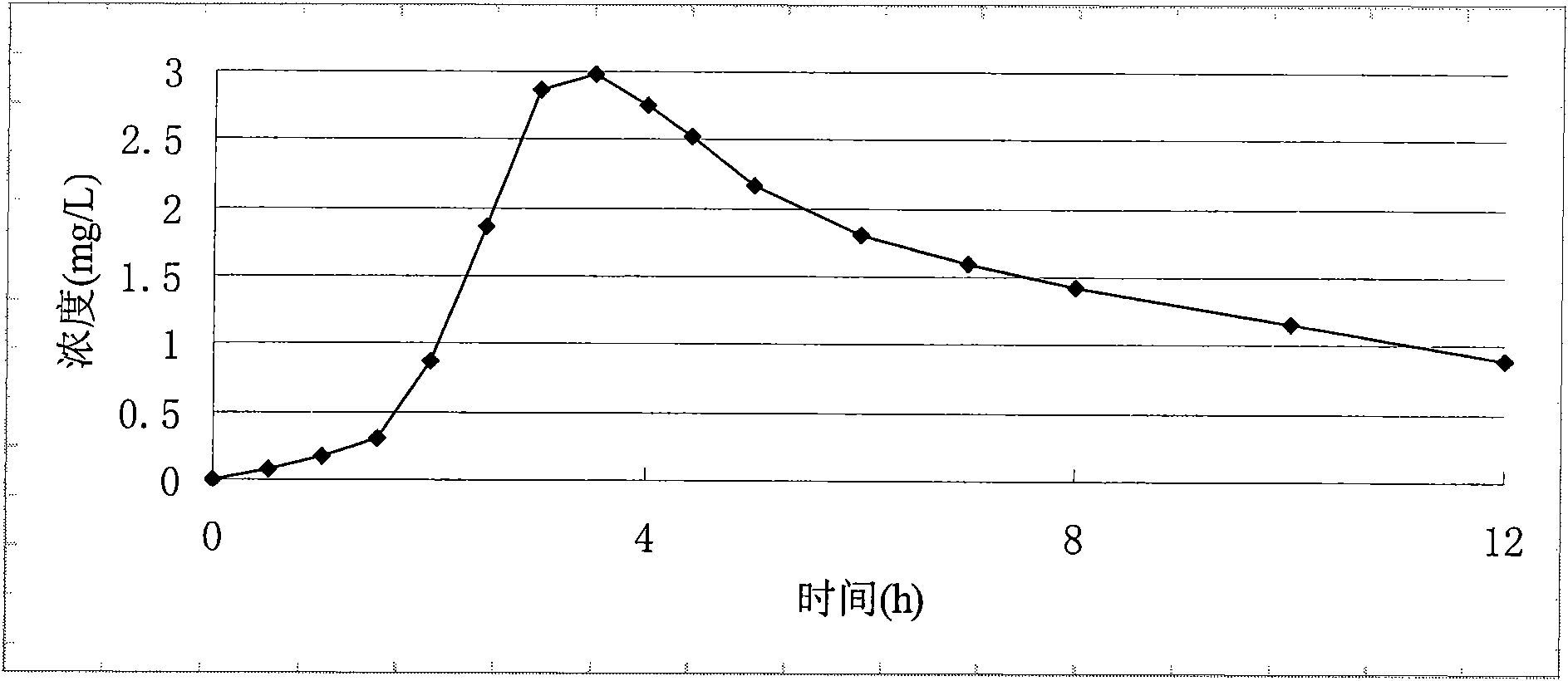
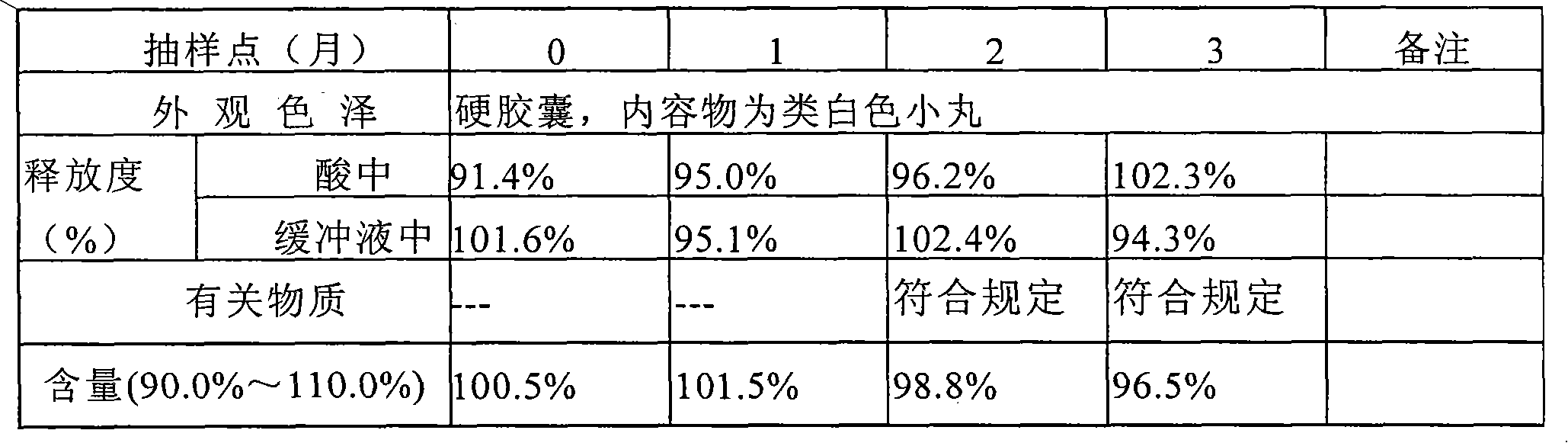
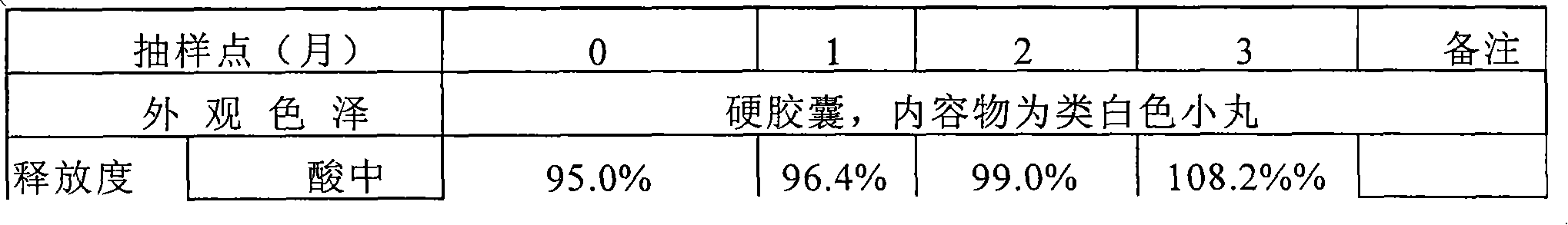
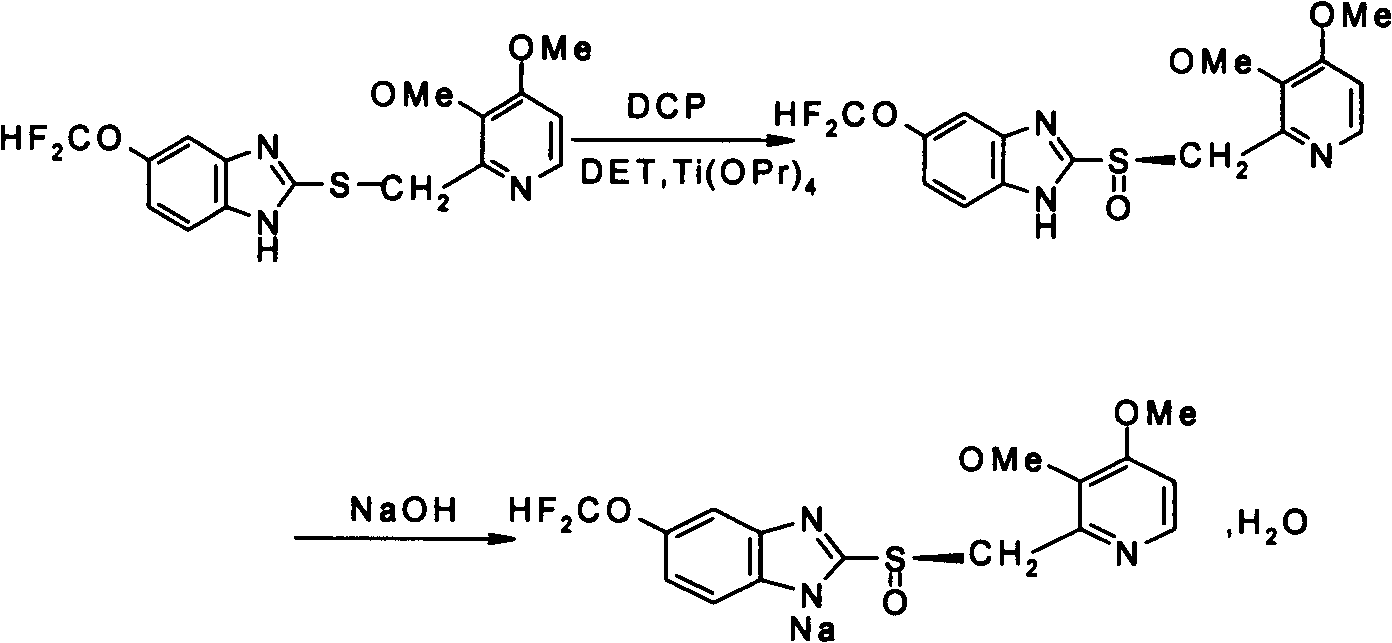
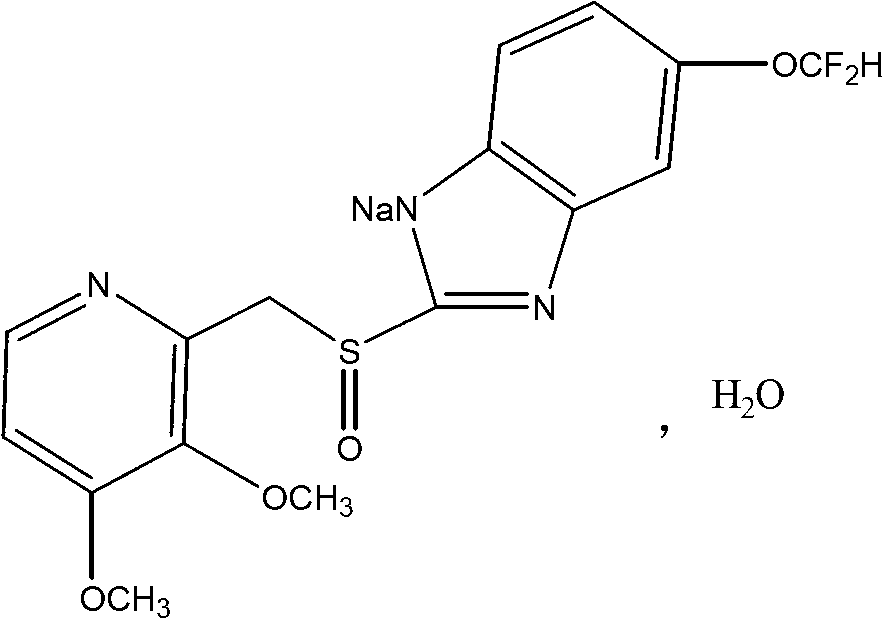
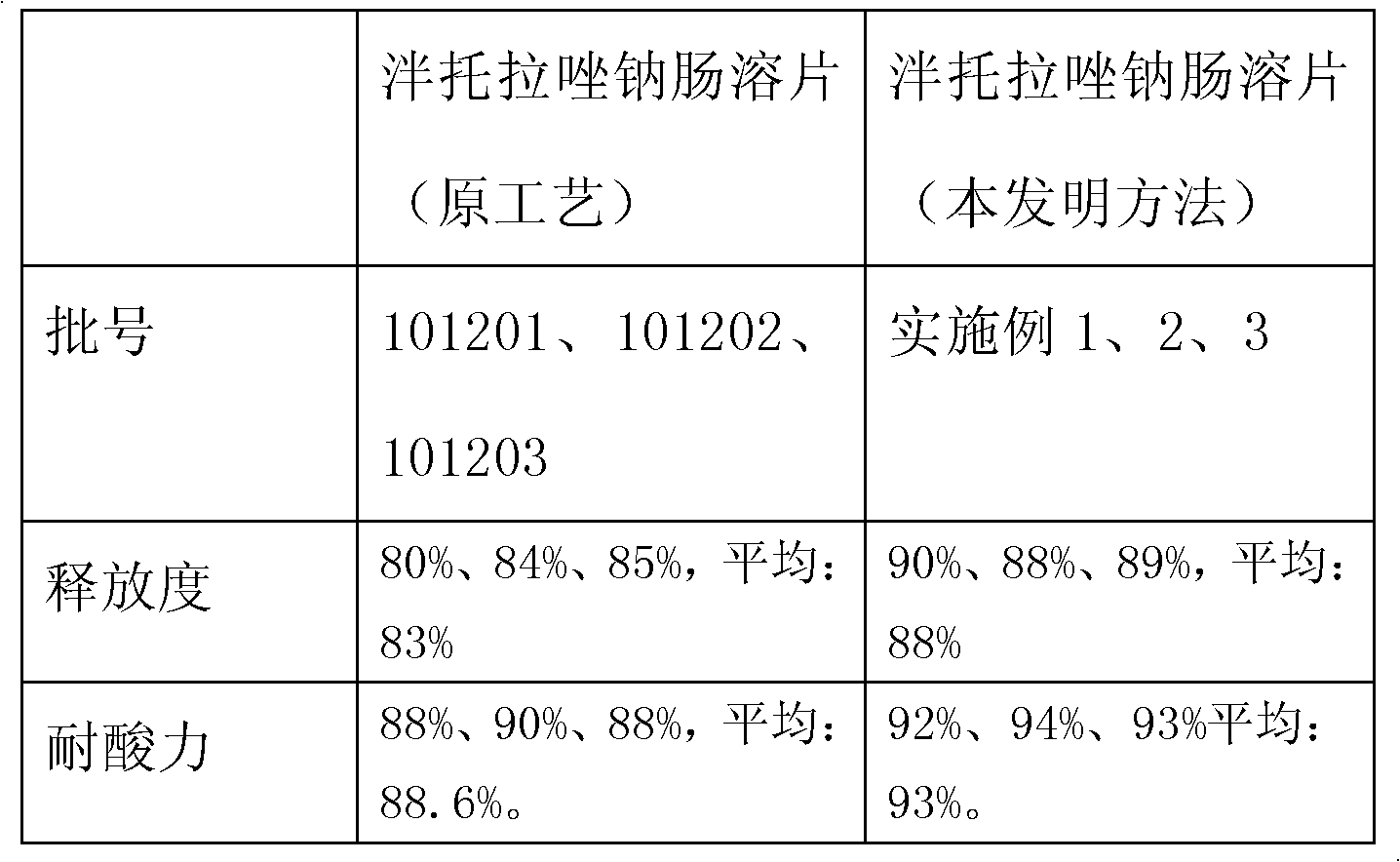
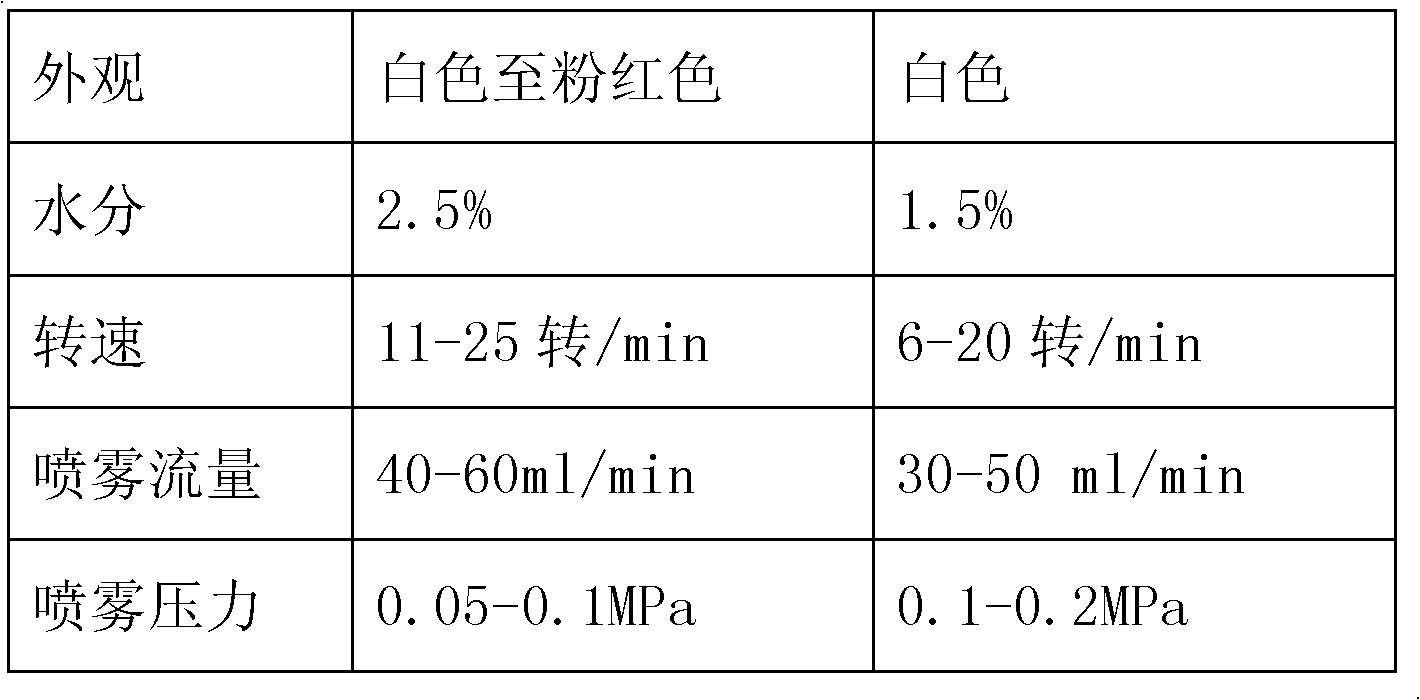
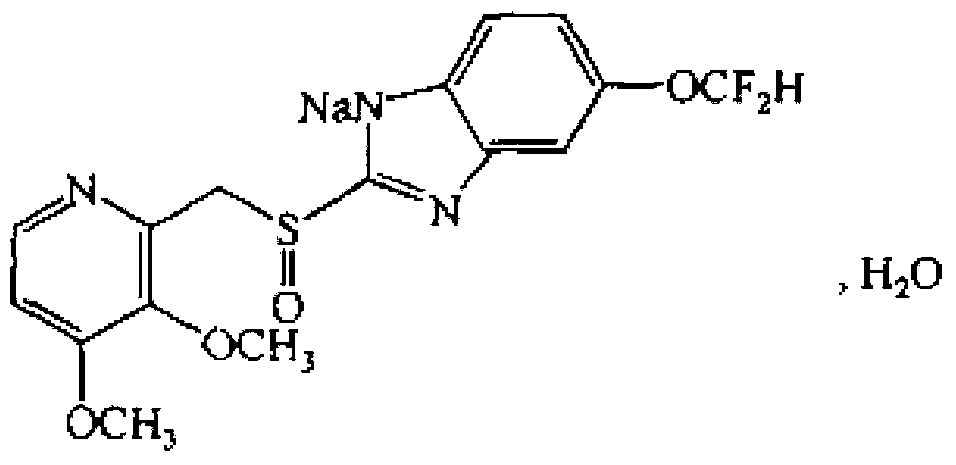
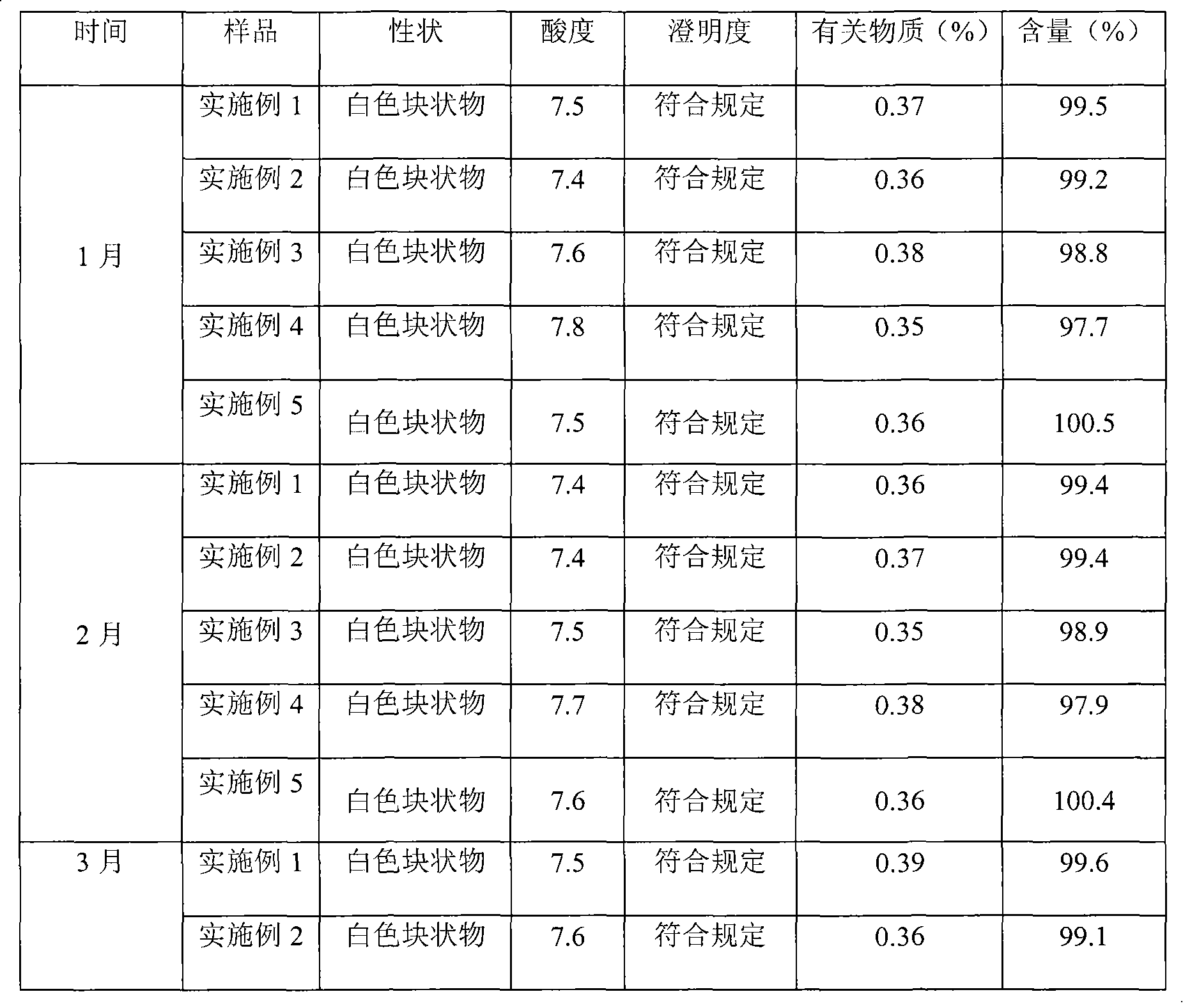
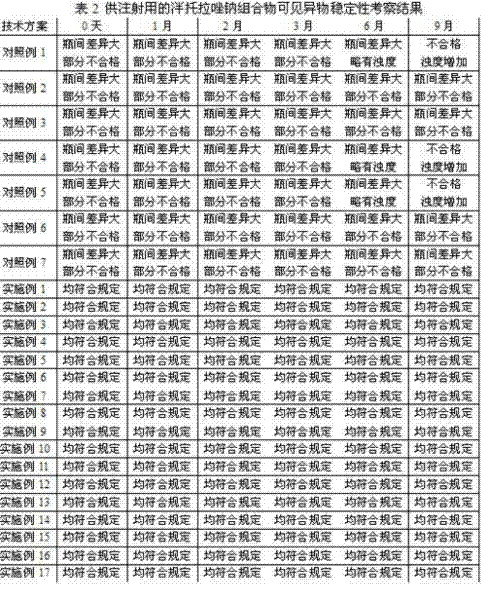
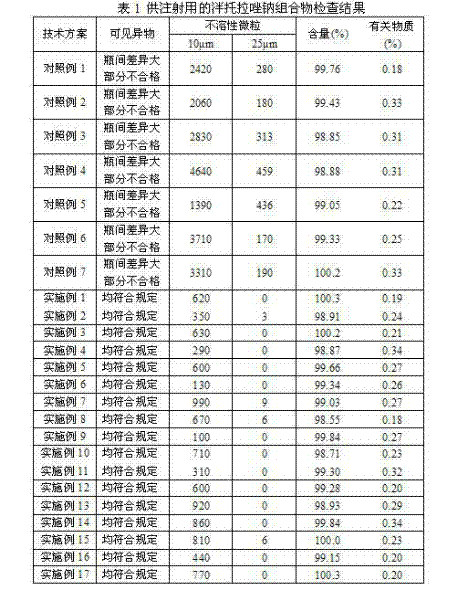
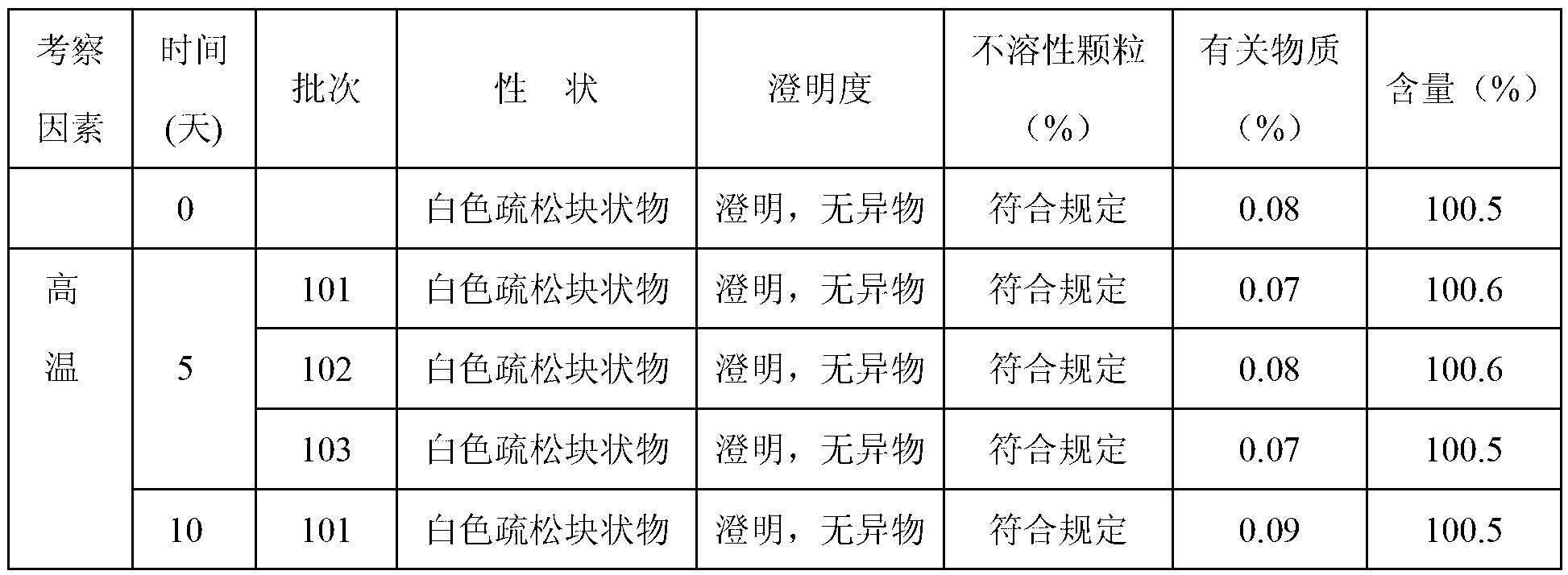
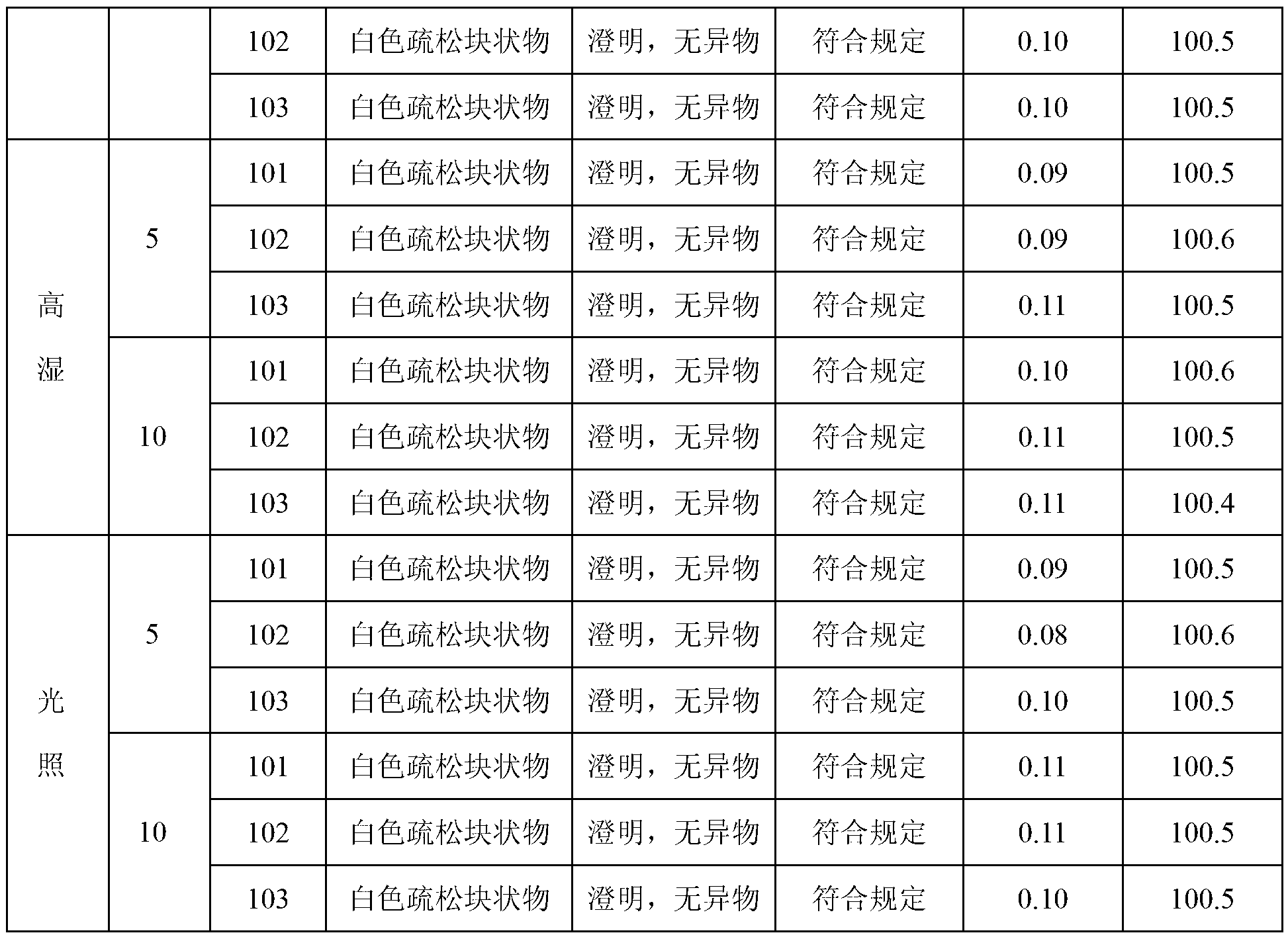
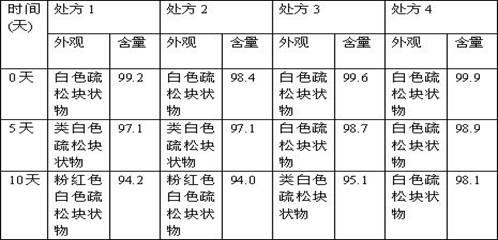
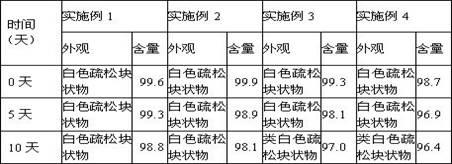
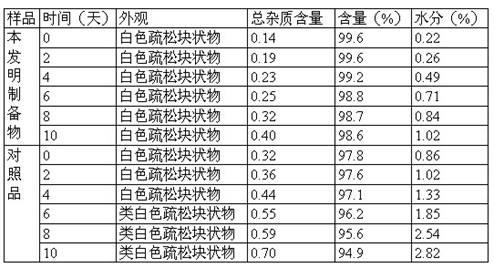
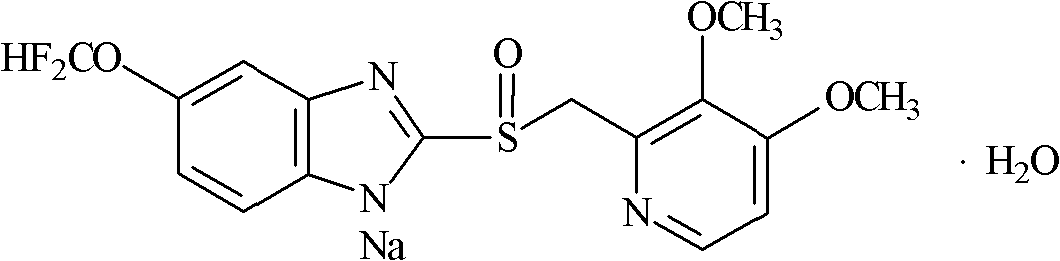
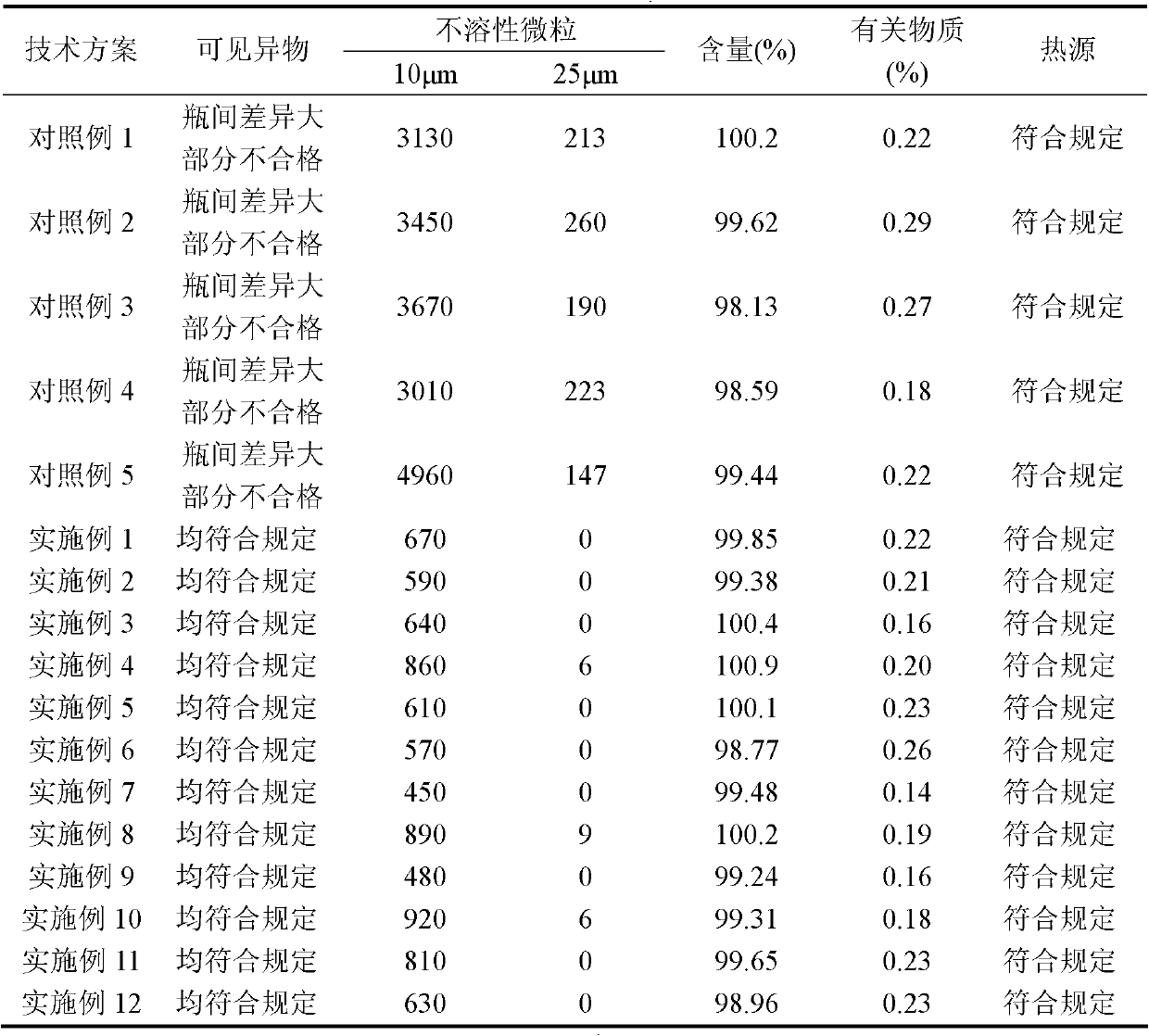
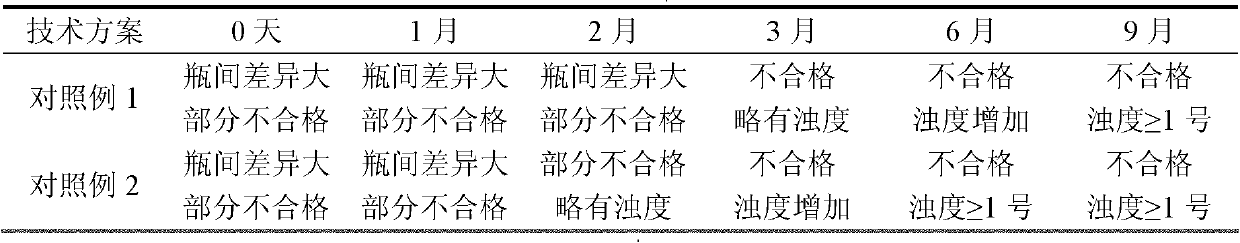
Patents
Literature
171 results about "Pantoprazole Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
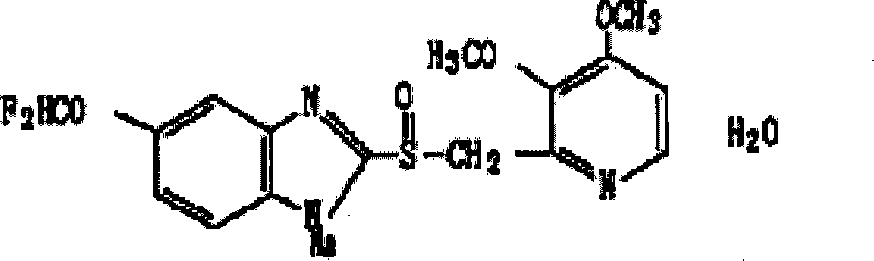
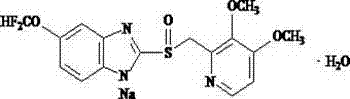
The sodium salt form of a substituted benzimidazole with proton pump inhibitor activity. Pantoprazole is a lipophilic, weak base that crosses the parietal cell membrane and enters the acidic parietal cell canaliculus where it becomes protonated, producing the active metabolite sulfenamide, which forms an irreversible covalent bond with two sites of the H+/K+-ATPase enzyme located on the gastric parietal cell, thereby inhibiting both basal and stimulated gastric acid production.
