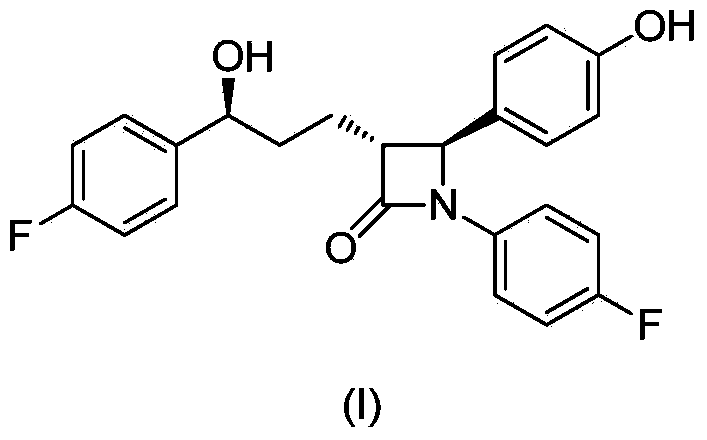
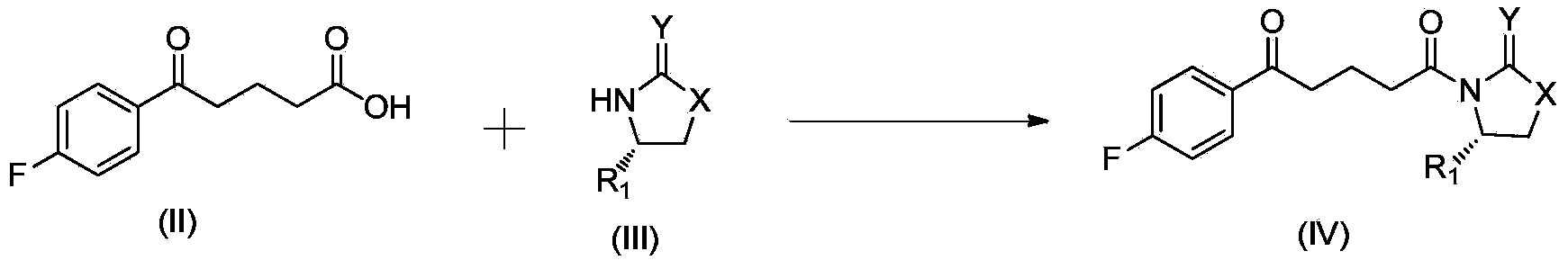Stereselective synthesis method for lipid-lowering drug ezetimibe
A hypolipidemic drug, stereoselective technology, applied in the production of bulk chemicals, organic chemistry and other directions, can solve the problem of no reported product optical purity, etc., and achieve the effects of improving optical purity, improving yield and purity, and improving chemical purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
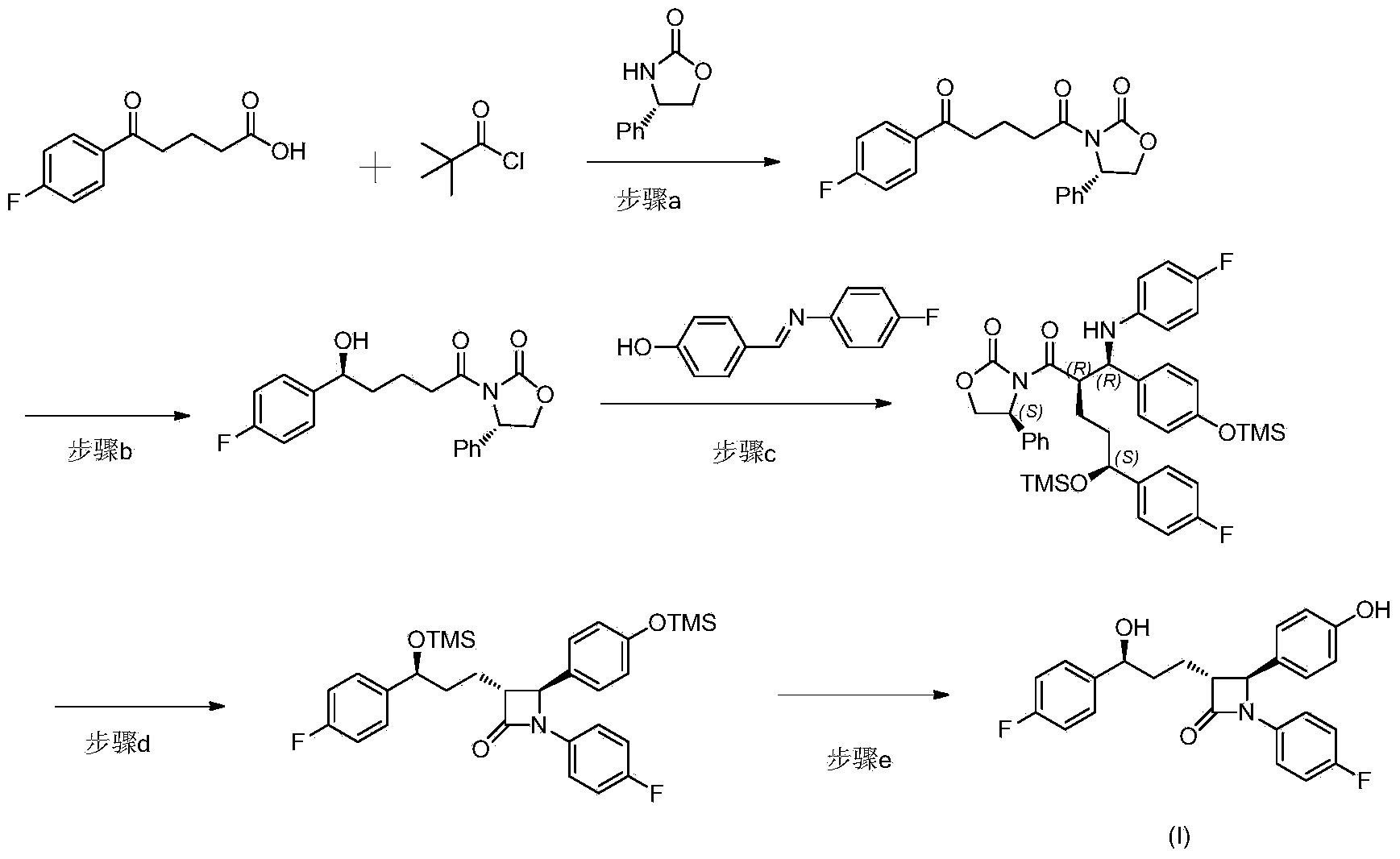
[0040] The synthesis of ezetimibe when the chiral prosthetic group is (S)-4-phenyl-2-oxazolidinone comprises the following steps:
[0041] Step (a), the reaction formula is as follows:
[0042]
[0043] Into a 500 mL three-neck round bottom flask was added compound II p-fluorobenzoyl butyric acid (20 g, 95.15 mmol), dichloromethane (100 mL) and triethylamine (23 mL, 165 mmol), and the mixture was stirred at room temperature for 5 min . Pivaloyl chloride (11.3 mL, 91.75 mmol) was added slowly over 30 minutes and stirring was continued for 1 hour. Add chiral prosthetic compound IIIa(S)-4-phenyl-2-oxazolidinone (10g, 61.3mmol), 4-(N,N-dimethylamino)pyridine (1.6g, 13mmol) and dry N,N-Dimethylformamide (10 mL), heated to reflux of