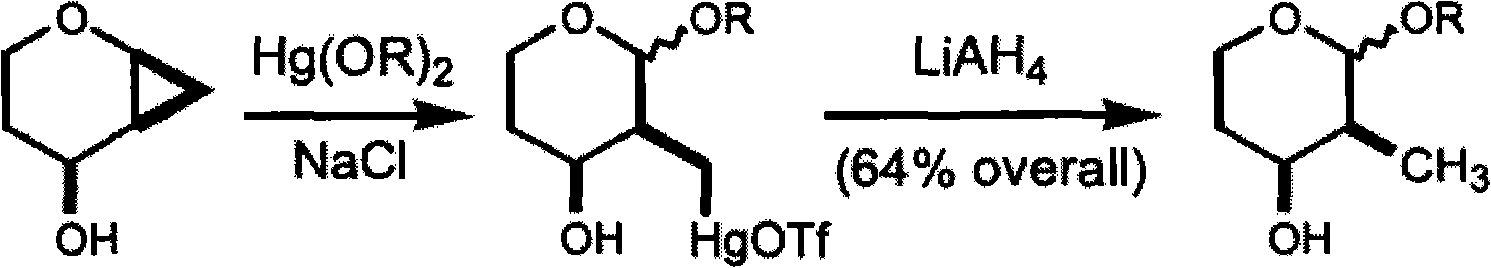
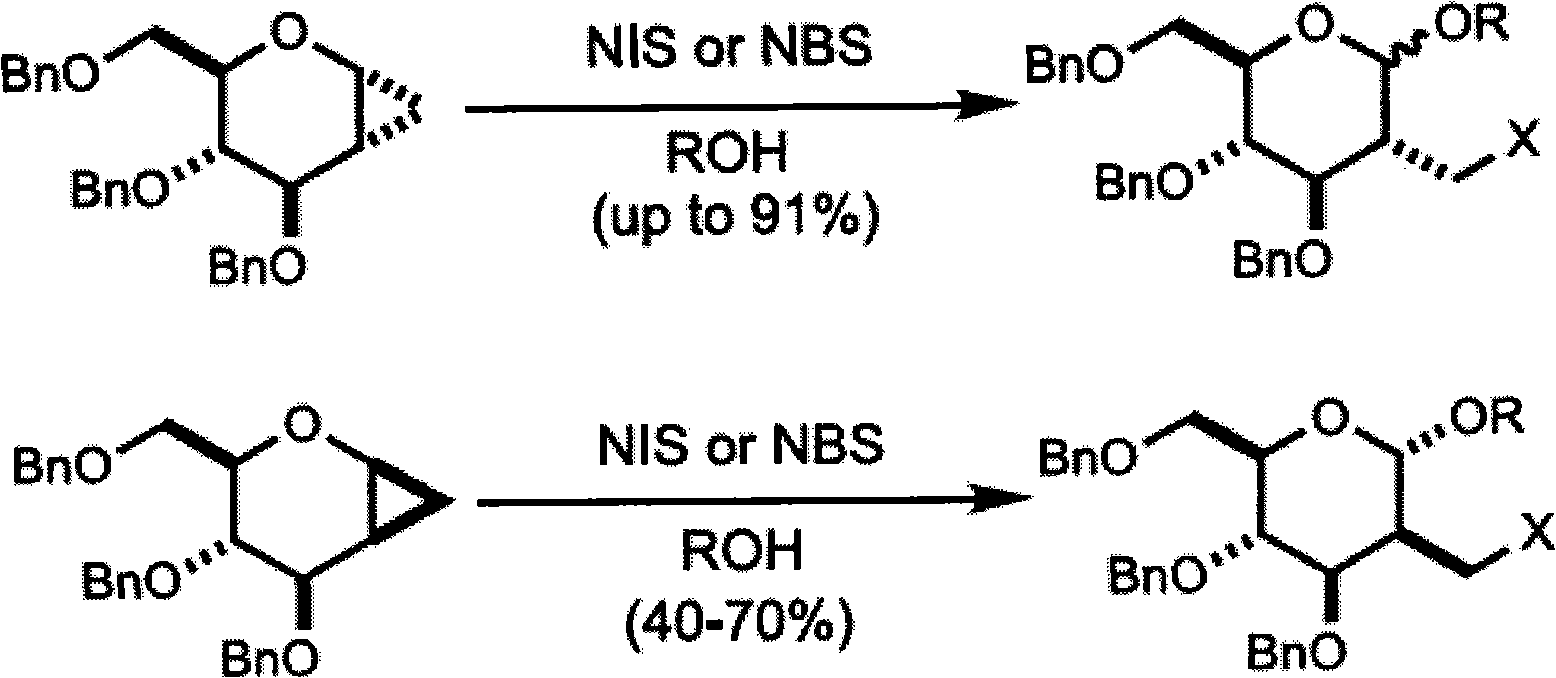
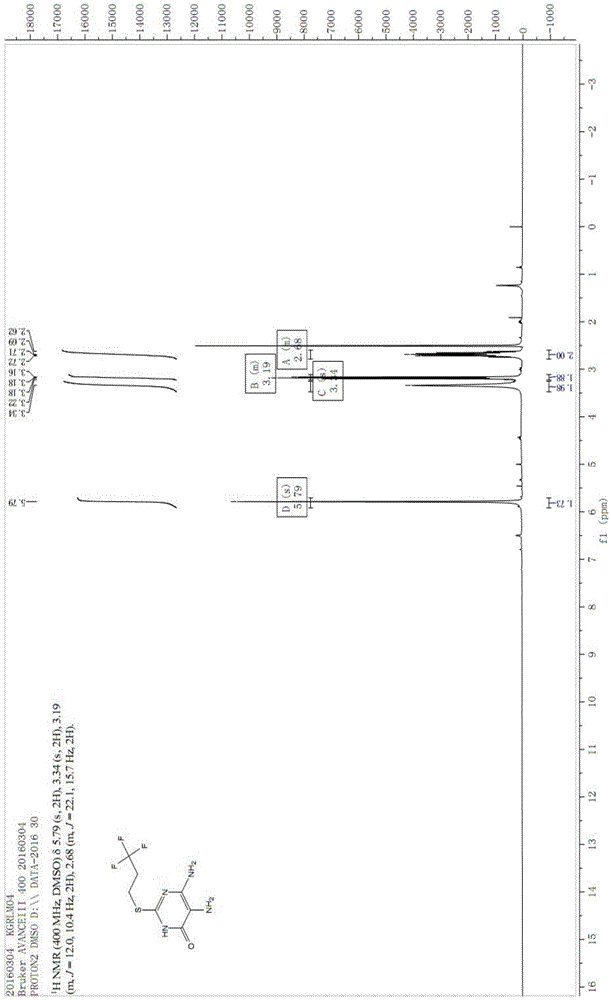
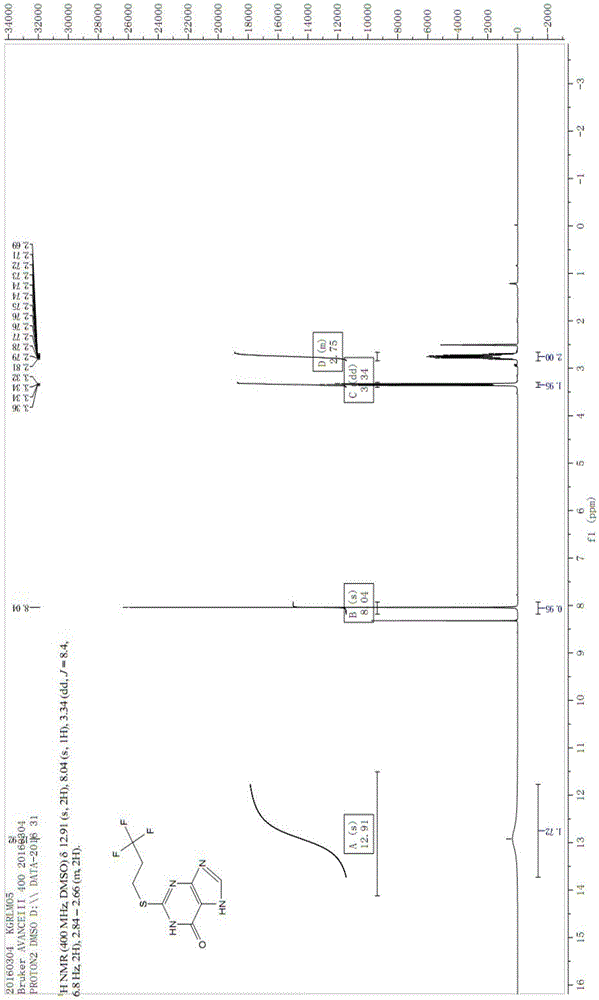
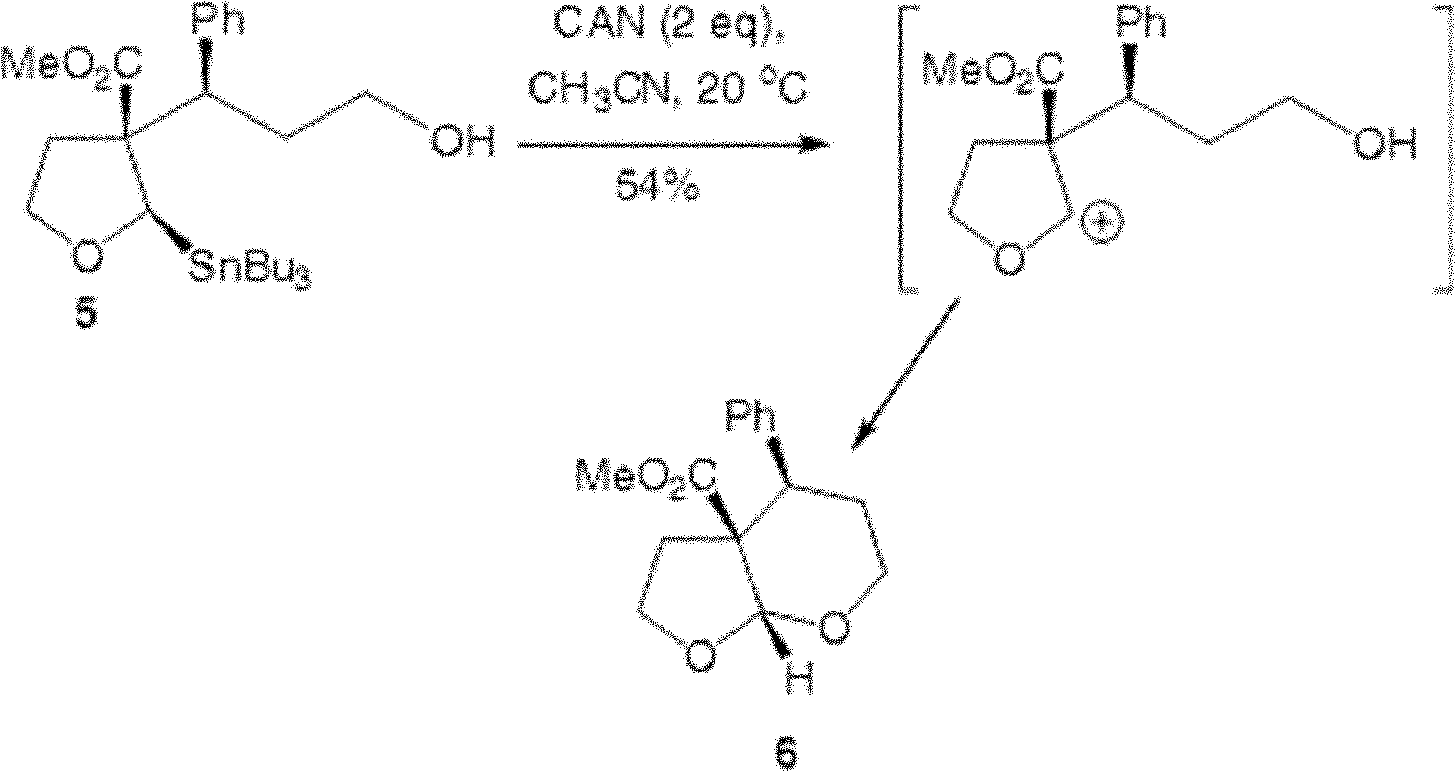
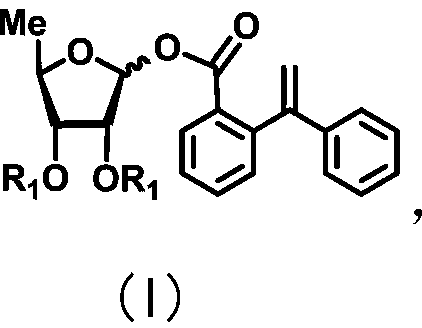
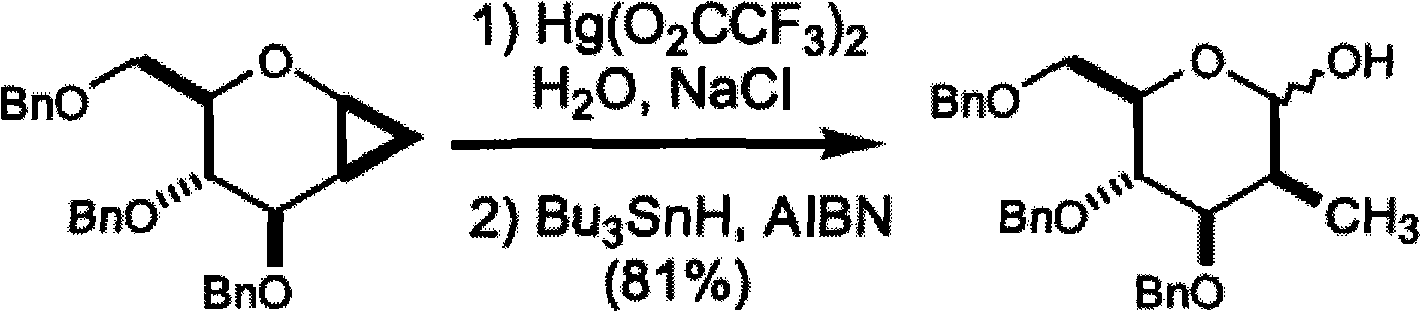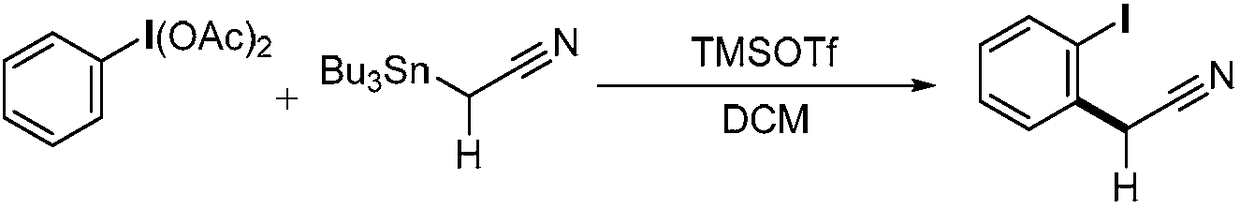Patents
Literature
75 results about "Trimethylsilyl trifluoromethanesulfonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trimethylsilyl trifluoromethanesulfonate is a trifluoromethanesulfonate derivate with a trimethylsilyl R-group. It has similar reactivity to trimethylsilyl chloride, and is also used often in organic synthesis.