Pantoprazole multiparticulate formulations
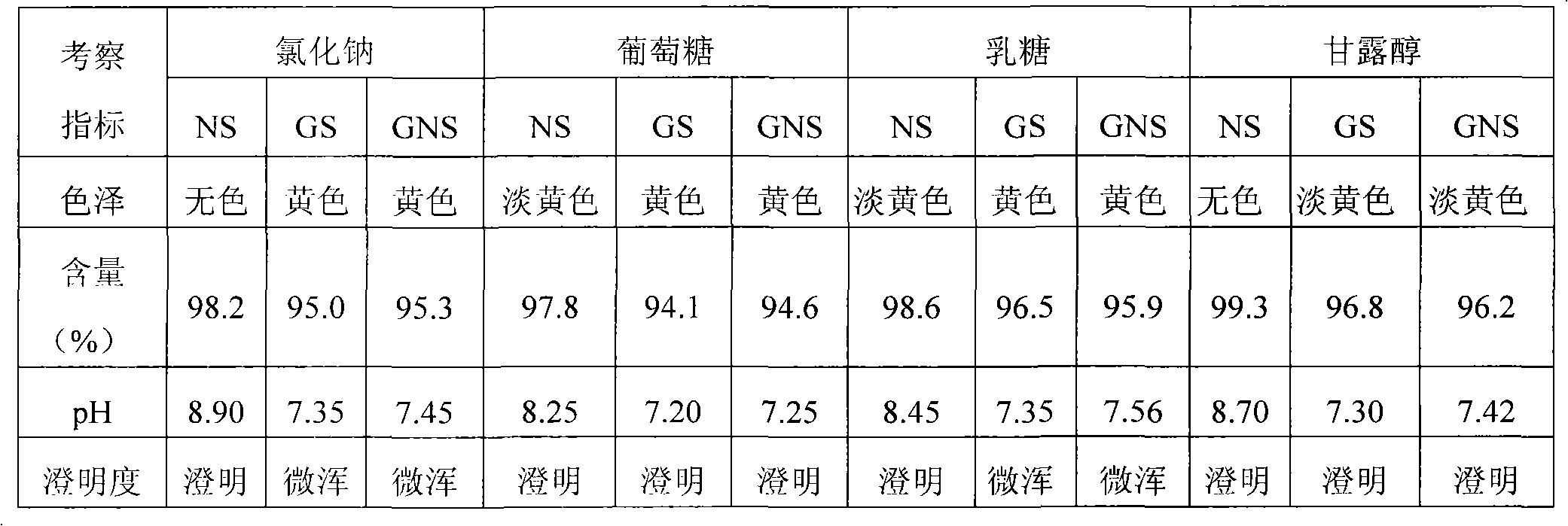
ActiveUS20050129761A1Low variabilityProlong the action timeBiocideDispersion deliveryMedicineEnantiomer
Pantoprazole sodium multiparticulates are described which avoid sticking to nasogastric and gastronomy tubes. The pantoprazole multiparticulates have a spheroid core of pantoprazole or an enantiomer thereof, or a salt thereof, a surfactant, and a distintegrant; a sub coat which is comprised of hydroxypropyl methylcellulose (hypromellose) and water, an enteric coat on the sub-coat, and a final seal coat over the enteric coat, which is composed of hydroxypropyl methylcellulose (hypromellose) and water.
Owner:WYETH LLC
Pantoprazole sodium freeze-dried powder injection and preparing method thereof
ActiveCN101229138ASimple recipeLittle side effectsPowder deliveryOrganic active ingredientsSolubilityMANNITOL/SORBITOL
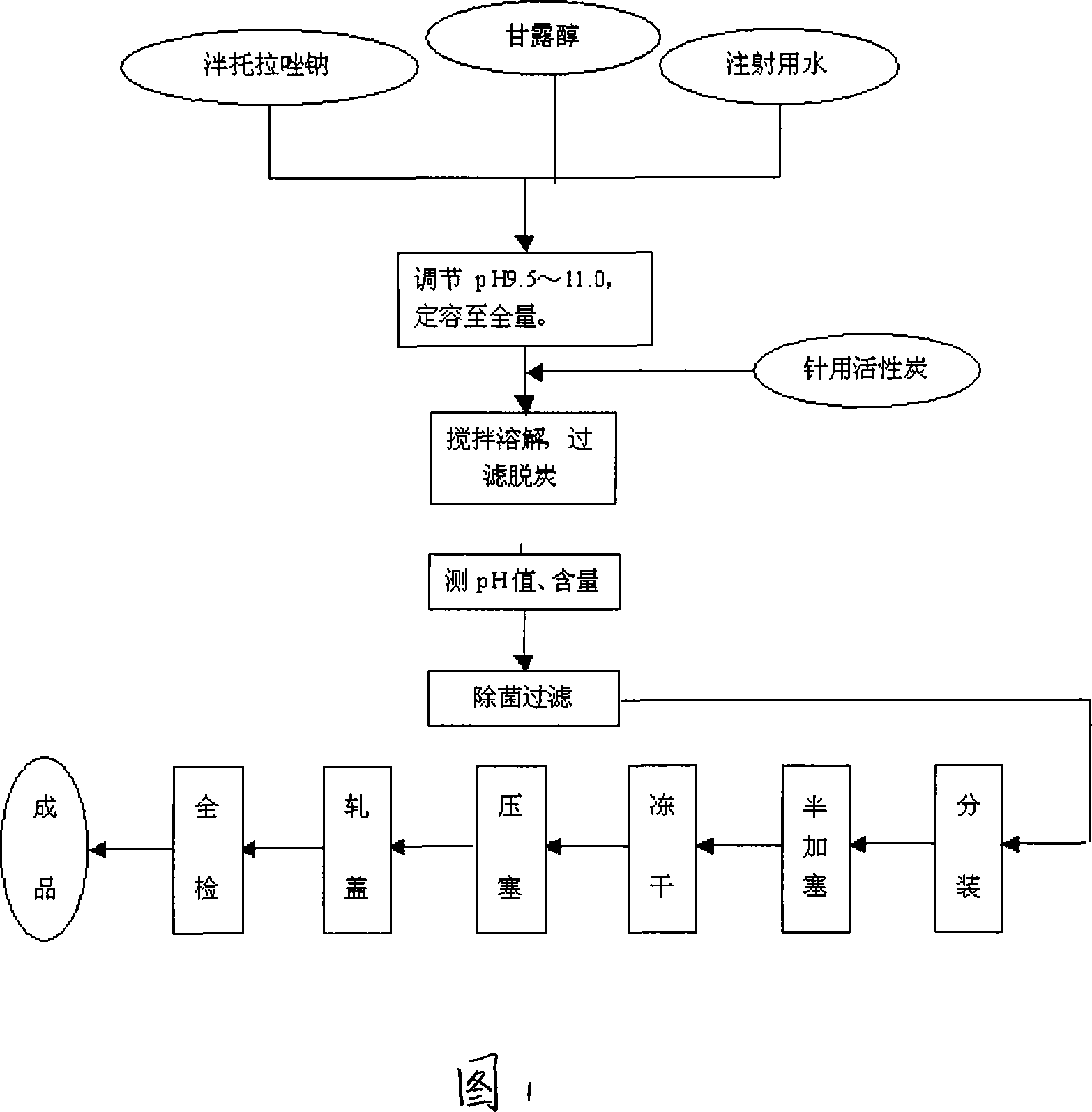