dichloromethane for about 7 hours. After cooling to room temperature, the entire batch was slowly transferred to a flask containing 2N sulfuric acid (80 mL) with stirring, and stirring was continued for 30 min, the layers were separated, and the mi...
Embodiment 2
[0054] The synthesis of ezetimibe when the chiral prosthetic group is (S)-4-phenyl-2-thiazolidinone comprises the following steps:
[0055] Step (a), the reaction formula is:
[0056]
[0057] Into a 500 mL three-neck round bottom flask were added p-fluorobenzoylbutyric acid (20 g, 95.15 mmol), dichloromethane (100 mL) and triethylamine (23 mL, 165 mmol), and the mixture was stirred at room temperature for 5 minutes. Pivaloyl chloride (11.3 mL, 91.75 mmol) was added slowly over 30 minutes and stirring was continued for 1 hour. Add the chiral prosthetic group (S)-4-phenyl-2-thiazolidinone (with (S)-4-phenyl-1,3-thiazolidine-2-thione as raw material, according to the literature Phosphorus, Sulfur and Silicon and the Related Elements, 2011, 186(7), prepared by the method provided in 1563–1571) (11g, 61.3mmol), 4-(N,N-dimethylamino)pyridine (1.6g, 13mmol) and dry N,N-Dimethylformamide (10 mL), heated to reflux of dichloromethane for about 8 hours. After cooling to room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com