board. The preparation method comprises the following steps: preparing mixed liquid B from tetrahydrothiophene, 4-acetamido-3-nitrobenzoic acid, N,N-dimethyl formamide, polyhexamethylene adipamide and hexamethylenetetramine; preparing mixed liquid D from polyvinyl chloride, N,N-dimethyl formamide, 1-(3-pyridyl)-3-(dimethylamino)-2-propylene-1-ketone; preparing mixed liquid F from tert-butyl peracetate, chloroform and N,N-dimethyl glycinamide; preparing mixed liquid H from the mixed liquid B, the mixed liquid D, the mixed liquid F, methyl isobutyrate, polyethylene glycol and chloroform; ejecting distilled water into a container with liquid nitrogen to prepare ice ball particles, and putting the ice ball particles with the particle size being 50 to 100 microns into a mold cavity of a mold, and performing compaction; and pouring the mixed liquid H into the mold, freezing and shaping the mold in the liquid nitrogen for 6 hours, and removing the chloroform and the ice ball particles through vacuum freeze drying to obtain the function board for adsorbing the parathion-methyl.
Owner:北京益净环保设备科技有限公司
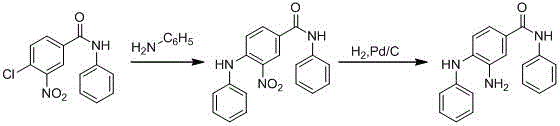
A kind of synthetic method of n-methyl-4-(methylamino)-3-nitrobenzamide
InactiveCN104356022BWide variety of sourcesLow priceOrganic compound preparationCarboxylic acid amides preparationCarbylamine reactionSynthesis methods
Owner:SHANGHAI INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com