The invention aims at providing a pantoprazole sodium freeze-dried powder injection and comprises pantoprazole sodium and mannitol with the weight ratio of 1: 2 to 5. The invention is simple in formula and little in side effect; products prepared by the method are plump in appearance, good in complex solubility and excellent in quality with the adoption of an advanced freezing and drying process.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Pantoprazole sodium freeze dried injection and preparation method thereof
InactiveCN101011397AReduce dosageIncrease dosagePowder deliveryOrganic active ingredientsInorganic saltsFreeze-drying
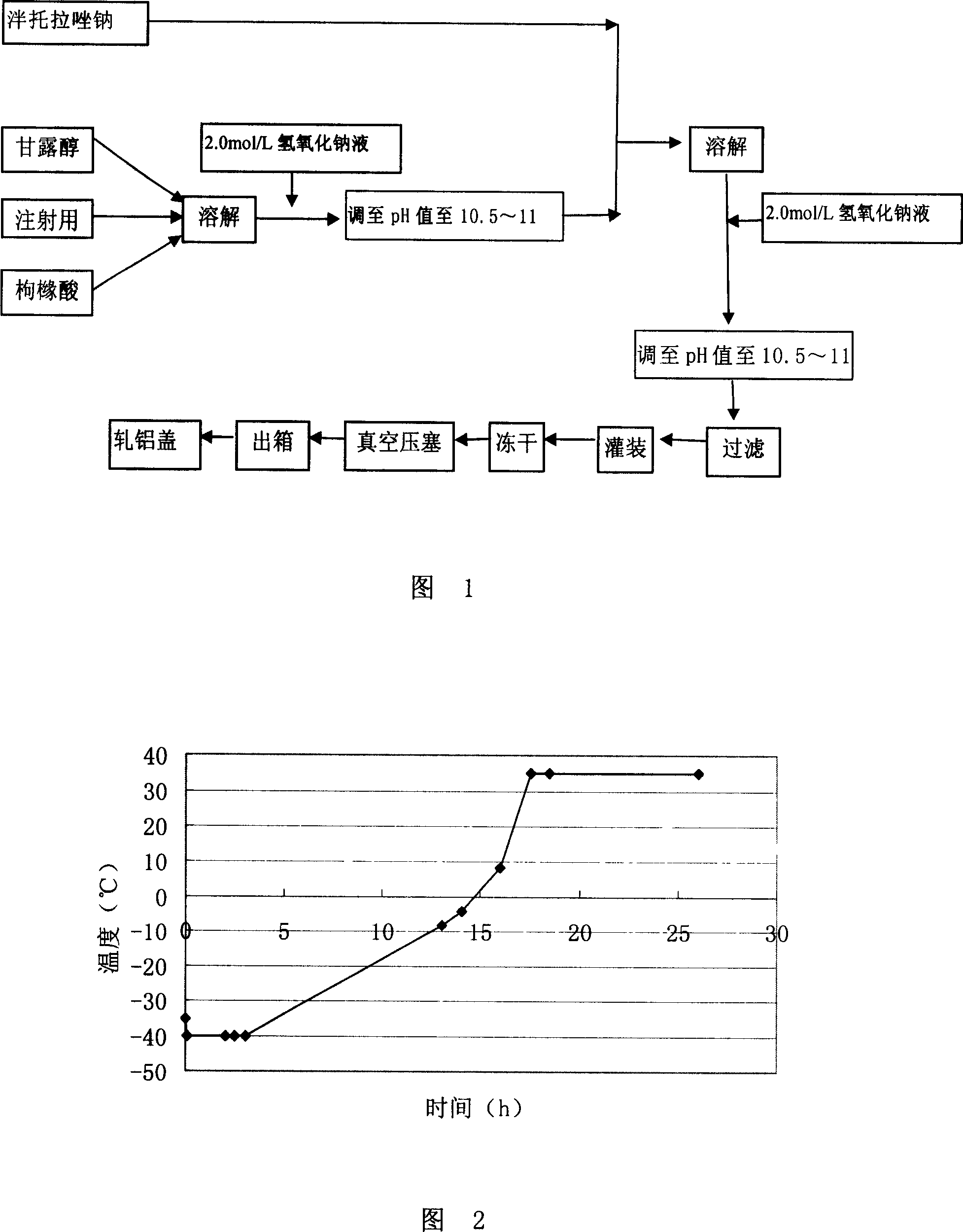
The invention relates to a method for preparing batoracosodium freeze dried, whose pH value is 9.5-11.5. And the invention comprises 1 deal of batoracosodium, 0.5-1 deals of supporting agent, 0-0.06 deals of weak-acid strong-alkali salt, and some inorganic alkali. And the preparation comprises that 1, preparing materials; 2, dissolving the supporting agent and weak-acid strong-alkali salt via injection water, using inorganic salt to adjust the pH value to 9.5-11.5, adding batoracosodium, dissolving and using inorganic salt to adjust the pH value to 9.5-11.5; 3, filtering; 4, freezing and drying to obtain the final product. The invention can be used treat peptic ulcer, ulcer bleed, or the like.