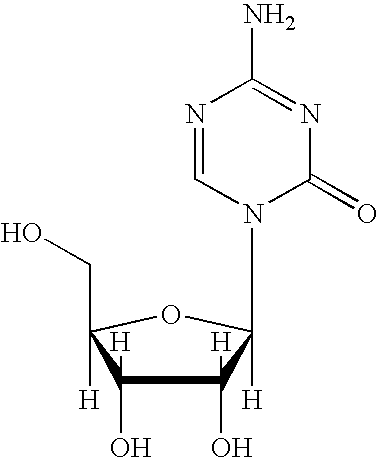
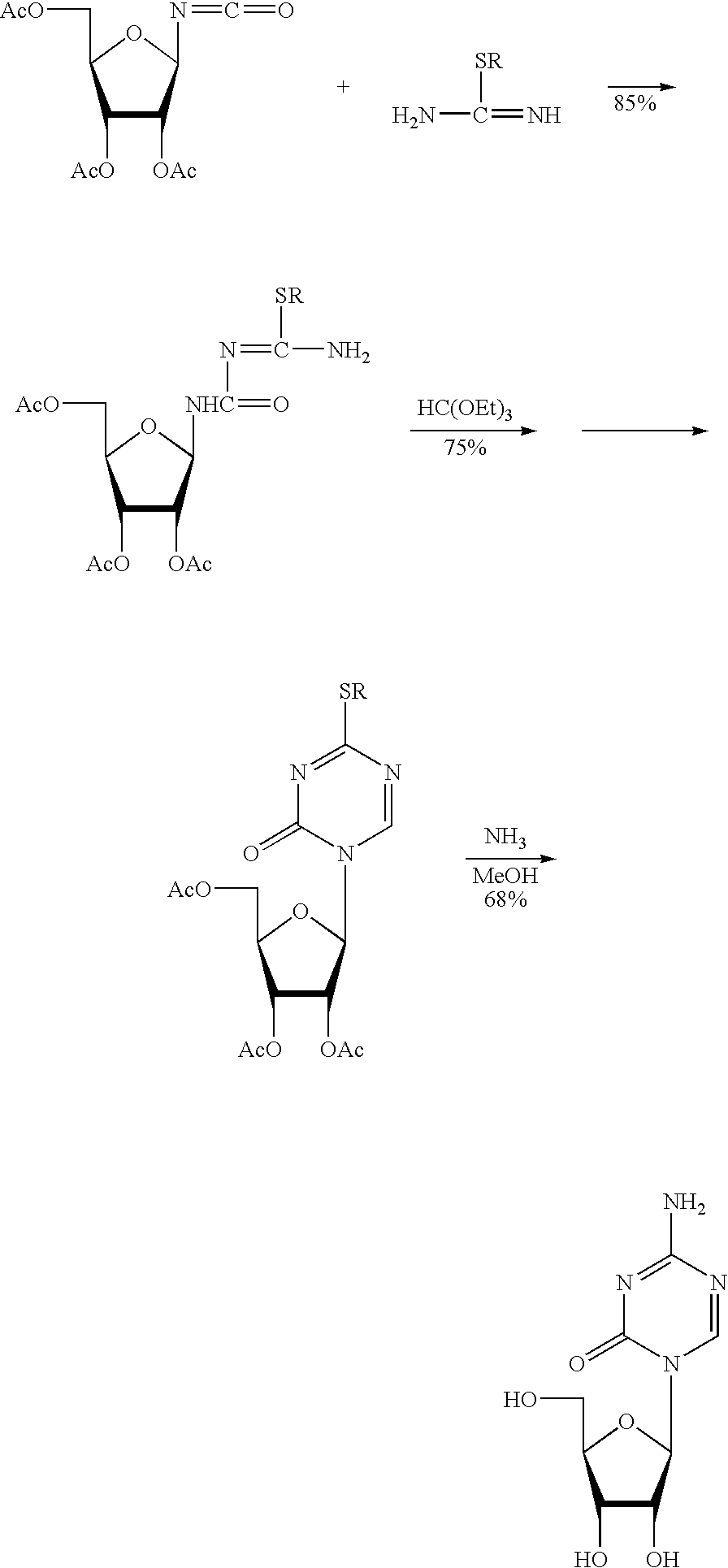
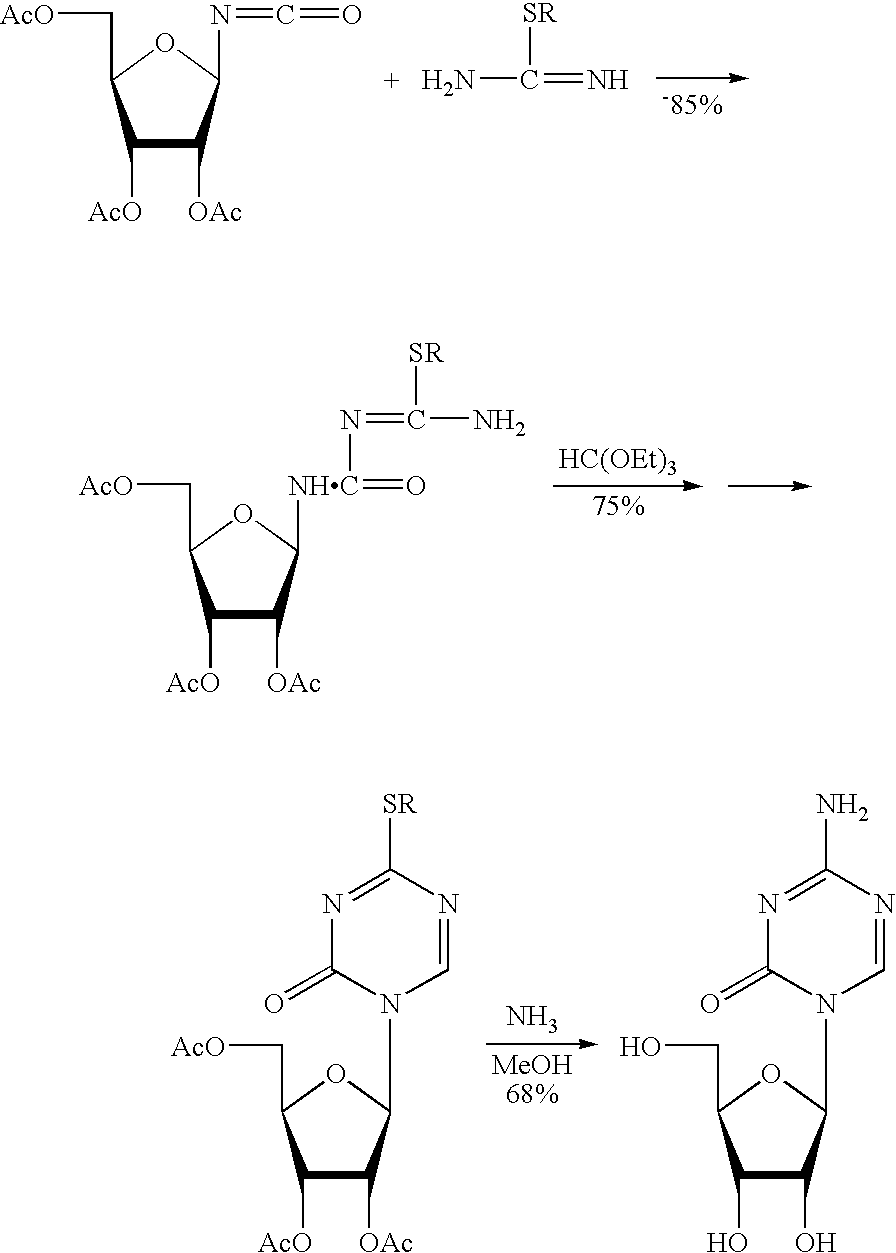
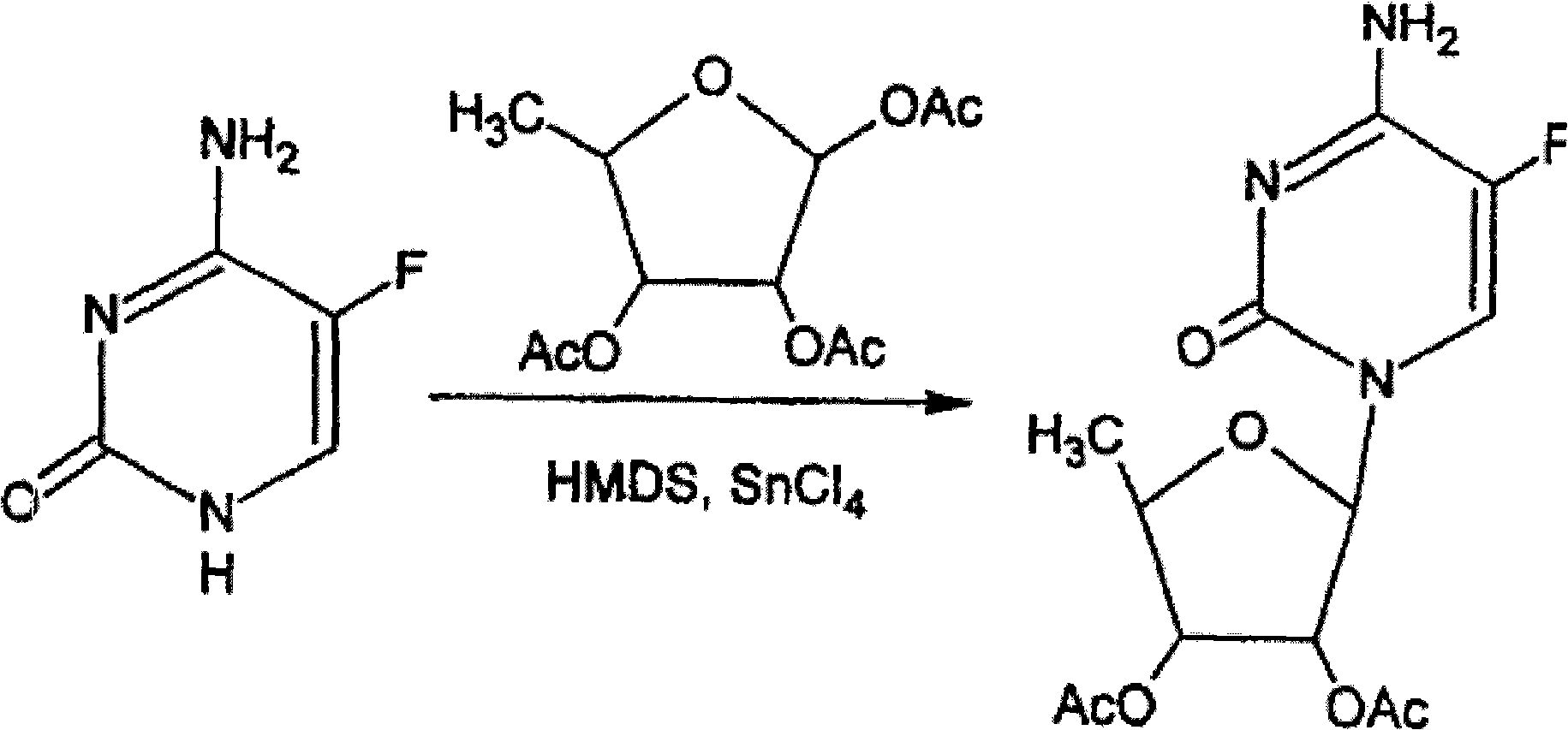
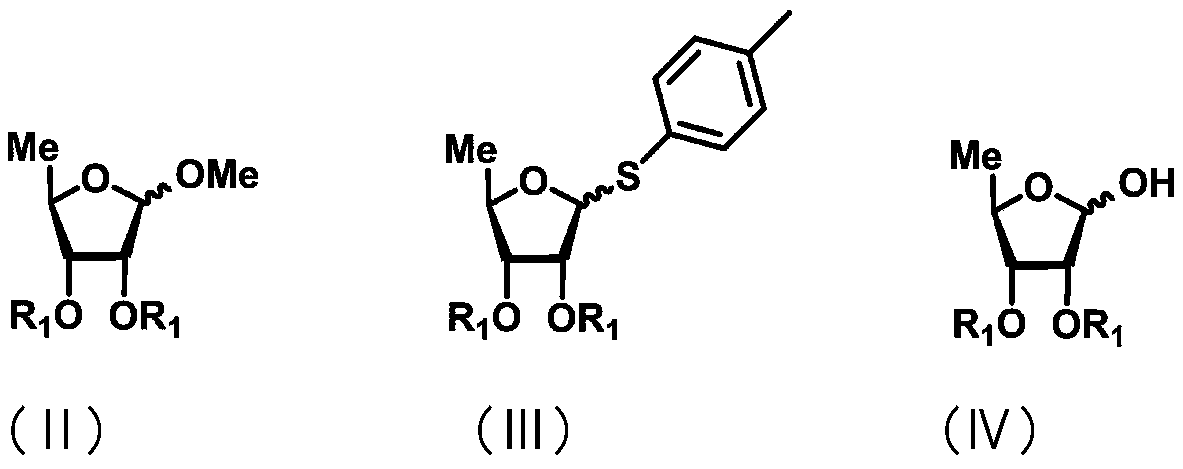
Synthesis of 5-azacytidine
InactiveUS7038038B2Amenable to scale-upAvoiding hydrolysis of the s-triazine ringBiocideSugar derivativesTrimethylsilyl trifluoromethanesulfonateCoupling
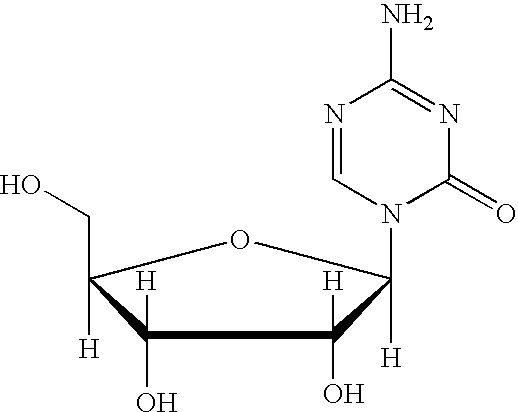
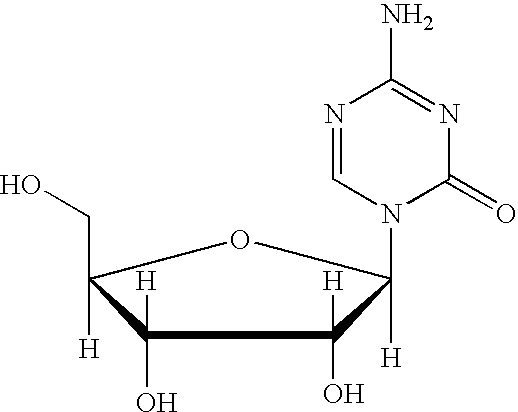
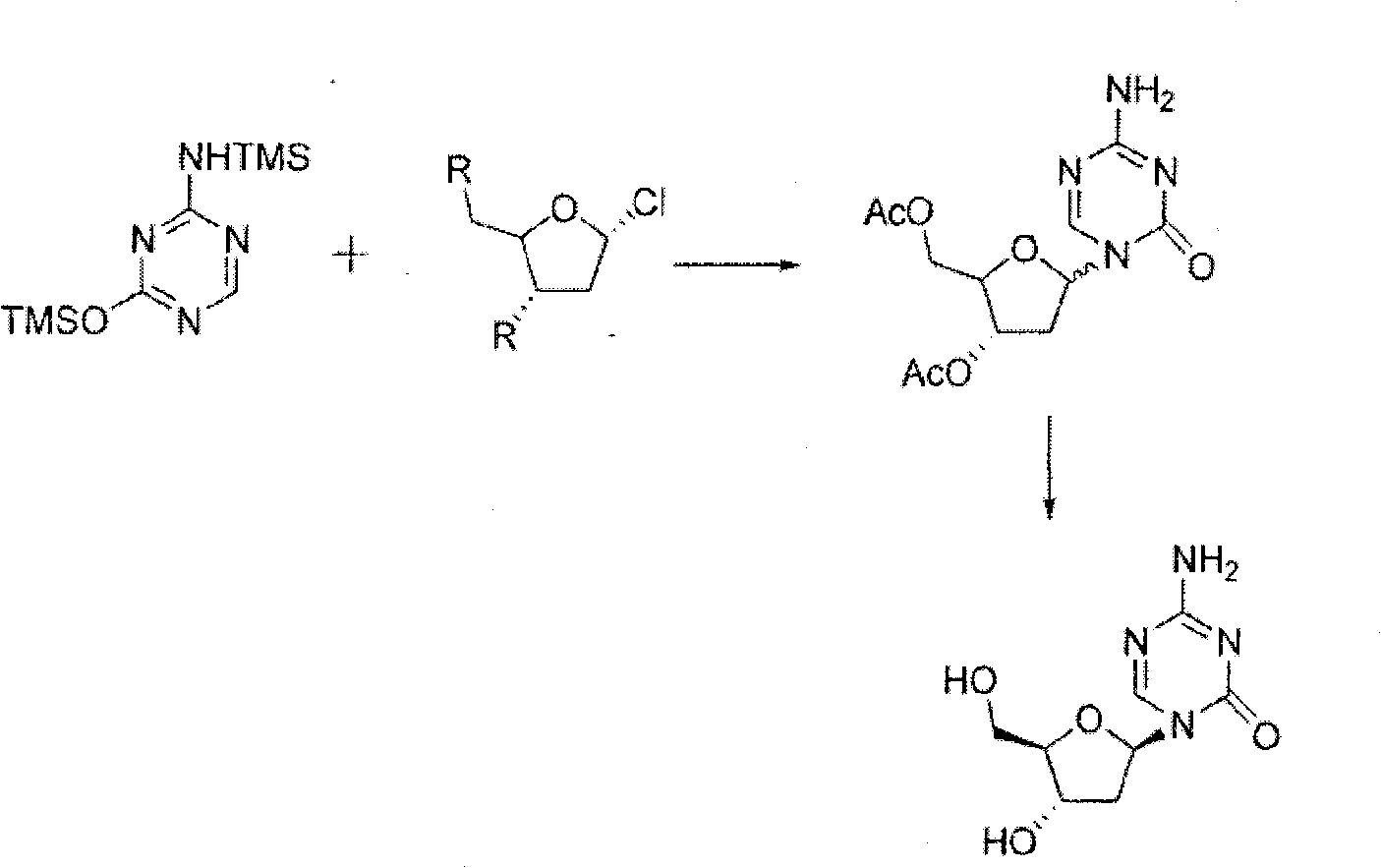
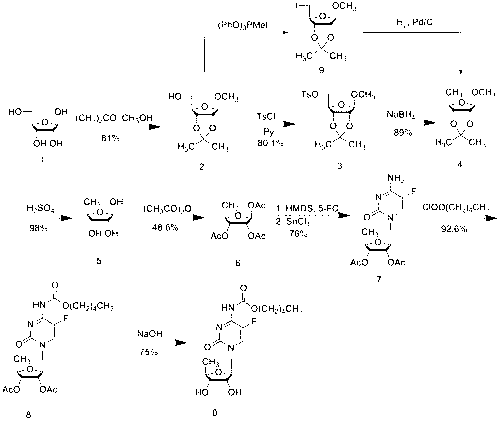
The present invention provides a method for the preparation of 5-azacytidine, wherein 5-azacytidine is represented by the structure: The method involves the silylation of 5-azacytosine, followed by the coupling of silylated 5-azacytosine to a protected β-D-ribofuranose derivative. The coupling reaction is catalyzed by trimethylsilyl trifluoromethanesulfonate (TMS-Triflate).
