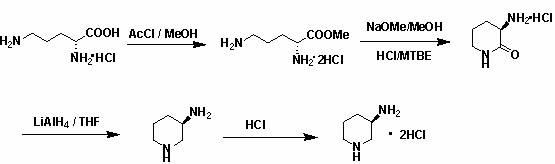
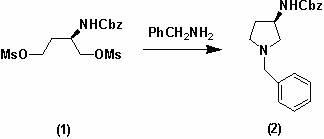
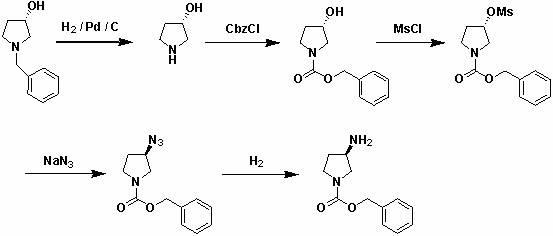
General preparation method of optical-activity 3-aminopyrrolidine, 3-alkyl amino piperidine and derivatives thereof
A technology of aminopyrrolidine and aminopiperidinane, which is applied in the field of general preparation of derivatives, can solve the problems of low final product yield, expensive raw materials, waste of starting materials, etc., and achieve low equipment requirements, post-treatment and purification The effect of simple operation and low price
Inactive Publication Date: 2011-01-26
WISDOM PHARM CO LTD
View PDF13 Cites 24 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The first step of the method involves kinetic resolution, wasting a lot of starting material
In summary, in the existing method for synthesizing 3-aminopyrrolidine, 3-aminopiperidinane and derivatives thereof, there are various deficiencies, such as expensive raw materials, harsh reaction conditions, The final product yield is low, etc.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a general preparation method of optical-activity 3-aminopyrrolidine, 3-alkyl amino piperidine and derivatives thereof by using compounds with optical activity as an initial raw material. The compounds can be simply expressed in the following molecular structure shown in the specification, wherein n is 1, 2 or 3, R1 or R2 can independently present a hydrogenous or amino-group protection group, and * presents a chiral centre with optical activity and can be R configuration or S configuration. The 3-aminopyrrolidine, 3-alkyl amino piperidine and derivatives thereof, which have optical activity, are generally used for synthesizing chiral medicaments or pesticides in the development and the production of medicaments. The invention has the advantages of cheap and easily obtainable raw materials, simple and convenient process operation, high safety, low cost and little environmental pollution and is suitable for industrial production.
Description
technical field The invention relates to the technical field of preparation of chiral drugs and their optically active intermediates, in particular to a general preparation method for optically active 3-aminopyrrolidine, 3-aminopiperidinane and their derivatives. Background technique Optically active 3-aminopyrrolidine and its derivatives are key intermediates in the synthesis of chiral drugs or pesticides. For example, patent WO2004013097 describes their application in the synthesis of Vinylpyrrolidone-Cephaloridine drugs. These compounds have highly effective antibacterial activity, especially antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa. In the patent US4854418, they are used as important intermediates for the synthesis of quinolone antibacterial drugs, and quinolone compounds are well known to have strong antibacterial properties against Gram-negative bacteria. Optically active 3-aminopiperidinane and i...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D207/14C07D211/56C07D223/12
CPCY02P20/55
Inventor 杨登贵邱小龙桑大永邹平
Owner WISDOM PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com