Synthetic method of (R)-2-methyl-4-nitro-1-butanol
A synthesis method, the technology of nitro-n-butyraldehyde, is applied in the field of synthesis of -2-methyl-4-nitro-1-butanol to achieve cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
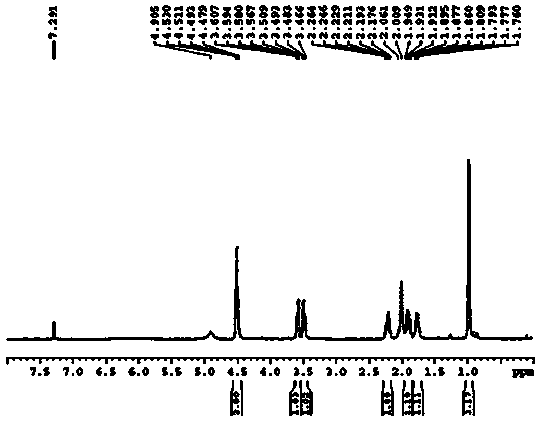
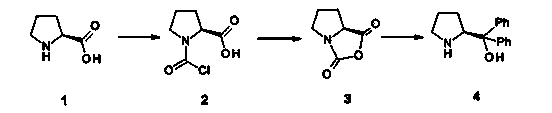
[0025] (1) Add 120.0g (1.042mol) (S)-proline to 1.2L tetrahydrofuran (THF), cool down to 15°C, slowly add 648 ml phosgene toluene solution (COCl 2 1.93mol / L, 1.25mol), stirred for 1 hour, then raised the temperature to 40°C, stirred for 30 minutes, then lowered the temperature to 20°C, concentrated the reaction solution to 160ml by distillation under reduced pressure, added 1.2L tetrahydrofuran, and then lowered the temperature to 0°C , then added dropwise the triethylamine of 145.2ml (1.042mol) in 15 minutes, stirred for 30 minutes, filtered, and the filter residue was washed with tetrahydrofuran, collected the filtrate and the washings, and removed the solvent by distillation under reduced pressure to obtain the product 139.6g (S)- Proline-N-carboxy-anhydride (namely compound 3), the yield is 95%.
[0026] (2) Cool 1.6L of phenylmagnesium chloride (3.2mol) in tetrahydrofuran (2mol / L) to -10°C, and slowly add 600mL of (S)-proline-N-carboxy-anhydride (139.6g , 0.99mol) in t...
Embodiment 2
[0030] (1) Add 12.0g (0.1mol) (S)-proline into 120ml tetrahydrofuran, cool down to 20°C, slowly add 54ml phosgene toluene solution (COCl 2 1.93mol / L, 0.1mol), stirred for 0.5 hours, then raised the temperature to 30°C, stirred for 45 minutes, then lowered the temperature to 20°C, concentrated the reaction solution to 16ml by distillation under reduced pressure, added 120ml of tetrahydrofuran, and then lowered the temperature to 5°C, Then 6.67ml (0.05mol) of triethylamine was added dropwise within 15 minutes, stirred for 30 minutes, filtered, the filter residue was washed with tetrahydrofuran, the filtrate and washings were collected, and the solvent was distilled off under reduced pressure to obtain the product 11.9g (S)-proline Amino acid-N-carboxy-anhydride in the ring (namely compound 3), the yield is 80.9%.
[0031](2) Cool 106ml of phenylmagnesium chloride solution in tetrahydrofuran (2mol / L, 0.2mol) to -10°C, and slowly add 51.15mL of (S)-proline-N-carboxy-anhydride (1...
Embodiment 3
[0035] (1) Add 12.0g (0.1mol) (S)-proline into 120ml tetrahydrofuran, cool down to 15°C, slowly add 81 ml phosgene toluene solution (COCl 2 1.93mol / L, 0.15mol), stirred for 0.5 hours, then raised the temperature to 40°C, stirred for 30 minutes, then lowered the temperature to 15°C, concentrated the reaction solution to 16ml by distillation under reduced pressure, added 120ml of tetrahydrofuran, and then lowered the temperature to 0°C, Then within 15 minutes, 16.68ml (0.125mol) of triethylamine was added dropwise, stirred for 30 minutes, filtered, the filter residue was washed with tetrahydrofuran, the filtrate and washings were collected, and the solvent was distilled off under reduced pressure to obtain the product 12.7g (S)-proline Amino acid-N-carboxy-anhydride in the ring (namely compound 3), the yield is 86.3%.
[0036] (2) Cool 159ml of phenylmagnesium chloride solution in tetrahydrofuran (2mol / L, 0.315mol) to -10°C, and slowly add 54.58mL of (S)-proline-N-carboxy-anhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com