Owner:PHARMION
Liquid Chemical for Forming Protecting Film
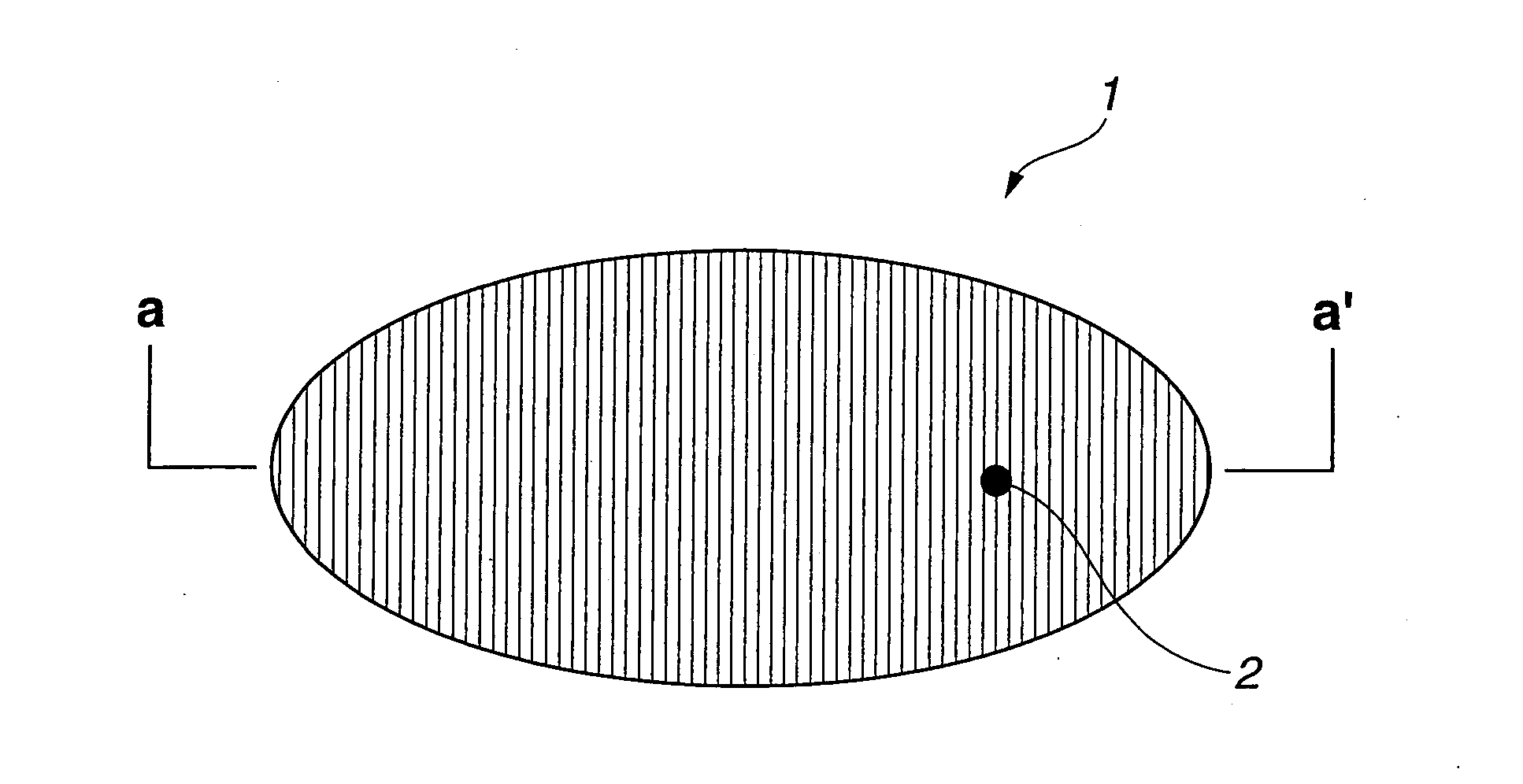
ActiveUS20120017934A1Improve waterproof performanceReduce capillary forceNon-ionic surface-active compoundsOther chemical processesTrimethylsilyl trifluoromethanesulfonateCompound a
Disclosed is a liquid chemical for forming a water-repellent protecting film at least on a surface of a recessed portion of an uneven pattern at the time of cleaning a wafer having a finely uneven pattern at its surface and containing silicon at least a part of the uneven pattern. This liquid chemical contains a silicon compound A represented by the general formula: R1aSi(H)bX4-a-b and an acid A, the acid A being at least one selected from the group consisting of trimethylsilyl trifluoroactate, trimethylsilyl trifluoromethanesulfonate, dimethylsilyl trifluoroactate, dimethylsilyl trifluoromethanesulfonate, butyldimethylsilyl trifluoroactate, butyldimethylsilyl trifluoromethanesulfonate, hexyldimethylsilyl trifluoroacetate, hexyldimethylsilyl trifluoromethanesulfonate, octyldimethylsilyl trifluoroactate, octyldimethylsilyl trifluoromethanesulfonate, decyldimethylsilyl trifluoroacetate and decyldimethylsilyl trifluoromethanesulfonate.
Owner:CENT GLASS CO LTD
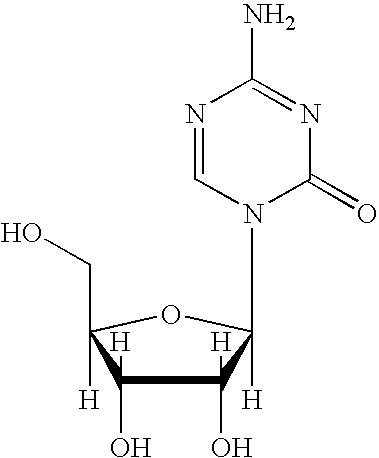
Synthesis of 5-Azacytidine
InactiveUS20060247432A1Amenable to scale-upAvoiding hydrolysis of the s-triazine ringSugar derivativesCarbohydrate active ingredientsTrimethylsilyl trifluoromethanesulfonateFuran
The present invention provides a method for the preparation of 5-azacytidine, wherein 5-azacytidine is represented by the structure: The method involves the silylation of 5-azacytosine, followed by the coupling of silylated 5-azacytosine to a protected β-D-ribofuranose derivative. The coupling reaction is catalyzed by trimethylsilyl trifluoromethanesulfonate (TMS-Triflate).
Owner:PHARMION
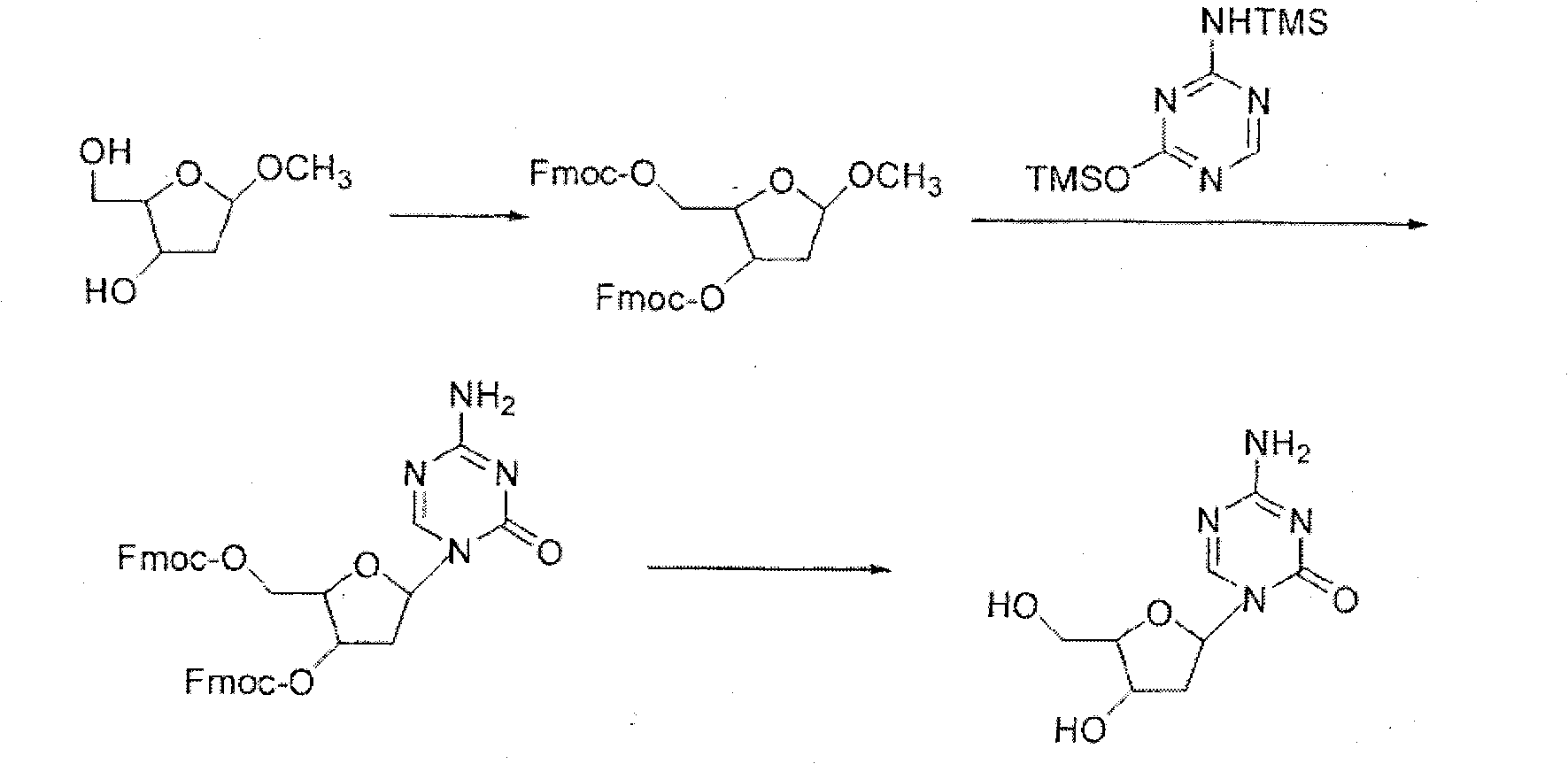
Decitabine synthesis and industrial production method
ActiveCN102827224AAvoid the problem of excessive heavy metalsControl volume and reaction conditionsSugar derivativesSugar derivatives preparationGlucosideMethanol
The invention relates to a decitabine synthesis and industrial production method, which comprises the following steps of: using 2-Deoxy-D-ribose as a raw material, performing a reaction between the raw material and methanol to obtain glucoside, protecting 3,5-dihydroxy by the use of 9-fluorenylmethyloxycarbonyl, reacting with hydrogen chloride to obtain 1-chlorflurecol sugar, performing a reaction between 1-chlorflurecol sugar and silanized 5-azacytosine, carrying out deprotection, and refining to obtain decitabine. The invention is characterized in that stannic chloride is not required during the condensation reaction between 1-chlorflurecol sugar and silanized 5-azacytosine so as to avoid the problem of excessive contents of heavy metals in pharmaceutical materials; and simultaneously the amount of trimethylsilyl trifluoromethanesulfonate and reaction conditions are controlled so as to increase the body burden of beta in the product.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for preparing 2-C-acetonyl-2-deoxy-D-galactopyranose and derivatives of 2-C-acetonyl-2-deoxy-D-galactopyranose
InactiveCN102070676AReduce pollutionEasy to operateSugar derivativesSugar derivatives preparationMethyl groupPyran
The invention belongs to the technical field of organic chemistry and pharmaceutical chemistry, and particularly relates to a method for preparing 2-C-acetonyl-2-deoxy-D-galactopyranose and derivatives of the 2-C-acetonyl-2-deoxy-D-galactopyranose. In the method, a 1,2-deoxy-7-C-acetyl-alpha-D-galactopyranose derivative reacts with glycosylated acceptors of alcohol, thioalcohol, amino acid derivatives, monosaccharide derivatives and the like in the presence of one of trimethylsilyl trifluoromethanesulfonate, boron trifluoride ether, anhydrous aluminum trichloride, anhydrous bismuth trichloride and anhydrous zinc chloride to generate the 2-C-acetonyl-2-deoxy-D-galactopyranose and the derivatives thereof. The method has the advantages of mild reaction condition, good reaction selectivity, high yield and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Preparation method of Cangrelor intermediate
InactiveCN106008632AReduce processing costsLow costSugar derivativesSugar derivatives preparationThioureaNitration
The invention discloses a preparation method of a Cangrelor intermediate. The preparation method comprises the following steps of enabling ethyl cyanoacetate and thiourea to perform closed-loop reaction to generate a product under the alkaline condition, reacting the product and trifluoropropane under the alkaline condition, performing nitration reaction and reduction reaction, and performing closed-loop reaction with formic acid; chlorinating the generated product, and performing condensation reaction on the product and 2-(thiomethyl)ethylamine under the alkaline condition; reacting the obtained product and 1,2,3,5-tetraacetyl-beta-D-ribofuranose under the actions of alkylating agent and TMSOTF (trimethylsilyl trifluoromethanesulfonate), and hydrolyzing the product under the alkaline condition, so as to obtain the Cangrelor intermediate. The preparation method has the advantages that the silica-gel column chromatography is not needed, so that the technology cost is greatly reduced; the carbon disulfide, fuming nitric acid or concentrated sulfuric acid is not used in the preparation process, so that any danger is avoided; the noble metal hydrogenating reducing agent is not needed, so that the cost is reduced, and the operation danger is decreased; the operation is easy, safe and reliable, and the preparation method is suitable for large-scale industrial production.