Owner:LIVZON PHARM GRP INC
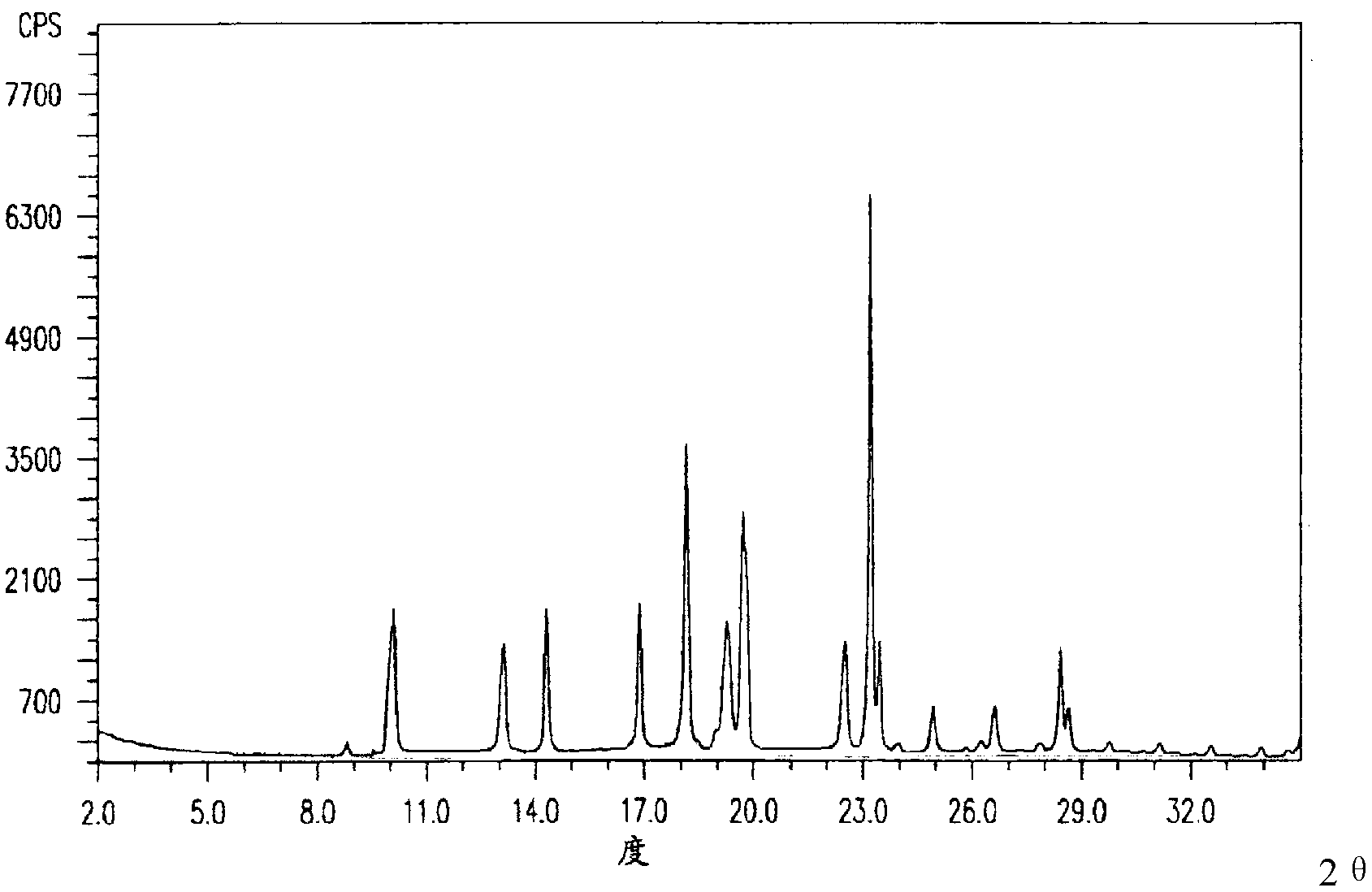
Pantoprazole sodium compound and pharmaceutical composition thereof
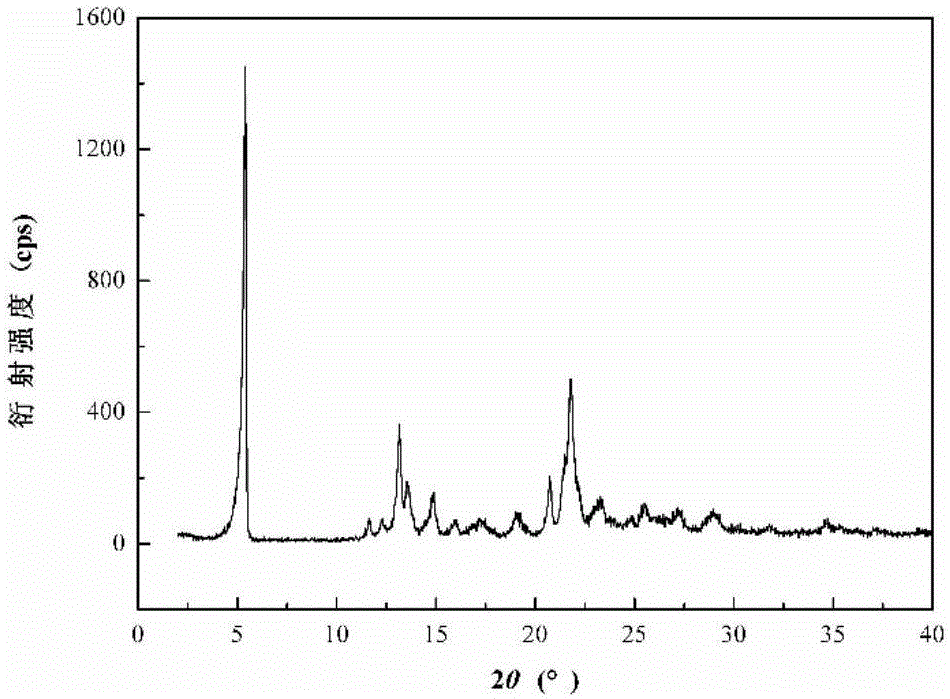
ActiveCN102351844AStable moisture contentNo changeOrganic active ingredientsPowder deliveryFreeze-dryingX-ray
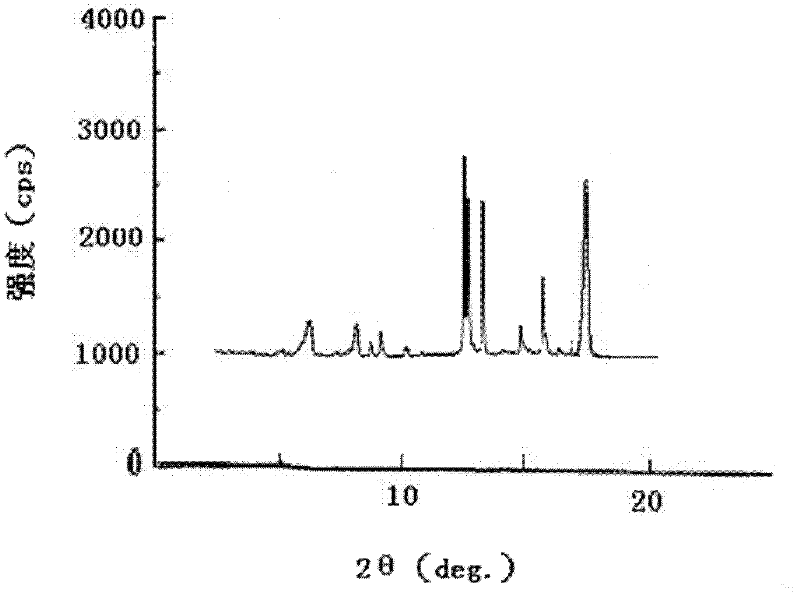
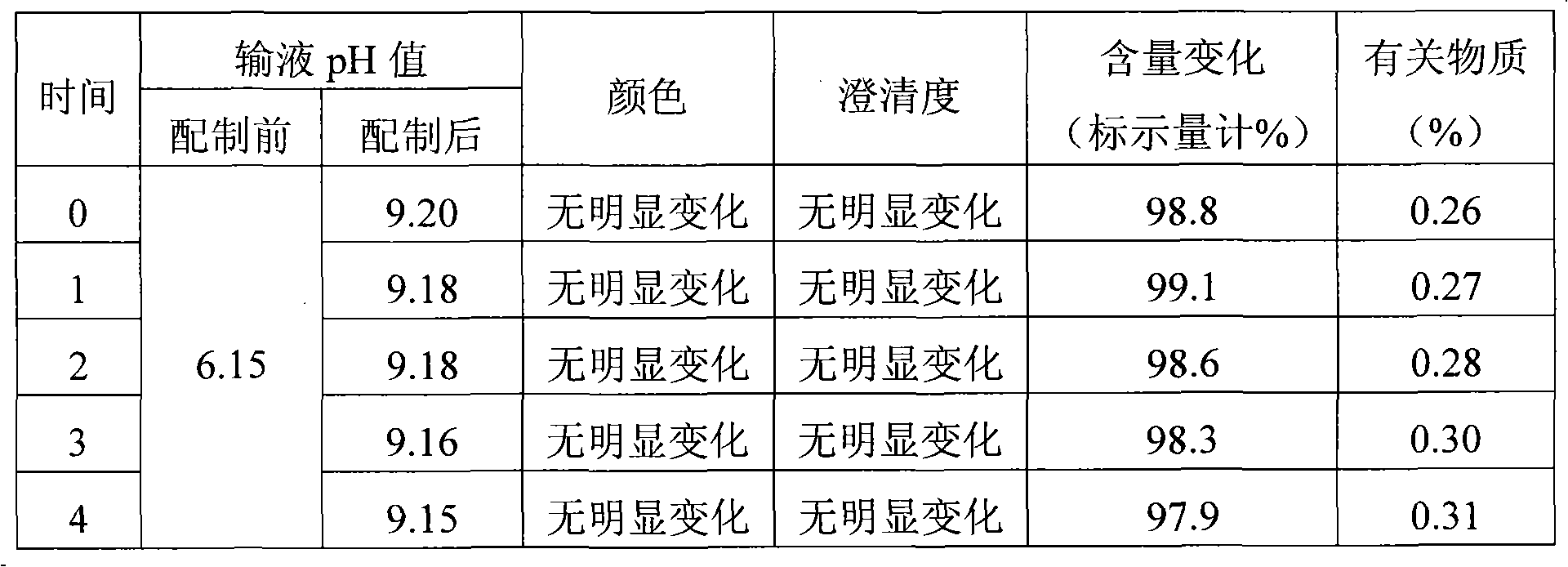
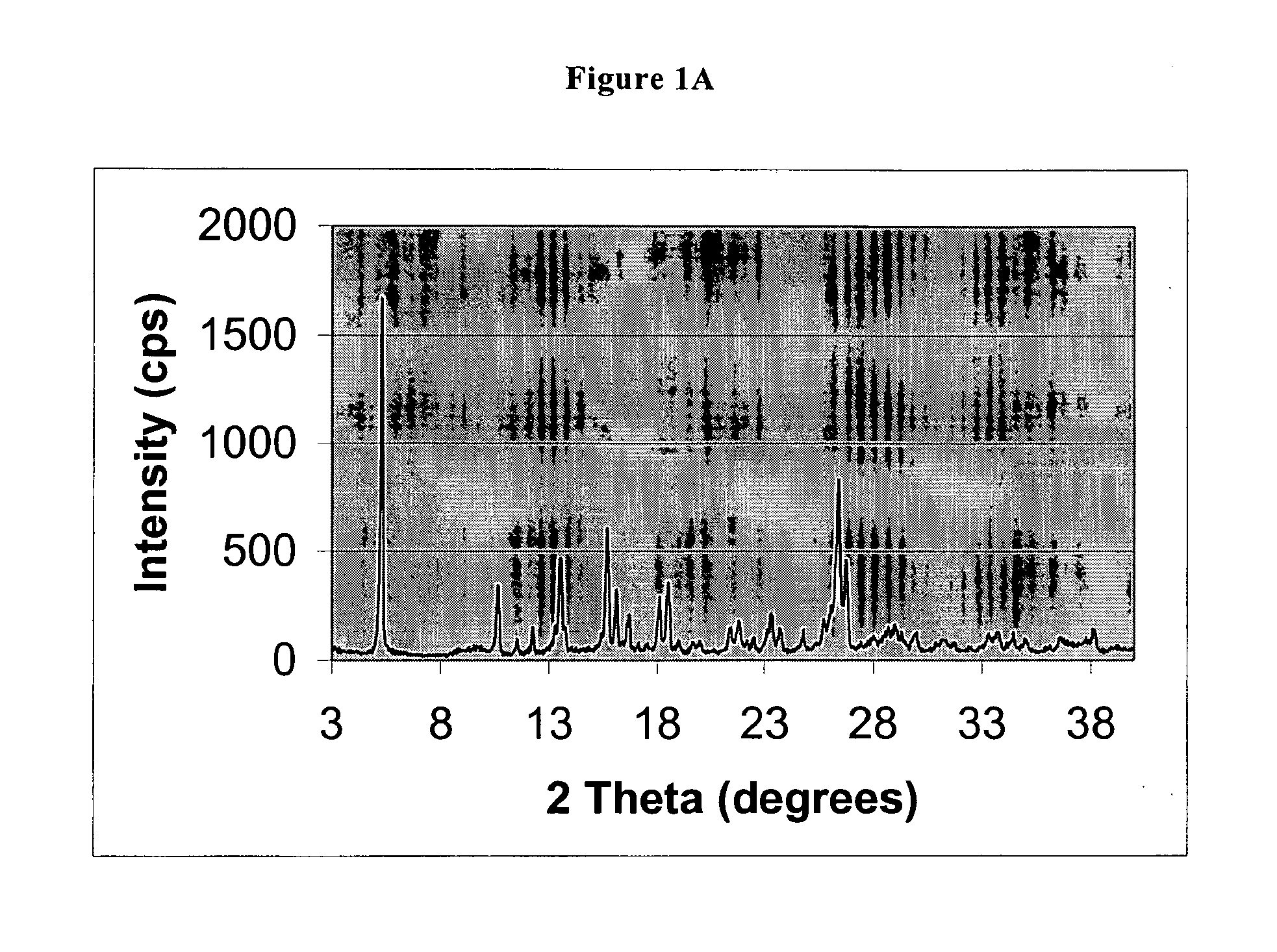
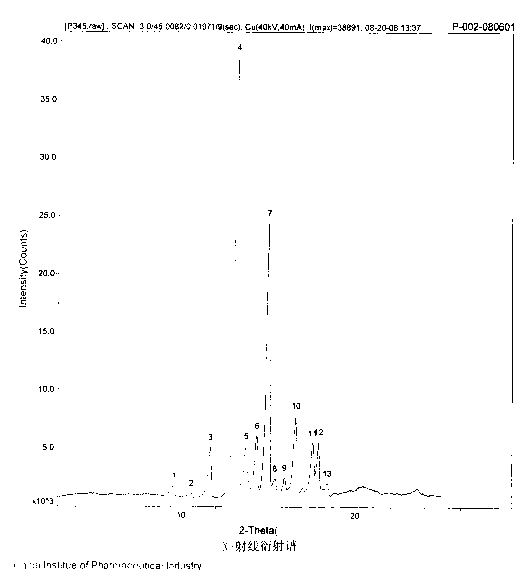
The invention discloses a pantoprazole sodium compound, which is crystal. In X-ray powder diffraction pattern obtained through Cu-Kalpha ray measurement, the characteristic peaks of the pantoprazole sodium compound are shown in positions where 2theta is 12.5, 12.6, 13.2, 16.2 and 17.3. The pantoprazole sodium compound can be used together with multiple freeze-drying supporting agents, the prepared freeze-dried powder injection has the advantages of good redissolution, good transparency after redissolution, low impurity content and the like; and moreover, the use amount of the freeze-drying supporting agent is lower, thus saving the pharmaceutical cost and improving the stability of a drug preparation. The invention also discloses a pharmaceutical composition. The pharmaceutical composition comprises a pharmaceutical active ingredient and pharmaceutical auxiliary materials, wherein the pharmaceutical active ingredient is the pantoprazole sodium compound. The stability of the pharmaceutical composition is obviously superior to that of commercial products, and especially, the stability duration of the pharmaceutical composition after being matched with common infusion fluid is prolonged, thus facilitating the clinical application.
