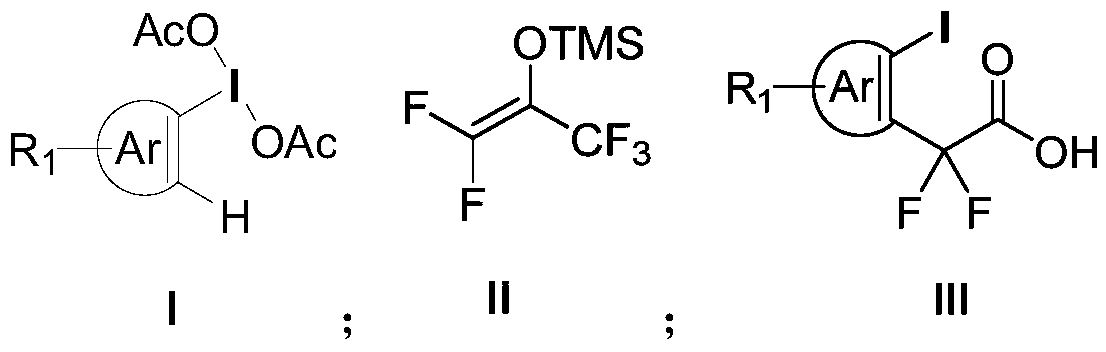
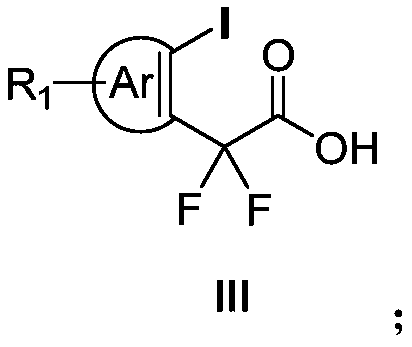
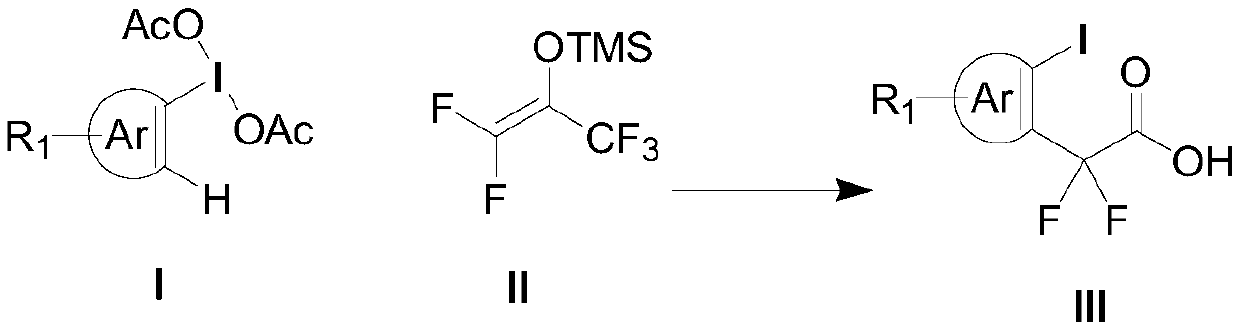
Aryl iodine compound containing carboxydifluoromethylene at ortho-position and preparation method thereof
A technology of carboxydifluoromethylene and aryl iodide, which is applied in the field of organic chemical synthesis, can solve the problems of high price and poor functional group compatibility, and achieve the effects of easy separation, mild reaction conditions, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] N 2 Under protection, redistilled dichloromethane (5 mL) was added to a 25 ml reaction tube, then 161 mg (0.5 mmol) of iodobenzene diacetate was added, and then 180 microliters of trimethylsilyl trifluoromethanesulfonate was added (TMSOTf, 1.0mmol), the reaction solution was stirred at room temperature for 5min, and finally 220mg (1.0mmol) of pentafluoroacetone enol silicon ether was added at -78°C, stirred for 5min, then warmed to -50°C and reacted for 2h, using a thin The reaction process was tracked by layer chromatography. After the reaction was completed, saturated sodium bicarbonate solution (3ml) was added to quench the reaction, and the temperature was slowly raised to room temperature, then extracted with dichloromethane (3mL×3), the organic phase was dried with anhydrous sodium sulfate, and vacuum After concentration, it was dissolved in KOH (560mg) in tetrahydrofuran / water (1:1) solution, reacted at room temperature for 12h, adjusted the pH value ...
Embodiment 2
[0055]
[0056] N 2 Under protection, redistilled dichloromethane (5 mL) was added to a 25 ml reaction tube, then 168 mg (0.5 mmol) of iodobenzene p-methyldiacetate was added, and then 180 microliters of trimethyl trifluoromethanesulfonate was added base silicon ester (TMSOTf, 1.0mmol), the reaction solution was stirred at room temperature for 5min, and finally 220mg (1.0mmol) of pentafluoroacetophenone enol siloxane was added at -78°C, stirred for 5min, then heated to -50°C and React for 2h, track the reaction progress with thin layer chromatography, add saturated sodium bicarbonate solution (3ml) to quench the reaction after the reaction is over, slowly warm up to room temperature, then extract with dichloromethane (3mL×3), the organic phase is washed with anhydrous Dry over sodium sulfate, concentrate in vacuo and dissolve in KOH (560mg) in tetrahydrofuran / water (1:1) solution, react at room temperature for 12h, adjust the pH value to 2-3 with dilute hydrochloric acid af...
Embodiment 3
[0064]
[0065] N 2 Under protection, redistilled dichloromethane (5 mL) was added to a 25 ml reaction tube, then 189 mg (0.5 mmol) of iodobenzene p-tert-butyldiacetate was added, and then 180 microliters of trifluoromethanesulfonate tri Methyl silicon ester (TMSOTf, 1.0mmol), the reaction solution was stirred at room temperature for 5min, and finally 220mg (1.0mmol) of pentafluoroacetophenone enol silicon ether was added at -78°C, stirred for 5min, and then heated to -50°C And react for 2h, track the reaction process with thin-layer chromatography, add saturated sodium bicarbonate solution (3ml) to quench the reaction after the end of the reaction, slowly warm up to room temperature, then extract with dichloromethane (3mL × 3), the organic phase with Dry over sodium sulfate, dissolve in KOH (560mg) in tetrahydrofuran / water (1:1) solution after vacuum concentration, react at room temperature for 12h, adjust the pH value to 2-3 with dilute hydrochloric acid after the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com