Owner:北京信益泰医药科技开发有限公司
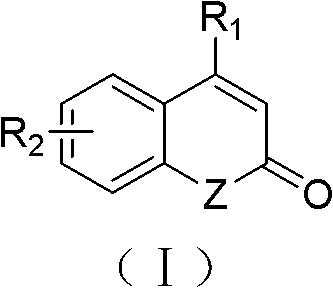
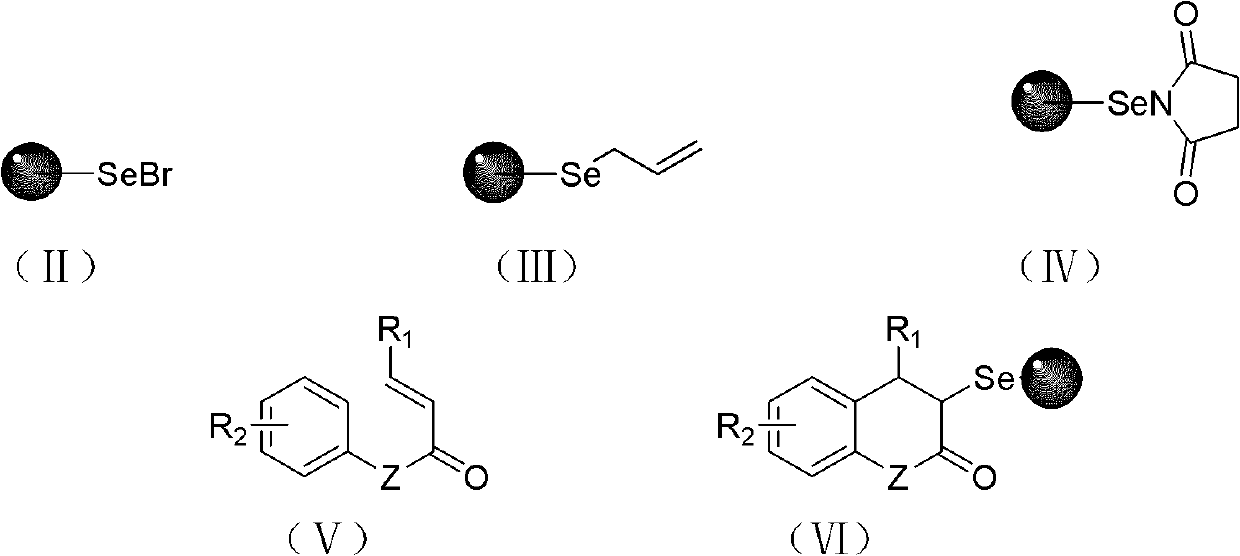
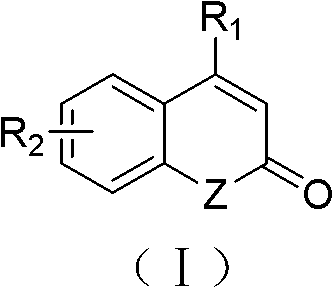
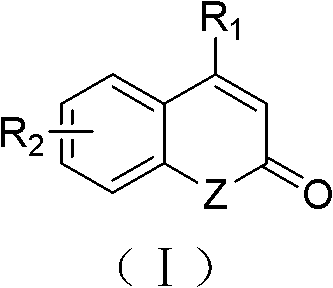
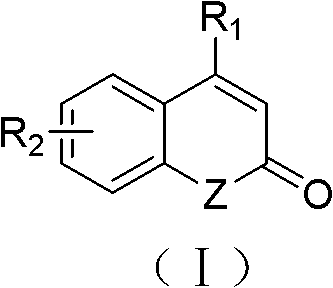
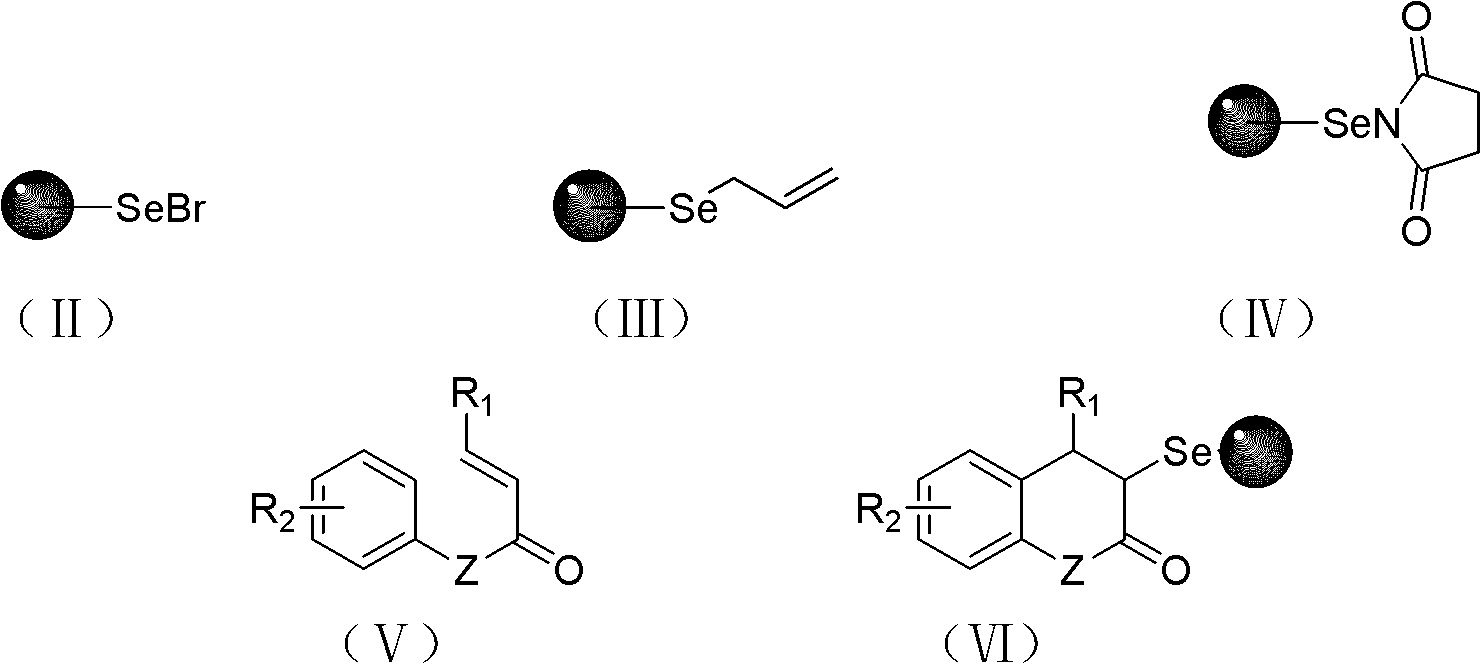
Solid-phase synthesis method of coumarin and analogue thereof
InactiveCN102532015AHigh yieldOvercome the disadvantage of low yield of cyclization reactionOrganic chemistryPolystyreneKetone
The invention relates to a solid-phase synthesis method of coumarin and an analogue (I) thereof and belongs to the field of organic chemistry. The method comprises the following steps: 1) taking 1% of cross-linked polystyrene resin as a carrier to prepare a polystyrene-supported seleno-succimide reagent (III); 2) using the III to induce phenyl acrylate (V) to perform intramolecular cyclization under the catalysis of trimethylsilyl trifluoromethanesulfonate so as to form 3-polystyrene-supported seleno-3,4-dihydro-benzopyran-2-ketone (VI); and 3) performing oxidation elimination on the VI via an oxidant so as to directly get the coumarin (I) without further separation. When the phenyl acrylate (V) is replaced by N-phenyl acrylamide, the analogue of the coumarin, namely a 2-quinolone compound, can be prepared through the same steps. The solid-phase synthesis method disclosed by the invention has the advantages of easily available raw materials, good product yield, high purity, simplicityand convenience in operation, simple post-treatment and great industrial application prospects.
Owner:YUNNAN UNIV
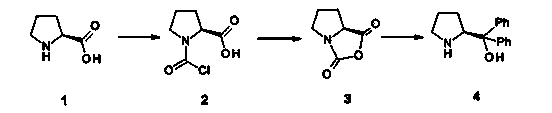
Synthetic method of (R)-2-methyl-4-nitro-1-butanol
InactiveCN103483201AIncrease splitLow costOrganic chemistryOrganic compound preparationTrimethylsilyl trifluoromethanesulfonatePyrrolidine
The invention discloses a synthetic method of (R)-2-methyl-4-nitro-1-butanol. The method comprises the following steps that firstly, (S)-alpha, alpha-diphenyl-pyrrolidinemethanol is reacted with trimethylsilyl trifluoromethanesulfonate under the catalyzing action of triethylamine to obtain (S)-2-(1, 1-diphenyl-1-trimethylsilanolate) methyl pyrrolidine, and secondly, (S)-2-(1, 1-diphenyl-1- trimethylsilanolate) methyl pyrrolidine is reacted with acetaldehyde, 3-nitrobenzoic acid and nitro ethylene in a mixing mode to obtain (S)-2-methyl-4-nitro n-butanal, and the (S)-2-methyl-4-nitro n-butanal is reduced to obtain (R)-2-methyl-4-nitro-1-butanol. By means of the method, the (R)-2-methyl-4-nitro-1-butanol can be obtained directly instead of racemate, the racemate is prevented from being resolved, cost is saved, and the yield is 50%.
Owner:CHEMFUTURE PHARMATECH JIANGSU
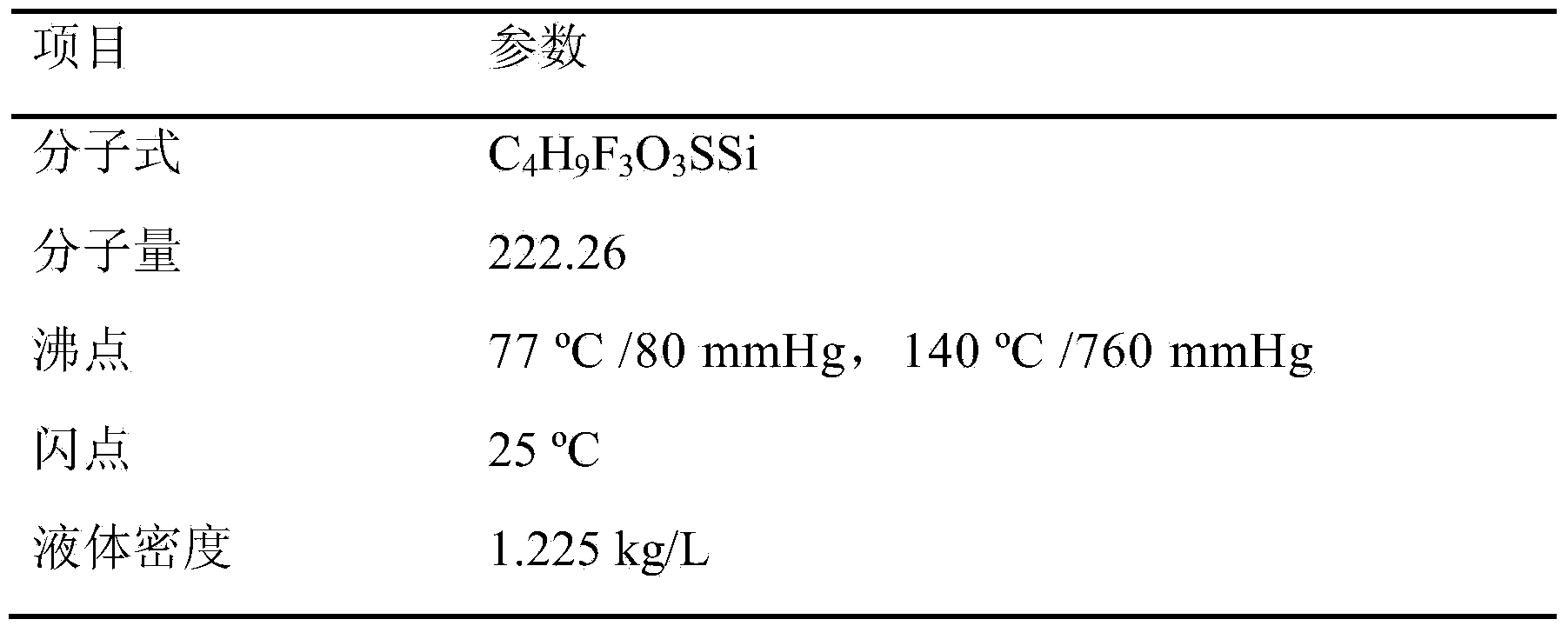
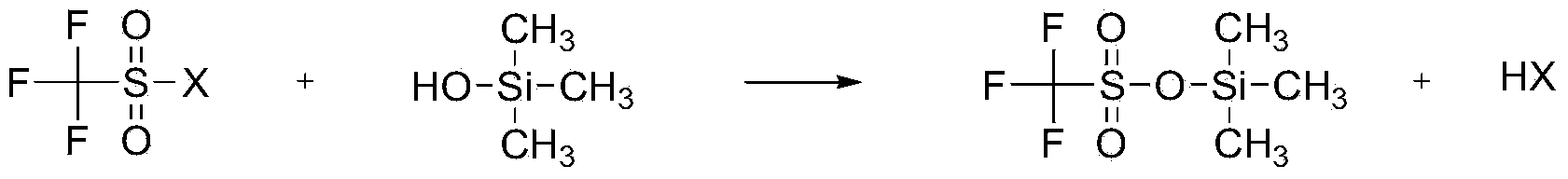
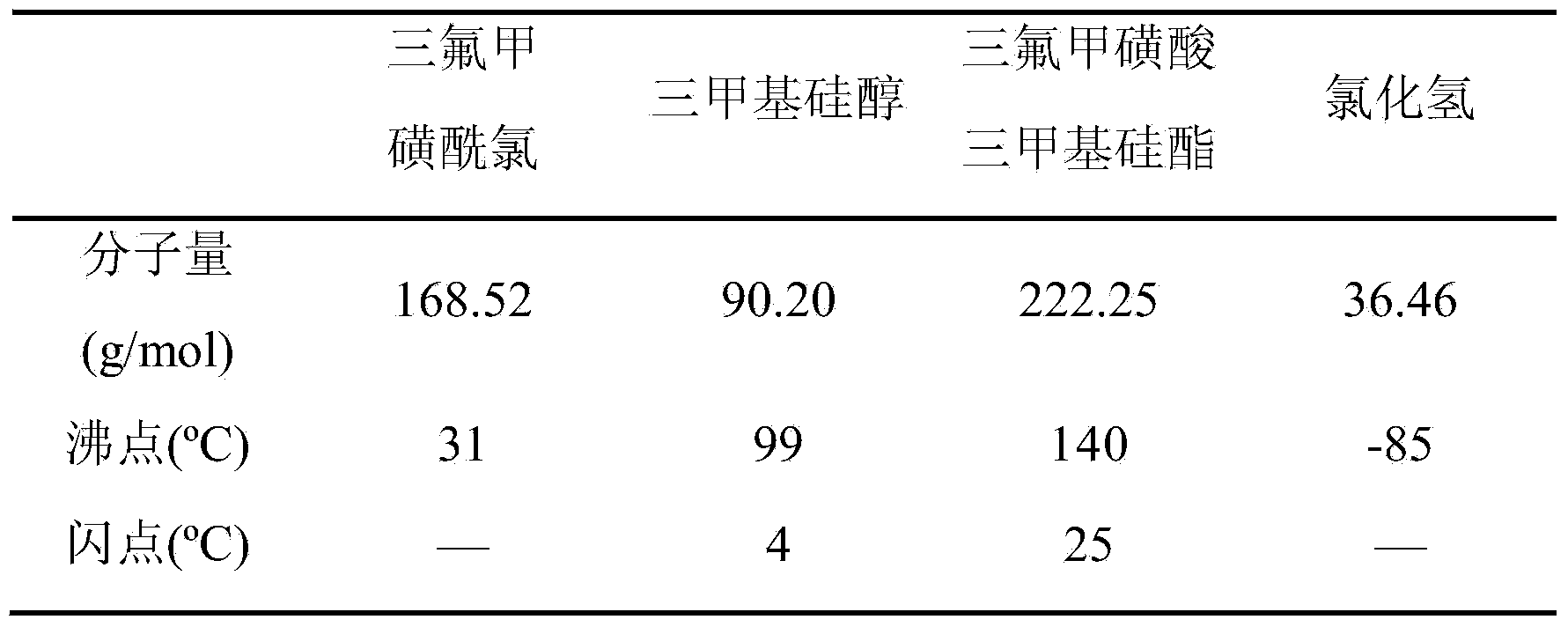
Preparation method of Trimethylsilyl trifluoromethanesulfonate
ActiveCN103665017AHigh quality contentLow mass contentSilicon organic compoundsTrimethylsilyl trifluoromethanesulfonateAlkyl transfer
The invention relates to a preparation method of Trimethylsilyl trifluoromethanesulfonate, and belongs to the field of a fine chemical industry. The preparation method comprises the following specific steps: a compound of which the general formula is CF3SO2X reacts with hydroxytrimethylsilane, wherein X is Cl, F or OSO2CF3, and hydroxytrimethylsilane is used as a silicon alkylation reagent of the reaction; the reduced pressure distillation is carried out on a reaction mixture after the reaction is finished to collect Trimethylsilyl trifluoromethanesulfonate of which the mass content is higher than 99.0%, wherein the mole ratio of the CF3SO2X to the hydroxytrimethylsilane in the reaction is (1.00: 0.80) to (1.00: 1.60), the reaction time is 1 to 10 hours, the reduced pressure distillation is carried out on the reaction mixture after the reaction is finished, and the pressure is 0.005 to 0.030MPa.