Owner:江西新先锋医药有限公司
Freeze-dried powder injection of pantoprazole sodium and its preparation
ActiveCN1679563ALittle side effectsImprove stabilityOrganic active ingredientsPowder deliveryDisodium EdetateFreeze-drying
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Pantoprazole sodium enteric tablet and preparation method thereof
InactiveCN101461809AOne-sided bright and tidyQuality improvementOrganic active ingredientsDigestive systemPantoprazole SodiumSilicon dioxide
The invention relates to a pantoprazole sodium enteric-coated tablet and a preparation method thereof. The enteric-coated tablet is prepared by a pantoprazole sodium plain tablet which is coated with an insulating layer and an enteric coating layer, and the pantoprazole sodium plain tablet contains 0.5 to 5 percent of silicon dioxide and 0.5 to 5 percent of talcum powder. The method solves the tablet pressing problem of extremely easy sticking during the pressing of the pantoprazole sodium plain tablet.
Owner:YAOPHARMA CO LTD
Pantoprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN102085190AShorten the secondary drying timeGood lookingOrganic active ingredientsPowder deliveryCLARITYFreeze-drying
The invention relates to a pantoprazole sodium freeze-dried powder injection and a preparation method thereof. The powder injection is prepared from pantoprazole sodium and mannitol, wherein the consumption ratio of the pantoprazole sodium to the mannitol is (1:0.8)-(1:1.6), and the PH value is 10.5-11.0. In the invention, by lowering the pre-freezing temperature, properly lowering the freezing temperature, maintaining the lowered freezing temperature for a proper time, properly shortening two-stage drying time and carrying out other adjustment processes, good appearance and quality of the product can be kept under the condition that the content of the mannitol is low, the processes are reliable and feasible, and the effect is obvious. The prepared product has low content of related substances and has controllable quality, and the freeze-dried product has good clarity and formability after being redissolved.
Owner:HAINAN JINRUI PHARMA CO LTD
Pantoprazole sodium enteric-coated pellet
ActiveCN101596165AImprove stabilityAbsorb evenlyOrganic active ingredientsDigestive systemBioavailabilityPantoprazole Sodium
The invention discloses a pantoprazole sodium enteric-coated pellet which contains the following components in percentage by weight from inside to outside: 20-60 percent of blank core pellet, 4-38 percent of medicinal layer containing pantoprazole sodium and one or more medicinal excipients, 2-15 percent of isolated layer and 15-26 percent of enteric-coated layer. The invention also discloses a preparation method and an application of the enteric-coated pantoprazole sodium pellet. The pantoprazole sodium enteric-coated capsule has the advantages of better stability, uniform absorption, smaller difference of bioavailability among individuals, and the like.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
Method for preparing and purifying (L)-pantoprazole sodium
The invention provides a method for preparing (L)-pantoprazole sodium, which comprises the following steps of: oxidizing 5-difluoromethoxy-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]thio}-1H-benzimidazole by using 3,5-diisopropylbenzene hydroperoxide under the catalysis of a tetraisopropyl titanate, D-(-)-diethyl tartrate and N,N-diisopropylethylamine system to obtain S-(-)-5-difluoromethoxy-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]sulfinyl}-1H-benzimidazole, namely (L)-pantoprazole, refining the (L)-pantoprazole, and preparing a salt to obtain the (L)-pantoprazole sodium.
Owner:HC SYNTHETIC PHARMA CO LTD
Pantoprazole sodium freeze-drying medicinal composition for injection and preparation method thereof
ActiveCN101810588AImprove stabilityEliminate side effectsPowder deliveryOrganic active ingredientsFreeze-dryingSulfite salt
The invention relates to a pantoprazole sodium freeze-drying medicinal composition for injection and a preparation method thereof. The pantoprazole sodium freeze-drying medicinal composition for injection comprises the following components in part by weight: 1 part of pantoprazole sodium, 0.01 to 0.1 part of mannitol, 0.02 to 0.03 part of natrium adetate, 0.07 to 0.10 part of sodium sulfite and 0 to 0.1 part of sodium citrate. In the method, the stability of the solution of the pantoprazole sodium is improved, related matters of the solution of the pantoprazole sodium in the process of preparation, packaging or freeze-drying during preparation are not increased obviously, the content of the related matters is not reduced obviously; the prepared pantoprazole sodium freeze-drying powder injection is good in stability in the process of transportation and storage; solution mixed with the injection during clinical use can be placed for a long time, so that the clinical use is more convenient; and simultaneously, hidden troubles of the medication safety of patients due to the increase of impurities (related matters) and the problem of the curative effect on the patients due to content reduction are reduced greatly.
Owner:福建康成医药有限公司
Polymorphs of pantoprazole sodium salt and process for the preparation thereof
Novel crystalline forms of pantoprazole sodium salt solvate with ketone solvents, a process for the preparation thereof, the use of the forms for the purification of pantoprazol, pharmaceutical compositions therefrom and the use thereof in therapy.
Owner:DIPHARMA SPA
Pantoprazole compound, preparation methods and pharmaceutical preparations thereof
ActiveCN102796078AGood resolubilityHigh clarityOrganic active ingredientsPowder deliverySolubilityDrug compound
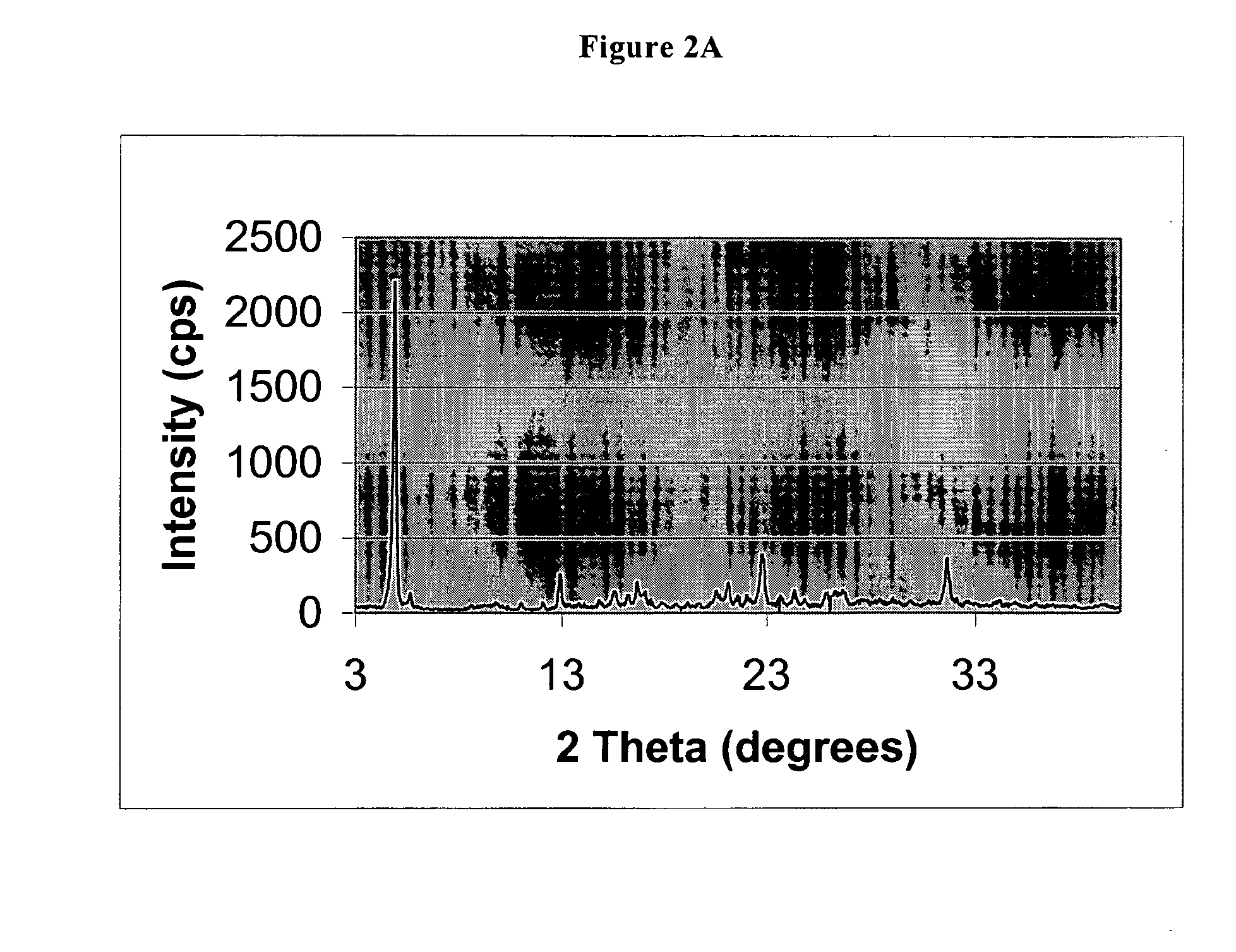
The invention belongs to the technical field of pharmaceutical compounds, and relates to a pantoprazole sodium compound entity, especially a pantoprazole sodium crystal form, preparation methods and pharmaceutical preparations thereof. The pantoprazole sodium compound is crystal, and measured by X-diffraction powder diffraction, and the diffraction pattern has the following diffraction angles (2Theta) in turn: 9.5 degrees, 10.4 degrees, 11.6 degrees, 13.1 degrees, 13.8 degrees, 14.2 degrees, 15.0 degrees, 15.3 degrees, 15.9 degrees, 16.5 degrees, 17.5 degrees, 18.0 degrees and 18.2 degrees. The pantoprazole sodium compound entity may be associated with a variety of lyophilization supporting agents and the prepared lyophilized powder for injection has good solubility, good clarity and low content of related substances, etc. simultaneously the use level of the used lyophilization supporting agent is relatively less, the cost of the products is reduced, and the stability and quality of the products are improved.
Owner:杭州澳亚生物技术股份有限公司
Pantoprazole sodium enteric-coated tablet and preparation method thereof
InactiveCN102626398AImprove acid resistanceFacilitated releaseOrganic active ingredientsDigestive systemAcrylic resinDissolution
The invention provides a pantoprazole sodium enteric-coated tablet, which consists of the following components in parts by weight: 1 part of a pantoprazole sodium plain film, 0.1-0.5 part of an isolating layer and 0.5-1 part of an enteric-coated layer, wherein the isolating layer consists of hydroxypropyl methylcellulose and an alkali in the weight part ratio of 1:5-5:1; and the enteric-coated layer is prepared from 0.5-1 part of acrylic resin. The invention provides a preparation method of the pantoprazole sodium enteric-coated tablet. Parameters such as spray speed, spray pressure, the rotating speed of a coating pan and the like are improved through process parameters of the isolating layer, an enteric liquid and a coating, so that the prepared pantoprazole sodium enteric-coated tablet has high acid tolerance and dissolution rate, the acid tolerance of a finished product is over 90 percent, the dissolution rate is over 75 percent, and the pantoprazole sodium enteric-coated tablet is accordant with and superior to the requirements of the Chinese Pharmacopoeia on the enteric tablet. The product has the advantages of stable quality, convenience for storing and transporting and contribution to clinical application. The method is simple, is suitable for industrial production, and has high application value.
