Novel technology for synthesis of capecitabine
A capecitabine and synthetic process technology, applied in the field of medicinal chemistry, can solve problems such as long route and complicated operation, and achieve the effects of reducing environmental pollution, convenient post-processing, and improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
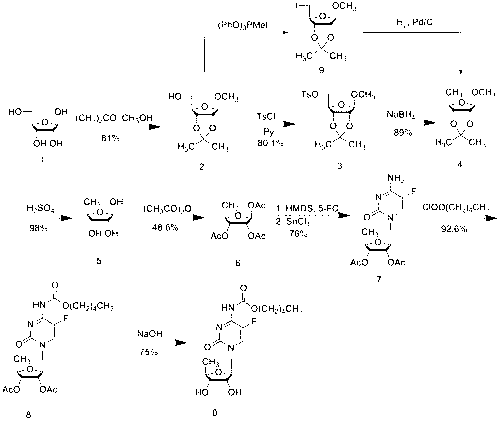
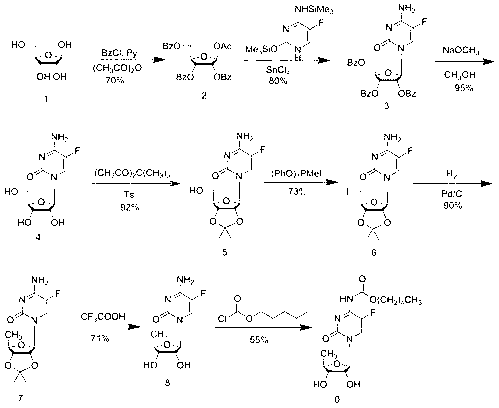
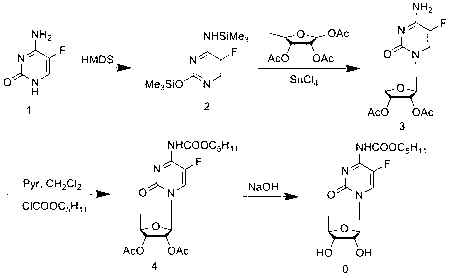
[0043] Synthesis of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (3):
[0044] Add 332.1g of trimethylsilyl-protected 5-fluorocytosine (1) into 1200mL of dichloromethane, stir to dissolve, then add 345.9g of 5-deoxy-1,2,3-tri-O-acetyl-D- Ribofuranose (2), stirred for a while, partially dissolved, and added dropwise 322.4g (96.2mmol) trimethylsilyl trifluoromethanesulfonate (TMSOTf) in 300mL DCM solution to the above reaction solution at room temperature, controlled temperature 20~ Between 25°C, after dropping, stir at room temperature for about 15h, TLC monitors that the reaction is complete (developer: DCM:MeOH=15:1), control the temperature at 20°C, add 3900ml saturated sodium bicarbonate solution to the reaction solution, and Stir at low temperature for 1 h, separate the liquids, extract the aqueous phase twice with 1300 mL of dichloromethane, combine the organic phases, wash once with 20% aqueous sodium chloride solution, once with saturated brine, dry over anhydrous sodium...
Embodiment 2
[0046] 2',3'-Di-O-acetyl-5'-deoxy-5-fluoro- N Synthesis of -[(pentyloxy)carbonyl]cytidine (4):
[0047] Dissolve 108 g of intermediate 3 (5'-deoxy-2',3'-di-O-acetyl-5-fluorocytidine) in 900 mL of anhydrous CH at room temperature 2 Cl 2 Add 51.9g of anhydrous pyridine, cool and control the temperature between -5~-10°C, add dropwise a solution of 69.2g of n-pentyl chloroformate dissolved in 100mL of anhydrous DCM to the above reaction solution, dropwise, and react at room temperature 1h, TLC monitoring (developing solvent: DCM; MeOH=15:1) After the reaction of intermediate 3 is complete, add 75mL of methanol, stir for 15min, add 330mL of pure water for extraction, and then extract the aqueous layer twice with 110mL of DCM, combine the organic phase, 20 % sodium chloride aqueous solution was washed once, saturated sodium chloride aqueous solution was washed once, dried over anhydrous sodium sulfate, the desiccant was filtered off, and the solvent was evaporated under reduced pr...
Embodiment 3
[0049] Synthesis of capecitabine:
[0050] Intermediate 4 (2',3'-di-O-acetyl-5'-deoxy-5-fluoro- N -[(pentyloxy)carbonyl]cytidine) 110g was dissolved in 330mL of methanol, the temperature was controlled between -15~-20°C, and 120mL of methanol solution containing 13.4g of sodium methoxide was added dropwise, and the temperature was controlled to react 15min, TLC monitoring (developing agent: DCM:MeOH=50:1,) the reaction is complete, add 2N hydrochloric acid dropwise to the above reaction solution at low temperature to adjust the pH of the reaction solution to 5~6, evaporate methanol under reduced pressure at 40°C, add Extract with 1000mL DCM, wash the organic phase once with distilled water, wash the organic phase once with 20% aqueous sodium chloride solution, dry over anhydrous sodium sulfate, filter off the desiccant, distill off the solvent under reduced pressure at 40°C to obtain 98.3g of crude product, and wash with 500mL acetic acid Ethyl ester was recrystallized and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com