Owner:PERIC SPECIAL GASES CO LTD
Chemical solution for formation of protective film
ActiveCN102934207AExcellent water repellencyReduced capillary forceSemiconductor/solid-state device manufacturingPhotosensitive material processingTectorial membraneTrimethylsilyl trifluoromethanesulfonate
Disclosed is a chemical solution for forming a water-repellent protective film (10) on at least the surfaces of concaved parts in a fine concave-convex pattern (2) that is formed on the surface of a wafer (1) containing a silicon atom in at least a part thereof, during the washing of the wafer (1). The chemical solution comprises a silicon compound (A) represented by the general formula: R1 aSi(H)bX4-a-b and an acid (A), wherein the acid (A) comprises at least one compound selected from the group consisting of trimethylsilyltrifluoroacetate, trimethylsilyltrifluoromethanesulfonate, dimethylsilyl- trifluoroacetate, dimethylsilyltrifluoromethanesulfonate, butyldimethylsilyltrifluoroacetate, butyldimethyl- silyltrifluoromethanesulfonate, hexyldimethylsilyl- trifluoroacetate, hexyldimethylsilyltrifluoro- methanesulfonate, octyldimethylsilyltrifluoroacetate, octyldimethylsilyltrifluoromethanesulfonate, decyldimethyl- silyltrifluoroacetate and decyldimethylsilyl- trifluoromethanesulfonate.
Owner:CENT GLASS CO LTD
Novel technology for synthesis of capecitabine
InactiveCN103288905ASuitable for industrialized mass productionHigh yieldSugar derivativesSugar derivatives preparationSodium methoxideMeth-
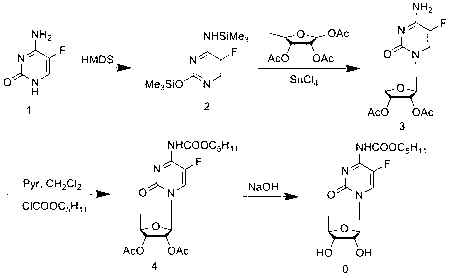
The invention relates to a novel technology for synthesis of capecitabine. The technology is characterized in that: 5-fluorocytosine protected by trimethyl silicon is taken as raw material; and the capecitabine is obtained after condensation, esterification and deacetylation. The Reaction sequence is more economically reasonable, the synthetic route is short, the cost is low, the operation is simplified, the yield is high, the synthetic period is short, the quality of intermediates can be controlled, solvents used in reaction are few, pollution to the environment is little, and the technology is suitable for industrial production. Comparing the technology with the prior art for capecitabine production, trimethylsilyl trifluoromethanesulfonate (TMSOTf) which replaces a heavy metal agent stannic chloride is used as a condensing agent for glycosylation (condensation), and a sodium methoxide / methanol system replaces an ammonia gas / methanol system for deacetylation, so that the production yield is increased, and heavy metal residues of the products and the environmental pollution are reduced. The overall yield of the technology of the invention reaches 59%, the purity of the production is high and meets the standards of the United States Pharmacopeia.
Owner:北京博时安泰科技发展有限公司
Preparation method of 2-C-acetonyl-2-deoxy-glucoside compounds
InactiveCN101591363AReduce pollutionEasy to operateEsterified saccharide compoundsSugar derivativesMethylene DichlorideReaction temperature
The invention belongs to the fields of organic chemistry and pharmacochemistry, in particular to a preparation method of 2-C-acetonyl-2-deoxy-glucoside compounds. The preparation method comprises the following steps: sequentially adding 1, 2-deoxy-7-C-acetyl-alpha-D-glucopyranose derivatives, receptors, such as alcohol, and solvents, such as methylene dichloride into the same reactor under the protection of nitrogen, controlling the reaction temperature between -20 DEG C and the room temperature, and adding catalysts, such as trimethylsilyl triflate to generate 2-C-acetonyl-2-deoxy-beta-D-glucopyranose compounds through reaction. The preparation method has the advantages of simple operation, mild reaction condition, good stereoselectivity and less environment pollution.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Liquid chemical for forming protecting film
ActiveUS9228120B2Improve waterproof performanceReduce capillary forceOrganic detergent compounding agentsNon-ionic surface-active compoundsTrimethylsilyl trifluoromethanesulfonateCompound a
Disclosed is a liquid chemical for forming a water-repellent protecting film at least on a surface of a recessed portion of an uneven pattern at the time of cleaning a wafer having a finely uneven pattern at its surface and containing silicon at least a part of the uneven pattern. This liquid chemical contains a silicon compound A represented by the general formula: R1aSi(H)bX4-a-b and an acid A, the acid A being at least one selected from the group consisting of trimethylsilyl trifluoroactate, trimethylsilyl trifluoromethanesulfonate, dimethylsilyl trifluoroactate, dimethylsilyl trifluoromethanesulfonate, butyldimethylsilyl trifluoroactate, butyldimethylsilyl trifluoromethanesulfonate, hexyldimethylsilyl trifluoroacetate, hexyldimethylsilyl trifluoromethanesulfonate, octyldimethylsilyl trifluoroactate, octyldimethylsilyl trifluoromethanesulfonate, decyldimethylsilyl trifluoroacetate and decyldimethylsilyl trifluoromethanesulfonate.
Owner:CENT GLASS CO LTD
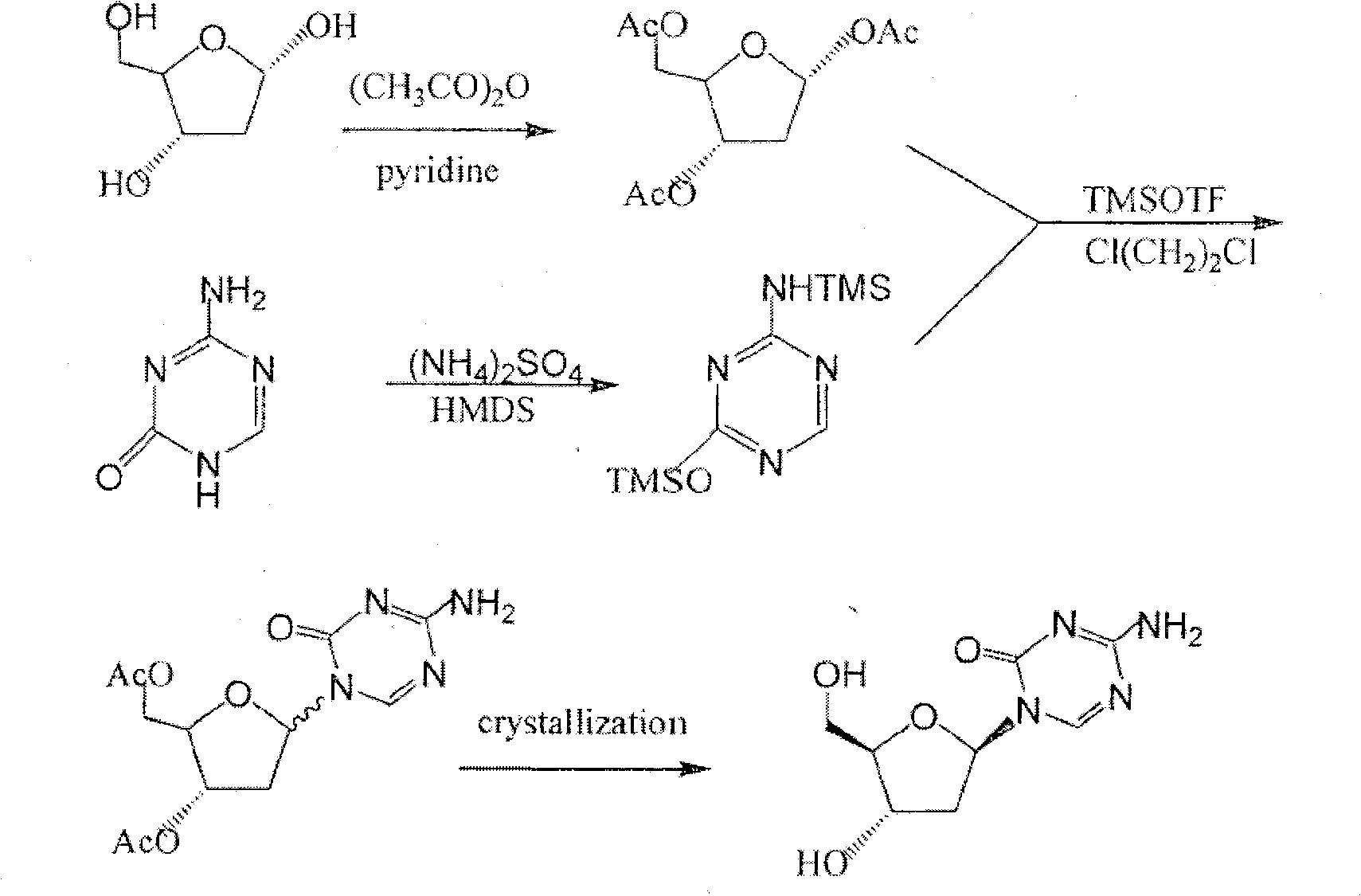
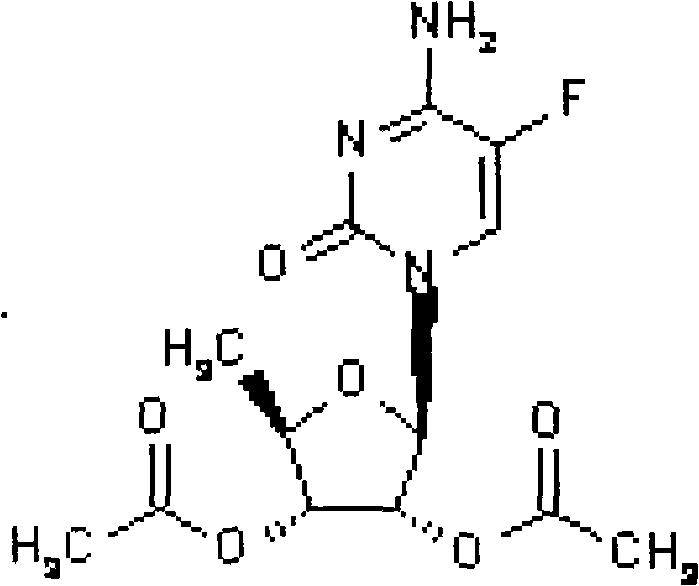
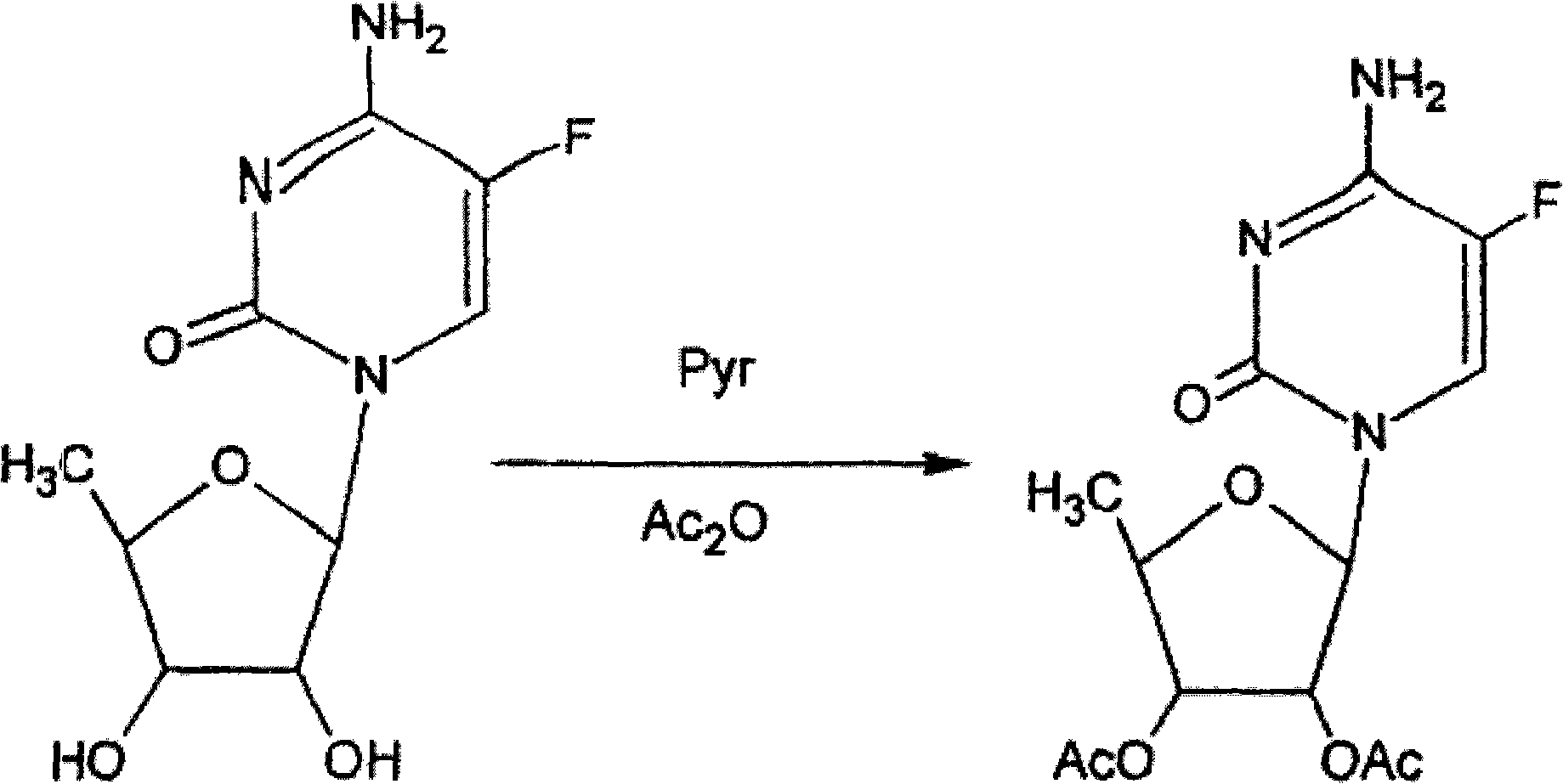
Preparation method of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine
InactiveCN102250175ASugar derivativesSugar derivatives preparationTrimethylsilyl trifluoromethanesulfonate5-fluorocytidine
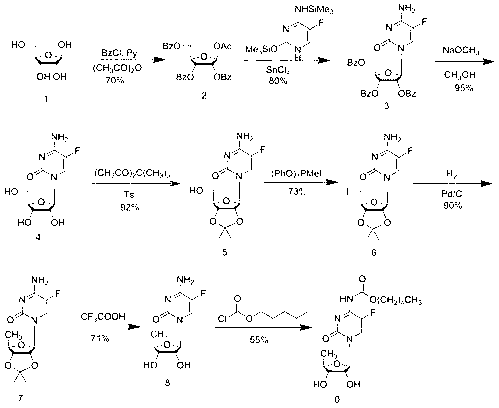
The invention provides a preparation method of capecitabine intermediate 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, which is suitable for actual industrial big production and has the advantages of high yield and quality and fine stability. The preparation method of capecitabine intermediate comprises the step of innovatively using a trifluoromethanesulfonic acid trimethylsilyl ester catalyst as the silanization agent of 5-fluorocytosine. The method has the advantages of high yield and good quality, and the process is simple and easy to operate.