Owner:SHANGHAI TENRY PHARMCEUTICAL CO LTD
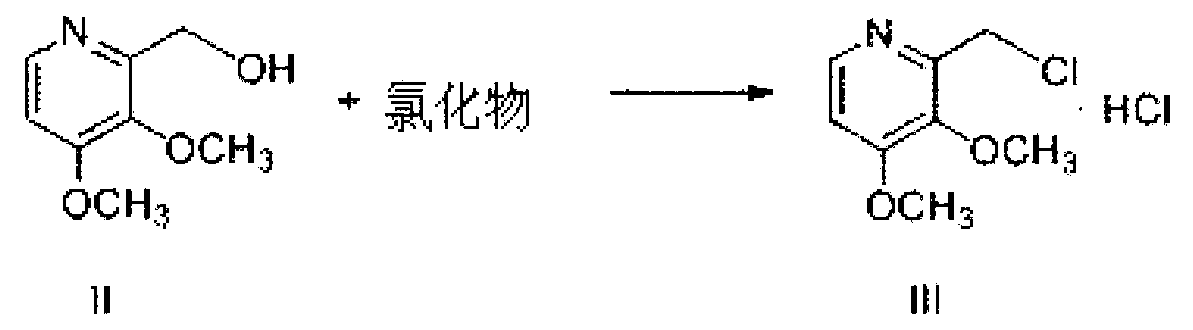
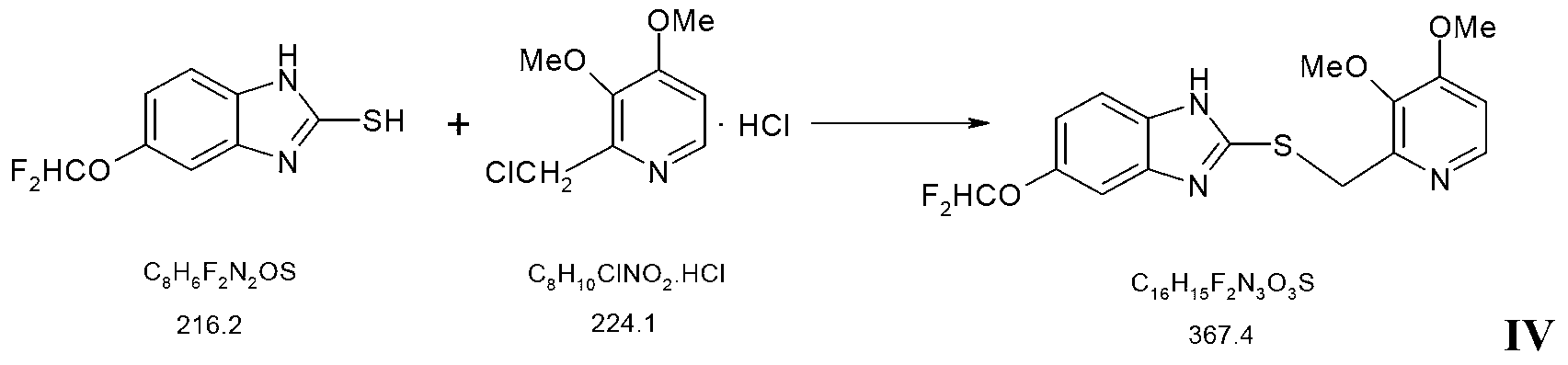
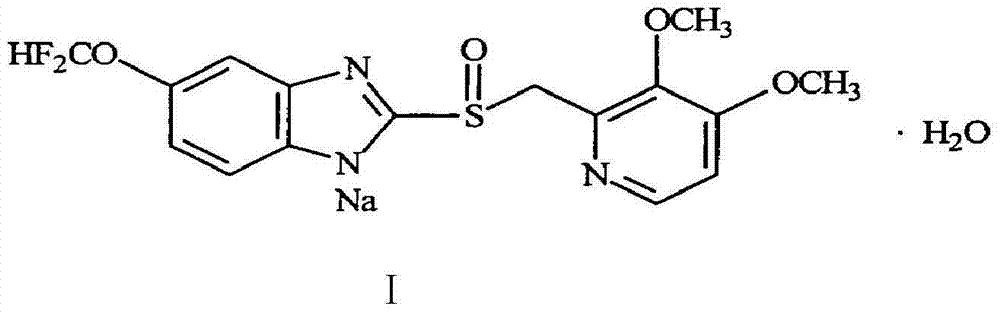
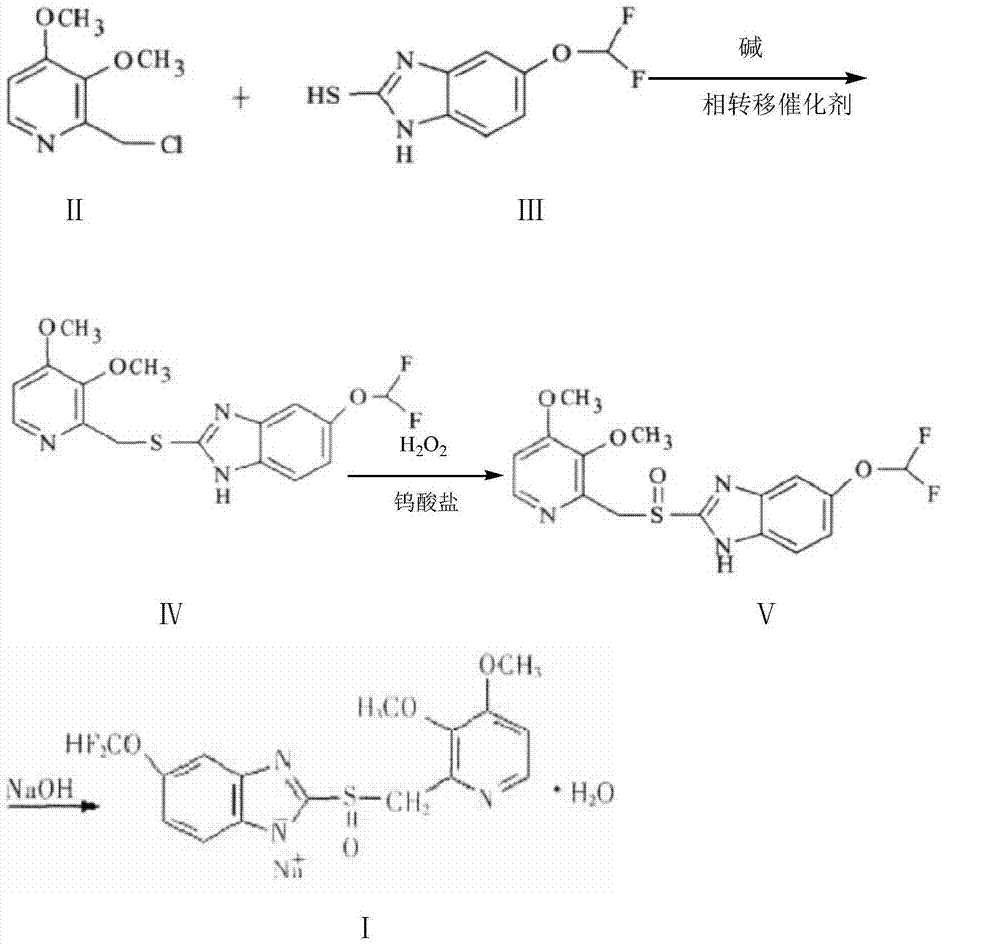
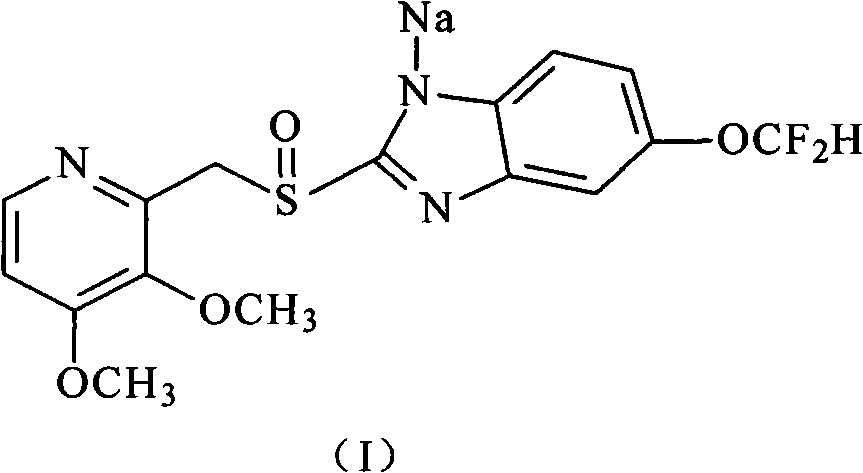
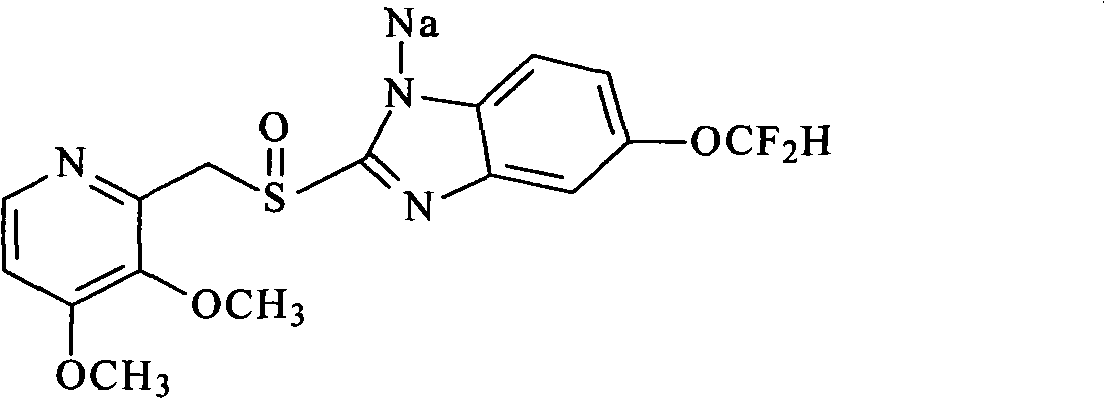
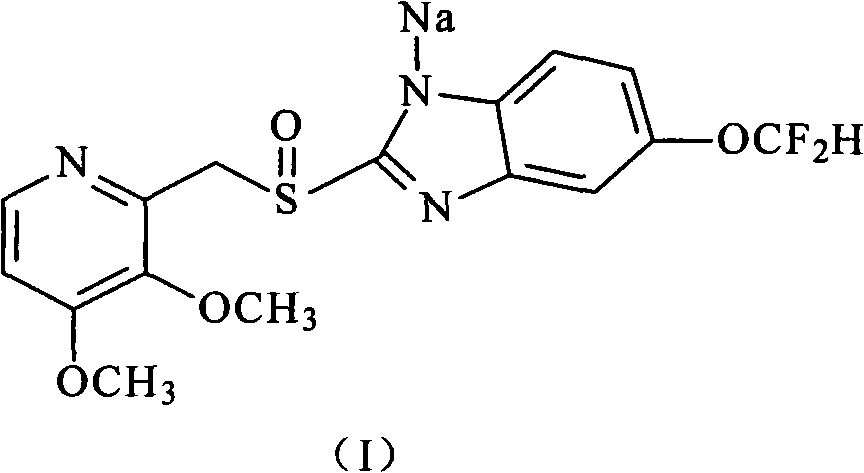
Pantoprazole sodium and preparation method thereof
The invention relates to pantoprazole sodium and a preparation method thereof and in particular relates to a method for preparing the pantoprazole sodium. The preparation method comprises the following steps of: (1) with 2-hydroxymethyl-3,4-dimethoxyl pyridine (II) as a starting material, generating 2-chloromethyl-3,4-dimethoxyl pyridine hydrochloride (III) under the action of chlorides; (2) carrying out condensation on the obtained compound (III) and 5-difluoromethoxyl-2-sulfydryl-1H-benzimidazole in the presence of inorganic base to generate 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfenyl-1H-benzimidazole (IV); (3) oxidizing the obtained compound (IV) by using an oxidant to generate 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfinyl-1H-benzimidazole; (4) enabling the 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfinyl-1H-benzimidazole to react with sodium hydroxide to generate a salt, namely the pantoprazole sodium (I); and optionally (5) refining the prepared pantoprazole sodium. According to the preparation method, the prepared pantoprazole sodium product has high purity.
Owner:CHENGDU TIANTAISHAN PHARMA
Pantoprazole sodium freeze-dried preparation for injection and preparation method thereof
ActiveCN102670524AImprove stabilityWell formedOrganic active ingredientsPowder deliveryFreeze-dryingD-Glucose
The invention relates to a pantoprazole sodium freeze-dried preparation for injection and a preparation method thereof. The freeze-dried preparation is formed by preparing components containing the following parts by weight into liquor for freeze drying after dissolving into water for injection: 60 to 80 parts of pantoprazole sodium, 100 to 300 parts of glycocoll, 50 to 100 parts of glucose and aright amount of sodium hydroxide. According to the pantoprazole sodium freeze-dried preparation for the injection, which is disclosed by the invention, the problem of stability of a preparation is solved; and moreover, an adverse reaction existing in a traditional product is lowered.
Owner:NANJING CHIA TAI TIANQING PHARMA
Pantoprazole sodium liposomes freeze-dry preparations and method of preparing the same
InactiveCN101249073ASolve the problem of quality stabilitySmall toxicityOrganic active ingredientsDigestive systemFreeze-dryingCholesterol
The invention discloses a freeze-dried preparation of pantoprazole sodium liposomes, wherein pantoprazole sodium is encapsulated in antioxidant-containing liposomes made of soybean lecithin and cholesterol. The freeze-dried preparation is administered intravenously with stable quality, less toxicity and high effectiveness.
Owner:HAINAN LINGKANG PHARMA CO LTD
Pantoprazole sodium enteric-coated tablet and preparation method thereof
ActiveCN103006613AImprove stabilityReduced stabilityOrganic active ingredientsDigestive systemAdhesivePantoprazole Sodium
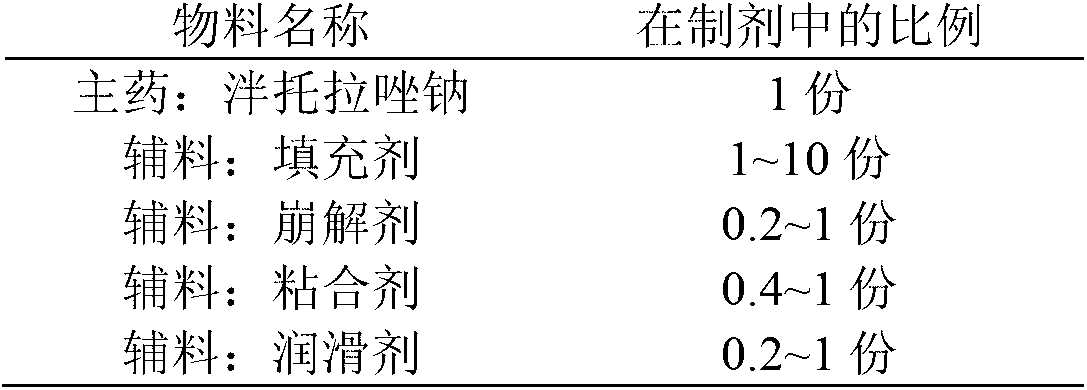
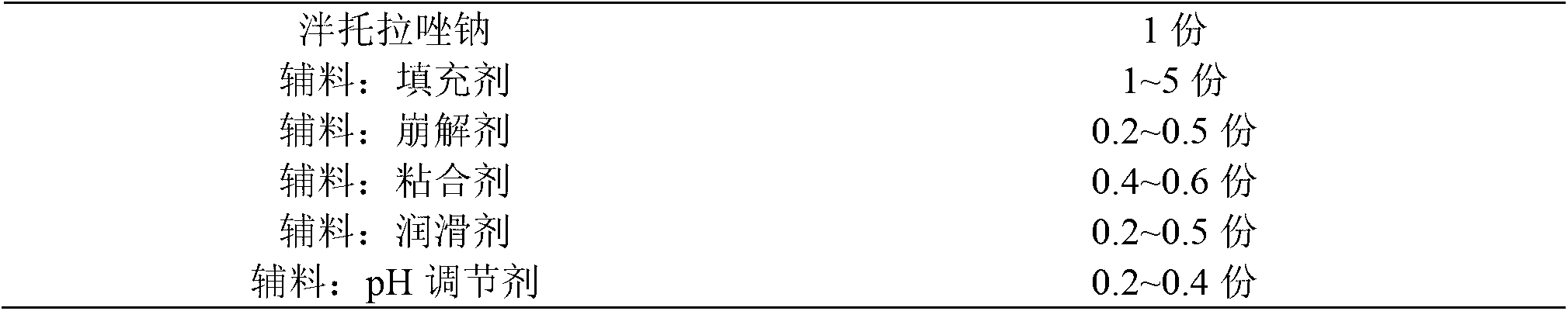
The invention provides a pantoprazole sodium enteric-coated tablet and a preparation method thereof. The pantoprazole sodium enteric-coated tablet comprises a pantoprazole sodium tablet, an isolated layer and an enteric-coated layer, wherein the pantoprazole sodium tablet comprises main drug pantoprazole sodium and auxiliaries; and the auxiliaries include a filler, a disintegrant, a lubricating agent, an adhesive, a pH regulating agent, and the like. The pantoprazole sodium enteric-coated tablet accelerates the disintegration time of the pantoprazole sodium enteric-coated tablet and solves the problem of drug instability during a storage period.