Owner:NANJING VARSAL MEDICINE TECH DEV
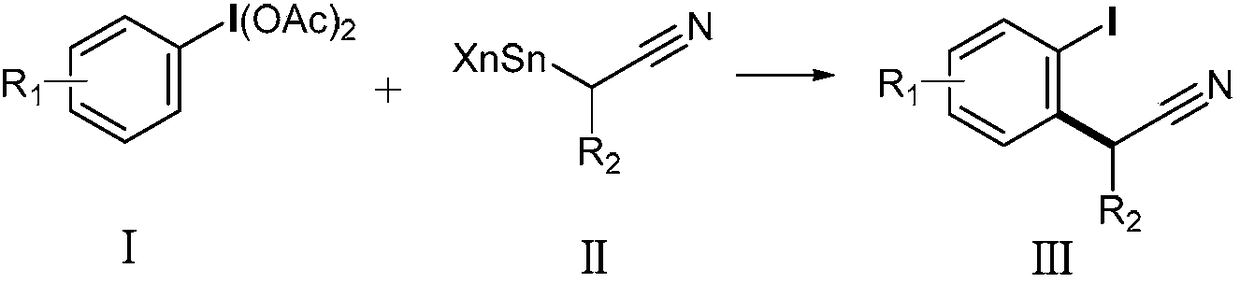
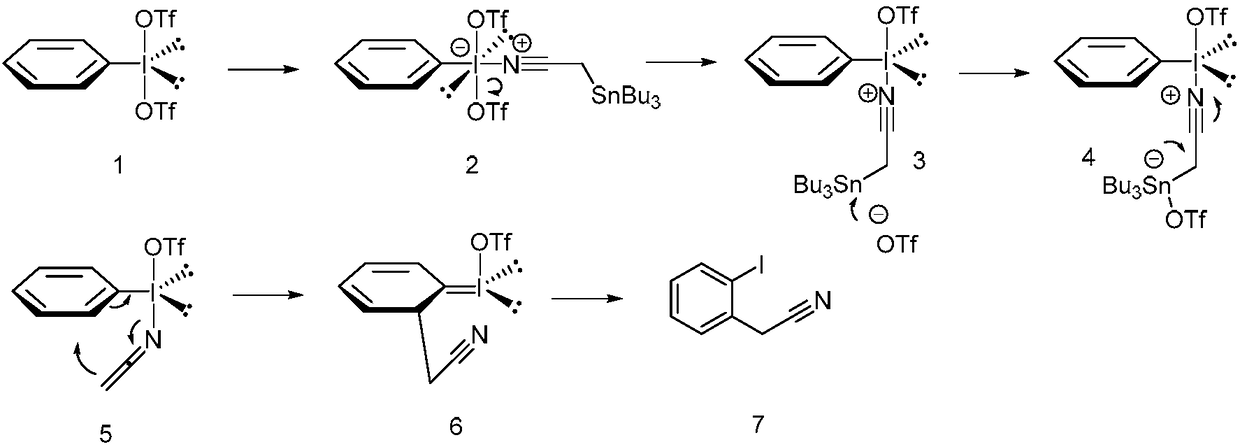
Method for preparing alpha-aryl nitrile compound
ActiveCN108409602AEasy to separateMild reaction conditionsGroup 4/14 element organic compoundsCarboxylic acid nitrile preparationArylTrimethylsilyl trifluoromethanesulfonate
The invention discloses a method for preparing an alpha-aryl nitrile compound, and the method comprises rearrangement reaction of a diacetoxyl aryl iodide represented by the formula (I) and an alpha-tin-substituted nitrile compound represented by the formula (II) in the presence of trimethylsilyl trifluoromethanesulfonate to synthesize the alpha-aryl nitrile compound represented by the formula (III). The method has mild reaction conditions, good selectivity, high yield, easy separation of products and simple operation and other advantages.
Owner:ZHEJIANG NORMAL UNIVERSITY
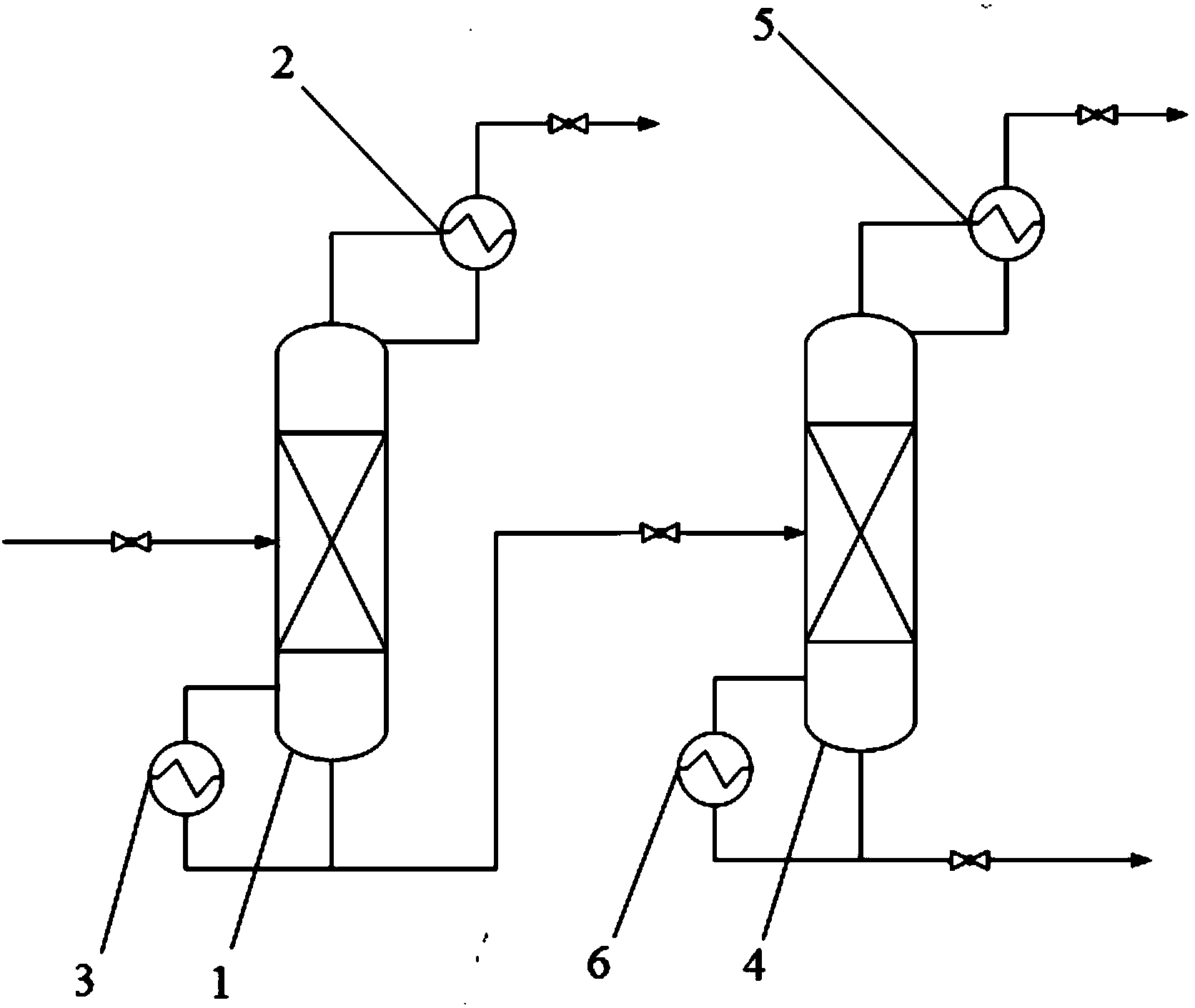
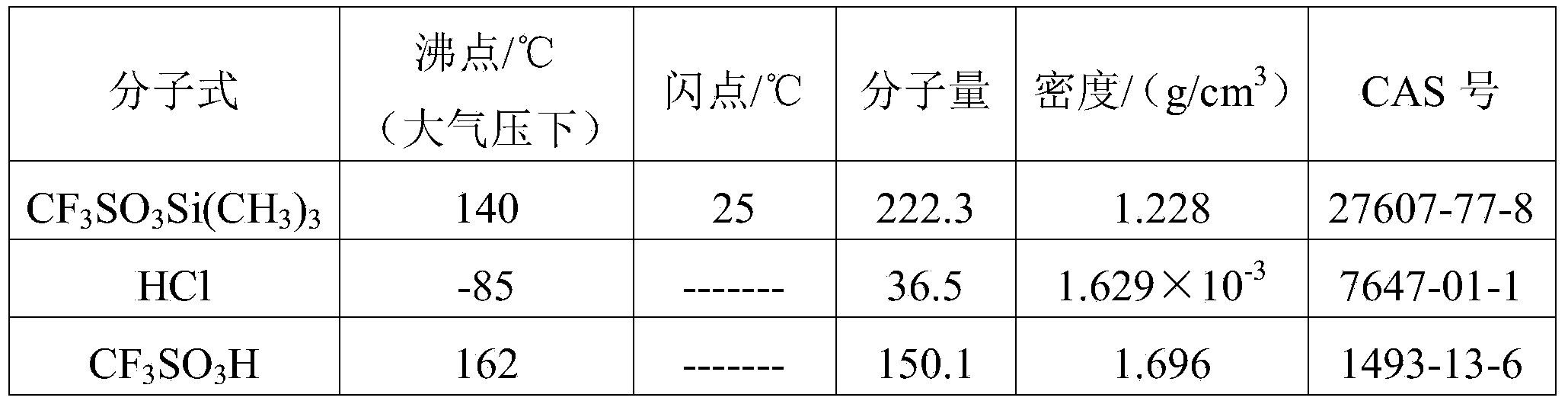
TMSOTf (trimethylsilyl trifluoromethanesulfonate) purifying method
InactiveCN104262376AEfficient and safe removalComplete recycling measuresSilicon organic compoundsRefluxTrimethylsilyl trifluoromethanesulfonate
The invention relates to a TMSOTf (trimethylsilyl trifluoromethanesulfonate) purifying method and belongs to the technical field of fine chemical engineering. The method comprises steps as follows: HCL is removed in an HCL-removing rectifying tower under conditions that the pressure of a tower kettle ranges from -0.098 MPa to -0.002 MPa, the temperature of the tower kettle ranges from 40 DEG C to 138 DEG C, the temperature of the tower top ranges from 20 DEG C to 120 DEG C, the feed quantity ranges from 5 m<3> / h to 500 m<3> / h, and the reflux ratio ranges from 200 to 2000; then CF3SO3H is removed in a CF3SO3H-removing rectifying tower under conditions that the pressure of a tower kettle ranges from -0.099 MPa to -0.008 MPa, the temperature of the tower kettle ranges from 20 DEG C to 100 DEG C, the temperature of the tower top ranges from 0 DEG C to 80 DEG C, and the reflux ratio ranges from 200 to 1000; the temperature of each tower top is lower than that of each tower kettle. The energy consumption of the method is obviously lower than that of a repeated distillation method, impurity recovery measures are complete, impurity gases, namely, the CF3SO3H and the HCL, can be removed in an efficient, low-cost and pollution-free manner, and purer TMSOTf can be obtained.
Owner:718TH RES INST OF CHINA SHIPBUILDING INDAL CORP
Method for stereoselective preparation of derivatives of pyrane
InactiveCN102180923AReduce pollutionEasy to operateBiocideSugar derivativesReaction temperatureSolvent
The invention belongs to the technical field of organic chemistry and pharmaceutical chemistry, and in particular relates to a method for stereoselective preparation of derivatives of pyrane. The method comprises the following steps of: under the protection of inert gases, adding derivatives of 1-p-tolylthio-2-C-glyoxyl-alpha-D-glucopyranose, derivatives of 1-thiophenyl-2-C- glyoxyl-alpha-D-glucopyranose or derivatives of 1-ethylthio p-tolyl-2-C-glyoxyl-alpha-D-glucopyranose, which serve as reactants, into a reactor, adding alcohol, phenol, trimethylsilyl azide or monosaccharide derivatives, adding a solvent such as methylene chloride to dissolve the raw materials, and performing the reaction at the reaction temperature of between 40 DEG C below zero and room temperature in the presence of a catalyst which is N-iodosuccinimide, or N-bromosuccinimide, or copper bromide and tetrabutylammonium bromide, or iodine, bromine simple substance or N-iodosuccinimide and trimethylsilyl triflate, or trifluoromethanesulfonic silver, or paratoluenesulfonic acid or trifluoromethanesulfonic acid to obtain the derivatives of pyrane. The method has the advantages of simple operation, high selectivity, low cost, light environmental pollution and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Method for preparing decitabine
InactiveCN101712708ARaw materials are easy to getThe total yield of the preparation process is highSugar derivativesAntineoplastic agentsTrimethylsilyl trifluoromethanesulfonateDecitabine
The invention discloses a method for preparing decitabine. The method comprises the following steps of: using 2-deoxy-D-ribose as a raw material; protecting the raw material by a pivaloyl group, and then reacting the raw material with alkylated 5-azacytosine under the action of a proper amount of a catalyst namely trimethylsilyl trifluoromethanesulfonate; performing hydrolysis to prepare a crude product of the decitabine without purification by a chromatography column; and performing recrystallization to prepare pure decitabine under the condition of a proper solvent. The method for preparing the decitabine greatly improves the total yield, greatly reduces the product cost, and is more suitable for large-scale industrial production.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
Uracil nucleoside derivative and method for preparing doxifluridine medicine by using uracil nucleoside derivative
ActiveCN111072734AMild reaction systemEfficient responseSugar derivativesSugar derivatives preparationBenzoic acidFormic Acid Esters
The invention discloses a 5-deoxy-D-ribofuranose 1-[2-(1-styryl) benzoate] derivative as shown in a general formula (I) and a preparation method of the derivative, wherein the structure of the generalformula (I) is as follows, and the invention discloses a method for preparing a uracil nucleoside derivative and an antitumor drug doxifluridine by taking the derivative as shown in the general formula (I) as a raw material. The 5-deoxy-D-ribofuranose 1-[2-(1-styryl) benzoate] derivative used as a reaction raw material can be activated as a glycosyl donor under the condition of a catalytic amountof lewis acid trimethylsilyl trifluoromethanesulfonate and N-iodosuccinimide. The method avoids traditional use of equivalent or excessive lewis acid, has a mild reaction system, has no other side reaction, has efficient reaction, and has a yield up to 98%.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
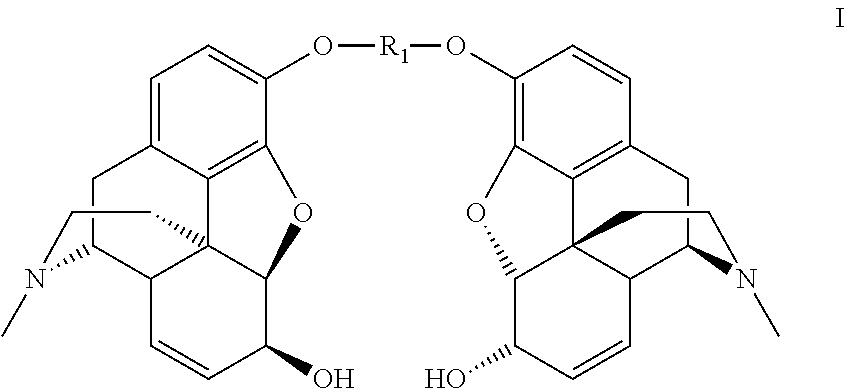
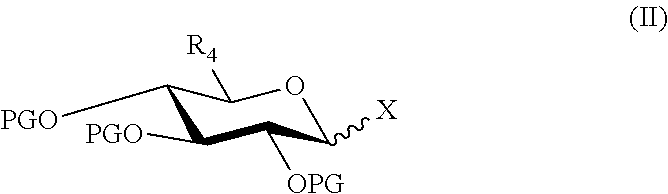
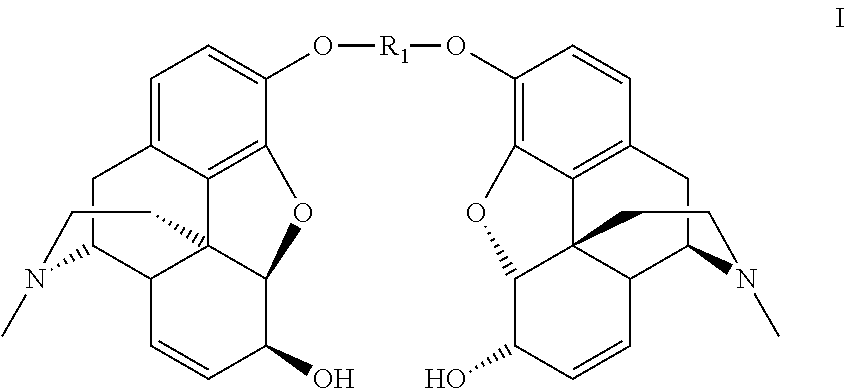
Synthesis of morphine-6-glucuronide or one of the derivatives thereof
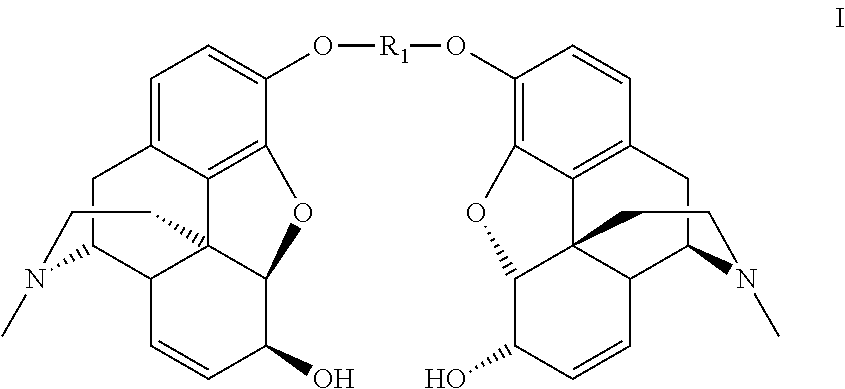
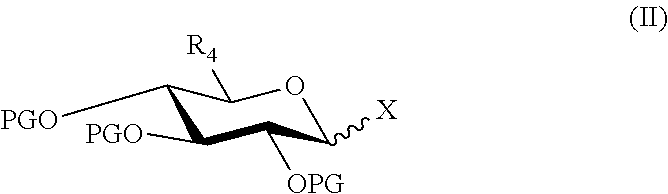
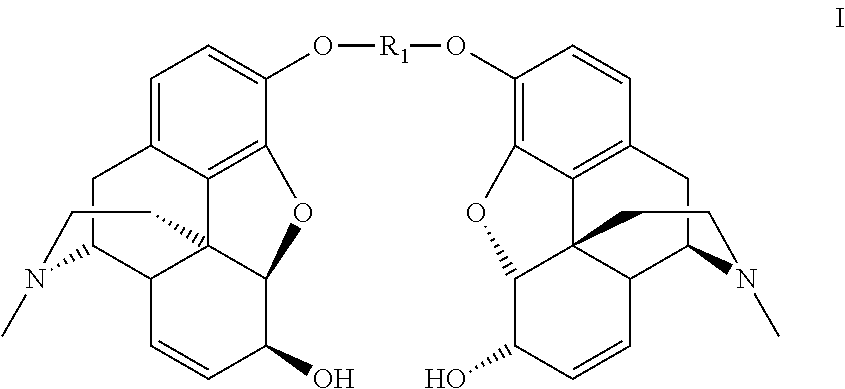
InactiveUS8258298B2High stereoselectivityNervous disorderOrganic chemistryTrimethylsilyl trifluoromethanesulfonateAromatic solvent
The disclosure relates to a method for preparing morphine-6-glucuronide or one of the deritives thereof comprising reacting a compound of formula (I):wherein R1 is as defined in the disclosure,with a glucuronic acid derivative of formula (II):wherein PG, X, and R4 are as defined in the disclosure,in the presence of an aromatic solvent and trimethylsilyl trifluoromethanesulfonate;(ii) reacting the product obtained in step (i) with a strong basic agent; and then (iii) recovering the product obtained in step (ii).
Owner:SANOFI SA
Preparation method of Cangrelor intermediate
InactiveCN106008633AReduce processing costsLow costSugar derivativesSugar derivatives preparationThioureaNitration
The invention discloses a preparation method of a Cangrelor intermediate. The preparation method comprises the following steps of enabling ethyl cyanoacetate and thiourea to perform closed-loop reaction to generate a product under the alkaline condition, reacting the product and trifluoropropane under the alkaline condition, performing nitration reaction and reduction reaction, and performing closed-loop reaction with formic acid; chlorinating the generated product, and performing condensation reaction on the product and 2-(thiomethyl)ethylamine under the alkaline condition; reacting the obtained product and 1,2,3,5-tetraacetyl-beta-D-ribofuranose under the actions of alkylating agent and TMSOTF (trimethylsilyl trifluoromethanesulfonate), and hydrolyzing the product under the alkaline condition, so as to obtain the Cangrelor intermediate. The preparation method has the advantages that the silica-gel column chromatography is not needed, so that the technology cost is greatly reduced; the carbon disulfide, fuming nitric acid or concentrated sulfuric acid is not used in the preparation process, so that any danger is avoided; the noble metal hydrogenating reducing agent is not needed, so that the cost is reduced, and the operation danger is decreased; the operation is easy, safe and reliable, and the preparation method is suitable for large-scale industrial production.
Owner:北京广博德赛医药技术开发有限责任公司 +1
Preparation method of trimethylsilyl trifluoromethanesulfonate
InactiveCN103588803AHigh purityHigh yieldSilicon organic compoundsTrimethylsilyl trifluoromethanesulfonateDistillation
The invention discloses a preparation method of trimethylsilyl trifluoromethanesulfonate, which comprises the following steps: adding anhydrous trifluoromethanesulfonic acid and anhydrous trimethylchlorosilane into a reaction vessel, and reacting at 10-30 DEG C for 12 hours while stirring under an inert gas protective atmosphere; and after the reaction is finished, performing reduced pressure distillation for 1-2 hours, and collecting the distillate of 125-135 DEG C, which is the trimethylsilyl trifluoromethanesulfonate. According to the method, no side reaction exists in the preparation process; the product is high in purity and yield and low in cost; and the purity of the product is up to 99.9%, and the yield of the product is up to 97.8%.
Owner:QINGDAO WINCHANCE TECH
Synthesis of morphine-6-glucuronide or one of the derivatives thereof
InactiveUS20110275820A1High stereoselectivityNervous disorderOrganic chemistryTrimethylsilyl trifluoromethanesulfonateAromatic solvents
The disclosure relates to a method for preparing morphine-6-glucuronide or one of the deritives thereof comprising reacting a compound of formula (I):wherein R1 is as defined in the disclosure,with a glucuronic acid derivative of formula (II):wherein PG, X, and R4 are as defined in the disclosure,in the presence of an aromatic solvent and trimethylsilyl trifluoromethanesulfonate;(ii) reacting the product obtained in step (i) with a strong basic agent; and then (iii) recovering the product obtained in step (ii).
Owner:SANOFI SA
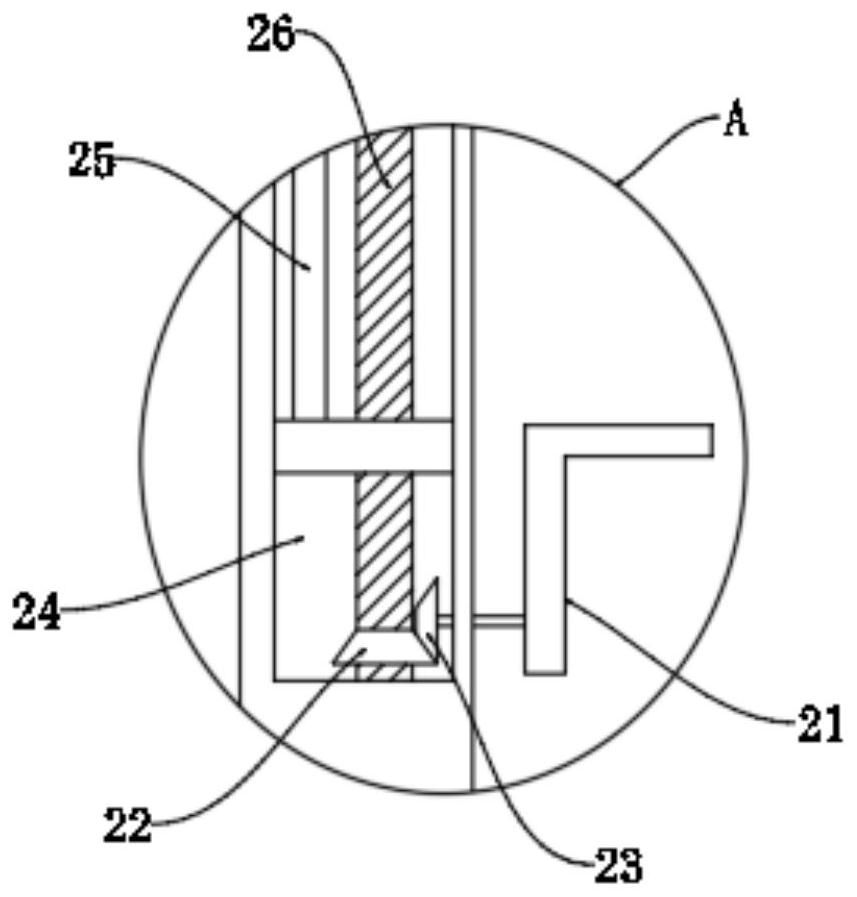
Method and equipment for producing trimethylsilyl trifluoromethanesulfonate
ActiveCN112094289AHigh purityLess impuritiesSilicon organic compoundsChemical/physical/physico-chemical stationary reactorsTrimethylsilyl trifluoromethanesulfonateTrimethylsilyl chloride
The invention relates to a method and equipment for producing trimethylsilyl trifluoromethanesulfonate, which belongs to the technical field of trimethylsilyl trifluoromethanesulfonate production. Themethod comprises the following steps of adding trifluoromethanesulfonic acid into a reaction kettle, dropwisely adding trimethylchlorosilane, reacting at 20-30 DEG C under 0.002-0.003 MPa for 8.5-10hours, carrying out reduced pressure distillation, and collecting 125-135 DEG C fractions, thereby obtaining the trimethylsilyl trifluoromethanesulfonate. The equipment comprises a reaction kettle anda quantitative tank, a fixed plate is fixed in the reaction kettle, a rotating rod is rotatably connected to the fixed plate, a motor fixed to the rotating rod is installed at the bottom of the reaction kettle, a stirring mechanism is arranged on the outer side of the rotating rod, and a titration opening is formed in the top of the reaction kettle and connected with a titration mechanism in a liquid inlet pipe at the bottom of the quantitative tank; astrip-shaped cavity is arranged on one side wall of thequantitative tank; a control mechanism connected with the titration mechanism is arranged in the strip-shaped cavity, and an injection port and a nitrogen filling port are formed in the other side wall of the strip-shaped cavity. The equipment can accurately control the trimethylchlorosilane titration speed, and the product purity is higher.
Owner:PERIC SPECIAL GASES CO LTD
Method for preparing loaded solid super acidic catalyst directly by microwave method
ActiveCN102614926AHigh catalytic hydrocracking activityGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationTrimethylsilyl trifluoromethanesulfonatePtru catalyst
The invention relates to a method for preparing a loaded solid super acidic catalyst directly by a microwave method, and belongs to a method for preparing a super acidic catalyst. The method comprises the following steps of: (1) mixing and stirring NaHCO3 and a carbon nano tube in a weight ratio of 1:500 fully, and roasting in a microwave reactor to obtain a Na-loaded catalyst carrier; (2) heating the Na-loaded catalyst carrier by microwave radiation, adding a mixed solution of antimony pentachloride (SbCl5) and trimethylsilyl trifluoromethanesulfonate (F3CSO3Si(CH3)3) dropwise, stirring and immersing to obtain a suspension mixed solution; (3) filtering the suspension mixed solution to obtain a solid precursor and a mixed solution; (4) putting the solid precursor into the microwave reactor, heating by the microwave radiation, and roasting to obtain a solid super acidic catalyst prototype; and (5) performing operation for 1 to 5 times by taking the solid super acidic catalyst prototype as a raw material according to the steps (2), (3) and (4) to obtain the solid-loaded super acidic catalyst. The method has the advantages that: the loaded solid super acidic catalyst prepared by the microwave method is high in catalytic hydrocracking activity and selectivity.