Owner:SHIJIAZHUANG HUAXIN PHARMA
Pantoprazole sodium composition for injection
ActiveCN102225063AWon't releaseAvoid reactionOrganic active ingredientsPowder deliveryForeign matterSide effect
The invention provides a pantoprazole sodium composition for injection. The composition contains pantoprazole sodium and disodium ethylene diamine tetraacetate that are in a proportion of 1:0.02-0.1 by weight. And the composition is prepared by the steps of: 1) liquid medicine preparation: placing pantoprazole sodium and disodium ethylene diamine tetraacetate in a preparation pot, adding water for injection and stirring to make the above agents dissolved and mixed well with the water, adjusting the pH value of the mixture to 10.5-12.5; 2) rubber stopper treatment; 3) aseptic filtration and separate packing; 4) vacuum freeze drying, thus obtaining the composition. For pantoprazole sodium medicaments extremely easy to react with exudates from a rubber stopper, the pantoprazole sodium composition for injection in the invention can simultaneously guarantee the visible foreign matters and insoluble particles in products meeting the requirement of an injection. According to the invention, quality level of the composition product is improved, and the hidden trouble that unqualified visible foreign matters and insoluble particles threat patient clinical medication safety can be avoided. Additionally, the composition of the invention has better curative effects and less clinical side effects.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
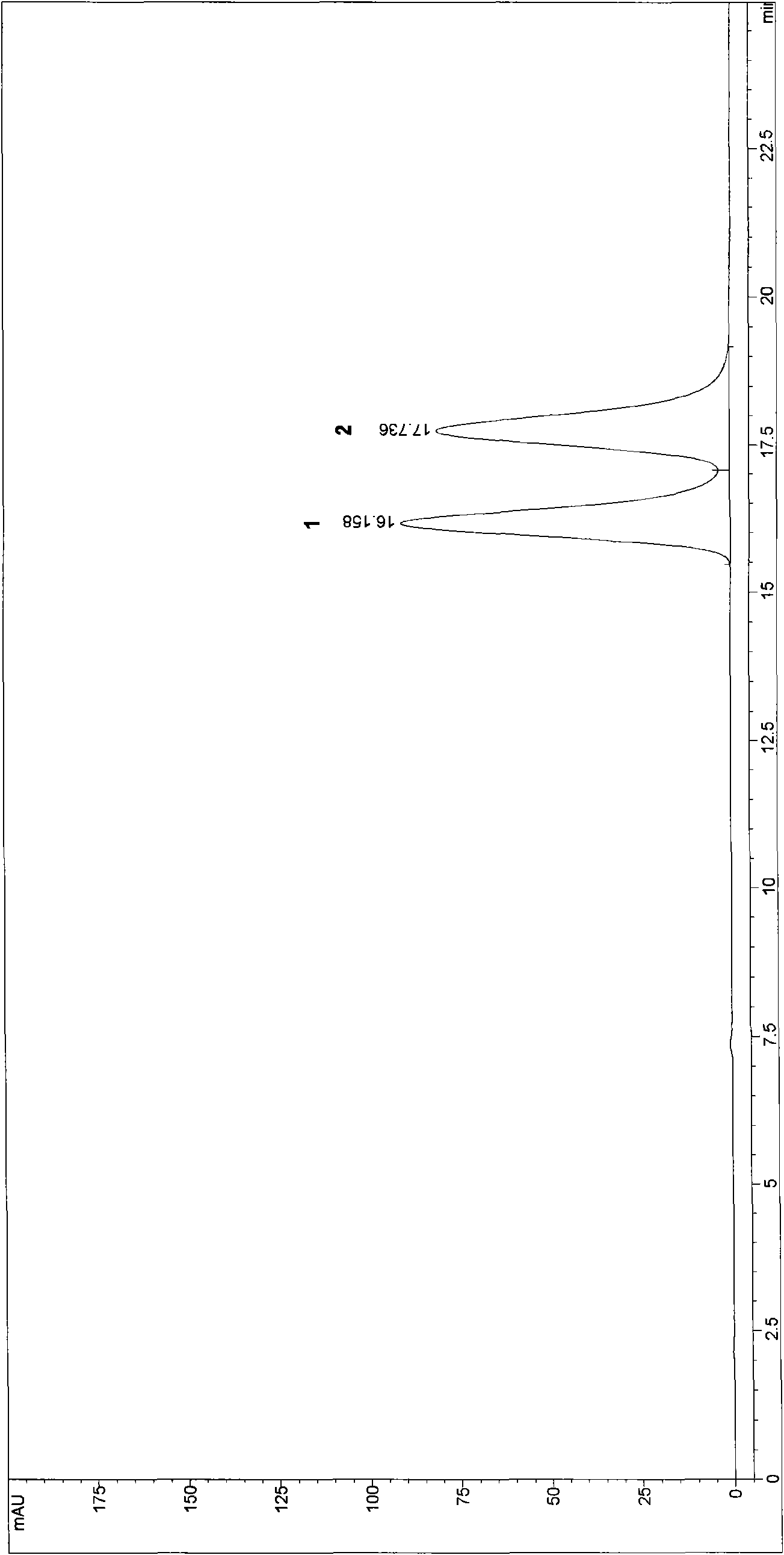
High performance liquid chromatography (HPLC) method for analyzing and separating optical isomer of pantoprazole sodium
InactiveCN102141547AEfficient analytical separationGuaranteed stabilityComponent separationCelluloseCarbamate
The invention relates to a high performance liquid chromatography (HPLC) method for analyzing and separating an optical isomer of pantoprazole sodium. Cellulose 3,5-dimethyl phenyl carbamate chiral column OD-RH (Chiralcel, 150mm*4.6mm, 5 microns) is used, and a mixed solvent of water-acetonitrile in certain proportion is used as a mobile phase for separating and analyzing the optical isomer of the pantoprazole sodium.
Owner:YANGTZE RIVER PHARM GRP CO LTD
An enteric coated mini-pill of pantoprazole sodium
ActiveCN1883460AHigh feasibilityImprove stabilityOrganic active ingredientsDigestive systemCellulosePantoprazole Sodium
The invention discloses pantoprazole sodium enteric pellets which comprise cores containing pantoprazole sodium and enteric coating containing cellulose substances. The invention also discloses the process for preparing the cores. The invention realize higher preparation stability and facilitated mass production.
Owner:HANGZHOU ZHONGMEI HUADONG PHARMA
Pharmaceutical composition of pantoprazole sodium and preparation method thereof
ActiveCN103054797ASpeed up absorption into the bloodReduce dosagePowder deliveryOrganic active ingredientsMANNITOL/SORBITOLGlucose polymers
The invention relates to pantoprazole sodium, and in particular relates to a pharmaceutical composition of pantoprazole sodium. The pharmaceutical composition contains pantoprazole sodium, sodium chloride and an excipient, wherein the weight ratio of pantoprazole sodium to sodium chloride and the excipient is 10:(1-5):(5-20), and preferably 10:(1-3):(8-16); and the excipient is at least one selected from mannitol, glucose, dextran, sorbitol and lactose, and preferably mannitol or sorbitol. The pharmaceutical composition of pantoprazole sodium is low in impurity content and high in stability and is applicable to clinical application.
Owner:HAINAN JINRUI PHARMA CO LTD
Pantoprazole sodium medicinal composition and preparation method thereof
ActiveCN102525960AImprove stabilityLow hygroscopicityPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
Owner:湖北美林药业有限公司
S-pantoprazole sodium composite for injection and preparation method thereof
ActiveCN102000034AImprove uniformityInhibition releasePowder deliveryOrganic active ingredientsEthylenediamineForeign matter
The invention provides an S-pantoprazole sodium composite for injection, which is prepared through a method comprising the following steps of: (1) preparing liquid medicine: selecting S-pantoprazole sodium and edetic acid or edetate as raw materials, wherein the weight ratio of the S-pantoprazole sodium and the edetic acid or the edetate is 1:0.01-0.1; weighing a right amount of the raw materials, placing into a preparation tank, adding water for the injection till the weight of a solution is 11.82-118.2 times more than that of the S-pantoprazole sodium, stirring for dissolution, uniformly mixing, and regulating the pH value of the solution at 10.0-12.5; (2) finishing active carbon; (3) absorbing; (4) carrying out aseptic filtration and split charging; and (5) carrying out vacuum freeze drying so as to obtain the S-pantoprazole sodium composite for the injection. The S-pantoprazole sodium composite for the injection and the preparation method thereof can both ensure that product heat sources, visible foreign matters and insoluble particles meet the requirements of injections for the injections, i.e. S-pantoprazole sodium preparing liquid medicine which has higher pH.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Pantoprazole sodium-containing enteric-coated tablet and preparation method thereof
ActiveCN103933005AReduce typesFacilitated releaseOrganic active ingredientsDigestive systemAlcoholFiller Excipient
The invention discloses a pantoprazole sodium-containing enteric-coated tablet and a preparation method thereof. The enteric-coated tablet is obtained by sequentially coating a tablet core with an isolating coating and an enteric coating, wherein the tablet core is formed by evenly mixing medicine-containing granules with a filler and a lubricant and then directly tabletting, and a preparation method of the medicine-containing granules comprises the following steps of: dissolving pantoprazole sodium into absolute ethyl alcohol, adding polacrilin potassium, drying and volatilizing to remove ethanol, and sieving dried materials. The varieties of auxiliary materials are fewer, the drug release is fast, the stability of the preparation is high, the production technology is simple, no sticking phenomenon exists in tabletting, and the industrial production is easy.
Owner:LIAONING NIRVANA PHARMA
S-pantoprazole sodium freeze-drying medicament composition and preparation method thereof
ActiveCN102688204AControl moisture contentImprove stabilityPowder deliveryOrganic active ingredientsFreeze-dryingPantoprazole Sodium
The invention discloses an s-pantoprazole sodium freeze-drying medicament composition and a preparation method thereof. The preparation method comprises the following steps of: preparing an un-freeze-dried solution from s-pantoprazole sodium, an excipient, a stabilizer and an acid-base regulator; and freeze-drying to obtain a freeze-dried composition with the water content of 10-20%. The freeze-drying medicament composition, prepared by the method disclosed by the invention, has greatly improved stability during storage and transport processes and is conductive to clinical use.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Process for preparing pantoprazole sodium for injection
ActiveCN101961309AIncrease profitReduce manufacturing costOrganic active ingredientsDigestive systemMANNITOL/SORBITOLHigh volume manufacturing
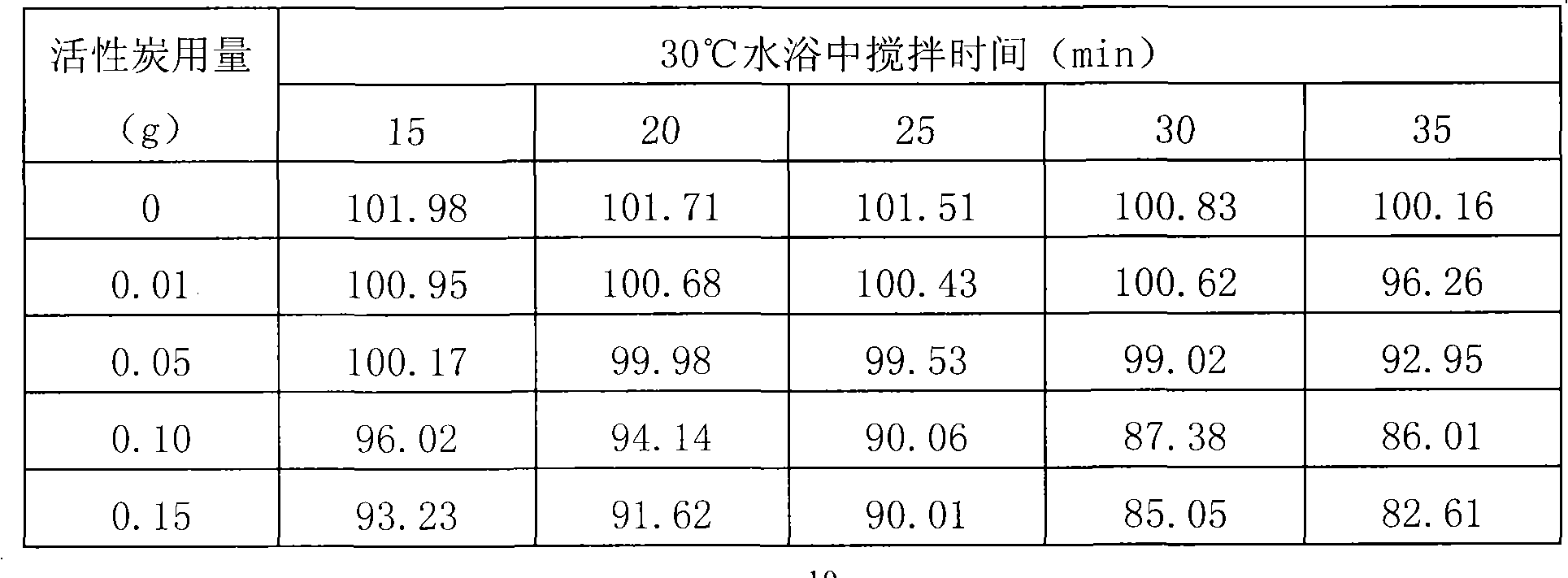
The invention relates to a preparation process (preparation method) for pantoprazole sodium for injection, overcomes the defect of the traditional preparation process for the pantoprazole sodium for injection, and provides a preparation process with low cost, high yield and more stable finished product quality. The process comprises the following steps of: (1) feeding materials according to 100 percent of formula quantity, adding mannitol into injection water in an amount which is about 15 percent of preparation quantity, dissolving the materials with stirring, adding medicinal carbon in an amount which is 0.2 percent (weight / volume) of preparation quantity into the solution, boiling the solution for 30 minutes, removing carbon and filtering the solution, cooling the filtered solution to between 10 and 20 DEG C, and introducing nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the solution for later use; (2) introducing the nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the 10-20 DEG C injection water in an amount which is about 60 percent of preparation quantity, adding the medicinal carbon in an amount which is 0.1 percent (weight / volume) of preparation quantity into the solution, stirring the solution uniformly, standing the solution for 20 minutes, removing carbon and filtering the solution; and (3) mixing the filtered solution, introducing the nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the solution for protecting, adjusting the pH value of the solution to between 10.0 and 10.8 by using 10 percent sodium hydroxide solution, adding 10-20 DEG C injection water to the preparation quantity, stirring the solution uniformly, retesting the pH value which should be between 10.0 and 10.8, filtering the solution by using filters with a 0.45mum filter element and a 0.22mum filter element respectively, and filling the product after the submitted semi-finished products are qualified.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Preparation method of pantoprazole sodium
The invention belongs to the technical field of medicines and particularly relates to a preparation method of pantoprazole sodium. Aiming at operation of condensation, oxidization and salification by adopting a one-pot process in the prior art, a phase transfer catalyst is added into the condensation process, so that the reaction speed and yield are increased. By virtue of catalytic oxidation of hydrogen peroxide in the presence of tungstate, the selectivity of the reaction is improved and the impurities are reduced. The method provided by the invention is simple to operate, the yield and the purity of the product are improved, and the method is suitable for industrial production.