Owner:CHINA UNIV OF MINING & TECH
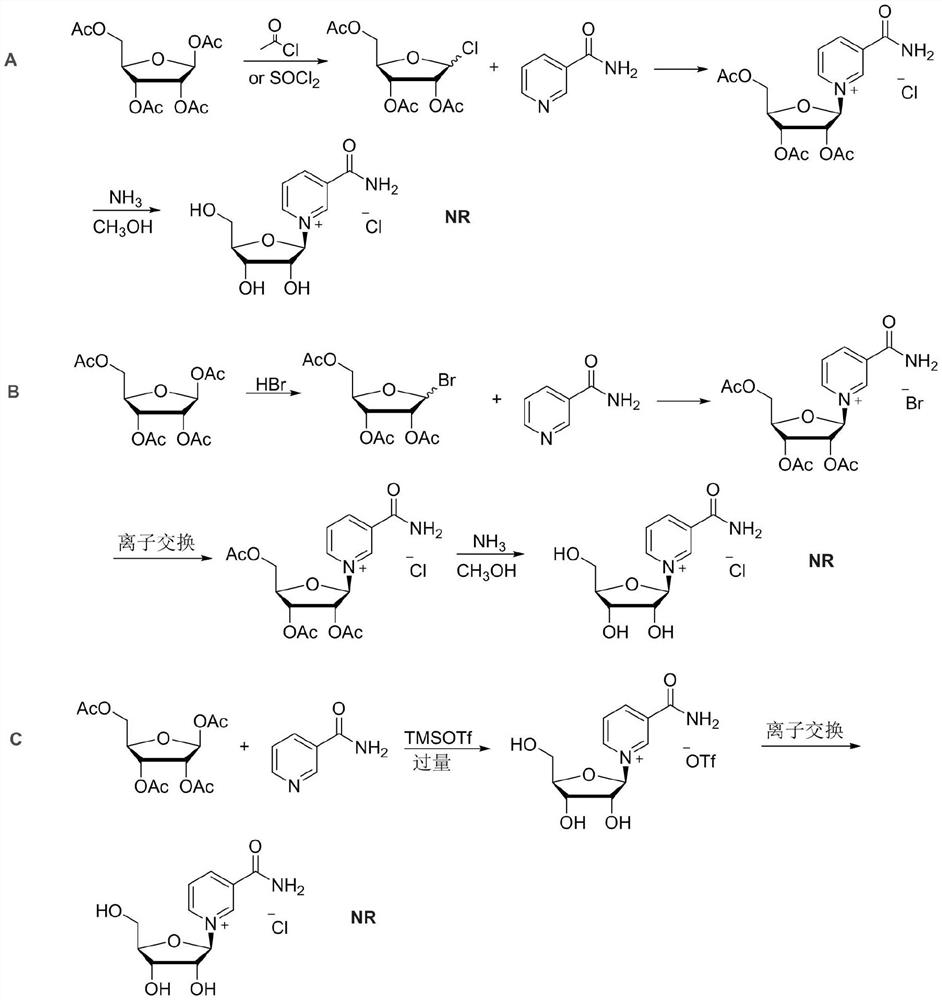
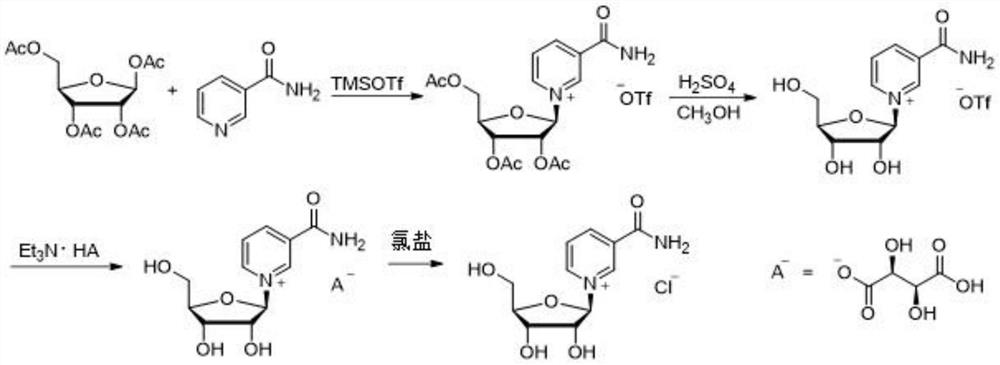
Synthesis method of nicotinamide chloride ribose
PendingCN114369129AImprove conversion rateHigh yieldSugar derivativesOrganic compound preparationChemical synthesisNicotinamide riboside
The invention relates to a synthesis method of chloridized nicotinamide ribose, which comprises the following steps: by taking tetraacetyl ribose as a raw material, activating the tetraacetyl ribose by adopting trimethylsilyl trifluoromethanesulfonate of which the amount is twice that of the tetraacetyl ribose, synthesizing trifluoromethanesulfonic acid acetyl nicotinamide ribose, deprotecting by using sulfuric acid, exchanging trifluoromethanesulfonic acid ions by using triethylamine tartrate, and synthesizing the chloridized nicotinamide ribose by using trimethylsilyl trifluoromethanesulfonate to obtain the chloridized nicotinamide ribose. And finally, reacting with soluble chlorine salt to exchange hydrogen tartrate into chloride ions to synthesize nicotinamide ribose chloride. According to the method, trimethylsilyl trifluoromethanesulfonate is adopted as an activating reagent of nicotinamide, and a sulfuric acid methanol solution is adopted as a deacetylation reagent, so that the high conversion rate of tetraacetyl ribose and the high yield of beta isomer are kept, and the production cost of nicotinamide chloride ribose is reduced. The method is high in yield, low in cost and good in stereoselectivity, residues of ions such as bromide ions and trifluoromethanesulfonate ions harmful to the human body are avoided, and the quality of the chloridized nicotinamide ribose product obtained through a chemical synthesis method is improved.
Owner:NINGBO XINKAI BIOTECH CO LTD
Solid-phase synthesis method of coumarin and analogue thereof
InactiveCN102532015BHigh yieldOvercome the disadvantage of low yield of cyclization reactionOrganic chemistryMeth-Polystyrene
The invention relates to a solid-phase synthesis method of coumarin and an analogue (I) thereof and belongs to the field of organic chemistry. The method comprises the following steps: 1) taking 1% of cross-linked polystyrene resin as a carrier to prepare a polystyrene-supported seleno-succimide reagent (III); 2) using the III to induce phenyl acrylate (V) to perform intramolecular cyclization under the catalysis of trimethylsilyl trifluoromethanesulfonate so as to form 3-polystyrene-supported seleno-3,4-dihydro-benzopyran-2-ketone (VI); and 3) performing oxidation elimination on the VI via an oxidant so as to directly get the coumarin (I) without further separation. When the phenyl acrylate (V) is replaced by N-phenyl acrylamide, the analogue of the coumarin, namely a 2-quinolone compound, can be prepared through the same steps. The solid-phase synthesis method disclosed by the invention has the advantages of easily available raw materials, good product yield, high purity, simplicity and convenience in operation, simple post-treatment and great industrial application prospects.
Owner:YUNNAN UNIV
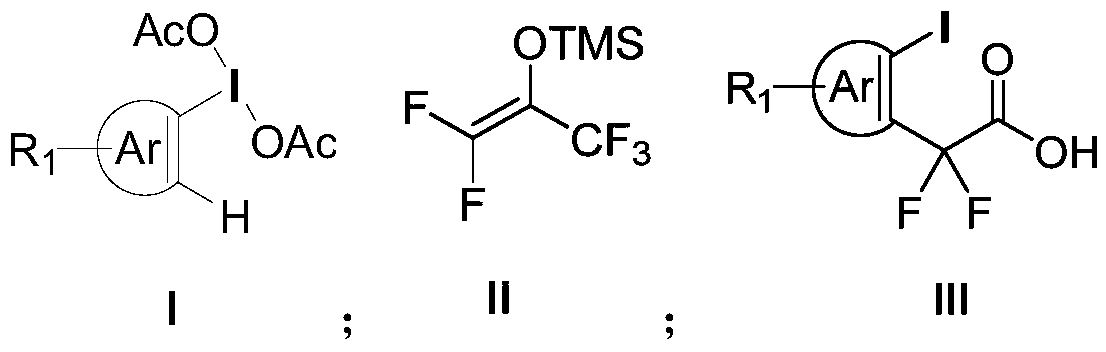
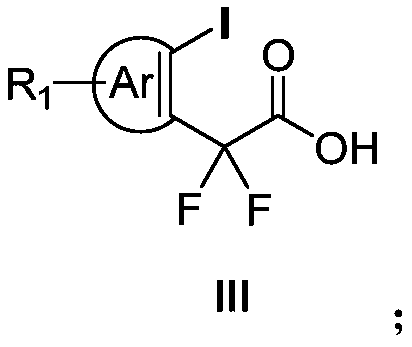
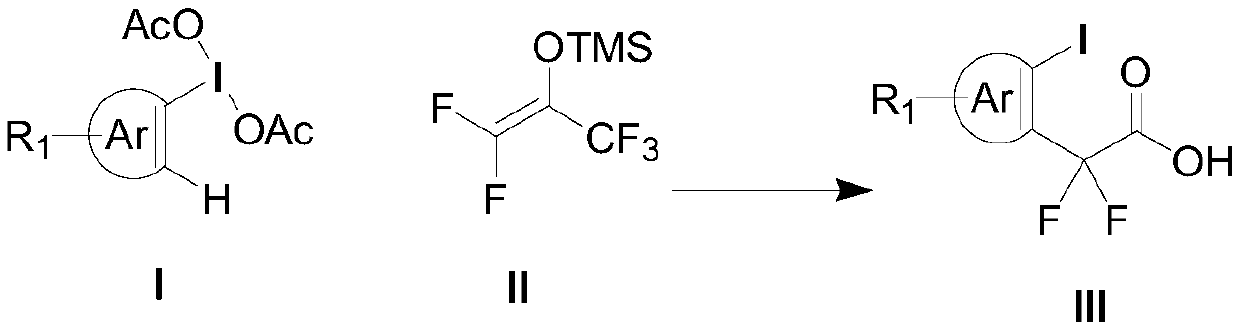
Aryl iodine compound containing carboxydifluoromethylene at ortho-position and preparation method thereof
ActiveCN109824501AEasy to separateMild reaction conditionsOrganic compound preparationCarboxylic compound preparationOrtho positionStructural formula
The invention discloses a preparation method of an aryl iodine compound containing carboxydifluoromethylene at ortho-position. The preparation method is characterized by including: at the presence oftrimethylsilyl trifluoromethanesulfonate, allowing aryl iodine diacetate shown as a structural formula (I) and pentafluoroacetone enol silicon ether shown as a structural formula (II) to be in rearrangement reaction, and hydrolyzing under strong base to obtain the aryl iodine compound containing methylene difluoroacetate and shown as a structural formula (III), wherein R1 is selected from hydrogen, halogen, alkyl, alkoxy, carbalkoxy, halogenated alkyl, halogenated alkoxy, carbalkoxy substituted alkyl, amino substituted alkyl, carbalkoxy and amino substituted alkyl, cyano or nitro, and Ar is selected from benzene ring, naphthalene ring and thiophene ring. The preparation method has the advantages of being mild in reaction condition, high in selectivity and yield, easy-to-separate in productand simple in operation.
Owner:ZHEJIANG NORMAL UNIVERSITY
Polypropylene composite material as well as preparation method and application thereof
ActiveCN113308046AIncrease in microscopic defectsLow elongation at breakTrimethylsilyl trifluoromethanesulfonatePolypropylene composites
The invention discloses a polypropylene composite material as well as a preparation method and application thereof, and the polypropylene composite material comprises the following components in parts by weight: 10-50 parts of polypropylene resin; 10 to 40 parts of polyformaldehyde resin; 1 to 3 parts of trimethylsilyl trifluoromethanesulfonate; 5 to 15 parts of starch; 10 to 20 parts of mica; and 20 to 30 parts of microbeads. The polypropylene resin and the polyformaldehyde resin are selected and blended to form the alloy, the incompatibility of the two phases enables the material to have phase separation in structure, and meanwhile, the starch, the mica and the microbeads are added to form a multiphase structure, so that the phase structure of the material is more dispersed, and the microdefects in the material are increased. Therefore, the elongation at break and the deflection of the material are greatly reduced, the brittleness of the material is remarkably improved, the application of the polypropylene composite material is further widened, and the polypropylene composite material can be used in some special occasions with high requirements on the brittleness of the material.
Owner:KINGFA SCI & TECH CO LTD +2
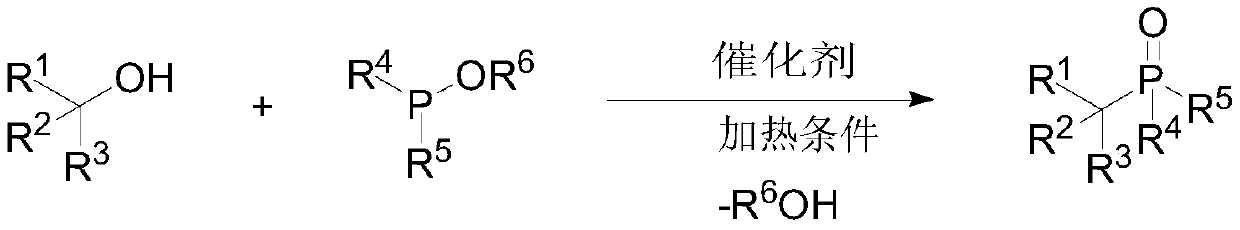
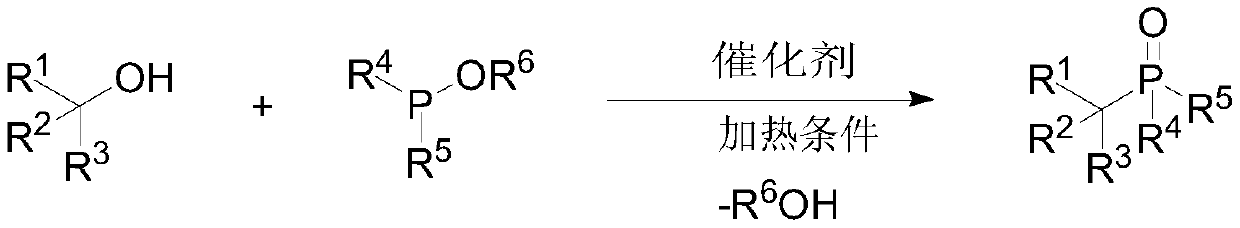
Preparation method of large-steric-hindrance alkyl substituted phosphite diester
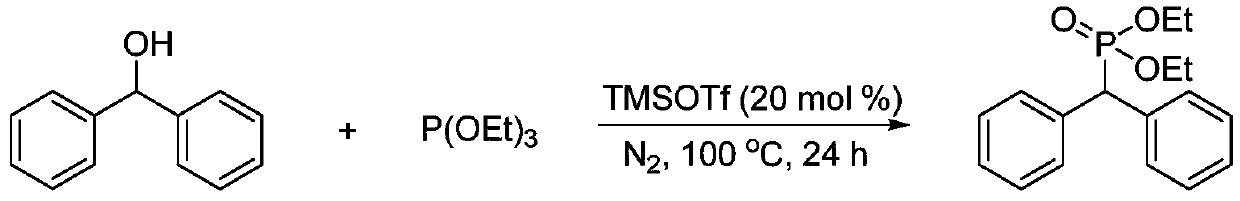
ActiveCN110922427AEasy to operateGood theoretical researchGroup 5/15 element organic compoundsPhosphorous acidPtru catalyst
The invention discloses a preparation method of large-steric-hindrance alkyl substituted phosphite diester, and relates to a novel method for preparing the large-steric-hindrance alkyl substituted phosphite diester compound which is difficult to synthesize by a known method through direct reaction of large-steric-hindrance alcohols and triphosphite by using trifluoromethanesulfonic acid trimethylsilyl ester as a catalyst. Therefore, the method can be a breakthrough for synthesizing the large-steric-hindrance alkyl substituted phosphite diester compound by an Arbuzov method, and is an improvedArbuzov method with high universality due to the fact that the method is also suitable for common primary alcohol raw materials. Specifically, according to the method, stable and low-toxicity large-steric-hindrance alcohols are used as raw materials; the method has the advantages of simple reaction conditions, easy operation and no need of a solvent, no transition metal is contained in the catalyst, the product has no transition metal residual hidden trouble, the byproduct is micromolecular alcohols such as ethanol, and almost no toxicity exists, so the method is a green synthesis method of large-steric-hindrance alkyl substituted diphosphite, and has good research value and synthesis application prospect.
Owner:WENZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com