Owner:桂林华信制药有限公司
Compound of pantoprazole sodium and a preparation method thereof
InactiveCN101514198AImprove stabilityImprove clinical efficacyOrganic chemistryDigestive systemPantoprazole SodiumPantoprazole
The invention relates to a compound of pantoprazole sodium and a preparation method thereof, belonging to the technical field of medicines. By the inventive method, the refined product of pantoprazole with high yield above 90% and high purity above 99.8% is obtained, thus improving the stability and clinical application effect of the pantoprazole sodium preparation. The method of the method is simple, easy to operate, and suitable for large industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
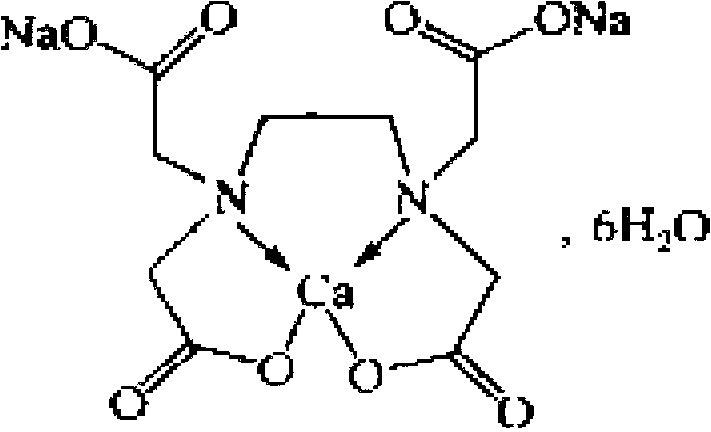
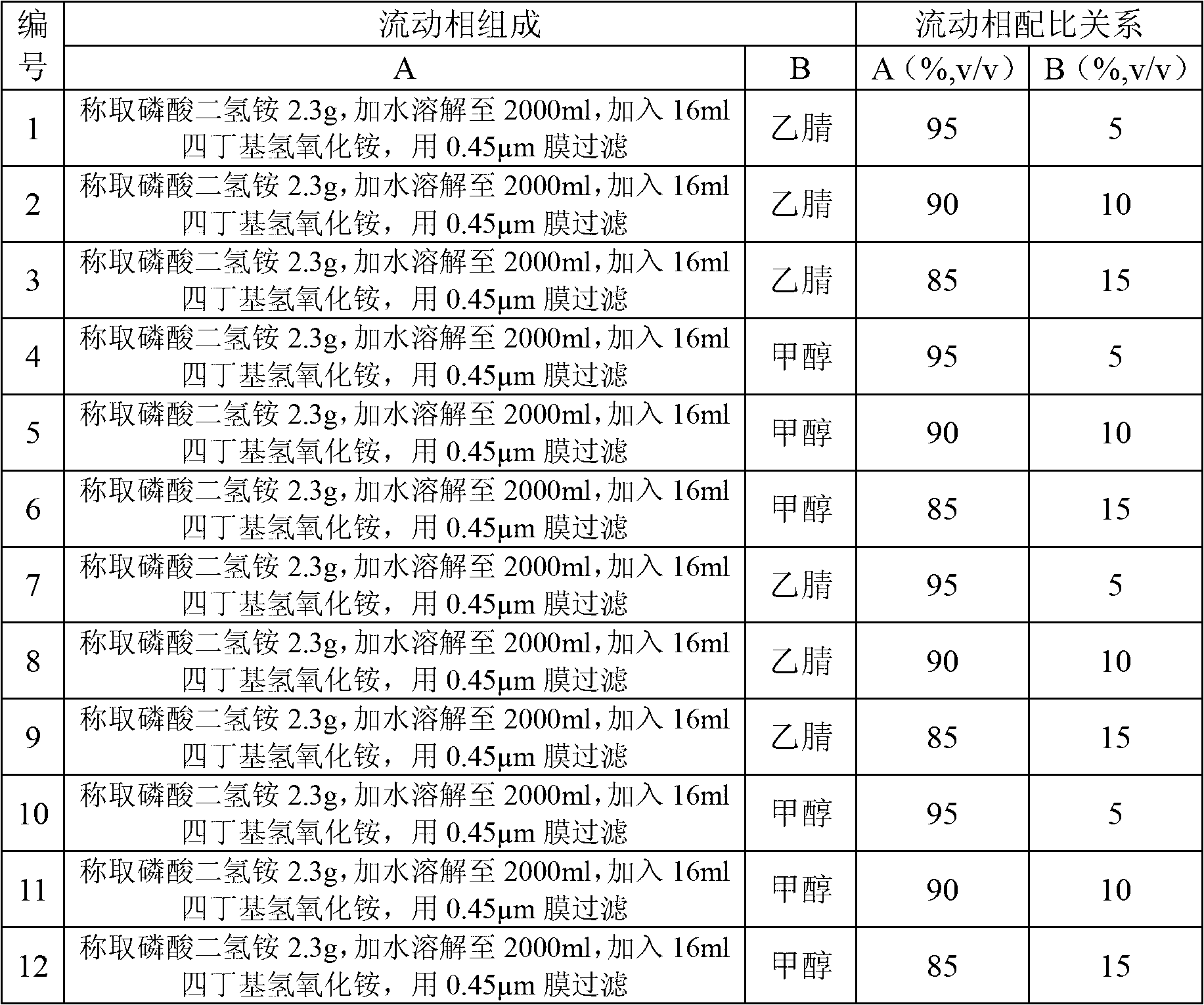
Detection method and content determining method of sodium calcium edetate in pantoprazole sodium for injecting
The invention belongs to the field of medicament analysis and particularly relates to a detection method and a content determining method for sodium calcium edetate as an accessory in pantoprazole sodium for injecting. The invention aims at solving the technical problem of providing a method with the advantages of simpleness in and convenience for operation, quickness and accuracy for detecting the sodium calcium edetate as the accessory in the pantoprazole sodium for injecting. HPLC (High Performance Liquid Chromatography) detection conditions are as follows: a stationary phase: octadecylsilane bonded silica gel is used as a filling agent; a mobile phase: in terms of volume, an ion pair buffer solution is 85-95 percent, acetonitrile is 5-15 percent and the pH value is adjusted to 2.2-2.6 with phosphoric acid; the flowing speed is 0.8-1.2 ml / min; the column temperature is 30-40DEG C; the detection wavelength is 250-260 nm; and the theoretical plate number is required not to be lower than 2000 calculated in terms of a sodium calcium edetate peak. The detection method has the advantages of simple and convenient operation, accurate and reliable determination result, stronger specificity and shorter detection time; and the retention time of a main peak is about 6 minutes.
Owner:CHENGDU BAIYU JINGELAI PHARMA CO LTD
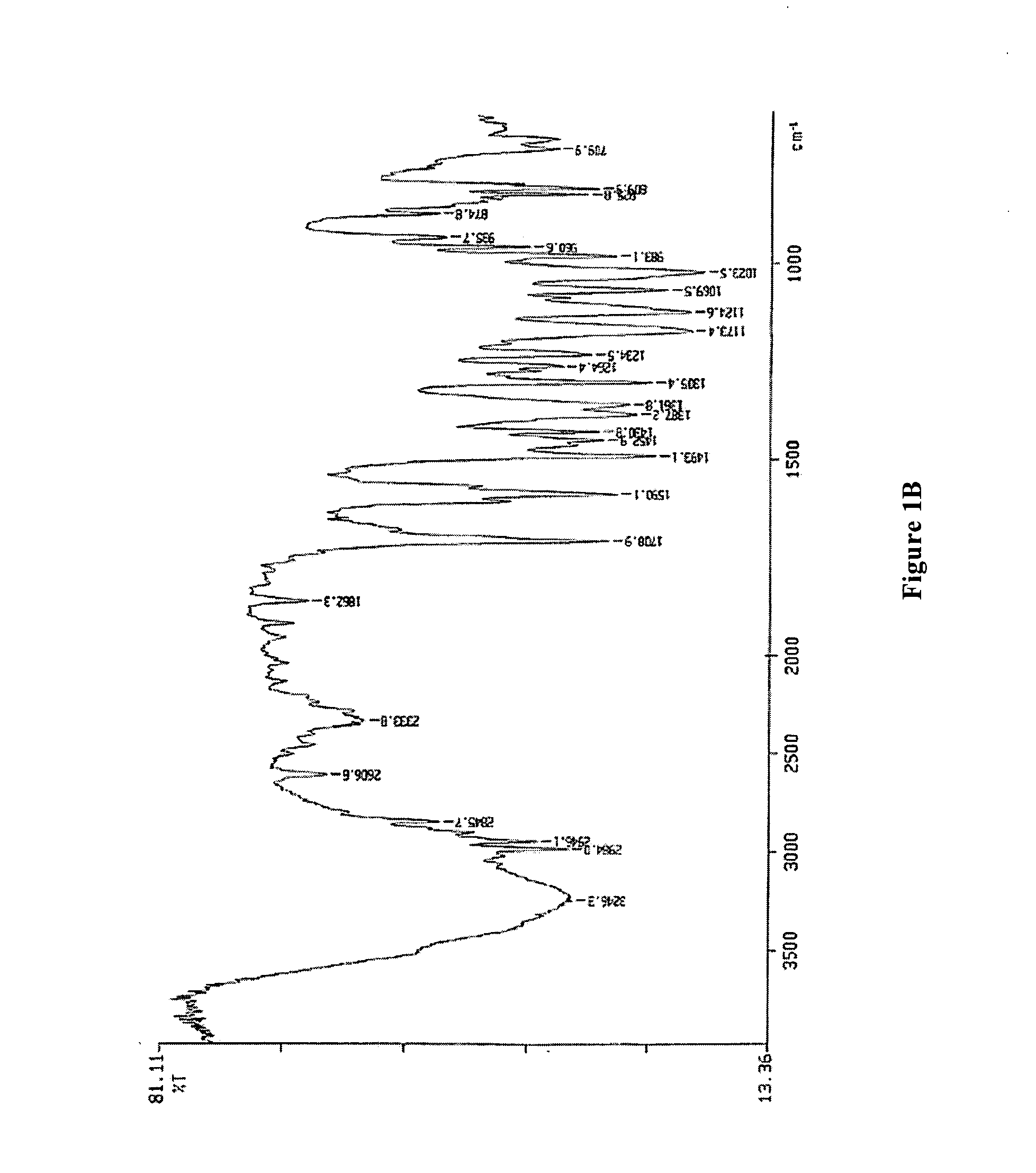
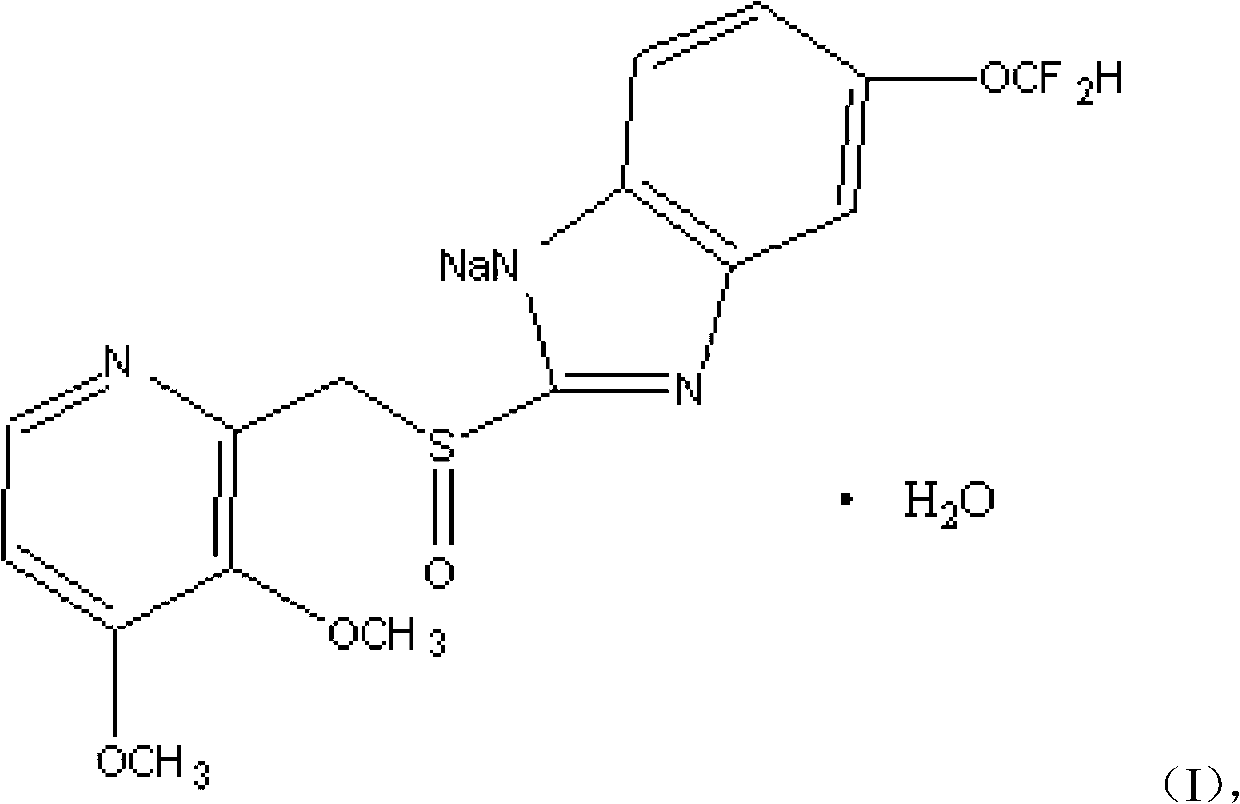
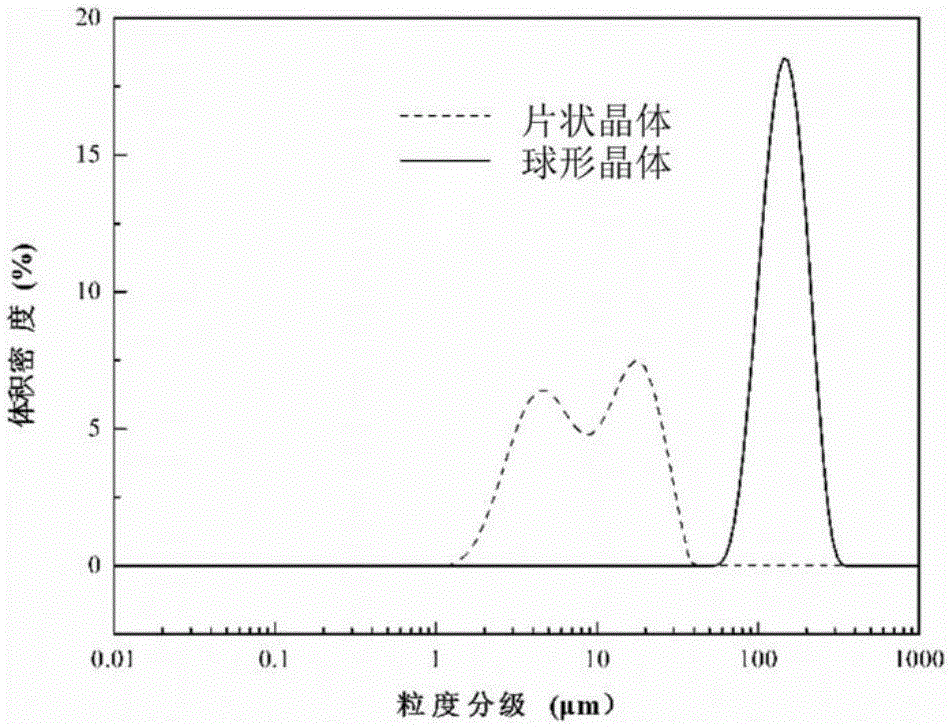
Pantoprazole sodium compound monohydrate sphaerocrystal and preparation method thereof
ActiveCN105111189AIncrease master granularityUniform particle size distributionOrganic active ingredientsOrganic chemistry methodsFiltrationSlurry
The invention relates to a pantoprazole sodium compound monohydrate sphaerocrystal and a preparation method thereof. The average particle size of the crystal is greater than 150 mu m, the coefficient of variation (C.V.) value is less than 0.32, and the bulk density is greater than 0.45 g / ml. The preparation method comprises the following steps: adding a pantoprazole sodium solid into a solvent to prepare a 0.2-0.3 g / ml solution at 40-50 DEG C; cooling the solution under stirring actions, keeping the temperature constant when the temperature drops to 10-25 DEG C, adding a bridging agent, and growing the crystal at constant temperature for 30-60 minutes; continuing cooling to 0-5 DEG C at the same stirring rate, and continuing stirring at the same stirring rate for 30-90 minutes; and carrying out vacuum filtration on the formed crystal slurry, and drying the filter cake at 50-60 DEG C to constant weight, thereby obtaining the pantoprazole sodium monohydrate sphaerocrystal. The method can directly perform tabletting, and is simple and controllable in operating conditions. The single-pass mole yield of the crystallization process is 90% or above, and the product purity is 99% or above.
Owner:TIANJIN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com